Abstract
Cell division is orchestrated by a complex protein network that aims to maintenance of genomic stability. Visualisation of mitotic protein–protein associations in space and time has been limited due to the lack of proper biochemical and easy‐to‐use imaging tools. Here we report adaptation of the in situ proximity ligation assay (is‐PLA) to study mitotic protein interactions with spatio‐temporal resolution. We examined the composition of the Chromosomal Passenger Complex (CPC) at various mitotic phases and after chemical treatments using is‐PLA with antibodies against the core CPC subunits Aurora B, INCENP, Survivin and Borealin. Our results support the notion that the core CPC functions as a single structural unit at centromeres in early mitosis and at central spindle after the onset of anaphase. Treatment of cells with the Aurora B inhibitor ZM447439 diminished the is‐PLA signals at centromeres suggesting that Aurora B activity contributes to structural maintenance and/or proper subcellular localization of the core CPC. Is‐PLA‐based analysis of interaction between INCENP and Polo‐like kinase 1 (Plk1) proposes that the kinase co‐travels with CPC during late mitosis. The data illustrates both the strengths and limitations of the is‐PLA in the analysis of mitotic macromolecule associations at sub‐organelle level.
Keywords: <i>In situ</i> proximity ligation assay, Chromosome passenger complex, INCENP, Aurora B, Plk1
Abbreviations
- CPC
chromosomal passenger complex
- INCENP
inner centromere protein
- is-PLA
in situ proximity ligation assay
- Plk1
Polo-like kinase 1
- RCA
rolling-circle amplification reaction
- SAC
spindle assembly checkpoint
1. Introduction
Cell division is composed of temporal sequence of events driven by a complex protein network that aims to the equal distribution of genomic material between the daughter cells. The process is safeguarded by the spindle assembly checkpoint (SAC), an evolutionarily conserved signalling cascade that monitors attachments between the spindle microtubules and kinetochores of chromosomes (Musacchio and Salmon, 2007). In the presence of attachment errors, SAC delays chromosome segregation until the mistakes are corrected. Failures in SAC signalling can cause loss or gain of chromosomes (aneuploidy) that has been implicated in tumorigenesis (Holland and Cleveland, 2009).
One essential protein compilation working during mitosis is the Chromosomal Passenger Complex (CPC) consisting of four core proteins: the catalytic subunit Aurora B, and three non‐enzymatic subunits Inner Centromere Protein (INCENP), Survivin and Borealin. CPC has been shown to regulate many mitotic events such as chromosome condensation, microtubule‐kinetochore attachments, SAC signalling, and cytokinesis (Jeyaprakash et al., 2007; Tanaka, 2005; Vagnarelli and Earnshaw, 2004). The subcellular targeting, activity, and stability of the CPC is thought to depend on the presence of all core subunits in the complex. Depletion of any core CPC subunit leads to loss of the complex function and mitotic defects (Carmena et al., 2009). CPC contributes to mitotic events mainly via the action of Aurora B on its substrates, such as Hec1 and MCAK (DeLuca et al., 2006; Lan et al., 2004; Tien et al., 2004).
The localization and function of CPC subunits are though to be intimately linked. After establishment of the CPC in early mitotic cells, the complex binds to chromosome arms and inner centromeres to regulate chromosome condensation, microtubule‐kinetochore attachments and SAC signalling. At metaphase‐to‐anaphase transition, CPC subunits co‐translocate to microtubules of the central spindle and later to midbody to control cytokinesis. The composition of the CPC at specific mitotic stages has remained controversial. Previous studies have proposed that besides the core CPC various subcomplexes consisting of Aurora B–INCENP pair (Gassmann et al., 2004), or a ternary complex of Plk1, Aurora B and INCENP (Goto et al., 2006) may exist in mitotic cells. More recent data, based on the crystal structure of CPC, implies that the core CPC subunits are tightly bound together and therefore the complex is believed to operate as a single structural unit (Jeyaprakash et al., 2007).
Here, we employed in situ proximity ligation assay (is‐PLA) to examine whether CPC composition varies from one mitotic phase to another or between different subcellular localizations. Is‐PLA is a novel method for detection of protein–protein interactions based on the antibody‐mediated recognition of target antigens in close proximity (Fredriksson et al., 2002; Gullberg et al., 2003). Is‐PLA was first described as a sensitive mechanism for protein measurement in solution (Fredriksson et al., 2002) and thereafter its use has broadened to various cell biological applications. Is‐PLA enables the detection of protein–protein interactions with high spatio‐temporal resolution making it advantageous compared to standard biochemical methods. Furthermore, is‐PLA is faster and much simpler method compared to other imaging based techniques used for visualisation of protein–protein interactions, such as Förster/Fluorescence Resonance Energy Transfer (FRET). Finally, is‐PLA provides high sensitivity and it is claimed to enable the detection of transient protein–protein interactions at single‐molecule resolution (Gustafsdottir et al., 2005; Söderberg et al., 2006).
2. Results and discussion
Is‐PLA is based on two recognition events aiming to visualise an interaction between two chosen target molecules (Figure 1A). First, the proteins of interest are recognized by two primary antibodies binding to their target epitopes. This is followed by detection of the primary antibodies with proximity probes (is‐PLA probes) composed of secondary antibodies conjugated to connector oligonucleotides (Figure 1a). If the proteins are in close proximity, connector oligonucleotides are linked in a hybridization step by two more added oligonucleotides that are partially complementary to the connectors (Figure 1b). In an following enzymatic ligation step, the two hybridized oligonucleotides form a circular DNA strand that functions as a template in the rolling‐circle amplification reaction (RCA) that produces a long single‐stranded DNA molecule (Figure 1c and d). Lastly, the RCA product is hybridized with fluorescently labelled oligonucleotides (Figure 1e) enabling the microscopic detection of the fluorescent signals arising from the protein–protein interaction (Söderberg et al., 2006, 2008).
Figure 1.
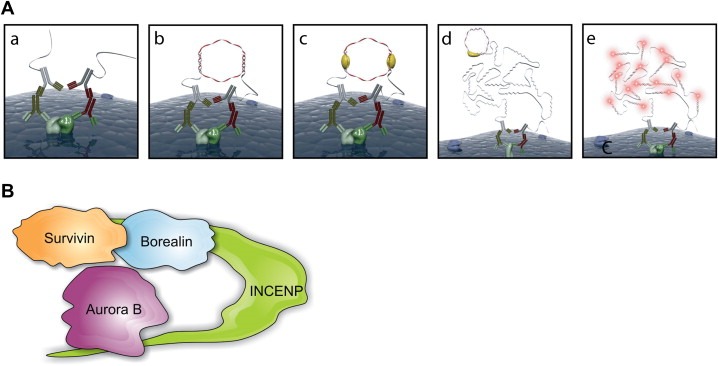
(A) Principle of the is‐PLA (see text for details). Panels (a–e) correspond to the different steps in the in situ proximity ligation protocol (images by courtesy of the Olink AB). (B) Schematic model of the core CPC.
To determine interactions between different CPC subunit pairs (Figure 1B) at various mitotic phases, we utilised is‐PLA with CPC subunit specific antibodies. To verify that the antibodies against Aurora B, Survivin, INCENP and Borealin work as expected, we first visualised the antibody labelling patterns in mitotic cells using standard immunofluorescence method. With each antibody, we detected a punctuated staining pattern on the centromeric region of chromosomes corresponding to the reported localization of these core CPC proteins (Cooke et al., 1987). The Figure 2A shows representative cells stained with anti‐Borealin and anti‐Survivin antibodies. When we performed is‐PLA using anti‐Borealin and anti‐Survivin antibodies, a similar labelling pattern was observed (Figure 2B). We detected comparable is‐PLA signals at centromeres with all tested CPC subunit pairs Aurora B–INCENP, Aurora B–Survivin, Aurora B–Borealin, and Survivin–INCENP (results not shown). INCENP–Borealin pair was not tested due to the overlapping species specificities of the available antibodies. To demonstrate the specificity of the is‐PLA signals, we removed CPC from the centromeres by silencing INCENP (Carmena and Earnshaw, 2003; Honda et al., 2003). As expected, Borealin–Survivin is‐PLA signals (Figure 2B) and those of all other CPC pairs tested (Figure 2D–E) were abolished from the centromeres upon INCENP silencing. As a negative control, we performed is‐PLA on Aurora B–CenpF pair (Figure 2C). Aurora B and CenpF localize to different subregions of the centromere–kinetochore complex (Adams et al., 2001; Liao et al., 1995; Rattner et al., 1993; Zhu et al., 1995) and have not been reported to interact. As expected, no is‐PLA signals were detected for the Aurora B–CenpF pair (Figure 2C). These results demonstrate that the known interactions between CPC proteins can be visualised at the expected subcellular region using the is‐PLA. Analysis of the is‐PLA signals can be performed using various imaging methods including determination of the signal intensities within the target areas and calculation of the number of is‐PLA signals in the target regions. The samples were analysed using Metamorph software for the signal intensities and Blobfinder software (Allalou and Wählby, 2009) that automatically detects and calculates the number of is‐PLA signals in specified areas. In our hands both methods gave similar results indicating loss of CPC subunit is‐PLA signals upon INCENP silencing (Figure 2D–E).
Figure 2.
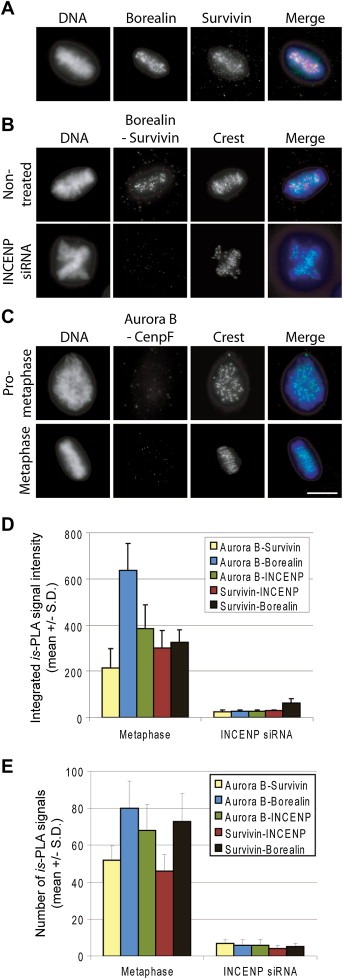
The is‐PLA labelling pattern of CPC subunit pairs in Hela cells. (A) Immunofluorescence labelling pattern of endogenous Borealin and Survivin at metaphase. (B) Borealin–Survivin Is‐PLA signals in non‐treated and INCENP silenced mitotic cells. (C) Aurora B–CenpF is‐PLA signals at prometaphase and metaphase served as a negative control. In the merge panel, is‐PLA signals (red), DAPI stained chromosomes (blue) and kinetochores (Crest marker, green) are shown. The bar equals to 10 μm. Quantification of indicated CPC subunit pair is‐PLA signal intensities (D) and signal numbers (E) from the target areas in control metaphase and INCENP silenced cells (n = 5–6 cells per each group).
Next we investigated if the CPC subunit associations vary between different mitotic phases. In these experiments, we analysed mitotic cells at prophase, prometaphase, metaphase, anaphase and late telophase. We tested all CPC subunit pair combinations, except INCENP–Borealin. Figure 3A shows the is‐PLA labelling pattern of INCENP–Survivin protein pair in the above mentioned mitotic stages. Is‐PLA signals localized to centromeres from the breakdown of the nuclear envelope to the onset of anaphase, and to the spindle midzone and midbody at anaphase and late telophase, respectively. This staining pattern was completely reproducible and identical with the known subcellular localization of the CPC (Carmena et al., 2009). Moreover, the same results were obtained with all tested CPC subunit pairs (data not shown). These results suggest that the core CPC composed of all four core subunits exist throughout mitosis. This does not however exclude the possibility that some subunits could associate with each other outside the core complex. Nevertheless, silencing of INCENP diminished is‐PLA signals from all CPC subunit pairs, which proposes that the cells possess only the core CPC compilations or that formation of separate subunit pairs depends on the presence of intact core CPC.
Figure 3.
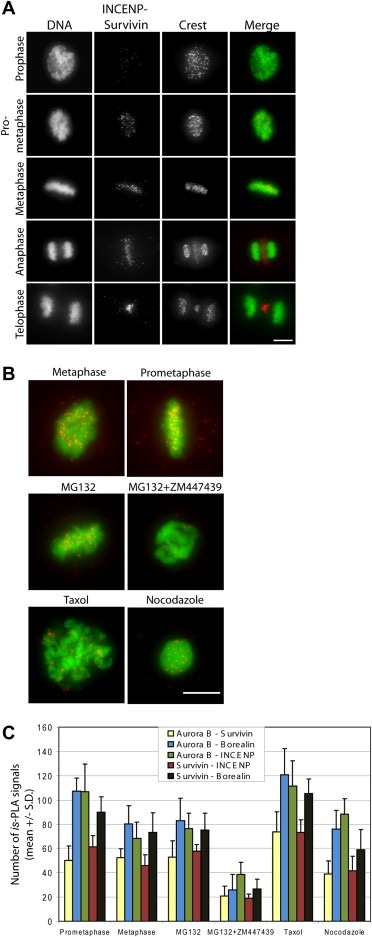
Is‐PLA labelling pattern of INCENP–Survivin pair in different phases of mitosis in Hela cells and after various drug treatments. (A) The INCENP–Survivin pair showed a dotted labelling pattern at centromeres in pre‐anaphase cells and at the spindle midzone in anaphase/telophase cells corresponding to the known subcellular localization of the proteins. In the merge panel, is‐PLA signals (red) and DAPI stained chromosomes (DNA, green) are shown. (B) Overlay images of is‐PLA INCENP–Survivin signals (red) and DAPI stained chromosomes (DNA, green) from non‐treated (metaphase and prometaphase panels) and drug treated mitotic cells. The bars equal to 10 μm. (C) Quantification of the number of is‐PLA signals from Blobfinder analysis of cells subjected to the indicated treatments (n = 6 cells per each group).
In order to test if alterations in Aurora B kinase activity change the binding properties of individual CPC subunits, we suppressed the kinase activity using a small molecule inhibitor ZM447439 (Ditchfield et al., 2003). The drug induces a premature exit from mitosis by interfering with Aurora B dependent SAC signalling. To keep cells in mitosis, the populations were pre‐treated for 1 h with the proteasome inhibitor MG132, which causes cell cycle arrest at metaphase downstream of the SAC. Then the cells were incubated in the presence of ZM447439 for 1 h before fixation and processing for is‐PLA. The samples were analysed using Blobfinder software. Analysis showed that the inhibition of Aurora B kinase activity in early mitotic cells caused a significant reduction in the number of is‐PLA signals from each CPC protein pair examined compared to controls (P < 0.001, Figure 3B and C). To correlate this result with Aurora B protein levels, we first measured anti‐Aurora B antibody signal intensities at centromere regions of chromosomes in cells treated with MG132 or with a combination of MG132 and ZM447439A using the Blobfinder software. The average Aurora B signal intensity at centromeres was reduced by 33.3 ± 17.0% in cells co‐treated with MG132 and ZM447439 compared to MG132 alone (P < 0.05). At the whole cell level, however, the Aurora B protein levels were not significantly altered by the co‐treatment with ZM447439 and MG132 compared to MG132 alone (average Aurora B signal intensity in cells was reduced by 15.8 ± 12.0%, P > 0.05). Together this proposes that in the presence of ZM447439 the kinase is slightly mislocalized from the inner centromeres of pre‐anaphase cells. In our earlier study, we demonstrated that the mobile fraction of INCENP at centromeres was significantly reduced upon Aurora B inhibition (Ahonen et al., 2009) that together with the current observations suggest a role for Aurora B activity in the maintenance of the structural integrity of CPC at centromeres. We also analysed whether hyperactivation of SAC by microtubule interacting drugs nocodazole and taxol affect CPC composition. We detected slightly more is‐PLA signals from CPC associations in cells treated with taxol compared to nonperturbed metaphase cells or nocodazole treated cells (Figure 3B–C, P < 0.01). Interestingly, microtubules have been suggested to contribute to Aurora B kinase activation in vitro (Rosasco‐Nitcher et al., 2008). Therefore our results may reflect a higher activation level of Aurora B kinase in the presence of stabilised microtubules that in turn may be translated into increased number of CPCs in cells. Alternatively, the observed difference may denote better accessibility of the primary antibodies to CPC in the presence of stabilised microtubules.
Finally, we wanted to determine whether more transient mitotic interactions, such as those between INCENP and Polo‐like kinase 1 (Plk1) (Goto et al., 2006), and between Aurora B and its substrates Hec1 (Cheeseman et al., 2002; Tien et al., 2004) and BubR1 (Rancati et al., 2005) can be detected with PLA methodology (Figure 4). The performance of these antibodies was validated in our previous studies (Ahonen et al., 2009; Kukkonen‐Macchi et al., in press). First, we examined the possible interaction between INCENP and Plk1 that has been implicated in metaphase–anaphase transition (Goto et al., 2006). Only very few INCENP – Plk1 is‐PLA signals were present in early mitotic cells proposing that the two proteins interact at low levels in pre‐anaphase cells (Figure 4A). However, after anaphase onset the number and intensity of the INCENP – Plk1 is‐PLA signals increased notably at the central spindle and midbody suggesting more frequent association rate. This result proposes that Plk1 co‐travels with the CPC during late mitosis after it is first recruited to the kinetochore/centromere by INCENP (Goto et al., 2006). Analysis of is‐PLA signals from Aurora B – Hec1 (Figure 4B) and Aurora B – BubR1 protein pairs indicated no signals above the background at any mitotic phase examined. This proposes that certain kinase–substrate associations may be too infrequent in nature to be visualised using is‐PLA.
Figure 4.
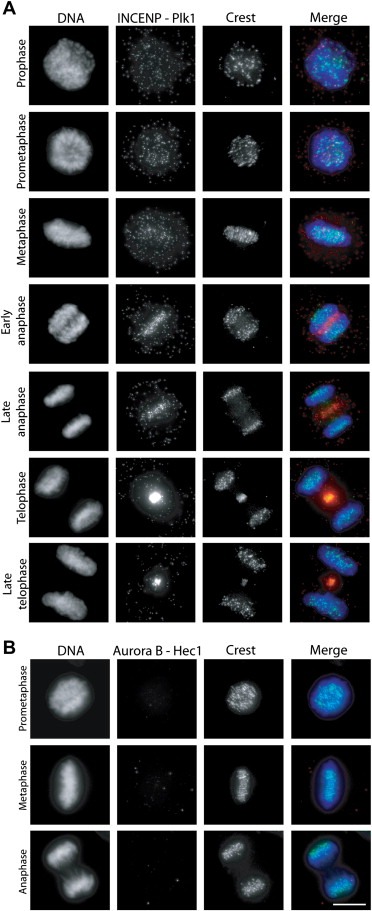
Detection of interactions between CPC subunits and mitotic co‐factors. (A) INCENP–Plk1 interactions visualised using is‐PLA at different mitotic phases. (B) Aurora B–Hec1 interactions visualised using is‐PLA at different mitotic phases. In the merge panels, is‐PLA signals (red), DAPI stained chromosomes (DNA, blue) and kinetochores (Crest marker, green) are shown. The bar equals to 10 μm.
Potential limiting factors of the methodology include the availability and specificity of antibodies against the proteins of interest. Moreover, the protein–protein interaction may mask the one or both of the epitopes of interest used in detection of the PLA signal. Also the sample preparation is a critical step in is‐PLA as the cell permeabilisation, fixation and blocking protocols utilised may influence the relative localization and spacing of proteins, or cause protein conformational changes that shift the relative position of the target epitopes of is‐PLA. Another possible caveat of is‐PLA relates to the production of long RCA chains that do not reside at the exact location of the target proteins, which may hamper use of the method in fine co‐localization studies. Furthermore, the distance requirement to create is‐PLA signals between two target epitopes is relatively long being 10–30 nm (Söderberg et al., 2008). This may allow creation of is‐PLA signals between two adjacent complexes carrying the same target epitopes. In context of CPC analysis this is however very unlikely due to the size of the complex and the fact that all tested CPC subunit pairs produced the same is‐PLA labelling pattern and the various negative controls gave no visible signals.
In summary, we have demonstrated that is‐PLA is a fast and sensitive method to examine relatively stable mitotic protein–protein interactions in space and time via antibody‐mediated recognition of the target proteins. Our results on the composition of the core CPC strengthens the notion that the complex works as a single structural unit throughout mitosis to ensure the fidelity of chromosome segregation and cytokinesis. The data also suggests that the activity of Aurora B kinase is essential for the assembly and/or maintenance of CPC as well as for the proper subcellular localization of the complex. Finally, with the method we were able to get new insights about the known association between Plk1 and INCENP, which is currently being further explored.
3. Materials and methods
3.1. Reagents, cell culture and transfection
Cell culture media, supplements, and chemicals were purchased from Sigma (St Louis, MO) unless stated otherwise. HeLa cells were purchased from ATCC and cultured in DMEM supplemented with 10% fetal bovine serum (Autogen Bioclear, Wiltshire, UK), 20 mM HEPES, 0.1 mM non‐essential amino acids, 1 mM sodium pyruvate, 2 mM l‐glutamine, and 0.1 mg/ml penicillin/streptomycin. Cells were grown at 37 °C in a humidified incubator with 5% CO2. For siRNA transfection, subconfluent Hela cells were transfected with INCENP siRNA using Oligofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. 48 h after transfections the cells were either processed for proximity ligation assay or immunofluorescence.
3.2. Antibodies and chemicals
The following primary antibodies were used at the dilutions indicated: rabbit anti‐Aurora B (1:1000, Abcam, Cambridge, UK), mouse anti‐Aurora B (AIM1) (1:2000, BD Transduction Labs), mouse anti‐Survivin (1:1000, Novus biologicals, Littleton, CO), rabbit anti‐Survivin (1:1000, Abcam), rabbit anti‐INCENP (1:1000, a kind gift from Dr. E. Nigg), rabbit anti‐Borealin (1:1000, a kind gift from Dr. W. Earnshaw), mouse anti‐BuBR1 (1:200, Abcam), mouse anti‐CenpF (1:200, BD Biosciences, San Jose, CA), mouse anti‐Hec1 (1:200, Abcam), human autoimmune serum crest (1:200, Antibodies Inc., Davies, CA) and mouse anti‐Plk1 (1:200, Abcam). Secondary antibodies were Alexa Fluor® antibodies (1:400, Invitrogen) against each species at 488, 555 and 647 nm. Antibodies were diluted in MBST containing 5% boiled normal goat serum (bngs). Nocodazole (Sigma) was used at 3 μM, MG132 at 20 μM, Paclitaxel (Invitrogen) at 0.6 μM and ZM447439 (Tocris Bioscience, Bristol, UK) at 20 μM concentration.
3.3. Immunofluorescence
Hela cells growing on coverslips were fixed and permeabilized with 2% paraformaldehyde and 0.5% Triton X‐100 in PHEM for 15 min. The coverslips were rinsed in 10 mM MOPS, pH 7.4, 150 mM NaCl, 0.05% Tween‐20 (MBST) and blocked in 20% bngs in MBST overnight at +4 °C or 1 h at RT. Cells were immunolabelled with primary and secondary antibodies for 1 h at RT, and DNA was counterstained with DAPI. Coverslips were mounted on microscope slides in Vectashield (Vector laboratories).
3.4. In situ proximity ligation assay
Is‐PLAs were performed according to the Olink Bioscience's protocol using Duolink™ is‐PLA reagents with minor changes. After blocking and primary antibody staining (as in immunofluorescence) the is‐PLA probes anti‐mouse MINUS and anti‐rabbit PLUS were diluted 1/5 in 1× Antibody dilution stock and incubated for 1.5 h at +37 °C in a pre‐heated humidity chamber. Subsequent hybridizations, ligations and detections were performed using Duolink Detection Kit according to the manufacturer's protocol, except final washing step was done 2× 5 min in MBST and 1× 5 min in dH2O.
3.5. Imaging
Fluorescence microscope images were acquired as Z‐stacks using Zeiss Axiovert 200 M microscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany) equipped with a Plan‐Apochromat 63×/1.4 oil objective and Orca‐ER camera (Hamamatsu Photonics Norden, Solna, Sweden). Images were processed with the MetaMorph imaging software (Molecular Devices, Downingtown, PA) and is‐PLA signal dots were counted using Blobfinder image analysis software (The Centre for Image Analysis, Uppsala University, Sweden).
Acknowledgements
We thank Tuomo Hokkanen and Olink Bioscience for assistance in image art preparation. This study was supported by the Academy of Finland grant to M.J.K. (CoE for Translational Genome‐Scale Biology), and by the Finnish Cancer Organisations and the Foundation for the Finnish Cancer Institute. M.V. was also supported by the Turku Graduate School of Biomedical Sciences, University of Turku, Finland. We thank Drs. W. Earnshaw and E. Nigg as well as Olink Bioscience for providing materials for the study. The authors declare no conflict of interest.
Vuoriluoto Mariaana, Laine Leena J., Saviranta Petri, Pouwels Jeroen, Kallio Marko J., (2011), Spatio‐temporal composition of the mitotic Chromosomal Passenger Complex detected using in situ proximity ligation assay, Molecular Oncology, 5, doi: 10.1016/j.molonc.2010.10.002.
References
- Adams, R.R. , Carmena, M. , Earnshaw, W.C. , 2001. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 11, 49–54. [DOI] [PubMed] [Google Scholar]
- Ahonen, L.J. , Kukkonen, A.M. , Pouwels, J. , Bolton, M.A. , Jingle, C.D. , Stukenberg, P.T. , Kallio, M.J. , 2009. Perturbation of Incenp function impedes anaphase chromatid movements and chromosomal passenger protein flux at centromeres. Chromosoma. 118, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allalou, A. , Wählby, C. , 2009. BlobFinder, a tool for fluorescence microscopy image cytometry. Comput. Methods Programs Biomed. 94, 58–65. [DOI] [PubMed] [Google Scholar]
- Carmena, M. , Earnshaw, W.C. , 2003. The cellular geography of aurora kinases. Nat. Rev. Mol. Cell Biol. 4, 842–854. [DOI] [PubMed] [Google Scholar]
- Carmena, M. , Ruchaud, S. , Earnshaw, W.C. , 2009. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr. Opin. Cell Biol. 21, 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman, I.M. , Anderson, S. , Jwa, M. , Green, E.M. , Kang, J. , Yates, J.R. , Chan, C.S. , Drubin, D.G. , Barnes, G. , 2002. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 111, 163–172. [DOI] [PubMed] [Google Scholar]
- Cooke, C.A. , Heck, M.M. , Earnshaw, W.C. , 1987. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J. Cell Biol. 105, 2053–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca, J.G. , Gall, W.E. , Ciferri, C. , Cimini, D. , Musacchio, A. , Salmon, E.D. , 2006. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 127, 969–982. [DOI] [PubMed] [Google Scholar]
- Ditchfield, C. , Johnson, V.L. , Tighe, A. , Ellston, R. , Haworth, C. , Johnson, T. , Mortlock, A. , Keen, N. , Taylor, S.S. , 2003. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161, 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson, S. , Gullberg, M. , Jarvius, J. , Olsson, C. , Pietras, K. , Gustafsdottir, S.M. , Ostman, A. , Landegren, U. , 2002. Protein detection using proximity-dependent DNA ligation assays. Nat. Biotechnol. 20, 473–477. [DOI] [PubMed] [Google Scholar]
- Gassmann, R. , Carvalho, A. , Henzing, A.J. , Ruchaud, S. , Hudson, D.F. , Honda, R. , Nigg, E.A. , Gerloff, D.L. , Earnshaw, W.C. , 2004. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 166, 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, H. , Kiyono, T. , Tomono, Y. , Kawajiri, A. , Urano, T. , Furukawa, K. , Nigg, E.A. , Inagaki, M. , 2006. Complex formation of Plk1 and INCENP required for metaphase–anaphase transition. Nat. Cell Biol. 8, 180–187. [DOI] [PubMed] [Google Scholar]
- Gullberg, M. , Fredriksson, S. , Taussig, M. , Jarvius, J. , Gustafsdottir, S. , Landegren, U. , 2003. A sense of closeness: protein detection by proximity ligation. Curr. Opin. Biotechnol. 14, 82–86. [DOI] [PubMed] [Google Scholar]
- Gustafsdottir, S.M. , Schallmeiner, E. , Fredriksson, S. , Gullberg, M. , Söderberg, O. , Jarvius, M. , Jarvius, J. , Howell, M. , Landegren, U. , 2005. Proximity ligation assays for sensitive and specific protein analyses. Anal. Biochem. 345, 2–9. [DOI] [PubMed] [Google Scholar]
- Holland, A.J. , Cleveland, D.W. , 2009. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat. Rev. Mol. Cell Biol. 10, 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, R. , Korner, R. , Nigg, E.A. , 2003. Exploring the functional interactions between Aurora B, INCENP, and Survivin in mitosis. Mol. Biol. Cell. 14, 3325–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash, A.A. , Klein, U.R. , Lindner, D. , Ebert, J. , Nigg, E.A. , Conti, E. , 2007. Structure of a Survivin–Borealin–INCENP core complex reveals how chromosomal passengers travel together. Cell. 131, 271–285. [DOI] [PubMed] [Google Scholar]
- Kukkonen-Macchi, A., Sicora, O., Kaczynska, K., Oetken-Lindholm, C., Pouwels, J., Laine, L., Kallio, M.J. Loss of p38γ MAPK induces pleiotropic mitotic defects and massive cell death. J. Cell Science, in press. [DOI] [PubMed]
- Lan, W. , Zhang, X. , Kline-Smith, S.L. , Rosasco, S.E. , Barrett-Wilt, G.A. , Shabanowitz, J. , Hunt, D.F. , Walczak, C.E. , Stukenberg, P.T. , 2004. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 14, 273–286. [DOI] [PubMed] [Google Scholar]
- Liao, H. , Winkfein, R.J. , Mack, G. , Rattner, J.B. , Yen, T.J. , 1995. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J. Cell Biol. 130, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio, A. , Salmon, E.D. , 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8, 379–393. [DOI] [PubMed] [Google Scholar]
- Rancati, G. , Crispo, V. , Lucchini, G. , Piatti, S. , 2005. Mad3/BubR1 phosphorylation during spindle checkpoint activation depends on both Polo and Aurora kinases in budding yeast. Cell Cycle. 4, 972–980. [DOI] [PubMed] [Google Scholar]
- Rattner, J.B. , Rao, A. , Fritzler, M.J. , Valencia, D.W. , Yen, T.J. , 1993. CENP-F is a ca. 400 kDa kinetochore protein that exhibits a cell-cycle dependent localization. Cell Motil. Cytoskeleton. 26, 214–226. [DOI] [PubMed] [Google Scholar]
- Rosasco-Nitcher, S.E. , Lan, W. , Khorasanizadeh, S. , Stukenberg, P.T. , 2008. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science. 319, 469–472. [DOI] [PubMed] [Google Scholar]
- Söderberg, O. , Gullberg, M. , Jarvius, M. , 2006. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods. 3, 995–1000. [DOI] [PubMed] [Google Scholar]
- Söderberg, O. , Leuchowius, K.J. , Gullberg, M. , Jarvius, M. , Weibrecht, I. , Larsson, L.G. , Landegren, U. , 2008. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods. 45, 227–232. [DOI] [PubMed] [Google Scholar]
- Tanaka, T.U. , 2005. Chromosome bi-orientation on the mitotic spindle. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 360, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien, A.C. , Lin, M.H. , Su, L.J. , Hong, Y.R. , Cheng, T.S. , Lee, Y.C. , Lin, W.J. , Still, I.H. , Huang, C.Y. , 2004. Identification of the substrates and interaction proteins of aurora kinases from a protein–protein interaction model. Mol. Cell. Proteomics. 3, 93–104. [DOI] [PubMed] [Google Scholar]
- Vagnarelli, P. , Earnshaw, W.C. , 2004. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 113, 211–222. [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Mancini, M.A. , Chang, K.H. , Liu, C.Y. , Chen, C.F. , Shan, B. , Jones, D. , Yang-Feng, T.L. , Lee, W.H. , 1995. Characterization of a novel 350-kilodalton nuclear phosphoprotein that is specifically involved in mitotic-phase progression. Mol. Cell. Biol. 15, 5017–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]


