Abstract
Vitamin D is a fat‐soluble steroid hormone, which is essential to health and for which epidemiological studies suggest a role in autoimmune disease, infections, cardiovascular disease and cancer. It is ingested in foods such as oily fish and supplements, so that average levels vary between countries, but most individuals worldwide make most of their vitamin D as a result of the effects of sun exposure on the skin. Many studies in different populations around the world have in recent years shown that sub‐optimal levels of vitamin D (<70 nmol/L) are common. A series of epidemiological studies have suggested that low vitamin D levels increase the risk of cancers, particularly of the breast and gastrointestinal tracts, so that there has been much interest in understanding the effects of vitamin D on cancer cells. Vitamin D binds to the vitamin D receptor (VDR) resulting in transcription of a number of genes playing a role in inhibition of MAPK signalling, induction of apoptosis and cell‐cycle inhibition, and therefore vitamin D has anti‐proliferative and pro‐apoptotic effects in cells of many lineages. It also has suppressive effects on adaptive immunity and is reported to promote innate immunity. Here we review data on vitamin D and melanoma. There are in vitro data, which suggest that vitamin D has the same anti‐proliferative effects on melanoma cells as have been demonstrated in other cells. We have reported data to suggest that vitamin D levels at diagnosis have a role in determining outcome for melanoma patients. There is a curious relationship between melanoma risk and sun exposure where sunburn is causal but occupational sun exposure is not (at least in temperate climes). Seeking to understand this, we discuss data, which suggest (but by no means prove) that vitamin D might also have a role in susceptibility to melanoma. In conclusion, much remains unknown about vitamin D in general and certainly about vitamin D and melanoma. However, the effects of avoidance of suboptimal vitamin D levels on cancer cell proliferation are likely to be beneficial to the melanoma patient. The possible results of high vitamin D levels on the immune system remain unclear however and a source of some concern, but the data support the view that serum levels in the range 70–100 nmol/L might be a reasonable target for melanoma patients as much as for other members of the population.
Keywords: Melanoma, Vitamin D, Cancer cell proliferation, Prevention
1. Vitamin D and health
Vitamin D is a fat‐soluble steroid hormone, which binds to a receptor (the vitamin D receptor, VDR) via which it mediates its genetic effects (Reichrath et al., 2007a). Vitamin D has additional rapid action effects, which are mediated by effects on signalling pathways. Although much is now known about the role of vitamin D in determining health and the means by which it does so, much remains to be understood. This review takes an overview of what is known about vitamin D and health, as a background to considering the very much smaller amount of data on vitamin D and melanoma. Egan has discussed some of the limited data on vitamin D and melanoma (Egan, 2009).
It has long been known that vitamin D is crucial to bone health (the “classical” effects) but more recently it has been recognized to have pleiotropic effects, and is now held to play a role in autoimmune diseases, cardiovascular disease, susceptibility to infections and cancer and even in physiological ageing in animal studies (Keisala et al., 2009). Its pleiotropic effects are suggested perhaps by the work of Autier et al. who reported evidence of reduced overall mortality in individuals who took part in 18 randomized clinical trials of vitamin D supplementation (Autier and Gandini, 2007).
There are dietary sources of vitamin D (predominantly oily fish): it is postulated that the vitamin D originally derives primarily from synthesis by plankton, and is then concentrated by fish in the food chain (Holick, 2003). The content of vitamin D in fish therefore varies considerably so that there are, for example, high levels in oriental tuna and low levels in sole liver (Holick, 2003). Dietary sources also include supplements such as multivitamins or cod liver oil, and in some countries such as the USA (Holick, 2003), foods may be fortified.
Rickets, which resulted from vitamin D deficiency in industrial countries, has been recognized since the 17th century (Holick, 1981), and in the 19th century was known as the “English Disease”. It was recognized in the 1920s that rickets was caused by vitamin D deficiency and the condition was so common in the USA during the 1930s and 1940s, that many foods were fortified with synthetic vitamin D; even foods such as hot dogs, sodas, bread and beer (Holick, 2003). Synthetic vitamin D is produced by irradiation of yeasts (vitamin D2) or lanolin (vitamin D3). After World War II, vitamin D fortification was not sufficiently monitored and an outbreak of vitamin D intoxification in children, leading to hypercalcaemia, lead to the abandonment of fortification in many European countries (Holick, 2004). Rickets started to recur, in very recent years, especially in darker‐skinned peoples living in less sunny countries, contributing to a resurgence of interest in the role of vitamin D in skeletal health and indeed in health generally.
As national diets vary considerably, the dietary contribution to serum levels is very variable. In countries where oily fish are a large part of the diet such as in Scandinavia and Japan, higher levels are expected, particularly compared with many other countries such as Scotland and Spain where intake is low. Even within countries however there is much variation in dietary patterns, understanding and assessing variation may be difficult: wild salmon, for example, is said to have higher levels of vitamin D than does farmed salmon. The amount of vitamin D available from diet is also influenced by the preparation of the food, with smoked herring containing approximately 4 μg (160 IU) per 100 g and raw herring 40 μg (1600 IU) per 100 g. As mentioned above, food fortification policies also differ considerably between countries. Thus in many countries such as the UK, the diet is profoundly lacking in vitamin D.
Vitamin D supplementation has been shown to increase serum vitamin D levels as we reported in a study of melanoma cases (Newton‐Bishop et al., 2009), but compliance with taking vitamin supplements is only moderate (48–95%) even in the controlled setting of randomized control trials (RCT) (Autier and Gandini, 2007). A 2009 study suggested that although supplements and fortified food explained some of the differences in intake between populations, they contributed relatively little to overall intake compared with the usual diet (Flynn et al., 2009). Flynn et al reported expected variation in levels between European countries with different diets. So, for high vitamin D consumers at the 95th percentile within each country, dietary intake ranged from 3.1 μg/d (<150 IU, Spain) to 17.3 μg/d (approx 750 IU, Finland) in men, and from 2.4 μg/d (100 IU, Spain) to 10.5 μg/d (400 IU, Finland) in women. There was no suggestion of excessively high levels in people with relatively high levels of intake in the diet who were also taking supplements. The higher levels observed in Finland, especially in men, were associated with a high‐fish diet and vitamin D fortification of milk, which is semi‐mandatory there (Flynn et al., 2009), but even in a Scandinavian country like Finland where the local diet is traditionally rich in oily fish the vitamin D daily intake (if taken as 400 IU) was still only sufficient in 5% of Finnish women (Flynn et al., 2009).
The principal source of vitamin D for most humans is therefore derived from a pro‐vitamin in the epidermis, called 7‐dehydrocholesterol, which requires photoactivation by ultraviolet B (UVB) in the range 290–315 nm (Holick, 2003). The skin is both the site of crucial steps in the synthesis of active metabolites of vitamin D, and a target organ, where it regulates growth and differentiation of cells (Reichrath, 2007b). In the winter however, less UVB penetrates the ozone layer so that beyond a latitude of 35°, little vitamin D synthesis occurs at that time of the year (Holick, 2003). In northern climes such as Canada, the period of time during which vitamin D synthesis does not occur is protracted: from October to March (Cantor and Shinohara, 2009). Following photoactivation, a series of further modifications occur in the skin, the liver and then the kidney to form hormonally active calcitriol (1α,25‐dihydroxyvitamin D3) (Figure 1). The precursors are transported between the skin and the liver, the kidney and other tissues by the carrier protein, vitamin D binding protein. Tissues other than the kidney are known also to be capable of the last hydroxylation step (Reichrath, 2007b) to produce calcitriol. Levels of calcitriol are closely regulated via this activation process but also by inactivation processes in which the cytochrome P450 isoform CYP24A1 plays a major part. Levels of CYP24A1 also vary considerably between cells of different lineage, so that in keratinocytes for example they are high and the enzyme results in limitation of vitamin D levels in the skin whereas the level of CYP24A1 is low in macrophages (Bikle, 2009). The CYP24A1 gene is very responsive to VDR regulation and it is suggested that variation in transcription of CYP24A1 between cells will modulate variation in the effects of vitamin D between cells.
Figure 1.
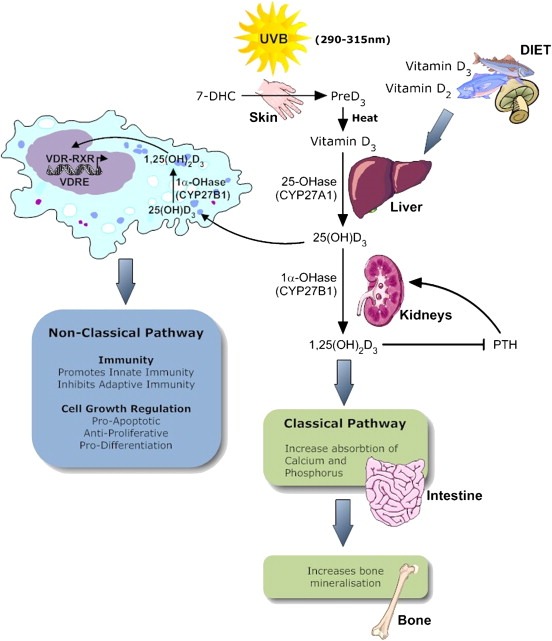
Vitamin D Metabolism and Biological effects. Sun exposure (UVB) results in photoactivation of 7‐dehydrocholesterol to pro‐D3(cholecalciferol), then heat results in production of Vit D3. Endogenous and dietary Vit D3 along with dietary D2 (ergocalciferol) are metabolized by the liver and kidney to 1,25(OH)2D3 (1,25 dihydrocholecalciferol) via the enzymes 25‐OHase (25‐hydroxylase) and 1αOHase (1α‐hydroxylase) encoded by the genes CYP27A1 and CYP27B1 respectively. 1,25(OH)2D3 has a negative feedback on PTH secretion and it's classical effects are on intestinal absorption and bone mineralization. Many cells have the ability to synthesis 1,25 dihydrocholecalciferol locally, resulting in possible apocrine and paracrine non‐classical effects on a variety of tissue and immune cells. Vitamin D forms a heterodimer with retinoid X receptor and binds to the nuclear vitamin D receptor (VDR) resulting in effects on gene expression.
We will refer henceforth to 1α,25‐dihydroxyvitamin D3, as “vitamin D”.
2. Cellular effects of vitamin D
Vitamin D has non‐genetic and genetic effects and, as above, the latter are mediated by binding to the vitamin D receptor (VDR), which is predominantly nuclear and is a member of the nuclear receptor superfamily. It is thought that interaction of the genetic and non‐genetic pathways occurs, but the non‐genetic effects are less well understood, and interpretation of published data is made even more complex by the presence of tissue‐specific variation in cellular responses to vitamin D (Campbell et al., 2010).
When vitamin D binds to the VDR, conformational changes occur in the ligand‐binding domain which result in modification of co‐regulatory molecules (such as DRIP, SRC, hairless and β‐catenin in the skin (Bikle, 2010)), and ultimately in both modification of chromatin and transcription. Transcription generally requires that the activated VDR binds to so‐called vitamin D response elements (VDREs) in target genes. The VDR forms a heterodimer, especially with the retinoid X receptor (RXR), which also binds to 9‐cis‐retinoic acid, a metabolite of vitamin A present in the serum.
The downstream target genes of VDR are many and therefore vitamin D has pleiotropic effects. Gene expression pathways have been generated in different cell types but the data are still relatively limited (Fleet, 2008). Vitamin D target genes include genes that may be upregulated, such as those coding for osteopontin, RANKL, Calbindin‐9k, IGFBP and β3 integrin, and others that are downregulated (including interleukin genes IL1 and IL12, TNFa, IFN‐γ and EGF‐R) (Huotari and Herzig, 2008) and TERT (telomerase) (Hansen et al., 2001). Other authors have listed other signalling pathways downstream of the VDR (Hansen et al., 2001; Seifert et al., 2004), reviewed by Deeb et al. (2007). The literature is large and this review will not attempt to summarise the findings other than to comment that vitamin D appears to inhibit proliferation of cells, to increase apoptosis and to increase differentiation of cells in a number of ways. The review by Deeb et al. (2007) summarises the genetic and non‐genetic effects as follows: “vitamin D appears to inhibit MAPK signalling through suppression of the epidermal growth factor receptor pathway (EGFR) (a pathway that is upregulated in melanoma), and insulin‐like growth factor. It induces apoptosis via the IGFR1/PI3K‐Akt signalling pathway and by inhibiting telomerase. It increases apoptosis by downregulating BCL2, inducing BAX and activating caspase cleavage and it results in perturbation of cell‐cycle progression by targeting p27 for degradation and via MYC. It increases the expression of E‐cadherin, which ultimately leads to repression of the proto‐oncogene of MYC”. The pathways targeted by vitamin D are therefore those known to be activated in melanoma (and in many cancers) so these data provide support for the view that vitamin D might have an anti‐cancer effect on melanoma cells.
Further evidence that vitamin D might have an anti‐cancer effect for melanoma would result from demonstration of changes in expression of genes downstream of vitamin D which are anti‐proliferative or pro‐apoptotic in an in vitro model. The genes which were demonstrated to show most upregulation in prostate cancer cells after incubation with vitamin D were CYP24A1, IGFBP‐3 (which encodes insulin growth factor binding protein 3) and DUSP10 (dual specificity phosphatase 10), which inactivates MAPK signalling and thereby may mediate in part the anti‐proliferative effects of vitamin D (Krishnan et al., 2003). Apart from Reichrath's work showing induction of CYP24A1 mRNA in melanoma cell lines in response to vitamin D (discussed later), we are not aware of other similar work done specifically in melanoma cells as yet.
Animal studies of VDR knockout mice showed that the mice were smaller than the controls. The older VDR knockout mice also had a reduced lifespan, wrinkled skin (probably due to reduced fat deposits), vestibular problems (Keisala et al., 2009), and reduced expression of NF‐κB, FGF‐23, p53, klotho and IGF1R.
The “classical” targets of vitamin D are osteoblasts, cells of the small intestine and kidney cells, so that for many years the only significant effects of vitamin D deficiency were thought to be on the bones (Figure 1). In more recent times however, additional major affects have been established, including anti‐proliferative effects on cells and effects on the immune system. The possible relationship between vitamin D levels and melanoma may relate to these effects.
The anti‐proliferative effects of vitamin D have been demonstrated in vitro in cells of many types such that vitamin D analogues have been used to treat a number of cancers, albeit with as yet limited success. In skin diseases characterized by immunologically driven hyperproliferation such as psoriasis however vitamin D analogues such as calcipotriene have proved effective and are widely used. It has proved more difficult to use vitamin D analogues effectively to treat cancer partly because of hypercalcaemic toxicity of high doses of vitamin D analogues, but also at least in part postulated to be because of genetic changes in the cancer cells which reverse its anti‐proliferative effects (Holick, 2008).
3. Vitamin D and the immune system
A number of different immuno‐competent immune cells express the VDR such as activated T‐ and B‐lymphocytes and dendritic cells, and it is known that vitamin D plays a role in macrophage function (Holick, 2008). Overall, vitamin D is thought to inhibit adaptive immunity and to promote innate immunity (Bikle, 2009). The incidence of a number of diseases postulated to be immunologically mediated appears to be negatively correlated with sunlight exposure, such as multiple sclerosis (Smolders et al., 2008). These epidemiological observations lead to the suggestion that vitamin D may play a role in autoimmune diseases. The immune effects of vitamin D appear to be complex but many appear to result in suppression of adaptive immune responses, particularly of the T helper (Th1)‐driven immune response (Froicu et al., 2006), probably mediated in part by reduced antigen presentation by dendritic cells (Enioutina et al., 2009). Moreover, there are data to suggest that vitamin D, at least in VDR knockout mice, reduces the Th1 activation in proportion to Th2 responses (Cantorna et al., 2004). Vitamin D also appears to increase regulatory T cells (Tregs) (Urry et al., 2009) with increased Foxp3 expression, resulting in blockage of Th1 responses (Bikle, 2009). Th1 activation is crucial for cell‐mediated immune responses, which are held to be important for anti‐cancer responses in man, and indeed many authors propose that a Th2 dominant response to cancer has an adverse effect. There is therefore a view that high levels of vitamin D might be harmful for cancer patients as a result of suppression of host mediated adaptive immune responses to cancer cells.
The effects of vitamin D on immunity however are complex. In colonic epithelium, vitamin D is reported to play a role in the control of the innate immune system (Froicu and Cantorna, 2007), and there is a reasonable amount of evidence to suggest that innate immunological responses to tuberculosis are dependent on vitamin D. Thus there is also a possibility that higher vitamin D levels could promote beneficial innate immunological responses to cancer cells. The innate immune responses involve sensing of foreign antigens (such as bacterial antigens) via toll‐like receptors (TLR), and the induction of cell killing, in part via the release of reactive oxygen species (Bikle, 2009). Vitamin D appears to be involved in these responses and it is of note that TLR agonists such as imiquimod have activity against melanoma in which the drug appears capable of inducing apoptosis (Schon et al., 2004). There are some data additionally that the drug may modify the Th1/Th2 balance (Green et al., 2008).
That vitamin D clearly has a number of effects on the immune system is therefore both a concern for cancer patients (if the data are relevant which suggest that higher doses of vitamin D suppress Th1 responses), and a hope, if promotion of innate immunity results from increased vitamin D levels.
4. Serum levels of vitamin D and the determinants of those levels
If vitamin D is important to health, then what are the optimal levels in the serum and how can they be achieved?
Vitamin D levels are measured in nmol/L or ng/ml by different laboratories but we will use nmol/L in this article. Although 1α,25‐dihydroxyvitamin D3 is the active form of vitamin D, it has a short half life (<4 h) and therefore laboratories measure 25‐hydroxyvitamin D. Levels of 25‐hydroxyvitamin D are linearly inversely correlated with parathyroid hormone (PTH) levels. Although most commercial assays have an upper level of 125 nmol/L, people out in the sun all the time such as lifeguards may have levels higher than 250 nmol/L (Holick, 2004). Vitamin D intoxication occurs at more than 325 nmol/L. It is unclear what the optimal level of vitamin D might be, but PTH levels have been reported to plateau at around 78 nmol/L (although this figure is contested (Bjorkman et al., 2009)) so that the lower limit of optimal is usually quoted non‐the‐less as around 78 nmol/L. In our own hospital laboratory in Leeds a level of 60 nmol/L is judged to be the lower end of the optimal range and others have suggested a level of around 75 nmol/L (Holick, 2004; Ingraham et al., 2008). There are some data to suggest that, in study populations at least, vitamin D levels tend to remain fairly steady over time (Hofmann et al., 2010) although with some seasonal variation as we have observed in melanoma patients (Figure 2). Despite the reports of blood levels in lifeguards described above, serum levels of vitamin D are said to be tightly regulated (Huotari and Herzig, 2008) which suggests that maintaining an “optimal” level is important to human health.
Figure 2.
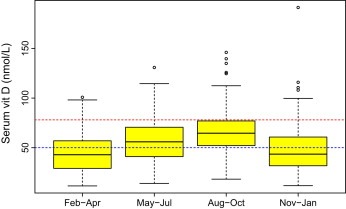
Variation in serum vitamin D levels by season of venepuncture in the Leeds Melanoma Cohort. A level of 78 nmol/L indicated by red line is the level that PTH stabilisation is expected and <50 nmol/L below the blue line is used by some studies to define vitamin D insufficient which illustrates how many melanoma patients have sub‐optimal levels at diagnosis.
As most humans depend on UV‐induced synthesis of vitamin D, those with black or Asian skin living in less sunny areas of the world tend to have lower levels. For white skinned people, there has been the view expressed that comparatively little sun exposure such as “5–15 min of exposure between 1000 and 1500 h during the spring, summer and autumn” is usually enough sun exposure for individuals with type II or III skin (Holick, 2004). In recent times however, many authors from around the world have reported sub‐optimal levels in many different populations as summarized in Table 1. The high prevalence of sub‐optimal levels might suggest that in real life rather than under laboratory conditions, rather more sun exposure is required. Furthermore, within white skinned populations, somewhat surprisingly, we and others have shown that in the very fair, vitamin D levels are actually lower than in brunettes for example (Glass et al., 2009; Randerson‐Moor et al., 2009), which is probably behavioural, related to the sun avoidance measures required to avoid sunburn. There may therefore be particular difficulties for fair skinned peoples in achieving optimal vitamin D levels.
Table 1.
Vitamin D serum levels in various populations at various latitudes.
| Country of study | Latitude | Population | Number studied | Serum level in nmol/L | Factors influencing serum levels | Reference |
|---|---|---|---|---|---|---|
| Norway | 65–71°N | Older females 44–59 years | 300 | Overall<37.5 13.7%<50.0 38%Jan/Feb<37.5 23.3% | Low UV exposureVit D intake significant predictor of serum Vit D level (p = .0003) | (Brustad et al., 2003) |
| Denmark | 55°N | Perimenopausal females 45–58 years | 2016 | <25 7%<50 39.7% | Avoidance of direct sun, no vitamin supplements, low dietary Vit d | (Brot et al., 2001) |
| Netherlands | 52°N | Older male and female longitudinal aging study | 1235 | <25 10.6%<50 47% | Mean age 75.4 ± 6.5 years | (Snijder et al., 2006) |
| United Kingdom | 51°N | Caucasian females | 1414 | <25 3.78%<50 25.73%<75 57.29% | Fair skinned | (Glass et al., 2009) |
| United Kingdom | 50–57°N | 45 year old white male and female | 7437 | Winter/Spring<25 15.5%<40 46.6%<75 87.1% | Seasonal variation, regional variation from North to south UK. Higher levels with vitamin supplements or oily fish intake | (Hypponen and Power, 2007) |
| Summer/Autumn<25 3.2%<40 15.4%<75 60.9% | ||||||
| Italy | 45°N | Postmenopausal females screening osteoporosis clinic | 570 | <30 28%Winter (Nov–May)<30 38.5%Summer (Jun–Nov)<30 12.5% | Most frequent in women >70 years <30 51% May–Dec | (Bettica et al., 1999) |
| France | 43–51°N | General urban | 1569 | North<30 29%Mediterranean Coast<30 7%North43 ± 21South 81 ± 27 | Low Vit D intake, less daytime sunshine in north. PTH rises with Vit D < 78 nmol/L | (Chapuy et al., 1997) |
| Canada | 43°N | Healthy 18–30 years, mixed ethnicity | 107 | Wintertime<25 25.5%<50 73.6%<75 93.4% | Skin pigment inverse relationship with Vit D levels | (Gozdzik et al., 2008) |
| USA | 32–45°N | Males, healthy, six U.S. centres | 1606 | <50 26%<75 72% | Increased rate of deficiency if >80 years, BMI >25, low Vit D intake, N latitude, | (Orwoll et al., 2009) |
| USA | 25–47°N | NHANES III Non‐institutionalised, adolescents and adults population | 18,875 | Winter/median 32°N<25 1–5%<50 13–40%<62.5 25–57%Summer/median 39°N<25 <1–3%<50 8–34%<62.5 21–58% | Insufficiency more prevalent in Mexican Americans and non‐Hispanic blacks compared with non‐Hispanic whites | (Looker et al., 2002) |
| USA | 25–47°N | NHANES III Non‐institutionalised, African American women (1546) and white women (1426) 15–49 years | 2972 | African American<25 12.2 ± 1.7%≤37.5 42.4 ± 3.1%White American<25 4.2 ± 0.7%≤37.5 4.2 ± 0.7% | Determinants of serum levels in African American: no supplements, diet, season, BMI, urban living, oral contraceptive | (Nesby‐O'Dell et al., 2002) |
| Australia Queensland | 27°S | General female population <60 years of age | 167 | Winter/spring<50 40.5% | Latitude and seasonal variation explained 1/5 of variation | (van der Mei et al., 2007) |
| Geelong | 38°S | 561 | <50 37.4% | |||
| Tasmania | 41–43°S | 432 | <50 67.3% |
1 ng/mL = 2.5 nmol/L. NHANES = National Health and Nutrition Examination Surveys; BMI = Body mass index.
Vitamin D is stored in fat: indeed it is stored there in the summer and released during the winter months. Individuals with a high body mass index (BMI) tend to have lower serum levels of vitamin D which is postulated to be explained by excess fat acting as an irreversible sink for vitamin D (Holick, 2004) although there is also some support for the view that overweight individuals sunbathe less (Kull et al., 2009). Inactivity (Brock et al., 2010) and low intake as well as obesity, were recently reported as determinants of low levels in an Australian sample.
Levels are also genetically determined. Genetic associations with African ancestry were shown to correlate with lower vitamin D levels (Signorello et al., 2010), and recent genome wide association studies have identified single nucleotide polymorphisms (SNPs) near genes coding for pathways involved in cholesterol synthesis, hydroxylation, and vitamin D transport as determinants of vitamin D levels (Ahn et al., 2010; Wang et al., 2010).
Vitamin D levels are therefore related to skin type (reported tendency to burn), BMI, diet, supplementation, patterns of sun exposure (and therefore likely latitude) and genetic variation. It is not therefore easy as yet to give practical advice to different populations, which will enable individuals to achieve “optimal” levels whilst avoiding skin cancer.
5. The epidemiology of vitamin D and cancer
Epidemiological studies of the incidence of cancers in relation to latitude and altitude by Apperly (1941) and then by Garland and Garland (1980) first suggested a relationship between vitamin D and death rates from cancers, particularly breast and colon cancer.
There are few data on the role of vitamin D in survival from melanoma other than those published by our group, which are discussed below. There are a series of studies however in other cancers which are broadly summarized in Table 2: indeed the literature is extensive and cannot reasonably be exhaustively covered here. The studies have been “association” studies, in which the relationship between lower vitamin D levels and cancer risk is reported (and these are essentially retrospective studies therefore subject to bias) and cohort studies. In cohort studies healthy participants either have their vitamin D levels measured at entry to the study or have their intake estimated and the long‐term risk of cancer determined. Vitamin D levels tend to be higher in thinner healthier individuals and it must be said that observational or association studies of either type are always subject to the worry that the association seen with vitamin D might be merely a marker for other causal health related factors which are not identified.
Table 2.
Examples of epidemiological data looking at associations between vitamin D levels and cancer.
| Population | Cancer type | Number of cases/controls | Vitamin D benefit | Comment | Reference | |
|---|---|---|---|---|---|---|
| (a) Cancer risk | ||||||
| Prospective case‐cohort study (nested in osteoporotic fractures in men study) | U.S.A. All ethnicity, 1217 non‐Hispanic whites | Prostate | 297/1351≥65 years | No | No association found between Vit D serum levels and subsequent risk of prostate cancer | (Barnett et al., 2010) |
| Prospective case‐control study (nested within prostate, lung, colorectal and ovarian cancer screening trial) | U.S.A. non‐hispanic white | Prostate | 749/781 | No | No association with Vit D and decreased risk of prostate cancer, higher circulating levels may be associated with increased risk of aggressive disease | (Ahn et al., 2008) |
| Prospective case‐control study (nested European prospective investigation into cancer and nutrition) | Europeans | Prostate | 652/752 | No | The risk of prostate cancer did not vary significantly with Vit D levels | (Travis et al., 2009) |
| Prospective case‐control study (nested α‐tocopherol, β‐carotene prevention study) | Finnish | Prostate | 296/296 | No | No difference in Vit D levels between cases and controls | (Faupel‐Badger et al., 2007) |
| Prospective case‐control study (nested nutritional prevention cancer trial) | U.S.A. Caucasians | Prostate | 83/166 | No | No difference in prostate cancer risk | (Jacobs et al., 2004) |
| Prospective case‐control study (nested European prospective investigation into cancer and nutrition) | Europeans | Colorectal | 1248/1248 | Yes | Strong inverse association between levels of pre‐diagnostic Vit D levels and risk of colorectal cancer | (Jenab et al., 2010) |
| Prospective case‐control study (nested nurses health study) | U.S.A. | Colorectal | 193/386 | Yes | 25(OH)D levels but not 1,25‐dihyrdoxyvit D levels were associated in an inverse linear manner with risk of colorectal cancer (distal colon and rectal) | (Feskanich et al., 2004) |
| Prospective case‐control study (nested α‐tocopherol, β‐carotene prevention study) | Finnish men | Colorectal | 146/292 | Yes | 25(OH)D levels but not 1,25‐dihyrdoxyvit D levels were associated in an inverse linear manner with risk of colorectal cancer (most marked for rectal cancer 55 cases; RR by quartile = 1.00, .93, .77, .37; trend P = 0.06) | (Tangrea et al., 1997) |
| Prospective case‐control study (nested cancer prevention study‐II nutrition cohort) | U.S.A. | Breast | 516/516 | No | No association between 25(OH)D levels and breast cancer risk | (McCullough et al., 2009) |
| Case series | U.K. Caucasians | Breast | 279 | Yes | Serum levels of 25(OH)D are significantly higher in patients with early stage breast cancer than those with locally advanced or metastatic | (Palmieri et al., 2006) |
| Prospective study (third national health and nutrition examination survey) | U.S.A. | Breast | 28 breast cancer deaths | No | No association between Vit D levels and risk of death from breast cancer | (Freedman et al., 2007) |
| Prospective case‐control study (nested nurses health study) | U.S.A. | Breast | 701/724 | Trend to benefit | Higher levels of both 25(OH)D (RR 0.73) and 1,25(OH)2D (RR 0.76) were associated with non significant lower risk of breast cancer. For both metabolites, the association was stronger ≥60 years | (Bertone‐Johnson et al., 2005) |
| Pool nested case‐control study (Cohort consortium vitamin D pooling project of rarer cancers) | Multiple geographical location | Pancreatic | 952/1333 | Negative benefit | High 25(OH)D level (≥100 nmol/L) was associated with a significant 2‐fold increase in pancreatic cancer risk overall (OR = 2.12) | (Stolzenberg‐Solomon et al., 2010) |
| Prospective case‐control study (nested α‐tocopherol, β‐carotene prevention study) | Finnish Male smokers | Pancreatic | 200/400 | Negative benefit | 3‐fold increase risk of pancreatic cancer with high Vit D levels (highest versus lowest quintile, >65.5 versus <32.0 nmol/L: OR, 2.92; 95% CI, 1.56–5.48, Ptrend = 0.001) | (Stolzenberg‐Solomon et al., 2006) |
| (b) Outcome | ||||||
| Prospective study (nested physicians health study) | U.S.A. | Prostate | 492/664 | Yes | Pre‐diagnostic Vit D levels tended to be inversely associated with risk of aggressive prostate cancer at diagnosis, especially men >65 years | (Li et al., 2007) |
| Association study | Norway | Prostate | 160 | Yes | Serum Vit D levels 50–80 nmol/L and >80 compared to <50 had better prognosis (cause specific mortality) (RR0.33; RR0.16) | (Tretli et al., 2009) |
| Prospective cohort study | Japan | Colorectal | 257 | Yes | Higher Vit D levels were associated with better overall survival (HR, 0.91, P = .027) | (Mezawa et al., 2010) |
The epidemiological data listed in Table 2 are certainly nonetheless persuasive for some cancers. Other authors have reported studies designed to bring together the evidence for particular cancers. Chen et al. (2010), recently reported a meta‐analysis of 11 studies on vitamin D intake, 7 studies on circulating 25(OH)D levels, 3 studies of circulating 1α,25(OH)2D levels, and 15 studies on calcium intake and breast cancer risk. From the meta‐analysis, there was a significant inverse relationship between vitamin D intake and breast cancer risk, with an overall relative risk (RR) of high versus low vitamin D intake for breast cancer of 0.91 (95% CI = 0.85–0.97). The highest quantile of circulating 25(OH)D was found to be associated with a 45% (OR = 0.55, 95% CI = 0.38–0.80) decrease in breast cancer when compared with the lowest quantile. No significant association for the circulating 1α,25(OH)2D level and breast cancer was found (OR = 0.99, 95% CI = 0.68–1.44). For calcium, a 19% (RR = 0.81, 95% CI = 0.72–0.90) decrease in breast cancer risk was found for those with the highest quantile of calcium intake compared to the lowest quantile. These results provide good evidence that vitamin D and calcium have a chemopreventive effect against breast cancer. Rhee et al. (2009) also recently reviewed a number of studies of colorectal cancer and concluded that prospective studies showed fairly uniform reduction in risk in relation to higher vitamin D levels. In an important, broader study reported by Giovannucci et al. (2006) a model which predicted vitamin D levels was used to compute a predicted 25(OH)D level for each of 47,800 men in the Health Professionals Cohort. 4286 incident cancers (excluding organ‐confined prostate cancer and non‐melanoma skin cancer) and 2025 deaths from cancer were then observed prospectively and correlated with predicted vitamin D levels. In multivariable models, an increment of 25 nmol/L in predicted 25(OH)D level was associated with a 17% reduction in total cancer incidence (multivariable relative risk [RR] = 0.83, 95% confidence interval [CI] = 0.74–0.92), a 29% reduction in total cancer mortality (RR = 0.71, 95% CI = 0.60–0.83), and a 45% reduction in digestive‐system cancer mortality (RR = 0.55, 95% CI = 0.41–0.74). The absolute annual rate of total cancer was 758 per 100,000 men in the bottom decile of predicted 25(OH)D, and 674 per 100,000 men for the top decile. The respective rates were 326 per 100,000 and 277 per 100,000 for total cancer mortality, and 128 per 100,000 and 78 per 100,000 for digestive‐system cancer mortality. The authors concluded that low levels of vitamin D may be associated with increased cancer incidence and mortality in men, particularly for digestive‐system cancers. There was no protective effect observed for melanoma, indeed there was a non‐significant positive relationship, but the measure used to predict vitamin D level in the cohort was derived from such variables as skin colour and latitude of residency which would likely confound such a test as skin color and latitude of residence are risk factors for melanoma.
The only conclusive evidence that supplemental vitamin D should be adopted as a means of prevention, would be long‐term data showing benefit in the context of randomized clinical trials. The difficulties are numerous however in reaching this goal and the data so far are of limited value. A study from the Women's Health Initiative, for example, failed to report evidence of reduced risk of colon cancer in 36,000 women randomised to receiving 400 IU of vitamin D and calcium per day for an average of 7 years (Wactawski‐Wende et al., 2006): however the study was criticized for too small doses, poor compliance and inadequate control for vitamin D in the placebo group (Bikle, 2009). There was some evidence for a preventive effect for all cancers in a short study of 1179 women in residential homes given 1100 IU vitamin D and calcium daily over four years (Lappe et al., 2007).
6. Vitamin D and melanocytes: in vitro data
There are data on in vitro models of melanoma in relation to added vitamin D but they are few in comparison with those produced in cells of other cell lineages. We have therefore started by summarizing the type of evidence of anti‐proliferative effects reported, accepting that this cannot be an exhaustive list (Table 3).
Table 3.
In vitro studies looking at the effect of vitamin D on cancer cells.
| Type of cancer | Model studied | Cell line used | Vitamin D or analogue | Findings | Conclusion | Reference |
|---|---|---|---|---|---|---|
| Prostate | Mouse model | PC‐3 | 16‐23‐D3 | Mean tumour volume 15% smaller than in controls – modest anti‐proliferative effect | Antiproliferative | (Schwartz et al., 1995) |
| Prostate | Cell culture | Human Tissue samples | 1,25(OH)2D3 | Benign and malignant epithelial cells growth was inhibited with half maximal inhibition at 1 nmol and complete inhibition at 25 nmol | Antiproliferative | (Peehl et al., 1994) |
| Prostate | Cell culture | DU 145, PC‐3, LNCaP | 1,25(OH)2D 16‐23‐D3 | PC‐3, LNCaP were poorly invasive ± Vit d, PC‐3 cells – highly invasive and markedly inhibited by 1,25(OH)2D and 16‐23‐D3, dose and time dependent | Decreased Invasiveness | (Schwartz et al., 1997) |
| Breast | Cell culture | MCF‐7, BT‐20 | 1,25(OH)2D3 | Reduced proliferation irrespective of oestrogen status of cell line | Probably Antiproliferative | (Chouvet et al., 1986) |
| Breast | Rats | MCF‐7 | Calcipotriol, (MC903), 1,25(OH)2D3, 1α (OH)D3 | 1α (OH)D3 (0.25, 1.25 μg/kg) response rate (rr) 25% (rr = 50% regression of tumour) but ↑Ca+2. Calcipotriol 17% rr without severe ↑Ca+2 | Antiproliferative | (Colston et al., 1992) |
| Breast | RatsCell culture | Nitrosomethyl urea induced rat mammary tumours MCF‐7 cell line | 1,25(OH)2D3 EB1089 | ↑Ca+2 No significant inhibition of tumour progression with 1,25(OH)2D3 (0.5 μg/kg). EB1089 tumour suppression no ↑Ca+2. EB1089 increased expression of TRPM‐2 apoptosis gene, ↑p21,↑p53, ↓bcl‐2 in MCF‐7 cell cultures | Antiproliferative analogue in vivo Possible role in apoptosis in vitro | (James et al., 1996) |
| Colon | Cell culture | HT‐29 (human colon carcinoma cell line HT‐29 differentiates into functional enterocytes) | 1,25(OH)2D3 | Promotes differentiation of HT‐29 cells as measured by maltase activity | Prodifferenciation | (Brehier and Thomasset, 1988) |
| Colon | Cell culture | HT‐29 Biopsy normal mucosa and ademoas | 1,25(OH)2D3 Synthetic Analogues | Inhibit proliferation (CCPR, Ki‐67) in normal and premalignant rectal epithelium and suppress growth HT‐29 colon cell line | Antiproliferative | (Thomas et al., 1992) |
| Colon | Cell culture | SW480 | 1,25(OH)2D3 and Multiple nonhypercalcemic synthetic analogues | 1α,25(OH)2D3 induces E‐cadherin and modulates beta‐catenin‐TCF‐4 target genes promoting the differentiation of colon carcinoma cells | Pro differentiation | (Palmer et al., 2001) |
| Pancreas | Cell culture and mice | GER xenografts immunodeficient mice | EB1089 | GER cells dose dependent growth inhibition. No ↑p53. In vivo 5 μg/kg 3/week significant growth inhibition | Antiproliferative | (Colston et al., 1997) |
| Pancreas | Cell culture | AsPc‐1, BxPc‐3, T3M‐4 | EB1089, CB1093 | Growth arrest correlated with increased proportion of cells in G0/GI phase | Antiproliferativeno antiapoptotic effect | (Pettersson et al., 2000) |
| Pancreas | Cell culture | BxPC‐3Hs700THs766TAsPC‐1 | 1,25(OH)2D3, 25(OH)2D3 | 25(OH)D3 inhibited the growth of 3/4 pancreatic cell lines that correlated with induction of p21, p27 and cell‐cycle arrest at the G1/S checkpoint | Antiproliferative | (Schwartz et al., 2004) |
16‐23‐D3 = Dihydroxy‐16‐ene‐23‐yne‐cholecalciferol; 1,25(OH)2D = 1,25(OH)2D3 = 1,25‐dihydroxyvitamin D3 = calcitriol; EB1089 = 1(S),3(R)‐dihydroxy‐20(R)‐(5′‐ethyl‐5′hydroxy‐hepta‐1′(E),3′(E)‐dien‐1′‐yl)‐9,10‐secopregna‐5(Z),7(E),10(19)‐triene; TRMP‐2 = Testosterone‐repressed prostatic message‐2 also known as clusterin; CCPR = Crypt cell production rate.
Vitamin D was shown to inhibit proliferation and induce differentiation in melanoma cells in the late 1990s (Danielsson et al., 1998, 1999), and variable degrees of inhibition of proliferation were reported more recently (Reichrath et al., 2007b). Subsequently however certain melanoma cell lines have been demonstrated to be vitamin D resistant. The Reichrath group compared a vitamin D resistant melanoma cell line (SKMel 5) and a vitamin D responsive melanoma cell line (MeWo cells) to explore determinants of vitamin D resistance. They showed that both cell lines had mRNA expression for the VDR gene and metabolizing enzymes such as CYP24A1 (24‐OHase). However, in response to vitamin D only the MeWo cells responded with a dose dependent increase in mRNA for the VDR and CYP24A1 genes as well as suppressing cell line proliferation (up to approximately 50%). (Seifert et al., 2004) The conclusion of the Reichrath group was that unresponsiveness to vitamin D was mediated by failure of VDR‐mediated transcription. After a breast cancer research group reported that amplification at chromosome 20q13.2 (the location of the 24‐OHase gene, CYP24A1) might explain vitamin D resistance in breast cancer cells (Albertson et al., 2000), the Reichrath group sought amplification of the CYP24A1 in vivo using Southern blotting in a small number of fresh tissue cutaneous malignant melanoma samples but did not demonstrate upregulation of 24‐OHase (Reichrath et al., 2004). Further work in other melanoma resistant and responsive cell lines to study vitamin D anti‐proliferative effects, has also demonstrated support for a defect in VDR‐mediated transcription (Reichrath et al., 2007b).
There are, in summary, some in vitro data, to suggest that vitamin D has anti‐proliferative effects on cultured melanoma cells, as it is reported to have in other cancers, but the data are limited and further research is needed.
7. Survival from melanoma
A relatively simple way to look at a potential relationship between vitamin D and cancer mortality is to correlate death rates from that cancer by ultraviolet flux, or at least latitude as a surrogate. This would, for melanoma, be extremely difficult to assess, given that both vitamin D levels and melanoma risk are related to factors such as skin colour and sun exposure. In 2005 however, Berwick et al. (2005) reported a study in which the presence of solar elastosis (dermal sun damage) in melanoma excision specimens appeared to be associated with a better prognosis for melanoma patients. One interpretation of this finding was that chronic sun damage might induce a less aggressive form of melanoma, but another possible explanation is that vitamin D levels related to chronic sun exposure might be protective for relapse even if sun exposure is related to its aetiology.
If the anti‐proliferative effect of vitamin D was important in modifying outcome for melanoma patients, we might first postulate that prognosis would be better in countries with higher sun exposure than in countries with less. In fact, outcome for patients diagnosed with melanoma in Australia is better than for those diagnosed in the UK. Both groups of melanoma patients are genetically similar as the melanoma population in Australia is largely of UK origin ethnically as seen in analyses reported in the GenoMEL genome wide association study (Bishop et al., 2009). We therefore thought it possible to compare determinants of outcome in the two populations with similar genetic background but potentially different environmental influences. We therefore carried out a study collaboratively between cancer registries in the UK and Australia (Downing et al., 2008) to better understand the determinants of outcome in both countries. Patients diagnosed with invasive melanoma between 1993 and 2003 in Yorkshire (n = 4170) and New South Wales (NSW, n = 30,520) were identified from cancer registry databases and prognostic information (age, sex, socioeconomic background, tumour site and Breslow thickness) was extracted. Age‐standardised incidence rates, 5‐year relative survival and relative excess risk of death were calculated. Between 1993–1995 and 2001–2003, the incidence of melanoma increased in both areas. These increases were mainly seen in patients with tumours with a Breslow thickness ≤1 mm. Five‐year relative survival was 86.9% (95% CI 85.2–88.5) in Yorkshire and 88.6% (95% CI 88.1–89.1) in NSW. Compared with that observed in NSW, survival in Yorkshire was lower for males and for those living in the most deprived areas. After adjustment for differences in prognostic factors, the relative excess risk of death in Yorkshire compared to that in NSW reduced from 1.36 (95% CI 1.20–1.53) to 1.11 (95% CI 0.99–1.23). There was a small residual suggestion of reduced risk for death in Australia, but differences in tumour thickness appeared to be the most important factor. We therefore concluded that the small difference in outcome for melanoma patients in the UK and Australia likely results from earlier presentation with thinner tumours in Australia where the surveillance for early detection of skin cancers is heavily promoted. Thus this study provided no real support for a protective effect of vitamin D, if one assumes indeed that vitamin D levels are on average higher in Australian melanoma patients than in UK patients.
Nonetheless we explored the possible effects of vitamin D level in UK melanoma patients. A pilot retrospective study of 271 patients with melanoma suggested that vitamin D may protect against recurrence of melanoma (Newton‐Bishop et al., 2009). We tested these findings in a survival analysis in a cohort of 872 patients recruited to the Leeds Melanoma Cohort (median follow‐up, 4.7 years). In the retrospective study, self‐reports of taking vitamin D supplements were non‐significantly correlated with a reduced risk of melanoma relapse (odds ratio = 0.6; 95% CI, 0.4–1.1; P = .09). Non‐relapsers had higher mean 25‐hydroxyvitamin D3 levels than relapsers (49 versus 46 nmol/L; P = .3; not statistically significant). In the cohort (prospective) study, higher 25‐hydroxyvitamin D3 levels were associated with lower Breslow thickness at diagnosis (P = .002), and were independently protective of relapse and death: the hazard ratio for relapse‐free survival (RFS) was 0.79 (95% CI, 0.64–0.96; P = .01) for a 20 nmol/L increase in serum level. A survival curve for the cohort with longer follow up than previously reported is shown in Figure 3. There was evidence of interaction between the vitamin D receptor gene (VDR) BsmI genotype and serum 25‐hydroxyvitamin D3 levels on RFS in the data reported in 2009. We concluded that the results from the retrospective study were consistent with a role for vitamin D in melanoma outcome. The cohort study tested this hypothesis, providing evidence that higher 25‐hydroxyvitamin D3 levels, at diagnosis, were associated with both thinner tumours and better survival from melanoma, independent of Breslow thickness. The data urgently need to be validated in additional mature sample sets and importantly the effects of vitamin D level in the follow up period must be investigated.
Figure 3.
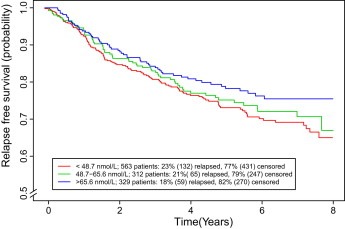
Up to date (Sept 2010) Kaplan‐Meier curves for relapse free survival probabilities for serum vitamin D levels (nmol/L) at interview (categorized based on tertile cutoff) in the Leeds Melanoma Cohort. The text box shows the overall proportion of relapsers by vitamin D category.
Nonetheless in the interim the data do suggest a relationship between higher vitamin D levels and thinner tumours and better outcome for melanoma patients in the UK. At the beginning of this section we described a comparative study between patients diagnosed with melanoma in Australia and the UK, and when we published these data, we concluded that the difference between the two countries with regards to outcome was related to Breslow thickness. In view of our more recent data in which tumour thickness was related to vitamin D level (Newton‐Bishop et al., 2009), we cannot exclude the possibility that outcome is better for melanoma patients in Australia presenting with thinner tumours at least in part, as a result of postulated higher vitamin D levels in that sunnier country.
We will next discuss the very limited data on the relationship between vitamin D and melanoma susceptibility.
8. Vitamin D and susceptibility to melanoma
Although epidemiological studies have established a clear relationship between sun exposure and melanoma risk (Gandini et al., 2005), the nature of this relationship has proved complex. Indeed the complexities (largely related to the lack of a simple dose/risk relationship between sun exposure and melanoma risk) have complicated public education programmes internationally. We will explore possible explanations for these complexities and speculate as to whether vitamin D might play a role.
For most carcinogens there is a dose/risk relationship, so that higher levels of exposure are associated with higher levels of risk. The analogy for melanoma would be that the more sun exposure a fair skinned person has through life, the higher would be the risk of melanoma. One would then predict that outdoor workers would be at greatest risk of melanoma. In many studies however there was no evidence of such relationship, and indeed there is a suggestion from individual studies and from a meta‐analysis that occupational sun exposure might actually be associated with a lower risk (Gandini et al., 2005). We reported a pooled data analysis, which did not show a protective effect of measures of total lifetime sun exposure, but confirmed that it was sun bathing which was associated with the highest risk at all latitudes. For studies in temperate climes there was no measurable relationship between total lifetime sun exposure and risk (Chang et al., 2009). At all latitudes, a history of sunburn was consistently associated with increased risk (sunburn being a marker both of genetically determined susceptibility and exposure). Again therefore intermittent sun exposure associated with sun bathing and sunburn was confirmed as the dominant risk factor for melanoma rather than cumulative sun exposures at most latitudes.
This complex relationship has not yet been explained but seminal animal work by Kripke and colleagues has lead to the view that sunburn related immune suppression is important for carcinogenesis for melanoma (Jeevan et al., 1992; Noonan et al., 1981; Wang et al., 1990). The strong relationship between sunburn history and melanoma risk for humans, coupled with the lack of a simple dose response relationship between sun exposure and risk, suggested also that there is something particular about inflammation related to sunburn, and melanoma tumourigenesis. For melanoma, chronic sun exposure (in this hypothesis) would be less important, at least in temperate climates, than it is for non‐melanoma skin cancer.
There is some evidence that chronic sun exposure protects from sunburn by inducing photoadaption with sun exposure related tanning and epidermal thickening affording some protection. The most prevalent current hypothesis is that the indoor worker (not adapted to sun exposure) suffers sunburn during holidays, which induces DNA damage and immunosuppression, leading over time to an increased risk of melanoma on intermittently covered body sites. The outdoor worker, or people habitually exposed to moderate sun exposure, may become photoadapted so that burning (and melanoma) are less likely consequences.
We have recently reported a large case‐control study of melanoma in the north of England (Newton‐Bishop et al., 2010). In this study we showed that the phenotypes associated with a tendency to sunburn and reported sunburn at ≥20 years of age were associated with increased melanoma risk. Overall higher average weekend sun exposure in warmer months was however actually protective for melanoma (OR 0.67, 95% CI 0.50–0.89 for highest versus lowest tertile of exposure) (Newton‐Bishop et al., 2010), lending some support for the hypothesis that moderate sun exposure can actually be protective for melanoma.
What is the explanation for a possible protective effect of habitual sun exposure? Would it result from photoadaption, or because sun exposure increases vitamin D levels? There are limited data to inform this but there are some which suggest that vitamin D itself has photoprotective effects (Damian et al., 2010; Gorman et al., 2007; Gupta et al., 2007). Conflicting data exist however that vitamin D may actually be a mediator via which sunburn results in systemic immunosuppression (Gorman et al., 2007). Mason et al. (2010) have reported evidence that vitamin D reduces UV‐induced DNA damage in vitro and in mouse and human skin, and reduces UV‐induced immunosuppression as a result, although other data suggest that vitamin D has an immunosuppressive effect when analogues were applied topically to irradiated skin (Damian et al., 2010). A recent review published by Mason's group describes the complexity of what is currently known about the putative role of vitamin D as a cutaneous defence molecule (Dixon et al., 2010). We would suggest that the complexity of those responses is such that currently the data are difficult to interpret.
If vitamin D was playing a role in melanoma prevention, then although sunburn is causal for melanoma, vitamin D intake might be protective. The possible role of vitamin D as reported dietary intake in melanoma risk was reported by Gandini et al. (2009) in a meta‐analysis. They excluded one study on the basis of heterogeneity and in the remaining studies showed some evidence of a protective effect for highest verses lowest intake, SRR 0.63 (95% CI 0.42–0.94). A small previous study had failed to show lower levels of vitamin D in melanoma patients than in healthy individuals (Reichrath and Querings, 2004), and a tiny study from 1992 showed a 5% lower level of vitamin D in 23 healthy donors who later went on to develop a melanoma (Cornwell et al., 1992). This was, not surprisingly, a non‐significant effect but shows the long‐standing interest in the field at least. Asgari et al. (2009) however explored this in a large prospective cohort. They examined whether dietary and supplemental vitamin D intake was associated with melanoma risk among 68,611 men and women who were participants in the Vitamins and Lifestyle cohort study. Participants reported dietary vitamin D intake over the preceding year and 10‐year use of multivitamin and individual vitamin D supplements on a baseline questionnaire. After follow‐up through 2006, 455 incident melanomas were identified through linkage to the Surveillance, Epidemiology, and End Results cancer registry. There was a suggestion in these data of a decreased risk for high supplemental use RR = 0.77 (95% CI 0.34–1.72) compared with the lowest quartile, but they did not see a protective effect of combined dietary and supplemental intake (1.05, 95% CI = 0.79–1.40). There was therefore no support from this large study for the suggestion that vitamin D levels might be protective for melanoma, but in this study the authors were not able to correct for known risk factors of melanoma risk such as reported sunburn or sun exposure. Overall therefore the data available to assess the role of vitamin D and melanoma risk are few.
The global burden of disease attributed to sunlight exposure is estimated to be only 0.1% of the total and the likely negative health effects resulting from very low levels of vitamin D as a result of very low levels of sun exposure, are estimated to be much greater (Lucas et al., 2008) therefore the advice to avoid excessive sun exposure must be carefully delivered. The epidemiology relevant to primary prevention of melanoma currently remains incompletely understood. The above summarized data suggest that sunburn is aetiological for melanoma, and that sunny holidays and sun bathing are causal. It also suggests that chronic sun exposure is much less clearly related to risk in many temperate areas of the world, such as Europe, compared with hot countries such as Australia. Given that occupational sun exposure has been reported as protective for melanoma, that vitamin D is reported to have anti‐proliferative effects in melanoma cells, and the reported photoprotective and anti‐proliferative effects of vitamin D, interest has arisen in a possible role for vitamin D in melanoma prevention. If vitamin D was preventive for melanoma then one might hypothesise that melanoma incidence would be higher in areas of the world where sun exposure is less but this relationship would likely be confounded by the effect of sun exposure itself and therefore there is no easy means of using incidence data to address this possibility.
In order to further investigate this hypothesis, a number of groups looked at inheritance of polymorphisms in the gene coding for VDR as determinants of melanoma risk (Halsall et al., 2004; Han et al., 2006; Li et al., 2008; Moon et al., 2006; Santonocito et al., 2007). We in Leeds also looked at these and reported the data in a meta‐analysis in conjunction with published data from other smaller data sets (2159 cases and 2429 controls) (Randerson‐Moor et al., 2009). In the meta‐analysis, the VDR FokI T allele was associated with increased melanoma risk OR 1.19 (95% CI 1.05, 1.35), and the BsmI A allele was associated with a reduced risk OR 0.82 (95% CI 0.71, 0.94), in each instance assuming a dominant model. The association between the FokI T allele and melanoma was confirmed in a second Leeds case (299) control (560) series, OR 1.42 (1.06–1.91), p = .02. In our reported study however there was no significant case‐control difference in vitamin D levels. These association study data therefore provide some genetic evidence to support for the view that vitamin D might have an effect on susceptibility to melanoma.
In summary then, there are as yet no clear answers as to whether vitamin D might have a role in protection of melanoma but there are sufficient data to suggest that we should continue to explore the possibility. There is no doubt however that sunburn causes melanoma and the incidence of melanoma continues to increase worldwide. Public health advice must therefore remain that sunburn MUST be avoided.
9. Future research
In 1, 2 we have summarized what we do know and what we don't know about vitamin D but the reality is that there remains much to learn.
The optimal levels of vitamin D required to prevent cancer or to improve outcomes are unclear. Indeed, although it is known with some certainty that low levels of vitamin D are associated with myocardial infarction, congestive heart disease and calcific aortic stenosis, the exact doses recommended are unclear (Zittermann and Gummert, 2010). The shape of the dose response curves for risk of cardiovascular disease (Wang et al., 2008) and for better survival for breast cancer patients (Goodwin et al., 2009), are both suggestive of a U‐shaped effect. That is that there are concerns that high doses of vitamin D may also be harmful, and there is an urgent need to understand the effects of higher blood levels, particularly on host immune responses to melanoma and cancer in general.
The evidence however is persuasive that levels lower than around 75 nmol/L are sub‐optimal for many aspects of health and should be avoided. Research directed towards better establishing optimal levels is urgently needed and understanding how those levels should be attained in phenotypically variable individuals. It is established that there is genetic variation in genes coding for key proteins such as the vitamin D binding protein (Wang et al., 2010) which affect vitamin D levels, and that there is variation due to BMI, age, skin colour, diet and sun exposure. We therefore do not know as yet how best to advise different populations as to how to balance the need to avoid melanoma with the need to avoid vitamin D deficiency. It is clear however that rapid progress is now taking place in understanding these factors which will likely lead to improved health internationally in the relatively near future.
10. Recommendations currently for melanoma patients
In the UK at least, it has been shown that melanoma patients around the time of diagnosis, commonly have sub‐optimal levels of vitamin D, and moreover that lower levels of vitamin D were both associated with thicker tumours and poorer survival even when adjusted for thickness and other factors having an effect on outcome such as age and sex (Newton‐Bishop et al., 2009). Whilst it will require a randomized clinical trial to establish whether increasing blood levels of vitamin D after diagnosis might improve survival, the established harmful effects of sub‐optimal levels of vitamin D on health in general (Autier and Gandini, 2007) lead us to the view that melanoma patients should avoid vitamin D deficiency. A common practice is for melanoma patients to reduce their sun exposure after a diagnosis and this is sensible, as sunburn‐induced immunosuppression is potentially harmful, and the patient wishes to reduce their probability of another primary. However, if they do so, it is likely that their vitamin D levels will fall. In a small study reported by McCombie et al. (2009) the majority of patients who had had non‐melanoma skin cancer had levels lower than 50 nmol/L after winter even in Sydney. It would seem sensible therefore to supplement the diet with vitamin D.
Many experts suggest that a minimum of 1000 IU of vitamin D daily is necessary to achieve serum levels of around 70 nmol/L (Holick et al., 2007; Reichrath, 2007a). The National Academy of Sciences suggests somewhat lower levels ranging from 200 IU up till 50 years of age and 400 IU for older individuals, (these levels are usually found in multivitamin tablets). It seems to us however that there is still much to learn about what are optimal levels and how they might be achieved at different latitudes, in people of different ages, skin types, diets and BMI. In the healthy population many authors have suggested that sun exposure is the best means of maintaining healthy levels of vitamin D. We have demonstrated however, as have others, that people with fair skin (those at risk of melanoma) tend to have lower levels of vitamin D than other “white skinned” people (Glass et al., 2009; Malvy et al., 2000) which has been attributed to sun avoidance (Malvy et al., 2000), probably in order to avoid a burn. We would therefore recommend that fair skinned melanoma patients, and probably all fair skinned peoples at risk of melanoma (those with red hair, increased numbers of melanocytic naevi, previous melanoma or a family history of melanoma), should avoid sun exposure sufficient to avoid burning and to supplement their diet with vitamin D. As high levels of vitamin D may have currently unforeseen effects on melanoma patients, we would recommend aiming for serum levels around 70 nmol/L rather than aiming for very high levels. Clearly however urgent research is needed to establish the optimal level and how it might be achieved. Good dietary sources of vitamin D include oily fish, fortified foods, cod liver oil and dietary supplements in multivitamins. Vitamin D3 is thought to be more effective than vitamin D2. Mushrooms are the only vegan source of vitamin D other than the sun. On‐line information for melanoma patients can be found at www.genomel.org.
Field Sinead and Newton‐Bishop Julia A., (2011), Melanoma and vitamin D, Molecular Oncology, 5, doi: 10.1016/j.molonc.2011.01.007.
References
- Ahn, J. , Peters, U. , Albanes, D. , Purdue, M.P. , Abnet, C.C. , Chatterjee, N. , 2008. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J. Natl. Cancer Inst.. 100, 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, J. , Yu, K. , Stolzenberg-Solomon, R. , Simon, K.C. , McCullough, M.L. , Gallicchio, L. , 2010. Genome-wide association study of circulating vitamin D levels. Hum. Mol. Genet.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson, D.G. , Ylstra, B. , Segraves, R. , Collins, C. , Dairkee, S.H. , Kowbel, D. , 2000. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat. Genet.. 25, 144–146. [DOI] [PubMed] [Google Scholar]
- Apperly, F. , 1941. The relation of solar radiation to cancer mortality in North America. Cancer Res.. 1, 191–195. [DOI] [PubMed] [Google Scholar]
- Asgari, M.M. , Maruti, S.S. , Kushi, L.H. , White, E. , 2009. A cohort study of vitamin D intake and melanoma risk. J. Invest. Dermatol.. 129, 1675–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autier, P. , Gandini, S. , 2007. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch. Intern. Med.. 167, 1730–1737. [DOI] [PubMed] [Google Scholar]
- Barnett, C.M. , Nielson, C.M. , Shannon, J. , Chan, J.M. , Shikany, J.M. , Bauer, D.C. , 2010. Serum 25-OH vitamin D levels and risk of developing prostate cancer in older men. Cancer Causes Contr.. 21, 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone-Johnson, E.R. , Chen, W.Y. , Holick, M.F. , Hollis, B.W. , Colditz, G.A. , Willett, W.C. , 2005. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol. Biomarkers Prev.. 14, 1991–1997. [DOI] [PubMed] [Google Scholar]
- Berwick, M. , Armstrong, B.K. , Ben-Porat, L. , Fine, J. , Kricker, A. , Eberle, C. , 2005. Sun exposure and mortality from melanoma. J. Natl. Cancer Inst.. 97, 195–199. [DOI] [PubMed] [Google Scholar]
- Bettica, P. , Bevilacqua, M. , Vago, T. , Norbiato, G. , 1999. High prevalence of hypovitaminosis D among free-living postmenopausal women referred to an osteoporosis outpatient clinic in northern Italy for initial screening. Osteoporos. Int.. 9, 226–229. [DOI] [PubMed] [Google Scholar]
- Bikle, D. , 2009. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab.. 94, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle, D.D. , 2010. Vitamin D and the skin. J. Bone Miner Metab.. 28, 117–130. [DOI] [PubMed] [Google Scholar]
- Bishop, D.T. , Demenais, F. , Iles, M.M. , Harland, M. , Taylor, J.C. , Corda, E. , 2009. Genome-wide association study identifies three loci associated with melanoma risk. Nat. Genet.. 41, 920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman, M. , Sorva, A. , Tilvis, R. , 2009. Responses of parathyroid hormone to vitamin D supplementation: a systematic review of clinical trials. Arch. Gerontol. Geriatr.. 48, 160–166. [DOI] [PubMed] [Google Scholar]
- Brehier, A. , Thomasset, M. , 1988. Human colon cell line HT-29: characterisation of 1,25-dihydroxyvitamin D3 receptor and induction of differentiation by the hormone. J. Steroid Biochem.. 29, 265–270. [DOI] [PubMed] [Google Scholar]
- Brock, K. , Huang, W.Y. , Fraser, D.R. , Ke, L. , Tseng, M. , Stolzenberg-Solomon, R. , 2010. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J. Steroid Biochem. Mol. Biol.. 121, 462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot, C. , Vestergaard, P. , Kolthoff, N. , Gram, J. , Hermann, A.P. , Sorensen, O.H. , 2001. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br. J. Nutr.. 86, (Suppl. 1) S97–S103. [DOI] [PubMed] [Google Scholar]
- Brustad, M. , Sandanger, T. , Wilsgaard, T. , Aksnes, L. , Lund, E. , 2003. Change in plasma levels of vitamin D after consumption of cod-liver and fresh cod-liver oil as part of the traditional north Norwegian fish dish “Molje”. Int. J. Circumpolar Health. 62, 40–53. [DOI] [PubMed] [Google Scholar]
- Campbell, F.C. , Xu, H. , El-Tanani, M. , Crowe, P. , Bingham, V. , 2010. The yin and yang of vitamin D receptor (VDR) signaling in neoplastic progression: operational networks and tissue-specific growth control. Biochem. Pharmacol.. 79, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor, H. , Shinohara, M.L. , 2009. Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat. Rev. Immunol.. 9, 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna, M.T. , Zhu, Y. , Froicu, M. , Wittke, A. , 2004. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am. J. Clin. Nutr.. 80, 1717S–1720S. [DOI] [PubMed] [Google Scholar]
- Chang, Y.M. , Barrett, J.H. , Bishop, D.T. , Armstrong, B.K. , Bataille, V. , Bergman, W. , 2009. Sun exposure and melanoma risk at different latitudes: a pooled analysis of 5700 cases and 7216 controls. Int. J. Epidemiol.. 38, 814–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuy, M.C. , Preziosi, P. , Maamer, M. , Arnaud, S. , Galan, P. , Hercberg, S. , 1997. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int.. 7, 439–443. [DOI] [PubMed] [Google Scholar]
- Chen, P. , Hu, P. , Xie, D. , Qin, Y. , Wang, F. , Wang, H. , 2010. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res. Treat.. 121, 469–477. [DOI] [PubMed] [Google Scholar]
- Chouvet, C. , Vicard, E. , Devonec, M. , Saez, S. , 1986. 1,25-Dihydroxyvitamin D3 inhibitory effect on the growth of two human breast cancer cell lines (MCF-7, BT-20). J. Steroid Biochem.. 24, 373–376. [DOI] [PubMed] [Google Scholar]
- Colston, K.W. , Chander, S.K. , Mackay, A.G. , Coombes, R.C. , 1992. Effects of synthetic vitamin D analogues on breast cancer cell proliferation in vivo and in vitro. Biochem. Pharmacol.. 44, 693–702. [DOI] [PubMed] [Google Scholar]
- Colston, K.W. , James, S.Y. , Ofori-Kuragu, E.A. , Binderup, L. , Grant, A.G. , 1997. Vitamin D receptors and anti-proliferative effects of vitamin D derivatives in human pancreatic carcinoma cells in vivo and in vitro. Br. J. Cancer. 76, 1017–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell, M.L. , Comstock, G.W. , Holick, M.F. , Bush, T.L. , 1992. Prediagnostic serum levels of 1,25-dihydroxyvitamin D and malignant melanoma. Photodermatol. Photoimmunol. Photomed.. 9, 109–112. [PubMed] [Google Scholar]
- Damian, D.L. , Kim, Y.J. , Dixon, K.M. , Halliday, G.M. , Javeri, A. , Mason, R.S. , 2010. Topical calcitriol protects from UV-induced genetic damage but suppresses cutaneous immunity in humans. Exp. Dermatol.. 19, e23–30. [DOI] [PubMed] [Google Scholar]
- Danielsson, C. , Fehsel, K. , Polly, P. , Carlberg, C. , 1998. Differential apoptotic response of human melanoma cells to 1 alpha, 25-dihydroxyvitamin D3 and its analogues. Cell Death Differ.. 5, 946–952. [DOI] [PubMed] [Google Scholar]
- Danielsson, C. , Torma, H. , Vahlquist, A. , Carlberg, C. , 1999. Positive and negative interaction of 1,25-dihydroxyvitamin D3 and the retinoid CD437 in the induction of human melanoma cell apoptosis. Int. J. Cancer. 81, 467–470. [DOI] [PubMed] [Google Scholar]
- Deeb, K.K. , Trump, D.L. , Johnson, C.S. , 2007. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat. Rev. Cancer. 7, 684–700. [DOI] [PubMed] [Google Scholar]
- Dixon, K.M. , Sequeira, V.B. , Camp, A.J. , Mason, R.S. , 2010. Vitamin D-fence. Photochem. Photobiol. Sci.. 9, 564–570. [DOI] [PubMed] [Google Scholar]
- Downing, A. , Yu, X.Q. , Newton-Bishop, J. , Forman, D. , 2008. Trends in prognostic factors and survival from cutaneous melanoma in Yorkshire, UK and New South Wales, Australia between 1993 and 2003. Int. J. Cancer. 123, 861–866. [DOI] [PubMed] [Google Scholar]
- Egan, K.M. , 2009. Vitamin D and melanoma. Ann. Epidemiol.. 19, 455–461. [DOI] [PubMed] [Google Scholar]
- Enioutina, E.Y. , Bareyan, D. , Daynes, R.A. , 2009. TLR-induced local metabolism of vitamin D3 plays an important role in the diversification of adaptive immune responses. J. Immunol.. 182, 4296–4305. [DOI] [PubMed] [Google Scholar]
- Faupel-Badger, J.M. , Diaw, L. , Albanes, D. , Virtamo, J. , Woodson, K. , Tangrea, J.A. , 2007. Lack of association between serum levels of 25-hydroxyvitamin D and the subsequent risk of prostate cancer in Finnish men. Cancer Epidemiol. Biomarkers Prev.. 16, 2784–2786. [DOI] [PubMed] [Google Scholar]
- Feskanich, D. , Ma, J. , Fuchs, C.S. , Kirkner, G.J. , Hankinson, S.E. , Hollis, B.W. , 2004. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol. Biomarkers Prev.. 13, 1502–1508. [PubMed] [Google Scholar]
- Fleet, J.C. , 2008. Molecular actions of vitamin D contributing to cancer prevention. Mol. Aspects Med.. 29, 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn, A. , Hirvonen, T. , Mensink, G.B. , Ocke, M.C. , Serra-Majem, L. , Stos, K. , 2009. Intake of selected nutrients from foods, from fortification and from supplements in various European countries. Food Nutr. Res.. 53, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, D.M. , Looker, A.C. , Chang, S.C. , Graubard, B.I. , 2007. Prospective study of serum vitamin D and cancer mortality in the United States. J. Natl. Cancer Inst.. 99, 1594–1602. [DOI] [PubMed] [Google Scholar]
- Froicu, M. , Cantorna, M.T. , 2007. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol.. 8, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froicu, M. , Zhu, Y. , Cantorna, M.T. , 2006. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 117, 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini, S. , Sera, F. , Cattaruzza, M.S. , Pasquini, P. , Picconi, O. , Boyle, P. , 2005. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer. 41, 45–60. [DOI] [PubMed] [Google Scholar]
- Gandini, S. , Raimondi, S. , Gnagnarella, P. , Dore, J.F. , Maisonneuve, P. , Testori, A. , 2009. Vitamin D and skin cancer: a meta-analysis. Eur. J. Cancer. 45, 634–641. [DOI] [PubMed] [Google Scholar]
- Garland, C.F. , Garland, F.C. , 1980. Do sunlight and vitamin D reduce the likelihood of colon cancer?. Int. J. Epidemiol.. 9, 227–231. [DOI] [PubMed] [Google Scholar]
- Giovannucci, E. , Liu, Y. , Rimm, E.B. , Hollis, B.W. , Fuchs, C.S. , Stampfer, M.J. , 2006. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst.. 98, 451–459. [DOI] [PubMed] [Google Scholar]
- Glass, D. , Lens, M. , Swaminathan, R. , Spector, T.D. , Bataille, V. , 2009. Pigmentation and vitamin D metabolism in Caucasians: low vitamin D serum levels in fair skin types in the UK. PLoS One. 4, e6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin, P.J. , Ennis, M. , Pritchard, K.I. , Koo, J. , Hood, N. , 2009. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J. Clin. Oncol.. 27, 3757–3763. [DOI] [PubMed] [Google Scholar]
- Gorman, S. , Kuritzky, L.A. , Judge, M.A. , Dixon, K.M. , McGlade, J.P. , Mason, R.S. , 2007. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+ CD25+ cells in the draining lymph nodes. J. Immunol.. 179, 6273–6283. [DOI] [PubMed] [Google Scholar]
- Gozdzik, A. , Barta, J.L. , Wu, H. , Wagner, D. , Cole, D.E. , Vieth, R. , 2008. Low wintertime vitamin D levels in a sample of healthy young adults of diverse ancestry living in the Toronto area: associations with vitamin D intake and skin pigmentation. BMC Public Health. 8, 336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, D.S. , Dalgleish, A.G. , Belonwu, N. , Fischer, M.D. , Bodman-Smith, M.D. , 2008. Topical imiquimod and intralesional interleukin-2 increase activated lymphocytes and restore the Th1/Th2 balance in patients with metastatic melanoma. Br. J. Dermatol.. 159, 606–614. [DOI] [PubMed] [Google Scholar]
- Gupta, R. , Dixon, K.M. , Deo, S.S. , Holliday, C.J. , Slater, M. , Halliday, G.M. , 2007. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J. Invest. Dermatol.. 127, 707–715. [DOI] [PubMed] [Google Scholar]
- Halsall, J.A. , Osborne, J.E. , Potter, L. , Pringle, J.H. , Hutchinson, P.E. , 2004. A novel polymorphism in the 1A promoter region of the vitamin D receptor is associated with altered susceptibility and prognosis in malignant melanoma. Br. J. Cancer. 91, 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. , Colditz, G.A. , Hunter, D.J. , 2006. Polymorphisms in the MTHFR and VDR genes and skin cancer risk. Carcinogenesis. [DOI] [PubMed] [Google Scholar]
- Hansen, C.M. , Binderup, L. , Hamberg, K.J. , Carlberg, C. , 2001. Vitamin D and cancer: effects of 1,25(OH)2D3 and its analogs on growth control and tumorigenesis. Front. Biosci.. 6, D820–D848. [DOI] [PubMed] [Google Scholar]
- Hofmann, J.N. , Yu, K. , Horst, R.L. , Hayes, R.B. , Purdue, M.P. , 2010. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol. Biomarkers Prev.. 19, 927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick, M.F. , 1981. The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. J. Invest. Dermatol.. 77, 51–58. [DOI] [PubMed] [Google Scholar]
- Holick, M.F. , 2003. Vitamin D: a millennium perspective. J. Cell Biochem.. 88, 296–307. [DOI] [PubMed] [Google Scholar]
- Holick, M.F. , 2004. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr.. 80, 1678S–1688S. [DOI] [PubMed] [Google Scholar]
- Holick, M.F. , 2008. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol. Aspects Med.. 29, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick, M.F. , Chen, T.C. , Lu, Z. , Sauter, E. , 2007. Vitamin D and skin physiology: a D-lightful story. J. Bone Miner. Res.. 22, (Suppl. 2) V28–V33. [DOI] [PubMed] [Google Scholar]
- Huotari, A. , Herzig, K.H. , 2008. Vitamin D and living in northern latitudes – an endemic risk area for vitamin D deficiency. Int. J. Circumpolar Health. 67, 164–178. [DOI] [PubMed] [Google Scholar]
- Hypponen, E. , Power, C. , 2007. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am. J. Clin. Nutr.. 85, 860–868. [DOI] [PubMed] [Google Scholar]
- Ingraham, B.A. , Bragdon, B. , Nohe, A. , 2008. Molecular basis of the potential of vitamin D to prevent cancer. Curr. Med. Res. Opin.. 24, 139–149. [DOI] [PubMed] [Google Scholar]
- Jacobs, E.T. , Giuliano, A.R. , Martinez, M.E. , Hollis, B.W. , Reid, M.E. , Marshall, J.R. , 2004. Plasma levels of 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and the risk of prostate cancer. J. Steroid Biochem. Mol. Biol.. 89–90, 533–537. [DOI] [PubMed] [Google Scholar]
- James, S.Y. , Mackay, A.G. , Colston, K.W. , 1996. Effects of 1,25 dihydroxyvitamin D3 and its analogues on induction of apoptosis in breast cancer cells. J. Steroid Biochem. Mol. Biol.. 58, 395–401. [DOI] [PubMed] [Google Scholar]
- Jeevan, A. , Ullrich, S.E. , Dizon, V.V. , Kripke, M.L. , 1992. Supernatants from ultraviolet-irradiated keratinocytes decrease the resistance and delayed-type hypersensitivity response to Mycobacterium bovis bacillus Calmette–Guerin in mice and impair the phagocytic ability of macrophages. Photodermatol. Photoimmunol. Photomed.. 9, 255–263. [PubMed] [Google Scholar]
- Jenab, M. , Bueno-de-Mesquita, H.B. , Ferrari, P. , van Duijnhoven, F.J. , Norat, T. , Pischon, T. , et al., 2010. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. BMJ 340, b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keisala, T. , Minasyan, A. , Lou, Y.R. , Zou, J. , Kalueff, A.V. , Pyykko, I. , 2009. Premature aging in vitamin D receptor mutant mice. J. Steroid Biochem. Mol. Biol.. 115, 91–97. [DOI] [PubMed] [Google Scholar]
- Krishnan, A.V. , Peehl, D.M. , Feldman, D. , 2003. Inhibition of prostate cancer growth by vitamin D: regulation of target gene expression. J. Cell Biochem.. 88, 363–371. [DOI] [PubMed] [Google Scholar]
- Kull, M. , Kallikorm, R. , Lember, M. , 2009. Body mass index determines sunbathing habits: implications on vitamin D levels. Intern. Med. J.. 39, 256–258. [DOI] [PubMed] [Google Scholar]
- Lappe, J.M. , Travers-Gustafson, D. , Davies, K.M. , Recker, R.R. , Heaney, R.P. , 2007. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am. J. Clin. Nutr.. 85, 1586–1591. [DOI] [PubMed] [Google Scholar]
- Li, H. , Stampfer, M.J. , Hollis, J.B. , Mucci, L.A. , Gaziano, J.M. , Hunter, D. , 2007. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 4, e103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Liu, Z. , Wang, L.E. , Gershenwald, J.E. , Lee, J.E. , Prieto, V.G. , 2008. Haplotype and genotypes of the VDR gene and cutaneous melanoma risk in non-Hispanic whites in Texas: a case-control study. Int. J. Cancer. 122, 2077–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker, A.C. , Dawson-Hughes, B. , Calvo, M.S. , Gunter, E.W. , Sahyoun, N.R. , 2002. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 30, 771–777. [DOI] [PubMed] [Google Scholar]
- Lucas, R.M. , McMichael, A.J. , Armstrong, B.K. , Smith, W.T. , 2008. Estimating the global disease burden due to ultraviolet radiation exposure. Int. J. Epidemiol.. 37, 654–667. [DOI] [PubMed] [Google Scholar]
- Malvy, D.J. , Guinot, C. , Preziosi, P. , Galan, P. , Chapuy, M.C. , Maamer, M. , 2000. Relationship between vitamin D status and skin phototype in general adult population. Photochem. Photobiol.. 71, 466–469. [DOI] [PubMed] [Google Scholar]
- Mason, R.S. , Sequeira, V.B. , Dixon, K.M. , Gordon-Thomson, C. , Pobre, K. , Dilley, A. , 2010. Photoprotection by 1alpha, 25-dihydroxyvitamin D and analogs: further studies on mechanisms and implications for UV-damage. J. Steroid Biochem. Mol. Biol.. 121, 164–168. [DOI] [PubMed] [Google Scholar]
- McCombie, A.M. , Mason, R.S. , Damian, D.L. , 2009. Vitamin D deficiency in Sydney skin cancer patients. Med. J. Aust.. 190, 102 [DOI] [PubMed] [Google Scholar]
- McCullough, M.L. , Stevens, V.L. , Patel, R. , Jacobs, E.J. , Bain, E.B. , Horst, R.L. , 2009. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res.. 11, R64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezawa, H. , Sugiura, T. , Watanabe, M. , Norizoe, C. , Takahashi, D. , Shimojima, A. , 2010. Serum vitamin D levels and survival of patients with colorectal cancer: post-hoc analysis of a prospective cohort study. BMC Cancer. 10, 347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, S. , Holley, S. , Bodiwala, D. , Luscombe, C.J. , French, M.E. , Liu, S. , 2006. Associations between G/A1229, A/G3944, T/C30875, C/T48200 and C/T65013 genotypes and haplotypes in the vitamin D receptor gene, ultraviolet radiation and susceptibility to prostate cancer. Ann. Hum. Genet.. 70, 226–236. [DOI] [PubMed] [Google Scholar]
- Nesby-O'Dell, S. , Scanlon, K.S. , Cogswell, M.E. , Gillespie, C. , Hollis, B.W. , Looker, A.C. , 2002. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Clin. Nutr.. 76, 187–192. [DOI] [PubMed] [Google Scholar]
- Newton-Bishop, J.A. , Beswick, S. , Randerson-Moor, J. , Chang, Y.M. , Affleck, P. , Elliott, F. , 2009. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. J. Clin. Oncol.. 27, 5439–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton-Bishop, J.A., Chang, Y.M., Elliott, F., Chan, M., Leake, S., Karpavicius, B., Haynes, S., Fitzgibbon, E., Kukalizch, K., Randerson-Moor, J., Elder, D.E., Bishop, D.T., Barrett, J.H., 2010. Relationship between sun exposure and melanoma risk for tumours in different body sites in a large case-control study in a temperate climate. Eur J Cancer. [DOI] [PMC free article] [PubMed]
- Noonan, F.P. , De Fabo, E.C. , Kripke, M.L. , 1981. Suppression of contact hypersensitivity by UV radiation and its relationship to UV-induced suppression of tumor immunity. Photochem. Photobiol.. 34, 683–689. [PubMed] [Google Scholar]
- Orwoll, E. , Nielson, C.M. , Marshall, L.M. , Lambert, L. , Holton, K.F. , Hoffman, A.R. , 2009. Vitamin D deficiency in older men. J. Clin. Endocrinol. Metab.. 94, 1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, H.G. , Gonzalez-Sancho, J.M. , Espada, J. , Berciano, M.T. , Puig, I. , Baulida, J. , 2001. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J. Cell Biol.. 154, 369–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri, C. , MacGregor, T. , Girgis, S. , Vigushin, D. , 2006. Serum 25-hydroxyvitamin D levels in early and advanced breast cancer. J. Clin. Pathol.. 59, 1334–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peehl, D.M. , Skowronski, R.J. , Leung, G.K. , Wong, S.T. , Stamey, T.A. , Feldman, D. , 1994. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res.. 54, 805–810. [PubMed] [Google Scholar]
- Pettersson, F. , Colston, K.W. , Dalgleish, A.G. , 2000. Differential and antagonistic effects of 9-cis-retinoic acid and vitamin D analogues on pancreatic cancer cells in vitro. Br. J. Cancer. 83, 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerson-Moor, J.A. , Taylor, J.C. , Elliott, F. , Chang, Y.M. , Beswick, S. , Kukalizch, K. , 2009. Vitamin D receptor gene polymorphisms, serum 25-hydroxyvitamin D levels, and melanoma: UK case-control comparisons and a meta-analysis of published VDR data. Eur. J. Cancer. 45, 3271–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichrath, J. , 2007. Sunlight, skin cancer and vitamin D: what are the conclusions of recent findings that protection against solar ultraviolet (UV) radiation causes 25-hydroxyvitamin D deficiency in solid organ-transplant recipients, xeroderma pigmentosum, and other risk groups?. J. Steroid Biochem. Mol. Biol.. 103, 664–667. [DOI] [PubMed] [Google Scholar]
- Reichrath, J. , 2007. Vitamin D and the skin: an ancient friend, revisited. Exp. Dermatol.. 16, 618–625. [DOI] [PubMed] [Google Scholar]
- Reichrath, J. , Querings, K. , 2004. No evidence for reduced 25-hydroxyvitamin D serum level in melanoma patients. Cancer Causes Contr.. 15, 97–98. [DOI] [PubMed] [Google Scholar]
- Reichrath, J. , Rafi, L. , Rech, M. , Meineke, V. , Tilgen, W. , Seifert, M. , 2004. No evidence for amplification of 25-hydroxyvitamin D-1alpha-OHase (1alpha-OHase) or 1,25-dihydroxyvitamin D-24-OHase (24-OHase) genes in malignant melanoma (MM). J. Steroid Biochem. Mol. Biol.. 89-90, 163–166. [DOI] [PubMed] [Google Scholar]
- Reichrath, J. , Lehmann, B. , Carlberg, C. , Varani, J. , Zouboulis, C.C. , 2007. Vitamins as hormones. Horm. Metab. Res.. 39, 71–84. [DOI] [PubMed] [Google Scholar]
- Reichrath, J. , Rech, M. , Moeini, M. , Meese, E. , Tilgen, W. , Seifert, M. , 2007. In vitro comparison of the vitamin D endocrine system in 1,25(OH)2D3-responsive and -resistant melanoma cells. Cancer Biol. Ther.. 6, 48–55. [DOI] [PubMed] [Google Scholar]
- Rhee, H.V. , Coebergh, J.W. , Vries, E.D. , 2009. Sunlight, vitamin D and the prevention of cancer: a systematic review of epidemiological studies. Eur. J. Cancer Prev.. [DOI] [PubMed] [Google Scholar]
- Santonocito, C. , Capizzi, R. , Concolino, P. , Lavieri, M.M. , Paradisi, A. , Gentileschi, S. , 2007. Association between cutaneous melanoma, Breslow thickness and vitamin D receptor BsmI polymorphism. Br. J. Dermatol.. 156, 277–282. [DOI] [PubMed] [Google Scholar]
- Schon, M.P. , Wienrich, B.G. , Drewniok, C. , Bong, A.B. , Eberle, J. , Geilen, C.C. , 2004. Death receptor-independent apoptosis in malignant melanoma induced by the small-molecule immune response modifier imiquimod. J. Invest. Dermatol.. 122, 1266–1276. [DOI] [PubMed] [Google Scholar]
- Schwartz, G.G. , Hill, C.C. , Oeler, T.A. , Becich, M.J. , Bahnson, R.R. , 1995. 1,25-Dihydroxy-16-ene-23-yne-vitamin D3 and prostate cancer cell proliferation in vivo. Urology. 46, 365–369. [DOI] [PubMed] [Google Scholar]
- Schwartz, G.G. , Wang, M.H. , Zang, M. , Singh, R.K. , Siegal, G.P. , 1997. 1 alpha,25-Dihydroxyvitamin D (calcitriol) inhibits the invasiveness of human prostate cancer cells. Cancer Epidemiol. Biomarkers Prev.. 6, 727–732. [PubMed] [Google Scholar]
- Schwartz, G.G. , Eads, D. , Rao, A. , Cramer, S.D. , Willingham, M.C. , Chen, T.C. , 2004. Pancreatic cancer cells express 25-hydroxyvitamin D-1 alpha-hydroxylase and their proliferation is inhibited by the prohormone 25-hydroxyvitamin D3. Carcinogenesis. 25, 1015–1026. [DOI] [PubMed] [Google Scholar]
- Seifert, M. , Rech, M. , Meineke, V. , Tilgen, W. , Reichrath, J. , 2004. Differential biological effects of 1,25-dihydroxyVitamin D3 on melanoma cell lines in vitro. J. Steroid Biochem. Mol. Biol.. 89–90, 375–379. [DOI] [PubMed] [Google Scholar]
- Signorello, L.B. , Williams, S.M. , Zheng, W. , Smith, J.R. , Long, J.R. , Cai, Q. , 2010. Blood vitamin D levels in relation to genetic estimation of African ancestry. Cancer Epidemiol. Biomarkers Prev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders, J. , Damoiseaux, J. , Menheere, P. , Hupperts, R. , 2008. Vitamin D as an immune modulator in multiple sclerosis, a review. J. Neuroimmunol.. 194, 7–17. [DOI] [PubMed] [Google Scholar]
- Snijder, M.B. , van Schoor, N.M. , Pluijm, S.M. , van Dam, R.M. , Visser, M. , Lips, P. , 2006. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J. Clin. Endocrinol. Metab.. 91, 2980–2985. [DOI] [PubMed] [Google Scholar]
- Stolzenberg-Solomon, R.Z. , Vieth, R. , Azad, A. , Pietinen, P. , Taylor, P.R. , Virtamo, J. , 2006. A prospective nested case-control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Res.. 66, 10213–10219. [DOI] [PubMed] [Google Scholar]
- Stolzenberg-Solomon, R.Z. , Jacobs, E.J. , Arslan, A.A. , Qi, D. , Patel, A.V. , Helzlsouer, K.J. , 2010. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: cohort consortium vitamin D pooling project of rarer cancers. Am. J. Epidemiol.. 172, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangrea, J. , Helzlsouer, K. , Pietinen, P. , Taylor, P. , Hollis, B. , Virtamo, J. , 1997. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Contr.. 8, 615–625. [DOI] [PubMed] [Google Scholar]
- Thomas, M.G. , Tebbutt, S. , Williamson, R.C. , 1992. Vitamin D and its metabolites inhibit cell proliferation in human rectal mucosa and a colon cancer cell line. Gut. 33, 1660–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis, R.C. , Crowe, F.L. , Allen, N.E. , Appleby, P.N. , Roddam, A.W. , Tjonneland, A. , 2009. Serum vitamin D and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition (EPIC). Am. J. Epidemiol.. 169, 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretli, S. , Hernes, E. , Berg, J.P. , Hestvik, U.E. , Robsahm, T.E. , 2009. Association between serum 25(OH)D and death from prostate cancer. Br. J. Cancer. 100, 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry, Z. , Xystrakis, E. , Richards, D.F. , McDonald, J. , Sattar, Z. , Cousins, D.J. , 2009. Ligation of TLR9 induced on human IL-10-secreting tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J. Clin. Invest.. 119, 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mei, I.A. , Ponsonby, A.L. , Engelsen, O. , Pasco, J.A. , McGrath, J.J. , Eyles, D.W. , 2007. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ. Health Perspect.. 115, 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wactawski-Wende, J. , Kotchen, J.M. , Anderson, G.L. , Assaf, A.R. , Brunner, R.L. , O'Sullivan, M.J. , 2006. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N. Engl. J. Med.. 354, 684–696. [DOI] [PubMed] [Google Scholar]
- Wang, C.Q. , Kripke, M.L. , Fidler, I.J. , 1990. Ultraviolet-B-irradiated mouse epidermis releases mediators that stimulate melanoma cells. Photodermatol. Photoimmunol. Photomed.. 7, 128–135. [PubMed] [Google Scholar]
- Wang, T.J. , Pencina, M.J. , Booth, S.L. , Jacques, P.F. , Ingelsson, E. , Lanier, K. , 2008. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 117, 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T.J. , Zhang, F. , Richards, J.B. , Kestenbaum, B. , van Meurs, J.B. , 2010. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 376, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittermann, A. , Gummert, J.F. , 2010. Sun, vitamin D, and cardiovascular disease. J Photochem Photobiol B. [DOI] [PubMed] [Google Scholar]


