Abstract
In the initial period after melanoma was recognised as a disease entity in the early 1800's, it was subclassified on the basis of its presumed origin (from a precursor naevus, from a melanocytic precursor lesion acquired during adult life or in previously blemish‐fee skin). In 1967 the eminent American pathologist, Dr Wallace Clark, proposed a histogenetic classification for melanoma in which the disease was subdivided predominantly on the basis of histopathological features of the intra‐epidermal component of the tumour adjacent to any dermal invasive component. The subtypes were superficial spreading melanoma (SSM), lentigo maligna melanoma (LMM) and nodular melanoma (NM). Whilst additional entities, including acral lentiginous melanoma, mucosal melanoma, desmoplastic melanoma and naevoid melanoma have since been recognised, SSM, LMM and NM remain in the latest (2006) version of the WHO melanoma classification. Clark's histogenetic classification has been criticised because the criteria upon which it is based include clinical features (such as the site of the melanoma) and non‐tumourous histopathological features (such as the character of the associated epidermis and the degree of solar elastosis) and also because of overlap in defining features, lack of an independent association with patient outcome and minimal relevance as a determinant of clinical management. However, such criticisms fail to acknowledge its importance in highlighting the myriad of clinical and histological guises of melanoma, which if not recognized by clinicians and pathologists will inevitably lead to a delay in diagnosis and a concomitant adverse clinical outcome. Recently, mutually exclusive oncogenic mutations in melanomas involving NRAS (15–20%), BRAF (50%), CKIT (2%), and GNAQ/GNA11 (50% of uveal melanomas) have been identified. This might herald the beginning of a new molecular classification of melanoma in which the biologically distinct subsets share a common oncogenic mechanism, behave clinically in a similar fashion and require similar clinical management. These discoveries are already being successfully exploited as therapeutic targets in clinical trials of metastatic melanoma patients with promising activity. Whilst there remains much to be discovered in this rapidly evolving field, there is already great optimism that more rational and effective therapies for melanoma patients will soon be widely available.
Keywords: BRAF, Classification, Diagnosis, Melanoma, Pathology, Prognosis, Staging, Targeted therapy, Treatment, Molecular biology
Highlights
Traditional Clark histogenetic melanoma classification is clinicopathological.
Clark classification highlights guises of melanoma and important for diagnosis.
Mutually exclusive oncogenic mutations in melanoma recently identified.
Molecular classification of melanoma emerging but will be refined in future.
BRAF and CKIT mutations being exploited as therapeutic targets.
1. Introduction
The traditional clinicopathological classification scheme for melanoma was based on gross clinical and pathological features and includes four main subtypes – superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma and acral lentiginous melanoma. The classification was based on the pioneering work of Wallace Clark, Vincent McGovern, Martin Mihm, Richard Reed and others in the late 1960's and 1970's (Arrington et al., 1977; Clark, 1967; Clark et al., 1969; McGovern, 1970). Since then a number of less common melanoma subtypes, such as desmoplastic melanoma (Chen et al., 2008; McCarthy et al., 2004; Scolyer and Thompson, 2005; Shaw et al., 2006) and naevoid melanoma, (Zembowicz et al., 2001) have been recognised and further characterized. These were included in the latest (2006) version of the World Health Organisation's authoritative “blue book” entitled “Pathology and Genetics: Tumours of the Skin” (Table 1) (LeBoit et al., 2006).
Table 1.
The melanoma subtypes listed in the current (2006) World Health Organisation's publication pathology and genetics: tumours of the skin.
| ‐ Superficial spreading melanoma |
| ‐ Nodular melanoma |
| ‐ Lentigo maligna melanoma |
| ‐ Acral lentiginous melanoma |
| ‐ Desmoplastic melanoma |
| ‐ Melanoma arising from a blue naevus |
| ‐ Melanoma arising in a congenital naevus |
| ‐ Melanoma of childhood |
| ‐ Naevoid melanoma |
| ‐Persistent melanoma |
It was originally suggested that each of the major melanoma subtypes was associated with a different prognosis and a characteristic clinical behaviour (Clark et al., 1969; McGovern, 1970; Mihm et al., 1971). However, more complex analyses of larger datasets performed over the subsequent 40 years with more powerful statistical methods, including the recent large evaluation of melanoma prognostic factors by the American Joint Committee on Cancer (AJCC) Melanoma Taskforce (Balch et al., 2009; Gershenwald et al., 2010; Soong et al., 2010), have shown otherwise. It is known well known that the prognosis for a patient with clinically localised primary cutaneous melanoma is principally determined by tumour thickness but other features also have an independent prognostic effect; these include other primary tumour characteristic such as its mitotic rate, the presence of ulceration, anatomical site and, to a lesser extent, Clark level of invasion and patient characteristics such as age and gender (Azzola et al., 2003; Francken et al., 2004; Scolyer et al., 2003). The major melanoma subtypes do not have independent prognostic significance. Furthermore, current recommendations for the clinical management of clinically localised primary cutaneous melanoma, including the width of excision margins and the appropriateness of sentinel lymph node biopsy, are made primarily on the basis of tumour thickness although other factors are also taken into account (Scolyer et al., 2008; Thompson et al., 2005). Nevertheless, melanoma subtype is not a major consideration influencing the treatment of the primary melanoma site. Similarly, the WHO subtype of melanoma does not influence the clinical management of melanoma patients with metastatic disease. For these reasons, some authorities have advocated abandoning the use of melanoma subclassification altogether. However, in our view, there are convincing reasons why this course of action would be entirely inappropriate. These reasons are discussed in more detail below.
During the past decade, critical molecular alterations in melanomas have been identified. Mutations in kinases in the mitogen‐activated protein (MAP) kinase signal transduction pathway have been found in about 70% of melanomas and their clinicopathological associations have recently been described (Curtin et al., 2005; Bauer et al., 2011). Furthermore, these oncogenic mutations in melanoma are now being exploited as therapeutic targets (Arkenau et al., 2010; Flaherty et al., 2010, 2010, [Link]). This has brought new relevance to melanoma classification because it has become important to identify subgroups of melanomas that share molecular pathogenic mechanisms and behave in a similar clinical fashion. The ultimate objective is to identify patients who are likely to benefit from targeted therapies. A comparison of the traditional clinicopathological melanoma classification with a classification based on the somatic mutation status reveals remarkable similarities. For example, melanomas associated with prominent solar damage (lentigo maligna melanomas) commonly have NRAS and sometimes c‐KIT mutations, while superficial spreading melanomas that arise in the skin of intermittently sun‐exposed areas often have BRAF mutations (Bauer et al., 2011, 2010, 2005, 2006, 2008). Whilst there remains much to be discovered in this rapidly evolving field and a definitive molecular‐based classification for melanoma is yet to emerge as a consequence of these recent discoveries, there is great optimism that more rational and effective therapies for melanoma patients, based on their mutation status will soon be generally available.
2. Historical perspective
To understand the present and what may lie ahead, it is often useful and informative to examine the past. This is certainly the case with regard to melanoma classification. Melanoma is a disease that has affected mankind for a long time. It has been identified in Inca mummies entombed in Peru more than 2000 years ago, and was probably known to the ancient Greek physician and father of modern medicine Hippocrates in the 5th century BC (Davis et al., 2004) Although not recognised as such at the time, the British surgeon John Hunter is credited with being the first person to operate on a patient with melanoma with curative intent (Davis et al., 2004). The excised tumour, from the neck of an adult female, is preserved in the Hunterian Museum of the Royal College of Surgeons in London. The large circumscribed tumour, which macroscopically appears black in parts, was examined histologically in 1967 and confirmed to be melanoma, most probably a metastasis. It is a widely held belief that the French physician Rene Laennec was the first to formally describe melanoma as a disease entity when he presented a lecture in Paris in 1804 and subsequently published his findings in a Bulletin in 1806. However, others have suggested that the French surgeon Dupuytren was the first to recognise melanoma and apparently he himself also claimed this honour (Davis et al., 2004). In 1820, the British general practitioner William Norris published the first report in the English language literature on melanoma. Jean Cruveilhier, a French anatomist and pathologist, produced illustrated descriptions of both primary and metastatic melanoma from autopsies in his atlas titled Anatomie Pathologique du Corps Humains published in 1829. In 1840, the British surgeon Samuel Cooper stated that advanced melanoma was untreatable and the best chance for benefit was to remove the disease at an early stage: these profound observations have remained true, at least in major part, until very recently.
In 1864, Sir James Paget noted that melanoma (“cancer”) “could develop in a mole”. In 1892, his British surgical colleague Jonathan Hutchinson documented that melanoma could also arise in pigmented lesions that were acquired during adult life, which he initially termed senile freckles. When documenting and illustrating a case of a malignant nodule developing in the upper cheek/lower eyelid region of a female patient in 1894, he suggested “lentigo melanosis” was a more apt designation for such lesions. The excised amelanotic nodule was examined pathologically by his son (Jonathan Hutchinson, Jr) who identified a malignant tumour composed of spindle shaped cells resembling a sarcoma which he therefore called melanotic sarcoma. In 1912, the French dermatologist Dubreuilh, provided further, more detailed descriptions of the pigmented lesions described by Hutchinson as lentigo melanosis and coined the term melanose circonscrite precancereuse: this subsequently became widely used in the English dermatology literature in its Latinised form, melanosis circumscripta praecancerosa (Klauder and Beerman, 1955). Furthermore, in perhaps the first formal melanoma classification, Dubreuilh noted at this time that melanoma could arise in a naevus, in a pigmented skin lesion appearing in adult life (melanosis circumscripta praecancerosa) or in previously blemish free skin (melanoma d'emblee) (Table 2).
Table 2.
A melanoma classification scheme reported by the French dermatologist Dubreuilh in 1912.
| Melanoma subtype | Synonym |
|---|---|
| Melanoma arising in a naevus | |
| Melanoma arising in a pigmented skin lesion appearing in adult life | melanose circonscrite precancereuse |
| melanosis circumscripta praecancerosa | |
| Melanoma arising in previously blemish‐free skin | Melanoma d'emblee |
In January 1967, a committee of Australian pathologists, chaired by Vincent McGovern, published their recommendations for melanoma classification and terminology (Committee of Australian Pathologists, 1967). They classified melanoma on the basis of both clinical and histological features according to the lesion from which it was thought to arise. These categories were melanoma arising in a: junctional naevus, compound naevus, Hutchinson's melanotic freckle (now usually referred to as lentigo maligna), pre‐malignant melanosis (now usually termed superficial spreading melanoma insitu) or previously blemish‐free skin as well as “other” and “indeterminate” categories. Interestingly, Hutchinson's melanotic freckle (now referred to as lentigo maligna and listed as a type of insitu melanoma in the current version of the WHO classification), was not listed as a form of intra‐epidermal melanoma and was categorised amongst the benign melanocyic tumours. They also recommended that the adjective “malignant’ always be used when referring to melanoma, a recommendation we do not support, as discussed further below.
3. Terminology
Since it was first recognised as an entity in the latter part of the 18th century, melanoma has been referred to in a variety of ways, many of which are mentioned above. Such terms have included melanotic sarcoma (to describe a malignant tumour composed histologically of spindle shaped cells resembling a sarcoma) (Low, 1912), melanoblastoma (McGovern, 1952) (to describe highly pleomorphic and mitotically active melanomas composed of epithelioid cells) (personal communication, Martin C Mihm, Jr., 2011) and, in the German literature, chromatophoroma (Hertzler and Gibson, 1914). The term “juvenile melanoma” referred to a tumour that is now known to be benign (but which shares many histological features with melanoma). It is now usually referred to as a Spitz naevus (Crotty et al., 2002; Dahlstrom et al., 2004). From the 1950s onwards, the term “malignant melanoma” was increasingly used, but in recent years use of the simple term “melanoma” has become more common.
While there may perhaps be some benefit in retaining the term “malignant” in relation to melanoma, it can be a source of considerable confusion and uncertainty because there is no known form of melanoma that is not malignant. Continued use of the term creates doubt and uncertainty in the minds of many patients, their relatives, nurses, medical students and even some doctors. We therefore suggest that the time has come to completely abandon the use of the terminology “malignant melanoma” and refer to it simply as melanoma.
Most importantly, the term malignant melanoma implies that some melanomas are not malignant, i.e. that they are benign. This is certainly not the case, although sometimes the pathologist cannot be certain whether a melanocytic tumour is malignant or not (Scolyer et al., 2010; Zembowicz and Scolyer, 2011). If there is a benefit, it is that it does indicate that the tumour is malignant, an important point that is sometimes not otherwise appreciated by patients. It is our experience, for example, that when eliciting a history from a patient he or she will often state that a number of “melanomas” have been removed in the past, when in fact the lesions removed were benign melanocytic naevi. Such patients clearly believe that their recently diagnosed “melanoma” is benign, and do not appreciate the serious prognostic implications of that diagnosis.
In our view, the well established term “melanoma” (derived from the Greek words “melas” meaning black and “oma” meaning tumour) is generally accepted and understood and a “malignant” description seems both unnecessary and confusing. It would seem to be time to make a clean break, accept the obvious, and abandon the term “malignant” melanoma completely.
There have also been numerous terminologies used for pre‐malignant and insitu melanomas and these have also caused much confusion (and continue to do so). The lesion now generally known as lentigo maligna has previously been referred to as Hutchinson's freckle, senile freckle, melanose circonscrite precancereuse, and Dubreuilh's melanosis circumscripta precancerosa. Terminology for lesions now classified as superficial spreading melanoma have included pagetoid melanoma and pre‐malignant melanosis and, confusingly, on some occasions such lesions were also included under the broad categories of melanose circonscrite precancereuse and Dubreuilh's melanosis circumscripta precancerosa. Thankfully, such terminology has also been allowed to pass gracefully into the pages of history.
4. The Clark clinicopathological melanoma classification/histogenetic melanoma subtypes
In 1967, Wallace Clark published his landmark publication on the histogenetic classification of melanoma that still remains the basis of the current version of the WHO classification of melanoma used today (Clark et al., 1969). He described three types of melanoma (lentigo maligna melanoma, superficial spreading melanoma and nodular melanoma) which were distinguished principally on the basis of the features of the intra‐epidermal component of the tumour peripheral to (rather than overlying) any dermal invasive component. The histological features upon which the subtypes are distinguished include mostly architectural features of the insitu melanoma (including the degree of pagetoid or lentiginous growth) and also some features of the non‐tumourous component such as changes in the thickness and character of the epidermis and the presence and degree of accompanying solar elastosis. To some extent, clinical features such as the anatomical site of the tumour and age of the patient also contributed to the histogenetic subtype and for this reason Clark's histogenetic subtyping of melanoma is usually referred to as a clinco‐pathological classification scheme.
In his original description, Clark suggested that there were differing prognoses associated with the different melanoma subtypes, a suggestion supported by evidence that he and others presented in 1969 and that subsequently presented by McGovern (Clark et al., 1969; McGovern, 1970; Mihm et al., 1971). In their 1969 publication, Clark et al also showed that the prognosis of melanoma was related to the depth of invasion which they categorised according to the level of the skin involved (now known as Clark levels) (Clark et al., 1969; McGovern, 1970; Mihm et al., 1971). Although it had been documented previously that the depth of invasion was associated with prognosis in melanoma (including by Allen and Spitz in 1953) (Allen and Spitz, 1953), this was prior to the publication of Alexander Breslow's landmark paper on the prognostic significance of tumour thickness in 1970 (Breslow, 1970).
In 1970, Vincent McGovern published his classification; this was similar to that proposed by Clark albeit using slightly different terminology: melanoma arising in Hutchinson's melanotic freckle (“associated with lentigo maligna” in Clark's terminology), melanoma arising in pre‐malignant melanosis (Clark: “superficial spreading melanoma”) and nodular melanoma (Clark: “nodular melanoma”) (McGovern, 1970).
At the International Pigment Cell Conference and International Cancer Conference held concurrently in Sydney in 1972, an international group of pathologists, chaired by Vincent McGovern, met to develop a consensus on the classification and histopathological reporting of melanoma. The classification, published in 1973 (McGovern et al., 1973), was based on Clark's original proposal and listed two forms of insitu melanoma: Hutchinson's melanotic freckle and superficial spreading melanoma, non‐invasive; and three forms of invasive melanoma: i) melanoma, invasive, with adjacent intra‐epidermal component of Hutchinson's melanotic freckle type, ii) melanoma, invasive, with adjacent intra‐epidermal component of superficial spreading type and iii) melanoma, invasive, without adjacent intra‐epidermal component (Table 3).
Table 3.
The 1972 Sydney classification of melanoma and list of synonyms reportedly in common use at that time.
| Recommended Terminology | Synonyms |
|---|---|
| Hutchinson's melanotic freckle | Huchinson's freckle |
| Senile freckle | |
| melanose circonscrite precancereuse | |
| Dubreuilh's melanosis circumscripta praecancerosa | |
| Superficial spreading melanoma, non‐invasive | Pagetoid melanoma |
| Premalignant melanosis | |
| melanose circonscrite precancereuse | |
| Dubreuilh's melanosis circumscripta praecancerosa | |
| In‐situ melanoma | |
| Melanoma, invasive, with adjacent intra‐epidermal component of Hutchinson's melanotic freckle | Lentigo maligna melanoma |
| Melanoma, invasive, with adjacent intra‐epidermal component of superficial spreading type | Superficial spreading melanoma |
| Pagetoid melanoma | |
| Melanoma with insitu component | |
| Melanoma, invasive, without adjacent intra‐epidermal component | Nodular melanoma |
| Melanoma d'emblee | |
In 1977, Arrington, Reed and colleagues described the features of melanomas involving acral skin as a distinct subtype of melanoma that has subsequently become known as acral lentiginous melanoma (Arrington et al., 1977).
The 1972 Sydney classification was revised at an international workshop held in Sydney in 1982 and subsequently published (McGovern et al., 1986). The original categories of the 1972 Sydney classification were retained (albeit with some slightly different terminologies) and new categories were added: malignant melanoma with an adjacent component of acral lentiginous type, malignant melanoma with an adjacent component of mucosal lentiginous type and malignant melanoma of unclassifiable histogenetic type (Table 4).
Table 4.
The revised Sydney classification of melanoma and a list of common synonyms published in 1986.
| Recommended Terminology | Synonyms |
|---|---|
| Malignant melanoma with an adjacent component of superficial spreading type | Superficial spreading melanoma |
| Pagetoid melanoma | |
| Malignant melanoma with an adjacent component of lentigo maligna type | Lentigo maligna melanoma |
| Malignant melanoma of Hutchinson's melanotic freckle type | |
| Malignant melanoma with an adjacent component of acral lentiginous type | Plantar‐ palmer‐, subunual lentiginous melanoma |
| Acral lentiginous melanoma | |
| Malignant melanoma with an adjacent component of mucosal lentiginous type | Mucosal lentiginous melanoma |
| Malignant melanoma with no adjacent component | Nodular melanoma |
| Malignant melanoma of unclassifiable histogenetic type | |
Since that time, a number of additional clinically and histologically distinct subtypes of melanoma have been described including desmoplastic melanoma (Jain and Allen, 1989), naevoid melanoma (Zembowicz et al., 2001) and melanomas arising in association with a blue naevus (sometimes inappropriately referred to as a malignant blue naevus) (Martin et al., 2009).
In the current (2006) version of the WHO Classification of Skin Tumours, the four major histogenetic subtypes are listed along with six other subtypes (Table 1) (LeBoit et al., 2006).
4.1. Clinical and pathological characteristics of the major melanoma histogenetic subtypes
Pathologically, the major histogenetic subtypes of melanoma are primarily distinguished on the basis of features of the intra‐epidermal component of the tumour adjacent to any dermal invasive component. When present, the latter does not show features that are specific for the various major melanoma histogenetic subtypes.
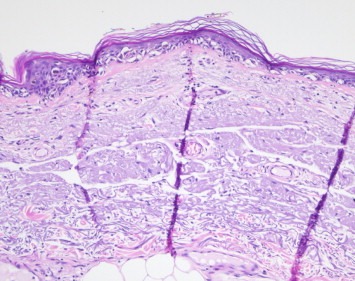
4.1.1. Superficial spreading melanoma (Figure 1)
Figure 1.

Superficial spreading melanoma.
Superficial spreading melanomas (SSM) usually occur in younger patients (median age: 5th decade) than nodular melanomas (NM) or lentigo maligna melanomas (LMM). They typically involve intermittently sun‐exposed anatomical sites such as the trunk, back and extremeties. SSM usually presents as a flat slowly growing irregular lesion with variegated pigmentation that enlarges in a radial manner unless dermal invasion supervenes; this is usually signified clinically by the presence of a raised area.
Histologically, the intra‐epidermal portion of SSM is characterised by the presence of large pleomorphic epithelioid melanocyte showing nested and single cell upward migration (Pagetoid epidermal invasion) (Figure 1). Other features include circumscription, variable epidermal thickening and prominent intracytoplasmic melanisation.
4.1.2. Nodular melanoma (Figure 2)
Figure 2.
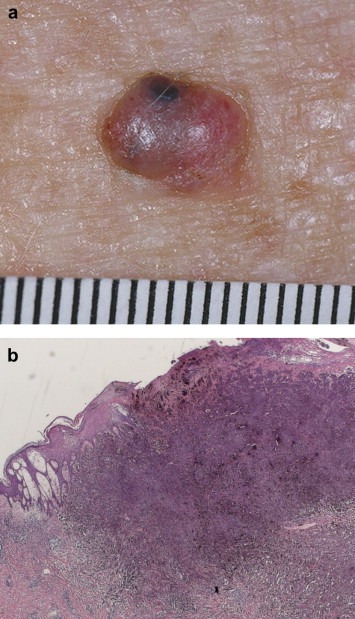
a & b. Nodular melanoma (Figure 2. a courtesy of Prof Scott Menzies copyright retained by Prof Scot Menzies).
Nodular melanomas tend to occur in slightly older patients than SSM (median age: 7th decade). They can occur at any location and usually present as a rapidly expanding nodule that may show ulceration and haemorrhage. Clinically they may be confused with other cutaneous tumours such as basal cell carcinomas, particularly if they are amelanotic.
Histologically in NM, there is no epidermal component extending beyond the edges of the dermal component (a “cut off” of three rete ridges is used by some authors) (Figure 2).
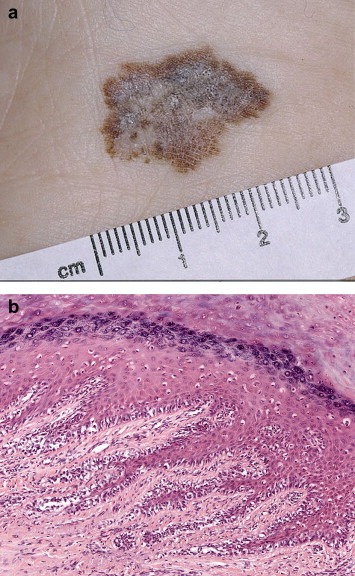
4.1.3. Lentigo maligna melanoma (Figure 3)
Figure 3.
Lentigo maligna melanoma insitu.
LMM usually involves chronically sun‐exposed cutaneous sites such as the head and neck region or forearm. It often presents as a large, variegated pigmented macule with irregular edges in an elderly patient (median age 8th decade), parts of which may become raised if dermal invasion supervenes.
LMM is characterized histologically by a lentiginous (back‐to‐back) proliferation of atypical melanocytes in severely sun damaged skin showing epidermal atrophy with loss of rete ridges, severe dermal solar elastosis and dermal thinning. Involvement of the superficial portion of skin adnexal structures and occasional multinucleate or giant melanocytes are often present. As the lesion progresses, the epidermal component often shows confluent lentiginous growth, nesting and pagetoid epidermal invasion, similar to that seen in superficial spreading melanoma.
4.1.4. Acral lentiginous melanoma (Figure 4)
Figure 4.
Lentigo maligna melanoma insitu.
ALMs, by definition, involve acral sites which are the palms, soles and subungual region. They usually present as slowly growing, variegated pigmented macules similar to LMM.
Histologically, ALM is usually characterized in its earliest recognisable form as single atypical melanocytes scattered along the junctional epidermal layer. The presence of an associated lymphocytic infiltrate partly obscuring the dermoepidermal junction can be a useful diagnostic clue. More advanced forms show confluent lentiginous and nested growth and some pagetoid epidermal invasion.
4.1.5. Other uncommon melanoma subtypes
Unlike the major melanoma subtypes mentioned above, desmoplastic melanoma is defined by the pathological features of its dermal component (Figure 5). The latter is formed by spindle‐shaped cells that are separated by stromal desmoplasia (new collagen formation). Depending on whether the desmoplastic component represents almost all or only part of the dermal invasive tumour, it can be further subdivided into pure and mixed subtypes. The overlying epidermis often shows evidence of a lentigo maligna or other epidermal melanocytic tumour. Naevoid melanomas are melanomas that histologically resemble a naevus, usually only at low power magnification, and can be divided into small cell (resembling a common naevus) (Figure 6) and Spitzoid (resembling a Spitz naevus) types. Other melanoma subtypes currently listed by the WHO are melanoma arising from a blue naevus, melanoma in a congenital naevus, melanoma in childhood and persistent melanoma. The latter refers to melanomas that recur locally following their incomplete removal (and should be distinguished from local recurrence of melanoma due to local metastasis).
Figure 5.
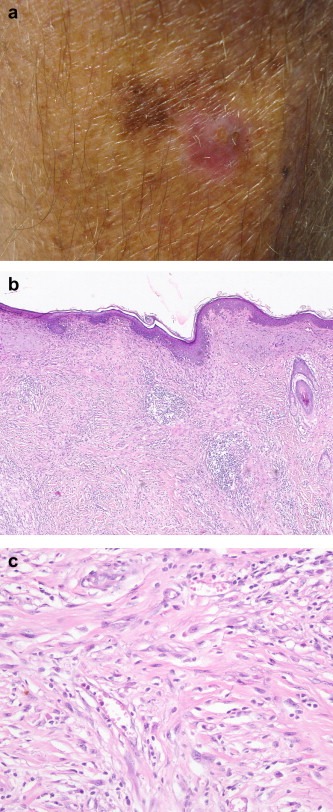
Lentigo maligna melanoma insitu.
Figure 6.
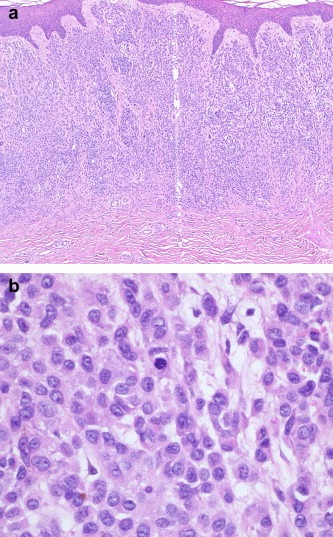
a & b. Naevoid melanoma.
To assess the usefulness of the traditional clinicopathological classification scheme of melanoma, as most recently espoused by the WHO, it is appropriate to step back and consider the definition, aims and purposes of disease classification.
5. The aims and purpose of disease classification
A disease classification scheme represents a set of categories into which all diseases and disease subtypes can be logically placed. The purpose of the classification determines its system of organisation. The primary goals of disease classification are to stratify diseases into biologically distinct subtypes using reproducible diagnostic criteria:
-
i.
share a common aetiological mechanism
-
ii.
behave clinically in a similar fashion, and
-
iii.
require similar clinical management
Another purpose of disease classification is to assist in the clinical recognition and pathological diagnosis of the condition.
Requirements for an optimal histopathological classification scheme include the following (Mooi and Krausz, 2007):
-
1.It is based on histopathological features that are:
-
a.well defined
-
b.reproducible
-
a.
-
2.Its defining features are:
-
a.always present in specific subgroups
-
b.always absent in other subgroups
-
c.mutually exclusive
-
d.properties of the tumour itself (not associated structures)
-
a.
-
3.
The subtypes differ from each other clinically
6. Limitations of the traditional clinicopathological classification of melanoma
The traditional clinicopathological melanoma classification, based on Clark's original proposal and its successors, including the current WHO classification, have been criticised because of their deficiencies when measured against some of the aforementioned benchmarks. Some of these deficiencies are discussed below.
The traditional classification scheme is not based purely on histopathological features. The anatomical site of the tumour must also be taken into account in determining the subcategory (particularly for acral tumours and, to some extent, those occurring in sun‐exposed sites) and, to a lesser extent, the age of the patient. Another criticism is that many of the histological features are shared by a number of melanoma subtypes. For example, pagetoid epidermal invasion is characteristic of superficial spreading melanoma but is also often present in advanced cases of lentigo maligna and acral lentiginous melanoma. The lentiginous growth pattern in lentigo maligna and acral lentiginous (and mucosal) melanomas is also very similar; they are distinguished based on the anatomical site of the tumour and changes in the associated structures. Another shortcoming of the classification scheme is that some of the diagnostic features are those related to the histopathological features of the non‐tumourous component (rather than the tumour itself). Examples include changes of the epidermis: atrophy with loss of rete ridges is characteristic of lentigo maligna whilst epidermal thickening is often present in superficial spreading melanoma and acral lentiginous melanoma, and is virtually always seen in Spitzoid melanoma. Furthermore, characteristics of the dermis are also important, such as severe solar elastosis in lentigo maligna. Another criticism is that it is possible to have more than one melanoma subtype present in a given tumour and indeed this may result in it being classified differently by different pathologists. For example, desmoplastic melanoma is often accompanied by a component of lentigo maligna in the overlying epidermis and naevoid melanomas often fulfil the criteria for nodular or, in some cases showing Spitzoid features, superficial spreading melanoma.
7. Influence of traditional clinicopathological melanoma classification on prognosis and management
It has further been suggested that the traditional clinicopathological classification scheme has little if any clinical relevance, particularly in terms of its influence on prognosis and in directing patient management. Indeed it is true that the major histogenetic subtype is not an independent predictor of outcome in patients with clinically localized primary cutaneous melanomas (Scolyer et al., 2008; Thompson et al., 2005). However, there are some notable exceptions. It is now known, for example, that the desmoplastic melanoma subtype, particularly its pure form, behaves more like a sarcoma than other subtypes of melanoma, with more frequent haematogenous (often pulmonary) metastasis than lymphatic (nodal) metastasis (Murali et al., 2010). In fact, as a consequence, sentinel node biopsy is not offered to patients with desmoplastic melanomas in some melanoma treatment centres because of the very low frequency of sentinel node positivity (Scolyer and Prieto, 2011). Furthermore, because of its propensity for local recurrence and the frequent difficulty in obtaining adequate excision margins, post operative radiotherapy is often administered in patients with desmoplastic melanoma (Chen et al., 2008). This provides another example of how management is impacted by melanoma subtype.
The periphery of the insitu component of lentigo maligna, acral lentiginous (and mucosal) melanomas is often discontinuous and poorly defined, with a gradual reduction in the density of the melanocytes and their degree of cytological atypia at the periphery of the lesion and the changes merging with those in the adjacent uninvolved skin (Scolyer et al., 2004). As a result, it is well known that the relationship of the tumour to the excision margins can be difficult to ascertain with certainty. This information, along with clinical features, must be taken into account when managing patients with these subtypes of melanoma, particularly as they typically involve cosmetically and functionally sensitive anatomical sites.
8. The traditional clinico‐pathological melanoma classification scheme is important for early diagnosis and melanoma disease control
The lynchpins of melanoma disease control are primary prevention, and early detection and treatment. One of the most important benefits of the traditional clinciopathological/histogenetic melanoma classification scheme is that it highlights the diverse clinical and morphological forms of melanoma which, if not known and recognized by clinicians and pathologists, will result in delayed diagnosis or misdiagnosis of melanoma and contribute to adverse clinical outcomes (McCarthy and Scolyer, 2004). For example, nodular melanomas may clinically resemble numerous other tumours such as basal cell carcinoma, dermatofibroma and seborrheoic keratosis and are often not clinically suspected or diagnosed until they are clinically advanced, thick tumours (Izikson et al., 2002). Amelanotic melanomas are also particularly prone to clinical misdiagnosis. Other subtypes of melanoma that are prone to clinical and pathlogical misdiagnosis include desmoplastic melanoma, naevoid melanoma and acral melanoma.
9. Emerging molecular subtypes of melanoma
In the early part of the 21st century there have been great technological advances that have facilitated new understanding of the molecular pathogenesis of a wide variety of diseases including melanoma (Thompson et al., 2009). In 2005, Curtin, Bastian and colleagues published their landmark study showing the presence of common oncogenic somatic gene mutations and amplifications in many human melanomas and their associations with the anatomical site of the primary tumour and the degree of chronic sun damage (Curtin et al., 2005).
Aberrant activation of the mitogen‐activated protein kinase (MAPK) pathway is present in over 80% of primary melanomas (Curtin et al., 2005, 2006, 2002, 2010, [Link], 2008, 2009, 2010), and mutations in proteins along the RAS‐RAF‐MEK‐ERK pathway are thought to be mutually exclusive (Curtin et al., 2006; Garrido and Bastian, 2010; Van Raamsdonk et al., 2009). Such mutations have been documented in all subtypes of melanoma, including cutaneous (50% BRAF, 15% NRAS, up to 17% CKIT chronic sun damage), mucosal (11% BRAF, 5% NRAS, 21% CKIT) and uveal (50% GNAQ) melanomas (Figure 7). Oncogenic NRAS also induces the phosphatidylinositol 3′ kinase (PI3K) cascade. MAPK signalling can lead to proliferation in transformed cells, but is also thought to induce a potent form of growth arrest, known as senescence, in normal melanocytes (Mooi and Peeper, 2006).
Figure 7.
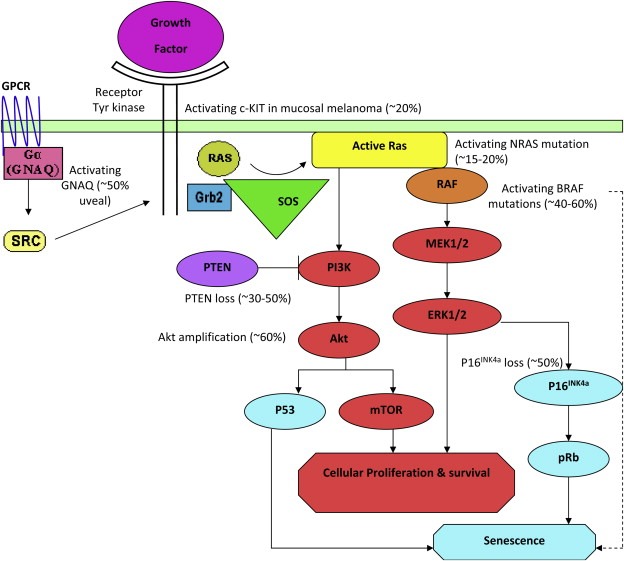
Activated oncogenic pathways important in melanoma (figure modified from Arkenau et al (Arkenau et al., 2010)). Oncogenic NRAS, BRAF, GNAQ and CKIT signal through the MAPK pathway. Oncogenic NRAS also induces the phosphatidylinositol 3′ kinase (PI3K) cascade. The approximate proportion of melanomas with mutations are shown. GPCR, G‐protein coupled receptor.
In 2002, BRAF somatic missense mutations were identified in a wide variety of human cancers, most resulting in constitutive activating of BRAF (Davies et al., 2002). Now, over 75 mutations in the BRAF gene have been identified, the most common mutations occurring in the activating segment of Exon 15. In the Australian population, approximately 74% are a point mutation in DNA resulting in a single amino acid substitution at Valine 600 to Glutamic acid (V600E) in the protein (Long et al., in press). A further 20% are Valine 600 to Lysine (V600K), and 6% are other genotypes. BRAF mutant melanomas are associated with young patient age (occurring in up to 86% of melanoma patients aged 20–30 years), trunk or limb primary site and low cumulative solar damage (Table 5). Whilst BRAF mutation status does not appear to affect prognosis at the time of diagnosis of the primary tumour, recent evidence suggests that BRAF mutant melanoma is associated with a poorer prognosis in metastatic melanoma patients.
Table 5.
Emerging molecular classification scheme for metastatic melanoma currently available.
| Mutation | Percentage of melanomas with mutation | Phenotypic associations | Targeted therapies currently available or in clinical development |
|---|---|---|---|
| BRAF | 50% | Young patient age | BRAFi |
| Intermittent sun‐exposed primary site | |||
| Trunk, limbs primary sites | |||
| High naevus counts | |||
| Few freckles | |||
| Tans easily | MEKi | ||
| Superficial spreading histogenetic type | |||
| See text for other morphologic associations | |||
| NRAS | 15–20% | Intermittent sun‐exposed sites (weak association) | ?MEKi |
| ?PI3Ki | |||
| Low or absent pagetoid epidermal scatter | ?AKTi | ||
| Peripheral circumscription | |||
| CKIT | 2% (10–20% of acral and mucosal melanomas) | Acral and mucosal sites | CKITi |
| Acral and mucosal lentiginous histogenetic melanoma types | other tyrosine kinase inhibitors | ||
| GNAQ/GNA11 | 50% of uveal melanomas | Uveal melanoma | Unknown |
| ?blue naevus‐like melanomas | |||
10. Similarities and differences between the molecular subtypes and traditional histogenetic subtypes
Just as the physical appearance of an individual is, to a major extent, determined by their inherited maternal and paternal genetic make up, the phenotypes of tumours are also determined by their genotype. As described above, melanomas can display a wide variety of architectural and cytological features and these, together with features of the host response, are key pathological diagnostic criteria for melanoma. The presence of many of these morphological features is associated with the presence of specific oncogenic mutations in melanoma. For example, BRAF mutant melanomas typically occur on skin sites intermittently exposed to the sun and histologically they characteristically show features of Clark's superficial spreading melanomas. They are also associated with the following pathological features: a high degree of Pagetoid spread, prominent nesting, heavy melanin pigmentation, large epithelioid cells, circumscription, epidermal thickening, and minimal solar elastosis (Broekaert et al., 2010; Viros et al., 2008). In contrast, KIT mutant melanomas usually show histological features of acral lentinginous and mucosal lentiginous melanomas (Curtin et al., 2005, 2006). However, nodular melanomas do not show characteristic molecular features. This suggests that nodular melanoma is probably not a subtype of the same taxonomic rank as the others (Broekaert et al., 2010).
11. Molecular subtypes and treatment of metastatic melanoma
Oncogenic mutations in melanoma are now being successfully exploited as therapeutic targets. The four known mutations (BRAF, GNAQ, CKIT, NRAS) affecting the MAPK pathway are largely mutually exclusive and are attractive targets for systemic therapies. As the field develops, more mutations that may be amenable to targeted therapies are emerging, e.g. mutation in the ERBB4 tyrosine receptor kinase has been reported in approximately 19% of melanomas. In addition, the clinicopathological phenotype corresponding to these mutations may provide a framework for selecting patients for different types of mutation testing, and thus targeted therapies.
The BRAF protein is a particularly attractive target for therapeutics because a) it is so prevalent in melanoma, b) it is a serine/threonine kinase with a kinase domain that is amenable to rationally designed blocking agents and c) it is not activated in normal cells. Inhibition of the mutant BRAF protein in melanoma shows promise in clinical trials of patients with BRAF mutant metastatic melanoma (Flaherty et al., 2010, 2010). Recent data from the phase 2 clinical trial of RG7204 (previously known as RO5185426/PLX‐4032) showed a 55% response rate in patients with BRAF mutant (V600E) metastatic melanoma and a progression free survival (PFS) of 6–7 months. This compares favourably with standard chemotherapy (DTIC/Dacarbazine) that has a response rate of <10% and a PFS of 1.6 months in more recently conducted randomised controlled clinical trials in metastatic melanoma. In addition, the BRAF inhibitor GSK2118436 has activity in melanoma brain metastases. In a subgroup of 10 patients with asymptomatic previously untreated brain metastases, 9 had a decrease of 20% or more in the size of their brain metastases. The toxicities of the BRAF inhibitors in humans are mild and well‐tolerated, however in a small but significant proportion of patients, there is an increased rate of cutaneous squamous cell carcinomas and keratoacanthomas. The mechanism of oncogenesis is unknown, although theories relate to paradoxical oncogenic activation of the MAPK pathway in keratinocytes.
MEK inhibitors are also showing encouraging activity in mutant BRAF metastatic melanoma. In preclinical models, the combination of BRAF and MEK inhibitors shows particular promise and is the subject of recently commenced clinical trials.
Similarly, patients with CKIT mutant metastatic melanoma have also been treated with CKIT inhibitors with some clinical responses (Garrido and Bastian, 2010).
Despite the encouraging response and PFS in early phase clinical trials of BRAF inhibitors for BRAF mutant metastatic melanoma, all but a very few patients relapse, some with aggressive progression and death (acquired resistance) (see article by Puzanov et al elsewhere in this issue). In addition, approximately 20–40% of patients do not respond at baseline (primary resistance), and complete remissions are extremely rare. Similarly, in other cancers, acquisition of drug resistance to kinase inhibitors has seriously restricted the duration of therapeutic response. It is likely that discovery of the mechanisms of resistance will lead to new rational and effective multiagent/multimodality therapeutic approaches.
12. Future directions
There have been great advances in our understanding of the molecular pathogenesis of melanoma during the past decade and these are already contributing to new ways to classify melanoma and treat patients with metastatic disease. In addition to the important information on mutation status, much complex data has also already been generated from RNA expression, proteomic and microRNA analysis of melanomas (see article by Michiels et al elsewhere in this issue). However, the clinical relevance of much of this information and how best to utilize it for melanoma classification and personalized patient care remain major challenges for the future. As the significance of these data and new discoveries become better understood it is likely that a refined melanoma classification will emerge that integrates currently known clinical, pathological and molecular data as well as yet undiscovered biological data. This will undoubtedly lead to more precise characterisation of biologically distinct subtypes of melanoma that can be utilised as a basis for personalized medicine for melanoma patients.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The clinical images were kindly provided by Prof Scott Menzies (Sydney Melanoma Diagnostic Centre). Assistance from colleagues at Melanoma Institute Australia and Royal Prince Alfred Hospital is also gratefully acknowledged.
Scolyer Richard A., Long Georgina V. and Thompson John F., (2011), Evolving concepts in melanoma classification and their relevance to multidisciplinary melanoma patient care, Molecular Oncology, 5, doi: 10.1016/j.molonc.2011.03.002.
References
- Allen, A.C. , Spitz, S. , 1953. Malignant melanoma; a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer. 6, 1–45. [DOI] [PubMed] [Google Scholar]
- Arkenau, H.T. , Kefford, R. , Long, G.V. , 2010. Targeting BRAF for patients with melanoma. Br. J. Cancer. 104, 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrington, J.H. , Reed, R.J. , Ichinose, H. , Krementz, E.T. , 1977. Plantar lentiginous melanoma: a distinctive variant of human cutaneous malignant melanoma. Am. J. Surg. Pathol.. 1, 131–143. [PubMed] [Google Scholar]
- Azzola, M.F. , Shaw, H.M. , Thompson, J.F. , Soong, S.J. , Scolyer, R.A. , Watson, G.F. , Colman, M.H. , Zhang, Y. , 2003. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer. 97, 1488–1498. [DOI] [PubMed] [Google Scholar]
- Balch, C.M. , Gershenwald, J.E. , Soong, S.J. , Thompson, J.F. , Atkins, M.B. , Byrd, D.R. , Buzaid, A.C. , Cochran, A.J. , Coit, D.G. , Ding, S. , Eggermont, A.M. , Flaherty, K.T. , Gimotty, P.A. , Kirkwood, J.M. , McMasters, K.M. , Mihm, M.C. , Morton, D.L. , Ross, M.I. , Sober, A.J. , Sondak, V.K. , 2009. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol.. 27, 6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, J. , Buttner, P. , Murali, R. , Okamoto, I. , Kolaitis, N.A. , Landi, M.T. , Scolyer, R.A. , Bastian, B.C. , 2011. BRAF mutations in cutaneous melanoma are independently associated with age, anatomic site of the primary tumor and the degree of solar elastosis at the primary tumor site. Pigment Cell Melanoma Res.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow, A. , 1970. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann. Surg.. 172, 902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert, S.M. , Roy, R. , Okamoto, I. , van den Oord, J. , Bauer, J. , Garbe, C. , Barnhill, R.L. , Busam, K.J. , Cochran, A.J. , Cook, M.G. , Elder, D.E. , McCarthy, S.W. , Mihm, M.C. , Schadendorf, D. , Scolyer, R.A. , Spatz, A. , Bastian, B.C. , 2010. Genetic and morphologic features for melanoma classification. Pigment Cell Melanoma Res.. 23, 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J.Y. , Hruby, G. , Scolyer, R.A. , Murali, R. , Hong, A. , Fitzgerald, P. , Pham, T.T. , Quinn, M.J. , Thompson, J.F. , 2008. Desmoplastic neurotropic melanoma: a clinicopathologic analysis of 128 cases. Cancer. 113, 2770–2778. [DOI] [PubMed] [Google Scholar]
- Clark, W.H. , 1967. A classification of malignant melanoma in man correlated with histogenesis and biologic behavior. Advanc. Biol. Skin. 8, 621–647. [Google Scholar]
- Clark, W.H. , From, L. , Bernardino, E.A. , Mihm, M.C. , 1969. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res.. 29, 705–727. [PubMed] [Google Scholar]
- Committee of Australian Pathologists 1967. Moles and malignant melanoma: terminology and classification. Med. J. Aust.. 1, 123–125. [DOI] [PubMed] [Google Scholar]
- Crotty, K.A. , Scolyer, R.A. , Li, L. , Palmer, A.A. , Wang, L. , McCarthy, S.W. , 2002. Spitz naevus versus spitzoid melanoma: when and how can they be distinguished?. Pathology. 34, 6–12. [DOI] [PubMed] [Google Scholar]
- Curtin, J.A. , Fridlyand, J. , Kageshita, T. , Patel, H.N. , Busam, K.J. , Kutzner, H. , Cho, K.H. , Aiba, S. , Brocker, E.B. , LeBoit, P.E. , Pinkel, D. , Bastian, B.C. , 2005. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med.. 353, 2135–2147. [DOI] [PubMed] [Google Scholar]
- Curtin, J.A. , Busam, K. , Pinkel, D. , Bastian, B.C. , 2006. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol.. 24, 4340–4346. [DOI] [PubMed] [Google Scholar]
- Dahlstrom, J.E. , Scolyer, R.A. , Thompson, J.F. , Jain, S. , 2004. Spitz naevus: diagnostic problems and their management implications. Pathology. 36, 452–457. [DOI] [PubMed] [Google Scholar]
- Davies, H. , Bignell, G.R. , Cox, C. , Stephens, P. , Edkins, S. , Clegg, S. , Teague, J. , Woffendin, H. , Garnett, M.J. , Bottomley, W. , Davis, N. , Dicks, E. , Ewing, R. , Floyd, Y. , Gray, K. , Hall, S. , Hawes, R. , Hughes, J. , Kosmidou, V. , Menzies, A. , Mould, C. , Parker, A. , Stevens, C. , Watt, S. , Hooper, S. , Wilson, R. , Jayatilake, H. , Gusterson, B.A. , Cooper, C. , Shipley, J. , Hargrave, D. , Pritchard-Jones, K. , Maitland, N. , Chenevix-Trench, G. , Riggins, G.J. , Bigner, D.D. , Palmieri, G. , Cossu, A. , Flanagan, A. , Nicholson, A. , Ho, J.W. , Leung, S.Y. , Yuen, S.T. , Weber, B.L. , Seigler, H.F. , Darrow, T.L. , Paterson, H. , Marais, R. , Marshall, C.J. , Wooster, R. , Stratton, M.R. , Futreal, P.A. , 2002. Mutations of the BRAF gene in human cancer. Nature. 417, 949–954. [DOI] [PubMed] [Google Scholar]
- Davis, N.C. , Shaw, H.M. , McCarthy, W.H. , 2004. Melanoma: an historical perspective. In Thomson J.F., Morton D.L., Kroon B.B.R.(Eds.), Cutaneous Melanoma. 1–12. [Google Scholar]
- Flaherty, K.T. , Hodi, F.S. , Bastian, B.C. , 2010. Mutation-driven drug development in melanoma. Curr. Opin. Oncol.. 22, 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty, K.T. , Puzanov, I. , Kim, K.B. , Ribas, A. , McArthur, G.A. , Sosman, J.A. , O'Dwyer, P.J. , Lee, R.J. , Grippo, J.F. , Nolop, K. , Chapman, P.B. , 2010. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med.. 363, 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francken, A.B. , Shaw, H.M. , Thompson, J.F. , Soong, S.J. , Accortt, N.A. , Azzola, M.F. , Scolyer, R.A. , Milton, G.W. , McCarthy, W.H. , Colman, M.H. , McGovern, V.J. , 2004. The prognostic importance of tumor mitotic rate confirmed in 1317 patients with primary cutaneous melanoma and long follow-up. Ann. Surg. Oncol.. 11, 426–433. [DOI] [PubMed] [Google Scholar]
- Garrido, M.C. , Bastian, B.C. , 2010. KIT as a therapeutic target in melanoma. J. Invest. Dermatol.. 130, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenwald, J.E. , Soong, S.J. , Balch, C.M. , 2010. 2010 TNM staging system for cutaneous melanoma and beyond. Ann. Surg. Oncol.. 17, 1475–1477. [DOI] [PubMed] [Google Scholar]
- Hertzler, A.E. , Gibson, E.T. , 1914. Melanoblastomas of the Foot (Chromatophoroma, Melanoma, Melanosarcoma). Ann. Surg.. 60, 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izikson, L. , Sober, A.J. , Mihm, M.C. , Zembowicz, A. , 2002. Prevalence of melanoma clinically resembling seborrheic keratosis: analysis of 9204 cases. Arch. Dermatol.. 138, 1562–1566. [DOI] [PubMed] [Google Scholar]
- Jain, S. , Allen, P.W. , 1989. Desmoplastic malignant melanoma and its variants. A study of 45 cases. Am. J. Surg. Pathol.. 13, 358–373. [DOI] [PubMed] [Google Scholar]
- Klauder, J.V. , Beerman, H. , 1955. Melanotic freckle (Hutchinson), melanose circonscrite precancereuse (Dubreuilh). AMA Arch. Derm.. 71, 2–10. [DOI] [PubMed] [Google Scholar]
- In LeBoit P.E., Burg G., Weedon D., Sarasain A.(Eds.), World Health Organization Classification of Tumours. IARC Press; Lyon: [Google Scholar]
- Long, G.V., Menzies A, M., Nagrial A, M., Haydu, L.E., Hamilton, A.L., Mann, G.J., Hughes, T.M., Thompson, J.F., Scolyer, R.A. and Kefford, R.F. Prognostic and Clinicopathologic Associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol, in press. [DOI] [PubMed]
- Low, V.W. , 1912. Multiple melanotic sarcomata of the skin, possibly secondary to a melanotic sarcoma of the skin, removed eighteen months ago. Proc. R Soc. Med.. 5, 104–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, R.C. , Murali, R. , Scolyer, R.A. , Fitzgerald, P. , Colman, M.H. , Thompson, J.F. , 2009. So-called "malignant blue nevus": a clinicopathologic study of 23 patients. Cancer. 115, 2949–2955. [DOI] [PubMed] [Google Scholar]
- McCarthy, S.W. , Scolyer, R.A. , 2004. Melanocytic lesions of the face: diagnostic pitfalls. Ann. Acad. Med. Singapore. 33, 3–14. [PubMed] [Google Scholar]
- McCarthy, S.W. , Scolyer, R.A. , Palmer, A.A. , 2004. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 36, 445–451. [DOI] [PubMed] [Google Scholar]
- McGovern, V. , 1952. Melanoblastoma. Med. J. Aust.. 1, 139–142. [DOI] [PubMed] [Google Scholar]
- McGovern, V.J. , 1970. The classification of melanoma and its relationship with prognosis. Pathology. 2, 85–98. [DOI] [PubMed] [Google Scholar]
- McGovern, V.J. , Mihm, M.C. , Bailly, C. , Booth, J.C. , Clark, W.H. , Cochran, A.J. , Hardy, E.G. , Hicks, J.D. , Levene, A. , Lewis, M.G. , Little, J.H. , Milton, G.W. , 1973. The classification of malignant melanoma and its histologic reporting. Cancer. 32, 1446–1457. [DOI] [PubMed] [Google Scholar]
- McGovern, V.J. , Cochran, A.J. , Van der Esch, E.P. , Little, J.H. , MacLennan, R. , 1986. The classification of malignant melanoma, its histological reporting and registration: a revision of the 1972 Sydney classification. Pathology. 18, 12–21. [DOI] [PubMed] [Google Scholar]
- Mihm, M.C. , Clark, W.H. , From, L. , 1971. The clinical diagnosis, classification and histogenetic concepts of the early stages of cutaneous malignant melanomas. N. Engl. J. Med.. 284, 1078–1082. [DOI] [PubMed] [Google Scholar]
- Mooi, W.J. , Krausz, T. , 2007. Pathology of Melanocytic Disorders second ed. Oxford University Press Inc; [Google Scholar]
- Mooi, W.J. , Peeper, D.S. , 2006. Oncogene-induced cell senescence–halting on the road to cancer. N. Engl. J. Med.. 355, 1037–1046. [DOI] [PubMed] [Google Scholar]
- Murali, R. , Shaw, H.M. , Lai, K. , McCarthy, S.W. , Quinn, M.J. , Stretch, J.R. , Thompson, J.F. , Scolyer, R.A. , 2010. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 116, 4130–4138. [DOI] [PubMed] [Google Scholar]
- Platz, A. , Egyhazi, S. , Ringborg, U. , Hansson, J. , 2008. Human cutaneous melanoma; a review of NRAS and BRAF mutation frequencies in relation to histogenetic subclass and body site. Mol. Oncol.. 1, 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolyer, R.A. , Prieto, V.G. , 2011. Melanoma pathology: important issues for clinicians involved in the multidisciplinary care of melanoma patients. Surg. Oncol. Clin. N. Am.. 20, 19–37. [DOI] [PubMed] [Google Scholar]
- Scolyer, R.A. , Thompson, J.F. , 2005. Desmoplastic melanoma: a heterogeneous entity in which subclassification as "pure" or "mixed" may have important prognostic significance. Ann. Surg. Oncol.. 12, 197–199. [DOI] [PubMed] [Google Scholar]
- Scolyer, R.A. , Shaw, H.M. , Thompson, J.F. , Li, L.X. , Colman, M.H. , Lo, S.K. , McCarthy, S.W. , Palmer, A.A. , Nicoll, K.D. , Dutta, B. , Slobedman, E. , Watson, G.F. , Stretch, J.R. , 2003. Interobserver reproducibility of histopathologic prognostic variables in primary cutaneous melanomas. Am. J. Surg. Pathol.. 27, 1571–1576. [DOI] [PubMed] [Google Scholar]
- Scolyer, R.A. , Thompson, J.F. , Stretch, J.R. , Sharma, R. , McCarthy, S.W. , 2004. Pathology of melanocytic lesions: new, controversial, and clinically important issues. J. Surg. Oncol.. 86, 200–211. [DOI] [PubMed] [Google Scholar]
- Scolyer, R.A. , Murali, R. , Satzger, I. , Thompson, J.F. , 2008. The detection and significance of melanoma micrometastases in sentinel nodes. Surg. Oncol.. 17, 165–174. [DOI] [PubMed] [Google Scholar]
- Scolyer, R.A. , Murali, R. , McCarthy, S.W. , Thompson, J.F. , 2010. Histologically ambiguous ("borderline") primary cutaneous melanocytic tumors: approaches to patient management including the roles of molecular testing and sentinel lymph node biopsy. Arch. Pathol. Lab. Med.. 134, 1770–1777. [DOI] [PubMed] [Google Scholar]
- Shaw, H.M. , Quinn, M.J. , Scolyer, R.A. , Thompson, J.F. , 2006. Survival in patients with desmoplastic melanoma. J. Clin. Oncol.. 24, e12 author reply e13 [DOI] [PubMed] [Google Scholar]
- Soong, S.J. , Ding, S. , Coit, D. , Balch, C.M. , Gershenwald, J.E. , Thompson, J.F. , Gimotty, P. , 2010. Predicting survival outcome of localized melanoma: an electronic prediction tool based on the AJCC melanoma database. Ann. Surg. Oncol.. 17, 2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J.F. , Scolyer, R.A. , Kefford, R.F. , 2005. Cutaneous melanoma. Lancet. 365, 687–701. [DOI] [PubMed] [Google Scholar]
- Thompson, J.F. , Scolyer, R.A. , Kefford, R.F. , 2009. Cutaneous melanoma in the era of molecular profiling. Lancet. 374, 362–365. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk, C.D. , Bezrookove, V. , Green, G. , Bauer, J. , Gaugler, L. , O'Brien, J.M. , Simpson, E.M. , Barsh, G.S. , Bastian, B.C. , 2009. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 457, 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk, C.D. , Griewank, K.G. , Crosby, M.B. , Garrido, M.C. , Vemula, S. , Wiesner, T. , Obenauf, A.C. , Wackernagel, W. , Green, G. , Bouvier, N. , Sozen, M.M. , Baimukanova, G. , Roy, R. , Heguy, A. , Dolgalev, I. , Khanin, R. , Busam, K. , Speicher, M.R. , O'Brien, J. , Bastian, B.C. , 2010. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med.. 363, 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viros, A. , Fridlyand, J. , Bauer, J. , Lasithiotakis, K. , Garbe, C. , Pinkel, D. , Bastian, B.C. , 2008. Improving melanoma classification by integrating genetic and morphologic features. PLoS Med.. 5, e120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembowicz, A. , Scolyer, R.A. , 2011. Nevus/Melanocytoma/Melanoma: an emerging paradigm for classification of melanocytic neoplasms?. Arch. Pathol. Lab. Med.. 135, 300–306. [DOI] [PubMed] [Google Scholar]
- Zembowicz, A. , McCusker, M. , Chiarelli, C. , Dei Tos, A.P. , Granter, S.R. , Calonje, E. , McKee, P.H. , 2001. Morphological analysis of nevoid melanoma: a study of 20 cases with a review of the literature. Am. J. Dermatopathol. 23, 167–175. [DOI] [PubMed] [Google Scholar]


