Abstract
The transcription factor SOX11 is a novel diagnostic marker for mantle cell lymphoma (MCL), distinguishing this aggressive tumor from potential simulators. Recent data also show that the level of SOX11 correlates to in vitro growth properties in MCL, as well as the clinical progression. We have previously shown that MCL‐associated pathways, such as Rb‐E2F, are dysregulated leading to decreased proliferation upon overexpression of SOX11, emphasizing the impact of SOX11 on MCL‐specific gene expression and growth control. However, it remains to be determined which growth regulatory pathways that are induced upon SOX11 knock‐down, leading to an increased cellular growth. Consequently, we established a model cell line with constitutive down‐regulation of SOX11. The highly proliferative features of this cell line were investigated by gene expression analysis, proliferation assay, cell cycle distribution and potential to induce tumors in NOD‐SCID mice. Our in vitro studies demonstrated a SOX11‐dependent regulation of MCL‐specific gene expression. In addition, we identified autotaxin (ATX) to be regulated by SOX11. Our results clearly showed a correlation between SOX11 level and cellular growth rate, which was dependent on ATX, as well as a direct relation between the level of SOX11 in tumorigenic cells and the growth rate of these tumors in NOD‐SCID mice.
Keywords: SOX11, Proliferation, Autotaxin, NOD-SCID mice, MCL, Z138
Highlights
A model cell line with constitutive down‐regulation of the SOX11 has been created.
The model cell line shows SOX11‐dependent regulation of MCL‐associated genes.
SOX11 knock‐down promotes a proliferative feature and altered cell cycle profile.
Increased growth can be reversed by the specific inhibition of autotaxin (ATX).
SOX11 knocked cells show a more aggressive behavior in vivo.
Abbreviations
- autotaxin
ATX
- counts per minute
cpm
- fetal bovine serum
FBS
- immunohistochemistry
IHC
- interference
iRNA
- intravenously
i.v
- mantle cell lymphoma
MCL
- phosphate buffered saline
PBS
- quantitative real-time polymerase chain reaction
Q-RT-PCR
- room temperature
RT
- scramble
SCR
- western blot
WB
1. Introduction
The neural transcription factor SOX11 (Penzo‐Mendez, 2010) has recently gained great interest as a diagnostic marker for mantle cell lymphoma (MCL) (Chen et al., 2009; Dictor et al., 2009; Ek et al., 2008; Fernandez et al., 2010; Mozos et al., 2009; Wang et al., 2008). Apart from MCL, SOX11 is also present in a subset of Burkitt's lymphoma, B‐ and T‐cell lymphoblastic leukemia (Dictor et al., 2009; Mozos et al., 2009), and some hairy cell leukemias (Chen et al., 2009), while other major lymphoma subtypes, including FL, completely lack expression of SOX11. We have previously shown that SOX11 is epigenetically regulated, (Gustavsson et al., 2010) although the underlying events leading to a differential methylation pattern remain to be determined. However, SOX11 has different partnering transcription factors (Kuhlbrodt et al., 1998; Wiebe et al., 2003) and its function must consequently be discussed in the relevant cellular context. We have recently identified SOX11 as a growth regulatory molecule in MCL, as transient knock‐down and overexpression of SOX11 in vitro changed the proliferation rate (Gustavsson et al., 2010). In addition, clinical data suggest a correlation between SOX11 expression levels and the aggressiveness of disease. In fact, the expression of SOX11 in MCL is associated with improved survival (Wang et al., 2008), although other data point towards a different role of SOX11 in non‐indolent MCL (Fernandez et al., 2010). However, both these observations need to be confirmed in larger clinical cohorts.
In this study, we use the MCL cell line Z138 to create a model cell line (Z138‐SOX11knocked) with constitutively reduced levels of SOX11, using a retroviral transduction of specific shRNA. As control we used the same cell line carrying a scrambled shRNA sequence (Z138‐SCR). The established cell lines were characterized by determining their, (i) gene expression (ii) functional characteristics and (iii) in vivo tumor formation. Our data clearly demonstrates that SOX11 directly regulates a large number of genes, previously shown to be specifically expressed in MCL compared to non‐malignant B cell populations or other B cell lymphomas, i.e. MCL‐specific genes (Obrador‐Hevia et al., 2009). This is consistent with SOX11 being a disease‐defining antigen, both phenotypically and functionally. Validation was focused on these genes, known to be consistently deregulated in primary MCL (Obrador‐Hevia et al., 2009). Proliferation and cell cycle analysis further confirmed our previously suggested growth‐regulatory role of SOX11 (Gustavsson et al., 2010). For the first time, we show that this growth regulatory effect is, at least in part, mediated by the increased levels of the secreted plasma lysophospholipase D enzyme autotaxin (ATX), known to be associated with aggressiveness of disease (Braddock, 2010; Samadi et al., 2011). Of clinical interest, the increased proliferation of Z138‐SOX11knocked was translated into a more aggressive tumor behavior in vivo, as shown using NOD‐SCID mice.
2. Material and methods
2.1. Retroviral production
Two different iRNAs, (i) one targeting SOX11 mRNA (5′‐CAAGTATGTTGGTACGTTATGTT‐3′) (Eurofins MWG Operon, Ebersberg, Germany) and (ii) the other targeting a scrambled sequence (SCR) (5′‐AGTACTGCTTACGATACGGTTTT‐3′), were cloned into the retroviral vector, pRSMX_PG, a kind gift from Louis M Staudt, (NCI‐Bethesda, MD, USA), as previously described (Ngo et al., 2006). In brief, sequences were cloned into the vector between the two restriction sites BglI and HindIII. Retroviral production was performed in Phoenix cells (American Type Culture Collection (ATCC)) (Rockville, MD) cultivated in DMEM (Invitrogen, San Diego, CA, USA) supplemented with 2 mM l‐glutamine (Invitrogen) and 10% fetal bovine serum (FBS, Invitrogen). At day of transfection the cells were 80% confluent and the media was changed to also include 1% Sodium Pyruvate (Invitrogen) and 1% HEPES 1 M (10 mM final concentration) (Invitrogen). The cells were transfected with a cocktail of DNA vectors (retroviral vectors pRSMX_PG–SCR or pRSMX_PG–SOX11knocked, 10 μg, Gag pol (pcDNA3.MLVgp) 6 μg, RD114 coding viral envelop 5 μg). To enable satisfying transfection, Calcium Phosphate Transfection Kit (CAPHOS) (Sigma–Aldrich St. Louis, USA) was used, according to the manufacturer's protocol. The cell supernatant containing retroviruses was harvested after 48 and 72 h, filtered to remove cellular debris, and stored at −80 °C. The viral titre was determined using the GFP gene.
2.2. Infection of target cells
The MCL cell line Z138 (kindly provided by Dr. Martin Dyer, Leicester University) was used to create a model cellular system for constitutive SOX11 knock‐down. The cells were cultured under standard conditions (humidified atmosphere, 5% CO2, 37 °C) and maintained in R10 medium (RPMI‐1640, Thermo Scientific HyClone, Logan, UT, USA) supplemented with 10% FBS (Invitrogen) and 1% 2 mM l‐glutamine (Invitrogen). The cell cultures were kept in log phase at a density of 0.75–0.8 × 106 cells/ml. Viral transfection was carried out at a multiplicity of infection (MOI) of 2, during which the media was changed to R2 (RPMI‐1640 (Invitrogen) supplemented with 2% FBS (Invitrogen)), with the addition of 8 μg/ml polybrene (Sigma–Aldrich). After over‐night incubation, the modified cell lines were washed with PBS and cultivated in R10 for an additional 24 h before addition of 5–20 μg/ml puromycin (Sigma–Aldrich) to allow selection of successfully transfected cells. The virally transduced and modified versions of the Z138 wild type (WT) cell lines, carrying SOX11 specific or scrambled shRNA sequences are referred to as Z138‐SOX11knocked and Z138‐SCR, respectively. The WT Z138 cell line is referred to as Z138‐WT.
2.3. Verification of SOX11 levels by quantitative real time PCR (Q‐RT‐PCR)
Lysis of cells, synthesis and amplification of cDNA were performed, using the Fast SYBR® Green Cell‐to‐CT kit (Applied Biosystem, CA, USA), according to manufacturer's protocol. Briefly, 1 × 105 cells were washed in PBS, lysed and treated with DNAse for 5 min, the lysates were reverse‐transcribed with random primers and RT Enzyme Mix (Applied Biosystem) in SYBR® RT Buffer at 37 °C for 60 min, followed by enzyme inactivation at 95 °C for 5 min.
For the amplification of SOX11 cDNA, the PCR reaction was performed as described previously (Gustavsson et al., 2010). For confirmation of the six genes identified by DNA microarray data TaqMan‐based Q‐RT‐PCR was performed, using TaqMan® Fast Universal PCR Master Mix (Applied Biosystem) and the TaqMan assays (all from Applied Biosystem) Hs00165563_m1 (paraoxonase 2, PON2), Hs00189310_m1 (drebrin 1, DBN1), Hs00195010_m1 (FARP1), Hs00905125_m1 (autotaxin, ATX), Hs00191446_m1 (Homer homolog 3, HOMER3), Hs00544069_m1 (L1 cell adhesion molecule, L1CAM). 18S (Hs99999901_s1) was used as reference gene. All data were analyzed with 7500 software v2.0.3 (Applied Biosystem).
2.4. Protein purification, quantification and analysis
Cells were harvested, washed with PBS and incubated on ice in 200 μl lysis‐buffer (1% NP40 (Sigma–Aldrich)/Protease Inhibitor cocktail (Roche, Basel, Switzerland) in PBS) for 30 min and cellular debris were removed by centrifugation (16,000 × g, 4 °C for 15 min). The protein concentration in each sample was measured using the BCA Kit (Thermo Scientific), according the manufacturer's protocol. Briefly, samples were mixed with BCA reagents, incubated at 37 °C for 20 min, and the absorbance was measured at 562 nm with a series of BSA dilutions (5–100 μg/ml) used as standard. To perform western blot (WB) cell lysates, containing 25 μg of protein, were loaded on NuPAGE 10% Bis‐Tris gels (Invitrogen) and electrophoresis was performed under reducing conditions for ∼60 min at 100 V. The proteins were then blotted onto PVDF membrane (Amersham Hybond‐P, GE Healthcare, Uppsala, Sweden) for 40 min at 15 V. The PVDF membrane was blocked and washed with 5% milk/PBS over‐night prior to staining (45 min, room temperature (RT)) with the primary antibody rabbit‐anti‐SOX11 (Atlas Antibodies Stockholm, Sweden) and the endogenous control antibody rabbit‐anti‐GAPDH (Abcam Cambridge, UK). Detection was performed using HRP‐labeled swine anti‐rabbit antibody (Dako Glostrup, Denmark) and SuperSignal West Femto Max Sensitivity Substrate (Thermo Scientific) according to manufacturer's protocol. The luminescent signal was detected with the Fluor‐S MultiImager CCD camera and QuantityOne software (Bio‐Rad Laboratories, California, USA).
2.5. Gene expression analysis
One million cells were washed in PBS and re‐suspended in 200 μl Trizol (Invitrogen). Preparation of tRNA, preparation of HuGene ST 1.0 arrays and raw data extraction was performed as previously described (Gustavsson et al., 2010) and in accordance with the instruction of the manufacturer (Affymetrix Inc., Santa Clara, CA). Quantile normalization using RMA and quality control was performed in the Expression Console 1.0 software (Affymetrix Inc.). Genes that had a 1.2 fold change comparing Z138‐SCR vs. Z138‐SOX11knocked were selected for further analysis. Ingenuity Pathway Analysis (9.0 version 3206, Ingenuity Systems, Redwood City, CA) was used to extract information on cellular function and localization.
2.6. Assessment of cellular growth in relation to ATX expression
Cells were kept in log phase for 24 h prior to the experiment. Before addition of the ATX inhibitor, cells were extensively washed in PBS and diluted to 1 × 106 cells/ml in fresh R10 with (10 μM final concentration) or without the ATX inhibitor S32826 (Echelon Biosciences, Salt Lake City, USA). After additional 24 h, 50.000 cells/well were seeded in a 96 well plate, in triplicates. Proliferation assays were performed, as described previously (Ortega‐Paino et al., 2008).
2.7. Cell cycle analysis of Z138 and modified derivatives
The different cell lines were diluted with R10 to 0.2, 0.6, 0.8, 1 and 2 million cells/ml and cultivated in 24 well plates at 37 °C. After 24 h, cells were counted and cell cycle analysis performed. Briefly, approximately 400.000 cells were harvested, washed once, re‐suspended in 2 ml cold PBS (Sigma–Aldrich) followed by addition of 5 ml 96% ethanol (Altia Corporation, Rajamäki, Finland) and incubated over‐night at −20 °C. Fixated cells were spun down and re‐suspended in 500 μl PBS (Thermo Scientific Hyclone) with 50 μg/ml propidium iodide (PI, Sigma–Aldrich), 0.1 mg/ml RNAse (Fermentas Life Sciences, Burlington, ON, Canada) and 0.05% Triton X‐100 (Sigma Chemical Co., St Louis, USA). All samples were incubated 40 min at 37 °C, washed with PBS and analyzed on FACS Canto™ II (BD Biosciences, San Jose, CA, USA). Data were analyzed using FCS Express MultiCycle plug‐in model 6 (De Novo Software, LA CA, USA).
2.8. Transplantation of human tumor cells into NOD‐SCID mice
At least 3–7 days before transplantation, the culture media for Z138‐SOX11knocked and Z138‐SCR was supplemented with 2–5 μg/ml puromycin (Sigma–Aldrich) to select transfected cells. 18–24 h prior to transplantation the viability of cells was confirmed to be above 90% and cells were diluted to 800.000 cells/ml, to ensure exponential growth at time of transplantation. At the day of transplantation, cells were washed, re‐suspended in R10 and kept at RT until transplantation (1–3 h). Injections were performed at four different time points (each defined as one experiment) with equal number of animals injected with Z138‐SCR and Z138‐SOX11knocked in gender matched groups. Animal experimental procedures were performed strictly in accordance with the related University Ethics Committee (approval M229‐09). Briefly, six to ten weeks old NOD‐SCID mice (The Jackson Laboratory, Sacramento, California, USA, (bred at Lund University for generations)) were intravenously (i.v.) injected in the tail vein with 0.5, 5 or 10 × 106 cells (Z138‐SCR or Z138‐SOX11knocked) or R10 as control. After transplantation the mice were monitored daily and their weight controlled twice a week. Symptoms of tumor growth were identified 21–56 days after injection and included slow movements, hunchbacked feature, shaggy coat, weight loose, paralyzed hind legs and death. As soon as symptoms arouse the mice were sacrificed using carbon dioxide or cervical dislocation. Sacrificed mice were kept cold until dissection.
2.9. Analysis of tumor cells post transplantation
Organs from the mice were analyzed using three different methods: (i) flow cytometry, (ii) immunohistochemistry (IHC) and (iii) histological examination. (i) Single cell suspensions were prepared from visible tumors, liver, lungs, heart, kidneys, spleen and brain. Furthermore, cells from bone marrow and blood were collected. Cells were stained with CD19‐PE (Dako), CD38‐PE (BD Biosciences) and HLA‐DR‐APC (BD Biosciences) and analyzed using FACS Canto™ II (BD Biosciences). Gating on size and granularity, based on Z138‐WT cells, confirmed maintained scatter profile, comparing cells pre‐ and post‐transplantation. Similarly, analysis of CD19, CD38 and HLA‐DR confirmed a maintained phenotype; (ii) Tissues, facial tumors and brains were fixed in formalin for 48 h, paraffin embedded and analyzed using IHC. Briefly, 2‐4 μm‐thick paraffin tissue sections were deparaffinized and rehydrated in a water bath. Sections were incubated with the primary antibody rabbit‐anti‐SOX11 (Atlas Antibodies) or mice‐anti‐CD20 (Dako) at RT and the signal was detected with REAL EnVision detection system (Dako), containing the secondary goat‐anti‐rabbit/mouse (CD20) or goat‐anti‐rabbit (SOX11) antibody conjugated to a peroxidase conjugated polymer. The reaction was visualized by 3,3′‐diaminobenzidine (Dako) and counterstained with Mayers hematoxylin (Sigma–Aldrich). (iii) Histological examination was performed at National Veterinary Institute (SVA) in Uppsala, Sweden. At day 29 post injection, three mice were sent to SVA following the general procedures determined by the animal facility at Lund University. Upon arrival, the mice were sacrificed and tissue specimens collected at necropsy were fixed in neutral buffered formalin. The brain, bone marrow, heart, intestines, kidney, liver, lungs, lymph node, skeletal muscle, skin, spinal cord, spleen, testis and stomach were routinely processed for histology, embedded in paraffin, sectioned at 4 μm and stained with hematoxylin and eosin.
3. Results
3.1. Generation of a constitutively SOX11 silenced Z138 cell line
In order to study the genes and the corresponding biological functions regulated by SOX11, we modified the MCL cell line Z138 (Medeiros et al., 2006) through retroviral infection of the pRSMX vector (Ngo et al., 2006), carrying SOX11‐specific shRNA or a scrambled control. Four iRNA sequences targeting different regions of SOX11 mRNA were initially tested. SOX11‐specific iRNA sequence‐1 showed the most prominent effect on SOX11 knock‐down, as assessed by WB analysis (data not shown), and was selected for all the further analysis. The generated stable cell lines were named Z138‐SOX11knocked and Z138‐SCR, respectively. The down‐regulation of SOX11 mRNA and protein in Z138‐SOX11knocked compared to Z138‐SCR and Z138‐WT was confirmed by Q‐RT‐PCR and WB (Figure 1).
Figure 1.
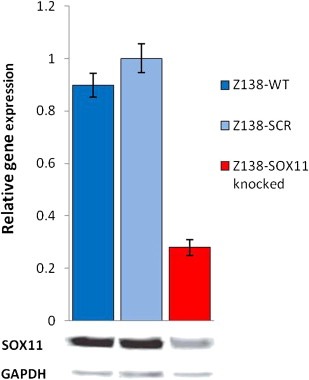
Verification of SOX11 mRNA and protein down‐regulation. Q‐RT‐PCR and WB confirms down‐regulation of SOX11 in Z138‐SOX11knocked compared to Z138‐SCR and Z138‐WT cells.
3.2. SOX11 regulate cellular growth rate
We have previously shown that transient knock‐down of SOX11 induced an increased growth rate (Gustavsson et al., 2010) and analysis of the established Z138‐SOX11knocked and Z138‐SCR cell lines confirmed that also constitutive knock‐down of SOX11 resulted in altered growth characteristics (Figure 2a). Of note, the cellular growth was assessed, using various cell densities and the difference in growth rate between Z138‐SOX11knocked and Z138‐SCR was most pronounced in log phase growth (Figure 2a). The difference in growth rate indicated that the cell cycle may be affected by the SOX11 knock‐down. This was confirmed by comparing frequencies of cells in G0/G1, S and G2/M phase, where the Z138‐SOX11knocked cell line had a low frequency of cells in G0 and an increased fraction (40–45%) of cells in S phase, compared to Z138‐SCR (25–30%) (Figure 2b).
Figure 2.
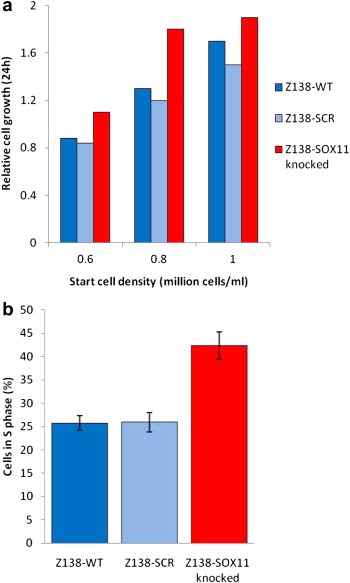
SOX11‐related differences in growth rate. (a) Growth rate was assessed through cell counting using tryptan blue exclusion and revealed a SOX11‐related difference in growth rate. (b) Cell cycle analysis confirmed an increased fraction of cells in S phase in Z138‐SOX11knocked compared to Z138‐SCR and Z138‐WT cells.
3.3. Gene expression analysis and TaqMan verification of target genes
To identify genes associated with altered SOX11 expression level, transcriptional analysis was performed. Bioinformatic analysis using fold change criteria (>1.2) identified 7000 genes with a differential expression in Z138‐SOX11knocked, compared to Z138‐SCR. Since SOX11 has been shown to identify MCL phenotypically, we focused on those differentially regulated transcripts that previously have been identified by us, as specifically over expressed in MCL (Ek et al., 2002, 2006). This procedure resulted in identification of 44 transcripts (Table 1), present in primary MCL tumors as supposed to normal tissue, as well as regulated upon SOX11 knock‐down in Z138. Furthermore, eight of these differentially regulated genes overlapped with a previously identified list of 30 genes, consistently deregulated in MCL compared to normal tissue or other lymphomas, as reviewed by Obrador‐Hevia et al. (2009) (marked in Table 1). Of note, similar to SOX11, several of the identified genes (Table 1) have important functions in development of the CNS or neuron function. These genes include, DBN1 (Kojima and Shirao, 2007; Wang et al., 2010), FARP1 (Zhuang et al., 2009), HOMER3 (Ehrengruber et al., 2004) and L1CAM (Schafer and Altevogt, 2010). This suggests that SOX11 regulate these genes, not only in MCL, but also in other tissues. Moreover, many of the identified genes are functionally involved in proliferation, apoptosis and migration, which may explain the functional effect seen upon SOX11 knock‐down (see Cellular function Table 1).
Table 1.
Genes deregulated upon SOX11 knock‐down.
| Symbol | Gene title | FC | Role in cellb | Localizationb |
|---|---|---|---|---|
| ATX, ENPP2a | Autotaxin, ectonucleotide pyrophosphatase/phosphodiesterase 2 | 1.77 | Chemotaxis, invasion, proliferationc | Plasma membrane |
| GNPDA1 | Glucosamine‐6‐phosphate deaminase 1 | 1.45 | Unknown | Cytoplasm |
| LTA | Lymphotoxin alpha (TNF superfamily, member 1) | 1.40 | Apoptosis, activation, proliferationc | Extracellular space |
| TRO | Trophinin | 1.33 | Adhesion, growth, proliferation | Plasma membrane |
| LGALS1 | Lectin, galactoside‐binding, soluble, 1 | 1.31 | Cell death, apoptosis, proliferationc | Extracellular space |
| STAT1 | signal transducer and activator of transcription 1, 91 kDa | 1.28 | Apoptosis, expression, proliferationc | Nucleus |
| HSPB1 | Heat shock 27 kDa protein 1 | 1.26 | Apoptosis, cell death, growthc | Cytoplasm |
| SRGN | Serglycin | 1.26 | Apoptosis, morphology, proliferationc | Extracellular space |
| IGSF6 | Immunoglobulin superfamily, member 6 | 1.26 | Unknown | Plasma membrane |
| IFNAR1 | interferon (alpha, beta and omega) receptor 1 | 1.25 | Activation, cell death, apoptosisc | Plasma membrane |
| VAV1 | vav 1 guanine nucleotide exchange factor | 1.24 | Proliferation, development, apoptosisc | Nucleus |
| EGR3 | Early growth response 3 | 1.24 | Apoptosis, development, proliferationc | Nucleus |
| SATB2 | SATB homeobox 2 | 1.23 | Migration, remodeling, development, invasion | Nucleus |
| ANXA2 | Annexin A2 | 1.23 | Binding, fusion, organizationc | Plasma membrane |
| FCER2 | Fc fragment of IgE, low affinity II, receptor for (CD23) | 1.23 | Apoptosis, activation, phagocytosisc | Plasma membrane |
| AOC3 | amine oxidase, copper containing 3 (vascular adhesion protein 1) | 1.23 | Migration, adhesion, cell rollingc | Plasma membrane |
| IL4R | Interleukin 4 receptor | −1.20 | Proliferation, differentiation, apoptosis3 | Plasma membrane |
| IL6 | Interleukin 6 (interferon, beta 2) | −1.23 | Differentiation, proliferation, apoptosisc | Extracellular space |
| CD22 | CD22 molecule | −1.24 | Activation, proliferation, apoptosisc | Plasma membrane |
| FCN1 | Ficolin (collagen/fibrinogen domain containing) 1 | −1.24 | Unknown | Extracellular space |
| HOXB2 | Homeobox B2 | −1.24 | Migration, differentiation | Nucleus |
| PON2a | Paraoxonase 2 | −1.24 | Plasma membrane | |
| HNF1A | HNF1 homeobox A | −1.25 | Transcription, apoptosis,proliferationc | Nucleus |
| MMP9 | Matrix metallopeptidase 9 | −1.26 | Migration, invasion, apoptosis, proliferation3 | Extracellular space |
| MAST4 | Microtubule associated serine/threonine kinase family member 4 | −1.26 | Unknown | Unknown |
| DLG5 | Discs, large homolog 5 x(Drosophila) | −1.27 | Plasma membrane | |
| PTPRG | Protein tyrosine phosphatase, receptor type, G | −1.28 | Growth, proliferation, colony formation, survival | Plasma membrane |
| ICK | Intestinal cell (MAK‐like) kinase | −1.28 | Unknown | Cytoplasm |
| L1CAMa | L1 cell adhesion molecule | −1.29 | Outgrowth, guidance, adhesion, migrationc | Plasma membrane |
| DBN1a | Drebrin 1 | −1.29 | cytoplasm | |
| KIAA1545 | HBV X‐transactivated gene 9 protein | −1.30 | ||
| OSBPL3 | Oxysterol binding protein‐like 3 | −1.37 | Unknown | Cytoplasm |
| FARP1a | FERM, RhoGEF (ARHGEF) and pleckstrin domain protein 1 | −1.37 | Unknown | Unknown |
| MERTK | c‐mer proto‐oncogene tyrosine kinase | −1.41 | Proliferation, apoptosis, phagocytosisc | Plasma membrane |
| NR4A1 | Nuclear receptor subfamily 4, group A, member 1 | −1.42 | Apoptosis, proliferation, cell deathc | Nucleus |
| SOX11a | SRY (sex determining region Y)‐box 11 | −1.43 | Expression in | Nucleus |
| GAB2 | GRB2‐associated binding protein 2 | −1.45 | Proliferation, differentiation, migration | Cytoplasm |
| AEBP1a | AE binding protein 1 | −1.45 | Proliferation | Nuclear |
| HOMER3a | homer homolog 3 (Drosophila) | −1.54 | Plasma membrane | |
| MDK | Midkine (neurite growth‐promoting factor 2) | −1.57 | Migration, proliferation, differentiationc | Extracellular space |
| ZNF711 | Zinc finger protein 711 | −1.65 | Unknown | Nucleus |
| CD27 | CD27 molecule | −1.67 | Apoptosis, proliferation, cytotoxicityc | Plasma membrane |
| RHOBTB3 | Rho‐related BTB domain containing 3 | −1.73 | Unknown | Unknown |
| ASAP2 | ArfGAP with SH3 domain, ankyrin repeat and PH domain 2 | −2.17 | Phagocytosis, migration, generation | Nucleus |
These genes are identified as MCL‐specific by Obrador‐Hevia et al. (2009).
As identified by Ingenuity Pathway Analysis 9.0 version 3206.
These descriptions are truncated due to limited space.
Based on their presence in primary MCL tumors and their relation to proliferation or neural development, six genes were selected for independent validation, using target‐specific TaqMan probes. These analyses confirmed a co variation with SOX11 expression levels for most genes, ATX being an exception showing a strong up‐regulation in response to the SOX11 knock‐down (Figure 3).
Figure 3.
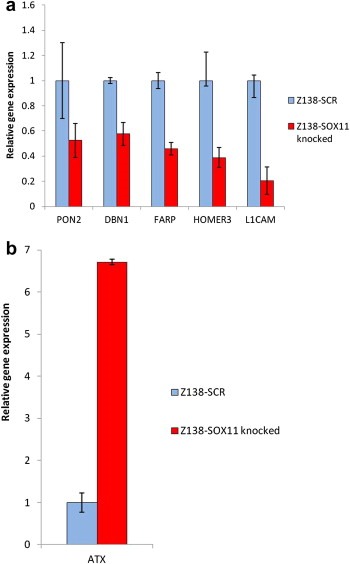
Technical verification of SOX11‐regulated genes. Verification of mRNA levels of six SOX11‐regulated genes were performed using TaqMan assays and revealed a co‐regulation of (a) DBN1, PON2, HOMER3, FARP and L1CAM with SOX11 while (b) ATX showed a major up‐regulation upon SOX11 knock‐down.
3.4. Functional role of ATX
The knock‐down of SOX11 resulted in an increase in proliferation and a shift in cell cycle distribution (Figure 2a and b). Consequently, we wanted to assess if ATX and its down‐stream effector molecules were involved in this growth regulatory effect. In brief, ATX is a phospholipase D (PLD) that generates lysophosphatidic acid (LPA) but also shingosine‐1‐phosphate (S1P) from lysophosphatidylcholine (LPC) and sphingo‐sylphosphorylcholine (SPC), respectively (Clair et al., 2003; Umezu‐Goto et al., 2002) and is thus a major regulator of cell proliferation.
To assess the role of ATX‐induced proliferation in Z138‐SOX11knocked and Z138‐SCR cells, the ATX‐specific inhibitor S32826 was used to specifically block the ATX‐related enzymatic activity. Cells were kept in log phase with or without the inhibitor for 48 h and growth rates were assessed by 3H‐thymidine incorporation. Repetitive experiments confirmed an increased growth rate of Z138‐SOX11knocked compared to Z138‐SCR cells, thus demonstrating a dependence on ATX for the increase in proliferation in Z138‐SOX11knocked cells (Figure 4).
Figure 4.
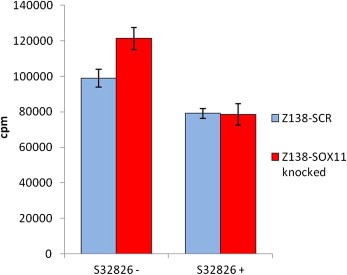
Proliferation measurement and ATX inhibition. Proliferation was assessed for Z138‐SOX11knocked compared to Z138‐SCR cell lines, confirming an increased growth rate for Z138‐SOX11knocked cells. The observed increase in proliferation comparing the Z138‐SOX11knocked and control cells could be completely inhibited using the ATX‐specific inhibitor S32826. The incorporation of 3H‐Thymidine is measured as counts per minute (cpm).
3.5. In vivo analysis of the tumorigenic potential of SOX11knocked MCL cells
To evaluate if the increased growth rate observed for the SOX11 knock‐down cells in vitro had any effect on the tumorigenic potential of cells in vivo Z138‐SOX11knocked and Z138‐SCR controls cells were i.v. transplanted into NOD‐SCID mice. Both Z138‐SOX11knocked and Z138‐SCR showed tumorigenic properties and generated tumors after three to eight weeks. A dissemination of tumor cells to CNS and brain was identified through histological examination, showing also extensive accumulation of lymphocytic cells in the meninges, around vessels in the brain, and in the spinal cord (Figure 5a). This finding was confirmed by flow cytometry of cells isolated from brain and tumors and by IHC, using staining for the human B cell marker CD20 (Figure 5b). The localization of the tumor cells into the CNS is consistent with the neurological symptoms that the mice show, including paralyzed hind legs. However, tumor cells were also identified in other organs, including liver and spleen (Table 2).
Figure 5.
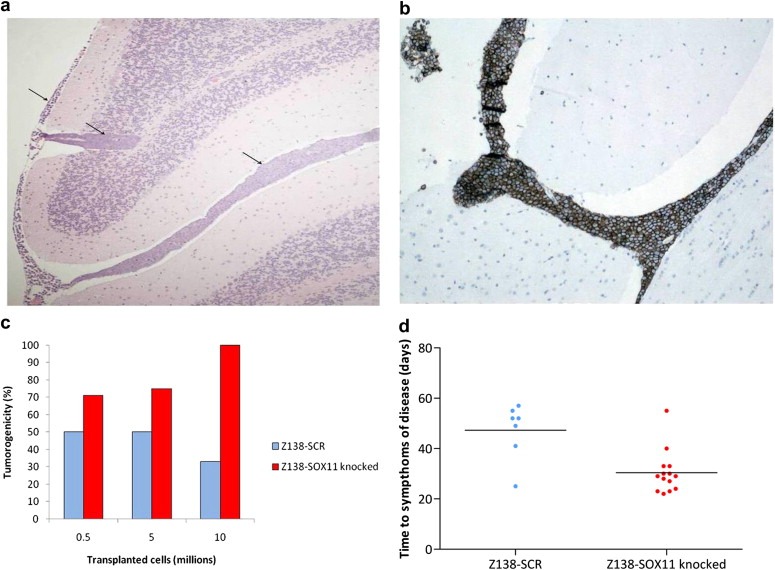
Establishment of tumors in vivo. The tumorigenicity of Z138‐SOX11knocked and Z138‐SCR cells was assessed in NOD‐SCID mice. (a) Histological examination of brain tissue confirmed involvement of the CNS (arrow‐heads) and (b) IHC analysis using antibodies targeting CD20 confirmed a human B cell origin of the established tumors. (c) A trend towards an increased tumorigenicity with reduced SOX11 expression was seen upon transplantation of tumor cells (0.5, 5 or 10 million cells). (d) Survival analysis showed a more rapid development of symptoms related to tumor growth for the Z138‐SOX11knocked compared to Z138‐SCR cells.
Table 2.
Identification of tumor cells in specific organs post transplantation.
| Organ | Identified/investigateda |
|---|---|
| Brain | 24/38 |
| Lung | 13/21 |
| Kidney | 4/21 |
| Spleen | 14/22 |
| Liver | 9/23 |
| Bone marrow | 9/18 |
| Blood | 2/3 |
Number of organs with identified tumor cells (Z138‐SCR or Z138‐SOX11knocked) using flow cytometry compared to the total number of specific organs analyzed.
Of note, a clear difference in tumorigenicity was detected between Z138‐SOX11knocked and Z138‐SCR in the majority of experiments, as determined by the frequency of animals showing symptoms of disease (Figure 5c). To further assess the different tumorigenic potentials, animals injected with Z138‐SOX11knocked and Z138‐SCR were monitored and time until symptoms of disease or occurrence of spontaneous death was compared. Three of four experiments showed a shorter time to symptoms of disease for animals injected with Z138‐SOX11knocked as compared to Z138‐SCR (Figure 5d).
4. Discussion
SOX11 has been recognized as an important diagnostic antigen (Chen et al., 2009; Dictor et al., 2009; Ek et al., 2008; Fernandez et al., 2010; Mozos et al., 2009; Wang et al., 2008) with a suppressor‐like function (Gustavsson et al., 2010) in MCL. Recent results have shown consistent changes in gene expression in primary MCL samples and MCL cell lines upon transient SOX11 knock‐down (Wang et al., 2010). In this study, we used the MCL‐derived cell line Z138 to create a model cell line with constitutively reduced levels of the transcription factor SOX11 (Z138‐SOX11knocked) and a corresponding control cell line (Z138‐SCR). MCL cell lines, including Z138, have previously been described as relevant models for investigations of MCL‐associated features (Amin et al., 2003). Z138 has been shown to be derived from a patient with blastoid transformation (Medeiros et al., 2006) and have features related to other MCL cell lines (Tucker et al., 2006), but is also characterized by a more rapid proliferation. Using this cell line and modifications thereof, we demonstrated that constitutive down‐regulation of SOX11 through viral integration of SOX11‐specific shRNA lead to an increased growth rate, measured by both cell counting and 3H‐Thymidine incorporation. Furthermore, cell cycle analysis revealed an increased frequency of the Z138‐SOX11knocked cells in S phase, as compared to Z138‐SCR. Of note, other MCL‐associated genes with a known growth promoting function, such as cyclin D1, have failed to induce changes in growth rate upon knock‐down, as shown by us (Ortega‐Paino et al., 2008) and others (Klier et al., 2008). This emphasizes the complexity of growth regulation but also the significance of SOX11 as a central player in growth control.
We have previously shown that overexpression of SOX11 led to dysregulation of signaling pathways normally used by MCL tumor cells, such as the Rb‐E2F pathway (Gustavsson et al., 2010). Here we show that the gene expression changes induced by SOX11 knock‐down are also tightly linked to MCL‐specific features. In a recent review by Obrador‐Hevia et al. a set of 30 MCL‐specific genes was identified, based on public data from gene expression analysis of primary MCL in at least two independent studies, regardless of the technical platforms used (Obrador‐Hevia et al., 2009). Among these 30 genes, SOX11 and seven additional genes were differentially regulated upon SOX11 down‐regulation. Of interest, many of the identified genes are important for neuron or normal brain function, emphasizing that the identified target genes not only are regulated by SOX11 in MCL, but also in other contexts, as for example during the fetal development of the central nervous system (CNS) (Ehrengruber et al., 2004; Kojima and Shirao, 2007; Penzo‐Mendez, 2010; Schafer and Altevogt, 2010; Zhuang et al., 2009).
In addition, to further analyze the growth regulatory role of SOX11, we focused on the novel finding that ATX is up‐regulated upon SOX11 knock‐down. The primary product of ATX is the lysophosphatidic acid 1‐acyl 2‐hydroxyl glycerol 3‐phosphate (LPA), which is the primary product of ATX‐catalyzed hydrolysis of circulating lysophospholipids, most often lysophoshatidylcholine (LPC) (Federico et al., 2008). LPA promotes growth, survival, differentiation and motility of a range of neuronal cells (Inoue et al., 2008) and is involved in tumor progression (Liu et al., 2009). It has been suggested that ATX is auto‐inhibited by its two products LPA and lysophospholipid S1P, regulating its own expression (van Meeteren and Moolenaar, 2007). Of note, ATX can also promote anti‐tumorigenic properties as it induces cyclic phosphatidic acid (cPA), which is associated with inhibition of cell growth (Kobayashi and Murakami‐Murofushi, 2001) through transphosphorylation of LPA (Tania et al., 2010). However, in lymphomas, ATX seems to be correlated with an increased tumor burden and has been proposed as serum biomarker to follow the clinical course of patients with follicular lymphoma (Masuda et al., 2008). Of note, using the ATX‐specific inhibitor S32826 (Ferry et al., 2008) we showed that the increase in proliferation upon SOX11 knock‐down could be reversed upon blockage of ATX activity. Thus, the increased SOX11‐mediated proliferation is ATX‐dependent.
As our model cell line showed increased growth rate upon SOX11 knock‐down and the literature suggests that increased levels of LPA promotes angiogenesis and tumor progression, we were also interested to assess the capability of tumor formation in vivo. Previous studies suggests that i.v. injections, using MCL cell lines such as GRANTA519 in immunodeficent mice, may promote systemic disease, involving liver tumors (Munger et al., 2006; Wang et al., 2007). Z138 has, however, previously been shown not to cause systemic disease in Rag‐2 deficient mice (Tucker et al., 2006). In our study, NOD‐SCID mice were used and characteristic symptoms of sickness, including neurological symptoms, were noted after three to eight weeks. Multiple analyses, including IHC staining, using the human B cell marker CD20, confirmed tumor growth in the CNS. This is consistent with data for human primary MCL, where involvement of the CNS often is associated with systemic disease and decreased survival (Gill et al., 2009; Valdez et al., 2002). In agreement with our in vitro data and previous clinical data (Wang et al., 2008), Z138‐SOX11knocked cells showed an increased tumorigenicity and shorter time to survival compared to Z138‐SCR control cells.
5. Conclusions
We have for the first time showed that constitutive SOX11 knock‐down in a MCL cell line promotes a highly proliferative feature, which can be reversed by the specific inhibition of ATX. This cell line, with a silenced SOX11 gene, was also shown to have an increased engraftment potential and a more aggressive behavior in vivo.
Authorship contributions
PC was involved in the design of the study, responsible for viral work and functional evaluation of modified cell lines in vitro and took active part in writing the manuscript. UA contributed to the design of the study, was responsible for the in vivo studies and evaluation of the data and took active part in writing the manuscript. VK took active part in the functional evaluation of modified cell lines. C.A.K.B contributed to the design of the study and revision of the manuscript. SE was responsible for the design of the study, interpretation of data and writing of the manuscript.
Conflicts of interest
SE and CAKB have previously filed a patent for the diagnostic, prognostic and therapeutic use of SOX11.
Acknowledgment
The study was supported by the Lund Institute of Technology (LTH), Bioinvent International AB, the Leukemia and Lymphoma Society (Grant No. R6189‐09), Cancerfonden (08/0285 and 09/0390), Smärtafonden (SSF/09‐05), CREATE Health, a strategic Center for Translational Cancer Research (www.createhealth.se), Stiftelsen Olle Engkvist Byggmästare and BioCARE – a strategic program for Cancer Research at Lund and Gothenburg Universities. We would like to acknowledge Eva Nilsson for introducing us to the NOD‐SCID work, Georg Hansson for his support with i.v. injections, Kristina Lövgren for immunohistochemistry staining and tissue preparation, Beata Lindqvist for lentiviral production, Ann‐Charlott Olsson for gene expression sample and array preparation and Ann‐Sofie Albrekt for bioinformatic support.
Conrotto Paolo, Andréasson Ulrika, Kuci Venera, Borrebaeck Carl A.K. and Ek Sara, (2011), Knock‐down of SOX11 induces autotaxin‐dependent increase in proliferation in vitro and more aggressive tumors in vivo , Molecular Oncology, 5, doi: 10.1016/j.molonc.2011.08.001.
Contributor Information
Paolo Conrotto, Email: paolo.conrotto@immun.lth.se.
Ulrika Andréasson, Email: ulrika.andreasson@immun.lth.se.
Venera Kuci, Email: venera.kuci@immun.lth.se.
Carl A.K. Borrebaeck, Email: carl.borrebaeck@immun.lth.se
Sara Ek, Email: sara.ek@immun.lth.se.
References
- Amin, H.M. , McDonnell, T.J. , Medeiros, L.J. , Rassidakis, G.Z. , Leventaki, V. , O'Connor, S.L. , Keating, M.J. , Lai, R. , 2003. Characterization of 4 mantle cell lymphoma cell lines. Arch. Pathol. Lab. Med.. 127, 424–431. [DOI] [PubMed] [Google Scholar]
- Braddock, D.T. , 2010. Autotaxin and lipid signaling pathways as anticancer targets. Curr. Opin. Investig. Drugs. 11, 629–637. [PubMed] [Google Scholar]
- Chen, Y.H. , Gao, J. , Fan, G. , Peterson, L.C. , 2009. Nuclear expression of sox11 is highly associated with mantle cell lymphoma but is independent of t(11;14)(q13;q32) in non-mantle cell B-cell neoplasms. Mod. Pathol.. 23, 105–112. [DOI] [PubMed] [Google Scholar]
- Clair, T. , Aoki, J. , Koh, E. , Bandle, R.W. , Nam, S.W. , Ptaszynska, M.M. , Mills, G.B. , Schiffmann, E. , Liotta, L.A. , Stracke, M.L. , 2003. Autotaxin hydrolyzes sphingosylphosphorylcholine to produce the regulator of migration, sphingosine-1-phosphate. Cancer Res.. 63, 5446–5453. [PubMed] [Google Scholar]
- Dictor, M. , Ek, S. , Sundberg, M. , Warenholt, J. , Gyorgy, C. , Sernbo, S. , Gustavsson, E. , Abu-Alsoud, W. , Wadstrom, T. , Borrebaeck, C. , 2009. Strong lymphoid nuclear expression of SOX11 transcription factor defines lymphoblastic neoplasms, mantle cell lymphoma and Burkitt's lymphoma. Haematologica. 94, 1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrengruber, M.U. , Kato, A. , Inokuchi, K. , Hennou, S. , 2004. Homer/Vesl proteins and their roles in CNS neurons. Mol. Neurobiol.. 29, 213–227. [DOI] [PubMed] [Google Scholar]
- Ek, S. , Hogerkorp, C.M. , Dictor, M. , Ehinger, M. , Borrebaeck, C.A. , 2002. Mantle cell lymphomas express a distinct genetic signature affecting lymphocyte trafficking and growth regulation as compared with subpopulations of normal human B cells. Cancer Res.. 62, 4398–4405. [PubMed] [Google Scholar]
- Ek, S. , Andreasson, U. , Hober, S. , Kampf, C. , Ponten, F. , Uhlen, M. , Merz, H. , Borrebaeck, C.A. , 2006. From gene expression analysis to tissue microarrays: a rational approach to identify therapeutic and diagnostic targets in lymphoid malignancies. Mol. Cell Proteomics. 5, 1072–1081. [DOI] [PubMed] [Google Scholar]
- Ek, S. , Dictor, M. , Jerkeman, M. , Jirstrom, K. , Borrebaeck, C.A. , 2008. Nuclear expression of the non B-cell lineage Sox11 transcription factor identifies mantle cell lymphoma. Blood. 111, 800–805. [DOI] [PubMed] [Google Scholar]
- Federico, L. , Pamuklar, Z. , Smyth, S.S. , Morris, A.J. , 2008. Therapeutic potential of autotaxin/lysophospholipase d inhibitors. Curr. Drug Targets. 9, 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez, V. , Salamero, O. , Espinet, B. , Sole, F. , Royo, C. , Navarro, A. , Camacho, F. , Bea, S. , Hartmann, E. , Amador, V. , Hernandez, L. , Agostinelli, C. , Sargent, R.L. , Rozman, M. , Aymerich, M. , Colomer, D. , Villamor, N. , Swerdlow, S.H. , Pileri, S.A. , Bosch, F. , Piris, M.A. , Montserrat, E. , Ott, G. , Rosenwald, A. , Lopez-Guillermo, A. , Jares, P. , Serrano, S. , Campo, E. , 2010. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res.. 70, 1408–1418. [DOI] [PubMed] [Google Scholar]
- Ferry, G. , Moulharat, N. , Pradere, J.P. , Desos, P. , Try, A. , Genton, A. , Giganti, A. , Beucher-Gaudin, M. , Lonchampt, M. , Bertrand, M. , Saulnier-Blache, J.S. , Tucker, G.C. , Cordi, A. , Boutin, J.A. , 2008. S32826, a nanomolar inhibitor of autotaxin: discovery, synthesis and applications as a pharmacological tool. J. Pharmacol. Exp. Ther.. 327, 809–819. [DOI] [PubMed] [Google Scholar]
- Gill, S. , Herbert, K.E. , Prince, H.M. , Wolf, M.M. , Wirth, A. , Ryan, G. , Carney, D.A. , Ritchie, D.S. , Davies, J.M. , Seymour, J.F. , 2009. Mantle cell lymphoma with central nervous system involvement: frequency and clinical features. Br. J. Haematol.. 147, 83–88. [DOI] [PubMed] [Google Scholar]
- Gustavsson, E. , Sernbo, S. , Andersson, E. , Brennan, D.J. , Dictor, M. , Jerkeman, M. , Borrebaeck, C.A. , Ek, S. , 2010. SOX11 expression correlates to promoter methylation and regulates tumor growth in hematopoietic malignancies. Mol. Cancer. 9, 187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, M. , Xie, W. , Matsushita, Y. , Chun, J. , Aoki, J. , Ueda, H. , 2008. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience. 152, 296–298. [DOI] [PubMed] [Google Scholar]
- Klier, M. , Anastasov, N. , Hermann, A. , Meindl, T. , Angermeier, D. , Raffeld, M. , Fend, F. , Quintanilla-Martinez, L. , 2008. Specific lentiviral shRNA-mediated knockdown of cyclin D1 in mantle cell lymphoma has minimal effects on cell survival and reveals a regulatory circuit with cyclin D2. Leukemia. 22, 2097–2105. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T. , Murakami-Murofushi, K. , 2001. Bioactions and synthesis of a novel lipid mediator, cyclic phosphatidic acid. World Rev. Nutr. Diet.. 88, 228–232. [DOI] [PubMed] [Google Scholar]
- Kojima, N. , Shirao, T. , 2007. Synaptic dysfunction and disruption of postsynaptic drebrin-actin complex: a study of neurological disorders accompanied by cognitive deficits. Neurosci. Res.. 58, 1–5. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt, K. , Herbarth, B. , Sock, E. , Enderich, J. , Hermans-Borgmeyer, I. , Wegner, M. , 1998. Cooperative function of POU proteins and SOX proteins in glial cells. J. Biol. Chem.. 273, 16050–16057. [DOI] [PubMed] [Google Scholar]
- Liu, S. , Umezu-Goto, M. , Murph, M. , Lu, Y. , Liu, W. , Zhang, F. , Yu, S. , Stephens, L.C. , Cui, X. , Murrow, G. , Coombes, K. , Muller, W. , Hung, M.C. , Perou, C.M. , Lee, A.V. , Fang, X. , Mills, G.B. , 2009. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 15, 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, A. , Nakamura, K. , Izutsu, K. , Igarashi, K. , Ohkawa, R. , Jona, M. , Higashi, K. , Yokota, H. , Okudaira, S. , Kishimoto, T. , Watanabe, T. , Koike, Y. , Ikeda, H. , Kozai, Y. , Kurokawa, M. , Aoki, J. , Yatomi, Y. , 2008. Serum autotaxin measurement in haematological malignancies: a promising marker for follicular lymphoma. Br. J. Haematol.. 143, 60–70. [DOI] [PubMed] [Google Scholar]
- Medeiros, L.J. , Estrov, Z. , Rassidakis, G.Z. , 2006. Z-138 cell line was derived from a patient with blastoid variant mantle cell lymphoma. Leuk. Res.. 30, 497–501. [DOI] [PubMed] [Google Scholar]
- Mozos, A. , Royo, C. , Hartmann, E. , De Jong, D. , Baro, C. , Valera, A. , Fu, K. , Weisenburger, D.D. , Delabie, J. , Chuang, S.S. , Jaffe, E.S. , Ruiz-Marcellan, C. , Dave, S. , Rimsza, L. , Braziel, R. , Gascoyne, R.D. , Sole, F. , Lopez-Guillermo, A. , Colomer, D. , Staudt, L.M. , Rosenwald, A. , Ott, G. , Jares, P. , Campo, E. , 2009. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. Haematologica. 94, 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger, C.M. , Vose, J.M. , Joshi, S.S. , 2006. Dendritic cell-based therapy for mantle cell lymphoma. Int. J. Oncol.. 28, 1337–1343. [PubMed] [Google Scholar]
- Ngo, V.N. , Davis, R.E. , Lamy, L. , Yu, X. , Zhao, H. , Lenz, G. , Lam, L.T. , Dave, S. , Yang, L. , Powell, J. , Staudt, L.M. , 2006. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 441, 106–110. [DOI] [PubMed] [Google Scholar]
- Obrador-Hevia, A. , Fernandez de Mattos, S. , Villalonga, P. , Rodriguez, J. , 2009. Molecular biology of mantle cell lymphoma: from profiling studies to new therapeutic strategies. Blood Rev.. 23, 205–216. [DOI] [PubMed] [Google Scholar]
- Ortega-Paino, E. , Fransson, J. , Ek, S. , Borrebaeck, C.A. , 2008. Functionally associated targets in mantle cell lymphoma as defined by DNA microarrays and RNA interference. Blood. 111, 1617–1624. [DOI] [PubMed] [Google Scholar]
- Penzo-Mendez, A.I. , 2010. Critical roles for SoxC transcription factors in development and cancer. Int. J. Biochem. Cell Biol.. 42, 425–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi, N. , Bekele, R. , Capatos, D. , Venkatraman, G. , Sariahmetoglu, M. , Brindley, D.N. , 2011. Regulation of lysophosphatidate signaling by autotaxin and lipid phosphate phosphatases with respect to tumor progression, angiogenesis, metastasis and chemo-resistance. Biochimie. 93, 61–70. [DOI] [PubMed] [Google Scholar]
- Schafer, M.K. , Altevogt, P. , 2010. L1CAM malfunction in the nervous system and human carcinomas. Cell Mol. Life Sci.. 67, 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tania, M. , Khan, A. , Zhang, H. , Li, J. , Song, Y. , 2010. Autotaxin: a protein with two faces. Biochem. Biophys. Res. Commun.. 401, 493–497. [DOI] [PubMed] [Google Scholar]
- Tucker, C.A. , Bebb, G. , Klasa, R.J. , Chhanabhai, M. , Lestou, V. , Horsman, D.E. , Gascoyne, R.D. , Wiestner, A. , Masin, D. , Bally, M. , Williams, M.E. , 2006. Four human t(11;14)(q13;q32)-containing cell lines having classic and variant features of mantle cell lymphoma. Leuk. Res.. 30, 449–457. [DOI] [PubMed] [Google Scholar]
- Umezu-Goto, M. , Kishi, Y. , Taira, A. , Hama, K. , Dohmae, N. , Takio, K. , Yamori, T. , Mills, G.B. , Inoue, K. , Aoki, J. , Arai, H. , 2002. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol.. 158, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez, R. , Kroft, S.H. , Ross, C.W. , Schnitzer, B. , Singleton, T.P. , Peterson, L.C. , Finn, W.G. , 2002. Cerebrospinal fluid involvement in mantle cell lymphoma. Mod. Patho.. 15, 1073–1079. [DOI] [PubMed] [Google Scholar]
- van Meeteren, L.A. , Moolenaar, W.H. , 2007. Regulation and biological activities of the autotaxin-LPA axis. Prog. Lipid. Res.. 46, 145–160. [DOI] [PubMed] [Google Scholar]
- Wang, M. , Zhang, L. , Han, X. , Yang, J. , Qian, J. , Hong, S. , Samaniego, F. , Romaguera, J. , Yi, Q. , 2007. Atiprimod inhibits the growth of mantle cell lymphoma in vitro and in vivo and induces apoptosis via activating the mitochondrial pathways. Blood. 109, 5455–5462. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Asplund, A.C. , Porwit, A. , Flygare, J. , Smith, C.I. , Christensson, B. , Sander, B. , 2008. The subcellular Sox11 distribution pattern identifies subsets of mantle cell lymphoma: correlation to overall survival. Br. J. Haematol.. 143, 248–252. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Bjorklund, S. , Wasik, A.M. , Grandien, A. , Andersson, P. , Kimby, E. , Dahlman-Wright, K. , Zhao, C. , Christensson, B. , Sander, B. , 2010. Gene expression profiling and chromatin immunoprecipitation identify DBN1, SETMAR and HIG2 as direct targets of SOX11 in mantle cell lymphoma. PLoS One. 5, e14085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe, M.S. , Nowling, T.K. , Rizzino, A. , 2003. Identification of novel domains within Sox-2 and Sox-11 involved in autoinhibition of DNA binding and partnership specificity. J. Biol. Chem.. 278, 17901–17911. [DOI] [PubMed] [Google Scholar]
- Zhuang, B. , Su, Y.S. , Sockanathan, S. , 2009. FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling. Neuron. 61, 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]


