Abstract
Lung carcinogenesis is a complex process in an unregulated inflammatory environment. Curcumin has been extensively investigated as a multi‐target anti‐tumor and anti‐inflammation compound. In this paper, we demonstrate a novel inflammation‐related mechanism for curcumin‐induced inhibition of lung tumor growth. We found that neutrophil elastase, an important regulator of inflammatory processes, directly triggered tumor cell proliferation in human lung adenocarcinoma A549 cells, and curcumin could completely suppress the excess tumor proliferation induced by neutrophil elastase. α1‐antitrypsin is synthesized by tumor cells and is the natural inhibitor of neutrophil elastase. We found that curcumin counteracted the decrease of α1‐antitrypsin induced by neutrophil elastase by inducing the promoter activity of α1‐antitrypsin and promoting its expression in A549 cells. The inhibition of neutrophil elastase‐induced proliferation by curcumin was dependent on the PI3K/Akt pathway. Knockdown of α1‐antitrypsin by siRNA further enhanced the tumor cell proliferation induced by neutrophil elastase and significantly blocked the anti‐proliferation effect of curcumin against neutrophil elastase. Curcumin remarkably inhibited the primary tumor growth of Lewis lung carcinoma (LLC) in C57BL/6 mice. We further showed that curcumin upregulated the level of α1‐antitrypsin in primary tumor tissue by promoting its local expression, and the protein level of neutrophil elastase in tumor tissue was obviously decreased in mice treated with curcumin. Overall, our results suggest that neutrophil elastase and α1‐antitrypsin play important roles in modulating lung tumor proliferation in inflammatory microenvironment and curcumin inhibits neutrophil elastase‐induced tumor proliferation via upregulating α1‐antitrypsin expression in vitro and in vivo.
Keywords: Curcumin, Tumor proliferation, α1-antitrypsin, Neutrophil elastase
Highlights
Neutrophil elastase (NE) directly triggers lung tumor cell proliferation.
Curcumin completely suppresses the excess tumor proliferation induced by NE.
Curcumin blocks NE‐induced tumor proliferation via upregulating α1‐antitrypsin in lung cancer.
Curcumin is a potential alternative against inflammation‐related tumor growth.
1. Introduction
In a wide variety of cancer types, lung cancer is respectively the first and the second leading cause of cancer death in males and in females globally, with only about 15% five‐year survival rates despite therapeutic advances in the diagnosis and treatment in recent decades (Jemal et al., 2011, 2009). Lung carcinogenesis is a complex process involving unregulated proliferation, apoptosis resistance, invasion, metastasis and angiogenesis in a deregulated inflammatory environment (Lee et al., 2008). The surrounding inflammation in the tumor microenvironment has many tumor‐promoting effects which accelerate the proliferation and survival of malignant cells, and promote angiogenesis and metastasis (Mantovani et al., 2008). A better understanding of molecular mechanisms involved in tumor‐associated inflammation may provide a new insight for improved diagnosis and drug development in the treatment of lung cancer.
Curcumin (diferuloylmethane), a polyphenolic natural product, is the main active component of tumeric derived from the rhizomes of Curcuma longa, which is used in cooking and traditional medicines (Goel et al., 2008). Curcumin has been extensively investigated as an anticancer compound which inhibits proliferation, invasion, angiogenesis and metastasis in various cancer cells and animal models including colon cancer, breast cancer, lung cancer and prostate cancer (Kunnumakkara et al., 2008, 2007; Wu et al., 2010). Increasing evidence indicates that curcumin has a range of molecular targets and influences numerous biochemical and molecular processes (Goel et al., 2008). Moreover, curcumin is safe for humans and there is no dose‐limiting toxicity when curcumin is administered at doses up to 10 g/day (Cheng et al., 2001). In addition to its anti‐tumor effect, curcumin also has anti‐inflammatory activity (Jurenka, 2009). Curcumin modulates the inflammatory response by downregulating the activity of cyclooxygenase‐2 (COX‐2), and inducible nitric oxide synthase (iNOS) enzymes, and suppressing nuclear factor kappa B (NF‐κB) activation (Surh et al., 2001). However, the molecular mechanisms underlying the anti‐tumor properties of curcumin within specific inflammatory microenvironment still remain elusive. In this paper, we demonstrate some other contributions of inflammation to lung cancer progression and the novel molecular mechanism of curcumin‐induced inhibition of lung tumor growth.
Neutrophils, as a component of the tumor microenvironment, only recently have been thought to play an important role in tumor growth and invasiveness (Gregory and Houghton, 2011). The presence of increased infiltrated neutrophils within tumors was significantly associated with a poorer clinical outcome in patients with bronchioloalveolar carcinoma (Cadranel et al., 1998). Neutrophil elastase released by activated neutrophils is the most potent neutrophil proteinase. The potential substrates of this protease include cytokines and cytokine receptors, which makes neutrophil elastase a potential regulator of the inflammatory process (Lungarella et al., 2008). Recent reports have shown that neutrophil elastase directly leads to uncontrolled tumor proliferation in lung adenocarcinoma mice model and lung epithelial tumor cells (Houghton et al., 2010). To our knowledge, there is no information available to address the effects of natural products on this key inflammatory molecule, neutrophil elastase. In the present study, we investigate the molecular mechanisms by which curcumin suppresses the tumor growth induced by neutrophil elastase. Our results suggest that curcumin could inhibit neutrophil elastase‐induced tumor proliferation by upregulating the expression of α1‐antitrypsin which is the natural inhibitor of neutrophil elastase in lung cancer in vitro and in vivo.
2. Materials and methods
2.1. Cell culture and treatment
Human lung adenocarcinoma cell lines A549 and Calu‐3 were used for in vitro experiments. The cells were maintained in Dulbecco's modified essential medium (DMEM, Gibco, NY, USA) plus 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified incubator with 5% CO2 in air at 37 °C. The cells were treated with different concentrations of neutrophil elastase (Merck Group, Darmstedt, Germany) and/or curcumin (Sigma–Aldrich Co., St Louis, Mo, USA) for 24 h and then evaluated as described later.
2.2. Lewis lung carcinoma mice model
Female C57BL/6 mice weighing 18–20 g were purchased from the Experimental Animal Center of Peking University (Grade II, Certificate No. 11‐00‐0004). Lewis lung carcinoma (LLC) was provided by Chinese Academy of Medical Sciences and maintained in C57BL/6 mice by the subcutaneous injection of 0.2 ml of homogenized tumor tissue suspension (tumor tissue (g): 0.9% sodium chloride (ml) = 1:3) into the left flank fold (Ma et al., 2011). Curcumin was administered orally via a gastric probe for 21 days after the inoculation of tumor cells. The mice were randomly divided into three groups, and respectively received the vehicle, curcumin 100 mg/kg/day or curcumin 300 mg/kg/day. On day 22, after anesthesia with ether and scarification, primary tumors and lungs were removed and weighed. All manipulations involving live mice were approved by the Animal Experiment Ethical Committee of Peking University. The sections of primary tumor tissue used for immunohistochemistry were rinsed with cold PBS, embedded in OCT compound (Sakura Finetek Japan Co., Tokyo, Japan), frozen in liquid nitrogen and finally stored at −80 °C. Other sections of the primary tumors were frozen at −80 °C for Western blot and quantitative real‐time PCR analysis.
2.3. Cell viability assay
Cell viability was determined using a Cell‐Counting Kit‐8 (CCK‐8, Dojindo Laboratories, Tokyo, Japan). A549 cells or Calu‐3 cells were seeded into 96‐well plates at the density of 5000 cells/well and allowed to adhere for 24 h. After incubation with neutrophil elastase and/or curcumin for 24 h, 10 μl of the CCK‐8 solution was added to each well, followed by incubation for 2 h at 37 °C. The resulting color was assayed at 450 nm using a microplate reader (Bio‐Rad Laboratories Inc., Hercules, CA, USA). LY294002 (Sigma–Aldrich) and U0126 (Sigma–Aldrich) were used in a subset of experiments.
2.4. Multiparametric proliferation assay by high content screening (HCS)
The BrdU and Ki‐67 Cell Proliferation Reagent Kit (Thermo Scientific, Pittsburgh, PA, USA) was used for simultaneous quantification of four fundamental parameters associated with cell proliferation: cell number, DNA content, BrdU incorporation and Ki‐67 expression in the same cell. Cell number and DNA content were quantified with DAPI staining. BrdU and Ki‐67 were determined with primary antibodies toward them and secondary antibodies conjugated with DyLight 488 and DyLight 549 respectively. Plates with fixed and stained A549 cells were analyzed using the ArrayScan VTI HCS Reader (Thermo Scientific) for automated imaging acquisition and analysis. Hundreds of cells were detected for each of the three microscopic fields per well.
2.5. Western blot analysis
Total protein was extracted with RIPA lysis buffer and equal amounts of proteins were subjected to 10% SDS‐PAGE for α1‐antitrypsin (1:500, Santa Cruz, CA, USA), neutrophil elastase (1:500, Santa Cruz), pAkt and Akt (1:1,000, Cell Signaling, Beverly, MA), pERK and ERK (1:1,000, Cell Signaling), p‐IKKβ and IKKβ (1:500, Bioworld, Louis Park, MN, USA), p‐p65 and p65 (1:500, Bioworld), Bcl‐2 (1:1,000, Bioworld), β‐actin (1:20,000, Sigma–Aldrich), GAPDH (1:10,000, Sigma–Aldrich), and 8% SDS‐PAGE for IRS‐1 (1:500, Santa Cruz), p‐mTOR and mTOR (1:1,000, Cell Signaling), then transferred to polyvinylidene difluoride membranes (Millipore Corp., MA, USA). The slides were fixed and incubated with primary antibodies overnight at 4 °C with gentle agitation, followed by secondary antibody reactions with AP‐labeled IgG (Santa Cruz). The detection was performed using the 3‐bromo‐4‐chloro‐5‐indolyl phosphate (BCIP) and nitro blue tetrazolium (NBT) reaction (Amersco, NJ, USA).
2.6. Immunofluorescence assay
A549 cells were plated on glass coverslips in 5% CO2 in air at 37 °C and then incubated in 40 nM of neutrophil elastase and 20 μM of curcumin for another 24 h. The coverslips were gently washed in PBS, fixed in 3.7% paraformaldehyde for 15 min, permeabilized in PBS containing 0.1% Triton X‐100 for 20 min and blocked in PBS containing 3% BSA for 30 min. The fixed cells were incubated with anti‐human α1‐antitrypsin (Santa Cruz) antibody overnight at 4 °C in a humid chamber, and then washed and incubated with the corresponding DyLight 594‐conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h at 37 °C. After a PBS wash, the cells were incubated with Hoechst 33342 (1 μg/ml, Sigma–Aldrich) for 5 min at room temperature. After several washes, stained specimens were viewed using a TCS‐SP5 laser scanning confocal microscope (Leica Microsystems, Wetzlar, Germany).
2.7. Quantitative real‐time PCR
TRIzol reagent (Invitrogen, Groningen, NL, USA) was used to isolate total RNA from A549 cells at 80% confluence and tumor tissues according to the manufacturer's instructions. Total RNA samples were prepared as previously described (Normandin et al., 2010) and retrotranscribed into first‐strand cDNAs using the QuantiTect reverse transcription kit (QIAGEN, Valencia, CA, USA). Quantitative real‐time PCR was performed as described previously (Normandin et al., 2010). The primers used to amplify human α1‐antitrypsin were 5′‐CCCCACCCAGAGTTGCTC‐3′ (sense) and 5′‐GGTTAGGTGACAGCGGGTC‐3′ (antisense) and those for mouse α1‐antitrypsin were 5′‐CGCAGAAGGTTAGCCCAGATCCATA‐3′ (sense) and 5′‐TATGCACAGCCTGGCTGAGCTTCA‐3′ (antisense). The primers for human β‐actin were 5′‐ GACGACATGGAGAAGATCTG‐3′ (sense) and 5′‐ATGGCGTGAGGGAGAGCATA‐3′ (antisense), and those for mouse β‐actin were 5′‐TGCGTGACATCAAAGAGAAG‐3′ (sense) and 5′‐GATGCCACAGGATTCCATA‐3′ (antisense).
2.8. Promoter cloning and dual‐luciferase reporter (DLR) assay
A 0.87‐kb genomic DNA fragment upstream of the α1‐antitrypsin start codon was amplified and cloned into the pGL3‐basic vector (Promega, Madison, Wisconsin, USA). Sequences of primers used in the amplification of the α1‐antitrypsin promoter were 5′‐GGTACCTCTCCAGGCAGGGCC‐3′ (sense) and 5′‐CTCGAGGCCCAGTTCCTGCCCACCCA‐3′ (antisense) (Morgan et al., 2009). A549 cells seeded into 24‐well plates were cotransfected with 200 ng of pAAT‐Luc and 20 ng of pRL‐TK vectors (as a transfection efficiency control, Promega) by Lipofectamine LTX (Invitrogen) according to standard instructions. After 24 h, cells were treated with neutrophil elastase and curcumin at the indicated concentrations for additional 24 h. Both firefly and Renilla luciferase activities in lysed cells were measured using the Dual‐Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions and an Infinite M200 plate reader (Tecan, Grödig, Australia). The ratio of firefly and Renilla luciferase activities represented the normalized firefly luciferase activity.
2.9. Small interfering RNA (siRNA) transfection
siRNA specific for the gene encoding α1‐antitrypsin was bought from Santa Cruz and was a pool of three target‐specific 19–25 nt siRNAs designed to knock down gene expression of α1‐antitrypsin. The scramble control siRNA (Santa Cruz) was used as the negative control. A549 cells were incubated with the mixture of siRNA and Lipofectamine 2000 (Invitrogen) for 6 h according to the general transfection procedure provided by the manufacturer. After transfection for 48 h, cells were subjected to CCK‐8 assay and Western blot analysis.
2.10. Immunohistochemistry
Ki‐67 expression was assessed as an evaluation of tumor cell proliferation. Tumor sections (6 μm) were incubated at 4 °C overnight with the antibody against Ki‐67 (1:200, Abcam Inc., Cambridge, MA, USA). Secondary antibody detection was performed with a Polink‐2 plus HRP anti‐rabbit DAB detection kit (Golden Bridge International Inc., Mukilteo, WA, USA). Detection was carried out using a DAB chromogen, which resulted in a positive brown stain. Instead of the primary antibody, normal rabbit serum was used as the negative control.
2.11. Statistical analysis
Experiments were repeated a minimum of three times. Data are expressed as mean ± SEM. Statistical significance of differences between means was determined by one‐way analysis of variance (ANOVA) followed by Dunnett's test or Newman–Keuls post hoc test (SPSS version 11.5 software). A value of P < 0.05 was considered to be statistically significant.
3. Results
3.1. Curcumin inhibited tumor cell proliferation induced by neutrophil elastase in A549 cells
To explore the anti‐proliferation effect of curcumin on lung cancer when neutrophil elastase is involved in vitro, we performed the following assays in human lung adenocarcinoma cell line A549. Dose‐response curves in A549 cells showed that modest concentrations of neutrophil elastase (20–160 nM) could enhance tumor cell viability, and among all the concentrations, 40 nM of neutrophil elastase showed the strongest effect and was selected for the subsequent experiments (Figure 1A). Without neutrophil elastase, a range of concentrations of curcumin (from 10 to 40 μM) significantly attenuated cell survival in a concentration‐dependent manner (Figure 1B). In the presence of 40 nM of neutrophil elastase, 20 μM and 40 μM of curcumin could completely suppress the excess proliferation induced by neutrophil elastase in A549 cells (Figure 1B). In the meantime, we found that 40 nM of neutrophil elastase also promoted cell proliferation in other human lung adenocarcinoma cells such as Calu‐3 cells (without K‐ras mutation), and curcumin inhibited neutrophil elastase‐induced proliferation to some extent (Figure 1C).
Figure 1.
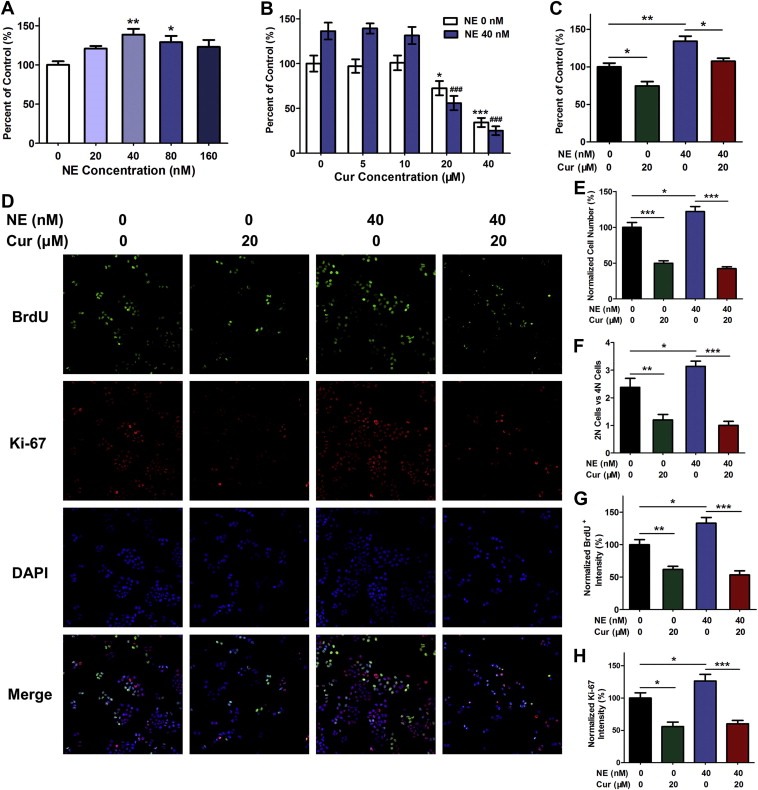
Curcumin prevented tumor cell proliferation induced by neutrophil elastase in A549 cells. (A) Cell viability detected by CCK‐8 assay for A549 cells stimulated with neutrophil elastase (NE). Data represent mean ± SEM, n = 3. *P < 0.05, **P < 0.01 vs. neutrophil elastase free group (control). (B) The effects of curcumin (Cur) on cell viability of A549 cells treated with or without neutrophil elastase. Data represent mean ± SEM, n = 3. *P < 0.05, ***P < 0.001 compared to the group without curcumin and neutrophil elastase. ###, P < 0.001 compared to the group with neutrophil elastase and without curcumin. (C) The effects of curcumin on the cell viability of Calu‐3 cells (K‐ras WT) induced by neutrophil elastase. Data represent mean ± SEM, n = 3. *P < 0.05, **P < 0.01. (D) Representative HCS images for BrdU, Ki‐67 and DAPI from A549 cells exposed to neutrophil elastase and curcumin. (E–H) Cell number (E), the ratio of the cells with 2N DNA content and those with 4N DNA content (F) and the quantification for BrdU (G) and Ki‐67 (H) were analyzed by HCS system. Data represent mean ± SEM, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001.
To further confirm the negative regulation of curcumin in neutrophil elastase‐induced cell survival, we conducted high content screening assay. Multiparameter fluorescent images (Figure 1D) and simultaneous quantifications of each parameter (Figure 1E–H) were obtained from the same microscopic field. 40 nM of neutrophil elastase increased tumor cell counts, however, in the presence of curcumin, this effect was overwhelmingly suppressed (Figure 1D, E). DNA content (2N vs. 4N) was determined by DAPI staining. Curcumin remarkably decreased the ratio of the cells with 2N DNA content and those with 4N DNA content, that was, relatively increased the cells with 4N DNA content, which indicated that curcumin might induce a G2/M cell‐cycle arrest to inhibit cell proliferation provoked by neutrophil elastase (Figure 1F). Neutrophil elastase resulted in an increase of BrdU incorporation and Ki‐67 content, which could be substantially diminished by curcumin (Figure 1D, G, H).
3.2. Curcumin upregulated α1‐antitrypsin expression in A549 cells provoked by neutrophil elastase
α1‐antitrypsin is a serine protease inhibitor (SERPIN) and it's the natural inhibitor of neutrophil elastase. The imbalance between α1‐antitrypsin and neutrophil elastase plays an important role in lung cancer progression (Sun and Yang, 2004). Given this, we predominantly focused on the regulation of α1‐antitrypsin by curcumin to investigate the mechanisms of the inhibition of neutrophil elastase‐induced proliferation by curcumin. Western blot was used to determine whether α1‐antitrypsin protein level was regulated by curcumin in vitro. Without neutrophil elastase co‐incubated, a range of concentrations of curcumin (from 5 to 40 μM) didn't affect α1‐antitrypsin expression in A549 cells. When co‐incubated with 40 nM of neutrophil elastase, curcumin promoted α1‐antitrypsin accumulation in A549 cells in a concentration‐dependent manner (Figure 2A, Supplementary Figure 1). As shown in Figure 2B, when neutrophil elastase was involved, the protein level of α1‐antitrypsin was obviously decreased, and 20 μM of curcumin counteracted the reduction of α1‐antitrypsin induced by neutrophil elastase. To further confirm the regulation of α1‐antitrypsin by curcumin, immunofluorescence and confocal microcopy were used to examine the subcellular distribution and protein level of α1‐antitrypsin. As shown in Figure 2C, α1‐antitrypsin in A549 cells exhibited a diffuse distribution pattern in both the cytoplasm and nucleus, and curcumin neutralized the decrease of α1‐antitrypsin induced by neutrophil elastase. Quantitative real‐time PCR showed that neutrophil elastase inhibited α1‐antitrypsin transcription and 20 μM of curcumin elevated α1‐antitrypsin mRNA levels in A549 cells treated with neutrophil elastase (Figure 2D). The α1‐antitrypsin promoter luciferase reporter assay illustrated that the upregulation of α1‐antitrypsin by curcumin in A549 cells treated with neutrophil elastase could be attributed to the induction of the promoter activity of α1‐antitrypsin by curcumin (Figure 2E).
Figure 2.
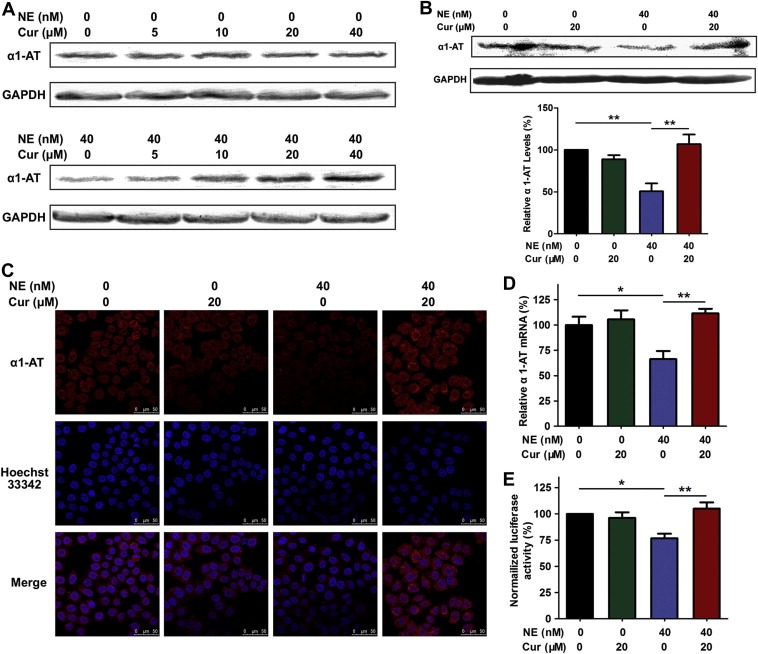
Curcumin‐induced α1‐antitrypsin expression in A549 cells provoked by neutrophil elastase. (A) Dose effects of curcumin (Cur) on α1‐antitrypsin (α1‐AT) protein levels in A549 cells when treated with or without neutrophil elastase (NE). (B) Western bolt of α1‐antitrypsin for 20 μM of curcumin‐treated and 40 nM of neutrophil elastase‐exposed A549 lysates. Data represent mean ± SEM, n = 3. **P < 0.01. (C) Confocal images for α1‐antitrypsin from A549 cells exposed to curcumin and neutrophil elastase. A549 cells were grown to 80% confluency and then incubated with 20 μM of curcumin and/or 40 nM of neutrophil elastase for 24 h. The cells were labeled by anti‐human α1‐antitrypsin antibody and DyLight 594‐conjugated secondary antibody (red) and Hoechst 33342 staining (blue). (D) Real‐time PCR for α1‐antitrypsin in A549 cells treated with curcumin and neutrophil elastase. Data represent mean ± SEM, n = 3. *P < 0.05, **P < 0.01. (E) The effect of curcumin on the normalized luciferase activity in A549 cells transfected with α1‐antitrypsin promoter when treated with neutrophil elastase. Data represent mean ± SEM, n = 3. *P < 0.05, **P < 0.01.
3.3. The counteraction of neutrophil elastase‐induced proliferation by curcumin was dependent on the PI3K/Akt pathway
In order to expand our observations on the regulation of α1‐antitrypsin by curcumin and assess downstream signaling and phenotype, we evaluated cell growth using selected signal pathway inhibitors and examined the protein levels of downstream signal molecules. Using the inhibitors of the PI3K pathway (LY294002) and the MEK–ERK pathway (U0126), we demonstrated that the negative regulation of neutrophil elastase‐induced proliferation by curcumin depended on the PI3K pathway but not on the MEK–ERK pathway in A549 cells (Figure 3A). Neutrophil elastase could directly induce lung tumor cell proliferation by degrading insulin receptor substrate‐1 (IRS‐1) which is an adapter protein of PI3K and then activating the PI3K pathway within the tumor cells (Houghton et al., 2010). With neutrophil elastase exposure, the protein level of IRS‐1 was decreased, the phosphorylation of Akt and mTOR which are the key downstream molecules of PI3K were elevated, and the phosphorylation of ERK was not affected. 20 μM of curcumin enhanced the protein level of IRS‐1, and reduced the phosphorylation of Akt and mTOR but did not affect the phosphorylation of ERK in A549 cells stimulated with neutrophil elastase (Figure 3B–F). Activation of Akt may act upstream of NF‐κB signaling pathway (El‐Rayes et al., 2006). We found that curcumin treatment significantly reduced neutrophil elastase‐induced phosphorylation of IKKβ and the p65 subunit of NF‐κB, and the expression of Bcl‐2 which is transcriptionally regulated by NF‐κB (Figure 3B, G–I). These results argued that curcumin inhibited IRS‐1 degradation induced by neutrophil elastase, suppressed the subsequent phosphorylation and activation of Akt and blocked the pro‐proliferation effect of neutrophil elastase.
Figure 3.
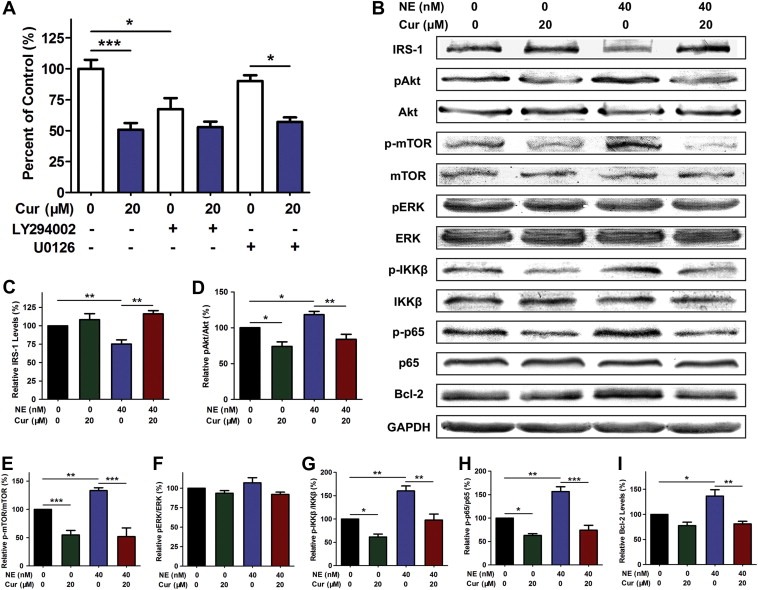
Curcumin attenuated neutrophil elastase‐induced proliferation depending on the PI3K/Akt pathway. (A) CCK‐8 assay for A549 cells stimulated with 40 nM of neutrophil elastase in the presence of 20 μM curcumin (Cur) and 10 μM LY294002 or 10 μM U0126, mean ± SEM, n = 3. *P < 0.05, ***P < 0.001. (B) Western blot for IRS‐1, pAkt, Akt, p‐mTOR, mTOR, pERK, ERK, p‐IKKβ, IKKβ, p‐p65, p65, Bcl‐2 and GAPDH in A549 cells treated with neutrophil elastase (NE) and curcumin. (C–I) Statistical data shown as the histograms of Western blot for IRS‐1 (C), the pAkt:Akt ratio (D), the p‐mTOR:mTOR ratio (E), the pERK:ERK ratio (F), the p‐IKKβ:IKKβ ratio (G), the p‐p65:p65 ratio (H) and Bcl‐2 (I) in A549 cells treated with neutrophil elastase and curcumin. Data represent mean ± SEM, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001.
3.4. α1‐antitrypsin‐targetted siRNA partially reduced the anti‐proliferation effect of curcumin in A459 cells exposed to neutrophil elastase
siRNA was used to further confirm the involvement of α1‐antitrypsin in curcumin‐induced inhibition of cell survival triggered by neutrophil elastase. When A549 cells were transfected with siRNA against α1‐antitrypsin for 48 h, the expression of α1‐antitrypsin was approximately reduced by 50% in comparison to the scramble siRNA control (Figure 4A). Transfection with α1‐antitrypsin siRNA significantly enhanced the cell viability (Figure 4B) and the phosphorylation activation of Akt (Figure 4C) in A549 cells stimulated with neutrophil elastase. The anti‐proliferation effect of curcumin was remarkably offset by the α1‐antitrypsin siRNA in A549 cells exposed to neutrophil elastase (Figure 4B). In addition, the inhibitory effect of curcumin on the phosphorylation of Akt was partially decreased in cells treated with neutrophil elastase (Figure 4C). These results strongly suggested that curcumin could inhibit neutrophil elastase‐induced proliferation through upregulation of α1‐antitrypsin expression in A549 cells.
Figure 4.
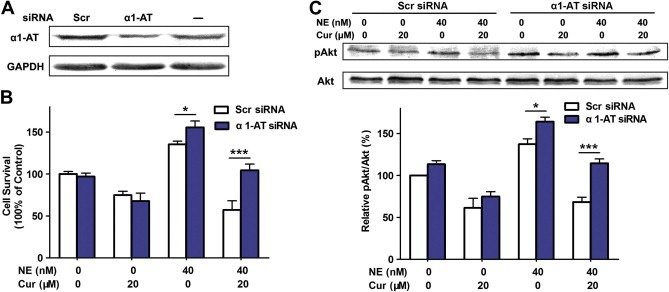
α1‐antitrypsin knockdown partially inhibited curcumin‐induced suppression of cell proliferation in A549 cells exposed to neutrophil elastase. (A) Western blot to detect the efficiency of α1‐antitrypsin (α1‐AT) knockdown. (B) Cell viability in A549 cells transfected with or without siRNA against α1‐antitrypsin. Scramble (Scr) siRNA was used as control. A549 cells were transfected with or without siRNA against α1‐antitrypsin for 24 h, and then incubated with or without 20 μM of curcumin (Cur) and 40 nM of neutrophil elastase (NE) for additional 24 h. Data represent mean ± SEM, n = 3. *P < 0.05, ***P < 0.001. (C) Western blot for pAkt and Akt in α1‐antitrypsin‐scilenced A549 cells exposed to neutrophil elastase and curcumin. Data represent mean ± SEM, n = 3. *P < 0.05, ***P < 0.001.
3.5. Curcumin inhibited primary tumor growth and lung metastasis in mice bearing Lewis lung carcinoma
Neutrophil elastase has been shown to directly mediate tumor growth in Lewis lung carcinoma model (Houghton et al., 2010). To determine the effects of curcumin on lung cancer in vivo, we introduced the Lewis lung carcinoma mouse model in C57BL/6 mice. As shown in Figure 5A, curcumin at a dose of 300 mg/kg reduced the primary tumor growth 21 days after flank injection of LLC cells (P < 0.01). The lung net weight (Figure 5B) and the number of lung metastasis (Figure 5C) were dramatically reduced in a dose‐dependent manner when treated with curcumin. To further confirm the inhibitory effect of curcumin on tumor growth, we identified tumor cell proliferation reductions in the groups treated with curcumin by Ki‐67 immunohistochemistry staining (Figure 5D).
Figure 5.
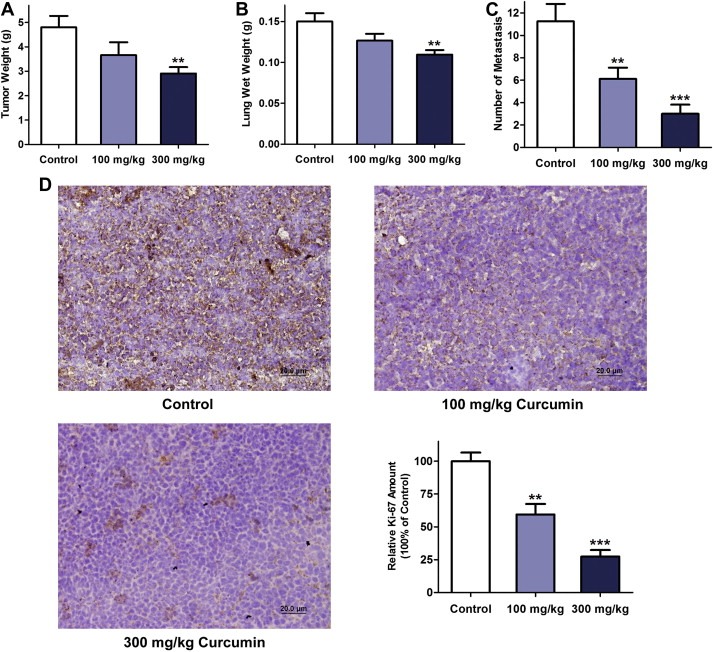
The effect of curcumin on the primary tumor growth and lung metastasis of Lewis lung carcinoma (LLC). (A) Primary tumor weights of mice treated with the vehicle (control), 100 mg/kg curcumin and 300 mg/kg curcumin for 21 days after subcutaneous injection of LLC cells. n = 8–10 mice per group, mean ± SEM, **P < 0.01 vs. control. (B) Lung net weights for each group. n = 8–10 mice per group, mean ± SEM, **P < 0.01 vs. control. (C) Gross LLC lung metastasis for the three groups. n = 8–10 mice per group, mean ± SEM, **P < 0.01, ***P < 0.001 vs. control. (D) Representative Ki‐67 immunohistochemistry images and statistical analysis of Ki‐67 expression in primary tumor tissue for each group. Data are expressed as the relative percentage of the value of Ki‐67 content for the untreated control group. n = 6, mean ± SEM, **P < 0.01, ***P < 0.001 vs. control.
3.6. Curcumin upregulated α1‐antitrypsin expression and inhibited the effect of neutrophil elastase in primary tumor tissue
Western bolt and quantitative real‐time PCR were used to determine the presence of α1‐antitrypsin in tumor tissues. The protein abundance of α1‐antitrypsin in the primary tumor in the flank fold was significantly increased at the higher concentration of curcumin (300 mg/kg, Figure 6A). α1‐antitrypsin mRNA level was consistently higher in mice treated with 300 mg/kg curcumin as determined by real‐time PCR, which indicated that cucumin could promote local expression of α1‐antitrypsin in primary tumor tissue (Figure 6B). As the natural inhibitor of neutrophil elastase, elevated α1‐antitrypsin might increase the degradation of neutrophil elastase. As shown in Figure 6C, the protein level of neutrophil elastase in tumor tissue was obviously decreased in mice treated with 300 mg/kg curcumin. Immunohistochemical analysis of Gr‐1 which is a maker of neutrophil showed equivalent neutrophil content in subcutaneously xenografted primary tumors in the three groups, which might exclude the possibility that the reduction of neutrophil elastase was attributed to decreased neutrophil infiltration induced by curcumin (Supplementary Figure 2). With the decrease of neutrophil elastase, the protein level of IRS‐1 was raised by curcumin in a dose‐dependent manner (Figure 6D). As the downstream molecules of PI3K, the phosphorylation of Akt and mTOR in tumor tissue was diminished in the groups treated with curcumin, while the phosphorylation of ERK was not affected (Figure 6D, E). Furthermore, the phosphorylation of IKKβ and p65, and the expression of Bcl‐2 in tumor tissues were significantly decreased in curcumin‐treated mice (Figure 6D, F). Overall, these results indicated that curcumin might inhibit neutrophil elastase‐induced activation of PI3K/Akt pathway via the upregulation of α1‐antitrypsin in primary tumor tissue.
Figure 6.
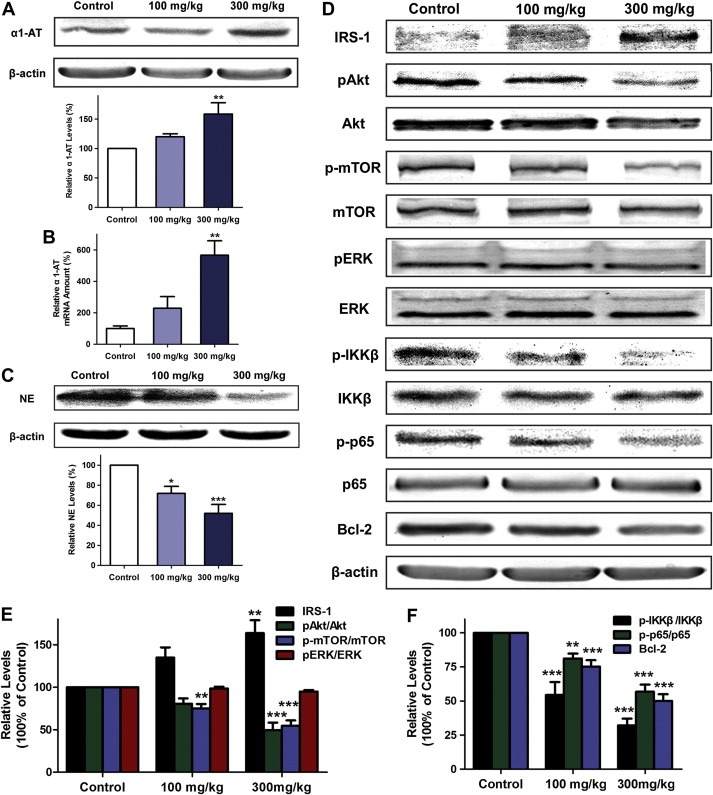
Curcumin‐induced increase of α1‐antitrypsin and decrease of neutrophil elastase in primary tumor tissue of C57BL/6 mice. (A) Western blot of α1‐antitrypsin (α1‐AT) in Lewis lung carcinoma tissue from the three groups. Data represent mean ± SEM, n = 6. **P < 0.01 as compared with control. (B) Quantitative real‐time PCR analysis for α1‐antitrypsin in tumor tissue. The mRNA level of α1‐antitrypsin was normalized to β‐actin. Data represent mean ± SEM, n = 3. **P < 0.01 as compared with control. (C) Western analysis of neutrophil elastase (NE) in tumor tissues. Data represent mean ± SEM, n = 6. *P < 0.05, ***P < 0.001 as compared with control. (D) Western blot for IRS‐1, pAkt, Akt, p‐mTOR, mTOR, pERK, ERK, p‐IKKβ, IKKβ, p‐p65, p65, Bcl‐2 and β‐actin in primary tumor tissues. (E) Statistical data shown as the histograms of Western blot for IRS‐1, the ratio of pAkt:Akt, p‐mTOR:mTOR and pERK:ERK. (F) Statistical data of Western blot for the p‐IKKβ:IKKβ ratio, the p‐p65:p65 ratio and Bcl‐2. Data represent mean ± SEM, n = 6. **P < 0.01, ***P < 0.001 as compared with control.
4. Discussion
In this decade, curcumin has been reported to inhibit tumor proliferation and metastasis, and induce the apoptosis of lung cancer in vitro and in vivo (Kunnumakkara et al., 2008). Curcumin suppresses the progression of K‐ras‐induced lung cancer progression in mice by inhibiting intrinsic and extrinsic inflammation and by direct anti‐tumor effects (Moghaddam et al., 2009). In addition, curcumin potentiates the anti‐tumor activity of gefitinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, in cell lines and xenograft mice model of non‐small cell lung cancer (NSCLC) through inhibition of proliferation, EGFR phosphorylation, and induction of EGFR ubiquitination (Lee et al., 2011). Many molecules are involved in the anti‐tumor activity of curcumin, including NF‐κB, COX‐2, iNOS, activator protein‐1 (AP‐1), tumor necrosis factor (TNF) and chemokines (Lin et al., 2009). As cancer progression is a complex process and its pathogenic mechanism remains somewhat elusive, and curcumin is considered as a multi‐target natural chemopreventive compound, the possibility that curcumin inhibits tumor development via other mechanisms cannot be excluded. The purpose of this study was to investigate the novel inflammation‐related mechanisms of curcumin for anti‐tumor activity in lung cancer.
Recent studies argue that chronic inflammatory pulmonary diseases, such as emphysema are highly associated with increased risk of lung cancer, independent of smoking (Koshiol et al., 2009). Neutrophil elastase as one of the most potent proteinases released by neutrophils is considered responsible for the elastolytic damage in emphysema (Fingleton, 2010). Neutrophil elastase may explain the link between emphysema and lung cancer to some extent. Recent reports showed that modest levels of neutrophil elastase led to uncontrolled proliferation of A549 lung epithelial tumor cells (Houghton et al., 2010). We found that neutrophil elastase treatment (20–160 nM) enhanced the viability of A549 cells and 40 nM of neutrophil elastase showed the strongest activity. The concentrations of neutrophil elastase used in our study were in agreement of a previous report that neutrophil elastase was released from activated neutrophils which resulted in modest concentrations (about 50 nM) of neutrophil elastase just beyond the cell surface (Liou and Campbell, 1996). Our results indicated that 20 μM and 40 μM of curcumin could completely suppress the excess enhancement of cell viability induced by neutrophil elastase in A549 cells. Notably, it was recently reported that tumor‐entrained neutrophils (TENs) induced by the primary tumor accumulated in the circulation and the premetastatic lung, and inhibited metastatic seeding of the tumor cells in the lungs by generating H2O2 in mouse models of breast cancer (Granot et al., 2011). Therefore, the role of neutrophil elastase which is generally considered the major effector of neutrophil function in tumor cell invasion and distant metastasis formation especially worth investigating.
Next, we explored whether curcumin inhibited neutrophil elastase‐induced cell proliferation by HCS analysis. HCS is an efficient technique combining multi‐channel quantitative fluorescence microscopy and automatic image analysis (Rausch, 2006). Our data related to four important hallmarks of proliferation including cell number, DNA content, BrdU incorporation and Ki‐67 expression. We found that neutrophil elastase elevated the BrdU incorporation and Ki‐67 content in A549 cells and the treatment of curcumin significantly neutralized their increase induced by neutrophil elastase. By DNA content analysis, the inhibitory effect of curcumin on cell proliferation stimulated by neutrophil elastase might be related to the inducement of a G2/M cell‐cycle arrest by curcumin. Our results are consistent with previous reports that curcumin is known as a G2/M cell‐cycle blocker (Weir et al., 2007; Zheng et al., 2004).
As α1‐antitrypsin is the specific and main inhibitor of neutrophil elastase, we assumed that α1‐antitrypsin might mediate the inhibitory effect of curcumin on neutrophil elastase‐induced proliferation in vitro. α1‐antitrypsin is synthesized mainly in liver, but also in extra‐hepatic tissues and cells, including carcinoma cells (Zelvyte et al., 2002). Furthermore, α1‐antitrypsin as a member of SERPIN is a glycoprotein which neutralizes the effects of proteases including neutrophil elastase in several organ systems, especially in lungs (Sun and Yang, 2004). Upon binding to neutrophil elastase, α1‐antitrypsin activates cleavage within the reactive site loop (RSL) in a suicide action (Hopkins et al., 1997). Evidence indicates that the level of α1‐antitrypsin is significantly elevated in the skin of NE−/− mice as compared to NE+/+ mice in a mouse model of the autoimmune disease bullous pemphigoid (Liu et al., 2000). We found that A549 cells produced α1‐antitrypsin and the treatment of curcumin might neutralize the decrease of α1‐antitrypsin induced by neutrophil elastase using Western blot and confocal microcopy. It's known that deficiency of circulating α1‐antitrypsin is highly associated with lung inflammation, especially with the early onset of pulmonary emphysema (Jones, 1974). In addition, clinical findings have indicated that α1‐antitrypsin which is elevated in serum of cancer patients is a serum biomarker for the diagnoses of lung cancer and prostate cancer (El‐Akawi et al., 2010; Patz et al., 2007). To explain the regulation of α1‐antitrypsin by curcumin, we examined the gene expression of α1‐antitrypsin by real‐time PCR and the α1‐antitrypsin promoter activity by luciferase reporter assay. We found that the accumulation of α1‐antitrypsin induced by curcumin might be attributed to the induction of the promoter activity of α1‐antitrypsin by curcumin and the subsequent promotion of its expression in A549 cells stimulated by neutrophil elastase.
Previous studies showed that curcumin inhibits PI3K/Akt/mTOR signaling in various tumor cells (Choi et al., 2008; Johnson et al., 2009; Yu et al., 2008). Our present data demonstrates that the inhibition of neutrophil elastase‐induced proliferation by curcumin is also depending on the PI3K pathway in A549 cells. Recently, it was shown that neutrophil elastase released by activated neutrophils within the lung is taken up by adjacent epithelial tumor cells and degrades IRS‐1 which is a binding partner of the p85 regulatory subunit of PI3K and induces the hyperactivity of the PI3K pathway which leads to uncontrolled tumor cell proliferation (Houghton et al., 2010). Our results showed that the increase of IRS‐1 and the decrease of phosphorylation activation of Akt and mTOR induced by neutrophil elastase were significantly inhibited by curcumin in A549 cells. Inhibition of the NF‐κB pathway has been thought to mediate the anti‐tumor effect of curcumin, and IKK which is the downstream target of Akt phosphorylates IκB, leading to the activation NF‐κB, which regulates the expression of key proteins involved in growth and survival such as Bcl‐2 and Bcl‐XL (El‐Rayes et al., 2006; Kamat et al., 2009; Prakobwong et al., 2011). Therefore, we explored whether curcumin could inhibit the NF‐κB pathway and found that curcumin treatment remarkably suppressed neutrophil elastase‐induced phosphorylation and activation of IKKβ and the p65 subunit of NF‐κB, and the expression of Bcl‐2 which is regulated by NF‐κB.
To further confirm that curcumin‐induced α1‐antitrypsin plays a crucial role in tumor cell proliferation triggered by neutrophil elastase, we used siRNA to specifically downregulate α1‐antitrypsin in A549 cells. Our data showed that knockdown of α1‐antitrypsin further enhanced the tumor cell proliferation induced by neutrophil elastase. In addition, the anti‐proliferation effect of curcumin was significantly blocked by the α1‐antitrypsin siRNA in neutrophil elastase‐triggered A549 cells.
To evaluate the effects of curcumin on lung cancer in vivo, we established the mouse model of Lewis lung carcinoma in C57BL/6 mice. It was reported that oral administration of curcumin inhibited mediastinal lymph node metastasis of orthotopically implanted Lewis lung carcinoma (Ichiki et al., 2000). Our results suggested that curcumin at doses up to 300 mg/kg significantly reduced the primary tumor weight and cell proliferation of primary tumors and inhibited LLC metastasis from primary tumor to lung in a dose‐dependent manner.
We found that curcumin promoted local expression of α1‐antitrypsin in primary tumor tissue using real‐time PCR and Western blot, which was in accordance with the anti‐proliferation effect of curcumin in a dose‐dependent manner. α1‐antitrypsin inhibited tumor growth and reduced tumor angiogenesis in nude mice bearing LLC cells and comparative analysis of the human tumors and normal tissues of origin showed that decreased local expression of α1‐antitrypsin was favorable for tumor growth (Huang et al., 2004). α1‐antitrypsin reduces [3H]‐thymidine incorporation and tissue inhibitor of metalloproteinases‐1 (TIMP‐1) levels in melanoma cells ME1477 in vitro (Zelvyte et al., 2002). The role of α1‐antitrypsin in cancer development has been supported by some laboratory data, however, the molecular mechanism underlying the role of α1‐antitrypsin in tumor proliferation is not well understood. The main function of α1‐antitrypsin is inhibiting neutrophil elastase. Our results indicated that the protein level of neutrophil elastase in tumor tissue was obviously decreased in mice treated with curcumin, which might be due to the upregulation of α1‐antitrypsin by curcumin. However, the contribution of α1‐antitrypsin to cancer development is somewhat controversial. In contrast to its effects against tumor growth, α1‐antitrypsin has been shown to be related to poor prognosis in lung adenocarcinoma (Higashiyama et al., 1992) and block the inhibitory effect of polymorphonuclear neutrophil (PMN) on cell proliferation and invasiveness in lung cancer HCC cells (Zelvyte et al., 2004). Thus, to unequivocally confirm the role of α1‐antitrypsin in primary tumor growth and metastasis in vivo, it's necessary to establish stable α1‐antitrypsin knockdown cell line using the shRNA lentiviral vector to perform mouse xenograft assays and determine the subsequent change of neutrophil elastase in response to inhibiting α1‐antitrypsin in vivo.
Taken as a whole, these data suggest that the stimulatory effects of curcumin on α1‐antitrypsin are crucial for the inhibition of neutrophil elastase‐induced proliferation by curcumin. However, the molecules and signal pathways that modulate the expression of α1‐antitrypsin remain exclusive and it is important to explore the mechanisms that regulate α1‐antitrypsin as a SERPIN highly related to lung inflammation and lung cancer. In addition, it remains to be investigated the role of α1‐antitrypsin in curcumin‐induced suppression of tumor growth in other xenograft mouse model of lung cancer.
5. Conclusions
This study demonstrates that α1‐antitrypsin as an inhibitor of neutrophil elastase modulates tumor proliferation in inflammatory microenvironment and curcumin blocks neutrophil elastase‐induced tumor proliferation via upregulating α1‐antitrypsin expression in lung cancer in vitro and in vivo. This provides a new inflammation‐related mechanism of curcumin against tumor proliferation and suggests the potential of curcumin to treat other inflammation‐related diseases in lung such as emphysema.
Supporting information
Supplementary data
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81020108031, 30572202, 30973558, 91129727, 30901815 and 30901803), the Major Specialized Research Fund from the Ministry of Science and Technology in China (No. 2009ZX09103‐144), and the Research Fund from Ministry of Education of China (111 Project, No. B07001).
Supplementary material 1.
1.1.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.molonc.2012.03.005.
Xu Yan, Zhang Jingjie, Han Jing, Pan Xueyang, Cao Yajun, Guo Hao, Pan Yan, An Yu, Li Xuejun, (2012), Curcumin inhibits tumor proliferation induced by neutrophil elastase through the upregulation of α1‐antitrypsin in lung cancer, Molecular Oncology, 6, doi: 10.1016/j.molonc.2012.03.005.
References
- Cadranel, J. , Bellocq, A. , Antoine, M. , Flahault, A. , Philippe, C. , Crestani, B. , Bernaudin, J.F. , Mayaud, C. , Milleron, B. , Baud, L. , 1998. Neutrophil alveolitis in bronchioloalveolar carcinoma – induction by tumor-derived interleukin-8 and relation to clinical outcome. Am. J. Pathol.. 152, 83–92. [PMC free article] [PubMed] [Google Scholar]
- Cheng, A.L. , Hsu, C.H. , Lin, J.K. , Hsu, M.M. , Ho, Y.F. , Shen, T.S. , Ko, J.Y. , Lin, J.T. , Lin, B.R. , Ming-Shiang, W. , Yu, H.S. , Jee, S.H. , Chen, G.S. , Chen, T.M. , Chen, C.A. , Lai, M.K. , Pu, Y.S. , Pan, M.H. , Wang, Y.J. , Tsai, C.C. , Hsieh, C.Y. , 2001. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res.. 21, 2895–2900. [PubMed] [Google Scholar]
- Choi, B.H. , Kim, C.G. , Lim, Y. , Shin, S.Y. , Lee, Y.H. , 2008. Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett.. 259, 111–118. [DOI] [PubMed] [Google Scholar]
- El-Akawi, Z.J. , Abu-Awad, A.M. , Sharara, A.M. , Khader, Y. , 2010. The importance of alpha-1 antitrypsin (alpha1-AT) and neopterin serum levels in the evaluation of non-small cell lung and prostate cancer patients. Neuroendocrinol. Lett.. 31, 113–116. [PubMed] [Google Scholar]
- El-Rayes, B.F. , Ali, S. , Ali, I.F. , Philip, P.A. , Abbruzzese, J. , Sarkar, F.H. , 2006. Potentiation of the effect of erlotinib by genistein in pancreatic cancer: the role of Akt and nuclear factor-kappaB. Cancer Res.. 66, 10553–10559. [DOI] [PubMed] [Google Scholar]
- Fingleton, B. , 2010. Inflammatory proteinase slips into tumor cells. Nat. Med.. 16, 161–163. [DOI] [PubMed] [Google Scholar]
- Granot, Z. , Henke, E. , Comen, E.A. , King, T.A. , Norton, L. , Benezra, R. , 2011. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 20, 300–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel, A. , Kunnumakkara, A.B. , Aggarwal, B.B. , 2008. Curcumin as "Curecumin": from kitchen to clinic. Biochem. Pharmacol.. 75, 787–809. [DOI] [PubMed] [Google Scholar]
- Gregory, A.D. , Houghton, A.M. , 2011. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res.. 71, 2411–2416. [DOI] [PubMed] [Google Scholar]
- Higashiyama, M. , Doi, O. , Kodama, K. , Yokouchi, H. , Tateishi, R. , 1992. An evaluation of the prognostic significance of alpha-1-antitrypsin expression in adenocarcinomas of the lung: an immunohistochemical analysis. Br. J. Cancer. 65, 300–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, P.C. , Chang, W.S. , Wardell, M.R. , Stone, S.R. , 1997. Inhibitory mechanism of serpins. Mobility of the C-terminal region of the reactive-site loop. J. Biol. Chem.. 272, 3905–3909. [DOI] [PubMed] [Google Scholar]
- Houghton, A.M. , Rzymkiewicz, D.M. , Ji, H. , Gregory, A.D. , Egea, E.E. , Metz, H.E. , Stolz, D.B. , Land, S.R. , Marconcini, L.A. , Kliment, C.R. , Jenkins, K.M. , Beaulieu, K.A. , Mouded, M. , Frank, S.J. , Wong, K.K. , Shapiro, S.D. , 2010. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med.. 16, 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Campbell, S.C. , Nelius, T. , Bedford, D.F. , Veliceasa, D. , Bouck, N.P. , Volpert, O.V. , 2004. Alpha1-antitrypsin inhibits angiogenesis and tumor growth. Int. J. Cancer. 112, 1042–1048. [DOI] [PubMed] [Google Scholar]
- Ichiki, K. , Mitani, N. , Doki, Y. , Hara, H. , Misaki, T. , Saiki, I. , 2000. Regulation of activator protein-1 activity in the mediastinal lymph node metastasis of lung cancer. Clin. Exp. Metastasis. 18, 539–545. [DOI] [PubMed] [Google Scholar]
- Jemal, A. , Bray, F. , Center, M.M. , Ferlay, J. , Ward, E. , Forman, D. , 2011. Global cancer statistics. CA Cancer J. Clin.. 61, 69–90. [DOI] [PubMed] [Google Scholar]
- Jemal, A. , Siegel, R. , Ward, E. , Hao, Y. , Xu, J. , Thun, M.J. , 2009. Cancer statistics, 2009. CA Cancer J. Clin.. 59, 225–249. [DOI] [PubMed] [Google Scholar]
- Johnson, S.M. , Gulhati, P. , Arrieta, I. , Wang, X. , Uchida, T. , Gao, T. , Evers, B.M. , 2009. Curcumin inhibits proliferation of colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer Res.. 29, 3185–3190. [PMC free article] [PubMed] [Google Scholar]
- Jones, S.H. , 1974. Pulmonary emphysema and alpha1-antitrypsin deficiency. Phys. Ther.. 54, 579–583. [DOI] [PubMed] [Google Scholar]
- Jurenka, J.S. , 2009. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern. Med. Rev.. 14, 141–153. [PubMed] [Google Scholar]
- Kamat, A.M. , Tharakan, S.T. , Sung, B. , Aggarwal, B.B. , 2009. Curcumin potentiates the antitumor effects of Bacillus Calmette-Guerin against bladder cancer through the downregulation of NF-kappaB and upregulation of TRAIL receptors. Cancer Res.. 69, 8958–8966. [DOI] [PubMed] [Google Scholar]
- Koshiol, J. , Rotunno, M. , Consonni, D. , Pesatori, A.C. , De Matteis, S. , Goldstein, A.M. , Chaturvedi, A.K. , Wacholder, S. , Landi, M.T. , Lubin, J.H. , Caporaso, N.E. , 2009. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PLoS One. 4, e7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara, A.B. , Anand, P. , Aggarwal, B.B. , 2008. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett.. 269, 199–225. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara, A.B. , Guha, S. , Krishnan, S. , Diagaradjane, P. , Gelovani, J. , Aggarwal, B.B. , 2007. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res.. 67, 3853–3861. [DOI] [PubMed] [Google Scholar]
- Lee, J.M. , Yanagawa, J. , Peebles, K.A. , Sharma, S. , Mao, J.T. , Dubinett, S.M. , 2008. Inflammation in lung carcinogenesis: new targets for lung cancer chemoprevention and treatment. Crit. Rev. Oncol. Hematol.. 66, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.Y. , Lee, Y.M. , Chang, G.C. , Yu, S.L. , Hsieh, W.Y. , Chen, J.J. , Chen, H.W. , Yang, P.C. , 2011. Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: the versatile adjuvant for gefitinib therapy. PLoS ONE. 6, e23756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S.S. , Lai, K.C. , Hsu, S.C. , Yang, J.S. , Kuo, C.L. , Lin, J.P. , Ma, Y.S. , Wu, C.C. , Chung, J.G. , 2009. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett.. 285, 127–133. [DOI] [PubMed] [Google Scholar]
- Liou, T.G. , Campbell, E.J. , 1996. Quantum proteolysis resulting from release of single granules by human neutrophils: a novel, nonoxidative mechanism of extracellular proteolytic activity. J. Immunol.. 157, 2624–2631. [PubMed] [Google Scholar]
- Liu, Z. , Zhou, X. , Shapiro, S.D. , Shipley, J.M. , Twining, S.S. , Diaz, L.A. , Senior, R.M. , Werb, Z. , 2000. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell. 102, 647–655. [DOI] [PubMed] [Google Scholar]
- Lungarella, G. , Cavarra, E. , Lucattelli, M. , Martorana, P.A. , 2008. The dual role of neutrophil elastase in lung destruction and repair. Int. J. Biochem. Cell Biol.. 40, 1287–1296. [DOI] [PubMed] [Google Scholar]
- Ma, B. , Pan, Y. , Song, Q. , Tie, L. , Zhang, Y. , Xiao, Y. , Zhang, J. , Han, J. , Xu, Y. , Xiang, Y. , Yu, H.M. , Li, X.J. , 2011. The effect of topiramate on tumor-related angiogenesis and on the serum proteome of mice bearing Lewis lung carcinoma. Eur. J. Pharmacol.. 663, 9–16. [DOI] [PubMed] [Google Scholar]
- Mantovani, A. , Allavena, P. , Sica, A. , Balkwill, F. , 2008. Cancer-related inflammation. Nature. 454, 436–444. [DOI] [PubMed] [Google Scholar]
- Moghaddam, S.J. , Barta, P. , Mirabolfathinejad, S.G. , Ammar-Aouchiche, Z. , Garza, N.T. , Vo, T.T. , Newman, R.A. , Aggarwal, B.B. , Evans, C.M. , Tuvim, M.J. , Lotan, R. , Dickey, B.F. , 2009. Curcumin inhibits COPD-like airway inflammation and lung cancer progression in mice. Carcinogenesis. 30, 1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, K. , Chappell, S. , Guetta-Baranes, T. , Morley, S. , Kalsheker, N. , 2009. The alpha-1-antitrypsin gene promoter in human A549 lung derived cells, and a novel transcription initiation site. Int. J. Biochem. Cell Biol.. 41, 1157–1164. [DOI] [PubMed] [Google Scholar]
- Normandin, K. , Peant, B. , Le Page, C. , de Ladurantaye, M. , Ouellet, V. , Tonin, P.N. , Provencher, D.M. , Mes-Masson, A.M. , 2010. Protease inhibitor SERPINA1 expression in epithelial ovarian cancer. Clin. Exp. Metastasis. 27, 55–69. [DOI] [PubMed] [Google Scholar]
- Patz, E.F. , Campa, M.J. , Gottlin, E.B. , Kusmartseva, I. , Guan, X.R. , Herndon, J.E. , 2007. Panel of serum biomarkers for the diagnosis of lung cancer. J. Clin. Oncol.. 25, 5578–5583. [DOI] [PubMed] [Google Scholar]
- Prakobwong, S. , Gupta, S.C. , Kim, J.H. , Sung, B. , Pinlaor, P. , Hiraku, Y. , Wongkham, S. , Sripa, B. , Pinlaor, S. , Aggarwal, B.B. , 2011. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis. 32, 1372–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch, O. , 2006. High content cellular screening. Curr. Opin. Chem. Biol.. 10, 316–320. [DOI] [PubMed] [Google Scholar]
- Sun, Z. , Yang, P. , 2004. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol.. 5, 182–190. [DOI] [PubMed] [Google Scholar]
- Surh, Y.J. , Chun, K.S. , Cha, H.H. , Han, S.S. , Keum, Y.S. , Park, K.K. , Lee, S.S. , 2001. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res.. 480-481, 243–268. [DOI] [PubMed] [Google Scholar]
- Weir, N.M. , Selvendiran, K. , Kutala, V.K. , Tong, L. , Vishwanath, S. , Rajaram, M. , Tridandapani, S. , Anant, S. , Kuppusamy, P. , 2007. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol. Ther.. 6, 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S.H. , Hang, L.W. , Yang, J.S. , Chen, H.Y. , Lin, H.Y. , Chiang, J.H. , Lu, C.C. , Yang, J.L. , Lai, T.Y. , Ko, Y.C. , Chung, J.G. , 2010. Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 cells through ER stress and caspase cascade- and mitochondria-dependent pathways. Anticancer Res.. 30, 2125–2133. [PubMed] [Google Scholar]
- Yu, S. , Shen, G. , Khor, T.O. , Kim, J.H. , Kong, A.N. , 2008. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol. Cancer Ther.. 7, 2609–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelvyte, I. , Sjogren, H.O. , Janciauskiene, S. , 2002. Effects of native and cleaved forms of alpha1-antitrypsin on ME 1477 tumor cell functional activity. Cancer Detect. Prev.. 26, 256–265. [DOI] [PubMed] [Google Scholar]
- Zelvyte, I. , Stevens, T. , Westin, U. , Janciauskiene, S. , 2004. alpha1-antitrypsin and its C-terminal fragment attenuate effects of degranulated neutrophil-conditioned medium on lung cancer HCC cells, in vitro. Cancer Cell Int.. 4, 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, M. , Ekmekcioglu, S. , Walch, E.T. , Tang, C.H. , Grimm, E.A. , 2004. Inhibition of nuclear factor-kappaB and nitric oxide by curcumin induces G2/M cell cycle arrest and apoptosis in human melanoma cells. Melanoma Res.. 14, 165–171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


