Abstract
Research with high throughput technologies has propitiated the segmentation of different types of tumors into very small subgroups characterized by the presence of very rare molecular alterations.
The identification of these subgroups and the apparition of new agents targeting these infrequent alterations are already affecting the way in which clinical trials are being conducted with an increased need to identify those patients harboring specific molecular alterations.
In this review we describe some of the currently ongoing and future studies at the Institut Gustave Roussy that aim for the identification of potential therapeutic targets for cancer patients with the incorporation of high throughput technologies into daily practice including aCGH, next generation sequencing and the creation of a software that allows for target identification specific for each tumor. The initial intention is to enrich clinical trials with cancer patients carrying certain molecular alterations in order to increase the possibility of demonstrating benefit from a targeted agent. Mid and long term aims are to facilitate and speed up the process of drug development as well as to implement the concept of personalized medicine.
Keywords: High throughput technologies, Metastatic cancer, Personalized medicine, Translational research
Highlights
Cancers are divided into very small subgroups carrying a rare molecular alteration.
Most of the new drugs are targeting these infrequent events.
Trials are testing the use of high throughput technologies in personalized medicine.
Circulating tumor cells and virtual cell program could optimize genomic testing.
Future clinical research will focus in target identification at a patients level.
1. Introduction
The emergence of high throughput technologies has allowed the stratification of the most common diseases into rare molecular segments. As an illustration, lung adenocarcinomas are now classified according to the presence of EGFR mutation, EML4‐ALK translocation, K‐Ras mutation or Her2 amplification (Paez et al., 2004; Soda et al., 2007; Slebos et al., 1990; Kern et al., 1990). The same situation is being met in breast cancer where luminal breast cancers can be divided in PIK3CA mutated, FGFR1 amplified, BRCA1/2 mutated, and so forth (Bachman et al., 2004; Reis‐Filho et al., 2006; Lancaster et al., 1996). The implication of such segmentation is the fact that most of the drugs now need to be developed in molecular segments that account for around 2–10% of a frequent disease. This profound change has led to the development of the concept of stratified medicine where the size population targeted by a drug is small and where the expected level of efficacy for each drug is very high (for more on this topic see Hoelder et al., 2012). This concept is not only restricted to the field of tumor cell biology, but also applies to host biology. As an example, there are emerging data suggesting that VEGFA serum levels could differentiate the degree of bevacizumab efficacy in breast cancer (Miles et al., 2010). Also, there is accumulating evidence that genetic polymorphisms on immune genes could define the magnitude of anticancer response (Zitvogel et al., 2011).
Overall, the concept that each disease or population could be segmented in sub‐stratum generates major questions regarding both clinical research and health care organization. The first question relates to the optimal way to set up a molecular screening program. Indeed, the need to enrich clinical therapeutic trials with patients presenting a certain alteration requires performing molecular screening to identify such patients eligible for targeted agent (see also Verweij et al., 2012). The second question relates to how to deal with tumor cell complexity. Considering that further segmentations will generate very rare populations, it is going to become unfeasible to test drugs in such very small groups. Hypotheses that do not directly relate to the drug should therefore be developed. One perspective could be to test the tools for target identification, and not longer the drugs themselves (see Garay, 2012). Finally, a major issue relates to the model of implementation for each molecular testing. Should these tests need to be performed within the context of an academic center and supervised by health care organizations or should be better done in private central labs? Moreover, which technologies are compatible with the daily practice needs? Also, on a related but germane issue, due to molecular screening, the spectrum of the cost of care in personalized medicine is expected to shift to diagnostic work up, a fact that also needs to be taken into consideration (see Philips et al., this issue).
A number of topics relevant to personalized cancer medicine are discussed in this issue of Molecular Oncology. From the basic biology of cancer (De Palma and Hanahan, 2012) to drug development (Hoelder et al, 2012), with connected themes, such as immunotherapy (Gajewski, 2012) and imaging (Kircher et al., 2012). Overall, these reviews take a glance into what the future of cancer medicine may look like. To try to set these perspective papers into the background of current initiatives, in the present paper we will review ongoing clinical research programs related to personalized medicine that are in progress at the Institut Gustave Roussy. We will discuss their impact and what perspectives they are expected to generate. The paper will only focus on tumor cell‐related personalized medicine, and will not address aspects related to the host or toxicities.
2. Molecular screening programs
As previously mentioned, most of the phase I/II trials now evaluate agents that target a specific protein. A significant number of these trials require for inclusion only those patients who present with a specific molecular alteration. We have previously reported that investigation of a single molecular alteration for each patient is suboptimal and generates a high rate of screen failure (Andre et al., 2011). In order to allow screening for a higher number of molecular alterations, and to drive as many patients as possible in clinical trials testing new targeted agents, we have generated an internal molecular screening program that consists in running multiplex technologies for patients with different types of metastatic cancers, to identify potential targets for each patient. This program overall involves 6 clinical trials and it is expected to include more than 2000 patients over the next 3 years. Three of these 6 programs are described with more detail below.
2.1. Molecular screening for metastatic breast cancer patients
The SAFIR01 trial (clinicaltrials.gov identifier: NCT01414933) aims to identify a targetable molecular alteration in patients with metastatic breast cancer. The trial design is reported in Figure 1. This program is sponsored by the cooperative group of French Cancer Centers (UNICANCER) and funded by the French National Cancer Institute (NCI). In this study, a biopsy is being performed in a metastatic site for each patient after signature of an informed consent and at the moment of stable disease or a partial response during an ongoing treatment. Array CGH and a panel of hot spot mutations on PIK3CA/AKT are done for each case. The primary endpoint is the percentage of patients entered into a clinical trial based on the molecular alterations detected. Overall 400 patients are planned. In November 2011, over 160 patients had already been included, after only 4 months being open for enrollment, indicating a high level of expectation from both medical oncologists and patients for such an approach. Interestingly, in addition to identify rare molecular alterations like FGFR1 amplification, PIK3CA mutations etc… some other more unusual alterations for breast cancer have been found, including EGFR and PDK1 amplifications, that could lead to the discovery of potentially new indications for already existing targeted agents (EGFR inhibitor and mTOR inhibitor, respectively).
Figure 1.
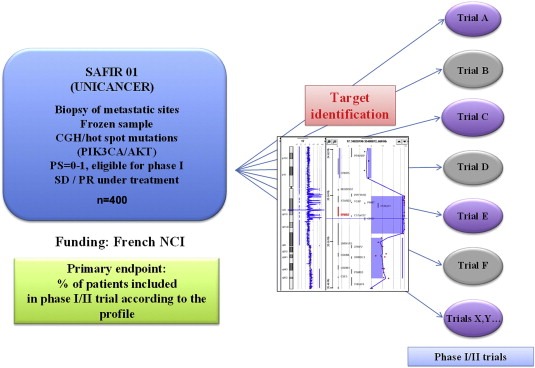
SAFIR01 schema.
2.2. Molecular screening in patients eligible for phase I trials
Since most of the phase I trials try to detect early signal for efficacy of targeted agents, there is a strong rationale to propose a molecular selection for patients eligible for these studies. Based on this, we have launched a trial called MOSCATO (Molecular Screening Approach for Treatment Optimization). The design is reported in Figure 2. This trial will include overall 600 patients over the next three years who will beneficiate from molecular profiling including array CGH and a panel of hot spots mutations in 96 amplicons from a biopsy performed in the metastatic site. Mutations will be detected using the Sanger method. Patients will be driven into specific phase I trials according to the presence of a molecular alteration. The primary endpoint is the efficacy of such approach measured as improvement in progression‐free survival (PFS) with the use of high throughput technologies is improved as compared to PFS on the prior line of treatment as described in a previously presented study (Von Hoff et al., 2010). This trial started in November 2011, and is funded by the Gustave Roussy Foundation.
Figure 2.
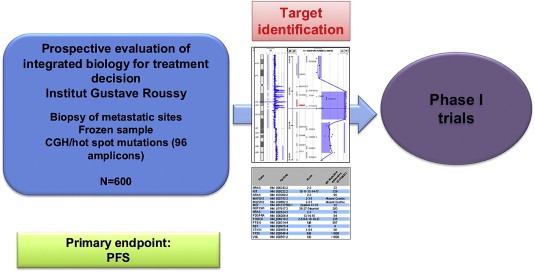
MOSCATO trial schema.
2.3. Molecular screening for lung cancer and melanoma patients
The MSN (Melanoma, Small cell, Non‐small cell lung cancers) trial aims at analyzing tumor samples in 400 lung cancers (small cell and non‐small cell types) and 150 melanomas before starting chemotherapy treatment. The primary objective is to assess differences in molecular profiling (by mRNA and microRNA) between responders and non‐responder patients in order to identify mechanisms of resistance to treatments. Also, for non‐small cell carcinomas the presence of ERCC1 amplification and EGFR mutations will be determined, as well as their impact in response to treatment. In the case of melanoma patients, BRAF, N‐Ras, c‐kit and p16 mutations will be evaluated. This study started in 2009 and so far has recruited a total of 246 and 81 patients with lung cancer and melanoma, respectively. In this case, molecular profiling is performed in either the primary tumor or the metastatic site, based on sample availability.
3. Implementation of next generation sequencing in molecular screening programs
As previously mentioned, the current approach for sequencing consists in using the Sanger method approach on a variable number of genes according to the need for each type of tumor. This approach presents nevertheless two major drawbacks. First, it allows the sequencing of only a limited number of genes per patient, leading to a suboptimal portrait. Second, it takes quite a significant amount of time for each sequence analysis. In order to increase the throughput of sequencing both in terms of base per patient and in number of patients per week, we are planning to implement next generation sequencing in the previously mentioned programs. For this purpose we have selected the Ion Torrent Technology based on its capacity, its length of each run and the possibility to develop an standard operating procedure (SOP) compatible with an eventual Clinical Laboratory Improvement Amendment (CLIA) certification. The goal is to implement it in our personalized medicine programs in March–April 2012 for the sequencing of 500 genes with a 10% sensitivity. The expected turnover will be 15 days for 16 patients per week. Beside the use of the Ion Torrent technology, our institution has also acquired both one Illumina and 454 Roche sequencing platforms for research purposes.
4. Expected impact of molecular screening programs
By running these large molecular screening programs, we expect to derive six sets of information. First, the primary goal of some trials (like SAFIR01 from Section 2.1) will be to enrich clinical trials with patients presenting a specific molecular alteration. In this situation, the objective is clearly to speed‐up drug development through the inclusion of informative patients in early therapeutic trials. Some other trials like the MOSCATO study (described in the Section 2.2) will use the molecular screening approach to generate preliminary data about the efficacy of high throughput technologies in terms of patient's outcome by comparing PFS before and after having performed high throughput technologies for each patient. Besides enrichment of phase I/II clinical trials with patients harboring specific alterations, this molecular screening program will have other major impacts. In the first place, it will allow creating the structure within the institution to propose molecular testing for patients with metastatic cancer and to implement the use of high throughput technologies for daily practice (Arnedos et al., 2011). This will require re‐organization of the radiology, biology and bioinformatics departments. Second, it will also potentiate the discovery of rare molecular segments that are not the target of drug development. As an example, the breast cancer patients described before for whom EGFR and FGFR2 amplifications have been found since they are very rare events in this type of tumors and because of this very low frequency, there is no plan for drug development on these molecular segments. Running high throughput technologies in a high number of patients will allow identifying a few patients who present such very rare alterations and to propose them a targeted agent in the context of a compassionate program. The occurrence of response in these few patients would allow drug repositioning in these rare indications. The molecular screening program will also allow generating a unique database of >2000 patients with metastatic cancer. This database will include both clinical and molecular data and the samples could be used for next generation technologies. Finally, since this database will match patient and tumor characteristics to the response to targeted agents, this program will be the baseline for the first validation steps of the virtual cell (see Section 5).
5. Towards a virtual cell
As previously mentioned, the current cancer segmentation includes rare diseases whose frequency is still compatible with the performance of clinical trials testing drugs in the population presenting the molecular alteration. Nevertheless, the use of high throughput technologies together with the eventual sub‐segmentations within specific subgroups will make each tumor unique regarding its molecular profile. In this context where each new disease segment will become more infrequent, it seems very unlikely that the next generation of trials in the metastatic setting will test new drugs in specific segments, given the low frequency of the indication. One perspective could be to further evaluate the methods for target identification at the tumor level, and no longer the drugs. Several approaches are being developed to identify the right target at the sample level (Mani et al., 2008; Chen et al., 2011; Barabasi et al., 2011) (see also O'Donnel and Ratain, 2012). We plan to develop a virtual cell that will map the main oncogenes and their interactions. The molecular profile of each sample will be entered in the software that will define the phenotype induced by the genomic alterations. In this virtual cell, each mutation, amplification or deletion will be attributed a specific weight and will activate the protein. This will define the virtual cell of a given cancer patients. The software will then test a panel of drug combination to evaluate which one leads to optimal effect on cell proliferation.
The long‐term perspective of the present personalized medicine program will be to assess whether the use of such virtual cell could improve outcome in patients with metastatic cancer. The expected trial is reported in Figure 4 and will address the efficacy of high throughput technologies interpreted using virtual cell. Four sets of data are needed before launching this trial: (i) we need to show feasibility of NGS in daily practice; (ii) report preliminary evidence for high throughput technologies in the trials previously mentioned; (iii) the virtual cell program needs to be completed and (iv) we need to show efficacy of this virtual cell both in preclinical models and in the retrospective analysis of the 2000 patients included in the 6 molecular screening programs.
Figure 4.
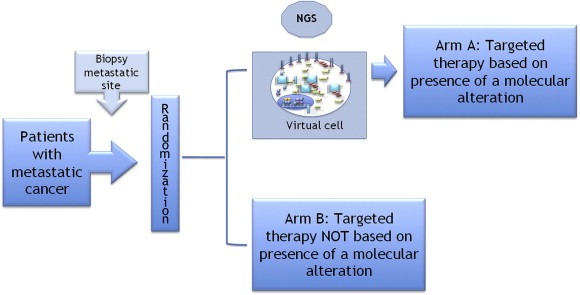
Design of the clinical trial to assess the use of the virtual cell to improve outcome in patients with metastatic cancer. Patients with advanced tumors will be subjected to a biopsy of the metastatic site and randomized to be treated or not according to NGS and virtual cell results.
6. Developing technologies to optimize genomic testing
6.1. Fine‐tuning genomic test by the use of functional testing
Although genomic analyses of metastatic samples will undoubtedly provide some advances in the field of personalized medicine, their use is being restricted by the fact that each mutation does not lead to activation, and that some pathways could be activated although no mutation is observed. The development of functional assays could solve this issue. Several functional assays are being developed that quantify either kinase activation or DNA repair. Regarding kinase activation, the reverse phase protein microarray (RPPA) has led the path since it allows quantification of phosphor‐proteins. The principle is to perform high throughput ELISA. Although very useful for characterization of populations, this tool is not currently appropriate for personalized medicine since it requires several dozens of samples or analysis (Gonzalez‐Angulo et al., 2010). More recent technologies have aimed at quantifying kinase activation, no longer phosphor‐proteins, at the sample level (Miduturu et al., 2011; Zhang et al., 2007; Karaman et al., 2008). In line with these goals, we are developing three tools to quantify kinase activity. This kinome array technology will be used to identify which kinase pathway is activated at baseline and to monitor bioactivity of targeted agents. The hypothesis of further clinical development will be that quantifying kinase activity could improve prediction obtained with genomic analyses.
6.2. Detecting genomic alterations in circulating tumor cells
Developing molecular characterization of circulating tumor cells (CTC) will lead to two major advances. First, it provides molecular characterization for treatment decisions, even in patients for which tumor biopsy is not feasible. This represents a significant proportion of patients with metastatic cancers, including patients with lung, pancreatic cancers. Second, it allows monitoring the molecular alteration in the blood during treatment exposure.
In the past decade technology advances enabled the detection of rare CTCs that have prospectively demonstrated to have prognostic significance for breast, colorectal, prostate and lung patients with advanced disease. In addition to prognostic utility, CTCs have emerged as an attractive alternative to tumor tissue for biomarker analysis that might help addressing some of the challenges described above. CTCs are easily accessible from a blood draw without risk and inconvenience to the patient compared to a fresh biopsy. Another facet of CTCs as a surrogate diagnosis tissue is the idea that CTCs could constitute a “liquid biopsy” and provide real time information on current state of the disease.
For several years our team has developed the ISET (Isolation by Size Epithelial Tumor, RareCell) system to quantify CTCs (Farace et al., 2011). More recently, we have also developed new technologies to characterize those CTCs detected with ISET. As illustration, we succeed to identify gene amplification using this approach (Figure 3). Based on this recent technological advance, we plan developing three programs. The overall goal of this program will be to characterize disease and monitor treatment efficacy. We will start from characterization of a few genes, then move to more complex characterization that includes functional DNA repair test, phospho‐proteins and high throughput technologies.
Figure 3.
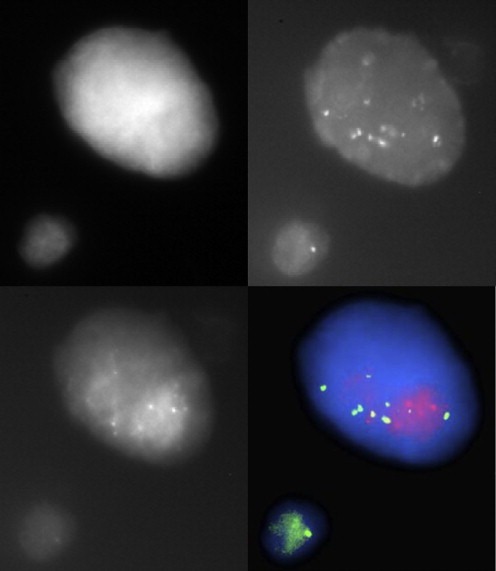
Example of FISH assay in a CTC isolated by ISET filtration in a HER2‐amplified patient. Cells are stained with FISH probes against HER2 (red dots) and a centromere probe (green dots).
First, we will propose this approach to select and monitor patients who present a gene amplification or translocation. This is being implemented in clinical trials testing new targeted agents in patients presenting with EML4/ALK translocation and FGFR1, FGFR2, EGFR, ERBB2 and MET amplifications. The goal will be to use this technology to identify gene alterations at baseline. Patients will be driven to clinical trials accordingly. This program will be complementary to the molecular screening program presented in the specific aim I. After patients have been included, we will monitor treatment efficacy using this new technology. In the second program, we will implement DNA repair test and quantification of phospho‐proteins in the CTC. This approach will allow us to broad the number of targets as compared to the 1st part. Finally, we will perform DNA extraction and CGH array, then NGS in single cancer cells isolated from ISET filters. In the long term, it should allow us proposing a more complex characterization of tumor cells without requiring any biopsy.
7. Developing platforms for molecular analyses
The French NCI has developed 28 platforms for molecular analyses in France that are publicly financed allowing each French resident to have access to molecular testing for free including those single gene tests that are required for the administration of a targeted agent. One of the major questions will be whether such a model will apply for high throughput technologies. In the previously mentioned SAFIR trial (Section 2.1), 4 of these 28 molecular platforms are running the high throughput molecular analyses. One of the goals for these academic platforms, including ours, will then be to implement NGS with SOP compatible for clinical use to obtain CLIA certification in order to propose service at the international level.
8. Conclusion
In the present document, we have presented ongoing and future prospective trials at the Institut Gustave Roussy that aim the identification of potential therapeutic targets for cancer patients. These studies will, in a first step, provide patients presenting specific molecular alterations for phase I/II clinical trials. Nevertheless, their long‐term goal is to prepare large studies that will test the benefit of high throughput technologies and personalized medicine. The tools that will allow such paradigm shift are the implementation of high throughput technologies and, more importantly, the development of software for target identification at the patient's level.
Although this presentation of ongoing programs on personalized medicine focus on tumor cell‐derived biomarkers, they are being integrated with personalized approaches in the field of immunotherapy and treatment toxicities. In these two latter fields, we are developing predictive biomarkers on host response to conventional or new agents.
Arnedos Monica, André Fabrice, Farace Françoise, Lacroix Ludovic, Besse Benjamin, Robert Caroline, Soria Jean Charles, Eggermont Alexander M.M., (2012), The challenge to bring personalized cancer medicine from clinical trials into routine clinical practice: The case of the Institut Gustave Roussy, Molecular Oncology, 6, doi: 10.1016/j.molonc.2012.02.008.
References
- Andre, F. , Delaloge, S. , Soria, J.C. , 2011. Biology-driven phase II trials: what is the optimal model for molecular selection?. J. Clin. Oncol.. 29, (10) 1236–1238. [DOI] [PubMed] [Google Scholar]
- Arnedos, M. , 2011 September. High throughput molecular analyses to select patients for targeted agents. Eur. J. Cancer. 47, (Suppl. 1) S1–S760. [Google Scholar]
- Bachman, K.E. , 2004. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol. Ther.. 3, (8) 772–775. [DOI] [PubMed] [Google Scholar]
- Barabasi, A.L. , Gulbahce, N. , Loscalzo, J. , 2011. Network medicine: a network-based approach to human disease. Nat. Rev. Genet.. 12, (1) 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , 2011. A novel paradigm for potential drug-targets discovery: quantifying relationships of enzymes and cascade interactions of neighboring biological processes to identify drug-targets. Mol. Biosyst.. 7, (4) 1033–1041. [DOI] [PubMed] [Google Scholar]
- De Palma, M. , Hanahan, D. , 2012. The biology of personalized cancer medicine: Facing individual complexities underlying hallmark capabilities. Mol. Oncol.. 6, (2) 111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farace, F. , 2011. A direct comparison of cell search and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br. J. Cancer.. 105, (6) 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski, T.F. , 2012. Cancer immunotherapy. Mol. Oncol.. 6, (2) 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay, J.P. , Gray, J.W. , 2012. Omics and therapy – A basis for precision medicine. Mol. Oncol. 6, (2) 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Angulo, A.M. , Hennessy, B.T. , Mills, G.B. , 2010. Future of personalized medicine in oncology: a systems biology approach. J. Clin. Oncol.. 28, (16) 2777–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelder, S. , Clarke, P.A. , Workman, P. , 2012. Discovery of small molecule cancer drugs: Successes, challenges and opportunities. Mol. Oncol.. 6, (2) 155–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman, M.W. , 2008. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol.. 26, (1) 127–132. [DOI] [PubMed] [Google Scholar]
- Kern, J.A. , 1990. p185neu expression in human lung adenocarcinomas predicts shortened survival. Cancer Res.. 50, (16) 5184–5187. [PubMed] [Google Scholar]
- Kircher, M.F. , Hricak, H. , Larson, S.M. , 2012. Molecular imaging for personalized cancer care. Mol. Oncol.. 6, (2) 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, J.M. , 1996. BRCA2 mutations in primary breast and ovarian cancers. Nat. Genet.. 13, (2) 238–240. [DOI] [PubMed] [Google Scholar]
- Mani, K.M. , 2008. A systems biology approach to prediction of oncogenes and molecular perturbation targets in B-cell lymphomas. Mol. Syst. Biol.. 4, 169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miduturu, C.V. , 2011. High-throughput kinase profiling: a more efficient approach toward the discovery of new kinase inhibitors. Chem. Biol.. 18, (7) 868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles, D. , 2010. Plasma biomarker analyses in the AVADO phase III Randomized study of first-line bevacizumab + Docetaxel in patients with human Epidermal Growth Factor Receptor (HER) 2-Negative metastatic breast cancer. Cancer Res.. 70, (24, Suppl. 2) [Google Scholar]
- O'Donnell, P.H. , Ratain, M.J. , 2012. Germline pharmacogenomics in oncology: Decoding the patient for targeting therapy. Mol. Oncol.. 6, (2) 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez, J.G. , 2004. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 304, (5676) 1497–1500. [DOI] [PubMed] [Google Scholar]
- Reis-Filho, J.S. , 2006. FGFR1 emerges as a potential therapeutic target for lobular breast carcinomas. Clin. Cancer Res.. 12, (22) 6652–6662. [DOI] [PubMed] [Google Scholar]
- Slebos, R.J. , 1990. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N. Engl. J. Med.. 323, (9) 561–565. [DOI] [PubMed] [Google Scholar]
- Soda, M. , 2007. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 448, (7153) 561–566. [DOI] [PubMed] [Google Scholar]
- Verweij, J. , de Jonge, M. , Eskens, F. , Sleijfer, S. , 2012. Moving molecular targeted drug therapy towards personalized medicine: Issues related to clinical trial design. Mol. Oncol. 6, (2) 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff, D.D. , 2010. Pilot study using molecular profiling of patients' tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol.. 28, (33) 4877–4883. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , 2007. Molecular imaging of Akt kinase activity. Nat. Med.. 13, (9) 1114–1119. [DOI] [PubMed] [Google Scholar]
- Zitvogel, L. , Kepp, O. , Kroemer, G. , 2011. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat. Rev. Clin. Oncol.. 8, (3) 151–160. [DOI] [PubMed] [Google Scholar]


