Abstract
Radiotherapy is today used in about 50% of all cancer patients, often in multidisciplinary approaches. With major advance in radiotherapy techniques, increasing knowledge on tumor genetics and biology and the continuous introduction of specifically targeted drugs into combined radio‐oncological treatment schedules, individualization of radiotherapy is of high priority to further improve treatment outcomes, i.e. to increase long‐term tumor cure and/or to reduce chronic treatment toxicity. This review gives an overview on the importance of predictive biomarkers for the field of radiation oncology. The current status of knowledge on potential biomarkers of tumor hypoxia, tumor cell metabolism, DNA repair, cancer stem cells and biomarkers for combining radiotherapy with inhibition of the epidermal growth factor receptor using monoclonal antibodies is described.
Keywords: Radiotherapy, Individualized treatment, Predictive biomarker
1. Introduction
Radiotherapy is an important modality in the treatment of cancer patients applied either alone or in combination with other modes of treatment, i.e. surgery or chemotherapy. External beam radiotherapy significantly contributes to cure or palliation of many cancer patients and is utilized in approximately 40–50% of all cases (Bamberg et al., 2004; Delaney et al., 2005). Curative radiotherapy aims to permanently control a primary tumor and regional lymph node metastases without unacceptable normal tissue toxicity. It is well recognized that all patients are individual and respond differently to the standard radiotherapy applying total radiation doses in 30–35 daily 2 Gy‐fractions within 6–7 weeks in many tumor entities. While most of the efforts to optimize radiotherapy have aimed to improve treatment from the physical and technological standpoint such as precision in dose delivery, optimization of treatment plans, etc. (Sonke and Belderbos, 2010), less progress has been made to include patients' individual biological characteristics into treatment decision. There are multiple radiobiological factors that may contribute either alone or in combination to the differential response of tumors to radiation therapy. Among them, the number of cancer stem cells, their intrinsic cellular radiosensitivity, repopulation and reoxygenation capacity during the course of radiotherapy, repair of radiation‐induced damage, and tumor hypoxia have been extensively investigated in experimental and clinical studies for many years (Baumann et al., 2011, 2009, 1998, 2001, 1993, 1988, 2007). It appears promising to integrate this biological information to further improve radiotherapy and thereby treatment outcome. Such treatment concept of applying the optimal therapy for each patient is referred to individualized or personalized treatment. For this, reliable predictive biologically rational markers are required that guide radiation oncologists to optimal dose prescription, selection of fractionation schemes and of combined treatments for each individual patient. Nowadays several pre‐treatment parameters such as tumor histology, stage, grade and performance status are used in clinical practice to prescribe the best available treatment, however, tumor biological parameters are rarely taken into account. Usually a tumor undergoes anatomical and biological changes during treatment, which may further help to optimize treatment of an individual patient if reliable tests to predict early tumor response to therapy are available. Such predictive tests are necessary because clearly not all patients will benefit from the same treatment interventions.
With the considerable progress in non‐invasive functional and molecular imaging, the tools for treatment individualization based not only on morphological criteria but also on biological information such as hypoxic tumor status, metabolic and proliferative activity before and during treatment are becoming available (Bussink et al. 2011). Development, validation and integration of imaging biomarkers using CT, PET and MRI to improve radiotherapy are therefore an important task for further preclinical and clinical research.
2. Predictive biomarkers and therapy individualization
The primary aim of a predictive biomarker is to accurately determine the outcome of a given treatment. Ideally both, tumor and surrounding normal tissue responses can be assessed. If a biomarker is to be measured during the course of treatment, the accurate prediction should be made early enough to permit treatment interventions. This underlines the causative relationship between a biological factor and therapy outcome, i.e. measures to change biomarker expression lead to the corresponding change in treatment response. In other words, a strong association between biomarker and treatment outcome should result from a similar direct treatment effect on both, biomarker level and endpoint of therapy. In contrast, prognostic markers may show an association with patient outcome independent of treatment. Therefore prognostic markers do not allow designing a treatment strategy for an individual patient.
There are two strategies on utilization of predictive biomarkers for individualized treatment. First, a biomarker can be used to select the primary treatment for an individual patient. For example, whereas a patient with a hypoxic tumor can benefit from radiotherapy combined with hypoxia‐targeted therapy, such a combined treatment may cause an unjustified elevated risk of drug‐related side effects and increased treatment costs in a patient with a non‐hypoxic tumor. Secondly, biomarkers can be used for early assessment of treatment efficacy and as a result can be applied to decide to continue, to modify or to interrupt initial treatment. Similarly, it can be speculated (see below) that outcome of radiotherapy may be improved using hypoxia‐modifying treatments in a patient with residual tumor hypoxia and no benefit may be expected in a patient showing no evidence of residual hypoxia.
Prior to implementation of an individualized treatment in the routine clinical practice several criteria must be met:
-
1.
The treatment or therapeutic intervention must be clinically approved.
-
2.
Evidence of the utility of a biomarker to predict patient outcome after a specific therapy is provided by a prospective clinical trial.
-
3.
A standardized protocol of acquisition and evaluation of the predictive information must be available.
These criteria predetermine a long way for a biomarker to be introduced in daily clinical practice. In addition, validation may not be universal for each tumor entity and specific treatment, which may require an independent testing.
In Medical Oncology a number of biomarkers for treatment decision have been implemented into clinical routine For example, HER‐2 and hormone receptor status assessed in biopsy of breast cancer patients is currently used for selection of adjuvant chemo‐ hormonal and antibody treatment (Harbeck et al. 2010). KRAS mutational status is integrated into treatment of colorectal cancer to assign patients for anti‐EGFR monoclonal antibody therapies with KRAS wild‐type tumors (Amado et al. 2008) and a gene signature to predict the metastatic potential and thereby decide for or against the use of adjuvant chemotherapy is currently validated in a prospective randomized trial in breast cancer patients (Bueno‐de‐Mesquita et al., 2007; Slodkowska and Ross, 2009). Although experimental and clinical studies have suggested a number of promising biomarkers for radiotherapy, these are not yet introduced in the routine clinical use. In this review we outline several examples of the use of potential predictive biomarkers in selection process of personalized radiotherapy.
3. Radiotherapy individualization based on hypoxia markers
Experimental and clinical data demonstrate that tumor hypoxia plays an important role in malignant progression and resistance to radiotherapy (Bristow and Hill, 2008; Lunt et al., 2009; Vaupel and Mayer, 2007). A number of different mechanisms might explain the association of tumor hypoxia with poor treatment outcome including the oxygen effect, i.e. three times higher radioresistance of cells in the absence of oxygen compared to normoxic cells, acute and chronic changes in gene expression and hypoxia‐driven selection of resistant clones during carcinogenesis. There are several ways to tackle tumor hypoxia such as to increase blood oxygen by breathing higher oxygen concentrations before and during irradiation, to specifically radiosensitize hypoxic cells using oxygen‐mimicking drugs or to directly kill hypoxic cells using bioreductive compounds (Begg et al., 2011). Controlled clinical trials during the last several decades indicated that some of these strategies to overcome tumor hypoxia had proved to improve the effect of radiotherapy (Overgaard, 2007) and other methods are currently being assessed in large international multicentre studies (Overgaard 2011; Kaanders et al., 2002a). Because human tumors show considerable heterogeneity in the extent of hypoxia (Nordsmark et al., 2007), markers to identify hypoxic tumors that can be responsive to hypoxia‐overcoming treatments are required. While many experimental and clinical studies demonstrated a prognostic value of various hypoxia‐associated parameters for local tumor control after radiotherapy (Evans and Koch, 2003; Vaupel and Mayer, 2007), only few clinical investigations concluded that hypoxia markers could be used as predictive tests (Rischin et al., 2006; Toustrup et al., 2011; Kaanders et al., 2002b; Overgaard et al., 2005).
Kaanders and colleagues measured the pre‐treatment hypoxic fraction using pimonidazole hypoxia marker in tumor biopsies from head and neck cancer patients in a Phase II trial (Kaanders et al., 2002b). Patients were treated either with radiotherapy or surgery or with ARCON, which combines accelerated radiotherapy to counteract tumor repopulation with carbogen breathing and nicotinamide to reduce chronic and acute hypoxia, respectively (Kaanders et al., 2002c). In this study the extent of pimonidazole binding varied between 0.3% and 17%, indicating that human tumors of the same histology exhibit a differential degree of hypoxia. Locoregional tumor control was significantly lower for patients who had a higher hypoxic fraction. This association disappeared in the subgroup of patients treated with ARCON, suggesting that pimonidazole binding can predict treatment outcome in head and neck cancer patients and may be used as biomarker to select patients for hypoxia‐modifying treatments.
Another study by Overgaard et al. provides evidence of a predictive value of osteopontin protein, which has been shown to be associated with tumor hypoxia (Overgaard et al., 2005). Although the relation between osteopontin and hypoxia is not completely understood, the inverse correlation between osteopontin and von Hippel‐Lindau (VHL) gene expression suggests that osteopontin may be modulated by VHL (Le et al., 2003). The latter regulates the oxygen‐dependent ubiquitination and proteolysis of the HIF‐1α (hypoxia‐inducible transcription factor‐1), which activates the transcription of numerous genes essential for adaptive responses to hypoxia (Majmundar et al., 2010). It has been also shown that hypoxia stimulated secretion of osteopontin in vitro and that plasma osteopontin levels negatively correlated with tumor oxygen partial pressure (pO2) in head and neck cancer patients (Le et al., 2003; Sorensen et al., 2005). Osteopontin was measured in plasma samples collected from patients with head and neck cancer in a Phase III randomized clinical trial comparing conventional radiotherapy with the same radiotherapy plus the hypoxic radiosensitizer nimorazole. Osteopontin concentration showed high variability between the patients (range 12–1382 μg/L). High plasma concentrations of osteopontin, i.e. high levels of hypoxia, were associated with poor outcome after radiotherapy alone. The outcome could be significantly improved by the combination of radiotherapy with nimorazole. In contrast, use of nimorazole was not beneficial in patients with low or intermediate plasma concentrations of osteopontin. The authors concluded that high osteopontin concentrations predicted clinically relevant, modifiable hypoxia‐induced resistance to radiotherapy, and this finding might help to identify patients who will benefit from treatment with a hypoxia modifier such as nimorazole during radiotherapy. Similar findings were obtained for a set of hypoxia‐responsive genes in this group of patients, i.e. in patients with tumors classified as “more hypoxic” combining of nimorazole with radiotherapy increased locoregional tumor control and prolonged disease‐specific survival to the same level as in patients with “less” hypoxic tumors (Toustrup et al., 2012). The authors considered 15‐gene hypoxia classifier as a more reliable estimate of tumor hypoxia than osteopontin concentration. Also in contrast to the findings of Overgaard et al. (2005), prognostic and predictive significance of plasma osteopontin could not be confirmed in a recent large Phase III randomized clinical trial in head and neck cancer patients comparing chemoradiotherapy with or without hypoxic‐cell cytotoxin tirapazamine (Lim et al., 2012). However, interpretation of these data is limited by the fact that the overall result of the study was negative, i.e. there was no improvement of outcome by tirapazamine. The advantage of osteopontin or gene signatures as biomarkers over pimonidazole is that they can easily be obtained from plasma samples (osteopontin) or tumor tissue (gene signature), whereas the evaluation of pimonidazole requires an injection of the marker before biopsy.
Similarly, Rischin and colleagues used 18F‐misonidazole PET imaging to measure hypoxia before treatment and at weeks 4–5 during treatment in patients with head and neck cancer randomized in a Phase II trial for chemoradiotherapy alone or in combination with tirapazamine (Rischin et al., 2006). Baseline tumor hypoxia, i.e. high 18F‐misonidazole uptake, was associated with a high risk of locoregional failure after chemoradiotherapy alone. Conversely, a considerable improvement in locoregional control was found in patients with hypoxic tumors treated with the tirapazamine‐containing regimen. Tirapazamine, however, could not improve the outcome of chemoradiotherapy in non‐hypoxic tumors. Interestingly, in this study all patients with residual hypoxia in the mid‐treatment experienced locoregional failure, whereas less than a half of the tumors without residual hypoxia recurred after chemoradiotherapy. This suggests that hypoxia‐targeted interventions may further improve treatment outcome. However, as mentioned above the negative results of the clinical trial that tested the benefit of combining tirapazamine with cisplatin and radiotherapy in unselected head and neck cancer patients underlies the need of biomarkers to carefully select patients for such treatments (Rischin et al., 2010).
Experimental data from our laboratory support the evaluation of biomarkers during the course of fractionated irradiation because they may provide better prognostic or predictive information for the outcome of radiotherapy than pre‐treatment parameters (Yaromina et al., 2011). In an exploratory study that included 6 different human head and neck squamous cell carcinoma xenografts, we found that the tumor microenvironment may undergo important changes during irradiation. The residual pimonidazole hypoxic volume measured at 2 weeks during fractionated irradiation varied between 5 and 19 mm3 and was associated with radioresistance to clinically relevant fractionated radiotherapy (Figure 1). In contrast, pre‐treatment hypoxic volumes did not show this correlation. Although this study so far only demonstrates a prognostic value of the pimonidazole hypoxic volume, it underlines the importance of testing the significance of tumor micromilieu‐related biomarkers not only prior to treatment but also during the course of radiotherapy. This finding is consistent with the clinical results reported by Rischin et al. (see above) (Rischin et al., 2006) and preliminary results of an ongoing clinical trial conducted in our department (Zips et al., unpublished data). This trial suggests that tumor hypoxia as detected using PET and the hypoxia marker 18F‐misonidazole shows better prognostic value when measured during the first weeks of radiotherapy than before treatment.
Figure 1.
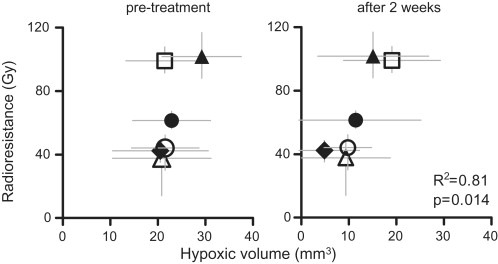
Pimonidazole hypoxic volume (±standard deviation) after 2 weeks of fractionated irradiation shows association with radioresistance to therapy with 30 fractions over 6 weeks in 6 different human tumor xenografts: ∆‐UT‐SCC‐15; ♦‐XF354; ○‐UT‐SCC‐14; ●‐FaDu; □‐SAS; ▲‐UT‐SCC‐5. The radioresistance represents dose to cure 50% of tumors (TCD50 with 95% confidence interval) after 30 fractions over 6 weeks. Adapted from (Yaromina et al., 2011).
Overall, there is evidence showing that important differences in the level of hypoxia exist between patients and that this variation can be detected and may be used for the selection of the optimal treatment, here for the use of combined radiotherapy and modifiers of hypoxia. There are a number of limitations of these studies that restrict the implementation of the above biomarkers in radiotherapy. For example, it is essential to use randomized patient populations to ensure that the patient groups are comparable, to assess biomarker in the majority of patients to limit selection biases, to prospectively state a hypothesis including statistical methods, power calculations and sample size (Van Schaeybroeck et al., 2011). Future studies that are performed in a randomized setting with an intervention to modify hypoxia are therefore needed to validate predictive hypoxia biomarkers.
4. Radiotherapy individualization based on FDG‐PET
The non‐invasive or minimally invasive nature of molecular imaging techniques allows serial evaluation of changes in tumor biological characteristics. Molecular imaging therefore is suitable to provide not only pre‐treatment parameters but also allows early assessment of treatment effects. In most studies performed to date, however, treatment response using imaging has been evaluated after the end of therapy and the potential clinical utility for early response assessment during therapy has not been addressed systematically (Schoder et al., 2009). The increasing use and availability of PET in radiotherapy will make it feasible to incorporate PET imaging predictive tests into clinical practice if validation studies confirm the utility of specific PET tracers.
The PET tracer 18F‐FDG is one of the best studied biomarkers in experimental and clinical investigations. It has been introduced for individual patients for staging and response monitoring and is currently tested for its predictive potential in clinical trials (Mac Manus and Hicks, 2008). 18F‐FDG intensity on a PET image represents the extent of glucose uptake by highly metabolically active malignant cells. A number of clinical studies demonstrated independent prognostic information of quantitative assessment of 18F‐FDG uptake before or early during treatment for the outcome of (chemo‐) radiotherapy in various tumor entities (Hentschel et al., 2011; Bussink et al., 2011). Despite these findings there is no evidence yet on the use of 18F‐FDG‐PET imaging for selection of patients for individualized radiotherapy. Because of the heterogeneous distribution of 18F‐FDG uptake within the tumor, one approach to utilize 18F‐FDG‐PET for specific treatment is to apply inhomogeneous radiation doses to the tumor (Bentzen, 2005), i.e. tumor regions with elevated 18F‐FDG avidity are irradiated with higher doses and standard or reduced doses are delivered to the rest of the tumor. The biological rationale for this strategy is that high 18F‐FDG uptake reflects tumor regions with high cell density and may identify the areas of radioresistant tumor cells owing to hypoxia or other mechanisms of radioresistance (Burgman et al., 2001; Pugachev et al. 2005; Rajendran et al., 2004), although preclinical data are partly contradictory (Bruechner et al., 2009). The hypothesis of a more aggressive tumor phenotype within high FDG‐avid tumor regions is also supported by the observation that local recurrences after radiotherapy often occur within the irradiated target volume with the highest 18F‐FDG uptake (Abramyuk et al., 2009; Aerts et al., 2009; Madani et al., 2007; van den Bogaard et al., 2011). The results of an experimental study performed in our laboratory showed that an increase of single radiation dose had a greater effect on local control in tumors with high pre‐therapeutic 18F‐FDG uptake than in tumors with low 18F‐FDG uptake in a human head and neck squamous cell carcinoma model (Schutze et al., 2007). This finding supports inhomogeneous dose distributions or dose‐escalation in individual patients based on 18F‐FDG uptake in different subvolumes of the tumors. The data suggest that dose should not be decreased in low 18F‐FDG uptake areas where the dose–response relationship seems to be very shallow but rather, dose should be increased in high uptake areas where at low doses the local tumor control probability is poor but the dose–response relationship appears to be steep. This suggestion is further supported by preliminary results showing that the dose–response curve for local tumor control is also steeper after fractionated irradiation in tumors with high pre‐treatment 18F‐FDG uptake (Schutze et al., unpublished data). In contrast, the same was not observed if tumors were stratified by 18F‐FDG uptake 2 weeks after fractionated irradiation.
Clinical radiotherapy treatment planning studies have demonstrated the feasibility to selectively escalate the dose to the tumor areas with increased tracer uptake in several cancers (Jingu et al., 2010; Schwartz et al., 2005). In a Phase I clinical trial the dose could be homogeneously escalated inside 18F‐FDG‐PET‐defined contour and treatment was well tolerated in patients with head and neck tumors (Madani et al., 2007). Furthermore, in a recent Phase I clinical trial a dose‐escalation in 18F‐FDG regions and dose painting by numbers approach (i.e. dose prescription to voxels as a function of signal intensity of the corresponding voxel in a biological image) was used for adaptation of radiation treatment volumes based on 18F‐FDG‐PET/CT‐ detected biological and anatomical changes at the end of the second week of treatment. With acceptable acute toxicity, the approach resulted in a significant reduction of target volumes in head and neck cancer patients (Duprez et al., 2011). The feasibility of boosting areas with residual 18F‐FDG uptake at mid‐treatment or later during radiotherapy in lung cancer is, however, controversial (Feng et al., 2009; Gillham et al., 2008).
Overall, these studies confirm a value of 18F‐FDG‐PET in monitoring of early treatment response and show the feasibility of integrating PET for radiotherapy optimization before initiation of treatment and/or early during therapy. The optimal time points of imaging, i.e. before or during treatment, for accurate response prediction and thereby for modifications or adaptation of treatment protocols need to be defined. In a number of clinical studies it has been shown that changes in 18F‐FDG uptake during (chemo‐) radiotherapy may better correlate with treatment outcome than baseline measurements. In esophageal cancer 18F‐FDG uptake decreased after 2 weeks of pre‐operative chemoradiotherapy and this decrease was by a factor of 2 more pronounced in histopathologic responders than in non‐responders (Wieder et al., 2004). Moreover, metabolic changes at this time point significantly correlated with patient survival. 18F‐FDG uptake or decrease in uptake during the first two weeks of chemoradiation showed a significant association with treatment outcome in head and neck cancer patients (Brun et al., 2002; Hentschel et al., 2011). In contrast, other clinical studies found prognostic significance of pre‐treatment 18F‐FDG uptake (Lee et al., 2007, 2008). Similarly, inconsistent results have been reported on the role of baseline and longitudinal 18F‐FDG‐PET in predicting treatment outcomes in lung cancer patients (Huang et al., 2011; Sasaki et al., 2005). These findings suggest that serial 18F‐FDG‐PET might be used to identify non‐responders early during (chemo‐) radiotherapy, allowing for early treatment modifications. Additional research is also required to define optimal quantification of 18F‐FDG uptake. Equally important is to take normal tissue reactions into account including biomarkers of late toxicity. Clearly, prospective clinical trials in well defined populations of patients are needed to test whether redistribution or boosting of radiation dose to the high 18F‐FDG uptake tumor subvolumes results in improved radiotherapy outcome. An ongoing randomized Phase II clinical trial in the Netherlands investigates whether boosting of radiation dose to the subvolume of the primary tumor with high pre‐treatment 18F‐FDG uptake (50% SUVmax) results in a better local progression‐free survival than escalating the dose to the primary tumor as a whole in lung cancer patients (NCT01024829). Another registered Belgian randomized Phase II clinical trial will compare standard intensity‐modulated radiotherapy (IMRT) with adaptive 18F‐FDG‐PET‐voxel intensity based IMRT or volumetric‐modulated arc therapy using repetitive per‐treatment planning 18F‐FDG‐PET/CT scans for head and neck cancer (NCT01341535).
5. Markers of DNA repair
Radiotherapy induces a variety of DNA lesions. The majority of the lesions can be repaired by the affected cell, while unrepaired damage is expected to cause cell death or mutations. Among markers for DNA repair, the most promising candidate biomarkers for tumor radioresponse are proteins involved in the repair of DNA double‐strand breaks, the most lethal DNA lesions. One of the best investigated markers for DNA double‐strand breaks is γH2AX, a histone protein that is phosphorylated after the induction of double‐strand breaks, forms foci around the break and recruits other DNA repair proteins. γH2AX foci can be visualized in tissue sections by immunofluorescence staining. There is accumulating evidence that the number of foci correlates closely with the number of DNA double‐strand breaks and that the number of residual foci in tumor or normal tissue cells 24 h after irradiation correlates with radiosensitivity (Banath et al., 2004; Kasten‐Pisula et al., 2005; Klokov et al., 2006; Menegakis et al., 2009; Mirzayans et al., 2006; Wada et al., 2005). It has been shown in head and neck squamous cell carcinoma lines that clonogenic cell survival curves after irradiation with different doses can be predicted by the number of residual γH2AX foci (Menegakis et al., 2009). In vivo, however, further parameters have to be considered, especially the impact of the tumor micromilieu on local tumor control (vide supra). In a group of patients with cancer of the uterine cervix, hypoxic tumor areas expressed more background γH2AX foci. In the same group of patients, no impairment of local tumor control could be shown in patients with more hypoxic tumors that at the same time expressed an overall lower residual γH2AX signal (Olive et al., 2010). However, using a co‐localization assay of γH2AX and hypoxia in head and neck squamous cell carcinoma xenografts, evaluation of γH2AX foci in perfused tumor areas could predict local tumor control. Especially, the steepness of the relationship between the number of residual foci and applied radiation dose turned out to be the best predictor (Menegakis et al., 2011). This assay is currently not automated and therefore time‐ and labor‐intensive. However, it will be further validated and adapted for the needs of higher throughput in the clinics.
Recent retrospective clinical data also support the use of other markers like the overexpression of the DNA repair protein Ku80 alone or in combination with other DNA repair proteins (Moeller et al., 2011; Soderlund Leifler et al., 2010). A good example for the utilization of DNA repair biomarkers for individualized radiotherapy is the combination of radiation treatment with inhibitors of DNA repair in tumors with low radiation‐induced marker expression, i.e. with effective repair of DNA damage. The poly(ADP‐ribose)‐polymerase‐1 (PARP‐1) is a nuclear metalloenzyme involved in the recognition of single‐strand DNA breaks and in the facilitation of DNA repair, which, if left unrepaired, can develop into potentially lethal double‐strand breaks at replication (Powell et al., 2010). PARP inhibitors are very promising drugs that impair the repair of DNA damage and enhance tumor response to radiation in various preclinical in vivo tumor models including lung, breast, colorectal, and head and neck cancers (Albert et al., 2007; Calabrese et al., 2004; Khan et al., 2010; Wang et al., 2011). Enhancement ratios of 1.4–2.2 could be achieved in growth delay assays. It has been shown that PARP inhibition resulted in accumulation of γH2AX foci in lung tumors (Albert et al., 2007), suggesting that this marker might be a candidate biomarker to identify patients for such combined treatments. Several PARP inhibitors have entered clinical studies in combination with chemotherapeutic agents. However, clinical data on toxicity and potential benefit of combining PARP inhibitors with radiotherapy are lacking so far. Also for normal tissue, DNA repair marker may be helpful to select patients with a high risk of severe radiation toxicity. Here, especially Single Nucleotide Polymorphisms (SNPs) or genetic variations of DNA repair genes are candidate biomarkers (Andreassen et al., 2003; Werbrouck et al., 2009). However, all clinical data available so far, are retrospective and partly contradictory. Thus, validation in larger prospective cohorts and optimization and homogenizations of the applied assays is highly warranted (Parliament and Murray, 2010).
6. Cancer‐stem‐cell markers
The current understanding of cancer stem cells (CSC) defines them as a small subpopulation of all tumor cells that have the unique capacity to cause a tumor recurrence by their infinite potential to generate progeny of daughter cells. Thus, tumor cure probability after radiotherapy is highly dependent on inactivation or survival of CSC (Baumann et al., 2009b). Biologically advanced techniques have opened the possibility of ex‐vivo sorting of tumor cells by their expression of specific markers. Using such techniques, subpopulations of tumor cell with high versus low marker expression can be selected and tested for their tumorigenicity, i.e. stemness. Using such techniques, there is some evidence of a different radiosensitivity between CSC and non‐CSC, which is not the focus of this review and is discussed elsewhere (Baumann et al., 2008; Koch et al., 2010; Krause et al., 2011). There are several datasets showing a correlation of the expression of putative stem cell markers with outcome of radiotherapy, e.g. a correlation of immunohistochemically detected CD133+ cells with resistance to combined radiochemotherapy and decreased survival in atypical teratoid/rhabdoid tumors and an increase of the CD133+ fraction in tumor recurrences (Chiou et al., 2008). Higher tumorigenicity of the CD133+ has been confirmed in vivo as well as a higher growth rate, invasiveness, sphere formation ability, differentiation capability and clonogenic survival after irradiation in vitro. Also in glioma and rectal cancer patients, correlations between CD133 expression and efficacy of radiotherapy or combined treatment have been shown (Murat et al., 2008; Pallini et al., 2008; Wang et al., 2009; Zeppernick et al., 2008).
One of the best investigated putative CSC markers is CD44. For this marker, subpopulations of head and neck squamous cell carcinoma cell expressing CD44 show a higher tumorigenicity then subpopulations without CD44 expression, indicating that CSC are enriched in the CD44 positive fraction (Prince et al., 2007). In a very stringent translational study on patients with early laryngeal cancer who were treated with primary radiotherapy, gene signatures were tested as potential biomarkers for permanent local tumor control after treatment (de Jong et al., 2010). A significant correlation of CD44 mRNA expression as well as CD44 immunohistochemical score with local tumor control after radiotherapy was shown in a hypothesis‐driven approach. Confirmative studies using a data‐driven approach, i.e. with inclusion of all probes with at least 20% of the samples having a minimum fold change greater than 1.35 and a p‐value for log‐ratio variation of <0.01, resulted in CD44 being the most significant marker discriminating between cures and recurrences. In laryngeal cancer cell lines, the CD44 expression correlated with in vitro plating efficiency, a marker for the percentage of clonogenic tumor cells, but not with intrinsic radiosensitivity of the clonogenic tumor cells in vitro. Although clonogenic cells in vitro do not necessarily reflect cancer stem cells in vivo (Baumann et al., 2009b), these data support that CD44 correlates with the number and not with the intrinsic radiosensitivity of CSC (Baumann and Krause, 2010). Also functional in vivo data are in line with this hypothesis, implying a significant impact of the pre‐treatment number of CSC on local tumor control after single dose or fractionated irradiation in head and neck cancer models (Yaromina et al., 2007).
The data provide a basis for further development of CSC‐based biomarkers for radiotherapy, which might be used to select patients for more intensified therapies, e.g. increase of radiation dose to the whole tumor or to the tumor regions expressing high levels of the CSC marker, or for combination with potential CSC‐targeted treatments. Biomarkers of CSC are currently limited to the very homogeneous subgroup of patients with small, early‐stage laryngeal cancer. Adaptation to the much more heterogeneous group of patients with advanced head and neck tumors of other regions will need to include more biological and clinical information. Such factors will likely be the size of the tumor, which is expected to correlate with the absolute number of CSC (Baumann et al., 2008; Hill and Milas, 1989), and parameters of the tumor micromilieu, especially hypoxia (Baumann et al., 2008; Koch et al., 2010).
7. Radiotherapy individualization based on EGFR status
The Epidermal Growth Factor Receptor (EGFR) is overexpressed in many human tumors and is a promising target for anti‐cancer therapies (Salomon et al., 1995). Pharmacological inhibitors of EGFR are available and are currently used in the treatments of cancer patients. The simultaneous application of the anti‐EGFR antibody cetuximab has been proven to improve locoregional tumor control and survival compared to radiotherapy alone in patients with head and neck squamous cell carcinomas (Bonner et al., 2006, 2010). However, recent data have shown that this combined treatment as well as a triple combination of radiochemotherapy and cetuximab are not superior to standard radiochemotherapy (Ang et al., 2011; Caudell et al., 2008) and that toxicity may be enhanced by simultaneous radiotherapy and cetuximab treatment (Giro et al., 2009; Walsh et al., 2011). In addition, preclinical and clinical data show a wide variability of tumor responses to this treatment schedule, with impressive responses in individual tumors and no response in other tumors (Gurtner et al., 2011; Krause et al., 2009). In light of these data, predictive biomarkers are warranted to appropriately select patients with the highest probability to benefit from this therapy and to avoid unnecessary adverse effects in patient cohorts with low chances of benefit. Preclinical in vivo data give first hints on such biomarkers. In our laboratory nearly all investigated head and neck tumor models showed growth delay after cetuximab treatment (Gurtner et al., 2011). This growth delay translated in some, but not in all tumor models into improved permanent local tumor control. The curative effect from combining radiotherapy with cetuximab significantly correlated with the magnitude of EGFR gene expression measured by fluorescence‐in‐situ‐hybridization, suggesting that genetic EGFR expression might be used to select tumors for such combined radiotherapy. These results will be validated in further experiments and additional markers will be investigated with the aim to identify promising biomarkers that may be introduced into clinical trials.
8. Conclusions
The introduction of individualized treatment into clinical practice is of great importance because it gives us the chance to improve tumor cure rates by treatment intensification in patients who are likely to respond while preventing the use of ineffective therapies with the associated normal tissue toxicity in other patients. The encouraging improvements of anti‐cancer treatments, better understanding of biological mechanisms of tumor resistance to therapies including radiation along with the rapid development of molecular imaging have necessitated the development of biomarkers to predict a group of patients with maximal benefit from specific treatments. Although some of the results of predictive biomarker studies are promising, none of the markers have yet been adequately validated to justify widespread introduction into clinical practice. Studies performed to date have often been underpowered and are mostly observational or retrospective subgroup analyses from larger clinical trials based on available tumor material or blood samples from heterogeneous population of patients. Sound data from prospective randomized clinical trials testing relevant interventions and incorporating putative predictive markers for these interventions are therefore required before implementation of predictive tools into routine clinical use can be achieved. A collaborative effort of biologists, clinicians and physicists is called for successful and unhampered development, validation and utilization of predictive biomarker for individualized treatment.
Acknowledgments
The authors are supported by grants of the Deutsche Forschungsgemeinschaft (DFG, Ba1433), the Federal Ministry of Education and Science, Germany (BMBF 03ZIK/OncoRay), and by the European Funds for Regional Development (EFRE), State excellence initiative of Saxony.
Yaromina Ala, Krause Mechthild, Baumann Michael, (2012), Individualization of cancer treatment from radiotherapy perspective, Molecular Oncology, 6, doi: 10.1016/j.molonc.2012.01.007.
References
- Abramyuk, A. , Tokalov, S. , Zophel, K. , Koch, A. , Szluha Lazanyi, K. , Gillham, C. , Herrmann, T. , Abolmaali, N. , 2009. Is pre-therapeutical FDG-PET/CT capable to detect high risk tumor subvolumes responsible for local failure in non-small cell lung cancer?. Radiother Oncol. 91, 399–404. [DOI] [PubMed] [Google Scholar]
- Aerts, H.J. , van Baardwijk, A.A. , Petit, S.F. , Offermann, C. , Loon, J. , Houben, R. , Dingemans, A.M. , Wanders, R. , Boersma, L. , Borger, J. , Bootsma, G. , Geraedts, W. , Pitz, C. , Simons, J. , Wouters, B.G. , Oellers, M. , Lambin, P. , Bosmans, G. , Dekker, A.L. , De Ruysscher, D. , 2009. Identification of residual metabolic-active areas within individual NSCLC tumours using a pre-radiotherapy (18)Fluorodeoxyglucose-PET-CT scan. Radiother Oncol. 91, 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert, J.M. , Cao, C. , Kim, K.W. , Willey, C.D. , Geng, L. , Xiao, D. , Wang, H. , Sandler, A. , Johnson, D.H. , Colevas, A.D. , Low, J. , Rothenberg, M.L. , Lu, B. , 2007. Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res. 13, 3033–3042. [DOI] [PubMed] [Google Scholar]
- Amado, R.G. , Wolf, M. , Peeters, M. , Van Cutsem, E. , Siena, S. , Freeman, D.J. , Juan, T. , Sikorski, R. , Suggs, S. , Radinsky, R. , Patterson, S.D. , Chang, D.D. , 2008. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 26, 1626–1634. [DOI] [PubMed] [Google Scholar]
- Andreassen, C.N. , Alsner, J. , Overgaard, M. , Overgaard, J. , 2003. Prediction of normal tissue radiosensitivity from polymorphisms in candidate genes. Radiother Oncol. 69, 127–135. [DOI] [PubMed] [Google Scholar]
- Ang, K.K. , Zhang, Q.E. , Rosenthal, D.I. , Nguyen-Tan, P. , Sherman, E.J. , Weber, R.S. , Galvin, J.M. , Schwartz, D.L. , El-Naggar, A.K. , Gillison, M.L. , Jordan, R. , List, M.A. , Konski, A.A. , Thorstad, W.L. , Trotti, A. , Beitler, J.J. , Garden, A.S. , Spanos, W.J. , Yom, S.S. , Axelrod, R.S. , 2011. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC). - ASCO ASCO annual meeting. Journal of clinical Oncology. p. abstract 5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberg, M. , Molls, M. , Sack, H. , 2004. Radioonkologie. W. Zuckschwerdt Verlag GmbH, Germering/ München. [Google Scholar]
- Banath, J.P. , Macphail, S.H. , Olive, P.L. , 2004. Radiation sensitivity, H2AX phosphorylation, and kinetics of repair of DNA strand breaks in irradiated cervical cancer cell lines. Cancer Res. 64, 7144–7149. [DOI] [PubMed] [Google Scholar]
- Baumann, M. , Herrmann, T. , Koch, R. , Matthiessen, W. , Appold, S. , Wahlers, B. , Kepka, L. , Marschke, G. , Feltl, D. , Fietkau, R. , Budach, V. , Dunst, J. , Dziadziuszko, R. , Krause, M. , Zips, D. , 2011. Final results of the randomized phase III CHARTWEL-trial (ARO 97-1) comparing hyperfractionated-accelerated versus conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC). Radiother Oncol. [DOI] [PubMed] [Google Scholar]
- Baumann, M. , Krause, M. , 2010. CD44: a cancer stem cell-related biomarker with predictive potential for radiotherapy. Clin Cancer Res. 16, 5091–5093. [DOI] [PubMed] [Google Scholar]
- Baumann, M. , Krause, M. , Hill, R. , 2008. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 8, 545–554. [DOI] [PubMed] [Google Scholar]
- Baumann, M. , Krause, M. , Thames, H. , Trott, K. , Zips, D. , 2009. Cancer stem cells and radiotherapy. Int J Radiat Biol. 1–12. [DOI] [PubMed] [Google Scholar]
- Baumann, M. , Krause, M. , Thames, H. , Trott, K. , Zips, D. , 2009. Cancer stem cells and radiotherapy. Int J Radiat Biol. 85, 391–402. [DOI] [PubMed] [Google Scholar]
- Begg, A.C. , Stewart, F.A. , Vens, C. , 2011. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 11, 239–253. [DOI] [PubMed] [Google Scholar]
- Bentzen, S.M. , 2005. Theragnostic imaging for radiation oncology: dose-painting by numbers. Lancet Oncol. 6, 112–117. [DOI] [PubMed] [Google Scholar]
- Bonner, J.A. , Harari, P.M. , Giralt, J. , Azarnia, N. , Shin, D.M. , Cohen, R.B. , Jones, C.U. , Sur, R. , Raben, D. , Jassem, J. , Ove, R. , Kies, M.S. , Baselga, J. , Youssoufian, H. , Amellal, N. , Rowinsky, E.K. , Ang, K.K. , 2006. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 354, 567–578. [DOI] [PubMed] [Google Scholar]
- Bonner, J.A. , Harari, P.M. , Giralt, J. , Cohen, R.B. , Jones, C.U. , Sur, R.K. , Raben, D. , Baselga, J. , Spencer, S.A. , Zhu, J. , Youssoufian, H. , Rowinsky, E.K. , Ang, K.K. , 2010. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 11, 21–28. [DOI] [PubMed] [Google Scholar]
- Bristow, R.G. , Hill, R.P. , 2008. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 8, 180–192. [DOI] [PubMed] [Google Scholar]
- Bruechner, K. , Bergmann, R. , Santiago, A. , Mosch, B. , Yaromina, A. , Hessel, F. , Hofheinz, F. , van den Hoff, J. , Baumann, M. , Beuthien-Baumann, B. , 2009. Comparison of [18F]FDG uptake and distribution with hypoxia and proliferation in FaDu human squamous cell carcinoma (hSCC) xenografts after single dose irradiation. Int J Radiat Biol. 85, 772–780. [DOI] [PubMed] [Google Scholar]
- Brun, E. , Kjellen, E. , Tennvall, J. , Ohlsson, T. , Sandell, A. , Perfekt, R. , Wennerberg, J. , Strand, S.E. , 2002. FDG PET studies during treatment: prediction of therapy outcome in head and neck squamous cell carcinoma. Head Neck. 24, 127–135. [DOI] [PubMed] [Google Scholar]
- Bueno-de-Mesquita, J.M. , van Harten, W.H. , Retel, V.P. , van't Veer, L.J. , van Dam, F.S. , Karsenberg, K. , Douma, K.F. , van Tinteren, H. , Peterse, J.L. , Wesseling, J. , Wu, T.S. , Atsma, D. , Rutgers, E.J. , Brink, G. , Floore, A.N. , Glas, A.M. , Roumen, R.M. , Bellot, F.E. , van Krimpen, C. , Rodenhuis, S. , van de Vijver, M.J. , Linn, S.C. , 2007. Use of 70-gene signature to predict prognosis of patients with node-negative breast cancer: a prospective community-based feasibility study (RASTER). Lancet Oncol. 8, 1079–1087. [DOI] [PubMed] [Google Scholar]
- Burgman, P. , Odonoghue, J.A. , Humm, J.L. , Ling, C.C. , 2001. Hypoxia-Induced increase in FDG uptake in MCF7 cells. J Nucl Med. 42, 170–175. [PubMed] [Google Scholar]
- Bussink, J. , Kaanders, J.H. , van der Graaf, W.T. , Oyen, W.J. , 2011. PET-CT for radiotherapy treatment planning and response monitoring in solid tumors. Nat Rev Clin Oncol. 8, 233–242. [DOI] [PubMed] [Google Scholar]
- Calabrese, C.R. , Almassy, R. , Barton, S. , Batey, M.A. , Calvert, A.H. , Canan-Koch, S. , Durkacz, B.W. , Hostomsky, Z. , Kumpf, R.A. , Kyle, S. , Li, J. , Maegley, K. , Newell, D.R. , Notarianni, E. , Stratford, I.J. , Skalitzky, D. , Thomas, H.D. , Wang, L.Z. , Webber, S.E. , Williams, K.J. , Curtin, N.J. , 2004. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst. 96, 56–67. [DOI] [PubMed] [Google Scholar]
- Caudell, J.J. , Sawrie, S.M. , Spencer, S.A. , Desmond, R.A. , Carroll, W.R. , Peters, G.E. , Nabell, L.M. , Meredith, R.F. , Bonner, J.A. , 2008. Locoregionally advanced head and neck cancer treated with primary radiotherapy: a comparison of the addition of cetuximab or chemotherapy and the impact of protocol treatment. Int J Radiat Oncol Biol Phys. 71, 676–681. [DOI] [PubMed] [Google Scholar]
- Chiou, S.H. , Kao, C.L. , Chen, Y.W. , Chien, C.S. , Hung, S.C. , Lo, J.F. , Chen, Y.J. , Ku, H.H. , Hsu, M.T. , Wong, T.T. , 2008. Identification of CD133-positive radioresistant cells in atypical teratoid/rhabdoid tumor. PLoS One. 3, e2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong, M.C. , Pramana, J. , van der Wal, J.E. , Lacko, M. , Peutz-Kootstra, C.J. , de Jong, J.M. , Takes, R.P. , Kaanders, J.H. , van der Laan, B.F. , Wachters, J. , Jansen, J.C. , Rasch, C.R. , van Velthuysen, M.L. , Grenman, R. , Hoebers, F.J. , Schuuring, E. , van den Brekel, M.W. , Begg, A.C. , 2010. CD44 expression predicts local recurrence after radiotherapy in larynx cancer. Clin Cancer Res. 16, 5329–5338. [DOI] [PubMed] [Google Scholar]
- Delaney, G. , Jacob, S. , Featherstone, C. , Barton, M. , 2005. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 104, 1129–1137. [DOI] [PubMed] [Google Scholar]
- Duprez, F. , De Neve, W. , De Gersem, W. , Coghe, M. , Madani, I. , 2011. Adaptive dose painting by numbers for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 80, 1045–1055. [DOI] [PubMed] [Google Scholar]
- Evans, S.M. , Koch, C.J. , 2003. Prognostic significance of tumor oxygenation in humans. Cancer Lett. 195, 1–16. [DOI] [PubMed] [Google Scholar]
- Feng, M. , Kong, F.M. , Gross, M. , Fernando, S. , Hayman, J.A. , Ten Haken, R.K. , 2009. Using fluorodeoxyglucose positron emission tomography to assess tumor volume during radiotherapy for non-small-cell lung cancer and its potential impact on adaptive dose escalation and normal tissue sparing. Int J Radiat Oncol Biol Phys. 73, 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillham, C. , Zips, D. , Ponisch, F. , Evers, C. , Enghardt, W. , Abolmaali, N. , Zophel, K. , Appold, S. , Holscher, T. , Steinbach, J. , Kotzerke, J. , Herrmann, T. , Baumann, M. , 2008. Additional PET/CT in week 5-6 of radiotherapy for patients with stage III non-small cell lung cancer as a means of dose escalation planning?. Radiother Oncol. 88, 335–341. [DOI] [PubMed] [Google Scholar]
- Giro, C. , Berger, B. , Bolke, E. , Ciernik, I.F. , Duprez, F. , Locati, L. , Maillard, S. , Ozsahin, M. , Pfeffer, R. , Robertson, A.G. , Langendijk, J.A. , Budach, W. , 2009. High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: results of a survey in EORTC institutes. Radiother Oncol. 90, 166–171. [DOI] [PubMed] [Google Scholar]
- Gurtner, K. , Deuse, Y. , Butof, R. , Schaal, K. , Eicheler, W. , Oertel, R. , Grenman, R. , Thames, H. , Yaromina, A. , Baumann, M. , Krause, M. , 2011. Diverse effects of combined radiotherapy and EGFR inhibition with antibodies or TK inhibitors on local tumour control and correlation with EGFR gene expression. Radiother Oncol. 99, 323–330. [DOI] [PubMed] [Google Scholar]
- Harbeck, N. , Pegram, M.D. , Ruschoff, J. , Mobus, V. , 2010. Targeted Therapy in Metastatic Breast Cancer: The HER2/neu Oncogene. Breast Care (Basel). 5, 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel, M. , Appold, S. , Schreiber, A. , Abolmaali, N. , Abramyuk, A. , Dorr, W. , Kotzerke, J. , Baumann, M. , Zophel, K. , 2011. Early FDG PET at 10 or 20 Gy under chemoradiotherapy is prognostic for locoregional control and overall survival in patients with head and neck cancer. Eur J Nucl Med Mol Imaging. 38, 1203–1211. [DOI] [PubMed] [Google Scholar]
- Hill, R.P. , Milas, L. , 1989. The proportion of stem cells in murine tumors. Int J Radiat Oncol Biol Phys. 16, 513–518. [DOI] [PubMed] [Google Scholar]
- Huang, W. , Zhou, T. , Ma, L. , Sun, H. , Gong, H. , Wang, J. , Yu, J. , Li, B. , 2011. Standard uptake value and metabolic tumor volume of (1)F-FDG PET/CT predict short-term outcome early in the course of chemoradiotherapy in advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 38, 1628–1635. [DOI] [PubMed] [Google Scholar]
- Jingu, K. , Ariga, H. , Kaneta, T. , Takai, Y. , Takeda, K. , Katja, L. , Narazaki, K. , Metoki, T. , Fujimoto, K. , Umezawa, R. , Ogawa, Y. , Nemoto, K. , Koto, M. , Mitsuya, M. , Matsufuji, N. , Takahashi, S. , Yamada, S. , 2010. Focal dose escalation using FDG-PET-guided intensity-modulated radiation therapy boost for postoperative local recurrent rectal cancer: a planning study with comparison of DVH and NTCP. BMC Cancer. 10, 127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaanders, J.H. , Bussink, J. , van der Kogel, A.J. , 2002. ARCON: a novel biology-based approach in radiotherapy. Lancet Oncol. 3, 728–737. [DOI] [PubMed] [Google Scholar]
- Kaanders, J.H. , I.W.K., Marres, H.A. , Ljunkvist, A.S. , Pop, L.A. , van den Hoogen, F.J. , de Wilde, P.C. , Bussink, J. , Raleigh, J.A. , van der Kogel, A.J. , 2002. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Research. 62, 7066–7074. [PubMed] [Google Scholar]
- Kaanders, J.H. , Pop, L.A. , Marres, H.A. , Bruaset, I. , van den Hoogen, F.J. , Merkx, M.A. , van der Kogel, A.J. , 2002. ARCON: experience in 215 patients with advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 52, 769–778. [DOI] [PubMed] [Google Scholar]
- Kasten-Pisula, U. , Tastan, H. , Dikomey, E. , 2005. Huge differences in cellular radiosensitivity due to only very small variations in double-strand break repair capacity. Int J Radiat Biol. 81, 409–419. [DOI] [PubMed] [Google Scholar]
- Khan, K. , Araki, K. , Wang, D. , Li, G. , Li, X. , Zhang, J. , Xu, W. , Hoover, R.K. , Lauter, S. , O'Malley, B. , Lapidus, R.G. , Li, D. , 2010. Head and neck cancer radiosensitization by the novel poly(ADP-ribose) polymerase inhibitor GPI-15427. Head Neck. 32, 381–391. [DOI] [PubMed] [Google Scholar]
- Kim, S.Y. , Roh, J.L. , Kim, M.R. , Kim, J.S. , Choi, S.H. , Nam, S.Y. , Lee, S.W. , Kim, S.B. , 2007. Use of 18F-FDG PET for primary treatment strategy in patients with squamous cell carcinoma of the oropharynx. J Nucl Med. 48, 752–757. [DOI] [PubMed] [Google Scholar]
- Klokov, D. , MacPhail, S.M. , Banath, J.P. , Byrne, J.P. , Olive, P.L. , 2006. Phosphorylated histone H2AX in relation to cell survival in tumor cells and xenografts exposed to single and fractionated doses of X-rays. Radiother Oncol. 80, 223–229. [DOI] [PubMed] [Google Scholar]
- Koch, U. , Krause, M. , Baumann, M. , 2010. Cancer stem cells at the crossroads of current cancer therapy failures–radiation oncology perspective. Semin Cancer Biol. 20, 116–124. [DOI] [PubMed] [Google Scholar]
- Krause, M. , Gurtner, K. , Deuse, Y. , Baumann, M. , 2009. Heterogeneity of tumour response to combined radiotherapy and EGFR inhibitors: differences between antibodies and TK inhibitors. Int J Radiat Biol. 85, 943–954. [DOI] [PubMed] [Google Scholar]
- Krause, M. , Yaromina, A. , Eicheler, W. , Koch, U. , Baumann, M. , 2011. Cancer stem cells: targets and potential biomarkers for radiotherapy. Clin Cancer Res. 17, 7224–7229. [DOI] [PubMed] [Google Scholar]
- Le, Q.T. , Sutphin, P.D. , Raychaudhuri, S. , Yu, S.C. , Terris, D.J. , Lin, H.S. , Lum, B. , Pinto, H.A. , Koong, A.C. , Giaccia, A.J. , 2003. Identification of osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomas. Clin Cancer Res. 9, 59–67. [PMC free article] [PubMed] [Google Scholar]
- Lee, S.W. , Nam, S.Y. , Im, K.C. , Kim, J.S. , Choi, E.K. , Ahn, S.D. , Park, S.H. , Kim, S.Y. , Lee, B.J. , Kim, J.H. , 2008. Prediction of prognosis using standardized uptake value of 2-[(18)F] fluoro-2-deoxy-d-glucose positron emission tomography for nasopharyngeal carcinomas. Radiother Oncol. 87, 211–216. [DOI] [PubMed] [Google Scholar]
- Lim, A.M. , Rischin, D. , Fisher, R. , Cao, H. , Kwok, K. , Truong, D. , McArthur, G.A. , Young, R.J. , Giaccia, A. , Peters, L. , Le, Q.T. , 2012. Prognostic Significance of Plasma Osteopontin in Patients with Locoregionally Advanced Head and Neck Squamous Cell Carcinoma Treated on TROG 02.02 Phase III Trial. Clin Cancer Res. 18, 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt, S.J. , Chaudary, N. , Hill, R.P. , 2009. The tumor microenvironment and metastatic disease. Clin Exp Metastasis. 26, 19–34. [DOI] [PubMed] [Google Scholar]
- Mac Manus, M. , Hicks, R.J. , 2008. The use of positron emission tomography (PET) in the staging/evaluation, treatment, and follow-up of patients with lung cancer: a critical review. Int J Radiat Oncol Biol Phys. 72, 1298–1306. [DOI] [PubMed] [Google Scholar]
- Madani, I. , Duthoy, W. , Derie, C. , De Gersem, W. , Boterberg, T. , Saerens, M. , Jacobs, F. , Gregoire, V. , Lonneux, M. , Vakaet, L. , Vanderstraeten, B. , Bauters, W. , Bonte, K. , Thierens, H. , De Neve, W. , 2007. Positron emission tomography-guided, focal-dose escalation using intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 68, 126–135. [DOI] [PubMed] [Google Scholar]
- Majmundar, A.J. , Wong, W.J. , Simon, M.C. , 2010. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 40, 294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegakis, A. , Eicheler, W. , Yaromina, A. , Thames, H.D. , Krause, M. , Baumann, M. , 2011. Residual DNA double strand breaks in perfused but not in unperfused areas determine different radiosensitivity of tumours. Radiotherapy Oncology. in press [DOI] [PubMed] [Google Scholar]
- Menegakis, A. , Yaromina, A. , Eicheler, W. , Dorfler, A. , Beuthien-Baumann, B. , Thames, H.D. , Baumann, M. , Krause, M. , 2009. Prediction of clonogenic cell survival curves based on the number of residual DNA double strand breaks measured by gammaH2AX staining. Int J Radiat Biol. 85, 1032–1041. [DOI] [PubMed] [Google Scholar]
- Mirzayans, R. , Severin, D. , Murray, D. , 2006. Relationship between DNA double-strand break rejoining and cell survival after exposure to ionizing radiation in human fibroblast strains with differing ATM/p53 status: implications for evaluation of clinical radiosensitivity. Int J Radiat Oncol Biol Phys. 66, 1498–1505. [DOI] [PubMed] [Google Scholar]
- Moeller, B.J. , Yordy, J.S. , Williams, M.D. , Giri, U. , Raju, U. , Molkentine, D.P. , Byers, L.A. , Heymach, J.V. , Story, M.D. , Lee, J.J. , Sturgis, E.M. , Weber, R.S. , Garden, A.S. , Ang, K.K. , Schwartz, D.L. , 2011. DNA repair biomarker profiling of head and neck cancer: Ku80 expression predicts locoregional failure and death following radiotherapy. Clin Cancer Res. 17, 2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murat, A. , Migliavacca, E. , Gorlia, T. , Lambiv, W.L. , Shay, T. , Hamou, M.F. , de Tribolet, N. , Regli, L. , Wick, W. , Kouwenhoven, M.C. , Hainfellner, J.A. , Heppner, F.L. , Dietrich, P.Y. , Zimmer, Y. , Cairncross, J.G. , Janzer, R.C. , Domany, E. , Delorenzi, M. , Stupp, R. , Hegi, M.E. , 2008. Stem cell-related "self-renewal" signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 26, 3015–3024. [DOI] [PubMed] [Google Scholar]
- Nordsmark, M. , Eriksen, J.G. , Gebski, V. , Alsner, J. , Horsman, M.R. , Overgaard, J. , 2007. Differential risk assessments from five hypoxia specific assays: The basis for biologically adapted individualized radiotherapy in advanced head and neck cancer patients. Radiother Oncol. 83, 389–397. [DOI] [PubMed] [Google Scholar]
- Olive, P.L. , Banuelos, C.A. , Durand, R.E. , Kim, J.Y. , Aquino-Parsons, C. , 2010. Endogenous and radiation-induced expression of gammaH2AX in biopsies from patients treated for carcinoma of the uterine cervix. Radiother Oncol. 94, 82–89. [DOI] [PubMed] [Google Scholar]
- Overgaard, J. , 2007. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 25, 4066–4074. [DOI] [PubMed] [Google Scholar]
- Overgaard, J. , 2011. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck–a systematic review and meta-analysis. Radiother Oncol. 100, 22–32. [DOI] [PubMed] [Google Scholar]
- Overgaard, J. , Eriksen, J.G. , Nordsmark, M. , Alsner, J. , Horsman, M.R. , 2005. Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol. 6, 757–764. [DOI] [PubMed] [Google Scholar]
- Overgaard, J. , Hansen, H.S. , Overgaard, M. , Bastholt, L. , Berthelsen, A. , Lindelov, B. , Jorgensen, K. , 1998. A randomized double-blind phase III study of nimorazole a a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiotherapy Oncology. 46, 135–146. [DOI] [PubMed] [Google Scholar]
- Pallini, R. , Ricci-Vitiani, L. , Banna, G.L. , Signore, M. , Lombardi, D. , Todaro, M. , Stassi, G. , Martini, M. , Maira, G. , Larocca, L.M. , De Maria, R. , 2008. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin Cancer Res. 14, 8205–8212. [DOI] [PubMed] [Google Scholar]
- Parliament, M.B. , Murray, D. , 2010. Single nucleotide polymorphisms of DNA repair genes as predictors of radioresponse. Semin Radiat Oncol. 20, 232–240. [DOI] [PubMed] [Google Scholar]
- Petersen, C. , Zips, D. , Krause, M. , Schone, K. , Eicheler, W. , Hoinkis, C. , Thames, H.D. , Baumann, M. , 2001. Repopulation of FaDu human squamous cell carcinoma during fractionated radiotherapy correlates with reoxygenation. Int J Radiat Oncol Biol Phys. 51, 483–493. [DOI] [PubMed] [Google Scholar]
- Powell, C. , Mikropoulos, C. , Kaye, S.B. , Nutting, C.M. , Bhide, S.A. , Newbold, K. , Harrington, K.J. , 2010. Pre-clinical and clinical evaluation of PARP inhibitors as tumour-specific radiosensitisers. Cancer Treat Rev. 36, 566–575. [DOI] [PubMed] [Google Scholar]
- Prince, M.E. , Sivanandan, R. , Kaczorowski, A. , Wolf, G.T. , Kaplan, M.J. , Dalerba, P. , Weissman, I.L. , Clarke, M.F. , Ailles, L.E. , 2007. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 104, 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugachev, A. , Ruan, S. , Carlin, S. , Larson, S.M. , Campa, J. , Ling, C.C. , Humm, J.L. , 2005. Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys. 62, 545–553. [DOI] [PubMed] [Google Scholar]
- Rajendran, J.G. , Mankoff, D.A. , O'Sullivan, F. , Peterson, L.M. , Schwartz, D.L. , Conrad, E.U. , Spence, A.M. , Muzi, M. , Farwell, D.G. , Krohn, K.A. , 2004. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 10, 2245–2252. [DOI] [PubMed] [Google Scholar]
- Rischin, D. , Hicks, R.J. , Fisher, R. , Binns, D. , Corry, J. , Porceddu, S. , Peters, L.J. , 2006. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 24, 2098–2104. [DOI] [PubMed] [Google Scholar]
- Rischin, D. , Peters, L.J. , O'Sullivan, B. , Giralt, J. , Fisher, R. , Yuen, K. , Trotti, A. , Bernier, J. , Bourhis, J. , Ringash, J. , Henke, M. , Kenny, L. , 2010. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 28, 2989–2995. [DOI] [PubMed] [Google Scholar]
- Salomon, D.S. , Brandt, R. , Ciardiello, F. , Normanno, N. , 1995. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 19, 183–232. [DOI] [PubMed] [Google Scholar]
- Sasaki, R. , Komaki, R. , Macapinlac, H. , Erasmus, J. , Allen, P. , Forster, K. , Putnam, J.B. , Herbst, R.S. , Moran, C.A. , Podoloff, D.A. , Roth, J.A. , Cox, J.D. , 2005. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol. 23, 1136–1143. [DOI] [PubMed] [Google Scholar]
- Schoder, H. , Fury, M. , Lee, N. , Kraus, D. , 2009. PET monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med. 50, (Suppl 1) 74S–88S. [DOI] [PubMed] [Google Scholar]
- Schutze, C. , Bergmann, R. , Yaromina, A. , Hessel, F. , Kotzerke, J. , Steinbach, J. , Baumann, M. , Beuthien-Baumann, B. , 2007. Effect of increase of radiation dose on local control relates to pre-treatment FDG uptake in FaDu tumours in nude mice. Radiother Oncol. 83, 311–315. [DOI] [PubMed] [Google Scholar]
- Schwartz, D.L. , Ford, E.C. , Rajendran, J. , Yueh, B. , Coltrera, M.D. , Virgin, J. , Anzai, Y. , Haynor, D. , Lewellen, B. , Mattes, D. , Kinahan, P. , Meyer, J. , Phillips, M. , Leblanc, M. , Krohn, K. , Eary, J. , Laramore, G.E. , 2005. FDG-PET/CT-guided intensity modulated head and neck radiotherapy: a pilot investigation. Head Neck. 27, 478–487. [DOI] [PubMed] [Google Scholar]
- Slodkowska, E.A. , Ross, J.S. , 2009. MammaPrint 70-gene signature: another milestone in personalized medical care for breast cancer patients. Expert Rev Mol Diagn. 9, 417–422. [DOI] [PubMed] [Google Scholar]
- Soderlund Leifler, K. , Queseth, S. , Fornander, T. , Askmalm, M.S. , 2010. Low expression of Ku70/80, but high expression of DNA-PKcs, predict good response to radiotherapy in early breast cancer. Int J Oncol. 37, 1547–1554. [DOI] [PubMed] [Google Scholar]
- Sonke, J.J. , Belderbos, J. , 2010. Adaptive radiotherapy for lung cancer. Semin Radiat Oncol. 20, 94–106. [DOI] [PubMed] [Google Scholar]
- Sorensen, B.S. , Hao, J. , Overgaard, J. , Vorum, H. , Honore, B. , Alsner, J. , Horsman, M.R. , 2005. Influence of oxygen concentration and pH on expression of hypoxia induced genes. Radiother Oncol. 76, 187–193. [DOI] [PubMed] [Google Scholar]
- Toustrup, K. , Sorensen, B.S. , Lassen, P. , Wiuf, C. , Alsner, J. , Overgaard, J. , 2012. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol. 102, 122–129. [DOI] [PubMed] [Google Scholar]
- Toustrup, K. , Sorensen, B.S. , Nordsmark, M. , Busk, M. , Wiuf, C. , Alsner, J. , Overgaard, J. , 2011. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 71, 5923–5931. [DOI] [PubMed] [Google Scholar]
- Van Schaeybroeck, S. , Allen, W.L. , Turkington, R.C. , Johnston, P.G. , 2011. Implementing prognostic and predictive biomarkers in CRC clinical trials. Nat Rev Clin Oncol. 8, 222–232. [DOI] [PubMed] [Google Scholar]
- Vaupel, P. , Mayer, A. , 2007. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 26, 225–239. [DOI] [PubMed] [Google Scholar]
- Wada, S. , Van Khoa, T. , Kobayashi, Y. , Funayama, T. , Ogihara, K. , Ueno, S. , Ito, N. , 2005. Prediction of cellular radiosensitivity from DNA damage induced by gamma-rays and carbon ion irradiation in canine tumor cells. J Vet Med Sci. 67, 1089–1095. [DOI] [PubMed] [Google Scholar]
- Walsh, L. , Gillham, C. , Dunne, M. , Fraser, I. , Hollywood, D. , Armstrong, J. , Thirion, P. , 2011. Toxicity of cetuximab versus cisplatin concurrent with radiotherapy in locally advanced head and neck squamous cell cancer (LAHNSCC). Radiother Oncol. 98, 38–41. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Mason, K.A. , Ang, K.K. , Buchholz, T. , Valdecanas, D. , Mathur, A. , Buser-Doepner, C. , Toniatti, C. , Milas, L. , 2011. MK-4827, a PARP-1/-2 inhibitor, strongly enhances response of human lung and breast cancer xenografts to radiation. Invest New Drugs. [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Chen, Z.G. , Du, C.Z. , Wang, H.W. , Yan, L. , Gu, J. , 2009. Cancer stem cell marker CD133+ tumour cells and clinical outcome in rectal cancer. Histopathology. 55, 284–293. [DOI] [PubMed] [Google Scholar]
- Werbrouck, J. , De Ruyck, K. , Duprez, F. , Veldeman, L. , Claes, K. , Van Eijkeren, M. , Boterberg, T. , Willems, P. , Vral, A. , De Neve, W. , Thierens, H. , 2009. Acute normal tissue reactions in head-and-neck cancer patients treated with IMRT: influence of dose and association with genetic polymorphisms in DNA DSB repair genes. Int J Radiat Oncol Biol Phys. 73, 1187–1195. [DOI] [PubMed] [Google Scholar]
- West, C.M. , Davidson, S.E. , Roberts, S.A. , Hunter, R.D. , 1993. Intrinsic radiosensitivity and prediction of patient response to radiotherapy for carcinoma of the cervix. Br J Cancer. 68, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieder, H.A. , Brucher, B.L. , Zimmermann, F. , Becker, K. , Lordick, F. , Beer, A. , Schwaiger, M. , Fink, U. , Siewert, J.R. , Stein, H.J. , Weber, W.A. , 2004. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 22, 900–908. [DOI] [PubMed] [Google Scholar]
- Withers, H.R. , Taylor, J.M. , Maciejewski, B. , 1988. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 27, 131–146. [DOI] [PubMed] [Google Scholar]
- Yaromina, A. , Krause, M. , Thames, H. , Rosner, A. , Krause, M. , Hessel, F. , Grenman, R. , Zips, D. , Baumann, M. , 2007. Pre-treatment number of clonogenic cells and their radiosensitivity are major determinants of local tumour control after fractionated irradiation. Radiother Oncol. 83, 304–310. [DOI] [PubMed] [Google Scholar]
- Yaromina, A. , Kroeber, T. , Meinzer, A. , Boeke, S. , Thames, H. , Baumann, M. , Zips, D. , 2011. Exploratory study of the prognostic value of microenvironmental parameters during fractionated irradiation in human squamous cell carcinoma xenografts. Int J Radiat Oncol Biol Phys. 80, 1205–1213. [DOI] [PubMed] [Google Scholar]
- Zeppernick, F. , Ahmadi, R. , Campos, B. , Dictus, C. , Helmke, B.M. , Becker, N. , Lichter, P. , Unterberg, A. , Radlwimmer, B. , Herold-Mende, C.C. , 2008. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 14, 123–129. [DOI] [PubMed] [Google Scholar]


