Abstract
The integral membrane channel protein aquaporin (AQP) is aberrantly expressed with oncogenic characteristics in various human cancers. In this study, we analyzed the expression pattern of all subtypes of AQPs, and found that 8 out of 13 AQPs expressed in melanoma cells. To understand the role of aberrant expression of AQP in this disease, we over‐expressed AQP3 and AQP9 in human melanoma WM266.4 cells and found that both AQPs significantly increased the chemoresistance of WM266.4 cells to arsenite. Functional studies showed that AQP3 and AQP9 can inhibit cell apoptosis induced by arsenite through down‐regulating p53 and up‐regulating Bcl‐2 and XIAP. Our data suggest the implication of APQ in melanoma progression and that the over‐expression of AQP3 and AQP9 contributes to the chemoresistance of melanoma to arsenite.
Keywords: AQP, Melanoma, Arsenite, Chemotherapy
Highlights
We identify the expression pattern of all subtypes of AQP in melanoma cells.
Over‐expression of AQP3 and AQP9 increases the chemoresistance of melanoma cells to arsenite.
AQP has no effect on melanoma cell growth.
The mechanism of AQP affecting melanoma chemoresistance is relevant to apoptosis regulated by p53, Bax, Bcl‐2, and XIAP.
1. Introduction
Melanoma is currently the sixth most common cancer in caucasian men and women in the United States. It is by far the deadliest skin cancer, and the incidence rate of melanoma is increasing faster than any other cancer in the world. Primary cutaneous melanoma has an excellent outcome with early surgical intervention (5‐year survival rate is 89%) but regional and distant metastatic disease have a much more dismal prognosis (5‐year survival rate is 65% and 16%, respectively) (Howell Jr. et al., 2010; Jemal et al., 2009). For those patients who will develop locally advanced and metastatic melanoma, current treatment options are limited. Thus, the overall poor mortality rates and high resistance to therapy emphasize the need for a better understanding of this disease.
The integral membrane channel proteins, aquaporins (AQPs), consist of 13 members (AQP0‐12) in mammals that are expressed in a variety of tissues. AQPs were originally identified as water‐ permeating channels in the plasma membrane (Knepper et al., 2004). Although the central function of AQPs is to facilitate transportation of small molecules, such as solutes and possibly gasses (Knepper et al., 2004), several AQPs have been proven to regulate physiological cell functions, including osmoregulation and energy metabolism (Ishibashi et al., 2011), and involve many essential biological processes such as proliferation, apoptosis, differentiation and metastasis (Nico and Ribatti, 2011; Papadopoulos et al., 2008; Verkman et al., 2008).
In this study, we screened the expression of 13 AQPs in a number of melanoma cells consisting of primary and metastatic tumor, and we found that 8 of 13 AQPs were differentially expressed in these melanoma cells. To learn the pathological role of aberrant expression of AQP in melanoma, we over‐expressed AQP3 and AQP9 in human melanoma WM266.4 cells respectively and proved that AQP3 and AQP9 significantly increased chemoresistance of WM266.4 cells to arsenite. Study of the mechanistics suggested that AQP3 and AQP9 reduced the apoptosis in response to arsenite through down‐regulating p53 and Bax while up‐regulating Bcl‐2 and XIAP. Our results provide a novel insight into better understanding the mechanism of chemoresistance and developing more efficacious drugs for treating melanoma.
2. Materials and methods
2.1. Cell culture
Human 293TN cells (System Bioscience, CA, USA) and human melanoma WM266.4 cells (ATCC, VA, USA) were cultured with Dulbecco's Modified Eagle's Medium (DMEM) and Minimum Essential Media (MEM) containing 10% (v/v) fetal bovine serum (FBS). The remainder of the melanoma cell lines were established from clinical melanoma samples as described previously (Liu et al., 2008) and were cultured in RPMI 1640 medium supplemented with 10% FBS. All cell culture supplies were purchased from Invitrogen (CA, USA) and cells were maintained under standard conditions.
2.2. RNA isolation and Reverse Transcription PCR (RT‐PCR)
Total RNA was extracted using Trizol reagent (Invitrogen) according to manufacturer's instruction. Briefly, cells were dissolved in 1 mL of Trizol reagent, then 100 μL of 1‐bromo‐3‐chloropropane (BCP) solution (Molecular Research Center, Inc. OH, USA) was added and vortexed vigorously. After centrifugation for 10 min at 14,000 rpm and 4 °C, the upper aqueous phase was transferred to a new tube and an equal volume of isopropanol (Sigma–Aldrich, MO, USA) was added for precipitation. After washing pellets using 75% ethanol, RNA was dried and dissolved in nuclease free water. Two micrograms of total RNA were used for cDNA synthesis with high capacity cDNA reverse transcriptase (Applied Biosystems, CA, USA). Random primers were used for mRNA reverse transcription. The reverse transcription reaction was performed at 37 °C for 2 h by incubating the following mixture: 2 μg of total RNA, 2 μmol of primer, 2 μL of 10× buffers, 0.8 μL of 100 mM dNTP, and up to 20 μL of nuclease‐free water. The PCR reaction was performed for 30 cycles consisting of a denaturation step for 15 s at 94 °C, annealing step for 30 s at 58 °C, and extension for 1 min at 72 °C. The primers for AQP subtype detection is shown in Supplementary Table 1. Electrophoresis by using Agarose gel was finally applied to analyze the expression of AQPs in melanoma cells.
2.3. Lentivector construction and packaging
To construct the AQP3 and AQP9 over‐expression lentivectors, the AQP3 and AQP9 open reading frames (ORFs) were amplified using genomic DNA, then the PCR products were ligated to T vectors, followed by digestion with BamH1 and Mlu1 (Promega, WI, USA) and ligation with the self‐ inactivating transfer vector plasmid pWPXL. The primers used for the AQP constructs are shown in Supplementary Table 1. A total of 2 μg of constructs was transfected with 10 μg of packaging plasmids mixture (System Bioscience, CA, USA) into 293TN cells. The lentivector‐containing supernatants were collected and concentrated by PEG precipitation (System Bioscience) at 48 h after transfection then were stored at −80 °C. To establish the stable clones with expressed target genes, WM266.4 cells were infected by the designated lentivectors then were sorted by flow cytometry after 72 h.
2.4. Cell viability assay
Tissue culture microtiter 96 well plates were seeded at a density of 1000 cells per well. WM266.4 control and AQP3/AQP9 over‐expressed WM266.4 cells were treated with sodium arsenite (Sigma–Aldrich) or the vehicle for 48 h. Cell viability was determined using the WST‐1 cell viability assay (Roche, IN, USA). The plates were mixed by a shaker for 30 min, and then the absorbance was measured at 630 nm by a plate reader (Biotek, VT, USA).
2.5. Cell proliferation assay
For the cell proliferation assay, a total of 2 × 105 cells was plated in a 3.5 cm dish and cultured under the standard condition. The cells were counted at different time points using a hemocytometer and the cell growth curve was drawn according to the cell numbers at designated time points.
2.6. Cell apoptosis
For the apoptosis assay, 5 × 105 AQP3/AQP9 over‐expressed WM266.4 cells and the control cells were exposed to 8 μM of sodium arsenite for 24 h, then cell apoptosis was detected using apoptosis kit (BD science, NJ, USA). Briefly, the cells were collected and washed before re‐suspending in Binding Buffer at a concentration of 1 × 106 cells/mL. One hundred microliters of the solution (1 × 105 cells) were transferred to a 5 mL culture tube, then 5 μL of PE Annexin V and 5 μL 7‐AAD were added. The cells were gently vortexed and incubated for 15 min at RT (25 °C) in the dark. Binding Buffer (1×; 400 μL) was added to each tube and the samples were analyzed by flow cytometry within 1 h.
2.7. Western blot
Cells were lysed by RIPA buffer (Sigma–Aldrich) and total protein was quantified with the BCA protein assay kit (Thermo Fisher Scientific, MA, USA). Proteins were segregated on a 10% SDS‐PAGE gel and then transferred to polyvinylidene difluoride (PVDF) membranes (Bio‐Rad, CA, USA). The membrane was blocked with 5% degreased milk and then incubated with mouse anti‐human α‐tubulin and P53 monoclonal antibody (Santa Cruz, CA, USA), rabbit anti‐human XIAP, BCL‐2 and Bax polyclonal antibody (Milipore, MA, USA) or rabbit anti‐human phosphorylated AKT, ERK monoclonal antibody and rabbit anti‐human JNK, and STAT3 polyclonal antibody (Abcam, MA, USA) at 4 °C overnight. Membranes were then washed with TBST (Tris‐buffered saline containing 0.1% Tween 20, both from Bio‐Rad). Peroxidase‐linked secondary antibody, goat anti‐mouse IgG or goat anti‐rabbit IgG (Santa Cruz), were incubated with the membranes for 1 h at room temperature. After washing using TBST, the enhanced chemiluminescent substrate for detection of HRP (Thermo Fisher Scientific) was applied and images were captured by a Fujifilm Las‐3000 imager (Fujifilm, Japan).
3. Results
3.1. The expression pattern of AQP subtypes in melanoma cell lines
The expression of AQP subtypes in melanocyte cells was evaluated previously (Boury‐Jamot et al., 2006); however, their expression in melanoma has not yet been studied. To characterize AQP subtypes expression in melanoma, RT‐PCR was used to assess the expression of AQP0‐12 in 14 melanoma cell lines consisting of 2 primary melanomas, 6 muscle metastases, 5 lymph node metastases and 1 brain metastasis. Most of AQP subtypes (except AQP0, 2, 6, 10 and 12) were detected in melanoma cells with different expression patterns (Table 1). All cell lines expressed AQP4, 13/14 of cell lines expressed AQP11, 12/14 of cells expressed AQP1 and AQP3, 11/14 of cells expressed AQP5, 8/14 of cells expressed AQP7, 7/14 of cells expressed AQP9, and 5/14 of cells expressed AQP8. Our results showed that over 50% of melanoma cell lines expressed AQP1, 3, 4, 5, 7, 9 and 11.
Table 1.
AQPs expression in melanoma cells
| Cells | Type | AQP0 | AQP1 | AQP2 | AQP3 | AQP4 | AQP5 | AQP6 | AQP7 | AQP8 | AQP9 | AQP10 | AQP11 | AQP12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCC80a | pm | ◇ | ◆ | ◇ | ◆ | ◆ | ◆ | ◇ | ◆ | ◇ | ◇ | ◇ | ◆ | ◇ |
| MCC070 | pm | ◇ | ◆ | ◇ | ◆ | ◆ | ◆ | ◇ | ◆ | ◆ | ◇ | ◇ | ◆ | ◇ |
| C8161.9 | mm | ◇ | ◆ | ◇ | ◆ | ◆ | ◇ | ◇ | ◆ | ◆ | ◆ | ◇ | ◆ | ◇ |
| MCC12L | mm | ◇ | ◇ | ◇ | ◇ | ◆ | ◇ | ◇ | ◇ | ◇ | ◆ | ◇ | ◆ | ◇ |
| WM266.4 | mm | ◇ | ◆ | ◇ | ◆ | ◆ | ◆ | ◇ | ◆ | ◇ | ◆ | ◇ | ◆ | ◇ |
| 5U50 | mm | ◇ | ◆ | ◇ | ◆ | ◆ | ◆ | ◇ | ◇ | ◇ | ◇ | ◇ | ◆ | ◇ |
| MCC63 | mm | ◇ | ◆ | ◇ | ◆ | ◆ | ◆ | ◇ | ◇ | ◆ | ◆ | ◇ | ◆ | ◇ |
| MCC66c | mm | ◇ | ◆ | ◇ | ◆ | ◆ | ◆ | ◇ | ◆ | ◆ | ◆ | ◇ | ◆ | ◇ |
| MCC83 | ln | ◇ | ◆ | ◇ | ◆ | ◆ | ◆ | ◇ | ◆ | ◇ | ◇ | ◇ | ◆ | ◇ |
| MCC80b | ln | ◇ | ◆ | ◇ | ◆ | ◆ | ◆ | ◇ | ◆ | ◆ | ◇ | ◇ | ◆ | ◇ |
| MCC072 | ln | ◇ | ◆ | ◇ | ◆ | ◆ | ◇ | ◇ | ◇ | ◇ | ◆ | ◇ | ◆ | ◇ |
| MCC89 | ln | ◇ | ◆ | ◇ | ◆ | ◆ | ◆ | ◇ | ◇ | ◇ | ◆ | ◇ | ◆ | ◇ |
| MCC067 | ln | ◇ | ◇ | ◇ | ◆ | ◆ | ◆ | ◇ | ◆ | ◇ | ◇ | ◇ | ◆ | ◇ |
| MCC57 | bm | ◇ | ◆ | ◇ | ◆ | ◆ | ◆ | ◇ | ◇ | ◇ | ◇ | ◇ | ◇ | ◇ |
| Ratio | 0 | 12/14 | 0 | 12/14 | 100 | 11/14 | 0 | 8/14 | 5/14 | 7/14 | 0 | 13/14 | 0 |
Note: ◇: Not expression; ◆: Expression.
Pm: primary melanoma; mm: muscle metastatic melanoma; ln: lymph node metastatic melanoma; bm: brain metastatic melanoma.
3.2. AQP3 and AQP9 over‐expression attenuates the apoptotic effect of arsenite on WM266.4 cells
Resistance to apoptosis is a prominent feature of melanoma, and current effective chemotherapeutics available for the treatment of melanoma are lacking. Recently, arsenite has become an attractive therapeutic molecule due to its apoptotic effect on melanoma cells (Chowdhury et al., 2009; Chen et al., 2010; Hiwatashi et al., 2010), and a phase 2 clinical trial is underway using arsenic trioxide combined with other anti‐tumor medicines to treat metastatic melanoma patients (Bael et al., 2008). To learn the pathological role of the aberrant expression of AQP subtypes in melanoma, we over‐expressed AQP3 and AQP9 in WM266.4 cells through lentiviral transfection. As Fig. 1 shows, AQP3 is dominantly distributed in the cytoplasm, while AQP9 seems to be evenly distributed throughout the whole cell. Additionally, we found the similar expression patterns of AQP3 and 9 in 293T cells (data is not shown).
Figure 1.
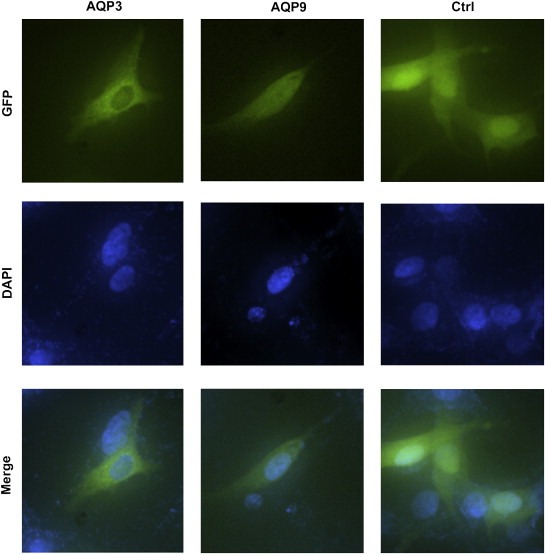
Over‐expression of AQP3 and AQP9 in WM266.4 cells. The fusion proteins GFP‐ AQP3 and GFP‐AQP9 were detected by a fluorescence microscope. AQP3 was found to be dominantly expressed in the cytoplasm, while AQP9 seemed to be evenly distributed throughout the whole cell.
Next, we examined the impacts of AQP3 and AQP9 on the arsenite therapeutic activity in WM266.4 cells. The sorted AQP3 and AQP9 over‐expressed and the control cells were exposed to arsenite for 48 h. The results showed that AQP3 and AQP9 significantly increased the resistance of WM266.4 cells to arsenite (Fig. 2A). To further study the mechanism of this action, we examined the roles of AQP3 and AQP9 in cell proliferation and apoptosis. Our results showed that both AQPs have no significant effects on cell growth (Fig. 2B) but dramatically blocked cell apoptosis induced by arsenite (Fig. 2C and Table 2).
Figure 2.
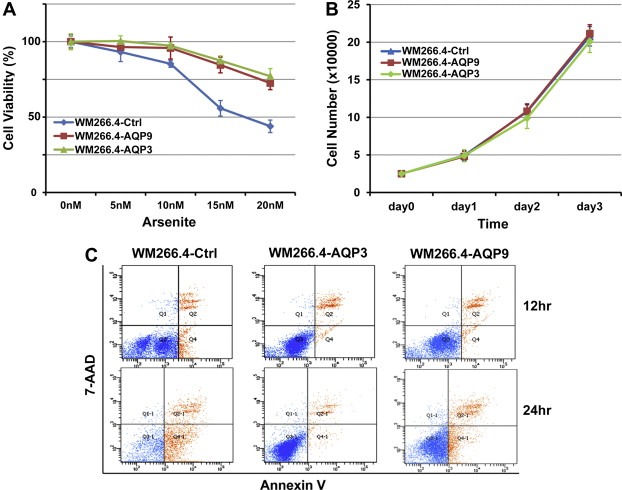
(A) AQP3 and AQP9 over‐expression significantly inhibits the chemotherapeutic effect of arsenite in WM266.4 cells. (B) AQP3 and AQP9 over‐expression has no significant effect on cell growth. (C) AQP3 and AQP9 over‐expression significantly inhibits the apoptosis of WM266.4 cells induced by arsenite.
Table 2.
Apoptotic cell induced by arsenite (%)
| Cells | Arsenite | |
|---|---|---|
| 12 h | 24 h | |
| WM266.4‐Ctrl | 9.4 | 60.0 |
| WM266.4‐AQP3 | 1.1 | 3.1 |
| WM266.4‐AQP9 | 2.0 | 13.0 |
3.3. AQP3 and AQP9 affect the expression of apoptosis‐associated genes
Previous research suggests that AQP over‐expression affects some cell signaling pathways associated with cell apoptosis (Zhang et al., 2010; Woo et al., 2008; Kang et al., 2008). To determine the mechanistic basis of AQP3 and AQP9 inhibiting apoptosis in WM266.4 cells, we examined the activities of several cell signaling pathways, including ERK, AKT, JNK, and STAT, but the results suggested that neither AQP3 nor AQP9 over‐expression could significantly affect these pathways (Fig. 3A). In turn, we detected the effect of AQP3 and AQP9 on the expression of selected apoptosis‐associated genes, including Bcl‐2, XIAP, p53 and BAX. The results suggested that AQP3 and AQP9 can significantly promote the expression of Bcl‐2 and XIAP, but reduce the expression of p53. Additionally, AQP3 but not AQP9 can inhibit the expression of BAX (Fig. 3B) in WM266.4 cells.
Figure 3.
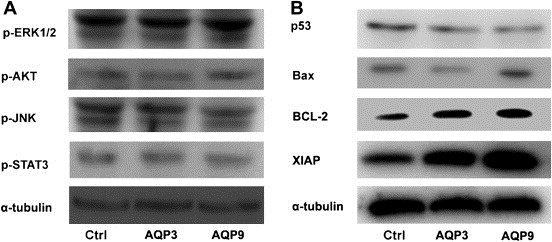
AQP3 and AQP9 affect the expression of apoptosis associated genes. (A) Neither AQP3 nor AQP9 over‐expression affects the activity of several apoptosis associated cell signaling pathway proteins (ERK, AKT, JNK, STAT et al.). (B) Both AQP3 and AQP9 over‐expression promote the expression of BCL‐2 and XIAP while decreasing the expression of p53; AQP3 but not AQP9 over‐expression also inhibits the expression of Bax in WM266.4 cells.
4. Discussion
Boury‐Jamot et al. detected the expression of AQP subtypes (AQP0‐10) in human melanocytes, finding that the melanocytes only expressed AQP1 (Boury‐Jamot et al., 2006). However, to our knowledge, there are still no reports showing the expression profile of AQPs in melanoma cells. In this study, we first evaluated the expression of all AQP subtypes in 14 melanoma cells, and proved that the expression patterns of AQPs in melanoma cells and melanocytes are different; melanoma cells express AQP3, 4, 5, 7, 8, 9 and 11, in addition to AQP1. These results implied a pathological function of aberrantly expressed AQPs in melanoma cells.
The roles of AQPs in tumor biology are attracting more attention. Until now, among AQPs, only AQP1, 3, 4, 5, 7, 8 and 9 have been observed for aberrant expression in many types of cancers and carcinomas (Verkman et al., 2008). In particular, AQP1 has been exclusively studied in tumor biology. AQP1 is expressed in vascular endothelial cells throughout the body, except in the normal central nervous system (Bondy et al., 1993). Recent research suggested that AQP1 can facilitate tumor angiogenesis and metastasis. Verkman et al. proved that tumor growth was reduced due to impaired tumor angiogenesis causing extensive tumor necrosis in an AQP1‐null mouse model (Saadoun et al., 2005). Consistently, Hu et al. found that microvessels in the tumors of AQP1‐null mice were of much lower density (Hu and Verkman, 2006), and AQP1 was strongly expressed in proliferating tumor microvessels in human and rat (Endo et al., 1999; Saadoun et al., 2002). Additionally, Hu et al. found that tumor cell migration and metastatic potential greatly increased in two mouse tumor cell lines with AQP1 expression as compared to the same cell lines without AQP1 expression (Hu and Verkman, 2006). Jiang et al. found that AQP1 could facilitate tumor cell metastasis in colon cancer cells (Jiang, 2009), and Otterbach et al. demonstrated a tight correlation between AQP1 expression and high tumor grade and poor prognosis in invasive breast carcinoma (Otterbach et al., 2008).
The aquaglyceroporin (transporting water and glycerol) AQP3 is expressed at the basolateral membrane of epithelial cells in the kidney collecting duct, airways, and intestine, as well as in bladder, conjunctiva, and cornea (Verkman, 2005). Recently, Hara‐Chikuma et al. demonstrated that AQP3 expression was increased in skin carcinomas (Hara‐Chikuma and Verkman, 2008a), and they also found that AQP3‐konckout mice were resistant to skin tumor formation possibly through reducing cell glycerol content and inhibiting the biosynthesis of ATP (Hara‐Chikuma and Verkman, 2008b). Additionally, Ismail et al. (Ismail et al., 2009) demonstrated that inhibition of AQP3 increases the sensitivity of prostate cancer cells to chemotherapy. In mammalian skin, AQP3 was expressed strongly in plasma membranes of the basal epidermal cell layer (Hara‐Chikuma and Verkman, 2008a), but melanocytes do not express AQP3 at all (Boury‐Jamot et al., 2006). In this study, we found that most of the melanoma cells expressed AQP3, which suggested that AQP3 might play an important role in the development of melanoma. AQP9 is another aquaglyceroporin, and it was highly expressed in malignant brain tumor (Warth et al., 2007), but the expression in hepatocellular carcinoma was declined (Jablonski et al., 2007). In this study, for the first time, we identify that AQP9 is up‐regulated in melanoma cells.
Previous research showed that AQPs could affect the expression of tumor suppressor genes or oncogenes and impact the activation of cell signaling pathways. Woo et al. first proved that AQP5 over‐expression in 3T3 cells significantly elevated the Ras signaling pathway (Woo et al., 2008). Similarly, Zhang et al. showed that AQP5 over‐expression in lung cancer induced the expression of several oncogenes including PCNA, c‐Myc and MUC5AC mucin and activated the EGFR/ERK/P38 MAPK signaling pathway (Zhang et al., 2010). Kang et al. also proved that AQP5 over‐expression in colon cancer increased the phosphorylation of ERK1/2 (Kang et al., 2008). In this study, after over‐expressing AQP3 and AQP9 in WM266.4 cells through lentiviral transfection, we examined the activities of several signaling pathways, including MAPK, JNK, AKT and STAT3; however, our results showed that the over‐expression of both AQPs has no significant impact on these pathways. This result is partially consistent with previous results showing a lack of clear effect of AQP3 on the MAPK signaling pathway (Kang et al., 2008).
Our results showed that AQP3 and AQP9 over‐expression increases the resistance of WM266.4 cells to arsenite. To further explore the mechanistic basis of this action, we found that AQP3 and AQP9 can promote the expression of two antiapoptotic genes, Bcl‐2 (Cory and Adams, 2002) and XIAP (Srinivasula et al., 2001; Riedl et al., 2001), and inhibit the expression of p53, one of the most well‐known pro‐apoptotic genes. Additionally AQP3 but not AQP9 can suppress the expression of Bax, another pro‐apoptotic gene (Cory and Adams, 2002). Except several studies debated whether AQPs located in the mitochondrial membrane were involved in apoptotic process by modulating water movement (Jablonski et al., 2007; Yang et al., 2006), there are very few reports to study the mechanism of AQPs regulating apoptotic genes. We found that AQP3‐ and 9‐GFP fusion proteins were distributed in the nucleus and/or cytoplasm as shown in Fig. 1, which implies that the anti‐apoptotic properties of AQPs, especially for the interactivities with apoptotic genes, may be distinct from their essential roles as water transporters; however, further functional investigations have to be implemented before drawing such a conclusion.
In summary, we have for the first time described the expression of AQPs in melanoma cells and have identified several members of the AQP family to be aberrantly expressed in melanoma. Furthermore, we proved that AQP3 and AQP9 inhibit the therapeutic effect of arsenite in melanoma by up‐regulating the expression of antiapoptotic genes Bcl‐2 and XIAP while down‐regulating the expression of proapoptotic gene P53 and Bax. In conclusion, our study provides an insight into exploring the chemoresistant basis for melanoma treatment.
Supporting information
The following are the Supplementary material related to this article:
Supplementary data
Acknowledgment
This study is supported by the USAMCI start‐up fund awarded to Dr. Yaguanag Xi.
Supplementary material 1.
1.1.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.molonc.2011.11.001.
Gao Lin, Gao Yanhui, Li Xiaobo, Howell Paul, Kumar Rajeev, Su Xiulan, Vlassov Alexander V., Piazza Gary A., Riker Adam I., Sun Dianjun, Xi Yaguang, (2012), Aquaporins mediate the chemoresistance of human melanoma cells to arsenite, Molecular Oncology, 6, doi: 10.1016/j.molonc.2011.11.001.
Contributor Information
Dianjun Sun, Email: hrbmusdj@163.com.
Yaguang Xi, Email: xi@usouthal.edu.
References
- Bael, T.E. , Peterson, B.L. , Gollob, J.A. , 2008. Phase II trial of arsenic trioxide and ascorbic acid with temozolomide in patients with metastatic melanoma with or without central nervous system metastases. Melanoma Res.. 18, 147–151. [DOI] [PubMed] [Google Scholar]
- Bondy, C. , Chin, E. , Smith, B.L. , Preston, G.M. , Agre, P. , 1993. Developmental gene expression and tissue distribution of the CHIP28 water-channel protein. Proc. Natl. Acad. Sci. U S A. 4500–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boury-Jamot, M. , Sougrat, R. , Tailhardat, M. , Le Varlet, B. , Bonte, F. , Dumas, M. , Verbavatz, J.M. , 2006. Expression and function of aquaporins in human skin: is aquaporin-3 just a glycerol transporter?. Biochim. Biophys. Acta. 1758, 1034–1042. [DOI] [PubMed] [Google Scholar]
- Chen, M.J. , Yang, P.Y. , Ye, Y.Z. , Hu, D.N. , Chen, M.F. , 2010. Arsenic trioxide induces apoptosis in uveal melanoma cells through the mitochondrial pathway. Am. J. Chin. Med.. 38, 1131–1142. [DOI] [PubMed] [Google Scholar]
- Chowdhury, R. , Chowdhury, S. , Roychoudhury, P. , Mandal, C. , Chaudhuri, K. , 2009. Arsenic induced apoptosis in malignant melanoma cells is enhanced by menadione through ROS generation, p38 signaling and p53 activation. Apoptosis. 14, 108–123. [DOI] [PubMed] [Google Scholar]
- Cory, S. , Adams, J.M. , 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2, 647–656. [DOI] [PubMed] [Google Scholar]
- Endo, M. , Jain, R.K. , Witwer, B. , Brown, D. , 1999. Water channel (aquaporin 1) expression and distribution in mammary carcinomas and glioblastomas. Microvasc. Res.. 58, 89–98. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma, M. , Verkman, A.S. , 2008. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol. Cell Biol.. 28, 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma, M. , Verkman, A.S. , 2008. Roles of aquaporin-3 in the epidermis. J. Invest. Dermatol.. 128, 2145–2151. [DOI] [PubMed] [Google Scholar]
- Hiwatashi, Y. , Tadokoro, H. , Henmi, K. , Arai, M. , Kaise, T. , Tanaka, S. , Hirano, T. , 2010. Antiproliferative and anti-invasive effects of inorganic and organic arsenic compounds on human and murine melanoma cells in vitro. J. Pharm. Pharmacol.. 63, 1202–1210. [DOI] [PubMed] [Google Scholar]
- Howell, P.M. , Li, X. , Riker, A.I. , Xi, Y. , 2010. MicroRNA in melanoma. Ochsner J.. 10, 83–92. 21603362 [Google Scholar]
- Hu, J. , Verkman, A.S. , 2006. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. Faseb J.. 20, 1892–1894. [DOI] [PubMed] [Google Scholar]
- Ishibashi, K. , Kondo, S. , Hara, S. , Morishita, Y. , 2011. The evolutionary aspects of aquaporin family. Am. J. Physiol. Regul. Integr. Comp. Physiol.. 300, R566–R576. [DOI] [PubMed] [Google Scholar]
- Ismail, M. , Bokaee, S. , Morgan, R. , Davies, J. , Harrington, K.J. , Pandha, H. , 2009. Inhibition of the aquaporin 3 water channel increases the sensitivity of prostate cancer cells to cryotherapy. Br. J. Cancer. 100, 1889–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski, E.M. , Mattocks, M.A. , Sokolov, E. , Koniaris, L.G. , Hughes, F.M. , Fausto, N. , Pierce, R.H. , McKillop, I.H. , 2007. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett.. 250, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal, A. , Siegel, R. , Ward, E. , Hao, Y. , Xu, J. , Thun, M.J. , 2009. Cancer statistics, 2009. CA Cancer J. Clin.. 59, 225–249. [DOI] [PubMed] [Google Scholar]
- Jiang, Y. , 2009. Aquaporin-1 activity of plasma membrane affects HT20 colon cancer cell migration. IUBMB Life. 61, 1001–1009. [DOI] [PubMed] [Google Scholar]
- Kang, S.K. , Chae, Y.K. , Woo, J. , Kim, M.S. , Park, J.C. , Lee, J. , Soria, J.C. , Jang, S.J. , Sidransky, D. , Moon, C. , 2008. Role of human aquaporin 5 in colorectal carcinogenesis. Am. J. Pathol.. 173, 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepper, M.A. , Nielsen, S. , Agre, Peter , 2004. 2003 Nobel Prize winner in chemistry. J. Am. Soc. Nephrol.. 15, 1093–1095. [DOI] [PubMed] [Google Scholar]
- Liu, S. , Ren, S. , Howell, P. , Fodstad, O. , Riker, A.I. , 2008. Identification of novel epigenetically modified genes in human melanoma via promoter methylation gene profiling. Pigment Cell Melanoma Res.. 21, 545–558. [DOI] [PubMed] [Google Scholar]
- Nico, B. , Ribatti, D. , 2011. Role of aquaporins in cell migration and edema formation in human brain tumors. Exp. Cell Res.. [DOI] [PubMed] [Google Scholar]
- Otterbach, F. , Callies, R. , Kimmig, R. , Schmid, K.W. , Bankfalvi, A. , 2008. Aquaporin 1 expression in invasive breast carcinomas. Pathologe. 29, (2) 357–362. [DOI] [PubMed] [Google Scholar]
- Papadopoulos, M.C. , Saadoun, S. , Verkman, A.S. , 2008. Aquaporins and cell migration. Pflugers Arch.. 456, 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl, S.J. , Renatus, M. , Schwarzenbacher, R. , Zhou, Q. , Sun, C. , Fesik, S.W. , Liddington, R.C. , Salvesen, G.S. , 2001. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 104, 791–800. [DOI] [PubMed] [Google Scholar]
- Saadoun, S. , Papadopoulos, M.C. , Davies, D.C. , Bell, B.A. , Krishna, S. , 2002. Increased aquaporin 1 water channel expression in human brain tumours. Br. J. Cancer. 87, 621–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun, S. , Papadopoulos, M.C. , Hara-Chikuma, M. , Verkman, A.S. , 2005. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 434, 786–792. [DOI] [PubMed] [Google Scholar]
- Srinivasula, S.M. , Hegde, R. , Saleh, A. , Datta, P. , Shiozaki, E. , Chai, J. , Lee, R.A. , Robbins, P.D. , Fernandes-Alnemri, T. , Shi, Y. , Alnemri, E.S. , 2001. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 410, 112–116. [DOI] [PubMed] [Google Scholar]
- Verkman, A.S. , Hara-Chikuma, M. , Papadopoulos, M.C. , 2008. Aquaporins–new players in cancer biology. J. Mol. Med. (Berl). 86, 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman, A.S. , 2005. More than just water channels: unexpected cellular roles of aquaporins. J. Cell Sci.. 118, 3225–3232. [DOI] [PubMed] [Google Scholar]
- Warth, A. , Mittelbronn, M. , Hulper, P. , Erdlenbruch, B. , Wolburg, H. , 2007. Expression of the water channel protein aquaporin-9 in malignant brain tumors. Appl. Immunohistochem. Mol. Morphol.. 15, 193–198. [DOI] [PubMed] [Google Scholar]
- Woo, J. , Lee, J. , Kim, M.S. , Jang, S.J. , Sidransky, D. , Moon, C. , 2008. The effect of aquaporin 5 overexpression on the Ras signaling pathway. Biochem. Biophys. Res. Commun.. 367, 291–298. [DOI] [PubMed] [Google Scholar]
- Yang, B. , Zhao, D. , Verkman, A.S. , 2006. Evidence against functionally significant aquaporin expression in mitochondria. J. Biol. Chem.. 281, 16202–16206. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Chen, Z. , Song, Y. , Zhang, P. , Hu, J. , Bai, C. , 2010. Expression of aquaporin 5 increases proliferation and metastasis potential of lung cancer. J. Pathol.. 221, 210–220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the Supplementary material related to this article:
Supplementary data


