Abstract
Since the early clinical studies of cancer immunotherapy, the question arose as to whether it was possible to combine it with standard cancer treatments, mostly chemotherapy. The answer, now, is past history. The combined use of immunotherapy and chemotherapy is not only possible but, in certain cases, can be advantageous, depending on the drug, the dose and the combination modalities. In order to find the best synergisms between the two treatments and to turn weak immunotherapeutic interventions into potent anticancer instruments, it is mandatory to understand the complex mechanisms responsible for the positive interactions between chemotherapy and immunotherapy. In this article, we review the current knowledge on mechanisms involved in the immunostimulating activity of chemotherapy and summarize the main studies in both mouse models and patients aimed at exploiting such mechanisms for enhancing the response to cancer immunotherapy.
Keywords: Cancer, Immunotherapy, Chemotherapy, Clinical trials
Highlights
Chemotherapy strongly enhances anticancer efficacy of immunotherapeutic strategies.
Three mechanisms of synergism have been hypothesized:
ldquo;subtractive
rdquo;,
ldquo;immunogenic
rdquo; and
ldquo;propulsive
rdquo;.
Chemo‐immunotherapy can effectively be used as secondary prevention against tumor relapse.
Abbreviations
- ACT
adoptive cell transfer
- AIRC
Association for Research against Cancer
- Ara‐C
cytosine arabinoside
- CEA
carcinoembryonic antigen
- CLR
C‐type lectin receptor
- CRT
calreticulin
- CTX
cyclophosphamide
- DC
dendritic cells
- DLI
donor lymphocyte infusion
- DTIC
dacarbazine
- GVHD
graft versus host disease
- HMGB1
High‐mobility group box 1 protein
- HSC
hematopoietic stem cell
- HSF2
stress response factor
- IFN
interferon
- IL
interleukin
- IP‐10
chemokine (C‐X‐C motif) ligand‐10
- MBL1
mannose binding lectin
- MCP‐1
monocyte chemotactic protein‐1
- MDSC
myeloid‐derived suppressor cells
- PRR
pattern recognition receptor
- SCT
stem cell transplant
- TBI
total body irradiation
- Th1
T helper 1
- Th17
T helper 17
- TLR
toll‐like receptor
- TMZ
temozolomide
- Treg
regulatory T cells
1. Introduction
Recently, a renewed interest has been focused on combining chemotherapy with immunotherapy for the treatment of cancer patients. Such interest is mostly explained by the discovery of new effects of certain chemotherapeutic agents, which suggest novel rationales for a different and selected use in combination with immunotherapy, including cancer vaccines (Emens, 2010; Moschella et al., 2010; Sistigu et al., 2011).
The history of pharmacology is scattered by the discovery of drugs whose therapeutic use changed from time to time. Anticancer drugs are no exception. The first studies on the toxic effect of mustard gas (sulfur mustards), chemical compounds used as poisoning gas during World War I, led to the discovery of their anticarcinogenic activity in experimental models (Berenblum, 1929). The results obtained by Berenblum were unexpected and suggested the first “repositioning” of the activity of this class of compounds. The anticancer activity of mustard gas derivatives was definitely proved after the so called “Disaster at Bari”, the only European city to experience an accidental chemical warfare in the course of World War II (Disaster at Bari by Glenn B. Infield). Studies performed by Goodman and Philips, including observations on the casualties produced in Bari harbor by the explosion of the Liberty ship John Harvey, which was carrying mustard gas, were published after war time (Gilman and Philips, 1946), demonstrating that these compounds could exert cytotoxic actions on a variety of tissues, particularly related to the degree of their proliferative activity. Many studies have been subsequently conducted leading to the discovery of other types of alkylating agents, many of which are in clinical practice today. Cyclophosphamide (CTX) was the major drug under experimental and clinical investigation (Comparative clinical and biological effects of alkylating agents. Monograph of the New Academy of Sciences; 1958). Many other chemical compounds were added to the antineoplastic drugs armamentarium over the years, all having the goal of directly and selectively interfering with the growth of malignant cells. Most of these compounds are not, however, devoid of side effects, among which the most significant are the myelo‐ and immunosuppressive activities. Because of these side effects, which impair the proliferative and effector functions of peripheral T cells, CTX and methotrexate are currently used for the treatment of many autoimmune diseases, and for the same reason they have been historically regarded as detrimental to antitumor immunity. This dogma was challenged at the beginning of the 1970s suggesting a second repositioning of the pharmacologic activity of these compounds (Maguire and Ettore, 1967; Vadlamudi et al., 1971). Several authors subsequently showed that combination therapy consisting of CTX and adoptive immunotherapy induced complete regression of experimental large tumors (Cheever et al., 1980; Greenberg et al., 1980; North, 1982). Noticeably, Robert North described the toxic effect of CTX against a T lymphocyte subpopulation endowed with immune suppressive activity (North, 1982). North's studies were particularly far‐sighted as he envisaged both the presence of a T cell population, which will be later characterized as CD4+CD25+ regulatory T cells (Treg) (Sakaguchi et al., 1995), and the selective sensitivity of this population to the toxic effect of CTX, as recently proved by Ghiringhelli et al. (2004).
Following the way paved by North, the combination of CTX and adoptive immunotherapy has been tested in several experimental settings to revert tumor‐induced tolerance for cancer treatment. The first results were extremely encouraging and made it possible to turn the adoptive transfer of tumor‐immune lymphocytes (which was barely effective) into a highly efficient tool to eradicate developing tumors in animal models first (Greenberg et al., 1985; Rosenberg et al., 1986; Proietti et al., 1998) and in melanoma patients later (Rosenberg et al., 2011).
In the following years, several reports have shown that, depending on the dosage and timing of administration, other chemotherapeutic drugs as well as irradiation (Dummer et al., 2002) can display either immunosuppressive or immunopotentiating effects and can augment the antitumor efficacy of adoptive and active immunotherapy. In particular, along with CTX, doxorubicin, docetaxel and paclitaxel were shown to augment the activity of tumor vaccines in HER‐2/neu tolerized mice (Machiels et al., 2001) as well as in established tumor models (Chopra et al., 2006; Chu et al., 2006). Of note, the co‐administration of CTX and paclitaxel was shown to synergize to further slow tumor growth (Pfannenstiel et al., 2010).
The main milestones in combining chemotherapy with immunotherapy on the basis of the identification of multiple biologic effects of anticancer drugs are depicted in Figure 1.
Figure 1.
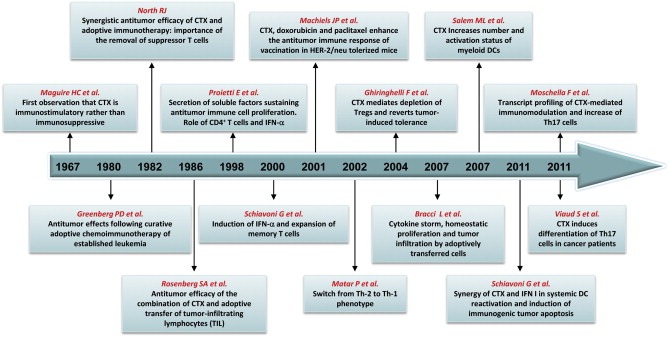
Selected reports highlighting the mechanisms whereby cyclophosphamide (CTX) enhances the antitumor efficacy of immunotherapy in animal models and patients.
In the present article, we review the current knowledge on mechanisms involved in the immunostimulating activity of chemotherapy and summarize the main studies in both mouse models and patients aimed at exploiting such mechanisms for enhancing the response to cancer immunotherapy. Taking into account the most recently described effects of chemotherapy on cells of the immune system and data obtained in animal models and clinical trials; we discuss some new perspectives for combining certain chemotherapeutic agents with immunotherapy, which may lead to a prolonged antitumor response in patients and to new opportunities for the development of effective therapeutic cancer vaccines.
2. Mechanisms involved in the immunostimulating activity of certain chemotherapeutic drugs
Initially, the first experimental models were preferentially based on the use of CTX as chemotherapeutic agent and the adoptive cell transfer (ACT) of lymphocytes endowed with specific antitumor activity as immunotherapeutic strategy (Rosenberg et al., 1986; Proietti et al., 1998; Vierboom et al., 2000). Although a simple vaccination of naive mice with a tumor cell lysate was in most cases able to prevent the growth of a subsequently implanted related tumor, thus demonstrating the development of specific tumor immunity, the transfer of spleen cells from immunized mice to mice bearing an established related tumor was totally ineffective unless mice were treated with one dose of CTX just before splenocyte infusion (Proietti et al., 1998). This simple model of adoptive immune cell transfer has allowed the demonstration of several key concepts: i) passively transferred antitumor immunity could not expand and become effective in a tumor‐bearing host without CTX pre‐treatment; ii) CTX did not synergize with ACT by simply reducing tumor burden. In fact, the simulation of tumor burden reduction, leaving a reduced number of tumor cells together with cell debris was not as effective as the treatment with CTX in association with ACT (Proietti et al., 1998); iii) three elements were essential to induce an effective antitumor response: lymphocytes with antitumor specificity, CTX and tumor antigens (Figure 2); and, iv) CTX could be effective only if administered before ACT in a well‐defined time window (Moschella et al., 2011).
Figure 2.
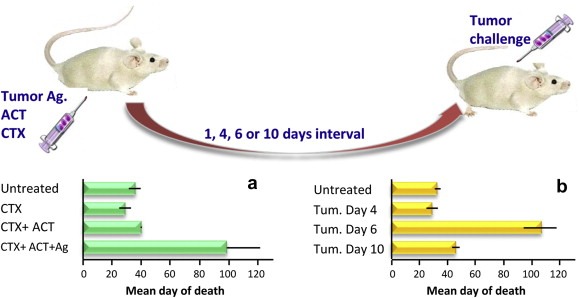
Illustration of the elements responsible for an effective antitumor immune response: cyclophosphamide (CTX), adoptive transfer of tumor‐immune lymphocytes (ACT) and tumor lysate (Ag) must be combined to reject a subsequent tumor challenge (panel a). Six days is the time interval necessary to mature an effective antitumor response to reject tumor (panel b). The detailed results of the experiment are reported elsewhere (Proietti et al., 1998).
A great deal of work has been done in recent years to elucidate the mechanisms responsible for the phenomena described above, whose ensemble can be summarized in three working hypotheses: the “subtractive” hypothesis that takes into account primarily the selective toxic effect of chemotherapy on cells responsible for the immunosuppressive activity; the “immunogenic” hypothesis that highlights the ability of some chemotherapeutic agents to increase the immunogenicity of tumor cells; and, the “propulsive” hypothesis that considers the homeostatic reaction of the body to the toxic effect of chemotherapy (Figure 3).
Figure 3.
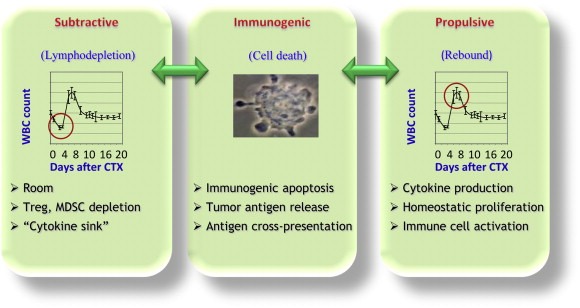
Illustration of the 3 main working hypotheses on the mechanisms of the positive interaction between chemotherapy and immunotherapy.
2.1. The subtractive hypothesis
Lymphodepletion, produced by different chemotherapeutic agents or by radiotherapy, has been considered the key event to facilitate the success of immunotherapeutic strategies against cancer. Actually, lymphodepletion can act through the contribution of different mechanisms. When therapeutic strategies of ACT are used, lymphodepletion makes room for the transferred tumor‐immune cells (Maine and Mule, 2002). Indeed, a lymphodepleted host can easily accept syngeneic lymphocyte transfusion. Transfused cells will find a reduced competition with resident cells for the available “resources” (Freitas et al., 1996). Endogenous cellular elements acting as sinks for cytokines are decreased and cytokines become more available to the needs of the adoptively transferred cells (Gattinoni et al., 2005a). Nonetheless, the reduction of immunosuppressive T lymphocytes has been considered, up to now, the leading mechanism by which chemotherapy unleashes and potentiates the antitumor immunity. The idea that progressive tumors can escape immune recognition and destruction, by actively establishing an immune tolerance involving immunosuppressive T lymphocytes, stemmed from the works of North's group in the 1980s (Berendt and North, 1980). North also described the selective toxic activity of CTX against “immunosuppressive T lymphocytes cells” (North, 1982), which have been later characterized as a CD4+ T cell subset expressing CD25, the α‐chain of the interleukin (IL)‐2 receptor (Jonuleit et al., 2001; Sakaguchi et al., 1995; Stephens and Mason, 2000).
CD4+CD25+ Treg represent 5–10% of the peripheral T lymphocyte pool in mice and humans, and are essential to limit the normal immune responses and prevent autoimmunity (Zou, 2006). Treg express CTLA‐4, GITR, and FoxP3 and inhibit CD8+ T cell responses in an IL‐2‐dependent manner through either direct cell–cell contact or by IL‐10 or TGF‐β secretion. Treg have been shown to suppress antitumor immune responses and accumulate in the peripheral blood and tumor microenvironment of patients with different types of cancer (Nizar et al., 2009).
Following North's paths, many groups have shown in preclinical models that treatment with low‐dose CTX reduces the number of CD4+CD25+ Treg, allowing tumor immunity to develop (Ercolini et al., 2005; Ghiringhelli et al., 2004; Lutsiak et al., 2005). Later on, Ghiringhelli showed that CTX not only depleted Treg in the blood and lymphoid organs of tumor‐bearing animals, but also decreased the number of Treg infiltrating tumor beds (Roux et al., 2008). Moreover, different authors demonstrated a direct effect of CTX on Treg cells. Mafosfamide, a CTX precursor, proved to be selective in vitro for T cells expressing FoxP3, a Treg specific marker (Kasprowicz et al., 2005). A single i.p. administration of CTX (100 mg/kg) directly affected the expression levels of GITR and FoxP3, thus impairing Treg functionality in addition to decreasing their numbers (Lutsiak et al., 2005). Finally, since Tregs control NK‐mediated antitumor immunity (Ghiringhelli et al., 2006), CTX restores innate killing activities both in mice (Terme et al., 2008) and in humans (Ghiringhelli et al., 2007).
Fewer studies have been performed on the Treg cell depleting activity of other chemotherapeutic agents, but some drugs, like paclitaxel in non‐small cell lung cancer, fludarabine in B cell chronic lymphocytic leukemia, gemcitabine and FOLFOX4 in metastatic colorectal carcinoma, have been observed to produce a significant reduction of Tregs in a relevant number of patients.
More controversial is the interaction between chemotherapy and myeloid‐derived suppressor cells (MDSC). These cells markedly expand in both tumor‐bearing mice and cancer patients and suppress T cell‐mediated immune responses through increased nitric oxide and arginase production (Gabrilovich and Nagaraj, 2009). An increase in MDSC numbers was described in breast cancer patients treated with dose dense doxorubicin–CTX (Diaz‐Montero et al., 2009). In preclinical models, CTX was shown to induce the expansion of MDSC in tumor‐free mice (Salem et al., 2010), but in combination with IL‐12 was able to decrease the number of MDSCs in comparison to non‐treated mice (Malvicini et al., 2011). Conversely, standard dose gemcitabine and 5‐Fluorouracil can eliminate MDSC, thereby enhancing the activity of CD8+ T cells and NK cells (Le et al., 2009; Vincent et al., 2010). Also cisplatin, given prior to vaccination, can decrease levels of peripheral MDSC in tumor‐bearing mice (Tseng et al., 2008).
2.2. The immunogenic hypothesis
Some chemotherapeutic agents are more efficient at inhibiting the growth of established syngeneic tumors in immunocompetent mice than in athymic littermates, demonstrating that an intact immune system enhances the therapeutic efficacy of conventional anticancer treatments (Apetoh et al., 2007a). A growing body of evidence is indicating that the adaptive and innate immune systems make a decisive contribution to the anticancer effects of conventional cancer treatments so that the number of remaining cells (minimal residual disease) may be kept in control.
The intervention of some chemotherapeutic drugs on the immune system is substantially triggered by modulating the expression of tumor antigens or influencing the expression of molecules that regulate antigen processing and presentation. The immunostimulating activity depends also on the specific tumor.
In murine models of acute myelogenous leukemia as well as in primary cultured human AML, cytosine arabinoside (ara‐C) increases the expression of B7‐1 and B7‐2 and decreases the expression of B7‐H1, thus enhancing CD8+ T cell‐mediated killing (Vereecque et al., 2004). Melphalan and mitomycin‐C (Sojka et al., 2000) were also found to induce the up‐regulation of costimulatory B7 molecules in human cancer cells. Five‐fluorouracil (5‐Fu) and dacarbazine can in vitro sensitize melanoma cells to antigen‐specific CTL lysis through fas‐ or perforin–granzyme‐mediated pathways (Yang and Haluska, 2004). A single dose of CTX can suppress mesothelioma growth in mice by sensitizing tumor cells to TRAIL‐dependent CD8+ T cell‐mediated apoptosis (van der Most et al., 2009).
Five‐Fu can also enhance the expression of carcinoembryonic antigen (CEA) in colon and breast carcinoma cells (Correale et al., 2011), and 5′‐aza‐2′deoxycytidine can induce the expression of a variety of cancer testis antigens and/or upregulate cell surface MHC Class I expression in distinct tumor cell lines (renal cell carcinoma, ovarian carcinoma, glioma, and melanoma), rendering them more susceptible to antigen‐specific CD8+ T cell‐mediated lysis (Adair and Hogan, 2009; Coral et al., 2002; Fonsatti et al., 2007; Natsume et al., 2008).
Paclitaxel, a drug belonging to the family of taxanes, was found to alter the cytokine profile within the tumor site in vivo, with increased monocyte chemotactic protein‐1 (MCP‐1) and chemokine (C‐X‐C motif) ligand‐10 (IP‐10) levels and decreased levels of IL‐1α. These modulations seem to correlate with a reduced inhibition of dendritic cells (DC) maturation in vivo and enhanced numbers of tumor infiltrating CD4+ and CD8+ T cells (Zhong et al., 2007). Paclitaxel, as well as cisplatin and doxorubicin, can make tumor cells permeable to granzyme B92, sensitizing them to T cell‐mediated lysis (Ramakrishnan et al., 2010).
A relevant body of evidence is now disclosing new mechanisms by which chemotherapy can trigger anticancer immune responses. In fact, apoptosis, which is considered a non‐inflammatory process to eliminate chemotherapy‐injured tumor cells, has been discovered to become, in some cases, immunogenic through the translocation to cell surface of calreticulin (CRT). In response to some cell death inducers, and in particular anthracyclines and ionizing irradiation, CRT can translocate from the lumen of the endoplasmic reticulum to the surface of cells at an early pre‐apoptotic stage (Obeid et al., 2007b). As a consequence, when tumor cells are treated for a few hours with anthracyclines and then injected subcutaneously into mice, they become highly efficient in inducing a specific DC and antitumor T cell response (Casares et al., 2005). Cell surface expression of CRT greatly enhances the uptake of dying tumor cells by DC. Importantly, CRT is necessary, but not sufficient, for the immune response induced by apoptosis.
Further studies have revealed that the alarmin high‐mobility group box 1 protein (HMGB1), a toll like receptor 4 (TLR4) ligand, is a nuclear protein that is released from anthracyclin‐treated dying cells during late‐stage apoptosis. Depletion of HMGB1 from dying tumor cells with siRNAs or neutralization of HMGB1 with specific antibodies abolishes the TLR4‐dependent, DC‐mediated presentation of antigens from dying tumor cells in vitro and in vivo. Therefore, HMGB1 release is required for the immunogenicity of cell death through its effect on TLR4.
In conclusion, CRT is essential for engulfment and subsequent DC maturation, whereas the HMGB1–TLR4 interaction is required for optimal processing and presentation of tumor antigens from the dying tumor cells to T lymphocytes (Apetoh et al., 2007a). The clinical relevance of this pathway is revealed by a TLR4 polymorphism leading to a single amino acid substitution (asp299gly) in the TLR4 extracellular domain. This polymorphism reduces the binding of HMGB1 to human TLR4 and inhibits HMGB1 mediated DC‐T cell interactions. A cohort of breast cancer patients with this polymorphism of TLR4 had a higher risk of disease relapse after adjuvant treatment with anthracycline‐based chemotherapy (Apetoh et al., 2007b). Similar findings have been reported for advanced colon cancer patients treated with oxaliplatin (Tesniere et al., 2010). Lastly, another drug (i.e., CTX) proved to induce CRT exposure in vivo and to increase tumor cell immunogenicity in murine models, thus joining the list of the compounds inducing immunogenic apoptosis (Schiavoni et al., 2011).
2.3. The propulsive hypothesis
For several years the rationale for combining chemotherapy and immunotherapy was mostly based on the impairment of Treg cell activity due to drug‐mediated lymphopenia. Indeed, although CTX depletes Tregs in vivo, some authors have shown that these cells can repopulate the periphery (Moschella et al., 2011; Powell et al., 2007), highlighting the transient nature of this phenomenon and its possible limiting importance for a potential impact on the effectiveness on immunotherapy treatments. Moreover, even in the genetic absence of Treg cells, a lymphodepleting non‐myeloablative regimen substantially augmented CD8+ T cell reactivity to self‐tissue and tumor (Gattinoni et al., 2005b), thus suggesting that other mechanisms concur in the enhancement of immune responses by chemotherapy.
It is now well‐known that lymphopenia is followed by a spontaneous expansion of the remaining T cells in the periphery to restore the original T cell pool size and maintain homeostasis (Mackall et al., 1997). It has not been fully elucidated how our body can count blood cells; nevertheless, immediately after chemotherapy‐induced lymphodepletion, the cell number rises over the baseline (Figure 4), a phenomenon known as rebound overshoot, which correlates with immune function recovery (Braun and Harris, 1981; Wu et al., 2004; Zaragoza et al., 2011).
Figure 4.
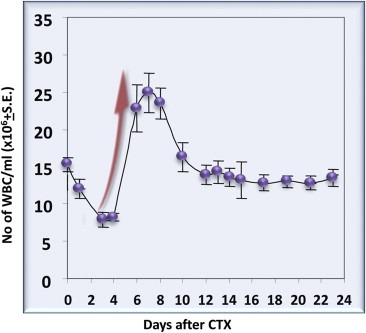
Cyclophosphamide (CTX)‐induced rebound overshoot of white blood cells after a single 100 mg/kg CTX injection of mice. The detailed results of the experiment are reported elsewhere (Proietti et al., 1998).
A very simple experiment, performed by our group in 1998, gave a clear demonstration that lymphocyte proliferation is driven by soluble factors produced in vivo by bone marrow (BM) cells three days after CTX injection (Proietti et al., 1998) (Figure 5). Of interest, the time of production of these factors matched with the nadir of WBC count, right before homeostatic proliferation recovers the WBC number.
Figure 5.
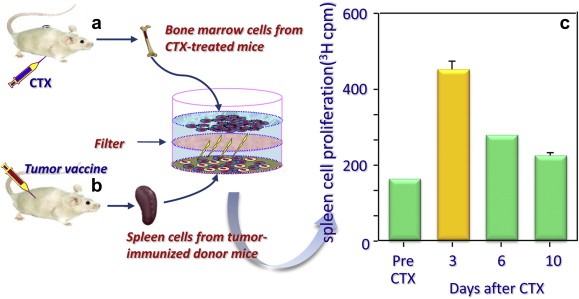
Demonstration of the release by bone marrow cells from cyclophosphamide (CTX)‐treated mice of soluble factors responsible for the proliferation of tumor‐immune lymphocytes in vitro. Bone marrow cells were harvested from mice at different times after CTX treatment (a) and put in culture, without cell contact, with splenocytes deriving from mice vaccinated against tumor antigens. (b). After several days, splenocytes were pulsed with 3H thimidine to measure cell proliferation induced by soluble factor released by BM cells. Of note, the optimal proliferation was achieved 3 days after in vivo CTX treatment. The detailed results of the experiment are reported elsewhere (Proietti et al., 1998).
The same mechanisms responsible for the rebound overshoot have been shown to act also on adoptively transferred tumor‐immune cells, inducing their proliferation, activation and migration into secondary lymphoid organs and into the tumor bed. The proliferation of adoptively transferred cells involved both CD8+ T and B lymphocytes and correlated with elevated and sustained levels of tumor‐specific serum antibodies and increased frequency of tumor‐specific IFN‐γ‐producing CD8+ T cells (Bracci et al., 2007). More recently, the humoral immune response to an EGF‐based cancer vaccine was proven to be significantly boosted by high‐dose CTX and doxorubicin (Montero et al., 2009).
Many studies have been carried out to characterize the different cell populations targeted by CTX‐induced factors and the immunopotentiating factors themselves.
The demonstration that the injection of anti‐interferon α/β (type I IFN) antibodies inhibited the antitumor efficacy of chemo‐immunotherapy suggested that type I IFN could be induced by CTX and played an important role in the overall antitumor response (Proietti et al., 1998). Subsequent studies showed that type I IFN was indeed induced in vivo soon after the administration of a single dose of CTX (100 mg/kg) and that this cytokine was responsible for the expansion of memory CD4+ and CD8+ T cells (Schiavoni et al., 2000). CTX‐induced IFN‐α/β was also shown to mediate the restoration of an activated polyfunctional helper phenotype in tumor‐specific adoptively transferred CD4+ T cells, thus preventing a tumor‐driven dysfunctional phenotype of effector T cells, characterized by selective down‐regulation of IL‐7 receptor, heightened apoptosis, and poor antitumor efficacy (Ding et al., 2010).
More recently, CTX and type I IFN were demonstrated to synergize through systemic DC reactivation (Schiavoni et al., 2011). Interestingly, CTX‐lymphodepleting activity spared BM DC precursors, thus facilitating the recovery of an immature DC pool in the periphery and modulating the balance between DC subsets, leading to the preferential expansion of CD8α+ DC. The effects of CTX on DC homeostasis, were shown to be mediated by endogenous type I IFN (Schiavoni et al., 2011). These findings corroborated previous observations that CTX‐induced lymphodepletion was responsible for the marked expansion of immature DC in peripheral blood during the rebound phase (Salem et al., 2009, 2007).
Further studies showed that CTX has a profound and selective cytotoxic activity on CD8+ resident DCs, known to contribute to peripheral tolerance, but not on skin‐derived migratory DCs or plasmacytoid DCs in lymph nodes and spleen, causing an imbalance among these DC subsets. CTX treatment also increased the potency of DCs in antigen presentation and cytokine secretion (Nakahara et al., 2010).
Moreover, CTX was shown to induce a pre‐apoptotic surface translocation of CRT and the release of HMGB1 by tumor cells, which represent a prerequisite for an adequate engulfment of tumor apoptotic material and optimal CD8α+ T cell cross priming by DC (Schiavoni et al., 2011).
As it was shown that acute IFN‐α treatment promotes the proliferation of dormant hematopoietic stem cells (HSCs) in vivo, whereas chronic activation of the IFN‐α pathway in HSCs impairs their function (Essers et al., 2009), it is possible to envisage that IFN‐α, acutely induced by a single CTX administration, can mediate the proliferation of BM precursors as well as the peripheral rebound overshoot. On the other hand, the chronic exposure to chemotherapy would mediate lymphodepletion and immunosuppression through persistent IFN‐α release.
A number of other chemotherapy drugs, including vinblastine, methotrexate, mitomycin‐C, vincristine, doxorubicin, 5‐aza‐2′‐deoxycytidine and paclitaxel, have been reported to enhance DC maturation and functions (Kaneno et al., 2009; Shurin et al., 2009).
In the attempt to further characterize the CTX‐induced cytokine milieu driving homeostatic proliferation and the immunostimulatory effect on different cell populations, the expression of a selected number of cytokine‐encoding genes was analyzed in the bone marrow of tumor‐bearing mice at different times after CTX treatment. Real‐time PCR analysis showed the early and transient induction of hematopoietic growth factors, including GM‐CSF and IL‐1β. GM‐CSF is known to induce myelorestoration following chemotherapy‐induced myelosuppression (Dempke et al., 2000). Of note, also the transcript levels of IL‐2 were augmented by CTX and the combination of IL‐1β and IL‐2 was shown to strongly enhance myelorestoration when given after CTX treatment in a mouse model (Proietti et al., 1993).
Real time PCR analyses demonstrated also that CTX administration actively induces the expression of cytokines regulating homeostatic expansion, such as IL‐7, IL‐15 and IL‐21, which share (along with IL‐2) the common gamma‐chain receptor. Although each of these cytokine have overlapping functions because of their ability to activate common signal transducer and shared STAT family members, individual cytokines may possess unique or selective activities. IL‐15 is involved in mediating the stimulation of memory‐phenotype CD8+ T cells by type I IFN (Zhang et al., 1998). IL‐21 can synergize with either IL‐7 or IL‐15 in driving the proliferation of CD8+ T cells, in inducing the differentiation of B cells into plasma cells and in enhancing the activity of NK cells (Zeng et al., 2005). IL‐7 has been shown to play a pivotal role for homeostatic expansion of naive CD8+ and CD4+ T cells in lymphopenic hosts and for CD8+ T cell survival in normal hosts (Schluns et al., 2000). Noteworthy, the neutralization of IL‐7 by specific monoclonal antibody greatly reduced the homing of transferred lymphocytes to the secondary lymphoid organs of CTX‐treated mice (Bracci et al., 2007). The importance of the increased expression of IL‐7 and IL‐15 for the effectiveness of combined therapies is also suggested by the finding that the antitumor efficacy of the combination of a sublethal total body irradiation (TBI) with the adoptive transfer of CD8+ T cells was impaired in mice deficient of both cytokines (Gattinoni et al., 2005a). Moreover, the systemic administration of IL‐2, IL‐7, IL‐15 and IL‐21 was shown to be capable of augmenting tumor regression mediated by adoptively transferred T cells in a dose‐dependent manner (Klebanoff et al., 2011).
Considering that the γ chain of the IL‐7 receptor is expressed only at low levels in regulatory T cells (Seddiki et al., 2006), all these observations suggest that the CTX‐induced homeostatic cytokines may act synergistically in promoting the proliferation/activation of effector/memory T cells following CTX‐mediated lymphodepletion but not that of Treg cells, leading to a temporary imbalance of effector/Treg cells favoring effective immunity and disadvantaging tolerance.
Such an imbalance in CD4+ T‐cell subtypes was demonstrated by the simultaneous analysis of the kinetic of recovery of Treg, T helper 17 (Th17), T helper (Th1) and activated CD25+CD4+Foxp3− T lymphocyte after CTX‐mediated lymphodepletion. Th17, Th1 and activated T cells showed a pronounced expansion, while Treg cells exhibited a lower and more delayed rebound (Moschella et al., 2011).
Of note, the CTX‐mediated polarization of T helper cells toward a Th1 phenotype, the T helper subtype typically considered to be the most important for tumor rejection, has been shown in different experimental settings. In fact, CTX treatment induced increased expression of Th1 cytokines (IL‐2 and IFN‐γ), while decreasing the level of IL‐4 transcripts and, in association with adoptive cell transfer, induced increased plasma levels of IFN‐γ while reducing that of IL‐10 (Bracci et al., 2007). A Th2 to Th1 shift in cytokine production was also shown in tumor‐bearing rats treated with low‐dose CTX (Matar et al., 2002). Regarding Th17 cells, a recently characterized lineage distinct from Th1 or Th2 subsets and characterized by the secretion of IL‐17A, their role in controlling tumor growth is currently under debate. In fact, some studies found no significant correlation of Th17 with patient clinic‐pathological characteristics or survival (Zhang et al., 2010), while in other studies Th17 correlated with improved cancer prognosis (Sfanos et al., 2008; Ye et al., 2010). Moreover, Martin‐Orozco and colleagues demonstrated that IL‐17A‐deficient mice are more susceptible to developing lung melanoma and that the adoptive transfer of tumor‐specific Th17 cells prevented tumor development exhibiting a stronger therapeutic efficacy than Th1 cells (Martin‐Orozco et al., 2009). Indeed, in both tumor models and cancer patients CTX was able to markedly promote the differentiation of Th17 cells (Moschella et al., 2011; Viaud et al., 2011).
3. Molecular aspects of chemotherapy‐induced immunomodulation
At a glance, it would seem very difficult to reconcile all the above mentioned effects of CTX on both the tumor and the host (Figure 6) in one simple mechanistic model. The use of high throughput technologies, such as transcriptomic and proteomic approaches, allowed a comprehensive characterization of CTX‐mediated immunomodulation (Moschella et al., 2011). Gene expression profiling demonstrated that CTX modulates the expression of about a thousand of genes in bone marrow, spleen and PBLs of tumor‐bearing mice. Down‐regulated genes were shown to be involved in cell division, RNA processing, metabolic processes, establishment of cellular localization and chromosome segregation, representing the biosynthetic, metabolic and cell cycle arrest mediated by CTX toxicity (Moschella et al., 2011). Up‐regulated genes were related to various aspects of immune regulation and activation. In particular, 1–2 days post‐treatment, CTX induced the expression of numerous danger signals, including genes associated to DNA repair, cell death, autophagy and drug resistance (DNA‐damage‐inducible transcript 4, clusterin, HIF1A, ATG7 and HSP70‐2) and CRT, whose exposure is required for the immunogenicity of cell death (Obeid et al., 2007a). Moreover, CTX treatment induced the up‐regulation of two alarmins (defensin‐alpha‐4 and defensin‐beta‐2), acting on immature DCs as ligands for Toll‐like receptor 4 (TLR4) (Biragyn et al., 2002), of the stress response factor (HSF2) and of several soluble and cell associated pattern recognition receptors (PRR), responsible for the recognition of pathogens and apoptotic/necrotic cells (Moschella et al., 2011). Up‐regulated PRRs included complement component C1q (Ogden et al., 2001), complement protein properdin, ficolin A and several C‐type lectin receptors (CLRs), such as mannose binding lectin (MBL1) (Nauta et al., 2003; Ogden et al., 2001), DCIR (Klechevsky et al., 2010), SIGNR1, Dectin‐1 (Weck et al., 2008) and Dectin‐2 (Robinson et al., 2009). In addition, CTX induced the up‐regulation of genes involved in CLR signal‐transduction pathway (SYK, BCL‐10) and of the transcription factor NF‐κB that regulate proinflammatory gene expression (Gringhuis et al., 2009). Triggering of several PRRs simultaneously can induce diverse innate immune responses, which provides the diversity that is required to shape an effective adaptive immune response. Accordingly, factors involved in inflammatory responses and Th1 and Th17 differentiation (nitric oxide synthase 2, IL‐1β, IL‐17, IL‐18, IFN‐γ) were increased post‐treatment. At the same time, CTX treatment augmented the expression of several genes encoding for chemokines (CCL21, CXCL2, CXCL7, CXCL12) and chemokine receptors (CCR6, CCR9 and IL8RB), of genes modulating lymphocyte proliferation and activation, such as APRIL and BAFF, and of growth factors acting on different cell populations (FGF1, HBEGF, IGF1, PDGFB, VEGFA) (Moschella et al., 2011). The model stemming from this data is that CTX induces an immunogenic apoptosis not only of tumor cells but also of host BM and spleen cells and that the perception of danger signals by PRRs leads to the promotion of hematopoiesis and activation of the immune response (Figure 7). When an immune intervention (vaccination or adoptive immunotherapy) is performed during the recovery phase that follows the damage, also tumor‐specific immune cells can take advantage of the drug‐mediated immunomodulation.
Figure 6.
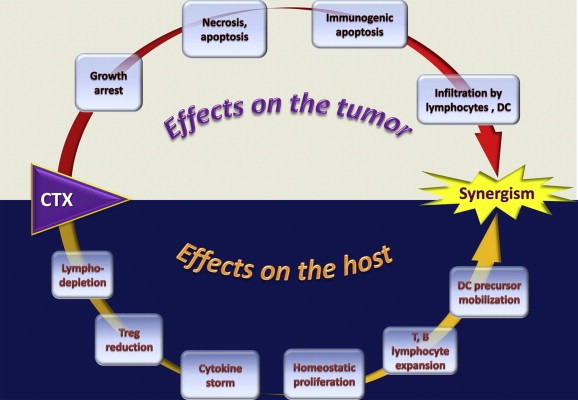
Schematic representation of the multiple direct and indirect effects of cyclophosphamide (CTX) in association with immunotherapy on both the host and the tumor, leading to a synergistic anticancer activity.
Figure 7.
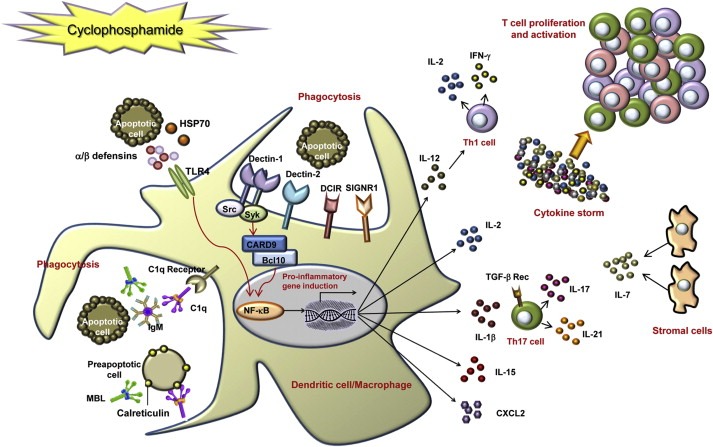
Model of cyclophosphamide (CTX)‐mediated immunomodulation on the basis of genes up‐regulated by treatment. CTX treatment of tumor‐bearing mice results in the killing of proliferating cells, including tumor and host bone marrow and spleen cells. Pre‐apoptotic and dead cells release or expose danger signals such as HSP70, alarmins (defensin‐alpha‐4 and defensin‐beta‐2) and calreticulin. The latter could be recognized by the soluble pattern recognition receptors (PRRs) C1q and mannose binding lectin (MBL), thus triggering phagocytosis of apoptotic cells. The secretion of HSP70 and defensin‐beta‐2, through the activation of TLR4, may act directly on immature DC, inducing their maturation and up‐regulation of costimulatory molecules, via activation of NF‐κB. Apoptotic cells may be recognized also by membrane PRRs, such as dectin‐1, dectin‐2, DCIR and SIGNR1. After ligand binding, the C‐type lectin dectin‐1 activates the transcription factor NF‐κB through a Syk kinase‐dependent signaling pathway, leading to the induction of genes encoding proinflammatory cytokines and chemokines. Up‐regulated cytokines, including IL‐1β, IL‐2, IL‐7, IL‐15, IL‐17, IL‐21 and IFN‐γ (produced either by DC or by T cells or by stromal cells) may therefore induce Th1 and Th17 polarization and, altogether, T cell proliferation and activation (Moschella et al., 2011).
Such a response could be interpreted as a hormetic response. Hormesis is a biological phenomenon whereby a beneficial effect (improved health, stress tolerance, growth or longevity) results from exposure to low doses of an agent that is otherwise toxic or lethal when given at higher doses (Calabrese et al., 2007). It is a biphasic biological phenomenon that can result from cellular repair processes following a disruption in homeostasis (i.e., toxicity). The repair process fixes the damage caused by the stressor and is then followed by an overcompensatory response which would be seen as a stimulation. This effect has been described for a large number of chemicals and radiation and also the immune system can be seen as a hormetic system, in which the stressor is the antigen and proliferation is the hormetic response (Calabrese, 2005). The classical hormetic model where low‐dose exposure to a toxic compound produces a stimulatory response and high‐dose exposure an inhibitory one, would correspond for CTX to a model where a single treatment before immunotherapy is immunostimulatory while repeated treatments are immunosuppressive.
Of note, the massive modulation of gene expression by CTX is early and transient, being activated already 1 day after treatment and finished by day 5 (Moschella et al., 2011), pointing out that the immunotherapeutic intervention, which may include the use of cancer vaccines, should be performed early after treatment, to consent to the chemotherapy‐induced “hormetic response” to condition and enhance the antitumor immunity. This concept is consistent with results obtained in mouse tumors models, where CTX could strongly synergize with the adoptive cell transfer of tumor‐immune lymphocytes only if the vaccine itself was administered 1 day after drug‐treatment, at the time of the detection of a chemotherapy‐induced cytokine/chemokine “storm” (Moschella et al., 2010, 2011). Thus, all this suggests that the propulsive force of chemotherapy can be successfully exploited at an early time after drug administration for improving the response to immunotherapy in cancer patients.
4. Clinical studies of combination therapies
Today, a large amount of preclinical studies performed by our group as well as by others strongly suggest the possibility of taking advantage of chemotherapy‐activated homeostatic mechanisms to boost the tumor‐specific cellular immunity. The results of some clinical studies may support this concept. Here, we will not review all the many clinical studies based on combining chemotherapy with immunotherapy, which have been the subject of some comprehensive recent review articles (Emens, 2010). We only summarize the results of some selected clinical studies which may support the rationale of exploiting the propulsive role of chemotherapy for enhancing the response to vaccines and other immunotherapy interventions. Some studies have shown that different kinds of immunotherapy in close proximity to cytotoxic chemotherapy can increase the immune response against cancer. This concept is further indirectly supported by a recent observation that in chemotherapy treated breast cancer patients, influenza vaccine administered at day 4 after chemotherapy discontinuation induced a significantly better antibody response than in patients vaccinated at day 16 (Meerveld‐Eggink et al., 2011). The use of chemotherapy administration just before immunotherapy with immune‐enhancing purposes can be defined as “immune‐adjuvant chemotherapy”.
Most clinical trials of chemotherapy and immunotherapy combination have been conducted in patients with melanoma undergoing ACT of tumor‐specific lymphocytes and CTX. Rosenberg's group has steadily moved in the direction of the ACT getting progressively more encouraging results. Trial after trial he obtained that the administration of a heavy non‐myeloablative lymphodepleting regimen consisting of CTX and fludarabine before the adoptive transfer of antitumor effector cells could lead to a dramatic increase in the persistence of transferred cells in vivo and objective cancer regression in 49–72% of patients with metastatic melanoma, with complete responses ongoing beyond 3–7 years (Rosenberg et al., 2011). More recently, the same group demonstrated that new approaches using autologous cells genetically engineered to express conventional or chimeric T‐cell receptors can mediate cancer regression in patients with metastatic melanoma, synovial sarcoma, neuroblastoma and refractory lymphoma (Rosenberg et al., 2011).
The importance of CTX pre‐treatment was highlighted also in a recent trial in chemotherapy‐refractory chronic lymphocytic leukemia or relapsed B cell acute lymphoblastic leukemia patients treated with autologous T cells modified to express 19‐28z, an antigen receptor specific to the B cell lineage antigen CD19. The short‐term persistence of infused T cells was enhanced by prior (2 days) administration of a relatively high dose (3 gr/sqm) CTX. Further analyses revealed rapid trafficking of modified T cells to tumor and retained ex vivo cytotoxic potential of CD19‐targeted T cells retrieved 8 days after infusion. Of particular relevance, this trial supports the concept that immune‐adjuvant chemotherapy can be administered also in chemotherapy‐refractory patients to overcome tumor resistance occurring after prolonged chemotherapy treatments (Brentjens et al., 2011).
Finally, the crucial relevance of timing between chemotherapy and cell infusion was demonstrated by Foà and colleagues in a recent study in hematologic patients who have undergone an allogeneic stem cell transplant (SCT) procedure and had evidence of resistant/residual disease. These patients underwent one or two cycles of donor lymphocyte infusion (DLI) already 2 days after a chemotherapeutic treatment (usually DLI is given at least 15 days after chemotherapy). Four of the six patients who received DLI after chemotherapy experienced for the first time a clinical picture of graft versus host disease (GVHD), although they had previously undergone one or more cycles of standard DLI; in these four patients, the onset of GVHD was associated with a hematological complete remission (Torelli et al., 2010).
A more limited number of studies have been performed in cancer patients using chemotherapeutic drugs along with cell‐based vaccination. A wide number of drugs have been tested with different modalities of combination with vaccine. Most cellular vaccines consisted of tumor cells engineered to secrete GM‐CSF administered early after treatment with therapeutic or low‐dose chemotherapy. Different types of cancer have been studied so far (breast, colon, pancreas, prostate cancers) showing a chemotherapy‐dependent increase of antitumor cellular immunity, as recently reviewed (Emens, 2010).
Although several experimental studies demonstrate that moderately low‐dose chemotherapy may positively affect DC differentiation/maturation and that it can be efficiently combined with DC‐based vaccination (Asavaroengchai et al., 2002; Salem et al., 2007; Schiavoni et al., 2011), only one clinical study reported that in patients with glioblastoma, immune therapy with DC vaccination after radiation and temozolomide (TMZ) resulted in tumor‐specific immune responses that were associated with prolonged survival (Fadul et al., 2011).
Only a few studies have been completed with the sequential combination of chemotherapy and peptide vaccines. Recently, Sampson and colleagues showed that a dose‐intensified TMZ treatment of glioblastoma patients in combination with experimental EGFRvIII‐targeted peptide vaccine, although increasing the proportion of immunosuppressive Treg cells, produced humoral and delayed‐type hypersensitivity responses of greater magnitude with respect to vaccine alone or vaccine in combination with standard dose TMZ and eradicated EGFRvIII‐expressing tumor cells in nearly all patients (Sampson et al., 2011). A phase I/II trial in resected melanoma patients demonstrated that pre‐treatment of resected stage IIIb–IV melanoma patients with standard dose dacarbazine (DTIC) 1 day before the administration of a gp100 and Melan A peptide vaccine induced high tumor‐specific cellular immune responses and a progressive widening of T cell receptor repertoire diversity accompanied by high avidity which correlated with a disease‐free survival of more than 6 years only in patients treated with DTIC vaccine combination (Nistico et al., 2009; Palermo et al., 2010).
5. Conclusions and perspectives
The history of cancer immunotherapy has been characterized by alternate cycles of optimism and discouragement. With the recent approval of new types of immunotherapy treatments for certain categories of cancer patients (i.e., a monoclonal antibody, Ipilimumab, for melanoma and a cellular vaccine, Provenge, for prostate cancer), we are now encouraged to move ahead with new preclinical and clinical studies of cancer immunotherapy, taking also into consideration the better understanding on how immunologic interventions, including cancer vaccines, can be successfully combined with other anticancer treatments. Likewise, we now understand much better the potential relevance of some cellular and molecular markers of the response to both immunotherapy and chemotherapy in certain categories of cancer patients and all this can significantly contribute to the development of more effective and personalized antitumor therapies.
During the last decade, parallel flows of knowledge have shed new light on both the understanding of immunostimulating effects of some anticancer chemotherapeutic drugs and on the importance of the immune system activity in controlling the development and the progression of cancer. This new information has supplied the rationale for using certain chemotherapeutic drugs to achieve a favorable modulation of the antitumor immune response and to amplify the response to therapeutic anticancer immune treatments.
The initial rationale for combining chemotherapy and immunotherapy was based essentially on the selective cytotoxic activity of CTX against Treg but was gradually implemented by the discovery of the concomitant involvement of homeostatic proliferation and of immunogenic apoptosis.
This new complex framework of the mechanisms of synergistic interaction between chemotherapy and immunotherapy can now let us draw much more articulate immunotherapeutic strategies which take into account the wide panel of effects of chemotherapy directed to both the stimulation of the host immune response and the modification of the tumor microenvironment.
The direct consequence of this new vision is that it is possible today to combine chemotherapy and immunotherapy not only in advanced cancer patients, where the tumor‐induced immunosuppressive activity has a dominant role, but also in tumor‐free or resected patients, where the goal is to restimulate a vanished tumor‐specific immune response to erase the minimal residual disease and prevent tumor recurrence. Moreover, the new knowledge on the effects of certain chemotherapy agents and on the functional properties of some types of DC, such as those generated in the presence of IFN‐α (IFN‐DC) exhibiting a special capability to kill certain tumor cells and take up apoptotic bodies and induce antigen cross‐presentation and potent cross‐priming of CD8 T cells (reviewed by Rizza et al., 2011), suggests possible novel combination therapies in patients bearing metastatic lesions. In particular, the recent discovery of the stimulating activity of chemotherapy on DC maturation/activation and of immunogenic apoptosis of tumor cells permits to envisage a possible association of chemotherapy with direct intratumoral injection of autologous DC, to achieve a potent systemic antitumor immune response.
In conclusion, the ensemble of the new findings in the field of immune‐adjuvant chemotherapy supports the following two main types of therapeutic approaches of combined anticancer immunotherapy:
immunotherapy of patients with established tumors by using chemotherapy just before ACT of tumor‐immune lymphocytes, which represents the extension of the studies originally performed by Rosenberg's group in patients with advanced melanoma;
immunotherapy based on the use of chemotherapy just before vaccination with defined tumor antigens, including DC‐vaccines exhibiting some potential advantage for inducing a prolonged antitumor immune response. (This approach could results in a major clinical impact in patients with minimal residual disease).
Both approaches can selectively exploit the recently described effects of chemotherapy, including its propulsive role in enhancing the antitumor immune response to immunotherapy.
Acknowledgments
Most of the researches by the authors were supported by grants from the Italian Association for Research against Cancer (AIRC), from the Italian Ministry of Health (Integrated Project on Oncology) and from “ISS Project for Alliance against Cancer”.
We are grateful to Ms. Rosina Bellizzi and Annamaria Fattapposta for helpful secretarial support and to Ms. Annamaria De Conteris for the graphical assistance.
Proietti Enrico, Moschella Federica, Capone Imerio, Belardelli Filippo, (2012), Exploitation of the propulsive force of chemotherapy for improving the response to cancer immunotherapy, Molecular Oncology, 6, doi: 10.1016/j.molonc.2011.11.005.
Contributor Information
Enrico Proietti, Email: enrico.proietti@iss.it.
Federica Moschella, Email: federica.moschella@iss.it.
Imerio Capone, Email: imerio.capone@iss.it.
Filippo Belardelli, Email: filippo.belardelli@iss.it.
References
- Adair, S.J. , Hogan, K.T. , 2009. Treatment of ovarian cancer cell lines with 5-aza-2'-deoxycytidine upregulates the expression of cancer-testis antigens and class I major histocompatibility complex-encoded molecules. Cancer Immunol. Immunother.. 58, 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh, L. , Ghiringhelli, F. , Tesniere, A. , Criollo, A. , Ortiz, C. , Lidereau, R. , 2007. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol. Rev.. 220, 47–59. [DOI] [PubMed] [Google Scholar]
- Apetoh, L. , Ghiringhelli, F. , Tesniere, A. , Obeid, M. , Ortiz, C. , Criollo, A. , 2007. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med.. 13, 1050–1059. [DOI] [PubMed] [Google Scholar]
- Asavaroengchai, W. , Kotera, Y. , Mule, J.J. , 2002. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc. Natl. Acad. Sci. U S A. 99, 931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenblum, I. , 1929. The modifying influence of dychloroethyl sulphide on the induction of tumours in mice by tar. J. Pathol. Bacteriol.. 32, 424–434. [Google Scholar]
- Berendt, M.J. , North, R.J. , 1980. T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J. Exp. Med.. 151, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biragyn, A. , Ruffini, P.A. , Leifer, C.A. , Klyushnenkova, E. , Shakhov, A. , Chertov, O. , 2002. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 298, 1025–1029. [DOI] [PubMed] [Google Scholar]
- Bracci, L. , Moschella, F. , Sestili, P. , La Sorsa, V. , Valentini, M. , Canini, I. , 2007. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin. Cancer Res.. 13, 644–653. [DOI] [PubMed] [Google Scholar]
- Braun, D.P. , Harris, J.E. , 1981. Effects of combination chemotherapy on immunoregulatory cells in peripheral blood of solid tumor cancer patients: correlation with rebound overshoot immune function recovery. Clin. Immunol. Immunopathol.. 20, 193–214. [DOI] [PubMed] [Google Scholar]
- Brentjens, R.J. , Riviere, I. , Park, J.H. , Davila, M.L. , Wang, X. , Stefanski, J. , 2011. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese, E.J. , 2005. Hormetic dose-response relationships in immunology: occurrence, quantitative features of the dose response, mechanistic foundations, and clinical implications. Crit. Rev. Toxicol.. 35, 89–295. [DOI] [PubMed] [Google Scholar]
- Calabrese, E.J. , Bachmann, K.A. , Bailer, A.J. , Bolger, P.M. , Borak, J. , Cai, L. , 2007. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol.. 222, 122–128. [DOI] [PubMed] [Google Scholar]
- Casares, N. , Pequignot, M.O. , Tesniere, A. , Ghiringhelli, F. , Roux, S. , Chaput, N. , 2005. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med.. 202, 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever, M.A. , Greenberg, P.D. , Fefer, A. , 1980. Specificity of adoptive chemoimmunotherapy of established syngeneic tumors. J. Immunol.. 125, 711–714. [PubMed] [Google Scholar]
- Chopra, A. , Kim, T.S. , InSug, O.S. , Martinez, D. , Cohen, E.P. , 2006. Combined therapy of an established, highly aggressive breast cancer in mice with paclitaxel and a unique DNA-based cell vaccine. Int. J. Cancer. 118, 2888–2898. [DOI] [PubMed] [Google Scholar]
- Chu, Y. , Wang, L.X. , Yang, G. , Ross, H.J. , Urba, W.J. , Prell, R. , 2006. Efficacy of GM-CSF-producing tumor vaccine after docetaxel chemotherapy in mice bearing established Lewis lung carcinoma. J. Immunother.. 29, 367–380. [DOI] [PubMed] [Google Scholar]
- Coral, S. , Sigalotti, L. , Altomonte, M. , Engelsberg, A. , Colizzi, F. , Cattarossi, I. , 2002. 5-Aza-2'-deoxycytidine-induced expression of functional cancer testis antigens in human renal cell carcinoma: immunotherapeutic implications. Clin. Cancer Res.. 8, 2690–2695. [PubMed] [Google Scholar]
- Correale, P. , Botta, C. , Cusi, M. , Del Vecchio, M. , De Santi, M. , Gori Savellini, G. , 2011. Cetuximab+/- chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int. J. Cancer. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Dempke, W. , Von Poblozki, A. , Grothey, A. , Schmoll, H.J. , 2000. Human hematopoietic growth factors: old lessons and new perspectives. Anticancer Res.. 20, 5155–5164. [PubMed] [Google Scholar]
- Diaz-Montero, C.M. , Salem, M.L. , Nishimura, M.I. , Garrett-Mayer, E. , Cole, D.J. , Montero, A.J. , 2009. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother.. 58, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Z.C. , Blazar, B.R. , Mellor, A.L. , Munn, D.H. , Zhou, G. , 2010. Chemotherapy rescues tumor-driven aberrant CD4+ T-cell differentiation and restores an activated polyfunctional helper phenotype. Blood. 115, 2397–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer, W. , Niethammer, A.G. , Baccala, R. , Lawson, B.R. , Wagner, N. , Reisfeld, R.A. , 2002. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J. Clin. Invest.. 110, 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens, L.A. , 2010. Chemoimmunotherapy. Cancer J.. 16, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercolini, A.M. , Ladle, B.H. , Manning, E.A. , Pfannenstiel, L.W. , Armstrong, T.D. , Machiels, J.P. , 2005. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J. Exp. Med.. 201, 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers, M.A. , Offner, S. , Blanco-Bose, W.E. , Waibler, Z. , Kalinke, U. , Duchosal, M.A. , 2009. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 458, 904–908. [DOI] [PubMed] [Google Scholar]
- Fadul, C.E. , Fisher, J.L. , Hampton, T.H. , Lallana, E.C. , Li, Z. , Gui, J. , 2011. Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy. J. Immunother.. 34, 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonsatti, E. , Nicolay, H.J. , Sigalotti, L. , Calabro, L. , Pezzani, L. , Colizzi, F. , 2007. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2'-deoxycytidine in cutaneous melanoma: immunotherapeutic implications. Clin. Cancer Res.. 13, 3333–3338. [DOI] [PubMed] [Google Scholar]
- Freitas, A.A. , Agenes, F. , Coutinho, G.C. , 1996. Cellular competition modulates survival and selection of CD8+ T cells. Eur. J. Immunol.. 26, 2640–2649. [DOI] [PubMed] [Google Scholar]
- Gabrilovich, D.I. , Nagaraj, S. , 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol.. 9, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni, L. , Finkelstein, S.E. , Klebanoff, C.A. , Antony, P.A. , Palmer, D.C. , Spiess, P.J. , 2005. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med.. 202, 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni, L. , Klebanoff, C.A. , Palmer, D.C. , Wrzesinski, C. , Kerstann, K. , Yu, Z. , 2005. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest.. 115, 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli, F. , Larmonier, N. , Schmitt, E. , Parcellier, A. , Cathelin, D. , Garrido, C. , 2004. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol.. 34, 336–344. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli, F. , Menard, C. , Martin, F. , Zitvogel, L. , 2006. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol. Rev.. 214, 229–238. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli, F. , Menard, C. , Puig, P.E. , Ladoire, S. , Roux, S. , Martin, F. , 2007. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother.. 56, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman, A. , Philips, F.S. , 1946. The biological actions and therapeutic applications of the B-chloroethyl amines and sulfides. Science. 103, 409–415. [PubMed] [Google Scholar]
- Greenberg, P.D. , Cheever, M.A. , Fefer, A. , 1980. Detection of early and delayed antitumor effects following curative adoptive chemoimmunotherapy of established leukemia. Cancer Res.. 40, 4428–4432. [PubMed] [Google Scholar]
- Greenberg, P.D. , Kern, D.E. , Cheever, M.A. , 1985. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+,2- T cells. Tumor eradication does not require participation of cytotoxic T cells. J. Exp. Med.. 161, 1122–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis, S.I. , den Dunnen, J. , Litjens, M. , van der Vlist, M. , Wevers, B. , Bruijns, S.C. , 2009. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat. Immunol.. 10, 203–213. [DOI] [PubMed] [Google Scholar]
- Jonuleit, H. , Schmitt, E. , Stassen, M. , Tuettenberg, A. , Knop, J. , Enk, A.H. , 2001. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med.. 193, 1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneno, R. , Shurin, G.V. , Tourkova, I.L. , Shurin, M.R. , 2009. Chemomodulation of human dendritic cell function by antineoplastic agents in low noncytotoxic concentrations. J. Transl. Med.. 7, 58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprowicz, D.J. , Droin, N. , Soper, D.M. , Ramsdell, F. , Green, D.R. , Ziegler, S.F. , 2005. Dynamic regulation of FoxP3 expression controls the balance between CD4+ T cell activation and cell death. Eur. J. Immunol.. 35, 3424–3432. [DOI] [PubMed] [Google Scholar]
- Klebanoff, C.A. , Gattinoni, L. , Palmer, D.C. , Muranski, P. , Ji, Y. , Hinrichs, C.S. , 2011. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin. Cancer Res.. 17, 5343–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky, E. , Flamar, A.L. , Cao, Y. , Blanck, J.P. , Liu, M. , O'Bar, A. , 2010. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 116, 1685–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, H.K. , Graham, L. , Cha, E. , Morales, J.K. , Manjili, M.H. , Bear, H.D. , 2009. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int. Immunopharmacol.. 9, 900–909. [DOI] [PubMed] [Google Scholar]
- Lutsiak, M.E. , Semnani, R.T. , De Pascalis, R. , Kashmiri, S.V. , Schlom, J. , Sabzevari, H. , 2005. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 105, 2862–2868. [DOI] [PubMed] [Google Scholar]
- Machiels, J.P. , Reilly, R.T. , Emens, L.A. , Ercolini, A.M. , Lei, R.Y. , Weintraub, D. , 2001. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res.. 61, 3689–3697. [PubMed] [Google Scholar]
- Mackall, C.L. , Hakim, F.T. , Gress, R.E. , 1997. Restoration of T-cell homeostasis after T-cell depletion. Semin. Immunol.. 9, 339–346. [DOI] [PubMed] [Google Scholar]
- Maguire, H.C. , Ettore, V.L. , 1967. Enhancement of dinitrochlorobenzene (DNCB) contact sensitization by cyclophosphamide in the guinea pig. J. Invest. Dermatol.. 48, 39–43. [DOI] [PubMed] [Google Scholar]
- Maine, G.N. , Mule, J.J. , 2002. Making room for T cells. J. Clin. Invest.. 110, 157–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvicini, M. , Ingolotti, M. , Piccioni, F. , Garcia, M. , Bayo, J. , Atorrasagasti, C. , 2011. Reversal of gastrointestinal carcinoma-induced immunosuppression and induction of antitumoural immunity by a combination of cyclophosphamide and gene transfer of IL-12. Mol. Oncol.. 5, 242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Orozco, N. , Muranski, P. , Chung, Y. , Yang, X.O. , Yamazaki, T. , Lu, S. , 2009. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 31, 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matar, P. , Rozados, V.R. , Gervasoni, S.I. , Scharovsky, G.O. , 2002. Th2/Th1 switch induced by a single low dose of cyclophosphamide in a rat metastatic lymphoma model. Cancer Immunol. Immunother.. 50, 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerveld-Eggink, A. , de Weerdt, O. , van der Velden, A.M. , Los, M. , van der Velden, A.W. , Stouthard, J.M. , 2011. Response to influenza virus vaccination during chemotherapy in patients with breast cancer. Ann. Oncol.. 22, 2031–2035. [DOI] [PubMed] [Google Scholar]
- Montero, E. , Valdes, M. , Avellanet, J. , Lopez, A. , Perez, R. , Lage, A. , 2009. Chemotherapy induced transient B-cell depletion boosts antibody-forming cells expansion driven by an epidermal growth factor-based cancer vaccine. Vaccine. 27, 2230–2239. [DOI] [PubMed] [Google Scholar]
- Moschella, F. , Proietti, E. , Capone, I. , Belardelli, F. , 2010. Combination strategies for enhancing the efficacy of immunotherapy in cancer patients. Ann. N. Y. Acad. Sci.. 1194, 169–178. [DOI] [PubMed] [Google Scholar]
- Moschella, F. , Valentini, M. , Arico, E. , Macchia, I. , Sestili, P. , D'Urso, M.T. , 2011. Unraveling cancer chemoimmunotherapy mechanisms by gene and protein expression profiling of responses to cyclophosphamide. Cancer Res.. 71, 3528–3539. [DOI] [PubMed] [Google Scholar]
- Nakahara, T. , Uchi, H. , Lesokhin, A.M. , Avogadri, F. , Rizzuto, G.A. , Hirschhorn-Cymerman, D. , 2010. Cyclophosphamide enhances immunity by modulating the balance of dendritic cell subsets in lymphoid organs. Blood. 115, 4384–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsume, A. , Wakabayashi, T. , Tsujimura, K. , Shimato, S. , Ito, M. , Kuzushima, K. , 2008. The DNA demethylating agent 5-aza-2'-deoxycytidine activates NY-ESO-1 antigenicity in orthotopic human glioma. Int. J. Cancer. 122, 2542–2553. [DOI] [PubMed] [Google Scholar]
- Nauta, A.J. , Raaschou-Jensen, N. , Roos, A. , Daha, M.R. , Madsen, H.O. , Borrias-Essers, M.C. , 2003. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur. J. Immunol.. 33, 2853–2863. [DOI] [PubMed] [Google Scholar]
- Nistico, P. , Capone, I. , Palermo, B. , Del Bello, D. , Ferraresi, V. , Moschella, F. , 2009. Chemotherapy enhances vaccine-induced antitumor immunity in melanoma patients. Int. J. Cancer. 124, 130–139. [DOI] [PubMed] [Google Scholar]
- Nizar, S. , Copier, J. , Meyer, B. , Bodman-Smith, M. , Galustian, C. , Kumar, D. , 2009. T-regulatory cell modulation: the future of cancer immunotherapy?. Br. J. Cancer. 100, 1697–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North, R.J. , 1982. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J. Exp. Med.. 155, 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid, M. , Panaretakis, T. , Joza, N. , Tufi, R. , Tesniere, A. , van Endert, P. , 2007. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ.. 14, 1848–1850. [DOI] [PubMed] [Google Scholar]
- Obeid, M. , Tesniere, A. , Ghiringhelli, F. , Fimia, G.M. , Apetoh, L. , Perfettini, J.L. , 2007. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med.. 13, 54–61. [DOI] [PubMed] [Google Scholar]
- Ogden, C.A. , deCathelineau, A. , Hoffmann, P.R. , Bratton, D. , Ghebrehiwet, B. , Fadok, V.A. , 2001. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med.. 194, 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo, B. , Del Bello, D. , Sottini, A. , Serana, F. , Ghidini, C. , Gualtieri, N. , 2010. Dacarbazine treatment before peptide vaccination enlarges T-cell repertoire diversity of melan-a-specific, tumor-reactive CTL in melanoma patients. Cancer Res.. 70, 7084–7092. [DOI] [PubMed] [Google Scholar]
- Pfannenstiel, L.W. , Lam, S.S. , Emens, L.A. , Jaffee, E.M. , Armstrong, T.D. , 2010. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol.. 263, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, D.J. , de Vries, C.R. , Allen, T. , Ahmadzadeh, M. , Rosenberg, S.A. , 2007. Inability to mediate prolonged reduction of regulatory T Cells after transfer of autologous CD25-depleted PBMC and interleukin-2 after lymphodepleting chemotherapy. J. Immunother.. 30, 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti, E. , Greco, G. , Garrone, B. , Baccarini, S. , Mauri, C. , Venditti, M. , 1998. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J. Clin. Invest.. 101, 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti, E. , Tritarelli, E. , Gabriele, L. , Testa, U. , Greco, G. , Pelosi, E. , 1993. Combined interleukin 1 beta/interleukin 2 treatment in mice: synergistic myelostimulatory activity and protection against cyclophosphamide-induced myelosuppression. Cancer Res.. 53, 569–576. [PubMed] [Google Scholar]
- Ramakrishnan, R. , Assudani, D. , Nagaraj, S. , Hunter, T. , Cho, H.I. , Antonia, S. , 2010. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J. Clin. Invest.. 120, 1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza, P. , Capone, I. , Moretti, F. , Proietti, E. , Belardelli, F. , 2011. IFN-alpha as a vaccine adjuvant: recent insights into the mechanisms and perspectives for its clinical use. Expert Rev. Vaccines. 10, 487–498. [DOI] [PubMed] [Google Scholar]
- Robinson, M.J. , Osorio, F. , Rosas, M. , Freitas, R.P. , Schweighoffer, E. , Gross, O. , 2009. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med.. 206, 2037–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, S.A. , Spiess, P. , Lafreniere, R. , 1986. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 233, 1318–1321. [DOI] [PubMed] [Google Scholar]
- Rosenberg, S.A. , Yang, J.C. , Sherry, R.M. , Kammula, U.S. , Hughes, M.S. , Phan, G.Q. , 2011. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res.. 17, 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux, S. , Apetoh, L. , Chalmin, F. , Ladoire, S. , Mignot, G. , Puig, P.E. , 2008. CD4+CD25+ Tregs control the TRAIL-dependent cytotoxicity of tumor-infiltrating DCs in rodent models of colon cancer. J. Clin. Invest.. 118, 3751–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi, S. , Sakaguchi, N. , Asano, M. , Itoh, M. , Toda, M. , 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol.. 155, 1151–1164. [PubMed] [Google Scholar]
- Salem, M.L. , Al-Khami, A.A. , El-Naggar, S.A. , Diaz-Montero, C.M. , Chen, Y. , Cole, D.J. , 2010. Cyclophosphamide induces dynamic alterations in the host microenvironments resulting in a Flt3 ligand-dependent expansion of dendritic cells. J. Immunol.. 184, 1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem, M.L. , Diaz-Montero, C.M. , Al-Khami, A.A. , El-Naggar, S.A. , Naga, O. , Montero, A.J. , 2009. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I: C). J. Immunol.. 182, 2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem, M.L. , Kadima, A.N. , El-Naggar, S.A. , Rubinstein, M.P. , Chen, Y. , Gillanders, W.E. , 2007. Defining the ability of cyclophosphamide preconditioning to enhance the antigen-specific CD8+ T-cell response to peptide vaccination: creation of a beneficial host microenvironment involving type I IFNs and myeloid cells. J. Immunother.. 30, 40–53. [DOI] [PubMed] [Google Scholar]
- Sampson, J.H. , Aldape, K.D. , Archer, G.E. , Coan, A. , Desjardins, A. , Friedman, A.H. , 2011. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol.. 13, 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavoni, G. , Mattei, F. , Di Pucchio, T. , Santini, S.M. , Bracci, L. , Belardelli, F. , 2000. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 95, 2024–2030. [PubMed] [Google Scholar]
- Schiavoni, G. , Sistigu, A. , Valentini, M. , Mattei, F. , Sestili, P. , Spadaro, F. , 2011. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res.. 71, 768–778. [DOI] [PubMed] [Google Scholar]
- Schluns, K.S. , Kieper, W.C. , Jameson, S.C. , Lefrancois, L. , 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol.. 1, 426–432. [DOI] [PubMed] [Google Scholar]
- Seddiki, N. , Santner-Nanan, B. , Martinson, J. , Zaunders, J. , Sasson, S. , Landay, A. , 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med.. 203, 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfanos, K.S. , Bruno, T.C. , Maris, C.H. , Xu, L. , Thoburn, C.J. , DeMarzo, A.M. , 2008. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin. Cancer Res.. 14, 3254–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin, G.V. , Tourkova, I.L. , Kaneno, R. , Shurin, M.R. , 2009. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J. Immunol.. 183, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistigu, A. , Viaud, S. , Chaput, N. , Bracci, L. , Proietti, E. , Zitvogel, L. , 2011. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin. Immunopathol.. 33, 369–383. [DOI] [PubMed] [Google Scholar]
- Sojka, D.K. , Donepudi, M. , Bluestone, J.A. , Mokyr, M.B. , 2000. Melphalan and other anticancer modalities up-regulate B7-1 gene expression in tumor cells. J. Immunol.. 164, 6230–6236. [DOI] [PubMed] [Google Scholar]
- Stephens, L.A. , Mason, D. , 2000. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25- subpopulations. J. Immunol.. 165, 3105–3110. [DOI] [PubMed] [Google Scholar]
- Terme, M. , Chaput, N. , Combadiere, B. , Ma, A. , Ohteki, T. , Zitvogel, L. , 2008. Regulatory T cells control dendritic cell/NK cell cross-talk in lymph nodes at the steady state by inhibiting CD4+ self-reactive T cells. J. Immunol.. 180, 4679–4686. [DOI] [PubMed] [Google Scholar]
- Tesniere, A. , Schlemmer, F. , Boige, V. , Kepp, O. , Martins, I. , Ghiringhelli, F. , 2010. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 29, 482–491. [DOI] [PubMed] [Google Scholar]
- Torelli, G.F. , Natalino, F. , Barberi, W. , Maggio, R. , Peragine, N. , De Propris, M.S. , 2010. Clinical responses in allografted acute leukaemia patients with resistant disease using a combined chemo-immunotherapeutic treatment strategy. Br. J. Haematol.. 151, 86–89. [DOI] [PubMed] [Google Scholar]
- Tseng, C.W. , Hung, C.F. , Alvarez, R.D. , Trimble, C. , Huh, W.K. , Kim, D. , 2008. Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin. Cancer Res.. 14, 3185–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi, S. , Padarathsingh, M. , Bonmassar, E. , Goldin, A. , 1971. Effect of combination treatment with cyclophosphamide and isogeneic or allogeneic spleen and bone marrow cells in leukemic (L1210) mice. Int. J. Cancer. 7, 160–166. [DOI] [PubMed] [Google Scholar]
- van der Most, R.G. , Currie, A.J. , Cleaver, A.L. , Salmons, J. , Nowak, A.K. , Mahendran, S. , 2009. Cyclophosphamide chemotherapy sensitizes tumor cells to TRAIL-dependent CD8 T cell-mediated immune attack resulting in suppression of tumor growth. PLoS One. 4, e6982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecque, R. , Saudemont, A. , Quesnel, B. , 2004. Cytosine arabinoside induces costimulatory molecule expression in acute myeloid leukemia cells. Leukemia. 18, 1223–1230. [DOI] [PubMed] [Google Scholar]
- Viaud, S. , Flament, C. , Zoubir, M. , Pautier, P. , Lecesne, A. , Ribrag, V. , 2011. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res.. 71, 661–665. [DOI] [PubMed] [Google Scholar]
- Vierboom, M.P. , Bos, G.M. , Ooms, M. , Offringa, R. , Melief, C.J. , 2000. Cyclophosphamide enhances anti-tumor effect of wild-type p53-specific CTL. Int. J. Cancer. 87, 253–260. [DOI] [PubMed] [Google Scholar]
- Vincent, J. , Mignot, G. , Chalmin, F. , Ladoire, S. , Bruchard, M. , Chevriaux, A. , 2010. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res.. 70, 3052–3061. [DOI] [PubMed] [Google Scholar]
- Weck, M.M. , Appel, S. , Werth, D. , Sinzger, C. , Bringmann, A. , Grunebach, F. , 2008. hDectin-1 is involved in uptake and cross-presentation of cellular antigens. Blood. 111, 4264–4272. [DOI] [PubMed] [Google Scholar]
- Wu, Z. , Bensinger, S.J. , Zhang, J. , Chen, C. , Yuan, X. , Huang, X. , 2004. Homeostatic proliferation is a barrier to transplantation tolerance. Nat. Med.. 10, 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S. , Haluska, F.G. , 2004. Treatment of melanoma with 5-fluorouracil or dacarbazine in vitro sensitizes cells to antigen-specific CTL lysis through perforin/granzyme- and Fas-mediated pathways. J. Immunol.. 172, 4599–4608. [DOI] [PubMed] [Google Scholar]
- Ye, Z.J. , Zhou, Q. , Gu, Y.Y. , Qin, S.M. , Ma, W.L. , Xin, J.B. , 2010. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J. Immunol.. 185, 6348–6354. [DOI] [PubMed] [Google Scholar]
- Zaragoza, B. , Evaristo, C. , Kissenpfennig, A. , Libri, V. , Malissen, B. , Rocha, B. , 2011. Cell-to-cell interactions and signals involved in the reconstitution of peripheral CD8 T(CM) and T(EM) cell pools. PLoS One. 6, e17423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, R. , Spolski, R. , Finkelstein, S.E. , Oh, S. , Kovanen, P.E. , Hinrichs, C.S. , 2005. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med.. 201, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Sun, S. , Hwang, I. , Tough, D.F. , Sprent, J. , 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 8, 591–599. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.L. , Li, J. , Mo, H.Y. , Qiu, F. , Zheng, L.M. , Qian, C.N. , 2010. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol. Cancer. 9, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, H. , Han, B. , Tourkova, I.L. , Lokshin, A. , Rosenbloom, A. , Shurin, M.R. , 2007. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin. Cancer Res.. 13, 5455–5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, W. , 2006. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol.. 6, 295–307. [DOI] [PubMed] [Google Scholar]


