Abstract
Lysophosphatidic acid (LPA) augments proliferation and metastasis of various cancer cells. We recently identified a critical role of the Rho/ROCK pathway for LPA‐induced proteolytic enzyme expression and cancer cell progression. In the present study, we elucidate the underlying mechanisms by which LPA induces Rho activation and subsequent cellular invasion, and the reversal of these effects by resveratrol. We observed that both Gi and G13 contribute to LPA‐induced EGFR activation. The activated EGFR in turn initiates a Ras/Rho/ROCK signaling cascade, leading to proteolytic enzyme secretion. Further we provide evidence that resveratrol inhibits EGFR phosphorylation and subsequent activation of a Ras/Rho/ROCK signaling. Therefore, we demonstrate a mechanistic cascade of LPA activating EGFR through Gi and G13 thus inducing a Ras/Rho/ROCK signaling for proteolytic enzyme expression and ovarian cancer cell invasion, as well as interference of the cascade by resveratrol through blocking EGFR phosphorylation.
Keywords: Lysophosphatidic acid, EGFR, Ovarian cancer, Resveratrol, Invasion
Highlights
-
►
LPA‐induced EGFR transactivation and its inhibition for ovarian cancer progression.
-
►
EGFR aggravates ovarian cancer progression through proteolytic enzyme secretion.
-
►
A Gi and G13/EGFR/Ras/Rho/ROCK signaling cascade for proteolytic enzyme secretion.
-
►
Reseveratrol inhibits EGFR activation and ovarian cancer invasion.
-
►
Resveratrol increases the therapeutic potential of an EGFR inhibitor.
1. Introduction
Ovarian cancer is the fifth cause of cancer‐related death in women in North America and the leading cause of death from gynecological malignancies (Ozols et al., 2004; Siegel et al., 2012). Like many other types of cancer, the main reasons for the low survival rate for ovarian cancer patient are the lack of effective early detection and treatment, as well as the highly metastatic nature of disease (Gardner et al., 1995). Ovarian cancer disseminates throughout the peritoneal cavity with the main site of ovarian cancer metastasis being the omentum (Nieman et al., 2011). Lysophosphatidic acid (1‐ or 2‐acyl‐lysophosphatidic acid, LPA) is a biolipid mediator that is involved in various pathophysiological events, including ovarian cancer invasion and metastasis (Mills and Moolenaar, 2003). In ovarian cancer, LPA contributes to tumor development, progression, and metastasis and is increased in both plasma and ascites of ovarian patients compared to patients with benign ovarian masses and healthy subjects (Bese et al., 2010; Xu et al., 1998). Ovarian cancer cells also produce LPA, thereby maintaining a LPA‐rich microenvironment (Fang et al., 2002). LPA binds to a series of high‐affinity cell‐surface G protein‐coupled receptors (GPCRs). There are at least six GPCRs currently identified as LPA receptors, designated LPA1‐6 (Noguchi et al., 2009). Other putative GPCRs that seem to bind and respond to LPA also have been identified (Tabata et al., 2007; Murakami et al., 2008).
Resveratrol (3,4,5′‐trihydroxystilbene) is a naturally occurring phytoalexin produced by a wide variety of plants in response to stress, injury and fungal infection. Resveratrol has been shown to suppress diverse cellular events associated with cancer growth and progression (Jang et al., 1997). This phytoalexin compound has been shown to suppress LPA‐induced ovarian cancer cell migration through blocking the induction of HIF‐1α and VEGF (Park et al., 2007) by unknown mechanisms. The EGFR plays an oncogenic role in a number of human cancers, including ovarian cancer (Sheng and Liu, 2011). Recent studies demonstrate that the EGFR serves as an important mediator for LPA‐induced ovarian cancer cell migration and invasiveness. EGFR phosphorylation is required for LPA‐induce cyclooxygenase‐2 (COX‐2) expression to stimulate ovarian cancer cell migration (Jeong et al., 2008). In addition, EGF was shown to increase production of LPA, leading to aggravated ovarian cancer cell progression (Snider et al., 2010).
Recently, we uncovered an important role for RhoA in LPA‐induced proteolytic enzyme matrix metalloproteinase (MMP)‐9 and urokinase plasminogen activator (uPA) expression and ovarian cancer cell invasion (Jeong et al., 2012). This small GTPase is upregulated in metastatic peritoneal lesions compared to primary tumors of advanced ovarian carcinoma (Dolo et al., 1995) and plays an important role in the peritoneal dissemination of ovarian cancer cells (Horiuchi et al., 2008). To extend the knowledge on the molecular and cellular basis of LPA‐induced Rho activation and proteolytic enzyme expression, we demonstrate a critical role for LPA‐induced EGFR phosphorylation in activation of the Ras/Rho/ROCK signaling cascade. Resveratrol impinges on this cascade at the level of EGFR phosphorylation leading to reduction of proteolytic enzyme secretion and ovarian cancer cell invasion.
2. Materials and methods
2.1. Reagents
LPA was purchased from Avanti Polar Lipids (Alabaster, AL). Gefitinib was obtained from Selleck (Houston, TX). Pertussis Toxin (PTX) and resveratrol were acquired from Sigma (St. Louis, MO). All other reagents used were of the purest grade available.
2.2. Cell culture
Ovarian cancer cell SKOV‐3 and PA‐1 were purchased from American Type Culture Collection (Manassas, VA). SKOV‐3 cells were maintained in RPMI 1640 supplemented with 5% fetal bovine serum and 1% penicillin/streptomycin. PA‐1 cells were maintained in EMEM supplemented with 5% fetal bovine serum in a humidified atmosphere containing 5% CO2 at 37 °C.
2.3. siRNA transfection
G12 siRNA‐1 and G13 siRNA‐1 were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). G12 siRNA‐2 and G13 siRNA‐2 were purchased from Sigma (St. Louis, MO). Negative control siRNA were obtained from Invitrogen (Carlsbad, CA). Cells were transfected with siRNA by utilizing Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
2.4. RT‐PCR quantification
Isolation of total cellular RNA and reaction were performed as described previously (Park et al., 2011). Complementary DNA was amplified using an iQ5 Real‐Time PCR Detection System (Bio‐Rad Laboratories, Hercules, CA) with the following primer sets: MMP‐9, 5′‐GTGCCATGTAAATCCCCACT‐3′ (forward) and 5′‐CTCCACTCCTCCCTTTCCTC‐3′ (reverse); uPA, 5′‐GTGGCCAAAAGACTCTGAGG‐3′ (forward) and 5′‐GCCGTACATGAAGCAGTGTG‐3′ (reverse); and GAPDH, 5′‐ACAGTCAGCCGCATCTTCTT‐3′ (forward) and 5′‐ACGACCAAATCCGTTGACTC‐3′ (reverse).
2.5. Immunoblotting & immunoprecipitation
The cell lysates were prepared as described (Lee et al., 2006). Antibodies for EGFR, G12, G13 and GAPDH were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti‐Rho antibody was from Upstate Biotechnology (Lake Placid, NY). 200 μg of proteins in extraction buffer were incubated with 4 μg/ml anti‐EGFR antibody. The immunocomplex was precipitated with protein A Sepharose CL‐4B beads (Amersham Biosciences, Piscataway) overnight at 4 °C. The beads were washed with PBS containing Tween 20, resuspended in SDS buffer, and boiled for 5 min. The protein samples were then immunoblotted with anti‐p‐Tyr antibody. The immunoreactive bands were visualized with ECL (Thermo Fisher Scientific Inc., Rockford, IL) under ImageQuant 300 (GE Healthcare, Buckinghamshire, UK).
2.6. In vitro invasion assay
In vitro invasion assays were performed in triplicates using a 48‐well microchemotaxis chamber as described previously (Jeong et al., 2012). The values obtained were calculated using the number of invaded cells from three filters.
2.7. Rho activation assay
To assess Rho activation, the amount of RhoA‐GTP bound to the Rhotekin RBD was determined using the Rho Activation Assay Kit (Millipore, Temecula, CA) according to the manufacturer's instructions as described previously (Jeong et al., 2012).
2.8. Ras activation assay
The analysis of Ras activity was determined using Ras activation ELISA assay kit (Millipore, Temecula, CA) according to the manufacturer's instructions. Briefly, cell lysates were prepared with Mg2+Lysis/Wash buffer and calculated protein concentration with BCA protein assay. Glutathione‐coated wells were incubated with Raf‐1‐RBD, GST for 1 h at 4 °C. 50 μg of cell lysate was added to Raf‐1‐RBD‐coated wells and incubated for 1 h at room temperature (RT) with mild agitation, followed by incubation with Ras detection antibody and HRP antibody for 1 h, respectively. After treating with 50 μl of the chemiluminescent substrate, a luminometer was used to read results.
2.9. Statistics
Data are shown as means ± s.d. Differences between two groups were assessed using the Student's t‐test. Differences among three or more groups were evaluated by analysis of variance, followed by Bonferroni multiple comparison tests.
3. Results
3.1. EGFR is important for ovarian cancer cell invasion
Given that the EGFR is implicated in LPA‐induced gastric cancer cell motility and invasion (Shida et al., 2008) and that LPA stimulates MMP‐9 expression in PA‐1 and uPA expression in SKOV‐3 cells (Jeong et al., 2012), we first determined whether the EGFR governs LPA‐induced proteolytic enzyme secretion and invasion of these ovarian cancer cells. We observed that pretreatment with a selective EGFR inhibitor gefitinib significantly inhibited LPA‐induced MMP‐9 and uPA expression from PA‐1 (Figure 1A) and SKOV‐3 (Figure 1B) ovarian cancer cells. More importantly, this EGFR inhibitor also profoundly reduced LPA‐induced ovarian cancer invasiveness (Figure 1C and D). Therefore, these data strongly suggest that LPA‐induced EGFR activation is important for proteolytic enzyme secretion and ovarian cancer cell progression.
Figure 1.
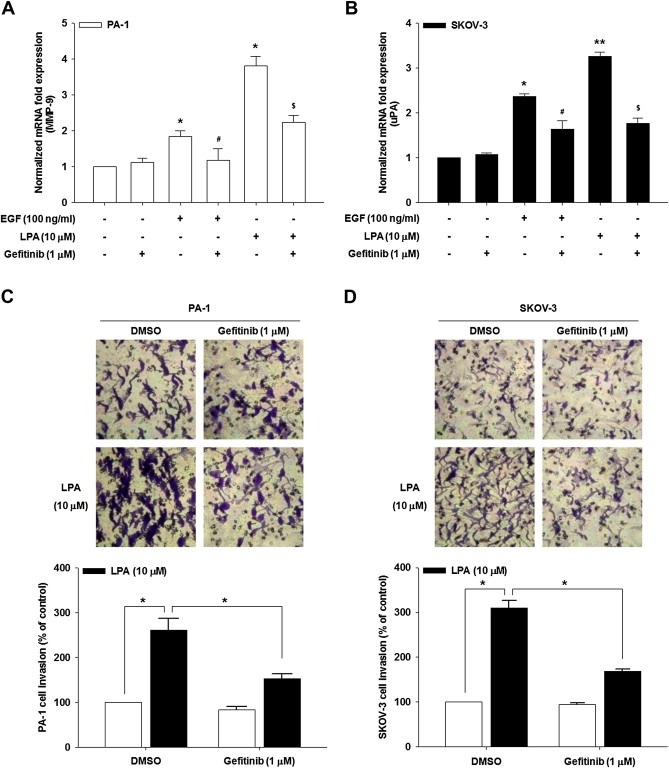
EGFR is important for proteolytic enzyme expression and ovarian cancer invasion. The serum‐starved PA‐1 (A) and SKOV‐3 (B) cells were pretreated with gefitinib for 1 h and then stimulated with LPA for 24 h. mRNA expression of MMP‐9 and uPA by quantitative RT‐PCR (Error bars, ± s.d. ∗p < 0.01 and ∗∗p < 0.001 vs control, #p < 0.05 vs EGF treatment only, $p < 0.05 vs LPA treatment only). The serum‐starved PA‐1 (C) and SKOV‐3 (D) cells were pretreated with gefitinib for 1 h and in vitro invasion was analyzed against LPA (Error bars, ± s.d. ∗p < 0.01). All experiments were repeated three times.
3.2. Gi and G13 are required for EGFR activation
Since LPA binds to GPCRs and various G proteins are implicated in LPA‐induced pathophysiological events (Oyesanya et al., 2010), we next determined which G protein is involved in LPA‐induced EGFR activation. Pretreatment of cells with a Gi selective inhibitor PTX profoundly inhibited LPA‐induced EGFR phosphorylation (Figure 2A). In contrast, PTX showed little effect on EGF‐induced EGFR‐phosphorylation. Further, PTX did not show any inhibitory effect on LPA‐induced activation of PKCδ that is located downstream of Gq (Sriwai et al., 2008) (Supplementary data S1). Next, we utilized siRNAs for G12 and G13 to determine which G protein is implicated in LPA‐induced EGFR activation. siRNA for each G protein specifically reduced corresponding G protein expression without any detectable effect on the other G protein (Figure 2C and Supplementary data S2B). Transfection of G13‐specific siRNA efficiently attenuated LPA‐induced EGFR phosphorylation (Figure 2B and Supplementary data S2A). Conversely, silencing G12 did not show any detectable effect on LPA‐induced EGFR activation. Therefore, these data strongly suggest that both Gi and G13 are required for LPA‐induced EGFR phosphorylation.
Figure 2.
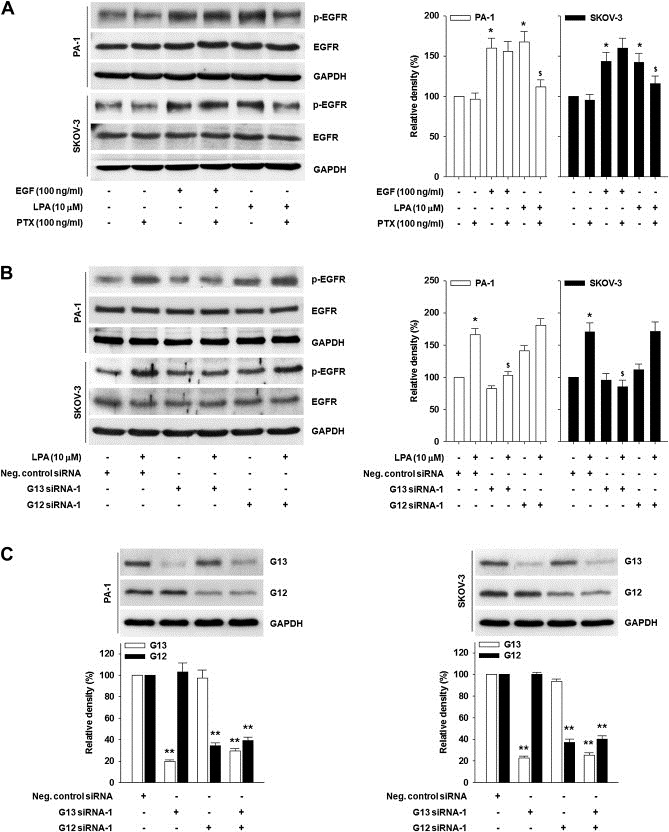
Both Gi and G13 are required for EGFR phosphorylation. (A) The serum‐starved cells were pretreated with PTX for 1 h and then stimulated with EGF or LPA for 5 min. EGFR phosphorylation by immunoprecipitation and immunoblotting. (B) The cells were transfected with indicated siRNA and then stimulated with or without LPA for 5 min. EGFR phosphorylation by immunoprecipitation and immunoblotting. Densitometric analysis is representative of three independent experiments. The histograms show the ratio between active and total EGFR protein levels (Error bars, ± s.d. *p < 0.05 vs control, $p < 0.05 vs LPA treatment only). (C) The cells were transfected with indicated siRNA. G13 and G12 expression by immunoblotting (Error bar, ± s.d. **p < 0.01 vs negative control siRNA only).
3.3. EGFR induces Ras and Rho activation
We have recently shown that the Ras/Rho/ROCK pathway is implicated in LPA‐induced proteolytic enzyme expression and ovarian cancer invasiveness (Jeong et al., 2012). Therefore we next questioned whether the EGFR is required for LPA‐induced Ras and Rho activation. Both LPA and EGF induced Ras (Figure 3A and B) and Rho activation (Figure 3C). However, pretreatment of the cells with gefitinib profoundly inhibited LPA‐ and EGF‐induced Ras (Figure 3A and B) and Rho activation (Figure 3C). Therefore, these data suggest that EGFR activation governs a LPA‐induced Ras/Rho signaling cascade.
Figure 3.
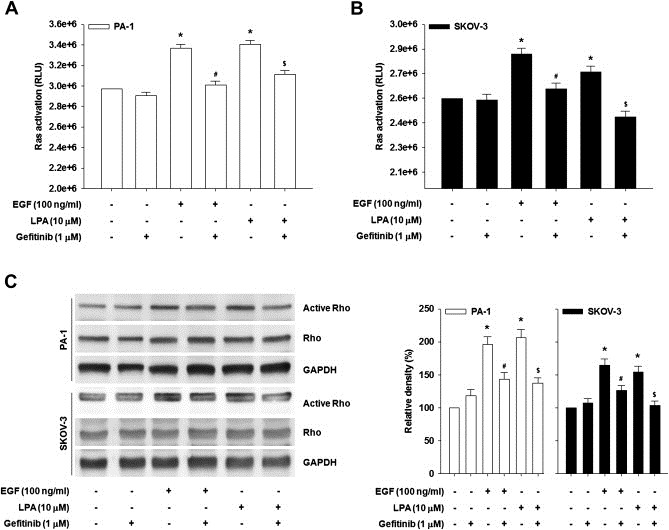
EGFR activates Ras and Rho. The serum‐starved PA‐1 (A) and SKOV‐3 (B) cells were pretreated with gefitinib for 1 h and then stimulated with LPA or EGF for 3 min. Ras activity was analyzed by Ras activation assay kit (Error bars, ± s.d. ∗p < 0.01 vs control, #p < 0.05 vs EGF treatment only, $p < 0.05 vs LPA treatment only). All experiments were repeated three times. (C) The serum‐starved cells were pretreated with gefitinib for 1 h and then stimulated with LPA or EGF for 3 min. Rho activity was analyzed by Rho GTP pull‐down assay. Densitometric analysis is representative of three independent experiments. The histograms show the ratio between active and total Rho protein levels (Error bars, ± s.d. *p < 0.01 vs control, #p < 0.05 vs EGF treatment only, $p < 0.05 vs LPA treatment only).
3.4. Resveratrol inhibits EGFR phosphorylation and ovarian cancer invasion
Resveratrol has been shown to interfere with a number of pathophysiological processes in cancer through inhibition of EGFR activation (Yance and Sagar, 2006). This naturally occurring phytoalexin also significantly inhibited LPA‐induced VEGF expression and angiogenesis (Park et al., 2007). Therefore, we determined whether resveratrol altered LPA‐ or EGF‐induced EGFR phosphorylation and downstream effects. Pretreatment of the cells with resveratrol significantly inhibited EGFR phosphorylation induced by either LPA or EGF (Figure 4A). In addition, resveratrol reduced LPA‐ and EGF‐induced MMP‐9 expression from PA‐1 cells (Figure 4B) and uPA expression from SKOV‐3 cells (Figure 4C). Consistently, resveratrol also significantly inhibited LPA‐ and EGF‐induced ovarian cancer invasion (Figure 4D). Strikingly, we observed increased inhibition of LPA‐induced proteolytic enzyme expression (Figure 4B and C) and ovarian cancer invasion (Figure 4D) when the cells were pretreated with both resveratrol and gefitinib compared to pretreatment with single agents. Therefore, these data strongly suggest that resveratrol reduces ovarian cancer cell invasion through preventing EGFR phosphorylation and potentiates the inhibitory effect of gefitinib on ovarian cancer cell invasion.
Figure 4.
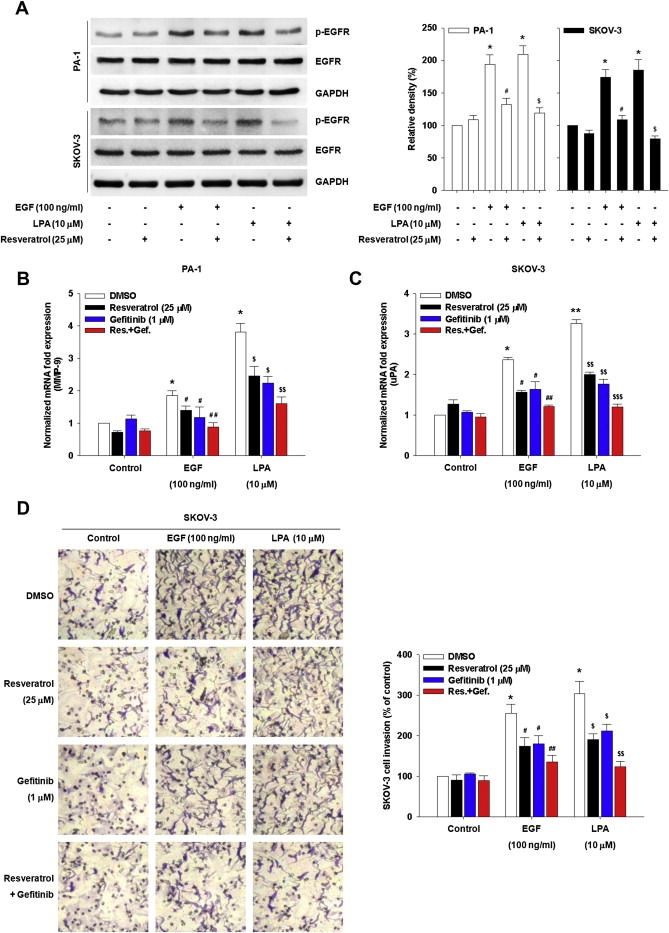
Resveratrol inhibits LPA‐induced EGFR phosphorylation and ovarian cancer cell invasion. (A) The serum‐starved cells were pretreated with resveratrol for 1 h and then stimulated with EGF or LPA for 5 min. Densitometric analysis is representative of three independent experiments. The histograms show the ratio between active and total EGFR protein levels (Error bars, ± s.d. *p < 0.05 vs control, #p < 0.05 vs EGF treatment only, $p < 0.05 vs LPA treatment only). The serum‐starved PA‐1 (B) and SKOV‐3 (C) cells were pretreated with indicated agents for 1 h and then stimulated with EGF and LPA for 24 h. mRNA expression of MMP‐9 (B) and uPA (C) by quantitative RT‐PCR (Error bars, ± s.d. *p < 0.01 and **p < 0.001 vs control, #p < 0.05 and ##p < 0.01 vs EGF treatment only, $p < 0.05, $$p < 0.01 and $$$p < 0.001 vs LPA treatment only). (D) The serum‐starved SKOV‐3 cells were pretreated with indicated agents for 1 h and in vitro invasion was analyzed against EGF or LPA (Error bars, ± s.d. *p < 0.01 vs control, #p < 0.05 and ##p < 0.01 vs EGF treatment only, $p < 0.05 and $$p < 0.01 vs LPA treatment only). All experiments were repeated three times.
4. Discussion
Recently we have shown that LPA induces ovarian cancer invasion through activation of a Ras/Rho/ROCK signaling and consequent proteolytic enzyme secretion (Jeong et al., 2012). In the present study, we elucidate the underlying mechanism by which LPA activates a Ras/Rho/ROCK signaling: LPA induces EGFR phosphorylation through both Gi and G13. In addition, we provide evidence that resveratrol has a strong inhibitory effect on EGFR phosphorylation, leading to suppression of LPA‐ and EGF‐induced ovarian cancer invasion. These findings suggest not only a pivotal role of the EGFR on LPA‐induced ovarian cancer invasiveness but support the addition of resveratrol to EGFR inhibitors for reducing ovarian cancer progression.
Accumulating data support a critical role of EGFR in LPA‐induced cancer cell progression. The EGFR is implicated in sphingosine kinase 1 up‐regulation to promote gastric cancer cell invasion (Shida et al., 2008). In addition, LPA‐induced EGFR activation increases epithelial ovarian cancer invasion through NF‐κB and VEGF expression (Dutta et al., 2011). We also previously provided evidence that LPA induces ovarian cancer cell migration through EGFR activation and subsequent COX‐2 expression (Jeong et al., 2008). In the present study, we explore another aspect of EGFR‐induced ovarian cancer invasion and demonstrate that the EGFR induces proteolytic enzyme expression through a Ras/Rho/ROCK signaling cascade. Although the EGFR is known to induce MMP‐9 (Cowden Dahl et al., 2007) and uPA (Mahabeleshwar et al., 2004; Simon et al., 1996) expression, this is the first report to our knowledge demonstrating the ability of EGFR to enhance proteolytic enzyme expression and cancer invasion through a Ras/Rho/ROCK signaling.
LPA has been demonstrated to link to different G proteins to induce ovarian cancer invasion. The G protein G13 but not G12 has been implicated in signaling from the LPA receptor to EGFR and Rho (Gohla et al., 1998). In support of these results, our present study shows the involvement of G13 in LPA‐induced EGFR phosphorylation and a Ras/Rho/ROCK signaling cascade. Congruently, we found that Gi also implicated in LPA‐induced EGFR phosphorylation and proteolytic enzyme expression. As we previously reported that Gi protein mediates LPA‐induced cell motility through EGFR phosphorylation and consequent COX‐2 expression (Jeong et al., 2008), Gi protein seems to have diverse downstream effectors for ovarian cancer progression as a consequence of EGFR activation: the Rho/ROCK signaling cascade and COX‐2. However, we do not rule out the possibility of that G13 contributes to COX‐2 expression.
Resveratrol has been shown to suppress EGFR‐induced breast cancer cell migration (Azios and Dharmawardhane, 2005). In addition, our previous data show that resveratrol inhibits LPA‐induced VEGF expression in ovarian cancer cells (Park et al., 2007). In the present study, we present evidence that this naturally occurring phytoalexin compound attenuates LPA‐induced ovarian cancer invasion through inactivation of EGFR and reduction of proteolytic enzyme expression. Intriguingly, resveratrol showed greater inhibitory effect on EGFR phosphorylation induced by LPA than that by EGF (Figure 4A). Since LPA transactivates EGFR through MMP and Src (Jeong et al., 2008; Prenzel et al., 1999), it might be possible that resveratrol has another targets implicated in LPA‐induced EGFR transactivation. In addition, we observed that combining resveratrol with gefitinib showed more significant suppression of LPA‐ or EGF‐induced secretion of proteolytic enzymes and ovarian cancer cell invasion than either compound alone, suggesting that resveratrol potentiates gefitinib to inhibition of EGFR activation. Therefore, combination of resveratrol with EGFR inhibitor might improve inhibitory effects on ovarian cancer cell invasion. In view of our present data, we present a working model (Figure 5), in which Gi and G13 are required for LPA‐induced EGFR activation and ovarian cancer progression that is efficiently attenuated by resveratrol and gefitinib.
Figure 5.
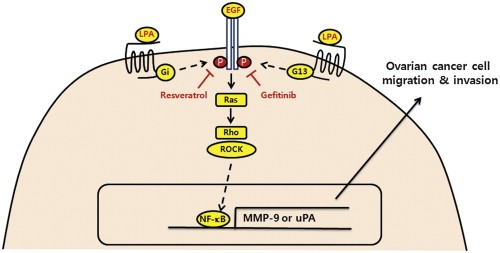
Working model for LPA‐induced EGFR transactivation inhibited by resveratrol. LPA activates EGFR through Gi and G13 subsequently induces Ras/Rho/ROCK activation, leading to lineage‐specific proteolytic enzyme expression. Resveratrol and gefitinib inhibit LPA‐ and EGF‐induced EGFR activation, proteolytic enzyme secretion and ovarian cancer cell progression.
Collectively, our results demonstrate that LPA activates EGFR phosphorylation through both Gi and G13 and that EGFR phosphorylation is required for LPA‐induced proteolytic enzyme expression and ovarian cancer invasion. We further show effective suppression of both EGF and LPA‐induced EGFR activation by resveratrol in ovarian cancer cells. Therefore, Gi and G13 as well as the EGFR may represent useful targets to reduce invasion and metastasis of ovarian cancer, potentially improving outcomes for this devastating disease.
5. Conflict of interest
The authors declare no conflict of interest.
Supporting information
The following are the supplementary data related to this article:
Supplementary data S1 PTX does not inhibit LPA‐induced PKCδ activation. The indicated cells were serum‐starved overnight and pretreated with PTX for 1 h, followed by LPA stimulation for 10 min. Immunoblotting analysis. Densitometric analysis shows the ratio between active and total PKCδ protein levels.
Supplementary data S2 G13 is required for EGFR phosphorylation. (A) The cells were transfected with indicated siRNA and then stimulated with or without LPA for 5 min. EGFR phosphorylation by immunoprecipitation and immunoblotting. Densitometric analysis is representative of three independent experiments. The histograms show the ratio between active and total EGFR protein levels (Error bars, ±s.d. *p < 0.05 vs control, $p < 0.05 vs LPA treatment only). (B) The cells were transfected with indicated siRNA. G13 and G12 expression by immunoblotting (Error bar, ±s.d. *p < 0.05, **p < 0.01 and ##p < 0.01 vs negative control siRNA only).
Acknowledgments
Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2011‐0015761 and 2010‐0022216).
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2012.10.001.
Jeong Kang Jin, Cho Kyung Hwa, Panupinthu Nattapon, Kim Hoon, Kang Jaeku, Park Chang Gyo, Mills Gordon B., Lee Hoi Young, (2013), EGFR mediates LPA‐induced proteolytic enzyme expression and ovarian cancer invasion: Inhibition by resveratrol, Molecular Oncology, 7, doi: 10.1016/j.molonc.2012.10.001.
References
- Azios, N.G. , Dharmawardhane, S.F. , 2005. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia. 7, 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bese, T. , Barbaros, M. , Baykara, E. , Guralp, O. , Cengiz, S. , Demirkiran, F. , Sanioglu, C. , Arvas, M. , 2010. Comparison of total plasma lysophosphatidic acid and serum CA-125 as a tumor marker in the diagnosis and follow-up of patients with epithelial ovarian cancer. J. Gynecol. Oncol.. 21, 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowden Dahl, K.D. , Zeineldin, R. , Hudson, L.G. , 2007. PEA3 is necessary for optimal epidermal growth factor receptor-stimulated matrix metalloproteinase expression and invasion of ovarian tumor cells. Mol. Cancer Res.. 5, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolo, V. , Adobati, E. , Canevari, S. , Picone, M.A. , Vittorelli, M.L. , 1995. Membrane vesicles shed into the extracellular medium by human breast carcinoma cells carry tumor-associated surface antigens. Clin. Exp. Metastasis. 13, 277–286. [DOI] [PubMed] [Google Scholar]
- Dutta, S. , Wang, F.Q. , Wu, H.S. , Mukherjee, T.J. , Fishman, D.A. , 2011. The NF-kappaB pathway mediates lysophosphatidic acid (LPA)-induced VEGF signaling and cell invasion in epithelial ovarian cancer (EOC). Gynecol. Oncol.. 123, 129–137. [DOI] [PubMed] [Google Scholar]
- Fang, X. , Schummer, M. , Mao, M. , Yu, S. , Tabassam, F.H. , Swaby, R. , Hasegawa, Y. , Tanyi, J.L. , LaPushin, R. , Eder, A. , Jaffe, R. , Erickson, J. , Mills, G.B. , 2002. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim. Biophys. Acta. 1582, 257–264. [DOI] [PubMed] [Google Scholar]
- Gardner, M.J. , Jones, L.M. , Catterall, J.B. , Turner, G.A. , 1995. Expression of cell adhesion molecules on ovarian tumour cell lines and mesothelial cells, in relation to ovarian cancer metastasis. Cancer Lett.. 91, 229–234. [DOI] [PubMed] [Google Scholar]
- Gohla, A. , Harhammer, R. , Schultz, G. , 1998. The G-protein G13 but not G12 mediates signaling from lysophosphatidic acid receptor via epidermal growth factor receptor to Rho. J. Biol. Chem.. 273, 4653–4659. [DOI] [PubMed] [Google Scholar]
- Horiuchi, A. , Kikuchi, N. , Osada, R. , Wang, C. , Hayashi, A. , Nikaido, T. , Konishi, I. , 2008. Overexpression of RhoA enhances peritoneal dissemination: RhoA suppression with Lovastatin may be useful for ovarian cancer. Cancer Sci.. 99, 2532–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, M. , Cai, L. , Udeani, G.O. , Slowing, K.V. , Thomas, C.F. , Beecher, C.W. , Fong, H.H. , Farnsworth, N.R. , Kinghorn, A.D. , Mehta, R.G. , Moon, R.C. , Pezzuto, J.M. , 1997. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 275, 218–220. [DOI] [PubMed] [Google Scholar]
- Jeong, K.J. , Park, S.Y. , Cho, K.H. , Sohn, J.S. , Lee, J. , Kim, Y.K. , Kang, J. , Park, C.G. , Han, J.W. , Lee, H.Y. , 2012. The Rho/ROCK pathway for lysophosphatidic acid-induced proteolytic enzyme expression and ovarian cancer cell invasion. Oncogene. 31, 4279–4289. [DOI] [PubMed] [Google Scholar]
- Jeong, K.J. , Park, S.Y. , Seo, J.H. , Lee, K.B. , Choi, W.S. , Han, J.W. , Kang, J.K. , Park, C.G. , Kim, Y.K. , Lee, H.Y. , 2008. Lysophosphatidic acid receptor 2 and Gi/Src pathway mediate cell motility through cyclooxygenase 2 expression in CAOV-3 ovarian cancer cells. Exp. Mol. Med.. 40, 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Park, S.Y. , Lee, E.K. , Park, C.G. , Chung, H.C. , Rha, S.Y. , Kim, Y.K. , Bae, G.U. , Kim, B.K. , Han, J.W. , Lee, H.Y. , 2006. Activation of hypoxia-inducible factor-1alpha is necessary for lysophosphatidic acid-induced vascular endothelial growth factor expression. Clin. Cancer Res.. 12, 6351–6358. [DOI] [PubMed] [Google Scholar]
- Mahabeleshwar, G.H. , Das, R. , Kundu, G.C. , 2004. Tyrosine kinase, p56lck-induced cell motility, and urokinase-type plasminogen activator secretion involve activation of epidermal growth factor receptor/extracellular signal regulated kinase pathways. J. Biol. Chem.. 279, 9733–9742. [DOI] [PubMed] [Google Scholar]
- Mills, G.B. , Moolenaar, W.H. , 2003. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer. 3, 582–591. [DOI] [PubMed] [Google Scholar]
- Murakami, M. , Shiraishi, A. , Tabata, K. , Fujita, N. , 2008. Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun.. 371, 707–712. [DOI] [PubMed] [Google Scholar]
- Nieman, K.M. , Kenny, H.A. , Penicka, C.V. , Ladanyi, A. , Buell-Gutbrod, R. , Zillhardt, M.R. , Romero, I.L. , Carey, M.S. , Mills, G.B. , Hotamisligil, G.S. , Yamada, S.D. , Peter, M.E. , Gwin, K. , Lengyel, E. , 2011. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med.. 17, 1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, K. , Herr, D. , Mutoh, T. , Chun, J. , 2009. Lysophosphatidic acid (LPA) and its receptors. Curr. Opin. Pharmacol.. 9, 15–23. [DOI] [PubMed] [Google Scholar]
- Oyesanya, R.A. , Greenbaum, S. , Dang, D. , Lee, Z. , Mukherjee, A. , Wu, J. , Dent, P. , Fang, X. , 2010. Differential requirement of the epidermal growth factor receptor for G protein-mediated activation of transcription factors by lysophosphatidic acid. Mol. Cancer. 9, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols, R.F. , Bookman, M.A. , Connolly, D.C. , Daly, M.B. , Godwin, A.K. , Schilder, R.J. , Xu, X. , Hamilton, T.C. , 2004. Focus on epithelial ovarian cancer. Cancer Cell. 5, 19–24. [DOI] [PubMed] [Google Scholar]
- Park, S.Y. , Jeong, K.J. , Lee, J. , Yoon, D.S. , Choi, W.S. , Kim, Y.K. , Han, J.W. , Kim, Y.M. , Kim, B.K. , Lee, H.Y. , 2007. Hypoxia enhances LPA-induced HIF-1alpha and VEGF expression: their inhibition by resveratrol. Cancer Lett.. 258, 63–69. [DOI] [PubMed] [Google Scholar]
- Park, S.Y. , Jeong, K.J. , Panupinthu, N. , Yu, S. , Lee, J. , Han, J.W. , Kim, J.M. , Lee, J.S. , Kang, J. , Park, C.G. , Mills, G.B. , Lee, H.Y. , 2011. Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression. Oncogene. 30, 1351–1359. [DOI] [PubMed] [Google Scholar]
- Prenzel, N. , Zwick, E. , Daub, H. , Leserer, M. , Abraham, R. , Wallasch, C. , Ullrich, A. , 1999. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 402, 884–888. [DOI] [PubMed] [Google Scholar]
- Sheng, Q. , Liu, J. , 2011. The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. Br. J. Cancer. 104, 1241–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida, D. , Fang, X. , Kordula, T. , Takabe, K. , Lepine, S. , Alvarez, S.E. , Milstien, S. , Spiegel, S. , 2008. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res.. 68, 6569–6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, R. , Naishadham, D. , Jemal, A. , 2012. Cancer statistics, 2012. CA Cancer J. Clin.. 62, 10–29. [DOI] [PubMed] [Google Scholar]
- Simon, C. , Juarez, J. , Nicolson, G.L. , Boyd, D. , 1996. Effect of PD 098059, a specific inhibitor of mitogen-activated protein kinase kinase, on urokinase expression and in vitro invasion. Cancer Res.. 56, 5369–5374. [PubMed] [Google Scholar]
- Snider, A.J. , Zhang, Z. , Xie, Y. , Meier, K.E. , 2010. Epidermal growth factor increases lysophosphatidic acid production in human ovarian cancer cells: roles for phospholipase D2 and receptor transactivation. Am. J. Physiol. Cell. Physiol.. 298, C163–C170. [DOI] [PubMed] [Google Scholar]
- Sriwai, W. , Zhou, H. , Murthy, K.S. , 2008. G(q)-dependent signalling by the lysophosphatidic acid receptor LPA(3) in gastric smooth muscle: reciprocal regulation of MYPT1 phosphorylation by Rho kinase and cAMP-independent PKA. Biochem. J.. 411, 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata, K. , Baba, K. , Shiraishi, A. , Ito, M. , Fujita, N. , 2007. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun.. 363, 861–866. [DOI] [PubMed] [Google Scholar]
- Xu, Y. , Shen, Z. , Wiper, D.W. , Wu, M. , Morton, R.E. , Elson, P. , Kennedy, A.W. , Belinson, J. , Markman, M. , Casey, G. , 1998. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 280, 719–723. [DOI] [PubMed] [Google Scholar]
- Yance, D.R. , Sagar, S.M. , 2006. Targeting angiogenesis with integrative cancer therapies. Integr. Canc. Ther.. 5, 9–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data S1 PTX does not inhibit LPA‐induced PKCδ activation. The indicated cells were serum‐starved overnight and pretreated with PTX for 1 h, followed by LPA stimulation for 10 min. Immunoblotting analysis. Densitometric analysis shows the ratio between active and total PKCδ protein levels.
Supplementary data S2 G13 is required for EGFR phosphorylation. (A) The cells were transfected with indicated siRNA and then stimulated with or without LPA for 5 min. EGFR phosphorylation by immunoprecipitation and immunoblotting. Densitometric analysis is representative of three independent experiments. The histograms show the ratio between active and total EGFR protein levels (Error bars, ±s.d. *p < 0.05 vs control, $p < 0.05 vs LPA treatment only). (B) The cells were transfected with indicated siRNA. G13 and G12 expression by immunoblotting (Error bar, ±s.d. *p < 0.05, **p < 0.01 and ##p < 0.01 vs negative control siRNA only).


