Abstract
The number of new cancer cases each year is projected to rise worldwide by about 70% by 2030 due to demographic changes alone, with the largest increases in the lower‐income countries. Wider adoption of specific aspects of westernized lifestyles would translate to still greater increases in certain cancer types. In many countries the burden of cancer and other non‐communicable diseases will add to communicable diseases and malnutrition to impose a “double burden” on the poorest. These trends represent major challenges to health, poverty, sustainable development and equality. Prevention is, however, possible based on implementing existing knowledge about risk factors and the natural history of the disease. Both primary and secondary cancer prevention offer therefore many opportunities to combat the projected increases. Tobacco control, reductions in obesity and physical inactivity, reduced consumption of alcohol, vaccination against hepatitis B and human papilloma viruses, safe sex, avoidance of environmental and occupational carcinogens and excessive sun exposure as well as the early detection and screening for breast, cervix and colorectal cancers would all make significant contributions. At the same time, for a number of major cancers (e.g., colon, prostate, kidney, pancreas, brain, lympho‐haematological malignancies) research is needed to identify as yet unknown risk factors whilst for existing prevention strategies additional work is needed on their implementation into health services. Finally, there is a remarkable opportunity for advances in understanding the molecular basis of carcinogenesis to provide new tools and insights into aetiology and prevention. It is only by complementing efforts to improve treatment with those aimed at prevention that the impending epidemic of this disease can be addressed.
Keywords: Cancer prevention, Cancer burden, Risk factors, Epidemiology
Highlights
-
►
The annual number of new cancer cases is projected to rise by 70% worldwide by 2030.
-
►
Improvements in treatment will not be adequate to combat this impending tragedy.
-
►
Cancer prevention is therefore a vital component of cancer control.
-
►
At least 50% of cancers could be prevented with current knowledge.
-
►
Interdisciplinary research will help identify new risk factors and evaluate interventions.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- HDI
Human Development Index
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- H. pylori
Helicobacter pylori
- HPV
human papillomavirus
- IARC
International Agency for Research on Cancer
- KS
Kaposi sarcoma
- KSHV
Kaposi sarcoma herpes virus
- NCDs
non-communicable diseases
- PSA
prostate-specific antigen
- UN
United Nations
- WHO
World Health Organization
1. Introduction
The burden of cancer as well as that of other non‐communicable diseases (NCDs) is increasing globally. NCDs (including cancer but also cardiovascular diseases, stroke, diabetes, chronic obstructive pulmonary disease (COPD), etc.) in 2005 were estimated to have caused more than 60% (35 million) of all deaths worldwide (United Nations, 2012). Without prevention and control actions, the figure is expected to increase to 41 million in 2015. This phenomenon is mainly a consequence of the so‐called epidemiologic transition, i.e., a shift from infectious to NCDs (Omran, 1971; Maule and Merletti, 2012). One consequence, certainly in the lower‐income countries, is the implausibility of treating our way out of the NCD epidemic.
In the present review we will focus on needs, knowledge and opportunities in cancer prevention cognisant of the intimate link between prevention of cancer and prevention of other NCDs. Cancer burden will be put in a global perspective and knowledge on established risk factors summarised. While cancer is a global problem it is not a uniform one. There are distinct patterns of the types of cancer at a regional and national level. These differences reflect heterogeneity in underlying risk factors and hence imply the need for cancer control strategies tailored to specific regional challenges. Any strategy that can prevent NCDs as a whole is, obviously, attractive, but some cancer‐specific preventive tools can be extremely effective and potentially cost‐effective.
2. The epidemiologic transition
The second part of the twentieth century witnessed enormous progress in improving health and survival around the world. Life expectancy at birth for the world population rose from 48 years in 1950–1955 to 68 years in 2005–2010 (United Nations, 2012). In a number of countries that have transited towards the highest levels of human development (e.g., Australia, Canada, France, Italy, Spain, Norway, Sweden, Switzerland, Israel, Japan and the Republic of Korea), life expectancy at birth exceeded 80 years in 2005–2010. Epidemiologic transition is characterised by initial declines in communicable diseases (Group I) followed by subsequent increases in crude and proportional mortality attributable to NCDs (Group II). Enormous disparities exist across regions in the stage of epidemiologic transition attained, but there are no countries where the present and future challenge posed by NCDs can be ignored.
Figure 1 shows the ranking of world regions in 2008 by the three main Groups of causes of death including, in addition to Group I and II, Group III, i.e., injuries. The non‐standardised (or crude) death rate reflects the burden to the population from the number of deaths from a disease or group of diseases. Conversely, age‐standardised rates account for the different age structures observed in different populations. Africa's mortality rate for Group I was nearly four‐times higher than in Asia and over 20‐fold higher than in more developed regions (excluding Eastern Europe). The relationship between regional rates for Group I deaths is not modified by age adjustment indicating that Africa's elevated mortality for Group 1 diseases is not attributable to a very young population but rather to other factors (i.e., very high mortality from malaria and diarrhoea in children and from HIV infection among young adults). If Africa's mortality rates due to communicable diseases were to be reduced to those observed in the longest‐lived world populations, the region would achieve a 17‐year increase in life expectancy at birth, from 55 to 72 years. Conversely, age‐adjustment substantially modifies the ratios between Group II deaths across regions (Figure 1). If the underlying population age structure was equal, Africa, developing Oceania (i.e., Oceania except Australia and New Zealand), and Eastern Europe would have the highest NCD mortality followed by Asia and Latin America. Perhaps contrary to common perception, more developed regions tend to have relatively low age‐standardised rates of NCDs. To a lesser extent, deaths from injuries follow a pattern similar to NCDs, with the highest age‐standardised mortality rates in Africa and Eastern Europe. It is, therefore, essential to bear in mind that less developed countries experience a “double burden” of infectious diseases and NCDs compared to more developed ones and are potentially extremely vulnerable to additional NCD increases.
Figure 1.
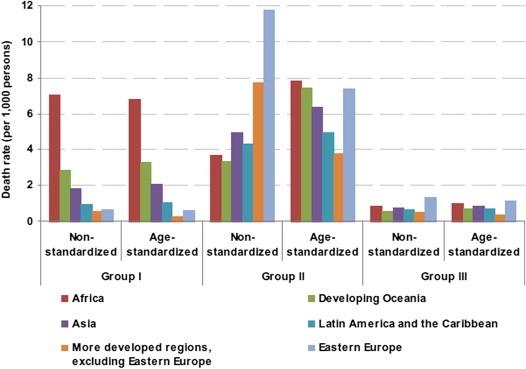
Non‐standardised and age‐standardised death rates by group of causes (see text) for selected regions, 2008 (United Nations, 2012).
The possibility to live a long and healthy life is a fundamental aspect of human development and the epidemiologic transition in more developed countries was associated with improved socioeconomic conditions (that in turn improved hygiene and nutrition) earlier than medical advances (Maule and Merletti, 2012). Medical advances in disease prevention and treatment came at a later stage but they can now, in principle, be made available globally.
3. Global cancer burden: mortality and incidence
This section reviews the current burden of cancer in 2008 and cancer projections for 2030 (GLOBOCAN http://globocan.iarc.fr) (Ferlay et al., 2010). It also provides the latest estimates on the number, and rates of global deaths from cancer. A description of the methods used to produce these estimates is provided in GLOBOCAN. Data are also discussed and presented according to the Human Development Index (HDI) groups (Bray et al., 2012a).
A total of 7.6 million deaths from cancer are estimated to have occurred in 2008 (21% of all NCD deaths). Premature death is a major consideration when evaluating the impact of cancer on a given population. Cancer accounted for 27% of NCD deaths below age 70 (World Health Organization, 2011).
Large variations in both cancer incidence and mortality are observed, overall and in relation to the major forms of cancer, in different regions of the world (Ferlay et al., 2010). Figure 2 presents the most frequent types of cancer diagnosis (based on the number of new cases per year) in each country, for men and women.
Figure 2.
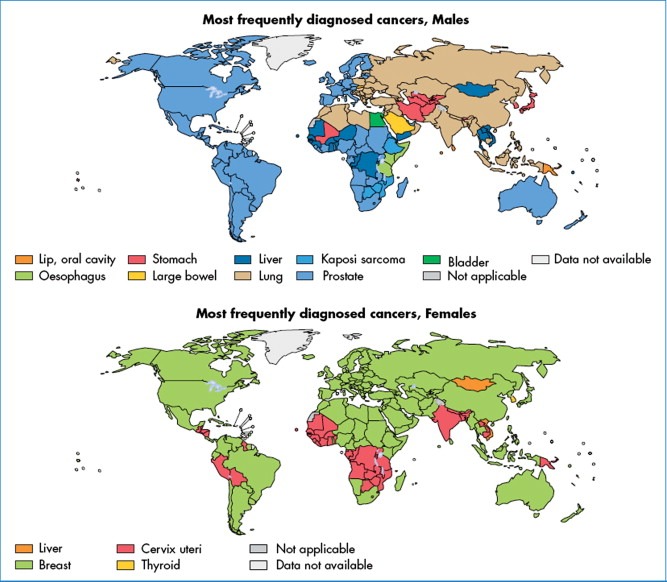
Most frequently diagnosed cancers worldwide, by country and sex, 2008 (GLOBOCAN).
The geographical variation in cancer distribution is mirrored on examination of the number of new cases and deaths for the most common cancers in relation to the HDI of countries (Bray et al., 2012a)(Figure 3). It is worth bearing in mind that the population in very high‐, high‐, medium‐, and low‐HDI countries was of very different size, i.e., 1.0, 0.9, 4.4 and 0.4 billion, respectively. Figure 3 is, therefore, only useful to evaluate the relative importance of different cancer types by HDI. In all countries, other than those in the low HDI category, men have a heavier burden of all types of cancer combined than women. The exception of low HDI countries is most likely explained by the high rates of cervical cancer among women in the African Region.
Figure 3.
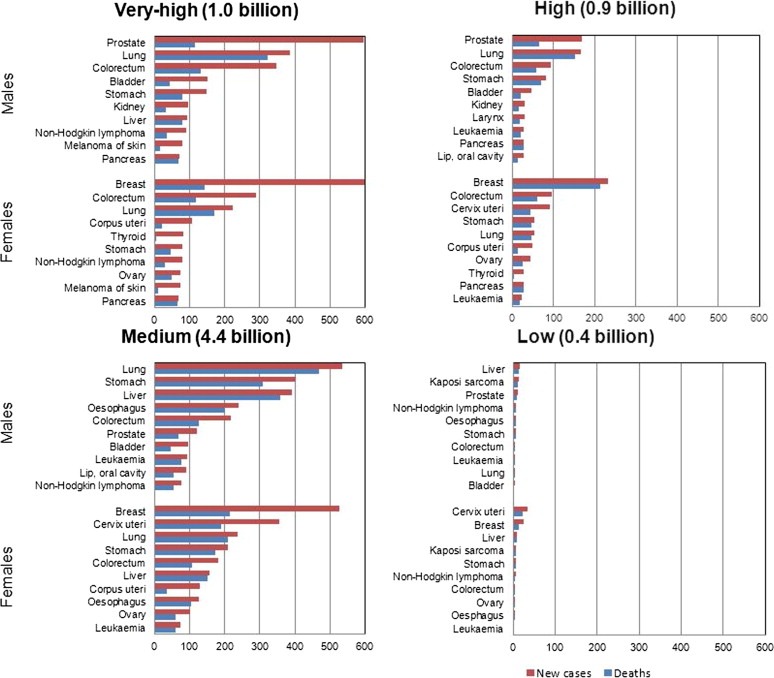
Total population and estimated annual number of new cases and deaths for the most common cancers, by Human Development Index groups and by sex, 2008 (GLOBOCAN).
Within high‐ and very high‐HDI countries, prostate and breast cancers are the most commonly diagnosed in males and females respectively, with lung and colorectal cancers representing the next most common type (Figure 3). These cancers also represent the most frequent types of cancer‐related deaths in these countries although lung cancer is the most common cause of cancer death in both sexes. Within low‐HDI countries, the absolute burden of cancer is lower, and while prostate and breast cancers remain among the most common diagnoses and types of cancer‐related deaths, cancers of the cervix, stomach, liver, and Kaposi sarcoma (KS) are also among the leading types – all of which are cancers with infection‐related aetiology. Medium‐HDI countries are intermediate with respect to their patterns of cancer burden, reflecting an on‐going transition from infection‐related cancers to those most frequently diagnosed in countries with the highest HDI. The three most common types of cancer in medium‐HDI countries are lung, stomach and liver cancers in males, and breast, cervix and lung cancer in females (Figure 3).
Concerning the overall prevalence of cancer in 2008, the estimated proportion of adults (>15 years old) living with cancer varies from one in 60 people in the very high HDI countries to just one in 450 in the low HDI countries (data not shown) (Bray et al., 2012b).
Future planning of service provision is an integral part of cancer control programmes. Considering the projected growth in cancer morbidity, important differences can be observed in relation to HDI groups. Without any changes in the prevalence and distribution of underlying known or putative risk factors (i.e., based only on anticipated demographic changes alone), between 10 and 11 million cancers will be diagnosed annually in 2030 in the low‐ and medium‐HDI countries (Figure 4). The estimated percentage increase in cancer incidence by 2030 (compared with 2008) will be greater in low‐ (93%) and medium‐HDI countries (78%) compared to high‐ (60%) and very high‐HDI (39%) countries.
Figure 4.
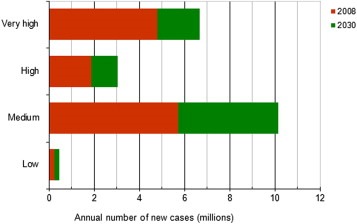
Estimated annual number of new cancer cases 2008 and predicted 2030, by Human Development Index groups (Bray et al., 2012a).
4. Risk factors for NCDs and cancer
For more than a decade the World Health Organization (WHO) has been raising awareness of the need to place higher priority on NCDs. This culminated in the United Nations (UN) high‐level meeting on NCDs in September 2011 and the Political Declaration that emerged. WHO is now engaged in establishing a Global Monitoring Framework for NCDs. The WHO Assembly in May 2012 agreed on the first global target: to reduce premature (30–70 years) mortality from NCDs by 25% by 2025 (“25 by 25”). The UN also called upon the WHO, in collaboration with the Member States, UN agencies (including IARC) and other relevant organisations, to prepare before the end of 2012 recommendations for a set of voluntary global targets and indicators. These should permit the monitoring of trends and thus an assessment of progress made in the implementation of national strategies and plans on NCDs.
There are different ways to establish priorities and achievable targets in the prevention of cancer and the focus of WHO has been on risk factors shared across the spectrum of key NCDs. The criteria for selecting risk factors included: 1) to be leading causes of disease globally or regionally; 2) not to be too specific (e.g., not a single environmental pollutant); 3) availability of reasonably complete data on exposure, risk levels, and evidence of causality; and 4) being potentially modifiable. Along these lines, Table 1 is a modified version of the widely quoted work by the Institute for Health Metrics and Evaluation, Seattle, USA (Ezzati et al., 2006). Table 1 is, however, restricted to risk factors for NCDs that are also relevant to cancer. For each risk factor, it also shows a theoretical‐minimum‐risk exposure that would be desirable to reach in any population.
Table 1.
Risk factors for cancer that are also leading causes of disease burden globally or regionally and are potentially modifiable (adapted from Ezzati et al., 2006).
| Risk factor | Minimum‐risk exposure | Cancer | Other major diseases |
|---|---|---|---|
| Smoking | No smoking | Cancer of the lung, upper aero‐digestive tract; liver; pancreas; cervix uteri; bladder; kidney; and leukaemia | IHD; stroke; COPD and other respiratory diseases |
| Alcohol | No use | Cancer of the upper aero‐digestive tract; liver, and breast | IDH; stroke; diabetes; cirrhosis |
| Overweight and obesity | BMI = 21SD 1 kg/m2 | Cancer of the colon; gallbladder; breast (post‐menopausal); endometrium; and kidney | IHD; stroke; diabetes; osteo‐arthritis |
| Physical inactivity | ≥2.5 h/wk | Cancer of the colon ‐rectum; breast; and prostate | IHD; diabetes; osteoporosis; osteoarthritis |
| Low fruit and vegetable intake | 600 gr/daySD 50 g | Cancer of the upper aero‐digestive tract; stomach; colon; and lung | IHD; stroke |
| Urban air pollution | 7.5 μg/m3 for PM2.51.5 μg/m3 for PM10 | Cancer of the lung | Mortality from respiratory and cardiovascular diseases |
| Indoor smoke from solid fuels | No solid fuel use | Cancer of the lung | COPD, respiratory infections in children |
| Unsafe sex | No unsafe sex | Cancer of the cervix, other anogenital tract, and oropharynx | HIV/AIDS; sexually‐transmitted diseases |
| Contaminated injections in health care setting | No contaminated injections | Liver cancer; non‐Hodgkin lymphoma | Infection with HBV; HCV; HIV, cirrhosis, |
BMI = body mass index; COPD: chronic obstructive pulmonary disease; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; IDH: ischemic heart disease; SD: standard deviation.
Not surprisingly, Table 1 includes many of the most important cancer risk factors given NCDs share key modifiable behavioural risk factors like tobacco use, the harmful use of alcohol, overweight and obesity, unhealthy diet, and lack of physical activity. There is, therefore, a substantial overlap between Table 1 and several past attempts to quantify lifestyle and environmental factors that contribute the most to cancer incidence and mortality. Danaei et al. (2005) used the same approach as in Ezzati et al. (2006) and estimated global mortality from 12 cancer types attributable to risk factors listed in Table 1. Altogether, the nine risk factors accounted for 35% of world cancer (37% in high‐income countries and 34% in low‐and‐middle‐income countries). Smoking was associated with a larger attributable fraction in high‐income countries than in low‐and‐middle‐income countries (29% versus 18%, respectively). A smaller difference was also reported for the fraction attributable to overweight and obesity (3% versus 1%). Cancer‐causing infections were not included in Danaei et al. (2005), except for an indirect mention to safe sex as a way to prevent cervical cancer and HIV.
In their seminal report on the causes of cancer Doll and Peto (1981) showed that each type of cancer that was common in one world population was rare in another. They argued that because these differences were not chiefly genetic, wherever one type of cancer was common there were likely to be potentially avoidable causes. The proportion of cancer theoretically avoidable was higher (approximately 75%, Table 2) than in Danaei et al. (2005). The difference was not accounted for completely by either the population under study (United States in 1978 versus world in 2001), the choice of minimal achievable risk level, or fractions attributed to individual risk factors. As an example, 30% (range of acceptable estimates: 25–40) of cancer deaths were attributed by Doll and Peto to tobacco smoking and 3% (range of acceptable estimates: 2–4) to alcohol drinking, compared to 21% and 5% in the report on the world (Danaei et al., 2005). The cancer fraction attributed to diet (35%; range of acceptable estimates: 10–70) by Doll and Peto would be probably considered too large nowadays but it had extremely wide range of acceptable estimates and encompassed, in its definition, different items from Table 1 (overweight and obesity; physical inactivity; and low fruit and vegetable intake). Doll and Peto's larger fraction of theoretically avoidable cancer depended in part on the fact that they included a broader range of cancer types and risk factors than in Danaei et al. (2005). Some risk factors are specific to certain cancer sites and not necessarily relevant to other NCDs or avoidable. An update of Doll and Peto's work by Julian Peto (2001) showed separate estimates for current smokers and non‐smokers. The cancer fraction attributed to smoking increased to 60% among current smokers. The fraction due to overweight was larger among non‐smokers (10%) than current smokers (4%) and so was the fraction of cancers presently unavoidable on account of insufficient knowledge (50% and 25%, respectively).
Table 2.
Doll and Peto's tabulation of proportions of all cancer deaths attributable to various different risk factors in the USA in 1978 (Doll and Peto, 1981).
| Factor or class of factors | % of all cancer deaths | Scope for updates | |
|---|---|---|---|
| Best estimate | Acceptable range | ||
| Tobacco | 30a | 25–40 | Going down in more developed and up in less developed countries |
| Alcohol | 3 | 2–4 | Much higher in some countries; it did not include breast cancer |
| Diet | 35 | 10–70 | Still uncertain but probably lower, even including overweight and obesity, physical activity, salt, and low fruit and vegetable intake |
| Food additives | <1 | −5 to 2b | |
| Reproductive and sexual behaviours | 7 | 1–13 | Increasingly important in many less developed countries in which childbearing is being delayed and family size reduced. Sexual behaviour should be partly moved to Infection |
| Occupation | 4 | 2–8 | |
| Pollution | 2 | <1–5 | |
| Industrial products | <1 | <1–2 | |
| Medicines and medical procedures | 1 | 0.5–3 | On the rise due to increases in use of ionising radiation in imaging and cancer treatment and of drugs with immunosuppressive and carcinogenic effects |
| Geophysical factorsc | 3 | 2–4 | It did not include radon |
| Infection | 10? | 1–? | Only HBV and EBV were well‐established carcinogens. Very different by countryd |
| Unknown | ? | ? | |
60% in current smokers (Peto, 2001).
Allowing for a possibly protective effect of antioxidants and other preservatives.
About 1%, not 3%, could reasonably be described as “avoidable” (see text). Geophysical factors also cause a much greater proportion of non‐fatal cancers (up to 30% of all cancers, depending on ethnic mix and latitude) because of the importance of UV light in causing the relatively non‐fatal basal cell and squamous cell carcinomas of sunlight‐exposed skin.
See Table 3 for current best estimates.
After Doll and Peto's report substantial knowledge of cancer causes has accumulated, particularly with respect to several chronic infections (de Martel et al., 2012) and the benefits of cancer screening are recognised (IARC, 2002, 2005; Segnan, 2010). A rigorous update of their estimates is beyond the scope of the present review but comments in Table 2 (Scope for updates) address the issues that would have to be revised to‐day. Doll and Peto's conclusions, however, still provide a strong reminder of the existence of many specific features of cancer aetiology and, hence, prevention opportunities.
5. Diversity in cancer aetiology and cancer prevention strategies
Cancer differs from the other NCDs by including a wide range of cancer sites and types that vary substantially with respect to their aetiology and natural history of disease. Although some risk factors, notably smoking, can increase the risk of a large number of cancer types (IARC, 2012a, 2012b, 2012c, 2012d) there is no universal risk factor for cancer. In addition, several different risk factors, including those affecting early‐stage and late‐stage, are typically involved in the causation of cancer in any given site (Day, 1990).
The International Agency for Research on Cancer (IARC) Monograph series has classified carcinogens for more than 40 years. Recently, IARC has provided up‐to‐date information on cancer sites associated with more than 100 carcinogenic agents (Cogliano et al., 2011). Initially, IARC classified an agent as carcinogenic to humans only when sufficient epidemiological evidence in humans supported a causal association. Scientific understanding of the mechanisms of carcinogenesis, accompanied by the development of assays for studying mechanistic events involved in carcinogenesis, have given researchers new ways of establishing whether an agent is carcinogenic. Agents that have been classified as carcinogenic to humans based on mechanistic and other relevant non‐human data include many that typically occur in complex exposures making it difficult for epidemiologic studies to attribute causality to specific components (Cogliano et al., 2011).
It needs to be noted in this context that for some cancer types the aetiology remains poorly understood and requires more research, preferably in regions with the highest or lowest incidence of those cancers. In addition, most cancers grow slowly and occur only decades after initiation. The full benefit of many primary prevention strategies, therefore, will often only be seen decades after their introduction. At the same time, the long induction time of many precancerous lesions and cancers allows early detection and screening (see section on Screening) and consequent prognostic improvements.
Avoidance of risk factors shared by cancer and NCDs (Table 1) is of paramount importance but should be complemented by additional approaches in order to diminish cancer burden, especially in low‐income countries. NCD risk factors mentioned above are highly relevant to the prevention of cancer of the lung (smoking), and fairly relevant to the one or other of cancers of the breast and colon (overweight and lack of physical activity, and alcohol). These three cancer sites have lower incidence and mortality in low‐income countries than in high‐income countries, but they nonetheless impose a heavy disease burden everywhere and are increasing in low‐income countries because of less favourable trends in smoking than in high‐resource countries (Giovino et al., 2012); and more recent changes in lifestyle and reproductive habits (decrease and postponement of childbearing). Because of the long induction time of cancers such prevention strategies should ideally be implemented now even in regions where those cancers are at present relatively uncommon, because the full extent of cancers related to those risk factors will appear only in the future and to reverse such a trend takes up to several decades.
Among the four cancer sites that show much more elevated incidence and mortality in low‐ than high‐income countries (cervical, liver, stomach, and oesophagus) all except cancer of the oesophagus are predominantly caused by chronic infections, although tobacco and/or alcohol consumption also play a role (IARC, 2012b).
5.1. Infections and cancer
Conservative estimates showed that about 2 million cancer cases per year (16% of the global cancer burden) are attributable to a few chronic infections (de Martel et al., 2012). This fraction is substantially larger in low‐resource countries (that include low‐ and medium‐HDI countries) (26%) than in high‐resource countries (8%) making the prevention or eradication of these infections a powerful tool to overcome inequalities in cancer incidence between poor and rich populations. Variations are also seen by continent and country (Table 3). The fraction of cancer attributable to infections is largest in sub‐Saharan Africa (33%) and China (26.1%) and smallest in Australia and New Zealand and in North America (≤4%).
Table 3.
Number of new cancer casesa in 2008 attributable to infectious agents, by geographic region (de Martel et al., 2012).
| Number of new cases in 2008 | Number attributable to infection | PAF (%) | |
|---|---|---|---|
| Africa | |||
| Sub‐Saharan Africa | 550,000 | 180,000 | 32.7% |
| North Africa and west Asia | 390,000 | 49,000 | 12.7% |
| Asia | |||
| India | 950,000 | 200,000 | 20.8% |
| Other central Asia | 470,000 | 81,000 | 17.0% |
| China | 2,800,000 | 740,000 | 26.1% |
| Japan | 620,000 | 120,000 | 19.2% |
| Other east Asia | 1,000,000 | 230,000 | 22.5% |
| America | |||
| South Americab | 910,000 | 150,000 | 17.0% |
| North America | 1,600,000 | 63,000 | 4.0% |
| Europe | 3,200,000 | 220,000 | 7.0% |
| Oceania | |||
| Australia and New Zealand | 130,000 | 4200 | 3.3% |
| Other Oceania | 8800 | 1600 | 18.2% |
| More developed regionsc | 5,600,000 | 410,000 | 7.4% |
| Less developed regionsd | 7,100,000 | 1,600,000 | 22.9% |
| World | 12,700,000 | 2,000,000 | 16.1% |
PAF = population attributable fraction.
Numbers are rounded to two significant digits.
Includes Mexico.
Total for Japan, North America, Europe, and Australia and New Zealand.
Total for all other regions.
The principal infectious agents, each responsible for approximately 5% of cancers worldwide, are human papillomavirus (HPV) (100% of cancer of the cervix, the majority of cancers of the ano‐genital tract in each sex, and between 13 and 56% of cancer of the oro‐pharynx depending upon the population); hepatitis B virus (HBV) and hepatitis C virus (HCV) (responsible for 77% of hepatocellular carcinoma worldwide); and Helicobacter pylori (H. pylori) (that causes 75% of non‐cardia carcinomas of the stomach) (de Martel et al., 2012).
The prevalence of cancer‐associated infections varies substantially in different populations in a way that closely resembles the geographic distribution of the incidence of the corresponding cancer types. The prevalence of cervical HPV infection in women, for instance, varies more than 10‐fold according to IARC population‐based HPV surveys: from less than 3% to more than 30% in some sub‐Saharan African populations (Franceschi et al., 2006; Keita et al., 2009). Large variations are also seen for H. pylori (EUROGAST, 1993), HBV, and HCV infection (Raza et al., 2007). The transmission of HCV infection has been substantially reduced in high‐income countries, where major epidemics had taken place in the last decades (e.g., Japan and Italy) but not so in many low‐resource countries (e.g., Egypt, Pakistan, Mongolia) where it is still mainly sustained by contaminated needles and unsafe transfusions (Raza et al., 2007).
Some infections globally less frequent than HPV, HBV, HCV, and H. pylori, are, however, extremely important in certain low‐income countries. One of the most frequent cancers in Africa (KS) is caused by a virus (Kaposi sarcoma herpes virus, KSHV) notably in combination with HIV‐induced immunosuppression (IARC, 2012b; de Martel et al., 2012). Epstein Barr virus (lymphoma and nasopharyngeal cancer) and some parasites are associated with a high cancer burden in parts of Asia and Africa (de Martel et al., 2012).
Estimates of infection‐attributable cancer in de Martel et al. (2012) did not separate the contribution of HIV from that of a few cancer‐associated viruses (KSHV, EBV, HPV, and, probably, HBV and HCV) whose carcinogenetic potential is greatly enhanced by HIV‐induced immunosuppression. HIV is one of the world's leading infectious killers, claiming more than 25 million lives over the past three decades. There were approximately 34 million people living with HIV in 2010. The introduction of highly active (combined) anti‐retroviral therapy in 1996 rapidly changed the outcome of the infection in Western countries making HIV infection a chronic, although still not curable, disease. By 2010, around 6.6 million people living with HIV were receiving antiretroviral therapy in low‐ and middle‐income countries, but over 7 million others are waiting for access to treatment. Although anti‐retroviral treatment led to a reduction of KS and non‐Hodgkin lymphomas, a beneficial effect on HPV‐associated cancer has not yet been seen, possibly because of the long latency of these malignancies (Franceschi et al., 2010). The burden of cancer in long‐living HIV‐positive individuals is certainly going to increase, and so should efforts to decrease cancer‐associated infections and high‐risk habits (smoking) among them.
The eradication of many cancer‐associated infections is possible and often affordable. Control measures include avoidance of contaminated blood and needles (HBV, HCV, HIV); safe sex practices (HBV and HIV); early detection of precancerous or cancerous lesions; or treatment of the infection (e.g., detection and treatment of HPV‐related precancerous cervical lesions in screening programmes and antibiotic treatment of H. pylori). The availability of highly effective and safe vaccines against HBV and HPV infection is a special asset. Historically, only clean water has performed better than vaccination to reduce disease burden (Andre et al., 2008). If high coverage can be achieved, vaccines can reduce inequalities in health more than other medical interventions. Immunisation programmes require funding for infrastructure, purchase of vaccines and adequate staffing. However, the mortality and morbidity prevented translates into long‐term savings and potential economic growth. In addition, “herd protection” of the unvaccinated occurs when a sufficient proportion of the population is immune (Andre et al., 2008).
Vaccination against HBV should prevent the majority of hepatocellular carcinomas in many world regions, as already observed in Taiwan (Chang, 2003) and being studied in an on‐going trial in The Gambia (Kirk et al., 2004). It is noteworthy that the carcinogenic aflatoxins, common dietary contaminants in HBV endemic areas, are more potent among chronic HBV carriers. Thus vaccination against HBV may also reduce the cancer risks associated with aflatoxins (Wild and Gong, 2010).
HPV16 and 18, the two oncogenic HPV types included in currently available vaccines, are responsible for at least 70% of cervical cancers in each world region (Guan et al., 2012). Findings from randomised vaccine trials suggested that the percentage of cervical cancer preventable by current HPV vaccines may be substantially higher than 70% because of cross‐protection against other oncogenic HPV types (Lehtinen et al., 2012). WHO recommended in 2009 that routine HPV vaccination of adolescent girls should be included in national immunisation programmes, provided cervical cancer constitutes a public health priority and cost effectiveness can be shown (World Health Organization, 2009). The price of HPV vaccines was initially far too high to be affordable in developing countries but it is rapidly decreasing. In addition, GAVI opened in April 2012 a funding window to support HPV vaccine introduction in GAVI‐eligible countries. The cost‐effectiveness of HPV vaccination programmes is therefore improving in both developing and developed countries provided high and equitable coverage of adolescent girls (>70%) can be achieved.
A remaining outstanding priority for research is to establish the most appropriate mechanism for H. pylori eradication and the impact on stomach cancer risk. IARC classified this bacterium as a human carcinogen in 1986, exposure is widespread globally, antibiotic treatments are available and yet to date the best prevention strategy remains undefined (IARC, 2012b).
5.2. Cancer and the environment
Environmental causes of cancer, encompassing environmental contaminants or pollutants, naturally occurring toxins, occupationally‐related exposures and radiation, can make substantial contributions to specific cancers or cancer clusters on a smaller scale. These exposures can also be amenable to low‐cost modification by regulation, thus reducing the burden of some very lethal cancers with straightforward operable measures. In addition, environmental causes add to health inequality in low‐income countries where it is often the most vulnerable groups that are affected most by those cancers.
Approximately 50 occupational agents and work‐related exposure circumstances are carcinogenic to humans (Cogliano et al., 2011), for example arsenic, benzene, chromium VI compounds, nickel compounds, polycyclic aromatic hydrocarbons, silica, soot, vinyl chloride, and several pesticides, and in occupations such as aluminium production, chimney sweeping, coke production, painters or rubber processing. In the United Kingdom, 5.3% of cancers were estimated to be attributable to occupation, including breast cancer due to shift work (Rushton et al., 2012). Occupation‐attributable fraction may be, however, higher in countries with less stringent standards of worker protection, less attention to industrial hygiene or with child labour.
Many of these occupation‐related chemicals or exposures also occur as environmental pollutants in air, soil or drinking water, at generally lower exposure levels than for workers but often appearing as highly localised pollution due to insufficient waste management or inadequate protection of the public or the environment. Outdoor air pollution due to industry or traffic has high local variability. Specifically in some low‐ and middle‐ income countries indoor air pollution is relevant for cancer prevention when solid fuels are used for cooking or heating in insufficiently ventilated places (Cogliano et al., 2011) (see also Table 1). Asbestos accounts for almost all of the mesothelioma burden worldwide today (IARC, 2012e), predominantly via occupational exposure, and is also accountable for at least as many lung cancers.
Naturally‐occurring carcinogenic chemicals are also among those which merit a regional priority for targeted cancer prevention. Aflatoxins contaminate grain and nuts in the field and during storage in humid environments (IARC, 2012e) and were estimated to have a causative role in 5–28% of all global hepatocellular cancers (Wild and Gong, 2010; Liu and Wu, 2010). Low‐cost methods to diminish aflatoxin contamination exist. Arsenic is another naturally‐occurring environmental carcinogen that can contribute to cancers of the lung, skin, and bladder (IARC, 2012e).
Ionising radiation, or more specifically X‐rays and gamma radiation, neutrons, and alpha‐ and beta‐particle emitting radionuclides, are risk factors for several cancer types (IARC, 2012d; UNSCEAR, 2011). Diagnostic X‐rays were estimated to contribute between 0.5 and 3% to the overall cancer burden in high‐income countries (Berrington de and Darby, 2004). Risk related to radon is high in miners and residential radon has been estimated to cause 2% of cancer deaths in Europe with particularly high risks among smokers (Darby et al., 2005). Protection against solar radiation and avoidance of UV tanning devices are effective cancer prevention strategies especially in populations of people with light‐coloured skin (IARC, 2012d).
6. Early diagnosis and screening
The majority of cancers have a long latent phase and are preceded by pre‐neoplastic lesions. Early detection and treatment of cancer or precancerous lesions allowed substantial declines in cancer mortality in high‐resource countries and would greatly improve survival in low‐resource countries where access to expensive cancer treatment is limited (Sankaranarayanan and Swaminathan, 2011). Firm evidence of efficacy of screening programmes in the reduction of cancer mortality exists for three cancer sites: the cervix uteri, breast, and colon‐rectum (IARC, 2002, 2005; Segnan, 2010).
Cervical cancer screening stands out compared to other screenings as it allows the recognition and treatment of precancerous lesions using relatively inexpensive and minimally invasive tests (Pap smear, visual inspection techniques, and HPV‐testing) that can be chosen according to different country settings. The superiority of HPV‐testing compared to cytology in terms of sensitivity, duration of negative predictive value and reproducibility of test results across different diagnostic laboratories has been demonstrated by a number of randomised clinical trials and prospective data in high‐ and low‐resource countries (Franceschi et al., 2011). A consensus exists that cytology has a better role to play in the additional evaluation (triage) of HPV‐positive women than as a primary screening test (Franceschi et al., 2011). Visual inspection methods are less sensitive and specific than either HPV‐testing or good‐quality cytology but they may be useful to establish a first cervical screening infrastructure in low‐resource countries and inform treatment after HPV‐testing. Problems remain, however, with respect to the cost of HPV testing and the management of HPV‐positive women. A simple and cheap HPV test, careHPV™ (Qiagen), has proved to be very accurate (Qiao et al., 2008) but, unfortunately, is not yet available.
Breast cancer screening is an obvious priority on account of the high frequency of the disease in high‐ and very high HDI countries and the rise of breast cancer in medium‐ and low‐HDI countries. The only screening test of demonstrated efficacy is mammography (IARC, 2002). Mammography‐based screening programmes are well‐established in high‐resource countries but major discrepancies exist in the literature on screening efficiency. The number of women who would need to be screened to prevent one death from breast cancer varied by 5‐fold according to different studies (Beral et al., 2011). The randomised evidence indicates, however, that in high‐HDI countries, around one breast cancer death would be prevented in the long term for every 400 women aged 50–70 years regularly screened over a 10‐year period (Beral et al., 2011). Mammography‐based screening, however, requires larger introduction and running costs than the tests available for cervical cancer screening and it is not consistently recommended in low‐ and medium‐HDI countries in which breast cancer incidence rates are lower than in high‐HDI countries. However, due to the substantial survival advantages of down‐staging, a broad range of strategies may be envisaged to anticipate breast cancer diagnosis according to different country settings (Harford, 2011). These may initially include improvements in breast cancer awareness among women and health workers; facilitation of access of women with clinically detectable lumps to high‐quality diagnostic facilities and of women with breast cancer to effective systemic treatment (Sankaranarayanan et al., 2011).
For colorectal cancer screening in high‐resource countries the issue is not whether to screen but rather how to screen. Randomised controlled trials showed that faecal occult blood tests and flexible sigmoidoscopy reduce colorectal cancer mortality, but there is much less evidence about the additional benefit from colonoscopy (Harris and Kinsinger, 2011; Segnan, 2010). Colorectal cancer screening using flexible sigmoidoscopy offers the double benefit of early cancer detection and removal of precancerous lesions (relatively large polyps). Analogous to breast cancer screening, transfer to low‐and medium‐ HDI‐countries is not yet advocated on account of the high cost and quality requirements of the screening process.
Evidence of mortality benefit from prostate cancer screening using prostate‐specific antigen (PSA) is weak. A large US‐based randomised controlled trial showed no significant reduction in prostate cancer mortality after a 13‐year follow up (Andriole et al., 2012). Another similar European‐based trial found a 20% mortality reduction, possibly linked to higher‐standard treatment of cases diagnosed within the intervention arm than in the control group (Wolters et al., 2010). Different use of PSA in the control group (85% and 24%, respectively, in US‐ and the Europe‐based study) may also partly explain the discrepancy between the two trials (D'Amico, 2012). Yet, the high uptake of PSA since the late 1980s in the United States and, later, in Australia, Canada, and many European countries led to a sharp rise in prostate cancer incidence rates followed by a much more modest mortality decline. It is of note that countries that did not start large‐scale screening showed some decline in prostate cancer mortality without the harm of over‐diagnosis (Shibata and Whittemore, 2001). Experts argued that large‐scale use of PSA was adopted prematurely (Brawley, 2009). Notably, contrary to the other effective cancer screening programmes, prostate cancer screening spread before consensus on the best treatment for localised prostate cancer. Many treatment options exist and some of these treatments are very expensive and have serious and long‐lasting side effects. As noted by Brawley (2009), every treatment looks good when more than 90% of men getting it do not need it.
Prostate cancer is the best known but not the only example of the potential risk of an intensive search for early lesions accompanied by a lack of adequate knowledge of the biology of the targeted cancer and the way to treat conservatively screen‐detected lesions. Cancer over‐diagnosis (i.e. diagnosis of a cancer that would otherwise not go on to cause symptoms or death, Welch and Black, 2010) occurs when there is a vast disease reservoir and activities leading to its detection. Detection can be intentional (e.g., visual inspection for the detection of melanoma and non‐melanoma skin cancer) or unintentional (detailed imaging of the brain, thorax, abdomen, and pelvis intended to evaluate symptoms that are not cancer‐specific).
Thyroid cancer is another important, though little discussed, example of possible over‐diagnosis. In high‐resource countries, small suspicious thyroid nodules are typically discovered by manual palpation of the gland or using ultrasound. Complaints that are especially often reported to the doctor by young women (e.g., weight variation, fatigue, irritability, etc) can also lead to the evaluation of the thyroid gland. Thyroid cancer incidence rates have been rapidly rising over the last two or three decades in many countries (Kilfoy et al., 2009; Dal Maso et al., 2011). Papillary carcinoma accounts for approximately 75% of thyroid cancer cases in high‐resource countries (Kilfoy et al., 2009; Dal Maso et al., 2011). In 2008, thyroid cancer was the ninth most frequent cancer in women and the sixteenth in men of all ages worldwide. However, in women aged 15–45 years thyroid cancer was the second most frequent cancer in more developed countries (age‐standardised incidence rate = 10.1/100000) and the third most frequent in less developed countries (age‐standardised incidence rate = 2.8/100000) (Table 4) (Ferlay et al., 2010). Only incidence rates of breast cancer and, in less developed countries, cervical cancer were more elevated in women aged 15–45 years. Incidence‐to‐mortality (I/M) ratios tend to be relatively favourable for all cancer types in young women, except for leukaemia in less developed countries, but I/M ratios for thyroid cancer were by far the largest: 1 death out of 203 incident cases in more developed countries and 1 out of 19 in less developed countries. It is noteworthy that, despite excellent survival, thyroid cancer treatment (thyroidectomy, radioactive iodine, and lifelong thyroid hormone replacement therapy) is associated with substantial side effects (Singer et al., 2012).
Table 4.
Age‐standardised incidence (I) and mortality (M) rates of the most frequent cancer types in women aged 15‐to‐44 years in more developed and less developed countries (GLOBOCAN).
| Cancer | No of new cancers | Age‐standardised incidence rate (per 100,000) | Age‐standardised mortality rate (per 100,000) | I/M ratio |
|---|---|---|---|---|
| a) More developed countries | ||||
| Breast | 78,001 | 27.7 | 4.1 | 7 |
| Thyroid | 26,251 | 10.1 | 0.0a | 203 |
| Cervix uteri | 25,638 | 9.7 | 1.9 | 5 |
| Melanoma of skin | 20,675 | 7.9 | 0.5 | 14 |
| Ovary | 11,105 | 4.1 | 1.0 | 4 |
| Colorectum | 10,896 | 3.9 | 1.1 | 4 |
| b) Less developed countries | ||||
| Breast | 207,679 | 16.1 | 4.3 | 4 |
| Cervix uteri | 152,496 | 11.8 | 3.8 | 3 |
| Thyroid | 36,282 | 2.8 | 0.1 | 19 |
| Ovary | 35,381 | 2.7 | 1.1 | 3 |
| Leukaemia | 30,735 | 2.4 | 1.9 | 1 |
| Colorectum | 29,076 | 2.3 | 1.1 | 2 |
Corresponding to an estimate of 129 deaths.
The existence of an especially large reservoir of slowly growing tumours in the thyroid gland is also demonstrated by the frequent detection of small papillary carcinoma in autopsy series (Riboli and Delendi, 1991). In a Finnish study of 101 thyroids in children and adults younger than 40 years, 36% harboured one foci of papillary carcinoma or more, with little variation by sex or age (Harach et al., 1985). Sixty‐seven percent of tumours were below 1 mm.
7. The future for prevention
Whilst innovative cancer treatments and improved access to affordable and effective cancer treatments are required (Farmer et al., 2010), it is difficult to envisage dealing with a doubling in cancer numbers in just two decades by improved efficacy, effectiveness or access to treatment alone, particularly in the low‐and medium‐HDI countries. This report illustrates what is known and what could be done if the evidence‐base for cancer prevention was put into practice now. Clearly such approaches need to be tailored to the particular challenges faced by a given country or region. By consequence, a focus only on shared common risk factors for NCDs (i.e., tobacco use, unhealthy diet, physical inactivity, and the harmful use of alcohol) would miss some special prevention opportunities (Wild, 2012).
In addition, whilst much could already be done, notably reducing tobacco‐ and infection‐associated cancers, there are significant gaps in knowledge which limit cancer prevention. First, for a number of common cancers the major risk factors remain unidentified. Second, in relation to screening, there is an inability to characterise which screen‐detected cancers will progress and result in morbidity and mortality for the patient, leading to a risk of over‐diagnosis. Third, whilst a number of prevention strategies have been demonstrated in clinical or community‐based trials, the implementation of these into health services remains sub‐optimal or incomplete. Each of these areas should be a priority for future research (Wild, 2012).
One area of great promise is to use the rapid advances in understanding carcinogenesis and the associated laboratory tools (e.g. biomarkers) to provide new opportunities for primary and secondary cancer prevention. Among these opportunities are: improved exposure assessment; elucidation of mechanistic pathways related to defined exposures; identification of molecular markers which indicate risk of disease progression; and stratification of cancer cases by molecular subtype in relation to specific exposures. An especially important development would be the identification of pre‐neoplastic conditions at stages that are still reversible and for which medical treatments may either be curative or at least able to stop disease progression. Such progress would diminish the current gap between rather expensive and potentially harmful screening approaches in cancer (i.e., early detection of neoplastic or pre‐neoplastic lesions that require surgical ablation) and the more effective screening approaches in cardio‐vascular diseases (i.e., detection and medical treatment of predisposing conditions such as hypertension, and hypercholesterolemia).
For these opportunities to be realised, however, basic science must be driven towards application to epidemiology and public health. Achieving this would require a multi‐faceted approach involving education and training, infrastructure (including co‐location of disciplines), resource allocation by funders and political prioritisation. Nevertheless, there is a responsibility at least as far as publically‐funded research is concerned, to translate the “common soil” of basic science to prevention in analogous fashion to the translation into better treatment, hence our call for “two‐way” translational cancer research (Wild, 2010, 2011). Policymakers and funders must prioritise translational research into the causes and prevention of cancer on a scale not yet seen by comparison to translational research from bench to beside. The longer‐term benefits of prevention in reducing years of life lost and the economic cost of cancer would counterbalance this investment.
This broader concept of translational cancer research and its potential to inform cancer prevention stands at an exciting but critical point in time. The causes of many cancers remain to be discovered, limiting primary prevention. Secondary prevention is available for some cancers, but improved management of individuals after a positive screening test is needed. Advances in basic science offer clear opportunities to unravel the complex aetiology of the disease and provide mechanism‐based entry points to disrupt the progression of the disease towards frank malignancy. However, if the benefits for society are to be realised then a fresh, innovative approach is required through interdisciplinary research and a robust, more balanced funding of research in cancer prevention and cancer treatment. To miss this opportunity will be to fail future generations, particularly those which are amongst the most vulnerable, commonly residing in poor countries.
Funding source
None.
Acknowledgements
The authors would like to acknowledge the helpful comments provided by Freddie Bray, Jacques Ferlay, David Forman, and Joachim Schüz and technical assistance by Ms Veronique Chabanis and Ms Laurence Marnat.
Franceschi Silvia, Wild Christopher P., (2013), Meeting the global demands of epidemiologic transition — The indispensable role of cancer prevention, Molecular Oncology, 7, doi: 10.1016/j.molonc.2012.10.010.
Contributor Information
Silvia Franceschi, Email: FranceschiS@iarc.fr.
Christopher P. Wild, Email: director@iarc.fr
References
- Andre, F.E. , Booy, R. , Bock, H.L. , 2008. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ.. 86, 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriole, G.L. , Crawford, E.D. , Grubb, R.L. , 2012. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J. Natl. Cancer Inst.. 104, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beral, V. , Alexander, M. , Duffy, S. , 2011. The number of women who would need to be screened regularly by mammography to prevent one death from breast cancer. J. Med. Screen.. 18, 210–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrington de, G.A. , Darby, S. , 2004. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 363, 345–351. [DOI] [PubMed] [Google Scholar]
- Brawley, O.W. , 2009. Prostate cancer screening; is this a teachable moment?. J. Natl. Cancer Inst.. 101, 1295–1297. [DOI] [PubMed] [Google Scholar]
- Bray, F. , Jemal, A. , Grey, N. , 2012. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol.. 13, 790–801. [DOI] [PubMed] [Google Scholar]
- Bray, F. , Ren, J.S. , Masuyer, E. , 2012. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int. J. Cancer 2012 Jul 3.. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chang, M.H. , 2003. Decreasing incidence of hepatocellular carcinoma among children following universal hepatitis B immunization. Liver Int.. 23, 309–314. [DOI] [PubMed] [Google Scholar]
- Cogliano, V.J. , Baan, R. , Straif, K. , 2011. Preventable exposures associated with human cancers. J. Natl. Cancer Inst.. 103, 1827–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico, A.V. , 2012. Prostate-cancer mortality after PSA screening. N. Engl. J. Med.. 366, 2229–2231. [DOI] [PubMed] [Google Scholar]
- Dal Maso, L. , Lise, M. , Zambon, P. , 2011. Incidence of thyroid cancer in Italy, 1991-2005: time trends and age-period-cohort effects. Ann. Oncol.. 22, 957–963. [DOI] [PubMed] [Google Scholar]
- Danaei, G. , Vander, H.S. , Lopez, A.D. , 2005. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 366, 1784–1793. [DOI] [PubMed] [Google Scholar]
- Darby, S. , Hill, D. , Auvinen, A. , 2005. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 330, 223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, N.E. , 1990. The Armitage-Doll multistage model of carcinogenesis. Stat. Med.. 9, 677–679. [DOI] [PubMed] [Google Scholar]
- de Martel, C. , Ferlay, J. , Franceschi, S. , 2012. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol.. 13, 607–615. [DOI] [PubMed] [Google Scholar]
- Doll, R. , Peto, R. , 1981. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst.. 66, 1191–1308. [PubMed] [Google Scholar]
- EUROGAST, 1993. An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST Study Group. Lancet. 341, 1359–1362. [PubMed] [Google Scholar]
- Ezzati, M. , Hoorn, S.V. , Lopez, A.D. , Danaei, G. , Rodgers, A. , Mathers, C.D. , Murray, C.J.L. , 2006. Comparative quantification of mortality and burden of disease attributable to selected risk factors. In Lopez A.D., Mathers C.D., Ezzati M., Jamison D.T., Murray C.J.L.(Eds.), Global Burden of Disease and Risk Factors. A co-publication of The World Bank and Oxford University Press; Washington: 241–396. [PubMed] [Google Scholar]
- Farmer, P. , Frenk, J. , Knaul, F.M. , 2010. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet. 376, 1186–1193. [DOI] [PubMed] [Google Scholar]
- Ferlay, J. , Shin, H.R. , Bray, F. , 2010. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 127, 2893–2917. [DOI] [PubMed] [Google Scholar]
- Franceschi, S. , Denny, L. , Irwin, K.L. , 2011. Eurogin 2010 roadmap on cervical cancer prevention. Int. J. Cancer. 128, 2765–2774. [DOI] [PubMed] [Google Scholar]
- Franceschi, S. , Herrero, R. , Clifford, G.M. , 2006. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int. J. Cancer. 119, 2677–2684. [DOI] [PubMed] [Google Scholar]
- Franceschi, S. , Lise, M. , Clifford, G.M. , 2010. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br. J. Cancer. 103, 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovino, G.A. , Mirza, S.A. , Samet, J.M. , 2012. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 380, 668–679. [DOI] [PubMed] [Google Scholar]
- Guan, P. , Howell-Jones, R. , Li, N. , 2012. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int. J. Cancer. 131, 2349–2359. [DOI] [PubMed] [Google Scholar]
- Harach, H.R. , Franssila, K.O. , Wasenius, V.M. , 1985. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 56, 531–538. [DOI] [PubMed] [Google Scholar]
- Harford, J.B. , 2011. Breast-cancer early detection in low-income and middle-income countries: do what you can versus one size fits all. Lancet Oncol.. 12, 306–312. [DOI] [PubMed] [Google Scholar]
- Harris, R. , Kinsinger, L.S. , 2011. Less is more: not "going the distance" and why. J. Natl. Cancer Inst.. 103, 1726–1728. [DOI] [PubMed] [Google Scholar]
- IARC, 2002. IARC Handbooks of Cancer Prevention Breast Cancer Screening. vol. 7, IARC Press; Lyon: [Google Scholar]
- IARC, 2005. IARC Handbooks of Cancer Prevention Cervix Cancer Screening. vol. 10, IARC Press; Lyon: [Google Scholar]
- IARC, 2012. Monographs on the Evaluation of Carcinogenic Risks to Humans A Review of Human Carcinogens: Pharmaceuticals. vol. 100A, International Agency for Research on Cancer; Lyon: Monographs on the Evaluation of Carcinogenic Risks to Humans [Google Scholar]
- IARC, 2012. Monographs on the Evaluation of Carcinogenic Risks to Humans A Review of Human Carcinogens: Biological Agents. vol. 100B, International Agency for Research on Cancer; Lyon: Monogr Eval Carcinog Risks Hum. International Agency for Research on Cancer. Monographs on the Evaluation of Carcinogenic Risks to Humans [PMC free article] [PubMed] [Google Scholar]
- IARC, 2012. Monographs on the Evaluation of Carcinogenic Risks to Humans A Review of Human Carcinogens: Arsenic, Metals, Fibres, and Dusts. vol. 100C, International Agency for Research on Cancer; Lyon: Monographs on the Evaluation of Carcinogenic Risks to Humans [PMC free article] [PubMed] [Google Scholar]
- IARC, 2012. Monographs on the Evaluation of Carcinogenic Risks to Humans A Review of Human Carcinogens: Radiation. vol. 100D, International Agency for Research on Cancer; Lyon: Monographs on the Evaluation of Carcinogenic Risks to Humans [Google Scholar]
- IARC, 2012. Monographs on the Evaluation of Carcinogenic Risks to Humans A Review of Human Carcinogens: Chemical Agents and Related Occupations. vol. 100F, International Agency for Research on Cancer; Lyon: Monographs on the Evaluation of Carcinogenic Risks to Humans [PMC free article] [PubMed] [Google Scholar]
- Keita, N. , Clifford, G.M. , Koulibaly, M. , 2009. HPV infection in women with and without cervical cancer in Conakry, Guinea. Br. J. Cancer. 101, 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilfoy, B.A. , Zheng, T. , Holford, T.R. , 2009. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 20, 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk, G.D. , Lesi, O.A. , Mendy, M. , 2004. The Gambia Liver Cancer Study: infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology. 39, 211–219. [DOI] [PubMed] [Google Scholar]
- Lehtinen, M. , Paavonen, J. , Wheeler, C.M. , 2012. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol.. 13, 89–99. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Wu, F. , 2010. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ. Health Perspect.. 118, 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maule, M. , Merletti, F. , 2012. Cancer transition and priorities for cancer control. Lancet Oncol.. 13, 745–746. [DOI] [PubMed] [Google Scholar]
- Omran, A.R. , 1971. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem. Fund. Q.. 49, 509–538. [PubMed] [Google Scholar]
- Peto, J. , 2001. Cancer epidemiology in the last century and the next decade. Nature. 411, 390–395. [DOI] [PubMed] [Google Scholar]
- Qiao, Y.L. , Sellors, J.W. , Eder, P.S. , 2008. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol.. 9, 929–936. [DOI] [PubMed] [Google Scholar]
- Raza, S.A. , Clifford, G.M. , Franceschi, S. , 2007. Worldwide variation in the relative importance of hepatitis B and hepatitis C viruses in hepatocellular carcinoma: a systematic review. Br. J. Cancer. 96, 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboli, E. , Delendi, M. , 1991. Autopsy in Epidemiology and Medical Research IARC Scientific Publication No 112 IARC Scientific Publications Series. International Agency for Research on Cancer; Lyon: [Google Scholar]
- Rushton, L. , Hutchings, S.J. , Fortunato, L. , 2012. Occupational cancer burden in Great Britain. Br. J. Cancer. 107, (Suppl. 1) S3–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan, R. , Ramadas, K. , Thara, S. , 2011. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. J. Natl. Cancer Inst.. 103, 1476–1480. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan, R. , Swaminathan, R. , 2011. Cancer Survival in Africa, Asia, the Caribbean and Central America (SurvCan) IARC Scientific Publication No 162 IARC Scientific Publications Series. International Agency for Research on Cancer; Lyon: [Google Scholar]
- In Segnan N., Patnick J., von Karsa L.(Eds.), European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis. first ed. European Commission, Publications Office of the European Union; Luxembourg: [Google Scholar]
- Shibata, A. , Whittemore, A.S. , 2001. Re: prostate cancer incidence and mortality in the United States and the United Kingdom. J. Natl. Cancer Inst.. 93, 1109–1110. [DOI] [PubMed] [Google Scholar]
- Singer, S. , Lincke, T. , Gamper, E. , 2012. Quality of life in patients with thyroid cancer compared with the general population. Thyroid. 22, 117–124. [DOI] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs Population Division, 2012. Changing Levels and Trends in Mortality: The Role of Patterns of Death by Cause United Nations publication; (ST/ESA/SER.A/318) [Google Scholar]
- UNSCEAR, 2011. Report of the United Nations Scientific Committee on the Effects of Atomic Radiation 2010. New York, United Nations.
- Welch, H.G. , Black, W.C. , 2010. Overdiagnosis in cancer. J. Natl. Cancer Inst.. 102, 605–613. [DOI] [PubMed] [Google Scholar]
- Wild, C.P. , 2010. OMICS technologies: an opportunity for "two-way" translation from basic science to both clinical and population-based research. Occup. Environ. Med.. 67, 75–76. [DOI] [PubMed] [Google Scholar]
- Wild, C.P. , 2011. Future research perspectives on environment and health: the requirement for a more expansive concept of translational cancer research. Environ. Health. 10, (Suppl. 1) S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild, C.P. , 2012. The role of cancer research in noncommunicable disease control. J. Natl. Cancer Inst.. 104, 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild, C.P. , Gong, Y.Y. , 2010. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 31, 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters, T. , Roobol, M.J. , Steyerberg, E.W. , 2010. The effect of study arm on prostate cancer treatment in the large screening trial ERSPC. Int. J. Cancer. 126, 2387–2393. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2009. Human papillomavirus vaccines. WHO position paper Wkly Epidemiol. Rec.. 84, 118–131. [PubMed] [Google Scholar]
- World Health Organization, 2011. Global Status Report on Noncommunicable Diseases 2010 World Health Organization; Geneva: [Google Scholar]


