Abstract
Background & Aim
Runt‐related transcription factor 3 (RUNX3) is a tumor suppressor gene that is expressed in gastric and other cancers including pancreatic cancer. However, the precise function of RUNX3 in pancreatic cancer has not been fully elucidated. In this study, we aimed to determine the effect of decreased RUNX3 expression in pancreatic cancer.
Methods
This study included 36 patients with primary pancreatic cancer, who had undergone pancreaticoduodenectomy. All patients were treated with 1000 mg/m2 gemcitabine after the surgery.
The pancreatic cancer cell lines PANC‐1, MIAPaCa‐2, BxPC‐3, SUIT‐2, and KLM‐1 were used for immunoblotting analysis of RUNX3 and multidrug resistance protein (MRP) expressions. Ectopic RUNX3 expression was achieved by cDNA transfection of the cells, and small interfering RNA (siRNA) against RUNX3 was used to knock down endogenous RUNX3. Cell growth in the presence of gemcitabine was assessed using the MTT assay.
Results
Patients with RUNX3‐positive and RUNX3‐negative pancreatic cancer had a median survival of 1006 and 643 days, respectively. Exogenous RUNX3 expression reduced the expression of MRP1, MRP2, and MRP5 in endogenous RUNX3‐negative cells, whereas RUNX3 siRNA increased the expressions of these genes in endogenous RUNX3‐positive cells. Exogenous RUNX3 expression decreased gemcitabine IC50 in RUNX3‐negative cells.
Conclusion
Loss of RUNX3 expression contributes to gemcitabine resistance by inducing MRP expression, thereby resulting in poor patient survival.
Keywords: Pancreaticoduodenectomy, Survival, Adjuvant chemotherapy, Runt-related transcription factor 3 (RUNX3)
Highlights
We categorized pancreatic cancer into 2 groups by RUNX3 expression.
We defined the staining grade as low or high by using 30% staining area as cutoff.
The prognosis was significantly poor in RUNX3‐negative subjects.
RUNX3 protein expression reduced the expression of multidrug‐resistant proteins.
RUNX3 expression increased gemcitabine sensitivity in pancreatic cancer cells.
Abbreviations
- ABC
ATP-binding cassette
- ABCC
ATP-binding cassette subfamily C
- CAT
chloramphenicol acetyltransferase
- cDNA
complementary DNA
- FBS
fetal bovine serum
- 5-FU
5-fluorouracil
- MRP
multidrug resistance protein
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- RUNX3
runt-related transcriptional factor 3
- SDS
sodium dodecyl sulfate
- siRNA
small interfering RNA
- TBS-T
Tris-buffered saline with Tween 20
- TGF-β
transforming growth factor-β
1. Introduction
Although pancreatic cancer has a low incidence rate of approximately 20 cases per 100,000 persons annually (Jemal et al., 2010), it is one of the most fatal cancers. In recent years, the outcome of patients with pancreatic cancer has improved following introduction of gemcitabine as standard therapy; however, the prognosis remains poor (Hidalgo, 2010).
Pancreatic cancer is often diagnosed at an advanced stage because of the lack of biomarkers for early detection. To date, there is no effective therapy for pancreatic cancer. The development of drug resistance presents a major problem in gemcitabine chemotherapy for pancreatic cancer, and elucidating the underlying molecular mechanisms of drug resistance would facilitate development of more effective therapeutic strategies (Arumugam et al., 2009).
One of the various molecular mechanisms postulated to underlie drug resistance in pancreatic cancer is decreased accumulation of anticancer drugs in cancer cells due to increased drug efflux (Bergman et al., 2003). Members of the ATP‐binding cassette (ABC) transporter superfamily are the major proteins involved in drug efflux (Dean et al., 2001). Multidrug resistance protein (MRP) 2, an ABC transporter, seems to play an important role in sensitivity to gemcitabine–cisplatin combination therapy in pancreatic cancer (Tanaka et al., 2011). Previous reports showed that MRP expression contributes in predicting the efficacy of chemotherapy (Konig et al., 2005). MRPs were upregulated in 5‐fluorouracil (5‐FU)‐resistant cancer cells and MRP5 expression influenced 5‐FU resistance in pancreatic carcinoma cells (Hagmann et al., 2009). Furthermore, a study showed a significant association between MRP5 and gemcitabine sensitivity in lung cancer (Oguri et al., 2006).
The pathogenesis of pancreatic ductal adenocarcinoma is induced by alteration in genes including K‐ras, p53, p16, and smad4 (Efthimiou et al., 2001; Giovannetti et al., 2006; Jaffee et al., 2002; Jones et al., 2008; Kern et al., 2002; Maitra and Hruban, 2008; Mimeault et al., 2005). K‐ras mutation is common in human pancreatic cancer (Giovannetti et al., 2006), and the transforming growth factor‐β (TGF‐β) signaling pathway is an important tumor‐suppressing signal (Nomoto et al., 2008; Subramaniam et al., 2009). The human runt‐related transcription factor 3 (RUNX3) gene, a tumor suppressor gene expressed in gastric cancers (Efthimiou et al., 2001), regulates cell growth and apoptosis as a downstream effector of TGF‐β signaling (Subramaniam et al., 2009). Previous reports showed that silencing mechanisms such as loss of heterozygosity and gene promoter hypermethylation result in loss of RUNX3 function (Bae and Choi, 2004; Li et al., 2002; Nomoto et al., 2008; Wada et al., 2004) and that RUNX3 expression is correlated with clinicopathological variables in pancreatic cancer (Li et al., 2004; Nomoto et al., 2008; Subramaniam et al., 2009). In this study, we investigated the correlation of RUNX3 expression with survival and gemcitabine sensitivity in human pancreatic cancer.
2. Materials and methods
2.1. Patients
Thirty‐six patients with primary pancreatic cancer, who underwent pancreaticoduodenectomy at the Okayama University Hospital, Okayama, Japan, between March 2004 and May 2009, were enrolled in this study. The patients received adjuvant chemotherapy after resection. Gemcitabine (Eli Lilly Co., Indianapolis, IN) was administrated at a starting dose of 1000 mg/m2 by a 30‐min intravenous infusion weekly for 3 out of 4 weeks. Pancreatic tissue samples were obtained from all patients after obtaining their informed consent as per institutional guidelines, and the study was approved by the Research Ethics Committee of Okayama University. For pathological tumor staging, we used the TNM classification system (Union for International Cancer Control [UICC]).
2.2. Pancreatic cancer tissue and immunohistochemistry
Pancreatic cancer and adjacent tissues were used in the analysis. None of the patients received chemotherapy before surgery.
Immunohistochemistry was performed on formalin‐fixed paraffin‐embedded sections. The sections were dewaxed and dehydrated. After rehydration, endogenous peroxidase activity was blocked for 30 min in a methanol solution containing 0.3% hydrogen peroxide. After antigen retrieval in citrate buffer, the sections were blocked overnight at 4 °C, and then probed with anti‐RUNX3 antibody (Abcam, Cambridge, MA). The primary antibody was detected with a biotinylated anti‐mouse antibody (Dako Japan, Tokyo, Japan). The signal was amplified via avidin–biotin complex formation and developed using diaminobenzidine. The sections were counterstained with hematoxylin and independently examined by 2 observers (T.T. and K.I.) blinded to the study. All discrepancies in RUNX3 expression evaluation were reviewed, and a consensus was reached. The cutoff for positive staining was set at 30% according to Li et al. (2004). The staining grade was defined as low or high. Samples showing negative or <30% staining were categorized as the low‐staining group, and samples with ≥30% staining area were categorized as the high‐staining group. To exclude misleading evaluation for nonspecific staining, we counted cells showing sharply defined staining as positive.
2.3. Gene expression profiling analysis in human pancreatic cancer
Correlations between the expressions of RUNX3 and MRPs were assessed using publicly available pancreatic cancer data sets from Oncomine (http://www.oncomine.org). Briefly, the mRNA expression profiles for RUNX3, MRP1, MRP2, and MRP5 were evaluated using RUNX3/234928_x_at, RUNX3/IMAGE:291478, MRP1/202804_at, MRP2/IMAGE:196387, and MRP5/226363_at, respectively.
2.4. Statistical analysis
Correlations between RUNX3 expression and clinicopathological variables were analyzed using the χ 2 test. Survival rates were calculated by the Kaplan–Meier method, and survival curves were compared using the log‐rank test. A Cox proportional hazard model was used to assess the influence of each variable on survival. For in vitro studies, each experiment was performed independently at least twice with similar results. The data were compared using Student's t‐test. The JMP software (SAS Institute Inc., Cary, NC) was used for the analyses. A P value of <0.05 was considered statistically significant.
2.5. Cell lines and cell culture
The human pancreatic cancer cell lines PANC‐1, MIAPaCa‐2, and BxPC‐3 were obtained from DS Pharma Biochemical Co., Ltd. (Osaka, Japan); SUIT‐2 from the Japanese Collection of Research Bioresources (Tokyo, Japan); and KLM‐1 from the Cell Resource Center of the Biomedical Research Institute of Development, Aging, and Cancer at Tohoku University (Sendai, Japan). PANC‐1 and MIAPaCa‐2 were maintained in Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA), and BxPC‐3, SUIT‐2, and KLM‐1 were maintained in RPMI 1640 (Sigma, St. Louis, MO). The media were supplemented with 10% heat‐inactivated fetal bovine serum (FBS) (Sigma), 1% nonessential amino acids (Sigma), 1% sodium pyruvate (Sigma), and 1% penicillin/streptomycin solution (Sigma). Cells were cultured at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. Before the experiment, cells were quiesced at subconfluence under restricted serum conditions by using 0.1% dialyzed FBS for 24 h.
2.6. Ectopic RUNX3 expression
A human RUNX3 construct was obtained by reverse transcriptase‐PCR‐based cloning of a normal human hepatocyte (Sanko Junyaku, Co. Ltd., Tokyo, Japan) (Nakanishi et al., 2011). Human RUNX3 and/or chloramphenicol acetyltransferase (CAT) (mock) constructs were transfected into PANC‐1, MIAPaCa‐2, and BxPC‐3 by using FuGENE™ 6 transfection reagent (Roche Diagnostics, Basel, Switzerland). At least 2 independent transfections were performed for each cell line. The cells were incubated under serum‐starved conditions for 24 h and used for the following experiments.
2.7. Immunoblot analysis
Cells were plated into 6‐well tissue culture plastic dishes and grown to confluence. Next, the cells were washed twice with cold phosphate‐buffered saline (PBS) and lysed with 150 μL of sample buffer (100 mM Tris–HCl [pH 6.8], 10% glycerol, 4% sodium dodecyl sulfate [SDS], 1% bromophenol blue, 10% β‐mercaptoethanol). The samples were resolved using SDS‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred onto Immobilon‐P™ membranes (Millipore Corporation, Bedford, MA). The membranes were blocked with the Tris‐buffered saline with Tween 20 (Sigma) (TBS‐T) buffer containing 1% bovine serum albumin for 1 h, and then incubated overnight with antibodies against RUNX3 (Abcam), MRP1 (Abcam), MRP2 (Abcam), MRP5 (Sigma), and α‐actin (Sigma) at 4 °C. The membranes were washed 3 times with TBS‐T, probed with horseradish peroxidase‐conjugated secondary antibody, and developed by enhanced chemiluminescence using an ECL Western Blotting Detection System (GE Healthcare, Buckinghamshire, UK).
2.8. MTT assay
Cell proliferation was assessed using the MTT (3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyl tetrazolium bromide) assay. Briefly, cells were grown in 96‐well plastic tissue culture dishes at a concentration of 104 cells/mL. After 24 h of quiescence, the cells were cultured for the indicated period with or without 10% FBS. Then, 10 μL of MTT (5 mg/mL in PBS) was added to each well, and the cells were incubated for an additional 4 h at 37 °C. The purple–blue formazan precipitate was dissolved using 100 μL of DMSO. Mitochondrial activity, which reflected cell viability, was evaluated by measuring the absorbance at 570 nm using a microplate reader (Bio‐Rad, Hercules, CA).
For evaluation of gemcitabine resistance, the cells were treated with various concentrations of gemcitabine for 72 h, and then, the MTT assay was performed as described previously in this section.
2.9. Gene silencing of RUNX3 by using small interfering RNA
RUNX3‐expressing SUIT‐2 and KLM‐1 cells were transfected with either scrambled negative control small interfering RNA (siRNA) or RUNX3 siRNA (Applied Biosystems, Foster City, CA) by using RNAiFect™ transfection reagent (Qiagen, Hilden, Germany). The cells were incubated for 24 h and then serum starved for 48 h. The MTT assay was performed as described in Section 2.8.
3. Results
3.1. RUNX3 expression in clinical samples improved the clinical course
Of the 36 patients enrolled in the study, 10 (28%) patients had RUNX3‐high pancreatic cancer (6 men and 4 women) and the remaining 26 (72%) patients had RUNX3‐low pancreatic cancer (15 men and 11 women).
Before resection, the cancers of all patients were staged using CT, MRI, and ultrasonography. There was no significant difference in the gender, age, UICC stage, pathologic T and N stage, histologic grade, and resection margin between the RUNX3‐high and RUNX3‐low groups (Table 1). Figure 1A shows representative images of RUNX3‐high and RUNX3‐low pancreatic cancer tissues. RUNX3 expression was observed in both the cytoplasm and nuclei. In one sample, some cells expressed RUNX3 in the cytoplasm, whereas other cells expressed RUNX3 in the nuclei.
Table 1.
Baseline characteristics and clinicopathological stage.
| RUNX3 expression | P value | |||
|---|---|---|---|---|
| High (n) | Low (n) | |||
| Age (y)a | 66.5 ± 10.5 | 64.7 ± 10.9 | 0.93 | |
| Gender | Male/female | 6/4 | 15/11 | 0.90 |
| UICC stage | IA | 0 | 2 | 0.65 |
| IB | 0 | 0 | ||
| IIA | 6 | 15 | ||
| IIB | 4 | 9 | ||
| III | 0 | 0 | ||
| IV | 0 | 0 | ||
| pT | 1 | 0 | 2 | 0.37 |
| 2 | 0 | 0 | ||
| 3 | 10 | 24 | ||
| 4 | 0 | 0 | ||
| pN | 0 | 6 | 17 | 0.76 |
| 1 | 4 | 9 | ||
| Histological grade | G1 | 3 | 10 | 0.29 |
| G2 | 7 | 12 | ||
| G3 | 0 | 4 | ||
| R | 0 | 9 | 24 | 0.82 |
| 1 | 1 | 2 | ||
Values are means ± SD.
Figure 1.
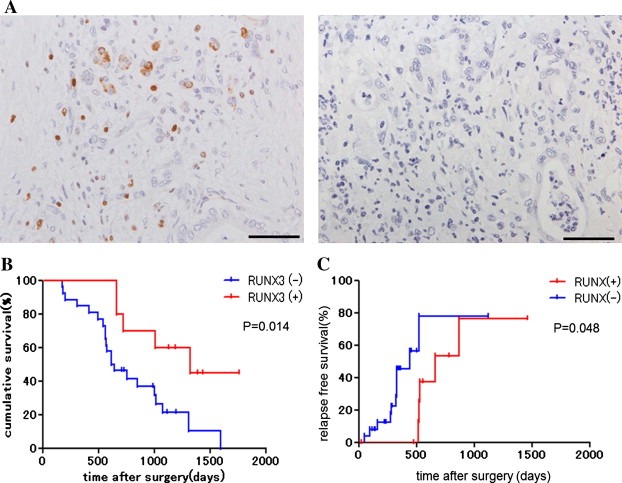
RUNX3 protein expression in pancreatic cancer samples. (A) RUNX3 protein expression in human pancreatic cancer tissue. RUNX3 was expressed in 10 of 36 (28%) patients. Scale bars: 100 μm. (B) Kaplan–Meier survival curves of gemcitabine‐treated patients. The survival of the 10 RUNX3‐high patients was significantly longer than that of the 26 RUNX3‐low patients (log‐rank test, P < 0.05). (C) Kaplan–Meier curves of time to recurrence of gemcitabine‐treated patients. The time to recurrence of the 10 RUNX3‐high patients was significantly longer than that of the 26 RUNX3‐low patients (log‐rank test, P < 0.05).
We investigated the association of RUNX3 expression with survival and time to recurrence. The RUNX3‐high and RUNX3‐low groups showed a median survival of 1322 days (range, 657–1757 days) and 627 days (range, 174–1590 days) (P = 0.014) (Figure 1B) and a median time to recurrence of 657 days (range, 22–1459 days) and 442 days (range, 49–1117 days) (P = 0.048) (Figure 1C), respectively.
3.2. RUNX3 was differentially expressed in pancreatic cancer cell lines
We determined RUNX3 expression in various pancreatic cancer cell lines by immunoblot analysis. RUNX3 protein was expressed in SUIT‐2 and KLM‐1 cells, but was undetectable in PANC‐1, MIAPaCa‐2, and BxPC‐3 cells (Supplementary Figure 1).
3.3. Ectopic RUNX3 expression decreased MRP protein expression
We analyzed ectopic RUNX3 expression in the endogenous RUNX3‐low pancreatic cancer cell lines: PANC‐1, MIAPaCa‐2, and BxPC‐3. RUNX3 protein expression was detected after transfection with the RUNX3 plasmid (Figure 2A).
Figure 2.
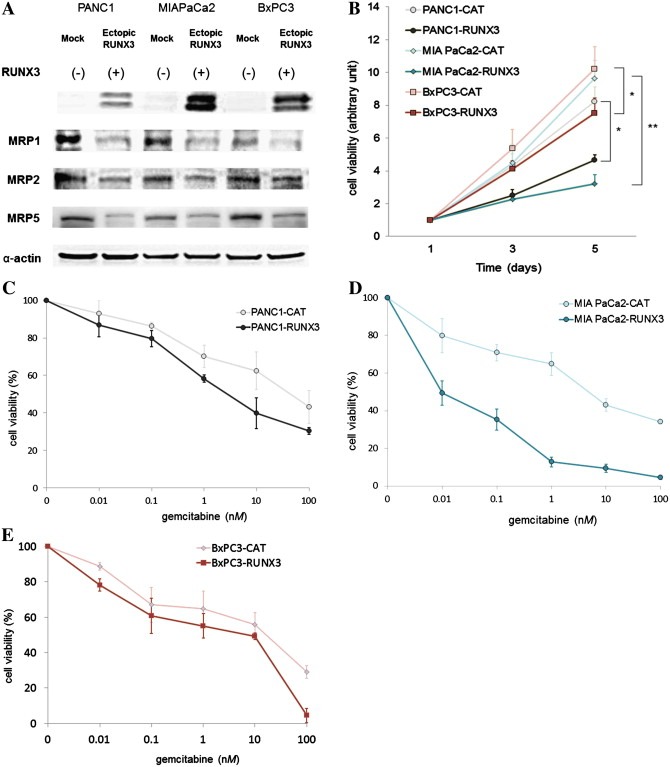
Ectopic RUNX3 protein expression in pancreatic cancer cell lines. Eukaryotic expression constructs for CAT (mock) and RUNX3 were introduced into PANC‐1, MIAPaCa‐2, and BxPC‐3 cells. (A) Immunoblot analysis of RUNX3 and MRP expression. Immunoblot analysis was performed using antibodies against RUNX3, MRP1, MRP2, MRP5, and α‐actin. Immunoblotting for α‐actin was done to verify equal loading. These 3 cell lines were originally endogenous RUNX3 negative. The left lane for each cell line represents mock‐transfected cancer cells, and the right lane represents RUNX3‐transfected cancer cells. Representative blots of more than 3 independent experiments. (B) Cell growth activity. Cell proliferative activities were measured by MTT assay. All results are expressed as ratios compared to day 1. Data represent the means ± SE of more than 3 independent experiments performed in triplicate. *P < 0.05; **P < 0.01, versus mock‐transfected cells (Student's t test). (C–E) Gemcitabine sensitivity. PANC‐1 (C), MIAPaCa‐2 (D), and BxPC‐3 (E) cell lines were treated with various concentrations of gemcitabine for 24 h. Cell viability was measured by MTT assay and expressed as a percentage relative to control cells. Data represent the means ± SE of more than 3 independent experiments performed in triplicate.
PANC‐1, MIAPaCa‐2, and BxPC‐3 cells expressed MRP1, MRP2, and MRP5. Strong MRP1 expression was observed in PANC‐1 and MIAPaCa‐2 at baseline. Exogenous RUNX3 expression generally decreased MRP expression in all 3 cell lines (Figure 2A). Expression of MRP1, MRP2, and MRP5 in RUNX3‐expressing PANC‐1, MIAPaCa‐2 and BxPC‐3 cells was weaker than that in control CAT‐expressing cells (Figure 2A).
3.4. Ectopic RUNX3 protein expression suppressed cell growth and increased gemcitabine sensitivity
Ectopic RUNX3 expression suppressed cell growth compared with control by 49%, 74%, and 29% in PANC‐1, MIAPaCa‐2, and BxPC‐3 cells, respectively, at 5 days after transfection (Figure 2B).
A chemosensitivity assay was performed on RUNX3‐ and CAT (mock)‐transfected PANC‐1, MIAPaCa‐2, and BxPC‐3 cells. RUNX3 expression enhanced gemcitabine sensitivity in all 3 cell lines; the gemcitabine IC50 decreased from 42 nM to 2.8 nM, from 4.0 nM to 0.01 nM, and from 17 nM to 7.8 nM in PANC‐1, MIAPaCa‐2, and BxPC‐3 cells, respectively (Figure 2C–E).
3.5. siRNA against RUNX3 increased cell growth and decreased gemcitabine sensitivity
After transfection with RUNX3 siRNA, RUNX3 expression was successfully knocked down in SUIT‐2 and KLM‐1 cells. RUNX3 siRNA‐treated cells exhibited increased MRP expression (Figure 3A). Moreover the growth of RUNX3 siRNA‐treated SUIT‐2 and KLM‐1 cells increased 8.1 and 3.2 times, respectively, over that of control siRNA‐treated cells at 5 days after transfection (Figure 3B). The gemcitabine sensitivity of RUNX3 siRNA‐treated cells decreased significantly; the gemcitabine IC50 increased from 11 nM to 61 nM and from 28 nM to 65 nM in SUIT‐2 and KLM‐1 cells, respectively (Figure 3C and D).
Figure 3.
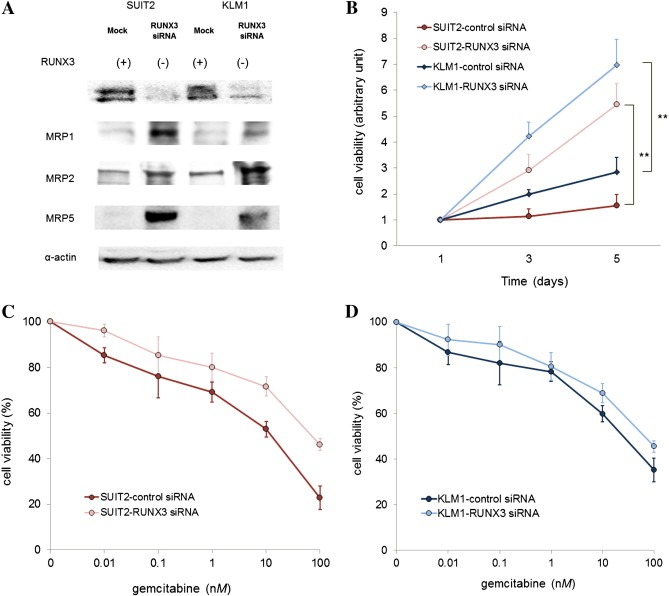
RUNX3 knockdown in pancreatic cancer cell lines. RUNX3 siRNA and control siRNA were transfected into the endogenous RUNX3‐positive cell lines SUIT‐2 and KLM‐1. (A) Immunoblot analysis of RUNX3 and MRP expression. Immunoblot analysis was performed using antibodies against RUNX3, MRP1, MRP2, MRP5, and α‐actin. Immunoblotting for α‐actin was done to verify equal loading. These 2 cell lines were originally endogenous RUNX3 positive. The left lane for each cell line represents mock‐transfected cancer cells, and the right lane represents RUNX3 siRNA‐transfected cancer cells. Representative blots of more than 3 independent experiments. (B) Cell growth activity. Cell proliferative activities were measured by MTT assay. Results are expressed as ratios compared to day 1. Data represent the means ± SE of more than 3 independent experiments performed in triplicate. **P < 0.01, versus control siRNA‐transfected cells (Student's t test). (C and D) Gemcitabine sensitivity. SUIT‐2 (C) and KLM‐1 (D) cell lines were treated with various concentrations of gemcitabine for 24 h. Cell viability was measured by MTT assay and expressed as a percentage relative to control cells. Data represent the means ± SE of more than 3 independent experiments performed in triplicate.
3.6. RUNX3 mRNA expression was inversely correlated with MRP mRNA expression
We evaluated the effect of ectopic RUNX3 expression on MRPs to elucidate the underlying mechanisms of RUNX3 expression‐induced chemosensitivity. We analyzed the Oncomine data sets to examine the correlation between the expression of RUNX3 and MRPs. The results revealed that RUNX3 mRNA expression was inversely correlated with MRP1 and MRP5 mRNA expression (MRP1: r = −0.40, P < 0.05; MRP5: r = −0.39, P < 0.05) (Figure 4). MRP2 mRNA expression had a trend correlating with RUNX3 mRNA expression (r = −0.20); however, this correlation was not statistically significant (Figure 4).
Figure 4.
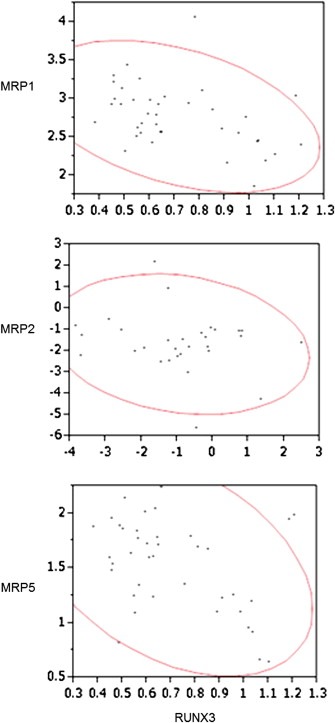
Correlation between RUNX3 and MRP expression in human pancreatic cancer data sets. The correlation between RUNX3 and MRP expression was analyzed using publically available microarray data sets (http://www.oncomine.org). The RUNX3, MRP1, MRP2, and MRP5 expression values (in arbitrary units) and 95% tolerance ellipse for pairs of variables were plotted.
4. Discussion
Runt domain family members are master regulators of gene expression in major developmental pathways (Subramaniam et al., 2009). RUNX3 is a downstream effector of the TGF‐β signaling pathway and plays a critical role in the regulation of cell proliferation and cell death (Subramaniam et al., 2009). Li et al. (2004) reported that TGF‐β1 repressed RUNX3 expression only in Colo‐357 pancreatic cancer cells and did not influence RUNX3 mRNA expression in the other cell lines studied. They suggested that the repressive effect of TGF‐β1 on RUNX3 expression was lost in tumors with gene alteration. Further, Ito (2011) reported that RUNX3 deficiency results in impaired TGF‐β signaling. It is possible that the TGF‐β pathway loses its repressive function in pancreatic cancer. More data are needed to elucidate the function of RUNX3 in pancreatic cancer.
Wada et al. (2004) reported that RUNX3 inactivation contributes to the tumorigenesis of bile duct and pancreatic carcinomas. Li et al. (2004) noted loss of RUNX3 expression in primary and metastatic pancreatic cancers. They reported that some primary pancreatic cancers lost RUNX3 expression and metastatic tumors either were completely devoid of or showed only very weak RUNX3 immunoreactivity. These findings suggest a tumor‐suppressive role of RUNX3 in pancreatic cancer. In the present study, we examined RUNX3 protein expression by immunohistochemistry in 36 primary pancreatic tumor samples and confirmed RUNX3 expression in 28% of the samples (Table 1). Previous reports demonstrated reduced RUNX3 expression in 71–75% of pancreatic cancer samples (Li et al., 2004; Subramaniam et al., 2009; Wada et al., 2004), which is concordant with our results. Li et al. reported differences in the location of RUNX3 protein between pancreatic cancer samples and normal pancreatic tissue (Li et al., 2004). However, they did not demonstrate the functional consequence of cytoplasmic translocation of RUNX3. In the present study, we observed RUNX3 expression in both the cytoplasm and nuclei. We did not observe any significant difference among the pancreatic cancer tissue samples. Moreover, a previous report showed RUNX3 to be inactivated in pancreatic cancer through either hemizygous deletion or hypermethylation and found no evidence of cytoplasmic mislocation (Chuang and Ito, 2010). Thus, the significance of mislocation of RUNX3 remains unclear. Additional data are necessary on the localization of RUNX3 in pancreatic cancer. The RUNX3‐high and RUNX3‐low groups showed no significant differences in patient characteristics and clinicopathological stages (Table 1), although Kaplan–Meier analysis demonstrated a significant difference in patient survival between the 2 groups (Figure 1B). A substantial difference in time to recurrence was also observed, with the RUNX3‐high group showing significantly longer relapse‐free survival than the RUNX3‐low group (Figure 1C).
Univariate analysis demonstrated that lymph node involvement, poor histological differentiation, and loss of RUNX3 expression were significant factors for survival (Supplementary Table 1). These factors were also identified as significant prognostic factors in the multivariate analysis (Table 2). A previous report showed that RUNX3 was frequently methylated in primary pancreatic cancer tissues, with hemizygous deletion occurring at locus 1p36 and the RUNX3‐inactivated cases showing worse survival (Nomoto et al., 2008). The study suggested that RUNX3 inactivation contributed to alteration of the TGF‐β signaling pathway and pancreatic cancer tumorigenesis. In concordance with this report, we observed a significant association between RUNX3 loss and poor prognosis among patients who had undergone an operation and who were treated with gemcitabine. Furthermore, RUNX3‐low patients treated with gemcitabine after the operation had significantly shorter time to recurrence than the RUNX3‐high patients.
Table 2.
Factors predictive of survival after pancreaticoduodenectomy—multivariate analysis.
| n | Risk ratio | 95% CI | P value | ||
|---|---|---|---|---|---|
| Age (y) | >60/≤60 | 25/11 | 1.85 | 0.66–6.22 | 0.25 |
| Gender | Male/female | 21/15 | 1.36 | 0.48–3.79 | 0.55 |
| UICC stage | IIB + IV/IA + IIA | 13/23 | 3.49 | 1.38–9.34 | 0.0083 |
| Histological grade | G3/G1 + G2 | 4/32 | 35.4 | 6.49–211.5 | 0.0001 |
| Resection margin | R1/R0 | 3/33 | 1.14 | 0.16–4.96 | 0.87 |
| RUNX3 expression | Low/high | 26/10 | 2.89 | 1.08–9.38 | 0.033 |
Some studies proposed an initial schedule of 7‐out‐of‐8‐weeks of gemcitabine administration (Burris et al., 1997; Rothenberg et al., 1996). We used a 3‐out‐of‐4‐week schedule because it is the commonly used administration schedule in Japan. This schedule is preferred because the effects of the 3‐out‐of‐4‐week schedule of gemcitabine administration without the initial consequent 7‐week administration were almost equal to that of the 3‐out‐of‐4‐weeks with initial 7‐out‐of‐8‐week administration in the treatment of pancreatic cancer (Poplin et al., 2009). Moreover, an initial 7‐out‐of‐8‐week administration reached the dose‐limiting toxicity in the phase I trial on Japanese patients with advanced pancreatic cancer (Okada et al., 2001).
We surmised that gemcitabine sensitivity was the main determinant of prognosis in pancreatic cancer because none of the patients who received adjuvant chemotherapy with gemcitabine showed any significant difference in baseline characteristics and clinicopathological stage in terms of RUNX3 expression status. We hypothesized that RUNX3 expression influences gemcitabine sensitivity in pancreatic cancer. Previous reports attributed drug resistance to several cellular processes, one of which is decreased accumulation of drugs within cancer cells due to increased drug efflux. Proteins mediating this drug efflux mostly belong to the superfamily of ABC transporters, particularly the ABCC family members (Konig et al., 2005). Although we assessed the correlation of MRP expression with RUNX3 expression in the current study, other factors have also been implicated in gemcitabine resistance. Multidrug resistance 1 (MDR1/ABCB1) and breast cancer resistance protein (BCRP/ABCG2) are also involved in the resistance against gemcitabine (Hong et al., 2009) (Chen et al., 2012). Previous reports showed that MRPs were associated with prognosis or/and relapse‐free survival in various cancers (Langer et al., 2010; Nakagawa et al., 2009; Plasschaert et al., 2005). Several reports have described chemoresistance and expression of MRPs in pancreatic cancer (Hagmann et al., 2009, 2010; Konig et al., 2005; Noma et al., 2008). These reports suggest an important role of MRPs in the gemcitabine resistance. Thus, we investigated MRP expression in pancreatic cancer cell lines, which we divided into 2 groups: RUNX3‐high and RUNX3‐low (Supplementary Figure 1).
We examined the expression of MRP subsets in the RUNX3‐negative and RUNX3‐positive cell lines by immunoblot analysis. Only MRP1, MRP2, and MRP5 correlated with RUNX3 expression. On the other hand, MRP1, MRP2, and MRP5 were expressed in the endogenous RUNX3‐negative cell lines (Figure 2A). In addition, a positive correlation was observed between these subtypes and RUNX3 in the Oncomine database. The cell lines showed various patterns of MRP expression, and exogenous RUNX3 expression generally suppressed MRP expression (Figure 2A). As expected, exogenous RUNX3 expression decreased cell proliferation (Figure 2B) and increased gemcitabine sensitivity in the endogenous RUNX3‐negative cell lines (Figure 2C–E). On the other hand, RUNX3 siRNA knocked down RUNX3 in the endogenous RUNX3‐positive pancreatic cancer cell lines. RUNX3 knockdown enhanced MRP expression in the endogenous RUNX3‐positive cell lines (Figure 3A), as well as increased the proliferation (Figure 3B) and decreased the gemcitabine sensitivity of the cells (Figure 3C and D). These results suggest that RUNX3 may regulate MRP expression. A previous study on gastric cancer reported that RUNX3 sensitized gastric cancer cells to chemotherapeutic drugs by downregulating MRP1 through inhibition of MRP1 promoter activity (Hagmann et al., 2010). Further, (Guo et al., 2005) established that RUNX3 directly controls MRP1 expression in EMSA and luciferase reporter assay. However, whether MRP2 and MRP5 expression is directly inhibited by RUNX3 is still unclear. Further study is warranted to elucidate the regulatory function of RUNX3 in MRP expression.
On the basis of these data, we analyzed MRP expression in pancreatic cancer tissues by using the Oncomine database to determine the molecules that contribute to gemcitabine sensitivity. Our results revealed an inverse relationship between MRP and RUNX3 expression (Figure 4).
The standard chemotherapy for metastatic pancreatic cancer patients may change in the future, because the efficiency of the chemotherapy with gemcitabine is limited. Conroy et al. report that the combination regimen consisting of oxialiplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX) is efficient for metastatic pancreatic cancer patients, with good performance status (Conroy et al., 2011). As MRPs could be involved in the drug resistance of the chemotherapeutic agent for FOLFIRINOX, RUNX3 expression may be a prognostic biomarker for FOLFIRINOX‐treated pancreatic cancer patients.
5. Conclusion
We found RUNX3 protein expression to be absent in 72% of pancreatic cancer samples. Loss of RUNX3 expression may upregulate MRP expression and may contribute to gemcitabine resistance, poor survival, and shorter time to recurrence. Moreover, re‐expression of RUNX3 downregulated MRP expression, suppressed the growth of pancreatic cancer cells, and resensitized the cells to gemcitabine. Taken together, these findings indicate that targeting the RUNX3 pathway may be a potential treatment modality for pancreatic cancer. Re‐expression of RUNX3 may help improve the chemosensitivity to gemcitabine in RUNX3‐low pancreatic cancer.
Grant support
This work was supported by the Japan Society for the Promotion of Science (Grant No. 23590975).
Conflict of interest
The authors have no conflicts of interest to disclose.
Author contributions
Hidenori Shiraha conceived the study design and drafted the manuscript. Shigeru Horiguchi performed the experiments with assistance from Teruya Nagahara, Jyunnro Kataoka, Masaya Iwamuro, Minoru Matsubara, Shinichi Nishina, Akinobu Takaki, Kazuhiro Nouso, Takehiro Tanaka, and Koichi Ichimura. Hironari Kato and Takahito Yagi assisted in the collection of pancreatic cancer tissues. Kazuhide Yamamoto provided financial support and participated in the analysis and discussion of results. All authors read and approved the final manuscript.
Supporting information
The following are the supplementary data related to this article:
Supplementary Figure 1RUNX3 protein expression in pancreatic cancer cell lines. Immunoblot analysis was performed using RUNX3 and α‐actin antibodies. Immunoblotting for α‐actin was done to verify equal loading. Representative blots of more than 3 independent experiments.
Supplementary data
Acknowledgments
We thank Yutaka Nakanishi and Shigetomi Tanaka for their valuable suggestions.
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2013.04.004.
Horiguchi Shigeru, Shiraha Hidenori, Nagahara Teruya, Kataoka Jyunnro, Iwamuro Masaya, Matsubara Minoru, Nishina Shinichi, Kato Hironari, Takaki Akinobu, Nouso Kazuhiro, Tanaka Takehiro, Ichimura Koichi, Yagi Takahito, Yamamoto Kazuhide, (2013), Loss of runt‐related transcription factor 3 induces gemcitabine resistance in pancreatic cancer, Molecular Oncology, 7, doi: 10.1016/j.molonc.2013.04.004.
References
- Arumugam, T. , Ramachandran, V. , Fournier, K.F. , Wang, H. , Marquis, L. , Abbruzzese, J.L. , Gallick, G.E. , Logsdon, C.D. , McConkey, D.J. , Choi, W. , 2009. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 69, 5820–5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, S.C. , Choi, J.K. , 2004. Tumor suppressor activity of RUNX3. Oncogene 23, 4336–4340. [DOI] [PubMed] [Google Scholar]
- Bergman, A.M. , Pinedo, H.M. , Talianidis, I. , Veerman, G. , Loves, W.J. , van der Wilt, C.L. , Peters, G.J. , 2003. Increased sensitivity to gemcitabine of P-glycoprotein and multidrug resistance-associated protein-overexpressing human cancer cell lines. Br. J. Cancer 88, 1963–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris, H.A. , Moore, M.J. , Andersen, J. , Green, M.R. , Rothenberg, M.L. , Modiano, M.R. , Cripps, M.C. , Portenoy, R.K. , Storniolo, A.M. , Tarassoff, P. , Nelson, R. , Dorr, F.A. , Stephens, C.D. , Von Hoff, D.D. , 1997. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 15, 2403–2413. [DOI] [PubMed] [Google Scholar]
- Chen, M. , Xue, X. , Wang, F. , An, Y. , Tang, D. , Xu, Y. , Wang, H. , Yuan, Z. , Gao, W. , Wei, J. , Zhang, J. , Miao, Y. , 2012. Expression and promoter methylation analysis of ATP-binding cassette genes in pancreatic cancer. Oncol. Rep. 27, 265–269. [DOI] [PubMed] [Google Scholar]
- Chuang, L.S. , Ito, Y. , 2010. RUNX3 is multifunctional in carcinogenesis of multiple solid tumors. Oncogene 29, 2605–2615. [DOI] [PubMed] [Google Scholar]
- Conroy, T. , Desseigne, F. , Ychou, M. , Bouche, O. , Guimbaud, R. , Becouarn, Y. , Adenis, A. , Raoul, J.L. , Gourgou-Bourgade, S. , de la Fouchardiere, C. , Bennouna, J. , Bachet, J.B. , Khemissa-Akouz, F. , Pere-Verge, D. , Delbaldo, C. , Assenat, E. , Chauffert, B. , Michel, P. , Montoto-Grillot, C. , Ducreux, M. , 2011. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825. [DOI] [PubMed] [Google Scholar]
- Dean, M. , Hamon, Y. , Chimini, G. , 2001. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 42, 1007–1017. [PubMed] [Google Scholar]
- Efthimiou, E. , Crnogorac-Jurcevic, T. , Lemoine, N.R. , 2001. Pancreatic cancer genetics. Pancreatology 1, 571–575. [DOI] [PubMed] [Google Scholar]
- Giovannetti, E. , Mey, V. , Nannizzi, S. , Pasqualetti, G. , Del Tacca, M. , Danesi, R. , 2006. Pharmacogenetics of anticancer drug sensitivity in pancreatic cancer. Mol. Cancer Ther. 5, 1387–1395. [DOI] [PubMed] [Google Scholar]
- Guo, C. , Ding, J. , Yao, L. , Sun, L. , Lin, T. , Song, Y. , Fan, D. , 2005. Tumor suppressor gene Runx3 sensitizes gastric cancer cells to chemotherapeutic drugs by downregulating Bcl-2, MDR-1 and MRP-1. Int. J. Cancer 116, 155–160. [DOI] [PubMed] [Google Scholar]
- Hagmann, W. , Jesnowski, R. , Faissner, R. , Guo, C. , Lohr, J.M. , 2009. ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Upregulation in 5-fluorouracil-resistant cells. Pancreatology 9, 136–144. [DOI] [PubMed] [Google Scholar]
- Hagmann, W. , Jesnowski, R. , Lohr, J.M. , 2010. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia 12, 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo, M. , 2010. Pancreatic cancer. N. Engl. J. Med. 362, 1605–1617. [DOI] [PubMed] [Google Scholar]
- Hong, S.P. , Wen, J. , Bang, S. , Park, S. , Song, S.Y. , 2009. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int. J. Cancer 125, 2323–2331. [DOI] [PubMed] [Google Scholar]
- Ito, K. , 2011. RUNX3 in oncogenic and anti-oncogenic signaling in gastrointestinal cancers. J. Cell. Biochem. 112, 1243–1249. [DOI] [PubMed] [Google Scholar]
- Jaffee, E.M. , Hruban, R.H. , Canto, M. , Kern, S.E. , 2002. Focus on pancreas cancer. Cancer Cell 2, 25–28. [DOI] [PubMed] [Google Scholar]
- Jemal, A. , Siegel, R. , Xu, J. , Ward, E. , 2010. Cancer statistics, 2010. CA Cancer J. Clin. 60, 277–300. [DOI] [PubMed] [Google Scholar]
- Jones, S. , Zhang, X. , Parsons, D.W. , Lin, J.C. , Leary, R.J. , Angenendt, P. , Mankoo, P. , Carter, H. , Kamiyama, H. , Jimeno, A. , Hong, S.M. , Fu, B. , Lin, M.T. , Calhoun, E.S. , Kamiyama, M. , Walter, K. , Nikolskaya, T. , Nikolsky, Y. , Hartigan, J. , Smith, D.R. , Hidalgo, M. , Leach, S.D. , Klein, A.P. , Jaffee, E.M. , Goggins, M. , Maitra, A. , Iacobuzio-Donahue, C. , Eshleman, J.R. , Kern, S.E. , Hruban, R.H. , Karchin, R. , Papadopoulos, N. , Parmigiani, G. , Vogelstein, B. , Velculescu, V.E. , Kinzler, K.W. , 2008. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321, 1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern, S.E. , Hruban, R.H. , Hidalgo, M. , Yeo, C.J. , 2002. An introduction to pancreatic adenocarcinoma genetics, pathology and therapy. Cancer Biol. Ther. 1, 607–613. [DOI] [PubMed] [Google Scholar]
- Konig, J. , Hartel, M. , Nies, A.T. , Martignoni, M.E. , Guo, J. , Buchler, M.W. , Friess, H. , Keppler, D. , 2005. Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. Int. J. Cancer 115, 359–367. [DOI] [PubMed] [Google Scholar]
- Langer, R. , Ott, K. , Feith, M. , Lordick, F. , Specht, K. , Becker, K. , Hofler, H. , 2010. High pretherapeutic thymidylate synthetase and MRP-1 protein levels are associated with nonresponse to neoadjuvant chemotherapy in oesophageal adenocarcinoma patients. J. Surg. Oncol. 102, 503–508. [DOI] [PubMed] [Google Scholar]
- Li, J. , Kleeff, J. , Guweidhi, A. , Esposito, I. , Berberat, P.O. , Giese, T. , Buchler, M.W. , Friess, H. , 2004. RUNX3 expression in primary and metastatic pancreatic cancer. J. Clin. Pathol. 57, 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q.L. , Ito, K. , Sakakura, C. , Fukamachi, H. , Inoue, K. , Chi, X.Z. , Lee, K.Y. , Nomura, S. , Lee, C.W. , Han, S.B. , Kim, H.M. , Kim, W.J. , Yamamoto, H. , Yamashita, N. , Yano, T. , Ikeda, T. , Itohara, S. , Inazawa, J. , Abe, T. , Hagiwara, A. , Yamagishi, H. , Ooe, A. , Kaneda, A. , Sugimura, T. , Ushijima, T. , Bae, S.C. , Ito, Y. , 2002. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 109, 113–124. [DOI] [PubMed] [Google Scholar]
- Maitra, A. , Hruban, R.H. , 2008. Pancreatic cancer. Annu. Rev. Pathol. 3, 157–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault, M. , Brand, R.E. , Sasson, A.A. , Batra, S.K. , 2005. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas 31, 301–316. [DOI] [PubMed] [Google Scholar]
- Nakagawa, T. , Ido, K. , Sakuma, T. , Takeuchi, H. , Sato, K. , Kubota, T. , 2009. Prognostic significance of the immunohistochemical expression of O6-methylguanine-DNA methyltransferase, P-glycoprotein, and multidrug resistance protein-1 in glioblastomas. Neuropathology 29, 379–388. [DOI] [PubMed] [Google Scholar]
- Nakanishi, Y. , Shiraha, H. , Nishina, S. , Tanaka, S. , Matsubara, M. , Horiguchi, S. , Iwamuro, M. , Takaoka, N. , Uemura, M. , Kuwaki, K. , Hagihara, H. , Toshimori, J. , Ohnishi, H. , Takaki, A. , Nakamura, S. , Kobayashi, Y. , Nouso, K. , Yagi, T. , Yamamoto, K. , 2011. Loss of runt-related transcription factor 3 expression leads hepatocellular carcinoma cells to escape apoptosis. BMC Cancer 11, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma, B. , Sasaki, T. , Fujimoto, Y. , Serikawa, M. , Kobayashi, K. , Inoue, M. , Itsuki, H. , Kamigaki, M. , Minami, T. , Chayama, K. , 2008. Expression of multidrug resistance-associated protein 2 is involved in chemotherapy resistance in human pancreatic cancer. Int. J. Oncol. 33, 1187–1194. [PubMed] [Google Scholar]
- Nomoto, S. , Kinoshita, T. , Mori, T. , Kato, K. , Sugimoto, H. , Kanazumi, N. , Takeda, S. , Nakao, A. , 2008. Adverse prognosis of epigenetic inactivation in RUNX3 gene at 1p36 in human pancreatic cancer. Br. J. Cancer 98, 1690–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguri, T. , Achiwa, H. , Sato, S. , Bessho, Y. , Takano, Y. , Miyazaki, M. , Muramatsu, H. , Maeda, H. , Niimi, T. , Ueda, R. , 2006. The determinants of sensitivity and acquired resistance to gemcitabine differ in non-small cell lung cancer: a role of ABCC5 in gemcitabine sensitivity. Mol. Cancer Ther. 5, 1800–1806. [DOI] [PubMed] [Google Scholar]
- Okada, S. , Ueno, H. , Okusaka, T. , Ikeda, M. , Furuse, J. , Maru, Y. , 2001. Phase I trial of gemcitabine in patients with advanced pancreatic cancer. Jpn. J. Clin. Oncol. 31, 7–12. [DOI] [PubMed] [Google Scholar]
- Plasschaert, S.L. , de Bont, E.S. , Boezen, M. , vander Kolk, D.M. , Daenen, S.M. , Faber, K.N. , Kamps, W.A. , de Vries, E.G. , Vellenga, E. , 2005. Expression of multidrug resistance-associated proteins predicts prognosis in childhood and adult acute lymphoblastic leukemia. Clin. Cancer Res. 11, 8661–8668. [DOI] [PubMed] [Google Scholar]
- Poplin, E. , Feng, Y. , Berlin, J. , Rothenberg, M.L. , Hochster, H. , Mitchell, E. , Alberts, S. , O'Dwyer, P. , Haller, D. , Catalano, P. , Cella, D. , Benson, A.B. , 2009. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 27, 3778–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg, M.L. , Moore, M.J. , Cripps, M.C. , Andersen, J.S. , Portenoy, R.K. , Burris, H.A. , Green, M.R. , Tarassoff, P.G. , Brown, T.D. , Casper, E.S. , Storniolo, A.M. , Von Hoff, D.D. , 1996. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann. Oncol. 7, 347–353. [DOI] [PubMed] [Google Scholar]
- Subramaniam, M.M. , Chan, J.Y. , Yeoh, K.G. , Quek, T. , Ito, K. , Salto-Tellez, M. , 2009. Molecular pathology of RUNX3 in human carcinogenesis. Biochim. Biophys. Acta 1796, 315–331. [DOI] [PubMed] [Google Scholar]
- Tanaka, M. , Okazaki, T. , Suzuki, H. , Abbruzzese, J.L. , Li, D. , 2011. Association of multi-drug resistance gene polymorphisms with pancreatic cancer outcome. Cancer 117, 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, M. , Yazumi, S. , Takaishi, S. , Hasegawa, K. , Sawada, M. , Tanaka, H. , Ida, H. , Sakakura, C. , Ito, K. , Ito, Y. , Chiba, T. , 2004. Frequent loss of RUNX3 gene expression in human bile duct and pancreatic cancer cell lines. Oncogene 23, 2401–2407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary Figure 1RUNX3 protein expression in pancreatic cancer cell lines. Immunoblot analysis was performed using RUNX3 and α‐actin antibodies. Immunoblotting for α‐actin was done to verify equal loading. Representative blots of more than 3 independent experiments.
Supplementary data


