Abstract
Malignant uveal melanoma severely damages eye function and is prone to metastasize to other organs. Tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL) is a promising agent to treat uveal melanoma because of its induction of apoptosis in cancer cells both at primary and metastatic sites. However, TRAIL therapy lacks tumor specificity in the current delivery systems for uveal melanoma treatment, thereby causing cytotoxiciy to normal tissues. To improve uveal melanoma specificity of adenovirus‐based TRAIL introduction, we used miRNA response elements (MREs) of miR‐34a, miR‐137 and miR‐182, which have been shown to have reduced expression in uveal melanoma cells, to regulate its expression. miR‐34a, miR‐137 and miR‐182 all had lower expression levels in uveal melanoma cell lines, compared with normal cells. MREs‐regulated luciferase activity was reduced in normal cell lines, but not significantly attenuated in uveal melanoma cells. The infection of MRE‐regulated TRAIL‐expressing adenoviral vector (Ad‐TRAIL‐3MREs) led to high level of TRAIL expression in uveal melanoma cell lines, but not in normal cells. Strong expression of TRAIL had a high anti‐tumor capacity by inducing apoptosis in uveal melanoma cells. In contrast, Ad‐TRAIL‐3MREs had no cytotoxicity to normal cell lines. Animal experiments further confirmed tumor‐suppressing effect of Ad‐TRAIL‐3MREs on uveal melanoma xenografts and its biosafety to hepatic tissues. Collectively, we constructed an MRE‐directed TRAIL‐expressing adenoviral vector and provided evidence that this vector possessed high anti‐tumor activity and uveal melanoma specificity.
Keywords: Uveal melanoma, Adenovirus, miRNA, TRAIL
Highlights
MREs of miR‐34a, miR‐137 and miR‐182 restrict TRAIL expression in uveal melanoma.
Ad‐TRAIL‐3MREs selectively induces apoptosis and reduce growth of uveal melanoma.
Ad‐TRAIL‐3MREs possesses no cytotoxicity to normal cells in vitro and in vivo.
1. Introduction
Uveal melanoma is the second most common ophthalmic malignancy in human and prone to metastasize to other organ such as liver (Bakalian et al., 2008). However, the survival of patients that suffer from metastasis benefits little from the current treatments, including photocoagulation, surgery and radiotherapy, probably at the cost of eye function (i.e. caused by ophthalmectomy). Their prognoses are quite poor and their survival is generally 2–7 months (Woodman, 2012). Therefore, alternative therapeutic strategies are urgently needed to treat malignant uveal melanoma.
Tumor gene therapy is a therapeutic strategy involving introducing specific genes into cancer cells to suppress its survival and/or growth. Various vectors have been developed to deliver these tumor suppressor genes. Among them, adenoviral vector is widely studied in preclinical and clinical trials, and even, has been clinically applied in China. For uveal melanoma, adenoviral vectors have been studied for more than a decade. Adenovirus can be used to inhibit the metastasis of uveal melanoma by expressing plasminogen activator inhibitor type 1 (PAI‐1) or interferon‐β (Alizadeh et al., 2003; Ma et al., 1997). Carlring and his colleagues utilized adenoviral vectors to stimulate immune response against uveal melanoma by delivering CD80 into tumor cells (Carlring et al., 2003). Recently, a conditionally replicative adenovirus, H101, was also demonstrated to suppress the growth of uveal melanoma, especially in combination with other anti‐tumor agents (Cun et al., 2012, 2012, 2012).
Tumor necrosis factor (TNF)‐related apoptosis‐inducing ligand (TRAIL) belongs to the superfamily of TNF. TRAIL binds two membrane‐located receptors, TRAIL‐R1 and TRAIL‐R2 (also known as DR‐4 and DR‐5, respectively), and subsequently induces receptor cross‐linking, caspase‐pathway activation and apoptosis (Pan et al., 1997, 1997). TRAIL has been well documented to induce apoptosis in various types of cancer cells, thereby serving as a promising biological agent for cancer treatment (Huang and Sheikh, 2007). Researchers have developed several vectors, such as recombinant adenovirus, to highly express TRAIL to suppress tumor growth (Carlo‐Stella et al., 2007; Chen et al., 2010). Like many other cancer types, uveal melanoma cells express TRAIL‐R1 and TRAIL‐R2, and thus, are susceptible to TRAIL‐stimulated apoptosis (Ren et al., 2004). Moreover, adenovirus‐based radioresponsive TRAIL expression was applied to eliminate radioresistant uveal melanoma cells (Zhou et al., 2010). However, TRAIL has also been evidenced to be toxic to liver tissue by enhancing Fas‐mediated apoptosis (Armeanu et al., 2003; Corazza et al., 2006), raising the risk of cytotoxicity to normal cells in future clinical treatment. In order to prevent normal cells from TRAIL‐induced cytotoxicity, the specificity of current delivery vector should be improved.
microRNAs (miRNAs) are endogenous non‐coding RNA with 20–22 nucleotides in length, binding miRNA response elements (MREs) located on the 3′ UTR of target mRNAs. In turn, miRNAs facilitate the degradation of the target mRNAs or suppress their translation, thereby negatively regulating their expression (Sontheimer and Carthew, 2005). Numerous publications have demonstrated that miRNAs have differential expression profiles between cancerous and normal tissues, and also, play multiply roles in promoting or suppressing the malignant phenotypes of cancer cells (Lujambio and Lowe, 2012). Regarding uveal melanoma, several miRNAs have been shown to be underexpressed in uveal melanoma cell lines and/or uveal melanoma samples. miR‐34a, which acts as a tumor‐suppressor in many other cancer types, have also been proven to compromise the ability of uveal melanoma cells to proliferate and migrate through inhibiting c‐MET expression (Yan et al., 2009). miR‐182 suppresses the growth of posterior uveal melanoma via decreasing MITF, BCL2 and cyclin D2 level (Yan et al., 2012). Restoration of miR‐137 expression induces cell cycle arrest by reducing MITF and CDK6 level in uveal melanoma cell lines (Chen et al., 2011). Compared with normal cells, all of these tumor‐suppressing miRNAs have decreased expression level in uveal melanoma cells (Chen et al., 2011, 2012, 2009).
In this study, we utilized the differential miRNA expression profile between uveal melanoma cells and normal cells to confer adenovirus‐based TRAIL expression with specificity. We inserted MREs of miR‐34a, miR‐137 and miR‐182 into the 3′ UTR of TRAIL‐coding sequence, generating a recombinant adenoviral vector Ad‐TRAIL‐3MREs. Subsequently, we evaluated the potency and specificity of this MRE‐regulated TRAIL expression system on uveal melanoma cells.
2. Methods and materials
2.1. Cell culture
ARPE‐19 human retinal pigmented epithelial cell line was purchased from American Type Culture Collection. L‐02 human normal liver cells were obtained from Shanghai Cell Collection (Shanghai, China). SP6.5 and OM431 human uveal melanoma cell lines were generously provided by Dr. Fan Wu (Massachusetts Eye Research and Surgery Institution, Boston, MA). SP6.5 and OM431 cells were both sensitive to TRAIL action (Li et al., 2005; Zhou et al., 2010). ARPE‐19 cells were cultured in ATCC‐formulated DMEM:F12 Medium (Catalog No. 30‐2006) with 10% fetal bovine serum (FBS), 4 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin in a 5% CO2 and humidified atmosphere at 37 °C. L‐02, SP6.5 and OM431 cells were cultured using DMEM supplemented with 10% FBS, 4 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin in a 5% CO2 and humidified atmosphere at 37 °C.
2.2. Northern blotting assay
Total RNA was extracted from ARPE‐19, L‐02, SP6.5 and OM431 cells with Trizol solution (Invitrogen). 15 μg RNA was loaded onto 15% TBE–urea gels, separated on a 15% urea–PAGE gel, transferred onto positively charged nylon membranes (GE Healthcare Life Sciences) and cross‐linked via UV irradiation. The RNA blots were subjected to hybridization with DIG‐labeled probe for miR‐34a, miR‐137 and miR‐182 (100 ng/ml; Exiqon, Denmark) overnight at 42 °C. After hybridization, membranes were washed with a low‐stringency buffer (2 × SSC plus 0.1% SDS). DIG Luminescent Detection Kit (Roche) was applied to detect the expression levels of miRNAs following the manufacturer's protocol. U6 RNA was used as a loading control.
2.3. Quantitative PCR (qPCR)
For detection of miR‐34a, miR‐137 and miR‐182, total RNA was extracted from uveal melanoma and normal cells with Trizol solution (Sigma–Aldrich). Reverse transcription reaction was performed with TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems) following the manufacturer's instructions. qPCR was performed using TaqMan® 2× Universal PCR Master Mix (Applied Biosystems) on CFX96™ Real‐Time PCR Detection System (Bio‐Rad Laboratories, CA) supplied with analytical software. U6 was used as loading control. The primers and probes specific for these miRNA were purchased from Invitrogen (# 4427012; # 4426961; # 4331182).
3 × 105 ARPE‐19, L‐02, SP6.5 and OM431 cells were planted in each well of 6‐well plates. Overnight, cells were infected with indicated adenoviruses of 10 MOI. 48 h later, total RNA were extracted with Trizol solution and transcribed into cDNAs using Rever Tra Ace qPCR RT Kit (Toyobo) according to the manufacturer's instructions. qPCR was performed using was performed using SYBR premix Ex Taq (Takara) on CFX96™ Real‐Time PCR Detection System (Bio‐Rad Laboratories, CA) supplied with analytical software. The involved primer sequences were as follows: TRAIL‐forward: 5′‐GACCTGCGTGCTGATC‐3′; TRAIL‐reverse: 5′‐TAAAAGAAGATGACAG‐3′; GAPDH‐forward: 5′‐TCAGTGGTGGACCTGA‐3′, GAPDH‐reverse: 5′‐TGCTGTAGCCAA ATTC‐3′.
2.4. Luciferase reporter assay
Paired primers containing 2 copies of MREs of miR‐34a, miR‐137 or miR‐182 were annealed and inserted into psiCheck2 (Promega, WI) at the site of XhoI and NotI to construct MRE‐regulated luciferase reporter plasmids psiCheck2‐miR‐34a, psiCheck2‐miR‐137 or psiCheck2‐miR‐182, respectively. To construct psiCheck2‐3MREs, whose luciferase expression was regulated simultaneously by MREs of miR‐34a, miR‐137 and miR‐182, a DNA fragment containing 2 copies of these MREs was synthesized and inserted into psiCheck2 at the site of XhoI and NotI. The sequences of inserted MREs of miRNAs were described in Table 1.
Table Table 1.
miRNA response elements (MREs) for uveal melanoma‐specific downregulated miRNAs.
| miRNA | Sequences of primers or DNA fragments |
|---|---|
| miR‐34a | Forward: TCGAGACAAACACCCACTGCCACAAACACCCACTGCCACAAACACCGCReverse: GGCCGCGGTGTTTGTGGCAGTGGGTGTTTGTGGCAGTGGGTGTTTGTC |
| miR‐137 | Forward: TCGAGACAAACACCAGCAATAACAAACACCAGCAATAACAAACACCGCReverse: GGCCGCGGTGTTTGTTATTGCTGGTGTTTGTTATTGCTGGTGTTTGTC |
| miR‐182 | Forward: TCGAGACAAACACCTTGCCAAACAAACACCTTGCCAAACAAACACCGCReverse: GGCCGCGGTGTTTGTTTGGCAAGGTGTTTGTTTGGCAAGGTGTTTGTC |
| miR‐34a + miR‐137 + miR‐182 | CTCGAGGATATCACAAACACCCACTGCCACAAACACCCACTGCCACAAACACCAGCAATAACAAACACCAGCAATAACAAACACCTTGCCAAACAAACACCTTGCCAAACAAACACCGATATCGCGGCCGC |
MREs of indicated miRNAs are underscored.
5 × 104 ARPE‐19, L‐02, SP6.5 and OM431 cells were transfected with 200 ng psiCheck2‐miR‐34a, psiCheck2‐miR‐137, psiCheck2‐miR‐182 or psiCheck2‐3MREs using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. 48 h later, cells were harvested with lysis buffer. Firefly and Renilla luciferase activities were measured with the Dual‐Luciferase reporter system (Promega, WI) following the manufacturer's instructions.
2.5. Adenovirus construction
The sequence of human TRAIL used in this study is available from NCBI website (NM_003810.3). Ad‐EGFP and Ad‐TRAIL were generously gifted by Youguang Zhao (General Hospital of Chengdu Military Area Command of Chinese PLA, Chengdu, China). The details on the two adenoviral vectors were available from the previous publications (Zhao et al., 2011). Ad‐TRAIL‐3MREs was generated as follows: The DNA fragment used for constructing psiCheck2‐3MREs was digested by EcoRV and subsequently inserted into the same site of pShuttle‐CMV‐TRAIL, which was also kindly gifted by Youguang Zhao (Zhao et al., 2011), to generate pShuttle‐CMV‐TRAIL‐3MREs. pShuttle‐CMV‐TRAIL‐3MREs and pAdEasy were cotransfected into HEK‐293 cells. After plague purification for three times and PCR‐based identification, recombinant adenovirus Ad‐TRAIL‐3MREs was harvested and purified using CsCl gradient centrifugation method. The titers of Ad‐EGFP, Ad‐TRAIL and Ad‐TRAIL‐3MREs were quantified with TCID50 method on HEK‐293 cells and represented as plaque‐forming units per milliliter (pfu/ml). The structures of Ad‐EGFP, Ad‐TRAIL and Ad‐TRAIL‐3MREs were shown in Figure 2A.
Figure 2.
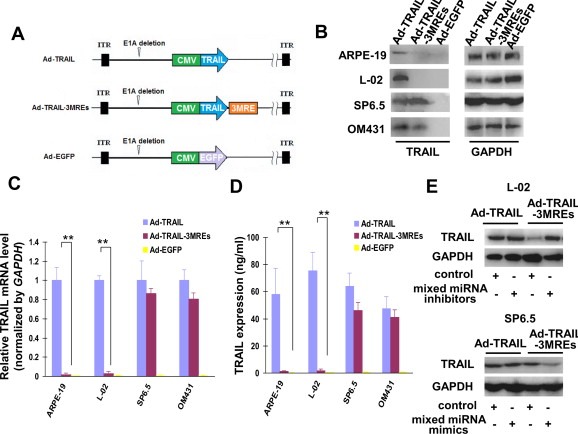
MREs‐regulated and adenovirus‐mediated TRAIL expression exhibited uveal melanoma specificity. (A) 2 copies of miR‐34a, miR‐137 and miR‐182 MREs were inserted immediately after the stop codon of a TRAIL‐coding open reading frame on a replication‐defect adenoviral vector Ad‐TRAIL to generate Ad‐TRAIL‐3MREs. Ad‐EGFP was used as a control. (B) Normal cells (ARPE‐19 and L‐02) and uveal melanoma cells (SP6.5 and OM431) were treated with Ad‐EGFP, Ad‐TRAIL and Ad‐TRAIL‐3MREs of 10 MOI. Proteins were extracted 48 h after adenovirus infection and detected for TRAIL expression with GAPDH as loading control. (C) Total RNA was also extracted from the same cells. TRAIL mRNA was subsequently quantified by qPCR with GAPDH as endogenous reference. (D) The production of excreted TRAIL protein was also evaluated in the same cells by ELISA assay. qPCR and ELISA assays were both repeated in triplicate and means ± SD were shown. (E) L‐02 and SP6.5 cells were transfected with mixed miRNA mimics or control (30 nM), followed by indicated adenovirus infection (10 MOI). 48 h later, TRAIL expression was determined with GAPDH as endogenous reference by immunoblotting.
2.6. Immunoblotting assay
3 × 105 ARPE‐19, L‐02, SP6.5 and OM431 cells were infected with 10 MOI of Ad‐EGFP, Ad‐TRAIL or Ad‐TRAIL‐3MREs. 48 h later, cells were lysized with M‐PER® Mammalian Protein Extraction Reagent (Thermo Scientific). Tumor xenografts and livers were immediately harvested from the nude mice after sacrifice and lysized with M‐TER® Tissue Protein Extraction Reagent (Thermo Scientific, IL). The samples were separated using 10–12% polyacrylamide gel electrophoresis and transferred onto 0.45 μm nitrocellulose membranes. After being blocked with 5% fat‐free dry milk for 1 h, the membrane was incubated with mouse monoclonal anti‐human TRAIL antibody (1:400, sc‐8440, Santa Cruz), mouse monoclonal anti‐cleaved caspase 8 (Asp384) antibody (1:1000, #9748, Cell Signaling Technology), rabbit monoclonal anti‐cleaved caspase 9 (Asp330) antibody (1:1000, #7237, Cell Signaling Technology), rabbit monoclonal anti‐cleaved caspase 3 (Asp175) antibody (1:1000, #9664, Cell Signaling Technology), rabbit monoclonal anti‐cleaved PARP antibody (Asp214) (1:1000, #5625, Cell Signaling Technology), rabbit polyclonal anti‐human BID antibody (1:1000, #2002, Cell Signaling Technology), and mouse monoclonal anti‐human GAPDH antibody (1:1500, sc‐81545, Santa Cruz) at 4 °C. Overnight, the membrane was incubated with the corresponding secondary antibody for 1 h and finally visualized with SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, IL).
2.7. Detection of TRAIL by ELISA
3 × 105 ARPE‐19, L‐02, SP6.5 and OM431 cells were infected with 10 MOI of Ad‐EGFP, Ad‐TRAIL or Ad‐TRAIL‐3MREs. 48 h later, the cell culture supernatants were harvested and two‐antibody sandwich ELISA assay were performed according to the procedures described previously (Lub‐de Hooge et al., 2005). The used antibodies are monoclonal mouse anti‐human TRAIL antibody (MAB3751, R&D Systems), biotinylated polyclonal goat anti‐human TRAIL antibody (BAF375, R&D Systems) and HRP‐conjugated avidin (A‐115, R&D Systems). The absorbance was read at 450 nm. A standard curve was used to determine the concentration of TRAIL.
2.8. miRNA mimics and inhibitors
mirVana™ miRNA mimics, a group of small and chemically modified double‐stranded RNAs that mimic endogenous miRNAs, was used to increase miRNA activity for miRNA functional analysis. mirVana™ miRNA inhibitors, which are single‐stranded RNAs designed to bind and suppress specific endogenous miRNA, were also applied for the confirmation of miRNA‐based regulation of adenoviral TRAIL expression.
mirVana™ mimics (#4464066) and inhibitors (#4464084) of miR‐34a, miR‐137 or miR‐182 (Invitrogen) were mixed thoroughly and added to the indicated cells at the concentration of 30 nM (10 nM for each mimic or inhibitor) using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer's instructions. Overnight, the cells were infected with indicated adenoviruses of 10 MOI.
2.9. Cell viability assay
1 × 103 ARPE‐19, L‐02, SP6.5 and OM431 cells were infected with the indicated MOI of Ad‐EGFP, Ad‐TRAIL or Ad‐TRAIL‐3MREs. 6 days later, 10 μl 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) (5 mg/ml) was added to the cell cultures. 4 h later, MTT was replaced with 150 μl dimethyl sulfoxide (DMSO). The spectrophotometric absorbance was measured on a model 550 microplate reader (Bio‐Rad Laboratories, Hercules, CA) at 570 nm with a reference wavelength of 655 nm. Cell viability = absorbance value of adenovirus‐infected cells/absorbance value of control cells.
2.10. Apoptosis analysis by cytometry
3 × 105 ARPE‐19, L‐02, SP6.5 and OM431 cells were infected with 10 MOI of Ad‐EGFP, Ad‐TRAIL or Ad‐TRAIL‐3MREs. 48 h later, cells were harvested and Annexin V‐PE Apoptosis Detection Kit (K128‐100, BioVision) was used for apoptosis detection according to the manufacturer's instructions. The percentage of apoptotic cells was evaluated by fluorescence‐activated cell sorting (FACS) analysis (Aria™ II, BD).
2.11. Apoptosis detection by Hoechst 33342 staining
3 × 105 L‐02 and SP6.5 cells were infected with 10 MOI of Ad‐EGFP, Ad‐TRAIL or Ad‐TRAIL‐3MREs. 48 h later, the cells were incubated with Hoechst 33342 (25 μg/ml, Thermo Scientific) for 30 min and observed under fluorescence microscope (Olympus).
2.12. Animal experiments
Animal experiment procedures were approved by the Committee on the Use and Care on Animals in Zhangjiang High Technology Park (Shanghai, China). Subcutaneous SP6.5 tumor xenograft was established by injecting 5 × 106 cells at the flanks of 4‐ to 6‐week‐old male BALB/c nude mice (Institute of Animal Center, Chinese Academy of Sciences, Shanghai, China). 24 mice were randomly divided into 4 groups (n = 6). The mice were intravenously injected with 100 μl PBS with 1 × 109 pfu of Ad‐TRAIL, Ad‐TRAIL‐3MREs, Ad‐EGFP or 100 μl PBS alone. The injections were repeated every other day for 3 times with a total dosage of 3 × 109 pfu of adenoviruses. Tumor diameter was measured by periodic measurements with calipers and volume. Tumor volume (mm3) = maximal length (mm) × perpendicular width (mm)2/2.
To test the hepatoxicity resulting from off‐target TRAIL expression mediated by adenovirus, 20 six‐week‐old male BALB/c mice (n = 5) were administered with 1 × 109 pfu of different adenoviruses via intravenous injection every other day for three times. 600 ml blood was harvested by cardiac puncture on Day 12. After incubation with 12 U of heparin, serum concentration of Alanine aminotransferase (ALT) was evaluated using ALT Activity Assay Kit (ab105134, Abcam).
2.13. Histological staining and TUNEL assay
One mouse was sacrificed from each group in the subcutaneous SP6.5 tumor model 7 days after adenovirus administration. Tumor and liver tissues were harvested and fixed with 10% formalin. Streptavidinbiotin peroxidase complex‐based histological staining was performed to detect the expression of TRAIL. The antigen retrieval procedure was performed by heating the samples in Dako antigen retrieval solution containing 10 mM EDTA (pH 8.0) with a pressure cooker. Goat polyclonal anti‐TRAIL antibody (1:150; sc‐6079, Santa Cruz Biotechnology, CA) was used and slides were counterstained with hematoxylin (Sigma–Aldrich).
Apoptosis was detected in the fixed tumor samples with TACS‐XL In Situ Apoptosis Detection Kit (R&D Systems) according to the manufacturer's instructions.
2.14. Statistical analysis
The statistical tests in this manuscript were two‐tailed student's t‐test. Differences were considered as statistically significant (*) when P < 0.05 and statistically very significant (**) when P < 0.01.
3. Results
3.1. Uveal melanoma cells have reduced levels of miR‐34a, miR‐137 and miR‐182
Expression profiles of miR‐34a, miR‐137 and miR‐182 were investigated in ARPE‐19, L‐02, SP6.5 and OM431 cells both by northern blotting and qPCR assays. Consistent with the previously published data, expression of miR‐34a, miR‐137 and miR‐182 were all reduced in SP6.5 and OM431 uveal melanoma cells (P < 0.01) in comparison with normal retinal epithelial cells (ARPE‐19) and liver cells (L‐02) (Figure 1A and B). The significant difference in the abundance of miR‐34a, miR‐137 and miR‐182 between uveal melanoma and normal cells potentially ensured uveal melanoma‐specific expression of exogenous genes by using them.
Figure 1.
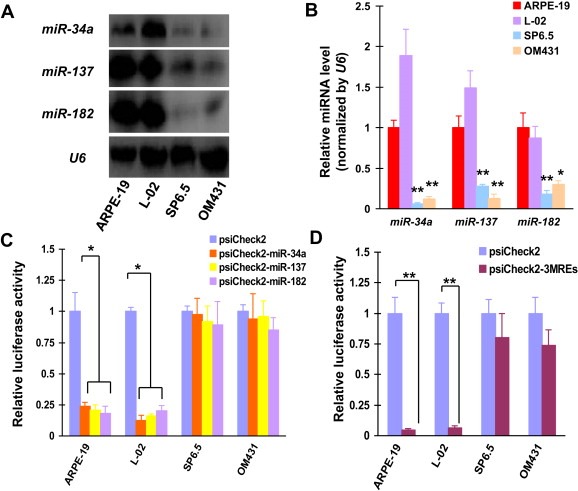
Uveal melanoma‐specific gene expression due to MREs of miR‐34a, miR‐137 and miR‐182. (A) Northern blotting was performed to detect the abundance of miR‐34a, miR‐137 and miR‐182 in ARPE‐19, L‐02, SP6.5 and OM431 cell lines. U6 was used as a loading control. (B) The levels of miR‐34a, miR‐137 and miR‐182 were also detected by qPCR in uveal melanoma and normal cell lines. Means ± SD of three independent experiments were shown. (C) Luciferase activity was detected 48 h after psiCheck2, psiCheck2‐miR‐34a, psiCheck2‐miR‐137 and psiCheck2‐miR‐182 transfection of normal and uveal melanoma cell lines. These experiments were performed in triplicate and means ± SD was presented. (D) Cells were also transfected with psiCheck2‐3MREs, followed by quantification of luciferase activity.
3.2. miRNA response elements (MREs) of miR‐34a, miR‐137 and miR‐182 targeted gene expression to uveal melanoma cells
To determine if MREs of miR‐34a, miR‐137 and miR‐182 were able to regulate uveal melanoma‐specific gene delivery, ARPE‐19, L‐02, SP6.5 and OM431 cells were transfected with luciferase reporter plasmids whose expression was regulated by these MREs. Luciferase activity assay indicated that there was no significant difference in luciferase expression between MRE‐regulated reporter‐ and control reporter‐transfected SP6.5 and OM431 cells (Figure 1C). Notably, luciferase expression was greatly suppressed in MRE‐regulated reporter‐transfected ARPE‐19 and L‐02 cells (P < 0.05) (Figure 1C). These results showed that the application of MREs of miR‐34a, miR‐137 and miR‐182 allowed exogenous genes to be expressed selectively in uveal melanoma cells.
3.3. Simultaneous application of MREs of miR‐34a, miR‐137 and miR‐182 conferred exogenous gene expression with a higher specificity to uveal melanoma
In order to enhance the specificity to uveal melanoma cells of MREs‐directed gene delivery, MREs of miR‐34a, miR‐137 and miR‐182 were inserted a single vector to regulate the expression of exogenous gene (psiCheck2‐3MREs). Luciferase assays showed that luciferase expression was suppressed more potently in psiCheck2‐3MREs‐transfected ARPE‐19 (95.2%) and L‐02 (93.7%) cells, compared with that transfected with psiCheck2‐miR‐34a (ARPE‐19: 76.0%, P < 0.05; L‐02: 81.6%, P < 0.05), psiCheck2‐miR‐137 (ARPE‐19: 79.2%, P < 0.05; L‐02: 83.6%, P < 0.05) or psiCheck2‐miR‐182 alone (ARPE‐19: 81.8%, P < 0.05; L‐02: 79.9%, P < 0.05; Figure 1C and D), suggesting an additive inhibitory effect of combined usage of 3 MREs on gene expression in normal cells. In contrast, using MREs of these 3 miRNAs resulted in only a moderate reduction of luciferase expression in psiCheck2‐3MREs‐transfected SP6.5 and OM431 cells (19.8% and 26.3%, P > 0.05, Figure 1D), showing that the potency of gene expression in uveal melanoma cells was not significantly compromised when multiple MREs were inserted into the adenovirus vector.
These data revealed that combined application of MREs of miR‐34a, miR‐137 and miR‐182 ensured exogenous gene to have higher uveal melanoma specificity without significantly reducing its expression potency.
3.4. MREs of miR‐34a, miR‐137 and miR‐182 ensured TRAIL to be expressed specifically in uveal melanoma cells
MREs of miR‐34a, miR‐137 and miR‐182 were subsequently inserted into TRAIL‐expressing adenoviral vectors to regulate expression of this apoptosis‐inducing gene. A recombinant adenovirus was constructed by inserting 2 copies of MREs of miR‐34a, miR‐137 and miR‐182 immediately following TRAIL‐coding open reading frame (named as Ad‐TRAIL‐3MREs, Figure 2A). 10 MOI of Ad‐TRAIL, Ad‐TRAIL‐3MREs or Ad‐EGFP was infected on SP6.5, OM431, ARPE‐19 and L‐02 cells. 48 h later, TRAIL expression was detected in all the Ad‐TRAIL‐infected cell lines, demonstrating a non‐specific expression fashion (Figure 2B). Almost no TRAIL expression was detected in ARPE‐19 and L‐02 cells infected by Ad‐TRAIL‐3MREs. However, Ad‐TRAIL‐3MREs‐infected SP6.5 and OM431 uveal melanoma cells expressed a similar level of TRAIL protein to that infected by Ad‐TRAIL (Figure 2B), displaying a uveal melanoma selective expression profile.
qPCR and ELISA assays were also employed to confirm the expression profile of TRAIL mediated by different adenovirus. 48 h after the treatment of Ad‐TRAIL or Ad‐TRAIL‐3MREs, TRAIL expression was detected in all the Ad‐TRAIL‐infected cell lines and Ad‐TRAIL‐3MREs‐infected SP6.5 and OM431 cells (Figure 2C and D). Consistently, TRAIL expression was greatly suppressed in Ad‐TRAIL‐3MREs‐infected normal cell lines (Figure 2C and D).
Collectively, MREs was able to act as regulatory elements for TRAIL expression and enabled its expression to display a uveal melanoma‐specific expression profile.
3.5. Ad‐TRAIL‐3MREs‐mediated TRAIL expression is regulated by the levels of miR‐34a, miR‐137 or miR‐182
To confirm the expression of exogenous TRAIL was regulated by the abundance of miR‐34a, miR‐137 and miR‐182 in cells, we changed their expression profiles using synthetic miRNA inhibitors or mimics specific for miR‐34a, miR‐137 and miR‐182 and subsequently detected TRAIL levels by immunoblotting assay. 48 h after the treatment of mixed miRNA inhibitors, TRAIL expression was obviously elevated in L‐02 cells which originally have high levels of endogenous miR‐34a, miR‐137 and miR‐182 (Figure 2E). Also, TRAIL expression was suppressed in SP6.5 melanoma cells when miR‐34a, miR‐137 and miR‐182 levels were reduced by mixed miRNA mimics (Figure 2E).
These data indicated that Ad‐TRAIL‐3MREs‐mediated TRAIL expression stringently depended on the abundance of the corresponding miRNAs in the infected cells.
3.6. Ad‐TRAIL‐3MREs reduced the survival of uveal melanoma cells without cytotoxicity to normal cells
MTT assay was performed to evaluate the survival of cells tranduced with different adenoviral vectors of indicated MOIs. 7 days after adenovirus administration, cell viability was determined in ARPE‐19, L‐02, SP6.5 and OM431 cells. Ad‐TRAIL infection significantly suppressed the survival of normal cells, ARPE‐19 (IC50 = 9.71 ± 1.34 MOI) and L‐02 (IC50, 9.30 ± 0.55 MOI). Nevertheless, viability of ARPE‐19 and L‐02 cells was almost not affected by the transduction of Ad‐TRAIL‐3MREs (IC50 > 100 MOI, P < 0.01 at MOI of 10, 20, 50 and 100), indicating a cytotoxicity‐free property of this MREs‐regulated recombinant adenovirus to normal cells (Figure 3).
Figure 3.
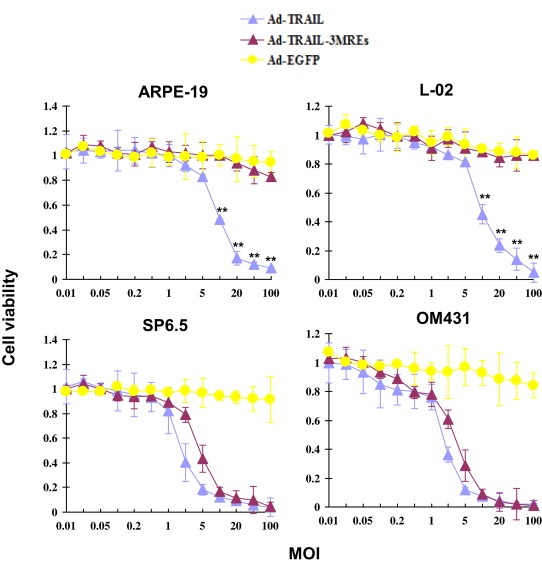
Ad‐TRAIL‐3MREs reduced the survival of uveal melanoma cells without significant cytotoxicity to normal cells. ARPE‐19, L‐02, SP6.5 and OM431 cells were transduced with Ad‐TRAIL, Ad‐TRAIL‐3MREs or Ad‐EGFP at indicated MOIs. The viability was measured by MTT assay 7 days after adenovirus infection. Means ± SD of three independent experiments were shown. ** P < 0.01.
Both Ad‐TRAIL and Ad‐TRAIL‐3MREs efficiently suppressed the viability of uveal melanoma cells. Intriguingly, there was no significant difference in survival‐suppressing activity between Ad‐TRAIL and Ad‐TRAIL‐3MREs on SP6.5 (Ad‐TRAIL IC50 = 1.77 ± 0.25 MOI vs Ad‐TRAIL‐3MREs IC50 = 4.41 ± 0.67 MOI, P > 0.05) and OM431 (Ad‐TRAIL IC50 = 1.64 ± 0.13 MOI vs Ad‐TRAIL‐3MREs IC50 = 2.93 ± 0.40 MOI, P > 0.05 at all MOIs) cell lines, demonstrating that using MREs did not significantly compromise the anti‐tumor potency of TRAIL‐expressing adenoviral vectors (Figure 3).
In conclusion, Ad‐TRAIL‐3MREs suppressed the survival of uveal melanoma cells without significant cytotoxicity to normal cells.
3.7. Ad‐TRAIL‐3MREs induced apoptosis in uveal melanoma cells, but not normal cells
Given that TRAIL suppresses survival of cancer cells primarily by inducing apoptosis, activation of apoptotic pathway was detected in cells transduced with Ad‐TRAIL, Ad‐TRAIL‐3MREs and Ad‐EGFP. Immunoblotting assays revealed that cleavage of caspase 3, caspase 8, caspase 9, BID and PARP occurred both in ARPE‐19 and SP6.5 cells infected with Ad‐TRAIL (Figure 4A). However, Ad‐TRAIL‐3MREs only triggered the cleavage of these apoptosis‐related molecules in SP6.5 cells (Figure 4A).
Figure 4.
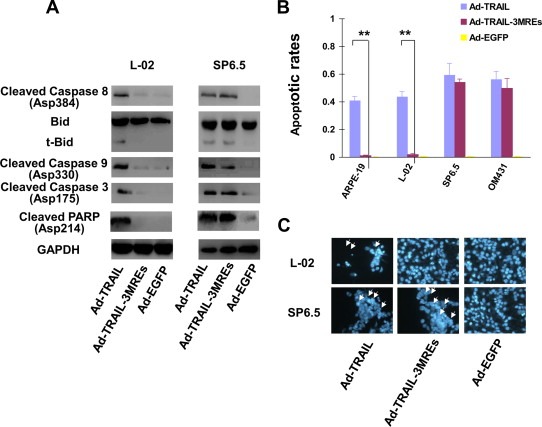
Ad‐TRAIL‐3MREs infection induced apoptosis in uveal melanoma cells. (A) Cleaved fragments of caspase 3, 8 and 9, and PARP, as well as truncated Bid, was detected by immunoblotting assays, in order to confirm the activation of apoptotic pathway. GAPDH was selected as endogenous controls. (B) Apoptotic rates were determined in ARPE‐19, L‐02, SP6.5 and OM431 cells 48 h after infection with Ad‐TRAIL, Ad‐TRAIL‐3MREs or Ad‐EGFP of 10 MOI by FACS‐based detection of Annexin V positivity. Means ± SD of three separate experiments were represented. (C) Apoptosis was detected in L‐02 and SP6.5 cells with Hoechst 33342 staining (×200). Arrows indicated the apoptotic cells with nuclear condensation.
The percentages of cells undergoing apoptosis were also evaluated by detecting Annexin V expression. In SP6.5 and OM431 cell lines, high percentages of cells underwent apoptosis both under the infection of 10 MOI Ad‐TRAIL and Ad‐TRAIL‐3MREs (varying between 49.9% and 59.3%, Figure 4B). In normal cell lines, Ad‐TRAIL, rather than Ad‐TRAIL‐3MREs, induced extensive apoptotic events (Figure 4B). Consistently, nuclear condensation was extensively observed in Ad‐TRAIL‐treated L‐02 and SP6.5 cells as well as Ad‐TRAIL‐3MREs‐treated SP6.5 cells. There was no significant difference in nuclear morphology between Ad‐TRAIL‐3MREs‐ and Ad‐EGFP‐treated L‐02 cells (Figure 4C).
Collectively, Ad‐TRAIL‐3MREs potently induced apoptosis in uveal melanoma cells and spared normal cells from this event.
3.8. Ad‐TRAIL‐3MREs reduced the growth of uveal melanoma in vivo
To further confirm the anti‐tumor capacity of Ad‐TRAIL‐3MREs, a subcutaneous SP6.5 uveal melanoma model was established in nude BALB/c mice. When tumors become palpable, Ad‐TRAIL, Ad‐TRAIL‐3MREs, Ad‐EGFP or PBS was injected into the tail vein of mice. Tumor diameters were periodically measured and their volumes were calculated according to the formula described in the section of Material and methods.
35 days after the first injection, uveal melanoma growth was reduced by 71.7% in Ad‐TRAIL‐treated mice. Similarly, Ad‐TRAIL‐3MREs administration suppressed the growth of SP6.5 tumor xenografts by 61.2%. There was no significant difference in their anti‐tumor activity in vivo between the two TRAIL‐expressing adenoviruses (P > 0.05 at all the indicated time points; Figure 5A and B).
Figure 5.
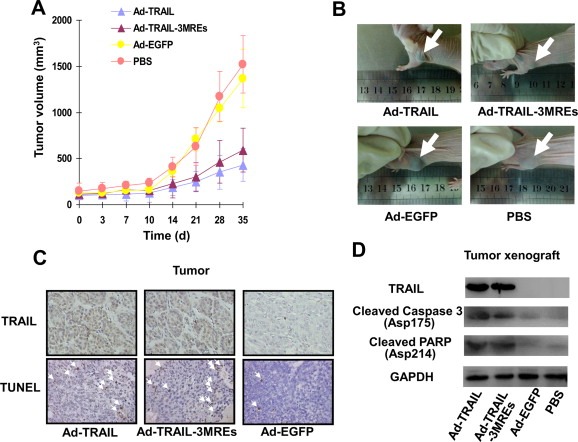
Ad‐TRAIL‐3MREs suppressed the growth of uveal melanoma xenotransplants by inducing apoptosis. (A) 5 × 106 SP6.5 uveal melanoma cells was inoculated into the flanks of male BALB/c nude mice (4 group; n = 6). 3 × 109 pfu of Ad‐TRAIL, Ad‐TRAIL‐3MREs, Ad‐EGFP or PBS were administrated via tail vein injection. The diameters of tumors were periodically measured until Day 35. Means ± SD of tumor sizes were shown. (B) The representative photographs of the SP6.5 tumor‐bearing mice treated with Ad‐TRAIL, Ad‐TRAIL‐3MREs, Ad‐EGFP or PBS were shown. The arrows indicated the tumors. (C) TRAIL expression was investigated in the tumor samples from the mice treated with indicated adenoviruses by immunohistological staining. TUNEL assay was also performed to detect the apoptotic events in tumors. The representative images were shown (×200). White arrows indicated apoptotic cells. (D) Immunoblotting assay showed the expression of TRAIL and apoptotic pathway‐related proteins, including cleaved caspase 3 and PARP, in the tumor xenografts. GAPDH was used as loading control.
Tumor sections from mice infected with adenoviruses were stained with anti‐TRAIL antibody. Both in Ad‐TRAIL‐ and Ad‐TRAIL‐3MREs‐infected groups, strong TRAIL expression was detected in SP6.5 tumor xenografts (Figure 5C). Furthermore, TUNEL assays indicated that apoptosis extensively occurred in SP6.5 tumors infected with Ad‐TRAIL and Ad‐TRAIL‐3MREs (Figure 5C).
Consistently, immunoblotting assays confirmed that tumor xenografts expressed high level of TRAIL protein in Ad‐TRAIL‐ and Ad‐TRAIL‐3MREs‐injected mice and apoptotic pathways were activated in these samples (Figure 5D).
3.9. Ad‐TRAIL‐3MREs prevented liver tissue from TRAIL‐related cytotoxicity in mice
In liver tissues of mice, Ad‐TRAIL‐3MREs expressed no detectable TRAIL protein, whereas Ad‐TRAIL infection led to extensive TRAIL expression, revealed by immunohistological staining (Figure 6A), indicating that application of MREs prevented the liver tissues from exogenous TRAIL expression in vivo (Figure 6A). Immunoblotting assays further confirmed that Ad‐TRAIL expressed high level of TRAIL, and as a result, activated apoptotic pathway in liver tissues of mice. There was no detectable TRAIL expression or activation of apoptotic pathway in hepatic cells from Ad‐TRAIL‐3MREs‐administered mice (Figure 6B).
Figure 6.
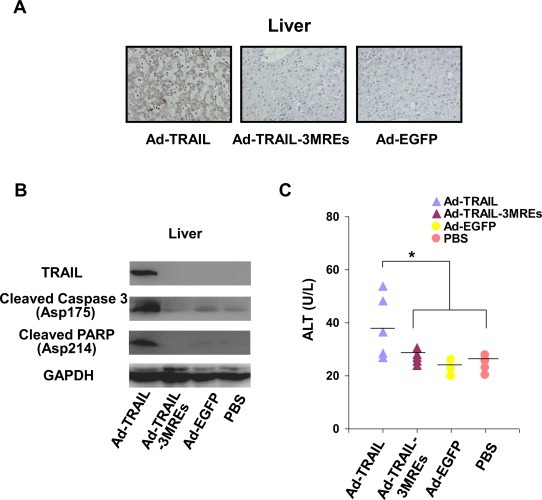
Ad‐TRAIL‐3MREs prevented liver tissue from cytotoxicity in mice. (A) TRAIL expression was investigated in liver tissues of the mice treated with indicated adenoviruses or PBS by immunohistological staining. The representative images were shown (×200). (B) Immunoblotting assay showed the expression of TRAIL and apoptotic pathway‐related proteins, including cleaved caspase 3 and PARP, in liver tissues of tumor‐bearing mice treated by adenoviruses. GAPDH was used as loading control. (C) 3 × 109 pfu of indicated adenoviruses or PBS was intravenously injected into 20 tumor‐free mice (4 group; n = 5). Subsequently, their blood was harvested to evaluate ALT levels. Means ± SD were shown.
To compare the cytotoxicity of Ad‐TRAIL and Ad‐TRAIL‐3MREs to liver tissue, another 20 tumor‐free mice were intravenously injected with adenoviruses used above, followed by sacrifice and blood harvesting. Serum level of alanine aminotransferase (ALT) was subsequently evaluated to test the damage of adenoviruses to hepatic cells. ALT level was significantly elevated in Ad‐TRAIL‐infected mice (Ad‐TRAIL: 38.8 ± 11.9 U/L, P < 0.05; Figure 6C). Ad‐TRAIL‐3MREs administration had no significant effect on ALT level, in comparison with PBS‐ and Ad‐EGFP‐treated mice (Ad‐TRAIL‐3MREs: 26.9 ± 2.5 U/L; Ad‐TRAIL: 22.8 ± 3.0 U/L; PBS: 24.5 ± 2.9 U/L, P > 0.05; Figure 6C). Increased level of ALT in blood indicated that liver cells underwent damages in Ad‐TRAIL‐infected mice.
4. Discussion
We evaluated the expression level of miR‐34a, miR‐137 and miR‐182 in uveal melanoma cell lines, SP6.5 and OM431, and normal cell lines, ARPE‐19 and L‐02. Consistent with the data previously published, the levels of the 3 miRNAs were all reduced in uveal melanoma cells, compared with normal cells. Yan et al. reported that miR‐34a expression was not detectable in M17, M21, M23 and SP6.5 melanoma cell lines by Northern blotting analysis. In contrast, uveal melanocyte cell line D‐87 had high level of miR‐34a. Lack of miR‐34a expression was also confirmed in patient‐derived uveal melanoma samples (Yan et al., 2009). miR‐137, which has been well established as tumor‐suppressing miRNA in a variety of cancer types (Kozaki et al., 2008; Liang et al., 2013; Liu et al., 2011b; Silber et al., 2008; Zhao et al., 2012; Zhu et al., 2013), also had reduced expression level in uveal melanoma cell lines, M17, M23 and SP6.5, in comparison with primary uveal melanocyte um95 (Chen et al., 2011). As one of strongest inhibitors of melanoma cell proliferation (Poell et al., 2012), miR‐182 expression was also suppressed in melanoma cell lines (Yan et al., 2012). The differential expression profiles of miR‐34a, miR‐137 and miR‐182 allowed the application of their MREs to restrict the exogenous gene expression within melanoma cells, and meanwhile, prevent its expression in normal tissue‐derived cells.
Luciferase assays showed that MREs of miR‐34a, miR‐137 and miR‐182 were able to suppress the expression of exogenous gene in normal cells that highly express these miRNAs without significantly compromising its expression in uveal melanoma cell lines, suggesting that these miRNA‐responsive DNA elements are suitable regulators for melanoma‐targeting TRAIL expression. Oncolytic adenoviruses have been widely studied aimed for future clinical cancer treatment (Jiang et al., 2012; Liu et al., 2011a; Zhang et al., 2012). MREs of tumor‐suppressing miRNAs, such as let‐7, have been used to construct oncolytic adenoviruses that only replicated in cancer cells where the involved miRNAs had reduced expression levels, compared with normal cells. In this oncolytic adenovirus, the level of E1A gene that is responsible for adenovirus replication was regulated by 8 copies of let‐7 MREs, permitting replication and cytotoxicity of oncolytic adenoviruses in HCC cells with no or low let‐7 expression. Importantly, this MRE‐regulated oncolytic adenovirus had no influence on the viability of normal cell lines (Jin et al., 2011).
2 copies of MREs of miR‐34a, miR‐137 and miR‐182 were inserted immediately following the TRAIL‐coding sequence to generate a MRE‐regulated TRAIL‐expressing replication‐deficient adenoviral vector (Ad‐TRAIL‐3MREs). Although more than 2 copies of binding sites were used in some cases, 2 copies of binding sites are sufficient for this miRNA‐based TRAIL expression in adenoviral vector (Bo et al., 2013; Zhao et al., 2013). Sufficient transduction efficiency of adenoviral vectors to the involved cell lines is also important. By qPCR assays, we found that CAR (The primary cellular receptor of adenovirus) mRNA levels were high and similar in these cell lines (data not shown). High CAR level ensured that adenovirus‐mediated TRAIL can be highly expressed in the normal epithelial and uveal melanoma cell lines. Multidisciplinary approaches indicated that TRAIL expression was almost undetectable in Ad‐TRAIL‐3MREs‐infected normal cells. However, there was no significant difference in the potency of TRAIL expression between Ad‐TRAIL‐ and Ad‐TRAIL‐3MREs‐infected uveal melanoma cells. Changes in endogenous miRNA levels in cells were able to affect TRAIL expression by this vector. These data demonstrated that MREs were able to control the expression of TRAIL transgene dependent on the level of the corresponding miRNA in cells. In fact, this strategy has been preclinically studied for the treatment of bladder cancer and glioma (Bo et al., 2013; Zhao et al., 2013).
As expected, TRAIL expression induced apoptosis and reduced the survival in uveal melanoma cells. Previously, SP6.5 and OM431 cell lines have been shown to be sensitive to TRAIL‐induced apoptosis (Zhou et al., 2010). Cytometry‐based analysis revealed that OM431 expressed high level of TRAIL‐R1 (Li et al., 2005; Ren et al., 2004). Primary hepatocytes were also susceptible to TRAIL treatment (Armeanu et al., 2003; Corazza et al., 2006; Jo et al., 2000). For instances, 30% of primary liver cells underwent apoptosis under the treatment of 50 ng/ml TRAIL (Ozoren et al., 2000). Our data also showed the cytotoxicity of Ad‐TRAIL to normal liver cell, L‐02. Remarkably, Ad‐TRAIL‐3MREs exhibited no effect on the viability and apoptosis induction in normal cells. The above results demonstrated that MRE‐regulated TRAIL‐expressing adenoviral vectors have both high biosafety and anti‐tumor potency.
Animal experiments further confirmed the anti‐tumor activity of Ad‐TRAIL‐3MREs on uveal melanoma and biosafety to liver tissues. Growth of SP6.5 tumor xenografts was greatly suppressed with infection of Ad‐TRAIL‐3MREs and the potency is similar to that of Ad‐TRAIL. The application of MREs was evidenced not to significantly affect the anti‐tumor function of TRAIL‐expressing adenoviral vectors. Although the amount of adenoviruses that reach the tumor sites varies depending on the location of the tumors and serotypes of adenovirus used, adenovirus can pass the fenestrations that are widely distributed in the tumor vasculature due to its small size (70–90 nm). So adenoviral vectors injected into blood can reach the tumor sites and enter the tumor cells without significant hurdles. Furthermore, ALT level was evaluated to compare the hepotatoxicity from different adenoviruses in mice. Ad‐TRAIL‐3MREs caused no significant damage in hepatic cells, compared with PBS‐ or Ad‐EGFP vector‐treated animals, suggesting that using MRE is effective to prevent normal hepatic cells from TRAIL‐induced toxicity. Finally, histological staining also supported the conclusion that Ad‐TRAIL‐3MREs is a TRAIL delivery system with high specificity and efficiency.
In future, MREs of other miRNAs can also be used to improve the specificity of exogenous gene expression. For example, miR‐122 is the most abundant miRNA in normal liver cells and acts as a tumor‐suppressing miRNA (Ma et al., 2010). Its expression has been frequently reported to be reduced in liver cancer cells. Therefore, MRE of miR‐122 can also be applied to regulate the expression of exogenous genes for biosafety. In addition, cytokines other than TRAIL, such as DKK1 or IL‐24, can also function as therapeutic genes in this MRE‐regulated adenoviral vector (Luo et al., 2008; Wang et al., 2012).
Collectively, we constructed a uveal melanoma‐targeting TRAIL‐expressing adenoviral vector. Its specificity and anti‐tumor potency were experimentally verified using multidisciplinary approaches. We provided evidence that this novel strategy‐regulated adenovirus‐based gene therapy vector may be a promising biological agent and is worthy of a further clinical trial for uveal melanoma treatment.
Conflict of interest
No declaration.
Supporting information
Supplementary data
Acknowledgement
We appreciated generous providing of adenoviruses by Dr. Zhao in General Hospital of Chengdu Military Area Command of Chinese PLA, Chengdu, China. This study was supported by Science and Technology Innovation Fund for Small and Medium‐Sized Enterprises, the Ministry of Science and Technology (No. 11c26213304738), Science and Technology Innovation Fund for Small and Medium‐Sized Enterprises in Shanghai City (No. 1202H124400), and National Natural Sciences Foundation of China (No. 81001104).
Supplementary data 1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2013.08.003.
Liu Jia, Ma Leina, Li Caixin, Zhang Ziyu, Yang Guanghua, Zhang Wenwei, (2013), Tumor‐targeting TRAIL expression mediated by miRNA response elements suppressed growth of uveal melanoma cells, Molecular Oncology, 7, doi: 10.1016/j.molonc.2013.08.003.
Contributor Information
Guanghua Yang, Email: guanghuayang1227@gmail.com.
Wenwei Zhang, Email: wenweizhang@gmail.com.
References
- Alizadeh, H. , Howard, K. , Mellon, J. , Mayhew, E. , Rusciano, D. , Niederkorn, J.Y. , 2003. Reduction of liver metastasis of intraocular melanoma by interferon-beta gene transfer. Invest. Ophthalmol. Vis. Sci.. 44, 3042–3051. [DOI] [PubMed] [Google Scholar]
- Armeanu, S. , Lauer, U.M. , Smirnow, I. , Schenk, M. , Weiss, T.S. , Gregor, M. , Bitzer, M. , 2003. Adenoviral gene transfer of tumor necrosis factor-related apoptosis-inducing ligand overcomes an impaired response of hepatoma cells but causes severe apoptosis in primary human hepatocytes. Cancer Res.. 63, 2369–2372. [PubMed] [Google Scholar]
- Bakalian, S. , Marshall, J.C. , Logan, P. , Faingold, D. , Maloney, S. , Di Cesare, S. , Martins, C. , Fernandes, B.F. , Burnier, M.N. , 2008. Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin. Cancer Res.. 14, 951–956. [DOI] [PubMed] [Google Scholar]
- Bo, Y. , Guo, G. , Yao, W. , 2013. MiRNA-mediated tumor specific delivery of TRAIL reduced glioma growth. J. Neurooncol.. 112, 27–37. [DOI] [PubMed] [Google Scholar]
- Carlo-Stella, C. , Lavazza, C. , Locatelli, A. , Vigano, L. , Gianni, A.M. , Gianni, L. , 2007. Targeting TRAIL agonistic receptors for cancer therapy. Clin. Cancer Res.. 13, 2313–2317. [DOI] [PubMed] [Google Scholar]
- Carlring, J. , Shaif-Muthana, M. , Sisley, K. , Rennie, I.G. , Murray, A.K. , 2003. Apoptotic cell death in conjunction with CD80 costimulation confers uveal melanoma cells with the ability to induce immune responses. Immunology. 109, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Lou, W. , Shen, J. , Ma, L. , Yang, Z. , Liu, L. , Luo, J. , Qian, C. , 2010. Potent antitumor activity in experimental hepatocellular carcinoma by adenovirus-mediated coexpression of TRAIL and shRNA against COX-2. Clin. Cancer Res.. 16, 3696–3705. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Wang, J. , Shen, H. , Lu, J. , Li, C. , Hu, D.N. , Dong, X.D. , Yan, D. , Tu, L. , 2011. Epigenetics, microRNAs, and carcinogenesis: functional role of microRNA-137 in uveal melanoma. Invest. Ophthalmol. Vis. Sci.. 52, 1193–1199. [DOI] [PubMed] [Google Scholar]
- Corazza, N. , Jakob, S. , Schaer, C. , Frese, S. , Keogh, A. , Stroka, D. , Kassahn, D. , Torgler, R. , Mueller, C. , Schneider, P. , Brunner, T. , 2006. TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J. Clin. Invest.. 116, 2493–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cun, B. , Song, X. , Jia, R. , Zhao, X. , Wang, H. , Ge, S. , Fan, X. , 2012. Combination of oncolytic adenovirus and dacarbazine attenuates antitumor ability against uveal melanoma cells via cell cycle block. Cancer Biol. Ther.. 13, 77–84. [DOI] [PubMed] [Google Scholar]
- Huang, X. , Jia, R. , Zhao, X. , Liu, B. , Wang, H. , Wang, J. , Zhou, Y. , Cun, B. , Ge, S. , Fan, X. , 2012. Recombinant oncolytic adenovirus H101 combined with siBCL2: cytotoxic effect on uveal melanoma cell lines. Br. J. Ophthalmol.. 96, 1331–1338. [DOI] [PubMed] [Google Scholar]
- Huang, X. , Wang, L. , Zhang, H. , Wang, H. , Zhao, X. , Qian, G. , Hu, J. , Ge, S. , Fan, X. , 2012. Therapeutic efficacy by targeting correction of Notch1-induced aberrants in uveal tumors. PLoS One. 7, e44301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Sheikh, M.S. , 2007. TRAIL death receptors and cancer therapeutics. Toxicol. Appl. Pharmacol.. 224, 284–289. [DOI] [PubMed] [Google Scholar]
- Jiang, G. , Zhang, K. , Jiang, A.J. , Xu, D. , Xin, Y. , Wei, Z.P. , Zheng, J.N. , Liu, Y.Q. , 2012. A conditionally replicating adenovirus carrying interleukin-24 sensitizes melanoma cells to radiotherapy via apoptosis. Mol. Oncol.. 6, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, H. , Lv, S. , Yang, J. , Wang, X. , Hu, H. , Su, C. , Zhou, C. , Li, J. , Huang, Y. , Li, L. , Liu, X. , Wu, M. , Qian, Q. , 2011. Use of microRNA Let-7 to control the replication specificity of oncolytic adenovirus in hepatocellular carcinoma cells. PLoS One. 6, e21307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, M. , Kim, T.H. , Seol, D.W. , Esplen, J.E. , Dorko, K. , Billiar, T.R. , Strom, S.C. , 2000. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat. Med.. 6, 564–567. [DOI] [PubMed] [Google Scholar]
- Kozaki, K. , Imoto, I. , Mogi, S. , Omura, K. , Inazawa, J. , 2008. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in oral cancer. Cancer Res.. 68, 2094–2105. [DOI] [PubMed] [Google Scholar]
- Li, H. , Niederkorn, J.Y. , Neelam, S. , Alizadeh, H. , 2005. Resistance and susceptibility of human uveal melanoma cells to TRAIL-induced apoptosis. Arch. Ophthalmol.. 123, 654–661. [DOI] [PubMed] [Google Scholar]
- Liang, L. , Li, X. , Zhang, X. , Lv, Z. , He, G. , Zhao, W. , Ren, X. , Li, Y. , Bian, X. , Liao, W. , Liu, W. , Yang, G. , Ding, Y. , 2013. MicroRNA-137, an HMGA1 target, suppresses colorectal cancer cell invasion and metastasis in mice by directly targeting FMNL2. Gastroenterology. 144, 624–635 e624. [DOI] [PubMed] [Google Scholar]
- Liu, C. , Sun, B. , An, N. , Tan, W. , Cao, L. , Luo, X. , Yu, Y. , Feng, F. , Li, B. , Wu, M. , Su, C. , Jiang, X. , 2011. Inhibitory effect of survivin promoter-regulated oncolytic adenovirus carrying P53 gene against gallbladder cancer. Mol. Oncol.. 5, 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M. , Lang, N. , Qiu, M. , Xu, F. , Li, Q. , Tang, Q. , Chen, J. , Chen, X. , Zhang, S. , Liu, Z. , Zhou, J. , Zhu, Y. , Deng, Y. , Zheng, Y. , Bi, F. , 2011. miR-137 targets Cdc42 expression, induces cell cycle G1 arrest and inhibits invasion in colorectal cancer cells. Int. J. Cancer. 128, 1269–1279. [DOI] [PubMed] [Google Scholar]
- Lub-de Hooge, M.N. , de Vries, E.G. , de Jong, S. , Bijl, M. , 2005. Soluble TRAIL concentrations are raised in patients with systemic lupus erythematosus. Ann. Rheum. Dis.. 64, 854–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujambio, A. , Lowe, S.W. , 2012. The microcosmos of cancer. Nature. 482, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, J. , Xia, Q. , Zhang, R. , Lv, C. , Zhang, W. , Wang, Y. , Cui, Q. , Liu, L. , Cai, R. , Qian, C. , 2008. Treatment of cancer with a novel dual-targeted conditionally replicative adenovirus armed with mda-7/IL-24 gene. Clin. Cancer Res.. 14, 2450–2457. [DOI] [PubMed] [Google Scholar]
- Ma, D. , Gerard, R.D. , Li, X.Y. , Alizadeh, H. , Niederkorn, J.Y. , 1997. Inhibition of metastasis of intraocular melanomas by adenovirus-mediated gene transfer of plasminogen activator inhibitor type 1 (PAI-1) in an athymic mouse model. Blood. 90, 2738–2746. [PubMed] [Google Scholar]
- Ma, L. , Liu, J. , Shen, J. , Liu, L. , Wu, J. , Li, W. , Luo, J. , Chen, Q. , Qian, C. , 2010. Expression of miR-122 mediated by adenoviral vector induces apoptosis and cell cycle arrest of cancer cells. Cancer Biol. Ther.. 9, 554–561. [DOI] [PubMed] [Google Scholar]
- Ozoren, N. , Kim, K. , Burns, T.F. , Dicker, D.T. , Moscioni, A.D. , El-Deiry, W.S. , 2000. The caspase 9 inhibitor Z-LEHD-FMK protects human liver cells while permitting death of cancer cells exposed to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res.. 60, 6259–6265. [PubMed] [Google Scholar]
- Pan, G. , Ni, J. , Wei, Y.F. , Yu, G. , Gentz, R. , Dixit, V.M. , 1997. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 277, 815–818. [DOI] [PubMed] [Google Scholar]
- Pan, G. , O'Rourke, K. , Chinnaiyan, A.M. , Gentz, R. , Ebner, R. , Ni, J. , Dixit, V.M. , 1997. The receptor for the cytotoxic ligand TRAIL. Science. 276, 111–113. [DOI] [PubMed] [Google Scholar]
- Poell, J.B. , van Haastert, R.J. , de Gunst, T. , Schultz, I.J. , Gommans, W.M. , Verheul, M. , Cerisoli, F. , van Noort, P.I. , Prevost, G.P. , Schaapveld, R.Q. , Cuppen, E. , 2012. A functional screen identifies specific microRNAs capable of inhibiting human melanoma cell viability. PLoS One. 7, e43569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, D.H. , Mayhew, E. , Hay, C. , Li, H. , Alizadeh, H. , Niederkorn, J.Y. , 2004. Uveal melanoma expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptors and susceptibility to TRAIL-induced apoptosis. Invest. Ophthalmol. Vis. Sci.. 45, 1162–1168. [DOI] [PubMed] [Google Scholar]
- Silber, J. , Lim, D.A. , Petritsch, C. , Persson, A.I. , Maunakea, A.K. , Yu, M. , Vandenberg, S.R. , Ginzinger, D.G. , James, C.D. , Costello, J.F. , Bergers, G. , Weiss, W.A. , Alvarez-Buylla, A. , Hodgson, J.G. , 2008. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med.. 6, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer, E.J. , Carthew, R.W. , 2005. Silence from within: endogenous siRNAs and miRNAs. Cell. 122, 9–12. [DOI] [PubMed] [Google Scholar]
- Wang, B. , Liu, J. , Ma, L.N. , Xiao, H.L. , Wang, Y.Z. , Li, Y. , Wang, Z. , Fan, L. , Lan, C. , Yang, M. , Hu, L. , Wei, Y. , Bian, X.W. , Chen, D. , Wang, J. , 2012. Chimeric 5/35 adenovirus-mediated Dickkopf-1 overexpression suppressed tumorigenicity of CD44(+) gastric cancer cells via attenuating Wnt signaling. J. Gastroenterol.. [DOI] [PubMed] [Google Scholar]
- Woodman, S.E. , 2012. Metastatic uveal melanoma: biology and emerging treatments. Cancer J.. 18, 148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, D. , Dong, X.D. , Chen, X. , Yao, S. , Wang, L. , Wang, J. , Wang, C. , Hu, D.N. , Qu, J. , Tu, L. , 2012. Role of microRNA-182 in posterior uveal melanoma: regulation of tumor development through MITF, BCL2 and cyclin D2. PLoS One. 7, e40967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, D. , Zhou, X. , Chen, X. , Hu, D.N. , Dong, X.D. , Wang, J. , Lu, F. , Tu, L. , Qu, J. , 2009. MicroRNA-34a inhibits uveal melanoma cell proliferation and migration through downregulation of c-Met. Invest. Ophthalmol. Vis. Sci.. 50, 1559–1565. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Fang, L. , Zhang, Q. , Zheng, Q. , Tong, J. , Fu, X. , Jiang, X. , Su, C. , Zheng, J. , 2012. An oncolytic adenovirus regulated by a radiation-inducible promoter selectively mediates hSulf-1 gene expression and mutually reinforces antitumor activity of I(131)-metuximab in hepatocellular carcinoma. Mol. Oncol.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Li, Y. , Lou, G. , Zhao, L. , Xu, Z. , Zhang, Y. , He, F. , 2012. MiR-137 targets estrogen-related receptor alpha and impairs the proliferative and migratory capacity of breast cancer cells. PLoS One. 7, e39102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Li, Y. , Wang, L. , Yang, H. , Wang, Q. , Qi, H. , Li, S. , Zhou, P. , Liang, P. , Wang, Q. , Li, X. , 2013. microRNA response elements-regulated TRAIL expression shows specific survival-suppressing activity on bladder cancer. J. Exp. Clin. Cancer Res.. 32, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Li, Y. , Wang, Q. , Wang, L. , Yang, H. , Li, M. , 2011. Increased antitumor capability of fiber-modified adenoviral vector armed with TRAIL against bladder cancers. Mol. Cell Biochem.. 353, 93–99. [DOI] [PubMed] [Google Scholar]
- Zhou, Y. , Song, X. , Jia, R. , Wang, H. , Dai, L. , Xu, X. , Gu, P. , Ge, S. , Fan, X. , 2010. Radiation-inducible human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene therapy: a novel treatment for radioresistant uveal melanoma. Pigment Cell Melanoma Res.. 23, 661–674. [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Li, Y. , Shen, H. , Li, H. , Long, L. , Hui, L. , Xu, W. , 2013. miR-137 inhibits the proliferation of lung cancer cells by targeting Cdc42 and Cdk6. FEBS Lett.. 587, 73–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


