Abstract
Small proline‐rich repeat protein 3 (SPRR3) has been linked with the altered chemoradiosensitivity, however the underlying molecular mechanisms remain elusive. Here, we report that ectopic overexpression of SPRR3 enhanced the sensitivity of cells in response to DNA damage‐induced apoptosis via loss of mitochondrial membrane potential (MMP), and increasing activation of caspase 3 in human esophageal cancer cell lines. Conversely, siRNA knockdown of SPRR3 reduced apoptosis. We found that SPRR3 was localized in mitochondria and interacted with Bcl‐2 in vivo, thus facilitating Bax mitochondrial translocation and the subsequent release of cytochrome c, and thereby enhancing cell sensitivity to DNA damage stimuli. In clinical samples, expression of SPRR3 was associated with the pathologic response (P = 0.007 in radiotherapy group, P = 0.035 in preoperative radiotherapy group) and good survival of patients with locally advanced esophageal squamous cell carcinoma (ESCC, P = 0.008). Taken together, our results implicate that SPRR3 might serve as a radiation‐sensitive predictor of ESCC.
Keywords: SPRR3, Apoptosis, Bcl-2, Chemoradiosensitivity, Esophageal cancer
Highlights
SPRR3 expression was induced by DNA damage response.
SPRR3 increased DNA damage‐induced apoptosis via mitochondrial death pathway.
SPRR3 was localized in mitochondria and interacted with Bcl‐2 in vivo.
SPRR3 expression was associated with sensitivity in preoperative radiotherapy of ESCC.
1. Introduction
Esophageal cancer remains one of the most virulent malignancies with a 5‐year overall survival (OS) rate of under 20% (Jemal et al., 2010). Surgery is one of the standard treatments for patients with resectable tumors, but surgery alone is inadequate therapy for patients with T3 or node‐positive disease (Davies et al., 2010; Kleinberg and Forastiere, 2007). Preoperative chemoradiotherapy for stage III and IV of esophageal squamous cell carcinoma (ESCC) significantly increased the resectability rate, reduced postoperative recurrence and improved survival (Osaka et al., 2004; Stahl et al., 2009; Sato and Ando, 2011). Recently, functional screens have been directed at finding novel targets affecting sensitivity to chemoradiotherapy (Motoori et al., 2010; Maher et al., 2009), but it remains unclear whether these targets have direct roles in chemoradiosensitivity or offer prognostic value to clinicians.
Small proline‐rich repeat proteins (SPRRs) possess glutamine‐ and lysine‐rich head and tail domains and a proline‐rich core. SPRRs are located in an evolutionarily conserved genetic cluster, designated as the epidermal differentiation complex (EDC) at the chromosome 1q21 (Cabral et al., 2001, 2001, 2000). SPRRs were modulated by a variety of agents, both intrinsic developmental signals and extrinsic events (Cabral et al., 2001, 2001, 2008, 2010, 1999). Two of the SPRRs family members SPRR1A and SPRR2A were identified as stress‐inducible proteins regulated by gp130 signaling pathway in the heart (Pradervand et al., 2004). In vascular smooth muscle cells (VSMC), SPRR3 regulation was dependent on the integrin α1β1/collagen interaction in response to biomechanical stress (Pyle et al., 2008). These data indicated that SPRRs were also stress‐inducible proteins.
Our previous study showed that overexpression of exogenous SPRR3 significantly suppressed cell proliferation, and triggered cellular apoptosis partially through the activation of CDK11p46 in vivo (Zhang et al., 2008). Luthra et al. showed that decreased expression of EDC genes S100A2 and SPRR3 at chromosome 1q21 defines molecular subgroups of chemoradiotherapy response in esophageal adenocarcinoma. Patients with SPRR3 expression had a higher survival rate than those lacking SPRR3 expression (Luthra et al., 2006; Luthra et al., 2007), but the mechanism remains elusive. These findings suggest that SPRR3 may be involved in cellular apoptosis and chemoradiosensitivity of esophageal cancer.
A variety of extracellular stimuli and intracellular stresses promote cell death by triggering the intrinsic or mitochondrial death pathway (Hill et al., 2003). Apoptosis is a cellular process regulated by the balance of pro‐ and anti‐apoptotic proteins of the Bcl‐2 family. Resistance to apoptosis is an essential physiologic hallmark of cancer cells (Hanahan and Weinberg, 2011).
In this study, we found ectopic expression of SPRR3 sensitized cells to DNA damage‐induced apoptosis via mitochondrial apoptosis pathway in EC9706 cells. Notably, we found, for the first time, that SPRR3 was localized in mitochondria, interacted with Bcl‐2 in vivo. SPRR3, as a novel protein partner of Bcl‐2, enhanced cell sensitivity to DNA damage stimuli through promoting Bax mitochondrial translocation and the subsequent release of cytochrome c. Moreover, SPRR3 might act as an important predictor of esophageal cancer cell sensitivity to chemoradiation. These findings demonstrated that SPRR3 was involved in mitochondrial death pathways and contributed to the sensitivity of esophageal cancer cells in response to DNA damage‐induced apoptosis.
2. Materials and methods
2.1. Tissue specimens
Tissue specimens from 109 patients with locally advanced ESCC were analyzed. At recruitment, informed consent was obtained from each patient. This study was approved by the Institutional Review Board of the Chinese Academy of Medical Sciences Cancer Institute & Hospital. Clinical specimens were obtained from initial diagnostic biopsies of the tumor sites, fixed in 4% polyformaldehyde and completely embedded in paraffin.
2.2. Cell culture, transfection and RNA interference
The ESCC cell lines (KYSE series) were kindly provided by Dr. Yutaka Shimada (Shimada et al., 1992). Human ESCC cell line EC9706 was established in our laboratory. HEK293T cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). HEK293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM), whereas KYSE series and EC9706 cells were maintained in RPM1640 supplemented with 10% fetal bovine serum and antibiotics. Small interfering RNAs (siRNA) targeting SPRR3 (siSPRR3 #1 and siSPRR3 #2) and control siRNA (siControl) were used at 25 nmol/L (Qiagen, Hilden, Germany). The sequence for control siRNA: 5′‐ UUCUCCGAACGUGUCACGU‐3′. The sequence for SPRR3 siRNA: #1, 5′‐CACUCUGAAGAAUCCUGUA‐3′; #2, 5′‐GAAAAUAUUUGUUCCCACA‐3′. Transfections were done using HiperFect (Qiagen) for siRNAs and Lipofectamine™ 2000 (Invitrogen, Groningen, NL, USA) for plasmids according to the manufacturers' recommendations.
2.3. Plasmids
Human full length SPRR3 cDNA was inserted into the mammalian expression vectors pcDNA3.0 and pCDEF‐HA. pEGFP‐C1‐Bcl‐2 plasmid was provided by Dr. Quan Chen (Nankai University, China).
2.4. In vitro radiation‐survival colony assay
Cells were seeded in triplicate in six‐well plates. After 24 h, cells were exposed to different doses of X‐IR, followed by incubation at 37 °C for 9–12 days until the development of visible colonies. Cells were fixed and then stained with crystal violet, and colonies with >50 cells were counted. The surviving fraction (SF) was calculated as the ratio of the number of colonies formed/the number of cells plated × the plating efficiency (Sheridan et al., 2010).
2.5. Determination of apoptosis and mitochondrial membrane potential
Apoptosis were assessed quantitatively using Annexin V‐FITC (Roche Diagnostics, Mannheim, Germany) as previously described (Sheridan et al., 2010). Mitochondrial membrane potential was measured by flow cytometry with Rhodamine 123 at a final concentration of 10 nM.
2.6. Assessment of caspase 3 activity
Caspase 3 activity was measured by luminescent assay according to the manufacture's instruction. Briefly, add 100 μl of Caspase‐Glo®3/7 Reagent to each well of a white‐walled 96‐well plate containing 100 μl of blank, negative control cells or treated cells in culture medium. Gently mix contents of wells using a plate shaker at 500 rpm for 30 s. Incubate at room temperature for 30 min to 3 h. The luminescence of each sample was measured. Caspase 3 activity in each experiment was normalized to untreated cells.
2.7. Subcellular fractionation
Cytosolic and nuclear extracts were prepared according to the manufacturer's instructions (Pierce, Rockford, IL, USA). Cells were fractionated with digitonin lysis buffer (80 mM KCl, 250 mM sucrose, 0.02% digitonin, and 1 μg/ml of each of the protease inhibitors) for 5 min. Mitochondrial (pellet) and cytosolic (supernatant) fractions were obtained after centrifugation at 10,000 g for 5 min.
2.8. Immunofluorescence staining assay
Cells were grown on coverslips at medium density and fixed in 4% paraformaldehyde for 15 min, and permeabilized with 0.1% Triton X‐100 for 10 min at room temperature. Cells were then stained with rabbit anti‐SPRR3 antibody. After extensive washing, cells were counterstained with secondary FITC‐conjugated mouse anti‐rabbit IgG. Mitochondrial localization was assessed by the fluorescence dye MitoTracker Red CMXRos (Invitrogen) according to the manufacturer's instructions. Fluorescence images were obtained using Leica TCS SP2 confocal laser scanning microscope (Leica Microsystems, Wetzlar, Germany).
2.9. Immunoprecipitation and Western blot
Immunoprecipitation and Western blot were performed as described previously (Leung and Ngan, 2010). Antibodies were used as follows: caspase 3, PARP, Bax, Bcl‐2, cytochrome c, AIF and GFP (Santa Cruz, Delaware, CA, USA); α‐tubulin and β‐actin (Sigma–Aldrich, St. Louis, Mo, USA); anti‐HA (Tiangen, Beijing, China). SPRR3 antibody was made by our lab (Zhang et al., 2008).
2.10. Immunohistochemistry analysis
Immunohistochemistry analysis was performed as described previously (Luo et al., 2004). Paraffin sections (thickness 5 μm) from ESCC patients were treated as described previously followed by incubation with anti‐SPRR3 overnight at 4 °C, at a dilution of 1:10,000. For negative controls, the primary antibody was replaced by nonimmune serum. After immunostaining, the slides were examined independently by two pathologists blinded to both clinical and pathologic data. The value of the integral intensity was measured by Aperio's ImageScope software (Aperio,Vista, CA, USA).
2.11. Statistical analysis
We statistically evaluated experimental results using two‐tailed, two‐independent sample t tests. Survival curves are calculated by the Kaplan–Meier method and compared using the log‐rank test. All tests of significance were set at P < 0.05.
3. Results
3.1. SPRR3 expression was induced by DNA damage response
In this study, we first assessed DNA‐damaging agents such as chemotherapeutic drugs (cisplatin) or ionizing radiation (X‐IR) on SPRR3 expression in esophageal cancer cell lines. We observed a dose‐ and time‐ dependent increase of endogenous SPRR3 protein in the presence of DNA damage stimuli. The expression level of SPRR3 was hardly detectable in EC9706 cells, and SPRR3 expression was not induced after DNA damage stimuli in EC9706 cells (Supplementary Figure S1). As shown in Supplementary Figure S1A, increase of SPRR3 expression level in response to cisplatin treatment was evident over a wide range of cisplatin concentrations. SPRR3 was induced, and remained an elevated level until 24 h after cisplatin (10 μM) treatment in KYSE30 and KYSE180 cells. Moreover, SPRR3 was strongly induced after exposure to 2, 4 and 8 Gy of X‐IR at the indicated time in KYSE30 and KYSE180 cells. A single exposure remarkably increased SPRR3 at 4 h then remained an elevated level until 24 h after recovery in KYSE30 cells (Supplementary Figure S1B, middle panel). To explore the mechanisms of SPRR3 overexpression, we treated KYSE180 cells with cisplatin and (or) protein synthesis inhibitor cycloheximide and (or) proteasome inhibitor MG132 to block de novo synthesis and the ubiquitin‐proteasome degradation. As shown in Supplementary Figure S2, we found that treatment of cells with cycloheximide and (or) proteosome inhibitor MG132 did not alter the stability of SPRR3. SPRR3 was induced in cells treated with cisplatin and cycloheximide in KYSE180 cells. These data indicate that the overexpression of SPRR3 was not due to the protein stability in response to DNA damage, but the underlying mechanisms remain poorly understood and need further investigation.
3.2. Overexpression of SPRR3 moderately sensitizes cells in response to X‐irradiation
To evaluate the possible role of SPRR3 in DNA damage response, we first examined the expression of SPRR3 in esophageal cancer cell lines. The expression level of SPRR3 was low in KYSE150, KYSE410, KYSE510, EC9706 and Colo‐680N cells, but high in KYSE30, KYSE70, KYSE140, KYSE180 and KYSE450 cells (Figure 1A, left panel). Next, we determined the clonogenic survival of these cells after exposure to 2, 4 and 8 Gy of X‐IR. KYSE150 and KYSE510 cells were the most radioresistant since these cells were derived from patients who had received prior irradiation (Shimada et al., 1992). KYSE140 and KYSE410 cells exhibited very low capability for colony formation (data not shown). EC9706 cells were found to be intermediately radioresistant (Figure 1A, right panel). Therefore, we selected EC9706 cells for establishing SPRR3 stable transfectants. As shown in Figure 1B left panel, SPRR3 was markedly increased in SPRR3 transfectants (SP57 & SP59) as compared with empty vector controls (K9 & K10) in EC9706 cells. SPRR3 overexpression alone reduced cell survival as detected by colony formation assay (Figure 1B, right panel).
Figure 1.
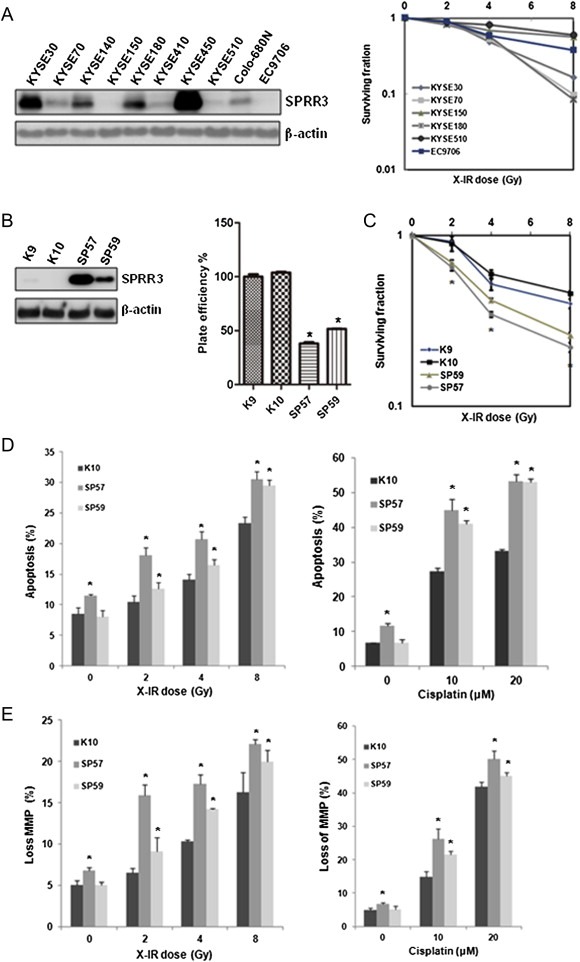
Overexpression of SPRR3 enhanced the sensitivity of esophageal cancer cells in response to DNA damage‐induced apoptosis. A) Endogenous SPRR3 was detected in esophageal cancer cell lines by Western blot (left panel), and clonogenic survival of these cells were determined after exposure to the indicated doses of X‐IR (right panel). EC9706 cells were comparatively more resistant to X‐IR‐induced cell death. B) EC9706 cells were stably transfected with pcDNA3.0‐SPRR3 or empty vector pcDNA3.0, respectively. SPRR3 transfectants (SP57 & SP59) and empty vector controls (K9 & K10) were analyzed for Western blot (left panel). Overexpression of SPRR3 inhibited cell growth in EC9706 cells (right panel). Cells were subjected to standard clonogenic assay. C) Overexpression of SPRR3 enhanced cell sensitivity to X‐IR. Clonogenic survival of cells was determined after exposure to the indicated doses of X‐IR. Data are expressed as Mean ± SD (*: P < 0.05). D) Cells were exposed to 2–8 Gy of X‐IR and allowed to recover for 72 h (left panel), or treated with cisplatin for 24 h (right panel), then subjected to Annexin V‐FITC and PI staining. Values are expressed as a percentage of Annexin V positive versus total cells. Data are expressed as Mean ± SD (*: P < 0.05). E) Effect of SPRR3 on DNA damage induced loss of mitochondrial membrane potential. Cells were treated with X‐IR or cisplatin as indicated and incubated for 30 min at 37 °C in the presence of Rhodamine 123, washed in phosphate‐buffered saline (PBS), and immediately analyzed by flow cytometry. Results are representative of three different experiments, and data are expressed as Mean ± SD (*: P < 0.05).
To determine if the status of SPRR3 in esophageal cancer cells influences their sensitivity to cytotoxic stimuli, we examined the effects of X‐IR on EC9706‐derived stable transfectants. The extent of radiosensitivity was significantly enhanced by overexpression of SPRR3 after X‐IR using colony formation assays (Figure 1C), indicating that overexpression of SPRR3 sensitized cells specifically to DNA damage response and associated with radiosensitivity of esophageal cancer cells.
3.3. Overexpression of SPRR3 increases DNA damage‐induced apoptosis via mitochondrial death pathway
To further study whether SPRR3 may influence cellular chemoradiosensitivity, we treated cells with X‐IR or cisplatin. Cells were exposed to 2–8 Gy of X‐IR and allowed to recover for 72 h. A significant increase in the percentage of apoptosis was observed in SP57 & SP59 cells compared with K10 cells. Similarly, sensitization to cisplatin treatment was observed (Figure 1D). In addition, we examined mitochondrial membrane potential (MMP) by flow cytometry in cells exposed to X‐IR or cisplatin, and found that overexpression of SPRR3 enhanced the breakdown of MMP following DNA damage stimuli (Figure 1E). Our results showed that overexpression of SPRR3 enhanced DNA damage‐induced apoptosis in esophageal cancer cells.
3.4. Overexpression of SPRR3 triggered caspase 3 activation
Caspases belong to a family of cysteine proteases that serve as major regulators of apoptosis. Initiator caspases, such as caspase 8, 9, 10, and 12, are activated by pro‐apoptotic signals. As shown in Figure 2A and B, compared with control cells, overexpression of SPRR3 could affect the activity of caspase 3 in DNA damage response. To exclude the effect of different cell lines, KYSE180 cells were transiently transfected with pcDNA3.0 or pcDNA3.0‐SPRR3 plasmids, and the effect of SPRR3 in DNA damage response were examined. As shown in Supplementary Figure S3, we found that overexpression of SPRR3 can also alter the activity of caspase 3 and PARP cleavage in cisplatin‐induced apoptosis. Moreover, we confirmed the activity of caspase 3 using luminescent assay. SPRR3 overexpressing cells accumulated significant amounts of the cleaved form of caspase 3 at the indicated dose after DNA damage response (Figure 2C). These results further indicated that overexpression of SPRR3 enhanced the sensitivity to DNA damage‐induced apoptosis.
Figure 2.
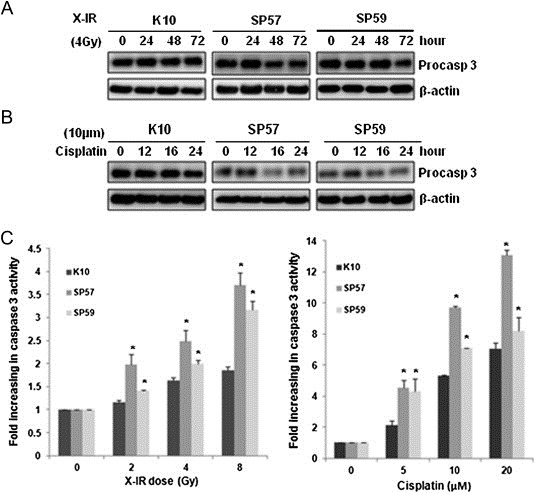
Overexpression of SPRR3 triggered caspase activation in response to DNA damage. A & B) Overexpression of SPRR3 enhances the processing of caspase 3. Cells were exposed to cisplatin (10 μΜ, A) or X‐IR (4 Gy, B) and analyzed after the indicated time by Western blot for the processing of caspase 3. β‐actin was probed as a loading control. C) Cells were treated with cisplatin or X‐IR as indicated. Caspase 3 activity was measured by luminescent assay. Data shown are Mean ± SD from multiple independent experiments (*: P < 0.05).
3.5. SPRR3 siRNA reduces DNA damage‐induced apoptosis
In order to further explore the functional role of SPRR3 in cellular apoptosis, KYSE30 cells were transfected with control or SPRR3‐specific siRNAs, and then treated with apoptosis inducer and analyzed by flow cytometry. The knockdown efficiency was examined by Western blot. Two siRNAs of SPRR3 showed similar silencing capacities (Supplementary Figure S4A). SPRR3 expression in SPRR3 siRNA‐transfected cells was lower than that in control siRNA‐transfected cells, although SPRR3 expression was induced by X‐IR (Supplementary Figure S4B). In order to verify the accuracy of gene specific siRNA, we have selected EC9706 cells that do not express SPRR3 and examined the impact of two siRNAs of SPRR3. As shown in Supplementary Figure S4C & D, two siRNAs of SPRR3 did not influence DNA damage‐induced apoptosis in EC9706 cells using Western blot and flow cytometry. Compared with control cells, silencing of SPRR3 reduces apoptosis (Figure 3A top panel). Notably, knockdown of the expression of SPRR3 decreased cell apoptosis under the treatment of cisplatin or X‐IR (Figure 3A middle and bottom panel). To investigate whether the pro‐apoptotic effect of SPRR3 is through the classical caspase‐dependent pathway, we examined the activation of PARP and caspase 3. The results indicated that changes of SPRR3 protein levels did alter the caspase 3 activation and PARP cleavage (Figure 3B), suggesting that SPRR3‐regulated apoptosis did involve in caspase‐mediated pathway. Moreover, we have selected KYSE450 cells which showed moderate SPRR3 expression, and knockdown of SPRR3 also decreased the caspase 3 activation and PARP cleavage in response to DNA damage (Supplementary Figure S4E).
Figure 3.
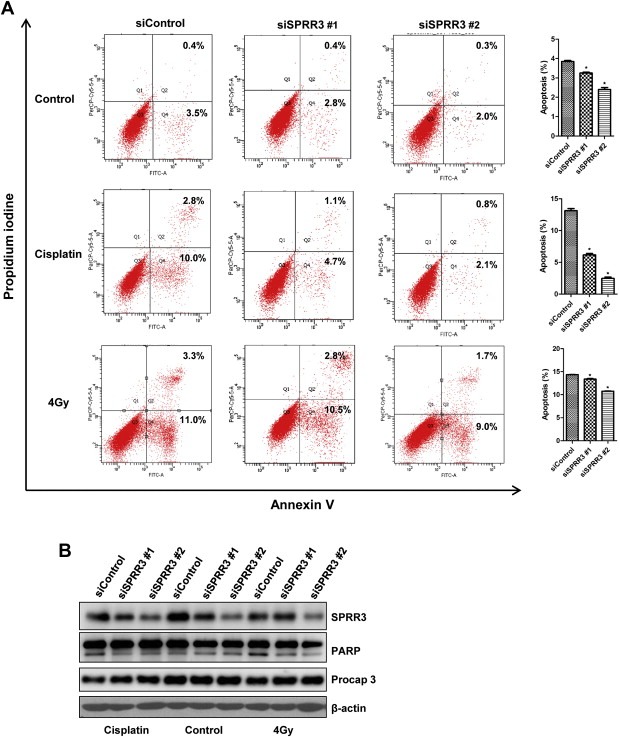
Knockdown of SPRR3 reduces DNA damage‐induced apoptosis. A) KYSE30 cells were transiently transfected with either control or SPRR3 siRNA as indicated. After 48 h, cells were treated with cisplatin or X‐IR. Cells were labeled with Annexin V‐FITC and PI, and analyzed by flow cytometry (left panel). Values are expressed as a percentage of Annexin V positive versus total cells. Data are expressed as Mean ± SD (*: P < 0.05, right panel). B) Total cell lysates from KYSE30 cells described in A) were prepared. The expression levels of SPRR3, activated PARP and caspase 3 were examined by Western blot. β‐actin was used as a loading control.
3.6. Localization and interaction of SPRR3 with Bcl‐2 in vivo
Previous studies showed that several proteins, including p53 and TAp73, were able to rapidly translocate to mitochondria upon exposure to genotoxic stress and triggered apoptosis (Marchenko et al., 2000). We also observed the localization of SPRR3 in cells. As shown in Figure 4A, exogenous/endogenous SPRR3 was detected predominantly in the cytoplasm, and localized in mitochondria. To further confirm the observation, we applied MitoTracker Red CMXRos as a marker of mitochondria, and found that SPRR3 was indeed localized in mitochondria (Figure 4B). Since the Bcl‐2 family proteins play major roles in regulating apoptosis, we then studied the interaction of SPRR3 with a major anti‐apoptotic factor, Bcl‐2. The physical interactions of SPRR3 and Bcl‐2 were confirmed by co‐immunoprecipitation in vivo (Figure 4C). Interaction between SPRR3 and Bcl‐2 was further confirmed by transient cotransfection of HA‐SPRR3 with GFP‐Bcl‐2 into HEK293T cells (Figure 4D).
Figure 4.
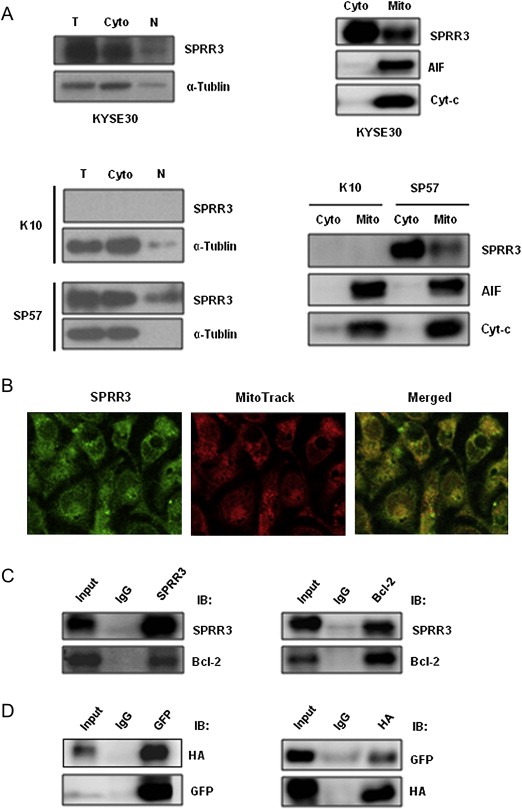
SPRR3 localizes in mitochondria and interacted with Bcl‐2 in vivo. A) Analysis of subcellular localization of SPRR3 by subcellular fractionation. Cells were subjected to subcellular fractionation (T, total extract; N, nuclear), and Western blot was performed with cytoplasm (Cyto) and mitochondrial (Mito) fractions. α‐Tubulin and AIF were used as cytosolic and mitochondrial marker proteins, respectively. B) Subcellular localization of SPRR3 protein. KYSE30 cells were incubated with 20 nM MitoTracker CMX‐Ros or anti‐SPRR3, and analyzed by confocal microscope images. C) Co‐immunoprecipitation of SPRR3 and Bcl‐2. Whole cell lysates from KYSE30 cells were prepared and immunoprecipitation was performed with anti‐SPRR3 followed by Western blot with antibodies against Bcl‐2 (left panel), or immunoprecipitated with antibodies against Bcl‐2 followed by Western blot with anti‐SPRR3 (right panel). Anti‐Mouse IgG or anti‐Rabbit IgG antibody was used as a negative control. D) HEK293T cells were transiently transfected with pCDEF‐HA‐SPRR3 and pEGFP‐C1‐Bcl‐2. Samples were subjected to immunoprecipitation with anti‐GFP or anti‐HA, and analyzed by Western blot using anti‐HA (left panel) and anti‐GFP (right panel) antibodies.
3.7. Overexpression of SPRR3 activates Bax and the release of cytochrome c
After cisplatin or X‐IR, we observed a slight decrease of Bcl‐2 protein level in SP57 & SP59 cells, whereas Bax protein was increased. Overexpression of SPRR3 activates p53 in SP57 cells in response to DNA damage (Figure 5A). Thus, we speculated Bax expression might be enhanced by SPRR3 via p53 pathway in response to DNA damage, but the underlying mechanisms remain poorly understood and need further investigation. Furthermore, we found that overexpression of SPRR3 triggered Bax to translocate to mitochondria, and caused an increase in the release of cytochrome c in cytoplasm in response to cisplatin or X‐IR (Figure 5B). AIF was used as a mitochondrial marker protein. Moreover, interaction of SPRR3 with Bcl‐2 was increased by co‐immunoprecipitation after cisplatin treatment (Figure 5C). Our data suggest that interaction of SPRR3 with Bcl‐2 might cause the change of ratio of Bcl‐2/Bax in cytoplasm. We further investigated whether overexpression of SPRR3 abolished the Bcl‐2‐mediated anti‐apoptosis. The implication of SPRR3 in attenuating the Bcl‐2‐mediated anti‐apoptotic signal induced by cisplatin was shown by transient co‐transfection of SPRR3 with Bcl‐2 into EC9706 cells (Figure 5D). The amount of cytochrome c slightly increased in the cytosolic fraction (Figure 5E). Thus, SPRR3 exerted a pro‐apoptotic effect and caused the accumulation of Bax at mitochondria as well as the release of cytochrome c during DNA damage‐induced apoptosis.
Figure 5.
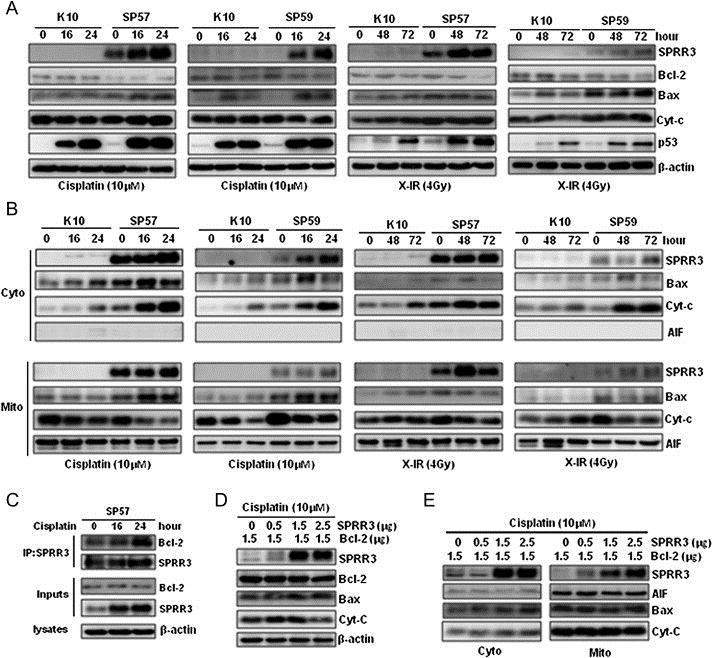
Overexpression of SPRR3 activates Bax and causes the release of cytochrome c. A) Cells were exposed to cisplatin (10 μM) or X‐IR (4 Gy) at the indicated time. Overexpression of SPRR3 increased the expression of Bax and p53 compared with controls. B) Overexpression of SPRR3 caused Bax mitochondria translocation and the release of cytochrome c. Cells were subjected to subcellular fractionation after treatment. The cytosolic (Cyto) and mitochondrial (Mito) fractions were analyzed by Western blot. C) Co‐immnunoprecipitation analysis showing that interaction of SPRR3 with Bcl‐2 was increased after cisplatin treatment. D & E) Overexpression of SPRR3 abolished the Bcl‐2‐mediated anti‐apoptosis. EC9706 cells were transiently cotransfected with various amounts of HA‐SPRR3 with constant amount of GFP‐Bcl‐2 expression plasmids. After transfection, cells were treated with 10 μM cisplatin for 24 h and harvested. Cells were subjected to subcellular fractionation, and detected by Western blot.
3.8. SPRR3 expression correlated with clinical outcome
To verify cell studies in clinical samples, we examined SPRR3 expression using immunohistochemistry analysis. One hundred and nine patients with locally advanced ESCC were treated with preoperative radiotherapy followed by surgery (n = 33) from 1986 to 2006 or radiotherapy alone (n = 76) from 2001 to 2007 at the Cancer Hospital Chinese Academy of Medical Sciences. The histological criteria for responses to radiotherapy were as follows: pathological complete responses (pathCR) defined as: (a) disappearance of the tumor lesion; or (b) absence of cancer cells in operative specimens. Partial response (PR) was defined as a reduction by 50% or more of cancer cells. Stable disease was indicated by a <50% reduction or <25% increase in tumor size. Progressive disease was defined as an increase of >25% in tumor size. Clinical characteristics of the patients are summarized in Table 1. As shown in Figure 6A, SPRR3 protein was mainly localized in cytoplasm using immunohistochemistry analysis. SPRR3 expression in response samples (n = 30 cases; 39.5%) was higher than that in the no response samples (n = 46 cases; 61.5%) in radiotherapy alone group (P = 0.007). We then divided the samples into two groups according to the expression level of SPRR3, one group with high SPRR3 expression (n = 26 cases; SPRR3 expression >50%) and the other group with low SPRR3 expression (n = 50 cases; SPRR3 expression <50%). SPRR3 expression was significantly associated with the good survival of patients with locally advanced ESCC (P = 0.008, Figure 6B). Moreover, SPRR3 expression was associated with esophageal cancer recurrence (P = 0.017, Figure 6C). Six cases were excluded since recurrence was unknown. These results suggest that SPRR3 expression is associated with patient outcome.
Table Table 1.
Patient Characteristics.
| Variables | Radiotherapy | Preoperative radiotherapy | |
|---|---|---|---|
| (n = 76) | (n = 33) | ||
| Age years | Median (range) | 65 (43–84) | 57 (38–70) |
| Sex F/M | n | 18/58 | 7/26 |
| Tumor location | |||
| Upper | n (%) | 36 (47) | 15 (45) |
| Middle | n (%) | 30 (40) | 16 (49) |
| Lower | n (%) | 10 (13) | 2 (6) |
| Tumor size, cm | Median (range) | 5 (3–12) | 6 (2–10) |
| T stage | |||
| T2 | n (%) | 22 (29) | 14 (42) |
| T3 | n (%) | 30 (39) | 14 (42) |
| T4 | n (%) | 24 (32) | 5 (16) |
| Clinic stage | |||
| II | n (%) | 51 (67) | 23 (70) |
| III | n (%) | 25 (33) | 10 (30) |
| LN metastasis | |||
| − | n (%) | 45 (59) | 20 (61) |
| + | n (%) | 31 (41) | 13 (40) |
| Radiotherapy (cGy) | Median (range) | 6000 (3000–8000) | 4000 (3800–6400) |
Note: LN: lymph node; T2, tumor invades muscularis propria; T3, tumor invades adventitia; T4, tumor invades surrounding tissue.
Figure 6.
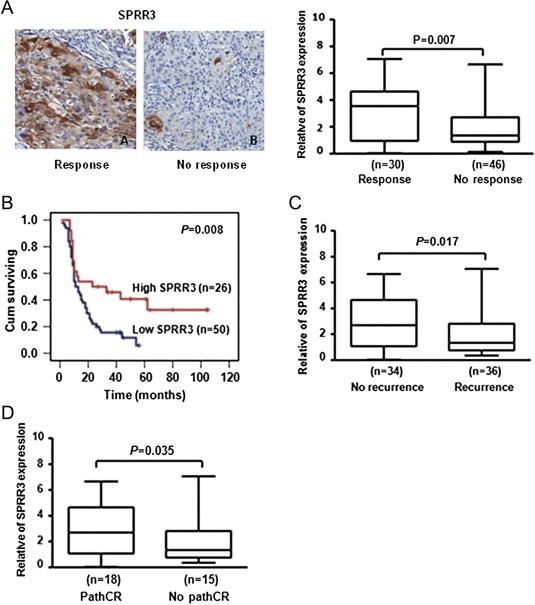
SPRR3 expression correlated with radiosensitivity of esophageal cancer. A) SPRR3 protein was detected by immunohistochemistry staining in the pretreatment biopsies in radiotherapy alone group (left panel). SPRR3 expression correlated with response of radiotherapy (P = 0.007, right panel). Response: cases with complete response and partial response after radiotherapy; No response: cases with stable disease and progress disease after radiotherapy. B) Overall survival of patients according to the expression of SPRR3 using Kaplan–Meier curve. Patients with SPRR3 expression (26 cases) had significantly higher survival rates than patients lacking SPRR3 expression (50 cases, P = 0.008). C) SPRR3 expression associated with esophageal cancer recurrence in radiotherapy alone group (P = 0.017). Six cases were excluded since recurrence was unknown. D) SPRR3 expression was associated with pathologic complete response to preoperative radiotherapy (P = 0.035). PathCR, complete pathologic response; No pathCR, less than complete pathologic response.
3.9. SPRR3 expression and pathologic response to preoperative radiotherapy
Since SPRR3 expression was related to radiosensitivity in ESCC samples, we further investigated whether SPRR3 might represent a pathologic complete response predictor in preoperative radiotherapy of ESCC. We reviewed SPRR3 expression by immunohistochemistry in 33 patients with preoperative radiotherapy followed by surgery. After surgery, the pathologic complete response (pathCR) assessed by pathologic examination of the operative specimen was 54.4% (18 of 33 patients). SPRR3 expression was associated with radiosensitivity in preoperative radiotherapy (P = 0.035, Figure 6D). These results implicated SPRR3 might serve as an important predictor of esophageal cancer cells sensitivity to radiation.
4. Discussion
SPRR3 was originally identified as a marker for the terminal squamous cell differentiation, and a precursor of the cornified envelope. The cornified envelope has a vital role in the barrier function of stratified squamous epithelia (Candi et al., 2005). SPRR3 was down‐regulated coordinately in ESCC, esophageal adenocarcinoma, anal cancer and gastric cancer compared with corresponding normal epithelium (Luo et al., 2004; Kimchi et al., 2005; Zucchini et al., 2001). SPRR3 significantly suppressed cell proliferation, and has been shown to suppress the tumorigenicity of ESCC. However, SPRR3 was strongly up‐regulated and involved in the tumorigenesis of colorectal and breast cancer (Cho et al., 2010; Kim et al., 2012). The differential expression of SPRR3 in cancers might be due to different tissue‐specific expression patterns. SPRR genes were differentially expressed in response to the external damaging insults. It has recently been reported that SPRR3 is up‐regulated in celiac disease and also induced by prolonged exposure to cyclic strain (Pyle et al., 2010; Bracken et al., 2008). In this study, we found that endogenous SPRR3 was induced in response to DNA damage stimuli including cisplatin and X‐IR in KYSE30 and KYSE180 cells. Exogenous SPRR3 remarkably enhances DNA damage‐induced apoptosis in EC9706 cells. Our results indicated that SPRR3 not only functions as a structural protein of cornified envelope but also plays an important role in DNA damage‐induced apoptosis in ESCC.
Cellular radiosensitivity is the relative susceptibility of cells, tissues or organs to the injurious action of radiation. Radiosensitivity is directly proportional to the rate of cell division and inversely proportional to the degree of cell differentiation. Sensitivity to chemoradiotherapy is probably mediated by a variety of genes including p53, Bcl‐2, S100A2, RUNX3 and NF‐κB in esophageal cancer (Wang et al., 2001; Sakakura et al., 2007; Izzo et al., 2006), but the molecular mechanisms involved in sensitivity of chemoradiotherapy need to be further investigated. Our previous study showed that overexpression of exogenous SPRR3 triggered cellular apoptosis partially through the activation of CDK11p46 in vivo (Zhang et al., 2008). In this study, we showed a novel mechanism of SPRR3 in DNA damage‐induced apoptotic response. Firstly, overexpression of SPRR3 moderately sensitizes esophageal cancer cells to DNA damage‐induced apoptosis via mitochondrial apoptotic pathway. Secondly, RNAi‐mediated “knockdown” of SPRR3 in KYSE30 and KYSE450 cells reduced sensitivity to cisplatin and X‐IR.
Apoptosis is a cellular process regulated by the balance of pro‐ and anti‐apoptotic proteins of the Bcl‐2 family (Adams and Cory, 2007). We here found that SPRR3 is a novel interacting partner of Bcl‐2. In response to DNA damage, overexpression of SPRR3 had a slight effect on the level of Bcl‐2, but the interaction of SPRR3 with Bcl‐2 was significantly increased. Overexpression of SPRR3 increased the expression of Bax, and promoted Bax translocation to mitochondria. Bax is a cytosolic protein that undergoes a death signal‐induced conformational change which affects mitochondrial permeability, causes the release of cytochrome c and leads to caspase activation (Tsujimoto, 1998). Thus, we speculated that overexpression of SPRR3 caused the change of distribution of Bax in cell and triggered mitochondrial death pathway. Moreover, we found, for the first time, that SPRR3 was localized in mitochondria. We have further analyzed the sequence of SPRR3, but did not find any mitochondrial signal peptide in the SPRR3 protein, thus how SPRR3 localizes to mitochondria is still unknown.
Preoperative chemoradiotherapy has been preformed to increase the resectability of locally advanced esophageal cancer (Gasast et al., 2010; Spigel et al., 2010). Stahl et al. showed that preoperative radiotherapy improved 3‐year survival rate from 27.7% to 47.4% (P = 0.007) for the locally advanced (T3‐4NXM0) esophageal adenocarcinoma (Stahl et al., 2009). Clinical characteristics such as primary location, histological type, classification and TNM stage are unable to predict differences in the biologic behavior of patients receiving preoperative chemoradiotherapy (Chirieac et al., 2005). PathCR has been used as a marker for the efficacy of preoperative chemoradiotherapy schedules in several cancers (Darnton et al., 2003). If preoperative chemoradiotherapy is not effective, patients might miss the chance of surgery, and suffer from toxicity or progression of disease without substantial benefit because of metastasis. Moreover, recurrence is frequently associated with the acquisition of chemoradioresistance by tumors and failures in chemoradiotherapy. Thus, there is an urgent need for new biomarkers that can reliably predict the efficacy of preoperative chemoradiotherapy. Recently, serum proteomic profiling was tried to be used as a means of predicting sensitivity to chemoradiotherapy of esophageal cancer (Hayashida et al., 2005). In this study, by using biopsy specimens obtained before radiotherapy, we found that SPRR3 expression was significantly correlated with pathologic response in treatment with preoperative radiotherapy followed by surgery or radiotherapy alone (P = 0.035 and P = 0.007, respectively). The pathCR was achieved for 39.5% and 54.4% of patients, respectively. Our data are also consistent with microarray analyses of chemoradiation‐resistant esophageal adenocarcinoma in which SPRR3 is downregulated (Luthra et al., 2006, 2007). Loss of the 1q21.3 region was associated with shorter OS duration (Maru et al., 2009). Coincident with this, we found that low SPRR3 expression was associated with poor OS in radiotherapy alone group (P = 0.008). Further study on larger independent patient sets would be necessary to confirm this observation.
5. Conclusions
In summary, we demonstrated that SPRR3 enhanced the sensitivity of esophageal cancer cells in response to DNA damage‐induced apoptosis via mitochondrial death pathway and SPRR3 might be a radiation‐sensitive factor and a prognostic molecule that might potentially guide preoperative therapy.
Conflict of interest
The authors declare no conflict of interest.
Financial support
This study was supported by the National Basic Research Program of China (2011CB504205, 2013CB911004) and National Natural Science Foundation of China (81101740, 81130043).
Supporting information
The following is the supplementary data related to this article:
Supplementary data
Acknowledgments
We thank Dr. Quan Chen for the generous gifts of pEGFP‐C1‐Bcl‐2 plasmid, and Dr. Yutaka Shimada for the generous gifts of ESCC cell lines (KYSE series).
Supplementary data 1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2013.05.005.
Luo Aiping, Chen Hongyan, Ding Fang, Zhang Yu, Wang Mingrong, Xiao Zefen, Liu Zhihua, (2013), Small proline‐rich repeat protein 3 enhances the sensitivity of esophageal cancer cells in response to DNA damage‐induced apoptosis, Molecular Oncology, 7, doi: 10.1016/j.molonc.2013.05.005.
Contributor Information
Zefen Xiao, Email: xiaozefen@sina.com.
Zhihua Liu, Email: liuzh@cicams.ac.cn.
References
- Adams, J.M. , Cory, S. , 2007. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 26, 1324–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken, S. , Byrne, G. , Kelly, J. , Jackson, J. , Feighery, C. , 2008. Altered gene expression in highly purified enterocytes from patients with active coeliac disease. BMC. Genomics. 9, 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral, A. , Voskamp, P. , Cleton-Jansen, A.M. , South, A. , Nizetic, D. , Backendorf, C. , 2001. Structural organization and regulation of the small proline-rich family of cornified envelope precursors suggest a role in adaptive barrier function. J. Biol. Chem.. 276, 19231–19237. [DOI] [PubMed] [Google Scholar]
- Cabral, A. , Sayin, A. , de Winter, S. , Fischer, D.F. , Pavel, S. , Backendorf, C. , 2001. SPRR4, a novel cornified envelope precursor: UV-dependent epidermal expression and selective incorporation into fragile envelopes. J. Cell Sci.. 114, 3837–3843. [DOI] [PubMed] [Google Scholar]
- Candi, E. , Paci, M. , Oddi, S. , Paradisi, A. , Paradisi, A. , Guerrieri, P. , Melino, G. , 2000. Ordered structure acquisition by the N- and C- terminal domains of the small proline-rich 3 protein. J. Cell Biochem.. 77, 179–185. [DOI] [PubMed] [Google Scholar]
- Candi, E. , Schmidt, R. , Melino, G. , 2005. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol.. 6, 328–340. [DOI] [PubMed] [Google Scholar]
- Chirieac, L.R. , Swisher, S.G. , Ajani, J.A. , Komaki, R.R. , Correa, A.M. , Morris, J.S. , Roth, J.A. , Rashid, A. , Hamilton, S.R. , Wu, T.T. , 2005. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 103, 1347–1355. [DOI] [PubMed] [Google Scholar]
- Cho, D.H. , J.o, Y.K. , Roh, S.A. , Na, Y.S. , Kim, T.W. , Jang, S.J. , Kim, Y.S. , Kim, J.C. , 2010. Upregulation of SPRR3 promotes colorectal tumorigenesis. Mol. Med.. 16, 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnton, S.J. , Archer, V.R. , Stocken, D.D. , Mulholland, P.J. , Casson, A.G. , Ferry, D.R. , 2003. Preoperative mitomycin, ifosfamide, and cisplatin followed by esophagectomy in squamous cell carcinoma of the esophagus: pathologic complete response induced by chemotherapy leads to long-term survival. J. Clin. Oncol.. 21, 4009–4015. [DOI] [PubMed] [Google Scholar]
- Davies, L. , Lewis, W.G. , Arnold, D.T. , Escofet, X. , Blackshaw, G. , Gwynne, S. , Evans, M. , Roberts, S.A. , Appadurai, I. , Crosby, T.D. , 2010. Prognostic significance of age in the radical treatment of oesophageal cancer with surgery or chemoradiotherapy: a prospective observational cohort study. Clin. Oncol. (R Coll. Radiol.). 22, 578–585. [DOI] [PubMed] [Google Scholar]
- Demetris, A.J. , Specht, S. , Nozaki, I. , Lunz, J.G. , Stolz, D.B. , Murase, N. , Wu, T. , 2008. Small proline-rich proteins (SPRR) function as SH3 domain ligands, increase resistance to injury and are associated with epithelial-mesenchymal transition (EMT) in cholangiocytes. J. Hepatol.. 48, 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasast, A.V. , van Hagen, P. , Hulshof, M. , Richel, D. , van Berge Henegouwen, M.I. , Nieuwenhuijzen, G.A. , Plukker, J.J. , Bonenkamp, E.W. , Steyerberg, H.W. , 2010. Effect of preoperative concurrent chemoradiotherapy on survival of patients with resectable oesophageal or esophagogastric junction cancer: results from a multicenter randomized phase III study. J. Clin. Oncol.. 28, 15s (suppl; abstr 4004) [Google Scholar]
- Hanahan, D. , Weinberg, R.A. , 2011. Hallmarks of cancer: the next generation. Cell. 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Hayashida, Y. , Honda, K. , Osaka, Y. , Hara, T. , Umaki, T. , Tsuchida, A. , 2005. Possible prediction of chemoradiosensitivity of esophageal cancer by serum protein profiling. Clin. Cancer Res.. 11, 8042–8077. [DOI] [PubMed] [Google Scholar]
- Hill, M.M. , Adrain, C. , Martin, S.J. , 2003. Portrait of a killer: the mitochondrial apoptosome emerges from the shadows. Mol. Interv.. 3, 19–26. [DOI] [PubMed] [Google Scholar]
- Izzo, J.G. , Malhotra, U. , Wu, T.T. , Ensor, J. , Luthra, R. , Lee, J.H. , Swisher, S.G. , Liao, Z. , Chao, K.S. , Hittelman, W.N. , Aggarwal, B.B. , Ajani, J.A. , 2006. Association of activated transcription factor nuclear factor kappab with chemoradiation resistance and poor outcome in esophageal carcinoma. J. Clin. Oncol.. 24, 748–754. [DOI] [PubMed] [Google Scholar]
- Jemal, A. , Siegel, R. , Xu, J. , Ward, E. , 2010. Cancer Statistics, 2010. CA. Cancer J. Clin.. 60, 277–300. [DOI] [PubMed] [Google Scholar]
- Kim, J.C. , Yu, J.H. , Cho, Y.K. , Jung, C.S. , Ahn, S.H. , Gong, G. , Kim, Y.S. , Cho, D.H. , 2012. Expression of SPRR3 is associated with tumor cell proliferation in less advanced stages of breast cancer. Breast Cancer Res. Treat.. 133, 909–916. [DOI] [PubMed] [Google Scholar]
- Kimchi, E.T. , Posner, M.C. , Park, J.O. , Darga, T.E. , Kocherginsky, M. , Karrison, T. , Hart, J. , Smith, K.D. , Mezhir, J.J. , Weichselbaum, R.R. , Khodarev, N.N. , 2005. Progression of Barrett's metaplasia to adenocarcinoma is associated with the suppression of the transcriptional programs of epidermal differentiation. Cancer Res.. 65, 3146–3154. [DOI] [PubMed] [Google Scholar]
- Kleinberg, L. , Forastiere, A.A. , 2007. Chemoradiation in the management of oesophageal cancer. J. Clin. Oncol.. 25, 4110–4117. [DOI] [PubMed] [Google Scholar]
- Leung, T.H. , Ngan, H.Y. , 2010. Interaction of TAp73 and breast cancer-associated gene 3 enhances the sensitivity of cervical cancer cells in response to irradiation-induced apoptosis. Cancer Res.. 70, 6486–6496. [DOI] [PubMed] [Google Scholar]
- Luo, A. , Kong, J. , Hu, G. , Liew, C.C. , Xiong, M. , Wang, X. , Ji, J. , Wang, T. , Zhi, H. , Wu, M. , Liu, Z. , 2004. Discovery of Ca2+-relevant and differentiation-associated genes downregulated in esophageal squamous cell carcinoma using cDNA microarray. Oncogene. 23, 1291–1299. [DOI] [PubMed] [Google Scholar]
- Luthra, R. , Wu, T.T. , Luthra, M.G. , Izzo, J. , Lopez-Alvarez, E. , Zhang, L. , Bailey, J. , Lee, J.H. , Bresalier, R. , Rashid, A. , Swisher, S.G. , Ajani, J.A. , 2006. Gene expression profiling of localized esophageal carcinomas: association with pathologic response to preoperative chemoradiation. J. Clin. Oncol.. 24, 259–267. [DOI] [PubMed] [Google Scholar]
- Luthra, M.G. , Ajani, J.A. , Izzo, J. , Ensor, J. , Wu, T.T. , Rashid, A. , Zhang, L. , Phan, A. , Fukami, N. , Luthra, R. , 2007. Decreased expression of gene cluster at chromosome 1q21 defines molecular subgroups of chemoradiotherapy response in esophageal cancers. Clin. Cancer Res.. 13, 912–919. [DOI] [PubMed] [Google Scholar]
- Maher, S.G. , Gillham, C.M. , Duggan, S.P. , Smyth, P.C. , Miller, N. , Muldoon, C. , O'Byrne, K.J. , Sheils, O.M. , Hollywood, D. , Reynolds, J.V. , 2009. Gene expression analysis of diagnostic biopsies predicts pathological response to neoadjuvant chemoradiotherapy of esophageal cancer. Ann. Surg.. 250, 729–737. [DOI] [PubMed] [Google Scholar]
- Marchenko, N.D. , Zaika, A. , Moll, U.M. , 2000. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J. Biol. Chem.. 275, 16202–16212. [DOI] [PubMed] [Google Scholar]
- Maru, D.M. , Luthra, R. , Correa, A.M. , White-Cross, J. , Anandasabapathy, S. , Krishnan, S. , Guha, S. , Komaki, R. , Swisher, S.G. , Ajani, J.A. , Hofstetter, W.L. , Rashid, A. , 2009. Frequent loss of heterozygosity of chromosome 1q in esophageal adenocarcinoma: loss of chromosome 1q21.3 is associated with shorter overall survival. Cancer. 115, 1576–1585. [DOI] [PubMed] [Google Scholar]
- Motoori, M. , Takemasa, I. , Yamasaki, M. , Komori, T. , Takeno, A. , Miyata, H. , Takiguchi, S. , Fujiwara, Y. , Yasuda, T. , Yano, M. , Matsuura, N. , Matsubara, K. , Monden, M. , Mori, M. , Doki, Y. , 2010. Prediction of the response to chemotherapy in advanced esophageal cancer by gene expression profiling of biopsy samples. Int. J. Oncol.. 37, 1113–1120. [DOI] [PubMed] [Google Scholar]
- Osaka, Y. , Takagi, Y. , Tsuchida, A. , Hoshino, S. , Tachibana, S. , Shinohara, M. , 2004. Concurrent preoperative chemoradiotherapy for stage III or IV esophageal squamous carcinoma. Oncol. Rep.. 12, 1121–1126. [PubMed] [Google Scholar]
- Pradervand, S. , Yasukawa, H. , Muller, O.G. , Kjekshus, H. , Nakamura, T. , St Amand, T.R. , Yajima, T. , Matsumura, K. , Duplain, H. , Iwatate, M. , Woodard, S. , Pedrazzini, T. , Ross, J. , Firsov, D. , Rossier, B.C. , Hoshijima, M. , Chien, K.R. , 2004. Small proline-rich protein 1A is a gp130 pathway- and stress-inducible cardioprotective protein. EMBO. J.. 23, 4517–4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle, A.L. , Atkinson, J.B. , Pozzi, A. , Reese, J. , Eckes, B. , Davidson, J.M. , Crimmins, D.L. , Young, P.P. , 2008. Regulation of the atheroma-enriched protein, SPRR3, in vascular smooth muscle cells through cyclic strain is dependent on integrin alpha1beta1/collagen interaction. Am. J. Pathol.. 173, 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle, A.L. , Li, B. , Maupin, A.B. , Guzman, R.J. , Crimmins, D.L. , Olson, S. , Atkinson, J.B. , Young, P.P. , 2010. Biomechanical stress induces novel arterial intima-enriched genes: implications for vascular adaptation to stress. Cardiovasc. Pathol.. 19, e13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakura, C. , Miyagawa, K. , Fukuda, K.I. , Nakashima, S. , Yoshikawa, T. , Kin, S. , Nakase, Y. , Ida, H. , Yazumi, S. , Yamagishi, H. , Okanoue, T. , Chiba, T. , Ito, K. , Hagiwara, A. , Ito, Y. , 2007. Frequent silencing of RUNX3 in esophageal squamous cell carcinomas is associated with radioresistance and poor prognosis. Oncogene. 26, 5927–5938. [DOI] [PubMed] [Google Scholar]
- Sato, M. , Ando, N. , 2011. Neoadjuvant chemotherapy followed by surgery as standard treatment forstage II + III thoracic esophageal squamous cell carcinoma in Japan. Nippon Geka. GakkaiZasshi. 112, 104–110. [PubMed] [Google Scholar]
- Sheridan, C. , Brumatti, G. , Elgendy, M. , Brunet, M. , Martin, S.J. , 2010. An ERK-dependent pathway to Noxa expression regulates apoptosis by platinum-based chemotherapeutic drugs. Oncogene. 29, 6428–6441. [DOI] [PubMed] [Google Scholar]
- Shimada, Y. , Imamura, M. , Wagata, T. , Yamaguchi, N. , Tobe, T. , Shimada, Y. , Imamura, M. , Wagata, T. , Yamaguchi, N. , Tobe, T. , 1992. Characterization of 21 newly established esophageal cancer cell lines. Cancer. 69, 277–284. [DOI] [PubMed] [Google Scholar]
- Spigel, D.R. , Greco, F.A. , Meluch, A.A. , Lane, C.M. , Farley, C. , Gray, J.R. , Clark, B.L. , Burris, H.A. , Hainsworth, J.D. , 2010. Phase I/II trial of preoperative oxaliplatin, docetaxel, and capecitabine with concurrent radiation therapy in localized carcinoma of the esophagus or gastroesophageal junction. J. Clin. Oncol.. 28, 2213–2219. [DOI] [PubMed] [Google Scholar]
- Stahl, M. , Walz, M.K. , Stuschke, M. , Meyer, H.J. , Riera-Knorrenschild, J. , Langer, P. , Engenhart-Cabillic, R. , Bitzer, M. , Königsrainer, A. , Budach, W. , Wilke, H. , 2009. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J. Clin. Oncol.. 7, 851–856. [DOI] [PubMed] [Google Scholar]
- Tesfaigzi, J. , Carlson, D.M. , 1999. Expression, regulation, and function of the SPRR family of proteins. A review. Cell Biochem. Biophys.. 30, 243–265. [DOI] [PubMed] [Google Scholar]
- Tsujimoto, Y. , 1998. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria?. Gene Cells. 3, 697–707. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Li, J. , Booher, R.N. , Kraker, A. , Lawrence, T. , Leopold, W.R. , Sun, Y. , 2001. Radiosensitization of p53 mutant cells by PD0166285, a novel G(2) checkpoint abrogator. Cancer Res.. 61, 8211–8217. [PubMed] [Google Scholar]
- Zhang, Y. , Feng, Y.B. , Shen, X.M. , Chen, B.S. , Du, X.L. , Luo, M.L. , Cai, Y. , Han, Y.L. , Xu, X. , Zhan, Q.M. , Wang, M.R. , 2008. Exogenous expression of Esophagin/SPRR3 attenuates the tumorigenicity of esophageal squamous cell carcinoma cells via promoting apoptosis. Int. J. Cancer. 122, 260–266. [DOI] [PubMed] [Google Scholar]
- Zucchini, C. , Biolchi, A. , Strippoli, P. , Solmi, R. , Rosati, G. , Del Governatore, M. , Milano, E. , Ugolini, G. , Salfi, N. , Farina, A. , Caira, A. , Zanotti, S. , Carinci, P. , Valvassori, L. , 2001. Expression profile of epidermal differentiation complex genes in normal and anal cancer cells. Int. J. Oncol.. 19, 1133–1141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following is the supplementary data related to this article:
Supplementary data


