Abstract
Gene therapy and antibody approaches are crucial auxiliary strategies for hepatocellular carcinoma (HCC) treatment. Previously, we established a survivin promoter‐regulated oncolytic adenovirus that has inhibitory effect on HCC growth. The human sulfatase‐1 (hSulf‐1) gene can suppress the growth factor signaling pathways, then inhibit the proliferation of cancer cells and enhance cellular sensitivity to radiotherapy and chemotherapy. I131‐metuximab (I131‐mab) is a monoclonal anti‐HCC antibody that conjugated to I131 and specifically recognizes the HAb18G/CD147 antigen on HCC cells. To integrate the oncolytic adenovirus‐based gene therapy and the I131‐mab‐based radioimmunotherapy, this study combined the CArG element of early growth response‐l (Egr‐l) gene with the survivin promoter to construct a radiation‐inducible enhanced promoter, which was used to recombine a radiation‐inducible oncolytic adenovirus as hSulf‐1 gene vector. When I131‐mab was incorporated into the treatment regimen, not only could the antibody produce radioimmunotherapeutic effect, but the I131 radiation was able to further boost adenoviral proliferation. We demonstrated that the CArG‐enhanced survivin promoter markedly improved the proliferative activity of the oncolytic adenovirus in HCC cells, thereby augmenting hSulf‐1 expression and inducing cancer cell apoptosis. This novel strategy that involved multiple, synergistic mechanisms, including oncolytic therapy, gene therapy and radioimmunotherapy, was demonstrated to exert an excellent anti‐cancer outcome, which will be a promising approach in HCC treatment.
Keywords: Oncolytic adenovirus, Radiation-inducible promoter, Human sulfatase-1, Radioimmunotherapy, Hepatocellular carcinoma
Highlights
-
►
A radiation‐induced oncolytic adenovirus is constructed as hSulf‐1 gene vector.
-
►
I131‐metuximab boosts oncolytic adenoviral proliferation in hepatocellular carcinoma.
-
►
I131‐metuximab increases adenovirus‐mediated hSulf‐1 expression in cancer cells.
-
►
Oncolytic adenovirus and I131‐metuximab synergistically increase antitumor effect.
1. Introduction
Gene therapy has become a prominent auxiliary measure for the comprehensive treatment of hepatocellular carcinoma (HCC) (Wrzesinski et al., 2011). A series of conditionally replicative viruses have been developed for transgene vectors, which can replicate specifically within tumor cells and result in oncolytic effect. As such, these are referred to as oncolytic viruses (Russell et al., 2012; Chen et al., 2012; Onimaru et al., 2010). The most common way to achieve tumor‐restricted replication of a virus is to employ a tumor‐specific promoter to regulate viral replication genes, which ensures the safety and effectiveness of the therapeutic strategy (Onimaru et al., 2010; Wong et al., 2012; Liu et al., 2012). Multiple tissue‐ or cell‐specific promoters have been used to control the specific replication of viruses (Doloff et al., 2011; Ma et al., 2009; Zhang et al., 2012; Banerjee et al., 2004; Xu et al., 2012). In our preliminary studies, we utilized an oncolytic adenovirus regulated by the survivin promoter, and the specificity of its replication and its oncolytic activity in cancer cells were investigated (Liu et al., 2011). The oncolytic adenovirus could acquire high replicative activity and express anti‐tumor transgene in survivin‐positive tumor cells, and exhibited an enhanced ability to kill cancer cells with a minimal impact on normal cells. Such a therapeutic strategy avoids some common issues in tumor gene therapy, such as low expression of target genes and a lack of tumor targeting. However, due to the potent malignancy and high proliferative capacity of HCC cells, oncolysis mediated by the virus and the anti‐tumor effects of the target genes were insufficient to contain the growth of the cancer cells. Thus, further optimization of the regulatory strategy for vector targeting in gene therapy for HCC is required, and these future studies should improve the specificity and safety of this therapeutic system.
In addition to targeted gene therapy strategies using oncolytic viruses, targeted antibody therapy is also an indispensable method for the comprehensive treatment of HCC. CD147 is a transmembrane protein and upregulated in a variety of tumors, which functions to promote tumor invasion and metastasis (Wang et al., 2010; Cui et al., 2012). From HCC cDNA expression library, a transmembrane glycoprotein, HAb18G, was identified and confirmed to represent a new member of CD147 family (Xu et al., 2007). HAb18G/CD147 displays selective high levels of expression in HCC tissues (75%) and is negative in normal liver tissues (Dai et al., 2009; Gou et al., 2009). As a result, a highly specific anti‐HCC monoclonal antibody was engineered to target HAb18G/CD147. The antibody was conjugated with a radioisotope to generate I131‐metuximab (I131‐mab), also named Licartin, which was officially approved for clinical application in 2007 by the State Food and Drug Administration of China (SFDA) and was intended for hepatic artery intervention to facilitate local radioimmunotherapy (RIT) in HCC treatment (Wu et al., 2010, 2012). Multi‐center clinical trials in China demonstrated that the use of an arterial infusion of I131‐mab through a transcatheter was safe and effective for HCC treatment and that this treatment could significantly postpone recurrence after liver transplantation for HCC (Wu et al., 2010; Chen et al., 2006).
Based on oncolytic gene therapy and I131‐mab targeted therapy, in this study, the CArG radiation‐sensitive element within the regulatory region of early growth response‐l (Egr‐l) gene was fused to the survivin promoter to generate a novel tumor‐specific radiation‐inducible promoter. This promoter was used to construct an oncolytic adenovirus as the human sulfatase‐1 (hSulf‐1) gene vector. When I131‐mab was applied in targeted therapy, I131 released beta and gamma rays to activate the radiation‐inducible promoter, thereby further initiating viral replication, boosting viral oncolytic capability and increasing anti‐tumor gene expression level. These effects were synergistic with the anti‐tumor activity of I131‐mab, which has been shown to highly improve the HCC treatment outcome. Therefore, this treatment strategy optimizes a vector‐based gene therapy for HCC and integrates multiple activities to enhance anti‐tumor efficacy, thereby augmenting the specificity and safety of the treatment system and delivering good prospects for therapeutic application.
2. Materials and methods
2.1. Activity verification of the radiation‐inducible tumor‐selective promoter
According to the 5′‐UTR sequence of the wild‐type survivin promoter (Surp), the primers for the core promoter sequence were designed (Sense: 5′‐cgGCTAGCcatagaaccagag‐3′; antisense: 5′‐gaAGATCTgccgccgccgccacct‐3′). DNA was extracted from HepG2 cells to serve as template for the survivin promoter amplification. The 1 kb amplification fragment was cloned into the NheI and BglII sites of the pGL3‐Basic plasmid to generate pGL3‐Surp. A fragment containing 9 tandem repeats of the CArG regulatory element (CCATATAAGG) of the Egr‐1 gene was artificially synthesized with NheI cohesive ends at both termini, and inserted upstream of the survivin promoter to serve as an enhancer, thereby generating pGL3‐eSurp.
One hundred thousand cells were harvested from individual cultures of the normal fibroblast cell line MRC‐5, normal liver cell line L02, and HCC cell lines MHCC97H, SMMC‐7721, and BEL‐7404 (Shanghai Institute for Biological Science, Chinese Academy of Science, Shanghai, China). The harvested cells were lysed with M‐PER™ Mammalian Protein Extraction Reagent (PIERCE, Rockford, IL) to obtain proteins, which were examined by Western blotting to determine survivin expression. The cells were seeded into a 24‐well plate at a concentration of 1 × 105 cells/well and incubated for 24 h prior to co‐transfection with 20 ng pRL‐TK and 200 ng of each of the following plasmids: pGL3‐Basic, pGL3‐Control, pGL3‐Surp, and pGL3‐eSurp. The cells were then incubated for an additional 24 h. Next, I131‐metuximab (Chengdu Huasun Group Inc., Ltd., Chengdu, China) was added at 10 μCi/well, and the cells were cultured for another 24 h. Cell lysate was examined according to instructions provided with the Dual‐Luciferase® Reporter Assay System Kit (Promega Corporation, Madison, WI) to determine the luciferase activity. These experiments were repeated independently 3 times. The corresponding results were expressed as the ‘mean value ± standard deviation’. The results from pGL3‐Control were used to normalize the relative promoter activities of pGL3‐Surp and pGL3‐eSurp.
2.2. Construction and recombination of oncolytic adenoviruses
Based on our previous oncolytic adenovirus (AdSurp‐P53) regulated by the survivin promoter (Liu et al., 2011) and hSulf‐1‐expressing adenovirus Ad5‐hSulf1 (Ji et al., 2011), the chimeric survivin promoter containing CArG enhancer (eSurp) was enzymatically released from pGL3‐eSurp to replace the wild‐type survivin promoter in Ad.Surp‐P53, the hSulf‐1 gene and the enhanced green fluorescent protein (EGFP) reporter gene were amplified from Ad5‐hSulf1 and Ad5‐EGFP to replace the P53 gene in AdSurp‐P53. The next recombination events generated a novel oncolytic adenovirus, Ad.eSurp‐hSulf1, which is regulated by a radiation‐inducible promoter, along with a series of control adenoviruses, including AdSurp‐hSulf1, AdSurp‐EGFP, and Ad.eSurp‐EGFP. All adenoviruses were amplified in HEK293 cells and purified using the cesium chloride gradient centrifugation method prior to being subjected to the TCID50 assay for the determination of virus titer (LaBarre and Lowy, 2001).
2.3. Replication and gene expression of oncolytic adenoviruses
Normal and HCC cells were seeded into 24‐well plates at a concentration of 1 × 105 cells/well and infected with adenoviruses (Ad.eSurp‐EGFP, AdSurp‐EGFP, or Ad5‐EGFP) at a multiplicity of infection (MOI) of 1 pfu/cell and cultured for 24 h. I131‐mab was added at 10 μCi/well, and the cells were incubated for another 24 h. The EGFP‐positive cells were counted under a fluorescence microscope to calculate the corresponding percentage ratios.
Normal and HCC cells were seeded into 24‐well plates at a concentration of 1 × 105 cells/well and infected with adenoviruses (Ad.eSurp‐hSulf1, AdSurp‐hSulf1, or Ad5‐hSulf1) at an MOI of 1 pfu/cell and cultured for 24 h. I131‐mab was added at 10 μCi/well, and the cells were incubated for another 24 h. The cells were harvested and lysed using M‐PER™ Mammalian Protein Extraction Reagent (PIERCE) to recover proteins, which were then subjected to Western blotting using anti‐E1a and anti‐hSulf‐1 antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) to determine the expression of these proteins.
2.4. Cytotoxicity experiments
Methyl thiazolyl tetrazolium (MTT) assay was used to determine the cytotoxic effects of the viruses on different cells. Normal and HCC cells in the logarithmic growth phase were harvested and seeded into 96‐well plates at a concentration of 1 × 104 cells/well and then incubated for 24 h. The Ad.eSurp‐hSulf1, AdSurp‐hSulf1, and Ad5‐hSulf1 adenoviruses were individually inoculated into each well at MOIs of 0, 0.5, 1, 5, 10, 50, and 100 pfu/cell, with each MOI performed in 12 replicates. After 24 h, 9 out of 12 replicating wells were supplemented with I131‐mab at concentrations of 0.1, 0.5, or 1 μCi/well, with each concentration performed in 3 replicates. The cells were allowed to continue growing for 48 h before discarding the media and adding 100 μl of 0.1 mol/L PBS to each well, along with supplementation of MTT Regent M2128 (Sigma–Aldrich Shanghai Trading Co., Shanghai, China) at 10 μl/well to reach a final concentration of 0.5 mg/ml. The plates were then incubated for 4 h before the solubilization solution (10% SDS in 0.01 mol/L HCl) was added at 100 μl/well. The plates were incubated overnight, and absorbance values at wavelength 570 nm were determined with a reference of 655 nm. The cell survival curves were then plotted.
2.5. Animal HCC xenograft experiments
Sixty healthy, purebred, male BALB/C nude mice at 4 weeks of age were obtained from the Shanghai SLAC Laboratory Animal Center of Chinese Academy of Sciences (Shanghai, China). One hundred microliters of MHCC97H cell suspension containing 1 × 106 cells was subcutaneously injected into the right flank of mouse. On day 10 post‐inoculation, a tumorigenic rate of 100% and xenograft diameters of 0.5–0.8 cm were observed. After removing 3 individuals harboring extra‐large tumors and 3 individuals harboring minuscule tumors, the remaining 54 animals were randomly divided into the following 9 groups of 6 animals each: the Ad.eSurp‐hSulf1, Ad.eSurp‐hSulf1 + I131‐mab, Ad.eSurp‐EGFP, Ad.eSurp‐EGFP + I131‐mab, AdSurp‐hSulf1, AdSurp‐EGFP, Ad5‐hSulf1, I131‐mab, and control groups. On days 1, 3, 5, 7, and 9, each animal in virus‐treated groups was administered a multipoint injection of the corresponding adenovirus at a dose of 2 × 108 pfu in a 100 μl. On days 4 and 10, each animal in I131‐mab‐treated groups was intraperitoneally injected with 10 μCi I131‐mab. Each animal in the control group was simultaneously injected with 100 μl saline. Following these treatments, the tumor sizes were measured and calculated every week using the following formula: maximum diameter × minimum diameter2 × 0.5. In addition, the tumor inhibition rates were calculated using the following formula: (tumor volume in the control group – tumor volume in the treatment group)/tumor volume in the control group × 100%.
2.6. HCC xenograft histopathology
At the end of observation period, the mice were sacrificed by anesthetization. The tumor specimens were collected and fixed in 10% neutral‐buffered formalin prior to paraffin‐embedded sectioning. H&E staining was performed to assist in tumor histology observation. The immunohistochemical streptavidin‐peroxidase (S‐P) conjugated method was used to reveal the expression of E1a and hSulf‐1 in tumor tissues with the anti‐E1a and anti‐hSulf‐1 antibodies (Santa Cruz Biotechnology, Inc.) and S‐P kit (Fuzhou Maixin Biotechnology Development Co., Fuzhou, China), and the terminal deoxynucleotidyl transferase‐mediated dUTP nick end labeling (TUNEL) was performed to detect apoptotic cells with the In Situ Cell Death Detection Kit (Fuzhou Maixin Biotechnology Development Co.). Each slice was enumerated under 5 fields of high magnification to determine the proportion of positive cells, which was expressed as a percentage; a specimen was considered to be positive if it had greater than 10% positive cells.
2.7. Statistical treatments
The experimental data are expressed as ‘mean ± standard deviation’ and statistically analyzed using the chi‐square test, student's t test, and a two‐way analysis of variance (ANOVA) according to the properties of the data. Statistical analyses were performed using the software PASW Statistics 18.0, and p values < 0.05 represented significant differences.
3. Results
3.1. Radiation‐induced enhancement of survivin promoter activity in HCC cells
The normal cell lines MRC‐5 and L02 were negative for survivin expression, while the HCC cell lines MHCC97H, SMMC‐7721 and BEL‐7404 showed positive survivin expression. Under normal growth conditions, both the wild‐type survivin promoter (Surp) and its enhanced version (eSurp) in normal cells exhibited a basal level of activity. In contrast, the levels of Surp and eSurp activity were significantly higher in HCC cell lines (p < 0.01; Table 1). Surp and eSurp activity showed no differences in the same cell line (Figure 1A). Twenty‐four hours after adding I131‐mab into medium at 10 μCi/well, Surp activity showed no obvious changes, whereas that of eSurp registered a significant increase (p < 0.01; Figure 1B).
Table 1.
Relative activity of luciferase under the control of radiation‐inducible survivin promoter.
| Cell lines | pGL3‐Basic | pGL3‐Surp | pGL3‐eSurp | pGL3‐Surp+I131‐mab | pGL3‐eSurp+I131‐mab | pGL3‐Control |
|---|---|---|---|---|---|---|
| MRC‐5 | 11.26 ± 2.87 | 15.18 ± 11.01 | 16.78 ± 5.01 | 15.04 ± 8.40 | 30.91 ± 9.03 | 951.20 ± 128.84 |
| L02 | 11.17 ± 6.72 | 11.23 ± 9.86 | 19.28 ± 7.90 | 12.54 ± 3.24 | 62.23 ± 17.91 | 830.43 ± 277.05 |
| MHCC97H | 72.48 ± 15.39 | 2149.50 ± 122.67 | 2326.74 ± 506.07 | 2212.39 ± 350.82 | 4416.36 ± 550.85 | 5059.78 ± 238.68 |
| SMMC‐7721 | 40.49 ± 23.40 | 532.59 ± 95.92 | 476.56 ± 31.19 | 481.75 ± 31.58 | 1381.71 ± 231.57 | 1539.25 ± 43.84 |
| BEL‐7404 | 118.10 ± 37.81 | 2615.01 ± 580.18 | 3051.25 ± 975.41 | 2234.68 ± 672.15 | 5832.62 ± 570.22 | 6068.23 ± 871.91 |
Figure 1.

Relative activity of the radiation‐inducible enhanced survivin promoter examined by luciferase assay. Normal and HCC cells were seeded into 24‐well plates at a concentration of 105 cells/well and transfected with luciferase plasmids containing the wild‐type survivin promoter (Surp) or the radiation‐inducible enhanced survivin promoter (eSurp), without (A) or with (B) adding I131‐mab into medium. The harvested cells were examined by dual‐luciferase reporter assay to determine the luciferase expression. The results from pGL3‐Control which contains cytomegaoviyns (CMV) promoter were used to normalize the relative activities of promoters in pGL3‐Surp and pGL3‐eSurp; *p < 0.05; **p < 0.01.
3.2. Efficient viral replication and gene expression in HCC cells
L02 and MHCC97H cells were infected with the EGFP‐expressing adenoviruses at an MOI of 1 pfu/cell and observed under microscope. EGFP expression was positive in only a small number of L02 cells infected with Ad.eSurp‐EGFP, Ad.Surp‐EGFP, or Ad5‐EGFP (Figure 2). But the HCC cells harboring Ad.eSurp‐EGFP or AdSurp‐EGFP showed much higher EGFP levels than the cells containing Ad5‐EGFP. Furthermore, following supplementation with I131‐mab, the HCC cells harboring Ad.eSurp‐EGFP showed further elevated EGFP expression, while the cells containing AdSurp‐EGFP displayed no apparent change in EGFP expression level.
Figure 2.
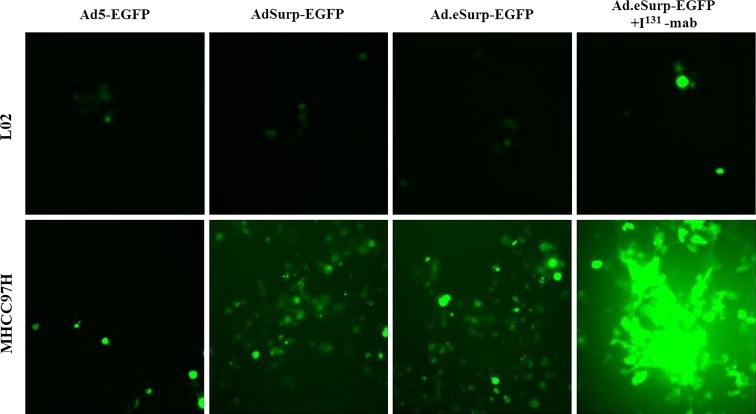
Tumor‐selective replication of oncolytic adenoviruses carrying the EGFP gene in HCC cells. L02 and MHCC97H cells in 24‐well plates at 1 × 105 cells/well were infected with the indicated adenoviruses expressing EGFP at an MOI of 1 pfu/cell. Replicates of virus‐infected cells was added I131‐mab at 10 μCi/well. The EGFP‐positive cells were observed under a fluorescence microscope.
The hSulf‐1 expression was low in the parental normal cells and did not change significantly following infection with hSulf‐1‐expressing adenoviruses, in comparison to uninfected cells, and supplementation with I131‐mab also did not affect hSulf‐1 expression. Furthermore, the E1a gene demonstrated relatively weak expression in normal cells treated with Ad.eSurp‐hSulf1 or AdSurp‐hSulf1. However, hSulf‐1 is not expressed in the parental HCC cells, but showed weak expression following infection with Ad5‐hSulf1, and highly expressed when infected with Ad.eSurp‐hSulf1 and AdSurp‐hSulf1. Such elevation of hSulf‐1 expression was especially pronounced when Ad.eSurp‐hSulf1 infection was combined with I131‐mab treatment (Figure 3). The finding that E1a expression was consistent with that of hSulf‐1 indicated that Ad.eSurp‐hSulf1 and AdSurp‐hSulf1 had strong replicative capacities in HCC cells; in addition, following the induction of I131‐mab radiation, the replicative capacity of Ad.eSurp‐hSulf1 was further stimulated.
Figure 3.

Tumor‐selective replication of oncolytic adenoviruses carrying the hSulf‐1 gene in HCC cells. Normal and HCC cells in 24‐well plates at 1 × 105 cells/well were infected with the indicated adenoviruses expressing hSulf‐1 at an MOI of 1 pfu/cell. Replicates of virus‐infected cells was added I131‐mab at 10 μCi/well. Expression of E1a and hSulf‐1 were examined by Western blotting. Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was used as a loading control. Densitometry analysis was performed to show E1a and hSulf‐1 expression levels, normalized with GAPDH density; *p < 0.05; **p < 0.01.
3.3. Cytotoxic effect of hSulf‐1‐expressing oncolytic adenovirus combined with I131‐mab on HCC cells
Because Ad.eSurp‐hSulf1, AdSurp‐hSulf1, and Ad5‐hSulf1 demonstrated no replicative activity and only induced weak hSulf‐1 expression in normal cells, no significant suppression of cell proliferation in normal cells was observed. Only when the MOI value was increased to 100 pfu/cell did the cell viability fall slightly to 90–95%. In contrast, Ad.eSurp‐hSulf1 and AdSurp‐hSulf1 replicated in cancer cells, demonstrated oncolytic activity, increased hSulf‐1 expression, and significantly suppressed HCC cell proliferation. When the MOI reached 100 pfu/cell, the survival rate of HCC cells had dropped to 50–70% of that of uninfected controls (p < 0.01). Ad5‐hSulf1 showed no obvious inhibition of cancer cells. Next, when the cells were also treated with different concentrations of I131‐mab. Because the normal cells did not express HAb18G/CD147, no clear effect of I131‐mab on cell growth was detected. The HCC cells highly expressed HAb18G/CD147, which enabled them to capture and bind I131‐mab. Moreover, cancer cell growth inhibition was enhanced with increasing concentrations of I131‐mab; when the I131‐mab concentration reached 1.0 μCi/well, the survival rate of cancer cells was decreased to 80–85% of the control level without treatment of I131‐mab (p < 0.05; Figure 4A).
Figure 4.
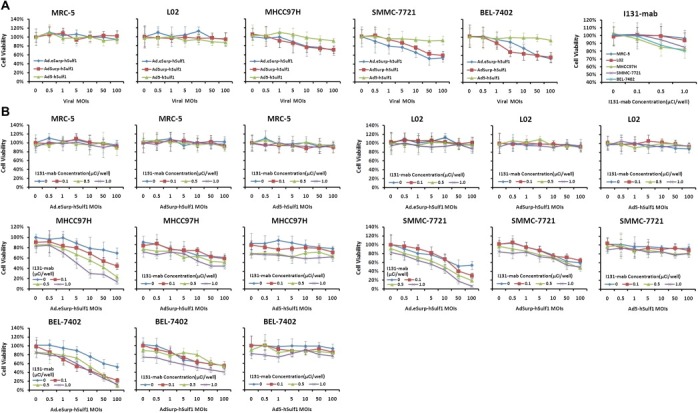
Selectively killing effect of hSulf‐1‐expressing oncolytic adenovirus combined with I131‐mab on HCC cells. Normal and HCC cells in 96‐well plates at 1 × 104 cells/well were inoculated with the indicated adenoviruses at different MOIs or/and supplemented with I131‐mab at different concentrations. Then all replicates of cells were detected by MTT assay. The absorbance values were determined at wavelength 570 nm with a reference of 655 nm. Cell viability was showed with the survival curves. (A), Cells were individually treated with adenoviruses or I131‐mab. (B), Cells were jointly treated with adenoviruses and I131‐mab; *p < 0.05; **p < 0.01.
When different MOIs of adenoviruses and different concentrations of I131‐mab were used in combination to treat cells, there was no substantial effect on the growth of normal cells. However, in HCC cell lines, Ad.eSurp‐hSulf1 displayed potent tumor inhibition as the viral MOI value and the I131‐mab concentration increased. At viral MOI of 10 pfu/cell and I131‐mab concentration of 1.0 μCi/well, the cancer cell viability diminished to approximately 10% of that of the untreated controls (p < 0.01; Figure 4B). In addition, AdSurp‐hSulf1 also demonstrated impressive tumor inhibition; when viral MOI was equal to 10 pfu/cell and I131‐mab concentration was 1.0 μCi/well, the cancer cell viability dropped to 40–50% of that of the untreated controls (p < 0.01). Ad5‐hSulf1 demonstrated the weakest inhibitory effect; when viral MOI was equal to 10 pfu/cell and I131‐mab concentration was 1.0 μCi/well, the cancer cell viability was between 70% and 80% of that of (p < 0.05).
3.4. Inhibitory effect of oncolytic adenovirus with I131‐mab on HCC xenografts
MHCC97H cells were subcutaneously implanted into BALB/C nude mice and treated with adenoviruses or/and I131‐mab. On day 35 post‐treatment initiation, the observations were halted because the tumor size of the control group had exceeded 8000 mm3, which was the threshold stipulated by the ethics committee for animal experiments. At this time point, the anti‐tumor efficacy of Ad.eSurp‐hSulf1 + I131‐mab treatment was the greatest, as the corresponding tumor growth was completely repressed with an inhibition rate of 96.20% compared to that of the control group (Figure 5; Table 2). The inhibition rates were followed by that of AdSurp‐hSulf1 77.16%, Ad.eSurp‐hSulf1 74.32%, Ad.eSurp‐EGFP + I131‐mab 55.67%, Ad5‐hSulf1 46.04%, Ad.Surp‐EGFP 39.18%, Ad.eSurp‐EGFP 34.93%, and I131‐mab 16.40%. When I131‐mab was not used, the efficacy of AdSurp‐hSulf1 and Ad.eSurp‐hSulf1 treatments was similar, but better than that of Ad5‐hSulf1 treatment (p < 0.0001, versus AdSurp‐hSulf1; p = 0.0002 versus Ad.eSurp‐hSulf1). In addition, the efficacy of Ad.eSurp‐EGFP and AdSurp‐EGFP treatments was similar. Although the anti‐tumor effect of I131‐mab treatment alone was low, this anti‐tumor activity was significantly enhanced when I131‐mab was used in combination with hSulf‐1‐ or EGFP‐expressing adenoviruses driven by eSurp promoter (p < 0.0001: Ad.eSurp‐hSulf1 + I131‐mab versus Ad.eSurp‐hSulf1; p = 0.0150: Ad.eSurp‐EGFP + I131‐mab versus Ad.eSurp‐EGFP). Compared the effect of treatment with single anti‐tumor mechanism, hSulf‐1 expression mediated by Ad5‐hSulf1 had the greatest influence on tumors, followed by the oncolytic effect of Ad.eSurp‐EGFP mediated by its specific replicative activity in tumor cells, and the efficacy of I131‐mab treatment alone was the poorest.
Figure 5.
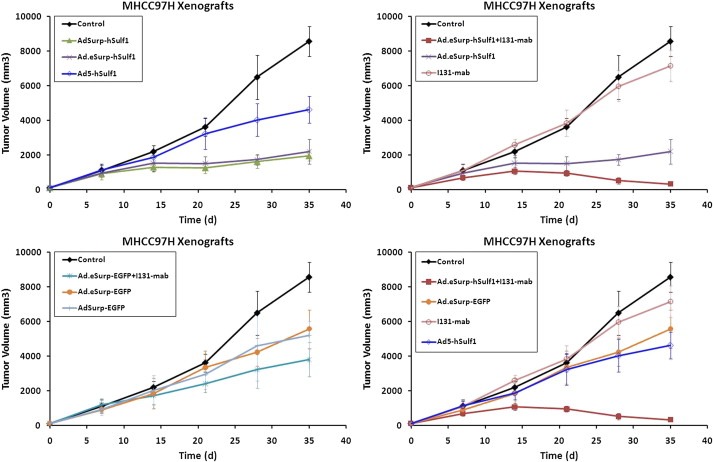
Inhibitory effect of the indicated oncolytic adenoviruses with or without I131‐mab on HCC xenografts in nude mice. MHCC97H cells (1 × 106) were subcutaneously implanted into BALB/C nude mice to establish xenograft tumors, followed by treatments of intratumorally injections of the indicated adenoviruses together with or without intraperitoneally injections of I131‐mab. The tumor sizes at the indicated time points were measured and calculated using the formula “maximum diameter × minimum diameter2 × 0.5”; *p < 0.05; **p < 0.01.
Table 2.
Xenograft tumor volume after treated with oncolytic adenovirus and/or I131‐mab at the indicated time points.
| Group | 0 d | 7 d | 14 d | 21 d | 28 d | 35 d |
|---|---|---|---|---|---|---|
| Ad.eSurp‐hSulf1+I131‐mab | 119.00 ± 21.27 | 693.48 ± 161.05 | 1068.74 ± 205.40 | 953.82 ± 153.39 | 522.28 ± 184.35 | 324.98 ± 123.31 |
| AdSurp‐hSulf1 | 110.15 ± 26.24 | 916.51 ± 336.01 | 1299.45 ± 268.79 | 1262.92 ± 313.85 | 1615.64 ± 361.94 | 1954.19 ± 292.84 |
| Ad5‐hSulf1 | 114.41 ± 26.85 | 1145.20 ± 259.71 | 1879.99 ± 399.63 | 3231.53 ± 912.88 | 4026.08 ± 941.89 | 4616.73 ± 771.64 |
| Ad.eSurp‐hSulf1 | 119.72 ± 19.67 | 940.47 ± 220.05 | 1521.60 ± 454.13 | 1501.99 ± 407.91 | 1725.73 ± 317.49 | 2197.21 ± 713.13 |
| Ad.eSurp‐EGFP + I131‐mab | 113.33 ± 23.04 | 1186.47 ± 346.37 | 1723.17 ± 510.29 | 2409.58 ± 501.68 | 3210.95 ± 1056.37 | 3792.58 ± 983.52 |
| Ad.eSurp‐EGFP | 115.31 ± 39.75 | 915.85 ± 339.30 | 1848.25 ± 875.15 | 3343.89 ± 944.20 | 4223.23 ± 794.89 | 5567.47 ± 1109.4 |
| AdSurp‐EGFP | 109.84 ± 30.67 | 942.83 ± 382.58 | 2019.04 ± 852.16 | 2951.38 ± 854.99 | 4607.38 ± 1512.07 | 5203.52 ± 805.69 |
| I131‐mab | 102.68 ± 19.88 | 1091.65 ± 352.34 | 2595.42 ± 315.49 | 3849.96 ± 763.12 | 5975.61 ± 893.53 | 7152.58 ± 913.79 |
| Control | 102.09 ± 29.48 | 1111.56 ± 386.67 | 2190.88 ± 349.69 | 3611.64 ± 510.58 | 6488.74 ± 1270.63 | 8555.62 ± 869.45 |
3.5. Cancer cell apoptosis induced by viral replication and hSulf‐1 gene expression
The xenograft specimens from nude mice were obtained to perform pathological examinations. Hematoxylin and eosin (H&E) staining showed cancer cells grew exuberantly in the control group with clear atypia and no major tumor necrosis. Whereas, the I131‐mab and Ad5‐hSulf1 groups displayed smaller zones of cancer cell necrosis that exhibited nuclear pyknosis, karyorrhexis and karyolysis as well as increased eosinophilic cytoplasm. Moreover, the Ad.eSurp‐hSulf1 + I131‐mab and Ad.eSurp‐hSulf1 groups demonstrated larger necrotic areas in the cancerous lesions. Immunohistochemical labeling of E1a in cancer tissues indicated viral replication, and hSulf‐1 expression was measured to reflect transgene expression levels (Figure 6). The TUNEL method was performed to evaluate cancer cells undergoing apoptosis. The results from these analyses demonstrated that the E1a expression level in the Ad.eSurp‐hSurlf1 + I131‐mab group was significantly higher than that observed in the Ad.eSurp‐hSulf1 group, and the I131‐mab and Ad5‐hSulf1 groups demonstrated negative E1a expression. hSulf‐1 was expressed at highest level in the Ad.eSurp‐hSulf1 + I131‐mab group, followed by the Ad.eSurp‐hSulf1 group and at lowest level in the Ad5‐hSulf1 group. The apoptosis was consistent with E1a and hSulf‐1 expression, such that the Ad.eSurp‐hSulf1 + I131‐mab, Ad.eSurp‐hSulf1, Ad5‐hSulf1, and I131‐mab groups showed, in descending order, decreasing levels of apoptosis, and the control group showed very few scattered apoptotic cells.
Figure 6.
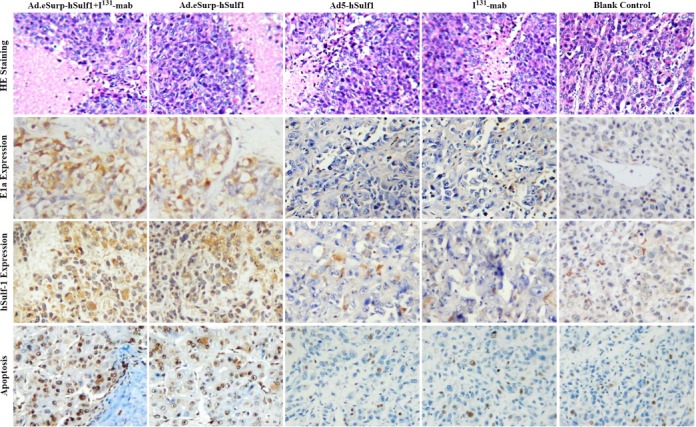
Adenovirus‐mediated expression of E1a and hSulf‐1 and adenovirus‐induced apoptosis in HCC xenograft tumor cells. The formalin‐fixed, paraffin‐embedded sections of HCC xenograft specimens were stained by H&E staining to show cancer tissue necrosis, stained by immunohistochemistry to show E1a and hSulf‐1 expression, and stained by TUNEL method to show cell apoptosis.
4. Discussion
HCC is characterized by rapid proliferation, high malignancy, poor sensitivity to chemotherapy and radiotherapy, and rapid progression. Gene therapy has recently become a part of comprehensive treatment for HCC (Hanahan and Weinberg, 2011). The sulfation of heparin sulfate proteoglycans (HSPGs) directly mediates the activation of receptor tyrosine kinases in the regulatory pathways of many growth factors (Ji et al., 2011). Importantly, cancer cell proliferation depends on the activation of these growth factor signaling pathways emanating from their cognate receptors. The hSulf‐1 protein is a membrane‐localized sulfatase that can desulfate HSPGs, influence a variety of HSPG‐related binding processes between the extracellular signaling molecules and their corresponding receptors, and, as such, suppress the activation of heparin‐binding growth factor and cytokine signaling pathways (Yoneda et al., 2012; Lai et al., 2004; Yang et al., 2011). In most normal human tissues, hSulf‐1 demonstrates stable expression. However, in the vast majority of human cancers, including those of breast, pancreatic, kidney, and liver cancers, the expression of hSulf‐1 is down‐regulated (Khurana et al., 2011; Narita et al., 2007). Loss of heterozygosity, methylation of CpG islands and histone modifications at upstream regulatory sequences in the hSulf‐1 gene may represent the main causes for the inactivation of hSulf‐1 (Chen et al., 2009; Staub et al., 2007). Re‐expression of hSulf‐1 in tumor cells can be initiated by gene transfection, which decreases the proliferation, migration and invasion of cancer cells, and enhances their sensitivity to chemotherapy and radiotherapy. Currently, hSulf‐1 gene therapy has achieved noteworthy efficacy for liver, ovarian, and pancreatic cancers (Ji et al., 2011; Abiatari et al., 2006). Therefore, hSulf‐1 is an effective tumor inhibitory factor and may represent an effective anti‐tumor candidate gene. Therapeutic strategies targeting the hSulf‐1 gene can simultaneously restrain the proliferation and migration of tumor cells and their vascular supply, which may function to control unlimited tumor growth and tumor angiogenesis (Liu et al., 2012; Ji et al., 2011). In this study, the hSulf‐1 gene was transferred into HCC cells by non‐replicative adenovirus (Ad5‐hSulf1) and inhibited cancer cell proliferation to some extent and suppressed xenograft tumor growth in nude mice. However, the results revealed that HCC cell lines undergo strong proliferation, and low expression of hSulf‐1 gene driven by non‐replicative adenovirus showed difficulty to achieve satisfactory tumor suppression, the tumors also grew up gradually.
Monoclonal antibody‐targeted therapy has great potential in clinical cancer treatment. The specific or related antigens of tumor cells, certain growth factors and their cognate receptors, certain oncogene products, and specific signaling pathways of cancer cell development can be exploited for cancer therapy. Monoclonal antibodies targeting these molecules can directly block the signaling pathways associated with cancer cell growth, or indirectly be used in target tumor therapy by operating as carriers of radionuclides, drugs, and toxins (Dienstmann et al., 2012; Prabhu et al., 2011). I131‐mab is a monoclonal anti‐cancer antibody that specifically targets the HAb18G/CD147 antigen of HCC cells and is artificially conjugated to the I131 radioisotope. It was approved by SFDA for use in hepatic artery interventional embolization to treat HCC. Analyses of its RIT mechanisms revealed that I131‐mab binds to the HAb18G/CD147 antigen on HCC cell surface, thereby blocking this target molecule, abolishing downstream signal transduction and suppressing tumor proliferation and metastasis (Xu et al., 2007; Zhu et al., 2009). Meanwhile, the antigen–antibody binding was shown to result in the targeted delivery of radioactive I131 to cancer tissues, whereby I131 releases beta and gamma rays to directly kill cancer cells. Moreover, the killing radius associated with this treatment modality is expanded due to ray penetration, which demonstrates the dual effects of targeted RIT for the effective control of tumor growth. The efficacy of I131‐mab treatment has produced some positive results for HCC patients, but it remains controversial used as a single agent. Modern biological tumor treatment model is comprehensive, therefore, it is important to explore superior ways to utilize I131‐mab and investigate the outcomes of different combination strategies involving I131‐mab for HCC treatment.
Because the expression of apoptotic inhibitory factor survivin is highly specific to malignant tumors, most of the common tumors in humans, including lung, liver, colon, pancreatic, prostate, and breast cancers and non‐Hodgkin's lymphoma, express high levels of survivin. Moreover, due to its relationship with tumor recurrence, metastasis, and overall poor prognosis of cancer patients, survivin represents a candidate target for cancer therapy (Fraunholz et al., 2012; Hu et al., 2011). By employing the survivin promoter to regulate adenoviral E1a expression, we constructed tumor‐selective replicative oncolytic adenovirus. Our experiments confirmed the high replicative capacity of this virus and its ability to mediate the expression of anti‐cancer genes at high levels in a variety of survivin‐positive tumor cells. The viral oncolytic and cytotoxic effect on cancer cells were significant, despite having minimal effect on normal cells (Liu et al., 2011). Thus, our survivin promoter‐regulated oncolytic adenovirus demonstrated an increased specificity and safety and represents a potential future vector for cancer gene therapy.
Based on the aforementioned characteristics of cancer therapeutic approaches, the integration of I131‐mab treatment with the delivery of an oncolytic adenovirus will likely generate greater anti‐tumor effect. However, this combination may only be additive but may not exert synergistic anti‐tumor effect. Previous studies demonstrated that 6 highly conserved base repeat sequences (CCATATAAGG) exist within the upstream regulatory sequences of Egr‐1, and these sequences can directly sense stimulation by ionizing radiation to induce the expression of downstream genes and are therefore termed CArG radiation‐sensitive elements (Xiong et al., 2012; Bickenbach et al., 2008). A synthetic DNA sequence containing several CArG elements exerted radiation‐inducible effect as the wild‐type Egr‐1 promoter (Greco et al., 2002; Scott et al., 2000), which suggested that the CArG element could function as a radiation‐inducible molecular switch in gene therapy and could thereby represent an ideal strategy to combine radiation and gene therapy. We fused the CArG radiation‐sensitive element, which would function as an enhancer, with survivin promoter to form a novel radiation‐inducible enhanced promoter which was used to control adenoviral replication. Our study demonstrated that the radiation‐inducible promoter‐regulated adenovirus could specifically replicate in tumor cells and mediated higher levels of hSulf‐1 expression when combined with I131‐mab, the radiation released by I131 initiate adenoviral replication, which could emerge multiple mechanisms to produce synergistic anti‐tumor efficacy.
In this study, the generated radiation‐inducible enhanced promoter (eSurp) and the wild‐type survivin promoter (Surp) were demonstrated to have high activity in HCC cells. When I131‐mab was added to the cells, the radiation significantly increased the eSurp activity but had minimal effect on Surp activity, indicating that the CArG enhancer in eSurp played an important role. Following experiments using the EGFP reporter gene and detection of E1a and hSulf‐1 gene expression, we discovered that the CArG‐containing promoter significantly improved the replicative activity of eSurp‐regulated oncolytic adenovirus (Ad.eSurp‐hSulf1) in HCC cells and stimulated the expression of anti‐tumor transgene. Moreover, in vitro studies and experiments in a nude mouse xenograft model further confirmed the therapeutic characteristics of Ad.eSurp‐hSulf1, including its high replication and efficient expression of anti‐tumor transgene in HCC cells, which resulted in potent tumor inhibition and an excellent anti‐cancer outcome when combined with I131‐mab via the induction of cancer cell apoptosis. The combination of Ad.eSurp‐hSulf1 and I131‐mab therapy produced a new strategy for biological treatment that boasts synergistic cooperation between immune‐radiation treatment and viral gene therapy.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors sincerely thank Jianzhong Gu, Shanghai SLAC Experimental Animal Center, Chinese Academy of Sciences, for his help with animal studies. This work was supported by grants from the National Natural Science Foundation of China (No. 81172303, 81172019).
Zhang Yan, Fang Lin, Zhang Quan'an, Zheng Qin, Tong Jinlong, Fu Xiaohui, Jiang Xiaoqing, Su Changqing, Zheng Junnian, (2013), An oncolytic adenovirus regulated by a radiation‐inducible promoter selectively mediates hSulf‐1 gene expression and mutually reinforces antitumor activity of I131‐metuximab in hepatocellular carcinoma, Molecular Oncology, 7, doi: 10.1016/j.molonc.2012.10.007.
Contributor Information
Changqing Su, Email: suchangqing@gmail.com.
Junnian Zheng, Email: jnzheng@xzmc.edu.cn.
References
- Abiatari, I. , Kleeff, J. , Li, J. , Felix, K. , Büchler, M.W. , Friess, H. , 2006. Hsulf-1 regulates growth and invasion of pancreatic cancer cells. J. Clin. Pathol. 59, 1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, N.S. , Rivera, A.A. , Wang, M. , Chow, L.T. , Broker, T.R. , Curiel, D.T. , Nettelbeck, D.M. , 2004. Analyses of melanoma-targeted oncolytic adenoviruses with tyrosinase enhancer/promoter-driven E1A, E4, or both in submerged cells and organotypic cultures. Mol. Cancer Ther. 3, 437–449. [PubMed] [Google Scholar]
- Bickenbach, K.A. , Veerapong, J. , Shao, M.Y. , Mauceri, H.J. , Posner, M.C. , Kron, S.J. , Weichselbaum, R.R. , 2008. Resveratrol is an effective inducer of CArG-driven TNF-alpha gene therapy. Cancer Gene Ther. 15, 133–139. [DOI] [PubMed] [Google Scholar]
- Chen, Z.N. , Mi, L. , Xu, J. , Song, F. , Zhang, Q. , Zhang, Z. , Xing, J.L. , Bian, H.J. , Jiang, J.L. , Wang, X.H. , Shang, P. , Qian, A.R. , Zhang, S.H. , Li, L. , Li, Y. , Feng, Q. , Yu, X.L. , Feng, Y. , Yang, X.M. , Tian, R. , Wu, Z.B. , Leng, N. , Mo, T.S. , Kuang, A.R. , Tan, T.Z. , Li, Y.C. , Liang, D.R. , Lu, W.S. , Miao, J. , Xu, G.H. , Zhang, Z.H. , Nan, K.J. , Han, J. , Liu, Q.G. , Zhang, H.X. , Zhu, P. , 2006. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine 131I metuximab injection: clinical phase I/II trials. Int. J. Radiat. Oncol. Biol. Phys. 65, 435–444. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Fan, J.Q. , Li, J. , Li, Q.S. , Yan, Z. , Jia, X.K. , Liu, W.D. , Wei, L.J. , Zhang, F.Z. , Gao, H. , Xu, J.P. , Dong, X.M. , Dai, J. , Zhou, H.M. , 2009. Promoter hypermethylation correlates with the Hsulf-1 silencing in human breast and gastric cancer. Int. J. Cancer 124, 739–744. [DOI] [PubMed] [Google Scholar]
- Chen, N.G. , Szalay, A.A. , Buller, R.M. , Lauer, U.M. , 2012. Oncolytic viruses. Adv. Virol. 2012, 320206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, H.Y. , Guo, T. , Wang, S.J. , Zhao, P. , Dong, Z.S. , Zhang, Y. , Jiang, J.L. , Chen, Z.N. , Yu, X.L. , 2012. Dimerization is essential for HAb18G/CD147 promoting tumor invasion via MAPK pathway. Biochem. Biophys. Res. Commun. 419, 517–522. [DOI] [PubMed] [Google Scholar]
- Dai, J.Y. , Dou, K.F. , Wang, C.H. , Zhao, P. , Lau, W.B. , Tao, L. , Wu, Y.M. , Tang, J. , Jiang, J.L. , Chen, Z.N. , 2009. The interaction of HAb18G/CD147 with integrin alpha6beta1 and its implications for the invasion potential of human hepatoma cells. BMC Cancer 9, 337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstmann, R. , Markman, B. , Tabernero, J. , 2012. Application of monoclonal antibodies as cancer therapy in solid tumors. Curr. Clin. Pharmacol. 7, 137–145. [DOI] [PubMed] [Google Scholar]
- Doloff, J.C. , Jounaidi, Y. , Waxman, D.J. , 2011. Dual E1A oncolytic adenovirus: targeting tumor heterogeneity with two independent cancer-specific promoter elements, DF3/MUC1 and hTERT. Cancer Gene Ther. 18, 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraunholz, I. , Rödel, C. , Distel, L. , Rave-Fränk, M. , Kohler, D. , Falk, S. , Rödel, F. , 2012. High survivin expression as a risk factor in patients with anal carcinoma treated with concurrent chemoradiotherapy. Radiat. Oncol. 7, 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou, X. , Ru, Q. , Zhang, H. , Chen, Y. , Li, L. , Yang, H. , Xing, J. , Chen, Z. , 2009. HAb18G/CD147 inhibits starvation-induced autophagy in human hepatoma cell SMMC7721 with an involvement of Beclin 1 down-regulation. Cancer Sci. 100, 837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco, O. , Marples, B. , Dachs, G.U. , Williams, K.J. , Patterson, A.V. , Scott, S.D. , 2002. Novel chimeric gene promoters responsive to hypoxia and ionizing radiation. Gene Ther. 9, 1403–1411. [DOI] [PubMed] [Google Scholar]
- Hanahan, D. , Weinberg, R.A. , 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Hu, H. , Li, Z. , Chen, J. , Wang, D. , Ma, J. , Wang, W. , Li, J. , Wu, H. , Li, L. , Wu, M. , Qian, Q. , Chen, J. , Su, C. , 2011. P16 reactivation induces anoikis and exhibits antitumour potency by downregulating Akt/survivin signalling in hepatocellular carcinoma cells. Gut 60, 710–721. [DOI] [PubMed] [Google Scholar]
- Ji, W. , Yang, J. , Wang, D. , Cao, L. , Tan, W. , Qian, H. , Sun, B. , Qian, Q. , Yin, Z. , Wu, M. , Su, C. , 2011. hSulf-1 gene exhibits anticancer efficacy through negatively regulating VEGFR-2 signaling in human cancers. PLoS One 6, e23274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana, A. , Liu, P. , Mellone, P. , Lorenzon, L. , Vincenzi, B. , Datta, K. , Yang, B. , Linhardt, R.J. , Lingle, W. , Chien, J. , Baldi, A. , Shridhar, V. , 2011. HSulf-1 modulates FGF2- and hypoxia-mediated migration and invasion of breast cancer cells. Cancer Res. 71, 2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarre, D.D. , Lowy, R.J. , 2001. Improvements in methods for calculating virus titer estimates from TCID50 and plaque assays. J. Virol. Methods 96, 107–126. [DOI] [PubMed] [Google Scholar]
- Lai, J.P. , Chien, J.R. , Moser, D.R. , Staub, J.K. , Aderca, I. , Montoya, D.P. , Matthews, T.A. , Nagorney, D.M. , Cunningham, J.M. , Smith, D.I. , Greene, E.L. , Shridhar, V. , Roberts, L.R. , 2004. hSulf1 Sulfatase promotes apoptosis of hepatocellular cancer cells by decreasing heparin-binding growth factor signaling. Gastroenterology 126, 231–248. [DOI] [PubMed] [Google Scholar]
- Liu, C. , Sun, B. , An, N. , Tan, W. , Cao, L. , Luo, X. , Yu, Y. , Feng, F. , Li, B. , Wu, M. , Su, C. , Jiang, X. , 2011. Inhibitory effect of Survivin promoter-regulated oncolytic adenovirus carrying P53 gene against gallbladder cancer. Mol. Oncol. 5, 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Fu, X. , Ji, W. , Liu, K. , Bao, L. , Yan, Y. , Wu, M. , Yang, J. , Su, C. , 2012. Human sulfatase-1 inhibits the migration and proliferation of SMMC-7721 hepatocellular carcinoma cells by downregulating the growth factor signaling. Hepatol. Res. 10.1111/j.1872-034X.2012.01080.x [DOI] [PubMed] [Google Scholar]
- Liu, J. , Fang, L. , Cheng, Q. , Li, L. , Su, C. , Zhang, B. , Pei, D. , Yang, J. , Li, W. , Zheng, J. , 2012. The effects of G250 promoter controlled conditionally replicative adenovirus expressing Ki67-siRNA on renal cancer cell. Cancer Sci. 10.1111/j.1349-7006.2012.02380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J. , He, X. , Wang, W. , Huang, Y. , Chen, L. , Cong, W. , Gu, J. , Hu, H. , Shi, J. , Li, L. , Su, C. , 2009. E2F promoter-regulated oncolytic adenovirus with p16 gene induces cell apoptosis and exerts antitumor effect on gastric cancer. Dig. Dis. Sci. 54, 1425–1431. [DOI] [PubMed] [Google Scholar]
- Narita, K. , Chien, J. , Mullany, S.A. , Staub, J. , Qian, X. , Lingle, W.L. , Shridhar, V. , 2007. Loss of HSulf-1 expression enhances autocrine signaling mediated by amphiregulin in breast cancer. J. Biol. Chem. 282, 14413–14420. [DOI] [PubMed] [Google Scholar]
- Onimaru, M. , Ohuchida, K. , Nagai, E. , Mizumoto, K. , Egami, T. , Cui, L. , Sato, N. , Uchino, J. , Takayama, K. , Hashizume, M. , Tanaka, M. , 2010. Combination with low-dose gemcitabine and hTERT-promoter-dependent conditionally replicative adenovirus enhances cytotoxicity through their crosstalk mechanisms in pancreatic cancer. Cancer Lett. 294, 178–186. [DOI] [PubMed] [Google Scholar]
- Prabhu, S. , Boswell, C.A. , Leipold, D. , Khawli, L.A. , Li, D. , Lu, D. , Theil, F.P. , Joshi, A. , Lum, B.L. , 2011. Antibody delivery of drugs and radionuclides: factors influencing clinical pharmacology. Ther. Deliv. 2, 769–791. [DOI] [PubMed] [Google Scholar]
- Russell, S.J. , Peng, K.W. , Bell, J.C. , 2012. Oncolytic virotherapy. Nat. Biotechnol. 30, 658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, S.D. , Marples, B. , Hendry, J.H. , Lashford, L.S. , Embleton, M.J. , Hunter, R.D. , Howell, A. , Margison, G.P. , 2000. A radiation-controlled molecular switch for use in gene therapy of cancer. Gene Ther. 7, 1121–1125. [DOI] [PubMed] [Google Scholar]
- Staub, J. , Chien, J. , Pan, Y. , Qian, X. , Narita, K. , Aletti, G. , Scheerer, M. , Roberts, L.R. , Molina, J. , Shridhar, V. , 2007. Epigenetic silencing of HSulf-1 in ovarian cancer: implications in chemoresistance. Oncogene 26, 4969–4978. [DOI] [PubMed] [Google Scholar]
- Wang, B. , Xu, Y.F. , He, B.S. , Pan, Y.Q. , Zhang, L.R. , Zhu, C. , Qu, L.L. , Wang, S.K. , 2010. RNAi-mediated silencing of CD147 inhibits tumor cell proliferation, invasion and increases chemosensitivity to cisplatin in SGC7901 cells in vitro. J. Exp. Clin. Cancer Res. 29, 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, H.H. , Jiang, G. , Gangeswaran, R. , Wang, P. , Wang, J. , Yuan, M. , Wang, H. , Bhakta, V. , Müller, H. , Lemoine, N.R. , Wang, Y. , 2012. Modification of the early gene enhancer-promoter improves the oncolytic potency of adenovirus 11. Mol. Ther. 20, 306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzesinski, S.H. , Taddei, T.H. , Strazzabosco, M. , 2011. Systemic therapy in hepatocellular carcinoma. Clin. Liver Dis. 15, 423–441. (vii-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. , Yang, Y.F. , Ge, N.J. , Shen, S.Q. , Liang, J. , Wang, Y. , Zhou, W.P. , Shen, F. , Wu, M.C. , 2010. Hepatic arterial iodine-131-labeled metuximab injection combined with chemoembolization for unresectable hepatocellular carcinoma: interim safety and survival data from 110 patients. Cancer Biother. Radiopharm. 25, 657–663. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Yang, Y.F. , Ge, N.J. , Shen, S.Q. , Liang, J. , Wang, Y. , Zhou, W.P. , Shen, F. , Wu, M.C. , 2012. Hepatic artery injection of 131I-labelled metuximab combined with chemoembolization for intermediate hepatocellular carcinoma: a prospective nonrandomized study. Eur. J. Nucl. Med. Mol. Imaging 39, 1306–1315. [DOI] [PubMed] [Google Scholar]
- Xiong, J. , Sun, W.J. , Wang, W.F. , Liao, Z.K. , Zhou, F.X. , Kong, H.Y. , Xu, Y. , Xie, C.H. , Zhou, Y.F. , 2012. Novel, chimeric, cancer-specific, and radiation-inducible gene promoters for suicide gene therapy of cancer. Cancer 118, 536–548. [DOI] [PubMed] [Google Scholar]
- Xu, J. , Xu, H.Y. , Zhang, Q. , Song, F. , Jiang, J.L. , Yang, X.M. , Mi, L. , Wen, N. , Tian, R. , Wang, L. , Yao, H. , Feng, Q. , Zhang, Y. , Xing, J.L. , Zhu, P. , Chen, Z.N. , 2007. HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol. Cancer Res. 5, 605–614. [DOI] [PubMed] [Google Scholar]
- Xu, C. , Sun, Y. , Wang, Y. , Yan, Y. , Shi, Z. , Chen, L. , Lin, H. , Lü, S. , Zhu, M. , Su, C. , Li, Z. , 2012. CEA promoter-regulated oncolytic adenovirus-mediated Hsp70 expression in immune gene therapy for pancreatic cancer. Cancer Lett. 319, 154–163. [DOI] [PubMed] [Google Scholar]
- Yang, J.D. , Sun, Z. , Hu, C. , Lai, J. , Dove, R. , Nakamura, I. , Lee, J.S. , Thorgeirsson, S.S. , Kang, K.J. , Chu, I.S. , Roberts, L.R. , 2011. Sulfatase 1 and sulfatase 2 in hepatocellular carcinoma: associated signaling pathways, tumor phenotypes, and survival. Genes Chromosomes Cancer 50, 122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda, A. , Lendorf, M.E. , Couchman, J.R. , Multhaupt, H.A. , 2012. Breast and ovarian cancers: a survey and possible roles for the cell surface heparan sulfate proteoglycans. J. Histochem. Cytochem. 60, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K.J. , Qian, J. , Wang, S.B. , Yang, Y. , 2012. Targeting Gene-Viro-Therapy with AFP driving Apoptin gene shows potent antitumor effect in hepatocarcinoma. J. Biomed. Sci. 19, 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H. , Yang, B. , Yang, X. , Wang, L. , Xu, J. , Liao, C. , Feng, Q. , Tang, H. , Hu, L. , Chen, Z. , Li, Y. , 2009. A novel antibody fragment targeting HAb18G/CD147 with cytotoxicity and decreased immunogenicity. Cancer Biol. Ther. 8, 1035–1044. [DOI] [PubMed] [Google Scholar]


