Abstract
Little is known on the expression of the tumour‐associated carbohydrate antigen sialyl‐Tn (STn), in bladder cancer. We report here that 75% of the high‐grade bladder tumours, presenting elevated proliferation rates and high risk of recurrence/progression expressed STn. However, it was mainly found in non‐proliferative areas of the tumour, namely in cells invading the basal and muscle layers. STn was also found in tumour‐adjacent mucosa, which suggests its dependence on a field effect of the tumour. Furthermore, it was not expressed by the normal urothelium, demonstrating the cancer‐specific nature of this antigen. STn expression correlated with that of sialyltransferase ST6GalNAc.I, its major biosynthetic enzyme. The stable expression of ST6GalNAc.I in the bladder cancer cell line MCR induced STn expression and a concomitant increase of cell motility and invasive capability. Altogether, these results indicate for the first time a link between STn expression and malignancy in bladder cancer. Hence, therapies targeting STn may constitute new treatment approaches for these tumours.
Keywords: Sialyl-Tn, Bladder cancer, Glycosylation, ST6GalNAc.I, Tumour-associated glycans, Proliferative bladder cancer
1. Introduction
Bladder cancer, the fifth most common cancer in Western society, is a growing concern, owing to increased incidence during the past years (Ploeg et al., 2009; van Rhijn et al., 2009). Most of the newly diagnosed bladder cancer cases are superficial, or low‐grade non‐muscle invasive papillary tumours, being conservatively treated by complete transurethral resection of the tumour (Babjuk et al., 2012). However, approximately half of the patients show a high‐percentage of recurrences and an elevated risk of progression to muscle invasive disease, which correlates with poor prognosis (Hussain et al., 2009). The risk of recurrence and/or progression is mostly determined by clinicopathological features (Babjuk et al., 2012). According to the European Organization for Research and Treatment of Cancer (EORTC), this group includes high grade (HG) papillary tumours and carcinoma in situ (CIS) and those with multifocal or recurrent lesions (Babjuk et al., 2012). The evaluation of the nuclear protein Ki‐67 (Ki‐67 proliferation index), an established marker of cell proliferation, is often used to enhance the prognostic accuracy of risk classification given by clinicopathological features (Margulis et al., 2009; Santos et al., 2003), since it is considered a surrogate biomarker of bladder cancer aggressiveness, disease recurrence and progression (Margulis et al., 2009; Santos et al., 2003).
Tumour resection followed by a schedule of intravesical instillations with live attenuated strains of Mycobacterium bovis (Bacillus Calmette–Guérin, BCG) is the standard adjuvant therapeutic option for high‐risk of recurrence/progression bladder tumours (Askeland et al., 2012; Babjuk et al., 2012). Although BCG has improved the management of high‐risk patients, 30–40% of cases either show intolerance or relapse after treatment (Yates and Roupret, 2011). Consequently, these patients require life‐long follow‐up and repeated courses of treatment making bladder cancer the costliest to treat among solid tumours (Askeland et al., 2012; Dovedi and Davies, 2009; Sievert et al., 2009). Upon therapeutic failure and/or muscle invasion, cystectomy is advocated for oncological control (Askeland et al., 2012; Dovedi and Davies, 2009; Sievert et al., 2009). Furthermore, at the moment there is a lack of specific biomarkers to target aggressive cell phenotypes and direct molecular‐based therapy, which may be used to avoid preventive cystectomy (Dovedi and Davies, 2009).
Vaccines using tumour‐associated glycans, in association with immunological boosters, are emerging as potential therapeutic strategies against cancer (Hakomori, 2001; Lakshminarayanan et al., 2012; Ryan et al., 2010; Sorensen et al., 2006). In the forefront of these antigens is sialyl‐Tn (STn; Neu5Acα2‐6GalNAcα‐O‐Ser/Thr) (Gilewski et al., 2007; Julien et al., 2009; Miles et al., 2011). STn has been mostly observed in tumour‐associated mucins due to their high number of potential O‐glycosylation sites (Clement et al., 2004; Conze et al., 2010; Julien et al., 2006; Marcos et al., 2011; Pinto et al., 2012). However, integrins (Clement et al., 2004) and CD44 (Julien et al., 2006), among other proteins, may also carry this posttranslational modification. Overexpression of STn antigen has been detected in breast (Leivonen et al., 2001), oesophagus (Ikeda et al., 1993), colon (Itzkowitz et al., 1989), pancreas (Kim et al., 2002), stomach (David et al., 1996; Marcos et al., 2011), endometrium (Inoue et al., 1991), and ovary (Numa et al., 1995) carcinomas, whereas low or no expression was observed in the respective normal tissues. STn overexpression was also reported in several cancer precursor lesions, such as esophageal dysplastic squamous epithelia (Itoh et al., 1996), gastric intestinal metaplasia (Baldus et al., 1998; Ferreira et al., 2006) and colonic moderate dysplasia (Cao et al., 1997).
STn is known to influence cell recognition by the immune system (Angata et al., 2007), affect processes as cell cycle, apoptosis, and actin cytoskeleton dynamics, decrease cell–cell aggregation and increase extra‐cellular adhesion, migration, invasion (David et al., 1996; Julien et al., 2006, 2005; Pinho et al., 2007) and metastization (Ozaki et al., 2012). In line with these observations, STn positive (STn+) cells have been frequently observed at the invasion front of tumours and in peritoneal and pleural effusions in ovarian cancer patients; yet they are less common in metastatic lesions than in primary tumours (Davidson et al., 2000). In gastric carcinomas, STn was correlated with the depth of invasion and metastization (Ikeda et al., 1993), and thus poor prognosis (Terashima et al., 1998). Conversely, STn was not correlated with the depth of invasion in studies concerning colorectal (Itzkowitz et al., 1989; Ogata et al., 1998) and breast cancers (Schmitt et al., 1995). However, some contradicting results have been presented regarding its association with metastasis and decreased survival in these cancers (Julien et al., 2012). Hence, a recent review suggests that the biological role of STn in tumour development may be dependent on each cancer type or sub‐type (Julien et al., 2012).
Despite these observations, there is little information regarding STn in the context of bladder cancer. Given its clinical relevance and the fact that there are available therapies based on this antigen, we addressed the presence of STn in bladder tumours and the mechanisms underlying its expression.
2. Materials and methods
2.1. Patient and sampling
Formalin‐fixed, paraffin embedded (FFPE) tissues were prospectively collected from 69 patients, mean age of 69 years (age range 45–89), who underwent transurethral resection (TUR) of the bladder tumour in the Portuguese Institute for Oncology of Porto (IPO‐Porto, Portugal), between July 2011 and May 2012. Based on urothelial carcinoma grading and staging criteria of the World Health Organization (WHO), three different groups were considered (Table 1), low‐grade (LG, n = 24) and high‐grade HG non muscle‐invasive (NMIBC, n = 26) and muscle‐invasive (MIBC, n = 19) bladder cancers. Of HG NMIBC, 21 were papillary tumours and 5 were carcinoma in situ (CIS). None of these patients had received prior adjuvant therapy. Six normal urothelium tissues of necropsied male individuals without bladder cancer history, within the same mean of age range, were also included.
Table 1.
STn expression in the healthy urothelium and in non‐muscle invasive (NMIBC) and muscle invasive (MIBC) bladder cancers of different clinicopathological natures.
| Total | STn expression | |
|---|---|---|
| Normal urothelium | 6 | |
| − | 6 (100%) | |
| + | − | |
| ++ | − | |
| +++ | − | |
| Total STn+ | 0 (0%) | |
| NMIBC | 50 | |
| Low‐grade papillary tumours | 24 | |
| − | 19 (79%) | |
| + | 5 (21%) | |
| ++ | − | |
| +++ | − | |
| Total STn+ | 5 (21%) | |
| High‐grade (CIS + papillary tumours) | 26 | |
| Carcinoma in situ (CIS) | 5 | |
| − | 4 (80%) | |
| + | 1 (20%) | |
| ++ | − | |
| +++ | − | |
| Total STn+ | 1 (20%) | |
| High‐grade papillary tumours | 21 | |
| − | 5 (24%) | |
| + | 9 (43%) | |
| ++ | 4 (19%) | |
| +++ | 3 (14%) | |
| Total STn+ | 16 (76%) | |
| MIBC | 19 | |
| − | 5 (26%) | |
| + | 11 (58%) | |
| ++ | 2 (11%) | |
| +++ | 1 (5%) | |
| Total STn+ | 14 (74%) |
−: No reactivity; +: ≤15%; ++: 15–30%; +++: 30–45% of the tumour.
Additionally, FFPE tissues from 16 radical cystectomy cases including the main lesion in each specimen, responsible for therapeutic decision, the adjacent mucosa, which may or may not include a concomitant tumour, and the ureter representing a distant mucosa, were also studied. Mucosa without visible histopathological alterations was defined as “histologically normal” mucosa.
All procedures were performed under the approval of the Ethics Committee of IPO‐Porto, after patient's informed consent. All clinicopathological information was obtained from patients' clinical records.
2.2. Tissue expression of STn and Ki‐67
FFPE tissue sections were screened for STn and Ki‐67 by immunohistochemistry using the avidin/biotin peroxidase method. Briefly, 3 μm sections were deparaffinised with xylene, rehydrated with graded ethanol series, microwaved for 15 min in boiling citrate buffer (10 mM Citric Acid, 0.05% Tween 20, pH 6.0), and exposed to 3% hydrogen peroxide in methanol for 20 min. The expression of STn was then evaluated using anti‐STn mouse monoclonal antibody, clone TKH2 (Kjeldsen et al., 1988), that identifies both single and clustered STn residues (Ogata et al., 1998), whereas Ki‐67 was evaluated using monoclonal mouse anti‐human Ki‐67 antibody, clone MIB‐1 (Dako). After blockage with BSA (5% in PBS), the antigens were identified with Vectastain Elite ABC peroxidase kit (Vector Lab) followed by incubation with 3,3‐diaminobenzidine tetrahydrochloride (DAB, Dako). Finally, the slides were counterstained with haematoxylin for 1 min. Positive and negative control sections of intestinal metaplasia were tested in parallel. The negative control sections were performed by adding BSA (5% in PBS) devoid of primary antibody. STn+ tissues were also treated with a neuraminidase from Clostridium perfringens (Sigma–Aldrich) as previously described by Marcos et al. (2011) in order to remove the sialic acid. The desialyated samples were thereafter screened for STn. The O‐acetylation of Neu5Ac residues in STn was evaluated after treatment with 100 mM NaOH at room temperature for 30 min as described by Ogata et al. (Ogata et al., 1998) prior to immunohistochemistry with antibody TKH2.
A semi‐quantitative approach was established to score the immunohistochemical labelling based on the intensity of staining and the percentage of cells that stained positively. The STn and Ki‐67 expression were assessed double‐blindly by two independent observers and validated by an experienced pathologist. Whenever there was a disagreement, the slides were reviewed, and consensus was reached. Tumours were classified as proliferative whenever Ki‐67 expression was higher than 18%, as described by Santos et al. (Santos et al., 2003).
2.3. Cell lines culture
The human bladder cancer cell line MCR and the transduced variants of MCR (MCRnc and MCRSTn+), were grown as described by Videira et al. (2009b).
2.4. Generation of STn+ bladder cancer cells
MCR cells were transduced with a retroviral vector generated with the ViraPower™ Lentiviral Expression System (Invitrogen), according to manufacturer's instructions. The whole coding region of human ST6GalNAc.I was PCR amplified and cloned in the pLenti6/V5 Directional TOPO cloning vector which drives the expression of inserted genes through the CMV promoter. A negative control retroviral vector was prepared with an empty plasmid. After transduction with negative control‐ or ST6GalNAc.I‐expressing vectors, MCR cells were selected with 4 μg ml−1 blasticidin. An additional immunomagnetic enrichment of the STn+ cells was performed by using mouse anti‐STn (HB‐STn1 clone from Dako), followed by the secondary antibody anti‐mouse IgG associated to paramagnetic microbeads (Miltenyi Biotec). The stable transduction of the enzyme was confirmed by evaluation of ST6GalNAc.I expression and activity. STn expression was determined by analysis of the mean fluorescence intensity (MFI) ± SE through flow cytometry analysis using monoclonal antibody TKH2.
2.5. Evaluation of STn expression in cell lines
For phenotypic characterization, cells were stained with 1:50 diluted anti‐STn TKH2 monoclonal antibody for 16 h at 4 °C, and 1:100 diluted goat fluorescein isothiocyanate (FITC)‐labelled anti‐mouse IgG (Dako) for 15 min at 4 °C in the dark and then acquired in a FacsCalibur Flow cytometer (Becton Dickinson). Data were analysed using the WinMDI v2.9 software (The Scripps Research Institute, San Diego, CA, USA).
2.6. Analysis of ST6GalNAc.I expression
RNA extraction from FFPE sections was performed after deparaffinization of the tissue using Absolutely RNA FFPE kit (Agilent technologies) while for cell lines it was used the GenElute Mammalian Total RNA Purification kit and DNAase treatment (Sigma), according to the manufacturer's instructions. The purity of RNA extracts was determined based on the A260/A280 ratio. Only ratios between 1.9 and 2.1 were considered further.
Approximately 250–500 ng of total RNA (1 μg for cell lines) was converted by reverse transcription into cDNA, using the random‐primers‐based High Capacity cDNA Archive Kit (Applied Biosystems). The expression levels of ST6GalNAc.I were determined by TaqMan assay (Applied Biosystems), the reference sequences detected by each primer/probe set and the Assay ID provided by the manufacturer were the following: ST6GalNAc1 (NM018414.2/Hs00300842_m1). Real time PCR was performed in a 7500 Fast Real‐Time PCR System using the TaqMan Universal PCR Master Mix Fast from Applied Biosystems, as described previously by Videira et al. (2007, 2009a). During the cDNA exponential amplification the product formation was proportional to the fluorescence emission resulting from the TaqMan probe degradation (van der Velden et al., 2003). The ST6GalNAc.I mRNA levels were normalized for the expression of β‐actin, which was taken as a suitable endogenous control for bladder cancer cells (Videira et al., 2007). The relative mRNA levels were calculated by adapting the 2−ΔΔCt formula (Livak and Schmittgen, 2001).
2.7. Evaluation of ST6GalNAc.I activity
MCR cell pellets were homogenized in H2O and the protein concentration was determined using the RC‐DC protein quantification kit (BioRad) according to the manufacturer's instructions. Sialyltransferase activity was assayed in whole cell homogenates as previously described by Dall'Olio et al. (1997) with some modifications. Briefly, the reaction mixture contained 80 mM sodium cacodylate buffer pH 6.5, 0.5% Triton X‐100, 6 μg μl−1 of asialo bovine submaxillary mucin (ABSM, prepared by acid desialylation of BSM) as acceptor substrate, 30 μM (1280 Bq) of CMP‐[14C]Sia (Amersham) and 2 μg μl−1 of homogenate proteins. Endogenous controls were prepared in the absence of acceptor substrate. The enzyme reactions were incubated at 37 °C for 2 h and the acid insoluble radioactivity was measured as previously described by Dall'Olio et al. (1997). The incorporation on endogenous substrates was subtracted.
2.8. Cell proliferation measurement
To study their proliferative capacity, cells were labelled with CellTrace™ CFSE Cell Proliferation Kit (Invitrogen). The MCR cells were resuspended into medium at final concentration of 1 × 106 cells ml−1 and incubated with 10 μM CFSE, following the manufacturer's instructions. Subsequently, the CFSE‐labelled cells were seeded into 24‐well microplates, incubated in a 5% CO2 incubator at 37 °C and harvested at 24, 48, 72 and 96 h post‐culture. Flow cytometry using a FACSCalibur (Becton–Dickinson) was performed and the data collected were analysed with ModFit LT 3.2 software (Verity Software House, Topsham, ME), allowing to assess the cell proliferation index (PI). The PI represents the average number of cells that were originated from a single cell of the parental generation. The parental generation was set based on the analysis of data obtained from the cells corresponding to the 24 h of culture.
2.9. Analysis of cell motility using a wound‐healing assay
Cell motility was tested in a wound‐healing migration assay. MCR cells were seed into 12‐well microplates and grown to confluency. A scratch was made in the monolayer with a sterile 200 μl pipette tip. After wounding, the suspended cells and debris were washed away and fresh medium was added. At 0 and 24 h after wounding, scratched regions were photographed with an inverted microscope equipped with a digital camera.
2.10. Invasion assay
Invasion assays were performed using BD Biocoat Matrigel™ invasion chambers, comprised by an 8‐μm diameter pore size filter coated with a thin layer of matrigel, and placed in a two‐compartment system in a 24‐well plate. Prior to each experiment, filters were re‐hydrated in serum‐free DMEM medium for 2 h at 37 °C. After detachment of subconfluent cells with trypsin/EDTA, cells were suspended in culture medium supplemented with 5% inactivated FBS, counted and seeded on the upper side of the matrigel‐coated filter at a density of 5 × 104 cells/well. After 24 h at 37 °C, filters were fixed in 4% paraformaldehyde and non‐invading cells, present on the upper side, were completely removed, to facilitate analysis. Cells that had invaded the underside of the filters were mounted in Vectashield+4′,6‐diamidino‐2‐phenylindole (DAPI, Vector Laboratories, CA, USA), and visualized through a Zeiss Axiovert 200M fluorescence microscope (Carl Zeiss, Germany). Invasive cells were scored in at least 12 microscopic fields (20× objective) when DAPI‐counterstained nuclei passed through the filter pores. Results are presented as means ± SD for each sample. Invasion levels are expressed as a ratio of the results obtained with the mock‐transfected control cell line.
2.11. Statistical analysis
Statistical analysis was performed using the Student's T‐test for unpaired samples. Differences were considered to be significant when p < 0.05. A chi‐square test was used to analyse correlations between clinicopathological features and STn and Ki‐67 expressions.
3. Results
3.1. Expression of STn in bladder tumours
STn expression in bladder tumours was evaluated by immunohistochemistry using mouse monoclonal antibody clone TKH2. As shown in Table 1, STn is not expressed in the healthy urothelium; conversely 46% of the bladder tumours presented cells with STn membrane and cytoplasmic staining (32/69) (Figure 1), demonstrating the tumour‐specific nature of this antigen. The removal of sialic acids from the tissue sections with a α‐neuraminidase impaired the recognition by TKH2 and confirmed STn expression.
Figure 1.
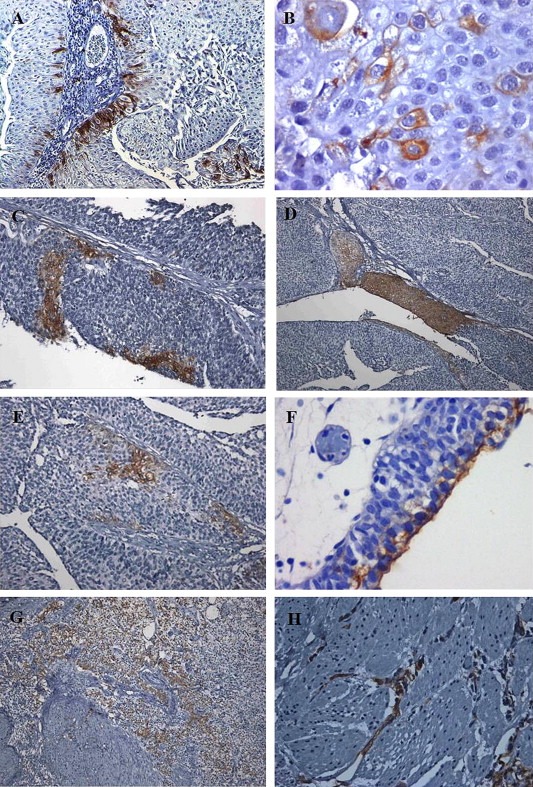
Expression of STn in FFPE bladder tumours. A) Low‐grade papillary tumour showing a predominance of STn+ cells in the basal layer; B) Magnification which shows tumour cells with membrane and cytoplasmic STn+ staining; C) High‐grade papillary tumour evidencing the focal nature of STn expression. Positive cells were found both in the basal layer and throughout the papillae; D) High‐grade papillary tumour showing locally extensive STn positivity; E) High‐grade papillary tumour evidencing STn+ in the basal layer; F) CIS showing STn+ in the cells facing the lumen of the bladder; G) MIBC showing locally extensive STn expression including at the muscle invasive front; H) MIBC highlighting STn+ cells invading the muscle layer.
STn expression was lower in low‐grade (LG) NMIBC (21% STn+ tumours; Figure 1A–B) compared to high‐grade lesions (HG; 67%), which include papillary tumours (76% STn+ tumours; Figure 1C–E), CIS (20% of STn+ tumours; Figure 1F), and MIBC (74% STn+ tumours; Figure 1G–H). Noteworthy, STn was absent from the majority of CIS (4/5; 80%) and showed an expression comparable to LG tumours. Altogether, these results highlight an association between the STn antigen and high grade NMIBC (p < 0.002; Figure 2) as well as with muscle invasive tumours (p < 0.03; Figure 2).
Figure 2.
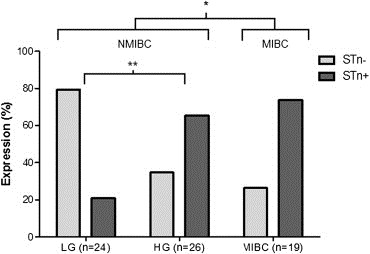
Association between STn expression and HG NMIBC and MIBC. The percentage of STn+ tumours was higher in HG when compared to LG and also in MIBC when compared to NMIBC (LG + HG). “*” p = 0.03; “**” p = 0.002 (Chi‐square test).
The O‐acetylation of sialic acid residues prevents TKH2 from recognizing STn antigens in certain tissues (Ogata et al., 1998). To exclude this possibility in bladder cancer, the slides were chemically de‐O‐acetylated prior to immunohistochemistry. This procedure did not alter STn expression patterns demonstrating that STn antigens were not encrypted by O‐acetylation.
3.1.1. Pattern and extension of STn expression in bladder tumours
The STn antigen presented a focal expression that for the majority of the STn positive cases (26/36) did not exceeded 15% of the tumour section (Table 1). Furthermore, in 25% of the STn positive cases (9/36) the antigen was detected in less than 5% of the tissue (data omitted from Table 1). Higher expression patterns were restricted to HG papillary NMIBC, where 27% of the cases (7/26) presented STn levels between 15% and 45% of the tumour section (Table 1) and locally diffuse staining (Figure 1C, D, G). STn was mainly observed in basal layer cells (75% of STn+ cases; Figure 1A, C–E), but it could be also detected throughout the papillae (Figure 1C–E) and cells of the luminal surface (Figure 1F) in cases presenting locally diffuse staining. STn was further observed in cells invading the basal (50% of STn+ of HG NMIBC; Figure 1C–E, G) and muscle layers (57% of STn+ MIBC; Figure 1G, H), suggesting a role in invasion.
3.1.2. STn antigen expression in advanced tumours and in the surrounding areas
The STn antigen was also evaluated in a series of radical cystectomy specimens which included the tumour used for therapeutic decision (termed “main tumour” in Figure 3) and the tumour‐adjacent mucosa. The ureters were included as distant mucosa (Figure 3). In agreement with the observations from Table 1, STn was detected in 69% (11/16) of all main tumours as well as in their adjacent mucosa (Figure 3), independently of their histological classification. Noteworthy, STn was absent from 90% of the distant mucosas of STn positive cases; the only exceptions being a ureter with pre‐neoplastic and another with a neoplastic lesions (Figure 3). These results point out that the STn+ tumour‐adjacent mucosa may display molecular changes similar to those of the main lesions. Thus, this antigen may be useful as a marker of field carcinogenesis in the bladder.
Figure 3.
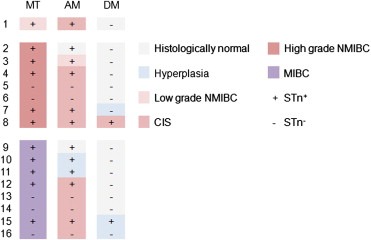
Expression pattern of STn in radical cystectomy specimens. Radical cystectomy specimens have been organized based on histological grade. They include the tumour responsible by the therapeutic decision termed “main tumour” (MT), an adjacent (AM) and distant mucosa (DM). The graphical matrix highlights that, whenever STn is expressed by the main tumour (13/16; 63%), it is always present in the adjacent mucosa (13/13, 100%). One preneoplastic and one neoplastic distant mucosa also expressed the antigen.
3.2. Expression of ST6GalNAc.I in bladder tumours
The presence of STn has been strongly associated with the overexpression of ST6GalNAc.I in several human malignancies. To assess this event in bladder tumours, mRNA levels of ST6GalNAc.I gene were analysed and normalized in relation to β‐actin, which proved to be a stable expressed gene in previous studies concerning bladder tumours (Videira et al., 2007). As shown by Figure 4, low gene expression levels were detected in tumours that did not express STn. In addition, the levels of ST6GalNAc.I increased with the expression of STn, and were significantly higher in the tumours with STn expression superior to 15%. Figure 4 also shows that this behaviour was similar in LG and HG tumours. However, as a result of higher STn expression, the average ST6GalNAc.I mRNA levels were more elevated in HG (53%) tumours than LG (9%). These observations suggest that overexpression of ST6GalNAc.I gene is one of the main events leading to STn expression in bladder tumours.
Figure 4.
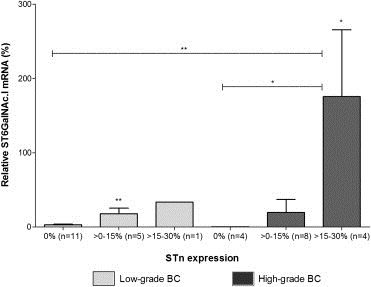
Association between ST6GalNAc.I and STn expression in LG and HG bladder tumours. The graph shows that ST6GalNAc.I expression is increased in STn+ tumours and increases further for more elevated STn expressions (>15–30% of the tumour section). This suggests that the overexpression of ST6GalNAc.I is one of the main mechanisms underlying the presence of STn in bladder cancers. Furthermore, it shows this event occurs in both LG and HG tumours. “*” p < 0.05; “**” p < 0.01 (Student's T‐test).
3.3. STn expression and tumour proliferation
As shown above, the expression pattern of STn correlates with HG tumours, known to present elevated proliferation rates (Margulis et al., 2009; Santos et al., 2003). To assess a possible association between STn and proliferation, 24 cases from the initial series of 69 bladder tumours, comprehending 12 LG and 12 HG tumours (7 NMIBC, none of them CIS, and 5 MIBC), were screened for STn and Ki‐67 expression. Tumours presenting Ki‐67 expression superior to 18% were classified as proliferative. As highlighted by the graphical matrix in Figure 5A, 8% (1/12) LG and 75% (9/12) HG cases showed elevated Ki‐67, confirming the higher proliferation of HG tumours (p < 0.0012). Similarly, Figure 5A also shows an association between proliferative phenotypes and STn expression (p < 0.001). However, in all STn positive cases, the examination of sequential sections revealed that STn antigen expression was mainly seen in areas that did not express Ki‐67 (Figure 5A), although some overlap was present in 25% of the cases (3/12; Figure 5B). This indicates that the STn antigen is mostly expressed in non‐proliferative areas of the tumour. Nevertheless, the majority of the non‐proliferative tumours also did not express STn (12/14), demonstrating an interdependence between both phenomena.
Figure 5.
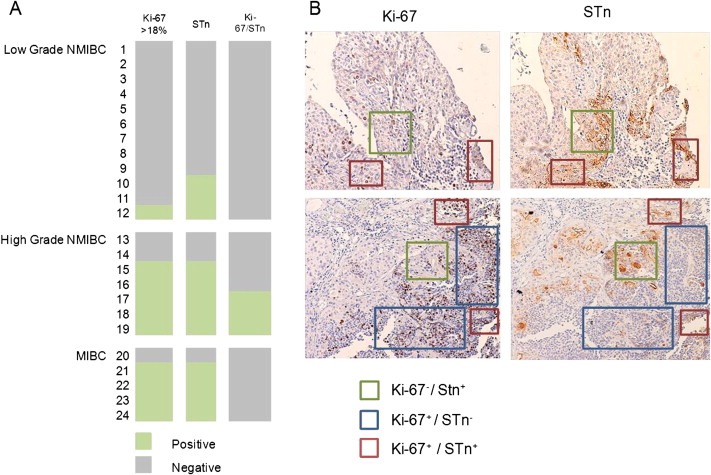
Expression of STn and Ki‐67 in bladder tumours. A) graphical matrix highlighting the association between proliferative tumours (Ki‐67 > 18%) and STn expression in bladder tumours. HG NMIBC and MIBC were considered to be proliferative tumours and a significant association was found between STn expression and tumours presenting proliferation phenotypes (p < 0.001; Chi‐square test). The notation “Ki‐67/STn” in the column more to the right refers to tumours presenting areas that appear to exhibit cells expressing both Ki‐67 and STn. B) Immunohistochemistry for Ki‐67 and STn highlighting Ki‐67−/STn+; Ki‐67+/STn−; and Ki‐67+/STn+ areas.
3.4. In vitro assessment of the biological significance of STn expression
3.4.1. Development of a high‐grade bladder cancer cell line overexpressing STn
To further corroborate the role of ST6GalNAc.I in the expression of STn antigen by bladder cancer cells, we induced the overexpression of ST6GalNAc.I in a bladder cancer cell line. The MCR bladder cell line, that showed negligible expression of ST6GalNAc.I and no STn (data not shown), was transduced with a lentivirus expressing the coding region of the human ST6GalNAc.I gene. The obtained cell line variant, herein named MCRSTn+, showed markedly increased expression of ST6GalNAc.I mRNA levels (Figure 6A). It also showed significantly higher sialyltransferase activity towards the ABSM, a substrate for the ST6GalNac.I enzyme, when compared with the negative control cell line (MCRnc) transduced with void lentivirus (Figure 6A). The overexpression of STn antigen by MCRSTn+ cell line variant was confirmed by flow cytometric analysis (Figure 6B).
Figure 6.
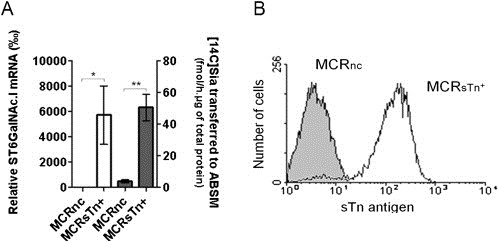
ST6GalNAc.I mRNA expression and sialyltransferase activity in bladder cancer MCR cell lines. A) ST6GalNAc.I expression and activity in MCR cell lines. The relative mRNA levels of ST6GalNAc.I (open bars) and sialyltransferase activity towards ABSM (grey bars) were analysed as described in the Material and methods section. Both, ST6GalNAc.I mRNA and sialyltransferase activity towards ABSM are negligible in negative control cells and markedly increased upon ST6GalNAc.I transduction. B) Flow cytometry analyses of transduced MCR cells. Both negative control (MCRnc in grey histogram) and ST6GalNAc.I‐transduced (MCRSTn+ in open histogram) cell lines were stained with the secondary antibody anti‐Ig's‐FITC following incubation with the primary antibody anti‐STn antigen. 90% of the ST6GalNAc.I‐transduced cells expressed the STn antigen (MFI = 216). The data are shown as a mean ± standard deviation of 3 independent studies. “*” p < 0.05, “**” p < 0.01 (Student's T‐test).
3.4.2. STn influence on cell proliferation, migration and invasion
STn expression was correlated with tumours with higher proliferative indexes (Figure 5). To assess the influence of STn in proliferation, MCR cells (MCRnc and MCRSTn+) were cultured for 48, 72 and 96 h and then evaluated in relation to their proliferation index. The comparison between the two cell line variants showed that the proliferation index of MCRSTn+ cells was generally higher than the index of MCRnc cells, although only statistically different at 72 h of culture (p < 0.05; Figure 7). However, this effect was no longer significant at 96 h of culture (Figure 7).
Figure 7.
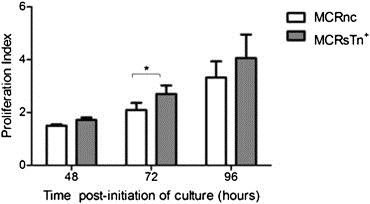
Comparison between the proliferation capacity of MCRnc and MCRSTn+ cells. The transduced MCR cells were labelled with CFSE and cultured for various periods of time (48, 72 and 96 h). The cells were harvested and analysed by flow cytometry with Modfit software, allowing the calculation of the proliferation index, which represents the average number of cells that was originated by a single cell of the parent generation. At the various periods of culture, MCRSTn+ cells show a higher proliferation index than the negative control, but this difference was only statistically significant at 72 h of culture. The data are presented as a mean ± standard deviation of 3 independent studies. “*” p < 0.05 (Student's T‐test).
STn positive cells were observed invading the basal and muscle layers (Figures 1 and 2) and in the adjacent mucosa of advanced stage bladder tumours (Figure 5), suggesting a correlation of STn with invasion and migration. Thus, the influence of STn expression in MCR cell invasion was assessed using the Matrigel invasion assay. Our results evidence that MCR cells transduced with ST6GalNAc.I (MCRSTn+) are approximately four folds more invasive than bladder cells transduced with the negative control (MCRnc; Figure 8A). The effect of STn expression on cell migration was estimated by a wound‐healing assay. Therefore, uniform scratches were made in confluent monolayers of MCRnc and MCRSTn+ cell lines and the capability of the cells to migrate and fill the scratches was monitored. As observed in Figure 8A, by 24 h after wounding, the MCRSTn+ cells had almost completely covered the empty space. Conversely, the negative control, MCRnc cells, displayed a large “gap”, thus demonstrating their lower capability to closure the wound. Our results evidence that MCR cells expressing STn present increased invasion and wound repair capacities.
Figure 8.
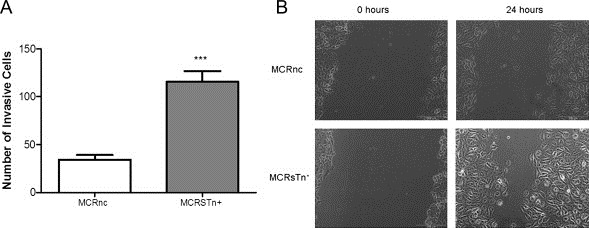
STn expression promotes MCR cells wound healing closure and invasion. A) Wound healing closure assay. Uniform scratches were made using a 200 μL pipette tip in confluent monolayers of MCRSTn+ and MCRnc cells. Cells were allowed to heal and the extent of closure was monitored by microscopic analysis. After 24 h culture, the MCRSTn+ cells had almost completely covered the wound, in clear contrast to negative control, MCRnc, where unoccupied space was still observed. B) Invasion assay. MCRSTn+ and MCRnc cells were incubated for 24 h, in the upper compartment of Matrigel invasion chambers, in complete DMEM medium and in the absence of other chemoattractants. Invasive cells were determined as described in Materials and methods. The data are presented as a mean ± standard deviation of 4 independent studies. “*” p < 0.001 (Student's T‐test).
4. Discussion
The STn antigen is highly expressed by several human carcinomas and preneoplastic lesions (Julien et al., 2012) and is explored as a tumour marker in serological assays (CA72‐4) (Reis et al., 2010).
Despite the clinical relevance of STn in human malignancies, scarce information is available about its role in bladder tumours. Over twenty years ago, Langkilde et al. (1992) addressed this antigen on series of transitional cell carcinomas (currently classified as high‐grade urothelial cell carcinomas according to current WHO guidelines (Babjuk et al., 2012)). Normal mucosal biopsy specimens from patients with non‐malignant bladder urologic diseases were included as controls. According to the authors, STn was not expressed by the control group, showed a very restricted pattern of expression in bladder tumours and no association with recurrence and progression. Subsequent in vitro studies found that mucins MUC1, MUC2 and MAUB (mucin antigen of the urinary bladder) isolated from bladder cancer cell lines carried STn (Bergeron et al., 1996, 1997). However, no evidence of such an expression was found in tumours. Herein, we readdressed this matter and found that the STn antigen was associated with advanced stage bladder tumours. More important, STn was absent in the healthy urothelium, which demonstrates its tumour‐associated nature. Since this study was performed on a recent prospective series it is not possible, at this point, to determine correlations with disease outcome. Nevertheless, STn was mainly expressed by HG papillary NMIBC, known for their elevated risk of recurrence and progression to muscle invasive disease and MIBC that encompass an elevated risk of metastization and present decreased overall survival (Babjuk et al., 2012). STn expression was further associated with elevated Ki‐67, a proliferation‐related molecule and a surrogate biomarker of increased risk to recurrence and progression in bladder tumours (Margulis et al., 2009; Santos et al., 2003). In addition, the majority of non‐proliferative tumours did not express STn, which demonstrates that the expression of the antigen is indeed a characteristic of proliferative tumours. Still, STn was mainly detected in non‐proliferative areas of the tumours. However, the STn antigen was frequently observed in areas of invasion of the basal and muscle layers, suggesting it may be associated with the process of cell migration and invasion. This reinforces the notion that STn is part of a malignant bladder cancer phenotype, as previously observed for other carcinomas (Clement et al., 2004; Julien et al., 2006; Ohno et al., 2006; Ozaki et al., 2012; Pinho et al., 2007). We also found the STn antigen in tumour‐adjacent mucosa, which may be explained by the migration of STn+ cells to the tumour surroundings. On the other hand, this may be a consequence of field carcinogenesis previously observed in bladder cancers (Jones et al., 2005; Palmeira et al., 2011). Nevertheless, the STn antigen holds potential as a biomarker of bladder disseminated disease.
STn is a product of an incomplete O‐glycosylation process due to the premature O‐6 sialylation of the glycoside GalNAcα1‐O‐Ser/Thr (Tn antigen) by ST6GalNAc.I (Marcos et al., 2004). In several epithelial tumours STn results from an increased ST6GalNAc.I expression and/or activity (Marcos et al., 2011; Sewell et al., 2006; Vazquez‐Martin et al., 2004). Previous studies have reported ST6GalNAc.I expression by the urothelium at the mRNA level (Yamamoto et al., 2003); however we and others (Langkilde et al., 1992) have not detected STn expression in the histologically healthy tissues. These observations suggest either the absence of the antigen or the insufficient sensitivity of the method. ST6GalNAc.I localization in the Golgi apparatus and the competitive action of other glycosyltransferases for the Tn antigen may also favour the extension of the O‐glycan chain in non‐pathological conditions. On the other hand we showed that the levels of STn in bladder tumours were correlated with the expression of ST6GalNAc.I, supporting this as a major molecular mechanism underlying STn biosynthesis in these tumours. Few cases presented STn expression associated with a basal level of ST6GalNAC.I, meaning that other factors may contribute to promote the biosynthesis of STn. A disorganization of secretory organelles (Sewell et al., 2006), somatic mutations in the gene Cosmc, encoding a molecular chaperone essential for O‐chain elongation (Ju et al., 2008), the down‐regulation/decreased activity of several other glycosyltransferases and/or the availability of sugar donors for biosynthesis, may also lead to STn overexpression. The integrated study of metabolic pathways, glycosyltransferases expression/activity, intra‐cellular ultrastructures and microenvironmental changes may further enlighten the molecular events leading to abnormal O‐glycosylation of bladder cancer proteins.
In addition we have screened HT1376, 5637, T24 and MCR bladder cancer cell lines and found neglectable levels of the STn antigen (data not shown). The same was previously observed in gastric (Ozaki et al., 2012; Pinho et al., 2007) and breast (Clement et al., 2004; Julien et al., 2005, 2006) cancers cell models, demonstrating that tumour cells may lose the ability to express this antigen in vitro. Microenvironmental factors may play a determinant role in the induction of STn biosynthesis, yet these events remain unknown. Following the association of STn with invasive cases, we elected the invasive bladder cancer cell line MCR to evaluate the biological role of STn in these tumours. We started by stably transducing the MCR cells with ST6GalNAc.I, which resulted in the overexpression of STn. The expression of STn did not promote a significant enhancement of MCR cell proliferation, which is agreement with observations made for breast (Clement et al., 2004; Julien et al., 2005, 2006) and gastric cancer models (Pinho et al., 2007). These findings associated with the absence of the antigen from most bladder tumours non‐proliferative areas strongly suggests that STn expression does not play a direct role in tumour proliferation.
On the other hand, STn expression significantly enhanced the migration and invasive capacity of MCR cells, demonstrating that this antigen plays an important role in bladder cancer cell invasion, as suggested by the observation of bladder tumours. Enhanced migration capabilities of STn+ cells on components of the extracellular matrix, such as fibronectin and collagen, have been described for other cancer cell lines (Julien et al., 2005, 2006; Pinho et al., 2007), and result, among several factors, from impaired integrin binding (Clement et al., 2004). In addition, STn expression has been shown to increase the invasion potential of tumour cells (Clement et al., 2004; Julien et al., 2006; Ohno et al., 2006; Ozaki et al., 2012; Pinho et al., 2007), supporting a similar role in bladder tumours. Further experiments are however required to clarify the molecular mechanisms underlying promotion of cancer cell invasion and migration. These findings reinforce however that alterations in the glycosylation patterns of cell‐surface proteins may strongly interfere with events like cell–cell adhesion, cell–matrix interaction, tumour growth, motility and invasion (Dall'Olio et al., 2012).
In resume, our work comprehensively describes the expression of the STn antigen in bladder cancer. Namely, it demonstrates the tumour‐specific nature of this type of glycosylation and its association with advanced, highly proliferative tumours, invasion and organ disseminated disease. Thus, the evaluation of STn antigen may add valuable information about the aggressiveness of proliferative tumours, complementing the information given by Ki‐67. Studies are ongoing in broader retrospective series to determine the association of STn with disease outcome and corroborate these findings. We are also devoted to the identification of the glycoproteins yielding STn, which is expected to bring insights about the role of this type of glycosylation in bladder carcinogenesis and provide novel therapeutic vectors. The antigen STn may also be monitored noninvasively in urine or serum using as is the case for other human carcinomas using the CA72‐4 test (Reis et al., 2010). This could allow decreasing the number of cystectomies in post‐surgery follow‐ups of patients with high‐grade tumours, a particularly critical matter for the elderly that constitute the majority of the cases.
Furthermore, the STn antigen is associated to high‐grade NMIBC which currently constitutes one of the main therapeutics concerns due to their elevated risk of recurrence/progression (Babjuk et al., 2012). Adjuvant immunotherapy with BCG has allowed to delay recurrence and decrease the risk of progression into muscle invasive disease (Babjuk et al., 2012); still more than half of the patients either recur within two‐years after TUR of the tumour or show intolerance to the treatment (Askeland et al., 2012; Yates and Roupret, 2011). Due to the lack of efficient therapies, upon therapeutic failure and/or muscle invasion, the patient is faced with cystectomy (Babjuk et al., 2012).
Carbohydrate antigens associated with advanced‐stage tumours and malignant phenotypes such as STn, are expressed at the cell surface and, therefore, available for antibody or lectin‐mediated recognition (Neutsch et al., 2012). Thus, these antigens may present an opportunity for the introduction of novel therapeutics, such as selective drug‐delivery approaches (Neutsch et al., 2012) or carbohydrate‐based immunotherapy (Heimburg‐Molinaro et al., 2011). An anti‐cancer vaccine named Theratope, comprehending a synthetic STn coupled to the immunogenic carrier keyhole limpet haemocyanin has already been developed (Julien et al., 2009; Miles et al., 2011; Sandmaier et al., 1999). Tests in animal models and humans for breast, ovarian, and colorectal cancers have showed that the antigen is safe and produces a strong immune response against these tumours (Julien et al., 2009, 2012; Miles et al., 2011). Even though Theratope failed to improve overall survival of metastatic breast cancer patients in a phase III clinical study, the design of the study disregarded the heterogeneous STn expression between patients (Miles et al., 2011), compromising the outcome (Julien et al., 2012; Zeichner, 2012). Thus, Theratope or other STn‐based vaccine designs may constitute valuable therapeutic options for STn positive advanced bladder tumours. However, given the low association of STn with more proliferative areas of the tumour, one is led to speculate that advanced stage bladder cancer patients may better benefit from the combination of anti‐STn immunotherapy and anti‐proliferative drugs. Furthermore, these approaches may allow targeting disseminated disease in the adjacent and distant mucosa from the main tumour.
Acknowledgements
This work was supported by Portuguese Foundation for Science and Technology (FCT) Postdoctoral grant SFRH/BPD/66288/2009 (José Alexandre Ferreira), PhD grant SFRH/BD/43399/2008 (Luis Lima), SFRH/BD/81860/2011 (Mariana Silva), SFRH/BD/45120/2008 (Paulo F. Severino) and by LPPC/Pfizer 2011 (Mylene Carrascal). This work was also financially supported by FCT (PTDC‐SAU‐ONC/112511/2009). FCT is co‐financed by European Social Fund (ESF) under Human Potential Operation Programme (POPH) from National Strategic Reference Framework (NSRF).
Ferreira José Alexandre, Videira Paula A., Lima Luís, Pereira Sofia, Silva Mariana, Carrascal Mylène, Severino Paulo F., Fernandes Elisabete, Almeida Andreia, Costa Céu, Vitorino Rui, Amaro Teresina, Oliveira Maria J., Reis Celso A., Dall'Olio Fabio, Amado Francisco, Santos Lúcio Lara, (2013), Overexpression of tumour‐ssociated carbohydrate antigen sialyl‐Tn in advanced bladder tumours, Molecular Oncology, 7, doi: 10.1016/j.molonc.2013.03.001.
Contributor Information
José Alexandre Ferreira, Email: alexandrecastroferreira@gmail.com, Email: jferreira@dq.ua.pt.
Paula A. Videira, Email: paula.videira@fcm.unl.pt
References
- Angata, T. , Tabuchi, Y. , Nakamura, K. , Nakamura, M. , 2007. Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology 17, 838–846. [DOI] [PubMed] [Google Scholar]
- Askeland, E.J. , Newton, M.R. , O'Donnell, M.A. , Luo, Y. , 2012. Bladder cancer immunotherapy: BCG and beyond. Adv. Urol. 2012, 181987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babjuk, M. , Oosterlinck, W. , Sylvester, R. , Kaasinen, E. , Bohle, A. , Palou-Redorta, J. , Roupret, M. , 2012. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Actas Urol. Esp. 36, 389–402. [DOI] [PubMed] [Google Scholar]
- Baldus, S.E. , Zirbes, T.K. , Monig, S.P. , Engel, S. , Monaca, E. , Rafiqpoor, K. , Hanisch, F.G. , Hanski, C. , Thiele, J. , Pichlmaier, H. , Dienes, H.P. , 1998. Histopathological subtypes and prognosis of gastric cancer are correlated with the expression of mucin-associated sialylated antigens: Sialosyl-Lewis(a), Sialosyl-Lewis(x) and sialosyl-Tn. Tumour. Biol. 19, 445–453. [DOI] [PubMed] [Google Scholar]
- Bergeron, A. , Champetier, S. , LaRue, H. , Fradet, Y. , 1996. MAUB is a new mucin antigen associated with bladder cancer. J. Biol. Chem. 271, 6933–6940. [DOI] [PubMed] [Google Scholar]
- Bergeron, A. , LaRue, H. , Fradet, Y. , 1997. Biochemical analysis of a bladder-cancer-associated mucin: structural features and epitope characterization. Biochem. J. 321, (3) 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. , Schlag, P.M. , Karsten, U. , 1997. Immunodetection of epithelial mucin (MUC1, MUC3) and mucin-associated glycotopes (TF, Tn, and sialosyl-Tn) in benign and malignant lesions of colonic epithelium: apolar localization corresponds to malignant transformation. Virchows Arch. 431, 159–166. [DOI] [PubMed] [Google Scholar]
- Clement, M. , Rocher, J. , Loirand, G. , Le, P.J. , 2004. Expression of sialyl-Tn epitopes on beta1 integrin alters epithelial cell phenotype, proliferation and haptotaxis. J. Cell Sci. 117, 5059–5069. [DOI] [PubMed] [Google Scholar]
- Conze, T. , Carvalho, A.S. , Landegren, U. , Almeida, R. , Reis, C.A. , David, L. , Soderberg, O. , 2010. MUC2 mucin is a major carrier of the cancer-associated sialyl-Tn antigen in intestinal metaplasia and gastric carcinomas. Glycobiology 20, 199–206. [DOI] [PubMed] [Google Scholar]
- Dall'Olio, F. , Malagolini, N. , Trinchera, M. , Chiricolo, M. , 2012. Mechanisms of cancer-associated glycosylation changes. Front. Biosci. 17, 670–699. [DOI] [PubMed] [Google Scholar]
- Dall'Olio, F. , Mariani, E. , Tarozzi, A. , Meneghetti, A. , Chiricolo, M. , Lau, J.T. , Facchini, A. , 1997. Expression of beta-galactoside alpha 2,6-sialyltransferase does not alter the susceptibility of human colon cancer cells to NK-mediated cell lysis. Glycobiology 7, 507–513. [DOI] [PubMed] [Google Scholar]
- David, L. , Carneiro, F. , Sobrinho-Simoes, M. , 1996. Sialosyl Tn antigen expression is associated with the prognosis of patients with advanced gastric cancer. Cancer 78, 177–178. [DOI] [PubMed] [Google Scholar]
- Davidson, B. , Berner, A. , Nesland, J.M. , Risberg, B. , Kristensen, G.B. , Trope, C.G. , Bryne, M. , 2000. Carbohydrate antigen expression in primary tumors, metastatic lesions, and serous effusions from patients diagnosed with epithelial ovarian carcinoma: evidence of up-regulated Tn and Sialyl Tn antigen expression in effusions. Hum. Pathol. 31, 1081–1087. [DOI] [PubMed] [Google Scholar]
- Dovedi, S.J. , Davies, B.R. , 2009. Emerging targeted therapies for bladder cancer: a disease waiting for a drug. Cancer Metastasis Rev. 28, 355–367. [DOI] [PubMed] [Google Scholar]
- Ferreira, B. , Marcos, N.T. , David, L. , Nakayama, J. , Reis, C.A. , 2006. Terminal alpha1,4-linked N-acetylglucosamine in Helicobacter pylori-associated intestinal metaplasia of the human stomach and gastric carcinoma cell lines. J. Histochem. Cytochem. 54, 585–591. [DOI] [PubMed] [Google Scholar]
- Gilewski, T.A. , Ragupathi, G. , Dickler, M. , Powell, S. , Bhuta, S. , Panageas, K. , Koganty, R.R. , Chin-Eng, J. , Hudis, C. , Norton, L. , Houghton, A.N. , Livingston, P.O. , 2007. Immunization of high-risk breast cancer patients with clustered sTn-KLH conjugate plus the immunologic adjuvant QS-21. Clin. Cancer Res. 13, 2977–2985. [DOI] [PubMed] [Google Scholar]
- Hakomori, S. , 2001. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv. Exp. Med. Biol. 491, 369–402. [DOI] [PubMed] [Google Scholar]
- Heimburg-Molinaro, J. , Lum, M. , Vijay, G. , Jain, M. , Almogren, A. , Rittenhouse-Olson, K. , 2011. Cancer vaccines and carbohydrate epitopes. Vaccine 29, 8802–8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain, M.H. , Wood, D.P. , Bajorin, D.F. , Bochner, B.H. , Dreicer, R. , Lamm, D.L. , O'Donnell, M.A. , Siefker-Radtke, A.O. , Theodorescu, D. , Dinney, C.P. , 2009. Bladder cancer: narrowing the gap between evidence and practice. J. Clin. Oncol. 27, 5680–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, Y. , Kuwano, H. , Baba, K. , Ikebe, M. , Matushima, T. , Adachi, Y. , Mori, M. , Sugimachi, K. , 1993. Expression of sialyl-Tn antigens in normal squamous epithelium, dysplasia, and squamous cell carcinoma in the esophagus. Cancer Res. 53, 1706–1708. [PubMed] [Google Scholar]
- Inoue, M. , Ogawa, H. , Tanizawa, O. , Kobayashi, Y. , Tsujimoto, M. , Tsujimura, T. , 1991. Immunodetection of sialyl-Tn antigen in normal, hyperplastic and cancerous tissues of the uterine endometrium. Virchows Arch. A Pathol. Anat. Histopathol. 418, 157–162. [DOI] [PubMed] [Google Scholar]
- Itoh, T. , Yonezawa, S. , Nomoto, M. , Ueno, K. , Kim, Y.S. , Sato, E. , 1996. Expression of mucin antigens and Lewis X-related antigens in carcinomas and dysplasia of the pharynx and larynx. Pathol. Int. 46, 646–655. [DOI] [PubMed] [Google Scholar]
- Itzkowitz, S.H. , Yuan, M. , Montgomery, C.K. , Kjeldsen, T. , Takahashi, H.K. , Bigbee, W.L. , Kim, Y.S. , 1989. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 49, 197–204. [PubMed] [Google Scholar]
- Jones, T.D. , Wang, M. , Eble, J.N. , MacLennan, G.T. , Lopez-Beltran, A. , Zhang, S. , Cocco, A. , Cheng, L. , 2005. Molecular evidence supporting field effect in urothelial carcinogenesis. Clin. Cancer Res. 11, 6512–6519. [DOI] [PubMed] [Google Scholar]
- Ju, T. , Lanneau, G.S. , Gautam, T. , Wang, Y. , Xia, B. , Stowell, S.R. , Willard, M.T. , Wang, W. , Xia, J.Y. , Zuna, R.E. , Laszik, Z. , Benbrook, D.M. , Hanigan, M.H. , Cummings, R.D. , 2008. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 68, 1636–1646. [DOI] [PubMed] [Google Scholar]
- Julien, S. , Adriaenssens, E. , Ottenberg, K. , Furlan, A. , Courtand, G. , Vercoutter-Edouart, A.S. , Hanisch, F.G. , Delannoy, P. , Le, B.X. , 2006. ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology 16, 54–64. [DOI] [PubMed] [Google Scholar]
- Julien, S. , Lagadec, C. , Krzewinski-Recchi, M.A. , Courtand, G. , Le, B.X. , Delannoy, P. , 2005. Stable expression of sialyl-Tn antigen in T47-D cells induces a decrease of cell adhesion and an increase of cell migration. Breast Cancer Res. Treat. 90, 77–84. [DOI] [PubMed] [Google Scholar]
- Julien, S. , Picco, G. , Sewell, R. , Vercoutter-Edouart, A.S. , Tarp, M. , Miles, D. , Clausen, H. , Taylor-Papadimitriou, J. , Burchell, J.M. , 2009. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br. J. Cancer 100, 1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien, S. , Videira, P.A. , Delannoy, P. , 2012. Sialyl-Tn in cancer: (how) did we miss the target?. Biomolecules 2, 435–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, G.E. , Bae, H.I. , Park, H.U. , Kuan, S.F. , Crawley, S.C. , Ho, J.J. , Kim, Y.S. , 2002. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology 123, 1052–1060. [DOI] [PubMed] [Google Scholar]
- Kjeldsen, T. , Clausen, H. , Hirohashi, S. , Ogawa, T. , Iijima, H. , Hakomori, S. , 1988. Preparation and characterization of monoclonal antibodies directed to the tumor-associated O-linked sialosyl-2-6 alpha-N-acetylgalactosaminyl (sialosyl-Tn) epitope. Cancer Res. 48, 2214–2220. [PubMed] [Google Scholar]
- Lakshminarayanan, V. , Thompson, P. , Wolfert, M.A. , Buskas, T. , Bradley, J.M. , Pathangey, L.B. , Madsen, C.S. , Cohen, P.A. , Gendler, S.J. , Boons, G.J. , 2012. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc. Natl. Acad. Sci. U. S. A. 109, 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkilde, N.C. , Wolf, H. , Clausen, H. , Kjeldsen, T. , Orntoft, T.F. , 1992. Nuclear volume and expression of T-antigen, sialosyl-Tn-antigen, and Tn-antigen in carcinoma of the human bladder. Relation to tumor recurrence and progression. Cancer 69, 219–227. [DOI] [PubMed] [Google Scholar]
- Leivonen, M. , Nordling, S. , Lundin, J. , von, B.K. , Haglund, C. , 2001. STn and prognosis in breast cancer. Oncology 61, 299–305. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. , Schmittgen, T.D. , 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Marcos, N.T. , Bennett, E.P. , Gomes, J. , Magalhaes, A. , Gomes, C. , David, L. , Dar, I. , Jeanneau, C. , DeFrees, S. , Krustrup, D. , Vogel, L.K. , Kure, E.H. , Burchell, J. , Taylor-Papadimitriou, J. , Clausen, H. , Mandel, U. , Reis, C.A. , 2011. ST6GalNAc-I controls expression of sialyl-Tn antigen in gastrointestinal tissues. Front. Biosci. (Elite. Ed.) 3, 1443–1455. [DOI] [PubMed] [Google Scholar]
- Marcos, N.T. , Pinho, S. , Grandela, C. , Cruz, A. , Samyn-Petit, B. , Harduin-Lepers, A. , Almeida, R. , Silva, F. , Morais, V. , Costa, J. , Kihlberg, J. , Clausen, H. , Reis, C.A. , 2004. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 64, 7050–7057. [DOI] [PubMed] [Google Scholar]
- Margulis, V. , Lotan, Y. , Karakiewicz, P.I. , Fradet, Y. , Ashfaq, R. , Capitanio, U. , Montorsi, F. , Bastian, P.J. , Nielsen, M.E. , Muller, S.C. , Rigaud, J. , Heukamp, L.C. , Netto, G. , Lerner, S.P. , Sagalowsky, A.I. , Shariat, S.F. , 2009. Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J. Natl. Cancer Inst. 101, 114–119. [DOI] [PubMed] [Google Scholar]
- Miles, D. , Roche, H. , Martin, M. , Perren, T.J. , Cameron, D.A. , Glaspy, J. , Dodwell, D. , Parker, J. , Mayordomo, J. , Tres, A. , Murray, J.L. , Ibrahim, N.K. , 2011. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist 16, 1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutsch, L. , Eggenreich, B. , Herwig, E. , Marchetti-Deschmann, M. , Allmaier, G. , Gabor, F. , Wirth, M. , 2012. Lectin bioconjugates trigger urothelial cytoinvasion – a glycotargeted approach for improved intravesical drug delivery. Eur. J. Pharm. Biopharm. 82, 367–375. [DOI] [PubMed] [Google Scholar]
- Numa, F. , Tsunaga, N. , Michioka, T. , Nawata, S. , Ogata, H. , Kato, H. , 1995. Tissue expression of Sialyl Tn antigen in gynecologic tumors. J. Obstet. Gynaecol. (Tokyo 1995) 21, 385–389. [DOI] [PubMed] [Google Scholar]
- Ogata, S. , Koganty, R. , Reddish, M. , Longenecker, B.M. , Chen, A. , Perez, C. , Itzkowitz, S.H. , 1998. Different modes of sialyl-Tn expression during malignant transformation of human colonic mucosa. Glycoconj. J. 15, 29–35. [DOI] [PubMed] [Google Scholar]
- Ohno, S. , Ohno, Y. , Nakada, H. , Suzuki, N. , Soma, G. , Inoue, M. , 2006. Expression of Tn and sialyl-Tn antigens in endometrial cancer: its relationship with tumor-produced cyclooxygenase-2, tumor-infiltrated lymphocytes and patient prognosis. Anticancer Res. 26, 4047–4053. [PubMed] [Google Scholar]
- Ozaki, H. , Matsuzaki, H. , Ando, H. , Kaji, H. , Nakanishi, H. , Ikehara, Y. , Narimatsu, H. , 2012. Enhancement of metastatic ability by ectopic expression of ST6GalNAcI on a gastric cancer cell line in a mouse model. Clin. Exp. Metastasis 29, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeira, C. , Lameiras, C. , Amaro, T. , Lima, L. , Koch, A. , Lopes, C. , Oliveira, P.A. , Santos, L. , 2011. CIS is a surrogate marker of genetic instability and field carcinogenesis in the urothelial mucosa. Urol. Oncol. 29, 205–211. [DOI] [PubMed] [Google Scholar]
- Pinho, S. , Marcos, N.T. , Ferreira, B. , Carvalho, A.S. , Oliveira, M.J. , Santos-Silva, F. , Harduin-Lepers, A. , Reis, C.A. , 2007. Biological significance of cancer-associated sialyl-Tn antigen: modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 249, 157–170. [DOI] [PubMed] [Google Scholar]
- Pinto, R. , Carvalho, A.S. , Conze, T. , Magalhaes, A. , Picco, G. , Burchell, J.M. , Taylor-Papadimitriou, J. , Reis, C.A. , Almeida, R. , Mandel, U. , Clausen, H. , Soderberg, O. , David, L. , 2012. Identification of new cancer biomarkers based on aberrant mucin glycoforms by in situ proximity ligation. J. Cell Mol. Med. 16, 1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploeg, M. , Aben, K.K. , Kiemeney, L.A. , 2009. The present and future burden of urinary bladder cancer in the world. World J. Urol. 27, 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis, C.A. , Osorio, H. , Silva, L. , Gomes, C. , David, L. , 2010. Alterations in glycosylation as biomarkers for cancer detection. J. Clin. Pathol. 63, 322–329. [DOI] [PubMed] [Google Scholar]
- Ryan, S.O. , Turner, M.S. , Gariepy, J. , Finn, O.J. , 2010. Tumor antigen epitopes interpreted by the immune system as self or abnormal-self differentially affect cancer vaccine responses. Cancer Res. 70, 5788–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmaier, B.M. , Oparin, D.V. , Holmberg, L.A. , Reddish, M.A. , MacLean, G.D. , Longenecker, B.M. , 1999. Evidence of a cellular immune response against sialyl-Tn in breast and ovarian cancer patients after high-dose chemotherapy, stem cell rescue, and immunization with Theratope STn-KLH cancer vaccine. J. Immunother. 22, 54–66. [DOI] [PubMed] [Google Scholar]
- Santos, L. , Amaro, T. , Costa, C. , Pereira, S. , Bento, M.J. , Lopes, P. , Oliveira, J. , Criado, B. , Lopes, C. , 2003. Ki-67 index enhances the prognostic accuracy of the urothelial superficial bladder carcinoma risk group classification. Int. J. Cancer 105, 267–272. [DOI] [PubMed] [Google Scholar]
- Schmitt, F.C. , Figueiredo, P. , Lacerda, M. , 1995. Simple mucin-type carbohydrate antigens (T, sialosyl-T, Tn and sialosyl-Tn) in breast carcinogenesis. Virchows Arch. 427, 251–258. [DOI] [PubMed] [Google Scholar]
- Sewell, R. , Backstrom, M. , Dalziel, M. , Gschmeissner, S. , Karlsson, H. , Noll, T. , Gatgens, J. , Clausen, H. , Hansson, G.C. , Burchell, J. , Taylor-Papadimitriou, J. , 2006. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J. Biol. Chem. 281, 3586–3594. [DOI] [PubMed] [Google Scholar]
- Sievert, K.D. , Amend, B. , Nagele, U. , Schilling, D. , Bedke, J. , Horstmann, M. , Hennenlotter, J. , Kruck, S. , Stenzl, A. , 2009. Economic aspects of bladder cancer: what are the benefits and costs?. World J. Urol. 27, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen, A.L. , Reis, C.A. , Tarp, M.A. , Mandel, U. , Ramachandran, K. , Sankaranarayanan, V. , Schwientek, T. , Graham, R. , Taylor-Papadimitriou, J. , Hollingsworth, M.A. , Burchell, J. , Clausen, H. , 2006. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology 16, 96–107. [DOI] [PubMed] [Google Scholar]
- Terashima, S. , Takano, Y. , Ohori, T. , Kanno, T. , Kimura, T. , Motoki, R. , Kawaguchi, T. , 1998. Sialyl-Tn antigen as a useful predictor of poor prognosis in patients with advanced stomach cancer. Surg. Today 28, 682–686. [DOI] [PubMed] [Google Scholar]
- van der Velden, V.H. , Hochhaus, A. , Cazzaniga, G. , Szczepanski, T. , Gabert, J. , van Dongen, J.J. , 2003. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia 17, 1013–1034. [DOI] [PubMed] [Google Scholar]
- van Rhijn, B.W. , Burger, M. , Lotan, Y. , Solsona, E. , Stief, C.G. , Sylvester, R.J. , Witjes, J.A. , Zlotta, A.R. , 2009. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur. Urol. 56, 430–442. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin, C. , Cuevas, E. , Gil-Martin, E. , Fernandez-Briera, A. , 2004. Correlation analysis between tumor-associated antigen sialyl-Tn expression and ST6GalNAc I activity in human colon adenocarcinoma. Oncology 67, 159–165. [DOI] [PubMed] [Google Scholar]
- Videira, P.A. , Calais, F.M. , Correia, M. , Ligeiro, D. , Crespo, H.J. , Calais, F. , Trindade, H. , 2009. Efficacy of bacille Calmette-Guerin immunotherapy predicted by expression of antigen-presenting molecules and chemokines. Urology 74, 944–950. [DOI] [PubMed] [Google Scholar]
- Videira, P.A. , Correia, M. , Malagolini, N. , Crespo, H.J. , Ligeiro, D. , Calais, F.M. , Trindade, H. , Dall'Olio, F. , 2009. ST3Gal.I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer 9, 357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira, P.A. , Ligeiro, D. , Correia, M. , Trindade, H. , 2007. Gene expression analysis in superficial bladder cancer: comparison of two suitable endogenous reference genes. Curr. Urol. 1, 145–150. [Google Scholar]
- Yamamoto, M. , Yamamoto, F. , Luong, T.T. , Williams, T. , Kominato, Y. , 2003. Expression profiling of 68 glycosyltransferase genes in 27 different human tissues by the systematic multiplex reverse transcription-polymerase chain reaction method revealed clustering of sexually related tissues in hierarchical clustering algorithm analysis. Electrophoresis 24, 2295–2307. [DOI] [PubMed] [Google Scholar]
- Yates, D.R. , Roupret, M. , 2011. Contemporary management of patients with high-risk non-muscle-invasive bladder cancer who fail intravesical BCG therapy. World J. Urol. 29, 415–422. [DOI] [PubMed] [Google Scholar]
- Zeichner, S.B. , 2012. The failed Theratope vaccine: 10 years later. J. Am. Osteopath. Assoc. 112, 482–483. [PubMed] [Google Scholar]


