Abstract
Background
Enumeration of circulating tumor cells (CTC) from whole blood permits monitoring of patients with breast carcinoma. Analysis of apoptosis & Bcl‐2 expression in CTC might add additional prognostic and predictive information. We estimated the degree of these markers in CTC from patients being treated for metastatic breast cancer.
Methods
Eighty‐three evaluable patients initiating a new therapy for metastatic breast cancer were enrolled. Whole blood was collected at baseline, at one of three short term time windows (24, 48, or 72 h) after initiating treatment, and at first follow‐up (3–5 weeks). CTC were isolated, enumerated, and expression of M30 and Bcl2 was determined using the CellSearch® System.
Results
At baseline, window, and 3–5 weeks post‐treatment, 41/80 (51%), 40/80 (50%) and 21/75 (28%) patients had ≥5 CTC, respectively. At baseline, the proportion of CTC‐apoptosis (M30) was inversely correlated with CTC number, and modestly inversely correlated with CTC‐Bcl‐2. As expected, higher CTC levels at baseline or first follow‐up were associated with worse prognosis. Surprisingly, in patients with elevated CTC, higher levels of CTC‐apoptosis were associated with worse prognosis, while higher CTC‐Bcl‐2 levels correlated with better outcomes.
Conclusions
CTC apoptosis and expression of Bcl‐2 can be analytically determined in patients with metastatic breast cancer and may have biological and clinical implications. Characterization of CTC for these and other markers could further increase the utility of CTC monitoring patients in clinical investigations of new anti‐neoplastic agents.
Keywords: Circulating tumor Cells, Breast cancer, Apoptosis, Bcl-2
Highlights
-
►
Apoptosis and Bcl‐2 were evaluated on CTC from patients with metastatic breast cancer.
-
►
40% and 60% of CTC undergo apoptosis and expressed Bcl‐2 at baseline.
-
►
CTC levels at baseline were inversely related to degree of apoptosis.
-
►
No relationship was noted between CTC levels and Bcl‐2 expression.
-
►
CTC‐phenotyping may predict clinical outcome beyond just enumeration of CTC.
Abbreviations
- CTC
Circulating Tumor Cells
- FISH
Fluorescent in situ hybridization
- PD
progressive disease
- PFS
progression free survival
- OS
overall survival
1. Introduction
Recent technological advances have provided the means to monitor circulating tumor cells (CTC) isolated from whole blood from patients with epithelial malignancies (Hayes and Smerage, 2008). CTC have been detected with a commercially available, automated rare cell detection system (CellSearch® System; Veridex, LLC. Raritan NJ) in approximately 30–50% of patients with metastatic colon, prostate and/or breast cancer (Cohen et al., 2009; Cristofanilli et al., 2004; de Bono et al., 2008). In each disease, elevated CTC levels at baseline are associated with shorter progression free and overall survival. In metastatic breast cancer, persistently elevated CTC levels (≥5 CTC/7.5 ml whole blood) after one cycle of treatment are associated with a poor prognosis (Cristofanilli et al., 2004), while a reduction in CTC levels indicates improved prognosis. Furthermore, elevation of CTC levels at any time during follow‐up predicts a high risk of subsequent progression. Furthermore, change in CTC levels is a more accurate indicator of prognosis than independent review of radiographic evidence of response (Budd et al., 2006; Hayes et al., 2006).
Not all patients with detectable CTC have a poor prognosis, suggesting that further characterization of CTC might provide even more specific information regarding their biologic, and clinical, significance (Cristofanilli et al., 2004). In addition, serial characterization of CTC might reflect the molecular evolution of cancer throughout its progression and thus represent a “real‐time,” non‐invasive “virtual” biopsy. Thus, characterization of clinically important biomarkers expressed by CTC is of biological and clinical interest (Fehm et al., 2010; Hayes et al., 2002; Meng et al., 2004b, 2006; Riethdorf et al., 2010).
Apoptosis (programmed cell death) within a tumor, before or as a function of therapy, might predict benefit from therapeutic agents (Russo et al., 2006). In this regard, early apoptosis can be detected via the monoclonal antibody (M30) directed against a neo‐epitope of cytokeratin 18 disclosed by caspase cleavage (Leers et al., 1999; Rossi et al., 2010). In a complementary fashion, the analysis of the anti‐apoptotic B‐cell lymphoma protein 2 (Bcl‐2) might also predict response to selected endocrine and chemotherapies (Callagy et al., 2008).
In this pilot clinical study, we investigated whether CTC apoptosis and Bcl‐2 status could be determined at baseline, within 24–72 h, and at first follow‐up (∼3–5 weeks) after initiation of a new therapy in patients with metastatic breast cancer.
2. Methods
We report this study according to the REMARK guidelines (see Figure 1 for REMARK diagram) (McShane et al., 2005).
Figure 1.
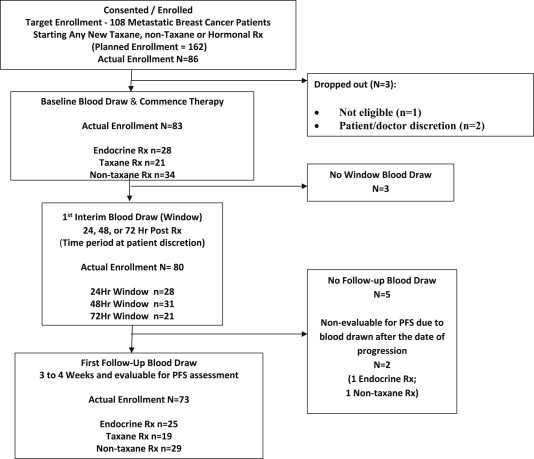
REMARK diagram of patient flow in the clinical study.
2.1. Study design and objectives
This was a prospective double‐blind multi‐center pilot study approved by the University of Michigan and Cleveland Clinic Institutional Review Boards. The primary objective of this study was to determine the feasibility of detecting apoptosis and Bcl‐2 expression in CTC at various time intervals (baseline, 24 h, 48 h, 72 h, and 3–4 weeks) in metastatic breast cancer patients starting a new endocrine or chemotherapy (taxane or non‐taxane‐based). Secondary objectives were designed to investigate the hypotheses that positive M30/apoptosis CTC staining correlated with positive clinical outcomes while positive Bcl‐2 CTC staining was associated with negative outcomes. We also proposed to correlate Bcl‐2 and M30 expression in CTCs with expression in tumor biopsy specimens, which will be reported in a separate manuscript.
2.2. Patient selection
All patients provided written informed consent prior to entry. Eligibility was limited to patients with progressive metastatic breast cancer and an ECOG status of 0–2 starting a new therapeutic regimen.
We designed this trial to address several hypotheses: 1) that apoptosis and Bcl‐2 could be successfully determined on CTC collected from patients with metastatic breast cancer; 2) that CTC levels, and apoptotic states, would change rapidly within the first 24–72 h after initiation of therapy; 3) that these early changes would differ according to systemic treatment type (endocrine therapy, taxane‐based or non‐taxane‐based chemotherapy); and 4) that either at baseline or at early follow‐up windows these markers might have different predictive roles regarding outcomes, in patients treated with different types of systemic therapies.
Accordingly, patients were assigned to a treatment cohort depending on their independently prescribed therapeutic regimen i.e., endocrine therapy (of any type), taxane‐based or non‐taxane‐based chemotherapeutic regimen. Endocrine therapies included tamoxifen, fulvestrant, megestrol acetate, or an aromatase inhibitor (anastrozole, exemestane, letrozole). Non‐taxane chemotherapies included capecitabine, carboplatin, cyclophosphamide, doxorubicin, etoposide, 5‐FU, gemcitabine, ixabepilone, oxaliplatin, or vinorelbine, given as single agents or in combination. Taxane‐based regimens included either paclitaxel, nanoalbumin bound‐paclitaxel, or docetaxel, alone or with other agents.
Our preclinical studies suggested that in vitro taxane‐induced apoptosis of cultured human breast cancer cell lines occurs quite early (12–24 h) (data not shown). Therefore, we addressed whether we could identify a rapid increase in CTC‐M30 (indicating apoptosis) after initiation of therapy. Patients elected to return for CTC testing within 24 h, 24–48 h, or 48–72 h (designated “window” time point) after administration of the first dose of the new regimen. Patient entry into each of these cohorts was based on their clinical situation and their preference and was not randomly assigned. When a specific window cohort was filled, accrual into that cohort stopped. Patients were then encouraged to select one of the remaining time windows available. Patient flow on the study is illustrated in Figure 1.
Original protocol‐planned accrual called for 9 patients with elevated CTC (≥5 CTC/7.5 ml whole blood) in each time/window cohort. Previous studies (Budd et al., 2006; Cristofanilli et al., 2004; Hayes et al., 2006) suggested that approximately 50% of patients in each cohort should have elevated CTC. Thus, we estimated that a minimum of 18 patients would be needed for each of nine treatment/window cohorts (total planned accrual = 162 patients). However, enrollment was suspended at n = 86 due to slower than expected accrual (Figure 1).
2.3. Patient staging and follow‐up
Prior to starting a new systemic treatment, all patients had CT and/or MRI scans of the chest and abdomen and a whole body scintigraphic bone scan. Follow‐up clinical evaluation was performed at day 1 of each subsequent cycle for at least three cycles. Cycle length was determined by the treating physician according to the type of treatment the patient received (endocrine therapy was generally considered a 4 week cycle, and chemotherapy was administered on either three or four week cycles), which was most commonly 3–4 weeks long. Clinicians were blinded to CTC results and to the independent assessment of radiographs, and all decisions regarding patient care were made using standard clinical and radiographic evaluation performed at the clinical sites.
2.4. Blood draw and processing
Blood collections were scheduled at baseline, window (24–72 h after the induction of therapy) and first follow‐up (∼3–5 weeks) time periods for enumeration of CTC.
2.5. CTC enumeration and characterization
At each time point, approximately 30 mL of whole blood were collected by drawing approximately 8 mL specimens into evacuated 10 mL blood draw tubes containing an anti‐coagulant and a cellular preservative (CellSave Preservative Tubes, Veridex, LLC. Raritan NJ). Specimens were maintained at room temperature, and processed within 96 h of collection. Upon receipt in the laboratory, the blood was pooled and divided into three aliquots of 7.5 mL. One aliquot was used for CTC enumeration + apoptosis (M30) expression (“apoptosis aliquot”) one for CTC enumeration + Bcl‐2 expression (“Bcl‐2 aliquot”), and one for CTC enumeration alone (“enumeration aliquot”), in that order of importance when an insufficient volume of blood was available for testing of all three.
CTC enumeration was performed using the CellSearch® System, which has been described previously (Allard et al., 2003, 2004; Cristofanilli et al., 2004). The CellSearch® CXC Kit (Veridex, LLC. Raritan NJ) was used for the enrichment and staining of CTC. The CXC kit differs from the CellSearch® Epithelial kit by the replacement of Cytokeratin 8,18, 19 conjugated to phycoerythrin (PE) with a fluorescein (FITC) conjugate. This change enables the subsequent characterization of user‐defined markers that appear at a relatively low antigen density (∼50,000 antigens/cell). Apoptosis in the CTC was evaluated using a monoclonal antibody M30 (Peviva, Stockholm, Sweden) (Hagg et al., 2002; Leers et al., 1999) and Bcl‐2 by monoclonal antibody Clone BCL‐2/100 (BD Pharmingen, San Diego, USA). Both antibodies were conjugated to phycoerythrin (PE).
Positive and background controls for the M30‐PE marker consisted of normal blood spiked with apoptotic MCF7 cells (MCF‐7 apo cells). After seven days in culture, permitting overgrowth, cells floating in the supernatant were harvested. These cells were fixed in 0.3% paraformaldehyde and subjected to flow cytometry to monitor time‐dependent increasing M30 and decreasing full‐length cytokeratin expression, consistent with cleavage of full‐length cytokeratin 18 and appearance of the M30 neoantigen (Carr, 2000; Rossi et al., 2010). The results were compared to flow cytometry for M30 and cytokeratin 18 analysis of the adherent cells in the same culture. Cytoplasmic M30 expression that occurred in cells that were observed to have a 50% decrease of cytokeratin 18 compared to non‐apoptotic adherent cells was chosen to signify truly apoptotic cells. Cytoplasmic M30 staining was observed in 50–70% of the cells produced in this process, and this level of M30 expression was used as a comparator for patient‐derived CTC‐M30 evaluation. Figure 2 illustrates CTC captured from blood collected from a patient with metastatic breast cancer that have been processed in the CellSearch® System for enumeration and M30 (Figure 2A) and Bcl‐2 (Figure 2B) staining. Patient derived CTC‐M30 evaluation was considered bi‐categorical: Positive or Negative.
Figure 2.
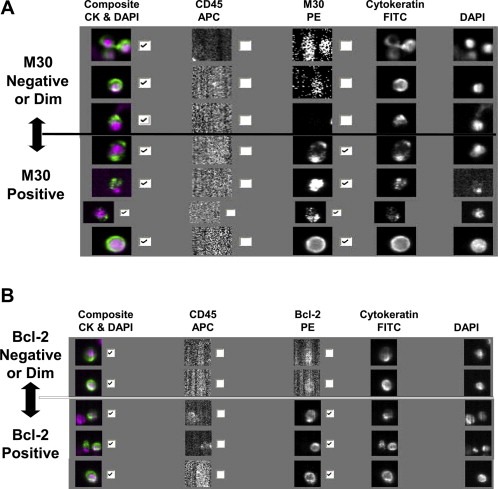
Composite figures of CTC analysis by CellSearch® System, illustrating the appearance of M30 (A) and Bcl‐2 (B) negative and positive CTC within a single blood specimen drawn from a patient with metastatic breast cancer. Check marks beside the composite represent cytokeratin positive, CD45 negative, nucleated cells, representing CTC. Check marks beside the composite for M30 and Bcl‐2 represent CTC that are positive for M30 (Figure 2A) or Bcl‐2 (Figure 2B). Smudged events in the unchecked frames represent cytokeratin positive, CD45 negative, nucleated cells that are negative for the respective markers.
To ensure that the fixative in the CellSearch® vacutainer tubes does not induce apoptosis, cultured MCF‐7 cells were harvested before confluence and spiked into either CellSave tubes or into 10 ml vacutainer tubes containing EDTA, and then processed immediately (time 0) or after 24, 48, 72, and 96 h at room temperature using the CellSearch® system. The positive control MCF‐7 apo cells were similarly prepared and processed.
For cells spiked into CellSave tubes, at time 0, 0.9% of the wild‐type MCF‐7 cells were positive for M30, compared to 53.3% of the MCF‐7 apo cells. At 24, 48, 72, and 96 h, 0.5%, 0.5%, 0.5%, and 0.9% of the wild type MCF‐7 cells were positive for M30, respectively, compared to 53.6%, 40.5%, 44%, 32.6%, and 41.4%, respectively, for the MCF‐7 apo cells. For cells spiked into EDTA‐containing tubes without fixative, 0.7% and 53.3% of the wild‐type and MCF‐7 apo cells, respectively, were positive for M‐30 at time 0. These data confirm that CellSave fixative does not induce apoptosis, and they support the use of CellSave tubes for blood collection in this and other studies of CTC‐M30.
Results from one or more aliquots at each of the different time points were not evaluable for several reasons, such as insufficient blood draw volume, shipping delays, technical failures and specimen irregularities. Anticipating such issues, it was predetermined that if there was insufficient blood volume, the enumeration alone aliquot was not evaluated, and priority was given to the phenotype aliquots (CTC‐M30, CTC‐Bcl‐2).
2.6. Statistical analysis
Descriptive statistics and/or frequency distributions were calculated for demographic and baseline clinical characteristics. Derivation of the threshold level (≥5 CTC per 7.5 mL of blood) for treatment decisions was described previously (Cristofanilli et al., 2004). Significance of trends in percentages of apoptotic and/or Bcl‐2 positive cells by various CTC groupings were tested using a non‐parametric test for trend across ordered groups. Median apoptosis and Bcl2 levels were compared using a non‐parametric two‐sample test on the equality of medians where the null hypothesis is that the k samples were drawn from populations with the same median. The chi‐squared statistic with a continuity correction was determined. The p‐values were not adjusted for multiple comparisons. Progression free survival (PFS) was defined as the interval from the date the CTC sample was drawn to the date of disease progression, death, or last follow‐up, whichever occurred first. Overall survival (OS) was determined from the date the CTC sample was drawn to the date of death or the date of last contact with the patient. Any CTC blood samples drawn after the date of progressive disease (PD) were excluded from the PFS analyzes. Separate Kaplan–Meier survival plots were generated based on CTC levels at baseline and follow‐up blood collections. Survival curves were compared using log‐rank testing. Cox proportional hazards regression was used to determine univariate and multivariate hazards ratios for PFS and OS.
3. Results
3.1. Patient demographics and CTC levels at baseline
Eighty‐six (86) patients were enrolled (16 at the Cleveland Clinic and 70 at the University of Michigan) between May 2005 and March 2008 (Figure 1). Three patients were not evaluable: one was not eligible, one withdrew prior to blood draw, and one had no follow‐up information beyond baseline blood draw. Table 1 provides demographic information for the 83 evaluable patients and shows the proportion of patients with ≥5 CTC in each of the three different aliquots at each time point (see Methods, Section 2.6: apoptosis aliquot, Bcl2 aliquot, enumeration aliquot). Because of various, anticipated specimen loss (see Methods), CTCs were evaluable at baseline in 66 (80%), 80 (96%), and 75 (90%) of the enumeration, apoptosis, and Bcl‐2 aliquots, respectively (Table 1).
Table 1.
Patient demographics and CTC levels.
| Characteristic (N = 83) | N (%) | N (%) with ≥5 CTC/7.5 mL at baseline | ||
|---|---|---|---|---|
| Enumeration aliquot | Apoptosis aliquot | Bcl‐2 aliquot | ||
| (N = 66) | (N = 80) | (N = 75) | ||
| All patients | – | 38 (58%) | 41 (51%) | 36 (48%) |
| Molecular phenotype of primary tumor | ||||
| ER+ and/or PR+ and Her2− | 45 (54%) | 22 (63%) | 26 (59%) | 21 (54%) |
| ER− and PR− and Her2− | 13 (16%) | 6 (60%) | 8 (62%) | 8 (62%) |
| Her2+a | 13 (16%) | 5 (50%) | 3 (27%) | 4 (36%) |
| Unknown | 12 (14%) | 5 (45%) | 4 (33%) | 3 (25%) |
| Fisher's exact p‐value | 0.734 | 0.136 | 0.208 | |
| Sites of metastasis | ||||
| Single site | 27 (33%) | 9 (43%) | 12 (46%) | 10 (42%) |
| Two sites | 33 (40%) | 17 (68%) | 19 (59%) | 15 (52%) |
| Three sites | 16 (19%) | 10 (67%) | 8 (50%) | 9 (56%) |
| Four or more sites | 7 (8%) | 2 (40%) | 2 (33%) | 2 (33%) |
| Fisher's exact p‐value | 0.269 | 0.577 | 0.708 | |
| Bone | 62 (75%) | 35 (70%) | 39 (66%) | 35 (64%) |
| Liver | 30 (36%) | 15 (60%) | 14 (48%) | 13 (45%) |
| Lung | 26 (31%) | 9 (41%) | 9 (36%) | 10 (42%) |
| Brain | 8 (10%) | 2 (33%) | 3 (38%) | 3 (38%) |
| Bone only | 20 (24%) | 9 (56%) | 12 (63%) | 10 (59%) |
| # of years between primary & metastatic diagnoses | ||||
| ≥2.0 Years | 50 (60%) | 21 (51%) | 21 (45%) | 19 (43%) |
| <=1.9 Years | 33 (40%) | 17 (68%) | 20 (61%) | 17 (55%) |
| Fisher's exact p‐value | 0.208 | 0.18 | 0.356 | |
| Treatment group | ||||
| Hormonal therapy | 28 (34%) | 11 (52%) | 13 (46%) | 11 (44%) |
| Non‐taxane therapy | 34 (41%) | 16 (62%) | 17 (53%) | 15 (50%) |
| Taxane therapy | 21 (25%) | 11 (58%) | 11 (55%) | 10 (50%) |
| Fisher's exact p‐value | 0.78 | 0.882 | 0.916 | |
| Line of therapy | ||||
| 1st line | 27 (33%) | 11 (58%) | 11 (46%) | 9 (38%) |
| 2nd line | 24 (29%) | 11 (58%) | 13 (54%) | 12 (57%) |
| 3rd or higher line | 32 (38%) | 16 (57%) | 17 (53%) | 15 (50%) |
| Fisher's exact p‐value | 1 | 0.883 | 0.398 | |
HER2+ = immunohistochemistry 3+or FISH >2.0.
Consistent with previous reports (Cristofanilli et al., 2004; Liu et al., 2009), approximately 50% of patients with metastatic breast cancer beginning a new treatment had elevated CTC (≥5 CTC/7.5 mL of whole blood). As also previously reported (Cristofanilli et al., 2004; Liu et al., 2009), no biological or clinical subgroup was more or less likely to have elevated CTC at baseline (Table 1).
3.2. CTC changes with treatment and correlation with clinical outcome
Table 2 provides the number of evaluable samples at each of the time points in all patients as well in each of the three treatment cohorts for the CTC enumeration (2A), apoptosis (2B) and Bcl‐2 (2C) aliquots separately. This table also shows the proportion of evaluable samples with ≥5 CTC. The largest number of patients had evaluable data from their apoptosis aliquot (Table 2B), thus, the data from the apoptosis aliquot was used for the comparisons described in this section. At baseline, 46%, 55%, and 53% of patients in the endocrine, taxane and non‐taxane cohorts, respectively, had elevated CTC levels (p = 0.88) (Table 2B). For all patients, 40 of 80 (50%) patients at the window time point had elevated CTC, which did not significantly differ from baseline (Table 2B). Because there were no significant differences between the proportion of patients with elevated CTC between the baseline and window draws, no correlations between CTC levels at 24, 48, or 72 h and progression free survival (PFS) were performed.
Table 2.
Patient cohorts and CTC levels.
| Time point | All patients | Endocrine therapy | Taxane therapy | Non‐taxane therapy |
|---|---|---|---|---|
| A. | # (%) patients with ≥5 CTC enumeration aliquot (CTC) | |||
| Baseline | 38/66 (58%) | 11/21 (52%) | 11/19 (58%) | 16/26 (62%) |
| 24 h | 13/25 (52%) | 2/7 (29%) | 4/7 (57%) | 7/11 (64%) |
| 48 h | 12/27 (44%) | 3/6 (50%) | 3/9 (33%) | 6/12 (50%) |
| 72 h | 10/19 (53%) | 5/8 (63%) | 2/3 (67%) | 3/8 (38%) |
| 24, 48, or 72 h | 35/71 (49%) | 10/21 (48%) | 9/19 (47%) | 16/31 (52%) |
| 1st follow‐up | 18/61 (30%) | 3/16 (19%) | 6/17 (35%) | 9/28 (32%) |
| B. | # (%) patients with ≥5 CTC apoptosis aliquot (M30) | |||
| Baseline | 41/80 (51%) | 13/28 (46%) | 11/20 (55%) | 17/32 (53%) |
| 24 h | 13/28 (46%) | 3/10 (30%) | 4/7 (57%) | 6/11 (55%) |
| 48 h | 16/31 (52%) | 4/9 (44%) | 5/9 (56%) | 7/13 (54%) |
| 72 h | 11/21 (52%) | 5/8 (63%) | 2/3 (67%) | 4/10 (40%) |
| 24, 48, or 72 h | 40/80 (50%) | 12/27 (44%) | 11/19 (58%) | 17/34 (50%) |
| 1st follow‐up | 21/75 (28%) | 9/26 (35%) | 6/19 (32%) | 6/30 (20%) |
| C. | # (%) patients with ≥5 CTC Bcl‐2 aliquot | |||
| Baseline | 36/75 (48%) | 11/25 (44%) | 10/20 (50%) | 15/30 (50%) |
| 24 h | 12/26 (46%) | 2/10 (20%) | 5/6 (83%) | 5/10 (50%) |
| 48 h | 11/28 (39%) | 4/9 (44%) | 2/7 (29%) | 5/12 (42%) |
| 72 h | 10/19 (53%) | 5/8 (63%) | 2/3 (67%) | 3/8 (38%) |
| 24, 48, or 72 h | 33/73 (45%) | 11/27 (41%) | 9/16 (56%) | 13/30 (43%) |
| 1st follow‐up | 21/69 (30%) | 9/25 (36%) | 4/16 (25%) | 8/28 (29%) |
Only 21 of 73 (27%) (2 patients progressed prior to first follow‐up blood draw) had elevated CTC at first follow‐up. The observed reduction in the proportion of patients with elevated CTC compared to baseline is consistent with prior studies (Cristofanilli et al., 2004; Liu et al., 2009) and strongly suggests response to systemic therapy (Table 2B). As expected (Cristofanilli et al., 2004; Liu et al., 2009), PFS for those patients with elevated CTC levels at first follow‐up was significantly shorter compared to patients who did not have elevated CTC (median PFS for elevated vs. not: 3.4 months vs. 6.4 months, p = 0.0097) (Figure 3).
Figure 3.
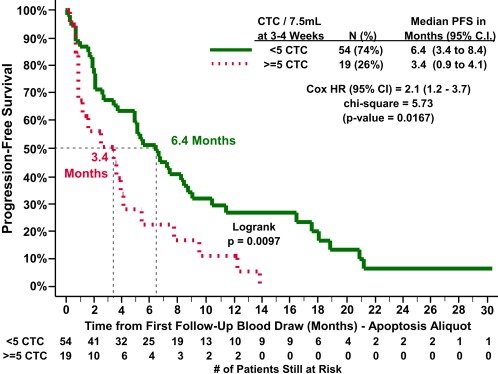
PFS according to CTC enumeration as determined in apoptosis aliquot at 1st follow‐up (∼3–5 weeks after the initiation of therapy) in 73 metastatic breast cancer patients who had blood drawn prior to evidence of progression. (solid line: CTC <5/7.5 ml whole blood; dotted line: ≥5 CTC/7.5 ml whole blood).
3.3. CTC apoptosis and Bcl‐2 phenotype
CTC apoptotic and Bcl‐2 status were determined at baseline, window, and first follow‐up (Table 3). Apoptosis was present in an average of 42% (median = 35%) of the CTCs in the 41 evaluable patients with ≥5 CTC at baseline compared to an average of 49% (median = 54%) and 41% (median = 38%) of CTC in the 40 and 21 patients with ≥5 CTC at window and first follow‐up, respectively (t‐test p‐values = 0.2791 and 0.9071, median p‐values = 0.318 and 0.788, respectively).
Table 3.
CTC phenotypes for apoptosis and Bcl‐2 according to time cohorts.
| M30 CTC sample | BCL2 CTC sample | ||||
|---|---|---|---|---|---|
| # (%) of patients with ≥5 CTC | % CTC M30+ | # (%) of patients with ≥5 CTC | % CTC Bcl‐2+ | ||
| Average | Median | Average | Median | ||
| Baseline | |||||
| 41/80 (51%) | 42% | 35% | 36/75 (48%) | 62% | 65% |
| Post‐treatment window (24, 48, or 72 h) | |||||
| 40/80 (50%) | 49% | 54% | 33/73 (45%) | 64% | 70% |
| Compared to baseline: | 0.2791* | 0.318** | Compared to baseline | 0.7889* | 0.402** |
| First follow‐up (3–4 weeks) | |||||
| 21/75 (28%) | 41% | 38% | 21/69 (30%) | 63% | 77% |
| Compared to baseline | 0.9071* | 0.788** | Compared to baseline | 0.8575* | 0.707** |
Two‐sample t‐test with equal variance p‐value.
Non‐parametric test for equality of medians p‐value.
An average of 62% (median = 65%) of CTCs were positive for Bcl‐2 expression in the 36 evaluable patients with ≥5 CTC at baseline compared to 64% (median = 70%) and 63% (median = 77%) of CTC in the 33 and 21 patients with ≥5 CTC at window and first follow‐up, respectively (t‐test p‐values = 0.7889 and 0.8575, median p‐values = 0.402 and 0.707, respectively). For all treatment cohorts combined, M30 positivity at baseline, an indication of apoptosis, was significantly inversely associated with higher numbers of CTC (Figure 4A; non‐parametric test for trend across ordered groups p < 0.001). Likewise, at window and first follow‐up evaluations, the percentage of cells positive for M30 was lower in patients with higher numbers of CTC, although these observations were not statistically significant (Table 3 and data not shown). These data suggest that as a result of biological forces (such as anoikis or treatment related phenomena), CTC may commence apoptosis before, at the time of extravasation, or during migration.
Figure 4.
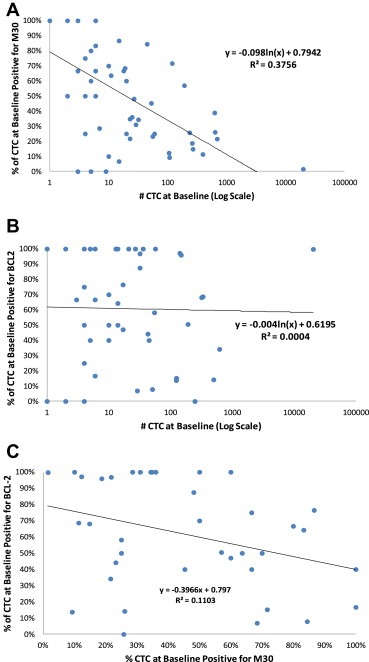
Association of CTC phenotypes and CTC levels at baseline and with each other. A. Association of apoptosis, analyzed by M30 expression, and number of CTC/7.5 ml whole blood in baseline blood draw of patients with ≥1 CTC at baseline. B. Association of Bcl‐2 and number of CTC/7.5 ml whole blood in baseline blood draw of patients with ≥1 CTC at baseline. C. Association of Bcl‐2 and apoptosis (analyzed by M30 expression) on CTC in baseline blood draw of patients with an average between aliquot tubes of ≥5 CTC at baseline.
Bcl‐2 was not associated with number of cells at baseline (Figure 4B; non‐parametric test for trend across ordered groups p = 0.415), window, or first follow‐up evaluations (Table 3 and data not shown). For patients with elevated CTC at baseline (an average of ≥5 CTC in both the Bcl‐2 and apoptosis aliquots), there was a weak, inverse relationship between the proportions of CTC positive for Bcl‐2 and apoptosis (Figure 4C, Pearson's correlation p = 0.04).
Table 4 provides CTC‐apoptosis and Bcl‐2 status by treatment cohorts at baseline, window, and first follow‐up. Treatment cohorts were too small to perform meaningful outcomes analyzes of M30 or Bcl‐2 staining, but the average proportion of CTC that were apoptotic in patients with ≥5 CTC did not change significantly from baseline to window or first follow‐up: 42%, 49%, and 41%, respectively. Likewise, the average proportion of CTC that were Bcl‐2 positive in patients with ≥5 CTC was unchanged at the three time‐points (62%, 64%, and 63%).
Table 4.
CTC phenotypes for apoptosis and Bcl‐2 according to treatment and time cohorts.
| Time point | Average (median) % CTC positive for marker in patients with ≥5 CTC | |||||||
|---|---|---|---|---|---|---|---|---|
| Apoptosis aliquot (M30) | Bcl‐2 aliquot | |||||||
| All | Endocrine therapy | Taxane therapy | Non‐taxane therapy | All | Endocrine therapy | Taxane therapy | Non‐taxane therapy | |
| Baseline | 42% (35%) | 45% (39%) | 48% (57%) | 35% (26%) | 62% (65%) | 68% (64%) | 62% (67%) | 57% (44%) |
| 24, 48, or 72 h | 49% (54%) | 59% (71%) | 54% (67%) | 39% (40%) | 64% (70%) | 65% (69%) | 69% (88%) | 59% (55%) |
| 1st follow‐up | 41% (38%) | 51% (50%) | 27% (25%) | 40% (34%) | 63% (77%) | 59% (61%) | 88% (92%) | 57% (66%) |
3.4. CTC‐M30 (apoptosis) and Bcl‐2 and clinical outcomes
The primary objective of this study was to investigate whether CTC‐Bcl‐2 and CTC‐M30 (apoptosis) could be analytically determined and whether these appeared to differ or change among the various treatment groups or time points. The study was not intended, conducted, nor powered to identify whether either of these two markers adds or complements CTC enumeration as a prognostic factor. However, in an exploratory secondary objective, we investigated the possibility of association between CTC‐M30 and CTC‐Bcl‐2 expression and outcomes. We compared PFS of patients with 0–4 CTC/7.5 ml whole blood with those who had ≥5 CTC/7.5 ml whole blood according to CTC‐M30 (Figure 5A) or CTC‐Bcl‐2 (Figure 5B). We arbitrarily selected a cut‐point of ≥50% of cells stained positive for each marker to be considered “positive.” Exploration of other cutoffs provided similar associations.
Figure 5.
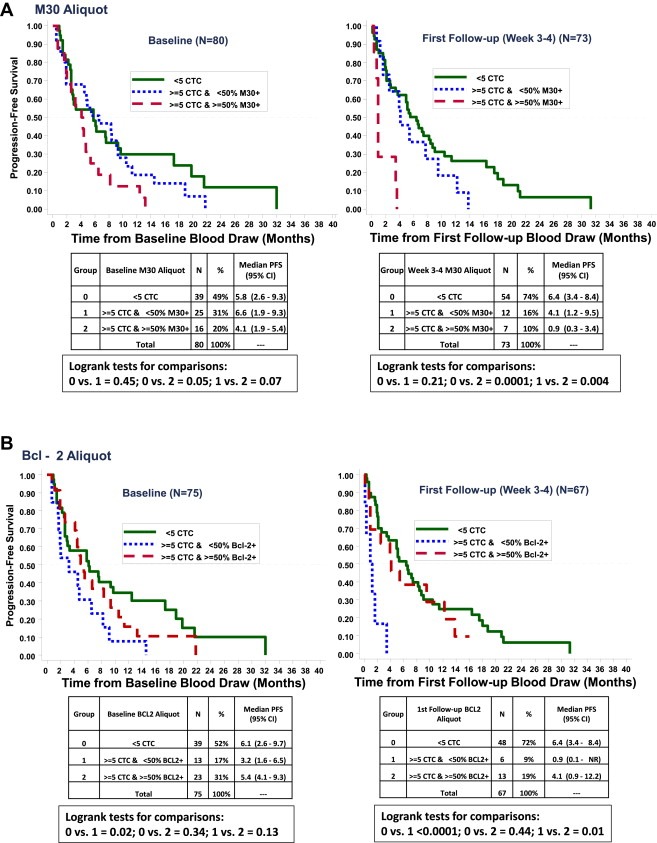
PFS according to CTC enumeration as determined at baseline and 1st follow‐up (∼3–5 weeks after the initiation of therapy). M30 and Bcl‐2 positivity was only considered in patients with ≥5 CTC. A cutoff of ≥50% CTC positive for apoptosis (5A) or Bcl‐2 (5B) was used to distinguish positive from negative for construction of the Kaplan–Meier curves. A. PFS according to CTC‐apoptosis (as determined by M30 staining). (solid line: CTC <5/7.5 ml whole blood; dotted line: ≥5 CTC/7.5 ml whole blood and M30 negative; dashed line: ≥5 CTC/7.5 ml whole blood and M30 positive). B. PFS according to CTC‐Bcl‐2. (solid line: CTC <5/7.5 ml whole blood; dotted line: ≥5 CTC/7.5 ml whole blood and Bcl‐2 negative; dashed line: ≥5 CTC/7.5 ml whole blood and Bcl‐2 positive).
We originally hypothesized that positive CTC‐M30 staining would be associated with favorable outcomes, since it should reflect non‐viable CTC. In contrast, as illustrated in Figure 5A, at baseline, patients who were considered CTC‐M30 “positive” (≥50% cells stained for M30) had a marginally significantly worse PFS than those with 0–4 CTC/7.5 ml whole blood (median PFS CTC‐M30 positive vs. 0–4 CTC = 4.1 vs. 5.8 months, p = 0.05) and trended to a worse prognosis than those with negative CTC‐M30 (<50% cells stained for M30; median PFS CTC‐M30 positive vs. CTC‐M30 negative = 4.1 vs. 6.6 months, p = 0.07). PFS for patients with CTC‐M30 negative was not apparently nor statistically different than for those with 0–4 CTC/7.5 ml whole blood (median PFS CTC‐M30 negative vs. 0–4 CTC = 6.6 vs. 5.8 months, p = 0.45). At first follow‐up, these observations were even more striking (Figure 5A). Median PFS for patients with positive CTC‐M30 was 0.9 months, compared to 6.4 and 4.1 months for patients with 0–4 CTC or negative CTC‐M30 (p = 0.004 for comparison of CTC‐M30 positive vs. negative, 0.0001 for CTC‐M30 positive vs. 0–4 CTC, and 0.21 for CTC‐M30 negative vs. 0–4 CTC).
We also hypothesized that CTC‐Bcl‐2 would be associated with worse prognosis, since pre‐clinical data suggest that Bcl‐2 prevents apoptosis and therefore should be associated with both worse natural history and resistance to cytotoxic therapies. Contrary to our pre‐study supposition, CTC‐Bcl‐2 was associated with more favorable outcomes (Figure 5B). At baseline, PFS for patients with low/negative CTC‐Bcl‐2 (<50% cells positive for Bcl‐2) was 3.2 months, compared to 5.4 and 6.1 months for patients with CTC‐Bcl‐2 positive or 0–4 CTC, respectively (p = 0.02 for comparison of CTC‐Bcl‐2 negative and 0–4 CTC; p = 0.13 and 0.34 for comparison of CTC‐Bcl‐2 negative vs. positive and for CTC‐Bcl‐2 positive vs. 0–4 CTC, respectively).
As with CTC‐M30, the data at first follow‐up were even more dramatic (Figure 5B). PFS for those with residual elevated CTC that were negative for Bcl‐2 was 0.9 months, compared to 4.1 and 6.4 months for those with CTC‐Bcl‐2 positive or 0–4 CTC (p = 0.01 for CTC‐Bcl‐2 negative vs. CTC‐Bcl‐2 positive; p < 0.0001 for CTC‐Bcl‐2 negative vs. 0–4 CTC; p = 0.44 for CTC‐Bcl‐2 positive vs. 0–4 CTC). Small numbers in this trial prohibit further comparison of outcomes according to a combination of CTC‐M30 and CTC‐Bcl‐2. However, median overall survival had not been reached and was >22 months for both the fifteen patients with high CTC‐M30 and low CTC‐Bcl‐2 and for the forty patients with 0–4 CTC (data not shown).
4. Discussion
In this study, we report successful integration of two potentially important cellular markers of breast cancer outcome, apoptosis and Bcl‐2, into an automated, fluorescently‐based immunomagnetic system to detect CTC in patients with metastatic breast cancer. Our results demonstrate that these markers can be monitored at baseline, within a few days of initiation, and at first clinical follow‐up after initiation of a new systemic therapy.
More than 40% of CTC were positive for M30/apoptosis at baseline. Blood was collected into vacutainer tubes containing a fixative (CellSave) (Allard et al., 2003), but our pre‐analytical study demonstrates that this fixative does not induce apoptosis. This observation suggests that many CTC undergo apoptosis either while still in the tumor or as a function of separation from the parent cancer. Moreover, the relative percent of apoptosis was inversely associated with CTC levels.
Several prior studies have demonstrated that CTC levels, when enumerated with the CellSearch® assay using strict criteria to define cellular events, are associated with poor clinical outcome in breast, colorectal, and prostate cancers (Budd et al., 2006; Cohen et al., 2008; Cristofanilli et al., 2004, 2005; de Bono et al., 2008; Hayes et al., 2006; Liu et al., 2009). Previously reported studies have demonstrated that rigorous and careful CTC evaluation, even if only for enumeration, is essential to ensure the specificity for bad outcome that we and others have demonstrated using the CellSearch® platform (Attard et al., 2011; Kraan et al., 2011; Ligthart et al., 2011). For example, reports regarding novel solid state CTC capture and separation techniques, such as with EpCAM‐coated microposts or non‐immunologic filters, have suggested intriguingly higher sensitivity rates in detecting CTC than have been reported with CellSearch® (Nagrath et al., 2007; Zheng et al., 2007). These techniques provide an opportunity to perform in situ cellular characterization for clinically important molecular markers (Maheswaran et al., 2008). However, clinical outcomes studies with these assays have not been reported and the analytical and clinical utility of these assays have not been confirmed with any acceptable level of evidence (Hayes et al., 1996; Simon et al., 2009; Teutsch et al., 2009). Our data suggest that some proportion of these cells may not be biologically viable. Indeed, Coumans et al. have demonstrated increased sensitivity using CellSearch® by broadening the definition of a CTC when enumerating events, but the specificity for cancer vs. normal subjects decreased (Coumans et al., 2010). If an image analysis algorithm was used to identify CTC, the more strict definitions of CTC provided the best specificity and resulted in the largest difference in survival.
We and others have reported successful phenotyping of CTC for a number of molecular markers, using CellSearch® and other platforms and assay strategies. In addition to EGFR mutations, these include HER2, urokinase plasminogen activator receptor, estrogen and androgen receptor, the receptor for insulin like growth factor, ERG and PTEN (Attard et al., 2009; de Bono et al., 2007; Fehm et al., 2008; Hayes et al., 2002; Ignatiadis et al., 2007; Kallergi et al., 2007; Maheswaran et al., 2008; Meng et al., 2004b, 2006). Most if not all of these studies have been, like ours, preliminary in nature and currently incorporation of phenotypic data into CTC evaluation has not gained widespread clinical application in breast or other epithelial malignancies (Harris et al., 2007).
We chose to phenotype for apoptosis and Bcl‐2 expression because of the obvious association between both markers and cell death, and the reported association between Bcl‐2 expression and breast cancer (Cory and Adams, 2002). In addition to their presumed association with prognosis, we also hypothesize that Bcl‐2 expression might be a marker for relative therapeutic resistance, and that serial apoptosis monitoring might be provide insight into the relative sensitivity of resistance to a therapeutic regime chosen for an individual patient.
A number of studies have addressed the potential clinical validity of monitoring circulating M30 protein levels (technically, the epitope of M30 on soluble cytokeratin 18 fragments in the circulation) in patients with a variety of epithelial malignancies, either before or after treatment (Ausch et al., 2009; Cummings et al., 2008; Dean et al., 2011; Hou et al., 2009; Koelink et al., 2009). More germane to the current study, CTC‐M30 has been studied in colon, renal cell, ovarian, and breast cancer (Behbakht et al., 2011; Fehm et al., 2006; Rossi et al., 2010, 2012). Our data are consistent with those of Rossi et al., suggesting a very high rate of CTC‐M30 before treatment (Rossi et al., 2010, 2012).
Fehm et al. reported that the presence of M30‐positive apoptotic DTC correlate with favorable response to therapy (Fehm et al., 2006). Our trial was not designed to address either the prognostic, predictive, or monitoring utility of baseline or changes in CTC‐Bcl2 or M30 with sufficient power, and we are unable to speculate whether we might have observed relative benefit or not in patient treatment cohorts according to baseline or serial CTC phenotypes. In exploratory analyzes, we observed that CTC levels decreased from baseline to first follow‐up in approximately one‐half of those who were initially elevated, consistent with response. However, we were unable to find any significant, or apparent, changes in either CTC number or phenotype between baseline and window blood draws, regardless of type of therapy.
In contrast to our pre‐study hypothesis, CTC‐Bcl‐2 positivity was associated with superior PFS, resembling that for patients without elevated CTC. The association between worse PFS and proportion of CTC that are positive for apoptosis or negative for Bcl‐2 is counter‐intuitive, and may be due to chance alone given the small numbers and heterogeneity of patients enrolled. Further, outcomes analyzes of this dataset are almost certainly confounded because of the manner in which patients were selected to address the primary aim of this study: the technical aspects of performing CTC‐apoptosis and CTC‐Bcl‐2 at different time windows and in different treatment subgroups. For example, although a recently published paper suggests Bcl‐2 is associated with worse prognosis, this marker has been found to predict favorable outcomes in patients with ER positive breast cancer who received adjuvant endocrine therapy (Albain et al., 2010; Callagy et al., 2008; Dowsett et al., 2010; Paik et al., 2004). Thus, we hypothesize that perhaps CTC‐apoptosis and Bcl‐2 might play a different role in endocrine refractory patients starting chemotherapy than they do for those with endocrine responsive disease. Since some of our patients had ER positive disease while others were ER negative, and some received endocrine therapy while others received chemotherapy, the association of CTC‐Bcl‐2 with outcomes in our study may be quite mixed.
We did not have sufficient power to perform even exploratory analyzes of CTC‐phenotype associations according to intrinsic subtype or type of treatment. However, as an illustrative case, we present a 59 year old patient with predominantly skeletal, ER and PR positive and HER2 negative metastatic invasive ductal carcinoma. Extraskeletal sites included a single pulmonary nodule and a small pleural effusion. Her only prior chemotherapy was adjuvant doxorubicin and cyclophosphamide given 7 years prior to the metastatic recurrence of her cancer, and she had only received letrozole and fulvestrant for metastatic disease prior to enrollment. On trial she initiated weekly paclitaxel. Baseline CTCs were remarkably elevated at 21,000/7.5 mL of whole blood, and four weeks later the level doubled to 43,000 CTC/7.5 mL of whole blood. She died of progressive visceral disease approximately 2‐1/2 months after the initiation of paclitaxel. It was notable that 98–100% of her CTCs at baseline and throughout her follow‐up were strongly positive for Bcl‐2 and were essentially negative for apoptosis, with M30 staining of 1–3% at all time points. Thus, even though the overall results enigmatically suggested that positive Bcl‐2 and negative M30 were associated with better outcomes, this patient provides a case in which these markers were associated with resistance to chemotherapy and rapid progression and death.
Therefore, the unexpected results may truly reflect cancer biology. The role that apoptosis and its control by Bcl‐2 play in the natural history of breast cancer is poorly understood, and, perhaps even more important, the differential sensitivity and resistance to different forms of therapy are conflicting. Our findings might relate to the curious association reported by many authors between increased proliferation, balanced by apoptosis, in primary breast cancers (Lipponen et al., 1994; Liu et al., 2001; Vakkala et al., 1999). Indeed, Meng et al. reported that one third of breast cancer patients who were asymptomatic and free of any evidence of recurrence for 7–22 years after primary and adjuvant therapy had detectable CTC, and they speculated that dormancy must be a balance of cell death and replenishment (Meng et al., 2004a).
Regardless of the phenotype data, the results from this pilot study re‐confirm the prognostic significance of CTC at baseline and first follow‐up for patients with metastatic breast cancer (Hayes and Smerage, 2008). It should be noted that we used a revised CTC capture and characterization assay (CellSearch® CXC kit) which, because of exchange of the fluorophores, is slightly less sensitive than the commercially available FDA‐approved kit. It is possible that this technical issue confounded the outcomes analysis. Nonetheless, as in other studies using the classic CellSearch® kit, in the current study approximately 50% of patients with progressive metastatic breast cancer had ≥5 CTC at baseline, decreasing to approximately 30% after one cycle of therapy, and those with residual CTC had a much worse prognosis. Thus, we do not believe that this concern is a valid confounder of the outcomes we observed relative to Bcl‐2 and M30 expression.
The clinical utility of reduction of CTC at first follow‐up is the objective of an ongoing prospective randomized clinical trial (Hayes and Smerage, 2008). We had hypothesized that we might detect either CTC level or CTC apoptosis changes even sooner (24, 48 or 72 h windows) in metastatic breast cancer patients starting a new systemic therapy. However, we were unable to observe, in either hormonally or chemotherapy‐treated patients, any meaningful changes in these parameters from baseline to window time points. Likewise, we did not detect any associations between CTC‐apoptosis or CTC‐Bcl‐2 at window and CTC at first follow‐up, nor between CTC‐phenotype at window and risk of subsequent progression.
5. Conclusions
We have demonstrated that CTC can be characterized for apoptotic and Bcl‐2 phenotypes, and that these results may have clinical implications. Further studies are needed to incorporate these assays into larger, more definitive trials as well as standard clinical practice.
Statement of conflicts of interest
Dr. Budd has received research support in the past from Immunicon. Drs. Hayes and Smerage received support from Immunicon, and more recently Veridex, LLC (the commercial vendor of CellSearch®) to support clinical and/or laboratory research support. Mr. Miller, Mrs. Repollet, and Drs. Doyle are or have been paid employees of Immunicon, Inc and/or Veridex, LLC. Prof Terstappen is a consultant of Veridex LLC and receives research funding from Veridex LLC.
Grant support
This work was supported by Immunicon, Inc. (now Veridex, LLC) and by the Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes on Sale™ (DFH).
Smerage Jeffrey B., Budd G. Thomas, Doyle Gerald V., Brown Marty, Paoletti Costanza, Muniz Maria, Miller M. Craig, Repollet Madeline I., Chianese David A., Connelly Mark C., Terstappen Leon W.W.M., Hayes Daniel F., (2013), Monitoring apoptosis and Bcl‐2 on circulating tumor cells in patients with metastatic breast cancer, Molecular Oncology, 7, doi: 10.1016/j.molonc.2013.02.013.
Presentations/publications: Data from this clinical trial have been reported at annual meetings (San Antonio Breast Cancer Symposium 2006 and American Society of Clinical Oncology 2008).
Contributor Information
Jeffrey B. Smerage, Email: smerage@med.umich.edu
G. Thomas Budd, Email: buddg@ccf.org.
Gerald V. Doyle, Email: doylegv@comcast.net
Marty Brown, Email: martybrn@med.umich.edu.
Costanza Paoletti, Email: pcostanz@med.umich.edu.
Maria Muniz, Email: mcmuniz@med.umich.edu.
M. Craig Miller, Email: CMiller3@ITS.jnj.com.
Madeline I. Repollet, Email: mrepollet@its.jnj.com
David A. Chianese, Email: DChianes@its.jnj.com
Mark C. Connelly, Email: MConnel1@its.jnj.com
Leon W.W.M. Terstappen, Email: l.w.m.m.terstappen@utwente.nl
Daniel F. Hayes, Email: hayesdf@umich.edu
References
- Albain, K.S. , Barlow, W.E. , Shak, S. , Hortobagyi, G.N. , Livingston, R.B. , Yeh, I.T. , Ravdin, P. , Bugarini, R. , Baehner, F.L. , Davidson, N.E. , Sledge, G.W. , Winer, E.P. , Hudis, C. , Ingle, J.N. , Perez, E.A. , Pritchard, K.I. , Shepherd, L. , Gralow, J.R. , Yoshizawa, C. , Allred, D.C. , Osborne, C.K. , Hayes, D.F. , 2010. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 11, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard, J. , Hayes, D.F. , Repollet, M. , Rao, C. , Herman, M.L. , Matera, J. , Miller, M.C. , Terstappen, L.W. , 2003. A cellular preservative improves the specificity and yield of circulating tumor cells in carcinoma patients. Proc. Am. Soc. Clin. Oncol. 22, 866 [Google Scholar]
- Allard, W.J. , Matera, J. , Miller, M.C. , Repollet, M. , Connelly, M.C. , Rao, C. , Tibbe, A.G. , Uhr, J.W. , Terstappen, L.W. , 2004. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 10, 6897–6904. [DOI] [PubMed] [Google Scholar]
- Attard, G. , Crespo, M. , Lim, A.C. , Pope, L. , Zivi, A. , de Bono, J.S. , 2011. Reporting the capture efficiency of a filter-based microdevice: a CTC is not a CTC unless it is CD45 negative-letter. Clin. Cancer Res. 17, 3048–3049. Author reply 3050 [DOI] [PubMed] [Google Scholar]
- Attard, G. , Swennenhuis, J.F. , Olmos, D. , Reid, A.H. , Vickers, E. , A'Hern, R. , Levink, R. , Coumans, F. , Moreira, J. , Riisnaes, R. , Oommen, N.B. , Hawche, G. , Jameson, C. , Thompson, E. , Sipkema, R. , Carden, C.P. , Parker, C. , Dearnaley, D. , Kaye, S.B. , Cooper, C.S. , Molina, A. , Cox, M.E. , Terstappen, L.W. , de Bono, J.S. , 2009. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 69, 2912–2918. [DOI] [PubMed] [Google Scholar]
- Ausch, C. , Buxhofer-Ausch, V. , Olszewski, U. , Schiessel, R. , Ogris, E. , Hinterberger, W. , Hamilton, G. , 2009. Circulating cytokeratin 18 fragment m65-a potential marker of malignancy in colorectal cancer patients. J. Gastrointest. Surg. 13, 2020–2026. [DOI] [PubMed] [Google Scholar]
- Behbakht, K. , Sill, M.W. , Darcy, K.M. , Rubin, S.C. , Mannel, R.S. , Waggoner, S. , Schilder, R.J. , Cai, K.Q. , Godwin, A.K. , Alpaugh, R.K. , 2011. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a gynecologic oncology group study. Gynecol. Oncol. 123, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd, G.T. , Cristofanilli, M. , Ellis, M.J. , Stopeck, A. , Borden, E. , Miller, M.C. , Matera, J. , Repollet, M. , Doyle, G.V. , Terstappen, L.W. , Hayes, D.F. , 2006. Circulating tumor cells versus imaging-predicting overall survival in metastatic breast cancer. Clin. Cancer Res. 12, 6403–6409. [DOI] [PubMed] [Google Scholar]
- Callagy, G.M. , Webber, M.J. , Pharoah, P.D. , Caldas, C. , 2008. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer 8, 153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, N.J. , 2000. M30 expression demonstrates apoptotic cells, correlates with in situ end-labeling, and is associated with Ki-67 expression in large intestinal neoplasms. Arch. Pathol. Lab. Med. 124, 1768–1772. [DOI] [PubMed] [Google Scholar]
- Cohen, S.J. , Punt, C.J. , Iannotti, N. , Saidman, B.H. , Sabbath, K.D. , Gabrail, N.Y. , Picus, J. , Morse, M. , Mitchell, E. , Miller, M.C. , Doyle, G.V. , Tissing, H. , Terstappen, L.W. , Meropol, N.J. , 2008. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 3213–3221. [DOI] [PubMed] [Google Scholar]
- Cohen, S.J. , Punt, C.J. , Iannotti, N. , Saidman, B.H. , Sabbath, K.D. , Gabrail, N.Y. , Picus, J. , Morse, M.A. , Mitchell, E. , Miller, M.C. , Doyle, G.V. , Tissing, H. , Terstappen, L.W. , Meropol, N.J. , 2009. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann. Oncol. 20, 1223–1229. [DOI] [PubMed] [Google Scholar]
- Cory, S. , Adams, J.M. , 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2, 647–656. [DOI] [PubMed] [Google Scholar]
- Coumans, F.A. , Doggen, C.J. , Attard, G. , de Bono, J.S. , Terstappen, L.W. , 2010. All circulating EpCAM+CK+CD45-objects predict overall survival in castration-resistant prostate cancer. Ann. Oncol. 21, 1851–1857. [DOI] [PubMed] [Google Scholar]
- Cristofanilli, M. , Budd, G.T. , Ellis, M.J. , Stopeck, A. , Matera, J. , Miller, M.C. , Reuben, J.M. , Doyle, G.V. , Allard, W.J. , Terstappen, L.W. , Hayes, D.F. , 2004. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791. [DOI] [PubMed] [Google Scholar]
- Cristofanilli, M. , Hayes, D.F. , Budd, G.T. , Ellis, M.J. , Stopeck, A. , Reuben, J.M. , Doyle, G.V. , Matera, J. , Allard, W.J. , Miller, M.C. , Fritsche, H.A. , Hortobagyi, G.N. , Terstappen, L.W. , 2005. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J. Clin. Oncol. 23, 1420–1430. [DOI] [PubMed] [Google Scholar]
- Cummings, J. , Hodgkinson, C. , Odedra, R. , Sini, P. , Heaton, S.P. , Mundt, K.E. , Ward, T.H. , Wilkinson, R.W. , Growcott, J. , Hughes, A. , Dive, C. , 2008. Preclinical evaluation of M30 and M65 ELISAs as biomarkers of drug induced tumor cell death and antitumor activity. Mol. Cancer Ther. 7, 455–463. [DOI] [PubMed] [Google Scholar]
- de Bono, J.S. , Attard, G. , Adjei, A. , Pollak, M.N. , Fong, P.C. , Haluska, P. , Roberts, L. , Melvin, C. , Repollet, M. , Chianese, D. , Connely, M. , Terstappen, L.W. , Gualberto, A. , 2007. Potential applications for circulating tumor cells expressing the insulin-like growth factor-I receptor. Clin. Cancer Res. 13, 3611–3616. [DOI] [PubMed] [Google Scholar]
- de Bono, J.S. , Scher, H.I. , Montgomery, R.B. , Parker, C. , Miller, M.C. , Tissing, H. , Doyle, G.V. , Terstappen, L.W. , Pienta, K.J. , Raghavan, D. , 2008. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 14, 6302–6309. [DOI] [PubMed] [Google Scholar]
- Dean, E.J. , Cummings, J. , Roulston, A. , Berger, M. , Ranson, M. , Blackhall, F. , Dive, C. , 2011. Optimization of circulating biomarkers of obatoclax-induced cell death in patients with small cell lung cancer. Neoplasia 13, 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett, M. , Cuzick, J. , Wale, C. , Forbes, J. , Mallon, E.A. , Salter, J. , Quinn, E. , Dunbier, A. , Baum, M. , Buzdar, A. , Howell, A. , Bugarini, R. , Baehner, F.L. , Shak, S. , 2010. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J. Clin. Oncol. 28, 1829–1834. [DOI] [PubMed] [Google Scholar]
- Fehm, T. , Becker, S. , Becker-Pergola, G. , Sotlar, K. , Gebauer, G. , Durr-Storzer, S. , Neubauer, H. , Wallwiener, D. , Solomayer, E.F. , 2006. Presence of apoptotic and nonapoptotic disseminated tumor cells reflects the response to neoadjuvant systemic therapy in breast cancer. Breast Cancer Res. 8, R60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehm, T. , Muller, V. , Aktas, B. , Janni, W. , Schneeweiss, A. , Stickeler, E. , Lattrich, C. , Lohberg, C.R. , Solomayer, E. , Rack, B. , Riethdorf, S. , Klein, C. , Schindlbeck, C. , Brocker, K. , Kasimir-Bauer, S. , Wallwiener, D. , Pantel, K. , 2010. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res. Treat. 124, 403–412. [DOI] [PubMed] [Google Scholar]
- Fehm, T. , Muller, V. , Alix-Panabieres, C. , Pantel, K. , 2008. Micrometastatic spread in breast cancer: detection, molecular characterization and clinical relevance. Breast Cancer Res. 10, (Suppl. 1) S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg, M. , Biven, K. , Ueno, T. , Rydlander, L. , Bjorklund, P. , Wiman, K.G. , Shoshan, M. , Linder, S. , 2002. A novel high-through-put assay for screening of pro-apoptotic drugs. Invest. New Drugs 20, 253–259. [DOI] [PubMed] [Google Scholar]
- Harris, L. , Fritsche, H. , Mennel, R. , Norton, L. , Ravdin, P. , Taube, S. , Somerfield, M.R. , Hayes, D.F. , Bast, R.C. , 2007. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J. Clin. Oncol. 25, 5287–5312. [DOI] [PubMed] [Google Scholar]
- Hayes, D.F. , Bast, R.C. , Desch, C.E. , Fritsche, H. , Kemeny, N.E. , Jessup, J.M. , Locker, G.Y. , Macdonald, J.S. , Mennel, R.G. , Norton, L. , Ravdin, P. , Taube, S. , Winn, R.J. , 1996. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J. Natl. Cancer Inst. 88, 1456–1466. [DOI] [PubMed] [Google Scholar]
- Hayes, D.F. , Cristofanilli, M. , Budd, G.T. , Ellis, M.J. , Stopeck, A. , Miller, M.C. , Matera, J. , Allard, W.J. , Doyle, G.V. , Terstappen, L.W. , 2006. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin. Cancer Res. 12, 4218–4224. [DOI] [PubMed] [Google Scholar]
- Hayes, D.F. , Smerage, J. , 2008. Is there a role for circulating tumor cells in the management of breast cancer?. Clin. Cancer Res. 14, 3646–3650. [DOI] [PubMed] [Google Scholar]
- Hayes, D.F. , Walker, T.M. , Singh, B. , Vitetta, E.S. , Uhr, J.W. , Gross, S. , Rao, C. , Doyle, G.V. , Terstappen, L.W. , 2002. Monitoring expression of HER-2 on circulating epithelial cells in patients with advanced breast cancer. Int. J. Oncol. 21, 1111–1117. [DOI] [PubMed] [Google Scholar]
- Hou, J.M. , Greystoke, A. , Lancashire, L. , Cummings, J. , Ward, T. , Board, R. , Amir, E. , Hughes, S. , Krebs, M. , Hughes, A. , Ranson, M. , Lorigan, P. , Dive, C. , Blackhall, F.H. , 2009. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am. J. Pathol. 175, 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatiadis, M. , Xenidis, N. , Perraki, M. , Apostolaki, S. , Politaki, E. , Kafousi, M. , Stathopoulos, E.N. , Stathopoulou, A. , Lianidou, E. , Chlouverakis, G. , Sotiriou, C. , Georgoulias, V. , Mavroudis, D. , 2007. Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J. Clin. Oncol. 25, 5194–5202. [DOI] [PubMed] [Google Scholar]
- Kallergi, G. , Mavroudis, D. , Georgoulias, V. , Stournaras, C. , 2007. Phosphorylation of FAK, PI-3K, and impaired actin organization in CK-positive micrometastatic breast cancer cells. Mol. Med. 13, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelink, P.J. , Lamers, C.B. , Hommes, D.W. , Verspaget, H.W. , 2009. Circulating cell death products predict clinical outcome of colorectal cancer patients. BMC Cancer 9, 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraan, J. , Sleijfer, S. , Strijbos, M.H. , Ignatiadis, M. , Peeters, D. , Pierga, J.Y. , Farace, F. , Riethdorf, S. , Fehm, T. , Zorzino, L. , Tibbe, A.G. , Maestro, M. , Gisbert-Criado, R. , Denton, G. , de Bono, J.S. , Dive, C. , Foekens, J.A. , Gratama, J.W. , 2011. External quality assurance of circulating tumor cell enumeration using the CellSearch((R)) system: a feasibility study. Cytometry B Clin. Cytom. 80, 112–118. [DOI] [PubMed] [Google Scholar]
- Leers, M.P. , Kolgen, W. , Bjorklund, V. , Bergman, T. , Tribbick, G. , Persson, B. , Bjorklund, P. , Ramaekers, F.C. , Bjorklund, B. , Nap, M. , Jornvall, H. , Schutte, B. , 1999. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J. Pathol. 187, 567–572. [DOI] [PubMed] [Google Scholar]
- Ligthart, S.T. , Coumans, F.A. , Attard, G. , Cassidy, A.M. , de Bono, J.S. , Terstappen, L.W. , 2011. Unbiased and automated identification of a circulating tumour cell definition that associates with overall survival. PLoS One 6, e27419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipponen, P. , Aaltomaa, S. , Kosma, V.M. , Syrjanen, K. , 1994. Apoptosis in breast cancer as related to histopathological characteristics and prognosis. Eur. J. Cancer 30A, 2068–2073. [DOI] [PubMed] [Google Scholar]
- Liu, M.C. , Shields, P.G. , Warren, R.D. , Cohen, P. , Wilkinson, M. , Ottaviano, Y.L. , Rao, S.B. , Eng-Wong, J. , Seillier-Moiseiwitsch, F. , Noone, A.M. , Isaacs, C. , 2009. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J. Clin. Oncol. 27, 5153–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. , Edgerton, S.M. , Moore, D.H. , Thor, A.D. , 2001. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin. Cancer Res. 7, 1716–1723. [PubMed] [Google Scholar]
- Maheswaran, S. , Sequist, L.V. , Nagrath, S. , Ulkus, L. , Brannigan, B. , Collura, C.V. , Inserra, E. , Diederichs, S. , Iafrate, A.J. , Bell, D.W. , Digumarthy, S. , Muzikansky, A. , Irimia, D. , Settleman, J. , Tompkins, R.G. , Lynch, T.J. , Toner, M. , Haber, D.A. , 2008. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane, L.M. , Altman, D.G. , Sauerbrei, W. , Taube, S.E. , Gion, M. , Clark, G.M. , 2005. Reporting recommendations for tumor marker prognostic studies (REMARK). J. Natl. Cancer Inst. 97, 1180–1184. [DOI] [PubMed] [Google Scholar]
- Meng, S. , Tripathy, D. , Frenkel, E.P. , Shete, S. , Naftalis, E.Z. , Huth, J.F. , Beitsch, P.D. , Leitch, M. , Hoover, S. , Euhus, D. , Haley, B. , Morrison, L. , Fleming, T.P. , Herlyn, D. , Terstappen, L.W. , Fehm, T. , Tucker, T.F. , Lane, N. , Wang, J. , Uhr, J.W. , 2004. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 10, 8152–8162. [DOI] [PubMed] [Google Scholar]
- Meng, S. , Tripathy, D. , Shete, S. , Ashfaq, R. , Haley, B. , Perkins, S. , Beitsch, P. , Khan, A. , Euhus, D. , Osborne, C. , Frenkel, E. , Hoover, S. , Leitch, M. , Clifford, E. , Vitetta, E. , Morrison, L. , Herlyn, D. , Terstappen, L.W. , Fleming, T. , Fehm, T. , Tucker, T. , Lane, N. , Wang, J. , Uhr, J. , 2004. HER-2 gene amplification can be acquired as breast cancer progresses. Proc. Natl. Acad. Sci. U S A 101, 9393–9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, S. , Tripathy, D. , Shete, S. , Ashfaq, R. , Saboorian, H. , Haley, B. , Frenkel, E. , Euhus, D. , Leitch, M. , Osborne, C. , Clifford, E. , Perkins, S. , Beitsch, P. , Khan, A. , Morrison, L. , Herlyn, D. , Terstappen, L.W. , Lane, N. , Wang, J. , Uhr, J. , 2006. uPAR and HER-2 gene status in individual breast cancer cells from blood and tissues. Proc. Natl. Acad. Sci. U S A 103, 17361–17365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagrath, S. , Sequist, L.V. , Maheswaran, S. , Bell, D.W. , Irimia, D. , Ulkus, L. , Smith, M.R. , Kwak, E.L. , Digumarthy, S. , Muzikansky, A. , Ryan, P. , Balis, U.J. , Tompkins, R.G. , Haber, D.A. , Toner, M. , 2007. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik, S. , Shak, S. , Tang, G. , Kim, C. , Baker, J. , Cronin, M. , Baehner, F.L. , Walker, M.G. , Watson, D. , Park, T. , Hiller, W. , Fisher, E.R. , Wickerham, D.L. , Bryant, J. , Wolmark, N. , 2004. A multi-gene RT-PCR assay using fixed, paraffin-embedded tumor tissue to predict the likelihood of breast cancer recurrence in node negative, estrogen receptor positive, tamoxifen-treated patients. N. Engl. J. Med. 351, 2817–2826. [DOI] [PubMed] [Google Scholar]
- Riethdorf, S. , Muller, V. , Zhang, L. , Rau, T. , Loibl, S. , Komor, M. , Roller, M. , Huober, J. , Fehm, T. , Schrader, I. , Hilfrich, J. , Holms, F. , Tesch, H. , Eidtmann, H. , Untch, M. , von Minckwitz, G. , Pantel, K. , 2010. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin. Cancer Res. 16, 2634–2645. [DOI] [PubMed] [Google Scholar]
- Rossi, E. , Basso, U. , Celadin, R. , Zilio, F. , Pucciarelli, S. , Aieta, M. , Barile, C. , Sava, T. , Bonciarelli, G. , Tumolo, S. , Ghiotto, C. , Magro, C. , Jirillo, A. , Indraccolo, S. , Amadori, A. , Zamarchi, R. , 2010. M30 neoepitope expression in epithelial cancer: quantification of apoptosis in circulating tumor cells by CellSearch analysis. Clin. Cancer Res. 16, 5233–5243. [DOI] [PubMed] [Google Scholar]
- Rossi, E. , Fassan, M. , Aieta, M. , Zilio, F. , Celadin, R. , Borin, M. , Grassi, A. , Troiani, L. , Basso, U. , Barile, C. , Sava, T. , Lanza, C. , Miatello, L. , Jirillo, A. , Rugge, M. , Indraccolo, S. , Cristofanilli, M. , Amadori, A. , Zamarchi, R. , 2012. Dynamic changes of live/apoptotic circulating tumour cells as predictive marker of response to sunitinib in metastatic renal cancer. Br. J. Cancer 107, 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, A. , Terrasi, M. , Agnese, V. , Santini, D. , Bazan, V. , 2006. Apoptosis: a relevant tool for anticancer therapy. Ann. Oncol. 17, (Suppl 7) vii115–vii123. [DOI] [PubMed] [Google Scholar]
- Simon, R.M. , Paik, S. , Hayes, D.F. , 2009. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J. Natl. Cancer Inst. 101, 1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teutsch, S.M. , Bradley, L.A. , Palomaki, G.E. , Haddow, J.E. , Piper, M. , Calonge, N. , Dotson, W.D. , Douglas, M.P. , Berg, A.O. , 2009. The evaluation of genomic applications in practice and prevention (EGAPP) initiative: methods of the EGAPP working group. Genet. Med. 11, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakkala, M. , Lahteenmaki, K. , Raunio, H. , Paakko, P. , Soini, Y. , 1999. Apoptosis during breast carcinoma progression. Clin. Cancer Res. 5, 319–324. [PubMed] [Google Scholar]
- Zheng, S. , Lin, H. , Liu, J.Q. , Balic, M. , Datar, R. , Cote, R.J. , Tai, Y.C. , 2007. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J. Chromatogr. A 1162, 154–161. [DOI] [PubMed] [Google Scholar]


