Abstract
In 2009 a prospective, randomized Phase II trial (NCT00842998) was initiated to evaluate the activity of HER2‐targeting agents without chemotherapy (CT) in HER2‐positive metastatic breast cancer (MBC) patients. The primary tumors of the patients enrolled in this study offered a unique opportunity to identify biomarkers that could predict durable clinical benefit from CT‐free anti‐HER2 therapy.
Patients with HER2‐positive MBC were randomized to trastuzumab or lapatinib as first‐line therapy. CT was added to anti‐HER2 therapy in patients failing to achieve tumor regression at the 8‐week evaluation and in those progressing at any time. Expression analysis of 105 selected genes was performed from formalin‐fixed paraffin‐embedded primary tumor samples. The research‐based PAM50 intrinsic subtypes were also identified. Additionally, quantitative HER2 (H2T) and p95HER2 (p95) protein expression were evaluated by HERmark® and VeraTag® assay, respectively. Predictors of persistence on protocol (PP) were studied by Cox univariate and multivariate analysis.
Nineteen patients were enrolled. Median overall survival was 43 months and median PP was 3.8 months (0.8–38.8+), with 4 patients (21.1%) persisting on single agent trastuzumab or lapatinib for longer than 12 mo (14.9–38.8 + mo). Seventeen patients were evaluable for PP. Gene expression analysis revealed that high expression of the 17q12‐21 amplicon genes HER2 and GRB7, and the PAM50 HER2‐enriched intrinsic profile, were significantly associated with longer PP. Conversely, high expression of luminal‐related genes such as PGR, MDM2 or PIK3CA, or the PAM50 luminal intrinsic profile correlated with reduced PP. Moreover, increasing H2T/p95 ratio was found to be significantly associated with longer PP (HR 0.56 per 2‐fold increase in H2T/p95, P = 0.0015).
Our data suggest that patients belonging to the “HER2‐enriched” subtype and/or having high H2T/p95 protein expression ratio are exquisitely sensitive to anti‐HER2 agents. MBC patients with these tumors could be candidates for studies aimed at establishing chemotherapy‐free regimens.
Keywords: Trastuzumab, Lapatinib, Chemotherapy-free, HER2-enriched, Microarray, Signature
Highlights
A subset of HER2‐positive breast cancer patients is exquisitely sensitive to anti‐HER2 therapy.
We identified biomarkers of response to anti‐HER2 therapy in patients not treated with concomitant chemotherapy.
The PAM50 HER2‐enriched gene expression profile was associated with clinical benefit from anti‐HER2 agents.
High HER2/p95 HER2 expression ratio also correlated with clinical benefit from anti‐HER2 agents.
Abbreviations
- CT
chemotherapy
- total HER2
(H2T)
- p95HER2
(p95)
- PP
persistence on protocol
- CR
complete remission
- PR
partial remission
- SD
stable disease
- PD
progressive disease
- ROR
risk of relapse
- OS
overall survival
- PFS
progression-free survival
- MR
minimal response
1. Introduction
Overexpression and/or amplification of the Human Epidermal Growth Factor Receptor 2 (HER2), found in 15–20% of breast cancers, is associated with adverse prognostic factors (Slamon et al., 1987; Yarden and Sliwkowski, 2001). However, the introduction of trastuzumab, the first monoclonal antibody specifically designed to target HER2‐positive (HER2+) breast cancer, has drastically improved the course of this disease both in the metastatic and adjuvant settings (Slamon et al., 2001; Marty et al., 2005; Romond et al., 2005; Piccart‐Gebhart et al., 2005). Furthermore, a number of additional compounds that target this receptor, such as the HER2/EGFR tyrosine kinase inhibitor lapatinib, have been found effective in HER2+ breast cancer (Geyer et al., 2006; Cortes et al., 2009; Baselga et al., 2012a; Verma et al., 2012).
Overall, anti‐HER2 agents are more effective when combined with chemotherapy (Pegram et al., 2004). However, the few clinical trials of single agent trastuzumab or lapatinib without chemotherapy showed that in the ∼25% of patients who responded to treatment, disease control duration approached that observed in trials where anti‐HER2 therapy was combined with chemotherapy (Vogel et al., 2002; Gomez et al., 2008). The identification of biomarkers that can predict which patients derive the largest benefit from chemotherapy‐free anti‐HER2 therapy would, therefore, significantly improve the management of this disease.
On these premises, in 2009 a phase II randomized chemotherapy‐free trial was initiated, consisting of first‐line therapy with either trastuzumab or lapatinib for previously untreated, HER2‐positive metastatic breast cancer patients (HERceptin LAPatinib‐HERLAP trial, NCT00842998). Because of slow accrual and emerging data regarding the efficacy of dual HER2 blockade (Blackwell et al., 2012), the HERLAP trial was closed with 19 patients recruited. However, despite this small number of patients, the HERLAP trial population provides a unique opportunity to study predictors of long‐term efficacy of single agent anti‐HER2 therapy. Here we report our findings based on gene expression and protein expression analyses correlated with benefit from single‐agent anti‐HER2 therapy.
2. Patients and methods
2.1. Patient population
The HERLAP study is a randomized, open label, phase II trial. Women with fluorescence in situ hybridization (FISH)‐proven HER2‐amplified metastatic breast cancer not previously treated with chemotherapy and/or anti‐HER2 agents for metastatic disease were eligible provided that they had “low burden” disease. This was defined by absence of carcinomatous lymphangitis and, in case of liver metastases, less than one third of the liver volume involved with disease. Baseline left ventricular ejection fraction (LVEF) had to be >55%. Furthermore, performance status (PS) had to be ≤ 2 according to the Eastern Cooperative Oncology Group (ECOG) definition. To be considered for the study women had to have at least one measurable lesion according to the Response Evaluation Criteria in solid Tumors (RECIST) version 1.0 (Therasse et al., 2000). Lymph nodal metastases were allowed if they were the only metastatic sites, provided that their diameter could be measured by a CT scan.
2.2. Treatments
After providing written informed consent, eligible patients were randomized to either trastuzumab (8 mg/kg on loading dose, followed by weekly trastuzumab at the dose of 2 mg/kg) or to oral lapatinib at the dose of 1500 mg daily with no interruptions. Treatment was administered for 8 weeks. After the first cycle of therapy (8 weeks), women underwent an imaging procedure to assess tumor response. Women who achieved minimal response (MR, any reduction in the sum of the largest diameters of the target lesions not fulfilling the definition of partial remission‐PR), partial PR or complete remission (CR) were allowed to proceed further on single agent therapy for another 8‐week cycle. Patients experiencing stable disease (SD) or progressing disease (PD) were considered protocol failures and continued the same anti‐HER2 agent and chemotherapy (three weekly docetaxel, weekly paclitaxel, or vinorelbine if they were on trastuzumab, and capecitabine, weekly paclitaxel or vinorelbine if they were on lapatinib) was added. Patients experiencing significant toxicity with one agent could be switched to the other agent in combination with chemotherapy and were considered protocol failures. Women continuing on single‐agent anti‐HER2 therapy were restaged every 8 weeks and allowed to proceed further if not progressing. Chemotherapy was added to anti‐HER2 therapy in women showing progressive disease.
2.3. Safety and dose‐reductions
Safety was assessed clinically and by laboratory exams every 3 weeks or shorter if needed. Left ventricular ejection fraction was assessed at the baseline and every 12 weeks thereafter during the whole exposure to anti‐HER2 therapy. No dose reductions were allowed for trastuzumab. In case of lapatinib‐related toxicity, the dose could be de‐escalated to 1250 mg/daily. Treatment was interrupted in those patients experiencing significant (grade III) lapatinib‐related toxicity after dose‐de‐escalation. Women not tolerating lapatinib were switched to trastuzumab plus chemotherapy.
2.4. Gene expression data
RNA was purified from formalin‐fixed paraffin‐embedded (FFPE) tissue using the Roche HighPure FFPE Micro Kit, and ∼100 ng of total RNA was used to measure expression of 110 selected genes using the nCounter Nanostring platform. The selected gene list includes the 50 PAM50 genes, genes associated with VEGF/hypoxia‐related biological processes (e.g. VEGF and ADM), proliferation/cell‐cycle (e.g. MKI67 and CCNB1), EGFR signaling (e.g. EGFR, ERBB4) and epithelial‐to‐mesenchymal transition (e.g. CLDN3 and EPCAM). Raw data was log base 2 transformed and normalized using 5 house‐keeping transcripts.
All tumors were assigned to an intrinsic molecular subtype of breast cancer (Luminal A, Luminal B, HER2‐enriched, Basal‐like) and the Normal‐like group using the PAM50 subtype predictor as previously described (Parker et al., 2009). For PAM50 platform‐to‐platform normalization, a total of 53 primary breast samples representing all the intrinsic molecular subtypes were also profiled using the same procedures as described above.
Eight genomic signatures were evaluated individually from the PAM50 subtype predictor output data (Parker et al., 2009; Nielsen et al., 2010; Prat et al., 2013). These signature were the correlation of each sample to each of the 5 PAM50 tumor centroids (Luminal A, Luminal B, HER2‐enriched, Basal‐like and Normal), the previously reported Proliferation score, which is the mean expression of 11 proliferation‐related genes (Nielsen et al., 2010), and the risk of relapse (ROR) score based on subtype only (ROR‐S), and based upon subtype and proliferation (ROR‐P).
All gene expression data has been deposited in GEO (accession number GSE46350).
2.5. HER2 and p95 protein expression quantification
Quantitative HER2 protein expression (H2T) was obtained using the HERmark assay. (Huang et al., 2010) The quantitative p95 protein assay which is specific for the active form of p95, was performed as previously described (Sperinde et al., 2010). Tumor average expression was reported in units of relative fluorescence per square millimeter of tumor (RF/mm2).
2.6. Statistical considerations
The original sample size calculation for the HERLAP trial is described in Supplementary data 1. For the current study, persistence on protocol (PP) was chosen ad hoc as the clinical outcome of interest. Persistence on protocol was defined as time from randomization to addition of chemotherapy or death from any cause. We considered this endpoint as a surrogate for benefit from single‐agent anti‐HER2 therapy. Additionally, we calculated progression‐free survival (PFS) as the interval from randomization to disease progression or death from any cause, and overall survival (OS) as the interval from randomization to death from any cause.
Analyses were conducted in January 2013, at a median overall follow‐up of 32 months (range 14–81 months). Expression values (genes or proteins) were analyzed as continuous variables. Where appropriate, continuous variables were dichotomized around their median values. Estimates of survival and PP were obtained by Kaplan–Meier curves and tests of differences by the log‐rank test. The association between gene expression, gene signature expression, HER2 and p95 protein levels and PP was assessed by Cox proportional regression analysis.
In the analyses, a Hazard Ratio (HR) < 1 indicated longer persistence on protocol (i.e. reduced probability of adding chemotherapy). All tests were two tailed. Due to the small sample size and the purely exploratory intent of this analysis, no correction was applied for multiple testing.
3. Results
3.1. Clinical outcome
A total of 19 patients were enrolled in the HERLAP trial. Nine and 10 patients were randomly assigned to single agent trastuzumab and lapatinib, respectively. A summary of the clinical‐pathological data of the patients is shown in Table 1. Two patients in the lapatinib arm discontinued protocol before the first evaluation due to grade 3 diarrhea (one patient), and grade 3 skin toxicity (one patient) that did not improve after dose reduction. Upon resolution of toxicities, both patients were switched to trastuzumab with chemotherapy consisting of vinorelbine (30 mg/m2 on days 1 and 8 every 3 weeks) in one, and in docetaxel (75 mg/m2 every 3 weeks) in the other. Seventeen patients were therefore evaluable for benefit from single‐agent anti‐HER2 therapy.
Table 1.
Clinical‐pathological characteristics of the patient population.
| Variable | Number (percentages o range) |
|---|---|
| Total enrolled | 19 |
| Median age (years) | 59 (36–76) |
| Stage at first diagnosis | |
| I/II | 11 (58) |
| III | 3 (16) |
| IV | 5 (26) |
| HER2 status | |
| HER2 3+/FISH+ | 16 (84) |
| HER2 2+/FISH+ | 3 (16) |
| ER and/or PgR status | |
| Positive | 12 (63) |
| Negative | 7 (37) |
| Mean Ki67 | 40 (14–70)a |
| Prior treatment | |
| Prior neo/adjuvant chemotherapy | 10 (53) |
| Adjuvant anthracycline | 10 (53) |
| Adjuvant taxane | 6 (32) |
| Adjuvant trastuzumab | 5 (26) |
| Adjuvant endocrine therapy | 7 (37) |
| Number of sites of metastatic disease | |
| 1 | 5 (26) |
| 2 | 7 (37) |
| 3 | 6 (32) |
| 4 | 1 (5) |
| Metastatic sites | |
| Liver | 4 (21) |
| Lung | 12 (63) |
| Lymph‐node/soft tissue | 16 (84) |
| Bone | 7 (37) |
| Effusions | 1 (5) |
| Type of site | |
| Visceral (liver + lung) | 14 (74) |
| Non visceral | 5 (26) |
| Treatment allocation | |
| Trastuzumab | 9 (47) |
| Lapatinib | 10 (53) |
FISH, fluorescence in‐situ hybridization, ER, estrogen receptor, PgR, progesterone receptor.
For 4 patients with de‐novo stage IV disease, Ki67 was not assessed on core biopsies. Women with de‐novo stage IV disease were considered as having DFS = 0.
At the time of data cutoff, two patients were still on protocol receiving single‐agent trastuzumab (persistence on protocol 14.9 and 38.8 months, respectively). Another two had achieved disease control that lasted at least 12 months or more. Finally, disease had not progressed in two patients who were considered protocol failures (one achieved a SD at the first evaluation and the other discontinued lapatinib because of toxicity before the first 8‐week evaluation). Of 8 patients in the lapatinib arm failing to show response at the 8‐week evaluation or whose disease progressed at any time during monotherapy, four received first‐line chemotherapy with capecitabine (2000 mg/m2/day for 14 days every three weeks) and three with vinorelbine (30 mg/m2 on days 1 and 8 every 3 weeks), both added to lapatinib (1250 mg/day). The remaining patient refused to undergo further HER2‐targeting therapy and received endocrine therapy with fulvestrant. Of 7 patients in the trastuzumab arm failing to show response at the 8‐week evaluation or with disease progression at any time during monotherapy, four received first‐line chemotherapy with docetaxel (75–100 mg/m2 every three weeks), and two with vinorelbine 30 mg/m2 on days 1 and 8 every 3 weeks), both added to trastuzumab (either 2 mg/kg weekly or 6 mg/kg every three weeks). The remaining patient refused chemotherapy and was treated with exemestane and trastuzumab.
In the overall population, median persistence on protocol, median progression‐free survival and median overall survival were 3.8 months (range 0.77–38.8+), 7.3 months (range 1.6–38.8+) and 43 months (14.0–81.0+), respectively. Analyses of the data from the 17 evaluable patients provided similar results (not shown).
In terms of tumor response, a total of 12 evaluable patients (71%) experienced at least a MR at 8 weeks, 2 (12%) experienced SD, and 3 (18%) experienced PD. No significant difference in terms of response at 8 weeks was observed according to treatment arm (75% and 67% MR or more with lapatinib and trastuzumab, respectively, P = 1.0).
3.2. Gene expression data
The PAM50 intrinsic molecular subtypes were found associated with persistence on protocol in the ITT population with the HER2‐enriched subtype showing an increased persistence on protocol than the other subtypes. Compared to patients with Luminal A and B tumors, patients with HER2‐enriched tumors showed a significant increase in persistence on protocol (Hazard Ratio for protocol discontinuation [HR] = 0.208, P = 0.044). Neither ER status by IHC nor HER2 FISH amplification ratio was found significantly associated with persistence on protocol.
Next, we looked for associations between the expression of each single gene, or the score of each signature, and persistence on protocol in the ITT population (Supplementary data 2). Among 113 variables (105 genes and 8 signatures), 11 (HER2, GRB7, CLDN4, RAB25, MAPT, CLDN7, EMP3, DDR1, MDM2, UBE2T and HER2‐enriched signature) and 20 (the 11 previous biomarkers and PIK3CA, DSP, RRAGD, SPINT2, BCL2, CDH3, VEGFA, PVRL3 and PGR) variables correlated with persistence on protocol in multivariate analyses (adjusted for treatment arm) with a P < 0.05 and P < 0.10, respectively. Conversely, high expression of genes associated with the Luminal subtypes, such as MAPT (also known as Tau), BCL2 and PGR was found associated with decreased persistence on protocol. Interestingly, low expression of genes associated with the epithelial‐to‐mesenchymal transition such as CLDN4 and CLDN7 were found significantly associated with a decreased persistence on protocol.
3.3. H2T and p95 protein expression analysis
H2T and p95 protein levels were successfully measured in 16 of the 17 patients analyzed for PP. Median expression values were 122.5 RF/sqmm (range 6.67–475) and 2.95 (range 0.60–8.23) for H2T and p95, respectively. Increasing log(H2T) correlated with longer PP (HR = 0.73 per 2‐fold increase in H2T; 95% CI 0.54–1.00; p = 0.046). Log(p95) was not correlated with PP by univariate analysis (p = 0.97), possibly due to the association of high p95 expression with high H2T expression. Due to this relationship, we then calculated the H2T/p95 ratio, which ranged from 1.97 to 88.17 (median value 46.60). This exploratory variable was associated with PP, with an HR of 0.56 per 2‐fold change in H2T/p95 (95% CI 0.37–0.84; p = 0.0015). This translates into a 44% reduction in the risk of adding chemotherapy for each 2‐fold increase in the ratio. Kaplan Meier analysis of PP by H2T/p95 ratio dichotomized around its median value showed a significantly longer PP for patients with higher values (HR = 0.24; 95% CI 0.074–0.75; p = 0.0089) with median PP of 2.0 months vs. 9.6 months for below vs. above the median, respectively (Figure 1). Because of the small sample size, we decided not to model a multivariate analysis including gene and protein expression data. However the median H2T/p95 expression ratio was lower in Luminal A/B (5.22) compared with HER2‐enriched tumors (57.72, p = 0.052) and with non‐Luminal/non HER2‐enriched tumors (65.57, p = 0.034). Table 2 summarizes the main features of the 4 patients experiencing persistence on protocol longer than 12 months.
Figure 1.
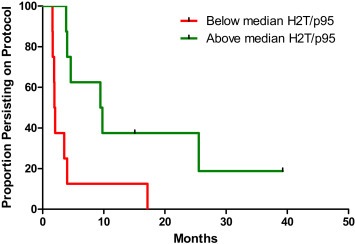
Kaplan Meier curves of persistence on protocol in patients with tumors showing H2T/p95 expression ratio values higher (solid line) or equal/lower (dotted line) than the median value of 46.60.
Table 2.
Features of the four patients achieving disease control lasting>12 months.
| Case | ER | PgR | Ki67 | HER2 statusa | PAM50 subtype | H2T/p95 | Treatment | Time on protocol (months) |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | Ratio | |||||
| 1 | 0 | 0 | Unk | 3+/FISH+ | HER2‐enriched | 67.62 | Trastuzumab | 38.8b |
| 2 | 97 | 0 | 32 | 3+/FISH+ | HER2‐enriched | 58.38 | Trastuzumab | 25.3 |
| 3 | 0 | 0 | 14 | 3+/FISH+ | HER2‐enriched | 18.75 | Lapatinib | 17.0 |
| 4 | 11 | 12 | 29 | 3+/FISH+ | Normal‐like | 88.17 | Trastuzumab | 14.9a |
Immunohistochemistry and Fluorescence in situ hybridization.
Still on treatment at the time of this analysis. Unk, unknown.
4. Discussion
The HERLAP trial was designed with the aim of evaluating potential biomarkers of long‐term benefit from HER2‐inhibition not confounded by concomitant chemo or endocrine therapy in the metastatic setting. Currently, data regarding the activity of chemotherapy‐free, anti‐HER2 therapy have been mainly generated from the preoperative setting (Gianni et al., 2012; Rimawi et al., 2013a; Prat and Baselga, 2013). In two randomized clinical trials, a significant proportion of HER2‐positive early breast cancer achieved primary disease eradication by multiple HER2 targeting alone, (Gianni et al., 2012) or in combination with endocrine therapy (Rimawi et al., 2013b). In the metastatic setting, trials testing the efficacy of anti‐HER2 therapy followed by addition of chemotherapy in a classical sequential fashion have provided results that have rather discouraged this approach (Hamberg et al., 2011; Inoue et al., 2010). Unlike these randomized studies, the HERLAP trial enrolled patients with metastatic disease (low‐burden) and allowed them to proceed on single‐agent anti‐HER2 therapy only if radiological signs of tumor regression could be registered after 8 weeks. Importantly, this design did not negatively affect the clinical outcomes of the patients enrolled in our study. In fact, their overall survival was similar to that reported with chemotherapy combined with single‐agent anti‐HER2 therapy (Marty et al., 2005; Baselga et al., 2012a). This suggests that the strategy of starting anti‐HER2 therapy and adding chemotherapy in non‐responding patients is safe and could be considered in clinical practice.
Overall, gene expression data suggest that the “HER2‐enriched” intrinsic subtype identified by gene expression analysis, or high ERBB2 and low ESR1 gene expression, can identify patients with tumors more likely to be successfully controlled by single agent trastuzumab or lapatinib. Conversely, tumors of the “luminal” subtype, or tumors with low expression of CLDN4 and CLDN7 (the so‐called “claudin‐low” subtype) (Prat et al., 2010), were less likely to benefit from anti‐HER2 treatment alone. Our data are corroborated by recent data showing that, in the neoadjuvant setting, HER2‐enriched tumors achieve higher pathological complete response rate following anti‐HER2 therapy compared to the other subtypes (Carey et al., 2013). Moreover, this study confirmed the heterogeneity of molecular subtypes among the tumors classified as HER2‐positive. This heterogeneity was further mirrored by the measurements of the total and truncated HER2 receptors in the primary tumors of the patients enrolled in our study. As expected, the levels of total HER2 positively correlate with benefit from anti‐HER2 therapy and high HER2 levels were coincided with a molecular HER2‐enriched signature. Our analysis has limitations due to the small number involved and the fact that a half of the patients received trastuzumab and the other half lapatinib. Combining the two groups was a necessary choice that we considered reasonable due to the fact that no difference emerged in the efficacy of the two treatments. The small sample size limited also the extent and quality of statistical analyses. However, our results reconcile a growing body of evidence suggesting that the heterogeneity of HER2‐positive breast cancer, which has been known for a long time (Perou et al., 2000), has a potentially substantial impact on clinical decision making. The most remarkable example regards co‐expression of hormone receptors that identifies a subgroup with a different clinical behavior, response to chemotherapy and potential for specific therapeutic strategies (Gianni et al., 2012; Rimawi et al., 2013a; Montemurro et al., 2012; Baselga et al., 2012b; Vaz‐Luis et al., 2013). Further studies will likely provide evidence of more complex biological heterogeneity and reveal a rationale for of specific treatment strategies. Along this line, although preliminary and needing confirmation, we believe that our data add an important piece of information in the increasingly diversified management of HER2‐positive breast cancer.
5. Conclusions
For the first time in the metastatic setting we show that a true “HER2‐enriched” gene expression status and/or a high ratio of H2T/p95 protein expression may represent a profile of clinical addiction to HER2 and a prerequisite for maximized benefit from targeting agents. HER2‐positive tumors deviating from this definition may need combined treatments (anti‐HER2 therapy with chemotherapy and/or perhaps endocrine therapy) to achieve optimal control.
Competing interests
FM has acted as a consultant for Hoffmann La Roche S.p.A and GlaxoSmithKline S.p.A. Uncompensated advisory role of AP for Nanostring Technologies. MS received speaker honoraria from GlaxoSmithKline. JS is an employee of LabCorp. JB had consultant/advisory roles at Genentech, Inc.
Supporting information
Supplementary data
Supplementary data
Acknowledgments
This work was partially funded by the BBVA Foundation. AP was funded by a Career Catalyst Grant from the Susan G. Komen Foundation and by the “Beca Roche en Onco‐Hematologia 2012”.
CPN is funded by Biennal AIRC Fellowship “Lorendana Gualandi Sabotti”
Supplementary data 1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2013.08.013.
Montemurro Filippo, Prat Aleix, Rossi Valentina, Valabrega Giorgio, Sperinde Jeff, Peraldo-Neia Caterina, Donadio Michela, Galván Patricia, Sapino Anna, Aglietta Massimo, Baselga José and Scaltriti Maurizio, (2014), Potential biomarkers of long‐term benefit from single‐agent trastuzumab or lapatinib in HER2‐positive metastatic breast cancer, Molecular Oncology, 8, doi: 10.1016/j.molonc.2013.08.013.
References
- Baselga, J. , Cortes, J. , Kim, S.B. , 2012. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med.. 366, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga, J. , Bradbury, I. , Eidtmann, H. , 2012. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 379, 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell, K.L. , Burstein, H.J. , Storniolo, A.M. , 2012. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J. Clin. Oncol.. 30, 2585–2592. [DOI] [PubMed] [Google Scholar]
- Carey, L.A. , Berry, D. , Ollila, D. , 2013. Clinical and translational results of CALGB 40601: a neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer. J. Clin. Oncol.. 31, 500 Meeting Abstracts [Google Scholar]
- Cortes, J. , Baselga, J. , Petrella, T. , 2009. Pertuzumab monotherapy following trastuzumab-based treatment: activity and tolerability in patients with advanced HER2- positive breast cancer. J. Clin. Oncol.. 27, 1022 Meeting Abstracts [DOI] [PubMed] [Google Scholar]
- Geyer, C.E. , Forster, J. , Lindquist, D. , 2006. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med.. 355, 2733–2743. [DOI] [PubMed] [Google Scholar]
- Gianni, L. , Pienkowski, T. , Im, Y.H. , 2012. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol.. 13, 25–32. [DOI] [PubMed] [Google Scholar]
- Gomez, H.L. , Doval, D.C. , Chavez, M.A. , 2008. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J. Clin. Oncol.. 26, 2999–3005. [DOI] [PubMed] [Google Scholar]
- Hamberg, P. , Bos, M.M. , Braun, H.J. , 2011. Randomized phase II study comparing efficacy and safety of combination-therapy trastuzumab and docetaxel vs. sequential therapy of trastuzumab followed by docetaxel alone at progression as first-line chemotherapy in patients with HER2+ metastatic breast cancer: HERTAX trial. Clin. Breast Cancer. 11, 103–113. [DOI] [PubMed] [Google Scholar]
- Huang, W. , Reinholz, M. , Weidler, J. , 2010. Comparison of central HER2 testing with quantitative total HER2 expression and HER2 homodimer measurements using a novel proximity-based assay. Am. J. Clin. Pathol.. 134, 303–311. [DOI] [PubMed] [Google Scholar]
- Inoue, K. , Nakagami, K. , Mizutani, M. , 2010. Randomized phase III trial of trastuzumab monotherapy followed by trastuzumab plus docetaxel versus trastuzumab plus docetaxel as first-line therapy in patients with HER2-positive metastatic breast cancer: the JO17360 trial group. Breast Cancer Res. Treat.. 119, 127–136. [DOI] [PubMed] [Google Scholar]
- Marty, M. , Cognetti, F. , Maraninchi, D. , 2005. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J. Clin. Oncol.. 23, 4265–4274. [DOI] [PubMed] [Google Scholar]
- Montemurro, F. , Rossi, V. , Rocca, M.C. , 2012. Hormone-receptor expression and activity of trastuzumab with chemotherapy in HER2-positive advanced breast cancer patients. Cancer. 118, 17–26. [DOI] [PubMed] [Google Scholar]
- Nielsen, T.O. , Parker, J.S. , Leung, S. , 2010. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin. Cancer Res.. 16, 5222–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, J.S. , Mullins, M. , Cheang, M.C. , 2009. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol.. 27, 1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram, M.D. , Konecny, G.E. , O'Callaghan, C. , 2004. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J. Natl. Cancer Inst.. 96, 739–749. [DOI] [PubMed] [Google Scholar]
- Perou, C.M. , Sorlie, T. , Eisen, M.B. , 2000. Molecular portraits of human breast tumours. Nature. 406, 747–752. [DOI] [PubMed] [Google Scholar]
- Piccart-Gebhart, M.J. , Procter, M. , Leyland-Jones, B. , 2005. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med.. 353, 1659–1672. [DOI] [PubMed] [Google Scholar]
- Prat, A. , Baselga, J. , 2013. Dual human epidermal growth factor receptor 2 (HER2) blockade and hormonal therapy for the treatment of primary HER2-positive breast cancer: one more step toward chemotherapy-free therapy. J. Clin. Oncol.. 31, 1703–1706. [DOI] [PubMed] [Google Scholar]
- Prat, A. , Parker, J.S. , Karginova, O. , 2010. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res.. 12, R68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat, A. , Cheang, M.C. , Martin, M. , 2013. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal a breast cancer. J. Clin. Oncol.. 31, 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimawi, M.F. , Mayer, I.A. , Forero, A. , 2013. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J. Clin. Oncol.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimawi, M.F. , Mayer, I.A. , Forero, A. , 2013. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J. Clin. Oncol.. 31, 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romond, E.H. , Perez, E.A. , Bryant, J. , 2005. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med.. 353, 1673–1684. [DOI] [PubMed] [Google Scholar]
- Slamon, D.J. , Clark, G.M. , Wong, S.G. , 1987. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 235, 177–182. [DOI] [PubMed] [Google Scholar]
- Slamon, D.J. , Leyland-Jones, B. , Shak, S. , 2001. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med.. 344, 783–792. [DOI] [PubMed] [Google Scholar]
- Sperinde, J. , Jin, X. , Banerjee, J. , 2010. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin. Cancer Res.. 16, 4226–4235. [DOI] [PubMed] [Google Scholar]
- Therasse, P. , Arbuck, S.G. , Eisenhauer, E.A. , 2000. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst.. 92, 205–216. [DOI] [PubMed] [Google Scholar]
- Vaz-Luis, I. , Winer, E.P. , Lin, N.U. , 2013. Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes?. Ann. Oncol.. 24, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, S. , Miles, D. , Gianni, L. , 2012. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med.. 367, 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, C.L. , Cobleigh, M.A. , Tripathy, D. , 2002. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol.. 20, 719–726. [DOI] [PubMed] [Google Scholar]
- Yarden, Y. , Sliwkowski, M.X. , 2001. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol.. 2, 127–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data


