Abstract
Dual specificity phosphatase 1 (DUSP1) and the transcription factor NF‐κB are implicated in prostate cancer since their expression levels are altered along this disease, although there are no evidences up to date demonstrating a crosstalk between them. In this report, we show for the first time that DUSP1 over‐expression in DU145 cells promotes apoptosis and decreases NF‐κB activity by blocking p65/NF‐κB nuclear translocation. Moreover, although DUSP1 impairs TNF‐α‐induced p38 MAPK and JNK activation, only the specific inhibition of p38 MAPK exerts the same effects than DUSP1 over‐expression on both apoptosis and NF‐κB activity. Consistently, DUSP1 promotes apoptosis and decreases NF‐κB activity in cells in which p38 MAPK is induced by TNF‐α treatment. These results demonstrate that p38 MAPK is specifically involved in DUSP1‐mediated effects on both apoptosis and NF‐κB activity. Interestingly, we show an inverse correlation between DUSP1 expression and activation of both p65/NF‐κB and p38 MAPK in human prostate tissue specimens. Thus, most of apparently normal glands, benign prostatic hyperplasia and low‐grade prostatic intraepithelial neoplasia samples show high DUSP1 expression and low levels of both nuclear p65/NF‐κB and activated p38 MAPK. By contrast, DUSP1 expression levels are low or even absent in high‐grade prostatic intraepithelial neoplasia and prostatic adenocarcinoma samples, whereas nuclear p65/NF‐κB and activated p38 MAPK are highly expressed in the same samples. Overall, our results provide evidence for a role of DUSP1 in the apoptosis of prostate cancer cells, through a mechanism involving the inhibition of p38 MAPK and NF‐κB. Furthermore, our findings suggest that the ratio between DUSP1 and p65/NF‐κB expression levels, rather than the individual expression of both molecules, is a better marker for diagnostic purposes in prostate cancer.
Keywords: Dual specificity phosphatase 1, NF-κB, Apoptosis, p38 MAPK, Prostate cancer
Highlights
DUSP1 promotes apoptosis in prostate cancer cells through the inhibition of p38 MAPK
DUSP1 impairs NF‐κB activity through a mechanism involving p38 MAPK inhibition.
DUSP1 expression inversely correlates with NF‐κB in human prostate tissue specimens.
The ratio DUSP1/NF‐κB can be useful for diagnostic purposes in prostate cancer.
Abbreviations
- DUSP1
Dual specificity phosphatase 1
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor κb
- TNF-α
tumor necrosis factor alpha
- PC
prostatic adenocarcinoma
- PIN
prostatic intraepithelial neoplasia
- BPH
benign prostatic hyperplasia
- PI
propidium iodide
- TMRM
tetramethylrhodamine methyl ester
- FITC
fluorescein isothiocyanate
- PARP
poly (ADP-ribose) polymerase
1. Introduction
Prostate cancer is the most common cancer in men and the second cause of male cancer death (Jemal et al., 2008). Clinical studies have shown that prostatic adenocarcinoma (PC) needs a long period to develop from prostatic intraepithelial neoplasia (PIN). Initial treatment of this disease is usually partial prostatectomy or radiation to remove or destroy the cancerous cells. However, in many cases, this cancer recurs in an androgen‐dependent fashion, and the androgen ablation is then the main therapy. Unfortunately, many patients fail this therapy and develop recurrent androgen‐independent prostate cancer. In these cases, the lack of effective therapies is related to poor understanding of the molecular mechanisms underlying the progression of this disease (Feldman and Feldman, 2001).
Although the complete mechanisms involved in the development and progression of prostate cancer are not fully understood, some of them include the mitogen‐activated protein kinases (MAPK) family (Dhillon et al., 2007). In particular, p38 MAPK has been previously shown to be involved in prostate cancer cells, where it controls diverse cellular functions (Boutros et al., 2008), including apoptosis, although its exact role in this disease appears to be complex. p38 MAPK activation is achieved by phosphorylation of its catalytic domain, and down‐regulation of its signalling is mediated by dephosphorylation of this domain by dual specificity phosphatases (DUSP) (Keyse, 2000). The founder member of this family, DUSP1, is an inducible phosphatase that has been increasingly related to cancer, although its role remains unclear (Keyse, 2008). Thus, DUSP1 is over‐expressed only in the early stages of several human tumors, including prostate, colon and bladder, whereas other cancers show significant DUSP1 expression even at late stages of the disease (Loda et al., 1996). Regarding prostate, higher levels of DUSP1 protein expression have been found in benign prostatic hyperplasia (BPH) and in PIN, whereas DUSP1 protein and mRNA expression decreases in parallel with tumor progression and is almost completely absent in high‐grade PC (Rauhala et al., 2005), as well as in metastasis to the lymph nodes derived from PC (Magi‐Galluzzi et al., 1997). These data suggest that DUSP1 regulates prostate cancer progression, although, up to date, there are no mechanistic studies supporting this hypothesis.
The nuclear factor κB (NF‐κB) signalling cascade is a second marker altered in prostate cancer. In a resting state, NF‐κB proteins are sequestered in the cytoplasm as homo‐ or heterodimers (usually the heterodimer p50/p65) by association with the IκB inhibitory proteins. Cellular stimulation by different molecules such as the pro‐inflammatory cytokine TNF‐α activates an IκB‐kinase (IκK) complex to phosphorylate the IκB proteins. Subsequent poly‐ubiquitination and proteasomal degradation of IκB leads to the translocation of NF‐κB dimers into the nucleus, where they bind to specific DNA sequences modulating expression of over 150 target genes (Oeckinghaus et al., 2011). Basal activation of NF‐κB has been observed in several cancers, and its effects have been linked to proliferation in the absence of growth signals and evasion of apoptosis (Naugler and Karin, 2008). Analysis of clinical prostate cancer samples have shown that p65/NF‐κB expression increases with the progression of the disease, being negative in normal prostate samples, low in BPH and low‐grade PIN, and elevated in high‐grade PIN and PC (Nunez et al., 2008; Royuela et al., 2008). This fact correlates with the increased NF‐κB basal activity observed in several prostate cancer cell lines, which can be enhanced by treatment with TNF‐α (Chopra et al., 2004; Rodriguez‐Berriguete et al., 2012). Moreover, NF‐κB activation plays an important role in prostate cancer cells, since the blockage of this pathway results in increased apoptosis (Rodriguez‐Berriguete et al., 2012).
As mentioned above, several studies with human prostate cancer specimens have shown that p65/NF‐κB levels increase as the tumor malignancy augments, whereas DUSP1 levels decrease, however there are no evidences of a connexion between both events so far. In other tissues, DUSP1 and its target p38 MAPK, have been shown to be NF‐κB regulators. In this sense, we and other groups have demonstrated that DUSP1 controls NF‐κB activation through a mechanism involving p38 MAPK in tumoral pituitary (Lasa et al., 2010) and pulmonary cells (King et al., 2009). Moreover, p38 MAPK has been shown to play a role in NF‐κB‐dependent gene expression in different cell types (Oeckinghaus et al., 2011). Here, we analyse whether DUSP1 also regulates NF‐κB activity in prostate cancer cells, as well as its role on apoptosis, and the mechanisms underlining this effect. Our data demonstrate for the first time that DUSP1 promotes apoptosis in DU145 cells, and we provide evidences that this effect is specifically mediated by the inhibition of p38 MAPK. In addition, our results show that DUSP1 over‐expression significantly decreases NF‐κB activity by blocking p65/NF‐κB nuclear translocation in DU145 cells, through a p38 MAPK‐dependent mechanism. Interestingly, we also demonstrate for the first time a significant inverse correlation between the levels of expression of DUSP1 and both nuclear p65/NF‐κB and activated p38 MAPK in samples from patients with BPH, high‐grade PIN lesions or PC.
In summary, our results demonstrate that re‐expression of DUSP1 in prostate cancer cells is a good strategy to induce apoptosis, which occurs through a mechanism involving the inhibition of the p38 MAPK/NF‐κB signalling pathway, and suggest that this phosphatase is a suitable tool to block tumor progression. Moreover, our data strongly suggest that the ratio between DUSP1 and p65/NF‐κB expression levels, rather than the individual expression of both molecules, represent a more reliable candidate marker for diagnostic purposes in prostate cancer.
2. Materials and methods
2.1. Cell lines, inhibitors, plasmids, transfection and luciferase assay
DU145 and PC3 prostate cancer cells were purchased from the American Tissue Culture Collection, (Rockville, MD, USA), and were cultured as recommended. The inhibitors SB203580, SB202190 and SP600125 were from Calbiochem (Merck Chemicals, Barcelona, Spain). The pCMV‐DUSP1 and the 3xNF‐κB‐TK‐Luc reporter plasmids were previously described (Lasa et al., 2010; Yano et al., 1987). Cells were transfected with the plasmids by using Fugene reagent (Promega, Biotech Iberica) as recommended. The NF‐κB reporter assays were performed as described (Chiloeches et al., 2008).
2.2. Cell extracts, western blot analysis, antibodies and quantitative RT‐PCR
Nuclear and total cell extracts preparation and Western blot analysis were performed as previously described with minor modifications (Chiloeches et al., 2008). The antibodies used were anti‐p65/NF‐κB, anti‐DUSP1, anti‐p38 MAPK, anti‐JNK1, and anti‐PARP (Santa Cruz Biotechnology, Heidelberg, Germany); anti‐phospho‐p38 MAPK (Cell Signalling Technology, Izasa S.A., Barcelona, Spain); anti‐phospho‐JNK (Promega, Promega Biotech Ibérica, Madrid, Spain); anti‐histone H3 (Abcam, Cambridge, UK); anti‐tubulin (Sigma Aldrich, Madrid, Spain); peroxidase‐conjugated secondary antibodies (GE Healthcare Europe GMBH, Barcelona, Spain).
Total RNA isolation and real‐time quantitative RT‐PCR validations were performed as previously described (Chiloeches et al., 2008). The gene‐specific primers were as follows: DUSP1: forward (5′‐CCT GAC AGC GCG GAA TCT‐3′), reverse (5′‐GAT TTC CAC CGG GCC AC‐3′); 18S: forward (5′‐CCA GTA AGT GCG GGT CAT AAG C‐3′), reverse (5′‐CCT CAC TAA ACC ATC CAA TCG G‐3′). ΔΔCt method was used to quantify changes in gene expression, and to calculate the relative changes normalized against the 18S gene.
2.3. Quantification of sub‐G1 DNA content and apoptosis assays
Quantification of cellular fractions with sub‐G1 DNA content was carried out by flow cytometry analysis of propidium iodide (PI)‐stained cells. Adherent and floating cells were collected after treatment, washed with ice‐cold PBS, fixed with 70% ice‐cold ethanol, and washed twice with PBS and treated with RNase (1 mg/ml). Cellular DNA was stained with PI (5 ng/ml; Sigma Aldrich) and cells were analysed by flow cytometric analysis. Percentages of cells in different cell cycle phases were calculated from DNA histograms. Cells with sub‐G1 DNA content were considered apoptotic.
Apoptosis was quantified by the Annexin V/PI dual staining assay. After transfection and/or treatment, both adherent and floating cells were incubated with a mixture of fluorescein isothiocyanate‐conjugated annexin V reagent (Annexin V‐FITC, Immunostep, Salamanca, Spain) and PI (3 mM). Samples were analysed by flow cytometry on a Beckton Dickinson FACScanto flow cytometer (BD Biosciences) and the percentages of Annexin V+/PI− marked cells were quantified. For TMRM (tetramethylrhodamine methyl ester) assay, both adherent and floating cells were loaded with TMRM reagent (50 nM, Invitrogen, Barcelona, Spain). The percentages of cells with low mitochondrial membrane potential were measured by flow cytometric analysis.
2.4. Experimental subjects and immunohistochemistry of prostate tissues
For immunohistochemical screening, we analysed the paraffin‐embedded samples from patients diagnosed of BPH (n = 9) or PC (n = 35), most of which had Gleason scores ≥7. Approval for the study was obtained from the local ethical committee. The apparently normal tissue as well as the low‐ and high‐grade PIN present in the selected samples was also analysed. Briefly, five‐micrometre sections were xylene‐treated and rehydrated in a graded ethanol series. Antigen retrieval was performed with 10 mM citrate buffer for 3 min. Endogenous fluorescence was quenched by incubation with 4% sodium borohydride in Tris buffered saline pH 7.6. Non‐specific immunoreactions were prevented by incubation with blocking solution (3% normal donkey serum, 0.05% triton X‐100 in Tris buffered saline, pH 7.5), and DUSP1, p65/NF‐κB or phospho‐p38 MAPK detection was achieved using specific antibodies diluted in blocking solution. After washing, samples were incubated for 5 min with a rabbit peroxidase‐conjugated secondary antibody (polimer‐based system Mas Vision, Master Diagnostica, Spain), and revealed using 3′‐diaminobenzidine substrate. Staining of nuclei was performed by using Caracci's hematoxylin, and negative controls were performed by incubating samples with non‐immune rabbit serum instead of primary antibodies. Sections were coverslipped using a permanent mounting medium (Entellan, Merck). The immunostaining was ranged into four categories based on the staining pattern in the majority of tumor cells in the whole section (0: negative; 1: weakly positive; 2–3: moderately positive; and 4: highly positive).
2.5. Statistical analysis
All data were expressed as means ± standard deviation. The student's t test was performed using the SSC‐Stat software (V2.18, University of Reading, United Kingdom). The statistical significance of difference between groups was expressed by asterisks (*0.01 < p < 0.05; **0.001 < p < 0.01; ***p < 0.001).
3. Results
3.1. DUSP1 promotes apoptosis in DU145 prostate cancer cells
DUSP1 is an inducible phosphatase that regulates apoptosis in different tumors, although the molecular mechanisms for its implication in this process remain controversial (Boutros et al., 2008). In order to investigate the role of this phosphatase on survival in prostate cancer cells, we over‐expressed DUSP1 in DU145 cell line, as these cells have very low endogenous levels of this phosphatase. DUSP1 over‐expression efficiency was analysed by measuring its mRNA and protein levels in extracts from cells transfected with a control plasmid or a plasmid encoding DUSP1. Our data showed a significant increase on both DUSP1 mRNA and protein expression levels (Figure 1A), thus validating our method for over‐expressing DUSP1 in DU145 cells.
Figure 1.
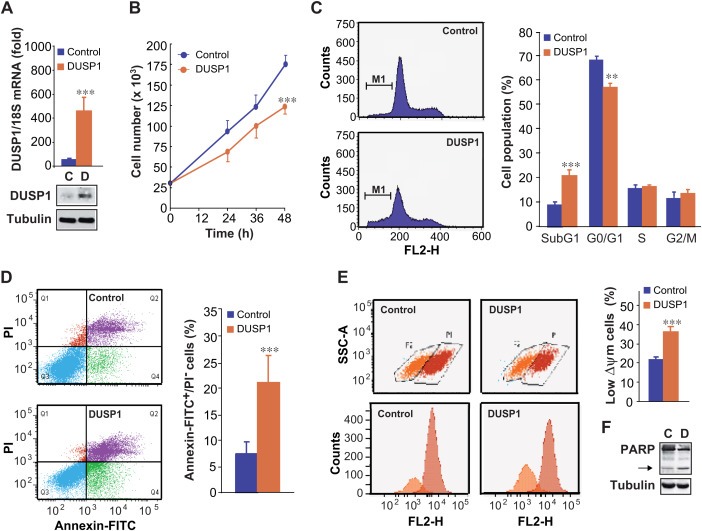
DUSP1 promotes apoptosis in DU145 cells. (A) Cells were transiently transfected for 48 h with a control vector (C) or a vector encoding DUSP1 (D), total RNA was isolated and the levels of DUSP1 mRNA were monitored by quantitative RT‐PCR. DUSP1 mRNA levels were normalized with 18S mRNA levels and the results were expressed as the fold change in mRNA expression. Total cellular protein lysates were analysed by western blotting with antibodies against DUSP1 and tubulin, to assess equal loading protein. (B) Cells were transfected for the indicated times as in (A), and counted. The asterisks show the statistical significance of differences between the groups: DUSP1 vs. control. (C) Cells were transfected for 48 h as in (A), and quantification of percentages in cell cycle phases was performed by PI staining. The graph shows the quantification of the results obtained from three independent experiments performed as in (C). (D) Cells were transfected for 48 h as in (A), and apoptosis was examined by the Annexin V/PI dual‐staining assay, as detected by flow cytometry. Annexin V+/PI‐ marked cells were considered apoptotic (Q4 population). The graph shows the quantification of the results obtained from four independent experiments performed as in (D). (E) Cells were transfected for 48 h as in (A) and apoptosis was determined by the TMRM assay, as detected by flow cytometry. The graph shows the quantification of the results obtained from four independent experiments performed as in (E). (F) Cells were transfected for 48 h as in (A), and western blotting was performed to analyse PARP cleavage expression, ensuring equal loading with tubulin. The arrow indicates cleaved PARP. FITC, Fluorescein isothiocyanate; PI, Propidium iodide; TMRM, tetramethylrhodamine methyl ester
To address the involvement of DUSP1 on apoptosis, we first examined the proliferation rate in DUSP1‐expressing cells, and our data showed that this phosphatase significantly reduced proliferation rate over time (Figure 1B). In order to distinguish between proliferation and survival, we next performed analysis of cell cycle to determine the percentage of cells in S and G2/M phases in DUSP1‐expressing cells. Our data revealed that DUSP1 over‐expression did not affect the percentage of cell population in either S or G2/M phases (Figure 1C). However, this phosphatase decreased the percentage of cells in G0/G1 phase and induced a significant increase on the sub‐G1 hypodiploid cell population, reaching a 20%, compared to the 8% observed in control cells transfected with the empty vector (Figure 1C). To confirm these results, we determined the level of phosphatidyl serine exposed outside the cell membrane by labelling DUSP1‐expressing cells with Annexin V‐FITC. Accordingly, our results revealed that DUSP1 produced a significant increase in basal levels of Annexin V+/PI− marked cells, indicative of apoptosis (Figure 1D). We also examined by TMRM staining the percentage of cells undergoing a decrease in mitochondrial membrane potential (ΔΨm), another characteristic parameter of the process of apoptosis. Our data showed that DUSP1 over‐expression significantly increased the population with low ΔΨm from 20% up to 38% (Figure 1E). Finally, we analysed the effects of DUSP1 on the caspase‐mediated cleavage of poly (ADP‐ribose) polymerase (PARP), and observed a significant increase of PARP cleavage in DUSP1‐expressing cells (Figure 1F).
To rule out cell‐type specific effects of DUSP1, we also studied its role on apoptosis in PC3 prostate cancer cells, which neither express this phosphatase in basal conditions, as observed in DU145 cells. As expected, DUSP1 expression was only detected after transfection with the plasmid encoding this phosphatase (Supplementary Figure S1A). Similarly to the effect observed in DU145 cells, DUSP1 significantly decreased the proliferation rate of PC3 cells over time (Supplementary Figure S1B). Moreover, this phosphatase also promoted apoptosis in these cells, increasing the population with low ΔΨm from 7.5% up to 27% (Supplementary Figure S1C).
Our results altogether show that DUSP1 promotes apoptosis in different prostate cancer cell lines, revealing a potential relevance of this phosphatase on prostate cancer progression.
3.2. The inhibition of p38 MAPK specifically induces apoptosis in DU145 cells
Previous reports have identified p38 MAPK as the major target for DUSP1 in different cell types (Lang et al., 2006). For this reason, we next analysed whether p38 MAPK was a target of DUSP1 also in prostate cancer cells. To carry out these experiments, we used TNF‐α as p38 MAPK inducer and, as expected, we observed that this cytokine induced p38 MAPK phosphorylation in a time‐dependent manner, while DUSP1 expression prevented this phosphorylation (Figure 2A). Since DUSP1 inhibited p38 MAPK in DU145 cells, we then studied whether the inactivation of this kinase by using its specific inhibitor, SB203580, exerted the same effects than DUSP1 over‐expression on cellular apoptosis. The inhibitory effect of SB203580 on p38 MAPK activity was confirmed by monitoring the levels of phospho‐MK2 in TNF‐α‐treated cells incubated with the inhibitor (Figure 2B). As we observed after DUSP1 over‐expression, a significant decrease in cell number was achieved upon 48 h of treatment with SB203580 (Figure 2C). Since previous reports had already shown that incubation of different prostate cancer cells in the presence of SB203580 inhibitor does not affect the distribution of cells either in the S or G2/M phases (Rodriguez‐Berriguete et al., 2012; Shin et al., 2012; Sun et al., 2010), we next determine the involvement of p38 MAPK on cellular apoptosis, by measuring the number of cells with low ΔΨm after incubation with the SB203580 inhibitor. Our data indicated that the population showing low ΔΨm significantly increased after incubation with the p38 MAPK inhibitor, compared to control cells (38% vs. 19%; Figure 2D). To verify that blockage of p38 MAPK induced apoptosis, we also examined the effect of SB203580 inhibitor on PARP cleavage. The obtained data confirmed that the abrogation of p38 MAPK activity achieved by incubation with the SB203580 inhibitor increased PARP cleavage (Figure 2E). The pro‐apoptotic role of p38 MAPK inhibition in DU145 cells was further investigated by using another specific pharmacological inhibitor of this kinase in these cells, SB202190 (Chen et al., 2012; Mehta et al., 2001). As expected, our data showed that this inhibitor exerted similar effects on cellular apoptosis than either the SB203580 inhibitor or DUSP1 over‐expression (Supplementary Figure S2).
Figure 2.
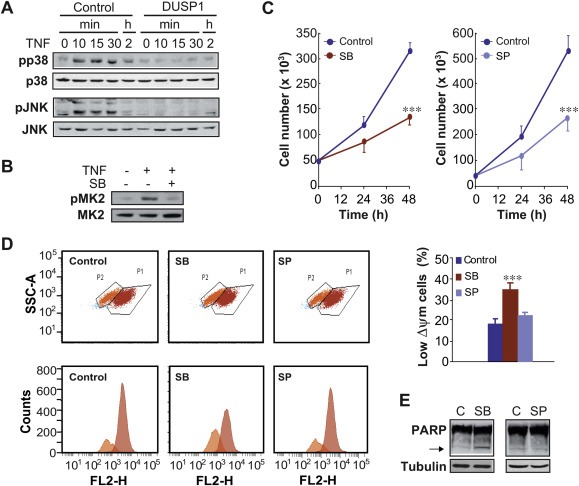
The inhibition of p38 MAPK specifically induces apoptosis in DU145 cells. (A) Cells were transfected for 48 h with a control vector or a vector encoding DUSP1, incubated with TNF‐α (10 ng/ml) for the indicated times and total cell lysates were analysed by western blotting with antibodies against both phosphorylated and total p38 MAPK or JNK. (B) Cells were incubated for 48 h with SB203580 (1 μM), and then treated for 15 min in the absence or presence of TNF‐α (10 ng/ml). Total cell lysates were analysed by western blotting with antibodies against both phosphorylated and total MAPKAPK‐2 (MK2). (C) Cells were incubated with either SB203580 (1 μM) or SP600125 (10 μM) and counted at the indicated times. The asterisks show the statistical significance of differences between the groups: SB vs. control or SP vs. control. (D) Cells were incubated for 48 h with either SB203580 (1 μM) or SP600125 (10 μM), and apoptosis was assayed by the TMRM assay. The graph shows the quantification of the results obtained from three independent experiments performed as in (D). (E) Cells were treated as in (D), and apoptosis was determined as in Figure 1(F). The arrow indicates cleaved PARP.
DUSP1 over‐expression could be regulating cellular apoptosis by affecting pathways different from p38 MAPK, such as JNK. For this reason, we analysed whether JNK was also a target of DUSP1 in DU145 cells. First, we observed that TNF‐α induced JNK phosphorylation in a time‐dependent manner and DUSP1 expression prevented it (Figure 2A). We then studied whether the inactivation of this kinase by using its specific inhibitor, SP600125, exerted the same effects than DUSP1 expression on cellular apoptosis. To this purpose, we examined the proliferation rate, and we observed that the SP600125 inhibitor caused a significant decrease in cell number after 48 h of treatment (Figure 2C). However, in contrast to what happened with p38 MAPK inhibition, the inactivation of JNK did not significantly affect either the percentage of population showing low ΔΨm (19.7% vs. 18.4%; Figure 2D), or the PARP cleavage (Figure 2E).
These results altogether demonstrate that the inhibition of p38 MAPK, but not that of JNK, and the over‐expression of DUSP1 exert the same effects on apoptosis in DU145 cells, suggesting that this phosphatase regulates this process by specifically inhibiting p38 MAPK.
3.3. DUSP1 induces apoptosis in DU145 cells through the inhibition of p38 MAPK
Since we had demonstrated that TNF‐α induced p38 MAPK activity in DU145 cells, and DUSP1 over‐expression inhibited it, we next analysed the effect of DUSP1‐mediated inhibition of p38 MAPK on the apoptosis of cells in which this kinase was activated by treatment with TNF‐α. To this purpose, we determined the sub‐G1 hypodiploid cell population in DUSP1‐expressing cells treated with TNF‐α. Our data showed that treatment with this cytokine of cells transfected with the empty vector did not significantly modify either the sub‐G1 population, or the percentage of cells in G0/G1 S and G2/M phases (Figure 3A). Moreover, similarly to the effect of DUSP1 in basal cells, this phosphatase did not affect cell population in either S or G2/M phases in TNF‐α‐treated cells, whereas it decreased the percentage of cells in G0/G1 phase, and increased the sub‐G1 hypodiploid cell population up to about 21% in TNF‐α‐treated cells (Figure 3A). To confirm these results, we determined the apoptosis level after labelling cells with Annexin V‐FITC under the same conditions. As expected, the results revealed that DUSP1 produced a significant increase in the levels of Annexin V+/PI− marked cells treated with TNF‐α, when compared to cells expressing the empty vector in which p38 MAPK was also activated by incubation with TNF‐α (Figure 3B). Finally, we also examined the percentage of cells with low ΔΨm, and our data confirmed that DUSP1 over‐expression significantly increased the number of apoptotic cells after TNF‐α treatment (Figure 3C).
Figure 3.
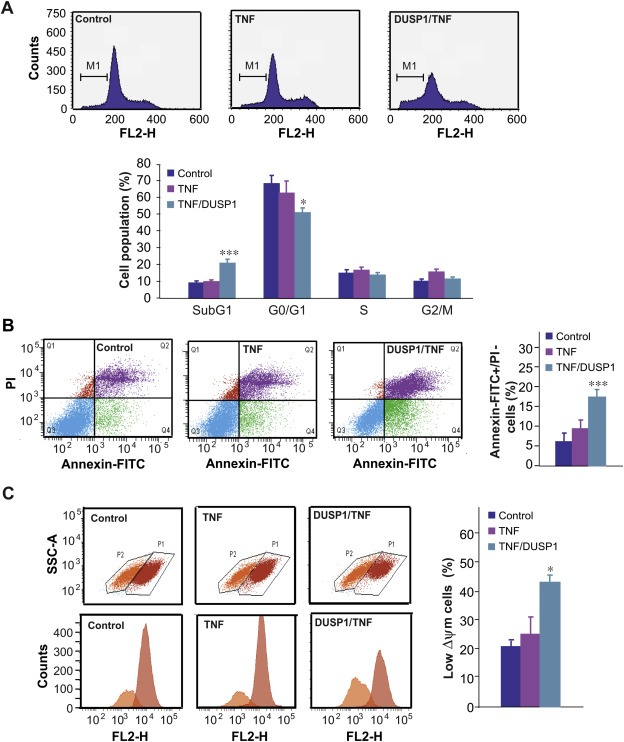
DUSP1 induces apoptosis in DU145 cells through the inhibition of p38 MAPK. (A) Cells were transfected with a control vector or a vector encoding DUSP1, incubated with TNF‐α (10 ng/ml) for 48 h, and quantification of percentages in cell cycle phases was assayed as in Figure 1(C). The graph shows the quantification of the results obtained from three independent experiments. (B) Cells were transfected and treated as in (A), and apoptosis was examined as in Figure 1(D). The graph shows the quantification of the results obtained from four independent experiments. (C) Cells were transfected and treated as in (A), and apoptosis was examined as in Figure 1(E). The graph shows the quantification of the results obtained from four independent experiments.
In order to analyse whether DUSP1 also induced apoptosis in TNF‐α‐treated PC3 cells, we carried out similar experiments to those performed in DU145 cells. Our results showed that DUSP1 prevented the phosphorylation of p38 MAPK induced by TNF‐α, and suggested that this kinase is the mediator of DUSP1 effects also in these cells (Supplementary Figure S1D). Moreover, similarly to what happened in DU145 cells, DUSP1 over‐expression also significantly reduced the proliferation rate (Supplementary Figure S1E), and promoted apoptosis (Supplementary Figure S1F) in TNF‐α‐treated PC3 cells.
Taken together, these data demonstrate that DUSP1 over‐expression induces apoptosis in prostate cancer cells through the inhibition of p38 MAPK.
3.4. DUSP1 regulates the NF‐κB pathway in DU145 cells through p38 MAPK inhibition
NF‐κB is an important survival regulator in prostate cancer cells (Chopra et al., 2004; Rodriguez‐Berriguete et al., 2012), which is activated by p38 MAPK in several cellular contexts (Baeza‐Raja and Munoz‐Canoves, 2004; Calleros et al., 2006; King et al., 2009; Lasa et al., 2010; Vanden Berghe et al., 1998). Because we had demonstrated that DUSP1 impaired p38 MAPK activity, and this inhibition promoted apoptosis in DU145 cells, we wondered whether the inhibition of p38 MAPK, achieved by either treatment with SB203580 or DUSP1 over‐expression, also prevented NF‐κB activity in these cells. To this purpose, we first performed experiments to determine the effect of the specific p38 MAPK inhibitor, SB230580, on NF‐κB activity. The results demonstrated that TNF‐α significantly increased NF‐κB activity by about four fold, and the treatment with the SB203580 inhibitor prevented both basal and TNF‐α‐induced NF‐κB activity (Figure 4A). To confirm the regulation of NF‐κB activity by p38 MAPK, we also measured p65/NF‐κB nuclear translocation in cells treated with the SB203580 inhibitor. As expected, TNF‐α increased p65/NF‐κB nuclear levels and SB203580 partially prevented this effect (Figure 4B). These results were verified by using another specific p38 MAPK inhibitor, SB202190, which was also able to impair the entry of p65/NF‐κB to the nucleus (Supplementary Figure S3).
Figure 4.
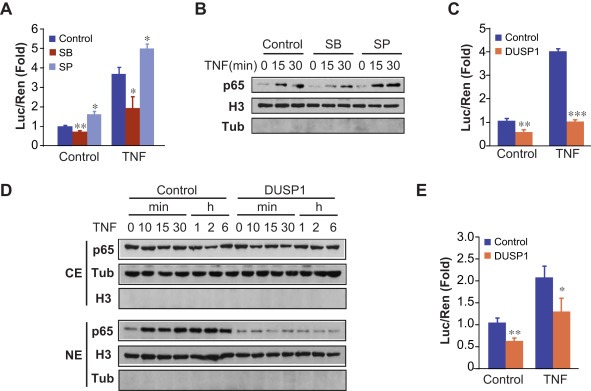
DUSP1 regulates the NF‐κB pathway in DU145 cells through p38 MAPK inhibition. (A) DU145 cells were transfected with the NF‐κB luciferase reporter plasmid and the pRL‐TK‐Renilla plasmid. Cells were incubated for 48 h with either SB203580 (1 μM) or SP600125 (10 μM), treated in the absence or presence of TNF‐α (10 ng/ml) during the last 6 h, and cell extracts were prepared and assayed for luciferase and renilla activities. The luciferase levels were normalized to those of renilla, and were expressed as the induction over the controls. (B) DU145 cells were incubated for 48 h with either SB203580 (1 μM) or SP600125 (10 μM) and then treated with TNF‐α (10 ng/ml) for the indicated times. Nuclear extracts were analysed by western blotting using antibodies against p65. The levels of tubulin and histone H3 were determined as controls to validate the integrity of the cytosolic and nuclear fractions, respectively. (C) DU145 cells were co‐transfected for 48 h with the plasmids described in (A) and a vector encoding DUSP1, then incubated with TNF‐α (10 ng/ml) for the last 6 h, processed and analysed as in (A). (D) DU145 cells were transfected for 48 h with a vector encoding DUSP1 and then incubated with TNF‐α (10 ng/ml) for the indicated times. The cytosolic extracts (CE) and the nuclear extracts (NE) were analysed by western blotting using antibodies against p65. The levels of tubulin and histone H3 were determined as controls to validate the integrity of the cytosolic and nuclear fractions, respectively. (E) PC3 cells were co‐transfected for 48 h with the plasmids described in (A) and a vector encoding DUSP1, then incubated with TNF‐α (10 ng/ml) for the last 6 h, processed and analysed as in (A).
Next, we studied whether the inhibition of p38 MAPK by DUSP1 over‐expression also affected NF‐κB activity. First, we demonstrated that over‐expression of this phosphatase significantly reduced NF‐κB activity by about 50% in basal conditions. Moreover, DUSP1 abolished the induction of NF‐κB achieved upon p38 MAPK activation by TNF‐α treatment (Figure 4C). Furthermore, we examined the effect of DUSP1 on TNF‐α‐induced p65/NF‐κB nuclear translocation, and found that DUSP1 over‐expression strongly reduced p65/NF‐κB nuclear levels (Figure 4D). Finally, to further confirm the role of DUSP1 in NF‐κB activation in prostatic tumor cells, we overexpressed DUSP1 in PC3 cells. Similarly to the effect observed in DU145 cells, DUSP1 over‐expression in PC3 cells significantly reduced NF‐κB‐dependent transcription, both in basal and TNF‐α‐treated cells (Figure 4E).
The observed partial effect of SB203580 inhibitor preventing TNF‐α‐induced NF‐κB activation and p65/NF‐κB nuclear translocation could be indicating that DUSP1 over‐expression would be regulating alternative pathways such as JNK, which in turn could affect NF‐κB activation. We next analyzed this possibility, and, opposite to the data observed after inhibition of p38 MAPK, our results showed that the blockage of JNK activation using the specific inhibitor SP600125 did not impair either TNF‐α‐mediated NF‐κB transcriptional activation (Figure 4A) or the translocation of p65/NF‐κB to the nucleus induced by the cytokine (Figure 4B). These data indicate that the inhibitory effect on NF‐κB activity achieved by DUSP1 over‐expression is not mediated by the abrogation of JNK activity induced by this phosphatase.
These results altogether demonstrate that DUSP1 negatively regulates NF‐κB activity in DU145 cells by decreasing p65/NF‐κB nuclear translocation, through a mechanism involving p38 MAPK inhibition.
3.5. DUSP1 expression inversely correlates with both nuclear p65/NF‐κB and activated p38 MAPK in human prostate tissue specimens
Our results strongly suggested that p65/NF‐κB activity is dependent on DUSP1 expression. To investigate the relevance of such regulation in samples from patients with prostate cancer, we analysed the distribution of DUSP1 and p65/NF‐κB in a series of human prostate tissue specimens. The results of DUSP1 or p65/NF‐κB immunohistochemical stainings appeared to be very similar between the cohorts. The majority of BPH and low‐grade PIN samples showed a weak expression of p65/NF‐κB (Figure 5A‐a, d), and a high DUSP1 expression (Figure 5A‐b, e). Conversely, a moderate‐high p65/NF‐κB expression was detected in high‐grade PIN (Figure 5A‐g), whereas DUSP1 expression was lower in the same samples (Figure 5A‐h). In addition, most of the invasive PC samples showed high p65/NF‐κB expression (Figure 5A‐j), reaching the maximum level in androgen‐resistant PC (Figure 5A‐m). By contrast, DUSP1 expression was very weak or even undetectable in these PC specimens (Figure 5A‐k,n). Activation of p65/NF‐κB in human samples was demonstrated by analysing its nuclear localization, which was evident in 77% of the specimens studied (Supplementary Figure S4). Considering all the samples tested (n = 35), the percentage of them showing an inverse correlation between p65/NF‐κB and DUSP1 expression levels was about 80% (Figure 5B). This inverse correlation was even more significant when considering only samples from patients developing the most aggressive disease (androgen‐resistant PC, positive node and metastatic samples). In these cases (n = 13), 92% of samples showed high levels of p65/NF‐κB expression and very low or even absent DUSP1 expression (Figure 5C).
Figure 5.
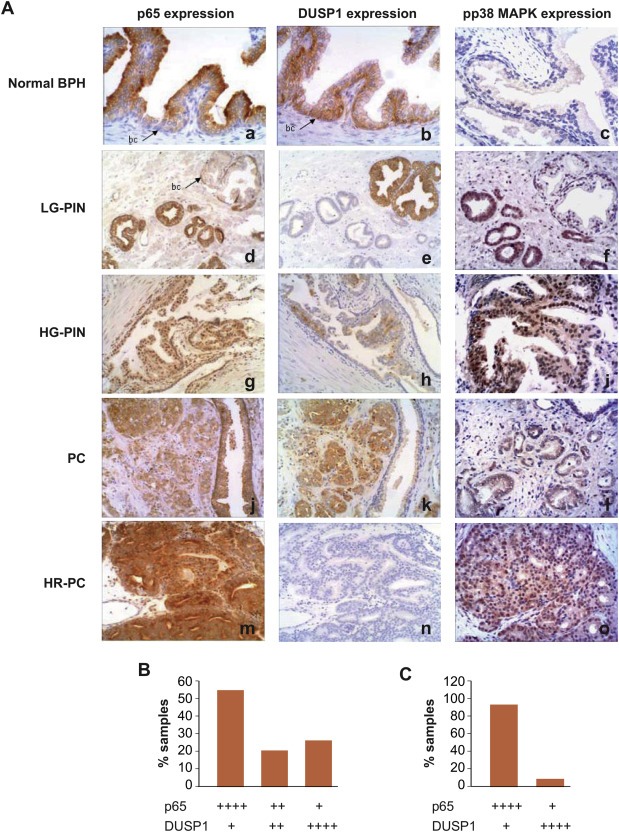
DUSP1 expression inversely correlates with both nuclear p65/NF‐κB and activated p38 MAPK in human prostate tissue specimens. (A) Immunohistochemical analysis of p65, DUSP1 and phospho‐p38 MAPK expression levels from human prostate cancer specimens. (B) Percentages of all samples tested (n = 35) expressing different levels of both p65 and DUSP1. (C) Percentages of samples with the worst prognosis (n = 13) expressing different levels of both p65 and DUSP1. The immunostaining intensities in (B) and (C) are indicated by crosses ranged into four categories based on the staining pattern in the majority of tumor cells in the whole section (+, weakly positive or absent; ++, moderately positive; and ++++, highly positive). BPH: benign prostatic hyperplasia; LG‐PIN: low‐grade prostatic intraepithelial neoplasia; HG‐PIN: high‐grade prostatic intraepithelial neoplasia; PC: prostatic adenocarcinoma; HR‐PC: hormone‐resistant prostatic adenocarcinoma.
Because our results in prostate cancer cells revealed that DUSP1 negatively regulates NF‐κB activity through a mechanism involving p38 MAPK inhibition, we also analysed the level of phospho‐p38 MAPK in the same samples in which the expression of both DUSP1 and p65/NF‐κB was studied. Our data indicated that the level of activated p38 MAPK was lower in BPH and low‐grade PIN samples (Figure 5A‐c, f), while most of the high‐grade PIN and invasive PC samples showed high levels of activated p38 MAPK (Figure 5A‐i, l, o).
Collectively, these results indicate an inverse correlation between DUSP1 expression levels and both p65/NF‐κB and p38 MAPK activity in human prostate tissue specimens. Interestingly, these data support our experiments in prostate cancer cells demonstrating that DUSP1 is an NF‐κB inhibitor, and suggest that the ratio between the expression levels of both proteins is an important marker for diagnostic purposes in prostate cancer.
4. Discussion
The phosphatase DUSP1 is becoming an important molecule in multiple types of human cancer due to its differential expression and to its regulation of the MAPK signalling pathways, which have been shown to be involved in this disease (Boutros et al., 2008). In prostate cancer, the expression of DUSP1 is high in BPH or PIN lesions, and low or even absent in high‐grade PC (Rauhala et al., 2005), although the relevance of this differential expression has been unknown so far. Here, we demonstrate by different approaches that the over‐expression of DUSP1 induces apoptosis in DU145 and PC3 cancer cells, which are in vitro models that resemble the late stage of PC. In agreement with our data, it has been shown that the up‐regulation of DUSP1 in several breast cancer cells is involved in the apoptosis induced by either the proteasome inhibitor Z‐LLF‐CHO (Orlowski et al., 2002) or nitric oxide (Pervin et al., 2003). In addition, DUSP1 over‐expression induces apoptosis in MCF7 breast cancer cells (Wang et al., 2007) and in colon cancer cells (Liu et al., 2008). Moreover, we have previously demonstrated that the induction of DUSP1 in pituitary tumor cells is crucial in thyroid hormone‐mediated apoptosis (Chiloeches et al., 2008). However, the role of DUSP1 in the apoptosis of cancer cells is controversial, since this phosphatase also seems to be important to prevent the cell death induced by chemotherapy agents in diverse tumor cells (Chattopadhyay et al., 2006; Wang et al., 2006; Wu et al., 2005).
The pro‐apoptotic role of DUSP1 demonstrated here is consistent with a recent report showing that photodynamic therapy of DU145 cells induces apoptosis and up‐regulates DUSP1 gene transcription (Kammerer et al., 2011), but is in contradiction with a report showing that this phosphatase blocks Fas Ligand‐induced apoptosis in these cells (Srikanth et al., 1999). Although these authors do not deeply analyse the mechanism underlying the anti‐apoptotic effect of DUSP1 in DU145 cells, the apparent controversy with our data could be explained by the fact that p38 MAPK seems not to be involved in the regulation of Fas Ligand‐induced apoptosis by this phosphatase (Srikanth et al., 1999). p38 MAPK has been identified as the major target for DUSP1 in different cellular contexts activated by the cytokine TNF‐α (Lang et al., 2006), although the role of this kinase on apoptosis in tumor cells is still controversial. In this report, we provide three lines of evidence demonstrating that DUSP1‐induced apoptosis in DU145 cells is mediated by the inhibition of p38 MAPK. (i) DUSP1 over‐expression abolishes TNF‐α‐induced activation of p38 MAPK. (ii) The specific inhibitors of p38 MAPK, SB203580 and SB202190, exert the same effects than the phosphatase on apoptosis. (iii) DUSP1 over‐expression also promotes apoptosis in cells in which p38 MAPK is activated by treatment with TNF‐α. Consistently with our data, it has been shown that this MAPK mediates cell survival in several tumor cells (Wagner and Nebreda, 2009). In reference to prostate cancer, our results are similar to those reported by other groups showing that the inhibition of p38 MAPK induces apoptosis in the androgen‐dependent LNCaP prostate cancer cells (Ricote et al., 2006; Rodriguez‐Berriguete et al., 2012). Moreover, knocking down p38 MAPK by specific siRNA significantly sensitizes LNCaP cells to docetaxel‐induced apoptosis through a p53‐dependent mechanism (Gan et al., 2011). Our data also demonstrate that JNK is not involved in the promotion of apoptosis induced by DUSP1. Although this phosphatase reduces TNF‐α‐induced activation of both p38 MAPK and JNK, the specific inhibitor of JNK, SP600125, does not promote apoptosis in these cells. These results are in agreement with other reports showing no effect of this kinase on apoptosis in DU145 cells (Curtin and Cotter, 2004; Ricote et al., 2006; Xiao et al., 2004). Despite the lack of effect of JNK on apoptosis in DU145 cells, we have observed that the JNK inhibitor highly decreases cell number. Our data are consistent with a previous report showing that this inhibitor affects proliferation by arresting cells in the G2/M phase of the cell cycle in several cell lines, including DU145 cells (Ennis et al., 2005).
On the other hand, the transcription factor NF‐κB is crucial in prostate tumors, since the increase of its activity has been shown to be responsible for the induction of tumor growth, evasion of apoptosis and promotion of metastasis (Naugler and Karin, 2008). Moreover, histopathological analysis of human prostate cancer specimens have demonstrated high levels of p65/NF‐κB expression in samples from patients with high‐grade PC, in comparison to normal prostate or BPH lesions (Nunez et al., 2008). Consistently, different studies have identified a constitutive activation of NF‐κB in several human PC cells (Gasparian et al., 2002). Here, we demonstrate a close relationship between NF‐κB and DUSP1, since over‐expression of this phosphatase inhibits NF‐κB activity in DU145 cells by affecting the p65/NF‐κB translocation to the nucleus. These results are similar to previous data showing that DUSP1 impairs NF‐κB activity in pituitary tumor cells (Chiloeches et al., 2008; Lasa et al., 2010) and pulmonary cells (King et al., 2009). The regulation of NF‐κB pathway by DUSP1 is not surprising considering that this protein is associated to the IκK proteins in different cell types (Kosaka et al., 1999; Mercurio et al., 1997; Woronicz et al., 1997). However, we have not detected any change in the activity of IκK α/β after DUSP1 over‐expression, and we have not seen any interaction between DUSP1 and IκK α/β in DU145 cells (data not shown). These findings strongly suggest that DUSP1 could inhibit NF‐κB activity in prostate cancer cells by an IκK‐independent mechanism, perhaps affecting the atypical NF‐κB pathway, in which p38 MAPK activates p65/NF‐κB through the phosphorylation and activation of the casein kinase 2 (Kato et al., 2003). This hypothesis is quite plausible since our data demonstrate that DUSP1 inhibits p38 MAPK in DU145 and PC3 cells, and the blockage of this kinase down‐regulates NF‐κB activity. In agreement with our results, previous studies have shown that p38 MAPK activation allows p65/NF‐κB nuclear translocation in several cell types (Baeza‐Raja and Munoz‐Canoves, 2004; Calleros et al., 2006; King et al., 2009; Lasa et al., 2010; Vanden Berghe et al., 1998). These data altogether open the possibility to study the interaction between DUSP1 and p38 MAPK/CK2/NF‐κB signalling pathway in prostate cancer cells.
The importance of the observed crosstalk between DUSP1 and NF‐κB in prostate cancer cells is supported by the analysis of the expression levels of both DUSP1 and p65/NF‐κB in the same clinical samples from patients with prostate cancer at different stages. Interestingly, we have found a strong inverse correlation between the expression of both DUSP1 and p65/NF‐κB proteins almost in all the groups of human specimens analysed (BPH, PIN and PC). We show that DUSP1 is highly expressed in BPH lesions, whereas p65/NF‐κB expression is low. Moreover, this correlation is progressively inversed with tumor malignancy, reaching a higher p65/NF‐κB expression and lower DUSP expression in high‐grade PC. As expected, our data also demonstrate an inverse correlation between the expression of both DUSP1 and activated p38 MAPK. These results altogether reinforce the relevance of our findings demonstrating the role of DUSP1 as NF‐κB inhibitor in prostate cancer. They also suggest that NF‐κB is down‐regulated in benign lesions as a result of the increased expression of DUSP1 and the decreased p38 MAPK activation, whereas NF‐κB levels augment in high‐grade PC samples, where the expression of DUSP1 is low or even absent, and the activation of p38 MAPK is increased. Interestingly, our data show a high correlation between the absence of DUSP1 and the level of androgen‐resistance in 92% of tested tumors, suggesting the existence of a crosstalk between DUSP1 and androgen receptor signalling pathways. Consistent with the low levels of DUSP1 in response to androgen ablation, this phosphatase has been found to be up‐regulated upon androgen treatment in LNCaP cells (Vaarala et al., 2012), although the implication of such regulation in prostate cancer remains to be established.
In conclusion, the results shown here demonstrate that re‐expression of DUSP1 is a good strategy to inhibit NF‐κB signalling in prostate cancer, and to decrease the survival of prostate cancer cells, contributing to reduce tumor progression. Moreover, our data strongly suggest that the ratio between DUSP1 and p65/NF‐κB expression levels, rather than the individual expression of both molecules, represents a more reliable marker for diagnostic purposes in prostate cancer.
5. Conclusions
The phosphatase DUSP1 promotes apoptosis in prostate cancer cells through the inhibition of p38 MAPK.
The phosphatase DUSP1 negatively regulates NF‐κB activity in prostate cancer cells by decreasing p65/NF‐κB nuclear translocation, through a mechanism involving p38 MAPK inhibition.
DUSP1 expression inversely correlates with nuclear p65/NF‐κB and p38 MAPK activity in human prostate tissue specimens. This suggests that the ratio between the expression levels of DUSP1 and p65/NF‐κB is an important marker for diagnostic purposes in prostate cancer.
Conflict of interest
The authors declare no relevant conflicts of interest.
Supporting information
Supplementary data
Acknowledgements
This work was supported by Fondo de Investigaciones Sanitarias (PI070832). We are grateful to Dr Fresno (Centro de Biología Molecular Severo Ochoa, Spain) and Dr Clark (Imperial College, UK) for providing 3xNF‐κB‐Luc and pCMV‐DUSP1plasmids, respectively. We thank Estrella Sánchez (IIBM, Spain) and Isabel Trabado (Universidad de Alcalá, Spain) for technical help.
Supplementary data 1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2013.08.012.
Gil-Araujo Beatriz, Toledo Lobo María-Val, Gutiérrez-Salmerón María, Gutiérrez-Pitalúa Julia, Ropero Santiago, Angulo Javier C., Chiloeches Antonio and Lasa Marina, (2014), Dual specificity phosphatase 1 expression inversely correlates with NF‐κB activity and expression in prostate cancer and promotes apoptosis through a p38 MAPK dependent mechanism, Molecular Oncology, 8, doi: 10.1016/j.molonc.2013.08.012.
References
- Baeza-Raja, B. , Munoz-Canoves, P. , 2004. p38 MAPK-induced nuclear factor-kappaB activity is required for skeletal muscle differentiation: role of interleukin-6. Mol. Biol. Cell. 15, 2013–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros, T. , Chevet, E. , Metrakos, P. , 2008. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol. Rev.. 60, 261–310. [DOI] [PubMed] [Google Scholar]
- Calleros, L. , Lasa, M. , Toro, M.J. , Chiloeches, A. , 2006. Low cell cholesterol levels increase NFkappaB activity through a p38 MAPK-dependent mechanism. Cell. Signal.. 18, 2292–2301. [DOI] [PubMed] [Google Scholar]
- Curtin, J.F. , Cotter, T.G. , 2004. JNK regulates HIPK3 expression and promotes resistance to Fas-mediated apoptosis in DU 145 prostate carcinoma cells. J. Biol. Chem.. 279, 17090–17100. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay, S. , Machado-Pinilla, R. , Manguan-Garcia, C. , Belda-Iniesta, C. , Moratilla, C. , Cejas, P. , Fresno-Vara, J.A. , de Castro-Carpeno, J. , Casado, E. , Nistal, M. , Gonzalez-Baron, M. , Perona, R. , 2006. MKP1/CL100 controls tumor growth and sensitivity to cisplatin in non-small-cell lung cancer. Oncogene. 25, 3335–3345. [DOI] [PubMed] [Google Scholar]
- Chen, W. , Liu, L. , Luo, Y. , Odaka, Y. , Awate, S. , Zhou, H. , Shen, T. , Zheng, S. , Lu, Y. , Huang, S. , 2012. Cryptotanshinone activates p38/JNK and inhibits Erk1/2 leading to caspase-independent cell death in tumor cells. Cancer Prev. Res. (Phila). 5, 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiloeches, A. , Sanchez-Pacheco, A. , Gil-Araujo, B. , Aranda, A. , Lasa, M. , 2008. Thyroid hormone-mediated activation of the ERK/Dual specificity phosphatase 1 pathway augments the apoptosis of GH4C1 cells by down-regulating nuclear factor-kappaB activity. Mol. Endocrinol.. 22, 2466–2480. [DOI] [PubMed] [Google Scholar]
- Chopra, D.P. , Menard, R.E. , Januszewski, J. , Mattingly, R.R. , 2004. TNF-alpha-mediated apoptosis in normal human prostate epithelial cells and tumor cell lines. Cancer Lett.. 203, 145–154. [DOI] [PubMed] [Google Scholar]
- Dhillon, A.S. , Hagan, S. , Rath, O. , Kolch, W. , 2007. MAP kinase signalling pathways in cancer. Oncogene. 26, 3279–3290. [DOI] [PubMed] [Google Scholar]
- Ennis, B.W. , Fultz, K.E. , Smith, K.A. , Westwick, J.K. , Zhu, D. , Boluro-Ajayi, M. , Bilter, G.K. , Stein, B. , 2005. Inhibition of tumor growth, angiogenesis, and tumor cell proliferation by a small molecule inhibitor of c-Jun N-terminal kinase. J. Pharmacol. Exp. Ther.. 313, 325–332. [DOI] [PubMed] [Google Scholar]
- Feldman, B.J. , Feldman, D. , 2001. The development of androgen-independent prostate cancer. Nat. Rev. Cancer. 1, 34–45. [DOI] [PubMed] [Google Scholar]
- Gan, L. , Wang, J. , Xu, H. , Yang, X. , 2011. Resistance to docetaxel-induced apoptosis in prostate cancer cells by p38/p53/p21 signaling. Prost.. 71, 1158–1166. [DOI] [PubMed] [Google Scholar]
- Gasparian, A.V. , Yao, Y.J. , Kowalczyk, D. , Lyakh, L.A. , Karseladze, A. , Slaga, T.J. , Budunova, I.V. , 2002. The role of IKK in constitutive activation of NF-kappaB transcription factor in prostate carcinoma cells. J. Cell Sci.. 115, 141–151. [DOI] [PubMed] [Google Scholar]
- Jemal, A. , Siegel, R. , Ward, E. , Hao, Y. , Xu, J. , Murray, T. , Thun, M.J. , 2008. Cancer statistics, 2008. CA: Cancer J. Clin.. 58, 71–96. [DOI] [PubMed] [Google Scholar]
- Kammerer, R. , Buchner, A. , Palluch, P. , Pongratz, T. , Oboukhovskij, K. , Beyer, W. , Johansson, A. , Stepp, H. , Baumgartner, R. , Zimmermann, W. , 2011. Induction of immune mediators in glioma and prostate cancer cells by non-lethal photodynamic therapy. PloS One. 6, e21834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, T. , Delhase, M. , Hoffmann, A. , Karin, M. , 2003. CK2 is a c-terminal IkappaB kinase responsible for NF-kappaB activation during the UV response. Molecular Cell.. 12, 829–839. [DOI] [PubMed] [Google Scholar]
- Keyse, S.M. , 2000. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 12, 186–192. [DOI] [PubMed] [Google Scholar]
- Keyse, S.M. , 2008. Protein phosphatases and cancer. Preface. Cancer Metastasis Rev.. 27, 121–122. [DOI] [PubMed] [Google Scholar]
- King, E.M. , Holden, N.S. , Gong, W. , Rider, C.F. , Newton, R. , 2009. Inhibition of NF-kappaB-dependent transcription by MKP-1: transcriptional repression by glucocorticoids occurring via p38 MAPK. J. Biol. Chem.. 284, 26803–26815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka, Y. , Calderhead, D.M. , Manning, E.M. , Hambor, J.E. , Black, A. , Geleziunas, R. , Marcu, K.B. , Noelle, R.J. , 1999. Activation and regulation of the IkappaB kinase in human B cells by CD40 signaling. Eur J Immunol. 29, 1353–1362. [DOI] [PubMed] [Google Scholar]
- Lang, R. , Hammer, M. , Mages, J. , 2006. DUSP meet immunology: dual specificity MAPK phosphatases in control of the inflammatory response. J. Immunol.. 177, 7497–7504. [DOI] [PubMed] [Google Scholar]
- Lasa, M. , Gil-Araujo, B. , Palafox, M. , Aranda, A. , 2010. Thyroid hormone antagonizes tumor necrosis factor-alpha signaling in pituitary cells through the induction of Dual specificity phosphatase 1. Mol. Endocrin.. 24, 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.X. , Wang, J. , Guo, J. , Wu, J. , Lieberman, H.B. , Yin, Y. , 2008. DUSP1 is controlled by p53 during the cellular response to oxidative stress. Mol. Cancer Res.: MCR. 6, 624–633. [DOI] [PubMed] [Google Scholar]
- Loda, M. , Capodieci, P. , Mishra, R. , Yao, H. , Corless, C. , Grigioni, W. , Wang, Y. , Magi-Galluzzi, C. , Stork, P.J. , 1996. Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Am. J. Pathol.. 149, 1553–1564. [PMC free article] [PubMed] [Google Scholar]
- Magi-Galluzzi, C. , Mishra, R. , Fiorentino, M. , Montironi, R. , Yao, H. , Capodieci, P. , Wishnow, K. , Kaplan, I. , Stork, P.J. , Loda, M. , 1997. Mitogen-activated protein kinase phosphatase 1 is overexpressed in prostate cancers and is inversely related to apoptosis. Lab. Invest.. 76, 37–51. [PubMed] [Google Scholar]
- Mehta, P.B. , Robson, C.N. , Neal, D.E. , Leung, H.Y. , 2001. Keratinocyte growth factor activates p38 MAPK to induce stress fibre formation in human prostate DU145 cells. Oncogene. 20, 5359–5365. [DOI] [PubMed] [Google Scholar]
- Mercurio, F. , Zhu, H. , Murray, B.W. , Shevchenko, A. , Bennett, B.L. , Li, J. , Young, D.B. , Barbosa, M. , Mann, M. , Manning, A. , Rao, A. , 1997. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 278, 860–866. [DOI] [PubMed] [Google Scholar]
- Naugler, W.E. , Karin, M. , 2008. NF-kappaB and cancer-identifying targets and mechanisms. Curr. Opin. Gen. Develop.. 18, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez, C. , Cansino, J.R. , Bethencourt, F. , Perez-Utrilla, M. , Fraile, B. , Martinez-Onsurbe, P. , Olmedilla, G. , Paniagua, R. , Royuela, M. , 2008. TNF/IL-1/NIK/NF-kappa B transduction pathway: a comparative study in normal and pathological human prostate (benign hyperplasia and carcinoma). Histopathology. 53, 166–176. [DOI] [PubMed] [Google Scholar]
- Oeckinghaus, A. , Hayden, M.S. , Ghosh, S. , 2011. Crosstalk in NF-kappaB signaling pathways. Nature Immun.. 12, 695–708. [DOI] [PubMed] [Google Scholar]
- Orlowski, R.Z. , Small, G.W. , Shi, Y.Y. , 2002. Evidence that inhibition of p44/42 mitogen-activated protein kinase signaling is a factor in proteasome inhibitor-mediated apoptosis. J. Biol. Chem.. 277, 27864–27871. [DOI] [PubMed] [Google Scholar]
- Pervin, S. , Singh, R. , Freije, W.A. , Chaudhuri, G. , 2003. MKP-1-induced dephosphorylation of extracellular signal-regulated kinase is essential for triggering nitric oxide-induced apoptosis in human breast cancer cell lines: implications in breast cancer. Cancer Res.. 63, 8853–8860. [PubMed] [Google Scholar]
- Rauhala, H.E. , Porkka, K.P. , Tolonen, T.T. , Martikainen, P.M. , Tammela, T.L. , Visakorpi, T. , 2005. Dual-specificity phosphatase 1 and serum/glucocorticoid-regulated kinase are downregulated in prostate cancer. Int. J. Cancer. 117, 738–745. [DOI] [PubMed] [Google Scholar]
- Ricote, M. , Garcia-Tunon, I. , Fraile, B. , Fernandez, C. , Aller, P. , Paniagua, R. , Royuela, M. , 2006. P38 MAPK protects against TNF-alpha-provoked apoptosis in LNCaP prostatic cancer cells. Apoptosis: Int. J. Prog. Cell Death. 11, 1969–1975. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Berriguete, G. , Fraile, B. , Paniagua, R. , Aller, P. , Royuela, M. , 2012. Expression of NF-kappaB-related proteins and their modulation during TNF-alpha-provoked apoptosis in prostate cancer cells. Prostate. 72, 40–50. [DOI] [PubMed] [Google Scholar]
- Royuela, M. , Rodriguez-Berriguete, G. , Fraile, B. , Paniagua, R. , 2008. TNF-alpha/IL-1/NF-kappaB transduction pathway in human cancer prostate. Hist. Histopath.. 23, 1279–1290. [DOI] [PubMed] [Google Scholar]
- Shin, D.Y. , Kim, G.Y. , Lee, J.H. , Choi, B.T. , Yoo, Y.H. , Choi, Y.H. , 2012. Apoptosis induction of human prostate carcinoma DU145 cells by Diallyl Disulfide via modulation of JNK and PI3K/AKT signaling pathways. Int. Journal of Molecular Sciences. 13, 14158–14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth, S. , Franklin, C.C. , Duke, R.C. , Kraft, R.S. , 1999. Human DU145 prostate cancer cells overexpressing mitogen-activated protein kinase phosphatase-1 are resistant to Fas ligand-induced mitochondrial perturbations and cellular apoptosis. Mol Cell Biochem. 199, 169–178. [DOI] [PubMed] [Google Scholar]
- Sun, B. , Zhang, X. , Yonz, C. , Cummings, B.S. , 2010. Inhibition of calcium-independent phospholipase A2 activates p38 MAPK signaling pathways during cytostasis in prostate cancer cells. Biochem Pharmacol. 79, 1727–1735. [DOI] [PubMed] [Google Scholar]
- Vaarala, M.H. , Hirvikoski, P. , Kauppila, S. , Paavonen, T.K. , 2012. Identification of androgen-regulated genes in human prostate. Molecular Medicine Reports. 6, 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Berghe, W. , Plaisance, S. , Boone, E. , De Bosscher, K. , Schmitz, M.L. , Fiers, W. , Haegeman, G. , 1998. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 273, 3285–3290. [DOI] [PubMed] [Google Scholar]
- Wagner, E.F. , Nebreda, A.R. , 2009. Signal integration by JNK and p38 MAPK pathways in cancer development. Nature Reviews. Cancer. 9, 537–549. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Yin, D.P. , Liu, Y.X. , Baer, R. , Yin, Y. , 2007. Dual specificity phosphatase 1/CL100 is a direct transcriptional target of E2F-1 in the apoptotic response to oxidative stress. Cancer Res. 67, 6737–6744. [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Xu, J. , Zhou, J.Y. , Liu, Y. , Wu, G.S. , 2006. Mitogen-activated protein kinase phosphatase-1 is required for cisplatin resistance. Cancer Res. 66, 8870–8877. [DOI] [PubMed] [Google Scholar]
- Woronicz, J.D. , Gao, X. , Cao, Z. , Rothe, M. , Goeddel, D.V. , 1997. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 278, 866–869. [DOI] [PubMed] [Google Scholar]
- Wu, W. , Pew, T. , Zou, M. , Pang, D. , Conzen, S.D. , 2005. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem. 280, 4117–4124. [DOI] [PubMed] [Google Scholar]
- Xiao, D. , Choi, S. , Johnson, D.E. , Vogel, V.G. , Johnson, C.S. , Trump, D.L. , Lee, Y.J. , Singh, S.V. , 2004. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 23, 5594–5606. [DOI] [PubMed] [Google Scholar]
- Yano, O. , Kanellopoulos, J. , Kieran, M. , Le Bail, O. , Israel, A. , Kourilsky, P. , 1987. Purification of KBF1, a common factor binding to both H-2 and beta 2-microglobulin enhancers. The EMBO Journal. 6, 3317–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


