Abstract
Targeted therapies, including antibodies, are becoming increasingly important in cancer therapy. Important limitations, however, are that not every patient benefits from a specific antibody therapy and that responses could be short‐lived due to acquired resistance. In addition, targeted therapies are quite expensive and are not completely devoid of side‐effects. This urges the need for accurate patient selection and response monitoring.
An important step towards personalizing antibody treatment could be the implementation of theranostics. Antibody theranostics combine the diagnostic and therapeutic potential of an antibody, thereby selecting those patients who are most likely to benefit from antibody treatment. This review focuses on the clinical application of theranostic antibodies in oncology. It provides detailed information concerning the suitability of antibodies for theranostics, the different types of theranostic tests available and summarizes the efficacy of theranostic antibodies used in current clinical practice. Advanced theranostic applications, including radiolabeled antibodies for non‐invasive functional imagining, are also addressed. Finally, we discuss the importance of theranostics in the emerging field of personalized medicine and critically evaluate recent data to determine the best way to apply antibody theranostics in the future.
Keywords: Theranostics, Antibody, Personalized medicine, Biomarker, Molecular imaging
Highlights
Importance of antibody theranostics in personalizing targeted cancer treatment.
Limitations of conventional theranostic tests.
Advantages of novel, advanced theranostic approaches.
Difference in theranostic applications for conjugated and unconjugated antibodies.
1. Introduction
Targeted therapies are becoming increasingly important for the treatment of cancer. These therapies are designed to specifically interfere with aberrant targets or pathways of cancer cells, which is in contrast to the generalized cytotoxic effects of standard chemotherapy (Teng et al., 2013). The two main types of targeted therapy are the monoclonal antibodies (mAbs) and tyrosine kinase inhibitors (TKIs). The major advantages of these targeted molecules are their tumor specificity and low toxicity profile. Important limitations, however, are that (i) not every patient benefits from a specific targeted treatment and (ii) responses could be short‐lived due to acquired resistance (Gerber, 2008). This urges the need for accurate patient selection and response monitoring.
A promising strategy towards improving personalized medicine is the implementation of theranostics. Theranostics combine the therapeutic (“thera”) and diagnostic (“nostic”) potentials of a certain compound. Prior to therapy, the compound is used in a diagnostic test to determine whether the drug will (potentially) exert a therapeutic effect, making it a powerful tool for personalizing cancer treatment. Because it operates on the molecular level, it could be more accurate for response prediction and/or monitoring than more general tumor characteristics such as tumor morphology (e.g. CT and MRI) or metabolism (e.g. 18F‐FDG‐PET).
This review focuses on the clinical application of theranostic antibodies in oncology. It provides detailed information concerning antibody suitability for theranostics, the different types of theranostic tests available and summarizes the efficacy of theranostic antibodies currently used in clinical practice. In addition, more advanced theranostic applications, including the use of radiolabeled antibodies are addressed. Finally, we discuss the importance of theranostics in the emerging field of personalized medicine and critically evaluate recent data to determine the best way to apply antibody theranostics in the future.
2. Antibodies used for theranostic applications
To date, several mAbs have been approved by the Food and Drug Administration (FDA) for the treatment of cancer. Antibodies specifically target antigens expressed on the cell membrane or ligands (e.g. bevacizumab, Section 4.3) and can either be administered unconjugated (Table 1) or conjugated to cytotoxic moieties such as radionuclides or toxins to increase efficacy (Table 2) (Gerber, 2008). Because target expression is usually a prerequisite for antibody response, one could base patient selection on the expression of the target antigen (Fleuren et al., 2011; Heskamp et al., 2013b). However, for many therapeutic antibodies mere target expression appeared not sufficient to predict response in all patients. For unconjugated or naked antibodies it is essential that, in addition to its presence on the tumor, the target antigen is also involved in tumor progression. But even when this is the case, tumors only rarely depend on a single regulatory pathway for growth and survival because of their molecular complexity and heterogeneity. Parallel activation of other receptors or mutations in downstream pathways are not rare events. This could at least in part explain the lack of response in tumors with apparent oncogenic target expression (De Palma and Hanahan, 2012). Because physiological factors (e.g. vascularization, hypoxia, intratumoral pressure) can impede adequate antibody targeting to the tumor, these should also be taken into account when predicting response (Heldin et al., 2004; Jain, 1999). In addition, the antibody Fc‐portion can evoke an additional anti‐tumor response by triggering antibody‐dependent cellular cytotoxicity (ADCC) or complement dependent cytotoxicity (CDC) processes. This underscores the complexity of finding a suitable predictive biomarker and indicate that theranostic applications should be interpreted with caution.
Table 1.
Registered unconjugated therapeutic antibodies for cancer treatment.
| Antibody | Antibody type | Target antigen | Antigen category | FDA‐approved indicationsa | Theranostic antibody test |
|---|---|---|---|---|---|
| Trastuzumab (Herceptin) | Humanized | HER2 | Growth factor | Breast cancer (HER2‐positive); as single agent or combined with chemotherapy for adjuvant or palliative treatmentGastric or gastro‐oesophageal junction carcinoma (HER2‐positive); as first‐line treatment in combination with cisplatin and capecitabine or 5‐fluorouracil | HER2 IHCc (e.g. HercepTest) |
| Cetuximabb (Erbitux) | Chimeric | EGFR | Growth factor | Head and neck cancer; combined with radiation therapy for initial treatment of locally or regionally advanced disease or as single agent for patients who failed prior platinum‐based therapyColorectal cancer (EGFR‐positive); palliative treatment of pretreated metastatic disease | Colorectal cancer: EGFR IHCc (e.g. EGFR pharmDx kit) |
| Panitumumabb (Vectibix) | Human | EGFR | Growth factor | Colorectal cancer (EGFR‐positive); as single agent for pretreated metastatic disease | EGFR IHCc (e.g. EGFR pharmDx kit) |
| Bevacizumab (Avastin) | Humanized | VEGF | Vascular target | Colorectal cancer; for first‐line and second‐line treatment of metastatic disease, in conjunction with 5‐fluorouracil‐based chemotherapyNon‐small cell lung cancer (NSCLC); first‐line treatment of advanced disease, in combination with carboplatin and paclitaxel, in patients who have not yet received chemotherapyGlioblastoma; as single agent in adult patients for second line treatmentRenal cell carcinoma (RCC); in conjunction with IFNα to treat metastatic disease | No (under development) |
| Rituximab (Rituxan) | Chimeric | CD20 | Hematopoetic differentiation antigen | Non‐Hodgkin's lymphoma (NHL) (CD20‐positive); for B cell NHL and maintenance therapy for untreated follicular NHLChronic lymphocytic leukemia | NHL: CD20 ELISA/flow cytometry |
EGFR: Epidermal Growth Factor Receptor; VEGF: Vascular Endothelial Growth Factor, IHC: immuno‐histochemistry, ELISA: enzyme‐linked immunosorbent assay.
Based on cancer.gov (January 2014) and (Scott et al., 2012).
Not recommended for colorectal cancer patients whose tumors express mutated KRAS.
In addition to IHC, the predictive value of HER2 and EGFR mRNA expression or gene amplification as measured by polymerase chain reaction (PCR) or fluorescence in situ hybridization (FISH) has also been studied in the clinic. Because this review focuses specifically on the theranostic applications of antibodies in oncology, we did not further consider these applications.
Table 2.
Conjugated therapeutic antibodies for cancer treatment.
| Antibody | Target antigen | Antibody type | Conjugate type | Emittera | Indicationsb,c | Theranostic antibody test |
|---|---|---|---|---|---|---|
| 90Y‐Ibritumomab tiuxetan (Zevalin) | CD20 | Murine | Radionuclide (yttrium‐90) | β | FDA: non‐Hodgkin's lymphoma (NHL); for relapsed or refractory, low‐grade or follicular B cell NHL or previously untreated follicular NHL in patients achieving a partial or complete response to first‐line chemotherapy | Safety: biodistribution and dosimetry prior to therapy (111In‐ Ibritumomab tiuxetan) |
| 131I‐Tositumomab (Bexxar) | CD20 | Murine | Radionuclide (iodine‐131) | β and γ | FDA: NHL (CD20‐positive); for relapsed or refractory, low‐grade, follicular or transformed NHL | CD20 ELISA/flow cytometrySafety: biodistribution and dosimetry prior to therapy (low‐dose Bexxar) |
| 177Lu/90Y ‐J591 | PSMA | Humanized | Radionuclide (lutetium‐177/yttrium‐90) | β and γ/β | Research: prostate cancer | Research: 111In‐J591 for response prediction |
| 177Lu –Girentuximab (cG250) | CAIX | Chimeric | Radionuclide (lutetium‐177) | β and γ | Research: renal cell carcinoma (RCC) | Research: 111In‐Girentuximab for response prediction |
| Gemtuzumab ozogamicin (Mylotarg) | CD33 | Humanized | Toxin (calicheamicin) | n.a. | FDA withdrawn: acute myeloid leukemia (AML) (CD33‐positive); for relapsed patients >60 years not suited for other chemotherapy | CD33 ELISA/flow cytometry |
| Trastuzumab emtansine (T‐DM1, Kadcyla) | HER2 | Humanized | Toxin (emtansine) | n.a. | FDA: breast cancer (HER2‐positive); metastatic | HER2 IHC (e.g. HercepTest)Research: 89Zr‐trastuzumab to exclude non‐responders |
β‐emission is solely used for radioimmunotherapy, while γ‐emission can be used for imaging.
FDA‐approved indications are specifically pointed out (FDA), others are under clinical investigation (research).
Based on cancer.gov (January 2014) and (Scott et al., 2012).
Not every overexpressed membranous tumor protein is a potential candidate for treatment with unconjugated antibodies as functional involvement is also required. Nevertheless, high target expression on tumor cells may be sufficient for predicting response for conjugated antibodies. However, also with conjugated antibodies, target accessibility should be taken into account and for these constructs it is of utmost importance to also determine target expression levels on non‐tumor tissue to avoid side‐effects.
3. Types of theranostic antibody tests
Because target expression is usually a prerequisite for response, it seems a logical first step to base patient selection on target expression. Although several comments can be made on this design (e.g. involvement of other pathways), detection of the target protein is expected to at least exclude primarily resistant patients. At present, several different diagnostic methods exist to screen for target expression, which all have their specific advantages and limitations as discussed below and summarized in Table 3.
Table 3.
Characteristics of theranostic antibody tests.
| Theranostic antibody assay | Standard use | Advantages | Disadvantages |
|---|---|---|---|
| Immunohistochemistry | Solid tumors | • Simple to perform • Low costs • Routinely available | • Requires invasive biopsies • No whole body target expression • No information about target accessibility • Variability (tissue handling/fixation) • Semi‐quantitative • Subjective interpretation • Membranous expression often hard to define |
| Enzyme‐linked immunosorbent assay (ELISA) | Hematological tumors | • Minimally invasive (blood sampling) • Simple to perform • Low costs • Routinely available • Can define membranous expression • Quantitative | • No whole body target expression • No information about target accessibility • Subjective interpretation (arbitrary thresholds) |
| Flow cytometry | Hematological tumors | • Minimally invasive (blood sampling) • Relatively simple to perform • Relatively low costs • Available in most laboratories • Can define membranous expression • Very accurate • Quantitative | • No whole body target expression • No information about target accessibility • Subjective interpretation (arbitrary thresholds) |
| Functional imaging (immuno‐PET/immuno‐SPECT) | Solid and hematological tumors | • Minimally invasive (IV injection) • Can define membranous expression • Very accurate • Quantitative • Whole body target expression • Target accessibility taken into account • Can be performed repetitively | • Specific equipment required • Specific expertise required • High costs • Tracers must be prepared shortly before injection |
PET: positron emission tomography, SPECT: single photon emission computed tomography, IV: intravenous.
3.1. Immunohistochemistry
Immunohistochemistry (IHC) is currently the most commonly used procedure to perform antibody theranostics for solid tumors. The antibody can be used to detect target expression on tumor tissue by IHC. When the section is scored positive for target expression, the same antibody can subsequently be used for therapy. A well‐known example of this approach is the HercepTest (Section 4.1). IHC has the advantages of being simple to perform, cheap, and readily available in most pathology laboratories. There are however numerous variables, such as tissue handling and fixation and antibody sensitivity and specificity. Because slides are not scored by automated computer systems, inter‐observer interpretation and variability can also affect the accuracy of test results (Cuadros and Villegas, 2009). Because antibodies can only target extracellularly‐expressed antigens, it would be appropriate to specifically screen for membranous expression. This is however difficult to determine by conventional IHC, often due to high cytoplasmic receptor expression. Other limitations include the lack of information obtained about in vivo target accessibility and the need for invasive biopsies. Moreover, receptor expression is determined on a limited number of sections of a single biopsy, often formalin‐fixed paraffin‐embedded (FFPE) archival tissue. Because tumors are usually very heterogeneous, patients often present with multiple lesions and target expression levels can change over time (for instance during therapy), multiple biopsies would be required for adequate antigen testing, which in most cases is clinically unfeasible.
3.2. Hematological analysis
For hematological cancers, immuno‐assays are commonly used for theranostic approaches, such as enzyme‐linked immunosorbent assay (ELISA) or flow cytometry. An ELISA uses antibodies to determine the amount of antigen in serum, plasma or other body fluids (Kenny and Dunsmoor, 1983). Flow cytometry is a laser‐based technology measuring antigen expression in single cells from a suspension. For biomarker detection with flow cytometry, antibodies can be used to determine the expression of a particular antigen on blood cell subtypes (Brown and Wittwer, 2000). A well‐known example of a hematological theranostic assay is CD20 testing by ELISA or flow cytometry prior to rituximab therapy (Section 4.4). ELISAs are in general quick and easy to perform, readily available, can define membranous expression levels and can be analyzed quantitatively. Flow cytometry measures single cells, is quantitative, very accurate and can measure specific membranous target expression. Disadvantages of both techniques include the subjective interpretation of results due to arbitrary threshold settings, so there is no straightforward difference between positive and negative samples. Also with these techniques, only a fraction of tumor cells is measured thus no whole body tumor expression pattern is determined.
3.3. Immuno‐PET/immuno‐SPECT
For both solid and hematological tumors, target expression levels can be determined in vivo using molecular imaging methods, such as immuno‐positron emission tomography (PET) and immuno‐single photon emission computed tomography (SPECT). For both techniques, an antibody is labeled with a radionuclide (positron or γ‐emitter) and injected intravenously. The 3D distribution of the radiolabeled antibody is subsequently visualized with a SPECT or PET scanner. With these molecular imaging techniques, several limitations of conventional IHC and serum assays can be circumvented. Advantages include non‐invasive screening and visualization of specific membranous target expression. Moreover, these whole body scans enable target visualization in the whole tumor, in multiple lesions simultaneously and take target accessibility into account (Figures 1 and 2). These scans can be performed repetitively to asses target modulation over time, for instance during (targeted) treatment. Because theranostic molecular imaging approaches are relatively new, they have not yet been implemented as standard diagnostic tests. Results from clinical trials are however encouraging, as for instance shown with indium‐111 (111In)‐labeled‐girentuximab imaging prior to lutetium‐177 (177Lu)‐girentuximab radioimmunotherapy (RIT) (Section 5.3). Disadvantages include the use of specific equipment and expertise, tracers should be prepared shortly before injection and costs are higher than with conventional methods (Heskamp et al., 2013b).
Figure 1.
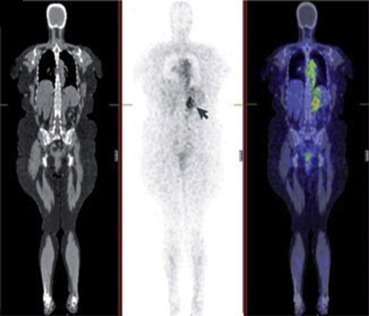
Whole body biodistribution of a radiolabeled antibody. Whole‐body coronal PET/CT images of 124I‐girentuximab, acquired 5 days after antibody infusion. The specific uptake of the antibody can be seen in the left renal tumor (arrow), which is expressing the CAIX antigen. The concentration of the radiolabeled antibody in a tumor can be determined quantitatively. Left: CT, middle: PET, right: fused PET/CT. This figure was originally published in Nat Rev Cancer; reprinted with permission (Scott et al., 2012) (adapted).
Figure 2.
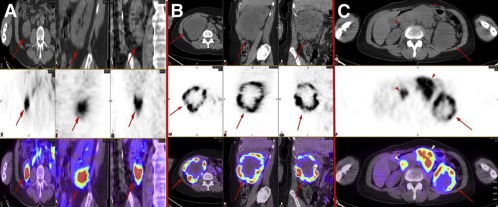
Heterogeneous distribution of 124I‐girentuximab in RCC lesions. (A and B) Axial, sagittal, and coronal CT (top), PET (middle), and fused PET/CT (bottom) images of a patient with clear cell renal cancer with relatively homogeneous intratumoral distribution of the radiolabeled antibody (arrows) (A) and a patient with large, centrally necrotic clear cell renal cancer with marked heterogeneity (arrows) (B). (C) Axial CT (top), PET (middle), and fused PET/CT (bottom) images of a patient with advanced clear cell renal cancer. Antibody distribution within the primary tumor is heterogeneous (arrows), whereas distribution within metastatic nodes is relatively homogeneous (arrowheads). This figure was originally published in JNM; reprinted with permission (Pryma et al., 2011) (adapted).
4. Theranostic antibodies in current clinical practice
4.1. Trastuzumab (Herceptin)
One of the most widely used theranostic approaches in clinical practice is the HercepTest to determine HER2 expression levels in breast cancer (BC) prior to trastuzumab therapy. HER2, also known as Neu or ErbB2, is a member of the epidermal growth factor receptor (EGFR) family and plays an important role in BC pathogenesis. HER2 is overexpressed in 20–30% of all BCs and trastuzumab showed activity only against HER2‐overexpressing tumors. An increasing number of studies suggest that HER2 status is more important than the histologic BC type (Cuadros and Villegas, 2009; Heskamp et al., 2013b). Notably, trastuzumab would not have been approved for BC treatment if studies were performed upon a general population of BC patients without prior identification of HER2 expression. HER2 is also overexpressed in subsets of ovarian, lung, gastric and oral cancers, and all newly diagnosed gastric cancers are also tested for HER2 by IHC to select patients eligible for trastuzumab therapy (de Mello et al., 2013; Meric‐Bernstam and Hung, 2006). Unfortunately, however, only 30% of HER2‐positive BCs actually respond to trastuzumab therapy. This may be explained by either inaccurate HER2 assessments or that these tumors not strictly depend on HER2‐mediated growth signaling, for instance due to mutations in downstream pathways (e.g. PI3K). Furthermore, HER2 expression can differ between primary tumors and metastases, and this technique does not take target accessibility into account (De Palma and Hanahan, 2012; Heskamp et al., 2013b).
Consequently, several approaches have been developed to determine HER2 expression levels in vivo, with promising results so far. Several clinical studies indicated that 111In‐trastuzumab SPECT and copper‐64 (64Cu)‐labeled or zirconium‐89 (89Zr)‐labeled trastuzumab PET are feasible to identify HER2‐positive lesions in patients with primary and metastatic BC (mBC) (Dijkers et al., 2010; Perik et al., 2006; Tamura et al., 2013). Although the exact predictive value of these imaging modalities for trastuzumab response remains to be investigated, they have great potential. In mice, 111In‐pertuzumab (a HER2 dimerization inhibitor) SPECT sensitively imaged HER2 downregulation during trastuzumab treatment in an in vivo human BC model, illustrating the feasibility of monitoring HER2 expression levels during HER2‐mediated treatment (McLarty et al., 2009).
4.2. Cetuximab (Erbitux) and panitumumab (Vectibix)
Cetuximab and panitumumab both target EGFR, also known as HER1. EGFR plays an important role in proliferation, migration and survival of tumor cells. Similar to HER2‐testing, also for EGFR a diagnostic IHC test (EGFR pharmDX Kit) was developed and approved to aid in identifying colorectal cancer (CRC) patients eligible for cetuximab or panitumumab treatment (Ensinger and Sterlacci, 2008). However, although EGFR is broadly expressed in metastatic CRC (mCRC), only 10% of patients respond to cetuximab. In those tumors with apparent EGFR expression but no response to cetuximab or panitumumab, additional signal transduction pathways can be active. Clinical studies indicated that tumors harboring mutations in KRAS, a downstream effector protein in the EGFR‐signaling cascade, show negligible responses to cetuximab despite elevated EGFR levels. Consequently, current clinical practice in CRC is to screen for KRAS mutations, and only KRAS wildtype tumors are considered for EGFR‐targeted therapies (De Roock et al., 2010). Other studies further demonstrated that the effect of cetuximab is independent of the degree of EGFR expression in the tumor. Remarkably, cetuximab even showed activity in EGFR‐negative CRC patients (Chung et al., 2005; Han, 2011). Thus, EGFR overexpression is not a reliable predictor of EGFR‐antibody efficacy. One potential explanation for this discrepancy could simply be inconsistent methodology and interpretation of EGFR IHC expression (section 3.1.). Several studies reported variability in EGFR staining depending on the tissue fixation technique used, possibly leading to false‐negative samples (Liu and Carpenter, 1993; Ong et al., 1990; Stein and Staros, 1996). Also, most EGFR analyses have been performed on archival primary tumor specimens that may have been kept for months to years before assessing EGFR levels. A dramatic decline in EGFR staining intensity has been reported with increased storage time of tissue samples, resulting in many more EGFR‐negative scores in older specimens (Atkins et al., 2004). An additional concern is that EGFR expression in metastatic tumor specimens can differ significantly from EGFR expression in the corresponding primary tumor (Johnson et al., 1997). Because of tissue availability, the primary tumor is frequently used to establish the patient's EGFR status, but it is the metastases that are being treated with cetuximab (Chung et al., 2005).
Also for EGFR, several attempts were made to visualize this receptor in vivo with molecular imaging techniques. Promising results were obtained with 111In‐cetuximab SPECT and 64Cu‐cetuximab and 89Zr‐cetuximab PET in in vivo cancer models, although liver and skin uptake was also observed (Aerts et al., 2009; Corcoran and Hanson, 2013; Hoeben et al., 2011). Moreover, skin toxicity appeared a surrogate marker for cetuximab activity, implying an anti‐tumor effect when skin EGFR saturation occurs (Burtness et al., 2005). Accordingly, with 111In‐cetuximab SPECT in lung carcinoma patients, tumors were only imaged when higher doses of cetuximab were administered (Divgi et al., 1991). A phase I trial investigating the predictive value of 89Zr‐cetuximab PET to cetuximab therapy in stage IV cancer patients was initiated recently, including a loading dose of 400 mg/m2 cetuximab (NCT00691548). Another recent study investigates the predictive value of 89Zr‐cetuximab PET prior to chemoradiation with cetuximab or cisplatin in head and neck cancer patients (Heukelom et al., 2013)(NCT01504815).
4.3. Bevacizumab (Avastin)
Bevacizumab is an antibody directed against Vascular Endothelial Growth Factor‐A (VEGF‐A), which plays a central role in inducing the formation of new blood vessels (angiogenesis). VEGF is a ligand that specifically binds to the VEGF receptor (VEGFR). Bevacizumab is currently approved (alone or combined with other drugs) for the treatment of mCRC, non‐small cell lung cancer (NSCLC), renal cell carcinoma (RCC) and recurrent glioblastoma. Response to bevacizumab is also observed in only a subset of patients and the overall clinical benefit is limited. Consequently, extensive clinical biomarker studies have been performed (Lambrechts et al., 2013). Many studies addressed the possible predictive role of circulating VEGF‐A levels by conventional methods, but inconsistent results were found. In NSCLC, breast, ovary, and endometrium cancer studies response rates in patients with high VEGF‐A levels were significantly higher in the bevacizumab arm than in the placebo arm. Many other studies however failed to observe such an effect (Lambrechts et al., 2013). A recent extensive meta‐analysis evaluating 1816 patients from phase III CRC, NSCLC, and RCC trials confirmed that pretreatment VEGF‐A levels are a prognostic rather than a predictive marker (Hegde et al., 2013). However, a novel ELISA with a preference to detect short VEGF‐A isoforms could be superior for response prediction. mBC and pancreatic cancer patients with high baseline VEGF‐A expression levels, as measured with this novel ELISA, exhibited improved progression‐free survival (PFS) and/or overall survival (OS) upon bevacizumab treatment (Lambrechts et al., 2013). Also in advanced gastric cancer, patients with high baseline plasma VEGF‐A levels exhibited improved OS compared to patients with low VEGF‐A levels (Van Cutsem et al., 2012). In randomized CRC, NSCLC, and RCC studies, no such correlation was observed, possibly due to differences in sampling techniques (Lambrechts et al., 2013). These results indicate that circulating VEGF levels may be of importance towards predicting bevacizumab response, but stress the importance of applying the appropriate methodology to assess target expression levels.
Several studies investigated the feasibility to visualize tumor VEGF expression in patients using molecular imaging techniques, and some even tried to link this to bevacizumab (or other angiogenesis inhibitors) response (Figure 3). 111In‐bevacizumab SPECT visualized VEGF expression in various malignancies, including CRC, RCC and melanoma and 89Zr‐bevacizumab PET visualized primary BC (Desar et al., 2010; Gaykema et al., 2013; Nagengast et al., 2011; Scheer et al., 2008). There was however no straightforward correlation between radiolabeled bevacizumab uptake and tumor VEGF‐A expression as determined by conventional methods (in situ hybridization/ELISA), suggesting that other factors play a role in bevacizumab targeting, such as vascular volume, vascular permeability and/or interstitial fluid pressure. In melanoma patients, bevacizumab treatment resulted in a decrease in 111In‐bevacizumab tumor targeting, which could be due to both therapeutic VEGF‐blocking and bevacizumab‐induced vascular changes, since bevacizumab‐induced vascular changes can hamper tumor uptake of antibodies (Arjaans et al., 2013; Heskamp et al., 2013a; Nagengast et al., 2011). Although these results indicate that bevacizumab imaging might aid in optimizing bevacizumab therapies, to date no studies have been performed to determine whether bevacizumab imaging is predictive to bevacizumab therapy.
Figure 3.
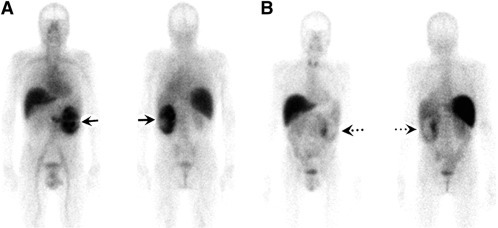
111In‐bevacizumab imaging in a RCC patient before and after anti‐angiogenic treatment. Anterior and posterior 111In‐bevacizumab scans at baseline (A) and after 4 weeks of treatment with the TKI sorafenib (angiogenesis inhibitor; 400 mg twice daily) (B). A marked decrease of 111In‐bevacizumab uptake is observed (arrows). This figure was originally published in JNM; reprinted with permission (Desar et al., 2010) (adapted).
4.4. Rituximab (Rituxan)
Rituximab was the first therapeutic mAb approved by the FDA for the treatment of cancer. Rituximab is directed against the cell surface marker CD20, which is expressed on B‐lymphocytes and approved for the treatment of non‐Hodgkin's lymphoma (NHL). Beneficial effects have also been reported in chronic lymphocytic leukemia (CLL) in combination with chemotherapy (Okroj et al., 2013). Also for rituximab, patients are selected based on CD20 expression levels, which can be measured by ELISA or flow cytometry. Again, its predictive value is not straightforward (Smith, 2003; Tran et al., 2010). While rituximab is effective in more than 50% of CD20‐positive follicular NHL patients, it is less effective in other CD20‐positive lymphoma subtypes, with activity in only 10–15% of CD20‐expressing small lymphocytic lymphomas (McLaughlin et al., 1998). This may partially be explained by interpretation of diagnostic results. For example, a positive flow cytometric sample is typically determined as having >30% of cells above the cut‐off. Thus even in a CD20‐positive lymphoma, many cells may have low or no expression. No or loss of CD20 expression is associated with rituximab resistance (Sugimoto et al., 2009). CLL and small lymphocytic lymphoma have faint CD20 staining, corresponding to a lower rituximab response than follicular lymphoma. Mantle cell and diffuse‐large‐cell lymphoma, however, have similar or even higher CD20 expression levels than follicular lymphoma, but lower response rates, thus staining intensity alone does not predict response (Smith, 2003). Therefore, other mechanisms must be involved affecting rituximab response, although the exact underlying resistance mechanisms are yet unknown.
Rituximab has been labeled with various radioactive compounds for either imaging or therapeutic RIT (Section 5.1), depending on the radioisotope used. Iodine‐131 (131I)‐labeled rituximab SPECT visualized CD20‐positive cells in NHL patients, although these studies primarily focused on hematologic toxicity due to subsequent rituximab‐RIT (Section 5.1) (Boucek and Turner, 2005; Leahy et al., 2006). In four patients with highly CD20‐positive primary central nervous system lymphomas (PCNSL), iodine‐123 (123I)‐labeled rituximab SPECT revealed very low tumor accumulation up to 48 h post‐injection, most likely because rituximab cannot pass the blood brain barrier (BBB), thus these tumors are unlikely to respond to rituximab treatment despite apparent CD20 expression (Dietlein et al., 2005). This exemplifies the importance of target accessibility instead of solely investigating target expression levels with conventional methods. Although there is at present no clinical study specifically linking pretreatment radiolabeled‐rituximab tumor uptake levels to rituximab therapy response, clinical imaging results so far indicate that molecular imaging approaches could be more predictive of response than conventional methods.
4.5. Antibodies against novel targets
Two novel promising targets for various cancers are the Insulin‐like Growth Factor‐1 Receptor (IGF‐1R) and MET (Fleuren et al., 2013; Pollak, 2012). Numerous clinical trials have been performed with various IGF‐1R antibodies in carcinomas and sarcomas. Unfortunately, despite some spectacular responses, the majority of patients are resistant to IGF‐1R‐targeted antibody therapy (Basu et al., 2011). However, these studies were performed upon a heterogeneous group of patients without prior identification of a predictive response biomarker. Nevertheless, IGF‐1R expression as determined by IHC cannot be an accurate predictor of response, since in Ewing sarcomas IGF‐1R is expressed in virtually all tumors, but the majority of patients are IGF‐1R‐therapy resistant (Scotlandi et al., 2011; van de Luijtgaarden et al., 2013). Molecular IGF‐1R imaging might be more adequate as exemplified in a preclinical bone sarcoma study. Here, 111In‐R1507 SPECT was able to differentiate the degree of response to the IGF‐1R antibody R1507, while IGF‐1R IHC could not, highlighting the importance of selecting the appropriate predictive test and warranting further clinical studies (Figure 4) (Fleuren et al., 2011).
Figure 4.
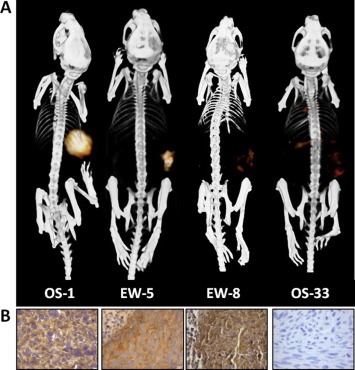
111In‐R1507 immuno‐SPECT and immunohistochemical IGF‐1R expression in mice with subcutaneous bone sarcoma xenografts. 111In‐R1507 immuno‐SPECT/CT scans (A) and IGF‐1R expression levels of corresponding tumors (B) in mice with bone sarcoma xenografts. Xenografts with high (OS‐1), intermediate (EW‐5), or no (EW‐8 and OS‐33) response to treatment with the IGF‐1R antibody R1507 were selected. Images were acquired 3 days post injection of 111In‐R1507. This figure was originally published in Clin Cancer Res; reprinted with permission (Fleuren et al., 2011) (adapted).
Although the majority of MET‐targeting drugs are TKIs, the MET antibody ornatuzumab (MetMab) showed promising (pre)clinical results (Catenacci et al., 2011). High immunohistochemical MET expression was an indicator for ornatuzumab efficacy in combined trials (Sharma and Adjei, 2011). At present, two MET‐antibodies have been radiolabeled (89Zr‐DN30 and 125I‐met3) and clearly visualized MET‐expressing xenografts in mice, showing the feasibility of MET‐imaging and encouraging further clinical (biomarker) studies (Hay et al., 2003; Perk et al., 2008).
5. Conjugated theranostic antibodies in current clinical practice
5.1. 90Y‐Ibritumomab tiuxetan (Zevalin) and 131I‐Tositumomab (Bexxar)
Zevalin and Bexxar are, like rituximab, antibodies directed against the CD20 antigen (Section 4.4) and approved for NHL treatment. Unlike rituximab, these are murine antibodies and conjugated to therapeutic β‐emitting radionuclides (yttrium‐90 (90Y) and 131I, respectively) for RIT. Although there are differences between Zevalin and Bexxar, such as different murine mAbs, radioisotopes and in vivo behavior, they share similar toxicity and efficacy profiles (Nowakowski and Witzig, 2006). Zevalin or Bexxar RIT is combined with unlabeled rituximab treatment to prevent excessive uptake of the radiolabeled antibodies in the spleen and bone marrow. One advantage of RIT over regular immunotherapy is its capacity to actually kill tumor cells instead of solely inhibiting proliferation. Secondly, the radioisotope delivers radiation not only to tumor cells binding the antibody, but also to neighboring cells inaccessible to the antibody or with insufficient antigen expression (cross‐fire effect). The additive efficacy of Zevalin and Bexxar RIT over unconjugated rituximab has been demonstrated in various studies. In a randomized control trial in patients with recurrent NHL, Zevalin RIT was well‐tolerated and had a higher rate of overall and complete responses compared to rituximab alone (Witzig et al., 2002b). Zevalin RIT was also effective in rituximab‐refractory NHL patients (Witzig et al., 2002a). Similar to rituximab, patients eligible for Zevalin or Bexxar treatment are primarily selected based on CD20 expression levels assessed by conventional methods. However, particularly for RIT, molecular imaging seems more appropriate, not only for assessing lymphoma targeting but also for evaluating the distribution of CD20‐positive cells in non‐tumor tissue (e.g. bone marrow) to estimate toxicity levels. Because 90Y is a pure therapeutic β‐emitter, it requires a surrogate radioisotope (γ‐emitter), such as 111In, for imaging. Thus for Zevalin imaging, ibritumomab tiuxetan can be labeled with 111In, while for Bexxar the 131I‐label can be used for both imaging and therapy since it emits both β and γ radiation. However, also for RIT, the predictive value of CD20 imaging is not straightforward. In eight NHL patients with biopsy‐proven CD20‐positivity, 111In‐ibritumomab tiuxetan scans revealed that patients with the highest quantitative concentration of lymph node radioactivity had a clinical and FDG‐PET/CT response upon Zevalin treatment (Jacobs et al., 2009). However, another study in 19 NHL patients showed no correlation between 111In‐ibritumomab tiuxetan uptake and Zevalin response (Gokhale et al., 2005). In 4 out of 6 patients with PCNSL, tumor 111In‐ibritumomab tiuxetan accumulation was observed up to 5 days post‐injection, in contrast to another study using 123I‐rituximab (Dietlein et al., 2005; Maza et al., 2009) (Section 4.4). The discrepancy was ascribed to delayed antibody accumulation in some PCNSL patients due to some remaining BBB function, which could only be visualized by 111In‐ibritumomab tiuxetan due to a longer physical half‐life, pointing out the importance of selecting the appropriate radionuclide. The study showed however no correlation between 111In‐ibritumomab tiuxetan tumor uptake and Zevalin response. Similar discordances have been reported for Bexxar scans (Sgouros et al., 2003). Because Zevalin and Bexxar are murine antibodies that might induce human anti‐mouse antibodies (HAMA), in general these agents are not administrated repeatedly. To obviate HAMA induction and allow repeated administration, several studies radiolabeled the chimeric antibody rituximab (131I‐rituximab and 177Lu‐rituximab) and investigated its RIT efficacy. In a phase I/II 177Lu‐rituximab trial of 29 lymphoma patients, activity (overall response and stable disease (SD)) was reported in 79% of patients (Forrer et al., 2013). In relapsed or refractory NHL studies, 131I‐rituximab RIT achieved high overall and complete response rates with minimal toxicity and repeated 131I‐rituximab RIT increased the response rate and duration compared to a single treatment (Kang et al., 2013; Leahy et al., 2006; Turner et al., 2003). Prior to 131I‐rituximab RIT, imaging of a tracer activity of 131I‐rituximab was performed for individualized dosimetry to monitor 131I‐rituximab distribution in these studies. Although this was primarily performed to assess expected toxicity, these scans often visualized target areas very clearly.
5.2. 177Lu‐J591 and 90Y‐J591
The J591 antibody is directed against the prostate‐specific membrane antigen (PSMA). PSMA expression is highly constricted to prostate epithelium and is upregulated in prostate cancer. Virtually all prostate cancers express PSMA and it is considered a prostate‐cancer restricted target. Although J591 can be administered unlabeled for regular immunotherapy, best responses are seen at relatively high J591 doses (≥100 mg) and the current opinion is that conjugation of J591 with toxins or radionuclides is a more promising approach (Morris et al., 2005). For RIT purposes, J591 has been labeled with 90Y or 177Lu in phase I studies in metastatic castraction‐resistant prostate cancer (CRPC) patients with promising results (Tagawa et al., 2013; Vallabhajosula et al., 2005b).
177Lu‐J591 can be used for both imaging and therapy because it emits both β and γ radiation, while for 90Y‐J591 imaging a surrogate radionuclide is required. A phase II study of 177Lu‐J591 in CRPC patients demonstrated accurate tumor targeting and prostate specific antigen (PSA) responses. Importantly, non‐invasive assessment of PSMA expression by imaging proved to be a predictive biomarker, since patients with poor PSMA‐imaging were less likely to respond to 177Lu‐J591 therapy (Tagawa et al., 2013). For 177Lu‐J591 and 90Y‐J591 RIT patient selection, 111In‐J591 imaging can be performed since distribution patterns are generally comparable and in this way no patient is exposed to a β‐emitter in the screening process (Kawano et al., 2010; Vallabhajosula et al., 2005b). Multiple administrations of 177Lu‐J591 or 90Y‐J591 were tolerated (Vallabhajosula et al., 2005a).
5.3. 177Lu‐girentuximab
Girentuximab binds to carbonic anhydrase IX (CAIX), a heat‐sensitive transmembranous glycoprotein, and is developed for the treatment of RCC. Previous studies reported almost ubiquitous expression (>90%) of CAIX in clear cell RCC (ccRCC). Girentuximab has been successfully labeled to 111In, 131I and 124I, though intra‐patient comparisons revealed higher uptake of residualizing 111In‐girentuximab preparations (Brouwers et al., 2005; Pryma et al., 2011). Thus for RIT, higher radiation doses can be guided to tumor lesions with residualizing radionuclides, such as 177Lu and 90Y. A phase I study in metastatic ccRCC patients reported that 177Lu‐girentuximab RIT is well‐tolerated, can be infused multiple times and may stabilize previously progressive metastatic ccRCC (Stillebroer et al., 2013). 111In‐girentuximab was used for diagnostic imaging, and only patients with visible CAIX‐targeting received high‐dose 177Lu‐girentuximab RIT (n = 23). Within this group, 78% of patients responded (1 partial response and 17 SD), which is superior over another study administering two consecutive 131I‐girentuximab treatments and indicates the predictive value of 111In‐girentuximab imaging (Brouwers et al., 2005).
5.4. Gemtuzumab ozogamicin (Mylotarg)
Mylotarg is a CD33‐mAb (gemtuzumab) linked to a toxin belonging to the class of calicheamicins. CD33 is expressed on the majority of myeloid leukemia cells, making it an attractive target for acute myeloid leukemia (AML). Mylotarg was developed because lintizumab, an unconjugated CD33 antibody, showed only modest clinical activity with or without chemotherapy. Mylotarg improved survival in a subset of AML patients when combined with standard chemotherapy, but the FDA requested withdrawal from the market due to the results of a phase III trial in AML (Jurcic, 2012). In that study, the addition of mylotarg to standard therapies resulted in no survival benefits and more toxicity (Petersdorf et al., 2013). Because CD33‐targeting might still be of interest in certain AML subsets, the introduction of predictive response biomarkers could aid in selecting those patients that could benefit from this treatment (Jurcic, 2012).
5.5. Trastuzumab emtansine (T‐DM1, Kadcyla)
T‐DM1 consists of the HER2‐antibody trastuzumab (Section 4.1) linked to the potent toxin mertansine (DM1). In phase II studies, T‐DM1 was active in HER2‐positive mBC patients refractory to trastuzumab and lapatinib and led to improved PFS compared to the combination of first‐line trastuzumab and docetaxel (Hurvitz et al., 2013; Krop and Winer, 2014). In a recent phase III trial in HER2‐positive mBC patients who previously received trastuzumab and a taxane, T‐DM1 improved the time to symptom worsening compared to capecitabine and lapatinib (7.1 vs. 4.6 months) (Verma et al., 2012). Based on the latter study, T‐DM1 was recently FDA‐approved for HER2‐positive mBC patients previously treated with trastuzumab. Similar to trastuzumab monotherapy, patients were selected based on HER2‐expression by conventional methods, although again HER2‐positivity is at best a prerequisite. Molecular HER2 imaging might be of more predictive value, which is the subject of an ongoing study investigating whether 89Zr‐trastuzumab PET/CT could identify HER2‐positive BC patients unlikely to benefit from T‐DM1 (NCT01565200). The early identification of non‐responders could not only aid in avoiding unnecessary side‐effects, but could also be cost‐effective because only patients likely to benefit from T‐DM1 treatment would be treated.
6. Summary and future prospects
Theranostics is a rapidly evolving approach in an era of personalized and precision medicine. Especially because the focus for treatment of most cancers is changing from relatively low‐cost chemotherapy to more expensive, targeted therapies, predictive markers are not only a clinical necessity but also an economic requirement. Application of an antibody for both diagnostic and therapeutic purposes is an elegant approach for predicting and monitoring therapy response. The first clinical studies, however, show some limitations, of which the majority lie in the type of diagnostic test used. Standard diagnostic tests such as IHC are performed upon a single, often archival FFPE tumor section and are unable to demonstrate whole tumor expression levels, multiple lesions or target accessibility. This could in part explain why these tests cannot accurately predict response in all patients. Other IHC shortcomings are inter‐observer subjectivity and the difficulty to define subcellular target localization. Functional antibody/antigen imaging modalities such as PET and SPECT can overcome all of these shortcomings by only showing membranous expression levels in all lesions. Moreover, these non‐invasive whole body scans can be carried out repetitively without the need for repeated invasive biopsies. Although besides target expression, especially for unconjugated antibody monotherapies, other growth compensatory mechanisms could influence the degree of response, this may be of less importance for conjugated antibodies due to additional therapeutic radiation or toxic effects. (Pre)clinical studies specifically addressing the predictive value of imaging biomarkers are however necessary, since not all radiolabeled antibodies have equally predictive value.
Altogether, we believe that antibody theranostics are definitely promising for the future, but close attention should be paid to the diagnostic test used for patient selection. Implementation of appropriate theranostics can significantly improve efficacy, efficiency and cost‐effectiveness of modern cancer treatments.
Fleuren Emmy D.G., Versleijen-Jonkers Yvonne M.H., Heskamp Sandra, van Herpen Carla M.L., Oyen Wim J.G., van der Graaf Winette T.A., Boerman Otto C., (2014), Theranostic applications of antibodies in oncology, Molecular Oncology, 8, doi: 10.1016/j.molonc.2014.03.010.
References
- Aerts, H.J. , Dubois, L. , Perk, L. , Vermaelen, P. , van Dongen, G.A. , Wouters, B.G. , Lambin, P. , 2009. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J. Nucl. Med.. 50, 123–131. [DOI] [PubMed] [Google Scholar]
- Arjaans, M. , Oude Munnink, T.H. , Oosting, S.F. , Terwisscha van Scheltinga, A.G. , Gietema, J.A. , Garbacik, E.T. , Timmer-Bosscha, H. , Lub-de Hooge, M.N. , Schroder, C.P. , de Vries, E.G. , 2013. Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res.. 73, 3347–3355. [DOI] [PubMed] [Google Scholar]
- Atkins, D. , Reiffen, K.A. , Tegtmeier, C.L. , Winther, H. , Bonato, M.S. , Storkel, S. , 2004. Immunohistochemical detection of EGFR in paraffin-embedded tumor tissues: variation in staining intensity due to choice of fixative and storage time of tissue sections. J. Histochem. Cytochem.. 52, 893–901. [DOI] [PubMed] [Google Scholar]
- Basu, B. , Olmos, D. , de Bono, J.S. , 2011. Targeting IGF-1R: throwing out the baby with the bathwater?. Br. J. Cancer. 104, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucek, J.A. , Turner, J.H. , 2005. Validation of prospective whole-body bone marrow dosimetry by SPECT/CT multimodality imaging in (131)I-anti-CD20 rituximab radioimmunotherapy of non-Hodgkin's lymphoma. Eur. J. Nucl. Med. Mol. Imaging. 32, 458–469. [DOI] [PubMed] [Google Scholar]
- Brouwers, A.H. , Mulders, P.F. , de Mulder, P.H. , van den Broek, W.J. , Buijs, W.C. , Mala, C. , Joosten, F.B. , Oosterwijk, E. , Boerman, O.C. , Corstens, F.H. , Oyen, W.J. , 2005. Lack of efficacy of two consecutive treatments of radioimmunotherapy with 131I-cG250 in patients with metastasized clear cell renal cell carcinoma. J. Clin. Oncol.. 23, 6540–6548. [DOI] [PubMed] [Google Scholar]
- Brown, M. , Wittwer, C. , 2000. Flow cytometry: principles and clinical applications in hematology. Clin. Chem.. 46, 1221–1229. [PubMed] [Google Scholar]
- Burtness, B. , Goldwasser, M.A. , Flood, W. , Mattar, B. , Forastiere, A.A. , Eastern Cooperative Oncology Group, 2005. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J. Clin. Oncol.. 23, 8646–8654. [DOI] [PubMed] [Google Scholar]
- Catenacci, D.V. , Henderson, L. , Xiao, S.Y. , Patel, P. , Yauch, R.L. , Hegde, P. , Zha, J. , Pandita, A. , Peterson, A. , Salgia, R. , 2011. Durable complete response of metastatic gastric cancer with anti-Met therapy followed by resistance at recurrence. Cancer Discov.. 1, 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, K.Y. , Shia, J. , Kemeny, N.E. , Shah, M. , Schwartz, G.K. , Tse, A. , Hamilton, A. , Pan, D. , Schrag, D. , Schwartz, L. , Klimstra, D.S. , Fridman, D. , Kelsen, D.P. , Saltz, L.B. , 2005. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J. Clin. Oncol.. 23, 1803–1810. [DOI] [PubMed] [Google Scholar]
- Corcoran, E.B. , Hanson, R.N. , 2013. Imaging EGFR and HER2 by PET and SPECT: a Review. Med. Res. Rev. 10.1002/med.21299 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Cuadros, M. , Villegas, R. , 2009. Systematic review of HER2 breast cancer testing. Appl. Immunohistochem. Mol. Morphol.. 17, 1–7. [DOI] [PubMed] [Google Scholar]
- de Mello, R.A. , Marques, A.M. , Araujo, A. , 2013. HER2 therapies and gastric cancer: a step forward. World J. Gastroenterol.. 19, 6165–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma, M. , Hanahan, D. , 2012. The biology of personalized cancer medicine: facing individual complexities underlying hallmark capabilities. Mol. Oncol.. 6, 111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roock, W. , Claes, B. , Bernasconi, D. , De Schutter, J. , Biesmans, B. , Fountzilas, G. , Kalogeras, K.T. , Kotoula, V. , Papamichael, D. , Laurent-Puig, P. , Penault-Llorca, F. , Rougier, P. , Vincenzi, B. , Santini, D. , Tonini, G. , Cappuzzo, F. , Frattini, M. , Molinari, F. , Saletti, P. , De Dosso, S. , Martini, M. , Bardelli, A. , Siena, S. , Sartore-Bianchi, A. , Tabernero, J. , Macarulla, T. , Di Fiore, F. , Gangloff, A.O. , Ciardiello, F. , Pfeiffer, P. , Qvortrup, C. , Hansen, T.P. , Van Cutsem, E. , Piessevaux, H. , Lambrechts, D. , Delorenzi, M. , Tejpar, S. , 2010. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol.. 11, 753–762. [DOI] [PubMed] [Google Scholar]
- Desar, I.M. , Stillebroer, A.B. , Oosterwijk, E. , Leenders, W.P. , van Herpen, C.M. , van der Graaf, W.T. , Boerman, O.C. , Mulders, P.F. , Oyen, W.J. , 2010. 111In-bevacizumab imaging of renal cell cancer and evaluation of neoadjuvant treatment with the vascular endothelial growth factor receptor inhibitor sorafenib. J. Nucl. Med.. 51, 1707–1715. [DOI] [PubMed] [Google Scholar]
- Dietlein, M. , Pels, H. , Schulz, H. , Staak, O. , Borchmann, P. , Schomacker, K. , Fischer, T. , Eschner, W. , Pogge von Strandmann, E. , Schicha, H. , Engert, A. , Schnell, R. , 2005. Imaging of central nervous system lymphomas with iodine-123 labeled rituximab. Eur. J. Haematol.. 74, 348–352. [DOI] [PubMed] [Google Scholar]
- Dijkers, E.C. , Oude Munnink, T.H. , Kosterink, J.G. , Brouwers, A.H. , Jager, P.L. , de Jong, J.R. , van Dongen, G.A. , Schroder, C.P. , Lub-de Hooge, M.N. , de Vries, E.G. , 2010. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther.. 87, 586–592. [DOI] [PubMed] [Google Scholar]
- Divgi, C.R. , Welt, S. , Kris, M. , Real, F.X. , Yeh, S.D. , Gralla, R. , Merchant, B. , Schweighart, S. , Unger, M. , Larson, S.M. , 1991. Phase I and imaging trial of indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J. Natl. Cancer Inst.. 83, 97–104. [DOI] [PubMed] [Google Scholar]
- Ensinger, C. , Sterlacci, W. , 2008. Implications of EGFR PharmDx kit for cetuximab eligibility. Expert Rev. Mol. Diagn.. 8, 141–148. [DOI] [PubMed] [Google Scholar]
- Fleuren, E.D. , Roeffen, M.H. , Leenders, W.P. , Flucke, U.E. , Vlenterie, M. , Schreuder, H.W. , Boerman, O.C. , van der Graaf, W.T. , Versleijen-Jonkers, Y.M. , 2013. Expression and clinical relevance of MET and ALK in Ewing sarcomas. Int. J. Cancer. 133, 427–436. [DOI] [PubMed] [Google Scholar]
- Fleuren, E.D. , Versleijen-Jonkers, Y.M. , van de Luijtgaarden, A.C. , Molkenboer-Kuenen, J.D. , Heskamp, S. , Roeffen, M.H. , van Laarhoven, H.W. , Houghton, P.J. , Oyen, W.J. , Boerman, O.C. , van der Graaf, W.T. , 2011. Predicting IGF-1R therapy response in bone sarcomas: immuno-SPECT imaging with radiolabeled R1507. Clin. Cancer Res.. 17, 7693–7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrer, F. , Oechslin-Oberholzer, C. , Campana, B. , Herrmann, R. , Maecke, H.R. , Mueller-Brand, J. , Lohri, A. , 2013. Radioimmunotherapy with 177Lu-DOTA-rituximab: final results of a phase I/II Study in 31 patients with relapsing follicular, mantle cell, and other indolent B-cell lymphomas. J. Nucl. Med.. 54, 1045–1052. [DOI] [PubMed] [Google Scholar]
- Gaykema, S.B. , Brouwers, A.H. , Lub-de Hooge, M.N. , Pleijhuis, R.G. , Timmer-Bosscha, H. , Pot, L. , van Dam, G.M. , van der Meulen, S.B. , de Jong, J.R. , Bart, J. , de Vries, J. , Jansen, L. , de Vries, E.G. , Schroder, C.P. , 2013. 89Zr-bevacizumab PET imaging in primary breast cancer. J. Nucl. Med.. 54, 1014–1018. [DOI] [PubMed] [Google Scholar]
- Gerber, D.E. , 2008. Targeted therapies: a new generation of cancer treatments. Am. Fam. Physician. 77, 311–319. [PubMed] [Google Scholar]
- Gokhale, A.S. , Mayadev, J. , Pohlman, B. , Macklis, R.M. , 2005. Gamma camera scans and pretreatment tumor volumes as predictors of response and progression after Y-90 anti-CD20 radioimmunotherapy. Int. J. Radiat. Oncol. Biol. Phys.. 63, 194–201. [DOI] [PubMed] [Google Scholar]
- Han, C.B. , M, J.T. , Li, F. , Zou, H.W. , 2011. Molecular markers for the prediction of anti-EGFR monoclonal antibody treatment efficacy in metastatic colorectal Cancer. J. Cancer Ther.. 2, 675–682. [Google Scholar]
- Hay, R.V. , Cao, B. , Skinner, R.S. , Wang, L.M. , Su, Y. , Resau, J.H. , Knudsen, B.S. , Gustafson, M.F. , Koo, H.M. , Vande Woude, G.F. , Gross, M.D. , 2003. Radioimmunoscintigraphy of human met-expressing tumor xenografts using met3, a new monoclonal antibody. Clin. Cancer Res.. 9, 3839S–3844S. [PubMed] [Google Scholar]
- Hegde, P.S. , Jubb, A.M. , Chen, D. , Li, N.F. , Meng, Y.G. , Bernaards, C. , Elliott, R. , Scherer, S.J. , Chen, D.S. , 2013. Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin. Cancer Res.. 19, 929–937. [DOI] [PubMed] [Google Scholar]
- Heldin, C.H. , Rubin, K. , Pietras, K. , Ostman, A. , 2004. High interstitial fluid pressure - an obstacle in cancer therapy. Nat. Rev. Cancer. 4, 806–813. [DOI] [PubMed] [Google Scholar]
- Heskamp, S. , Boerman, O.C. , Molkenboer-Kuenen, J.D. , Oyen, W.J. , van der Graaf, W.T. , van Laarhoven, H.W. , 2013. Bevacizumab reduces tumor targeting of antiepidermal growth factor and anti-insulin-like growth factor 1 receptor antibodies. Int. J. Cancer. 133, 307–314. [DOI] [PubMed] [Google Scholar]
- Heskamp, S. , van Laarhoven, H.W. , Oyen, W.J. , van der Graaf, W.T. , Boerman, O.C. , 2013. Tumor-receptor imaging in breast cancer: a tool for patient selection and response monitoring. Curr. Mol. Med.. 13, 1506–1522. [DOI] [PubMed] [Google Scholar]
- Heukelom, J. , Hamming, O. , Bartelink, H. , Hoebers, F. , Giralt, J. , Herlestam, T. , Verheij, M. , van den Brekel, M. , Vogel, W. , Slevin, N. , Deutsch, E. , Sonke, J.J. , Lambin, P. , Rasch, C. , 2013. Adaptive and innovative Radiation Treatment FOR improving Cancer treatment outcomE (ARTFORCE); a randomized controlled phase II trial for individualized treatment of head and neck cancer. BMC Cancer. 13, 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeben, B.A. , Molkenboer-Kuenen, J.D. , Oyen, W.J. , Peeters, W.J. , Kaanders, J.H. , Bussink, J. , Boerman, O.C. , 2011. Radiolabeled cetuximab: dose optimization for epidermal growth factor receptor imaging in a head-and-neck squamous cell carcinoma model. Int. J. Cancer. 129, 870–878. [DOI] [PubMed] [Google Scholar]
- Hurvitz, S.A. , Dirix, L. , Kocsis, J. , Bianchi, G.V. , Lu, J. , Vinholes, J. , Guardino, E. , Song, C. , Tong, B. , Ng, V. , Chu, Y.W. , Perez, E.A. , 2013. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol.. 31, 1157–1163. [DOI] [PubMed] [Google Scholar]
- Jacobs, S.A. , Harrison, A.M. , Swerdlow, S.H. , Foon, K.A. , Avril, N. , Vidnovic, N. , Joyce, J. , DeMonaco, N. , McCarty, K.S. , 2009. Radioisotopic localization of (90)Yttrium-ibritumomab tiuxetan in patients with CD20+ non-Hodgkin's lymphoma. Mol. Imaging Biol.. 11, 39–45. [DOI] [PubMed] [Google Scholar]
- Jain, R.K. , 1999. Transport of molecules, particles, and cells in solid tumors. Annu. Rev. Biomed. Eng.. 1, 241–263. [DOI] [PubMed] [Google Scholar]
- Johnson, G.A. , Mannel, R. , Khalifa, M. , Walker, J.L. , Wren, M. , Min, K.W. , Benbrook, D.M. , 1997. Epidermal growth factor receptor in vulvar malignancies and its relationship to metastasis and patient survival. Gynecol. Oncol.. 65, 425–429. [DOI] [PubMed] [Google Scholar]
- Jurcic, J.G. , 2012. What happened to anti-CD33 therapy for acute myeloid leukemia?. Curr. Hematol. Malig Rep.. 7, 65–73. [DOI] [PubMed] [Google Scholar]
- Kang, H.J. , Lee, S.S. , Byun, B.H. , Kim, K.M. , Lim, I. , Choi, C.W. , Suh, C. , Kim, W.S. , Nam, S.H. , Lee, S.I. , Eom, H.S. , Shin, D.Y. , Lim, S.M. , 2013. Repeated radioimmunotherapy with 131I-rituximab for patients with low-grade and aggressive relapsed or refractory B cell non-Hodgkin lymphoma. Cancer Chemother. Pharmacol.. 71, 945–953. [DOI] [PubMed] [Google Scholar]
- Kawano, M.O.J. , Vallabhajosula, S. , Tagawa, S. , Bander, N. , Goldsmith, S. , 2010. 111In-J591 scan to predict PSMA targeting and treatment response following RIT with 177Lu-J591 monoclonal antibody in patients with metastatic prostate cancer. J. Nucl. Med.. 51, (Suppl. 2) Abstract no. 173 [Google Scholar]
- Kenny, G.E. , Dunsmoor, C.L. , 1983. Principles, problems, and strategies in the use of antigenic mixtures for the enzyme-linked immunosorbent assay. J. Clin. Microbiol.. 17, 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krop, I. , Winer, E.P. , 2014. Trastuzumab emtansine: a novel antibody-drug conjugate for HER2-positive breast cancer. Clin. Cancer Res.. 20, 15–20. [DOI] [PubMed] [Google Scholar]
- Lambrechts, D. , Lenz, H.J. , de Haas, S. , Carmeliet, P. , Scherer, S.J. , 2013. Markers of response for the antiangiogenic agent bevacizumab. J. Clin. Oncol.. 31, 1219–1230. [DOI] [PubMed] [Google Scholar]
- Leahy, M.F. , Seymour, J.F. , Hicks, R.J. , Turner, J.H. , 2006. Multicenter phase II clinical study of iodine-131-rituximab radioimmunotherapy in relapsed or refractory indolent non-Hodgkin's lymphoma. J. Clin. Oncol.. 24, 4418–4425. [DOI] [PubMed] [Google Scholar]
- Liu, S.M. , Carpenter, G. , 1993. Differential heat stress stability of epidermal growth factor receptor and erbB-2 receptor tyrosine kinase activities. J. Cell Physiol. 157, 237–242. [DOI] [PubMed] [Google Scholar]
- Maza, S. , Kiewe, P. , Munz, D.L. , Korfel, A. , Hamm, B. , Jahnke, K. , Thiel, E. , 2009. First report on a prospective trial with yttrium-90-labeled ibritumomab tiuxetan (Zevalin) in primary CNS lymphoma. Neuro Oncol.. 11, 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarty, K. , Cornelissen, B. , Cai, Z. , Scollard, D.A. , Costantini, D.L. , Done, S.J. , Reilly, R.M. , 2009. Micro-SPECT/CT with 111In-DTPA-pertuzumab sensitively detects trastuzumab-mediated HER2 downregulation and tumor response in athymic mice bearing MDA-MB-361 human breast cancer xenografts. J. Nucl. Med.. 50, 1340–1348. [DOI] [PubMed] [Google Scholar]
- McLaughlin, P. , Grillo-Lopez, A.J. , Link, B.K. , Levy, R. , Czuczman, M.S. , Williams, M.E. , Heyman, M.R. , Bence-Bruckler, I. , White, C.A. , Cabanillas, F. , Jain, V. , Ho, A.D. , Lister, J. , Wey, K. , Shen, D. , Dallaire, B.K. , 1998. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J. Clin. Oncol.. 16, 2825–2833. [DOI] [PubMed] [Google Scholar]
- Meric-Bernstam, F. , Hung, M.C. , 2006. Advances in targeting human epidermal growth factor receptor-2 signaling for cancer therapy. Clin. Cancer Res.. 12, 6326–6330. [DOI] [PubMed] [Google Scholar]
- Morris, M.J. , Divgi, C.R. , Pandit-Taskar, N. , Batraki, M. , Warren, N. , Nacca, A. , Smith-Jones, P. , Schwartz, L. , Kelly, W.K. , Slovin, S. , Solit, D. , Halpern, J. , Delacruz, A. , Curley, T. , Finn, R. , O'Donoghue, J.,A. , Livingston, P. , Larson, S. , Scher, H.I. , 2005. Pilot trial of unlabeled and indium-111-labeled anti-prostate-specific membrane antigen antibody J591 for castrate metastatic prostate cancer. Clin. Cancer Res.. 11, 7454–7461. [DOI] [PubMed] [Google Scholar]
- Nagengast, W.B. , Hooge, M.N. , van Straten, E.M. , Kruijff, S. , Brouwers, A.H. , den Dunnen, W.F. , de Jong, J.R. , Hollema, H. , Dierckx, R.A. , Mulder, N.H. , de Vries, E.G. , Hoekstra, H.J. , Hospers, G.A. , 2011. VEGF-SPECT with (111)In-bevacizumab in stage III/IV melanoma patients. Eur. J. Cancer. 47, 1595–1602. [DOI] [PubMed] [Google Scholar]
- Nowakowski, G.S. , Witzig, T.E. , 2006. Radioimmunotherapy for B-cell non-Hodgkin lymphoma. Clin. Adv. Hematol. Oncol.. 4, 225–231. [PubMed] [Google Scholar]
- Okroj, M. , Osterborg, A. , Blom, A.M. , 2013. Effector mechanisms of anti-CD20 monoclonal antibodies in B cell malignancies. Cancer Treat Rev.. 39, 632–639. [DOI] [PubMed] [Google Scholar]
- Ong, G. , Gullick, W. , Sikora, K. , 1990. Oncoprotein stability after tumour resection. Br. J. Cancer. 61, 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perik, P.J. , Lub-De Hooge, M.N. , Gietema, J.A. , van der Graaf, W.T. , de Korte, M.A. , Jonkman, S. , Kosterink, J.G. , van Veldhuisen, D.J. , Sleijfer, D.T. , Jager, P.L. , de Vries, E.G. , 2006. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol.. 24, 2276–2282. [DOI] [PubMed] [Google Scholar]
- Perk, L.R. , Stigter-van Walsum, M. , Visser, G.W. , Kloet, R.W. , Vosjan, M.J. , Leemans, C.R. , Giaccone, G. , Albano, R. , Comoglio, P.M. , van Dongen, G.A. , 2008. Quantitative PET imaging of met-expressing human cancer xenografts with 89Zr-labelled monoclonal antibody DN30. Eur. J. Nucl. Med. Mol. Imaging. 35, 1857–1867. [DOI] [PubMed] [Google Scholar]
- Petersdorf, S.H. , Kopecky, K.J. , Slovak, M. , Willman, C. , Nevill, T. , Brandwein, J. , Larson, R.A. , Erba, H.P. , Stiff, P.J. , Stuart, R.K. , Walter, R.B. , Tallman, M.S. , Stenke, L. , Appelbaum, F.R. , 2013. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 121, 4854–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak, M. , 2012. The insulin receptor/insulin-like growth factor receptor family as a therapeutic target in oncology. Clin. Cancer Res.. 18, 40–50. [DOI] [PubMed] [Google Scholar]
- Pryma, D.A. , O'Donoghue, J.A. , Humm, J.L. , Jungbluth, A.A. , Old, L.J. , Larson, S.M. , Divgi, C.R. , 2011. Correlation of in vivo and in vitro measures of carbonic anhydrase IX antigen expression in renal masses using antibody 124I-cG250. J. Nucl. Med.. 52, 535–540. [DOI] [PubMed] [Google Scholar]
- Scheer, M.G. , Stollman, T.H. , Boerman, O.C. , Verrijp, K. , Sweep, F.C. , Leenders, W.P. , Ruers, T.J. , Oyen, W.J. , 2008. Imaging liver metastases of colorectal cancer patients with radiolabelled bevacizumab: lack of correlation with VEGF-A expression. Eur. J. Cancer. 44, 1835–1840. [DOI] [PubMed] [Google Scholar]
- Scotlandi, K. , Manara, M.C. , Serra, M. , Marino, M.T. , Ventura, S. , Garofalo, C. , Alberghini, M. , Magagnoli, G. , Ferrari, S. , Lopez-Guerrero, J.A. , Llombard-Bosch, A. , Picci, P. , 2011. Expression of insulin-like growth factor system components in Ewing's sarcoma and their association with survival. Eur. J. Cancer. 47, 1258–1266. [DOI] [PubMed] [Google Scholar]
- Scott, A.M. , Wolchok, J.D. , Old, L.J. , 2012. Antibody therapy of cancer. Nat. Rev. Cancer. 12, 278–287. [DOI] [PubMed] [Google Scholar]
- Sgouros, G. , Squeri, S. , Ballangrud, A.M. , Kolbert, K.S. , Teitcher, J.B. , Panageas, K.S. , Finn, R.D. , Divgi, C.R. , Larson, S.M. , Zelenetz, A.D. , 2003. Patient-specific, 3-dimensional dosimetry in non-Hodgkin's lymphoma patients treated with 131I-anti-B1 antibody: assessment of tumor dose-response. J. Nucl. Med.. 44, 260–268. [PubMed] [Google Scholar]
- Sharma, N. , Adjei, A.A. , 2011. In the clinic: ongoing clinical trials evaluating c-MET-inhibiting drugs. Ther. Adv. Med. Oncol.. 3, S37–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M.R. , 2003. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 22, 7359–7368. [DOI] [PubMed] [Google Scholar]
- Stein, R.A. , Staros, J.V. , 1996. Thermal inactivation of the protein tyrosine kinase of the epidermal growth factor receptor. Biochemistry. 35, 2878–2884. [DOI] [PubMed] [Google Scholar]
- Stillebroer, A.B. , Boerman, O.C. , Desar, I.M. , Boers-Sonderen, M.J. , van Herpen, C.M. , Langenhuijsen, J.F. , Smith-Jones, P.M. , Oosterwijk, E. , Oyen, W.J. , Mulders, P.F. , 2013. Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur. Urol.. 64, 478–485. [DOI] [PubMed] [Google Scholar]
- Sugimoto, T. , Tomita, A. , Hiraga, J. , Shimada, K. , Kiyoi, H. , Kinoshita, T. , Naoe, T. , 2009. Escape mechanisms from antibody therapy to lymphoma cells: downregulation of CD20 mRNA by recruitment of the HDAC complex and not by DNA methylation. Biochem. Biophys. Res. Commun.. 390, 48–53. [DOI] [PubMed] [Google Scholar]
- Tagawa, S.T. , Milowsky, M.I. , Morris, M. , Vallabhajosula, S. , Christos, P. , Akhtar, N.H. , Osborne, J. , Goldsmith, S.J. , Larson, S. , Taskar, N.P. , Scher, H.I. , Bander, N.H. , Nanus, D.M. , 2013. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res.. 19, 5182–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. , Kurihara, H. , Yonemori, K. , Tsuda, H. , Suzuki, J. , Kono, Y. , Honda, N. , Kodaira, M. , Yamamoto, H. , Yunokawa, M. , Shimizu, C. , Hasegawa, K. , Kanayama, Y. , Nozaki, S. , Kinoshita, T. , Wada, Y. , Tazawa, S. , Takahashi, K. , Watanabe, Y. , Fujiwara, Y. , 2013. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med.. 54, 1869–1875. [DOI] [PubMed] [Google Scholar]
- Teng, F.F. , Meng, X. , Sun, X.D. , Yu, J.M. , 2013. New strategy for monitoring targeted therapy: molecular imaging. Int. J. Nanomedicine. 8, 3703–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, L. , Baars, J.W. , Aarden, L. , Beijnen, J.H. , Huitema, A.D. , 2010. Pharmacokinetics of rituximab in patients with CD20 positive B-cell malignancies. Hum. Antibodies. 19, 7–13. [DOI] [PubMed] [Google Scholar]
- Turner, J.H. , Martindale, A.A. , Boucek, J. , Claringbold, P.G. , Leahy, M.F. , 2003. 131I-Anti CD20 radioimmunotherapy of relapsed or refractory non-Hodgkins lymphoma: a phase II clinical trial of a nonmyeloablative dose regimen of chimeric rituximab radiolabeled in a hospital. Cancer Biother. Radiopharm.. 18, 513–524. [DOI] [PubMed] [Google Scholar]
- Vallabhajosula, S. , Goldsmith, S.J. , Kostakoglu, L. , Milowsky, M.I. , Nanus, D.M. , Bander, N.H. , 2005. Radioimmunotherapy of prostate cancer using 90Y- and 177Lu-labeled J591 monoclonal antibodies: effect of multiple treatments on myelotoxicity. Clin. Cancer Res.. 11, 7195s–7200s. [DOI] [PubMed] [Google Scholar]
- Vallabhajosula, S. , Kuji, I. , Hamacher, K.A. , Konishi, S. , Kostakoglu, L. , Kothari, P.A. , Milowski, M.I. , Nanus, D.M. , Bander, N.H. , Goldsmith, S.J. , 2005. Pharmacokinetics and biodistribution of 111In- and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu?. J. Nucl. Med.. 46, 634–641. [PubMed] [Google Scholar]
- Van Cutsem, E. , de Haas, S. , Kang, Y.K. , Ohtsu, A. , Tebbutt, N.C. , Ming Xu, J. , Peng Yong, W. , Langer, B. , Delmar, P. , Scherer, S.J. , Shah, M.A. , 2012. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol.. 30, 2119–2127. [DOI] [PubMed] [Google Scholar]
- van de Luijtgaarden, A.C. , Versleijen-Jonkers, Y.M. , Roeffen, M.H. , Schreuder, H.W. , Flucke, U.E. , van der Graaf, W.T. , 2013. Prognostic and therapeutic relevance of the IGF pathway in Ewing's sarcoma patients. Target Oncol.. 8, 253–260. [DOI] [PubMed] [Google Scholar]
- Verma, S. , Miles, D. , Gianni, L. , Krop, I.E. , Welslau, M. , Baselga, J. , Pegram, M. , Oh, D.Y. , Dieras, V. , Guardino, E. , Fang, L. , Lu, M.W. , Olsen, S. , Blackwell, K. , Group, E.S. , 2012. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med.. 367, 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig, T.E. , Flinn, I.W. , Gordon, L.I. , Emmanouilides, C. , Czuczman, M.S. , Saleh, M.N. , Cripe, L. , Wiseman, G. , Olejnik, T. , Multani, P.S. , White, C.A. , 2002. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin's lymphoma. J. Clin. Oncol.. 20, 3262–3269. [DOI] [PubMed] [Google Scholar]
- Witzig, T.E. , Gordon, L.I. , Cabanillas, F. , Czuczman, M.S. , Emmanouilides, C. , Joyce, R. , Pohlman, B.L. , Bartlett, N.L. , Wiseman, G.A. , Padre, N. , Grillo-Lopez, A.J. , Multani, P. , White, C.A. , 2002. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J. Clin. Oncol.. 20, 2453–2463. [DOI] [PubMed] [Google Scholar]


