Abstract
Notch signaling in prominently involved in growth regulation in metazoan tissues. Because of this, Notch is often upregulated in cancer and current efforts point to developing drugs that block its activation. Notch receptor endocytosis towards acidic compartments is a recently appreciated determinant of signaling activation. Vacuolar H+ ATPase (V‐ATPase) is responsible for acidification of endocytic organelles and mutants in V‐ATPase subunit encoding genes in model organisms have been recently shown to display loss of Notch signaling. Here, we show that administration of BafilomycinA1 (BafA1), a highly specific V‐ATPase inhibitor decreases Notch signaling during Drosophila and Zebrafish development, and in human cells in culture. In normal breast cells, we find that BafA1 treatment leads to accumulation of Notch in the endo‐lysosomal system, and reduces its processing and signaling activity. In Notch‐addicted breast cancer cells, BafA1 treatment reduces growth in cells expressing membrane tethered forms of Notch, while sparing cells expressing cytoplasmic forms. In contrast, we find that V‐ATPase inhibition reduces growth of leukemia cells, without affecting Notch activatory cleavage. However, consistent with the emerging roles of V‐ATPase in controlling multiple signaling pathways, in these cells Akt activation is reduced, as it is also the case in BafA1‐treated breast cancer cells. Our data support V‐ATPase inhibition as a novel therapeutic approach to counteract tumor growth via signaling pathways regulated at the endo‐lysosomal level.
Keywords: V‐ATPase, Notch, BafilomycinA1, Endocytosis, Cancer
Highlights
V‐ATPase inhibition decreases Notch signaling during fly and fish development.
V‐ATPase inhibition reduces Notch signaling in normal and breast cancer cells.
V‐ATPase inhibition blocks degradation of membrane‐bound Notch forms.
V‐ATPase inhibition prevents Notch cleavage and nuclear translocation.
V‐ATPase inhibition reduces Akt signaling in breast cancer and T‐ALL cells.
1. Introduction
Tissue growth is regulated by a small number of signaling pathways universally conserved in metazoans. Enhanced growth signaling activity, due to an increasingly appreciated rooster of genetic lesions in genes encoding signaling components, is a hallmark of cancer.
Notch is at the center of a conserved signaling cascade mediating cell fate decisions, including those regulating tissue growth, during development and tissue homeostasis in metazoans. Notch activation in signal receiving cells relies on binding of the heterodimeric Notch receptor by ligands of Delta‐Serrate‐Lag‐2 (DSL) family expressed by neighboring signal‐sending cells. Ligand binding ultimately leads to production of a Notch form that is an efficient substrate for intramembrane protease γ‐secretase. Cleavage leads to release of the Notch intracellular domain (cNICD) which reaches the nucleus and signals (Bray, 2006).
Cleavage of Notch by γ‐secretase is thought to occur mostly at the plasma membrane. However, recent evidence points to the possibility of cleavage and activation in endosomes. In mouse and human cells, Notch has been found to be cleaved at the plasma membrane and in endosomes (Tagami et al., 2008). In Drosophila endocytic mutants that prevent entry of cargoes in endosomes, signaling is abolished. In contrast, in mutants that lead to accumulation of Notch in endosomes, Notch signaling is increased (Childress et al., 2006; Gallagher and Knoblich, 2006; Jaekel and Klein, 2006; Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005). Finally, activity of vacuolar ATPase, which is required for acidification of endocytic compartments, has been found to be necessary for activation of Notch signaling in both Drosophila and mammals (Lange et al., 2011; Sethi et al., 2010; Vaccari et al., 2010; Yan et al., 2009). Endocytic trafficking is also required to promote activation in signal‐sending cells, as Notch ligands need internalization to become competent for signaling (for review see Nichols et al., 2007).
As a consequence of the countless instances in which Notch operates during development and tissue homeostasis, Notch signaling alteration are observed in a wide array of developmental disorders and cancers. While congenital syndromes, as Alagille and a limited number of tumors, arise from partial loss of signaling activity, other syndromes and a large proportion of tumors, including the large majority of T‐cell acute lymphoblastic leukemias (T‐ALL), display enhanced Notch signaling activity (Agrawal et al., 2011; Klinakis et al., 2011; McDaniell et al., 2006; Nicolas et al., 2003; Oda et al., 1997; Puente et al., 2011; Stylianou et al., 2006; Weng et al., 2004). Consistent with the role of Notch as an oncogene, a number of T‐ALL and breast cancer cell lines possess mutations in Notch genes and are addicted to Notch signaling for growth (Robinson et al., 2011; Weng et al., 2004). In T‐ALL cells, growth is also promoted by ectopic activation of Akt, due to frequent loss of the negative regulator PTEN. Notch signaling and the Akt pathway appear to synergize, and mutational loss of PTEN is associated with human T‐ALL resistance to pharmacological inhibition of Notch signaling (Palomero et al., 2007). In addition, extensive evidence points to coregulation of the Notch and Akt/mTor signaling axes in breast cells and tumors, and in T‐ALL cells (Calzavara et al., 2008; Efferson et al., 2010; Guo et al., 2009; Meurette et al., 2009; Shepherd et al., 2013). Thus, the ability to inhibit both Notch and Akt signaling pharmacologically is likely to be the key to counteract oncogenic growth in these tumor contexts. We reasoned that the recently appreciated role of endocytic trafficking of Notch through acidic compartments for signaling activation could be harnessed to down‐modulate signaling. Inhibition of V‐ATPase activity is in principle a promising strategy also because of the recently reported involvement of V‐ATPase in regulation of mTor signaling (Bar‐Peled et al., 2012; Pena‐Llopis et al., 2011; Settembre et al., 2012; Zoncu et al., 2011).
Here, we find that pharmacologic inhibition of V‐ATPase decreases Notch signaling activity in vivo and in tissue culture cells. Upon treatment with specific V‐ATPase inhibitors, we find reduction of Notch signaling in both physiologic and pathologic conditions. We also observed reduction of signaling by the Akt/mTOR axis, which together with Notch, contributes to growth in certain tumor contexts. Our data suggest that pharmacologic inhibition of V‐ATPase could be beneficial to decrease pro‐growth signaling by the Notch and Akt/mTOR signaling pathways in breast tumors and T‐ALL leukemia.
2. Materials and methods
2.1. Drosophila strains
Fly strains used were Oregon R (OreR), E(spl)mβ‐lacZ (Nellesen et al., 1999), UAS shrub:GFP (Sweeney et al., 2006). rotund‐Gal4,TubGal80ts (Smith‐Bolton et al., 2009) driver, which is expressed in the wing pouch, was used to induce the expression of UAS shrub:GFP in larval wing imaginal discs. First instar larvae from rotund‐Gal4,TubGal80ts/UAS shrub:GFP flies were collected at 18 °C and then shifted at 29 °C for 24hr to induce expression in wing discs.
2.2. Compound treatments in flies
For drug treatment the following compounds were used: DAPT (Sigma), Leupeptin (Sigma), NH4Cl (Sigma), BafilomycinA1 (Calbiochem), ConcanamycinA (Calbiochem), Chloroquine (Sigma). They were all diluted in DMSO or ethanol and stock solutions for each compound were added to 0.5 ml of liquid yeast to a final concentration of 1 mM for DAPT and 2–4 μM for BafA1. Yeast containing the appropriate compound was added on the top of a low‐sugar fly food (Agarose 1%, Propionic Acid, 15% Sucrose, Tegosept, Ampicillin, water). Vials were kept overnight to allow evaporation of residual DMSO/ethanol. 20 females and 10 males were introduced into each vial, and kept at 25 °C for 5 days. Adults were removed, and progeny was allowed to progress in the presence of compound until eclosion. For experiments with rotund‐Gal4, TubGal80ts/UAS shrub:GFP flies, 20 females and 10 males were kept at 18 °C into vials containing drugs until first instar larvae appeared. Adults were removed and vials containing first instar larve were shifted to 29 °C until 3rd instar.
2.3. Zebrafish drug treatments
Zebrafish strains were maintained and bred according to the standard procedures and according to EU regulations on laboratory animals. We used embryos from the Notch‐responsive reporter line Tg(Tp1bglob:eGFP)ˆum14 (Parsons et al., 2009). To inhibit γ‐secretase, manually dechorionated zebrafish embryos at gastrulation (80–90% epiboly stage) were incubated O/N at 28.5 °C in the dark into 2 ml petri dish containing 0.4% DMSO in E3 medium (embryo water) with DAPT, (Sigma) at a final concentration of 50, 100 or 200 μM. To specifically block V‐ATPase proton pump activity, we incubated gastrulation, 1‐somite or 18 somites stage zebrafish embryos with Bafylomicin A1 inhibitor (Sigma) that was added directly to the fish medium at a final concentration of 50, 100 or 300 nM. As control, we incubated at the same temperature embryos in 0.4% DMSO in fish medium without drugs. In all treatments, the embryos were left in the incubation medium at 28.5 °C until 26–28 hpf stage. All phenotypes have been scored in n > 10 embryos for each condition and at least repeated twice. Treated and control embryos were then mounted in 3% methylcellulose solution and observed under a Nikon fluorescence stereomicroscope.
2.4. Cell culture
MCF10A cells were cultured in DMEM/F12 (1:1) supplemented with 5% Horse Serum (Invitrogen), 10 μg/ml Insulin, 0.5 μg/ml Hydrocortisone, 100 ng/ml cholera toxin (SIGMA) and freshly added 20 ng/ml EGF. HCC2218, HCC1187, and CCRF‐CEM cells (ATCC) were cultured in RPMI 1640 (Lonza) supplemented with 10% FBS and 1% l‐glutamine according to supplier's indications. HCC1599 (ATCC) was cultured in RPMI 1640 (Lonza) supplemented with 1% l‐glutamine and 20% FBS. DND‐41 was cultured in RPMI 1640 (Lonza) supplemented with 15% FBS SA, 2 mM glutamine. HCC1599 and DND‐41 cells require in our hands higher FBS concentrations, compared to the other cell lines, for optimal growth. All cells were cultured at 37 °C and 5% CO2 in a humidified incubator.
2.4.1. Drug treatment of cells
For drug treatments, cells were diluted to appropriate densities and treated by adding the drugs or DMSO directly into the medium to the indicated final concentrations. They were then mixed by gently rocking the cell culture vessel. DMSO was used at a final concentration of 0.5%. The cells were then seeded in appropriate cell culture vessels and placed in culture. All cells were cultured at 37 °C and 5% CO2 in a humidified incubator.
2.5. Lysotracker assays and DQ‐Red BSA assays
Wing discs from 3rd instar larvae dissected in Drosophila cell culture medium (M3, Sigma) were incubated in medium containing 1 μM Lysotracker (DND‐99, Molecular Probes) for 5 min at RT. After washing in fresh medium, the samples were mounted and imaged.
To label organelles with low internal pH in Zebrafish embryos, we added LysoTracker directly to the E3 medium at the concentration of 0.08 μM. In this experiment, 28 hpf Tg (Tp1bglob:eGFP)ˆum14 embryos were incubated 60 min at 28.5 °C with the LysoTracker prior mounting in 1% low‐melting agarose in E3 medium for confocal analysis.
To label MCF10‐A cells, they were seeded onto glass coverslips and cultured to approximately 70% confluence, at which point they were treated with DMSO, DAPT or BafA1 by adding the drugs directly into the wells so as to achieve the desired final concentrations. The medium was mixed and the cells placed under normal cell culture conditions for 3 h. Lysotracker or DQ‐BSA were then added directly into the medium to a final concentration of 1uM. The medium was mixed well and the cells placed in normal cell culture conditions for 30 min (Lysotracker) or 3 h (DQ‐BSA). They were then rinsed twice with DPBS and mounted immediately (Lysotracker) or fixed with 4% ParaFormAldehyde (4% PFA; DQ‐BSA) and mounted onto glass coverslips for confocal examination.
2.6. Notch translocation assay
MCF10A cells were seeded onto glass coverslips and grown to approximately 70–80% confluence. Notch translocation was stimulated by adding a EGTA directly into the wells to a final concentration of 10 mM. The cells were put back in normal culture conditions for 30 min after which the medium was decanted and cells rinsed twice with ice cold PBS 1X. The cells were then fixed for immunostaining. Where noted, MCF10A cells were pre‐treated for 3 h by adding the indicated drugs directly into the wells to the desired final concentrations.
2.7. Immunostainings
For Drosophila experiments, wing imaginal discs were dissected and treated as in [5]. Primary antibodies used were mouse anti β‐Gal [1:25; E7, Developmental Studies Hybridoma Bank (DSHB)], mouse anti‐Notch (1:50; C17.9C6 DHSB), rabbit anti‐Ubiquitin (1:1000; FK2 1:1000; Biomol), rabbit anti‐Avalanche (1:500; gift of D. Bilder).
For cell tissue culture experiments, cells were seeded onto glass coverslips and grown to desired confluence and then fixed for 10 min in 4% PFA at RT. After three washes in PBS 1X (5 min each), cells were permeabilized for 10 min in 0.1% Triton X‐100 diluted in PBS 1X. They were then incubated in 3% BSA dissolved in PBS 1X blocking solution for 30 min. The cells were then incubated at RT in primary antibody diluted in blocking solution for 1 h followed by 3 washes (5 min each). They were then incubated at RT with secondary antibody diluted in PBS 1X for 1 h followed by three washes (5 min each) with PBS 1X. Primary antibodies used were: mouse anti‐Lamp1/CD107a H4A3 (BD Pharmingen) at 1:1000, or rat anti‐Full length Notch1, 5B5 monoclonal antibody at 1:300 (Sigma). For both Drosophila wing discs and cells, cortical actin was stained using Rodamine‐Phalloidin (Sigma) diluted at 1:100 and the nuclei with DAPI (Sigma) diluted at 1:1000. The samples were then rinsed thrice with PBS 1X and then mounted using ProLong antifade‐Glycerol 1:1 solution. Samples were then analyzed and imaged using a Leica TCS SL confocal system. Digital images were processed using the Photoshop and ImageJ softwares without biased manipulations.
2.8. Western blot assays
For western blot assays, cells were seeded and treated by adding the drugs at indicated doses directly into the medium. The cells were then placed in normal culture conditions for 7 days after which they were collected. For adherent cells, the medium was decanted and cells rinsed twice with ice cold PBS 1X. They were then collected by scraping into 1 ml ice cold PBS 1X. Where indicated, MCF10A cells were EGTA‐treated in the presence of the drugs prior to collection. For collection, cells growing in suspension were centrifuged at 1200 rpm for 5 min. The medium was decanted and the pellet resuspended in 1 ml ice cold PBS 1X. The cells were centrifuged at full speed, at 4 °C for 5 min. The PBS was decanted and the pellets resuspended in appropriate volumes of RIPA buffer freshly supplemented protease inhibitor cocktail set III (Calbiochem). The cells were placed on ice for 30 min and vortexed at 10 min intervals while maintaining them on ice. The cell lysate was cleared by centrifugation at full speed, at 4 °C for 30 min. The supernatants were recovered and quantified by standard methods. Samples were denatured by adding β‐mercaptoethanol containing loading dye and by heating for 5 min at 98 °C. They were then resolved on 8% polyacrylamide gels. After blotting onto nitrocellulose membranes, the membranes were stained with rabbit anti‐cleaved Notch1 (Val1744, Cell Signaling) diluted at 1:500, Rat anti full length Notch1 (5B5 monoclonal antibody, Sigma) diluted at 1:500, Rabbit anti phospho‐S473‐Akt (Cell Signaling) at 1:1000, Rabbit anti pan‐Akt (Cell Signaling) at 1:1000, Rabbit anti‐p70 S6K (Cell Signaling) at 1:1000 or Rabbit anti phospho‐p70‐S6K (Cell Signaling) at 1:1000 primary antibodies. Normalization of cell extracts was performed by staining with mouse anti‐Vinculin (Sigma) antibody at 1:4000 or mouse anti‐tubulin (Sigma) at 1:10,000. Goat anti‐rabbit (Biorad), Goat anti‐mouse (Biorad) or Goat anti‐rat (GE Healthcare) HRP conjugated secondary antibodies were used. Signal was detected using Pierce ECL Western Blotting Substrate (Thermo Scientific), and imaged using a Chemidoc molecular imager (Biorad).
2.9. Population growth assay
Cells were counted and resuspended so as to have the desired cell numbers per 100 μL of the culture medium. They were then treated by adding the drugs directly into the medium to the desired final concentration. The cells were then seeded in triplicate into 96‐well plates by transferring 100 μl of the cell suspension into each well and treated when needed. As a blank, 100 μl of culture medium was also seeded in triplicate. The cells were placed under normal cell culture conditions and cell proliferation measurements taken on day 7 using WST‐1 cell proliferation assay (Roche) according to the manufacturer's instructions. Cell proliferation measures were taken by reading absorbance at 450 nm using a Wallac 1420 VICTOR plate reader (Perkin Elmer).
2.10. RT‐PCR
Total RNA from wing imaginal discs (40 discs per sample) or human cells was extracted using TRIZOL Reagent (Invitrogen) and RNeasy Mini Kit (Qiagen) following the manufacturer's instructions. Concentration and purity was determined by measuring optical density at 260 and 280 nm using a Nanodrop spectrophotometer. Total RNA was reverse transcribed using SuperScript VILO cDNA Synthesis kit (Invitrogen) according to the manufacturer's instructions. 5 ng of cDNA was amplified (in triplicate) in a reaction volume of 15 uL containing the following reagents: 7.5ul of TaqMan PCR Mastermix 2× No UNG (Applied Biosystems), 0.75 ul of TaqMan Gene expression assay 20× (Applied Biosystems). 300 nM of primers and 100 nM of Roche probes were used. RT‐PCR was carried out on the ABI/Prism 7900 HT Sequence Detector System (Applied Biosystems), using a pre‐PCR step of 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 60 s at 60 °C.
The following primers (5′‐3′) were used: Drosophila E(Spl)‐mβ: fwd gagtgcctgacccaggag, rvs cggtcagctccaggatgt. Drosophila E(Spl)‐ m7: fwd agcgacaacgagtctctgct, rvs ttaccagggacgccacac. Drosophila rpL32‐RA: fwd cggatcgatatgctaagctgt, rvs cgacgcactctgttgtcg. GFP: fwd gaagttcgagggcgacac, rvs ccgtcctccttgaagtcg. For human genes the following Applied Biosystem probes were used: Hes1: Hs00172878_m1, Hes2; Hs00219505_m1, Hes5; Hs01387463_g1, Hey1: Hs00232618‐m1, Hey2: Hs00232622_m1, Notch1: Hs00413187‐m1, Notch2: Hs00225747‐m1, Notch3: Hs00166432_m1, Notch4: Hs00270200_m1, Jag1: Hs00164982‐m1, Jag2: Hs00171432_m1, Delta1:Hs00194509_m1, Delta3: Hs00213561_m1, Delta4: Hs00184092_m1, Numb: Hs00377772‐m1, c‐Myc: Hs00153408‐m1, GADPH: Hs99999905‐m1.
Statistical analysis is based on Student's t‐test. Results are flagged with two asterisks when the P‐value is less than 0.01, and three asterisks when the P‐value is less than 0.001.
2.11. SiRNA knock‐downs
To knock down genes, siRNAs against desired genes were transfected into MCF10A cells using the lipofectamine RNAiMax transfection reagent (Life technologies) by following the manufacturer's instructions. Following transfection, the cells were placed under normal cell culture conditions for 72 h after which RNA was extracted for qPCR analysis. siRNA duplexes against PSENEN (D‐008057‐01‐0010), ATP6V0C (D‐017620‐03), ATP6V1A (D‐017590‐01) and ATP6V1F (D‐011930‐01) were purchased from Dharmacon, Thermo Scientific.
3. Results
Because Drosophila tissue mutant for non redundant V‐ATPase subunit encoding genes display loss of Notch signaling activation (Vaccari et al., 2010; Yan et al., 2009), we sought to test whether pharmacologically blocking the proton pump affected Notch signaling. To this end, we first determined the condition to inhibit V‐ATPase in vivo. We fed Drosophila larvae fresh yeast supplemented with ConcanamycinA (ConA) and BafA1 which specifically inhibit proton pumping, and with NH4Cl and Chloroquine, which reduce luminal pH independently of V‐ATPase as positive controls. As a negative control, we also fed animals with N‐[N‐(3,5‐Difluorophenacetyl)‐L‐alanyl]‐S‐phenylglycine butyl ester (DAPT), a GSI frequently used to block Notch signaling, and Leupetin, an inhibitor of lysosomal proteases (Fig. S1). Under these conditions, we tested the extent of acidification of the endolysosomal system in cells of the wing imaginal disc, an epithelial organ precursor of the adult wing, by culturing dissected discs with LysoTracker, a fluorescent acidophylic dye. In wing disc tissue of mock treated larvae, LysoTracker incorporates in acidified endosomes (Fig. S1A). In NH4Cl‐, Chloroquine‐, ConA‐, and BafA1‐fed animals, LysoTracker incorporation in discs is markedly reduced, consistent with decreased endolysosomal acidification (Fig. S1B‐E). As expected, LysoTracker incorporation is not reduced in discs of DAPT‐ and Leupetin‐fed animals (Fig. S1F‐G). These data indicate that endolysosomal acidification can be effectively inhibited pharmacologically in vivo. As BafA1, among the compounds that elicited a reduction of endolysosomal acidification, is a highly specific inhibitor of V‐ATPase (Bowman and Bowman, 2002), we selected it for further experiments.
We next asked whether pharmacologic inhibition of V‐ATPase reduces activation of Notch signaling in vivo (Figure 1). To this end, we fed larvae expressing the Notch reporter E(spl)mβ‐LacZ with BafA1 and assessed the extent of β‐gal expression in wing discs. E(spl)mβ‐LacZ reports expression of the Notch target gene E(spl)mβ along the dorso‐ventral boundary of the disc, corresponding to the future wing margin and in other parts of the discs in which Notch signaling is active and promotes cell growth (Figure 1A) (Nellesen et al., 1999). Compared to mock‐fed controls, discs from larvae fed with the DAPT exhibit a marked decrease of β‐gal expression by immunostaining (Figure 1B). This control experiment indicates that reduction of target gene expression can be achieved by feeding a known Notch signaling inhibitor and confirms previous evidence (Micchelli et al., 2003). Similarly, discs from BafA1‐fed larvae show a decrease of β‐gal expression by immunostaining (Figure 1C). Quantitative RT‐PCR (QPCR) using primers to detect endogenous expression of E(spl)mβ and E(spl)m7 indicates a more than 50% reduction of mRNA expression, an effect comparable to feeding DAPT (Figure 1D). These data indicate that pharmacologic inhibition of V‐ATPase activity in vivo leads to reduction of physiologic Notch signaling activity and support the recent finding that V‐ATPase might be part of the machinery controlling endolysosomal Notch activation.
Figure 1.
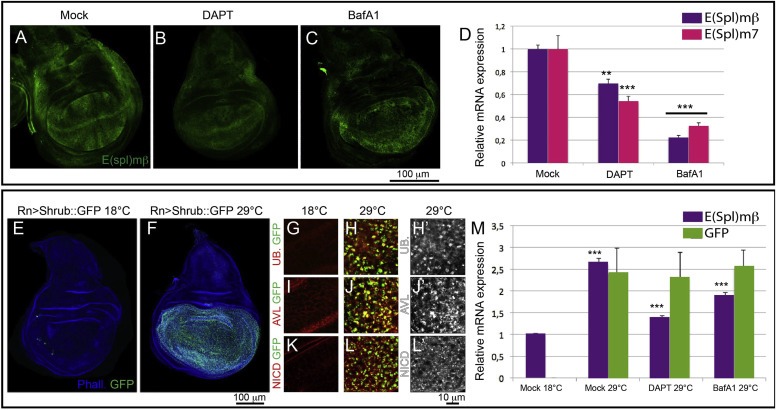
Reduced Notch signaling in Drosophila imaginal disc upon pharmacological inhibition of V‐ATPase. (A–C) Single confocal sections of 3rd instar wing imaginal discs from larvae expressing the Notch signaling reporter E(spl)mβ‐LacZ that have been fed with the indicated drug. Discs are stained with anti‐βGal to detect expression of the Notch target. Compared with discs from mock‐fed animals (A), discs from animals fed with DAPT (B) and BafA1 (C) show a significant decrease of Beta‐Gal expression. (D) Quantitative RT‐PCR on mRNA extracted from 3rd instar wing imaginal discs from flies fed with drugs as indicated. E(spl)mβ‐LacZ and E(spl)m7 show a 30–40% decrease upon γ‐secretase inhibition and more than 60% with V‐ATPase inhibitor BafA1. (E–F) Single confocal section of discs not expressing (E) or expressing (F) Shrub:GFP under the control of RnGAL4, a wing pouch specific driver. (G–L) High magnification confocal sections of wing pouch cells not expressing (G, I, K) or expressing (H, J, L) Shrub:GFP. Discs have been stained to detect Ubiquitin (G–H), the endosomal marker Avl (I–J) or the Notch intracellular domain (NICD; K‐L). In discs expressing Shrub:GFP, accumulation of ubiquitinated cargoes, including Notch, at endosomal sites is observed. Single channels for Ubiquitin, Avl and NICD are shown in H′, J′, L′. (M) Quantitative RT‐PCR on mRNA extracted from wing imaginal discs from Shrub:GFP expressing flies fed with drugs and induced as indicated. E(spl)mβ expression is 30% reduced upon feeding with BafA1 and 50% upon γ‐secretase inhibition. Comparable GFP expression levels indicate equal amounts of Shrub:GFP expressing cells in induced samples under different drug treatment.
We then wondered whether V‐ATPase inhibition is capable not only of reducing physiologic Notch signaling activation, but also the excess activation seen when endolysosomal Notch degradation is blocked. Chronic block of endosomal degradation during wing disc development is observed in mutants of Endosomal Sorting Required for Transport (ESCRT) genes, which control sorting of Notch and other degradative cargoes on the endosomal membrane. ESCRT mutants imaginal discs display ligand‐independent, but γ‐secretase dependent, Notch signaling activation (Herz et al., 2006; Hori et al., 2011; Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005). To block endolysosomal degradation in BafA1 feeding experiments, we expressed in wing discs a GFP‐tagged dominant negative VPS32 (Shrub:GFP) (Sweeney et al., 2006), a component of the ESCRT‐III complex. Expression of Shrub:GFP for 24 h during late wing disc development results in accumulation of Notch and ubiquitinated cargoes in endosomes, consistent with a block in endosomal sorting (Figure 1E–L). Such accumulation correlates with a more than 2.5 fold increase in expression of the Notch target E(spl)mβ, as revealed by QPCR on wing discs extracts (Figure 1M). Compared to mock‐fed controls, DAPT feeding of larvae expressing Shrub:GFP results in reduction of excess Notch signaling to almost uninduced levels. In comparison, by feeding with BafA1 we obtained an intermediate reduction of excess Notch signaling (Figure 1M). These data indicate that, similarly to γ‐secretase inhibition, V‐ATPase inhibition reduces Notch signaling in a model of pathological activation associated with defective endolysomal degradation.
Overall, our data so far indicate that pharmacologic inhibition of V‐ATPase, using the highly specific drug BafA1, leads to reduction of Notch signaling activity in vivo in Drosophila. We next wondered whether control of Notch signaling by V‐ATPase is exclusive of invertebrate development or whether is conserved in vertebrates. To test this, we assessed whether BafA1 reduces Notch signaling in developing Zebrafish embryos (Figure 2). To detect Notch signaling activity, we used fish transgenic for the Notch signaling reporter Tg(Tp1bglob:eGFP)ˆum14 in which EGFP expression is under the control of 12 Notch‐reponsive RBP‐Jk binding sites (Parsons et al., 2009). Compared to controls, embryos developing from gastrulation in presence of DAPT under these conditions display only minor defects (Figure 2A and B). However, as reported they show reduced Tg(Tp1bglob:eGFP)ˆum14 expression, indicating reduced Notch signaling activation (Figure 2A′ and B′; (Parsons et al., 2009)). Consistent with V‐ATPase inhibition, LysoTracker incorporation is lost in BafA1‐treated embryos (Fig. S2A‐B). However, compared with DAPT, treating with BafA1 at high doses results in strong developmental defects, including tail and trunk shortening (Fig. S2C‐E). These defects are consistent with those observed in Zebrafish insertional mutants of multiple V‐ATPase subunit genes (Amsterdam et al., 2004). Despite this, reduction of GFP expression is observed (Fig. S2D′). Treatment at 1‐somite stage results in milder defects and reduced GFP expression (Fig. S2F–F″). Treatment with BafA1 at the end of somitogenesis reduces the extent of defects further, while reduction of GFP expression remains comparable to that achieved with DAPT (Fig. S2G‐G″). BafA1 treatment at gastrulation with reduced dosage allows embryos to develop almost normally. Also under these conditions we still observe a reduction of GFP expression that is comparable to that achieved upon DAPT treatment (Figure 2A–C). Overall, these data indicate that V‐ATPase inhibition reduces Notch signaling activity in Zebrafish embryos, and suggest that Notch signaling depends on V‐ATPase activity also during vertebrate development.
Figure 2.
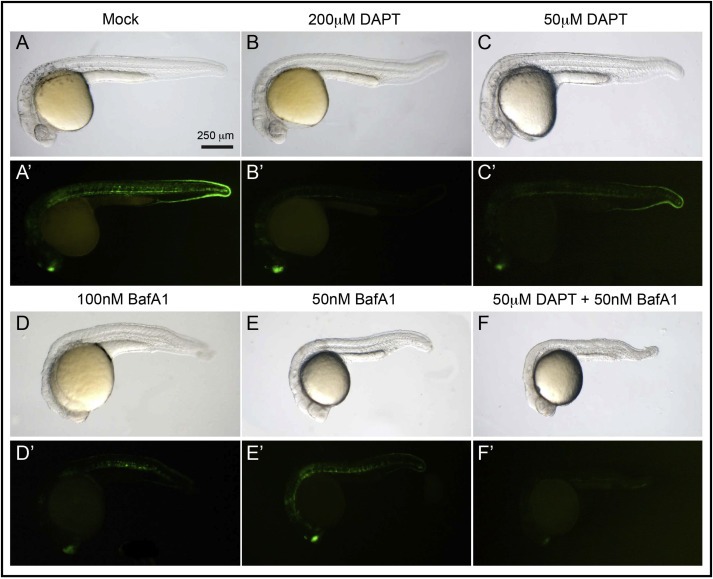
Impaired Notch‐signaling activity by V‐ATPase inhibition during vertebrate development. (A–E) Representative 28 hpf zebrafish embryos treated as at gastrulation as indicated and visualized in bright field, lateral view. Compared to mock‐treated embryo (A) embryos incubated with varying doses of DAPT (B–C) or BafA1 (D–E) or both (F) show mild morphological defects. (A′–F′) GFP levels associated to the expression of the Notch reporter line Tg(Tp1bglob:eGFP)ˆum14 visualized in the same embryos. Incubation with DAPT or BafA1 results in dose‐dependent reduction of GFP signal (B′–F′). Note that combination of both drugs at low dose leads to a reduction of GFP expression that is comparable to treatment with either of the drugs at high dose (compare B′ and D′ with F′).
We then tested ability of V‐ATPase to control Notch signaling activation in human cells. BafA1 treatment on MCF10A cells leads to a dose dependent block in lysosomal acidification and degradation activity (Fig S3A). MCF10A cells are derived from normal breast epithelium and endogenously express Notch receptors and ligands, but have low basal levels of signaling, as revealed by QPCR (Fig S3B). Expression of the endogenous Notch1 receptor can also be readily detected by immunofluorescence. In confluent cells, Notch1 localizes mostly to the plasma membrane, as visualized by counterstaining with the cortical actin marker phalloidin (Fig. S3C). Such subcellular localization is reminiscent of that observed in epithelial tissues of Drosophila (Vaccari and Bilder, 2005). Treatment with DAPT does not alter Notch1 localization, while addition of BafA1 for 3 h is sufficient to reduce LysoTracker incorporation and to greatly increase the portion of Notch1 that colocalizes with Lamp‐1, which marks late endosomes and lysosomes (Fig. S3C). These data indicate that inhibition of V‐ATPase activity is effective in MCF10A cells and leads to accumulation of Notch1 in lysosomes. Thus, endosomal trafficking of Notch and its high turnover by the endolysosomal system is observed not only in Drosophila, but also in human epithelial cells.
Notch signaling activation can be achieved in cultured cells by EGTA treatment. Extracellular Calcium chelation by EGTA leads to shedding of the extracellular domain of Notch, resulting in the production of a Notch molecule that is a substrate for γ‐secretase (Rand et al., 2000). To test whether BafA1 treatment interferes with Notch1 cleavage off membranes and nuclear translocation, we pretreated cells with BafA1, or DAPT as a control for 3 h, and then we activated Notch signaling by 30 min EGTA treatment. We then immunolocalized Notch1. In EGTA treated cells, Notch1 is relocalized to the nucleus (Figure 3A and A′). Consistent with loss of target activation upon DAPT pretreatment, Notch1 translocation form membranes to the nucleus is completely blocked (Figure 3B and B′). Pretreatment with BafA1 affect Notch1 nuclear translocation upon EGTA treatment reduces translocation partially and in a dose dependent fashion (Figure 3C and C′, D and D′, quantified in E). To test whether BafA1 prevents Notch cleavage and signaling activation upon Calcium chelation, we pretreated MCF10A cells for 7 days with drugs and then subjected them to EGTA treatment. Compared to mock‐treated controls, BafA1‐treated cells display a reduction of cleavage and Notch target activation, that is comparable to that achieved by DAPT (Figure 3F and G). Similar results were obtained by interfering against genes encoding obligate V‐ATPase subunits, or by treatment with Apicularen A, a V‐ATPase inhibitor structurally unrelated to BafA1 (Osteresch et al., 2012), excluding the possibility that the Notch phenotypes observed upon BafA1 treatment are due to off‐target effects (Fig. S4). Overall, these data indicate that V‐ATPase control of Notch signaling activation by V‐ATPase is conserved in human epithelial cells.
Figure 3.
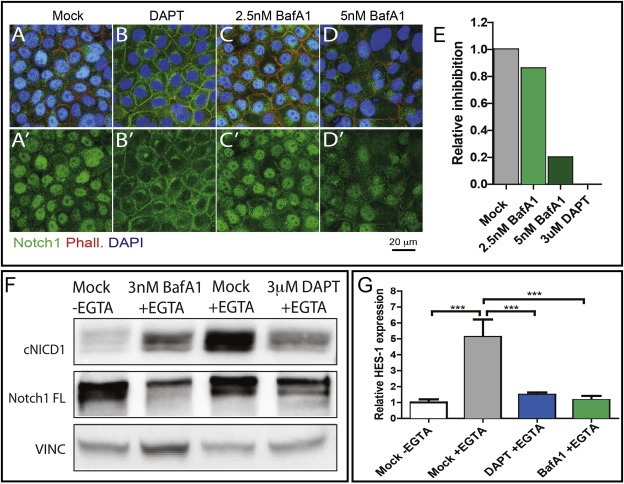
V‐ATPase inhibition reduces Notch signaling activation in human breast cells. (A–D) Single confocal section showing the subcellular localization of endogenous Notch1 in MCF10A upon EGTA stimulation in cells treated as indicated. Cells have been stained with an anti‐Notch1, Phalloidin and DAPI were used to highlight the cell cortices and nuclei. Nuclear Notch1 localization is completely inhibited by DAPT treatment and is significantly reduced by BafA1 treatment. Single channels for anti‐Notch1 are shown in A′–D′. Nuclear pixel intensity quantification of panel A′–D′ is shown in E. (F) Western blot analysis of full length Notch1 (300 KDa) and cleaved Notch1 (cNICD1) on extracts from MCF10A treated as indicated. Stimulation with EGTA leads to production of the γ‐secretase‐cleaved active form of Notch (cNICD1), which is reduced upon pretreatment with BafA1 and abolished upon pretreatment with DAPT. (G) Quantitative RT‐PCR to detect Hes1 expression levels in MCF10A cells. Cells were pretreated with DMSO, DAPT, or BafA1 and stimulated with EGTA. Upon BafA1 pretreatment we observe a 20% reduction of Hes1 expression.
Oncogenic Notch signaling is observed in a number to cancers, including breast cancers and leukemias (Robinson et al., 2011; Stylianou et al., 2006; Weng et al., 2004). To test whether V‐ATPase inhibition can counteract Notch signaling activation in breast cancer cells, we made use of three cell lines, HCC2218, HCC1599 and HCC1187, which harbor translocations in Notch receptor genes. In HCC2218 and HCC1599, translocations in the Notch1 gene result in the expression of membrane‐tethered constitutively active Notch1 forms. In HCC1187, a translocation in the Notch2 gene produces a cytoplasmic constitutively active form of Notch2 (Robinson et al., 2011). These lines have been shown to depend on Notch signaling for growth. However, only HCC2218 and HCC1599 are GSI sensitive, due to the fact that Notch1 is membrane‐tethered and thus activation still requires cleavage (Robinson et al., 2011). We treated both cell lines with DAPT and BafA1 and assessed proliferation and survival after 7 days of treatment (Figure 4). As expected, we observed that in HCC1187, which expresses GSI‐independent Notch2 fusion, growth and survival is not inhibited by DAPT, a similar behavior to that observed by treating MCF10A cells, whose growth does not depend on Notch signaling (Figure 4A and B). In contrast, HCC2218 and HCC1599, which are GSI‐sensitive, display a strong dose‐dependent growth reduction upon DAPT treatment compared to control (Figure 4C and D). These results confirm previous evidence (Robinson et al., 2011) and allow us to test the effect of BafA1 on Notch‐addicted breast cancer cells. Upon treating lines with high doses of BafA1, we observed strong growth and viability reduction in all cell lines tested (Fig. S5). However, by reducing BafA1 dosage to below 5 nM, we found reduction of growth and survival in HCC2218 and HCC1599 cells, and only to a minimal extent in HCC1187 cells, while MCF10A cells were unaffected (Figure 4A–D). We also observed that the effects of BafA1 in HCC2218 and HCC1599 are dose‐dependent and can be potentiated by co‐treatment with both DAPT and BafA1 (Figure 4C and D). Consistent with Notch signaling requirement in growth of the analyzed lines, Western blots to detect cNICD1 indicate that reduced growth upon BafA1 treatment correlates with reduction in Notch1 cleavage in HCC1599, and to a lesser extent in HCC2218 cells (Figure 4E and F). Since HCC1187 cells express a truncated Notch2, which is not recognized by anti‐cleaved Notch, we were unable to assess cleavage status in these cells. Finally, we monitored expression of the Notch1 target HES1 in HCC1599 cells and found that its expression is reduced upon BafA1 treatment, when compared to mock treated cells (Figure 4G). Overall, these data indicate that V‐ATPase inhibition reduces Notch‐dependent growth and survival in breast cancer cells, and that combined treatment with a GSI allows to reduce dosage of both drugs, while preserving efficacy.
Figure 4.
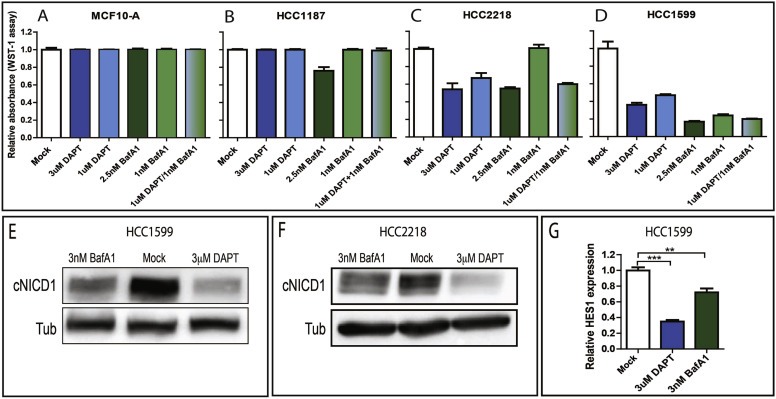
V‐ATPase inhibition impairs Notch signaling and associated growth in breast cancer cells. (A–D) Cell culture growth after 7 days upon treatment as indicated. Growth of normal breast MCF10A cells and breast cancer HCC1187 cells, which harbor a translocation leading to expression of a cytoplasmic active Notch truncation, is not affected by drug treatments. In contrast, growth of breast cancer HCC2218 and HCC1599 cells, which harbor translocations leading to expression of a membrane tethered, active Notch truncation, is sensitive to both DAPT and BafA1 in a dose‐sensitive fashion. Note that combination of both drugs at low dose in HCC2218 cells results in a growth inhibition that is comparable to treatment with either of the drugs at high dose. (E–F) Western blot analysis of cleaved Notch1 (cNICD1) on extracts from HCC1599 and HCC2218 treated for 7 days as indicated. cNICD1 production is strongly reduced upon pretreatment with BafA1 and DAPT. (G) Quantitative RT‐PCR to detect Hes1 expression levels in HCC1599 cells treated as indicated. Upon BafA1 pretreatment we observe a 25% reduction of Hes1 expression.
T‐ALL cell lines also have been characterized whose oncogenic potential is dependent on Notch. One of these, the DND‐41 cell line is GSI sensitive and possesses mutations in the Notch1 heterodimerization domain that lead to spontaneous activation in absence of ligands, and a deletion in the PEST domain of Notch1, that leads to NICD stabilization (Palomero et al., 2006, 2006, 2004, 2006). Because DND‐41 represents an established T‐ALL cell model of ligand‐independent Notch signaling, we tested whether signaling activity could be reverted by pharmacologic inhibition of V‐ATPase, as it is the case for breast cancer lines. Similarly to breast cells, we find that 7‐day treatment with BafA1 at low doses reduces proliferation and survival of DND‐41 cells in a dose‐dependent fashion, as is the case of DAPT treatments (Figure 5A). In addition, we observe that co‐treatment with both, at low dose, is more efficient than either single treatments at high dose (Figure 5A). However, western blot analysis showed only a very minor reduction of cNICD1 production upon BafA1 treatment (Figure 5B). Because growth in T‐cell leukemia lines is also prominently sustained by Akt/mTOR signaling (Guo et al., 2009; Palomero et al., 2007; Shepherd et al., 2013), upon drug treatment we also tested for Akt phosphorylation, an accepted read‐out of Akt signaling activation (Alessi et al., 1996; Sarbassov et al., 2005). In both DAPT and BafA1 treated cells, we find a noticeable reduction of Akt phosphorylation (Figure 5C). Consistent with an effect of V‐ATPase inhibition on Akt signaling in DND‐41 cells, we observed additive growth reduction by combining BafA1 or DAPT with SH6, a specific Akt inhibitor (Figure 5D). Similarly, breast cancer cells treated with BafA1 also display a reduction of Akt phosphorylation (Fig. S6A‐E). These data indicate that growth reduction by BafA1 is likely to be mediated also by the Akt pathway.
Figure 5.
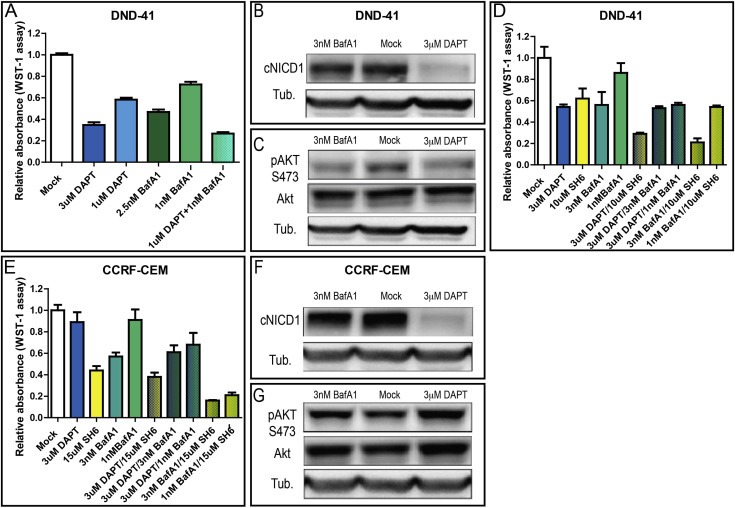
V‐ATPase inhibition reduces growth of T‐cell leukemia cell lines. (A) Cell culture growth after 7 days upon treatment as indicated. Growth of T‐cell leukemia DND‐41 cells, which harbor activating Notch1 mutations, is sensitive to both DAPT and BafA1 in a dose‐sensitive fashion. Note that combination of both drugs at low dose results in a growth inhibition that is comparable to treatment with either of the drugs at high dose. (B–C) Western blot analysis of cNICD1 (B) or of Akt and pAKT S473 (C) on extracts from DND‐41 cells treated as indicated. cNICD1 production is not reduced upon pretreatment with BafA1. In contrast, pAKT S473 levels are reduced upon pretreatment with BafA1 and DAPT. (D–E) Cell culture growth after 7 days upon treatment as indicated. Growth of T‐cell leukemia DND‐41 cells (D) and of CCRF‐CEM cells (E), which also harbor inactivating PTEN mutations, is sensitive to the Akt signaling inhibitor SH6, but not to GSIs. Note that combination of SH6 with BafA1 results in maximal growth inhibition. (F–G) Western blot analysis of cNICD1 (F) or of Akt and pAKT S473 (G) on extracts from CCRF‐CEM cells treated as indicated. cNICD1 production and pAKT levels are not reduced upon pretreatment with BafA1.
Similarly to DND‐41, CCRF‐CEM T‐ALL cell lines carry activating mutations in Notch1. As the breast cancer cells and the DND‐41 cells analyzed above, CCRF‐CEM T‐ALL cell also display elevated expression of target genes (Fig. S7A‐D). In addition, they possess inactivating mutations in PTEN, a negative regulator of the Akt pathway, which render their growth for the most part GSI insensitive, but sensitive to Akt inhibitors (Figure 5E; (Palomero et al., 2007)). Interestingly, V‐ATPase inhibition reduces growth in CCRF‐CEM cells, in a dose dependent fashion, alone or in combination with Akt inhibitors (Figure 5E). As it is the case of DND‐41 cells, compared to controls, no reduction of Notch1 cleavage is observed in CCRF‐CEM cells (Figure 5F). In contrast to DND‐41 cells, phospho‐Akt levels are very high and are not altered by drug treatments (Figure 5G). These data suggest that in T‐ALL lines displaying ectopic activation of Notch and Akt signaling, V‐ATPase inhibition might reduce growth in part by affecting Akt signaling, and in part by affecting other growth‐promoting pathways.
Is it possible that in CCRF‐CEM cells BafA1 treatment affects Akt signaling downstream of phopho‐Akt? Because V‐ATPase have been shown to be necessary for lysosomal mTOR activation (Zoncu et al., 2011), a process regulating nutrient sensing downstream of Akt, we tested whether treatment with drugs affected the phosphorylation of S6K, a target of mTOR (Fingar et al., 2002). Despite differences in levels of phospho‐S6K among cell lines, we observed no major alterations upon drug treatments (Fig S7E‐G). Such behavior is expected considering that we grow cultures in rich medium, and it indicates that V‐ATPase inhibition is not likely to affect mTOR activation in non starved cells. Because of the reported requirement of V‐ATPase for Wnt signaling activation (Buechling et al., 2010; Cruciat et al., 2010; Hermle et al., 2010), we finally tested whether growth of T‐ALL cells is sensitive to Wnt signaling inhibition by treating cells with ICG‐001 and IWR‐1, two small molecule Wnt signaling inhibitors (Chen et al., 2009; Emami et al., 2004). Using inhibitor doses that are not affecting growth in cultures of MCF10A cells, we observe significant reduction of growth in DND‐41, but not in CCRF‐CEM cells (Fig. S7H‐J). These data suggest that in the latter cell type V‐ATPase inhibition is likely to affect additional growth‐pathways that remain to be characterized.
4. Discussion
To date, no drug that block ectopic Notch activation has entered the clinic. This a major problem considering that it is estimated that up to 55–60% of T‐ALL possess mutations in Notch1 and that a number of cancers, including breast, lung, melanoma, medulloblastoma, colon, and ovarian cancers, possess oncogenic Notch activity (Miele et al., 2006; Roy et al., 2007). In our study, we find that block of V‐ATPase, either by treatment with the highly‐specific but structurally unrelated V‐ATPase inhibitors BafA1 and Apicularen‐A (Bowman and Bowman, 2002; Osteresch et al., 2012; Yoshimori et al., 1991), or by RNAi interference against V‐ATPase subunit genes, phenocopies in part the effect of a GSI.
Using genetic analysis in Drosophila, we and others recently proposed a role for V‐ATPase in promoting Notch signaling in signal‐receiving cells, possibly owing to a modulation of γ‐secretase activity, or of Notch receptor degradation (Vaccari et al., 2010; Yan et al., 2009). Such ligand‐independent effect is likely to be related to the described activation of Drosophila Notch in the endo‐lysosomal pathway (Herz et al., 2006, 2004, 2011, 2006, 2005, 2005, 2005, 2005, 2008, 2008). While it is possible that impairment of acidification also reduces trafficking of ligands, the fact that here we observe reduction of signaling activity regardless of ligand activity (i.e. in condition of endosomal sorting block, EGTA treatment or in the presence of ligand independent, receptor activating mutations) confirms that the effect of V‐ATPase is mostly on the Notch receptor and indicates that the potential therapeutic benefit of V‐ATPase inhibition might apply to cancers harboring activating alterations not only of Notch receptors, but also of their ligands.
As it is the case of GSIs, inhibition of Notch activations is only observed in breast cells expressing Notch forms retaining transmembrane portion, indicating that the effect of V‐ATPase inhibition on Notch depends on γ‐secretase activity. Even in these cells, inhibition of Notch activation is variable, ranging from comparable to GSIs, in the case of HCC1599 cells to mild, in HCC2218 cells, to very minor, in DND‐41 and CCRF‐CEM lines. Considering that HCC1599 appears to express more Notch1 than HCC2218 (Robinson et al., 2011), it is possible that V‐ATPase function is necessary to sustain high level of ligand‐independent Notch1 cleavage. A Notch level‐dependent effect is also supported by the fact that HCC1599 cells are more sensitive to BafA1 treatment than HCC2218 cells. Surprisingly, T‐ALL cells, while expressing high levels of mutant Notch1, display only minor reduction of Notch1 cleavage upon BafA1 treatment. Whether this reflect less reliance on the endo‐lysosomal system for cleavage of mutant Notch1 requires further study.
Recent evidence suggests that V‐ATPase activity also controls Wnt signaling (Buechling et al., 2010; Cruciat et al., 2010; Hermle et al., 2010). Consistent with an effect of V‐ATPase inhibition also on Wnt signaling, our experiments in Zebrafish reveal early development alterations that resemble the Wnt phenotypes observed by impairing V‐ATPase function in Xenopus embryos (Cruciat et al., 2010). Interestingly, crosstalk between Notch and Wnt signaling at multiple levels has been described in most metazoans (Sanders et al., 2009). Importantly, a number of tumors, including breast and melanoma display overactivation of both Notch and Wnt signaling (Ayyanan et al., 2006; Balint et al., 2005). In addition, Notch and Wnt cooperate during normal and tumor development of intestinal cells (Fre et al., 2005; van Es et al., 2005). Finally, stabilization of a downstream component of Wnt signaling has been shown to cause T‐ALL in mouse models (Guo et al., 2007). While more effort is required to assess whether reduced Notch signaling activity might be a consequence of Wnt signaling impairment, our observations in vertebrate development suggest that V‐ATPase inhibition might be effective in cancer types in which the Notch/Wnt axis operates.
In addition to Wnt, functional interaction of V‐ATPase subunits with mTOR pathway components has been recently described (Bar‐Peled et al., 2012; Pena‐Llopis et al., 2011; Settembre et al., 2012; Zoncu et al., 2011). mTOR signaling coordinates energy metabolism downstream of PTEN/Akt signaling, which is frequently altered together with Notch1, especially in T‐ALL (Palomero et al., 2007). Interestingly, PTEN loss or Akt inactivation have been reported to sensitize cells to BafA1 (Degtyarev et al., 2008). While we did not observe alterations of mTOR signaling, we showed that growth of HCC2218, DND‐41 and CCRF‐CEM cells is reduced upon treatment with Akt inhibitors and that Akt phosphorylation is reduced upon treatment of HCC2218, HCC1599 and DND‐41 with BafA1. These data confirm that Akt signaling is a major growth pathway in both breast and T‐ALL tumor cells and suggest that V‐ATPase inhibition might affect growth of cancer cells also by restraining Akt signaling by an unknown mechanism. Such a mechanism is unlikely to involve mTOR regulation, as we do not find it affected in treated cells. In CCRF‐CEM, we did not observe reduction of neither Notch nor Akt/mTOR signaling upon BafA1 treatment, indicating that a yet different pathway(s) might be affected. Interestingly, we find that in CCRF‐CEM cells growth is also not significantly reduced by Wnt signaling inhibition, suggesting that growth pathways other than Notch, Akt/mTOR and Wnt may be affected by V‐ATPase inhibition in cancer cells.
Increasing organelle pH is bound to result in a number of pleiotropic effects, chief among others a general reduction of protein degradation in lysosomes. However, drugs that ultimately buffer acidic environments are well‐tolerated and have been long present in the clinic. Indeed, Chloroquine and other mildly alkalizing drugs are currently used as anti‐parasitic and antiviral agents. Specific inhibitors of V‐ATPase as BafA1 have also been reported to possess anti‐parasitic properties (van Schalkwyk et al., 2010; Yoshimori et al., 1991). Consistent with the possibility of using V‐ATPase inhibitors in cancer therapy, we find that low nM doses of BafA1 are tolerated well by cells in culture over extended treatments. Interestingly, both Chloroquine and BafA1 appears to behave as anticancer agents: While it has been proposed that their anticancer activity depends on regulation of autophagy, the tumor inhibition mechanism is unclear (Fan et al., 2006; Lim et al., 2006; Maclean et al., 2008; Nakashima et al., 2003; Ohta et al., 1998; Sasaki et al., 2010; Wu et al., 2009). We propose that part of their anticancer effect might also be mediated by effects on pro‐growth Notch, Akt and possibly Wnt signaling.
Notch signaling can be pharmacologically inhibited using γ‐secretase inhibitors (GSIs), blocking antibodies or peptide inhibitors (Aste‐Amezaga et al., 2010; Moellering et al., 2009; Wu et al., 2010). GSIs display potent effect in vitro but carry high specific gut toxicity (Riccio et al., 2008; Wong et al., 2004), likely due their effect on all 4 human Notch paralogs and possibly other γ‐secretase targets. To overcome drug toxicity, clinical oncology is more and more moving towards the development of low‐dosage and multi‐drug regimens (Real et al., 2009). Since combining BafA1 with DAPT allow to achieve growth inhibition at lower GSI doses, we envisage that V‐ATPase inhibitors could be combined with GSIs and other specific Notch inhibitors to possibly curtail GSI toxicity, while preserving efficacy. In addition, because Notch controls growth together with other signaling pathways, a persistent pharmacologic inhibition of Notch is likely to induce the emergence of activating mutations in other pro‐growth pathways, as is likely to be the case in some T‐ALL cell lines (Palomero et al., 2007). Thus, multi‐drug regimens are also being designed, to inhibit more than one growth inducing pathway (Shepherd et al., 2013). Pharmacologic treatment with V‐ATPase inhibitors, in this regards, allows simultaneous reduction of Notch, Akt, at least another uncharacterized growth pathway and possibly Wnt, a promising prospect for use alone or in combination with existing inhibitors.
Curing signaling dysfunction in cancer rationally ultimately will require a detailed understanding of how signals are controlled and interact with each others. Endocytic trafficking is emerging as a major control and coordination hub for multiple cancer‐relevant signaling pathways relying on membrane‐associated factors. The work presented here represents a step in the direction of cogent pharmacological modulation of endocytic events to counteract oncogenic growth.
Supporting information
The following are the supplementary data related to this article:
Fig. S1 V‐ATPase inhibition specifically reduces endolysosomal acidification. (A–G) Single confocal sections of of the wing pouch portion of 3rd instar wing imaginal discs from Ore(R) flies. Flies have been fed with drugs at the concentrations listed above the panels and cultured discs have been subjected to incorporation of Lysotracker. Endolysosomal acidification is severely impaired in flies fed with NH4Cl, Chloroquine, ConA and BafA1, but DAPT or Leupeptin.
Fig. S2 Developmental defects and impaired Notch reporter expression in Zebrafish embryos by V‐ATPase inhibition. (A–B) High magnification confocal sections of the region of the intersomitic vessels of transgenic embryos treated with LysoTracker. Compared to a vehicle‐treated control (A), embryos treated with 300 nM BafA1 at somitogenesis (B) do not show staining (red), indicating inhibition of V‐ATPase activity. (C–G) Representative 28 hpf zebrafish embryos treated as at indicated developmental stages and visualized in bright field, lateral view. Compared to mock‐treated embryo (C) embryos incubated with DAPT (D) show mild morphological defects, while BafA1 treatment at early developmental stages show shorter body length and tail and trunk defects (E and F). BafA1 treatment after somitogenesis leads to only mild defects (G), as is the case of DAPT. (C′–G′) GFP levels associated to the expression of the Notch reporter line Tg(Tp1bglob:eGFP)ˆum14 visualized in the same embryos. Incubation with BafA1 results in a strong reduction of GFP expression (E′–G′) just as it occurs after incubation with DAPT (D′). (C″–G″) High magnification of the trunk region of embryos C′–G″.
Fig. S3 mRNA expression analysis of Notch pathway genes and Notch1 localization in MCF10A cells. (A) Lysotracker (top) and DQ‐BSA (bottom) incorporation in MCF10A cells treated as indicated. Treatment with BafA1 reduces lysosomal acidification and cargo degradation in a dose dependent fashion, compared to mock or DAPT treatment. (B) Quantitative RT‐PCR on mRNA from MCF10A cells showing relative expression of Notch receptors (Notch1‐4), ligands (Jag1‐2, Delta1,3‐4), targets (Hes1‐2,5, Hey1‐2) and the Notch inhibitor Numb. MCF10A cells express both Notch ligands and receptors and have low basal level of Notch signaling. (C) Single confocal section showing the subcellular localization of endogenous Notch1 in MCF10A using an anti‐Notch1 and anti‐Lamp1 to detect endolysosomal compartments (top). Counterstain with Phalloidin and DAPI was performed to identify cell and nuclear morphology (bottom). Notch1 is predominantly located at the plasma membrane in mock and DAPT‐treated cells. In BafA1‐treated cells, a large pool of Notch1 is present in the endolysosomal system as revealed by colocalization with Lamp‐1.
Fig. S4 Inhibition of Notch signaling by Apicularen A and knock‐down of V‐ATPase subunit encoding genes. (A) Western blot analysis of cleaved Notch1 (cNICD1) on extracts from MCF10A treated as indicated. Stimulation with EGTA leads to production of the γ‐secretase‐cleaved active form of Notch (cNICD1), which is strongly reduced upon 7‐days pretreatment with BafA1, or with Apicularen‐A (Api‐A), a V‐ATPase inhibitor structurally unrelated to BafA1. (B) Quantitative RT‐PCR to detect Hes1 expression levels in MCF10A cells. Cells were transfected with siRNA to knock down PSENEN, the gene encoding an obligate g‐secretase component, or the indicated V‐ATPase subunits in combination (triple‐i), or singly, and stimulated with EGTA. Relative to mock transfections, V‐ATPase knock‐downs reduce Notch signaling to a variable extent, with the strongest effect comparable to knock down of PSENEN. (C–F) Quantitative RT‐PCR to detect the expression levels of the indicated g‐secretase or V‐ATPase component upon control and knock‐down conditions in MCF10A cells. All knock‐down experiments reveal reduction of expression of the targeted genes by at least 90%.
Fig. S5 Dose‐dependent toxicity of BafA1 on tissue culture cells. MCF10A cells (A‐D), HCC1187 cells (E‐F) and DND41 cells (I‐L) were treated for 96 h with DMSO or increasing BafA1 concentrations, as indicated. The effect of BafA1 was assessed by wide field microscopy. BafA1 affects proliferation and viability of the cells in a dose‐dependent manner, ranging from no effect at 1 nM to cell death at 5 nM
Fig. S6 Akt phosphorylation is reduced upon drug treatments of breast cancer cells. (A–C) Western blot analysis of total Akt and active phospho‐Akt (pAKT S473) on extracts from MCF10A, HCC1599 and HCC2218 cells treated as indicated. pAKT S473 levels are strongly reduced upon pretreatment with BafA1 in HCC1599 and HCC2218 cells. (D and E) Cell culture growth after 7 days upon treatment as indicated. Growth of HCC2218 (E) is sensitive to the Akt signaling inhibitor SH6.
Fig S7 Expression profiling of Notch pathway components in breast cancer and T‐ALL cells, mTOR pathway activation upon drug treatments and Wnt inhibitors. (A‐D) Quantitative RT‐PCR on mRNA from HCC2218, HCC1599, DND‐41 and CCRF‐CEM cells showing relative expression of Notch receptors (Notch1‐4), ligands (Jag1‐2, Delta1,3‐4), targets (Hes1‐2,5, Hey1‐2, Myc) and the Notch inhibitor Numb. (E‐G) Western blot analysis of protein lysates from DND‐41, CCRF‐CEM and HCC1599 cell lines probed to detect the phosphorylated form of the mTOR target S6K (p‐S6K) and the total level of S6K. Tubulin or vinculin is used as a loading control. Despite variable levels of S6K in the different cell lines, only minor alterations are observed upon drug treatment. (H‐J) Dose‐dependent effect of the indicated Wnt inhibitors in MCF10A cells (H and I) and anti‐growth effect of low‐dose Wnt inhibitors in DND‐41 and CCRF‐CEM cell (J). DND‐41 cell culture growth is strongly reduced, while it is not in CCRF‐CEM cultures.
Acknowledgments
We thank Fen‐Biao Gao, Jim Posakony, David Bilder, Iswar Hariharan, Urban Lendhal and Pier Paolo Di Fiore for reagents. We also thank Nina Offenhauser, Marisa Oppizzi and Salvatore Pece for critically reading the manuscript. F.K. was supported in part by IIT@SEMM and S.D. was supported by a fellowship from Associazione Italiana Ricerca contro il Cancro. Research in the lab of T.V. is funded by a new unit start‐up grant from Associazione Italiana Ricerca contro il Cancro and by Telethon Italia. Work in the lab of T.V. is supported by grant# 6118 from Associazione Italiana Ricerca sul Cancro, and by grant# GGP13225 from Telethon Italia.
Supplementary data 1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2013.11.002.
Kobia Francis, Duchi Serena, Deflorian Gianluca and Vaccari Thomas, (2014), Pharmacologic inhibition of vacuolar H+ ATPase reduces physiologic and oncogenic Notch signaling, Molecular Oncology, 8, doi: 10.1016/j.molonc.2013.11.002.
References
- Agrawal, N. , Frederick, M.J. , Pickering, C.R. , Bettegowda, C. , Chang, K. , Li, R.J. , Fakhry, C. , Xie, T.-X. , Zhang, J. , Wang, J. , Zhang, N. , El-Naggar, A.K. , Jasser, S.A. , Weinstein, J.N. , Treviño, L. , Drummond, J.A. , Muzny, D.M. , Wu, Y. , Wood, L.D. , Hruban, R.H. , Westra, W.H. , Koch, W.M. , Califano, J.A. , Gibbs, R.A. , Sidransky, D. , Vogelstein, B. , Velculescu, V.E. , Papadopoulos, N. , Wheeler, D.A. , Kinzler, K.W. , Myers, J.N. , 2011. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 333, 1154–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi, D.R. , Andjelkovic, M. , Caudwell, B. , Cron, P. , Morrice, N. , Cohen, P. , Hemmings, B.A. , 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J.. 15, 6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Amsterdam, A. , Nissen, R.M. , Sun, Z. , Swindell, E.C. , Farrington, S. , Hopkins, N. , 2004. Identification of 315 genes essential for early zebrafish development. Proc. Natl. Acad. Sci. U. S. A.. 101, 12792–12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aste-Amezaga, M. , Zhang, N. , Lineberger, J.E. , Arnold, B.A. , Toner, T.J. , Gu, M. , Huang, L. , Vitelli, S. , Vo, K.T. , Haytko, P. , Zhao, J.Z. , Baleydier, F. , L'Heureux, S. , Wang, H. , Gordon, W.R. , Thoryk, E. , Andrawes, M.B. , Tiyanont, K. , Stegmaier, K. , Roti, G. , Ross, K.N. , Franlin, L.L. , Wang, F. , Chastain, M. , Bett, A.J. , Audoly, L.P. , Aster, J.C. , Blacklow, S.C. , Huber, H.E. , 2010. Characterization of Notch1 antibodies that inhibit signaling of both normal and mutated Notch1 receptors. PLoS One. 5, e9094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyanan, A. , Civenni, G. , Ciarloni, L. , Morel, C. , Mueller, N. , Lefort, K. , Mandinova, A. , Raffoul, W. , Fiche, M. , Dotto, G.P. , Brisken, C. , 2006. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc. Natl. Acad. Sci. U. S. A.. 103, 3799–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint, K. , Xiao, M. , Pinnix, C.C. , Soma, A. , Veres, I. , Juhasz, I. , Brown, E.J. , Capobianco, A.J. , Herlyn, M. , Liu, Z.J. , 2005. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J. Clin. Invest.. 115, 3166–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled, L. , Schweitzer, L.D. , Zoncu, R. , Sabatini, D.M. , 2012. Ragulator is a GEF for the Rag GTPases that signal amino Acid levels to mTORC1. Cell. 150, 1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, B.J. , Bowman, E.J. , 2002. Mutations in subunit C of the vacuolar ATPase confer resistance to bafilomycin and identify a conserved antibiotic binding site. J. Biol. Chem.. 277, 3965–3972. [DOI] [PubMed] [Google Scholar]
- Bray, S.J. , 2006. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol.. 7, 678–689. [DOI] [PubMed] [Google Scholar]
- Buechling, T. , Bartscherer, K. , Ohkawara, B. , Chaudhary, V. , Spirohn, K. , Niehrs, C. , Boutros, M. , 2010. Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr. Biol.. 20, 1263–1268. [DOI] [PubMed] [Google Scholar]
- Calzavara, E. , Chiaramonte, R. , Cesana, D. , Basile, A. , Sherbet, G.V. , Comi, P. , 2008. Reciprocal regulation of Notch and PI3K/Akt signalling in T-ALL cells in vitro. J. Cell Biochem.. 103, 1405–1412. [DOI] [PubMed] [Google Scholar]
- Chen, B. , Dodge, M.E. , Tang, W. , Lu, J. , Ma, Z. , Fan, C.-W. , Wei, S. , Hao, W. , Kilgore, J. , Williams, N.S. , Roth, M.G. , Amatruda, J.F. , Chen, C. , Lum, L. , 2009. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol.. 5, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress, J.L. , Acar, M. , Tao, C. , Halder, G. , 2006. Lethal giant discs, a novel C2-domain protein, restricts notch activation during endocytosis. Curr. Biol.. 16, 2228–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciat, C.M. , Ohkawara, B. , Acebron, S.P. , Karaulanov, E. , Reinhard, C. , Ingelfinger, D. , Boutros, M. , Niehrs, C. , 2010. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 327, 459–463. [DOI] [PubMed] [Google Scholar]
- Degtyarev, M. , De Maziere, A. , Orr, C. , Lin, J. , Lee, B.B. , Tien, J.Y. , Prior, W.W. , van Dijk, S. , Wu, H. , Gray, D.C. , Davis, D.P. , Stern, H.M. , Murray, L.J. , Hoeflich, K.P. , Klumperman, J. , Friedman, L.S. , Lin, K. , 2008. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J. Cell Biol.. 183, 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferson, C.L. , Winkelmann, C.T. , Ware, C. , Sullivan, T. , Giampaoli, S. , Tammam, J. , Patel, S. , Mesiti, G. , Reilly, J.F. , Gibson, R.E. , Buser, C. , Yeatman, T. , Coppola, D. , Winter, C. , Clark, E.A. , Draetta, G.F. , Strack, P.R. , Majumder, P.K. , 2010. Downregulation of Notch pathway by a gamma-secretase inhibitor attenuates AKT/mammalian target of rapamycin signaling and glucose uptake in an ERBB2 transgenic breast cancer model. Cancer Res.. 70, 2476–2484. [DOI] [PubMed] [Google Scholar]
- Emami, K.H. , Nguyen, C. , Ma, H. , Kim, D.H. , Jeong, K.W. , Eguchi, M. , Moon, R.T. , Teo, J.-L. , Oh, S.W. , Kim, H.Y. , Moon, S.H. , Ha, J.R. , Kahn, M. , 2004. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc. Natl. Acad. Sci. U. S. A.. 101, 12682–12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, C. , Wang, W. , Zhao, B. , Zhang, S. , Miao, J. , 2006. Chloroquine inhibits cell growth and induces cell death in A549 lung cancer cells. Bioorg. Med. Chem.. 14, 3218–3222. [DOI] [PubMed] [Google Scholar]
- Fingar, D.C. , Salama, S. , Tsou, C. , Harlow, E. , Blenis, J. , 2002. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev.. 16, 1472–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre, S. , Huyghe, M. , Mourikis, P. , Robine, S. , Louvard, D. , Artavanis-Tsakonas, S. , 2005. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 435, 964–968. [DOI] [PubMed] [Google Scholar]
- Gallagher, C.M. , Knoblich, J.A. , 2006. The conserved c2 domain protein lethal (2) giant discs regulates protein trafficking in Drosophila. Dev. Cell. 11, 641–653. [DOI] [PubMed] [Google Scholar]
- Guo, D. , Ye, J. , Dai, J. , Li, L. , Chen, F. , Ma, D. , Ji, C. , 2009. Notch-1 regulates Akt signaling pathway and the expression of cell cycle regulatory proteins cyclin D1, CDK2 and p21 in T-ALL cell lines. Leuk. Res.. 33, 678–685. [DOI] [PubMed] [Google Scholar]
- Guo, Z. , Dose, M. , Kovalovsky, D. , Chang, R. , O'Neil, J. , Look, A.T. , von Boehmer, H. , Khazaie, K. , Gounari, F. , 2007. Beta-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood. 109, 5463–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermle, T. , Saltukoglu, D. , Grunewald, J. , Walz, G. , Simons, M. , 2010. Regulation of Frizzled-dependent planar polarity signaling by a V‐ATPase subunit. Curr. Biol.. 20, 1269–1276. [DOI] [PubMed] [Google Scholar]
- Herz, H.M. , Chen, Z. , Scherr, H. , Lackey, M. , Bolduc, C. , Bergmann, A. , 2006. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development. 133, 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori, K. , Fostier, M. , Ito, M. , Fuwa, T.J. , Go, M.J. , Okano, H. , Baron, M. , Matsuno, K. , 2004. Drosophila deltex mediates suppressor of Hairless-independent and late-endosomal activation of Notch signaling. Development. 131, 5527–5537. [DOI] [PubMed] [Google Scholar]
- Hori, K. , Sen, A. , Kirchhausen, T. , Artavanis-Tsakonas, S. , 2011. Synergy between the ESCRT-III complex and Deltex defines a ligand-independent Notch signal. J. Cell Biol.. 195, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaekel, R. , Klein, T. , 2006. The Drosophila Notch inhibitor and tumor suppressor gene lethal (2) giant discs encodes a conserved regulator of endosomal trafficking. Dev. Cell. 11, 655–669. [DOI] [PubMed] [Google Scholar]
- Klinakis, A. , Lobry, C. , Abdel-Wahab, O. , Oh, P. , Haeno, H. , Buonamici, S. , van De Walle, I. , Cathelin, S. , Trimarchi, T. , Araldi, E. , Liu, C. , Ibrahim, S. , Beran, M. , Zavadil, J. , Efstratiadis, A. , Taghon, T. , Michor, F. , Levine, R.L. , Aifantis, I. , 2011. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 473, 230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, C. , Prenninger, S. , Knuckles, P. , Taylor, V. , Levin, M. , Calegari, F. , 2011. The H(+) vacuolar ATPase maintains neural stem cells in the developing mouse cortex. Stem Cells Dev.. 20, 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, J.H. , Park, J.W. , Kim, M.S. , Park, S.K. , Johnson, R.S. , Chun, Y.S. , 2006. Bafilomycin induces the p21-mediated growth inhibition of cancer cells under hypoxic conditions by expressing hypoxia-inducible factor-1alpha. Mol. Pharmacol.. 70, 1856–1865. [DOI] [PubMed] [Google Scholar]
- Maclean, K.H. , Dorsey, F.C. , Cleveland, J.L. , Kastan, M.B. , 2008. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J. Clin. Invest.. 118, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniell, R. , Warthen, D.M. , Sanchez-Lara, P.A. , Pai, A. , Krantz, I.D. , Piccoli, D.A. , Spinner, N.B. , 2006. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am. J. Hum. Genet.. 79, 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurette, O. , Stylianou, S. , Rock, R. , Collu, G.M. , Gilmore, A.P. , Brennan, K. , 2009. Notch activation induces Akt signaling via an autocrine loop to prevent apoptosis in breast epithelial cells. Cancer Res.. 69, 5015–5022. [DOI] [PubMed] [Google Scholar]
- Micchelli, C.A. , Esler, W.P. , Kimberly, W.T. , Jack, C. , Berezovska, O. , Kornilova, A. , Hyman, B.T. , Perrimon, N. , Wolfe, M.S. , 2003. Gamma-secretase/presenilin inhibitors for Alzheimer's disease phenocopy Notch mutations in Drosophila. Faseb J.. 17, 79–81. [DOI] [PubMed] [Google Scholar]
- Miele, L. , Golde, T. , Osborne, B. , 2006. Notch signaling in cancer. Curr. Mol. Med.. 6, 905–918. [DOI] [PubMed] [Google Scholar]
- Moberg, K.H. , Schelble, S. , Burdick, S.K. , Hariharan, I.K. , 2005. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev. Cell. 9, 699–710. [DOI] [PubMed] [Google Scholar]
- Moellering, R.E. , Cornejo, M. , Davis, T.N. , Del Bianco, C. , Aster, J.C. , Blacklow, S.C. , Kung, A.L. , Gilliland, D.G. , Verdine, G.L. , Bradner, J.E. , 2009. Direct inhibition of the NOTCH transcription factor complex. Nature. 462, 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, A. , Veraksa, A. , Bauer, A. , Rosse, C. , Camonis, J. , Artavanis-Tsakonas, S. , 2005. Regulation of Notch signalling by non-visual beta-arrestin. Nat. Cell Biol.. 7, 1191–1201. [DOI] [PubMed] [Google Scholar]
- Nakashima, S. , Hiraku, Y. , Tada-Oikawa, S. , Hishita, T. , Gabazza, E.C. , Tamaki, S. , Imoto, I. , Adachi, Y. , Kawanishi, S. , 2003. Vacuolar H+-ATPase inhibitor induces apoptosis via lysosomal dysfunction in the human gastric cancer cell line MKN-1. J. Biochem.. 134, 359–364. [DOI] [PubMed] [Google Scholar]
- Nellesen, D.T. , Lai, E.C. , Posakony, J.W. , 1999. Discrete enhancer elements mediate selective responsiveness of enhancer of split complex genes to common transcriptional activators. Dev. Biol.. 213, 33–53. [DOI] [PubMed] [Google Scholar]
- Nichols, J.T. , Miyamoto, A. , Weinmaster, G. , 2007. Notch signaling–constantly on the move. Traffic. 8, 959–969. [DOI] [PubMed] [Google Scholar]
- Nicolas, M. , Wolfer, A. , Raj, K. , Kummer, J.A. , Mill, P. , van Noort, M. , Hui, C.-c. , Clevers, H. , Dotto, G.P. , Radtke, F. , 2003. Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet.. 33, 416–421. [DOI] [PubMed] [Google Scholar]
- Oda, T. , Elkahloun, A.G. , Pike, B.L. , Okajima, K. , Krantz, I.D. , Genin, A. , Piccoli, D.A. , Meltzer, P.S. , Spinner, N.B. , Collins, F.S. , Chandrasekharappa, S.C. , 1997. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat. Genet.. 16, 235–242. [DOI] [PubMed] [Google Scholar]
- Ohta, T. , Arakawa, H. , Futagami, F. , Fushida, S. , Kitagawa, H. , Kayahara, M. , Nagakawa, T. , Miwa, K. , Kurashima, K. , Numata, M. , Kitamura, Y. , Terada, T. , Ohkuma, S. , 1998. Bafilomycin A1 induces apoptosis in the human pancreatic cancer cell line Capan-1. J. Pathol.. 185, 324–330. [DOI] [PubMed] [Google Scholar]
- Osteresch, C. , Bender, T. , Grond, S. , von Zezschwitz, P. , Kunze, B. , Jansen, R. , Huss, M. , Wieczorek, H. , 2012. The binding site of the V‐ATPase inhibitor apicularen is in the vicinity of those for bafilomycin and archazolid. J. Biol. Chem.. 287, 31866–31876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero, T. , Lim, W.K. , Odom, D.T. , Sulis, M.L. , Real, P.J. , Margolin, A. , Barnes, K.C. , O'Neil, J. , Neuberg, D. , Weng, A.P. , Aster, J.C. , Sigaux, F. , Soulier, J. , Look, A.T. , Young, R.A. , Califano, A. , Ferrando, A.A. , 2006. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc. Natl. Acad. Sci. U. S. A.. 103, 18261–18266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero, T. , Sulis, M.L. , Cortina, M. , Real, P.J. , Barnes, K. , Ciofani, M. , Caparros, E. , Buteau, J. , Brown, K. , Perkins, S.L. , Bhagat, G. , Agarwal, A.M. , Basso, G. , Castillo, M. , Nagase, S. , Cordon-Cardo, C. , Parsons, R. , Zúñiga-Pflücker, J.C. , Dominguez, M. , Ferrando, A.A. , 2007. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat. Med.. 13, 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, M.J. , Pisharath, H. , Yusuff, S. , Moore, J.C. , Siekmann, A.F. , Lawson, N. , Leach, S.D. , 2009. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech. Dev.. 126, 898–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Llopis, S. , Vega-Rubin-de-Celis, S. , Schwartz, J.C. , Wolff, N.C. , Tran, T.A. , Zou, L. , Xie, X.J. , Corey, D.R. , Brugarolas, J. , 2011. Regulation of TFEB and V-ATPases by mTORC1. Embo J.. 30, 3242–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente, X.S. , Pinyol, M. , Quesada, V. , Conde, L. , Ordóñez, G.R. , Villamor, N. , Escaramis, G. , Jares, P. , Beà, S. , González-Díaz, M. , Bassaganyas, L. , Baumann, T. , Juan, M. , López-Guerra, M. , Colomer, D. , Tubío, J.M.C. , López, C. , Navarro, A. , Tornador, C. , Aymerich, M. , Rozman, M. , Hernández, J.M. , Puente, D.A. , Freije, J.M.P. , Velasco, G. , Gutiérrez-Fernández, A. , Costa, D. , Carrió, A. , Guijarro, S. , Enjuanes, A. , Hernández, L. , Yagüe, J. , Nicolás, P. , Romeo-Casabona, C.M. , Himmelbauer, H. , Castillo, E. , Dohm, J.C. , de Sanjosé, S. , Piris, M.A. , de Alava, E. , San Miguel, J. , Royo, R. , Gelpí, J.L. , Torrents, D. , Orozco, M. , Pisano, D.G. , Valencia, A. , Guigó, R. , Bayés, M. , Heath, S. , Gut, M. , Klatt, P. , Marshall, J. , Raine, K. , Stebbings, L.A. , Futreal, P.A. , Stratton, M.R. , Campbell, P.J. , Gut, I. , López-Guillermo, A. , Estivill, X. , Montserrat, E. , López-Otín, C. , Campo, E. , 2011. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 475, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand, M.D. , Grimm, L.M. , Artavanis-Tsakonas, S. , Patriub, V. , Blacklow, S.C. , Sklar, J. , Aster, J.C. , 2000. Calcium depletion dissociates and activates heterodimeric notch receptors. Mol. Cell Biol.. 20, 1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real, P.J. , Tosello, V. , Palomero, T. , Castillo, M. , Hernando, E. , de Stanchina, E. , Sulis, M.L. , Barnes, K. , Sawai, C. , Homminga, I. , Meijerink, J. , Aifantis, I. , Basso, G. , Cordon-Cardo, C. , Ai, W. , Ferrando, A. , 2009. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat. Med.. 15, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio, O. , van Gijn, M.E. , Bezdek, A.C. , Pellegrinet, L. , van Es, J.H. , Zimber-Strobl, U. , Strobl, L.J. , Honjo, T. , Clevers, H. , Radtke, F. , 2008. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep.. 9, 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, D.R. , Kalyana-Sundaram, S. , Wu, Y.M. , Shankar, S. , Cao, X. , Ateeq, B. , Asangani, I.A. , Iyer, M. , Maher, C.A. , Grasso, C.S. , Lonigro, R.J. , Quist, M. , Siddiqui, J. , Mehra, R. , Jing, X. , Giordano, T.J. , Sabel, M.S. , Kleer, C.G. , Palanisamy, N. , Natrajan, R. , Lambros, M.B. , Reis-Filho, J.S. , Kumar-Sinha, C. , Chinnaiyan, A.M. , 2011. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nat. Med.. 17, 1646–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, M. , Pear, W.S. , Aster, J.C. , 2007. The multifaceted role of Notch in cancer. Curr. Opin. Genet. Dev.. 17, 52–59. [DOI] [PubMed] [Google Scholar]
- Sanders, P.G. , Munoz-Descalzo, S. , Balayo, T. , Wirtz-Peitz, F. , Hayward, P. , Arias, A.M. , 2009. Ligand-independent traffic of Notch buffers activated Armadillo in Drosophila. PLoS Biol.. 7, e1000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov, D.D. , Guertin, D.A. , Ali, S.M. , Sabatini, D.M. , 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 307, 1098–1101. [DOI] [PubMed] [Google Scholar]
- Sasaki, K. , Tsuno, N.H. , Sunami, E. , Tsurita, G. , Kawai, K. , Okaji, Y. , Nishikawa, T. , Shuno, Y. , Hongo, K. , Hiyoshi, M. , Kaneko, M. , Kitayama, J. , Takahashi, K. , Nagawa, H. , 2010. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer. 10, 370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi, N. , Yan, Y. , Quek, D. , Schupbach, T. , Kang, Y. , 2010. Rabconnectin-3 is a functional regulator of mammalian Notch signaling. J. Biol. Chem.. 285, 34757–34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre, C. , Zoncu, R. , Medina, D.L. , Vetrini, F. , Erdin, S. , Huynh, T. , Ferron, M. , Karsenty, G. , Vellard, M.C. , Facchinetti, V. , Sabatini, D.M. , Ballabio, A. , 2012. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. Embo J.. 31, 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, V.M. , Calvo, J.A. , Draheim, K.M. , Cunningham, L.A. , Hermance, N. , Beverly, L. , Krishnamoorthy, V. , Bhasin, M. , Capobianco, A.J. , Kelliher, M.A. , 2006. Notch1 contributes to mouse T-cell leukemia by directly inducing the expression of c-myc. Mol. Cell Biol.. 26, 8022–8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd, C. , Banerjee, L. , Cheung, C.W. , Mansour, M.R. , Jenkinson, S. , Gale, R.E. , Khwaja, A. , 2013. PI3K/mTOR inhibition upregulates NOTCH-MYC signalling leading to an impaired cytotoxic response. Leukemia. 27, 650–660. [DOI] [PubMed] [Google Scholar]
- Smith-Bolton, R.K. , Worley, M.I. , Kanda, H. , Hariharan, I.K. , 2009. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev. Cell. 16, 797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylianou, S. , Clarke, R.B. , Brennan, K. , 2006. Aberrant activation of notch signaling in human breast cancer. Cancer Res.. 66, 1517–1525. [DOI] [PubMed] [Google Scholar]
- Sweeney, N.T. , Brenman, J.E. , Jan, Y.N. , Gao, F.B. , 2006. The coiled-coil protein shrub controls neuronal morphogenesis in Drosophila. Curr. Biol.. 16, 1006–1011. [DOI] [PubMed] [Google Scholar]
- Tagami, S. , Okochi, M. , Yanagida, K. , Ikuta, A. , Fukumori, A. , Matsumoto, N. , Ishizuka-Katsura, Y. , Nakayama, T. , Itoh, N. , Jiang, J. , Nishitomi, K. , Kamino, K. , Morihara, T. , Hashimoto, R. , Tanaka, T. , Kudo, T. , Chiba, S. , Takeda, M. , 2008. Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1. Mol. Cell Biol.. 28, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, B.J. , Mathieu, J. , Sung, H.H. , Loeser, E. , Rorth, P. , Cohen, S.M. , 2005. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev. Cell. 9, 711–720. [DOI] [PubMed] [Google Scholar]
- Vaccari, T. , Bilder, D. , 2005. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev. Cell. 9, 687–698. [DOI] [PubMed] [Google Scholar]
- Vaccari, T. , Duchi, S. , Cortese, K. , Tacchetti, C. , Bilder, D. , 2010. The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development. 137, 1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari, T. , Lu, H. , Kanwar, R. , Fortini, M.E. , Bilder, D. , 2008. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J. Cell Biol.. 180, 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es, J.H. , van Gijn, M.E. , Riccio, O. , van den Born, M. , Vooijs, M. , Begthel, H. , Cozijnsen, M. , Robine, S. , Winton, D.J. , Radtke, F. , Clevers, H. , 2005. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 435, 959–963. [DOI] [PubMed] [Google Scholar]
- van Schalkwyk, D.A. , Chan, X.W. , Misiano, P. , Gagliardi, S. , Farina, C. , Saliba, K.J. , 2010. Inhibition of Plasmodium falciparum pH regulation by small molecule indole derivatives results in rapid parasite death. Biochem. Pharmacol.. 79, 1291–1299. [DOI] [PubMed] [Google Scholar]
- Weng, A.P. , Ferrando, A.A. , Lee, W. , Morris, J.P.t. , Silverman, L.B. , Sanchez-Irizarry, C. , Blacklow, S.C. , Look, A.T. , Aster, J.C. , 2004. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 306, 269–271. [DOI] [PubMed] [Google Scholar]
- Weng, A.P. , Millholland, J.M. , Yashiro-Ohtani, Y. , Arcangeli, M.L. , Lau, A. , Wai, C. , Del Bianco, C. , Rodriguez, C.G. , Sai, H. , Tobias, J. , Li, Y. , Wolfe, M.S. , Shachaf, C. , Felsher, D. , Blacklow, S.C. , Pear, W.S. , Aster, J.C. , 2006. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev.. 20, 2096–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin, M. , Tongngok, P. , Gensch, N. , Clemence, S. , Motoki, M. , Yamada, K. , Hori, K. , Taniguchi-Kanai, M. , Franklin, E. , Matsuno, K. , Baron, M. , 2008. Drosophila HOPS and AP-3 complex genes are required for a Deltex-regulated activation of notch in the endosomal trafficking pathway. Dev. Cell. 15, 762–772. [DOI] [PubMed] [Google Scholar]
- Wong, G.T. , Manfra, D. , Poulet, F.M. , Zhang, Q. , Josien, H. , Bara, T. , Engstrom, L. , Pinzon-Ortiz, M. , Fine, J.S. , Lee, H.J. , Zhang, L. , Higgins, G.A. , Parker, E.M. , 2004. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J. Biol. Chem.. 279, 12876–12882. [DOI] [PubMed] [Google Scholar]
- Wu, Y. , Cain-Hom, C. , Choy, L. , Hagenbeek, T.J. , de Leon, G.P. , Chen, Y. , Finkle, D. , Venook, R. , Wu, X. , Ridgway, J. , Schahin-Reed, D. , Dow, G.J. , Shelton, A. , Stawicki, S. , Watts, R.J. , Zhang, J. , Choy, R. , Howard, P. , Kadyk, L. , Yan, M. , Zha, J. , Callahan, C.A. , Hymowitz, S.G. , Siebel, C.W. , 2010. Therapeutic antibody targeting of individual Notch receptors. Nature. 464, 1052–1057. [DOI] [PubMed] [Google Scholar]
- Wu, Y.C. , Wu, W.K. , Li, Y. , Yu, L. , Li, Z.J. , Wong, C.C. , Li, H.T. , Sung, J.J. , Cho, C.H. , 2009. Inhibition of macroautophagy by bafilomycin A1 lowers proliferation and induces apoptosis in colon cancer cells. Biochem. Biophys. Res. Commun.. 382, 451–456. [DOI] [PubMed] [Google Scholar]
- Yan, Y. , Denef, N. , Schupbach, T. , 2009. The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev. Cell. 17, 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori, T. , Yamamoto, A. , Moriyama, Y. , Futai, M. , Tashiro, Y. , 1991. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem.. 266, 17707–17712. [PubMed] [Google Scholar]
- Zoncu, R. , Bar-Peled, L. , Efeyan, A. , Wang, S. , Sancak, Y. , Sabatini, D.M. , 2011. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 334, 678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Fig. S1 V‐ATPase inhibition specifically reduces endolysosomal acidification. (A–G) Single confocal sections of of the wing pouch portion of 3rd instar wing imaginal discs from Ore(R) flies. Flies have been fed with drugs at the concentrations listed above the panels and cultured discs have been subjected to incorporation of Lysotracker. Endolysosomal acidification is severely impaired in flies fed with NH4Cl, Chloroquine, ConA and BafA1, but DAPT or Leupeptin.
Fig. S2 Developmental defects and impaired Notch reporter expression in Zebrafish embryos by V‐ATPase inhibition. (A–B) High magnification confocal sections of the region of the intersomitic vessels of transgenic embryos treated with LysoTracker. Compared to a vehicle‐treated control (A), embryos treated with 300 nM BafA1 at somitogenesis (B) do not show staining (red), indicating inhibition of V‐ATPase activity. (C–G) Representative 28 hpf zebrafish embryos treated as at indicated developmental stages and visualized in bright field, lateral view. Compared to mock‐treated embryo (C) embryos incubated with DAPT (D) show mild morphological defects, while BafA1 treatment at early developmental stages show shorter body length and tail and trunk defects (E and F). BafA1 treatment after somitogenesis leads to only mild defects (G), as is the case of DAPT. (C′–G′) GFP levels associated to the expression of the Notch reporter line Tg(Tp1bglob:eGFP)ˆum14 visualized in the same embryos. Incubation with BafA1 results in a strong reduction of GFP expression (E′–G′) just as it occurs after incubation with DAPT (D′). (C″–G″) High magnification of the trunk region of embryos C′–G″.
Fig. S3 mRNA expression analysis of Notch pathway genes and Notch1 localization in MCF10A cells. (A) Lysotracker (top) and DQ‐BSA (bottom) incorporation in MCF10A cells treated as indicated. Treatment with BafA1 reduces lysosomal acidification and cargo degradation in a dose dependent fashion, compared to mock or DAPT treatment. (B) Quantitative RT‐PCR on mRNA from MCF10A cells showing relative expression of Notch receptors (Notch1‐4), ligands (Jag1‐2, Delta1,3‐4), targets (Hes1‐2,5, Hey1‐2) and the Notch inhibitor Numb. MCF10A cells express both Notch ligands and receptors and have low basal level of Notch signaling. (C) Single confocal section showing the subcellular localization of endogenous Notch1 in MCF10A using an anti‐Notch1 and anti‐Lamp1 to detect endolysosomal compartments (top). Counterstain with Phalloidin and DAPI was performed to identify cell and nuclear morphology (bottom). Notch1 is predominantly located at the plasma membrane in mock and DAPT‐treated cells. In BafA1‐treated cells, a large pool of Notch1 is present in the endolysosomal system as revealed by colocalization with Lamp‐1.
Fig. S4 Inhibition of Notch signaling by Apicularen A and knock‐down of V‐ATPase subunit encoding genes. (A) Western blot analysis of cleaved Notch1 (cNICD1) on extracts from MCF10A treated as indicated. Stimulation with EGTA leads to production of the γ‐secretase‐cleaved active form of Notch (cNICD1), which is strongly reduced upon 7‐days pretreatment with BafA1, or with Apicularen‐A (Api‐A), a V‐ATPase inhibitor structurally unrelated to BafA1. (B) Quantitative RT‐PCR to detect Hes1 expression levels in MCF10A cells. Cells were transfected with siRNA to knock down PSENEN, the gene encoding an obligate g‐secretase component, or the indicated V‐ATPase subunits in combination (triple‐i), or singly, and stimulated with EGTA. Relative to mock transfections, V‐ATPase knock‐downs reduce Notch signaling to a variable extent, with the strongest effect comparable to knock down of PSENEN. (C–F) Quantitative RT‐PCR to detect the expression levels of the indicated g‐secretase or V‐ATPase component upon control and knock‐down conditions in MCF10A cells. All knock‐down experiments reveal reduction of expression of the targeted genes by at least 90%.
Fig. S5 Dose‐dependent toxicity of BafA1 on tissue culture cells. MCF10A cells (A‐D), HCC1187 cells (E‐F) and DND41 cells (I‐L) were treated for 96 h with DMSO or increasing BafA1 concentrations, as indicated. The effect of BafA1 was assessed by wide field microscopy. BafA1 affects proliferation and viability of the cells in a dose‐dependent manner, ranging from no effect at 1 nM to cell death at 5 nM
Fig. S6 Akt phosphorylation is reduced upon drug treatments of breast cancer cells. (A–C) Western blot analysis of total Akt and active phospho‐Akt (pAKT S473) on extracts from MCF10A, HCC1599 and HCC2218 cells treated as indicated. pAKT S473 levels are strongly reduced upon pretreatment with BafA1 in HCC1599 and HCC2218 cells. (D and E) Cell culture growth after 7 days upon treatment as indicated. Growth of HCC2218 (E) is sensitive to the Akt signaling inhibitor SH6.
Fig S7 Expression profiling of Notch pathway components in breast cancer and T‐ALL cells, mTOR pathway activation upon drug treatments and Wnt inhibitors. (A‐D) Quantitative RT‐PCR on mRNA from HCC2218, HCC1599, DND‐41 and CCRF‐CEM cells showing relative expression of Notch receptors (Notch1‐4), ligands (Jag1‐2, Delta1,3‐4), targets (Hes1‐2,5, Hey1‐2, Myc) and the Notch inhibitor Numb. (E‐G) Western blot analysis of protein lysates from DND‐41, CCRF‐CEM and HCC1599 cell lines probed to detect the phosphorylated form of the mTOR target S6K (p‐S6K) and the total level of S6K. Tubulin or vinculin is used as a loading control. Despite variable levels of S6K in the different cell lines, only minor alterations are observed upon drug treatment. (H‐J) Dose‐dependent effect of the indicated Wnt inhibitors in MCF10A cells (H and I) and anti‐growth effect of low‐dose Wnt inhibitors in DND‐41 and CCRF‐CEM cell (J). DND‐41 cell culture growth is strongly reduced, while it is not in CCRF‐CEM cultures.


