Abstract
Sphingosine 1‐phosphate (S1P) plays important roles in cell proliferation, differentiation or survival mainly through its surface G‐protein‐coupled receptors S1P1−5. Bone represents the major site of metastasis for prostate cancer (CaP) cells, which rely on bone‐derived factors to support their proliferation and resistance to therapeutics.
In the present work we have found that conditioned medium (CM) from the MC3T3 osteoblastic cell line or primary murine and human osteoblast‐like cells, as well as co‐culture with MC3T3 stimulate proliferation of CaP lines in S1P‐dependent manner. In addition, osteoblastic‐derived S1P induces resistance of CaP cells to therapeutics including chemotherapy and radiotherapy. When S1P release from osteoblastic cells is decreased (inhibition of SphK1, knock‐down of SphK1 or the S1P transporter, Spns2 by siRNA) or secreted S1P neutralized with anti‐S1P antibody, the proliferative and survival effects of osteoblasts on CaP cells are abolished. Because of the paracrine nature of the signaling, we studied the role of the S1P receptors expressed on CaP cells in the communication with S1P secreted by osteoblasts. Strategies aimed at down‐regulating S1P1, S1P2 or S1P3 (siRNA, antagonists), established the exclusive role of the S1P/S1P1 signaling between osteoblasts and CaP cells.
Bone metastases from CaP are associated with osteoblastic differentiation resulting in abnormal bone formation. We show that the autocrine S1P/S1P3 signaling is central during differentiation to mature osteoblasts by regulating Runx2 level, a key transcription factor involved in osteoblastic maturation. Importantly, differentiated osteoblasts exhibited enhanced secretion of S1P and further stimulated CaP cell proliferation in a S1P‐dependent manner.
By establishing the dual role of osteoblast‐borne S1P on both osteoblastic differentiation and CaP cell proliferation and survival, we uncover the importance of S1P in the bone metastatic microenvironment, which may open a novel area of study for the treatment of CaP bone metastasis by targeting S1P.
Keywords: Sphingomab, Osteoblasts, Differentiation, Sphingosine kinase, Docetaxel, Radiotherapy
Highlights
We report a dual role for S1P in prostate cancer bone metastasis.
S1P signaling is essential for osteoblast differentiation.
Osteoblast‐borne S1P induce proliferation and resistance of prostate cancer cells.
1. Introduction
Sphingosine 1‐phosphate (S1P) is a bioactive lipid regulating pleiotropic activities in cancer biology including proliferation, survival, migration, inflammation, or angiogenesis (Maceyka et al., 2012; Mendelson et al., 2014; Pitson, 2011; Pyne and Pyne, 2010). S1P is generated from sphingosine, the pro‐apoptotic backbone component of all sphingolipids (Cuvillier, 2002), in a reaction mostly catalyzed by the sphingosine kinase‐1 (SphK1) isoform (Olivera et al., 1999). SphK1 is activated by various stimuli to produce S1P which promotes cell survival and proliferation (Maceyka et al., 2012). The balance between the levels of S1P and its metabolic precursors ceramide and sphingosine has been regarded as a rheostat that could determine whether a cell proliferates or dies (Cuvillier et al., 1996).
S1P is a ligand for five high‐affinity G‐coupled receptors (S1P1−5), which differ in their tissue distribution, and the specific tumorigenic and angiogenic effects, depending on the suite of S1P receptor subtypes expressed (Rosen et al., 2009). S1P is produced intracellularly by SphK1 and exerts its paracrine autocrine effects by being secreted into the tumor microenvironment by the cell surface transporter Spinster 2 (Spns2) (Fukuhara et al., 2012; Hisano et al., 2011; Kawahara et al., 2009; Nagahashi et al., 2013). Importantly, the biological effects of S1P can be mitigated by inhibition of SphK1 gene expression or enzymatic activity indicating that SphK1 plays a crucial role in the biological effects of S1P (Cuvillier, 2008) although recent studies using novel selective inhibitors of SphK1 argued against this conventional wisdom (Rex et al., 2013; Schnute et al., 2012).
Accumulating evidence links SphK1/S1P signaling with prostate cancer (CaP). SphK1 is overexpressed in primary tumor samples in comparison to non‐cancer tissues in patients undergoing surgery (Brizuela et al., 2012; Malavaud et al., 2010). In cellular and mouse tumor models, the SphK1/S1P metabolism is involved in resistance to chemotherapy (Pchejetski et al., 2008, 2005, 2009), radiotherapy (Nava et al., 2000; Pchejetski et al., 2010) and androgen effects (Dayon et al., 2009), suggesting that the S1P signaling system could be a potential therapeutic target (Cuvillier, 2008) (Sabbadini, 2011).
CaP metastasizes to the bone to predominantly produce an osteoblastic reaction (bone formation) by virtue of promoting proliferation and differentiation of mesenchymal progenitor cells into mature osteoblasts (Logothetis and Lin, 2005). Although S1P has been reported to regulate remodeling in normal bone physiology (Ishii et al., 2009, 2010, 2008, 2006), its role in the pathological context of bone metastasis has not been described to date. Because we have recently shown that the SphK1/S1P signaling is highly expressed in osteoblastic MC3T3 cells and stimulated by androgens (Martin et al., 2010), we investigated the putative role of S1P in the complex interactions between CaP and osteoblastic cells. Here we show that S1P produced by osteoblasts promotes CaP cell proliferation and resistance to standard therapeutics, a paracrine mechanism exclusively driven to the S1P receptor subtype 1 (S1P1). Concomitantly osteoblast‐borne S1P contributes to the osteoblastic differentiation, a hallmark of CaP bone metastasis, via an autocrine effect involving the osteoblastic S1P receptor subtype 3 (S1P3). Of note, differentiated osteoblasts further enhance proliferation and resistance to therapeutics of CaP cells in a S1P‐dependent manner.
There is a need to understand the molecular mechanisms underlying prostate cancer bone metastasis as there is no effective therapy. These results support the notion that osteoblastic S1P could be a mediator of the communication between osteoblastic and CaP cells in the bone microenvironment, and the potential clinical value of anti‐S1P antibodies (Sabbadini, 2011) which are currently in Phase 2 clinical trials for cancer, suggesting that metastatic CaP could be an important disease for S1P‐directed therapy.
2. Material and methods
2.1. Chemicals and reagents
Culture medium, serum, and antibiotics were from Invitrogen (Villebon sur Yvette, France). [γ‐32P]ATP, D‐erythro‐[3‐3H] sphingosine and [methyl‐3H] thymidine were from Perkin‐Elmer (Courtaboeuf, France). Silica gel 60 TLC plates were from VWR (Fontenay sous Bois, France). Sphingosine was from Biomol (Plymouth Meeting, PA). MTT was from Sigma (Saint‐Quentin Fallavier, France). VPC23019, W146 were from Avanti (Alabaster, AL, USA). JTE013 was from R&D Systems (Lille, France). Pharmacological SphK1 inhibitor SKI‐II (2‐(p‐hydroxyanilino)‐4‐(p‐chlorophenyl) thiazole) was from Calbiochem (Fontenay‐Sous‐Bois, France). The murine monoclonal anti‐S1P antibody, Sphingomab™, and its isotype mAb control, LT1017, were generated as described previously (O'Brien et al., 2009).
2.2. Cell culture
LNCaP and PC‐3 cell lines were from DSMZ (Braunschweig, Germany), 22rv1, MC3T3, NIH3T3 cell lines were from ATCC‐LGC Standards (Molsheim, France), DU‐145 cell line was kindly supplied by Dr Lazennec (Inserm U844, Montpellier, France). The C4‐2B cell line was from Viromed (Minnetonka, MN). C4‐2B is a castration‐resistant prostate cancer cell line isolated from the bone metastasis of a mouse xenograft inoculated with C4‐2 cells, a subline of LNCaP cells. Cells were cultured in α‐MEM (MC3T3), DMEM (NIH3T3), or RPMI 1640 (LNCaP, 22rv1, C4‐2B, PC‐3, DU‐145) containing 10% FBS at 37 °C in 5% CO2 humidified incubators. Cell lines were routinely verified by the following tests: morphology check by microscope, growth curve analysis, and mycoplasma detection (PlasmoTest™, Invivogen, Toulouse, France). All experiments were started with low‐passaged cells (<15 times).
2.3. Osteoblastic cell differentiation
MC3T3 cells were seeded at 105 cells/well in 6‐well plates and cultivated during 3 weeks. Medium was changed twice a week and differentiating medium (α‐MEM 10% FBS with 10 mM β‐glycerophosphate and 50 μg/ml l‐ascorbic acid) was added with or without treatment. To visualize osteoblastic differentiation, cells were stained for calcium deposition using the alizarin red S staining (Lin et al., 2001) or alkaline phosphatase (AP) activity was determined using p‐nitrophenyl phosphate (pNPP) as substrate (Sigma) as previously reported (Zunich et al., 2009).
2.4. Preparation of primary human femoral head osteoblastic cells (hOBs) and mouse calvaria osteoblastic cells (mOBs)
Bone chips from femoral head trabecular explants were obtained from a patient undergoing hip replacement surgery as approved by the institutional ethics committed of the Hôpitaux de Toulouse and primary osteoblasts were obtained as described elsewhere (Wang et al., 2009).
Calvaria were excised from 8 week‐old C57BL/6 mice (gift from Dr Chabot, Ms Mirey and Dr Mercier from CNRS UMR 5089, Toulouse, France) and primary osteoblasts were obtained as described elsewhere (Bakker and Klein‐Nulend, 2012).
Bone cells start to migrate from bone chips after 3–5 days and cells growing from bone fragments reach confluency after 11–15 days. At this time, cells were trypsinized and plated at 2.5 to 5 × 103 cells per cm2 in T25 or T75 flasks in culture medium (DMEM 1 g/L glucose + l‐glutamine supplemented with 100 U/mL penicillin, 50 μg/mL streptomycin sulfate, 50 μg/mL gentamicin, 1.25 μg/mL fungizone, 100 μg/mL ascorbate and 10% FBS). Medium was changed three times a week, and after approximately 7–10 days cells reached subconfluency, upon which they were be used for experiments. The average number of primary cells obtained lied between 4 × 106 and 6 × 106 cells.
2.5. Co‐culture assays
Tumor cells and osteoblastic cells were co‐cultured using a Transwell system 0.4‐μm pore size from Millipore (Nottingham, UK). A total of 3Χ105 (MC3T3) cells were seeded in the upper chamber of the Transwell system in the culture medium of tumor cells and co‐cultured with or without tumor cells in the lower chamber for 24 h.
2.6. Generation of conditioned medium
Mouse MC3T3 osteoblastic cells, primary calvaria mouse or human femoral head osteoblastic cells were seeded in 15 cm diameter plates (4 × 106). 24 h later, cells were washed 4 times with PBS 1X and 20 mL of RPMI 1640 was added. Cells were then incubated for 24 h and conditioned medium (CM) was collected and filtered. For proliferation and cell viability assays, CM was used at a concentration of 50% as previously reported (Blaszczyk et al., 2004; Lu et al., 2004; Wang et al., 2009).
2.7. Quantitative RT‐PCR (qRT‐PCR) analysis
Total RNA was extracted from cells using the RNeasy kit (Qiagen). The concentration and purity of the RNA were determined spectrophotometrically by measuring the absorbance at 260 nm and 280 nm using a NanoDrop (Thermo). The cDNA were synthetized by the reverse transcription of 1 μg total RNA using the SuperScript III First‐Strand Synthesis System (Invitrogen). The qPCR analysis of the samples was performed using the MESA GREEN qPCR MasterMix Plus kit (Eurogentec) and a ABIPrism 7500 cycler (Applied Biosystems) with mSphK1 primers (forward: 5′‐ACAGTGGGCACCTTCTTTC‐3′; reverse: 5′‐CTTCTGCACCAGTGTAGAGGC‐3′), mSphK2 (forward: 5′‐TAGATGGGGAGTTAGTGGAGTATG‐3′; reverse: 5′‐TGCTTTTAGGCTCGTTCAGG‐3′), mSpns2 (forward: 5′‐ATGATGTGCCTGGAATGC‐3′; reverse: 5′‐TCAGACTTTCACGGATGCAG‐3′), and two reference genes, YWHAZ (forward: 5′‐ACTTTTGGTACATTGTGGCTTCAA‐3′; reverse: 5′‐CCGCCAGCACACACCAATAT‐3′) and RNA polymerase II (RPII) (forward: 5′‐GCACCACGTCCAATGACAT‐3′; reverse: 5′‐GTGCGGCTGCTTCCATAA‐3′). Each qPCR was done in triplicate. Amplifications were performed starting with 10 min of denaturation at 95 °C, then 40 cycles of 15 s denaturation at 95 °C, and 60 s of primer specific annealing and specific extension at 60 °C. The results were normalized using the ΔΔCT method.
2.8. RNA interference experiments
The ON‐TARGET plus SMART‐pool of double‐stranded small interfering RNA directed against mouse SphK1 was from Dharmacon Thermo Scientific. Spns2 transient interference was achieved by double‐stranded mouse specific small interfering RNAs 5′‐ GCUAGUAGGUAUUGGUGAA (dT)(dT) ‐3′ (siSpns2a), 5′‐CUCAUCUUUGGAGCCAUUA (dT)(dT) ‐3′ (siSpns2b), and 5′‐CCAUCUUCAUCUGCCUCAU (dT)(dT) ‐3′ (siSpns2c) from Sigma. S1P1 transient interference was achieved by double‐stranded human specific small interfering RNAs 5′‐GCAGCUCGGUCUCUGACUA (dT)(dT) ‐3′ (siS1P1a) and 5′‐ GGAGAACAUCUUUGUCUUG (dT)(dT) ‐3′ (siS1P1b) from Dharmacon Thermo Scientific. S1P3 transient interference was achieved by double‐stranded human specific small interfering RNAs 5′‐ GAACGCAGCACUUCAGAAU (dT)(dT) ‐3′ (siS1P3a) and 5′‐ GGGAUGUGCUGGCUCAUUG (dT)(dT) ‐3′ (siS1P3b) from Dharmacon Thermo Scientific. Aleatory sequence scrambled siRNA was from Eurogentec (Angers, France). Transfections were carried out using the Lipofectamine 2000 reagent (Invitrogen) as previously reported (Brizuela et al., 2010).
2.9. Cell proliferation and viability assays
LNCaP, 22rv1, C4‐2B, PC‐3 and DU‐145 cells were plated in 6‐well plates (3 × 105 for LNCaP and 22rv1, 2 × 105 for C4‐2B and DU‐145 and 105 cells/well for PC‐3, respectively), then cultured for 24 h. 3H‐thymidine was added to the culture medium (1 μCi/mL) 6 h before the end of the experiment. Cells were washed once with ice‐cold PBS and 3 times with 10% trichloroacetic acid to cause the precipitation of DNA and proteins. The precipitate was solubilized in 0.3 N NaOH/1% SDS, and radioactivity was measured (Gomez‐Brouchet et al., 2007). Cell viability was determined by 3‐(4,5‐dimethylthiazol‐2‐yl‐)2,5‐diphenyltetrazolium (MTT) bromide assay (Cuvillier et al., 1999).
2.10. SphK1 activity and mass measurement of S1P
The protocols for the determination of SphK1 enzymatic activity and measurement of S1P release have been described in detail previously (Brizuela and Cuvillier, 2012).
2.11. X‐ray irradiation and radiation survival determination
Irradiation was carried out in a Faxitron Rx‐650 irradiator (Faxitron X‐ray Corporation, Lincolnshire, IL) at a dose rate of 0.48 Gy/min. Survival after irradiation was defined as the ability of the cells to maintain their clonogenic capacity and to form colonies. Cells were plated from exponentially growing cell culture at 1000–1500 cells/well in 6 well‐plates 18 h before irradiation. Single doses ranging from 2 to 6 Gy were delivered. Colonies were fixed and stained with crystal violet (2 mg/mL in 150 mM NaCl) for 20 min. Survival clones were counted 6 days after irradiation (Brizuela et al., 2012).
2.12. Western blot
Rabbit anti‐S1P1, rabbit anti‐S1P3 obtained from Novus (Cambridge, UK), rabbit anti‐S1P2 and rabbit anti‐Spns2 obtained from Sigma, mouse anti‐Runx2 was from Abcam (Cambridge, UK), and rabbit anti‐SphK1 (Pitson et al., 2003) were used as primary antibodies. Proteins were visualized by an enhanced chemiluminescence detection system (Pierce, Brebières, France) using anti‐rabbit or anti‐mouse horseradish peroxidase‐conjugated IgG (Bio‐Rad, Hercules, CA). Equal loading of protein was confirmed by probing the blots with the mouse anti‐tubulin (Santa Cruz, clone DM1A; Heidelberg, Germany) antibody. Densitometry quantitation was determined using Image J software (NIH, Bethesda, MD).
2.13. Histological analysis
Osteotic samples were analyzed following decalcification according to standard protocols. Sections were stained with hematoxylin & eosin (H&E) and SphK1 (1/600) in conditions previously reported (Brizuela et al., 2012; Malavaud et al., 2010).
2.14. Statistical analysis
The statistical significance of differences between the means was evaluated using the unpaired Student's t or the one‐way analysis of variance (ANOVA) tests. All statistical tests were two‐sided, and the level of significance was set at P < 0.05. Calculations were done using Prism 6 (GraphPad Software, San Diego, CA).
3. Results
3.1. Osteoblast‐borne S1P promotes prostate cancer cell proliferation
Osteoblasts facilitate progression of metastatic CaP in bone (Logothetis and Lin, 2005; Weilbaecher et al., 2011), and a wealth of studies classically using conditioned media (CM) from osteoblastic cells support the view that osteoblast‐derived growth factors promote cell proliferation in various CaP cell lines (Blaszczyk et al., 2004; Festuccia et al., 1999; Gleave et al., 1991; Lang et al., 1995; Lu et al., 2004; Millimaggi et al., 2006; Wang et al., 2009). We used CM from the fibroblastic‐like pre‐osteoblast MC3T3 cell line, which displays osteoblast characteristics analogous to in vivo bone formation (Quarles et al., 1992), primary murine osteoblast‐like cells (mOB) obtained from C57BL/6 calvaria and primary human femoral head osteoblastic cells (hOB) to determine whether or not S1P is an important growth factor present in the CM. In line with previous reports (Gleave et al., 1991; Lang et al., 1995; Wang et al., 2009), CM (final concentration, 50%) from MC3T3, mOB or hOB significantly increased the proliferation of a wide‐ranging panel of CaP cells whereas CM from NIH3T3, a murine embryonic fibroblast cell line, had no effect, establishing the specificity of osteoblastic cells (Figure 1A).
Figure 1.
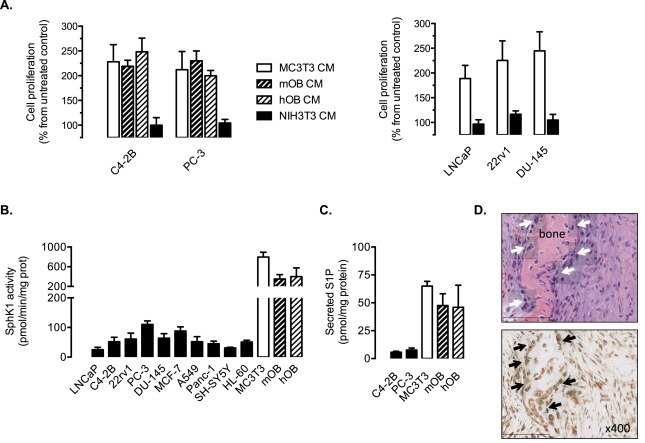
SphK1 is highly expressed in human and mouse osteoblastic cells, which secrete large amounts of S1P and stimulates cell proliferation in prostate cancer cell lines. A, cell proliferation was assessed in C4‐2B, PC‐3, LNCaP, 22rv1 and DU‐145 prostate cancer cells with 3H‐thymidine incorporation assay at 24 h in presence of CM (conditioned medium) from MC3T3 osteoblastic cells (MC3T3 CM), from primary calvaria mouse osteoblastic cells (mOB CM), from primary human femoral head osteoblastic cells (hOB CM), from NIH3T3 fibroblastic cells (NIH3T3 CM) or not (control). Columns, mean of at least 4 independent experiments; bars, SEM. B, basal SphK1 activity was quantified in various cancer cell lines (prostate, breast, lung, pancreas, neuroblastoma, leukemia), in non‐differentiated osteoblastic MC3T3 cells and primary osteoblasts from mouse calvaria (mOB) or primary human femoral head osteoblastic cells (hOB). Columns, mean of at least 4 independent experiments; bars, SEM. C, S1P levels were quantified in media from C4‐2B and PC‐3 prostate cancer cells, from non‐differentiated osteoblastic MC3T3 cells, from primary calvaria mouse osteoblastic cells (mOB) and from primary human femoral head osteoblastic cells (hOB). Columns, mean of at least 3 independent experiments; bars, SEM. D, H&E staining (upper panel) and SphK1 expression (brown, lower panel) on sections derived from an osteotic bone. Solid arrowheads show osteoblastic cells with specific presence of SphK1.
These results indicate that osteoblastic cells produce soluble factors that stimulate CaP cell proliferation. Because SphK1 was previously found highly expressed in MC3T3 cells and inducible by androgens (Martin et al., 2010), we investigated whether S1P could represent a putative osteoblast‐derived proliferating factor. Compared to various cancer cells including CaP cells such as LNCaP, C4‐2B, 22rv1, PC‐3 and DU145 or other cancer cell lineages (breast, lung, pancreas, neuroblastoma or leukemia), the basal SphK1 activity was found to be substantially expressed in MC3T3 cells as well as in mOB and hOB cells (Figure 1B). Importantly, significantly higher amounts of S1P were secreted by MC3T3, mOB and hOB cells in contrast to the metastatic C4‐2B and PC‐3 cells (Figure 1C).
To determine whether the in vitro findings of a strong expression of SphK1 in osteoblastic cells had any relevance to human physiology, sections from non‐tumoral osteotic bone lesions were stained for H&E and SphK1 (Figure 1D). H&E staining clearly showed the presence of osteoblasts (solid arrowheads), which were all strongly positive for SphK1 staining.
In order to establish that S1P secreted by osteoblastic cells contributed to CaP cell proliferation, we neutralized SphK1/S1P signaling in a variety of ways. First, the down‐regulation of osteoblastic MC3T3 cell SphK1 was achieved by siRNA strategy or pharmacological inhibition with SKI‐II, a SphK1 inhibitor (French et al., 2003). As shown in Figure 2A, basal SphK1 mRNA level and SphK1 activity were substantially decreased by SphK1 siRNA (siSphK1) as compared with scrambled siRNA in MC3T3 cells, with a concomitant decrease in S1P secretion (Figure 2B). A similar inhibitory effect on S1P secretion was achieved in presence of SKI‐II (Figure 2A and B). Cell proliferation in representative bone metastasis‐derived PC‐3 and C4‐2B cells was markedly reduced in presence of CM from MC3T3 cells transfected by siSphK1 or pre‐treated with the pharmacological inhibitor, SKI‐II (Figure 2C and D). Finally, we demonstrated the direct involvement of CM‐derived S1P by neutralizing S1P paracrine signaling in the CM using sphingomab™, a high affinity monoclonal anti‐S1P antibody (O'Brien et al., 2009; Visentin et al., 2006). As expected, the anti‐S1P mAb significantly and substantially blocked PC‐3 and C4‐2B cell proliferation when incubated with the CM from MC3T3, from primary murine (mOB) or human (hOB) osteoblastic cells (Figure 2C and D).
Figure 2.
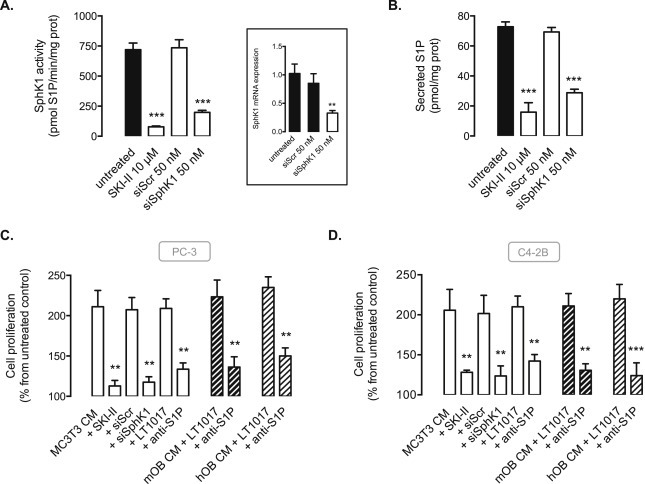
The inhibition of the osteoblast‐driven SphK1/S1P signaling blocks cell proliferation of PC‐3 and C4‐2B prostate cancer cells. A, SphK1 activity was quantified in osteoblastic MC3T3 cells untreated (black column) or treated with 10 μM SKI‐II SphK1 inhibitor, or transfected with 50 nM siScramble or 50 nM siSphK1 for 72 h. Columns, mean of at least 3 independent experiments; bars, SEM. ***, P < 0.001. Insert, cells were submitted to mRNA extraction and real time PCR analysis for murine SphK1. Columns, mean of at least 3 independent experiments; bars, SEM. ***, P < 0.001. B, secreted S1P levels were quantified in osteoblastic MC3T3 cells untreated or treated with 10 μM SKI‐II SphK1 inhibitor, or transfected with 50 nM siScramble or 50 nM siSphK1 for 72 h. Columns, mean of at least 3 independent experiments; bars, SEM. ***, P < 0.001. Cell proliferation was quantified in PC‐3 (C) and C4‐2B (D) cells with 3H‐thymidine incorporation assay at 24 h in absence (control) or in presence of CM from MC3T3 (empty columns), murine (mOB, thick hatched columns) or human (hOB, light hatched columns) primary osteoblasts untreated or treated with 10 μM SKI‐II SphK1 inhibitor, transfected for 72 h with 50 nM siScramble or 50 nM siSphK1, or in presence of CM treated with 50 μg/ml anti‐S1P antibody (Sphingomab™) or IgG control (LT1017). Columns, mean of at least 3 independent experiments; bars, SEM. **, P < 0.01.
3.2. Osteoblast‐derived S1P protects PC‐3 and C4‐2B cells from chemotherapy and radiotherapy
Taxane‐based chemotherapy (e.g., docetaxel) and radiotherapy represent conventional treatments for patients with progressive metastatic CaP (Sturge et al., 2011), we therefore examined whether osteoblastic‐borne S1P could be associated with a resistance of CaP cells to docetaxel and γ‐irradiation. Accordingly, the addition of CM from MC3T3 cells inhibited the loss of cell viability observed after treatment with docetaxel in both metastatic PC‐3 (Figure 3A) and C4‐2B cells (Figure 3B). Importantly, the prosurvival effect of CM from MC3T3 was significantly reversed when MC3T3 were transfected by siSphK1 or pre‐treated with the pharmacological inhibitor SKI‐II (Figure 3A and B). A similar effect was observed when S1P from CM from MC3T3 cells or primary murine (mOB) or human (hOB) osteoblastic cells was neutralized by the anti‐S1P antibody (Figure 3A and B), supporting the notion that S1P secreted by MC3T3 could act as a paracrine survival factor for CaP cells. Of note, the CM from MC3T3 also markedly enhanced survival after irradiation in PC‐3 and C4‐2B (Figure 3C). This radioprotective effect was due to osteoblastic S1P, as pharmacological inhibition of SphK1 by SKI‐II, knock‐down of SphK1 by siRNA or blocking of S1P by anti‐S1P antibody resensitized both cell lines to irradiation (Figure 3C).
Figure 3.
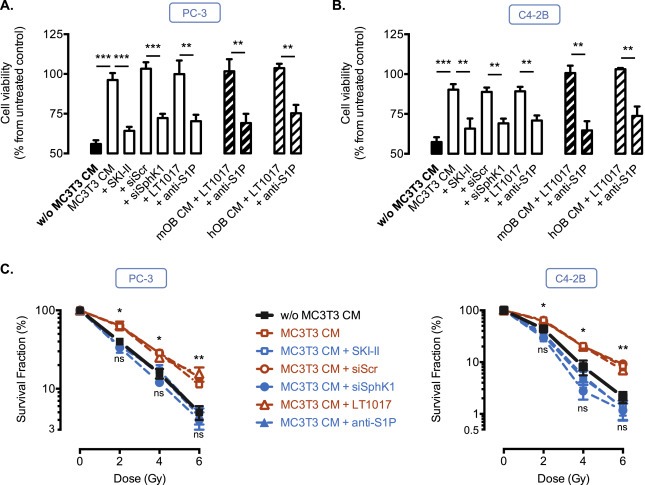
Osteoblast‐derived S1P protects PC‐3 and C4‐2B cells from chemotherapy and radiotherapy. PC‐3 (A) and C4‐2B (B) cells were untreated (control) or treated with 20 nM docetaxel for 24h in absence (w/o MC3T3‐CM, black column) or in presence of CM from MC3T3, murine (mOB, thick hatched columns) or human (hOB, light hatched columns) primary osteoblasts untreated or treated with 10 μM SKI‐II SphK1 inhibitor, transfected for 72h with 50 nM siScramble or 50 nM siSphK1, or in presence of CM treated with 50 μg/ml anti‐S1P antibody or IgG control (LT1017), and cell viability was assessed by MTT. Columns, mean of at least 4 independent experiments; bars, SEM. **, P < 0.01; ***, P < 0.001. C, PC‐3 (left) and C4‐2B (right) cells were irradiated from 2 to 6 Gy in absence (w/o MC3T3 CM) or in presence of CM from MC3T3 untreated or treated with 10 μM SKI‐II SphK1 inhibitor, transfected for 72h with 50 nM siScramble or 50 nM siSphK1, or in presence of CM treated with 50 μg/ml anti‐S1P antibody or IgG control (LT1017), and survival clones were counted 6 days later. Data are expressed as the percentage of survival fraction compared to non‐irradiated cells. Points, mean of at least 4 independent experiments; bars, SEM. The two‐tailed P values between the means of untreated (w/o MC3T3 CM) or MC3T3 CM treated cells are: ns, not significant; *, P < 0.05; **, P < 0.01.
3.3. Sphingosine 1‐phosphate receptor 1 (S1P1) expressed on prostate cancer cells is responsible for the effets of osteoblast‐derived S1P
To investigate which S1P receptor could mediate the proliferative and survival effects of osteoblast S1P, the targeting of the most commonly expressed S1P receptors in cancer cells (S1P1, S1P2 and S1P3) was carried out. All prostate cancer cell lines (PC‐3, C4‐2B, LNCaP, 22Rv1, DU‐145) expressed S1P1, S1P2 and S1P3 (data not shown). Cell proliferation in PC‐3 and C4‐2B cells was strongly reduced in presence of CM from MC3T3 pre‐treated with either VPC23019, an antagonist of both S1P1 and S1P3 receptors, or with W146, an antagonist of S1P1 (Figure 4A). On the contrary, the JTE013 compound did not have any impact on cell proliferation ruling out a role for S1P2 receptor (Figure 4A). To determine which of the two receptors, S1P1 or S1P3 is the dominant receptor involved in mediating the effects of extracellular S1P, the specific down‐regulation of S1P1 and S1P3 was achieved by siRNA strategy in PC‐3 cells with two different siRNAs tested and validated by assessing mRNA (data not shown) and protein expression of S1P1 and S1P3 (Figure 4B). Similar results were obtained in C4‐2B cells (data not shown). The prosurvival effect of CM from MC3T3 was strongly impaired when PC‐3 (Figure 4C) and C4‐2B cells (Figure 4D) were transfected by the two designed siRNAs against S1P1 (S1P1a or S1P1b) and then treated with docetaxel while siRNAs directed against S1P3 did not inhibit the prosurvival effects of S1P‐derived from MC3T3 in both prostate cancer cell lines. This result demonstrates that S1P1 receptor is responsible for the effects of bone‐derived S1P on both CaP cell proliferation and resistance to chemotherapy.
Figure 4.
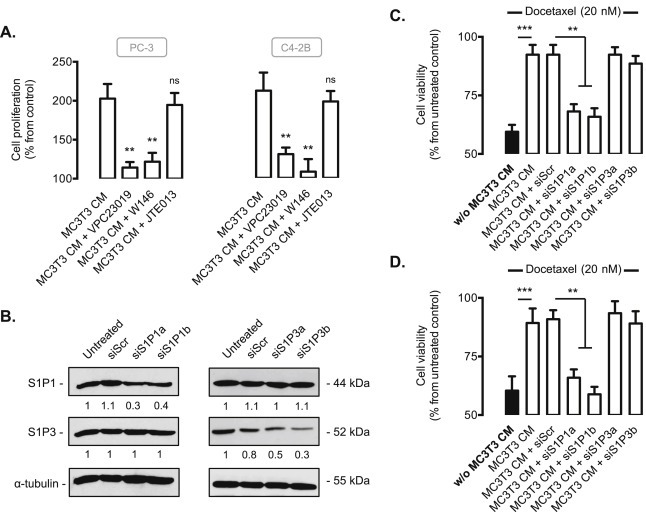
The effets of osteoblast‐derived S1P on cell proliferation and resistance to chemotherapy of prostate cancer cells involves S1P1 receptor. A, cell proliferation was assessed in PC‐3 and C4‐2B cells with 3H‐thymidine incorporation assay at 24 h in absence (control) or in presence of CM from MC3T3 untreated or treated with 2 μM VPC23019 (S1P1 and S1P3 antagonist), 2 μM W146 (S1P1 antagonist), or 2 μM JTE013 (S1P2 antagonist). Columns, mean of at least 3 independent experiments; bars, SEM. **, P < 0.01. B, PC‐3 cells were untransfected or transfected for 48 h with 50 nM of different siRNAs against S1P1 and S1P3 (siS1P1a, siS1P1b, siS1P3a, siS1P3b) or siScramble (siScr). Cell lysates were assayed for S1P1 and S1P3 expression by western blot analysis. Equal loading of protein was monitored using antibody to α‐tubulin. Similar results were obtained in three independent experiments. PC‐3 (C) and C4‐2B (D) untransfected or transfected for 48 h with 50 nM of different siRNAs against S1P1 and S1P3 (siS1P1a, siS1P1b, siS1P3a, siS1P3b) or siScramble (siScr) were treated with 20 nM docetaxel for an additional 24h in absence (w/o MC3T3‐CM, black column) or in presence of CM from MC3T3, and cell viability was then assessed by MTT. Columns, mean of at least 4 independent experiments; bars, SEM. **, P < 0.01; ***, P < 0.001.
3.4. Osteoblastic SphK1 activity and prostatic S1P1 receptor are necessary to stimulate cell proliferation and survival to therapeutics of PC‐3 and C4‐2B cells in co‐culture with MC3T3 cells
We next attempted to examine the interactions between osteoblastic cells and CaP in co‐culture experiments where MC3T3 cells were plated in the upper chamber with either C4‐2B or PC‐3 cells in the lower chamber (Figure 5A). When osteoblastic SphK1 was silenced with siSphK1, the proliferation of PC‐3 (Figure 5B, left) or C4‐2B (Figure 5B, right) cells was significantly inhibited. Moreover, when co‐cultures where treated with 20 nM docetaxel, the presence of MC3T3 cells (MC3T3 + siScr) protected PC‐3 (Figure 5B, left) or C4‐2B (Figure 5B, right) cells from its inhibitory effect. In contrast the silencing of osteoblastic SphK1 (M3T3 + siSphK1) markedly resensitized PC‐3 (Figure 5B, left) or C4‐2B (Figure 5B, right) cells to docetaxel, confirming the critical role of osteoblastic SphK1 in CaP cell survival. To support that S1P1 receptor expressed on CaP cells mediate the proliferative and prosurvival action of osteoblastic cells, PC‐3 cells silenced for S1P1 were co‐cultured in presence of MC3T3 treated or not with docetaxel. The transient knockdown of S1P1 receptor in PC‐3 cells with two different siRNAs (S1P1a or S1P1b) was associated with a lower proliferative rate (Figure 5C). In contrast to siScrambled treated cells, a resensitization to docetaxel treatment was observed when S1P1 receptor was silenced (Figure 5C). Altogether, these data support the notion that osteoblastic SphK1 activity and prostatic S1P1 receptor are necessary for cell proliferation and survival of CaP cells.
Figure 5.
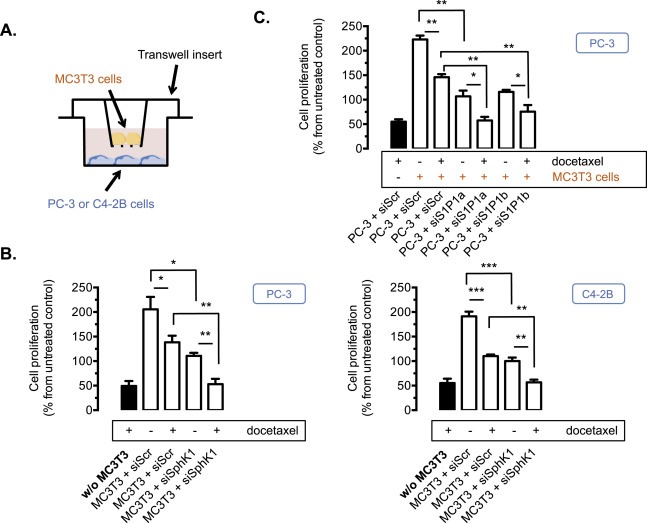
Osteoblastic SphK1 activity and prostatic S1P1 receptor are necessary to stimulate cell proliferation and promote survival to therapeutics of PC‐3 and C4‐2B cells in co‐culture with MC3T3 cells. A, a schematic diagram of the transwell culture system showing an upper chamber resting on the rim of a well that acts as the lower chamber. The upper chamber contains MC3T3 osteoblastic cells and its floor consists of a porous polycarbonate filter. The lower chamber contains C4‐2B or PC‐3 prostate cancer cells. B, osteoblastic MC3T3 cells untransfected or transfected for 72 h with 50 nM siSphK1 or siScramble (siScr) were treated with 20 nM docetaxel for an additional 24 h in absence (w/o MC3T3 CM, black column) or in presence of MC3T3 cells in the upper chamber, and cell proliferation of PC‐3 (left) and C4‐2B (right) cells was then assessed by with 3H‐thymidine incorporation assay. Columns, mean of at least 3 independent experiments; bars, SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001. C, prostate cancer PC‐3 cells untransfected or transfected for 48 h with 50 nM of two different siRNAs against S1P1 (siS1P1a, siS1P1b) or siScramble (siScr) for 72 h were treated or not with 20 nM docetaxel for an additional 24 h in absence (w/o MC3T3 cells, black column) or in presence of MC3T3 cells in the upper chamber, and cell proliferation of PC‐3 cells was then assessed by 3H‐thymidine incorporation assay. Columns, mean of at least 3 independent experiments; bars, SEM. *, P < 0.05; **, P < 0.01.
3.5. Spns2 invalidation inhibits PC‐3 cell proliferation and sensitizes to docetaxel in co‐culture with MC3T3 cells
When MC3T3 were treated with Spns2‐specific siRNAs, the expression of Spns2 protein decreased to less than 30% of the control with two of the three different siRNAs tested (siSpns2b and siSpns2c). The silencing of Spns2 was confirmed by qRT‐PCR analysis (Figure 6A). With regard to cell proliferation, the transient knockdown of Spns2 using the two different siSpns2b and siSpns2c siRNAs was associated with a significant inhibitory effect on PC‐3 cell proliferation (Figure 6B). When the co‐culture of PC‐3 with MC3T3 cells was treated with 20 nM docetaxel, a resensitization was found in osteoblastic cells transfected with the two Spns2 siRNAs (Figure 6B), with a major inhibition of cell proliferation similar to what was observed in PC‐3 cells treated with docetaxel in absence of MC3T3 cells (Figure 6B, black column). Overall, these data suggest that blocking secretion of S1P from osteoblastic cells can efficaciously block their proliferative and survival effects on metastatic CaP cells.
Figure 6.
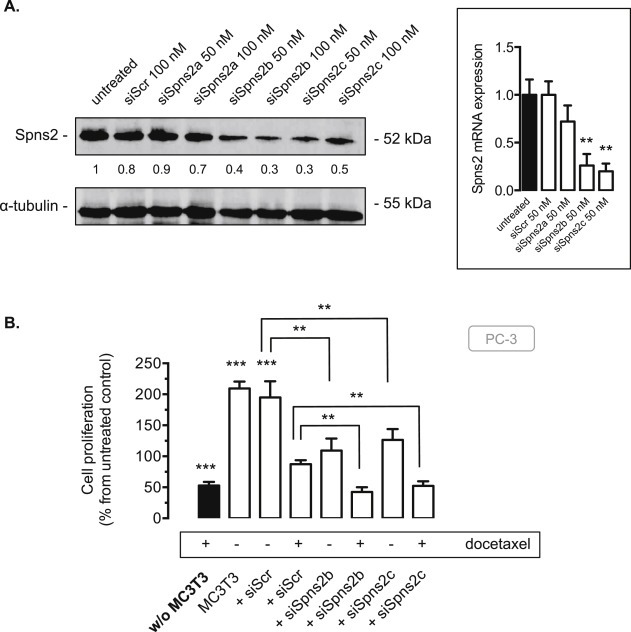
Spns2 knockdown inhibits PC‐3 cell proliferation and resensitizes PC‐3 to docetaxel in co‐culture with MC3T3 cells. A, MC3T3 cells were untransfected or transfected for 72 h with 50 nM or 100 nM of three different siRNAs against Spns2 (siSpns2a, siSpns2b, siSpns2c) or 100 nM siScramble (siScr). Osteoblastic cell lysates were assayed for Spns2 expression by western blot analysis. Equal loading of protein was monitored using antibody to α‐tubulin. Similar results were obtained in three independent experiments. Cells were submitted to mRNA extraction and real time PCR analysis for murine Spns2. Columns, mean of at least 3 independent experiments; bars, SEM. **, P < 0.01. B, osteoblastic MC3T3 cells untransfected or transfected for 72 h with 50 nM of two different siRNAs against Spns2 (siSpns2b, siSpns2c) or siScramble (siScr) were treated or not with 20 nM docetaxel for an additional 24 h in absence (w/o MC3T3 cells, black column) or in presence of MC3T3 cells in the upper chamber, and cell proliferation of PC‐3 cells was then assessed by with 3H‐thymidine incorporation assay. Columns, mean of at least 3 independent experiments; bars, SEM. **, P < 0.01; ***, P < 0.001.
3.6. The SphK1/S1P/S1P3 signaling axis is critical for osteoblastic maturation
Bone metastases from CaP are associated with accelerated osteoblastic activity (Charhon et al., 1983) where prostate cancer cells promote proliferation and differentiation of osteoblastic cells (Yang et al., 2001), thus resulting in abnormal bone formation. The potential involvement of SphK1/S1P signaling during osteoblastic differentiation was therefore investigated. Differentiation of pre‐osteoblasts into mature mineral‐producing osteoblasts resulted in a time‐dependent increase in SphK1 mRNA levels (data not shown) and enzymatic SphK1 activity (Figure 7A), associated with a higher amount of S1P secreted from osteoblastic cells (Figure 7A), suggesting that SphK1/S1P signaling could be critical in osteoblastic differentiation. Interestingly, during the differentiation process, changes in the S1P receptor expression pattern was found, specifically showing a marked loss of expression of S1P1 and S1P2 subtypes and a moderate increased expression of S1P3 (Figure 7B). The involvement of an autocrine SphK1/S1P/S1P3 signaling during osteoblastic differentiation was established by quantifying the alkaline phosphatase activity, a classical measure of osteoblast maturation, and Runx2 level, a key transcription factor involved in osteoblastic maturation. In 3‐week differentiated MC3T3 cells, the pharmacological inhibition of SphK1 with SKI‐II or the neutralization of exogenous S1P with anti‐S1P antibody strongly impaired alkaline phosphatase activity (Figure 7C) and bone matrix formation (data not shown). A similar inhibitory effect was observed when MC3T3 cells were incubated in presence of VPC23019, the antagonist of both S1P1 and S1P3 receptors. On the other hand, no effect was found in presence of W146, a specific antagonist of S1P1, thus ruling out a role for S1P1. Similarly, the JTE013 compound did not have any impact on alkaline phosphatase activity excluding a role for S1P2 receptor (Figure 7C). As expected, Runx2 expression was markedly reduced when MC3T3 cells undergoing differentiated were treated with SKI‐II, Sphingomab or VPC23019 but not with W146 nor JTE013 (Figure 7C). Collectively, these data establish the exclusive contribution of S1P3 receptor subtype in mediating the effect of increased secretion of S1P during osteoblastic maturation. Importantly, CM from mature osteoblasts further enhanced PC‐3 and C4‐2B cell proliferation compared to non‐differentiated osteoblasts. As anticipated, this enhanced cell proliferation was markedly abolished when S1P was neutralized with the anti‐S1P antibody (Figure 7D).
Figure 7.
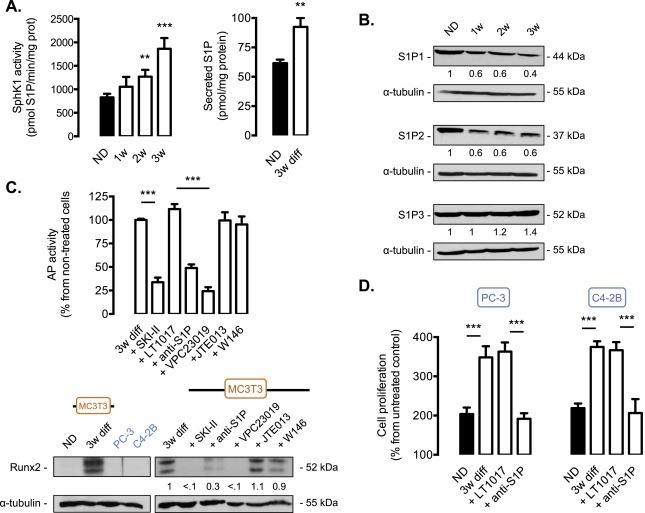
S1P is critical for the differentiation of pre‐osteoblasts into mature osteoblasts. A, MC3T3 murine osteoblastic cells were differentiated in presence of 10 mM of β‐glycerophosphate and 50 μg/ml of ascorbic acid and recovered every week during 3 weeks. Endogenous SphK1 activity (left) and secreted S1P (right) were measured in non‐differentiated MC3T3 cells or differentiated as indicated. Columns, mean of at least 4 independent experiments; bars, SEM. **, P < 0.01; ***, P < 0.001. B, cell lysates from non‐differentiated MC3T3 osteoblastic cells (ND), or differentiated for 1, 2 or 3 weeks were assayed for S1P1, S1P2 and S1P3 expression by western blot analysis. Equal loading of protein was monitored using antibody to α‐tubulin. Similar results were obtained in three independent experiments. C, alkaline phosphatase activity was quantified in osteoblastic MC3T3 differentiated for 3 weeks in absence (3w diff) or in presence of 10 μM SKI‐II SphK1 inhibitor, 50 μg/ml anti‐S1P antibody or IgG control (LT1017), 2 μM VPC23019 (S1P1 and S1P3 antagonist), 2 μM JTE013 (S1P2 antagonist) or 2 μM W146 (S1P1 antagonist). Columns, mean of at least 4 independent experiments; bars, SEM. ***, P < 0.001. Runx2 expression was assessed in non‐differentiated MC3T3 cells (ND) or differentiated for 3 weeks (3w diff) as well as in PC‐3 and C4‐2B cells (left). Cell lysates from MC3T3 differentiated for 3 weeks (3w diff) or in presence of 10 μM SKI‐II SphK1 inhibitor, 50 μg/ml anti‐S1P antibody or IgG control (LT1017), 2 μM VPC23019 (S1P1 and S1P3 antagonist), 2 μM JTE013 (S1P2 antagonist) or 2 μM W146 (S1P1 antagonist). were assayed for Runx2 expression by western blot analysis. Equal loading of protein was monitored using antibody to α‐tubulin. Similar results were obtained in three independent experiments. D, cell proliferation was quantified in PC‐3 (left) and C4‐2B (right) cells with 3H‐thymidine incorporation assay at 24 h in presence of CM (conditioned medium) from MC3T3 non‐differentiated (ND) MC3T3 osteoblastic cells (black column) and differentiated for 3 weeks treated with 50 μg/ml anti‐S1P antibody (Sphingomab™) or IgG control (LT1017). Columns, mean of at least 3 independent experiments; bars, SEM. ***, P < 0.001.
4. Discussion
CaP is the most commonly diagnosed cancer in males and the second leading cause of death from cancer in men (Jemal et al., 2009). A characteristic trait of CaP is its ability to metastasize to bone as the disease progresses with a bone‐forming (osteoblastic) phenotype, which is the result of stimulation of osteoblasts (Logothetis and Lin, 2005). Most patients with advanced CaP will experience complications ‐ known as skeletal‐related events (or SREs) — from bone metastases that are currently incurable (Sturge et al., 2011). Bone metastatic CaP cells grow at a more rapid rate than primary CaP (Jacobs, 1983), suggesting they are able to form a synergistic relationship with bone microenvironment, creating favorable conditions for their growth. A number of studies show that osteoblasts seem to be essential for tumor progression but only few osteoblast‐derived factors such as interleukin‐6 (IL‐6) (Lu et al., 2004; Wang et al., 2009), transforming growth factor‐β (TGF‐β) (Millimaggi et al., 2006) or insulin growth factor I (IGF‐I) (Ritchie et al., 1997) have been thus far described as growth factors able to stimulate proliferation of CaP cells.
In this report, we describe the contribution of sphingosine 1‐phosphate (S1P) as a paracrine growth factor released from osteoblastic cells to stimulate CaP proliferation and resistance to therapeutics and as an autocrine differentiation factor for osteoblasts themselves, thereby contributing to CaP metastases to bone.
Several studies establish that S1P induces CaP cell proliferation and resistance to therapeutics including docetaxel‐based chemotherapy, irradiation or androgen privation (Brizuela et al., 2012, 2009, 2010, 2008, 2005). Notwithstanding that S1P has been reported to regulate remodeling in normal bone physiology (Ishii et al., 2009, 2010, 2008, 2006), its role in the pathological context of bone metastasis has not been described prior to this report. Because we have recently shown that the SphK1/S1P signaling was markedly expressed in osteoblastic MC3T3 cells and up‐regulated by androgens (Martin et al., 2010), we investigated the potential role of S1P in the complex interactions between prostate cancer and osteoblastic cells.
Here we report for the first time that S1P from bone microenvironment can promote cell proliferation and survival of cancer cells. S1P‐borne osteoblast stimulates proliferation of metastatic CaP cells and protects them from conventional therapeutics (chemotherapy and radiotherapy). More precisely, we show that conditioned medium (CM) from the MC3T3 osteoblastic cell line or primary human or murine osteoblastic cells, stimulate proliferation and survival of bone metastatic PC‐3 and C4‐2B prostate cell lines in S1P‐dependent manner. When S1P released from osteoblastics cells is decreased by pharmacological inhibition of SphK1, the proliferative and survival effects of osteoblasts on PC‐3 and C4‐2B cells were markedly reduced. Even though recent studies using novel selective inhibitors of SphK1 argued against a role for SphK1 in cell proliferation (Rex et al., 2013; Schnute et al., 2012), similar data were obtained after the knock‐down of SphK1 and S1P transporter Spns2 by RNA interference strategies, or neutralization of secreted S1P through the use of an anti‐S1P antibody.
We demonstrate that S1P receptor subtype 1 (S1P1) expressed on CaP is important for cancer cell responses to the S1P secreted by osteoblastic cells as use of RNA interference or pharmacological antagonists failed to demonstrate a role of S1P2 or S1P3. Importantly, the co‐culture experiments corroborated the findings described with CM‐based experiments with regard to CaP cell proliferation and resistance to chemotherapy.
Certainly, the data presented in this work demonstrate that S1P has a dominant effect as a paracrine growth factor as a substantial inhibition of CM effects are negated by interfering with S1P signaling. In the bone microenvironment, CaP cells produce factors that promote the growth and differentiation of osteoblastic cells into mature osteoblasts, which in turn further stimulate CaP cell growth (Logothetis and Lin, 2005). It is important to note that we show for the first time that SphK1/S1P signaling is critical during differentiation to mature osteoblasts, notably by regulating Runx2 level, a key transcription factor involved in osteoblastic maturation. Indeed SphK1 expression and enzymatic activity as well as secretion of S1P increased during osteoblastic differentiation. Interestingly, the pattern of expression of S1P receptors appears to be modified during the differentiation of osteoblasts with a decrease in S1P1 and S1P2 expression contrasting with a relative increase in S1P3. Importantly, the more osteoblastic cells were differentiated into mature osteoblasts, the more S1P signaling was up‐regulated and the more proliferation of CaP cells was stimulated, suggesting that osteoblastic SphK1/S1P signaling may be central in the molecular mechanism driving the vicious interaction between osteoblastic and CaP cells in bone metastasis (Figure 8).
Figure 8.
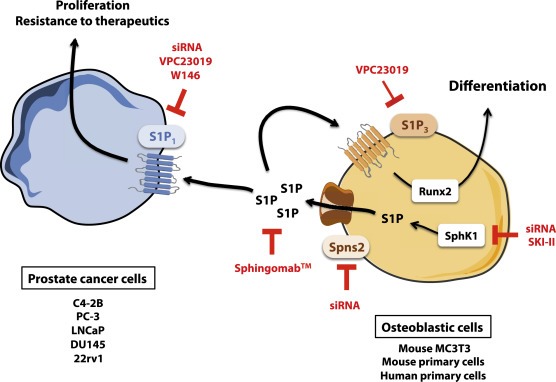
The autocrine and paracrine effects of osteoblast‐borne S1P with regard to osteoblasts and prostate cancer cells. Our findings suggest that osteoblastic cells secrete substantial amounts of S1P within the bone microenvironment through a high intrinsic SphK1 activity. Once secreted, S1P acts on S1P receptor subtype 1 (S1P1) to stimulate prostate cancer cell proliferation and resistance to the conventional treatments for bone metastatic patients, a mechanism that is further enhanced during the differentiation occuring during the natural process of prostate cancer bone metastasis. Genetic and pharmacological inhibition of release of S1P from osteoblasts or its binding to prostate cancer cells as well as neutralization of extracellular S1P by Sphingomab™ prevents the signaling between osteoblasts and tumor cells. Additionally, S1P is pivotal for the differentiation of pre‐osteoblasts into mature osteoblasts as the SphK1/S1P/S1P3 signaling axis regulates the expression of Runx2, the main transcription factor involved in osteoblastic differentiation.
Despite the fact that Sphingomab binds specifically S1P and does not recognize a wide variety of structurally related lipids such as ceramide 1‐phosphate (O'Brien et al., 2009; Visentin et al., 2006), it can cross‐react with dihydro S1P (dhS1P), which has been recently shown to exhibit (in contrast to S1P) an antitumor role during PhotoImmunoNanoTherapy by virtue of abrogating myeloid‐derived suppressor cells (MDSCs) in vitro and in animal models (Barth et al., 2013). Owing to the potential contrasting roles of S1P and dhS1P in PhotoImmunoNanoTherapy, the effects of Sphingomab on both S1P and dhS1P should be investigated in the future in this context.
In summary, together with other osteoblast‐related growth factors, S1P‐borne osteoblast cells can participate in the development of metastasis and resistance to therapy. Bone metastasis is a lethal form of CaP and presents considerable challenges for treatment. Interfering with the interactions between CaP cells and aspects of the bone microenvironment may hold the key to preventing the development of bone metastases or resensitize to therapeutics, and S1P may be a candidate as a target in the treatment of CaP bone metastasis. For example, a humanized version of the anti‐S1P monoclonal antibody is currently under development (Sabbadini, 2011), and is in Phase 2 clinical trials in cancer.
Conflict of interest statement
Authors would like to disclose that Dr. Roger A. Sabbadini is a founder of Lpath, and has stock in the company and is an inventor.
Acknowledgments
We thank Dr Ortega (CNRS, Institut de Pharmacologie et de Biologie Structurale) for her technical help in generating primary osteoblasts from mice, and Falek Zaifi (CHU Toulouse, Service d'Anatomopathologie) for her expertise in immunohistochemistry.
Brizuela Leyre, Martin Claire, Jeannot Pauline, Ader Isabelle, Gstalder Cécile, Andrieu Guillaume, Bocquet Magalie, Laffosse Jean-Michel, Gomez-Brouchet Anne, Malavaud Bernard, Sabbadini Roger A., Cuvillier Olivier, (2014), Osteoblast‐derived sphingosine 1‐phosphate to induce proliferation and confer resistance to therapeutics to bone metastasis‐derived prostate cancer cells, Molecular Oncology, 8, doi: 10.1016/j.molonc.2014.04.001.
References
- Bakker, A.D. , Klein-Nulend, J. , 2012. Osteoblast isolation from murine calvaria and long bones. Methods Mol. Biol. 816, 19–29. [DOI] [PubMed] [Google Scholar]
- Barth, B.M. , Shanmugavelandy, S.S. , Kaiser, J.M. , McGovern, C. , Altinoglu, E.I. , Haakenson, J.K. , Hengst, J.A. , Gilius, E.L. , Knupp, S.A. , Fox, T.E. , Smith, J.P. , Ritty, T.M. , Adair, J.H. , Kester, M. , 2013. PhotoImmunoNanoTherapy reveals an anticancer role for sphingosine kinase 2 and dihydrosphingosine-1-phosphate. ACS Nano. 7, 2132–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaszczyk, N. , Masri, B.A. , Mawji, N.R. , Ueda, T. , McAlinden, G. , Duncan, C.P. , Bruchovsky, N. , Schweikert, H.U. , Schnabel, D. , Jones, E.C. , Sadar, M.D. , 2004. Osteoblast-derived factors induce androgen-independent proliferation and expression of prostate-specific antigen in human prostate cancer cells. Clin. Cancer Res. 10, 1860–1869. [DOI] [PubMed] [Google Scholar]
- Brizuela, L. , Ader, I. , Mazerolles, C. , Bocquet, M. , Malavaud, B. , Cuvillier, O. , 2012. First evidence of sphingosine 1-phosphate lyase protein expression and activity downregulation in human neoplasm: implication for resistance to therapeutics in prostate cancer. Mol. Cancer Ther. 11, 1841–1851. [DOI] [PubMed] [Google Scholar]
- Brizuela, L. , Cuvillier, O. , 2012. Biochemical methods for quantifying sphingolipids: ceramide, sphingosine, sphingosine kinase-1 activity, and sphingosine-1-phosphate. Methods Mol. Biol. 874, 1–20. [DOI] [PubMed] [Google Scholar]
- Brizuela, L. , Dayon, A. , Doumerc, N. , Ader, I. , Golzio, M. , Izard, J.C. , Hara, Y. , Malavaud, B. , Cuvillier, O. , 2010. The sphingosine kinase-1 survival pathway is a molecular target for the tumor-suppressive tea and wine polyphenols in prostate cancer. FASEB J. 24, 3882–3894. [DOI] [PubMed] [Google Scholar]
- Charhon, S.A. , Chapuy, M.C. , Delvin, E.E. , Valentin-Opran, A. , Edouard, C.M. , Meunier, P.J. , 1983. Histomorphometric analysis of sclerotic bone metastases from prostatic carcinoma special reference to osteomalacia. Cancer. 51, 918–924. [DOI] [PubMed] [Google Scholar]
- Cuvillier, O. , 2002. Sphingosine in apoptosis signaling. Biochim. Biophys. Acta. 1585, 153–162. [DOI] [PubMed] [Google Scholar]
- Cuvillier, O. , 2008. Downregulating sphingosine kinase-1 for cancer therapy. Expert Opin. Ther. Targets. 12, 1009–1020. [DOI] [PubMed] [Google Scholar]
- Cuvillier, O. , Mayhew, E. , Janoff, A.S. , Spiegel, S. , 1999. Liposomal ET-18-OCH(3) induces cytochrome c-mediated apoptosis independently of CD95 (APO-1/Fas) signaling. Blood. 94, 3583–3592. [PubMed] [Google Scholar]
- Cuvillier, O. , Pirianov, G. , Kleuser, B. , Vanek, P.G. , Coso, O.A. , Gutkind, S. , Spiegel, S. , 1996. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 381, 800–803. [DOI] [PubMed] [Google Scholar]
- Dayon, A. , Brizuela, L. , Martin, C. , Mazerolles, C. , Pirot, N. , Doumerc, N. , Nogueira, L. , Golzio, M. , Teissié, J. , Serre, G. , Rischmann, P. , Malavaud, B. , Cuvillier, O. , 2009. Sphingosine kinase-1 is central to androgen-regulated prostate cancer growth and survival. PLoS One. 4, e8048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festuccia, C. , Bologna, M. , Gravina, G.L. , Guerra, F. , Angelucci, A. , Villanova, I. , Millimaggi, D. , Teti, A. , 1999. Osteoblast conditioned media contain TGF-beta1 and modulate the migration of prostate tumor cells and their interactions with extracellular matrix components. Int. J. Cancer. 81, 395–403. [DOI] [PubMed] [Google Scholar]
- French, K.J. , Schrecengost, R.S. , Lee, B.D. , Zhuang, Y. , Smith, S.N. , Eberly, J.L. , Yun, J.K. , Smith, C.D. , 2003. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 63, 5962–5969. [PubMed] [Google Scholar]
- Fukuhara, S. , Simmons, S. , Kawamura, S. , Inoue, A. , Orba, Y. , Tokudome, T. , Sunden, Y. , Arai, Y. , Moriwaki, K. , Ishida, J. , Uemura, A. , Kiyonari, H. , Abe, T. , Fukamizu, A. , Hirashima, M. , Sawa, H. , Aoki, J. , Ishii, M. , Mochizuki, N. , 2012. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 122, 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave, M. , Hsieh, J.T. , Gao, C.A. , von Eschenbach, A.C. , Chung, L.W. , 1991. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 51, 3753–3761. [PubMed] [Google Scholar]
- Gomez-Brouchet, A. , Pchejetski, D. , Brizuela, L. , Garcia, V. , Altie, M.F. , Maddelein, M.L. , Delisle, M.B. , Cuvillier, O. , 2007. Critical role for sphingosine kinase-1 in regulating survival of neuroblastoma cells exposed to amyloid-beta peptide. Mol. Pharmacol. 72, 341–349. [DOI] [PubMed] [Google Scholar]
- Hisano, Y. , Kobayashi, N. , Kawahara, A. , Yamaguchi, A. , Nishi, T. , 2011. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J. Biol. Chem. 286, 1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, M. , Egen, J.G. , Klauschen, F. , Meier-Schellersheim, M. , Saeki, Y. , Vacher, J. , Proia, R.L. , Germain, R.N. , 2009. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 458, 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, M. , Kikuta, J. , Shimazu, Y. , Meier-Schellersheim, M. , Germain, R.N. , 2010. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J. Exp. Med. 207, 2793–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, S.C. , 1983. Spread of prostatic cancer to bone. Urology. 21, 337–344. [DOI] [PubMed] [Google Scholar]
- Jemal, A. , Siegel, R. , Ward, E. , Hao, Y. , Xu, J. , Thun, M.J. , 2009. Cancer statistics, 2009. CA Cancer J. Clin. 59, 225–249. [DOI] [PubMed] [Google Scholar]
- Kawahara, A. , Nishi, T. , Hisano, Y. , Fukui, H. , Yamaguchi, A. , Mochizuki, N. , 2009. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 323, 524–527. [DOI] [PubMed] [Google Scholar]
- Lang, S.H. , Miller, W.R. , Habib, F.K. , 1995. Stimulation of human prostate cancer cell lines by factors present in human osteoblast-like cells but not in bone marrow. Prostate. 27, 287–293. [DOI] [PubMed] [Google Scholar]
- Lin, D.L. , Tarnowski, C.P. , Zhang, J. , Dai, J. , Rohn, E. , Patel, A.H. , Morris, M.D. , Keller, E.T. , 2001. Bone metastatic LNCaP-derivative C4-2B prostate cancer cell line mineralizes in vitro. Prostate. 47, 212–221. [DOI] [PubMed] [Google Scholar]
- Logothetis, C.J. , Lin, S.H. , 2005. Osteoblasts in prostate cancer metastasis to bone. Nat. Rev. Cancer. 5, 21–28. [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Zhang, J. , Dai, J. , Dehne, L.A. , Mizokami, A. , Yao, Z. , Keller, E.T. , 2004. Osteoblasts induce prostate cancer proliferation and PSA expression through interleukin-6-mediated activation of the androgen receptor. Clin. Exp. Metastasis. 21, 399–408. [DOI] [PubMed] [Google Scholar]
- Maceyka, M. , Harikumar, K.B. , Milstien, S. , Spiegel, S. , 2012. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22, 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavaud, B. , Pchejetski, D. , Mazerolles, C. , de Paiva, G.R. , Calvet, C. , Doumerc, N. , Pitson, S. , Rischmann, P. , Cuvillier, O. , 2010. Sphingosine kinase-1 activity and expression in human prostate cancer resection specimens. Eur. J. Cancer. 46, 3417–3424. [DOI] [PubMed] [Google Scholar]
- Martin, C. , Lafosse, J.M. , Malavaud, B. , Cuvillier, O. , 2010. Sphingosine kinase-1 mediates androgen-induced osteoblast cell growth. Biochem. Biophys. Res. Commun. 391, 669–673. [DOI] [PubMed] [Google Scholar]
- Mendelson, K. , Evans, T. , Hla, T. , 2014. Sphingosine 1-phosphate signalling. Development. 141, 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millimaggi, D. , Festuccia, C. , Angelucci, A. , D'Ascenzo, S. , Rucci, N. , Flati, S. , Bologna, M. , Teti, A. , Pavan, A. , Dolo, V. , 2006. Osteoblast-conditioned media stimulate membrane vesicle shedding in prostate cancer cells. Int. J. Oncol. 28, 909–914. [PubMed] [Google Scholar]
- Nagahashi, M. , Kim, E.Y. , Yamada, A. , Ramachandran, S. , Allegood, J.C. , Hait, N.C. , Maceyka, M. , Milstien, S. , Takabe, K. , Spiegel, S. , 2013. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 27, 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava, V.E. , Cuvillier, O. , Edsall, L.C. , Kimura, K. , Milstien, S. , Gelmann, E.P. , Spiegel, S. , 2000. Sphingosine enhances apoptosis of radiation-resistant prostate cancer cells. Cancer Res. 60, 4468–4474. [PubMed] [Google Scholar]
- O'Brien, N. , Jones, S.T. , Williams, D.G. , Cunningham, H.B. , Moreno, K. , Visentin, B. , Gentile, A. , Vekich, J. , Shestowsky, W. , Hiraiwa, M. , Matteo, R. , Cavalli, A. , Grotjahn, D. , Grant, M. , Hansen, G. , Campbell, M.A. , Sabbadini, R. , 2009. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J. Lipid Res. 50, 2245–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera, A. , Kohama, T. , Edsall, L. , Nava, V. , Cuvillier, O. , Poulton, S. , Spiegel, S. , 1999. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J. Cell Biol. 147, 545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pchejetski, D. , Boehler, T. , Brizuela, L. , Sauer, L. , Doumerc, N. , Golzio, M. , Salunkhe, V. , Teissie, J. , Malavaud, B. , Waxman, J. , Cuvillier, O. , 2010. FTY720 (Fingolimod) sensitizes prostate cancer cells to radiotherapy by inhibition of sphingosine kinase-1. Cancer Res. 70, 8651–8661. [DOI] [PubMed] [Google Scholar]
- Pchejetski, D. , Doumerc, N. , Golzio, M. , Naymark, M. , Teissie, J. , Kohama, T. , Waxman, J. , Malavaud, B. , Cuvillier, O. , 2008. Chemosensitizing effects of sphingosine kinase-1 inhibition in prostate cancer cell and animal models. Mol. Cancer Ther. 7, 1836–1845. [DOI] [PubMed] [Google Scholar]
- Pchejetski, D. , Golzio, M. , Bonhoure, E. , Calvet, C. , Doumerc, N. , Garcia, V. , Mazerolles, C. , Rischmann, P. , Teissie, J. , Malavaud, B. , Cuvillier, O. , 2005. Sphingosine kinase-1 as a chemotherapy sensor in prostate adenocarcinoma cell and mouse models. Cancer Res. 65, 11667–11675. [DOI] [PubMed] [Google Scholar]
- Pederson, L. , Ruan, M. , Westendorf, J.J. , Khosla, S. , Oursler, M.J. , 2008. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc. Natl. Acad. Sci. U S A. 105, 20764–20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitson, S.M. , 2011. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem. Sci. 36, 97–107. [DOI] [PubMed] [Google Scholar]
- Pitson, S.M. , Moretti, P.A. , Zebol, J.R. , Lynn, H.E. , Xia, P. , Vadas, M.A. , Wattenberg, B.W. , 2003. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 22, 5491–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne, N.J. , Pyne, S. , 2010. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer. 10, 489–503. [DOI] [PubMed] [Google Scholar]
- Quarles, L.D. , Yohay, D.A. , Lever, L.W. , Caton, R. , Wenstrup, R.J. , 1992. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J. Bone Miner Res. 7, 683–692. [DOI] [PubMed] [Google Scholar]
- Rex, K. , Jeffries, S. , Brown, M.L. , Carlson, T. , Coxon, A. , Fajardo, F. , Frank, B. , Gustin, D. , Kamb, A. , Kassner, P.D. , Li, S. , Li, Y. , Morgenstern, K. , Plant, M. , Quon, K. , Ruefli-Brasse, A. , Schmidt, J. , Swearingen, E. , Walker, N. , Wang, Z. , Watson, J.E. , Wickramasinghe, D. , Wong, M. , Xu, G. , Wesche, H. , 2013. Sphingosine kinase activity is not required for tumor cell viability. PLoS One. 8, e68328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, C.K. , Andrews, L.R. , Thomas, K.G. , Tindall, D.J. , Fitzpatrick, L.A. , 1997. The effects of growth factors associated with osteoblasts on prostate carcinoma proliferation and chemotaxis: implications for the development of metastatic disease. Endocrinology. 138, 1145–1150. [DOI] [PubMed] [Google Scholar]
- Rosen, H. , Gonzalez-Cabrera, P.J. , Sanna, M.G. , Brown, S. , 2009. Sphingosine 1-phosphate receptor signaling. Annu. Rev. Biochem. 78, 743–768. [DOI] [PubMed] [Google Scholar]
- Ryu, J. , Kim, H.J. , Chang, E.J. , Huang, H. , Banno, Y. , Kim, H.H. , 2006. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 25, 5840–5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbadini, R.A. , 2011. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br. J. Pharmacol. 162, 1225–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer, L. , Nunes, J. , Salunkhe, V. , Skalska, L. , Kohama, T. , Cuvillier, O. , Waxman, J. , Pchejetski, D. , 2009. Sphingosine kinase 1 inhibition sensitizes hormone-resistant prostate cancer to docetaxel. Int. J. Cancer. 125, 2728–2736. [DOI] [PubMed] [Google Scholar]
- Schnute, M.E. , McReynolds, M.D. , Kasten, T. , Yates, M. , Jerome, G. , Rains, J.W. , Hall, T. , Chrencik, J. , Kraus, M. , Cronin, C.N. , Saabye, M. , Highkin, M.K. , Broadus, R. , Ogawa, S. , Cukyne, K. , Zawadzke, L.E. , Peterkin, V. , Iyanar, K. , Scholten, J.A. , Wendling, J. , Fujiwara, H. , Nemirovskiy, O. , Wittwer, A.J. , Nagiec, M.M. , 2012. Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem. J. 444, 79–88. [DOI] [PubMed] [Google Scholar]
- Sturge, J. , Caley, M.P. , Waxman, J. , 2011. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat. Rev. Clin. Oncol. 8, 357–368. [DOI] [PubMed] [Google Scholar]
- Visentin, B. , Vekich, J.A. , Sibbald, B.J. , Cavalli, A.L. , Moreno, K.M. , Matteo, R.G. , Garland, W.A. , Lu, Y. , Yu, S. , Hall, H.S. , Kundra, V. , Mills, G.B. , Sabbadini, R.A. , 2006. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 9, 225–238. [DOI] [PubMed] [Google Scholar]
- Wang, G. , Haile, S. , Comuzzi, B. , Tien, A.H. , Wang, J. , Yong, T.M. , Jelescu-Bodos, A.E. , Blaszczyk, N. , Vessella, R.L. , Masri, B.A. , Sadar, M.D. , 2009. Osteoblast-derived factors induce an expression signature that identifies prostate cancer metastasis and hormonal progression. Cancer Res. 69, 3433–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilbaecher, K.N. , Guise, T.A. , McCauley, L.K. , 2011. Cancer to bone: a fatal attraction. Nat. Rev. Cancer. 11, 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Fizazi, K. , Peleg, S. , Sikes, C.R. , Raymond, A.K. , Jamal, N. , Hu, M. , Olive, M. , Martinez, L.A. , Wood, C.G. , Logothetis, C.J. , Karsenty, G. , Navone, N.M. , 2001. Prostate cancer cells induce osteoblast differentiation through a Cbfa1-dependent pathway. Cancer Res. 61, 5652–5659. [PubMed] [Google Scholar]
- Zunich, S.M. , Douglas, T. , Valdovinos, M. , Chang, T. , Bushman, W. , Walterhouse, D. , Iannaccone, P. , Lamm, M.L. , 2009. Paracrine sonic hedgehog signalling by prostate cancer cells induces osteoblast differentiation. Mol. Cancer. 8, 12 [DOI] [PMC free article] [PubMed] [Google Scholar]


