Abstract
To identify molecular markers indicative of response to tamoxifen and easily implemented in the routine setting, we recently reported three gene signatures that could stratify post‐menopausal tamoxifen‐treated, estrogen receptor‐positive (ER+) patients according to outcome in the adjuvant setting. Here, we evaluated the predictive potential of the total of 14 genes included in the 3 gene signatures using 2 hormone‐naïve Dutch ER+ cohorts of a total of 285 recurrent breast cancer patients treated with first‐line tamoxifen.
mRNA levels were measured by reverse transcriptase quantitative PCR (RT‐qPCR) and the length of progression‐free survival (PFS) was used as the primary endpoint. A Mann–Whitney U test was used to select for differentially expressed genes between tumors of patients who showed or did not show progressive disease within 6 months after start of tamoxifen treatment. Cox univariate and multivariate regression analysis for PFS were used to further assess their (independent) predictive potential.
Five (BCAR3, BCL2, ESR1, IGF1R, and NCOA1) of the 14 genes analyzed showed significantly higher mRNA levels in tumors of patients who showed no disease progression within 6 months. Only BCAR3, BCL2 and NAT1 were significantly associated with a favorable PFS in multivariate analysis that included the traditional predictive factors: age, dominant relapse site, disease‐free interval, ER and progesterone receptor (PGR), and adjuvant chemotherapy.
This study shows that BCAR3, BCL2 and NAT1 in particular exhibit predictive promise regarding the efficacy of tamoxifen treatment in recurrent disease, in addition to the previously shown favorable outcome in the adjuvant setting.
Keywords: Gene expression, Breast cancer, Endocrine therapy, Tamoxifen, Progression-free survival
Highlights
We recently published a promising predictive set of 14 genes for ER+ breast cancer.
We investigated the performance of these 14 genes in a new clinical cohort.
Three genes remained significant for both the adjuvant and 1st‐line clinical setting.
These three genes were measured reliable and robust in different laboratories.
Patients may benefit from the addition of BCAR3, BCL2 and NAT1 to standard measures.
Abbreviations
- DCIS
ductal carcinoma in situ
- EMC
Rotterdam Dutch cohort
- ER
estrogen receptor
- ER+
estrogen receptor-positive
- ERBB2
HER2/ERBB2 mRNA level
- ESR1
estrogen receptor alpha mRNA level
- IDC
infiltrating ductal carcinoma
- ILC
infiltrating lobular carcinoma
- LNN
lymph node-negative
- M1
patients which developed distant metastasis within 1 month after primary surgery
- M2
patients with SSCI
- MFS
metastasis-free survival
- PFS
progression-free survival
- PGR
progesterone receptor mRNA level
- PR
progesterone receptor
- RT-qPCR
reverse transcriptase quantitative PCR
- RUMC
Nijmegen Dutch cohort
- SCCI
supraclavicular-/cervical-/contralateral axillary/parasternal-/lymph nodes positive
1. Introduction
Tamoxifen is an effective anti‐estrogen treatment for patients with estrogen receptor‐positive (ER+) breast cancer in both adjuvant and recurrent disease. However, approximately 30% of such patients receiving adjuvant tamoxifen therapy experience recurrence within 15 years, and most patients with advanced disease will eventually develop tamoxifen resistance, highlighting the need for markers to stratify these patients for optimal treatment.
We recently published a promising set of gene signatures that could stratify post‐menopausal, tamoxifen‐treated, ER+ patients according to outcome in the adjuvant setting using tumor tissues from 108 post‐menopausal breast cancer patients. The gene expression of 59 literature‐based candidate genes was investigated using reverse‐transcribed quantitative‐PCR (RT‐qPCR), and the end‐point was clinically verified as recurrence to distant organs or to the ipsilateral breast (Lyng et al., 2013). The data revealed three gene signatures consisting of a total of 14 genes (AKT1, BCAR3, BCL2, CDKN1A, CGA, EGFR, ESR1, IGF1R, NAT1, NCOA1, NRG1, PRKCD, PRKCE and TFF1). The strongest prediction of outcome (75% accuracy) was obtained with a 2‐gene combination of BCL2 and CDKN1A and confirmed by independent examination of 4 previously‐reported microarray datasets of tamoxifen‐treated patient samples in the adjuvant setting (n = 503)(Chanrion et al., 2008; Loi et al., 2008; Ma et al., 2004; Zhang et al., 2009). The predictive value was further validated by comparing the ability of the genes to predict recurrence in an additional, previously‐published, cohort (Loi et al., 2007) consisting of both adjuvant tamoxifen‐treated and untreated patients (Lyng et al., 2013).
In an adjuvant setting such as the original study (Lyng et al., 2013), a marker might predict a tumors response to tamoxifen, but also inevitably addresses the tumors intrinsic aggressiveness (Ma et al., 2004). This is not a significant issue in the recurrent setting or in PFS analysis if a correction is made for disease‐free interval (time between surgery and the occurrence of metastasis), thus favoring this clinical set‐up for the discovery or validation of predictive markers regardless of their prognostic value.
In the current study, we investigated the performance of the 14 genes in a new clinical setting (recurrent vs. previously adjuvant) as well as in an independent laboratory, each using different sample handling and technical protocols. This study comprises a combined analysis of 285 endocrine treatment‐naïve ER+ breast cancer patients from two independent clinical cohorts (Rotterdam [EMC; n = 225] and Nijmegen [RUMC; n = 60]) in which hormone‐naïve patients at the time of clinically‐detected metastasis received tamoxifen as first‐line therapy, and for whom disease status was monitored during treatment. The main end‐point for this study was defined by the length of PFS.
2. Methods and materials
2.1. Patients
In this retrospective study, coded primary tumor tissues were used in accordance with the Code of Conduct of the Federation of Medical Scientific Societies in the Netherlands (http://www.federa.org/codes‐conduct). In the years the tumors were collected (1981–1996), tumor ER and PR levels were routinely determined in cytosolic extracts by ligand binding assay or enzyme immunoassay (Foekens et al., 1989). The cut‐off classifying primary breast tumors as ER‐ and/or PR‐positive was 10 fmol/mg cytosolic protein. The following inclusion and exclusion criteria were used: 1) Only female patients with measurable disease were evaluated; 2) all patients had undergone primary treatment (surgery with or without radiotherapy) for breast cancer; 3) only patients with ER protein‐positive primary tumors who received tamoxifen as first‐line therapy were included; 4) patients with residual disease diagnosed within 1 month after primary surgery were excluded; 5) patients with non‐invasive breast cancer or those who received neo‐adjuvant therapy or were exposed to hormonal adjuvant treatment were excluded; 6) patients with no response who were still alive or patients that stopped therapy for reasons other than progression (e.g. subjective or objective toxicity) were excluded if their follow‐up period during tamoxifen treatment was three months or less; 7) patients who previously experienced another cancer (except basal cell skin cancer or early‐stage cervical cancer stage Ia/Ib) were excluded; and 8) patients with over 100 mg frozen primary tumor samples yielding good quality RNA from sections containing at least 30% of the nuclei epithelial tumor cell origin and distributed uniformly over at least 70% of the section area were included.
2.2. Cohort description and clinical end point
The protocol to study biological markers associated with disease outcome was approved by the Medical Ethics Committee of the Erasmus Medical Center Rotterdam, The Netherlands (MEC 02.953). Of the 285 patients (n = 225 from Rotterdam (EMC) and n = 60 from Nijmegen (RUMC), 32 presented with distant metastasis at diagnosis or developed distant metastasis (including supraclavicular lymph node metastasis) within 1 month after primary surgery (M1‐patients). These 32 patients and the 253 patients who developed recurrence during follow‐up [25 patients with local‐regional relapse (LRR), 228 with distant metastasis] received tamoxifen as first‐line therapy. All patients were ER+ and endocrine therapy‐naive, while 38 received adjuvant chemotherapy. The median time between primary surgery and initiation of therapy was 24 months (range, 0–120 months). The median follow‐up of patients alive after primary surgery was 95 months (range, 9–240 months), and 45 months (range, 3–178 months) after start of first‐line tamoxifen therapy. For 182 patients (64%), disease progression was controlled by tamoxifen for at least 6 months after initiation of therapy. At the end of the follow‐up period, 268 (94%) patients had developed tumor progression and 222 (78%) had died. Relevant additional details on patient and tumor characteristics are summarized in Table 1. Details of the Rotterdam (EMC) and the Nijmegen (RUMC) Dutch cohorts are available online (Supplemental File 1). Postoperative follow‐up involved standard routine examinations as previously described (Martens et al., 2005; Meijer‐van Gelder et al., 2004). The date of diagnosis of recurrence was defined as that at confirmation of metastasis after symptoms reported by the patient or after detection of clinical signs at regular follow‐up. To evaluate PFS, the start of first‐line tamoxifen therapy was set at zero and the end point at the time of progression or the last date of follow‐up.
Table 1.
Clinical and biological factors of the patients and tumors used in the study.
| Clinical and biological factors | No. of patientsa | PFS ≤ 6 months | PFS > 6 months | P |
|---|---|---|---|---|
| All patients | ||||
| PFS ≤ 6 months | 102 | 102 | 0 | |
| PFS > 6 months | 182 | 0 | 182 | |
| Age at primary surgery | ||||
| ≤50 years | 79 | 31 | 48 | 0.37b |
| >50 years | 205 | 71 | 134 | |
| Age at start 1st line Tamoxifen | ||||
| ≤50 years | 59 | 25 | 34 | 0.16b |
| >50 years | 225 | 77 | 148 | |
| Menopausal status at primary surgery | ||||
| Pre‐menopausal | 65 | 29 | 36 | 0.07c |
| Post‐menopausal | 199 | 64 | 135 | |
| Peri‐menopausal | 11 | 6 | 5 | |
| Chirurgy primary tumor | ||||
| Ablatio | 177 | 59 | 118 | 0.25c |
| Lumpectomy | 107 | 43 | 64 | |
| Axillary dissection | ||||
| No | 19 | 9 | 10 | 0.32c |
| Yes | 265 | 93 | 172 | |
| Tumor grade | ||||
| Good/moderate | 51 | 15 | 36 | 0.24c |
| Poor | 148 | 58 | 90 | |
| Tumor size primary tumor | ||||
| ≤2 cm | 83 | 34 | 49 | 0.17b |
| >2–≤5 cm | 159 | 52 | 107 | |
| >5 cm + pT4 | 36 | 14 | 22 | |
| Unknown | 6 | 2 | 4 | |
| Histology primary tumor | ||||
| IDC | 177 | 64 | 113 | 0.53c |
| ILC | 27 | 8 | 19 | |
| IDC + ILC | 5 | 1 | 4 | |
| Nodal status primary tumor | ||||
| N0, no positive lymph nodes | 131 | 42 | 89 | 0.31c |
| N1 + N2, positive lymph nodes | 136 | 52 | 84 | |
| M‐stage primary tumor | ||||
| M0, no distant metastases present | 252 | 88 | 164 | 0.69c |
| M1, distant metastases present | 30 | 12 | 18 | |
| M2, SCCI | 2 | 2 | 0 | |
| ESR1 primary tumor | ||||
| Median | 6.03 | 5.00 | 6.55 | 0.002b |
| Interquartile range | 8.54 | 7.72 | 9.98 | |
| PGR primary tumor | ||||
| Median | 0.75 | 0.48 | 1.17 | 0.006b |
| Interquartile range | 2.78 | 1.96 | 3.57 | |
| ERBB2 primary tumor | ||||
| Median | 3.79 | 3.54 | 3.87 | 0.82b |
| Interquartile range | 5.19 | 5.37 | 5.20 | |
| Adjuvant systemic therapy | ||||
| None | 246 | 91 | 155 | 0.37c |
| Chemotherapy | 38 | 11 | 27 | |
| Endocrine therapy | 0 | 0 | 0 | |
| Adjuvant radio therapy | ||||
| No | 96 | 24 | 72 | 0.02c |
| Yes | 161 | 64 | 97 | |
| Disease‐free interval | ||||
| ≤1 yr | 78 | 42 | 36 | <0.001b |
| 1–3 yr | 113 | 38 | 75 | |
| >3 yr | 93 | 22 | 71 | |
| Dominant site of relapse | ||||
| Soft | 26 | 7 | 19 | 0.46c |
| Bone | 152 | 59 | 93 | |
| Viscera | 106 | 36 | 70 | |
Because of others and unknowns, numbers do not always add up to 284.
Abbreviations: EMC, Rotterdam Dutch cohort; RUMC, Nijmegen Dutch cohort; PFS, progression‐free survival; ESR1, estrogen receptor alpha mRNA level; PGR, progesterone receptor mRNA level; ERBB2, HER2/ERBB2 mRNA level; IDC, infiltrating ductal carcinoma; ILC, infiltrating lobular carcinoma; DCIS, ductal carcinoma in situ; SCCI, supraclavicular ‐/cervical ‐/contralateral axillary/parasternal‐/lymph nodes positive.
One patient, who was followed for only 3 months after start of first‐line tamoxifen treatment and showed no signs of recurrent disease during this time‐frame, was excluded for this analysis.
P for Mann–Whitney U test (2‐tailed).
P Fisher Exact Probability Test (2‐tailed).
2.3. RNA Extraction, cDNA synthesis and quantification by RT‐qPCR
Tissues from Nijmegen RUMC were transferred on dry ice to the Rotterdam EMC laboratory for processing. Detailed procedures for tissue processing, RNA extraction, cDNA synthesis and quantification of mRNA transcripts by RT‐qPCR have been described (Sieuwerts et al., 2005). In brief, only tumor tissues containing at least 30% invasive tumor cell nuclei were processed with RNA Bee (Tel Test, Thermo Fisher Scientific Inc.). After cDNA synthesis with the RevertAid H Minus First Strand cDNA Synthesis Kit from Thermo Fisher Scientific Inc, followed by an RNAse H step (Ambion, Life Technologies) to degrade the remaining RNA, qPCR reactions were performed using a Mx3000PTM Real‐Time PCR System (Agilent, Amsterdam, The Netherlands). PCR reactions were done in a final volume of 25 μL containing cDNA synthesized from 5 to 15 ng of total RNA. SYBR‐based assays were performed with 330 nM forward and reverse primer and 12.5 μL Absolute™ QPCR SYBR® Green mastermix containing ROX (Abgene Limited, Epsom, UK). After 15 min of denaturation and activation of the Taq‐DNA polymerase, PCR products were amplified in 35 cycles with 15 s of denaturing at 95 °C, 30 s of annealing at 62 °C, followed by data acquisition at 72 °C and 79 °C. For the Taqman Gene Expression assays from Applied BioSystems, all performed with Absolute™ QPCR Universal mastermix containing ROX from Abgene, PCR settings were as recommended by the manufacturer. Validations to ensure PCR specificity were done as described (Sieuwerts et al., 2005). In brief, when amplification rounds for a specific target exceeded 35 cycles for the SYBR‐based assays and 40 cycles for the Taqman Gene Expression assays, quantities were considered to be undetectable and were arbitrarily set at 50% of the lowest expression level measurable at the quantification detection threshold (Cq = 0.01). In addition to a negative genomic DNA control sample, a standard curve of a serially‐diluted cDNA breast tumor pool sample was included in each PCR plate to control PCR efficiency and harmonize the data between plates. Concentrations of target genes, expressed relative to the Dutch reference gene set consisting of hydroxymethylbilane synthase (HMBS), hypoxanthine phosphoribosyltransferase 1 (HPRT1) and TATA‐box binding protein (TBP), were quantified as follows: dCq mRNA target = 2Cq reference gene set−Cq target gene. To analyze the predictive value of gene combinations, we first centered dCq levels on zero and then added the dCq values of the individual genes in the gene combinations divided by the final number of genes in the combination. All primer sequences and Taqman Gene Expression assays are listed in Supplemental File 2.
2.4. Statistics
For statistical computations, STATA statistical package 12 (STATACorp, College Station, TX) and SPSS (IBM) version 20 were used. Differences in levels were assessed with the Mann–Whitney U test and between categorized variables with the Fisher Exact Probability Test, both using patient and tumor characteristics as grouping variables. The strength of associations between continuous variables was tested with the Spearman rank correlation (rs). The Cox proportional hazard model was used to calculate the hazard ratio (HR) and 95% confidence interval (CI) in the analyses of PFS, the latter defined as the time elapsed between the start of tamoxifen treatment for recurrent disease and the first detection of progressive disease. Visual inspection of the log minus log Cox regression plots was used to check the proportional hazards assumption. The Shapiro–Wilk test was used to test for normal distribution after log transformation. All P‐values are two‐sided and P < 0.05 was considered statistically significant.
3. Results
3.1. Reproducibility measuring the 14 genes in the independent Dutch laboratory
The RT‐qPCR reproducibility of the 14 genes comprising the 3 gene signature sets identified in the original study (Figure 1A) (Lyng et al., 2013) was investigated by transferring RNA of 10 patients stored since the original study approximately 6 years ago to the Dutch reference laboratory (EMC). At the original Danish site, cDNA synthesis, qPCR and settings were conducted as previously described (Lyng et al., 2013). In brief, qPCR was conducted in 96‐well plates containing a total volume of 25 μL (vs. 384‐well previously with 2 μL volume reactions). In the present study, cDNA synthesis, qPCR and settings were performed according to EMC's standard operation procedures (Sieuwerts et al., 2005) for kits, gene expression assays and reference gene set to normalize the data. As only 10 RNA samples were compared, a test for normality could not be conducted and non‐parametric Spearman Rank correlation tests were therefore performed to compare gene expression levels in these 10 RNA samples as measured in both laboratories. Despite the different settings, data correlated well (dCq Danish = 0.91 × dCq Dutch − 1.2, R2 = 0.64, n = 140, P < 0.0001, Figure 1B) and the RT‐qPCR assays were efficient (median 100%, range 94%–102%, Supplemental File 2). Specifics of the gene expression assays used in both laboratories and the resulting Spearman rank correlation coefficients and P‐values are given in Supplemental File 2.
Figure 1.
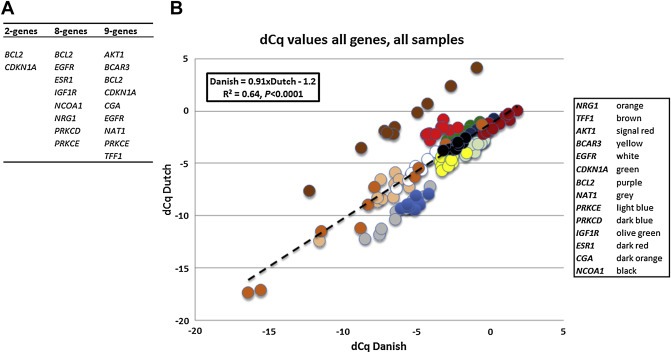
Reproducibility measuring the 14 genes in 10 RNA samples in the Danish and Dutch laboratories. A: Origin of the 14 gene transcripts studied. The 2‐, 8‐ and 9‐gene signatures were previously identified by various statistical analyses for the adjuvant setting (Lyng et al., 2013). B: Data of all 14 genes, measured in both the Dutch and Danish laboratories in 10 different clinical RNA samples. For both, data after normalization with the in‐house reference gene sets (dCq) are plotted. Note that only the values for TFF1 (brown dots) show a large range of values with high concordance between the laboratories, but with a relatively higher expression if measured with the Dutch gene expression assay.
3.2. Predictive value of the genes identified in the adjuvant setting translated to the recurrent setting
Similar to the original study in the adjuvant setting (Lyng et al., 2013), we first used single gene analysis to identify the genes most significantly (P < 0.05) associated with PFS in the recurrent setting. According the Shapiro–Wilk test, only the primary tumor levels of BCAR3, BCL2, NCOA1 and PRKCD showed normal distributions after log transformation. Therefore, we continued to use non‐parametric analyses, which showed that 5 of the 14 genes were significantly differentially expressed in tumors of patients with a short PFS (≤6 months) compared with those with a longer PFS (>6 months) (Table 2).
Table 2.
Predictive value of the 14 genes in the recurrent setting.
| Gene | P‐value∗/∗∗ | Higher expressed in |
|---|---|---|
| BCL2 | 9.4E‐04 | PFS > 6 months |
| ESR1 | 3.3E‐03 | PFS > 6 months |
| IGF1R | 8.8E‐03 | PFS > 6 months |
| BCAR3 | 2.3E‐02 | PFS > 6 months |
| NCOA1 | 4.9E‐02 | PFS > 6 months |
| EGFR | 3.1E‐01 | |
| NRG1 | 3.2E‐01 | |
| TFF1 | 3.5E‐01 | |
| PRKCE | 3.7E‐01 | |
| PRKCD | 3.8E‐01 | |
| CDNK1A | 4.8E‐01 | |
| AKT1 | 7.1E‐01 | |
| NAT1 | 7.2E‐01 | |
| CGA | 9.3E‐01 | |
| 2‐gene | 1.1E‐01 | |
| 8‐gene | 7.4E‐03 | PFS > 6 months |
| 9‐gene | 1.3E‐01 |
One hundred and two 102 patients showed progression of disease within 6 months after start of therapy, 182 patients showed no disease progression in this time frame. One patient, who was followed for only 3 months after start of tamoxifen treatment and showed no signs of recurrent disease during this time‐frame, was excluded for this analysis.
Grouping Variable: PFS > 6 months vs ≤ 6 months.
Kruskal–Wallis test.
Note that 4 of the remaining 9 non‐informative genes (AKT1, CGA, NAT1 and TFF1) were also not identified as being significantly associated with clinical outcome at the single gene level in the original study on adjuvant tamoxifen. These genes were part of the 2‐, 8‐ and 9‐gene prognostic tamoxifen signatures identified by various statistical analyses (Lyng et al., 2013). Only the 8‐gene signature, which harbors 4 of the 5 genes shown to be significant at the single gene level in the recurrent setting, was also significant in the recurrent setting (P = 0.0074), but this 8‐gene signature did not outperform BCL2 (P = 0.0009) as a single marker (Table 2). For the significant genes shown in Table 2, we divided the levels in 2 and 4 equal parts and, in an exploratory analysis, we calculated the positive and negative predictive values for the various quarter combinations. The results summarized in Table 3 show that high levels of the genes were more likely to predict a favorable clinical outcome.
Table 3.
Performance of the identified genes in the recurrent setting to predict outcome on tamoxifen therapy.
| Gene | Groups tested | PFS > 6 months | PPV | NPV | |
|---|---|---|---|---|---|
| Specificity | Sensitivity | ||||
| BCL2 | Q4 high vs Q1‐Q3 low | 88.2% | 32.4% | 83.1% | 42.3% |
| Q3/4 high vs Q1/2 low | 60.8% | 55.5% | 71.6% | 43.4% | |
| Q2‐Q4 high vs Q1 low | 31.4% | 76.9% | 66.7% | 43.2% | |
| ESR1 | Q4 high vs Q1‐Q3 low | 81.4% | 28.6% | 73.2% | 39.0% |
| Q3/4 high vs Q1/2 low | 54.9% | 52.7% | 67.6% | 39.4% | |
| Q2‐Q4 high vs Q1 low | 37.3% | 80.8% | 69.7% | 52.1% | |
| IGF1R | Q4 high vs Q1‐Q3 low | 82.4% | 29.1% | 74.6% | 39.4% |
| Q3/4 high vs Q1/2 low | 57.8% | 53.8% | 69.5% | 41.3% | |
| Q2‐Q4 high vs Q1 low | 32.4% | 78.0% | 67.3% | 45.2% | |
| BCAR3 | Q4 high vs Q1‐Q3 low | 82.4% | 29.1% | 74.6% | 39.4% |
| Q3/4 high vs Q1/2 low | 55.9% | 53.3% | 68.3% | 40.1% | |
| Q2‐Q4 high vs Q1 low | 31.4% | 78.0% | 67.0% | 44.4% | |
| NCOA1 | Q4 high vs Q1‐Q3 low | 81.4% | 27.5% | 72.5% | 38.6% |
| Q3/4 high vs Q1/2 low | 57.8% | 53.3% | 69.3% | 41.0% | |
| Q2‐Q4 high vs Q1 low | 32.4% | 78.6% | 67.5% | 45.8% | |
%PPV; percentage of patients with a positive test who had not progressed within 6 months.
%NPV: percentage of patients with a negative test who did progress within 6 months.
PFS; progression‐free survival.
Next, after stratification for study cohort, we performed Cox univariate and multivariate regression analyses for PFS as a function of the mRNA levels of the significant genes. High mRNA levels of ESR1, BCAR3, IGF1R and BCL2, but not NCOA1, were significantly associated with a favorable PFS (Table 4). Of the genes not identified as significantly associated with progression of disease within 6 months after start of therapy (Table 2), only mRNA levels of NAT1 were in addition significantly associated with PFS in this analysis (Table 4). Moreover, in Cox multivariate regression analysis, high BCL2 and NAT1 mRNA levels, when added separately to a base model that included the traditional predictive factors and adjuvant chemotherapy, were significantly associated with a favorable PFS (Table 4). BCL2 was also significant when analyzed as a continuous variable in both Cox univariate (HR = 0.75, 95% CI: 0.66–0.86, P < 0.0001) and multivariate (HR = 0.80, 95% CI: 0.69–0.93, P = 0.0033) regression analyses. The association of BCL2 (the 25% highest levels, Q4, versus Q1‐Q3) and NAT1 (the 75% highest levels, Q1‐Q3, versus Q4) with PFS is visualized in Kaplan–Meier curves (Figure 2). In addition to ESR1, which was already included in the base model, BCAR3 significantly added to the multivariate model, which is not surprising since most of the EMC samples analyzed in the present study were also used in our original study describing the predictive value of BCAR3 ( van Agthoven et al., 2009a ). In Table 4, only the dichotomized data resulting in the most significant associations of the different genes with PFS are shown. Data for all dichotomized cut‐off values based on the distribution of the mRNA values in quarters, including the genes not significantly differentially expressed in tumors of patients with a short PFS (≤6 months) compared with those with a longer PFS (>6 months), are shown in Supplemental File 3A.
Table 4.
Cox univariate and multivariate analysis for PFS in the recurrent setting.
| Factor | No. of patient | Univariate analysisa | Multivariate analysisa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||||
| Base model | |||||||||
| Age at start 1st line Tamoxifen | |||||||||
| ≤50 years | 59 | 1 | 1 | ||||||
| 50–70 years | 150 | 0.94 | 0.69 | 1.28 | 0.98 | 0.70 | 1.39 | ||
| >70 years | 76 | 0.85 | 0.60 | 1.22 | 6.7E‐01 | 0.94 | 0.63 | 1.40 | 9.5E‐01 |
| Disease‐free interval | |||||||||
| ≤1 yr | 78 | 1 | 1 | ||||||
| 1–3 yr | 113 | 0.71 | 0.52 | 0.96 | 0.65 | 0.47 | 0.89 | ||
| >3 yr | 94 | 0.54 | 0.40 | 0.75 | 1.0E‐03 | 0.50 | 0.36 | 0.69 | 1.6E‐04 |
| Dominant site of relapse | |||||||||
| Soft | 27 | 1 | 1 | ||||||
| Bone | 152 | 1.63 | 1.02 | 2.62 | 1.66 | 1.03 | 2.69 | ||
| Viscera | 106 | 1.54 | 0.95 | 2.50 | 9.7E‐02 | 1.78 | 1.08 | 2.92 | 7.2E‐02 |
| ESR1 primary tumor | |||||||||
| Q1/2 low | 143 | 1 | 1 | ||||||
| Q3/4 high | 142 | 0.78 | 0.61 | 1.00 | 4.9E‐02 | 0.76 | 0.58 | 0.98 | 3.6E‐02 |
| PGR primary tumor | |||||||||
| Q1/2 low | 142 | 1 | 1 | ||||||
| Q3/4 high | 143 | 0.73 | 0.57 | 0.93 | 1.0E‐02 | 0.72 | 0.57 | 0.93 | 1.0E‐02 |
| Adjuvant chemotherapy | |||||||||
| No | 247 | 1 | 1 | ||||||
| Yes | 38 | 0.98 | 0.68 | 1.40 | 9.1E‐01 | 1.02 | 0.69 | 1.52 | 9.1E‐01 |
| Additions to the base modelb | |||||||||
| BCL2 primary tumor | |||||||||
| Q1–Q3 low | 214 | 1 | 1 | ||||||
| Q4 high | 71 | 0.60 | 0.44 | 0.82 | 1.3E‐03 | 0.67 | 0.49 | 0.93 | 1.7E‐02 |
| BCAR3 primary tumor | |||||||||
| Q1–Q3 low | 214 | 1 | 1 | ||||||
| Q4 high | 71 | 0.66 | 0.50 | 0.88 | 4.5E‐03 | 0.69 | 0.51 | 0.92 | 1.1E‐02 |
| NAT1 primary tumor | |||||||||
| Q1 low | 73 | 1 | 1 | ||||||
| Q1–Q3 high | 212 | 0.68 | 0.51 | 0.89 | 5.8E‐03 | 0.70 | 0.52 | 0.94 | 1.9E‐02 |
| IGF1R primary tumor | |||||||||
| Q1 low | 73 | 1 | 1 | ||||||
| Q1‐Q3 high | 212 | 0.68 | 0.51 | 0.91 | 8.1E‐03 | 0.77 | 0.57 | 1.06 | 1.1E‐01 |
| NCOA1 primary tumor | |||||||||
| Q1–Q3 low | 214 | 1 | |||||||
| Q4 high | 71 | 0.79 | 0.59 | 1.06 | 1.2E‐01 | ||||
| Combined | |||||||||
| 1 | |||||||||
| BCL2 Q4 high | 71 | 0.70 | 0.50 | 0.97 | 3.0E‐02 | ||||
| BCAR3 Q4 high | 71 | 0.73 | 0.55 | 0.99 | 3.9E‐02 | ||||
| NAT1 Q1–Q3 high | 212 | 0.70 | 0.52 | 0.95 | 2.0E‐02 | ||||
| 3 genes combined | 20 | 0.45 | 0.26 | 0.78 | 4.0E‐03 | ||||
Stratified for study cohort.
Factors were separately introduced to the base multivariate model that included the following factors: age, disease‐free interval, dominant site of relapse, adjuvant chemotherapy and ER and PGR mRNA levels.
Figure 2.
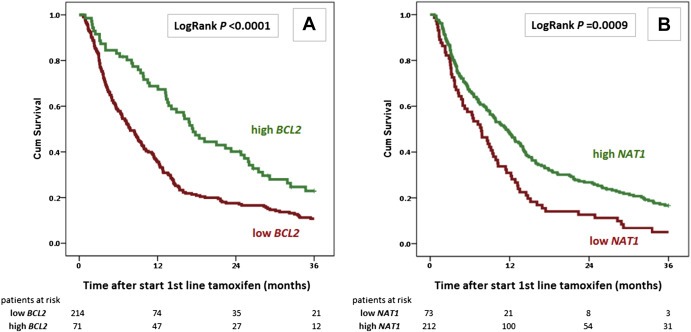
Kaplan–Meier curve for PFS as a function of BCL2 and NAT1 mRNA levels. Progression‐free survival (PFS) of 285 ER–positive breast cancer patients with recurrent disease treated with first‐line tamoxifen monotherapy. A: High BCL2, the 25% highest mRNA values; low BCL2, the 75% lowest mRNA values. B: High NAT1, the 75% highest mRNA values; low NAT1, the 25% lowest mRNA values. Patients at risk at 12‐month intervals and LogRank P‐values are indicated.
Finally, data for post‐menopausal patients only (n = 200) are shown in Supplemental File 3B, as this was the cohort studied in the adjuvant setting in the original study (Lyng et al., 2013). Restricting the analyses to post‐menopausal patients did only significantly alter the predictive outcome of our univariate findings as presented in Table 4 for 2 markers. For the sub cohort containing only post‐menopausal patients, also CGA and TFF1 were significant in both the univariate (Supplemental File 3B) and the multivariate setting (HR Q4 versus Q1‐Q3 for CGA; 1.49, 95% CI: 1.04–2.13, P = 0.031 and for TFF1: 0.69, 95% CI: 0.48–0.98, P = 0.037).
4. Discussion
In this study, we evaluated whether 14 genes previously identified in an adjuvant setting to be predictive of outcome with tamoxifen therapy could also be of clinical relevance in the advanced setting. In the adjuvant setting, in which the signatures were constructed, patients who did not receive tamoxifen were included as a control group (Lyng et al., 2013). In the advanced setting, however, patients cannot be withheld therapy and a control group can never be included. All the patients we studied were adjuvant hormonal therapy naïve and already had experienced a distant metastasis. Thus, all these patients were per definition poor prognosis cases and as such not a cohort suitable to study prognostic markers able to differentiate between good and poor prognosis patients. Furthermore, to correct for potential prognostic associations of markers, we also corrected for the length of disease‐free interval. We should realize that the molecular characteristics of the primary tumor may differ from those of the metastases and that ideally the metastatic tumors that need treatment should have been analyzed. However, obtaining biopsies from (all) available metastases is a cumbersome and painful procedure for the patients, and frequently not possible. Currently, in clinical practice the characteristics of the primary tumor (ER, PR and HER2 status) are used to guide treatment decisions in metastatic disease, although this is slowly changing as clinicians realize that better treatment decisions can be obtained by analyzing the metastasis themselves. Possibly in the future the molecular characterization of circulating tumor cells or cell‐free DNA will be feasible, such that treatment decisions of metastatic patients can be based on the molecular characteristics and molecular heterogeneity of the various metastases.
Current prognostic and predictive marker profiles for endocrine treatment of ER‐positive breast cancer seem to differ as to greater or lesser efficacy in high or low risk patients as well as to whether the marker profile predicts outcome over the short (1–5 year) or long term (over 5 years) (Dowsett et al., 2013; Sestak et al., 2013; Sgroi et al., 2013). Since certain genes may be useful only in a subpopulation of tamoxifen‐treated ER‐positive breast cancer patients, it was not surprising that not all genes identified as useful in the adjuvant setting were also informative in the recurrent setting. This was also demonstrated by our finding that low levels of CGA and high levels of TFF1 were only significantly associated with favorable PFS in this advanced setting when analyzed in the sub cohort of post‐menopausal patients only. Importantly, we found BCL2 and NAT1 to be predictive in a multivariate recurrent setting, together with ESR1 and BCAR3, which have previously been shown to be associated with a favorable outcome in tamoxifen therapy in the recurrent setting (van Agthoven et al., 2009a). Note that high levels of these genes were all more likely to correctly predict favorable versus unfavorable outcomes (Table 3). This might render these genes less clinically relevant when seeking markers predictive of poor response in patients who might benefit from other therapies. Nevertheless, stratification options to specifically select patients who are likely to respond favorably to tamoxifen may protect these patients from overtreatment. This is especially true in view of a recent meta‐analysis study showing that extended adjuvant tamoxifen is not associated with significantly reduced recurrence in unselected patients (Al‐Mubarak et al., 2014), demonstrating the need for better stratification.
To investigate whether the lack of association with PFS in the recurrent setting for the other genes was not simply due to an association of the genes with intrinsic aggressive behavior in the adjuvant setting, we also analyzed the pure prognostic value of our candidate genes. Affymetrix U133 microarray data of the single genes showed that 2 of the 13 genes present on the platform (BCAR3 and BCL2) were also significantly (P < 0.05) associated with shorter metastasis‐free survival (MFS) in our previously published untreated lymph node‐negative (LNN) ER+ cohort (Smid et al., 2008). No correlation with MFS was found for the other genes (Supplemental File 4), indicating that the lack of association with PFS in the recurrent setting for the other genes was not due to association with intrinsic aggressive behavior in the adjuvant setting. Indeed, all genes evaluated were selected based on the literature and their relation with tamoxifen treatment outcome. Genes uniformly identified to be associated with prognosis and solely with the ER+ phenotype were excluded to ensure a focus on genes that might prove to be purely predictive (Lyng et al., 2013). BCAR3, BCL2, ESR1 and NAT1 are therefore well‐documented in the field of prognostic and predictive markers.
Several studies have implicated high levels of the anti‐apoptotic oncogene BCL2 as a marker for active ER‐signaling, thus more likely to respond to anti‐estrogen therapies (Henriksen et al., 2009; Larsen et al., 2012). BCL2 is one of the 21 gene Oncotype DX RT‐qPCR assay used to predict which patients with LNN ER+ disease would benefit from adjuvant tamoxifen and/or chemotherapy (Albain et al., 2010; Paik, 2007; Paik et al., 2006). BCL2 is also part of the PAM50 gene set, a microarray‐based risk predictor for breast cancer based on intrinsic subtypes (Parker et al., 2009). A large analysis of data from 7230 primary breast cancers showed that BCL2 as a single marker had a strong influence on the established prognostic models, including the St. Gallen, the Nottingham Prognostic Index and the TNM models. Favorable clinicopathologic features and a strong correlation with ER and PR were suggested as the causes of superior survival in patients with BCL2‐positive breast cancer (Hwang et al., 2012). More specifically, Vaillant and colleagues demonstrated recently that targeting BCL2 with the BH3 mimetics ABT‐737 improves the response of xenografts from primary ER+ breast tumors to endocrine therapy and reduces tamoxifen‐induced endometrial hyperplasia, a strategy with potential clinical applicability utilizing BCL2 as a companion marker (Vaillant et al., 2013).
NAT1 was one of the 3 genes in the optimal 3‐gene combination that consisted of BCL2‐CDKN1A‐NAT1 in the adjuvant setting (Lyng et al., 2013). In addition, low levels of this gene have been indicated before in relation to tamoxifen resistance in the adjuvant setting and suggested NAT1 as a new ER‐responsive gene for breast cancer (Bieche et al., 2004; Kim et al., 2010). NAT1 (N‐acetyltransferase 1) encodes for a phase II drug‐metabolizing enzyme, which plays a role in the metabolism of tamoxifen (Bieche et al., 2004). It's mode of action therefore differs from that of BCL2. NAT1 is often methylated, resulting in a down‐regulated expression (as also observed in our studies), in tamoxifen‐resistant tumors compared to control cancers (Kim et al., 2010).
BCAR3 (breast cancer anti‐estrogen resistance gene 3) was identified in a search for genes involved in the development of estrogen resistance (van Agthoven et al., 1998; van Agthoven et al., 2009b). However, and in contrast to the function of BCL2, estrogen independence mediated by BCAR3 is transmitted through mechanisms distinct from the ER‐signaling pathway (Dorssers et al., 2005). BCAR3, as part of an intracellular signal transduction pathway that causes estrogen‐independent proliferation in human breast cancer cells, promotes cell motility and adhesion, processes required for cells to become metastatic (Oh et al., 2013; Wilson et al., 2013). Additional studies from our group demonstrated that high levels of BCAR3 were associated with clinical benefit and prolonged PFS (van Agthoven et al., 2009a), which has now been validated in a larger cohort for both the adjuvant (Danish cohort) and the recurrent settings (Dutch cohorts, which also include the original cohort used to identify BCAR3 as a predictive marker). Of note, high levels of BCAR3, BCL2 and NAT1 were also significantly associated with favorable PFS (P = 0.039, P = 0.030 and P = 0.020, respectively) when combined in the Cox multivariate regression analysis (Table 4), indicating their modes of action with respect to outcome on tamoxifen are indeed at least partly independent.
In summary, this study shows that BCL2, BCAR3 and NAT1, three genes with different modes of action, exhibit potential to predict favorable outcome of tamoxifen therapy in both the adjuvant and recurrent settings. The predictive power of the genes persisted despite different clinical settings (adjuvant vs. first‐line treatment, menopausal status, and adjuvant chemotherapy), clinical sampling (countries, laboratories) and molecular assays (cDNA synthesis protocol, qPCR assays, and normalization procedures). This study provides support for the findings of several other studies (Albain et al., 2009; Hwang et al., 2012; Kerr and Wittliff, 2011; Kolacinska et al., 2012; Linke et al., 2006; Lyng et al., 2013; Mangerini et al., 2012; Nehra et al., 2010; Paik, 2007; Sgroi et al., 2013; Tozlu et al., 2006; Wang et al., 2009) indicating that BCL2 is a biomarker for endocrine response. The addition of BCL2 (and NAT1 and BCAR3) to standard biological measures might be considered for future hormone receptor‐positive clinical studies.
Author contributions
AMS, MBL and VW performed the laboratory work.
AMS analyzed the data and wrote the manuscript.
MMvG curated the clinical data.
FS, JF, PS and JM provided the clinical specimens.
AMS and MBL designed the experiments.
AMS, MBL and HJD coordinated the study.
AMS, MBL, MMvG, FS, JF and HJD critically discussed the data and revised the manuscript.
All authors read and approved the final manuscript.
Conflicts of interest
The authors declare that they have no competing interests.
Supporting information
The following is the supplementary data related to this article:
Supplementary data
Acknowledgments
A.M.S. was supported by Cancer Genomics Netherlands (CGC.nl) and a grant for the Netherlands Organization of Scientific Research (NWO). M.B.L. and H.J.D. were supported by Danish Cancer Society, Danish Research Council, Sino‐Danish Breast Cancer Research Centre, Danish Center for Translational Breast Cancer Research (DCTB) and A Race Against Breast Cancer.
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2014.07.003.
Sieuwerts Anieta M., Lyng Maria B., Meijer-van Gelder Marion E., de Weerd Vanja, Sweep Fred C.G.J., Foekens John A., Span Paul N., Martens John W.M., Ditzel Henrik J., (2014), Evaluation of the ability of adjuvant tamoxifen‐benefit gene signatures to predict outcome of hormone‐naive estrogen receptor‐positive breast cancer patients treated with tamoxifen in the advanced setting, Molecular Oncology, 8, doi: 10.1016/j.molonc.2014.07.003.
References
- Al-Mubarak, M. , Tibau, A. , Templeton, A.J. , Cescon, D.W. , Ocana, A. , Seruga, B. , Amir, E. , 2014. Extended adjuvant tamoxifen for early breast cancer: a meta-analysis. PLoS One 9, e88238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albain, K.S. , Barlow, W.E. , Shak, S. , Hortobagyi, G.N. , Livingston, R.B. , Yeh, I.T. , Ravdin, P. , Bugarini, R. , Baehner, F.L. , Davidson, N.E. , Sledge, G.W. , Winer, E.P. , Hudis, C. , Ingle, J.N. , Perez, E.A. , Pritchard, K.I. , Shepherd, L. , Gralow, J.R. , Yoshizawa, C. , Allred, D.C. , Osborne, C.K. , Hayes, D.F. , Breast Cancer Intergroup of North, A2010. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 11, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albain, K.S. , Paik, S. , van't Veer, L. , 2009. Prediction of adjuvant chemotherapy benefit in endocrine responsive, early breast cancer using multigene assays. Breast 18, (Suppl. 3) S141–S145. [DOI] [PubMed] [Google Scholar]
- Bieche, I. , Girault, I. , Urbain, E. , Tozlu, S. , Lidereau, R. , 2004. Relationship between intratumoral expression of genes coding for xenobiotic-metabolizing enzymes and benefit from adjuvant tamoxifen in estrogen receptor alpha-positive postmenopausal breast carcinoma. Breast Cancer Res. 6, R252–R263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanrion, M. , Negre, V. , Fontaine, H. , Salvetat, N. , Bibeau, F. , Mac Grogan, G. , Mauriac, L. , Katsaros, D. , Molina, F. , Theillet, C. , Darbon, J.M. , 2008. A gene expression signature that can predict the recurrence of tamoxifen-treated primary breast cancer. Clin. Cancer Res. 14, 1744–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorssers, L.C. , van Agthoven, T. , Brinkman, A. , Veldscholte, J. , Smid, M. , Dechering, K.J. , 2005. Breast cancer oestrogen independence mediated by BCAR1 or BCAR3 genes is transmitted through mechanisms distinct from the oestrogen receptor signalling pathway or the epidermal growth factor receptor signalling pathway. Breast Cancer Res. 7, R82–R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett, M. , Sestak, I. , Lopez-Knowles, E. , Sidhu, K. , Dunbier, A.K. , Cowens, J.W. , Ferree, S. , Storhoff, J. , Schaper, C. , Cuzick, J. , 2013. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J. Clin. Oncol. 31, 2783–2790. [DOI] [PubMed] [Google Scholar]
- Foekens, J.A. , Portengen, H. , van Putten, W.L. , Peters, H.A. , Krijnen, H.L. , Alexieva-Figusch, J. , Klijn, J.G. , 1989. Prognostic value of estrogen and progesterone receptors measured by enzyme immunoassays in human breast tumor cytosols. Cancer Res. 49, 5823–5828. [PubMed] [Google Scholar]
- Henriksen, K.L. , Rasmussen, B.B. , Lykkesfeldt, A.E. , Moller, S. , Ejlertsen, B. , Mouridsen, H.T. , 2009. An ER activity profile including ER, PR, Bcl-2 and IGF-IR may have potential as selection criterion for letrozole or tamoxifen treatment of patients with advanced breast cancer. Acta Oncol. 48, 522–531. [DOI] [PubMed] [Google Scholar]
- Hwang, K.T. , Woo, J.W. , Shin, H.C. , Kim, H.S. , Ahn, S.K. , Moon, H.G. , Han, W. , Park, I.A. , Noh, D.Y. , 2012. Prognostic influence of BCL2 expression in breast cancer. Int. J. Cancer 131, E1109–E1119. [DOI] [PubMed] [Google Scholar]
- Kerr, D.A. , Wittliff, J.L. , 2011. A five-gene model predicts clinical outcome in ER+/PR+, early-stage breast cancers treated with adjuvant tamoxifen. Horm. Cancer 2, 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.J. , Kang, H.S. , Jung, S.Y. , Min, S.Y. , Lee, S. , Kim, S.W. , Kwon, Y. , Lee, K.S. , Shin, K.H. , Ro, J. , 2010. Methylation patterns of genes coding for drug-metabolizing enzymes in tamoxifen-resistant breast cancer tissues. J. Mol. Med. 88, 1123–1131. [DOI] [PubMed] [Google Scholar]
- Kolacinska, A. , Chalubinska, J. , Zawlik, I. , Szymanska, B. , Borowska-Garganisz, E. , Nowik, M. , Fendler, W. , Kubiak, R. , Pawlowska, Z. , Morawiec, Z. , Szemraj, J. , 2012. Apoptosis-, proliferation, immune function-, and drug resistance- related genes in ER positive, HER2 positive and triple negative breast cancer. Neoplasma 59, 424–432. [DOI] [PubMed] [Google Scholar]
- Larsen, M.S. , Bjerre, K. , Giobbie-Hurder, A. , Laenkholm, A.V. , Henriksen, K.L. , Ejlertsen, B. , Lykkesfeldt, A.E. , Rasmussen, B.B. , 2012. Prognostic value of Bcl-2 in two independent populations of estrogen receptor positive breast cancer patients treated with adjuvant endocrine therapy. Acta Oncol. 51, 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke, S.P. , Bremer, T.M. , Herold, C.D. , Sauter, G. , Diamond, C. , 2006. A multimarker model to predict outcome in tamoxifen-treated breast cancer patients. Clin. Cancer Res. 12, 1175–1183. [DOI] [PubMed] [Google Scholar]
- Loi, S. , Haibe-Kains, B. , Desmedt, C. , Lallemand, F. , Tutt, A.M. , Gillet, C. , Ellis, P. , Harris, A. , Bergh, J. , Foekens, J.A. , Klijn, J.G. , Larsimont, D. , Buyse, M. , Bontempi, G. , Delorenzi, M. , Piccart, M.J. , Sotiriou, C. , 2007. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J. Clin. Oncol. 25, 1239–1246. [DOI] [PubMed] [Google Scholar]
- Loi, S. , Haibe-Kains, B. , Desmedt, C. , Wirapati, P. , Lallemand, F. , Tutt, A.M. , Gillet, C. , Ellis, P. , Ryder, K. , Reid, J.F. , Daidone, M.G. , Pierotti, M.A. , Berns, E.M. , Jansen, M.P. , Foekens, J.A. , Delorenzi, M. , Bontempi, G. , Piccart, M.J. , Sotiriou, C. , 2008. Predicting prognosis using molecular profiling in estrogen receptor-positive breast cancer treated with tamoxifen. BMC Genomics 9, 239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng, M.B. , Laenkholm, A.V. , Tan, Q. , Vach, W. , Gravgaard, K.H. , Knoop, A. , Ditzel, H.J. , 2013. Gene expression signatures that predict outcome of tamoxifen-treated estrogen receptor-positive, high-risk, primary breast cancer patients: a DBCG study. PLoS One 8, e54078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X.J. , Wang, Z. , Ryan, P.D. , Isakoff, S.J. , Barmettler, A. , Fuller, A. , Muir, B. , Mohapatra, G. , Salunga, R. , Tuggle, J.T. , Tran, Y. , Tran, D. , Tassin, A. , Amon, P. , Wang, W. , Enright, E. , Stecker, K. , Estepa-Sabal, E. , Smith, B. , Younger, J. , Balis, U. , Michaelson, J. , Bhan, A. , Habin, K. , Baer, T.M. , Brugge, J. , Haber, D.A. , Erlander, M.G. , Sgroi, D.C. , 2004. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell 5, 607–616. [DOI] [PubMed] [Google Scholar]
- Mangerini, R. , Argellati, F. , Pfeffer, U. , Boccardo, F. , 2012. Effects of bicalutamide and 4OH-tamoxifen on androgen-regulated gene expression in the LNCaP cell line. Anticancer Res. 32, 5323–5329. [PubMed] [Google Scholar]
- Martens, J.W. , Nimmrich, I. , Koenig, T. , Look, M.P. , Harbeck, N. , Model, F. , Kluth, A. , Bolt-de Vries, J. , Sieuwerts, A.M. , Portengen, H. , Meijer-Van Gelder, M.E. , Piepenbrock, C. , Olek, A. , Hofler, H. , Kiechle, M. , Klijn, J.G. , Schmitt, M. , Maier, S. , Foekens, J.A. , 2005. Association of DNA methylation of phosphoserine aminotransferase with response to endocrine therapy in patients with recurrent breast cancer. Cancer Res. 65, 4101–4117. [DOI] [PubMed] [Google Scholar]
- Meijer-van Gelder, M.E. , Look, M.P. , Peters, H.A. , Schmitt, M. , Brunner, N. , Harbeck, N. , Klijn, J.G. , Foekens, J.A. , 2004. Urokinase-type plasminogen activator system in breast cancer: association with tamoxifen therapy in recurrent disease. Cancer Res. 64, 4563–4568. [DOI] [PubMed] [Google Scholar]
- Nehra, R. , Riggins, R.B. , Shajahan, A.N. , Zwart, A. , Crawford, A.C. , Clarke, R. , 2010. BCL2 and CASP8 regulation by NF-kappaB differentially affect mitochondrial function and cell fate in antiestrogen-sensitive and -resistant breast cancer cells. FASEB J.: Official Publication of the Federation of American Societies for Experimental Biology 24, 2040–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, M.J. , Yi, S.J. , Kim, H.S. , Kim, J.H. , Jeong, Y.H. , van Agthoven, T. , Jhun, B.H. , 2013. Functional roles of BCAR3 in the signaling pathways of insulin leading to DNA synthesis, membrane ruffling and GLUT4 translocation. Biochem. Biophys. Res. Commun. 441, 911–916. [DOI] [PubMed] [Google Scholar]
- Paik, S. , 2007. Development and clinical utility of a 21-gene recurrence score prognostic assay in patients with early breast cancer treated with tamoxifen. Oncologist 12, 631–635. [DOI] [PubMed] [Google Scholar]
- Paik, S. , Tang, G. , Shak, S. , Kim, C. , Baker, J. , Kim, W. , Cronin, M. , Baehner, F.L. , Watson, D. , Bryant, J. , Costantino, J.P. , Geyer, C.E. , Wickerham, D.L. , Wolmark, N. , 2006. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 24, 3726–3734. [DOI] [PubMed] [Google Scholar]
- Parker, J.S. , Mullins, M. , Cheang, M.C. , Leung, S. , Voduc, D. , Vickery, T. , Davies, S. , Fauron, C. , He, X. , Hu, Z. , Quackenbush, J.F. , Stijleman, I.J. , Palazzo, J. , Marron, J.S. , Nobel, A.B. , Mardis, E. , Nielsen, T.O. , Ellis, M.J. , Perou, C.M. , Bernard, P.S. , 2009. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 27, 1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak, I. , Dowsett, M. , Zabaglo, L. , Lopez-Knowles, E. , Ferree, S. , Cowens, J.W. , Cuzick, J. , 2013. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J. Natl. Cancer Inst. 105, 1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgroi, D.C. , Sestak, I. , Cuzick, J. , Zhang, Y. , Schnabel, C.A. , Schroeder, B. , Erlander, M.G. , Dunbier, A. , Sidhu, K. , Lopez-Knowles, E. , Goss, P.E. , Dowsett, M. , 2013. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 14, 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieuwerts, A.M. , Meijer-van Gelder, M.E. , Timmermans, M. , Trapman, A.M. , Garcia, R.R. , Arnold, M. , Goedheer, A.J. , Portengen, H. , Klijn, J.G. , Foekens, J.A. , 2005. How ADAM-9 and ADAM-11 differentially from estrogen receptor predict response to tamoxifen treatment in patients with recurrent breast cancer: a retrospective study. Clin. Cancer Res. 11, 7311–7321. [DOI] [PubMed] [Google Scholar]
- Smid, M. , Wang, Y. , Zhang, Y. , Sieuwerts, A.M. , Yu, J. , Klijn, J.G. , Foekens, J.A. , Martens, J.W. , 2008. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 68, 3108–3114. [DOI] [PubMed] [Google Scholar]
- Tozlu, S. , Girault, I. , Vacher, S. , Vendrell, J. , Andrieu, C. , Spyratos, F. , Cohen, P. , Lidereau, R. , Bieche, I. , 2006. Identification of novel genes that co-cluster with estrogen receptor alpha in breast tumor biopsy specimens, using a large-scale real-time reverse transcription-PCR approach. Endocr. Relat. Cancer 13, 1109–1120. [DOI] [PubMed] [Google Scholar]
- Vaillant, F. , Merino, D. , Lee, L. , Breslin, K. , Pal, B. , Ritchie, M.E. , Smyth, G.K. , Christie, M. , Phillipson, L.J. , Burns, C.J. , Mann, G.B. , Visvader, J.E. , Lindeman, G.J. , 2013. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell 24, 120–129. [DOI] [PubMed] [Google Scholar]
- van Agthoven, T. , Sieuwerts, A.M. , Meijer-van Gelder, M.E. , Look, M.P. , Smid, M. , Veldscholte, J. , Sleijfer, S. , Foekens, J.A. , Dorssers, L.C. , 2009. Relevance of breast cancer antiestrogen resistance genes in human breast cancer progression and tamoxifen resistance. J. Clin. Oncol. 27, 542–549. [DOI] [PubMed] [Google Scholar]
- van Agthoven, T. , van Agthoven, T.L. , Dekker, A. , van der Spek, P.J. , Vreede, L. , Dorssers, L.C. , 1998. Identification of BCAR3 by a random search for genes involved in antiestrogen resistance of human breast cancer cells. EMBO J. 17, 2799–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Agthoven, T. , Veldscholte, J. , Smid, M. , van Agthoven, T.L. , Vreede, L. , Broertjes, M. , de Vries, I. , de Jong, D. , Sarwari, R. , Dorssers, L.C. , 2009. Functional identification of genes causing estrogen independence of human breast cancer cells. Breast Cancer Res. Treat. 114, 23–30. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Belguise, K. , O'Neill, C.F. , Sanchez-Morgan, N. , Romagnoli, M. , Eddy, S.F. , Mineva, N.D. , Yu, Z. , Min, C. , Trinkaus-Randall, V. , Chalbos, D. , Sonenshein, G.E. , 2009. RelB NF-kappaB represses estrogen receptor alpha expression via induction of the zinc finger protein Blimp1. Mol. Cell Biol. 29, 3832–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, A.L. , Schrecengost, R.S. , Guerrero, M.S. , Thomas, K.S. , Bouton, A.H. , 2013. Breast cancer antiestrogen resistance 3 (BCAR3) promotes cell motility by regulating actin cytoskeletal and adhesion remodeling in invasive breast cancer cells. PLoS One 8, e65678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Sieuwerts, A.M. , McGreevy, M. , Casey, G. , Cufer, T. , Paradiso, A. , Harbeck, N. , Span, P.N. , Hicks, D.G. , Crowe, J. , Tubbs, R.R. , Budd, G.T. , Lyons, J. , Sweep, F.C. , Schmitt, M. , Schittulli, F. , Golouh, R. , Talantov, D. , Wang, Y. , Foekens, J.A. , 2009. The 76-gene signature defines high-risk patients that benefit from adjuvant tamoxifen therapy. Breast Cancer Res. Treat. 116, 303–309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following is the supplementary data related to this article:
Supplementary data


