Abstract
Brain metastasis is a devastating complication of cancer with unmet therapeutic needs. The incidence of brain metastasis has been rising in cancer patients and its response to treatment is limited due to the singular characteristics of brain metastasis (i.e., blood—brain‐barrier, immune system, stroma). Despite improvements in the treatment and control of extracranial disease, the outcomes of patients with brain metastasis remain dismal. The mechanisms that allow tumor cells to promulgate metastases to the brain remain poorly understood. Further work is required to identify the molecular alterations inherent to brain metastasis in order to identify novel therapeutic targets and explicate the mechanisms of resistance to systemic therapeutics. In this article, we review current knowledge of the unique characteristics of brain metastasis, implications in therapeutic resistance, and the possibility of developing biomarkers to rationally guide the use of targeted agents.
Keywords: Brain metastasis, Targeted therapy, Therapeutic resistance
Highlights
Brain metastasis is a devastating complication of cancer with unmet therapeutic needs.
Brain metastasis exhibits unique biologic characteristics that interfere with systemic therapeutics.
There is a need to identify the molecular mechanisms inherent to brain metastasis to improve their treatment.
Biomarkers for molecular diagnosis are required for a better managing of the disease.
1. Introduction
Brain metastasis is a dismal disease with still few therapeutic options. It is estimated that brain metastasis occurs in 20%–40% of advanced stage cancers (Barnholtz‐Sloan et al., 2004; Gavrilovic and Posner, 2005) and surpasses primary brain tumors in frequency (Maher et al., 2009). The annual incidence of brain metastasis in the United States is estimated to be around 200,000 cases (Barnholtz‐Sloan et al., 2004; Gavrilovic and Posner, 2005) and population‐based studies have predicted the diagnosis of 7–14 new brain metastases cases per 100,000 persons (Smedby et al., 2009). The rise in the incidence may be in part as a consequence of improved control of primary cancers and superior imaging methods (Frisk et al., 2012; Tabouret et al., 2012). Brain metastasis mainly occurs in patients with lung cancer, breast cancer and melanoma with frequencies of 40–50%, 20% and 10–20%, respectively (Barnholtz‐Sloan et al., 2004; Gavrilovic and Posner, 2005). Other primary solid tumors (e.g., colorectal cancer, bladder cancer, prostate cancer) do not tend to disseminate to the brain and maintain low frequencies.
The development of brain metastasis is an important clinical challenge associated with poor prognosis, neurological deterioration, and reduced quality of life (Gavrilovic and Posner, 2005; Stelzer, 2013). Despite the recent success of some therapies in the treatment of extracranial diseases, the outcome of patients developing brain metastasis has not been altered. Treatment of this fatal complication relies on palliative measures involving tumor resection and radiotherapy (i.e., stereotactic radiosurgery or whole brain radiotherapy) and survival is usually less than 12 months even with aggressive treatment (Gavrilovic and Posner, 2005; Stelzer, 2013).
Brain metastasis differs in many aspects from metastatic deposits originating from other organs. Several characteristics of the brain metastatic process (i.e., blood–brain barrier, immune system, stroma) and drug‐associated hurdles (i.e., intrinsic or secondary resistance) play important roles in the complex pathogenesis of brain metastasis and in the sensitivity and resistance to systemic therapeutics. Systemic therapeutic agents (either cytotoxic or targeted therapeutic agents) with confirmed clinical activity in extracranial disease may not be efficient against established brain metastases. In this manuscript, we discuss how understanding the singularities of brain metastases may offer opportunities to tackle their therapeutic resistance and develop novel biomarkers to rationally guide the use of targeted agents. The use of surgery and radiotherapy in the management of brain metastasis, as well as leptomeningeal disease, which may occur concomitantly with brain metastasis have been reviewed elsewhere (Ellis et al., 2012; Owonikoko et al., 2014; Scott and Kesari, 2013).
2. Brain metastasis constitutes a singular challenge
Metastasis is a multistage process in which malignant cells spread from the tumor of origin to colonize distant organs, following a sequence of steps (i.e., local invasion, intravasation, survival in circulation, extravasation and tissue colonization)(Chiang and Massague, 2008; Weigelt et al., 2005; Weil et al., 2005) (Figure 1). The mechanisms that allow tumor cells to colonize the brain are still not fully understood. Several molecular mechanisms contributing to brain metastasis have being revealed, as well as different classes of 'metastasis‐related genes' implicated in the development of brain secondary tumors (Bos et al., 2009; Grinberg‐Rashi et al., 2009; Nguyen et al., 2009; Salhia et al., 2014; Valiente et al., 2014). For example, in lung cancer the expression of HOXB9, LEF1, BPTF, CDH2, KIFC1 (Bos et al., 2009; Grinberg‐Rashi et al., 2009; Nguyen et al., 2009; Valiente et al., 2014) are associated with brain metastasis, and in breast cancer, the Notch pathway, expression of FOXC1, integrin alpha(v)beta(3) (αvβ3), and IGF1R are related to brain metastasis in preclinical models (Lorger et al., 2009; Nam et al., 2008; Ray et al., 2010; Saldana et al., 2013).
Figure 1.
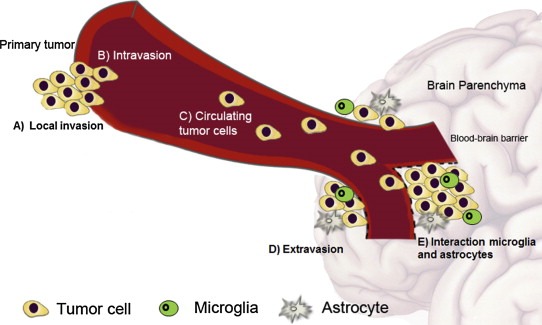
The process of brain metastasis formation. Tumor cells spread from the primary tumor or metastatic deposit to colonize the brain parenchyma, following a sequence of steps: A) local invasion, B) intravasation, C) tumor cells circulate in the bloodstream, D) extravasation into the brain parenchyma through the blood–brain barrier, E) interaction with stromal cells in the brain microenvironment (microglia, astrocytes).
Metastasis is an inefficient process. Most of the cancer cells that escape from solid tumors eventually die in the process of moving from the primary site to distant organs. The cells that survive may proliferate in the new microenvironment after periods of latency (Luzzi et al., 1998). In the case of brain colonization, cells encounter an additional hurdle since they have to cross the blood–brain barrier. Moreover, once in the brain parenchyma, tumor cells have to evade the brain immune system, invade through the brain stroma, degrading extracellular matrix components, and obtain blood supply through angiogenesis.
2.1. Blood—brain barrier
Brain metastasis may occur as a result of the hematogenous dissemination of tumor cells, either from a primary tumor or a metastatic deposit (Chiang and Massague, 2008). To seed the brain, tumor cells have to access the arterial circulation and bypass the blood–brain barrier, which is part of the neurovascular unit (Neuwelt et al., 2011). The blood–brain barrier forms a dynamic unit with different permeability ranges controlled by intracellular and intercellular signaling events (Neuwelt et al., 2011). Once the brain metastasis is established the blood–brain barrier is usually compromised, as can be observed by gadolinium enhancement of magnetic resonance imaging. The extent to which the blood–brain barrier is disrupted influences drug penetration in metastasis (Donelli et al., 1992). For example, the center of a metastatic lesion may present a disrupted blood–brain barrier, which is accessible to optimal drug concentration, whereas the periphery of a metastatic lesion may receive subtherapeutic dose concentrations leading to early development of resistance to treatment. Importantly and in addition, evidence suggests that systemic corticosteroids, which are used to treat peritumoral edema in patients with brain metastasis, can reestablish a disrupted blood–brain barrier, thereby preventing drug delivery and response to therapy (Posner, 1995).
The blood–brain barrier contributes to the inefficient process of metastasis (Lockman et al., 2010), but also provides a shelter for metastatic cells, protecting them from the immune response and systemic treatment (Luzzi et al., 1998). Hence on the one side the blood–brain barrier prevents the initiation of metastasis, but when the metastasis is established it facilitates the metastatic growth. The blood–brain barrier restricts delivery of therapeutic compounds (i.e., large molecules and hydrophilic drugs) to specific areas of the brain (Stewart, 1994); the exact extent to which this influences chemotherapeutic delivery and therapeutic intratumoral concentrations is unknown in many cases (Lockman et al., 2010).
2.2. Brain stroma and immune system
The brain has a distinct immune system (Hamilton and Sibson, 2013). The inflammatory cells of the brain involve perivascular mast cells and macrophages, microglia (which are believed to be the ‘brain‐resident’ macrophages) and astrocytes. The inflammatory response to brain metastasis consists primarily of the activation of microglial cells, which are the principal immune effectors and the main cell type of the innate immune system of the central nervous system (CNS) (Graeber, 2010; Noda et al., 2009). Metastatic tumor cells in the perivascular area or the brain parenchyma permit differential recruitment of circulating systemic immune cells to the metastatic site, though there is no systematic pattern (Blond et al., 2002; Campbell et al., 2002).
In response to brain tumors, astrocytes and microglial cells exert not only anti‐neoplastic effects, but also pro‐neoplastic effects on tumor cells invading the brain (Hamilton and Sibson, 2013). Evidence shows that interactions between tumor cells and cerebral microvascular endothelial cells regulate tumor growth and survival, whereas tumor cell interactions with astrocytes play a role in tumor response to therapy. Astrocytes protect tumor cells from the cytotoxic effects of chemotherapy (Langley and Fidler, 2013) and have been associated with upregulation of survival genes related to chemotherapy resistance (Kim et al., 2011). The interactions of host cells in the brain microenvironment and tumor cells may contribute to the development of metastasis and therapeutic resistance (Wood et al., 2014). The identification of the mechanisms through which tumor cells co‐operate with the microenvironment may reveal key molecules that might be used either as biomarkers or potential drug targets.
2.3. Angiogenesis and other modes of tumor vascularization
The development of new blood vessels is a hallmark of cancer and offers attractive opportunities as a therapeutic target in brain metastasis. The process of angiogenesis itself is an essential step for the formation and growth of metastatic tumors in the brain (Carmeliet and Jain, 2011). There is evidence showing differences in vascularization between primary tumors and brain metastasis (Lorger et al., 2009) and this may lead to diverse responses to antiangiogenic drugs. Besides angiogenesis, other modes of blood vessel recruitment can render tumors resistant to vascular endothelial growth factor (VEGF) inhibitors. These include vasculogenesis, in which a new vascular system is established; co‐option, where by cancer cells utilize contiguous existing blood vessels for growth; vasculogenic mimicry, where invasive and genetically dysregulated cancer cells form vascular networks; and intussusception, where intraluminal vessel pillar growth inside the lumen and the vessel split (Carmeliet and Jain, 2011; Kirschmann et al., 2012). These structural abnormalities in blood vessels may result in intra‐tumoral hypoxia, and impair perfusion of therapeutic agents.
Angiogenesis is orchestrated by pro‐ and anti‐angiogenic factors (Folkman, 2007) and is driven primarily by the VEGF, one of the most relevant factors in this phenomenon. In addition, integrins, in particular αvβ3, have been associated with brain metastasis (Brooks et al., 1994). The activation of integrin αvβ3 is critical for the growth of brain metastasis and the recruitment of supporting blood vessels within the brain have been shown to be independent of hypoxia (Lorger et al., 2009). Other angiogenesis‐associated growth factors have been studied in brain metastasis (e.g., placental growth factor, stromal cell‐derived factor 1 alpha, platelet‐derived growth factor, angiopoietin 1 and 2) (Avraham et al., 2014). Angiopoietin‐2, for example, has been shown to be involved in initial steps of the brain metastasis cascade. It seems to be correlated with blood–brain barrier disruption and colonization of cancer cells in the brain of breast cancer mouse models (Avraham et al., 2014). Collectively, these data point to angiogenesis as an interesting target in brain metastasis.
3. Treating brain metastasis systemically: old and novel approaches
Surgery and radiotherapy‐based approaches are the foundations for treatment of symptomatic brain metastasis (Ellis et al., 2012; Owonikoko et al., 2014; Scott and Kesari, 2013). Developments in imaging techniques are improving surgical performances in order to maximize tumor resection and spare normal brain tissue. On the other hand, the refinement of radiosurgery is likely to allow the reduction of CNS toxicity associated with whole brain radiotherapy.
The role of systemic therapy for the treatment of brain metastasis remains controversial. Historically, few systemic chemotherapeutic agents have shown activity in brain metastasis of solid tumors (Avril et al., 2004; Boogerd et al., 1992; Cortes et al., 2003; Dziadziuszko et al., 2003; Ebert et al., 2003; Franciosi et al., 1999; Fujita et al., 2000; Giorgio et al., 2005; Lin et al., 2004; Siena et al., 2010; Wong and Berkenblit, 2004). Currently, other chemotherapeutic and targeted agents have been investigated in the treatment of brain metastases, some of them in association with radiotherapy (Sperduto et al., 2013; Welsh et al., 2013).
The intrinsic therapeutic sensitivity of a given brain metastasis (as compared to that of extracranial disease) represents an important challenge for the care of cancer patients (Lesser, 1996). Some tumors are highly sensitive to chemotherapy (i.e., testicular germ cell tumor, gestational trophoblastic neoplasia). In these cases, chemotherapy is effective even when brain metastases are present. Thus far, the clinical experience with the use of cytotoxic and targeted systemic therapies for the treatment of brain metastases of lung cancers, breast cancers and melanoma, which are the most common cancers associated with brain metastasis, have shown little evidence of activity in response to a number of regimens (Table 1).
Table 1.
Systemic therapy with activity in brain metastasis from solid tumors.
| Tumor type | References | Cytotoxic | Targeted therapy | ||
|---|---|---|---|---|---|
| Antiangiogenic | Tyrosine kinase inhibitor | Others | |||
| Breast | (Rivera et al., 2006; Trudeau et al., 2006; Lin, 2013) | Cyclophosphamide, fluorouracil, methotrexate, doxorubicin, capecitabine, temozolomide, etoposide, and platinum agents | Bevacizumab | Lapatinib | |
| Lung | (Chiu et al., 2005; De Braganca et al., 2010; Jamal‐Hanjani and Spicer, 2012; Kim et al., 2009; Wu et al., 2007; Wu et al., 2013) | Cisplatin, etoposide, topotecan, temozolamide, pemetrexed, patupilone | Bevacizumab | Erlotinib, getifinib, crizotinib | |
| Melanoma | (Azer et al., 2014; Falchook et al., 2012; Long et al., 2012; Margolin et al., 2012; Rochet et al., 2011; van den Brom et al., 2013; Weber et al., 2011) | Temozolomide, fotemustine | – | Dabrafenib, vemurafenib | Ipilimumab, interleukin‐2 |
The use of novel targeted agents in brain metastasis from solid tumors is a promising approach, and can be explored either in the prevention setting or in already established brain metastasis (Steeg et al., 2011). Improvements in the loco‐regional control of brain metastasis has encouraged enrollment of patients with brain metastasis in early phase clinical trials (Marko and Weil, 2011). Currently, a growing number of clinical trials are targeting this patient population. Table 2 summarizes ongoing clinical trials for patients with solid cancers and brain metastasis receiving novel chemotherapy and targeted therapies.
Table 2.
Ongoing clinical trials testing novel therapeutic approaches for brain metastasis.
| Systemic therapy | ClinicalTrials.gov identifier | Phase of trial | Class of targeted therapy | Patient population with brain metastasis |
|---|---|---|---|---|
| Antiangiogenic | ||||
| Bevacizumab + WBRT | NCT01332929 | 1 | VEGF inhibitor | Solid tumors |
| Cilengitide + WBRT | NCT00884598 | 1 | Anti‐angiogenic small molecule targeting the integrins αvβ3, αvβ5 and α5β1 | Lung cancer (NSCLC or SCLC) |
| Bevacizumab + carboplatin | NCT01004172 | 2 | VEGF inhibitor | Breast cancer |
| Bevacizumab + cisplatin + etoposide | NCT01281696 | 2 | VEGF inhibitor | Breast cancer |
| Radiotherapy +/‐ Endostar Infusion | NCT01410370 | 2 | Antiangiogenic | Solid tumors |
| Tyrosine kinase inhibitors | ||||
| Erlotinib + pemetrexed + cisplatin | NCT01578668 | 2 | EGFR inhibitor | NSCLC |
| WBRT +/‐ erlotinib | NCT01518621 | 2 | EGFR inhibitor | NSCLC |
| Gefitinib + WBRT | NCT01363557 | 2 | EGFR inhibitor | EGFR‐mutant NSCLC |
| Icotinib + WBRT | NCT01516983 | 1/2. | EGFR inhibitor | EGFR‐mutant NSCLC |
| Icotinib + WBRT | NCT01514877 | 2 | EGFR inhibitor | EGFR‐mutant and non‐mutant NSCLC |
| 1) Neratinib or 2) neratinib + resection (candidates for surgery), 3) a. neratinib + capecitabine (no prior lapatinib treatment) or b. neratinib + capecitabine (prior lapatinib treatment) | NCT01494662 | 2 | HER2/EGFR inhibitor | HER2‐positive breast cancer |
| 1) Afatinib or 2) afatinib + vinorelbine or 3) investigator's choice of treatment | NCT01441596 | 2 | HER2/EGFR inhibitor | HER2‐positive breast cancer |
| Lapatinib + WBRT | NCT01218529 | 2 | HER2/EGFR inhibitor | Lung or breast cancer |
| Vemurafenib single arm | NCT01378975 | 2 | BRAF inhibitor | Melanoma BRAFV600 mutant |
| Dabrafenib ± trametinib | NCT01978236 | 2 | BRAF inhibitor/MEK inhibitor | Melanoma BRAFV600E or V600K mutant |
| Dabrafenib + trametinib | NCT02039947 | 2 | BRAF inhibitor/MEK inhibitor | Melanoma V600E, K, D or R mutant |
| Multikinase tyrosine kinase inhibitors/antiangiogenic | ||||
| Sunitinib + stereotactic Radiosurgery | NCT00981890 | 1 | Multikinase TKI/Antiangiogenic | Solid tumors |
| Capecitabine + WBRT → capecitabine + sunitinib** | NCT00570908 | 2 | Multikinase TKI/Antiangiogenic | Breast cancer |
| Sorafenib and radiation therapy ± temozolomide | NCT00639262 | 1 | Multikinase TKI/Antiangiogenic | Solid tumors |
| Sorafenib and stereotactic Radiosurgery | NCT01276210 | 1 | Multikinase TKI/Antiangiogenic | Solid tumors |
| Miscellaneous | ||||
| Iniparib + WBRT | NCT01551680 | 1 | PARP inhibitor | Solid tumors |
| Iniparib + irinotecan | NCT01173497 | 2 | PARP inhibitor | Triple negative breast cancer |
| ABT‐888 + WBRT | NCT00649207 | 2 | PARP inhibitor | Solid tumors |
| TPI‐287 | NCT01332630 | 2 | Taxane | Breast cancer |
| Everolimus + trastuzumab + vinorelbine | NCT01305941 | 2 | mTOR inhibitor | HER2‐positive breast cancer |
| BKM120 + capecitabine | NCT02000882 | 2 | PI3K inhibitor | Triple negative breast cancer |
| BKM120 + trastuzumab | NCT01132664 | 1 | PI3K inhibitor | HER2‐positive breast cancer with or without PIK3 signaling pathway alteration, previously failed trastuzumab |
| 1) GRN1005 or 2) GRN1005 + trastuzumab (if HER2+) | NCT01480583 | 2 | Taxane‐peptide conjugate | HER2‐negative and positive breast cancer |
| Trastuzumab + WBRT | NCT01363986 | 2 | Anti‐HER2 monoclonal antibody | HER2‐positive breast cancer |
Abbreviation: EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; PARP, poly ADP ribose polymerase; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; WBRT, whole brain radiotherapy.
3.1. Anti‐angiogenic agents
Several anti‐angiogenic therapeutic agents have been approved for clinical use in cancer. They comprise monoclonal antibodies (Banerjee and Kaye, 2012; Hurwitz et al., 2004; Johnson et al., 2013) or tyrosine kinase inhibitors (Motzer et al., 2013), most notably inhibiting the VEGF pro‐angiogenic signalling pathway. Treatment with VEGF inhibitors has demonstrated low objective response rates and has restricted increases in the time to progression and survival to a few months in the approved indications in cancer (Banerjee and Kaye, 2012; Hurwitz et al., 2004; Johnson et al., 2013; Motzer et al., 2013).
Bevacizumab (Avastin; Genentech/Roche, Basel, Switzerland) is a humanized monoclonal antibody that inhibits tumor angiogenesis by neutralizing the VEGF. It is part of standard therapy in non‐small cell lung cancer (NSCLC), colorectal cancer, gliomas and renal cell cancer and has been investigated in combination with chemotherapy in various solid tumors. In the last decade, patients with brain metastases were excluded from clinical trials testing bevacizumab because of hemorrhagic risk (Gordon et al., 2001). However, data from primary brain tumors have recently demonstrated that the use of bevacizumab has clinical activity and is safe in terms of CNS hemorrhage (Besse et al., 2010; Khasraw et al., 2012; Socinski et al., 2009; YS et al., 2012). Currently, there are data supporting the safety of bevacizumab in brain metastases from solid cancers (Cohen et al., 2009). In breast and lung cancers, case reports and retrospective analysis have suggested that bevacizumab might benefit patients with progressive brain metastases (De Braganca et al., 2010; Yamamoto et al., 2012). The results of ongoing clinical trials testing bevacizumab and novel anti‐angiogenic agents are eagerly awaited.
3.2. Small molecule tyrosine kinase inhibitors
Tyrosine kinase inhibitors may reach brain metastases more readily due to their low molecular weight and consequent ability to bypass the blood–brain barrier. A number of tyrosine kinase inhibitors have shown activity as a systemic therapeutic strategy for treating brain metastasis. In human epidermal growth factor receptor (HER2)‐positive breast cancer patients with established brain metastases, trastuzumab, a monoclonal antibody, apparently does not efficiently permeate the intact blood–brain barrier (Roy and Perez, 2009). Lapatinib‐based therapy (i.e., an orally active small molecule tyrosine kinase inhibitor of the epidermal growth factor receptor (EGFR) and HER2) has shown promising activity in association with capecitabine. Brain response rates were observed in as many as 40% of cases in previously treated patients (Lin et al., 2008, 2009) and reached 65% for patients with untreated brain metastases (Bachelot et al., 2013).
The EGFR tyrosine kinase inhibitors gefitinib and erlotinib have shown activity in brain metastases from NSCLC with activating EGFR mutations as reported in small retrospective studies (Eichler et al., 2010; Grommes et al., 2011; Porta et al., 2011) and preliminary evidence from clinical trials (Welsh et al., 2013; Wu et al., 2013). Objective responses in intracranial disease in these studies varied from 10% to 80%. Notably, erlotinib seems to reach higher cerebral spinal fluid concentrations compared to getifinib (Masuda et al., 2011). Data from a retrospective study have suggested that EGFR‐mutant patients who received erlotinib or getifinib had lower rates of brain metastasis progression, potentially indicating a ‘profilatic’ role for these targeted agents in the NSCLC setting (Heon et al., 2012). In NSCLC cancer treated with EGFR inhibitors, nearly all patients will develop secondary (‘acquired’) resistance to the EGFR inhibitor (Jackman et al., 2010) and in 50% of the cases, resistance will be attributed to a second‐site mutation in the threonine gatekeeper residue at position 790 (T790M) (Kobayashi et al., 2005). A clinical trial suggested that a new schedule of administration may overcome acquired resistance to erlotinib. Pulsatile high‐dose erlotinib was found to be effective against brain metastasis in patients who had progressed while on treatment with standard‐dose erlotinib (Grommes et al., 2011). In addition, crizotinib, an oral c‐MET/ALK/ROS1 tyrosine kinase inhibitor, is approved for ALK‐positive NSCLC (3–5% of all NSCLC). Of note, crizotinib has shown some clinical response in patients with brain metastasis (Shaw et al., 2013), although there is evidence that crizotinib does not easily penetrate the blood–brain barrier (Costa et al., 2011).
The RAS‐RAF‐MAPK signaling pathway has been identified as a target for melanoma patients. The specific small molecule inhibitors of BRAFV600E mutations, dabrafenib and vemurafenib, have shown activity in patients with brain metastases from melanoma (Falchook et al., 2012; Long et al., 2012; Rochet et al., 2011). In a phase 1 study with dabrafenib, nine of ten patients with melanoma and untreated brain metastases had reductions in the size of brain lesions (Falchook et al., 2012). A phase 2 trial reported 39% and 31% of intracranial responses in BRAFV600E mutant melanoma with either no previous brain treatment or with brain treatment, respectively (Long et al., 2012). Vemurafenib has shown evidence of activity in case reports (Rochet et al., 2011) and also in ongoing clinical trials enrolling previously treated metastatic melanoma patients with brain metastases (NCT01378975). In melanoma patients pre‐treated with vemurafenib, MEK1 mutation is a possible mechanism of secondary (‘acquired’) resistance (Wagle et al., 2011). Clinical trials are already testing agents simultaneously targeting multiple components of the BRAF/MAPK pathway for melanoma patients who develop brain metastasis (Table 2, NCT01978236, NCT02039947).
3.3. Immunomodulators
Ipilimumab is a monoclonal antibody that blocks the cytotoxic T‐lymphocyte antigen‐4 to increase anti‐tumor T‐cell responses. It has shown activity in patients with melanoma that has metastasized to the brain with no increase in the occurrence of CNS‐related toxicities (Hodi et al., 2008; Margolin et al., 2012; Schartz et al., 2010). Clinical activity in terms of long‐term stable disease or complete response has come from case reports or retrospective analyses of small clinical trials that did not exclude patients with small asymptomatic brain metastases (Schartz et al., 2010; Weber et al., 2011). This evidence has opened the ground for prospective studies using ipilimumab in the context of brain metastasis from melanoma (Di Giacomo et al., 2012; Margolin et al., 2012) and has led the U.S.‐expanded access program for ipilimumab to include melanoma patients with brain metastases.
4. Challenges for tackling brain metastasis
4.1. Tumor heterogeneity
Intra‐tumor spatial and temporal heterogeneity has been documented in metastatic brain tumors (Ding et al., 2010; Gerlinger et al., 2012; Navin et al., 2011; Shah et al., 2012; Sottoriva et al., 2013; Wu et al., 2012). The genomic and epigenomic basis for the development of metastasis to the brain from the primary tumor and the extent to which brain metastasis shares the genetic profile of the primary tumor remains largely unknown (Chiang and Massague, 2008; Weil et al., 2005).
Tumors are composed of assortments of cells (Navin et al., 2011; Shah et al., 2012), with subclones of cells harboring unique genomic alterations together with ubiquitous ones (i.e., genomic alterations common to all tumour cells). Genomic analyses have shown that many advanced tumors follow a branched, Darwinian evolutionary trajectory (Swanton, 2014). However, little is known about how intra‐tumor and inter‐tumor heterogeneity influence the development and progression of brain tumors. Understanding the contribution of spatial and temporal genetic heterogeneity and the role of subpopulations of cells that initiate brain metastasis is essential to deciphering the process of metastatic progression and identifying the subclones that will give rise to therapeutic resistance (Seoane and De Mattos‐Arruda, 2014).
4.2. Development of novel biomarkers that capture tumor dissemination to the brain
Circulating tumor cells (CTCs) that have detached from the primary tumor or metastases and circulate in the peripheral blood may constitute seeds for growth of metastases in different locations, and may be remarkable biomarkers that can demonstrate tumor dissemination to the brain (De Mattos‐Arruda et al., 2013; Mego et al., 2011). Zhang et al. identified a potential signature suggestive of the metastatic ability of CTC to colonize the brain (Zhang et al., 2013). CTCs not expressing the epithelial cell adhesion molecule marker, and consequently not captured by the FDA‐approved CellSearch platform, and expressing a signature of selected markers associated with brain metastasis (i.e., HER2+/EGFR+/HPSE+/Notch1+) were highly invasive and capable of generating brain and lung metastases in patient‐derived xenograft mouse models. The impact of CTC on the outcome of brain metastasis was evaluated in the single‐group phase 2 LANDSCAPE study, which analyzed CTCs from HER2‐positive metastatic breast cancer patients on a lapatinib‐based treatment. This is the first study that showed a correlation between CNS metastasis response, outcome and early CTC clearance in the setting of a targeted treatment regimen (Pierga et al., 2013).
Besides CTCs, plasma‐derived circulating tumor DNA (ctDNA) has introduced new modality that can be used to investigate the metastatic process, mechanisms of therapeutic resistance, and disease monitoring in cancer patients (Crowley et al., 2013; De Mattos‐Arruda et al., 2013). In brain tumors, however, the presence of ctDNA derived from plasma is very low mainly due to the location of the tumor and the presence of the blood–brain barrier (Chen et al., 2013; Lavon et al., 2010). Thus, it is not surprising that ctDNA was found only in approximately 10% of patients with brain tumors (Bettegowda et al., 2014).
Other circulating biomarkers such as microRNAs are being investigated and may add important information in terms of metastatic potential of cancers to the brain, or serve as diagnostic or prognostic tools (Nass et al., 2009; Camacho et al., 2013; Hwang et al., 2012; Li et al., 2012; McDermott et al., 2013; Zhao et al., 2013; Okuda et al., 2013). Interestingly, some microRNAs have been shown to be involved in the impairment of the blood–brain barrier facilitating the tumor colonization of the brain (Zhou et al., 2014).
5. Concluding remarks
The incidence of brain metastasis has been increasing in cancer patients and can limit improvements made by the use of new systemic treatments. The standard treatment for these patients remains radiotherapy‐based approaches or surgery, although in selected cases systemic therapy can be considered. Thus far, cytotoxic and specific targeted therapeutic agents have shown clinical activity against established brain metastases (e.g., ipilimumab, getifinib, and lapatinib used for BRAF600 mutant melanoma, EGFR mutant NSCLC and HER2‐positive breast cancer, respectively), but defining the precise clinical role of these agents needs to be determined.
Further work is also required to fully understand the mechanisms involved in the development and progression of brain metastasis, and in the identification of novel therapeutic targets that can curtail resistance to current available treatments. Resistance to systemic therapy depends on several factors, including the molecular and genomic characteristics of the primary cancer and the extracranial and intracranial metastatic deposits, the mechanisms that limit drug penetration into the brain, and the effectiveness of systemic targeted agents.
A full understanding of the molecular and genetic moieties and processes involved in brain metastasis may help plan treatment and counteract therapeutic resistance. Novel preclinical models will need to be generated, such as patient‐derived xenografts or transgenic models. Innovative clinical trials that investigate targeted therapy combined with approaches that increase drug delivery to the brain are warranted. Potential molecular biomarkers need to be identified for future clinical use parallel with new investigational treatment strategies.
Acknowledgments
The authors acknowledge financial support from Fundación Rafael del Pino, Yvonne Smolders for assistance in the figure and Johann Aaron for editing the manuscript.
Seoanea Joan and De Mattos-Arrudaa Leticia , ( 2014. ), Brain metastasis: New opportunities to tackle therapeutic resistance , Molecular Oncology , 8 , doi: 10.1016/j.molonc.2014.05.009.
References
- Avraham, H.K. , Jiang, S. , Fu, Y. , Nakshatri, H. , Ovadia, H. , Avraham, S. , 2014. Angiopoietin-2 mediates blood-brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J Pathol. 232, 369–381. [DOI] [PubMed] [Google Scholar]
- Avril, M.F. , Aamdal, S. , Grob, J.J. , Hauschild, A. , Mohr, P. , Bonerandi, J.J. , Weichenthal, M. , Neuber, K. , Bieber, T. , Gilde, K. , Guillem Porta, V. , Fra, J. , Bonneterre, J. , Saiag, P. , Kamanabrou, D. , Pehamberger, H. , Sufliarsky, J. , Gonzalez Larriba, J.L. , Scherrer, A. , Menu, Y. , 2004. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 22, 1118–1125. [DOI] [PubMed] [Google Scholar]
- Azer, M.W. , Menzies, A.M. , Haydu, L.E. , Kefford, R.F. , Long, G.V. , 2014. Patterns of response and progression in patients with BRAF-mutant melanoma metastatic to the brain who were treated with dabrafenib. Cancer. 120, 530–536. [DOI] [PubMed] [Google Scholar]
- Bachelot, T. , Romieu, G. , Campone, M. , Dieras, V. , Cropet, C. , Dalenc, F. , Jimenez, M. , Le Rhun, E. , Pierga, J.Y. , Goncalves, A. , Leheurteur, M. , Domont, J. , Gutierrez, M. , Cure, H. , Ferrero, J.M. , Labbe-Devilliers, C. , 2013. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 14, 64–71. [DOI] [PubMed] [Google Scholar]
- Banerjee, S. , Kaye, S.B. , 2012. Gynecological cancer: first-line bevacizumab for ovarian cancer–new standard of care?. Nat Rev Clin Oncol. 9, 194–196. [DOI] [PubMed] [Google Scholar]
- Barnholtz-Sloan, J.S. , Sloan, A.E. , Davis, F.G. , Vigneau, F.D. , Lai, P. , Sawaya, R.E. , 2004. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 22, 2865–2872. [DOI] [PubMed] [Google Scholar]
- Besse, B. , Lasserre, S.F. , Compton, P. , Huang, J. , Augustus, S. , Rohr, U.P. , 2010. Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res. 16, 269–278. [DOI] [PubMed] [Google Scholar]
- Bettegowda, C. , Sausen, M. , Leary, R.J. , Kinde, I. , Wang, Y. , Agrawal, N. , Bartlett, B.R. , Wang, H. , Luber, B. , Alani, R.M. , Antonarakis, E.S. , Azad, N.S. , Bardelli, A. , Brem, H. , Cameron, J.L. , Lee, C.C. , Fecher, L.A. , Gallia, G.L. , Gibbs, P. , Le, D. , Giuntoli, R.L. , Goggins, M. , Hogarty, M.D. , Holdhoff, M. , Hong, S.M. , Jiao, Y. , Juhl, H.H. , Kim, J.J. , Siravegna, G. , Laheru, D.A. , Lauricella, C. , Lim, M. , Lipson, E.J. , Marie, S.K. , Netto, G.J. , Oliner, K.S. , Olivi, A. , Olsson, L. , Riggins, G.J. , Sartore-Bianchi, A. , Schmidt, K. , Shih l, M. , Oba-Shinjo, S.M. , Siena, S. , Theodorescu, D. , Tie, J. , Harkins, T.T. , Veronese, S. , Wang, T.L. , Weingart, J.D. , Wolfgang, C.L. , Wood, L.D. , Xing, D. , Hruban, R.H. , Wu, J. , Allen, P.J. , Schmidt, C.M. , Choti, M.A. , Velculescu, V.E. , Kinzler, K.W. , Vogelstein, B. , Papadopoulos, N. , Diaz, L.A. , 2014. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 6, 224ra224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond, D. , Campbell, S.J. , Butchart, A.G. , Perry, V.H. , Anthony, D.C. , 2002. Differential induction of interleukin-1beta and tumour necrosis factor-alpha may account for specific patterns of leukocyte recruitment in the brain. Brain Res. 958, 89–99. [DOI] [PubMed] [Google Scholar]
- Boogerd, W. , Dalesio, O. , Bais, E.M. , van der Sande, J.J. , 1992. Response of brain metastases from breast cancer to systemic chemotherapy. Cancer. 69, 972–980. [DOI] [PubMed] [Google Scholar]
- Bos, P.D. , Zhang, X.H. , Nadal, C. , Shu, W. , Gomis, R.R. , Nguyen, D.X. , Minn, A.J. , van de Vijver, M.J. , Gerald, W.L. , Foekens, J.A. , Massague, J. , 2009. Genes that mediate breast cancer metastasis to the brain. Nature. 459, 1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, P.C. , Clark, R.A. , Cheresh, D.A. , 1994. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 264, 569–571. [DOI] [PubMed] [Google Scholar]
- Camacho, L. , Guerrero, P. , Marchetti, D. , 2013. MicroRNA and protein profiling of brain metastasis competent cell-derived exosomes. PLoS One. 8, e73790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, S.J. , Wilcockson, D.C. , Butchart, A.G. , Perry, V.H. , Anthony, D.C. , 2002. Altered chemokine expression in the spinal cord and brain contributes to differential interleukin-1beta-induced neutrophil recruitment. J Neurochem. 83, 432–441. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P. , Jain, R.K. , 2011. Molecular mechanisms and clinical applications of angiogenesis. Nature. 473, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W.W. , Balaj, L. , Liau, L.M. , Samuels, M.L. , Kotsopoulos, S.K. , Maguire, C.A. , Loguidice, L. , Soto, H. , Garrett, M. , Zhu, L.D. , Sivaraman, S. , Chen, C. , Wong, E.T. , Carter, B.S. , Hochberg, F.H. , Breakefield, X.O. , Skog, J. , 2013. BEAMing and droplet digital PCR analysis of mutant IDH1 mRNA in glioma patient serum and cerebrospinal fluid extracellular vesicles. Molecular therapy. Nucleic Acids. 2, e109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, A.C. , Massague, J. , 2008. Molecular basis of metastasis. N Engl J Med. 359, 2814–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, C.H. , Tsai, C.M. , Chen, Y.M. , Chiang, S.C. , Liou, J.L. , Perng, R.P. , 2005. Gefitinib is active in patients with brain metastases from non-small cell lung cancer and response is related to skin toxicity. Lung Cancer. 47, 129–138. [DOI] [PubMed] [Google Scholar]
- Cohen, M.H. , Shen, Y.L. , Keegan, P. , Pazdur, R. , 2009. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 14, 1131–1138. [DOI] [PubMed] [Google Scholar]
- Cortes, J. , Rodriguez, J. , Aramendia, J.M. , Salgado, E. , Gurpide, A. , Garcia-Foncillas, J. , Aristu, J.J. , Claver, A. , Bosch, A. , Lopez-Picazo, J.M. , Martin-Algarra, S. , Brugarolas, A. , Calvo, E. , 2003. Front-line paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology. 64, 28–35. [DOI] [PubMed] [Google Scholar]
- Costa, D.B. , Kobayashi, S. , Pandya, S.S. , Yeo, W.L. , Shen, Z. , Tan, W. , Wilner, K.D. , 2011. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 29, e443–445. [DOI] [PubMed] [Google Scholar]
- Crowley, E. , Di Nicolantonio, F. , Loupakis, F. , Bardelli, A. , 2013. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 10, 472–484. [DOI] [PubMed] [Google Scholar]
- De Braganca, K.C. , Janjigian, Y.Y. , Azzoli, C.G. , Kris, M.G. , Pietanza, M.C. , Nolan, C.P. , Omuro, A.M. , Holodny, A.I. , Lassman, A.B. , 2010. Efficacy and safety of bevacizumab in active brain metastases from non-small cell lung cancer. J Neurooncol. 100, 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mattos-Arruda, L. , Cortes, J. , Santarpia, L. , Vivancos, A. , Tabernero, J. , Reis-Filho, J.S. , Seoane, J. , 2013. Circulating tumour cells and cell-free DNA as tools for managing breast cancer. Nat Rev Clin Oncol. 10, 377–389. [DOI] [PubMed] [Google Scholar]
- Di Giacomo, A.M. , Ascierto, P.A. , Pilla, L. , Santinami, M. , Ferrucci, P.F. , Giannarelli, D. , Marasco, A. , Rivoltini, L. , Simeone, E. , Nicoletti, S.V. , Fonsatti, E. , Annesi, D. , Queirolo, P. , Testori, A. , Ridolfi, R. , Parmiani, G. , Maio, M. , 2012. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 13, 879–886. [DOI] [PubMed] [Google Scholar]
- Ding, L. , Ellis, M.J. , Li, S. , Larson, D.E. , Chen, K. , Wallis, J.W. , Harris, C.C. , McLellan, M.D. , Fulton, R.S. , Fulton, L.L. , Abbott, R.M. , Hoog, J. , Dooling, D.J. , Koboldt, D.C. , Schmidt, H. , Kalicki, J. , Zhang, Q. , Chen, L. , Lin, L. , Wendl, M.C. , McMichael, J.F. , Magrini, V.J. , Cook, L. , McGrath, S.D. , Vickery, T.L. , Appelbaum, E. , Deschryver, K. , Davies, S. , Guintoli, T. , Crowder, R. , Tao, Y. , Snider, J.E. , Smith, S.M. , Dukes, A.F. , Sanderson, G.E. , Pohl, C.S. , Delehaunty, K.D. , Fronick, C.C. , Pape, K.A. , Reed, J.S. , Robinson, J.S. , Hodges, J.S. , Schierding, W. , Dees, N.D. , Shen, D. , Locke, D.P. , Wiechert, M.E. , Eldred, J.M. , Peck, J.B. , Oberkfell, B.J. , Lolofie, J.T. , Du, F. , Hawkins, A.E. , O'Laughlin, M.D. , Bernard, K.E. , Cunningham, M. , Elliott, G. , Mason, M.D. , Thompson, D.M. , Ivanovich, J.L. , Goodfellow, P.J. , Perou, C.M. , Weinstock, G.M. , Aft, R. , Watson, M. , Ley, T.J. , Wilson, R.K. , Mardis, E.R. , 2010. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 464, 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelli, M.G. , Zucchetti, M. , D'Incalci, M. , 1992. Do anticancer agents reach the tumor target in the human brain?. Cancer Chemother Pharmacol. 30, 251–260. [DOI] [PubMed] [Google Scholar]
- Dziadziuszko, R. , Ardizzoni, A. , Postmus, P.E. , Smit, E.F. , Price, A. , Debruyne, C. , Legrand, C. , Giaccone, G. , 2003. Temozolomide in patients with advanced non-small cell lung cancer with and without brain metastases. a phase II study of the EORTC Lung Cancer Group (08965). Eur J Cancer. 39, 1271–1276. [DOI] [PubMed] [Google Scholar]
- Ebert, B.L. , Niemierko, E. , Shaffer, K. , Salgia, R. , 2003. Use of temozolomide with other cytotoxic chemotherapy in the treatment of patients with recurrent brain metastases from lung cancer. Oncologist. 8, 69–75. [DOI] [PubMed] [Google Scholar]
- Eichler, A.F. , Kahle, K.T. , Wang, D.L. , Joshi, V.A. , Willers, H. , Engelman, J.A. , Lynch, T.J. , Sequist, L.V. , 2010. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 12, 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, T.L. , Neal, M.T. , Chan, M.D. , 2012. The role of surgery, radiosurgery and whole brain radiation therapy in the management of patients with metastatic brain tumors. Int J Surg Oncol. 2012, 952345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchook, G.S. , Long, G.V. , Kurzrock, R. , Kim, K.B. , Arkenau, T.H. , Brown, M.P. , Hamid, O. , Infante, J.R. , Millward, M. , Pavlick, A.C. , O'Day, S.J. , Blackman, S.C. , Curtis, C.M. , Lebowitz, P. , Ma, B. , Ouellet, D. , Kefford, R.F. , 2012. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 379, 1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman, J. , 2007. Angiogenesis: an organizing principle for drug discovery? Nature reviews. Drug Discov. 6, 273–286. [DOI] [PubMed] [Google Scholar]
- Franciosi, V. , Cocconi, G. , Michiara, M. , Di Costanzo, F. , Fosser, V. , Tonato, M. , Carlini, P. , Boni, C. , Di Sarra, S. , 1999. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer. 85, 1599–1605. [PubMed] [Google Scholar]
- Frisk, G. , Svensson, T. , Backlund, L.M. , Lidbrink, E. , Blomqvist, P. , Smedby, K.E. , 2012. Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Cancer. 106, 1850–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, A. , Fukuoka, S. , Takabatake, H. , Tagaki, S. , Sekine, K. , 2000. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patients with brain metastases from non-small cell lung cancer. Oncology. 59, 291–295. [DOI] [PubMed] [Google Scholar]
- Gavrilovic, I.T. , Posner, J.B. , 2005. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 75, 5–14. [DOI] [PubMed] [Google Scholar]
- Gerlinger, M. , Rowan, A.J. , Horswell, S. , Larkin, J. , Endesfelder, D. , Gronroos, E. , Martinez, P. , Matthews, N. , Stewart, A. , Tarpey, P. , Varela, I. , Phillimore, B. , Begum, S. , McDonald, N.Q. , Butler, A. , Jones, D. , Raine, K. , Latimer, C. , Santos, C.R. , Nohadani, M. , Eklund, A.C. , Spencer-Dene, B. , Clark, G. , Pickering, L. , Stamp, G. , Gore, M. , Szallasi, Z. , Downward, J. , Futreal, P.A. , Swanton, C. , 2012. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 366, 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio, C.G. , Giuffrida, D. , Pappalardo, A. , Russo, A. , Santini, D. , Salice, P. , Blanco, G. , Castorina, S. , Failla, G. , Bordonaro, R. , 2005. Oral temozolomide in heavily pre-treated brain metastases from non-small cell lung cancer: phase II study. Lung Cancer. 50, 247–254. [DOI] [PubMed] [Google Scholar]
- Gordon, M.S. , Margolin, K. , Talpaz, M. , Sledge, G.W. , Holmgren, E. , Benjamin, R. , Stalter, S. , Shak, S. , Adelman, D. , 2001. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 19, 843–850. [DOI] [PubMed] [Google Scholar]
- Graeber, M.B. , 2010. Changing face of microglia. Science. 330, 783–788. [DOI] [PubMed] [Google Scholar]
- Grinberg-Rashi, H. , Ofek, E. , Perelman, M. , Skarda, J. , Yaron, P. , Hajduch, M. , Jacob-Hirsch, J. , Amariglio, N. , Krupsky, M. , Simansky, D.A. , Ram, Z. , Pfeffer, R. , Galernter, I. , Steinberg, D.M. , Ben-Dov, I. , Rechavi, G. , Izraeli, S. , 2009. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin Cancer Res. 15, 1755–1761. [DOI] [PubMed] [Google Scholar]
- Grommes, C. , Oxnard, G.R. , Kris, M.G. , Miller, V.A. , Pao, W. , Holodny, A.I. , Clarke, J.L. , Lassman, A.B. , 2011. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 13, 1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, A. , Sibson, N.R. , 2013. Role of the systemic immune system in brain metastasis. Mol Cell Neurosci. 53, 42–51. [DOI] [PubMed] [Google Scholar]
- Heon, S. , Yeap, B.Y. , Lindeman, N.I. , Joshi, V.A. , Butaney, M. , Britt, G.J. , Costa, D.B. , Rabin, M.S. , Jackman, D.M. , Johnson, B.E. , 2012. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 18, 4406–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi, F.S. , Oble, D.A. , Drappatz, J. , Velazquez, E.F. , Ramaiya, N. , Ramakrishna, N. , Day, A.L. , Kruse, A. , Mac Rae, S. , Hoos, A. , Mihm, M. , 2008. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nature clinical practice. Oncology. 5, 557–561. [DOI] [PubMed] [Google Scholar]
- Hurwitz, H. , Fehrenbacher, L. , Novotny, W. , Cartwright, T. , Hainsworth, J. , Heim, W. , Berlin, J. , Baron, A. , Griffing, S. , Holmgren, E. , Ferrara, N. , Fyfe, G. , Rogers, B. , Ross, R. , Kabbinavar, F. , 2004. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 350, 2335–2342. [DOI] [PubMed] [Google Scholar]
- Hwang, S.J. , Seol, H.J. , Park, Y.M. , Kim, K.H. , Gorospe, M. , Nam, D.H. , Kim, H.H. , 2012. MicroRNA-146a suppresses metastatic activity in brain metastasis. Mol Cells. 34, 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman, D. , Pao, W. , Riely, G.J. , Engelman, J.A. , Kris, M.G. , Janne, P.A. , Lynch, T. , Johnson, B.E. , Miller, V.A. , 2010. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 28, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal-Hanjani, M. , Spicer, J. , 2012. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res. 18, 938–944. [DOI] [PubMed] [Google Scholar]
- Johnson, D.R. , Leeper, H.E. , Uhm, J.H. , 2013. Glioblastoma survival in the United States improved after Food and Drug Administration approval of bevacizumab: a population-based analysis. Cancer. 119, 3489–3495. [DOI] [PubMed] [Google Scholar]
- Khasraw, M. , Holodny, A. , Goldlust, S.A. , DeAngelis, L.M. , 2012. Intracranial hemorrhage in patients with cancer treated with bevacizumab: the Memorial Sloan-Kettering experience. Ann Oncol. 23, 458–463. [DOI] [PubMed] [Google Scholar]
- Kim, J.E. , Lee, D.H. , Choi, Y. , Yoon, D.H. , Kim, S.W. , Suh, C. , Lee, J.S. , 2009. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 65, 351–354. [DOI] [PubMed] [Google Scholar]
- Kim, S.J. , Kim, J.S. , Park, E.S. , Lee, J.S. , Lin, Q. , Langley, R.R. , Maya, M. , He, J. , Kim, S.W. , Weihua, Z. , Balasubramanian, K. , Fan, D. , Mills, G.B. , Hung, M.C. , Fidler, I.J. , 2011. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia. 13, 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschmann, D.A. , Seftor, E.A. , Hardy, K.M. , Seftor, R.E. , Hendrix, M.J. , 2012. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res. 18, 2726–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, S. , Boggon, T.J. , Dayaram, T. , Janne, P.A. , Kocher, O. , Meyerson, M. , Johnson, B.E. , Eck, M.J. , Tenen, D.G. , Halmos, B. , 2005. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 352, 786–792. [DOI] [PubMed] [Google Scholar]
- Langley, R.R. , Fidler, I.J. , 2013. The biology of brain metastasis. Clin Chem. 59, 180–189. [DOI] [PubMed] [Google Scholar]
- Lavon, I. , Refael, M. , Zelikovitch, B. , Shalom, E. , Siegal, T. , 2010. Serum DNA can define tumor-specific genetic and epigenetic markers in gliomas of various grades. Neuro Oncol. 12, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser, G.J. , 1996. Chemotherapy of cerebral metastases from solid tumors. Neurosurg Clin N Am. 7, 527–536. [PubMed] [Google Scholar]
- Li, Z. , Gu, X. , Fang, Y. , Xiang, J. , Chen, Z. , 2012. microRNA expression profiles in human colorectal cancers with brain metastases. Oncol Lett. 3, 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, N.U. , Bellon, J.R. , Winer, E.P. , 2004. CNS metastases in breast cancer. J Clin Oncol. 22, 3608–3617. [DOI] [PubMed] [Google Scholar]
- Lin, N.U. , Carey, L.A. , Liu, M.C. , Younger, J. , Come, S.E. , Ewend, M. , Harris, G.J. , Bullitt, E. , Van den Abbeele, A.D. , Henson, J.W. , Li, X. , Gelman, R. , Burstein, H.J. , Kasparian, E. , Kirsch, D.G. , Crawford, A. , Hochberg, F. , Winer, E.P. , 2008. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 26, 1993–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, N.U. , Dieras, V. , Paul, D. , Lossignol, D. , Christodoulou, C. , Stemmler, H.J. , Roche, H. , Liu, M.C. , Greil, R. , Ciruelos, E. , Loibl, S. , Gori, S. , Wardley, A. , Yardley, D. , Brufsky, A. , Blum, J.L. , Rubin, S.D. , Dharan, B. , Steplewski, K. , Zembryki, D. , Oliva, C. , Roychowdhury, D. , Paoletti, P. , Winer, E.P. , 2009. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 15, 1452–1459. [DOI] [PubMed] [Google Scholar]
- Lin, N.U. , 2013. Breast cancer brain metastases: new directions in systemic therapy. Ecancermedicalscience. 7, 307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman, P.R. , Mittapalli, R.K. , Taskar, K.S. , Rudraraju, V. , Gril, B. , Bohn, K.A. , Adkins, C.E. , Roberts, A. , Thorsheim, H.R. , Gaasch, J.A. , Huang, S. , Palmieri, D. , Steeg, P.S. , Smith, Q.R. , 2010. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 16, 5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, G.V. , Trefzer, U. , Davies, M.A. , Kefford, R.F. , Ascierto, P.A. , Chapman, P.B. , Puzanov, I. , Hauschild, A. , Robert, C. , Algazi, A. , Mortier, L. , Tawbi, H. , Wilhelm, T. , Zimmer, L. , Switzky, J. , Swann, S. , Martin, A.M. , Guckert, M. , Goodman, V. , Streit, M. , Kirkwood, J.M. , Schadendorf, D. , 2012. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 1087–1095. [DOI] [PubMed] [Google Scholar]
- Lorger, M. , Krueger, J.S. , O'Neal, M. , Staflin, K. , Felding-Habermann, B. , 2009. Activation of tumor cell integrin alphavbeta3 controls angiogenesis and metastatic growth in the brain. Proc Natl Acad Sci U S A. 106, 10666–10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi, K.J. , MacDonald, I.C. , Schmidt, E.E. , Kerkvliet, N. , Morris, V.L. , Chambers, A.F. , Groom, A.C. , 1998. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 153, 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher, E.A. , Mietz, J. , Arteaga, C.L. , DePinho, R.A. , Mohla, S. , 2009. Brain metastasis: opportunities in basic and translational research. Cancer Res. 69, 6015–6020. [DOI] [PubMed] [Google Scholar]
- Margolin, K. , Ernstoff, M.S. , Hamid, O. , Lawrence, D. , McDermott, D. , Puzanov, I. , Wolchok, J.D. , Clark, J.I. , Sznol, M. , Logan, T.F. , Richards, J. , Michener, T. , Balogh, A. , Heller, K.N. , Hodi, F.S. , 2012. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 13, 459–465. [DOI] [PubMed] [Google Scholar]
- Marko, N.F. , Weil, R.J. , 2011. Medical oncology: patients with brain metastases in early-phase trials. Nat Rev Clin Oncol. 8, 390–391. [DOI] [PubMed] [Google Scholar]
- Masuda, T. , Hattori, N. , Hamada, A. , Iwamoto, H. , Ohshimo, S. , Kanehara, M. , Ishikawa, N. , Fujitaka, K. , Haruta, Y. , Murai, H. , Kohno, N. , 2011. Erlotinib efficacy and cerebrospinal fluid concentration in patients with lung adenocarcinoma developing leptomeningeal metastases during gefitinib therapy. Cancer Chemother Pharmacol. 67, 1465–1469. [DOI] [PubMed] [Google Scholar]
- McDermott, R. , Gabikian, P. , Sarvaiya, P. , Ulasov, I. , Lesniak, M.S. , 2013. MicroRNAs in brain metastases: big things come in small packages. J Mol Med (Berl). 91, 5–13. [DOI] [PubMed] [Google Scholar]
- Mego, M. , De Giorgi, U. , Dawood, S. , Wang, X. , Valero, V. , Andreopoulou, E. , Handy, B. , Ueno, N.T. , Reuben, J.M. , Cristofanilli, M. , 2011. Characterization of metastatic breast cancer patients with nondetectable circulating tumor cells. Int J Cancer. 129, 417–423. [DOI] [PubMed] [Google Scholar]
- Motzer, R.J. , Hutson, T.E. , Cella, D. , Reeves, J. , Hawkins, R. , Guo, J. , Nathan, P. , Staehler, M. , de Souza, P. , Merchan, J.R. , Boleti, E. , Fife, K. , Jin, J. , Jones, R. , Uemura, H. , De Giorgi, U. , Harmenberg, U. , Wang, J. , Sternberg, C.N. , Deen, K. , McCann, L. , Hackshaw, M.D. , Crescenzo, R. , Pandite, L.N. , Choueiri, T.K. , 2013. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 369, 722–731. [DOI] [PubMed] [Google Scholar]
- Nam, D.H. , Jeon, H.M. , Kim, S. , Kim, M.H. , Lee, Y.J. , Lee, M.S. , Kim, H. , Joo, K.M. , Lee, D.S. , Price, J.E. , Bang, S.I. , Park, W.Y. , 2008. Activation of notch signaling in a xenograft model of brain metastasis. Clin Cancer Res. 14, 4059–4066. [DOI] [PubMed] [Google Scholar]
- Nass, D. , Rosenwald, S. , Meiri, E. , Gilad, S. , Tabibian-Keissar, H. , Schlosberg, A. , Kuker, H. , Sion-Vardy, N. , Tobar, A. , Kharenko, O. , Sitbon, E. , Lithwick Yanai, G. , Elyakim, E. , Cholakh, H. , Gibori, H. , Spector, Y. , Bentwich, Z. , Barshack, I. , Rosenfeld, N. , 2009. MiR-92b and miR-9/9* are specifically expressed in brain primary tumors and can be used to differentiate primary from metastatic brain tumors. Brain Pathol. 19, 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navin, N. , Kendall, J. , Troge, J. , Andrews, P. , Rodgers, L. , McIndoo, J. , Cook, K. , Stepansky, A. , Levy, D. , Esposito, D. , Muthuswamy, L. , Krasnitz, A. , McCombie, W.R. , Hicks, J. , Wigler, M. , 2011. Tumour evolution inferred by single-cell sequencing. Nature. 472, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt, E.A. , Bauer, B. , Fahlke, C. , Fricker, G. , Iadecola, C. , Janigro, D. , Leybaert, L. , Molnar, Z. , O'Donnell, M.E. , Povlishock, J.T. , Saunders, N.R. , Sharp, F. , Stanimirovic, D. , Watts, R.J. , Drewes, L.R. , 2011. Engaging neuroscience to advance translational research in brain barrier biology. Nature reviews. Neuroscience. 12, 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, D.X. , Bos, P.D. , Massague, J. , 2009. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 9, 274–284. [DOI] [PubMed] [Google Scholar]
- Noda, M. , Seike, T. , Fujita, K. , Yamakawa, Y. , Kido, M. , Iguchi, H. , 2009. The role of immune cells in brain metastasis of lung cancer cells and neuron-tumor cell interaction. Rossiiskii fiziologicheskii zhurnal imeni I.M. Sechenova/Rossiiskaia akademiia nauk. 95, 1386–1396. [PubMed] [Google Scholar]
- Okuda, H. , Xing, F. , Pandey, P.R. , Sharma, S. , Watabe, M. , Pai, S.K. , Mo, Y.Y. , Iiizumi-Gairani, M. , Hirota, S. , Liu, Y. , Wu, K. , Pochampally, R. , Watabe, K. , 2013. miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res. 73, 1434–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owonikoko, T.K. , Arbiser, J. , Zelnak, A. , Shu, H.K. , Shim, H. , Robin, A.M. , Kalkanis, S.N. , Whitsett, T.G. , Salhia, B. , Tran, N.L. , Ryken, T. , Moore, M.K. , Egan, K.M. , Olson, J.J. , 2014. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 11, 203–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierga, J.Y. , Bidard, F.C. , Cropet, C. , Tresca, P. , Dalenc, F. , Romieu, G. , Campone, M. , Mahier Ait-Oukhatar, C. , Le Rhun, E. , Goncalves, A. , Leheurteur, M. , Domont, J. , Gutierrez, M. , Cure, H. , Ferrero, J.M. , Labbe-Devilliers, C. , Bachelot, T. , 2013. Circulating tumor cells and brain metastasis outcome in patients with HER2-positive breast cancer: the LANDSCAPE trial. Ann Oncol. 24, 2999–3004. [DOI] [PubMed] [Google Scholar]
- Porta, R. , Sanchez-Torres, J.M. , Paz-Ares, L. , Massuti, B. , Reguart, N. , Mayo, C. , Lianes, P. , Queralt, C. , Guillem, V. , Salinas, P. , Catot, S. , Isla, D. , Pradas, A. , Gurpide, A. , de Castro, J. , Polo, E. , Puig, T. , Taron, M. , Colomer, R. , Rosell, R. , 2011. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 37, 624–631. [DOI] [PubMed] [Google Scholar]
- Posner, J.B. , 1995. Neurologic Complications of Cancer F.A. Davis Co; Philadelphvia: 54–56. [Google Scholar]
- Ray, P.S. , Wang, J. , Qu, Y. , Sim, M.S. , Shamonki, J. , Bagaria, S.P. , Ye, X. , Liu, B. , Elashoff, D. , Hoon, D.S. , Walter, M.A. , Martens, J.W. , Richardson, A.L. , Giuliano, A.E. , Cui, X. , 2010. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 70, 3870–3876. [DOI] [PubMed] [Google Scholar]
- Rivera, E. , Meyers, C. , Groves, M. , Valero, V. , Francis, D. , Arun, B. , Broglio, K. , Yin, G. , Hortobagyi, G.N. , Buchholz, T. , 2006. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer. 107, 1348–1354. [DOI] [PubMed] [Google Scholar]
- Rochet, N.M. , Kottschade, L.A. , Markovic, S.N. , 2011. Vemurafenib for melanoma metastases to the brain. N Engl J Med. 365, 2439–2441. [DOI] [PubMed] [Google Scholar]
- Roy, V. , Perez, E.A. , 2009. Beyond trastuzumab: small molecule tyrosine kinase inhibitors in HER-2-positive breast cancer. Oncologist. 14, 1061–1069. [DOI] [PubMed] [Google Scholar]
- Saldana, S.M. , Lee, H.H. , Lowery, F.J. , Khotskaya, Y.B. , Xia, W. , Zhang, C. , Chang, S.S. , Chou, C.K. , Steeg, P.S. , Yu, D. , Hung, M.C. , 2013. Inhibition of type I insulin-like growth factor receptor signaling attenuates the development of breast cancer brain metastasis. PLoS One. 8, e73406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhia, B. , Kiefer, J. , Ross, J.T. , Metapally, R. , Martinez, R.A. , Johnson, K.N. , DiPerna, D.M. , Paquette, K.M. , Jung, S. , Nasser, S. , Wallstrom, G. , Tembe, W. , Baker, A. , Carpten, J. , Resau, J. , Ryken, T. , Sibenaller, Z. , Petricoin, E.F. , Liotta, L.A. , Ramanathan, R.K. , Berens, M.E. , Tran, N.L. , 2014. Integrated genomic and epigenomic analysis of breast cancer brain metastasis. PLoS One. 9, e85448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartz, N.E. , Farges, C. , Madelaine, I. , Bruzzoni, H. , Calvo, F. , Hoos, A. , Lebbe, C. , 2010. Complete regression of a previously untreated melanoma brain metastasis with ipilimumab. Melanoma Res. 20, 247–250. [DOI] [PubMed] [Google Scholar]
- Scott, B.J. , Kesari, S. , 2013. Leptomeningeal metastases in breast cancer. Am J Cancer Res. 3, 117–126. [PMC free article] [PubMed] [Google Scholar]
- Seoane, J. , De Mattos-Arruda, L. , 2014. The challenge of intratumour heterogeneity in precision medicine. J Intern Med. 10.1111/joim.12240 [DOI] [PubMed] [Google Scholar]
- Shah, S.P. , Roth, A. , Goya, R. , Oloumi, A. , Ha, G. , Zhao, Y. , Turashvili, G. , Ding, J. , Tse, K. , Haffari, G. , Bashashati, A. , Prentice, L.M. , Khattra, J. , Burleigh, A. , Yap, D. , Bernard, V. , McPherson, A. , Shumansky, K. , Crisan, A. , Giuliany, R. , Heravi-Moussavi, A. , Rosner, J. , Lai, D. , Birol, I. , Varhol, R. , Tam, A. , Dhalla, N. , Zeng, T. , Ma, K. , Chan, S.K. , Griffith, M. , Moradian, A. , Cheng, S.W. , Morin, G.B. , Watson, P. , Gelmon, K. , Chia, S. , Chin, S.F. , Curtis, C. , Rueda, O.M. , Pharoah, P.D. , Damaraju, S. , Mackey, J. , Hoon, K. , Harkins, T. , Tadigotla, V. , Sigaroudinia, M. , Gascard, P. , Tlsty, T. , Costello, J.F. , Meyer, I.M. , Eaves, C.J. , Wasserman, W.W. , Jones, S. , Huntsman, D. , Hirst, M. , Caldas, C. , Marra, M.A. , Aparicio, S. , 2012. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 486, 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, A.T. , Kim, D.W. , Nakagawa, K. , Seto, T. , Crino, L. , Ahn, M.J. , De Pas, T. , Besse, B. , Solomon, B.J. , Blackhall, F. , Wu, Y.L. , Thomas, M. , O'Byrne, K.J. , Moro-Sibilot, D. , Camidge, D.R. , Mok, T. , Hirsh, V. , Riely, G.J. , Iyer, S. , Tassell, V. , Polli, A. , Wilner, K.D. , Janne, P.A. , 2013. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 368, 2385–2394. [DOI] [PubMed] [Google Scholar]
- Siena, S. , Crino, L. , Danova, M. , Del Prete, S. , Cascinu, S. , Salvagni, S. , Schiavetto, I. , Vitali, M. , Bajetta, E. , 2010. Dose-dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: a multicenter phase II study. Ann Oncol. 21, 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedby, K.E. , Brandt, L. , Backlund, M.L. , Blomqvist, P. , 2009. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer. 101, 1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socinski, M.A. , Langer, C.J. , Huang, J.E. , Kolb, M.M. , Compton, P. , Wang, L. , Akerley, W. , 2009. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol. 27, 5255–5261. [DOI] [PubMed] [Google Scholar]
- Sottoriva, A. , Spiteri, I. , Piccirillo, S.G. , Touloumis, A. , Collins, V.P. , Marioni, J.C. , Curtis, C. , Watts, C. , Tavare, S. , 2013. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 110, 4009–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperduto, P.W. , Wang, M. , Robins, H.I. , Schell, M.C. , Werner-Wasik, M. , Komaki, R. , Souhami, L. , Buyyounouski, M.K. , Khuntia, D. , Demas, W. , Shah, S.A. , Nedzi, L.A. , Perry, G. , Suh, J.H. , Mehta, M.P. , 2013. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 85, 1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg, P.S. , Camphausen, K.A. , Smith, Q.R. , 2011. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 11, 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer, K.J. , 2013. Epidemiology and prognosis of brain metastases. Surg Neurol Int. 4, S192–S202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, D.J. , 1994. A critique of the role of the blood-brain barrier in the chemotherapy of human brain tumors. J Neurooncol. 20, 121–139. [DOI] [PubMed] [Google Scholar]
- Swanton, C. , 2014. Cancer evolution: the final frontier of precision medicine?. Ann Oncol. 25, 549–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabouret, E. , Chinot, O. , Metellus, P. , Tallet, A. , Viens, P. , Goncalves, A. , 2012. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 32, 4655–4662. [PubMed] [Google Scholar]
- Trudeau, M.E. , Crump, M. , Charpentier, D. , Yelle, L. , Bordeleau, L. , Matthews, S. , Eisenhauer, E. , 2006. Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada – Clinical Trials Group (NCIC-CTG). Ann Oncol. 17, 952–956. [DOI] [PubMed] [Google Scholar]
- Valiente, M. , Obenauf, A.C. , Jin, X. , Chen, Q. , Zhang, X.H. , Lee, D.J. , Chaft, J.E. , Kris, M.G. , Huse, J.T. , Brogi, E. , Massague, J. , 2014. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 156, 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brom, R.R. , de Vries, E.G. , Schroder, C.P. , Hospers, G.A. , 2013. Effect of vemurafenib on a V600R melanoma brain metastasis. Eur J Cancer. 49, 1795–1796. [DOI] [PubMed] [Google Scholar]
- Wagle, N. , Emery, C. , Berger, M.F. , Davis, M.J. , Sawyer, A. , Pochanard, P. , Kehoe, S.M. , Johannessen, C.M. , Macconaill, L.E. , Hahn, W.C. , Meyerson, M. , Garraway, L.A. , 2011. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 29, 3085–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, J.S. , Amin, A. , Minor, D. , Siegel, J. , Berman, D. , O'Day, S.J. , 2011. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma Res. 21, 530–534. [DOI] [PubMed] [Google Scholar]
- Weigelt, B. , Peterse, J.L. , van 't Veer, L.J. , 2005. Breast cancer metastasis: markers and models. Nat Rev Cancer. 5, 591–602. [DOI] [PubMed] [Google Scholar]
- Weil, R.J. , Palmieri, D.C. , Bronder, J.L. , Stark, A.M. , Steeg, P.S. , 2005. Breast cancer metastasis to the central nervous system. Am J Pathol. 167, 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh, J.W. , Komaki, R. , Amini, A. , Munsell, M.F. , Unger, W. , Allen, P.K. , Chang, J.Y. , Wefel, J.S. , McGovern, S.L. , Garland, L.L. , Chen, S.S. , Holt, J. , Liao, Z. , Brown, P. , Sulman, E. , Heymach, J.V. , Kim, E.S. , Stea, B. , 2013. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 31, 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, E.T. , Berkenblit, A. , 2004. The role of topotecan in the treatment of brain metastases. Oncologist. 9, 68–79. [DOI] [PubMed] [Google Scholar]
- Wood, S.L. , Pernemalm, M. , Crosbie, P.A. , Whetton, A.D. , 2014. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev. 40, 558–566. [DOI] [PubMed] [Google Scholar]
- Wu, C. , Li, Y.L. , Wang, Z.M. , Li, Z. , Zhang, T.X. , Wei, Z. , 2007. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer. 57, 359–364. [DOI] [PubMed] [Google Scholar]
- Wu, X. , Northcott, P.A. , Dubuc, A. , Dupuy, A.J. , Shih, D.J. , Witt, H. , Croul, S. , Bouffet, E. , Fults, D.W. , Eberhart, C.G. , Garzia, L. , Van Meter, T. , Zagzag, D. , Jabado, N. , Schwartzentruber, J. , Majewski, J. , Scheetz, T.E. , Pfister, S.M. , Korshunov, A. , Li, X.N. , Scherer, S.W. , Cho, Y.J. , Akagi, K. , MacDonald, T.J. , Koster, J. , McCabe, M.G. , Sarver, A.L. , Collins, V.P. , Weiss, W.A. , Largaespada, D.A. , Collier, L.S. , Taylor, M.D. , 2012. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature. 482, 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y.L. , Zhou, C. , Cheng, Y. , Lu, S. , Chen, G.Y. , Huang, C. , Huang, Y.S. , Yan, H.H. , Ren, S. , Liu, Y. , Yang, J.J. , 2013. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol. 24, 993–999. [DOI] [PubMed] [Google Scholar]
- Yamamoto, D. , Iwase, S. , Tsubota, Y. , Sueoka, N. , Yamamoto, C. , Kitamura, K. , Odagiri, H. , Nagumo, Y. , 2012. Bevacizumab in the treatment of five patients with breast cancer and brain metastases: Japan Breast Cancer Research Network-07 trial. OncoTargets Ther. 5, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YS, L. , WW, C. , CH, L. , LM, T. , DC, Y. , PF, W. , BB, C. , TC, C. , YF, T. , SM, H. , T, S. , AL, C. , 2012. Bevacizumab, etoposide, and cisplatin (BEEP) in brain metastases of breast cancer progressing from radiotherapy: results of the first stage of a multicenter phase II study. J Clin Oncol. 30, [Google Scholar]
- Zhang, L. , Ridgway, L.D. , Wetzel, M.D. , Ngo, J. , Yin, W. , Kumar, D. , Goodman, J.C. , Groves, M.D. , Marchetti, D. , 2013. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med. 5, 180ra148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, C. , Xu, Y. , Zhang, Y. , Tan, W. , Xue, J. , Yang, Z. , Lu, Y. , Hu, X. , 2013. Downregulation of miR-145 contributes to lung adenocarcinoma cell growth to form brain metastases. Oncol Rep. 30, 2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W. , Fong, M.Y. , Min, Y. , Somlo, G. , Liu, L. , Palomares, M.R. , Yu, Y. , Chow, A. , O'Connor, S.T. , Chin, A.R. , Yen, Y. , Wang, Y. , Marcusson, E.G. , Chu, P. , Wu, J. , Wu, X. , Li, A.X. , Li, Z. , Gao, H. , Ren, X. , Boldin, M.P. , Lin, P.C. , Wang, S.E. , 2014. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 25, 501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]


