Abstract
The CA125 assay detects circulating MUC16 and is one of the most widely used cancer biomarkers for the follow‐up of ovarian cancer. We previously demonstrated that detection of aberrant cancer‐associated glycoforms of MUC16 as well as MUC1 in circulation could improve the yield of these serum assays. Our aim was to refine ovarian cancer biomarkers by detection of aberrant glycoforms (Tn, STn, and T) of MUC16 and MUC1 in ovarian cancer tissue using Proximity Ligation Assays (PLA).
We studied two series of serous ovarian tumours, a pilot series of 66 ovarian tumours (27 cystadenomas, 16 borderline tumours and 23 adenocarcinomas) from Centro Hospitalar S. João, Porto and a validation series of 89 ovarian tumours (17 cystadenomas, 25 borderline tumours and 47 adenocarcinomas) from the Portuguese Institute of Oncology Francisco Gentil, Lisbon.
PLA reactions for MUC16/Tn, MUC16/STn, MUC1/Tn and MUC1/STn were negative in benign lesions but often positive in borderline and malignant lesions, in both series. An even better yield was obtained based on positivity for any of the four glyco‐mucin profiles, further increasing sensitivity to 72% and 83% in the two series, respectively, with 100% specificity. The strategy is designated glyco‐mucin profiling and provides strong support for development of PLA‐based serum assays for early diagnosis.
Keywords: Ovarian cancer, Proximity ligation assay, MUC16, MUC1, Glyco‐mucin profile
1. Introduction
Worldwide, ovarian cancer is the 7th cancer with highest incidence and the 8th cause of cancer death in women (Ferlay et al., 2012). The large majority of ovarian cancers are epithelial in origin and, within those, serous carcinomas constitute nearly 70% (Seidman et al., 2004) and represent the most relevant contributors to ovarian cancer mortality (Prat, 2012). Despite the downward trends in survival (Barnholtz‐Sloan et al., 2003; De Angelis et al., 2014) and in mortality rates (Hirabayashi and Marugame, 2009) observed in the last decades in many settings, there is a large heterogeneity in the across countries and an ample margin for improving diagnosis at treatable stages. This may be accomplished through the development of new strategies or combination of existing methods, capable to overcome limitations of those currently available.
The CA125 assay is the most successful cancer serum biomarker and it detects the large mucin MUC16 (Bast et al., 1981), which is heavily decorated with N and O‐glycans (Hattrup and Gendler, 2008). The CA125 assay is approved for monitoring ovarian cancer patients after treatment to predict recurrence but not for diagnostic purposes due to low specificity (Goonewardene et al., 2007). More recently, attempts were made to combine several tumor markers to increase sensitivity and specificity of cancer detection (Kondalsamy‐Chennakesavan et al., 2013), but CA125 is still the preferred biomarker (Cramer et al., 2011; Mai et al., 2011; Zhu et al., 2011). Several other membrane‐bound mucins, including MUC1 and MUC4, are overexpressed in ovarian carcinomas, although these are more widely expressed in other organs (Singh et al., 2008; Skates et al., 2004). Increased serum levels of MUC16 in non‐malignant gynecological conditions and diseases, especially in those that produce ascites, are serious limitations to the use of serum CA125 assay as a diagnostic tool. The CA125 assay is based on detection of MUC16 with monoclonal antibodies such as OC125 (Bast et al., 1981) and M11 (Nustad et al., 1996) that react with similar epitopes in the tandem repeat SEA region of protein (Bressan et al., 2013; Marcos‐Silva et al., 2014). We recently confirmed that these antibodies bind the protein backbone, albeit a particular conformational epitope, without substantial influence by glycosylation (Marcos‐Silva et al., 2014).
Mucins are characterized by dense decoration of O‐glycans and these O‐glycans themselves may serve as biomarkers. Thus, perhaps the most common phenotypic character of carcinoma cells is expression of truncated immature O‐glycans due to a variety of mechanisms leading to incomplete O‐glycosylation (Gill et al., 2011). Several studies showed expression of T (Galβ1‐3GalNAc‐α1‐O‐Ser/Thr), Tn (GalNAcα1‐O‐Ser/Thr) and STn (Neu5Acα2‐6GalNAcα1‐O‐Ser/Thr) in carcinoma cells in effusions (Davidson et al., 2000) and in tissue sections, where both Tn and STn correlated with higher histological grade and poorer survival (Ghazizadeh et al., 1997). In ovarian carcinomas STn antigen was detected on MUC16 using a sandwich ELISA on peritoneal fluid of ovarian cancer patients and co‐localizes with MUC16 on cancer tissues (Akita et al., 2012). Furthermore, we recently demonstrated that circulating MUC16 in ovarian cancer patients carry the STn glycosylation, and that selective detection of the STn MUC16 glycoform using an antibody capture array assay improved specificity of MUC16 compared to the CA125 assay (Chen et al., 2013). This suggests that detection of specific glycoforms of circulating mucins may represent a strategy towards improved biomarker assays. However, we identified sensitivity of the array assay as an obstacle in that the array assay performed equal or poorer than the routine CA125 assay in terms of overall sensitivity. This limits its use for early diagnosis regardless of the improved specificity.
Here, we have further studied the expression of glycoforms of mucins in ovarian cancer and developed highly sensitive mucin glycoform specific Proximity Ligation Assays (PLA). As a proof of concept we demonstrate that selective detection of Tn and STn glycoforms of MUC16 as well as MUC1 provide high specificity and sensitivity in tissue screening of ovarian serous neoplasia. We have previously demonstrated that PLA (Soderberg et al., 2006) can successfully be applied in tissue sections for detection of glycoforms of mucins (Conze et al., 2010), and we identified Tn/STn glycoforms of MUC1 in a large percentage of mucinous ovarian carcinomas (Pinto et al., 2012). We studied a total of 155 serous ovarian tumours (44 cystadenomas, 41 borderline tumours and 70 adenocarcinomas), encompassing a test and a validation series, using PLA probes for MUC1, MUC16, Tn, STn, and T antigens. Our results show that identification of MUC1 and MUC16 glycoforms with the Tn and STn glycans constitute a very promising biomarker signature to distinguish malignant/borderline serous ovarian tumours from benign lesions. The strategy is designated glyco‐mucin profiling and the results provide strong support for further development of PLA‐based serum assays with the potential for early diagnosis.
2. Materials and methods
2.1. Patient selection and tissue microarray construction
Pilot study: A series of 66 serous ovarian tumours, diagnosed between 2000 and 2012, was selected from the Pathology Department of Centro Hospitalar S. João, Porto, Portugal (CHSJ Porto series). The series was extracted from all ovarian lesions surgically removed in the same period (n = 1492, including occasional findings in hysterectomy specimens, metastasis, etc) and the 66 cases were selected on the basis of the quality/representativity of the histological material, clinical information and staging. Formalin‐fixed paraffin‐embedded histological sections were reviewed and the diagnosis confirmed. Patients' ages ranged from 25 to 88 years. Tumours were characterized for histologic type and stage (FIGO classification) (Supplementary Table S1).
From the 66 cases, 44 were arrayed in six Tissue Microarray (TMA) blocks with at least two tissue cores (1.5 mm in diameter) from each tumour sample. TMAs were built after careful review of hematoxilin & eosin stained sections by an experienced pathologist (LD) with selection of representative tumour areas. TMA blocks were designed and constructed according to rules previously described (Avninder et al., 2008; Simon et al., 2004). The remaining 22 cases were whole tissue sections. Overall, the CHSJ Porto series comprises 35% adenocarcinoma, 24% borderline tumours and 41% cystadenomas.
Validation study: For validation, we tested a second series in the same conditions. The validation series encompasses 89 serous ovarian tumours (17 cystadenomas, 25 borderline tumours and 47 adenocarcinomas) arrayed in 5 TMA blocks with 0.6 mm core diameter, obtained from Portuguese Institute of Oncology Francisco Gentil, Lisbon, Portugal (IPO Lisbon series) files. In this institution 90–100 cases of ovarian masses undergo surgery as primary treatment every year, and we retrieved serous tumour cases from years 2000–2013 on the basis of the quality/representativity of the histological material, clinical information and staging. Clinico‐pathological characteristics of IPO Lisbon series are shown in Supplementary Table S1. A flow chart representing the samples tested and the steps of the study is represented in Figure 1.
Figure 1.
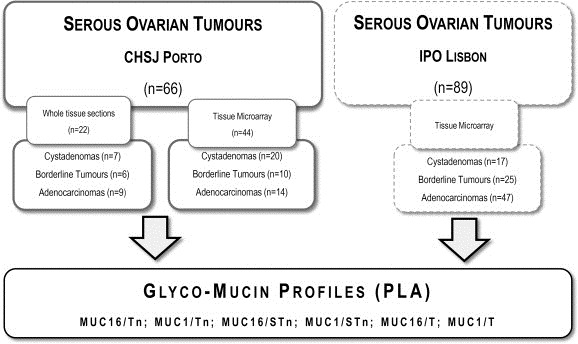
Flow chart of the study. The initial series (CHSJ Porto) consisted of 66 serous ovarian tumours in which we performed Proximity Ligation Assays (PLA) to define the glyco‐mucin profiles. To validate the results, PLA to the same glycol‐mucin pairs was performed in 89 serous ovarian tumours from another institution (IPO Lisbon).
Both pilot and validation studies were based on retrospective assessment of samples in which informed consent could not be obtained, but where research studies are authorized under the Portuguese Law.
2.2. Standard or direct immunohistochemistry
Tumours were immunostained with monoclonal antibodies for MUC16 (M11 and 5E11) (Marcos‐Silva et al., unpublished data), MUC1 (HMFG2) (Taylor‐Papadimitriou et al., 1981), Tn (5F4) (Mandel et al., 1991), STn (TKH2) (Kjeldsen et al., 1988), and T (3C9) (Zen et al., 1998). After deparaffinization, heat‐induced (98 °C) antigen retrieval was performed with citrate buffer (pH 6.0) (CE IVD by Thermo Scientific Lab Vision), and slides were incubated with hydrogen peroxide 3%. Antibodies were incubated undiluted (hybridoma culture supernatants) for 1h at room temperature. Primary antibodies were detected using a secondary antibody with HRP polymer (Cytomation Envision System HRP, DAKO, Carpinteria, CA) and visualization of the reaction was performed using diaminobenzidine according to the manufacturer's instructions. Since PLA assays require the use of two antibodies in a single reaction, efforts were made to homogenize IHC conditions.
An additional slide with positive tissues for each antibody were included in every set of reactions and used as positive control. Normal ovary/cystadenomas and other human tissues included in the TMAs were used as internal controls.
Immunohistochemistry was evaluated by two independent observers (SR and LD), who independently registered cytolocalization of staining and the percentage of tumor cells stained (0–10%, >10–25%, >25–50%, >50–75% and >75%). When less than 10% of the tumour cells were stained, cases were considered negative.
2.3. Proximity Ligation Assays on tissue sections
Validation of PLA assays was tested in the MUC16 expressing cell lines OVCAR3 wild‐type, which has elongated O‐glycans (Kui Wong et al., 2003), and OVCAR3 SimpleCells (SC), that express homogenous truncated Tn and STn O‐glycans due to knock‐out for the COSMC gene (Steentoft et al., 2013). This isogenic cell system provided a unique opportunity to validate PLA results based on MUC16 mucin (Supplementary Figure S1).
PLAs were performed using the Duolink in situ Detection Reagents Brightfield (Olink® Bioscience, Uppsala, Sweden) according to the manufacturer's instructions. Briefly, after deparaffinization and heat‐induced antigen retrieval, tissue slides were incubated with hydrogen peroxide 3% followed by incubation at 37 °C for 30 min with blocking solution in a humidity chamber.
The mAbs used to Mucins are IgG isotypes and therefore detected using an anti‐IgGγ specific conjugated PLA Probe PLUS (4.8 ng/μl). Antibodies for simple mucin‐type carbohydrate antigens T and Tn (both IgM) were detected using an anti‐IgM conjugated PLA Probe MINUS (0.0048 μg/μl). Primary antibodies were incubated in the same conditions used for IHC. For identification of MUC1 and MUC16 with STn a direct PLA assay was developed since our mAbs to STn is of IgG1 isotype similar to the mucin mAbs. mAbs to MUC1 and MUC16 were conjugated with PLA probe PLUS (concentration 0.005 μg/μl) and the mAb to STn with PLA probe MINUS (concentration 5 ng/μl). For direct PLA we used CA125 (Clone M11; DAKO, Carpinteria, CA) to detect MUC16 as conjugation of 5E11 with the PLA probe resulted in loss of activity. The conjugation of the antibodies was performed following the instructions of Duolink® In situ Probemaker.
Antibodies conjugated with PLA probes were hybridized for 1 h at 37 °C. Next, ligation was performed for 30 min at 37 °C and amplification was carried out for 120 min at 37 °C to produce rolling circle products, followed by incubation with HRP labelled probes and addition of the chromogen. Finally, sections were counterstained using hematoxilin, dehydrated, cleared and mounted for optical microscope analysis.
PLA results were evaluated by two observers (SR and LD), who independently registered cytolocalization of staining and the percentage of tumor cells stained (0–10%, >10–25%, >25–50%, >50–75% and >75%). When less than 10% of the tumour cells were stained, cases were considered negative.
2.4. Statistical analysis
We evaluated the expression of different PLAs or combinations of PLAs according to histological diagnosis and computed the sensitivity and specificity for distinguishing between malignant or borderline serous tumours and benign lesions (STATA, version 11; STATA Corp., College Station, Texas, USA).
3. Results
3.1. Pilot study ‐ expression of mucins and truncated O‐glycans in ovarian tumours
MUC16 and MUC1 were highly expressed in malignant and borderline lesions (MUC16 was expressed in 100% of the cases both with M11 and 5E11, MUC1 was observed in 87% of adenocarcinomas and 100% of borderline tumours) and less frequently expressed in benign lesions (85% for MUC16; 37% for MUC1). The Tn O‐glycan antigen was only expressed in adenocarcinomas (78%) and borderline lesions (13%), and not in benign lesions (0%). STn and T antigens were more frequently expressed in adenocarcinomas and borderline tumours. STn was expressed in 91% adenocarcinomas and 81% in borderline tumours, while T was expressed in 87% adenocarcinomas and 75% in borderline tumours. STn was, similarly to Tn, not expressed in benign lesions (0%), while the T antigen was expressed in 30% benign lesions. These results contrast with ST expression (in fact T and ST since detection was performed using 3C9 antibody after neuraminidase treatment of the sections that uncovers cryptic, sialylated T antigen) that was present in 100% of all tumour sub‐groups (data not shown). Details on the expression profile of ovarian tumours are presented in Supplementary Figure S2 and Supplementary Table S2.
3.2. Pilot study (CHSJ Porto series) – glyco‐mucin profiling of tissue sections by PLA
In positive cases, PLA signals were observed mainly in the apical membrane of tumour cells and mucus secretions, although some cytoplasmic signals were also identified.
PLA reactions for MUC16/Tn, MUC16/STn and MUC1/STn were negative in benign lesions but often positive in borderline lesions (19%, 31% and 25%, respectively) and even more frequently positive in malignant tumours (52%, 74% and 61% respectively). Expression of MUC1/Tn occurred only in malignant cases (61%). MUC16/T was expressed more frequently in malignant and borderline than in benign lesions (35%, 31% and 7%, respectively) and MUC1/T was more frequent in malignant (35%) than in borderline or benign lesions (6% and 4%, respectively) (Table 1). Images of brightfield PLA staining in situ and glyco‐mucin signatures of ovarian tumours are represented in Figure 2 and 3A, respectively. In a few cases we looked at serial sections trying to clarify if a single carbohydrate was using the two mucins as acceptors or if different areas/cells were building different glyco‐mucin profiles, and, as shown in Supplementary Figure S3, both events do occur.
Table 1.
Glyco‐Mucin profile obtained through PLA assays in 66 cases from CHSJ Porto series.
| % Positive | Adenocarcinoma/borderline vs. cystadenoma | ||||
|---|---|---|---|---|---|
| Adenocarcinoma | Borderline tumours | Cystadenoma | Sensitivity (%) | Specificity (%) | |
| MUC16/Tn | 52 | 19 | 0 | 38 | 100 |
| MUC16/STn | 74 | 31 | 0 | 56 | 100 |
| MUC16/T | 35 | 31 | 7 | 33 | 93 |
| MUC1/Tn | 61 | 0 | 0 | 36 | 100 |
| MUC1/STn | 61 | 25 | 0 | 46 | 100 |
| MUC1/T | 35 | 6 | 4 | 23 | 96 |
| MUC16/STn OR MUC1/STn | 83 | 44 | 0 | 67 | 100 |
| MUC16/STn OR MUC1/STn OR | |||||
| MUC16/Tn OR MUC1/Tn | 91 | 44 | 0 | 72 | 100 |
Figure 2.
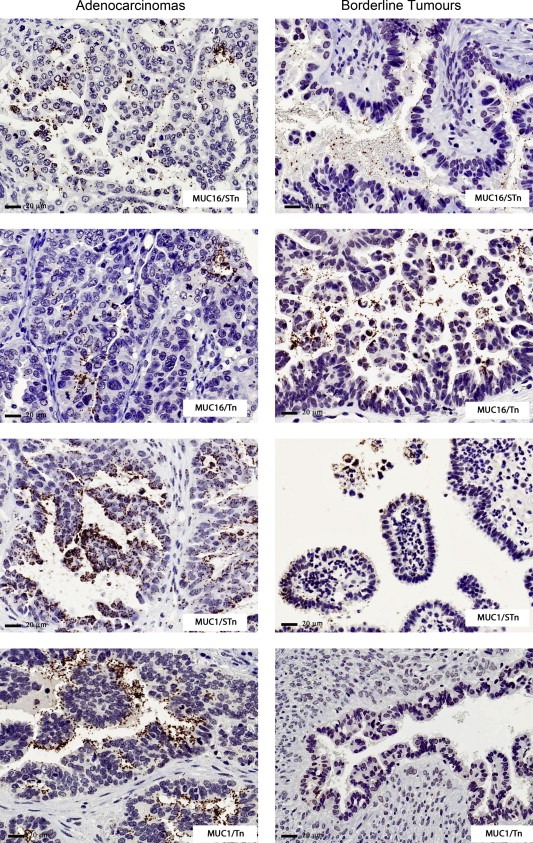
Glyco‐mucin profile of ovarian tumours in CHSJ Porto series. Examples of brightfield PLAs in adenocarcinomas and borderline lesions. The brown dots represent proximity signals between mucin/carbohydrate pairs, namely MUC16/Tn, MUC1/Tn, MUC16/STn and MUC1/ST.
Combinations of PLA results for different glycoforms of mucins increase or maximize sensitivity and specificity of the biomarkers when compared to mucins, glycans or even single glyco‐mucin pairs, to separate borderline and malignant serous adenocarcinomas from benign lesions. Positivity for MUC16/STn or MUC1/STn increased the sensitivity to 67% and specificity to 100%. An even better yield was obtained based on positivity for MUC16/STn, MUC1/STn, MUC16/Tn or MUC1/Tn, further increasing sensitivity to 72% maintaining the maximum value for specificity (Table 1). The positive predictive value for double or quadruple PLA combinations was 100%.
3.3. Validation study (IPO Lisbon series) ‐ glyco‐mucin profiling of tissue sections by PLA
The PLA results were similar to those obtained in the pilot study. PLA reactions for MUC16/Tn, MUC16/STn, MUC1/Tn, MUC1/STn and MUC1/T were negative in benign lesions but often positive in borderline (52%, 68%, 12%, 24% and 12%, respectively) and in malignant lesions (43%, 45%, 49%, 64% and 28%, respectively) (Table 2). MUC16/T was expressed in benign, borderline and malignant lesions (12%, 48% and 17%, respectively).
Table 2.
Glyco‐Mucin profile obtained through PLA assays in 89 cases from IPO Lisbon series.
| % Positive | Adenocarcinoma/borderline vs. cystadenoma | ||||
|---|---|---|---|---|---|
| Adenocarcinoma | Borderline tumours | Cystadenoma | Sensitivity (%) | Specificity (%) | |
| MUC16/Tn | 43 | 52 | 0 | 46 | 100 |
| MUC16/STn | 45 | 68 | 0 | 53 | 100 |
| MUC16/T | 17 | 48 | 12 | 28 | 88 |
| MUC1/Tn | 49 | 12 | 0 | 36 | 100 |
| MUC1/STn | 64 | 24 | 0 | 50 | 100 |
| MUC1/T | 28 | 12 | 0 | 22 | 100 |
| MUC16/STn OR MUC1/STn | 70 | 72 | 0 | 71 | 100 |
| MUC16/STn OR MUC1/STn OR | 85 | 80 | 0 | 83 | 100 |
| MUC16/Tn OR MUC1/Tn | |||||
Positivity for MUC16/STn or MUC1/STn increased the sensitivity to 71% and specificity to 100%. An even better yield was obtained based on positivity for MUC16/STn, MUC1/STn, MUC16/Tn or MUC1/Tn, further increasing sensitivity to 83% maintaining the maximum value for specificity (Table 2, Figure 3B). The positive predictive value for double or quadruple PLA combinations was 100%.
Figure 3.
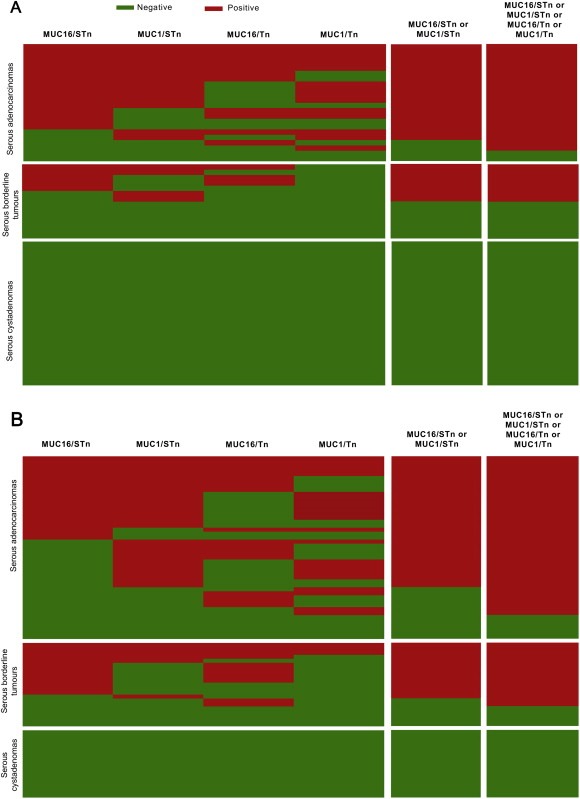
Glyco‐mucin signatures obtained by PLA (Green = Negative; Red = Positive): (A) Representation of PLA results in the 66 ovarian tumours of CHSJ Porto series; (B) Representation of PLA results in the 89 ovarian tumours of IPO Lisbon series. The two columns on the right depict combinations for PLA pairs.
4. Discussion
Our study provides strong support for use of PLAs to detect aberrant glycoforms of mucins and thereby improve cancer specificity of biomarker assays. We demonstrated that serous ovarian carcinomas selectively express the Tn and STn glycoforms of MUC16 and MUC1, and these glyco‐mucin profiles are likely to serve as more cancer‐specific biomarkers than the mucins themselves. We have also observed, in serial sections, that single tumours have areas/cells that use the two mucins as acceptors for addition of a glycan, whereas in other areas/tumours a preferred acceptor is used (Supplementary Figure 3). This observation is by itself very interesting since it adds to our previous observations showing that co‐localization does not imply generation of PLA signals (Pinto et al., 2012). In fact, it suggests that, at the single cell level, there might be an ambivalent usage of one or more than one mucin carriers according to still unclarified reasons. Future studies using triple‐binder PLA reactions, optimized in cell systems, will be a major step to clarify if mucins can carry single or multiple carbohydrate combinations at the single cell/molecule level and if this is a cell or “territory” phenomenon (Soderberg et al., 2006).
Our glyco‐mucin profiles support a previous microarray study from our group (Chen et al., 2013), where we glycoprofiled MUC16 and MUC1 in serum using antibody capture arrays and lectin profiling, but holds promise for improving the sensitivity because PLA has essentially unlimited amplification potential if a differential signal is available.
We found that four glyco‐mucin profiles, Tn and STn on the mucins MUC16 and MUC1, have the capacity to distinguish borderline and malignant serous tumours from benign lesions with a specificity of 100% and a sensitivity of 70–80% on tissues. These values were identified in an initial series of 66 cases from Porto, and validated on a second series of 89 cases from another Institution in Portugal – IPO Lisbon. At the tissue level a diagnostic panel based on MUC16 and MUC1 glycoforms clearly outperforms detection of MUC16, MUC1, or any of the glycoforms alone with respect to specificity.
The single antigen that provided specificity close to the PLA assays was the STn glycan structure. The STn O‐glycan is widely expressed in cancer and was originally identified as the B72.3 colorectal cancer antigen (Kjeldsen et al., 1988), although it was (and is still) unclear what the carrier protein molecule(s) for the B72.3 epitope are. Our previous studies in mucinous colorectal carcinomas suggested that at least part of the STn antigen is found on MUC2 mucin (Conze et al., 2010). The STn antigen was not expressed in benign cystadenomas, but it is found in many different adenocarcinomas including colon cancers. The MUC16 mucin provides the organ specificity to the combination assay of glyco‐mucin profile, since MUC16 is only found in the normal eye (Gipson, 2004) and pancreatic cancers (Haridas et al., 2011).
Our results hold promise for development of more specific serum biomarker assays based on CA125 and CA15‐3. In fact, previous studies have consistently concluded that MUC16, despite its limitations, was the better cancer biomarker (Cramer et al., 2011; Zhu et al., 2011). Most importantly, specificity in distinguishing malignant versus benign lesions is 75% for CA125 assay (Jacobs and Bast, 1989), much lower than the 100% value obtained with the combined set of biomarkers in both series.
Recent studies have added Human Epididymis secretory protein (HE4) into the Risk of Ovarian Malignancy Algorithm (ROMA) (Moore et al., 2010), reaching sensitivities around 90% in post‐menopausal women, and specificities that can go up to 97%, when coupled to CT‐scan (Stiekema et al., 2014). However, the additional value of including the HE4 in ovarian cancer detection is still under scrutiny (Van Gorp et al., 2011).
Although it clearly is not simple to extrapolate the current results based on tissue to assays based on serum, our current study clearly demonstrates that ovarian cancers produce MUC16 and MUC1 with aberrant Tn and STn glycosylation. We are furthermore encouraged by our previous microarray studies that confirmed circulation of the same STn glycoforms of MUC16 and MUC1 (Akita et al., 2012). In the array study we did not detect the Tn glycoforms, which we interpreted as being a result of clearance of non‐sialylated glycoforms by lectin receptors. Since we demonstrated here that ovarian cancers indeed express both the Tn and STn glycoforms our present results appear to support this conclusion. Thus, future development of a PLA‐based serum assay should focus on the STn glycoforms of MUC16 and MUC1. While the array assay clearly demonstrated that detection of the STn glycoforms of MU16 and MUC1 improved specificity compared to the standard CA125 and CA15‐3 serum assays it did not improve overall sensitivity. We believe that a PLA‐based assay may enhance sensitivity without loss of specificity of CA125 serum assay and thus has the potential for an improved biomarker assay with utility in early diagnosis.
The PLA approach has great prospects in the biomarker field for detection of glycoforms of glycoproteins in general, as we have previously demonstrated (Conze et al., 2010; Soderberg et al., 2006). Alternative methods, like Forster resonance energy transfer (FRET) have several limitations, including autofluorescence that is usually high in paraffin‐embedded tissue sections (Weibrecht et al., 2010). Recently, adaptations on the FRET methodology have allowed the identification of protein specific imaging of glycans on live cells, with very interesting applications to visualize glycosylation dynamics, but still without a conceivable application for biomarker identification in the clinic (Lin et al., 2014). Also recently, proteomics approaches identified combinations of mucin peptides, irrespective of its glycosylation, in pancreatic cyst fluid from pancreatic precursor lesions with high discrimination capacity (Jabbar et al., 2014). Glycomic approaches in serum of ovarian cancer patients have mostly identified N‐glycan profiles as candidate biomarkers (Kim et al., 2014). The advantages with PLA for analysis of serum levels of proteins are the high sensitivity, minute sample consumption (1–10 μl) (Fredriksson et al., 2002; Gullberg et al., 2004) and ability for multiplexing (Darmanis et al., 2010; Fredriksson et al., 2008). A related method, proximity extension assay (PEA) has been used to perform parallel analysis of 96 proteins in 96 samples using only 1 μl serum per sample (Assarsson et al., 2014).
A very important feature of our methodology is that it combines mucin identification with cancer‐associated glycan identification, each per se already in clinical use, but whose combination significantly increased the yield of sensitivity and specificity of detection of borderline and malignant serous adenocarcinomas versus benign lesions. One of the aims of ovarian cancer biomarkers is to detect by simple methods, ideally a serum assay, women that should be considered as candidates for a surgical approach. Particularly relevant is the follow‐up of women at high risk for ovarian cancer, like those with BRCA germline mutations (Karlan et al., 2014). Another major objective for all ovarian cancer biomarkers is to identify cystic lesions that should be targeted for a therapeutic/surgical approach. At this moment both borderline and invasive serous adenocarcinomas are mandatory candidates for surgery and therefore their identification prior to surgery would benefit all those that could be spared oophorectomy. On the other hand, early detection of borderline/malignant lesions would contribute to a decrease in late diagnosis, FIGO stages III–IV, a major determinant for poor survival (Assarsson et al., 2014; Curry et al., 2014).
In conclusion, we have developed a series of PLA assays for detection of glyco‐mucin profiles using a novel approach for glycopeptide identification, with the capability to detect mucins shed from cancer tissues. Through PLA assay, we have identified a panel of four glyco‐mucin profiles (MUC16/Tn, MUC16/STn, MUC1/Tn and MUC1/STn) with 100% specificity and 100% Positive Predictive Value for detection of borderline/malignant serous tumours of the ovary. The results show that ovarian cancer produces aberrantly glycosylated MUC16 and MUC1, which provides basis and further support for development of a PLA‐based serum assay with high specificity to improve CA125 and CA15‐3 serum assays. We propose to designate the approach glyco‐mucin profiling, and this would be applicable to other biomarkers based on proteins with O‐glycosylation. The multiple recognitions provided by PLA and PEA on multiplexed assays can generate signals with up to five antibody recognition events as previously shown in prostasomes detected in serum of aggressive prostate cancer patients (Tavoosidana et al., 2011). A multiplexed PLA, or PEA, assay that targets glyco‐mucin profiles brings hope to enhance specificity of biomarker assays in combination with enhanced sensitivity such that these serum biomarker assays may serve in early diagnosis/screening strategies.
Supporting information
The following are the supplementary data related to this article:
Supplementary data
Supplementary Figure S1 Immunohistochemistry staining pattern of mucins MUC16 and MUC1 and simple mucin‐type carbohydrate antigens Tn, STn, and T in serous adenocarcinomas, serous borderline tumours and cystadenomas from CHSJ Porto series.
Supplementary Figure S2 Expression and glyco‐mucin profile of wild‐type (WT) and SimpleCell (SC) (REF: EMBO paper) OVCAR‐3 isogenic cells. A. Immunofluorescence (IF) to evaluate expression of STn, Tn, T and MUC16. Cells were cultured on glass coverslips, and fixed with 4% paraformaldehyde during 20 min on ice. After fixation, cells were washed with PBS and incubated with primary antibodies TKH2 (STn), 5F4 (Tn), 3C9 (T) and M11 (MUC16), overnight at 4 °C. Bound antibodies were detected at RT with rabbit anti‐mouse conjugated with FITC (1:100). After washing with PBS, each sample was mounted with Duolink Mounting Medium with DAPI. Both cell lines are positive for MUC16, OVCAR‐3 SC show higher expression of STn and Tn and T antigen is completely negative in OVCAR3 SC, as expected. B. PLA assay was performed using the Duolink in situ Detection Reagents Fluorescence (Olink® Bioscience, Uppsala, Sweden) according to the manufacturer's instructions. The concentration for primary antibodies and PLA probes were the same described in material and methods. Our results show that MUC16 is a carrier of STn, Tn and T in OVCAR3 WT and that MUC16/STn and MUC16/Tn increase in OVCAR‐3 SC, whereas MUC16/T is completely negative. The IF and PLA staining were observed with a Zeiss microscope (Imager Z1), and images were acquired using the Axiovision software at 200× magnification. Scale bar, 20 μm. Samples were examined under a Zeiss Imager.Z1 Axio fluorescence microscope equipped with DAPI and Texas Red filters. Images were acquired using a Zeiss Axio cam MRm and the AxioVision Rel 4.8 software. The resulting images were modified using ImageJ software as follows: background with radius 4 was subtracted from the red channel of the RGB images and a maximum filter with radius 1 was applied. The result was intensity‐scaled to suit printing details.
Supplementary Figure S3 Glyco‐mucin profiles were evaluated in serial sections from three different cases. PLA assays show different profiles of overlap or absence of overlap. In the first example (A,B) we show an area where Tn is carried by MUC16 (B, 20×) but not by MUC 1 (A, 20×). In the second case (C,D) we show an area where STn is carried by MUC1 (C, 10×, insert 40×) but not by MUC 16 (D, 10×, insert 40×). Finally, in the third case (E,F) there is complete overlap between expression of STn in MUC1 (E, 40×) and in MUC16 (F, 40×).
Acknowledgements
The authors thank Joyce Taylor‐Papadimitriou and Joy Burchell for kind offer of antibody HMFG2. The authors also thank Fátima Carneiro, Director of the Department of Pathology of Centro Hospitalar S. João, for easy access to archived material. Grant Support: Support by Programa Operacional Ciência e Inovação 2010 do Quadro Comunitário de Apoio III and FEDER (PTDC/SAU‐ONC/117216/2010). IPATIMUP is an Associate Laboratory of the Portuguese Ministry of Science, Technology and Higher Education and partially supported by FCT. Project NORTE ‐ 07‐0124‐FEDER‐000024 co‐financed by Programa Operacional Regional do Norte (ON.2 – O Novo Norte), under Quadro de Referência Estratégico Nacional (QREN), by Fundo Europeu de Desenvolvimento Regional (FEDER). Lara Marcos‐Silva acknowledges also FCT for financial support through a PhD fellowship (SFRH/BD/60536/2009). Support by Kirsten og Freddy Johansen Fonden, A.P. Møller og Hustru Chastine Mc‐Kinney Møllers Fond til Almene Formaal, The Novo Nordisk Foundation, The Danish Research Councils, a programme of excellence from the University of Copenhagen, and The Danish National Research Foundation (DNRF107).
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2014.10.005.
Ricardo Sara, Marcos-Silva Lara, Pereira Daniela, Pinto Rita, Almeida Raquel, Söderberg Ola, Mandel Ulla, Clausen Henrik, Felix Ana, Lunet Nuno, David Leonor, (2015), Detection of glyco-mucin profiles improves specificity of MUC16 and MUC1 biomarkers in ovarian serous tumours, Molecular Oncology, 9, doi: 10.1016/j.molonc.2014.10.005.
References
- Akita, K. , Yoshida, S. , Ikehara, Y. , Shirakawa, S. , Toda, M. , Inoue, M. , Kitawaki, J. , Nakanishi, H. , Narimatsu, H. , Nakada, H. , 2012. Different levels of sialyl-Tn antigen expressed on MUC16 in patients with endometriosis and ovarian cancer. Int. J. Gynecol. Cancer. 22, 531–538. [DOI] [PubMed] [Google Scholar]
- Assarsson, E. , Lundberg, M. , Holmquist, G. , Bjorkesten, J. , Bucht Thorsen, S. , Ekman, D. , Eriksson, A. , Rennel Dickens, E. , Ohlsson, S. , Edfeldt, G. , Andersson, A.C. , Lindstedt, P. , Stenvang, J. , Gullberg, M. , Fredriksson, S. , 2014. Homogenous 96-Plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PloS one. 9, e95192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avninder, S. , Ylaya, K. , Hewitt, S.M. , 2008. Tissue microarray: a simple technology that has revolutionized research in pathology. J. Postgrad. Med. 54, 158–162. [DOI] [PubMed] [Google Scholar]
- Barnholtz-Sloan, J.S. , Schwartz, A.G. , Qureshi, F. , Jacques, S. , Malone, J. , Munkarah, A.R. , 2003. Ovarian cancer: changes in patterns at diagnosis and relative survival over the last three decades. Am. J. obstetrics Gynecol. 189, 1120–1127. [DOI] [PubMed] [Google Scholar]
- Bast, R.C. , Feeney, M. , Lazarus, H. , Nadler, L.M. , Colvin, R.B. , Knapp, R.C. , 1981. Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Invest. 68, 1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan, A. , Bozzo, F. , Maggi, C.A. , Binaschi, M. , 2013. OC125, M11 and OV197 epitopes are not uniformly distributed in the tandem-repeat region of CA125 and require the entire SEA domain. Dis. Markers. 34, 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K. , Gentry-Maharaj, A. , Burnell, M. , Steentoft, C. , Marcos-Silva, L. , Mandel, U. , Jacobs, I. , Dawnay, A. , Menon, U. , Blixt, O. , 2013. Microarray Glycoprofiling of CA125 improves differential diagnosis of ovarian cancer. J. Proteome Res. 12, 1408–1418. [DOI] [PubMed] [Google Scholar]
- Conze, T. , Carvalho, A.S. , Landegren, U. , Almeida, R. , Reis, C.A. , David, L. , Soderberg, O. , 2010. MUC2 mucin is a major carrier of the cancer-associated sialyl-Tn antigen in intestinal metaplasia and gastric carcinomas. Glycobiology. 20, 199–206. [DOI] [PubMed] [Google Scholar]
- Cramer, D.W. , Bast, R.C. , Berg, C.D. , Diamandis, E.P. , Godwin, A.K. , Hartge, P. , Lokshin, A.E. , Lu, K.H. , McIntosh, M.W. , Mor, G. , Patriotis, C. , Pinsky, P.F. , Thornquist, M.D. , Scholler, N. , Skates, S.J. , Sluss, P.M. , Srivastava, S. , Ward, D.C. , Zhang, Z. , Zhu, C.S. , Urban, N. , 2011. Ovarian cancer biomarker performance in prostate, lung, colorectal, and ovarian cancer screening trial specimens. Cancer Prev. Res. 4, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry, E.W. , Stronach, E.A. , Rama, N.R. , Wang, Y.Y. , Gabra, H. , El-Bahrawy, M.A. , 2014. Molecular subtypes of serous borderline ovarian tumor show distinct expression patterns of benign tumor and malignant tumor-associated signatures. Mod. Pathol. 27, 433–442. [DOI] [PubMed] [Google Scholar]
- Darmanis, S. , Nong, R.Y. , Hammond, M. , Gu, J. , Alderborn, A. , Vanelid, J. , Siegbahn, A. , Gustafsdottir, S. , Ericsson, O. , Landegren, U. , Kamali-Moghaddam, M. , 2010. Sensitive plasma protein analysis by microparticle-based proximity ligation assays. Mol. Cell. Proteomics. 9, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, B. , Berner, A. , Nesland, J.M. , Risberg, B. , Kristensen, G.B. , Trope, C.G. , Bryne, M. , 2000. Carbohydrate antigen expression in primary tumors, metastatic lesions, and serous effusions from patients diagnosed with epithelial ovarian carcinoma: evidence of up-regulated Tn and Sialyl Tn antigen expression in effusions. Hum. Pathol. 31, 1081–1087. [DOI] [PubMed] [Google Scholar]
- De Angelis, R. , Sant, M. , Coleman, M.P. , Francisci, S. , Baili, P. , Pierannunzio, D. , Trama, A. , Visser, O. , Brenner, H. , Ardanaz, E. , Bielska-Lasota, M. , Engholm, G. , Nennecke, A. , Siesling, S. , Berrino, F. , Capocaccia, R. , Group, E.-W. , 2014. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE–5-a population-based study. Lancet Oncol. 15, 23–34. [DOI] [PubMed] [Google Scholar]
- Ferlay, J. , Soerjomataram, I. , Ervik, M. , Dikshit, R. , Eser, S. , Mathers, C. , Rebelo, M. , Parkin, D.M. , Forman, D. , Bray, F. , 2012. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] International Agency for Research on Cancer; Lyon, France: 2013. Available from: http://globocan.iarc.fr (accessed on day/month/year) [Google Scholar]
- Fredriksson, S. , Gullberg, M. , Jarvius, J. , Olsson, C. , Pietras, K. , Gústafsdóttir, S.M. , Ostman, A. , Landegren, U. , 2002. Protein detection using proximity-dependent DNA ligation assays. Nat. Biotechnol. 20, 473–477. [DOI] [PubMed] [Google Scholar]
- Fredriksson, S. , Horecka, J. , Brustugun, O.T. , Schlingemann, J. , Koong, A.C. , Tibshirani, R. , Davis, R.W. , 2008. Multiplexed proximity ligation assays to profile putative plasma biomarkers relevant to pancreatic and ovarian cancer. Clin. Chem. 54, 582–589. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh, M. , Ogawa, H. , Sasaki, Y. , Araki, T. , Aihara, K. , 1997. Mucin carbohydrate antigens (T, Tn, and sialyl-Tn) in human ovarian carcinomas: relationship with histopathology and prognosis. Hum. Pathol. 28, 960–966. [DOI] [PubMed] [Google Scholar]
- Gill, D.J. , Clausen, H. , Bard, F. , 2011. Location, location, location: new insights into O-GalNAc protein glycosylation. Trends Cell Biol. 21, 149–158. [DOI] [PubMed] [Google Scholar]
- Gipson, I.K. , 2004. Distribution of mucins at the ocular surface. Exp. Eye Res. 78, 379–388. [DOI] [PubMed] [Google Scholar]
- Goonewardene, T.I. , Hall, M.R. , Rustin, G.J. , 2007. Management of asymptomatic patients on follow-up for ovarian cancer with rising CA-125 concentrations. Lancet Oncol. 8, 813–821. [DOI] [PubMed] [Google Scholar]
- Gullberg, M. , Gustafsdottir, S.M. , Schallmeiner, E. , Jarvius, J. , Bjarnegard, M. , Betsholtz, C. , Landegren, U. , Fredriksson, S. , 2004. Cytokine detection by antibody-based proximity ligation. Proc. Natl. Acad. Sci. USA. 101, 8420–8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haridas, D. , Chakraborty, S. , Ponnusamy, M.P. , Lakshmanan, I. , Rachagani, S. , Cruz, E. , Kumar, S. , Das, S. , Lele, S.M. , Anderson, J.M. , Wittel, U.A. , Hollingsworth, M.A. , Batra, S.K. , 2011. Pathobiological implications of MUC16 expression in pancreatic cancer. PloS one. 6, e26839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattrup, C.L. , Gendler, S.J. , 2008. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 70, 431–457. [DOI] [PubMed] [Google Scholar]
- Hirabayashi, Y. , Marugame, T. , 2009. Comparison of time trends in ovary cancer mortality (1990-2006) in the world, from the WHO Mortality Database. Jpn. J. Clin. Oncol. 39, 860–861. [DOI] [PubMed] [Google Scholar]
- Jabbar, K.S. , Verbeke, C. , Hyltander, A.G. , Sjovall, H. , Hansson, G.C. , Sadik, R. , 2014. Proteomic mucin profiling for the identification of cystic precursors of pancreatic cancer. J. Natl. Cancer Inst. 106, djt439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, I. , Bast, R.C. , 1989. The Ca-125 tumor-associated antigen - a review of the Literature. Hum. Reprod. 4, 1–12. [DOI] [PubMed] [Google Scholar]
- Karlan, B.Y. , Thorpe, J.D. , Watabayashi, K. , Drescher, C.W. , Palomares, M.R. , Daly, M. , Paley, P.J. , Hillard, P.J. , Andersen, M.R. , Anderson, G.L. , Drapkin, R. , Urban, N. , 2014. Use of CA125 and HE4 serum markers to predict ovarian cancer in elevated-risk women. Cancer Epidemiol. Biomarkers Prev. 23, 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H. , Park, C.W. , Um, D. , Baek, K.H. , Jo, Y. , An, H. , Kim, Y. , Kim, T.J. , 2014. Mass spectrometric screening of ovarian cancer with serum glycans. Dis. Markers. 2014, 634289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen, T. , Clausen, H. , Hirohashi, S. , Ogawa, T. , Iijima, H. , Hakomori, S. , 1988. Preparation and characterization of monoclonal antibodies directed to the tumor-associated O-linked sialosyl-2–--6 alpha-N-acetylgalactosaminyl (sialosyl-Tn) epitope. Cancer Res. 48, 2214–2220. [PubMed] [Google Scholar]
- Kondalsamy-Chennakesavan, S. , Hackethal, A. , Bowtell, D. , Australian Ovarian Cancer Study, G. Obermair, A. , 2013. Differentiating stage 1 epithelial ovarian cancer from benign ovarian tumours using a combination of tumour markers HE4, CA125, and CEA and patient's age. Gynecol. Oncol. 129, 467–471. [DOI] [PubMed] [Google Scholar]
- Kui Wong, N. , Easton, R.L. , Panico, M. , Sutton-Smith, M. , Morrison, J.C. , Lattanzio, F.A. , Morris, H.R. , Clark, G.F. , Dell, A. , Patankar, M.S. , 2003. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J. Biol. Chem. 278, 28619–28634. [DOI] [PubMed] [Google Scholar]
- Lin, W. , Du, Y. , Zhu, Y. , Chen, X. , 2014. A cis-membrane FRET-based method for protein-specific imaging of cell-surface glycans. J. Am. Chem. Soc. 136, 679–687. [DOI] [PubMed] [Google Scholar]
- Mai, P.L. , Wentzensen, N. , Greene, M.H. , 2011. Challenges related to developing serum-based biomarkers for early ovarian cancer detection. Cancer Prev. Res. 4, 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel, U. , Petersen, O.W. , Sorensen, H. , Vedtofte, P. , Hakomori, S. , Clausen, H. , Dabelsteen, E. , 1991. Simple mucin-type carbohydrates in oral stratified squamous and salivary gland epithelia. J. Invest. Dermatol. 97, 713–721. [DOI] [PubMed] [Google Scholar]
- Marcos-Silva, L. , Narimatsu, Y. , Halim, A. , Campos, D. , Yang, Z. , Tarp, M.A. , Pereira, P.J. , Mandel, U. , Bennett, E.P. , Vakhrushev, S.Y. , Levery, S.B. , David, L. , Clausen, H. , 2014. Characterization of binding epitopes of CA125 monoclonal antibodies. J. Proteome Res. 13, 3349–3359. [DOI] [PubMed] [Google Scholar]
- Moore, R.G. , Jabre-Raughley, M. , Brown, A.K. , Robison, K.M. , Miller, M.C. , Allard, W.J. , Kurman, R.J. , Bast, R.C. , Skates, S.J. , 2010. Comparison of a novel multiple marker assay vs the risk of malignancy index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am. J. Obstet. Gynecol. 203, (228) e221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nustad, K. , Bast, R.C. , Brien, T.J. , Nilsson, O. , Seguin, P. , Suresh, M.R. , Saga, T. , Nozawa, S. , Bormer, O.P. , de Bruijn, H.W. , Nap, M. , Vitali, A. , Gadnell, M. , Clark, J. , Shigemasa, K. , Karlsson, B. , Kreutz, F.T. , Jette, D. , Sakahara, H. , Endo, K. , Paus, E. , Warren, D. , Hammarstrom, S. , Kenemans, P. , Hilgers, J. , 1996. Specificity and affinity of 26 monoclonal antibodies against the CA 125 antigen: first report from the ISOBM TD-1 workshop. International Society for Oncodevelopmental Biology and Medicine. Tumour Biol. 17, 196–219. [DOI] [PubMed] [Google Scholar]
- Pinto, R. , Carvalho, A.S. , Conze, T. , Magalhaes, A. , Picco, G. , Burchell, J.M. , Taylor-Papadimitriou, J. , Reis, C.A. , Almeida, R. , Mandel, U. , Clausen, H. , Soderberg, O. , David, L. , 2012. Identification of new cancer biomarkers based on aberrant mucin glycoforms by in situ proximity ligation. J. Cell. Mol. Med. 16, 1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat, J. , 2012. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 460, 237–249. [DOI] [PubMed] [Google Scholar]
- Seidman, J.D. , Horkayne-Szakaly, I. , Haiba, M. , Boice, C.R. , Kurman, R.J. , Ronnett, B.M. , 2004. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int. J. Gynecol. Pathol. 23, 41–44. [DOI] [PubMed] [Google Scholar]
- Simon, R. , Mirlacher, M. , Sauter, G. , 2004. Tissue microarrays. Methods Mol. Med. 97, 377–389. [DOI] [PubMed] [Google Scholar]
- Singh, A.P. , Senapati, S. , Ponnusamy, M.P. , Jain, M. , Lele, S.M. , Davis, J.S. , Remmenga, S. , Batra, S.K. , 2008. Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. Lancet Oncol. 9, 1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skates, S.J. , Horick, N. , Yu, Y. , Xu, F.J. , Berchuck, A. , Havrilesky, L.J. , de Bruijn, H.W. , van der Zee, A.G. , Woolas, R.P. , Jacobs, I.J. , Zhang, Z. , Bast, R.C. , 2004. Preoperative sensitivity and specificity for early-stage ovarian cancer when combining cancer antigen CA-125II, CA 15-3, CA 72-4, and macrophage colony-stimulating factor using mixtures of multivariate normal distributions. J. Clin. Oncol. 22, 4059–4066. [DOI] [PubMed] [Google Scholar]
- Soderberg, O. , Gullberg, M. , Jarvius, M. , Ridderstrale, K. , Leuchowius, K.J. , Jarvius, J. , Wester, K. , Hydbring, P. , Bahram, F. , Larsson, L.G. , Landegren, U. , 2006. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods. 3, 995–1000. [DOI] [PubMed] [Google Scholar]
- Steentoft, C. , Vakhrushev, S.Y. , Joshi, H.J. , Kong, Y. , Vester-Christensen, M.B. , Schjoldager, K.T. , Lavrsen, K. , Dabelsteen, S. , Pedersen, N.B. , Marcos-Silva, L. , Gupta, R. , Bennett, E.P. , Mandel, U. , Brunak, S. , Wandall, H.H. , Levery, S.B. , Clausen, H. , 2013. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 32, 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiekema, A. , Lok, C.A. , Kenter, G.G. , van Driel, W.J. , Vincent, A.D. , Korse, C.M. , 2014. A predictive model combining human epididymal protein 4 and radiologic features for the diagnosis of ovarian cancer. Gynecol. Oncol. 132, 573–577. [DOI] [PubMed] [Google Scholar]
- Tavoosidana, G. , Ronquist, G. , Darmanis, S. , Yan, J. , Carlsson, L. , Wu, D. , Conze, T. , Ek, P. , Semjonow, A. , Eltze, E. , Larsson, A. , Landegren, U.D. , Kamali-Moghaddam, M. , 2011. Multiple recognition assay reveals prostasomes as promising plasma biomarkers for prostate cancer. Proc. Natl. Acad. Sci. USA. 108, 8809–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou, J. , Peterson, J.A. , Arklie, J. , Burchell, J. , Ceriani, R.L. , Bodmer, W.F. , 1981. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int. J. Cancer J. Int. du Cancer. 28, 17–21. [DOI] [PubMed] [Google Scholar]
- Van Gorp, T. , Cadron, I. , Despierre, E. , Daemen, A. , Leunen, K. , Amant, F. , Timmerman, D. , De Moor, B. , Vergote, I. , 2011. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the risk of ovarian malignancy algorithm. Br. J. Cancer. 104, 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibrecht, I. , Leuchowius, K.J. , Clausson, C.M. , Conze, T. , Jarvius, M. , Howell, W.M. , Kamali-Moghaddam, M. , Soderberg, O. , 2010. Proximity ligation assays: a recent addition to the proteomics toolbox. Expert Rev. Proteomics. 7, 401–409. [DOI] [PubMed] [Google Scholar]
- Zen, K. , Notarfrancesco, K. , Oorschot, V. , Slot, J.W. , Fisher, A.B. , Shuman, H. , 1998. Generation and characterization of monoclonal antibodies to alveolar type II cell lamellar body membrane. Am. J. Physiol. 275, L172–L183. [DOI] [PubMed] [Google Scholar]
- Zhu, C.S. , Pinsky, P.F. , Cramer, D.W. , Ransohoff, D.F. , Hartge, P. , Pfeiffer, R.M. , Urban, N. , Mor, G. , Bast, R.C. , Moore, L.E. , Lokshin, A.E. , McIntosh, M.W. , Skates, S.J. , Vitonis, A. , Zhang, Z. , Ward, D.C. , Symanowski, J.T. , Lomakin, A. , Fung, E.T. , Sluss, P.M. , Scholler, N. , Lu, K.H. , Marrangoni, A.M. , Patriotis, C. , Srivastava, S. , Buys, S.S. , Berg, C.D. , Team, P.P. , 2011. A framework for evaluating biomarkers for early detection: validation of biomarker panels for ovarian cancer. Cancer Prev. Res. 4, 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data
Supplementary Figure S1 Immunohistochemistry staining pattern of mucins MUC16 and MUC1 and simple mucin‐type carbohydrate antigens Tn, STn, and T in serous adenocarcinomas, serous borderline tumours and cystadenomas from CHSJ Porto series.
Supplementary Figure S2 Expression and glyco‐mucin profile of wild‐type (WT) and SimpleCell (SC) (REF: EMBO paper) OVCAR‐3 isogenic cells. A. Immunofluorescence (IF) to evaluate expression of STn, Tn, T and MUC16. Cells were cultured on glass coverslips, and fixed with 4% paraformaldehyde during 20 min on ice. After fixation, cells were washed with PBS and incubated with primary antibodies TKH2 (STn), 5F4 (Tn), 3C9 (T) and M11 (MUC16), overnight at 4 °C. Bound antibodies were detected at RT with rabbit anti‐mouse conjugated with FITC (1:100). After washing with PBS, each sample was mounted with Duolink Mounting Medium with DAPI. Both cell lines are positive for MUC16, OVCAR‐3 SC show higher expression of STn and Tn and T antigen is completely negative in OVCAR3 SC, as expected. B. PLA assay was performed using the Duolink in situ Detection Reagents Fluorescence (Olink® Bioscience, Uppsala, Sweden) according to the manufacturer's instructions. The concentration for primary antibodies and PLA probes were the same described in material and methods. Our results show that MUC16 is a carrier of STn, Tn and T in OVCAR3 WT and that MUC16/STn and MUC16/Tn increase in OVCAR‐3 SC, whereas MUC16/T is completely negative. The IF and PLA staining were observed with a Zeiss microscope (Imager Z1), and images were acquired using the Axiovision software at 200× magnification. Scale bar, 20 μm. Samples were examined under a Zeiss Imager.Z1 Axio fluorescence microscope equipped with DAPI and Texas Red filters. Images were acquired using a Zeiss Axio cam MRm and the AxioVision Rel 4.8 software. The resulting images were modified using ImageJ software as follows: background with radius 4 was subtracted from the red channel of the RGB images and a maximum filter with radius 1 was applied. The result was intensity‐scaled to suit printing details.
Supplementary Figure S3 Glyco‐mucin profiles were evaluated in serial sections from three different cases. PLA assays show different profiles of overlap or absence of overlap. In the first example (A,B) we show an area where Tn is carried by MUC16 (B, 20×) but not by MUC 1 (A, 20×). In the second case (C,D) we show an area where STn is carried by MUC1 (C, 10×, insert 40×) but not by MUC 16 (D, 10×, insert 40×). Finally, in the third case (E,F) there is complete overlap between expression of STn in MUC1 (E, 40×) and in MUC16 (F, 40×).


