Abstract
The synergistic interaction of two antibodies targeting the same protein could be developed as an effective anti‐cancer therapy. Human epidermal growth factor receptor 2 (HER2) is overexpressed in 20–25% of breast and gastric cancer patients, and HER2‐targeted antibody therapy using trastuzumab is effective in many of these patients. Nonetheless, improving therapeutic efficacy and patient survival is important, particularly in patients with HER2‐positive gastric cancer. Here, we describe the development of 1E11, a HER2‐targeted humanized monoclonal antibody showing increased efficacy in a highly synergistic manner in combination with trastuzumab in the HER2‐overexpressing gastric cancer cell lines NCI‐N87 and OE‐19. The two antibodies bind to sub‐domain IV of the receptor, but have non‐overlapping epitopes, allowing them to simultaneously bind HER2. Treatment with 1E11 alone induced apoptosis in HER2‐positive cancer cells, and this effect was enhanced by combination treatment with trastuzumab. Combination treatment with 1E11 and trastuzumab reduced the levels of total HER2 protein and those of aberrant HER2 signaling molecules including phosphorylated HER3 and EGFR. The synergistic antitumor activity of 1E11 in combination with trastuzumab indicates that it could be a novel potent therapeutic antibody for the treatment of HER2‐overexpressing gastric cancers.
Keywords: HER2, Gastric cancer, 1E11, Trastuzumab, Combination, Synergism
Highlights
We develop HER2 antibody, 1E11 synergistic with trastuzumab in gastric cancers.
1E11 and trastuzumab (TRA) bind to sub‐domain IV having non‐overlapping epitopes.
1E11 + TRA induce strong apoptosis compared to pertuzumab (PER) + TRA.
1E11 + TRA and PER + TRA reduce HER2 protein and down steam signaling.
1E11 + TRA shows superior antitumor activity to PER + TRA in xenograft models.
1. Introduction
Gastric cancer is one of the most common cancers, with approximately one million new cases diagnosed each year; it is the third leading cause of cancer death in both sexes worldwide (Ferlay et al., 2013). Despite advances in the prevention and treatment of gastric cancer and a decrease in mortality rates, the prognosis of patients with gastric cancer remains poor and few effective therapeutic options are available, in particular for advanced stages (Siegel et al., 2012). The 5 year survival rate in most parts of the world is approximately 20%. HER2 is a receptor tyrosine kinase and a member of the ErbB family, which includes EGFR, HER3, and HER4. This receptor family functions in the regulation of many essential cellular functions such as cell proliferation, differentiation, and apoptosis through homo‐ or heterodimerization and the activation of signal transduction pathways (Yarden and Sliwkowski, 2001). ErbB family proteins are overexpressed in several malignancies. In gastric cancer, overexpression of EGFR, HER2, and HER3 is correlated with poor prognosis (Garcia et al., 2003; Hayashi et al., 2008). The HER2‐targeting monoclonal antibody trastuzumab was first characterized in vitro and in vivo in 1992 (Kasprzyk et al., 1992) and was approved for the treatment of HER2‐overexpressing metastatic breast cancer in 1998. Recently, the ToGA trial (Trastuzumab for Gastric Cancer) demonstrated the survival benefit of trastuzumab in HER2‐overexpressing gastric cancer patients (Bang et al., 2010) after Food and Drug Administration approval of this antibody for the treatment of HER2‐positive metastatic gastric and gastroesophageal junction cancer.
Recent evidence suggests that particular combinations of noncompetitive antibodies targeting the same receptor increase antitumor activity in vitro and in vivo. One example is the combination of pertuzumab, which binds to sub‐domain II of the extracellular domain (ECD) of HER2, with trastuzumab, which binds to sub‐domain IV (Cai et al., 2008; Cho et al., 2003). Pertuzumab, which has limited antitumor activity as a single agent in HER2‐overexpressing breast cancer cells, shows increased efficacy in combination with trastuzumab (Nahta et al., 2004). The benefits of the pertuzumab and trastuzumab combination were further demonstrated in preclinical and clinical trials (Baselga et al., 2010; Scheuer et al., 2009). Pertuzumab has been approved for the treatment of HER2‐positive metastatic breast cancer in combination with trastuzumab (Baselga et al., 2012). Other HER2‐targeting antibodies showing better efficacy in combination than as single agents have been reported and have shown consistent downregulation of HER2 levels and beneficial combination effects in mouse models (Ben‐Kasus et al., 2009). The increased efficacy of antibody combinations has also been demonstrated with EGFR‐targeting antibodies (Friedman et al., 2005; Koefoed et al., 2011).
In the present study, we report the development of a novel HER2‐targeted antibody termed 1E11 and describe its anti‐cancer activities as a single agent and in combination with trastuzumab in preclinical models. 1E11 had moderate efficacy in HER2‐overexpressing NCI‐N87 and OE‐19 gastric cancer cells; however, its efficacy increased dramatically in vitro and in vivo when used in combination with trastuzumab, showing a highly synergistic effect. The binding site of 1E11 was localized to sub‐domain IV, at a distinct epitope different from that of trastuzumab. 1E11 induced apoptosis in combination with trastuzumab, which showed weak apoptotic activity as a single agent, and combination treatment with 1E11 and trastuzumab inhibited epidermal growth factor (EGF) and HRG1‐induced cell proliferation. The results of the present study suggest that HER2‐targeting antibody combinations are valid therapeutic strategies for the treatment of HER2‐overexpressing gastric cancer, and 1E11 is a strong synergistic partner for trastuzumab‐based combination treatments.
2. Materials and methods
2.1. Cell lines and materials
BT‐474, SK‐BR‐3, NCI‐N87, KATO‐III, Hs746T, HCC‐202, HCC‐1954, and MCF‐7 cells were purchased from American Type Culture Collection (ATCC). OE‐19 cells were obtained from the European Collection of Cell Culture (ECACC). MKN‐7 cells were from the Japanese Collection of Research Bioresources (JCRB), and MKN‐45, SNU‐216, MDA‐MB‐231, and MDA‐MB‐453 cells were from the Korean Cell Line Bank (KCLB). JIMT‐1 cells were gifted from Asan Medical Center in Seoul Korea. Cell culture media were Dulbecco's Modified Eagle's Medium (DMEM): F‐12 for BT‐474 cells, DMEM for Hs746T cells, and RPMI‐1640 for all other cell lines. All media were supplemented with 10% fetal bovine serum (FBS), and antibiotics and cells were cultured at 37 °C under 5% CO2. Trastuzumab and pertuzumab were produced by Genentech Incorporated, and palivizumab was produced by MedImmune, LLC. ChromPure human IgG (Jackson Immunoresearch Lab) was used as human IgG control antibody in in vitro assays. 1E11 and oz1E11 antibodies were produced using the 293F system (Invitrogen) and purified using protein‐A chromatography (GE Healthcare). The oz1E11 antibody (clone name: 1A12) consists of an optimized 1E11 antibody after humanization and affinity maturation. The endotoxin was removed with an Endotoxin removal kit (GenScript), and endotoxin levels were determined using an Endotoxin detection kit (GenScript). Human HER3 and HER4 proteins were purchased from R&D systems and rhesus HER2, mouse HER2, and rat HER2 proteins were purchased from Sino Biological Inc. Heregulin‐1 (HRG1) and EGF proteins were purchased from R&D systems. Other recombinant proteins were produced as secretion proteins using the 293F system and purified using protein‐A or Ni‐NTA chromatography (Qiagen Inc.) for Fc‐fused and His‐fused proteins, respectively.
2.2. Cell viability assay
Cells were seeded in 96‐well plates (Corning) in growth media containing 10% FBS and pre‐cultured for 24 h. The cells were treated with antibodies at the indicated concentrations and culture for 3–6 days. For ligand‐induced cell proliferation assays, NCI‐N87 cells were seeded and pre‐cultured for 24 h in 0.1% FBS media and treated with antibodies (10 μg/mL) for 1 h before the addition of ligand (200 ng/mL). Cell proliferation was assessed 3 days after the treatment. The WST (DoGen) or CellTiter‐Glo (Promega) assay was used to measure cell viability. Relative cell viability was calculated by dividing the absorbance of each well by the mean absorbance of PBS‐treated wells in each plate.
2.3. Surface plasmon resonance (SPR) analysis
For epitope binning, trastuzumab and pertuzumab were immobilized onto separate CM5 sensor chip surfaces (GE Healthcare) using amine coupling at approximately 1000 response units (RU). HER2‐ECD‐His (320 nM) and antibodies (1 μg/mL) were sequentially coupled by binding for 4 min and stabilization for 5 min at 50 μL/min flow rate. For affinity measurements, goat anti‐human IgG (γ) (Invitrogen) was immobilized onto a CM5 sensor chip using amine coupling, and antibodies were captured at approximately 50 RU. Then, HER2‐ECD‐His protein was injected at concentrations ranging from 0 to 640 nM. Sensorgrams were obtained at each concentration and evaluated using the BIAevaluation software.
2.4. Cell‐cycle and apoptosis analysis
NCI‐N87 and BT‐474 cells were treated with 10 μg/mL of antibodies in complete growth media. After 2 days of antibody treatment, cells were detached with trypsin and 1 × 105 cells were used for analysis. For cell‐cycle analysis, cells were fixed in 70% ethanol and resuspended in 33 μg/mL propidium iodide (PI) (Sigma–Aldrich Co.) supplemented with 1 mg/mL RNase A (Sigma–Aldrich Co.) and 10% Triton X‐100. Samples were incubated for 30 min and analyzed by flow cytometry using a Cytomics FC500 (Beckman Coulter Inc). For apoptosis analysis, detached cells were stained with annexin V and PI using the ApoScreen TM Annexin V Apoptosis kit (SouthernBiotech). Cell death was measured as cells staining positive for annexin V, PI, or both, as assessed by flow cytometry analysis.
2.5. Caspase‐3/7 activity test
Cells were treated as described for the cell viability assay with 10 μg/mL of antibodies for 24 h. The Caspase‐Glo 3/7 Assay (Promega) was used to measure caspase‐3/7 activity. Caspase‐3/7 activity was calculated as follows: Caspase‐3/7 activity = (luminescent unit of treatment well − luminescent unit of blank well)/(mean luminescent unit of control well − luminescent unit of blank well).
2.6. Downstream signaling
Cells were treated as described in Section 2.4 for 24 h, washed with ice‐cold PBS, and lysed in a cell lysis solution [50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP‐40, 0.1% sodium dodecyl sulfate, 1 mM NaF, 1 mM Na3VO4, 1 mM PMSF, and protease inhibitor cocktail (Sigma)]. Antibodies against HER2 (#4290), pHER2 (#2243), pHER3 (#4791), EGFR (#4267), pEGFR (#3777), AKT (#4691), pAKT (#4060), ERK (#4695), and pERK (#4370) were purchased from Cell Signaling Technology. Anti‐HER3 (sc‐285) antibody was purchased from Santa Cruz Biotechnology and anti‐GAPDH (AbC‐1001) antibody was purchased from AbClon. Horseradish peroxidase‐conjugated anti‐mouse (AbC‐5001) and anti‐rat (AbC‐5003) antibodies were purchased from AbClon. Bands were visualized using AbSignal (AbClon, AbC‐3001).
2.7. Antibody‐dependent cellular cytotoxicity (ADCC) assay
SK‐BR‐3 cells were used as target cells, and human peripheral blood mononuclear cells (PBMCs) purified from the blood of five healthy donors were used as effector cells. Target cells (10,000) were incubated for 15 min with the indicated antibodies followed by the addition of effector cells at a ratio of 1:50 (target:effector) and incubation for an additional 20 h at 37 °C under 5% CO2. ADCC was determined using the EZ‐LDH Cell Cytotoxicity Assay Kit (DoGen) according to the manufacturer's instructions. Cytotoxicity (%) was calculated using the following equation: (Experimental value – Effector Cell Spontaneous Control – Target Cell Spontaneous Control)/(Target Cell Maximum Control – Target Cell Spontaneous Control) × 100.
2.8. Optimization of 1E11 antibody
The humanized 1E11 antibody was developed by CDR‐grafting into human germline genes. The homologous human germline genes with highest sequence similarity were selected using IMGT/V‐QUEST (Brochet et al., 2008). The IGKV1‐39*01 and IGKJ1*01 genes for light chain and IGHV3‐48*03 and IGHJ4*01 genes for heavy chain were selected as acceptor sequences for the grafting of murine CDRs. One residue in heavy chain H47 according to the Kabat numbering scheme was back‐mutated to murine, since this site is responsible for stabilizing the CDR loop structure as well as modifying its position (Foote and Winter, 1992).
For affinity maturation of humanized 1E11, CDR3 residues in the heavy and/or light chain were randomized to 20 amino acids, and high affinity binders were selected using phage display. Selected clones were ranked based on the Koff value using SPR analysis with immobilized HER2‐ECD protein. The oz1E11 antibody (clone name: 1A12) has four amino acid mutations in CDR3 of the parental light chain and showed a >10‐fold improvement in Koff value.
2.9. NCI‐N87 and OE‐19 xenograft models
Athymic nude female mice (Daehan Biolink, Korea) were injected subcutaneously in the left flank area with 5 × 106 of NCI‐N87 or OE‐19 cells in Matrigel (BD Biosciences). Tumors were allowed to grow to approximately 200 mm3 or 500 mm3 in size, and mice were than randomized into groups. Animals received intraperitoneal administration of antibodies at the indicated doses twice weekly. Tumor volumes were calculated using the formula (L × W × W)/2, where “L” represents the larger tumor diameter and “W” represents the smallest tumor diameter. Animals were sacrificed, and the tumors were isolated and weighted after the termination of studies. Animals in the study group were also sacrificed if the average tumor volume was >3000 mm3. Tumor xenograft tissues were resected and processed as formalin‐fixed, paraffin‐embedded specimen sections. The TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) assay was performed to analyze apoptotic cell death. Cells were visualized under a light microscope.
All animal studies were conducted in accordance with the guidelines of the NIH “Guide for Care and Use of Animals” and an approved protocol received by the company's Institution Animal Care and Use Committee.
2.10. Statistical analysis
Statistical analysis was performed by Student unpaired t test to identify significant difference unless otherwise indicated. Differences were considered significant at a P value <0.05.
3. Results
3.1. 1E11 synergistically inhibits gastric cancer cell growth in combination with trastuzumab
Mouse monoclonal antibodies against the ECD of HER2 were developed using our improved hybridoma technology. These clones were used to assess cell viability in a panel of gastric cancer cell lines including NCI‐N87 and breast cancer cell lines including BT‐474. 1E11 was selected based on its affinity for HER2 and efficacy against HER2‐positive cancer cells, and was further developed into a mouse/human chimeric antibody with the IgG1 kappa format.
1E11 and trastuzumab showed moderate antiproliferative activity against HER2‐overexpressing gastric NCI‐N87 cells as single agents, whereas in combination they showed dramatically increased antiproliferative activity (Figure 1A). Pertuzumab did not show substantial antiproliferative activity as a single agent or in combination with trastuzumab in NCI‐N87 cells. The combination effect of 1E11 and trastuzumab was equivalent to that of trastuzumab and pertuzumab in HER2‐overexpresing BT‐474 breast cancer cells (Figure 1B).
Figure 1.
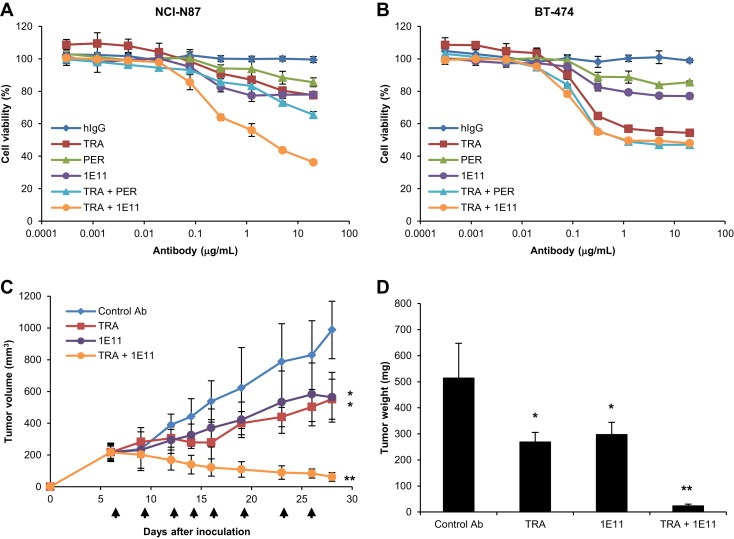
1E11 shows antiproliferative activity in combination with trastuzumab in a synergistic manner A, NCI‐N87 and B, BT‐474 cells were treated with hIgG, trastuzumab (TRA), pertuzumab (PER), and 1E11 as single agents and in combination with trastuzumab in a 1:1 ratio for 4 days. Cell viability was expressed as the mean ± SD (n = 3), and the 100% point was defined as the absorbance of the untreated well. C, Mice bearing NCI‐N87 xenograft tumors were treated in all experiments with a dose of 10 mg/kg of control antibody, trastuzumab, 1E11, or trastuzumab + 1E11. Palivizumab was used as the isotype control antibody. Administration days are indicated by arrows. Tumor volume (mm3) was expressed as mean ± SD (n = 5 mice/group). D, Tumor masses were isolated after measuring tumor weight. Statistically significant differences were determined by Student's t test. *, P < 0.01 versus the control group and **, P < 0.01 versus the trastuzumab‐treated group.
Combination effects were analyzed using the method of Chou and Talalay (Chou and Talalay, 1984) to obtain drug combination index (C.I.) values. The combination effects of drugs are defined as synergism, addition, and antagonism according to C.I. values <1.0, equal to 1.0, or >1.0, respectively. The combination effect of 1E11 and trastuzumab was determined as strong synergism with C.I. values of 0.03, 0.05, and 0.08 at ED50, ED75, and ED90, respectively.
The in vitro antiproliferative activity of 1E11 in combination with trastuzumab was confirmed in vivo in mice bearing NCI‐N87 xenograft tumors. Both trastuzumab and 1E11 significantly reduced tumor volume and weight compared to control antibody when used as single agents (Figure 1C and D); however, when used in combination, their antitumor activity was significantly higher than that of trastuzumab alone (P < 0.0001). The tumor growth inhibition (TGI) value of the antibody combination (95.1%) was markedly higher than those of trastuzumab and 1E11 as single agents (48.6% and 39.9%, respectively).
These results indicate that 1E11 in combination with trastuzumab inhibits HER2‐overexpressing gastric cancer cell growth in a synergistic manner.
3.2. 1E11 binds to domain IV of HER2 at a distinct epitope different from that of trastuzumab
To determine whether 1E11 binds to the same epitope as trastuzumab or pertuzumab, the binding of 1E11 to the HER2‐ECD that was occupied by trastuzumab or pertuzumab was analyzed by SPR. As shown in Figure 2A, 1E11 was able to bind a monomeric HER2‐ECD‐His protein captured by immobilized pertuzumab (Figure 2A). These data indicate that 1E11 binds to a distinct epitope that is different from the epitopes of trastuzumab or pertuzumab, and that it is capable of simultaneous binding to HER2 with the two other antibodies.
Figure 2.
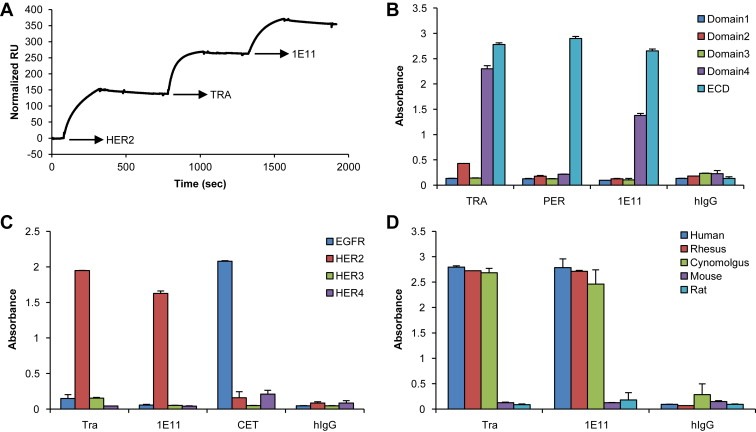
1E11 binds to a sub‐domain IV epitope different from that of trastuzumab A, Pertuzumab was immobilized on a CM5 sensor chip followed by sequential exposure to HER2‐ECD‐His, trastuzumab, and 1E11. B, The binding activities of antibodies were analyzed by ELISA using recombinant sub‐domain proteins. C, The ErbB family cross‐reactivity of 1E11 was analyzed using recombinant human ErbB proteins. Cetuximab (CET) was used as the control antibody for the EGFR protein. D, The species cross‐reactivity of 1E11 was analyzed using recombinant HER2‐ECD proteins of the indicated species.
Recombinant HER2‐ECD domain fragments were used for epitope mapping of the three reagents (Figure 2B), which showed that both 1E11 and trastuzumab bound to sub‐domain IV. However, pertuzumab did not bind to domain II of recombinant HER2, suggesting that it was not able to mimic its natural conformation. To further characterize the 1E11 epitope, the binding of 1E11 to 15‐mer peptides with a peptide–peptide overlap of 14 amino acids covering the whole HER2‐ECD was examined. However, no significant binding signals were detected for any of the peptides (data not shown). Taken together, these results indicated that 1E11 binds to a discontinuous or conformational epitope in sub‐domain IV.
Recombinant ErbB proteins were used to confirm that 1E11, similar to trastuzumab and pertuzumab, specifically binds HER2 and not the other human ErbB family members (Figure 2C). The species cross‐reactivity of 1E11 was determined by assessing the binding activity of 1E11 to HER2‐ECD proteins from five different species. Similar to trastuzumab and pertuzumab, 1E11 bound to monkey HER2 but not murine HER2 proteins (Figure 2D).
3.3. 1E11 increases tumor cell apoptosis in combination with trastuzumab
To determine the mechanism underlying the antitumor activity of 1E11, cell‐cycle progression and apoptosis were analyzed by flow cytometry. Treatment of NCI‐N87 cells with trastuzumab or 1E11 did not induce significant changes in the cell cycle either as single agents or in combination (Figure 3A). However, combination treatment with 1E11 and trastuzumab increased the sub‐G1 population of NCI‐N87 cells, indicating cell death.
Figure 3.
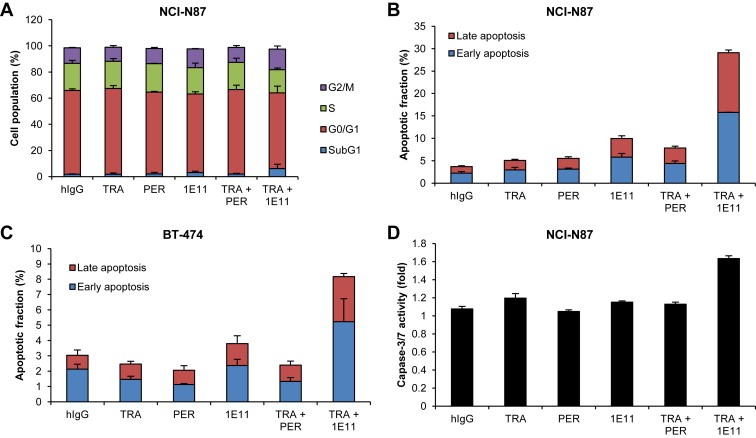
1E11 induces apoptosis in combination with trastuzumab A, NCI‐N87 cells were treated with antibodies for 48 h, stained with propidium iodide (PI), and DNA content was measured by flow cytometry. The relative cell populations in sub‐G1 (subdiploid), G1, S, and G2‐M phases are shown. B, NCI‐N87 and C, BT‐474 cells were treated with antibodies for 48 h and stained with annexin V‐phycoerythrin (PE) and PI. Cell death was assessed by flow cytometry. The percentage of cells staining positive for annexin V (early apoptosis) and annexin V and PI (late apoptosis) are shown. D, NCI‐N87 cells were treated with antibodies for 24 h and caspase‐3/7 activity was analyzed.
Assessment of cell apoptosis showed that 1E11 increased both the early and late apoptotic cell population compared to trastuzumab or pertuzumab single agent treatments, and this apoptotic activity was further increased in NCI‐N87 cells exposed to combination treatment with 1E11 and trastuzumab (Figure 3B). Trastuzumab alone did not induce apoptosis in NCI‐N87 cells even after a two‐fold increase in dose (data not shown). The effect of 1E11 was confirmed in the BT‐474 cell line, where it showed a similar effect as a single agent and in combination with trastuzumab (Figure 3C). To confirm that the cytotoxic effect of 1E11 is mediated by the induction of apoptosis, the activity of caspase‐3 and ‐7 was examined in NCI‐N87 cells. Caspase‐3/7 activity increased slightly after 24 h of treatment with trastuzumab as a single agent, whereas a 1.6‐fold increase was observed in response to combination treatment with 1E11 (Figure 3D). These results indicate that 1E11 exhibits antiproliferative activity by inducing apoptotic cell death, and its apoptotic activity is further increased in combination with trastuzumab in NCI‐N87 gastric cancer cells.
3.4. 1E11 inhibits ErbB family signaling and ligand‐induced cell proliferation in combination with trastuzumab
To elucidate the mechanism underlying the antiproliferative activity of 1E11 in combination with trastuzumab, the levels of the HER2 protein and related signaling molecules were measured. Combination treatment with 1E11 and trastuzumab or with pertuzumab and trastuzumab reduced total HER2 and phosphorylated HER2 levels (Figure 4A). Combination treatment decreased the levels of activated HER3 and EGFR without affecting total protein levels and significantly downregulated the activated form of the HER2 downstream factors AKT and ERK while the total protein levels remained unchanged. These results suggest that 1E11 in combination with trastuzumab inhibits the activity of ErbB family proteins and suppresses downstream signaling.
Figure 4.
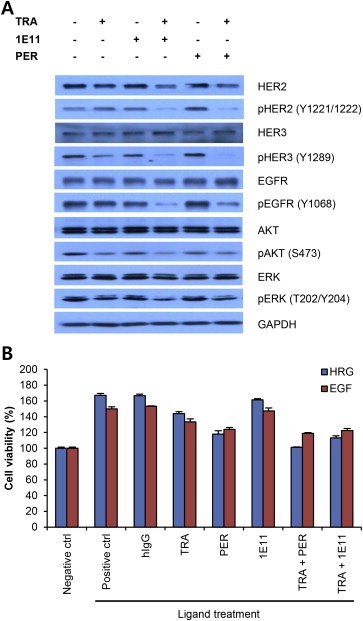
1E11 inhibits HER2 downstream signaling and heterodimerization‐induced cell proliferation A, NCI‐N87 cells were treated with 10 μg/mL of antibodies for 24 h under normal growth conditions, and changes in HER2 and HER3 downstream signaling were monitored by western blotting. Phosphorylated proteins were detected using antibodies against well‐characterized activation site residues. B, Cell growth inhibition in the presence of HRG1 or EGF was examined. Cell proliferation was assessed 3 days after the indicated treatments. Data points represent the mean ± SD (n = 3).
To evaluate the significance of ErbB family receptor signaling in the response of HER2‐overexpressing gastric cancer cells to antibody treatment, the antiproliferative activity of antibodies was analyzed under ligand‐induced dimerization conditions. The induction of cell proliferation by HRG1 and EGF in NCI‐N87 cells (Figure 4B) was not inhibited by trastuzumab or 1E11 as a single agent, whereas pertuzumab inhibited ligand‐induced cell proliferation as previously reported (Nahta et al., 2004). Combination treatment with 1E11 and trastuzumab inhibited ligand‐induced proliferation to a similar level as the combination of trastuzumab and pertuzumab, suggesting that 1E11 in combination with trastuzumab could compensate for the effect of pertuzumab. These results suggested that in addition to the inhibition of ErbB family dimerization, other mechanisms are involved in the effect of combination treatment with 1E11 and trastuzumab on the inhibition of cell proliferation in HER2‐overexpressing gastric cancer cells.
3.5. The antiproliferative activity of optimized 1E11 antibody (oz1E11) is dependent on HER2 expression
The oz1E11 antibody (clone name: 1A12) was developed by humanization and affinity maturation of 1E11. The binding affinity of oz1E11 for the ECD of HER2 (monomer) was 1.9 nM as determined by SPR, whereas that of the parental 1E11 antibody was 23 nM. The ADCC activity of oz1E11 was comparable to that of trastuzumab or pertuzumab in the lactate dehydrogenase (LDH) release assay performed using SK‐BR‐3 cells and PBMCs (Supplementary Figure 1) (Arnould et al., 2006).
The antiproliferative activity of oz1E11 was examined in seven gastric cancer and two breast cancer cell lines. The oz1E11 antibody showed moderate antiproliferative activity as a single agent in HER2‐overexpressing gastric cancer and breast cancer cells (Figure 5A), whereas in combination with trastuzumab, its antiproliferative activity was superior to that of pertuzumab or trastuzumab alone or in combination in HER2‐overexpressing NCI‐N87 and OE‐19 gastric cancer cell lines. Changes in cell viability in response to oz1E11 were correlated with HER2 levels in treated cells (Figure 5A), suggesting that the antiproliferative activity of oz1E11, both as a single agent and in combination with trastuzumab, is dependent on HER2 expression. The oz1E11 antibody showed high efficacy against HCC‐202 breast cancer cells, which are HER2‐positive but trastuzumab‐resistant, whereas it showed little activity against other trastuzumab‐resistant cell lines including HCC‐1954 (Supplementary Figure 2).
Figure 5.
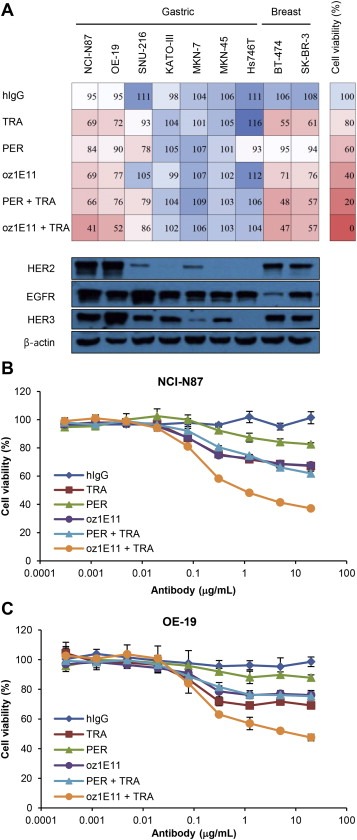
oz1E11 shows antiproliferative activity in HER2‐overexpressing gastric and breast cancer cells A, Human gastric and breast cancer cells were treated with 5 μg/mL of antibodies for 3 or 4 days and cell viability was determined (upper panel). The expressions of HER2, EGFR, and HER3 in 20 μg of total cell lysate were determined by western blotting (bottom panel). Dose‐effect curves of antibodies in NCI‐N87 (B) and OE‐19 (C) cells are shown. Cell viability was expressed as the mean ± SD (n = 3), and the 100% point was defined as the absorbance of the untreated well.
The antiproliferative activity of oz1E11 was confirmed in vivo in NCI‐N87 and OE‐19 xenograft models. Tumors were treated when reaching a volume of 200 mm3 (Figure 6A and B) or 500 mm3 (Figure 6C and D) to evaluate the effect of the antibody on TGI and regression. Combination treatment with oz1E11 and trastuzumab showed superior antitumor activity to that of each agent alone in both xenograft models. In the in vivo efficacy study using NCI‐N87 cells, pertuzumab showed increased antitumor activity when used in combination with trastuzumab, which was different from the results of the in vitro study. Both oz1E11 and pertuzumab caused complete regression of NCI‐N87 xenograft tumors when used in combination with trastuzumab (Figure 6A and C). However, in the OE‐19 xenograft model, only the combination of oz1E11 and trastuzumab completely inhibited tumor growth, and pertuzumab and trastuzumab in combination caused partial inhibition of tumor growth (Figure 6B and D). Isolated OE‐19 and NCI‐N87 xenograft tumors were subjected to the TUNEL assay, which showed a higher number of apoptotic cells in tissues treated with oz1E11 that was further increased in response to combination treatment with oz1E11 and trastuzumab (Supplementary Figure 4).
Figure 6.
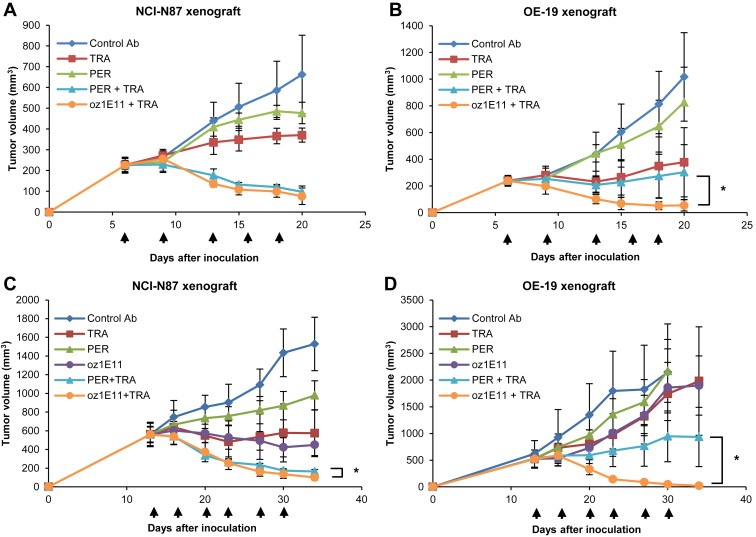
oz1E11 causes tumor regression in NCI‐N87 and OE‐19 gastric tumor xenograft models in combination with trastuzumab A, NCI‐N87 and B, OE‐19 cells were inoculated into mice (n = 6 mice/group each), and treatment with 10 mg/kg of antibody was started when tumor volumes reached approximately 200 mm3. For combination treatment, 10 mg/kg of each antibody was administered. C, NCI‐N87 (n = 5 mice/group) and D, OE‐19 (n = 3 mice/group) cells were inoculated into mice and antibody treatments were started when tumor volumes reached approximately 500 mm3. Mice received a dose of 20 mg/kg for single agent treatment and 10 mg/kg of each antibody for combination treatment. Administration days are indicated by arrows. Tumor volume (mm3) was expressed as the mean ± SD. Statistically significant differences were determined by Student's t test. *, P < 0.05 versus the pertuzumab and trastuzumab combination.
4. Discussion
HER2‐targeted therapy has shown promising results in the treatment of gastric cancer, and trastuzumab has been approved for the treatment of patients with HER2‐overexpressing gastric cancer. However, despite encouraging clinical results obtained with trastuzumab, the development of more potent targeted therapies for HER2‐positive gastric cancer is necessary to increase overall survival rates (Bang et al., 2010). Combination of non‐competing antibodies targeting receptor proteins can increase antitumor activity in vitro and in vivo (Kamat et al., 2008; Pedersen et al., 2010; Tvorogov et al., 2010; Zhang et al., 2010). Pertuzumab is the first approved antibody to be used in combination for dual targeting of the same protein in the treatment of metastatic breast cancer (Baselga et al., 2012). In the present study, efforts to identify a superior therapeutic antibody for HER2‐overexpressing gastric cancer resulted in the development of 1E11. 1E11 inhibits HER2‐overexpressing gastric cancer cell growth in in vitro and in vivo models in combination with trastuzumab, enhancing its apoptotic activity in a synergistic manner. Unlike pertuzumab and trastuzumab, 1E11 and trastuzumab bind to the same sub‐domain (IV) of HER2. To the best of our knowledge, this is the first antibody combination targeting the same sub‐domain of the HER2 protein with potential therapeutic application in the treatment of gastric cancer.
HER2/HER3 heterodimerization is one of the most common mechanisms triggering aberrant HER2 signaling in breast cancer (Baselga and Swain, 2009; Olayioye et al., 2000), and pertuzumab has shown antitumor activity through the inhibition of HER2/HER3 dimerization in non‐small cell lung cancer and breast cancer (Lee‐Hoeflich et al., 2008; Sakai et al., 2007). The NCI‐N87 cell line has previously been used as a model system for research on HER2‐overexpressing gastric cancer (Kim et al., 2008; Patel et al., 2009; Tanner et al., 2005; Yamashita‐Kashima et al., 2011). In the present study, NCI‐N87 cell proliferation was increased by the HER2 heterodimerizing ligands HRG1 and EGF, and treatment with pertuzumab efficiently inhibited ligand‐induced cell proliferation as demonstrated previously (Figure 4B). 1E11 in combination with trastuzumab inhibited ligand‐induced cell proliferation to a similar level as the pertuzumab/trastuzumab combination, suggesting that the effect of 1E11 in combination with trastuzumab is mediated by the inhibition of HER2 heterodimerization. Pertuzumab did not show significant antiproliferative activity as a single agent or in combination with trastuzumab in NCI‐N87 and OE‐19 cells. By contrast, the antiproliferative activity of 1E11 increased when used in combination with trastuzumab. These results suggest that blocking HER2 heterodimerization with EGFR or HER3 is not fully sufficient to induce cell death in HER2‐overexpressing gastric cancer.
One of the hallmarks of cancer is resistance to apoptosis (Hanahan and Weinberg, 2011; Susnow et al., 2009), and the induction of apoptosis is an important antitumor mechanism of therapeutic antibodies (Ben‐Kasus et al., 2007). The CD20‐targeting antibody rituximab activates caspase‐3 through a mechanism involving Src family kinases, and the EGFR‐targeting antibody cetuximab upregulates the pro‐apoptotic protein Bax and downregulates the anti‐apoptotic protein Bcl‐2 (Hofmeister et al., 2000; Huang et al., 1999). HER2 suppresses pro‐apoptotic Bad and Bim through AKT (Datta et al., 1997; Tanizaki et al., 2011), and AKT regulates several members of the forkhead family of transcription factors related to apoptosis (Chakrabarty et al., 2013; Real et al., 2005). Recently, translocation of HER2 to mitochondria was shown to contribute to trastuzumab resistance by decreasing the activity of cytochrome c oxidase in a HER2‐dependent manner (Ding et al., 2012). 1E11 significantly induced apoptosis in NCI‐N87 cells as single agent and in combination with trastuzumab (Figure 3B and C), and the apoptotic activity of 1E11 was confirmed in xenograft model (Supplementary Figure 4). As reported earlier (Nahta et al., 2004) and confirmed here, the apoptotic activity of trastuzumab is limited. Therefore, the synergistic increase in antitumor activity by the combination of 1E11 and trastuzumab could be attributed to the apoptotic activity of 1E11. The apoptotic cancer cell death induced by 1E11 and trastuzumab in combination could be mediated by the downregulation of HER2 and inhibition of PI3K‐AKT signaling. 1E11 and trastuzumab combination treatment increased effector caspase‐3/7 activity confirming that the apoptotic activity of 1E11 (Figure 3D). Trastuzumab alone induces cell‐cycle arrest in BT‐474 cells as previously reported (Brockhoff et al., 2007). However, in the present study, trastuzumab and 1E11 did not cause significant cell‐cycle arrest as single agents or in combination in NCI‐N87 cells.
Despite the important roles of sub‐domains II and IV of the HER2 extracellular region in ErbB family dimerization activation of downstream signaling pathways associated with cell growth (Burgess et al., 2003), the exact role of sub‐domain IV remains unknown. It has been reported that trastuzumab disrupts HER2/HER3 heterodimer‐formation under ligand‐independent conditions (Junttila et al., 2009); however, the direct interaction of HER2 with non‐ErbB family members such as CD44, MUC4, and IGF‐1R plays a role in tumor development and trastuzumab resistance (Balana et al., 2001; Bourguignon et al., 1997; Nagy et al., 2005). Pertuzumab inhibits HER2 and IGF‐1R dimerization in trastuzumab‐resistant breast cancer cells, although it does not significantly inhibit cell growth (Nahta et al., 2005). 1E11, in combination with trastuzumab, inhibited ligand‐induced cell proliferation to a similar level as pertuzumab, despite the fact that 1E11 binds to a non‐overlapping epitope in sub‐domain IV that is not associated with the dimerization arm (Figure 2A and B). Similar to previously reported antibody combinations, the 1E11 and trastuzumab combination could inhibit HER2 dimerization with other non‐ErbB2 family members, resulting in cancer cell apoptosis and/or downregulation of HER2 (Ben‐Kasus et al., 2009; Kamat et al., 2008; Pedersen et al., 2010). oz1E11 had antiproliferative effects against HCC‐202, a HER2‐positive but trastuzumab‐resistant breast cancer cell line, although the mechanism by which oz1E11 overcomes trastuzumab resistance in this particular cell line remains unclear. Further investigation is necessary to improve our understanding of the mode of action of oz1E11 (Supplementary Figure 2).
In conclusion, 1E11 showed synergistic antitumor activity in combination with trastuzumab in HER2‐positive human gastric cancer in vitro and in vivo. The antitumor activity of 1E11 was mediated by its apoptotic activity and the inhibition of HER2 homo‐and heterodimerization downstream signaling. To the best of our knowledge, 1E11 and trastuzumab is the first antibody combination targeting the same sub‐domain of HER2. This antibody combination could be a useful tool to improve our understanding of the biochemical properties of HER2 and a novel potent therapeutic strategy for the treatment of patients with HER2‐overexpressing gastric and breast cancer.
Supporting information
The following is the supplementary data related to this article:
Supplementary Figure 1. oz1E1 mediates antibody‐dependent cellular cytotoxicity of SK‐BR‐3. The antibody‐dependent cellular cytotoxicity of antibodies was measured using an assay that detects LDH released from lysed cells. Peripheral blood mononuclear cells were used as effector cells and HER2‐overexpressing SK‐BR‐3 cells were used as target cells with 1:50 target:effector ratio. Cytotoxicity was expressed as the mean ± SD (n = 3).
Supplementary Figure 2. oz1E11 shows antiproliferative activity in trastuzumab‐resistant breast cancer cells. A, Human breast cancer cells were treated with 5 μg/mL of antibodies for 3 or 4 days and cell viability was determined. B, A dose‐effect curve of antibodies in HCC‐202 cells is shown. Cell viability was expressed as the mean ± SD (n = 3), and the 100% point was defined as the absorbance of the untreated well.
Supplementary Figure 3. Expression of HER2 and EGFR in gastric and breast cancers. HER2 (A) and EGFR (B) expression levels were determined by flow cytometric analysis of 100,000 cells stained with 1 μg of trastuzumab or cetuximab for the detection of HER2 and EGFR, respectively. Data points are the mean ± SD (n = 2) of the mean fluorescence intensity (MFI).
Supplementary Figure 4. Apoptotic activity of oz1E11 in xenograft models. A, Tumor masses were isolated after the OE‐19 xenograft study described in Figure 6D that antibody treatments were started when tumor volumes reached approximately 500 mm3 . TUNEL assay was followed using these OE‐19 cell mass (B). C, Another TUNEL assay was conducted with isolated tumor masses from NCI‐N87 xenograft study that antibody treatments were started when tumor volumes reached approximated 500 mm3 (Figure 6C). *oz1E11‐A is another optimized antibody derived from 1E11.
Acknowledgments
The authors would like to thank all the researchers at AbClon Inc. for helpful discussions and collaborations, and Dr. Hyunbo Shim, Dr. Per Norlén and Dr. Fredrik Frejd for helpful discussions.
This study was supported by funds from the International Collaborative R&D Program (1415118385) of the Ministry of Trade, Industry & Energy (MOTIE) in the Republic of Korea.
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2014.09.007.
Ko Bong-Kook, Lee Sook-Yeon, Lee Young-Ha, Hwang In-Sik, Persson Helena, Rockberg Johan, Borrebaeck Carl, Park Dongeun, Kim Kyu-Tae, Uhlen Mathias, Lee Jong-Seo, (2015), Combination of novel HER2‐targeting antibody 1E11 with trastuzumab shows synergistic antitumor activity in HER2‐positive gastric cancer, Molecular Oncology, 9, doi: 10.1016/j.molonc.2014.09.007.
References
- Arnould, L.1 , Gelly, M. , Penault-Llorca, F. , Benoit, L. , Bonnetain, F. , Migeon, C. , Cabaret, V. , Fermeaux, V. , Bertheau, P. , Garnier, J. , Jeannin, J.F. , Coudert, B. , 2006. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism. Br. J. Cancer. 94, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balana, M.E. , Labriola, L. , Salatino, M. , Movsichoff, F. , Peters, C. , Charreau, E.H. , Elizalde, P.V. , 2001. Activation of ErbB-2 via a hierarchical interaction between ErbB-2 and type I insulin-like growth factor receptor in mammary tumor cells. Oncogene. 20, 34–47. [DOI] [PubMed] [Google Scholar]
- Bang, Y.J. , Van Cutsem, E. , Feyereislova, A. , Chung, H.C. , Shen, L. , Sawaki, A. , Lordick, F. , Ohtsu, A. , Omuro, Y. , Satoh, T. , Aprille, G. , Kulikov, E. , Hill, J. , Lehle, M. , Ruschoff, J. , Kang, Y.K. , ToGA Trial Investigators2010. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-esophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet. 376, 687–697. [DOI] [PubMed] [Google Scholar]
- Baselga, J. , Swain, S.M. , 2009. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer. 9, 463–475. [DOI] [PubMed] [Google Scholar]
- Baselga, J. , Gelmon, K.A. , Verma, S. , Wardley, A. , Conte, P. , Miles, D. , Bianchi, G. , Cortes, J. , McNally, V.A. , Ross, G.A. , Fumoleau, P. , Gianni, L. , 2010. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J. Clin. Oncol. 28, 1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga, J. , Cortes, J. , Kim, S.B. , Im, S.A. , Hegg, R. , Im, Y.H. , Roman, L. , Pedrini, J.L. , Pienkowski, T. , Knott, A. , Clark, E. , Benyunes, M.C. , Ross, G. , Swain, S.M. , CLEOPATRA Study Group2012. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Eng. J. Med. 366, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Kasus, T. , Schechter, B. , Sela, M. , Yarden, Y. , 2007. Cancer therapeutic antibodies come of age: targeting minimal residual disease. Mol. Oncol. 1, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Kasus, T. , Schechter, B. , Lavi, S. , Yarden, Y. , Sela, M. , 2009. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: relevance of receptor endocytosis. Proc. Natl. Acad. Sci. U S A. 106, 3294–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon, L.Y. , Zhu, H. , Chu, A. , Iida, N. , Zhang, L. , Hung, M.C. , 1997. Interaction between the adhesion receptor, CD44, and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J. Biol. Chem. 272, 27913–27918. [DOI] [PubMed] [Google Scholar]
- Brochet, X. , Lefrance, M.P. , Giudicelli, V. , 2008. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 36, W503–W508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhoff, G. , Heckel, B. , Schmidt-Bruecken, E. , Plander, M. , Hofstaedter, F. , Vollmann, A. , Diermeier, S. , 2007. Differential impact of Cetuximab, Pertuzumab and Trastuzumab on BT474 and SK-BR-3 breast cancer cell proliferation. Cell Prolif. 40, 488–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, A.W. , Cho, H.S. , Eigenbrot, C. , Ferguson, K.M. , Garrett, T.P. , Leahy, D.J. , Lemmon, M.A. , Sliwkowski, M.X. , Ward, C.W. , Yokoyama, S. , 2003. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol. Cell. 12, 541–552. [DOI] [PubMed] [Google Scholar]
- Cai, Z. , Zhang, G. , Zhou, Z. , Bembas, K. , Drebin, J.A. , Greene, M.I. , Zhang, H. , 2008. Differential binding patterns of monoclonal antibody 2C4 to the ErbB3-p185her2/neu and the EGFR-p185her2/neu complexes. Oncogene. 27, 3870–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty, A. , Bhola, N.E. , Sutton, C. , Ghosh, R. , Kuba, M.G. , Dave, B. , Chang, J.C. , Arteaga, C.L. , 2013. Trastuzumab-resistant cells rely on a HER2-PI3K-FoxO-survivin axis and are sensitive to PI3K inhibitors. Cancer Res. 73, 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, H.S. , Mason, K. , Ramyar, K.X. , Stanley, A.M. , Gabelli, S.B. , Denney, D.W. , Leahy, D.J. , 2003. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 421, 756–760. [DOI] [PubMed] [Google Scholar]
- Chou, T.C. , Talalay, P. , 1984. Quantitative analysis of dose-effect relationships: the combination effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22, 27–55. [DOI] [PubMed] [Google Scholar]
- Datta, S.R. , Dudek, H. , Tao, X. , Masters, S. , Fu, H. , Gotoh, Y. , Greenberg, M.E. , 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 91, 231–241. [DOI] [PubMed] [Google Scholar]
- Ding, Y. , Liu, Z. , Desai, S. , Zhao, Y. , Liu, H. , Pannell, L.K. , Yi, H. , Wright, E.R. , Owen, L.B. , Dean-Colomb, W. , Fodstad, O. , Lu, J. , LeDoux, S.P. , Wilson, G.L. , Tan, M. , 2012. Receptor tyrosine kinase ErbB2 translocates into mitochondria and regulates cellular metabolism. Nat. Commun. 3, 1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay, J. , Soerjomataram, I. , Ervik, M. , Dikshit, R. , Eser, S. , Mathers, C. , Rebelo, M. , Parkin, D.M. , Forman, D. , Bray, F. , 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] International Agency for Research on Cancer; Lyon, France: Available from: http://globocan.iarc.fr (accessed on day/month/year) [Google Scholar]
- Foote, J. , Winter, G. , 1992. Antibody framework residues affecting the conformation of the hypervariable loops. J. Mol. Biol. 224, 487–499. [DOI] [PubMed] [Google Scholar]
- Friedman, L.M. , Rinon, A. , Schechter, B. , Lyass, L. , Lavi, S. , Bacus, S.S. , Sela, M. , Yarden, Y. , 2005. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc. Natl. Acad. Sci. U S A. 102, 1915–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, I. , Vizoso, F. , Martin, A. , Sanz, L. , Abdel-Lah, O. , Raigoso, P. , Garcia-Muniz, J.L. , 2003. Clinical significance of the epidermal growth factor receptor and HER2 receptor in resectable gastric cancer. Ann. Surg. Oncol. 10, 234–241. [DOI] [PubMed] [Google Scholar]
- Hanahan, D. , Weinberg, R.A. , 2011. Hallmarks of cancer: the next generation. Cell. 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Hayashi, M. , Inokuchi, M. , Takagi, Y. , Yamada, H. , Kojima, K. , Kumagai, J. , Kawano, T. , Sugihara, K. , 2008. High expression of HER3 is associated with a decreased survival in gastric cancer. Clin. Cancer Res. 14, 7843–7849. [DOI] [PubMed] [Google Scholar]
- Hofmeister, J.K. , Cooney, D. , Coggeshall, K.M. , 2000. Clustered CD20 induced apoptosis: src-family kinase, the proximal regulator of tyrosine phosphorylation, calcium influx, and caspase 3-dependent apoptosis. Blood Cells Mol. Dis. 26, 133–143. [DOI] [PubMed] [Google Scholar]
- Huang, S.M. , Bock, J.M. , Harari, P.M. , 1999. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 59, 1935–1940. [PubMed] [Google Scholar]
- Junttila, T.T. , Akita, R.W. , Parsons, K. , Fields, C. , Lewis Phillips, G.D. , Friedman, L.S. , Sampath, D. , Sliwkowski, M.X. , 2009. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 15, 429–440. [DOI] [PubMed] [Google Scholar]
- Kamat, V. , Donaldson, J.M. , Kari, C. , Quadros, M.R. , Lelkes, P.I. , Chaiken, I. , Cocklin, S. , Williams, J.C. , Papazoglou, E. , Rodeck, U. , 2008. Enhanced EGFR inhibition and distinct epitope recognition by EGFR antagonistic mAbs C225 and 425. Cancer Biol. Ther. 7, 726–733. [DOI] [PubMed] [Google Scholar]
- Kasprzyk, P.G. , Song, S.U. , Di Fiore, P.P. , King, C.R. , 1992. Therapy of an animal model of human gastric cancer using a combination of anti-erbB-2 monoclonal antibodies. Cancer Res. 52, 2771–2776. [PubMed] [Google Scholar]
- Kim, S.Y. , Kim, H.P. , Kim, Y.J. , Oh do, Y. , Im, S.A. , Lee, D. , Jong, H.S. , Kim, T.Y. , Bang, Y.J. , 2008. Trastuzumab inhibits the growth of human gastric cancer cell lines with HER2 amplification synergistically with cisplatin. Int. J. Oncol. 32, 89–95. [PubMed] [Google Scholar]
- Koefoed, K. , Steinaa, L. , Soderberg, J.N. , Kjar, I. , Jacobsen, H.J. , Meijer, P.J. , Haurum, J.S. , Jensen, A. , Kragh, M. , Andersen, P.S. , Pedersen, M.W. , 2011. Rational identification of an optimal antibody mixture for targeting the epidermal growth factor receptor. MAbs. 3, 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Hoeflich, S.T. , Crocker, L. , Yao, E. , Pham, T. , Munroe, X. , Hoeflich, K.P. , Sliwkowski, M.X. , Stern, H.M. , 2008. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 68, 5878–5887. [DOI] [PubMed] [Google Scholar]
- Nagy, P. , Friedlander, E. , Tanner, M. , Kapanen, A.I. , Carraway, K.L. , Isola, J. , Jovin, T.M. , 2005. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a Herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 65, 473–482. [PubMed] [Google Scholar]
- Nahta, R. , Hung, M.C. , Esteva, F.J. , 2004. The HER2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 64, 2343–2346. [DOI] [PubMed] [Google Scholar]
- Nahta, R. , Yuan, L.X. , Zhang, B. , Kobayashi, R. , Esteva, F.J. , 2005. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer. Cancer Res. 65, 11118–11128. [DOI] [PubMed] [Google Scholar]
- Olayioye, M.A. , Neve, R.M. , Lane, H.A. , Hynes, N.E. , 2000. The ErbB signaling network: receptor dimerization in development and cancer. EMBO J. 19, 3159–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, D. , Bassi, R. , Hooper, A. , Prewett, M. , Hicklin, D.J. , Kang, X. , 2009. Anti-epidermal growth factor receptor monoclonal antibody cetuximab inhibits EGFR/HER-2 heterodimerization and activation. Int. J. Oncol. 34, 25–32. [PubMed] [Google Scholar]
- Pedersen, M.W. , Jacobsen, H.J. , Koefoed, K. , Hey, A. , Pyke, C. , Haurum, J.S. , Kragh, M. , 2010. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res. 70, 588–597. [DOI] [PubMed] [Google Scholar]
- Real, P.J. , Benito, A. , Cuevas, J. , Berciano, M.T. , de Juan, A. , Coffer, P. , Gomez-Roman, J. , Lafarga, M. , Lopez-Vega, J.M. , Fernandez-Luna, J.L. , 2005. Blockade of epidermal growth factor receptors chemosensitizes breast cancer cells through up-regulation of Bnip3L. Cancer Res. 65, 8151–8157. [DOI] [PubMed] [Google Scholar]
- Sakai, K. , Yokote, H. , Murakami-Murofushi, K. , Tamura, T. , Saijo, N. , Nishio, K. , 2007. Pertuzumab, a novel HER2 dimerization inhibitor, inhibits the growth of human lung cancer cells mediated by the HER3 signaling pathway. Cancer Sci. 98, 1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer, W. , Friess, T. , Burtscher, H. , Bossenmaier, B. , Endl, J. , Hasmann, M. , 2009. Strong enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 69, 9330–9336. [DOI] [PubMed] [Google Scholar]
- Siegel, R. , Naishadham, D. , Jemal, A. , 2012. Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29. [DOI] [PubMed] [Google Scholar]
- Susnow, N. , Zeng, L. , Margineantu, D. , Hockenbery, D.M. , 2009. Bcl-2 family proteins as regulators of oxidative stress. Semin. Cancer Biol. 19, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizaki, J. , Okamoto, I. , Fumita, S. , Okamoto, W. , Nishio, K. , Nakagawa, K. , 2011. Roles of BIM induction and surviving downregulation in lapatinib-induced apoptosis in breast cancer cells with HER2 amplification. Oncogene. 30, 4097–4106. [DOI] [PubMed] [Google Scholar]
- Tanner, M. , Hollmen, M. , Junttila, T.T. , Kapanen, A.I. , Tommola, S. , Soini, Y. , Helin, H. , Salo, J. , Joensuu, H. , Sihvo, E. , Elenius, K. , Isola, J. , 2005. Amplification of HER-2 in gastric cancer: association with topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann. Oncol. 16, 273–278. [DOI] [PubMed] [Google Scholar]
- Tvorogov, D. , Anisimov, A. , Zheng, W. , Leppanen, V.M. , Tammela, T. , Laurinavicius, S. , Holnthoner, W. , Helotera, H. , Holopainen, T. , Jeltsch, M. , Kalkkinen, N. , Lankinen, H. , Ojala, P.M. , Alitalo, K. , 2010. Effective suppression of vascular network formation by combination of antibodies blocking VEGFR ligand binding and receptor dimerization. Cancer Cell. 18, 630–640. [DOI] [PubMed] [Google Scholar]
- Yamashita-Kashima, Y. , Iijima, S. , Yorozu, K. , Furugaki, K. , Kurasawa, M. , Ohta, M. , Fujimoto-Ouchi, K. , 2011. Pertuzumab in combination with trastuzumab shows significant enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin. Cancer Res. 17, 5060–5070. [DOI] [PubMed] [Google Scholar]
- Yarden, Y. , Sliwkowski, M.X. , 2001. Untangling the ErbB signaling network. Nat. Rev. Mol. Cell Biol. 2, 127–137. [DOI] [PubMed] [Google Scholar]
- Zhang, A. , Shen, G. , Zhao, T. , Zhang, G. , Liu, J. , Song, L. , Wei, W. , Bing, L. , Wu, Z. , Wu, Q. , 2010. Augmented inhibition of angiogenesis by combination of HER2 antibody chA21 and trastuzumab in human ovarian carcinoma xenograft. J. Ovarian Res. 3, 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following is the supplementary data related to this article:
Supplementary Figure 1. oz1E1 mediates antibody‐dependent cellular cytotoxicity of SK‐BR‐3. The antibody‐dependent cellular cytotoxicity of antibodies was measured using an assay that detects LDH released from lysed cells. Peripheral blood mononuclear cells were used as effector cells and HER2‐overexpressing SK‐BR‐3 cells were used as target cells with 1:50 target:effector ratio. Cytotoxicity was expressed as the mean ± SD (n = 3).
Supplementary Figure 2. oz1E11 shows antiproliferative activity in trastuzumab‐resistant breast cancer cells. A, Human breast cancer cells were treated with 5 μg/mL of antibodies for 3 or 4 days and cell viability was determined. B, A dose‐effect curve of antibodies in HCC‐202 cells is shown. Cell viability was expressed as the mean ± SD (n = 3), and the 100% point was defined as the absorbance of the untreated well.
Supplementary Figure 3. Expression of HER2 and EGFR in gastric and breast cancers. HER2 (A) and EGFR (B) expression levels were determined by flow cytometric analysis of 100,000 cells stained with 1 μg of trastuzumab or cetuximab for the detection of HER2 and EGFR, respectively. Data points are the mean ± SD (n = 2) of the mean fluorescence intensity (MFI).
Supplementary Figure 4. Apoptotic activity of oz1E11 in xenograft models. A, Tumor masses were isolated after the OE‐19 xenograft study described in Figure 6D that antibody treatments were started when tumor volumes reached approximately 500 mm3 . TUNEL assay was followed using these OE‐19 cell mass (B). C, Another TUNEL assay was conducted with isolated tumor masses from NCI‐N87 xenograft study that antibody treatments were started when tumor volumes reached approximated 500 mm3 (Figure 6C). *oz1E11‐A is another optimized antibody derived from 1E11.


