Abstract
Deficiency of the tumour suppressor merlin leads to the development of schwannomas, meningiomas and ependymomas occurring spontaneously or as a part of the hereditary disease Neurofibromatosis type 2 (NF2). Merlin loss is also found in a proportion of other cancers like mesothelioma, melanoma, breast cancer and glioblastoma. The tumour suppressor/transcription factor p53 regulates proliferation, survival and differentiation and its deficiency plays a role in the development of many tumours. 53 can be negatively regulated by FAK, PI3K/AKT and MDM2 and possibly positively regulated by merlin in different cell lines. In this study we investigated the role of p53 in merlin‐deficient tumours. Using our in vitro model of primary human schwannoma cells we have previously demonstrated that FAK is overexpressed/activated and localises into the nucleus of schwannoma cells increasing proliferation. AKT is strongly activated via platelet‐derived growth factor (PDGF) – and insulin‐like growth factor 1 (IGF1) – receptors increasing survival. Here we investigated p53 regulation and its role in proliferation and survival of human primary schwannoma cells using western blotting, immunocytochemistry, immunohistochemistry and proliferation, survival and transcription factor assays. In human primary schwannoma cells p53 was found to be downregulated while MDM2 was upregulated leading to increased cell proliferation and survival. p53 is regulated by merlin involving FAK, AKT and MDM2. Merlin reintroduction into schwannoma cells increased p53 levels and activity, and treatment with Nutlin‐3, a drug which increases p53 stability by disrupting the p53/MDM2 complex, decreased tumour growth and reduced cell survival. These findings are important to dissect the mechanisms responsible for the development of merlin‐deficient tumours and to identify new therapeutic targets. We suggest that Nutlin‐3, possibly in combination with FAK or PI3K inhibitors, can be employed as a novel treatment for schwannoma and other merlin‐deficient tumours.
Keywords: Neurofibromatosis type 2 (NF2), Merlin, p53/MDM2, FAK, Proliferation and survival
Highlights
p53/MDM2 ratio is deregulated in schwannoma increasing tumour growth and survival.
p53 is regulated by merlin involving FAK, AKT and MDM2.
Combined merlin and Nutlin‐3 normalise p53 reducing schwannoma growth and survival.
Nutlin‐3 increases p53 stability and is a good drug candidate for schwannoma treatment.
Abbreviations
- AKT
Protein kinase B (PKB)
- FAK
Focal adhesion kinase
- GAPDH
Glyceraldehyde‐3‐Phosphate dehydrogenase
- IGF1
Insulin‐like growth factor 1
- MDM2
Mouse double minute 2 homolog
- NF2
Neurofibromatosis type 2
- PI3K
Phosphatidylinositide 3‐kinases
- PDGF
Platelet‐derived growth factor
- RhoGDI
Rho GDP‐dissociation inhibitor
- shRNA
short hairpin RNAs
- p53
protein 53
- p21
Waf1/Cip1‐cyclin‐dependent kinase inhibitor 1
1. Introduction
The loss of the tumour suppressor merlin is the main cause of the development of multiple low‐grade tumours of the nervous system e.g. schwannomas, meningiomas and ependymomas. These tumours may occur spontaneously or as a part of a hereditary disease Neurofibromatosis type 2 (NF2). Merlin mutations have also been observed in variety of other cancers including glioblastomas, mesotheliomas, renal and breast cancer ((Chen et al., 2011; Morrow et al., 2011) and cBioPortal). NF2 patients frequently develop bilateral vestibular schwannomas (VSs) leading to tinnitus, balance problems, bilateral hearing impairment and brain stem compression. Approximately 70–80% of NF2 patients have spinal schwannomas, 50–60% develop multiple meningiomas and a major portion of patients develop ependymomas, most of them spinal. Merlin localises to the cellular membrane (McClatchey and Giovannini, 2005) and in the nucleus (Li et al., 2010) and is involved in the signalling pathways regulating cell‐matrix adhesion, cell proliferation and survival (Zhou et al., 2011). As schwannomas are the most common merlin‐deficient tumours and the hallmark tumour for NF2, we have previously studied how merlin‐deficiency leads to tumour development using a human primary schwannoma in vitro model comprising human primary Schwann and schwannoma cells (Ammoun et al., 2008, 2012, 2011, 2000, 2005, 2011). As existing mice models only partly reflect human disease/tumours, using human primary cell and translation into early phase 0 trials has recently been recommended as an alternative (Blakeley et al., 2012).
Using our in vitro human schwannoma model, we have previously demonstrated that the Focal Adhesion Kinase (FAK) is overexpressed, activated and localised into the nucleus in human primary schwannoma cells (Ammoun et al., 2012) leading to increased cell proliferation and adhesion (Ammoun et al., 2012). Moreover, we observed that the Phosphatidylinositide 3‐Kinase/AKT (PI3K/AKT) pathway was strongly activated leading to increased cell survival (Ammoun et al., 2012). Thus merlin loss in schwannoma cells activates FAK and PI3K/AKT pathways which in turn have been shown to be involved in decreased p53 levels in cell lines (Lim et al., 2008; Mayo and Donner, 2002; Singh et al., 2013; Zhou et al., 2001). Therefore we investigated the effect of merlin loss on p53 activation in merlin‐deficient tumours. The tumour suppressor and transcription factor p53 negatively controls proliferation and survival by either gene regulation (Vogelstein et al., 2000) and/or by direct protein–protein signalling (Chylicki et al., 2000b) and can positively regulate cell differentiation (Chylicki et al., 2000a). Almost 50% of all tumours express mutated (Olivier et al., 2010) or inactivated p53 (Li and Lozano, 2013; Muller et al., 2011; Walerych et al., 2012).
Previous studies in vitro using cell lines showed that p53 levels and activity can be positively regulated by merlin through merlin‐mediated MDM2 degradation (Kim et al., 2004). However, depending on MDM2 conformation, MDM2 can have a dual effect on p53; either leading to p53 degradation or upregulation. It has been demonstrated that in order to keep p53 at physiological concentrations, non‐phosphorylated MDM2 binds p53 inducing its degradation via polyubiquitination (Ponnuswamy et al., 2012). In contrast, phosphorylated MDM2 on Ser395, leads to accumulation of MDM2 in the nucleolus (Gajjar et al., 2012) where it binds to p53 mRNA promoting p53 synthesis (Ponnuswamy et al., 2012).
In this study we investigated the mechanisms of p53 regulation and its role in schwannoma pathobiology. We show that p53 is downregulated and MDM2 upregulated in human primary schwannoma cells. MDM2 is active in downregulating p53 when the protein localises to the nucleus and in the cytoplasm but not into the nucleoli of schwannoma cells. We show that merlin reintroduction induces MDM2 accumulation in the nucleoli increasing p53 protein levels and activity. The simultaneous inhibition of p53 degradation with the p53/MDM2 complex inhibitor Nutlin‐3 has an additive effect. AKT contributes to p53 degradation probably via phosphorylation of MDM2 at Ser166 and Ser186 and consequent MDM2 localisation to the nucleus (Mayo and Donner, 2001; Singh et al., 2013; Zhou et al., 2001). Additionally we show that FAK, overexpressed and strongly activated in schwannoma (Ammoun et al., 2008, 2011), has a role in facilitating p53 deregulation possibly via formation of a MDM2/p53/FAK complex (Lim et al., 2008). Based on our observations we suggest p53 as a new therapeutic downstream target in merlin‐deficient tumours.
2. Material and methods
2.1. Cell cultures of human primary Schwann and schwannoma cells
The primary human Schwann and schwannoma cells were obtained as described previously (Rosenbaum et al., 2000) and cultured in the Growth Factor Medium (GFM): DMEM, 10% FCS, 0.5 μM forskolin, 10 nM β heregulin, 0.5 mM, 3‐isobutyl‐1‐methylxanthine (IBMX) and 2.5 μg/ml insulin. In every experiment, a minimum of three samples from three different individuals were included.
2.2. Inhibitors and chemicals
Wortmannin was purchased from Tocris Bioscience (Bristol, UK), DAPI and MG132 from Sigma (St. Louis, MO). Wortmannin and MG132 have previously been tested in our human primary schwannoma model and the protocol optimized (Ammoun et al., 2008, 2014, 2011).
2.3. shRNA knockdown
GIPZ‐shRNAmir lentiviral particles encoding a short hairpin RNA (shRNA) with non‐silencing sequence (mock) or sequences targeting FAK were used and infections were performed as previously described (Ammoun et al., 2012).
2.4. Re‐introduction of merlin
Mock (control adenovirus) and merlin wild type (recombinant adenovirus AdNF2) were a kind gift from J. Testa (Xiao et al., 2005). Cells were treated with virus for 24 h and then incubated with fresh GFM for additional 24 h.
2.5. TransAM™ p53 transcription factor assay
Schwannoma cells were infected with either mock (control adenovirus) or merlin wild type (recombinant adenovirus AdNF2) and p53 activity assay was performed according to the manufacturer (Active Motif, Tokyo, Japan).
2.6. Immunoblotting
Western blotting was performed as described by Kaempchen et al (Kaempchen et al., 2003). Primary antibodies from Cell Signalling (Danvers, MA.) included: phospho AKT (Ser473), cyclin D1, Cleaved Caspase 3 and FAK while antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA) were: anti‐survivin, anti‐p53, anti‐MDM2 and anti‐merlin (Santa Cruz, sc‐332). HRP‐conjugated secondary antibodies were purchased from Bio‐Rad (Hercules, CA, USA) and ECL‐plus from Amersham (Buckinghamshire, UK). We have used generic loading controls previously established for our system, RhoGDI (Anti‐RhoGDI antibody; Santa Cruz Biotechnology) (Hanemann et al., 2006) and GAPDH (anti‐GAPDH antibody; Millipore, Watford, UK). Images were scanned and processed using Corel Paint Shop Pro Photo XI software, and represent the original data. Western blot quantification analysis was performed using Quantity One software (Bio‐Rad).
2.7. Immunocytochemistry
Immunocytochemistry was performed as previously described (Flaiz et al., 2007; Kaempchen et al., 2003). Primary antibodies used for protein localization studies included: FAK (Cell signalling), p53 (Cell signalling), cleaved caspase 3 (Cell signalling), MDM2 (Santa Cruz), Ki67 (DACO), nucleolin (Abcam, Cambridge, UK) and merlin (Santa Cruz, sc‐332). Anti‐rabbit Cy3 (∼550 nm excitation, ∼570 nm emission) and anti‐mouse Cy2 (∼489 nm excitation, ∼506 nm emission) (Sigma) were used as labelled secondary antibodies. Alexa Fluor 488‐labelled phalloidin was used to visualize actin filaments (Molecular Probes, Eugene, OR) and DAPI for nuclear staining (Sigma; Poole, Dorset, UK). Multitrack imaging was performed using a Zeiss Confocal LSM510. To show specificity of FAK nuclear localization the cells were infected with shRNA FAK (Ammoun et al., 2012).
2.8. Immunohistochemistry
For tissue samples studies, local research ethics approval was obtained. Formalin fixed and paraffin embedded tissue samples from 5 cases of schwannomas were retrieved from the archives of the Department of Cellular & Anatomical Pathology, Derriford Hospital, Plymouth, UK. Cases were identified following a SNOMED search of the histology archives.
Paraffin‐embedded tissue samples (4 μm‐thick) were collected onto 3‐aminopropyltriethoxysilane‐coated glass slides, dewaxed in graded alcohols, then non‐specific peroxidase activity blocked with 3% hydrogen peroxide for 30 min. Antigen retrieval was performed microwaving sections for 30 min in 10 mM citrate buffer at pH 6.0. Sections were then incubated overnight with the following primary antibodies: MDM2 (SMP14, Santa Cruz ‐ 1:400); p53 (DO‐7 Dako – 1:4000) and p53 (7F5, Cell Signalling ‐ 1:200). Elite universal detection kit was used for detection (Vector, UK) and colour was developed with diaminobenzidine.
2.9. Cell proliferation and viability
Cells were cultured for 24–72 h in DMEM alone or DMEM containing Nutlin‐3 using the protocol previously described (Ammoun et al., 2008, 2012).
2.10. Data analysis
Student's two‐tailed t tests and ANOVA were used for statistical analysis. Experiments were performed in at least triplicates using at least three independent batches of cells from different individuals. Ns (not significant): P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001. Data are expressed as mean ± Standard Error (SEM).
3. Results
3.1. p53 protein level and activity is reduced in merlin‐deficient human primary schwannoma cells
By western blot, p53 overall protein amount was analysed in merlin‐deficient human primary schwannoma cells (NF2−/−) and compared to the merlin‐positive normal Schwann cells (NF2+/+). The resulting immunoreactive band showed a marked reduction in p53 protein levels in merlin‐negative cells compared to the merlin‐positive normal Schwann cells (Figure 1A). Immunofluorescence studies in Schwann cells (NF2+/+) localise p53 expression strongly to the nucleus (white arrows, red staining) but more weakly in the cytosol (yellow arrows) (Figure 1B). Weaker nuclear and cytosolic p53 staining was observed in schwannoma cells (NF2−/−) (Figure 1B). Also, immunostaining studies on paraffin‐embedded human schwannoma tissues showed very weak nuclear p53 staining only in some cells (Supplementary data 1). Merlin reintroduction significantly increased total p53 protein levels (Figure 1C left panel) and p53 nuclear accumulation (Figure 1C right panel). To check whether merlin reintroduction also increases p53 activity we investigated the expression of p21 Waf1/Cip1, used as p53 activity readout (Wu and Levine, 1997), and additionally performed TransAM™ p53 Transcription Factor assay using schwannoma cells from four different patients (NF2−/− 1–4). p21 Waf1/Cip1 levels (Figure 1D) and its nucleolar localisation (Figure 1E) as well as p53 activity (Figure 1F), significantly increased upon merlin reintroduction suggesting that merlin is involved in the regulation of p53 activity (Figure 7).
Figure 1.
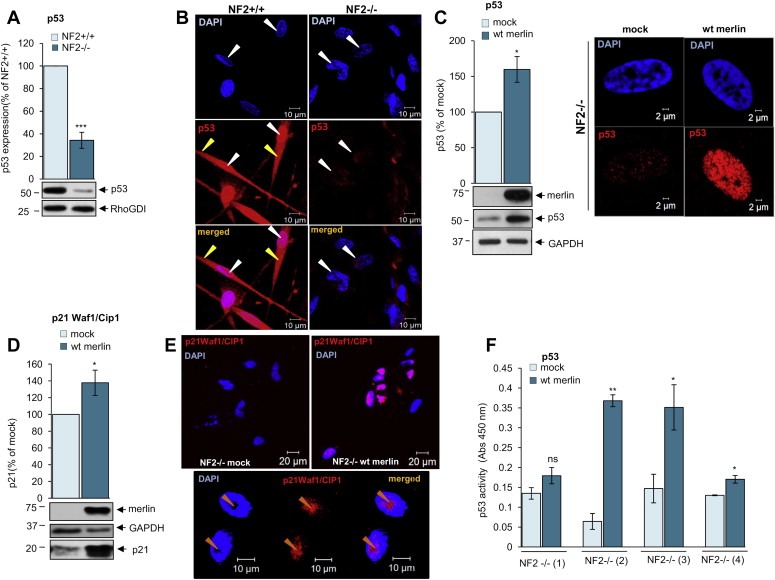
p53 levels and activity are decreased in schwannoma cells due to merlin deficiency. A. Western blot analysis demonstrates strong p53 expression in human primary Schwann (NF2+/+) and weak in human primary schwannoma (NF2−/−) cells. The levels of p53 are detected using anti‐p53 antibodies. ns (not significant): P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001., mean ± SEM is given. B. Immunofluorescent staining of p53 (red) and DAPI (blue) in human primary Schwann (NF2+/+, left panel) and schwannoma (NF2−/−, right panel) cells. A stronger nuclear staining of p53 in Schwann (NF2+/+) compared to schwannoma (NF2−/−) cells. p53 strongly localises to the nucleus (white arrows) and weaker the cytosol (yellow arrows). C–F. Analysis of the effect of merlin reintroduction on p53 levels (C left panel), nuclear localisation (C right panel) and p53 activity [(D) p21 Waf1/Cip1 expression, (E) p21 Waf1/Cip1 cellular localisation and, (F) p53 transcription factor assay]. Cells were infected with mock virus or wild type (wt) merlin containing virus. ns (not significant): P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001, mean ± SEM is given.
Figure 7.
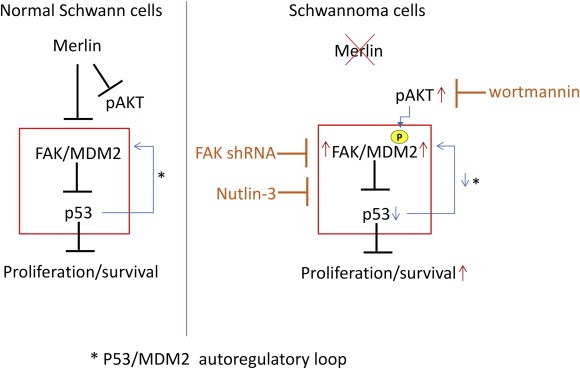
In merlin‐deficient schwannoma cells FAK is strongly overexpressed and activated, AKT activated and MDM2 overexpressed compared to normal merlin‐positive Schwann cells. This leads to decreased p53 stability/levels due to FAK/MDM2/pAKT mediated proteosomal degradation increasing proliferation and survival of schwannoma tumour cells. Nutlin‐3, p53/MDM2 complex formation inhibitor, wortmannin a PI3K/AKT inhibitor and FAK shRNA increase p53 stability/levels restoring proliferation and survival in schwannoma tumour cells. We suggest that Nutlin‐3, alone or in combination with PI3K/AKT or FAK inhibitors is a good drug candidate for treatment of schwannoma and other merlin‐deficient tumours.
3.2. p53 is negatively regulated by FAK/MDM2‐mediated proteosomal degradation
FAK promotes cell survival (Hennessy et al., 2005; Luo et al., 2003) indirectly via PI3K/AKT pathway (Reiske et al., 1999) and directly by interaction with p53 in the nucleus (Lim et al., 2008). Nuclear FAK regulates degradation of p53 by stabilising the MDM2‐p53 ubiquitination complex, resulting in increased export and proteasome‐mediated degradation of p53 (Lim et al., 2008). In human primary schwannoma cells, FAK is overexpressed and accumulates in the nucleus in an activated form leading to increased cell proliferation (Ammoun et al., 2008, 2012). FAK overexpression and nuclear localisation in schwannoma cells contributes to p53 proteosomal degradation (Lim et al., 2008). We used shRNA to knock‐down FAK in order to investigate whether its overexpression contributes to lower p53 levels in schwannoma. FAK shRNA knock‐down increased p53 protein levels (Figure 2A) and strongly potentiated nuclear accumulation of p53 in schwannoma cells (Figure 2B left and right panels). Additionally, treatment with the MDM2/p53 interaction inhibitor Nutlin‐3 highly increased p53 levels (Figure 2C,D) and potentiated p53 nuclear accumulation (Figure 2E). The maximum effect of Nutilin‐3 on p53 was observed either at 4 h incubation with 10 μM (Figure 2C) or at 24 h with 10 μM (Figure 2D).
Figure 2.
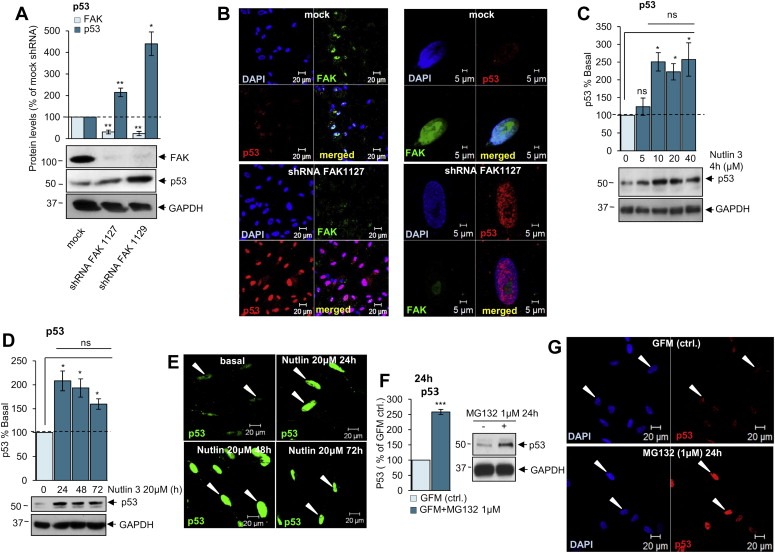
p53 is negatively regulated by FAK/MDM2 mediated proteosomal degradation. A. Western blot analysis shows that FAK knock down using FAK shRNA leads to increased p53 levels. B. Immunocytochemistry demonstrates that FAK shRNA increases nuclear staining of p53 (red) compared to mock shRNA. FAK staining is shown in green and nuclei in blue (DAPI). C, D. Western blot analysis shows that Nutlin‐3 increases p53 levels in schwannoma cells. E. Immunocytochemistry demonstrates increased nuclear staining of p53 in schwannoma cells upon treatment with Nutlin‐3 (20 μM, 24, 48 and 72 h). F. Western blot analysis showing the effect of proteasome inhibitor MG132 on p53 in schwannoma cells. Cells were incubated either with Growth Factor Medium (GFM) alone or GFM in combination with MG132 (1 μM) for 24 h. MG132 (1 μM) increased p53 levels in schwannoma cells. ns (not significant): P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001, mean ± SEM is given. G. Immunofluorescent staining of p53 (red) and DAPI in schwannoma cells. The cells were incubated with culture medium (GFM) alone (G upper panels) or in combination with MG132 (1 μM) (G lower panels) for 24 h. MG132 (1 μM) increased nuclear staining of p53 in schwannoma cells.
To check whether low p53 levels were due to proteosomal protein degradation we cultured schwannoma (NF2−/−) cells in Growth Factor Medium (GFM) with proteasome inhibitor MG132 (1 μM 24 h) (Figure 2F,G). MG132‐treated cells showed a 2.5 fold increase in p53 protein levels (Figure 2F) and p53 nuclear accumulation (Figure 2G), suggesting that proteasome‐mediated degradation does contribute to p53 deficiency in schwannoma cells. Taken together, these results demonstrate that p53 levels are decreased via FAK/MDM2‐mediated proteosomal degradation in merlin‐deficient schwannoma cells (Figure 7).
3.3. p53 is negatively regulated by AKT
AKT is strongly activated in schwannoma (Ammoun et al., 2012) and has been shown to phosphorylate MDM2 leading to MDM2 nuclear translocation and subsequent degradation of p53 (Mayo and Donner, 2001; Singh et al., 2013; Zhou et al., 2001). Thus, we investigated the role of AKT in schwannoma cells in the presence of low levels of p53; cells were treated with or without the PI3K inhibitor wortmannin (1 μM) or using 10% FCS as a control, for 10 min. This time point does not increase p53 and AKT transcription (Higami et al., 1998; Ouhtit et al., 2000; Sandrini et al., 2009) but increases AKT activity (Alvarez‐Moya et al., 2010; Zhang et al., 2005) (Figure 3C). The levels of active/phosphorylated AKT (pAKT) and p53 were monitored by western blotting (Figure 3A–C). We demonstrated that wortmannin (1 μM, 60 min) decreased AKT activity (Figure 3B) leading to increased levels of p53 (Figure 3A–D). Moreover, immunofluorescence staining showed increased accumulation of p53 in the nucleus in 100% of cells upon wortmannin treatment (Figure 3D). Thus, in schwannoma cells, the inhibiting AKT activity increased p53 stability (Fuchs et al., 1998). In summary, we demonstrated that in schwannoma cells the levels and activity of p53 can be increased by merlin reintroduction and the inhibition of PI3K/AKT, FAK and p53/MDM2 complex using wortmannin, FAK shRNA and Nutlin‐3 respectively (Figure 7).
Figure 3.
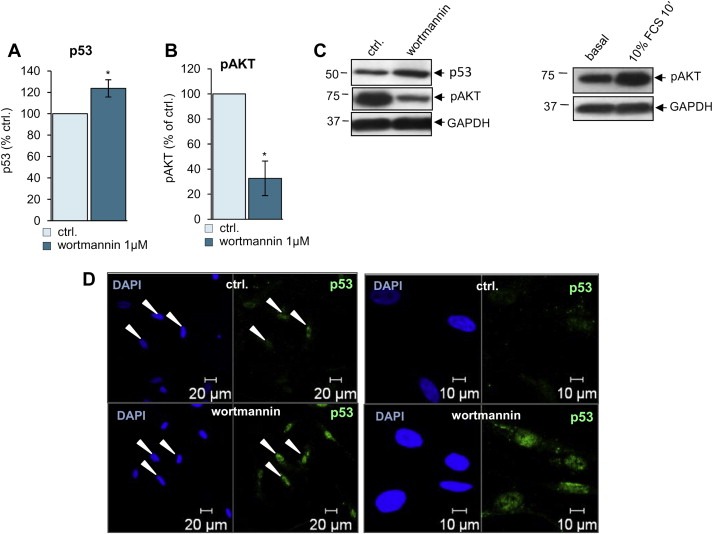
p53 is negatively regulated by AKT. A–C. Western blot analysis of p53 (A and C), pAKT (B and C) with 1 μM wortmannin and pAKT with 10% FCS 10 min (C right panel). Wortmannin (1 μM, 60 min) decreases activity/phosphorylation of AKT (pAKT) (B and C) leading to increased p53 (A and C). ns (not significant): P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001, mean ± SEM is given. D. Immunocytochemistry shows increased levels of p53 (green) in the nucleus (blue, DAPI) in schwannoma cells upon treatment with wortmannin 1 μM. White arrows indicate nuclei.
3.4. FAK and AKT inhibition increases MDM2 levels in the nucleus due to negative feedback from high levels of p53
Active/phosphorylated AKT promotes translocation of MDM2 into the nucleus (Mayo and Donner, 2001; Singh et al., 2013; Zhou et al., 2001) and FAK stabilizes MDM2/p53 complex formation in the nucleus (Lim et al., 2008). MDM2/p53 in the nucleus is involved in proteosomal degradation of p53, therefore we have investigated the effects of FAK, AKT and proteosomal degradation inhibitors/shRNA on MDM2 levels and cellular localisation.
We observed an increase in MDM2 levels upon FAK knockdown (Figure 4A) likely by activation of a negative feedback loop by increased p53 levels (Figure 2A, see also Figure 7) (Horn and Vousden, 2007; Ponnuswamy et al., 2012). Moreover, FAK shRNA leads to increased nuclear (Figure 4B left panel) and nucleolar accumulation of MDM2 (Figure 4B right panel, orange arrows). Schwannoma cells treated with wortmannin showed decreased AKT activity (Figure 4C lover panel), but increased levels of MDM2 (Figure 4C upper and lower panels) possibly due to the activation of a negative feedback induced by increased p53 levels observed upon the same treatment (Figure 3A, see also Figure 7) (Horn and Vousden, 2007; Ponnuswamy et al., 2012). Moreover, immunocytochemistry studies showed increased accumulation of MDM2 into the nuclei upon wortmannin treatment (Figure 4D, white arrows, red staining). Thus in schwannoma cells the inhibition of AKT activity leads to increased expression and stability of p53 (Fuchs et al., 1998) and the activation of a negative feedback loop leading to MDM2 upregulation (Horn and Vousden, 2007; Ponnuswamy et al., 2012). Next, we investigated whether proteosomal degradation inhibitor MG132 would effect MDM2 protein levels. We showed that proteosomal degradation inhibitor MG132 increased MDM2 levels approximately 5‐fold (Figure 4F) which is most likely due to the activation of the previously described negative feedback loop by elevated p53 observed upon the same treatment (Figure 2F, see also Figure 7) (Horn and Vousden, 2007; Ponnuswamy et al., 2012). MDM2 accumulated in the nucleus but not the nucleolus in MG132‐treated schwannoma cells (Figure 4F).
Figure 4.
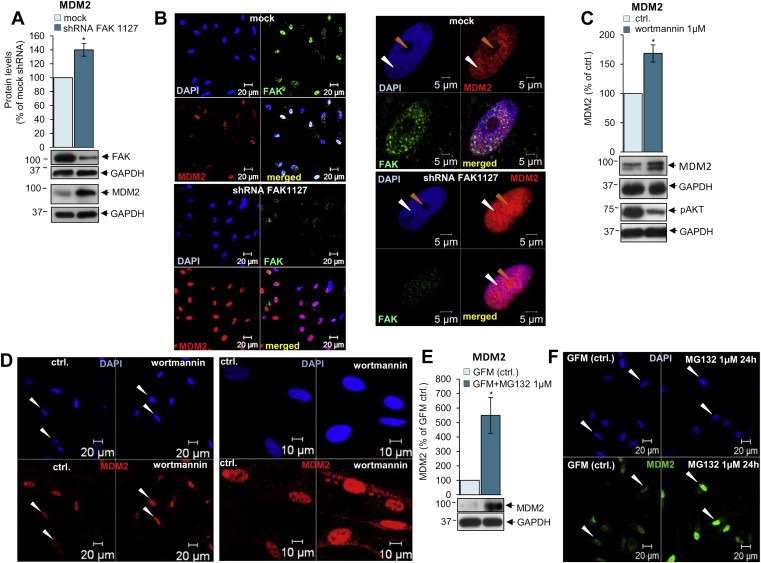
FAK and AKT inhibition increase MDM2 levels in the nucleus due to a negative feedback from elevated p53. A, B. Western blot (A) and immunocytochemistry (B) analysis shows that FAK knock down using FAK shRNA leads to increased MDM2 levels (A) and MDM2 nuclear localisation (B, red) compared to mock shRNA. MDM2 accumulates in the nucleoli (orange arrows) upon FAK shRNA (B right panel, compare upper and lower pictures). FAK staining is shown in green and MDM2 in red. Orange arrows indicate nucleoli within nuclear structure visualised with DAPI (blue) and white arrows indicate nucleus. The exposure time for all the pictures including p53 is identical. C. Western blot analysis of MDM2 and pAKT with and without 1 μM wortmannin treatment. Wortmannin (1 μM, 60 min) decreases activity/phosphorylation of AKT (pAKT) (lower picture) leading to increased MDM2 levels (lower picture and graph). ns (not significant): P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001, mean ± SEM is given. D. Immunocytochemistry shows increased nuclear (blue, DAPI) staining of MDM2 (red) in schwannoma cells upon treatment with wortmannin 1 μM. White arrows indicate nuclei. E. Western blot analysis showing the effect of proteasome inhibitor MG132 on MDM2 in schwannoma cells. Cells were incubated either with Growth Factor Medium (GFM) alone or GFM in combination with MG132 (1 μM) for 24 h ns (not significant): P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001, mean ± SEM is given. F. Immunofluorescent staining of MDM2 (green) and DAPI in schwannoma cells. The cells were incubated with culture medium (GFM) alone (G left panel) or in combination with MG132 (1 μM) for 24 h. MG132 (1 μM) increased nuclear staining of MDM2 in schwannoma cells. White arrows indicate nuclei.
3.5. Merlin reintroduction leads to the downregulation of FAK and accumulation of MDM2 into the nucleoli
Next, we investigated the effect of merlin reintroduction on FAK and MDM2 levels and their cellular localisation. MDM2 was strongly overexpressed in schwannoma cells compared to normal Schwann cells (Figure 5A) and the protein localised to the nucleus only in schwannoma cells (Figure 5B), while it localised to the cytosol (yellow arrows, green staining) in both Schwann and schwannoma cells (Figure 5B). Immunohistochemistry on human schwannoma tissues showed strong nuclear and cytosolic MDM2 staining in all the cells (Supplementary data 1). Additionally FAK co‐localised with MDM2 in the nuclei of schwannoma cells (Figure 5C, white arrows) fitting with Lim et.al's (2008) model of FAK/MDM2/p53 complex and p53 degradation. Merlin reintroduction into schwannoma cells leads to downregulation of FAK, suggesting that FAK overexpression is due to merlin deficiency in schwannoma cells (Figure 5D and (Poulikakos et al., 2006)). In contrast to Kim et al. (2004), we did not observe any MDM2 degradation upon merlin reintroduction (Figure 5E) thus suggesting a different mechanism of p53 regulation in primary human disease cells as opposed to cell lines. Instead, in line with the result using proteosome inhibitor MG132 and the described negative feedback loop, the levels of MDM2 increase even further upon merlin reintroduction (Figure 5E). To investigate whether merlin reintroduction leads to MDM2 translocation into the nucleoli (thus enabling MDM2 to bind p53 mRNA inducing its synthesis (Gajjar et al., 2012)), we performed additional immunocytochemistry staining of MDM2 before and after merlin reintroduction. We found that merlin reintroduction indeed leads to MDM2 accumulation in the nucleoli of all infected cells (Figure 5F) which would be the first step towards an increase in p53 synthesis. Nucleoli were visualised by nucleolin staining (Supplementary data 2).
Figure 5.
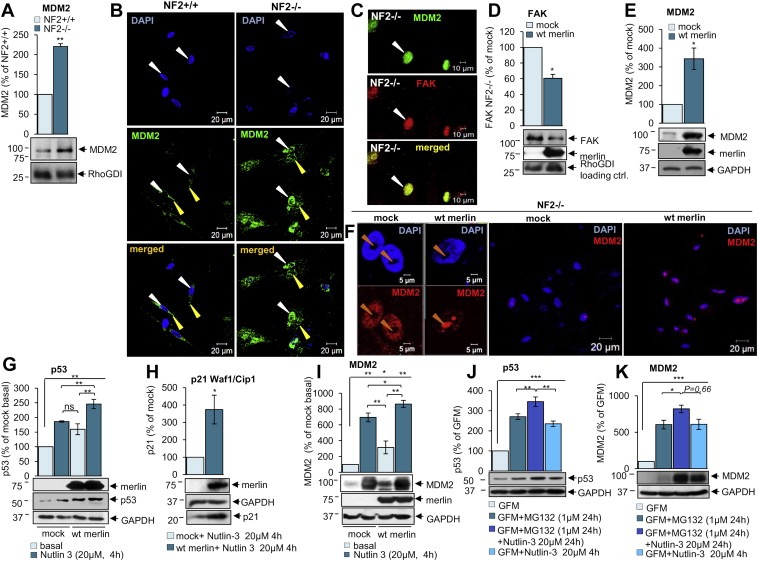
Merlin reintroduction leads to the downregulation of FAK and accumulation of MDM2 in the nucleoli. A, B. Western blotting (A) and immunocytochemistry (B) analysis of MDM2 expression and cellular localisation in human primary Schwann (NF2+/+) compared to schwannoma (NF2−/−) cells. MDM2 is overexpressed and schwannoma (NF2−/−) compared to Schwann cells (NF2+/+) (A). MDM2 nuclear staining is observed only in schwannoma (NF2−/−, B right panel) in contrast to Schwann cells (NF2+/+, B left panel). MDM2 cytosolic staining is observed weakly in both Schwann (NF2+/+) and schwannoma (NF2−/−) cells (B). The levels of MDM2 were detected using anti‐MDM2 antibodies. ns (not significant): P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001., mean ± SEM is given. C. Immunocytochemistry staining shows co‐localisation of MDM2 (green) and FAK (red) in the nuclei in schwannoma cells (NF2−/−). D, E. Merlin reintroduction decreases FAK (D) and increases MDM2 (E) levels in schwannoma cells demonstrated using western blotting analysis. F. Immunocytochemistry staining for MDM2 after merlin reintroduction in primary human schwannoma cells (NF2−/−). Merlin reintroduction increases MDM2 staining in the nucleoli observed in all schwannoma cells (F first and third pictures). Cells were infected with mock virus or wild type (wt) merlin containing virus. G, H, I. Western blot analysis on the effect of combined merlin re‐introduction in conjunction with Nutlin‐3 (20 μM) treatment on p53 (G), p21 Waf1/Cip1 (H) and MDM2 (I) levels in schwannoma cells. Cells were infected with mock virus or wild type (wt) merlin containing virus, treated with Nutlin‐3 (20 μM) for 4 h, followed 24 h incubation with DMEM. ns (not significant): P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001, mean ± SEM is given. J, K. Western blot analysis showing p53 (J) and MDM2 (K) after treatment with MG132 (1 μM) and Nutlin‐3 (20 μM) and combination of both components. Combination treatment of MG132 (1 μM) and Nutlin‐3 (20 μM) increases p53 levels stronger than single drugs alone (J). MDM2 follows the same pattern as p53 (K). ns (not significant): P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001, mean ± SEM is given.
As our data suggests that there is more active p53 after merlin reintroduction, due to MDM2 translocation into the nucleoli and increased p53 synthesis, we then tested whether merlin can potentiate the effects of MDM2/p53 complex inhibitor Nutlin‐3. Schwannoma cells were infected with either mock (GFP) adenovirus or wild‐type (wt) merlin containing adenovirus and then treated with MDM2/p53 complex inhibitor Nutlin‐3. We observed that both merlin reintroduction and Nutlin‐3 treatment of human primary schwannoma cells increased p53 levels and, when combined, the effect was potentiated (Figure 5G). p53 activity was also increased after merlin reintroduction and Nutlin‐3 treatment, as shown by increased levels of p53 downstream target p21 Waf1/Cip1 (Figure 5H). We also investigated the effect of Nutlin‐3 on MDM2 levels upon merlin reintroduction, as we did for p53. MDM2 levels increased even more upon merlin reintroduction with simultaneous Nutlin‐3 treatment which parallels the increase of p53 in the same conditions likely indicating the activation of a negative feedback loop increasing MDM2 to keep p53 levels in control (Figure 5I, See also Figure 7) (Horn and Vousden, 2007; Ponnuswamy et al., 2012).
In summary, in merlin‐deficient schwannoma cells, MDM2 localises to the nucleus and cytoplasm and very weakly to nucleoli (Figure 5F, first and second pictures), leading to p53 degradation reflected in weaker nuclear accumulation (Figure 1B) and a lower p53 expression in schwannoma compared to Schwann cells (Figure 1A). Merlin reintroduction resulted in increased MDM2 expression and strong accumulation in nucleoli which was observed in infected cells (100%) (Figure 5F first and third pictures) leading to p53 synthesis. Merlin increases p53 activity, shown using p21 Waf1/Cip1 increased expression and nucleolar localisation (Figure 1E) and p53 Transcription Factor assay (Figure 1F). We observed that merlin reintroduction leads to high levels of nucleolar MDM2 and simultaneous Nutlin‐3 treatment increase p53 levels even further. This could be due to a continuous degradation of MDM2‐bound p53 suggesting that simultaneous Nutlin‐3 treatment may be needed to keep p53 stable. To support this hypothesis we added proteasome inhibitor MG132 (1 μM, 24 h) to inhibit degradation together with Nutlin‐3 (20 μM, 24 h) to inhibit binding of MDM2 to p53 (Figure 5J,K). We show that combination treatment of MG132 and Nutlin‐3 significantly increases p53 levels more than the single drugs alone (Figure 5J). MDM2 follows the same pattern as p53 (Figure 5K) due to p53‐mediated feedback activation (Horn and Vousden, 2007; Ponnuswamy et al., 2012). Thus, continuous inhibition of MDM2/p53 complex by Nutlin‐3 and p53 activation by merlin is necessary to keep p53 stable (Figure 7).
3.6. Nutlin‐3 decreases proliferation and survival of schwannoma cells by increasing p53 and decreasing cyclin D1, survivin and pAKT levels
As p53 regulates cell proliferation and survival we investigated whether the increase of p53 upon Nutlin‐3 treatment would affect the levels of proliferation and survival/apoptosis markers such as cyclin D1, survivin and caspase 3, previously shown to be involved in schwannoma increased proliferation and survival (Ammoun et al., 2010, 2012, 2005). Nutlin‐3 decreased cyclin D1 (Figure 6A) and survivin (Figure 6B) and increased cleaved caspase 3 levels (Figure 6C). Accordingly, Nutlin‐3 treatment decreased schwannoma cell proliferation and led to decreased cell survival/increased cell death in a concentration‐dependent (Figure 6D) and time‐mediated manner (Figure 6E). Cell death caused by Nutlin‐3 correlates with caspase 3 activation (Figure 6F).
Figure 6.
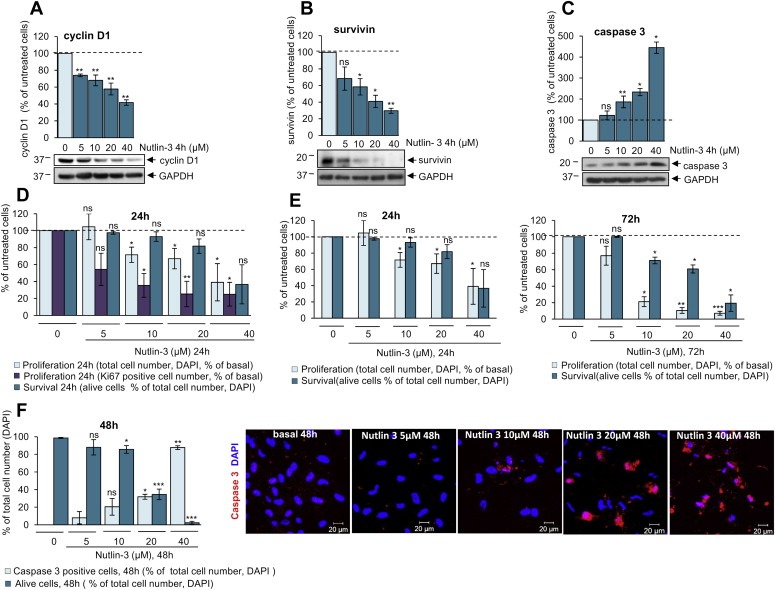
Nutlin‐3 decreases levels of cyclin D1 and survivin and increases levels of active caspase 3 leading to decreased proliferation and survival of human primary schwannoma cells. A, B, C. Nutlin‐3 (5, 10, 20, 40 μM, 4 h) decreases the levels of cyclin D1 (A) and survivin (B) and increases cleaved caspase 3 levels (B) in schwannoma cells shown by western blotting. ns (not significant): P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001, mean ± SEM is given. D, E. Proliferation and survival assay shows that Nutlin‐3 decreases schwannoma cell proliferation and leads to decreased cell survival in a concentration (D and E) and time dependent manner (E compare left and right panels). Total cell number and number of dead cells are monitored by DAPI staining (D–E), the number of proliferating cells by Ki67 (D). ns (not significant); P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001, mean ± SEM is given. F. Nutlin‐3‐triggered cell death of schwannoma cells is due to caspase 3 activation. Total cell number and number of dead cells is visualised by DAPI (blue) staining, caspase 3 positive cells by anti‐cleaved caspase 3 antibody (red).
Based on above results we suggest that p53 deficiency contributes to increased proliferation and survival in merlin‐deficient schwannoma cells (Figure 7).
4. Discussion
In this study we have investigated the regulation of p53/MDM2 feedback loop in merlin‐deficient human primary schwannoma cells and the role of merlin, FAK and AKT on p53 levels and activity. We demonstrate a decrease in levels of the tumour suppressor p53 and an increase in E3 ubiquitin‐protein ligase MDM2 levels in schwannoma cells compared to Schwann cells. Merlin reintroduction increases levels and activity of p53 shown by increased expression of p21 Waf1/Cip1 and p53 transcriptional activity (Wu and Levine, 1997). In contrast to previous results in NIH3T3 cells (Kim et al., 2004), no MDM2 degradation was observed in human primary schwannoma cells upon merlin reintroduction. Thus, we suggest that there must be another mechanism by which merlin regulates p53. We hypothesised that, rather than degrading MDM2, merlin may be involved in MDM2 intracellular localisation in human schwannoma cells, which, in turn, would increase p53 levels. According to previous publications, nucleolar MDM2 can bind to p53 mRNA, increasing p53 synthesis (Gajjar et al., 2012). Here we show that, indeed, merlin reintroduction leads to strong nucleolar accumulation of MDM2 in schwannoma cells. Additionally, we demonstrate that the proteasome inhibitor MG132 augments p53 levels. We thus suggest that p53 is relevant in merlin‐deficient tumours and that MDM2 contributes to p53 degradation (Horn and Vousden, 2007; Ponnuswamy et al., 2012).
As a response to increased p53 in the nucleus the levels of MDM2 are also elevated and a portion of MDM2 translocated to the cytosol where it can degrade increased p53 in order to keep p53 levels under control (Horn and Vousden, 2007; Ponnuswamy et al., 2012). Similarly, at higher concentrations of p53 upon Nutlin‐3 treatment, MDM2 levels increase which is likely due to p53‐induced negative feedback mechanism to protect cells from high, probably harmful, p53 levels (Horn and Vousden, 2007; Ponnuswamy et al., 2012). Once the steady state of p53 is obtained, the MDM2 levels drops, maintaining physiological p53 concentrations (Lev Bar‐Or et al., 2000). Thus merlin can increase nucleolar levels of MDM2 in schwannoma cells, followed by increased p53 activity. Active p53 can then be stabilised by the MDM2 inhibitor Nutlin‐3.
We then further investigated the mechanism by which merlin regulates MDM2/p53. FAK is known to accumulate in the nucleus and is overexpressed and activated in schwannoma cells leading to increased proliferation (Ammoun et al., 2008, 2012). Nuclear FAK decreases p53 levels by stabilisation of the MDM2/p53 ubiquitination complex, leading to increased export of p53 from the nucleus to the cytosol and p53 degradation (Lim et al., 2008). Using FAK shRNA we demonstrate that FAK knock‐down increases nuclear p53 levels. These data suggest that increased FAK accumulation in the nucleus plays an important role in MDM2‐mediated p53 degradation in human primary merlin‐deficient schwannoma cells. FAK knock‐down by shRNA leads to accumulation of MDM2 in the nucleoli and increases p53 levels and nuclear accumulation, similar to the effects of merlin reintroduction. In addition we showed that active AKT is indeed involved in p53 degradation as PI3K inhibitor wortmannin increases p53 levels whilst decreasing AKT phosphorylation/activity. In this study, we demonstrate for the first time, a link between FAK, as well as AKT, and decreased p53 levels in human primary schwannoma cells. Thus, merlin regulates p53 indirectly possibly via inhibition of FAK‐ and AKT‐mediated pathways (Kim et al., 2004; Lim et al., 2008; Mayo and Donner, 2001; Singh et al., 2013; Zhou et al., 2001) (Figure 7). Schwannomas are characterised by increased proliferation and survival via increased expression of cyclin D1 and survivin and decreased caspase pathway (Ammoun et al., 2010, 2014, 2000, 2005, 2003, 2011). Our results show that Nutlin‐3 decreases expression of pro‐proliferative cyclin D1 and the pro‐survival protein survivin. Moreover, Nutlin‐3 increases expression of pro‐apoptotic caspase 3. Importantly, schwannoma proliferation and cell survival are strongly impaired upon Nutlin‐3 treatment. We therefore suggest that targeting p53, via Nutlin‐3 alone or in combination with FAK/PI3K inhibitors, as good drug treatment option in schwannoma. Our strategy is to use our human in vitro model to re‐profile drugs which are already used, or have already been tested in clinical trials for other disease applications and, where possible, to use these pre‐approved drugs as a treatment for schwannomas and other merlin‐deficient tumours. We have proved that this strategy is possible by successfully translating a drug targeting a receptor tyrosine kinase, from our human in vitro model to phase 0 clinical trials. We suggest targeting p53 degradation in the future.
Conflict of interest statement
None declared.
Supporting information
The following are the supplementary data related to this article:
Supplementary data 1: Immunohistochemistry performed on human schwannoma tissues demonstrates a very weak nuclear p53 staining (left picture) in some cells and strong nuclear and cytosolic MDM2 staining (right picture) in all the cells.
Supplementary data 2: Upon merlin reintroduction MDM2 (blue staining) accumulates in the nucleoli (compare upper left and upper right panels). Nucleoli staining are shown in lower left and right panels (red staining) using anti‐nucleolin antibody. White arrows points nucleoli.
Acknowledgements
The authors thank Astrofund for support and Joseph Testa for merlin adenovirus.
1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2014.08.005.
Ammoun Sylwia, Schmid Marei Caroline, Zhou Lu, Hilton David A., Barczyk Magdalena, Hanemann Clemens Oliver, (2015), The p53/mouse double minute 2 homolog complex deregulation in merlin‐deficient tumours, Molecular Oncology, 9, doi: 10.1016/j.molonc.2014.08.005.
References
- Alvarez-Moya, B. , Lopez-Alcala, C. , Drosten, M. , Bachs, O. , Agell, N. , 2010. K-Ras4B phosphorylation at Ser181 is inhibited by calmodulin and modulates K-Ras activity and function. Oncogene. 29, 5911–5922. [DOI] [PubMed] [Google Scholar]
- Ammoun, S. , Cunliffe, C.H. , Allen, J.C. , Chiriboga, L. , Giancotti, F.G. , Zagzag, D. , Hanemann, C.O. , Karajannis, M.A. , 2010. ErbB/HER receptor activation and preclinical efficacy of lapatinib in vestibular schwannoma. Neuro Oncol. 12, 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoun, S. , Flaiz, C. , Ristic, N. , Schuldt, J. , Hanemann, C.O. , 2008. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 68, 5236–5245. [DOI] [PubMed] [Google Scholar]
- Ammoun, S. , Provenzano, L. , Zhou, L. , Barczyk, M. , Evans, K. , Hilton, D.A. , Hafizi, S. , Hanemann, C.O. , 2014. Axl/Gas6/NFkappaB signalling in schwannoma pathological proliferation, adhesion and survival. Oncogene. 33, 336–346. [DOI] [PubMed] [Google Scholar]
- Ammoun, S. , Schmid, M.C. , Ristic, N. , Zhou, L. , Hilton, D. , Ercolano, E. , Carroll, C. , Hanemann, C.O. , 2012. The role of insulin-like growth factors signaling in merlin-deficient human schwannomas. GLIA. 60, 1721–1733. [DOI] [PubMed] [Google Scholar]
- Ammoun, S. , Schmid, M.C. , Triner, J. , Manley, P. , Hanemann, C.O. , 2011. Nilotinib alone or in combination with selumetinib is a drug candidate for neurofibromatosis type 2. Neuro Oncol. 13, 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoun, S. , Schmid, M.C. , Zhou, L. , Ristic, N. , Ercolano, E. , Hilton, D.A. , Perks, C.M. , Hanemann, C.O. , 2011. Insulin-like growth factor-binding protein-1 (IGFBP-1) regulates human schwannoma proliferation, adhesion and survival. Oncogene. 31, [DOI] [PubMed] [Google Scholar]
- Blakeley, J.O. , Evans, D.G. , Adler, J. , Brackmann, D. , Chen, R. , Ferner, R.E. , Hanemann, C.O. , Harris, G. , Huson, S.M. , Jacob, A. , Kalamarides, M. , Karajannis, M.A. , Korf, B.R. , Mautner, V.F. , McClatchey, A.I. , Miao, H. , Plotkin, S.R. , Slattery, W. , Stemmer-Rachamimov, A.O. , Welling, D.B. , Wen, P.Y. , Widemann, B. , Hunter-Schaedle, K. , Giovannini, M. , 2012. Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis type 2. Am. J. Med. Genet. A. 158A, 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Mei, L. , Zhou, L. , Zhang, X. , Guo, C. , Li, J. , Wang, H. , Zhu, Y. , Zheng, Y. , Huang, L. , 2011. Moesin-ezrin-radixin-like protein (merlin) mediates protein interacting with the carboxyl terminus-1 (PICT-1)-induced growth inhibition of glioblastoma cells in the nucleus. Int. J. Biochem. Cell Biol. 43, 545–555. [DOI] [PubMed] [Google Scholar]
- Chylicki, K. , Ehinger, M. , Svedberg, H. , Bergh, G. , Olsson, I. , Gullberg, U. , 2000. p53-mediated differentiation of the erythroleukemia cell line K562. Cell growth & differentiation. Mol. Biol. J. Am. Assoc. Cancer Res. 11, 315–324. [PubMed] [Google Scholar]
- Chylicki, K. , Ehinger, M. , Svedberg, H. , Gullberg, U. , 2000. Characterization of the molecular mechanisms for p53-mediated differentiation. Cell growth & differentiation. Mol. Biol. J. Am. Assoc. Cancer Res. 11, 561–571. [PubMed] [Google Scholar]
- Flaiz, C. , Kaempchen, K. , Matthies, C. , Hanemann, C.O. , 2010. Actin-rich protrusions and nonlocalized GTPase activation in merlin-deficient schwannomas. J. Neuropathol. Exp. Neurol. 66, 608–616. [DOI] [PubMed] [Google Scholar]
- Fuchs, S.Y. , Adler, V. , Pincus, M.R. , Ronai, Z. , 1998. MEKK1/JNK signaling stabilizes and activates p53. Proc. Natl. Acad. Sci. U S A. 95, 10541–10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajjar, M. , Candeias, M.M. , Malbert-Colas, L. , Mazars, A. , Fujita, J. , Olivares-Illana, V. , Fahraeus, R. , 2012. The p53 mRNA-Mdm2 interaction controls Mdm2 nuclear trafficking and is required for p53 activation following DNA damage. Cancer Cell. 21, 25–35. [DOI] [PubMed] [Google Scholar]
- Hanemann, C.O. , Bartelt-Kirbach, B. , Diebold, R. , Kampchen, K. , Langmesser, S. , Utermark, T. , 2010. Differential gene expression between human schwannoma and control Schwann cells. Neuropathol. Appl. Neurobiol. 32, 605–614. [DOI] [PubMed] [Google Scholar]
- Hennessy, B.T. , Smith, D.L. , Ram, P.T. , Lu, Y. , Mills, G.B. , 2008. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nature Rev. Drug Discovery. 4, 988–1004. [DOI] [PubMed] [Google Scholar]
- Higami, Y. , Shimokawa, I. , Kishikawa, M. , Okimoto, T. , Ohtani, H. , Tomita, M. , Tsujino, A. , Ikeda, T. , 1998. Malignant peripheral nerve sheath tumors developing multifocally in the central nervous system in a patient with neurofibromatosis type 2. Clin. Neuropathol. 17, 115–120. [PubMed] [Google Scholar]
- Horn, H.F. , Vousden, K.H. , 2007. Coping with stress: multiple ways to activate p53. Oncogene. 26, 1306–1316. [DOI] [PubMed] [Google Scholar]
- Kaempchen, K. , Mielke, K. , Utermark, T. , Langmesser, S. , Hanemann, C.O. , 2014. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum. Mol. Genet. 12, 1211–1221. [DOI] [PubMed] [Google Scholar]
- Kim, H. , Kwak, N.J. , Lee, J.Y. , Choi, B.H. , Lim, Y. , Ko, Y.J. , Kim, Y.H. , Huh, P.W. , Lee, K.H. , Rha, H.K. , Wang, Y.P. , 2004. Merlin neutralizes the inhibitory effect of Mdm2 on p53. J. Biol. Chem. 279, 7812–7818. [DOI] [PubMed] [Google Scholar]
- Lev Bar-Or, R. , Maya, R. , Segel, L.A. , Alon, U. , Levine, A.J. , Oren, M. , 2000. Generation of oscillations by the p53-Mdm2 feedback loop: a theoretical and experimental study. Proc. Natl. Acad. Sci. U S A. 97, 11250–11255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Lozano, G. , 2013. Molecular pathways: targeting Mdm2 and Mdm4 in cancer therapy. Clin. Cancer Res. 19, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , You, L. , Cooper, J. , Schiavon, G. , Pepe-Caprio, A. , Zhou, L. , Ishii, R. , Giovannini, M. , Hanemann, C.O. , Long, S.B. , Erdjument-Bromage, H. , Zhou, P. , Tempst, P. , Giancotti, F.G. , 2010. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 140, 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, S.T. , Chen, X.L. , Lim, Y. , Hanson, D.A. , Vo, T.T. , Howerton, K. , Larocque, N. , Fisher, S.J. , Schlaepfer, D.D. , Ilic, D. , 2008. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol. Cell. 29, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, J. , Manning, B.D. , Cantley, L.C. , 2012. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 4, 257–262. [DOI] [PubMed] [Google Scholar]
- Mayo, L.D. , Donner, D.B. , 2001. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. U S A. 98, 11598–11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo, L.D. , Donner, D.B. , 2002. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem. Sci. 27, 462–467. [DOI] [PubMed] [Google Scholar]
- McClatchey, A.I. , Giovannini, M. , 2005. Membrane organization and tumorigenesis–the NF2 tumor suppressor. Merlin. Genes Dev. 19, 2265–2277. [DOI] [PubMed] [Google Scholar]
- Morrow, K.A. , Das, S. , Metge, B.J. , Ye, K. , Mulekar, M.S. , Tucker, J.A. , Samant, R.S. , Shevde, L.A. , 2011. Loss of tumor suppressor Merlin in advanced breast cancer is due to post-translational regulation. J. Biol. Chem. 286, 40376–40385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, P.A. , Vousden, K.H. , Norman, J.C. , 2011. p53 and its mutants in tumor cell migration and invasion. J. Cell Biol. 192, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier, M. , Hollstein, M. , Hainaut, P. , 2010. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2, a001008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouhtit, A. , Muller, H.K. , Davis, D.W. , Ullrich, S.E. , McConkey, D. , Ananthaswamy, H.N. , 2000. Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. Am. J. Pathol. 156, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnuswamy, A. , Hupp, T. , Fahraeus, R. , 2012. Concepts in MDM2 signaling: allosteric regulation and feedback loops. Genes Cancer. 3, 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos, P.I. , Xiao, G.H. , Gallagher, R. , Jablonski, S. , Jhanwar, S.C. , Testa, J.R. , 2011. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. [DOI] [PubMed] [Google Scholar]
- Reiske, H.R. , Kao, S.C. , Cary, L.A. , Guan, J.L. , Lai, J.F. , Chen, H.C. , 2011. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J. Biol. Chem. 274, 12361–12366. [DOI] [PubMed] [Google Scholar]
- Rosenbaum, C. , Kamleiter, M. , Grafe, P. , Kluwe, L. , Mautner, V. , Muller, H.W. , Hanemann, C.O. , 2000. Enhanced proliferation and potassium conductance of Schwann cells isolated from NF2 schwannomas can be reduced by quinidine. Neurobiol. Dis. 7, 483–491. [DOI] [PubMed] [Google Scholar]
- Sandrini, J.Z. , Trindade, G.S. , Nery, L.E. , Marins, L.F. , 2009. Time-course expression of DNA repair-related genes in hepatocytes of zebrafish (Danio rerio) after UV-B exposure. Photochem. Photobiol. 85, 220–226. [DOI] [PubMed] [Google Scholar]
- Singh, S. , Ramamoorthy, M. , Vaughan, C. , Yeudall, W.A. , Deb, S. , Palit Deb, S. , 2013. Human oncoprotein MDM2 activates the Akt signaling pathway through an interaction with the repressor element-1 silencing transcription factor conferring a survival advantage to cancer cells. Cell Death Differ. 20, 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermark, T. , Kaempchen, K. , Antoniadis, G. , Hanemann, C.O. , 2005. Reduced apoptosis rates in human schwannomas. Brain Pathol. 15, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermark, T. , Kaempchen, K. , Hanemann, C.O. , 2003. Pathological adhesion of primary human schwannoma cells is dependent on altered expression of integrins. Brain Pathol. 13, 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein, B. , Lane, D. , Levine, A.J. , 2000. Surfing the p53 network. Nature. 408, 307–310. [DOI] [PubMed] [Google Scholar]
- Walerych, D. , Napoli, M. , Collavin, L. , Del Sal, G. , 2012. The rebel angel: mutant p53 as the driving oncogene in breast cancer. Carcinogenesis. 33, 2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. , Levine, A.J. , 1997. Differential regulation of the p21/WAF-1 and mdm2 genes after high-dose UV irradiation: p53-dependent and p53-independent regulation of the mdm2 gene. Mol. Med. 3, 441–451. [PMC free article] [PubMed] [Google Scholar]
- Xiao, G.H. , Gallagher, R. , Shetler, J. , Skele, K. , Altomare, D.A. , Pestell, R.G. , Jhanwar, S. , Testa, J.R. , 2012. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol. Cell. Biol. 25, 2384–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , McQueen, T. , Schober, W. , Rassidakis, G. , Andreeff, M. , Konopleva, M. , 2005. Leukotriene B4 receptor inhibitor LY293111 induces cell cycle arrest and apoptosis in human anaplastic large-cell lymphoma cells via JNK phosphorylation. Leukemia. 19, 1977–1984. [DOI] [PubMed] [Google Scholar]
- Zhou, B.P. , Liao, Y. , Xia, W. , Zou, Y. , Spohn, B. , Hung, M.C. , 2001. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 3, 973–982. [DOI] [PubMed] [Google Scholar]
- Zhou, L. , Ercolano, E. , Ammoun, S. , Schmid, M.C. , Barczyk, M.A. , Hanemann, C.O. , 2011. Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/beta-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways. Neoplasia. 13, 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data 1: Immunohistochemistry performed on human schwannoma tissues demonstrates a very weak nuclear p53 staining (left picture) in some cells and strong nuclear and cytosolic MDM2 staining (right picture) in all the cells.
Supplementary data 2: Upon merlin reintroduction MDM2 (blue staining) accumulates in the nucleoli (compare upper left and upper right panels). Nucleoli staining are shown in lower left and right panels (red staining) using anti‐nucleolin antibody. White arrows points nucleoli.


