Abstract
Natural killer (NK) cells are innate lymphocytes with a refined ability to recognize transformed cells through a broad array of activating receptors in combination with stochastically expressed inhibitory receptors that recognize MHC‐class I. Recent advances in NK cell biology have revealed a high degree of functional plasticity that can be attributed to dynamic cell‐to‐cell interactions in concert with transcriptional and epigenetic reprogramming. Here, we discuss how new insights into the adaptive behavior of NK cells pave the way for next generation cell therapy based on guided differentiation and selective expansion of particularly cytotoxic NK cell subsets.
Keywords: NK cells, Immunotherapy, Killer cell immunoglobulin-like receptors, NKG2C, Adaptive
Highlights
Recent insights into the functional plasticity of human NK cells are discussed.
Guided differentiation and selective expansion hold promises for more specific NK cell therapy.
Manipulation of metabolic pathways may rescue the functional potential of NK cells during in vitro expansion.
1. Introduction
Immunotherapy has rapidly emerged to become a cornerstone in the treatment of a large variety of cancer types. Current approaches in immunotherapy include the relatively basic administration of cytokines, vaccines (Melero et al., 2014) and antibodies (Michaud et al., 2014) as well as more advanced manipulations involving in vitro expansion and infusion of specific T cell (Restifo et al., 2012) and natural killer (NK) cell subsets (Vivier et al., 2012). The natural cytotoxic potential of these lymphocytes may also be harnessed through the insertion of genetically engineered chimeric antigen receptors (CAR) or T cell receptors (TCR), specific for tumor associated and patient specific neo‐antigens, respectively (Gill and June, 2015; Schumacher and Schreiber, 2015). Currently, adoptive cell therapy platforms require enormous resources in order to facilitate tumor sequencing, bioinformatics and cell culture that fulfill the requirements for good manufacturing practice (GMP) conditions. It is likely, however, that refinement in these areas over the coming decade will enable the development of cost‐effective, high‐throughput platforms for large‐scale implementation of advanced cellular therapies in the clinic. In this review we focus on the development of the next generation of NK cell immunotherapy, based on new insights into their functional plasticity (Vivier et al., 2011).
2. NK cell‐based immunotherapy against cancer
NK cells were discovered in the mid 70′s based on their intrinsic “natural” capacity to kill tumor cells (Herberman et al., 1975; Kiessling et al., 1975). Mice deficient in key activating NK cell receptors are more prone to develop carcinogen‐induced tumors (Iguchi‐Manaka et al., 2008), highlighting the biological relevance of NK cells in immune surveillance. In humans, a population‐based functional screening of >3500 healthy individuals revealed an inverse correlation between NK cell cytotoxicity and the risk of developing cancer (Imai et al., 2000). Four decades of intense research have culminated in a rather detailed understanding of the biology of these potent cytotoxic lymphocytes, including their development and functional regulation by cytokines, and the broad array of activating and inhibitory receptors that they express (Cichocki et al., 2014). Insights into the molecular specificities of the missing self response, i.e. the ability of NK cells to sense the absence of self MHC class I molecules through stochastically expressed inhibitory receptors, suggest that NK cells may be particularly effective when transferred across HLA barriers (Karre, 2002; Ruggeri et al., 2002a; Valiante et al., 1997), in the context of allogeneic stem cell transplantation (Ruggeri et al., 2002a) or adoptive cell therapy (Miller et al., 2005). However, the research community has only recently begun to systematically address the potential role of NK cells in clinical settings. Currently, approximately 260 open studies are registered at ClinicalTrials.gov and the clinical translation of new insights in NK cell biology is an area of intense investigation.
Several recent reviews have covered historical landmarks of breakthroughs in NK cell biology (Cichocki et al., 2014), their functional regulation, mechanisms involved in maintenance of self tolerance (Goodridge et al., 2015; Kadri et al., 2015), as well as their role in the context of allogeneic stem cell transplantation (Cichocki et al., 2015). Other reviews have discussed strategies for ex vivo expansion (Pittari et al., 2015), de novo development of NK cells from induced pluripotent stem cells (iPSc) and human embryonic stem cells (hESC) (Eguizabal et al., 2014), genetic manipulation with CARs (Glienke et al., 2015), and prospects for using NK cells in both adult and pediatric hematological malignancies and solid tumors (Gras Navarro et al., 2015; Knorr et al., 2014; Leung, 2014; McDowell et al., 2015). In light of these, this review will focus entirely on the prospects for clinical translation of the most recent insights into the functional plasticity and adaptive behavior of NK cells. Several lines of evidence suggest that NK cells contribute to adaptive immunity both as mediators of memory responses (Min‐Oo et al., 2013) and in their ability to regulate T cell homeostasis (Cook et al., 2014; Waggoner et al., 2012). Thus, in addition to overcoming regulatory and technical challenges pertaining to donor selection, generation of sufficient NK cell numbers and choice of the target specificity for therapy, we believe it will be of outmost importance to consider the fundamental mechanisms involved in creating the vast repertoire diversity of NK cells as well as the heritability and persistence of the effector potential during in vivo homeostasis. Before outlining the emerging clinical possibilities of harnessing adaptive NK cells, we will briefly review recent insights into their differentiation and functional reprogramming.
3. NK cell differentiation
At birth, the repertoire of human NK cells is usually naïve and devoid of cells bearing the features of full functional maturity and terminal differentiation (Bjorkstrom et al., 2010; Le Garff‐Tavernier et al., 2010). Full development of mature phenotypic and functional NK cell profiles occurs only in response to environmental cues, from metabolism to infection, as observed with infection of mice raised under germ free conditions (Marcais et al., 2014). This development and accumulation of functional NK cells over time, likely occurs under persistent waves of environmental stimulation (Goodridge et al., 2015). The naïve state of NK cell differentiation is associated with high CD56 and CD62L expression, as well as expression of more broadly specific receptors, such as NKG2A and natural cytotoxicity receptors (NCRs) (Beziat et al., 2010; Bjorkstrom et al., 2010; Juelke et al., 2010). Moving along the spectrum of differentiation, cells are observed to progressively downregulate NKG2A and acquire CD16 as well as more specific inhibitory receptors, such as inhibitory killer cell immunoglobulin‐like receptors (KIR). KIR acquisition results in a narrower HLA‐I ligand specificity and determines the functional fate of the cell (Goodridge et al., 2015; Luetke‐Eversloh et al., 2013). As the NK cell population veers toward terminal differentiation, receptor expression profiles become less random and emerging patterns of surface expression suggest an accumulation of differentiated cells enriched by specific receptor interactions. Terminally differentiated NK cells display diminished responsiveness to cytokines, which correlates with the reduction of cytokine receptors, manifested both at the protein and transcriptional level (Beziat et al., 2010; Bjorkstrom et al., 2010). Concurrently, there is a gain in cytolytic potential, as they express higher levels of granzyme B and perforin and become particularly efficient in mediating antibody‐dependent cellular cytotoxicity (ADCC) (Lopez‐Verges et al., 2010), as well as in the capacity for cytokine production in response to stimulation through activating receptors (Luetke‐Eversloh et al., 2014b). It is during the approach towards terminal stages of maturation that the adaptive behavior of NK cells truly comes to light, and is most strikingly demonstrated during CMV infection in mice and humans alike (Cichocki et al., 2014).
4. Adaptive NK cell responses
Until recently, NK cells were believed to be short‐lived innate lymphocytes with no active involvement in the formation of immunological memory towards encountered pathogens. In recent years, this dogma has been challenged by compelling evidence of how viral infection shapes NK cell repertoires to provide specific protection against subsequent challenges (O'Leary et al., 2006, 2011, 2009, 2010). In mice, CMV infection results in the expansion of Ly49H+ NK cells which recognize the viral protein m157. Akin to memory CD8+ T cells, these cells proliferate faster and display stronger effector function upon re‐exposure to CMV (Sun et al., 2009). While such adaptive NK cell responses have been mostly documented in mice, there appears to be a similar plasticity in Rhesus Macaques (Reeves et al., 2015) and in the human NK cell compartment (Bjorkstrom et al., 2011), supporting the notion that NK cells exhibit adaptive behavior. In the human, CMV‐driven expansions of NKG2C+ NK cells have been documented in healthy individuals (Beziat et al., 2013; Guma et al., 2004) and in numerous clinical settings, including solid organ transplantation (SOT) (Lopez‐Verges et al., 2011) and hematopoietic stem cell transplantation (HSCT) (Della Chiesa et al., 2012; Foley et al., 2012). Elevated numbers of differentiated NKG2C+ NK cells have also been observed in the context of other virus infections, including hepatitis C, chikungunya and hantavirus, but only in CMV‐seropositive individuals (Beziat et al., 2012; Bjorkstrom et al., 2011; Petitdemange et al., 2011). The link between NKG2C‐mediated NK‐cell expansion and CMV infection indicates that acute or chronic virus infections may lead to subclinical reactivation of CMV that in turn triggers the expansion and long‐term persistence of NKG2C+ NK cells.
Expanded NKG2C+ NK cells have a differentiated phenotype and can be identified using an increasing collection of characteristic markers that include lower expression of CD62L, CD7, CD161, CD122, FcεR1γ, NKp30, NKp46, siglec‐7, siglec‐9 and higher expression of CD2, ILT2, CD57 and granzyme B (Luetke‐Eversloh et al., 2013). Another striking feature of adaptive NK cells is their preferential expression of inhibitory KIR specific for self HLA class I molecules (Beziat et al., 2013; Della Chiesa et al., 2012; Foley et al., 2012).
5. Molecular footprints in adaptive NK cells
In T cells, both lineage commitment and maintenance of memory is associated with epigenetic modification, which is subsequently retained in daughter cells during immune expansion as well as long‐term homeostatic cell divisions (Youngblood et al., 2013). Similar characteristics are observed in NK cells as they progress through cycles of differentiation and clonal expansion, providing a partial explanation for the long‐term stability of adaptive NK cells. Principal component analysis of the global epigenetic profiles of adaptive NK cells places them in close proximity to the memory subset of CD8 T cells (Luetke‐Eversloh et al., 2014b; Schlums et al., 2015). One way that the effect of epigenetic modification on function could be demonstrated directly was through the divergent IFN‐γ production observed in naïve (CD56bright) and mature (CD56dim) NK cells, despite there being an equivalent capacity for signaling between the two subsets in response to receptor stimulation (Luetke‐Eversloh et al., 2014a). These differences could be attributed to progressive epigenetic remodeling, where the more differentiated NK cells possess a greatly increased capacity to produce IFN‐γ owing to specific demethylation of the conserved non‐coding sequence (CNS1) of the IFN‐γ promoter (Luetke‐Eversloh et al., 2014b). In addition, adaptive NK cells have also been observed to produce high levels of TNF in response to target cell stimulation, possibly through related epigenetic mechanisms.
A deeper look into the relationship between CMV infection and its effect on the composition of the NK cell compartment revealed that adaptive NK cells frequently exhibit downregulation of the signaling adapter FcεR1γ (Zhang et al., 2013). The population of FcεR1γ negative NK cells showed enhanced functional responses to infected cells in the presence of virus specific antibodies. Broadening the scope, downregulation of multiple signaling intermediates, including SYK, DAB2 and EAT2, was shown to occur specifically in NK cells with adaptive features, and often in combination (Lee et al., 2015; Schlums et al., 2015). The downregulation of these markers was attributed to epigenetic silencing. Furthermore, transcription factors PLZF and IKZF2, which also correlate with epigenetic silencing, were identified through global epigenetic profiling (Schlums et al., 2015). As PLZF is known to interact with the promoter regions for SYK, EAT2 and FcεR1γ, its downregulation may also contribute to the change in their expression. Heterogeneous downregulation of these signaling intermediates, combined with more consistent downregulation of PLZF and modification in the CNS1 region of IFN‐γ provide molecular footprints of adaptive NK cell responses. Thus, the adaptive NK cell response is much more diverse than initially anticipated, involving waves of expansion of partially redundant phenotypes, where CD57+NKG2C+ subsets may only represent the tip of the iceberg.
6. Role of adaptive NK cells in host immunity
To date, there is limited knowledge as to whether NK cells directly control herpesvirus infections. Biron et al. reported a case with an isolated deficiency in NK cells, showing recurrent severe herpesvirus infections including CMV pneumonia (Biron et al., 1989). This patient later developed a bone marrow failure shown to be caused by GATA‐2 mutation (Mace et al., 2013). Patients presenting with isolated NK cell deficiency, adrenal insufficiency and herpesvirus susceptibility were shown to carry MCM4 autosomal recessive mutations, a gene involved in DNA replication (Gineau et al., 2012). Recently, a patient presenting an isolated NK cell deficiency, who died from VZV infection, was reported to carry a known founder mutation in RTEL1, a gene involved in telomere maintenance (Hanna et al., 2015). Evidently, these mutations in GATA2 and RTEL1 ultimately resulted in multiple cytopenias (Ballew et al., 2013; Collin et al., 2015). Furthermore, it has been postulated that NK cells might be the first lymphocyte subset affected during bone marrow failure. It therefore remains unclear whether herpesvirus susceptibility in these patients was due to NK deficiency itself or simply an early sign of bone marrow failure and a broader underlying immune defect. However, Kuijpers et al. reported a case of a young infant with a family history of fatal infections (Kuijpers et al., 2008). The three month‐old girl with a T−B+NK+ SCID phenotype presented with a CMV‐induced gastroenteritis that resolved spontaneously without anti‐viral treatment. Characterization of the immune response revealed a profound expansion of NKG2C+ NK cells with a restricted KIR‐repertoire that was normalized when the viral load declined. Although circumstantial, this case indicates the potential for NK cells to control acute CMV infection in the absence of T cells. Similarly, in the context of allogeneic HSCT, adaptive NK cells emerging in recipients early after transplant appear to protect from CMV reactivation (Davis et al., 2015).
Despite vigorous monitoring and pre‐emptive therapy, CMV infection and re‐activation remains a significant cause of morbidity and mortality in immunocompromised HSCT recipients (Ljungman, 2014). Intriguingly, however, the epidemiological link between CMV reactivation and relapse protection (Elmaagacli et al., 2011) has nurtured the idea that emergence of adaptive NK cells early after transplant may contribute to the elimination of leukemic cells. Although this concept is appealing, it is somewhat difficult to reconcile with the fact that adaptive NK cells express self‐specific inhibitory KIRs, which effectively abrogate recognition of HLA‐matched leukemic blasts (Liu et al., unpublished data). Further studies into the role of adaptive NK cells in determination of transplantation outcome are warranted, and it will be particularly interesting to dissect the role for HLA mismatch in this context, as certain combinations would result in transfer (or emergence) of large populations of highly cytotoxic NK cells mediating missing self responses.
7. Drivers of the adaptive NK cell response in the human
NKG2C and NKG2A are two transmembrane receptors expressed as heterodimers in association with CD94 (Brooks et al., 1997; Lazetic et al., 1996). Although these receptors both share specificity for the non‐classical class I molecule HLA‐E (Braud et al., 1998; Kaiser et al., 2005; Llano et al., 1998), they display opposing functional properties. CD94/NKG2A is an inhibitory receptor with two ITIM motifs while CD94/NKG2C associates with the adaptor molecule DAP12 and mediates activating signals (Lanier et al., 1998; Lazetic et al., 1996). NKG2A appears to have the stronger affinity for HLA‐E/peptide complexes and its inhibitory signal is dominant when the two receptors are co‐engaged on the same NK cell (Beziat et al., 2011; Kaiser et al., 2005).
The cellular mechanism that drives the expansion of NKG2C+ NK cells following HCMV infection remains elusive, as does the degree to which NKG2C is involved. NKG2C binding to HLA‐E is influenced by the peptide bound to the groove (Braud et al., 1998; Tomasec et al., 2000). It is therefore possible that the inhibitory receptor NKG2A and activating receptor NKG2C can distinguish between HLA‐E expressing different leader peptides and/or peptides derived from viral antigens and thus, may ultimately influence adaptive NK cell responses. Based on characteristics common to adaptive NK cells, expansions can also be seen independently of NKG2C, in NK cells expressing activating KIR (such as 2DS2, 2DS4) (Beziat et al., 2013). Further supporting this notion, a study of patients carrying a homozygous deletion of the NKG2C gene undergoing umbilical cord blood transplantation (UCBT) and experiencing CMV infection showed the emergence of adaptive NK cells characterized by the expression of activating KIRs (Della Chiesa et al., 2014). For most of the activating KIRs the natural ligands remain unknown (Ivarsson et al., 2014), albeit it is tempting to speculate that they recognize virally encoded ligands in a fashion similar to Ly49H‐mediated recognition of the CMV protein m157 (Arase et al., 2002). The discovery of a spectrum of adaptive NK cells, many of which lack NKG2C and activating KIRs (Schlums et al., 2015) (Béziat et al., unpublished observation), open up for the possibility that NKG2C merely serves as a marker of adaptive NK cells and that other cellular mechanisms drive their expansion.
Intriguingly, adaptive NK cells also exhibit a superior capacity for expansion in response to both CMV and Influenza antigens in the presence of seropositive plasma (Lee et al., 2015). This suggests the expansion of adaptive NK cells is driven at least in part by antibody‐mediated stimulation. Thus, in addition to signaling via NKG2C and activating KIRs, agonistic stimulation of CD16 by virus specific antibodies may be one such independent driver of adaptive NK cells. Notably, these insights have direct implications for the design of culturing protocols aiming to selectively expand adaptive NK cells.
8. Selective expansion of single‐KIR+ NK cells for cancer therapy
Transfer of NK cells across HLA barriers releases the cytotoxic potential of a functional repertoire that is otherwise restrained by interactions with self HLA class I in the donor (Ruggeri et al., 1999; Valiante et al., 1997; Yawata et al., 2008). This scenario has been observed to occur in certain specific donor‐recipient constellations in the context of partially mismatched allogeneic HSCT and has been linked to improved survival (Giebel et al., 2003; Miller et al., 2007; Ruggeri et al., 2002b; Symons et al., 2010). For such an NK cell‐mediated graft‐versus‐leukemia (GVL) effect to take place the recipient setting must lack any one of the three major KIR ligands present in the donor. For example, an HLA‐C1/C1 recipient must receive an HLA‐C2+ allograft, whereby donor‐derived KIR2DL1+ NK cells may contribute to the clearance of mismatched HLA‐C1+ leukemic cells (Figure 1). A major limitation, however, is the number of cells that can be isolated expressing 2DL1 (or KIR2DL3 for HLA‐C2/C2 recipients) as the sole inhibitory receptor (Fauriat et al., 2008). The genetic determination of the HLA‐C genotype provides no information about the size of the alloreactive NK cell repertoire for a given donor. An attractive solution to this problem would be to develop in vitro culturing protocols for selective expansion of the alloreactive NK cell subset (Figure 1).
Figure 1.

Selective expansion of alloreactive NK cell subsets. A schematic illustration of the expansion of single KIR2DL3+ NK cells from a C1/Bw4 donor. NK cells are educated (functionally tuned) against the HLA in the donor. Upon adoptive transfer into a recipient, NK cells expressing KIR2DL3 as their only inhibitory KIR sense the absence of the HLA‐C1 molecule and mediate alloreactivity against leukemic blasts. Adaptive NK cell responses to CMV leads to the expansion of NK cells expressing self‐specific KIR in vivo. Thus, it may be possible to mimic the effects of CMV and establish culture protocols that selectively expand NK cells of a given specificity. This would increase the number of alloreactive NK cells available for infusion into the patient.
Although there are many alternative strategies for ex vivo expansion of NK cells that are currently being explored in the clinic, few of these yield specific expansion of subsets expressing a single KIR. NKG2C+ adaptive NK cells represent a naturally occurring expansion of single KIR positive subsets, representing up to 75% of all NK cells in some healthy donors (Beziat et al., 2013). Expansions of NKG2C+ NK cells can be achieved in vitro by co‐culturing naïve NK cells with cell lines engineered to express HLA‐E at high levels or fibroblasts infected with CMV, supporting a direct role for NKG2C in driving adaptive NK cell responses (Charoudeh et al., 2013; Guma et al., 2006). CMV encodes several proteins that interfere with antigen processing and cause downregulation of classical MHC class I molecules (Mocarski, 2004). At the same time, the signal peptide of the CMV UL40 protein promotes surface expression of HLA‐E, providing stimulation to NKG2C+ NK cells (Prod'homme et al., 2012; Ulbrecht et al., 2000). However, in vitro expansion protocols based on infection with live viruses would face substantial challenge and are highly unlikely to pass regulatory GMP requirements. An alternative approach for recapitulating the expansion of NKG2C+KIR+ cells that occurs in vivo is to use HLA‐E transfected feeder cells devoid of other HLA‐I ligands (e.g. 721.221.AEH) (Beziat et al., 2013; Guma et al., 2006), a process potentially scalable to a GMP‐compliant production process. Likewise, a feeder‐free system based on latex beads or direct coating of agonists onto plates or culture bags utilizing distinct combination of cytokine with agonistic mAbs directed against specific activation receptors, such as anti‐CD94, anti‐NKG2C, anti‐NKp46, anti‐DNAM‐1, anti‐CD2, anti‐activating KIR, anti‐CD16 as well as pentamers of HLA‐E in complex with different leader sequences would provide an attractive platform for expansion and functional tuning (Figure 2).
Figure 2.
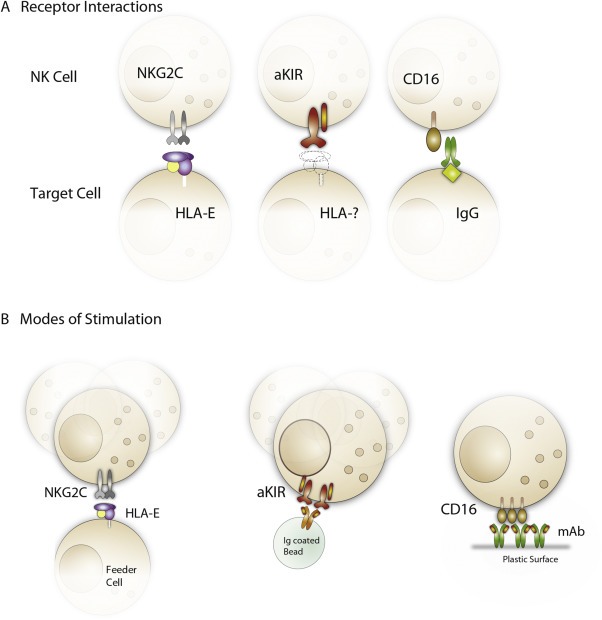
Strategies for achieving guided differentiation and selective skewing towards adaptive NK cells. A) Shown are potential pathways that stimulate growth of adaptive NK cells including NKG2C (left), activating KIRs (middle) and antibody‐based recognition of viral antigens (right). B) Guided differentiation and/or selective expansion of adaptive NK cells may be achieved by culturing NK cells together with genetically modified feeder cells (left) or through agonistic stimulation by antibody‐coated beads (middle) or plates/culture bags coated with the desired combination of antibodies (right). The three principal pathways that have been postulated for driving adaptive NK cells (NKG2C, activating KIR or antibody triggering of FcγRIIIa) could be targeted in either culturing platform.
In the above mentioned strategies to expand adaptive NK cells expressing a specific KIR, no distinct measure is taken in order to actively skew the KIR repertoire toward a given specificity. The mechanism behind the selective outgrowth of NK cells expressing self KIRs in such cultures is unknown and somewhat paradoxical, as one would expect cells that are not restricted through inhibitory interactions with self MHC to gain a proliferative advantage. One may speculate that education mediated through self KIR‐HLA interactions not only influences degranulation and IFN‐γ production, but also the ability of the cells to undergo proliferation. However this is unlikely to be the sole explanation, since mice lacking MHC class I mount similarly effective adaptive NK cell responses, which are fully capable of controlling MCMV infection, despite their NK cell compartment being profoundly hyporesponsive (Orr et al., 2010). Likewise, TAP‐deficient patients displaying low HLA class I expression can develop NKG2C+ adaptive NK cell responses that could potentially contribute to the immunity against CMV (Beziat et al., 2015). Notably, these patients displayed polyclonal KIR expression, demonstrating that the expression of self KIRs is not required for the expansion to take place. Perhaps a more likely explanation is that expression of self KIR provides a survival advantages during conditions of stress. Thus, during conditions with limited access to nutrients and oxygen, inhibitory input might protect the cell from activation‐induced cell death leading to a relative increase in cells with self KIRs (Felices et al., 2014).
Although it will be useful to define and optimize the culture conditions that leads to selective outgrowth of NK cells expressing self KIRs, alternative strategies, involving active manipulation should also be explored. For example, it may be possible to selectively inhibit unwanted populations through forced overexpression of specific HLA‐C molecules. Indeed, co‐culturing NK cells with lymphoblastoid cell lines genetically modified to express a given KIR ligand stimulated the outgrowth of NK cells expressing the mismatched KIR (Igarashi et al., 2004). Siegler et al. described a more direct strategy for selective expansion of NK cells, through enrichment of self KIR positive cells using a GMP‐compatible sorter (Siegler et al., 2010). The desired population was enriched to 98.6% purity and expanded 159‐ to 390‐fold in IL‐2 and IL‐15 over the course of three weeks. The final product displayed superior killing of AML blasts relative to bulk cultures and also in vivo efficacy in NOD/SCID mice. At present, there are no GMP‐compatible anti‐KIR antibodies available but once these reagents reach the market, pre‐expansion manipulation through FACS sorting may open up new possibilities to top‐feed cultures with precursor populations of adaptive NK cells. A potential caveat of this strategy is the possibility of uncontrolled skewing of the cultures down‐stream of the sort with outgrowth of for example NKG2A+ subsets.
9. Balancing numbers versus specificity
One outstanding challenge lies in identifying the ideal seeding population for expansion of NK cells of a given specificity. Like most forms of cell proliferation, the capacity of immune cells to proliferate generally decreases as the cells differentiate towards functional maturity (Weng, 2001; Weng et al., 1997), while target specificity increases. There are many mechanisms that underlie the poor proliferation of differentiated cells: the progressive loss of cytokine receptors, the shortening of telomeres in the absence of telomerase expression, and the induction of anergy. Thus, cytokine stimulation of a mixed population of NK cells tends to result in the preferential outgrowth of the relatively more immature NKG2A+ NK cells expressing polyclonal KIR repertoires (Figure 3A–B). It is obvious that the near uniform expression of inhibitory CD94/NKG2A receptors on such NK cells poses a significant limit to the clinical utility against a broad range of tumor cells expressing high levels of HLA‐E (de Kruijf et al., 2010; Gooden et al., 2011).
Figure 3.
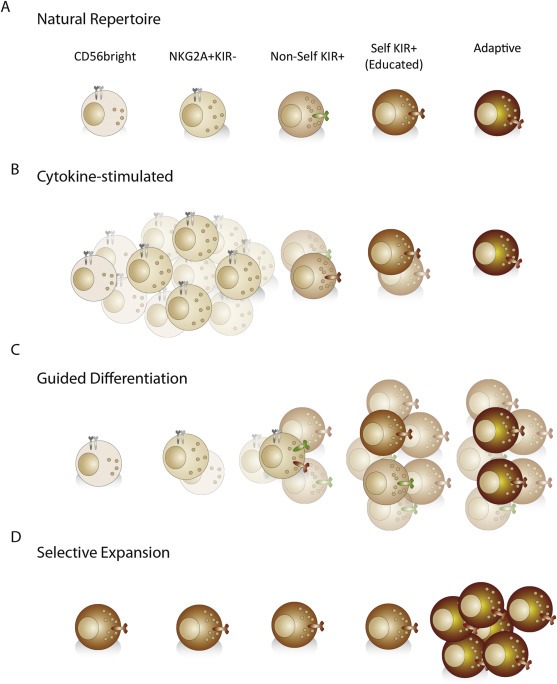
Balancing numbers versus specificity. A) The natural repertoire consists of cells at different stages of differentiation (CD56bright, CD56dim, NK cells expressing self and non‐self KIRs and terminally differentiated “adaptive” NK cells. B–D) The end result of the culture, in terms of cell populations, may vary depending on the strategy used. B) Large numbers of relatively naïve, NKG2A+ NK cells typically dominate following cultures in high doses of stimulatory cytokines. C, D) Shown are future potential protocols for generating large numbers of “adaptive” NK cells. C) It may be possible to define the conditions for guided differentiation of naïve NK cells into adaptive NK cells with desired KIR expression profiles. D) Alternatively, one may achieve selective expansion of pre‐existing adaptive NK cells or less differentiated precursors thereof.
In mice, selective proliferation and persistence of specific NK cells takes place during anti‐viral (MCMV) responses and typically follows an initial phase of proliferation of more broadly reactive bulk NK (Brown et al., 2001; Dokun et al., 2001), in a manner reminiscent of T cell effector expansion and subsequent contraction into a memory population. Mimicking this scenario in the human, CD56bright or NKG2A+KIR−CD56dim NK cells can be driven to undergo differentiation, involving acquisition of KIRs, under appropriate conditions including relevant cytokines and ligands (Bjorkstrom et al., 2010; Juelke et al., 2009; Romagnani et al., 2007) (Figure 3C). Such differentiation of CD56bright NK cells could supplement the total number of specific and highly functional NK cells. Since CD56bright NK cells and less mature NKG2A+ CD56dim NK cells tend to proliferate greatly under current protocols, the challenge is to guide the differentiation process towards the desired specificity. Deciphering the molecular mechanisms that govern the transitioning of NK cells through discrete stages of differentiation is likely to be very informative for future development of such protocols. In addition, it is possible that emerging platforms for NK cell differentiation from iPS cells may feed into strategies for guided differentiation of adaptive NK cell populations (Knorr et al., 2013).
Adaptive NK cells are poorly responsive to cytokine stimulation and appear to be unable to undergo further divisions in vitro. However, the currently ongoing mapping of their transcriptional and epigenetic profiles may open up new possibilities for selective outgrowth of this seemingly senescent subset, potentially involving the use of growth factors (Figure 3D). Although naïve NK cells were shown to be the dominant cellular source for the replenishment of adaptive NK cells during in vitro culture, the success rate of expanding large numbers of adaptive NK cells was actually higher in individuals with a pre‐existing adaptive subset (Beziat et al., 2013). These results suggested the existence of a primed NK cell intermediate that rapidly differentiates into adaptive cells with the same specificity as the original expansion.
A critical aspect to consider in the development of more selective culturing protocols is the choice of cytokines, potential combinations and the timing of feeding the cultures. Most current regimes employ fixed dosing and continuous supply of saturating levels of cytokines and nutrients. Adapted and sequential dosing schedules that are based on continuous monitoring of the cellular composition may be required to avoid outgrowth of unwanted subsets. IL‐15 is a commonly used cytokine for NK cell proliferation in therapeutic products as it promotes NK cell survival while activating and promoting proliferation. However, IL‐15 may not promote expression of telomerase in NK cells, which could potentially limit the long‐term proliferative response (Ouyang et al., 2007). In contrast, IL‐21 is known to induce telomerase expression in NK cells and could thereby overcome the telomere limitation and sustain longer culture periods (Lim et al., 2014). When IL‐21 was applied in the first week of culture, NK cells were found to have significantly longer telomeres than NK cells in continuous culture without IL‐21. Together with the attempt to direct differentiating CD56bright NK cells, the yield of selective NK cells would increase. Notably, parallel strategies are currently emerging in which the scheduling of combination of cytokines together with a structured rest, prime NK cells for more efficient function in response to subsequent challenges (Romee et al., 2012). Potentially such strategies could include co‐culture with feeder cells or beads with the aim of overcoming the hypo responsiveness of NK cells lacking self KIRs (Figure 4). Cytokine‐induced memory NK cells are currently being tested in the clinic (reviewed in Berrien‐Elliott et al., 2015).
Figure 4.
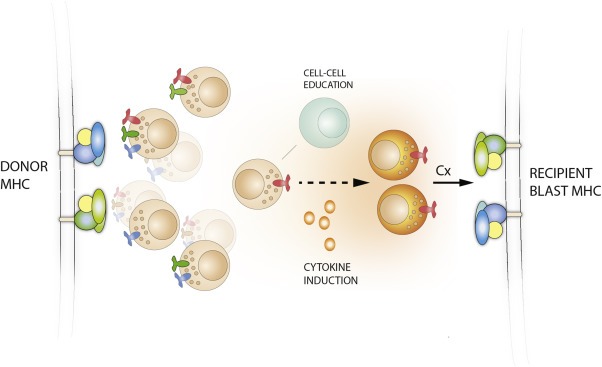
Overcoming NK cell tolerance. Strategies for scheduling cytokine stimulation may potentially be tailored to overcome the hypo responsiveness of NK cells lacking self‐KIRs. Such protocols could be used to prime uneducated NK cells for efficient missing self recognition in autologous and/or matched HLA class I settings as illustrated in the figure.
Another important point to consider in this context is the potential for retuning of NK cell function in vivo following transfer to the new host. It has been shown that educated NK cells loose functionality within 48 h post transfer into MHC‐deficient mice (Elliott et al., 2010; Joncker et al., 2010). Conversely, functional potential is regained in hyporesponsive NK cells by transfer to an educating environment. Whether these effects will influence the efficacy of adoptive NK cell therapy in the human remains unknown. It is possible that one may need to design treatment schedules with repeated infusions of functional NK cells rather than depending long‐term in vivo persistence of NK cells which have calibrated to the recipient and therefore lost their alloreactive potential.
10. Maintaining effector function by manipulating cell metabolism
Immune cell differentiation and function have been widely investigated, but only recently has immune cell metabolism emerged as a key regulatory mechanism behind these processes. As of now, the majority of the research has been focused on understanding T cell metabolism. It was previously believed that transcription factors and cytokines were the main determinants of T cell function and their differentiation into specific subsets. However it has now become clear that metabolic reprogramming due to environmental cues plays the central role in determining the fate of a T cell (Man and Kallies, 2015). These discoveries sparked an interest in deciphering NK cell metabolism.
The role of IL‐15 in NK cell homeostasis, activation and differentiation has been well documented, yet the molecular basis for the multifaceted functions of IL‐15 has only now been linked to the mammalian target of rapamycin (mTOR) (Donnelly et al., 2014; Marcais et al., 2014; Nandagopal et al., 2014). mTOR is a serine/threonine kinase which can form two protein complexes, mTORC1 and mTORC2, and has been shown to play a fundamental role in regulating T cells, dendritic cells and recently NK cells (Donnelly et al., 2014). One role of mTORC1 is to sense the microenvironment for nutrients such as glucose and amino acids, and in turn regulate NK cell metabolism, particularly through glycolysis. Donnely et al. have shown that as an NK cell is primed for activation, it increases its metabolic activity and undergoes metabolic reprogramming, primarily switching its source of energy acquisition from oxidative phosphorylation to glycolysis, which is required for both IFN‐γ and granzyme B production. Interestingly, NK cell activation and function are independently regulated. This was shown by inhibiting mTORC1 using rapamycin, resulting in smaller, yet activated NK cells (CD69 expression) exhibiting reduced glycolysis and consequently diminished IFN‐γ and granzyme B levels. Evidently, mTORC1 signaling leads to metabolic reprogramming and increased nutrient uptake in activated NK cells, and in turn modulates NK cell effector function through IFN‐γ and granzyme B production (Donnelly et al., 2014).
Marcais et al. investigated the regulation of mTOR itself, as well as the molecular machinery behind these metabolic changes in more detail. This study unearthed the role of IL‐15 in controlling mTORC1 activity and subsequently one of its downstream targets, STAT5. Interestingly, the dose of IL‐15 determines its downstream targets, accounting for its multifaceted role in NK cells. Only high concentrations of IL‐15 were able to induce mTORC1 activity, resulting in metabolic reprogramming to induce effector function. Furthermore, high levels of IL‐15 also induced the proliferative potential of NK cells. Low dose IL‐15, on the other hand, did not result in mTORC1 activation, but nonetheless resulted in STAT5 phosphorylation that maintained NK cell survival. Consequently, it is the amount of IL‐15 that determines its downstream targets and thus its effect on the NK cell (Marcais et al., 2014).
The importance of mTORC1 for NK effector function has also been investigated in mice infected with MCMV (Marcais et al., 2014; Nandagopal et al., 2014). Similar to IL‐15 treatment, MCMV infection in mice results mTORC1 activation, as seen through phosphorylation of S6 ribosomal protein (Nandagopal et al., 2014). In this setting, expression of effector molecules, mainly IFN‐γ and granzyme B, was greatly reduced following specific inhibition of mTORC1 signaling. This directly translated to NK cell cytotoxicity, where rapamycin treatment during MCMV infection abolished killing of tumor target cells. Evidently, mTORC1 plays a vital role in regulating NK cell effector function in both cytokine‐stimulated activation and in response to viral infection. As NK cells differentiate, their metabolism slows down resulting in metabolically resting, yet mature NK cells (Marcais et al., 2014). The same metabolic decrease is observed during NK cell expansion. Manipulating NK cell metabolism in order to maintain effector function is therefore an attractive method to expand mature, yet cytotoxic NK cells for adoptive therapy (Figure 5).
Figure 5.
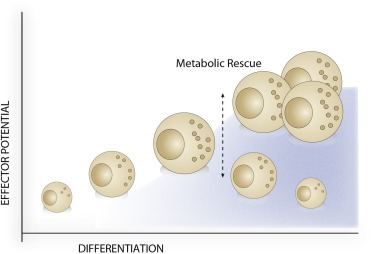
Manipulating metabolism to maintain effector potential. NK cells acquire effector potential during the initial stages of differentiation; followed by a decline in resting, mature NK cells due to a decrease in metabolic activity. Maintenance of NK cell metabolic activity during expansion and maturation could rescue the loss in effector potential, resulting in expanded, mature, and cytotoxic NK cells for use in adoptive cell therapy.
The change in metabolism that an NK cell undergoes as it moves along the spectrum of differentiation exhibits striking similarities to metabolic changes occurring during T cell differentiation. As a naïve T cell differentiates into an effector and eventually memory T cell, it moves from a catabolic to an anabolic and back to a catabolic state. Intriguingly, mTORC1 is activated during the anabolic state (effector T cell) and inhibited during the catabolic state (naïve and memory T cell) (Man and Kallies, 2015). Evidently, mTORC1 plays a vital role in determining the effector potential of both T cells and NK cells and mTORC1 itself, in turn, obtains cues from the environment. This correlates well with previous observations describing the surrounding environment shaping NK cell functionality. It has been well documented that the tumor microenvironment dampens NK cell activity through releasing immunosuppressive molecules (Baginska et al., 2013). However, malignant cells present in the surrounding environment can further suppress NK cell function by limiting the available nutrients, particularly glucose due to their increased glucose uptake. The same is true for sites of inflammation, where infiltrating immune cells will outcompete each other for the available nutrients (Donnelly et al., 2014). This further strengthens the need to manipulate NK cell metabolism to induce mTORC1 activation, effectively maintaining effector function in a variety of environments, during both expansion and subsequent infusion in adoptive NK cell therapy.
11. Concluding remarks
Adaptive NK cells in the human represent an increasing spectrum of defined NK cell subsets that have undergone in vivo proliferation and differentiation in response to pathogenic challenges. Although these cells have so far only been shown to expand in response to infection it may be possible to harness their unique properties in cell therapy against cancer. The development of in vitro protocols that mimic the pathogenic triggers and in so doing drive the expansion of single‐KIR+ NK cells would permit targeting of cancer in cell therapy across HLA barriers. We anticipate that this approach, with careful definition of donors and recipients, can be useful for patients with a HLA‐C1/C1 or C2/C2 genotype since it is possible to expand 2DL1‐ and 2DL3 NK cells respectively. While in vivo expansion of 3DL1 single positive NK cells is rarely observed in CMV‐seropositive patients, careful definition of 3DL1 and HLA‐BW4 phenotype can make a difference in efficacy of the effectors. Thus, an extended therapeutic strategy is probably required for HLA‐C1+/C2+/Bw4 ± patients, representing on average 40–50% of the population.
In parallel, it will be essential to consider the homeostatic regulation of NK cells, both during in vitro culture and following infusion into the patient. To date, this has been mostly studied in the context of tumor infiltrating lymphocytes (TIL), where evidence suggests that young TILs consisting of naïve effector cell populations are more effective in vivo (Gattinoni et al., 2012). This raises interesting questions concerning where, when and how long the effector response should take place to be as effective as possible. In terms of cytotoxic lymphocyte biology, this correlates to tumor‐homing ability, the maturation and maintenance (inheritability) of the cytotoxic effector potential and the survival of the effector cell. The extent to which these events are affected by the upstream culture conditions remains to be explored. New insights into how cytotoxic lymphocytes build potential may pave the way for manipulating pathways that shape the fate of the cells in the patient and thereby their efficacy against the tumor. Apart from refining culturing platforms to increase the effectiveness of NK cell therapy, a fundamental challenge in the field is to determine the therapeutic sequencing of cell therapy relative to other therapeutic strategies and the potential synergies that may be obtained through combinations.
Liu Lisa L., Pfefferle Aline, Yi Sheng Vincent Oei, Björklund Andreas T., Béziat Vivien, Goodridge Jodie P., Malmberg Karl-Johan, (2015), Harnessing adaptive natural killer cells in cancer immunotherapy, Molecular Oncology, 9, doi: 10.1016/j.molonc.2015.10.001.
This is a contribution to the special issue edited by Johanna Olweus, Cancer Immunotherapy.
References
- Arase, H. , Mocarski, E.S. , Campbell, A.E. , Hill, A.B. , Lanier, L.L. , 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 296, 1323–1326. [DOI] [PubMed] [Google Scholar]
- Baginska, J. , Viry, E. , Paggetti, J. , Medves, S. , Berchem, G. , Moussay, E. , Janji, B. , 2013. The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Front. Immunol. 4, 490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballew, B.J. , Joseph, V. , De, S. , Sarek, G. , Vannier, J.B. , Stracker, T. , Schrader, K.A. , Small, T.N. , O'Reilly, R. , Manschreck, C. , Harlan Fleischut, M.M. , Zhang, L. , Sullivan, J. , Stratton, K. , Yeager, M. , Jacobs, K. , Giri, N. , Alter, B.P. , Boland, J. , Burdett, L. , Offit, K. , Boulton, S.J. , Savage, S.A. , Petrini, J.H. , 2013. A recessive founder mutation in regulator of telomere elongation helicase 1, RTEL1, underlies severe immunodeficiency and features of Hoyeraal Hreidarsson syndrome. PLoS Genet. 9, e1003695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrien-Elliott, M.M. , Wagner, J.A. , Fehniger, T.A. , 2015. Human cytokine-induced memory-like natural killer cells. J. Innate Immun. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beziat, V. , Dalgard, O. , Asselah, T. , Halfon, P. , Bedossa, P. , Boudifa, A. , Hervier, B. , Theodorou, I. , Martinot, M. , Debre, P. , Bjorkstrom, N.K. , Malmberg, K.J. , Marcellin, P. , Vieillard, V. , 2012. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur. J. Immunol. 42, 447–457. [DOI] [PubMed] [Google Scholar]
- Beziat, V. , Descours, B. , Parizot, C. , Debre, P. , Vieillard, V. , 2010. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS One. 5, e11966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beziat, V. , Hervier, B. , Achour, A. , Boutolleau, D. , Marfain-Koka, A. , Vieillard, V. , 2011. Human NKG2A overrides NKG2C effector functions to prevent autoreactivity of NK cells. Blood. 117, 4394–4396. [DOI] [PubMed] [Google Scholar]
- Beziat, V. , Liu, L.L. , Malmberg, J.A. , Ivarsson, M.A. , Sohlberg, E. , Bjorklund, A.T. , Retiere, C. , Sverremark-Ekstrom, E. , Traherne, J. , Ljungman, P. , Schaffer, M. , Price, D.A. , Trowsdale, J. , Michaelsson, J. , Ljunggren, H.G. , Malmberg, K.J. , 2013. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 121, 2678–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beziat, v. , Sleiman, M. , Goodridge, J. , Kaarbo, M. , Liu, L. , Rollag, H. , Ljunggren, H.-G. , Zimmer, J. , Malmberg, K.-J. , 2015. Polyclonal expansion of NKG2C+ NK cells in TAP-deficient patients. Front. Immunol. 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron, C.A. , Byron, K.S. , Sullivan, J.L. , 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320, 1731–1735. [DOI] [PubMed] [Google Scholar]
- Bjorkstrom, N.K. , Lindgren, T. , Stoltz, M. , Fauriat, C. , Braun, M. , Evander, M. , Michaelsson, J. , Malmberg, K.J. , Klingstrom, J. , Ahlm, C. , Ljunggren, H.G. , 2011. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J. Exp. Med. 208, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkstrom, N.K. , Riese, P. , Heuts, F. , Andersson, S. , Fauriat, C. , Ivarsson, M.A. , Bjorklund, A.T. , Flodstrom-Tullberg, M. , Michaelsson, J. , Rottenberg, M.E. , Guzman, C.A. , Ljunggren, H.G. , Malmberg, K.J. , 2010. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 116, 3853–3864. [DOI] [PubMed] [Google Scholar]
- Braud, V.M. , Allan, D.S. , O'Callaghan, C.A. , Soderstrom, K. , D'Andrea, A. , Ogg, G.S. , Lazetic, S. , Young, N.T. , Bell, J.I. , Phillips, J.H. , Lanier, L.L. , McMichael, A.J. , 1998. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 391, 795–799. [DOI] [PubMed] [Google Scholar]
- Brooks, A.G. , Posch, P.E. , Scorzelli, C.J. , Borrego, F. , Coligan, J.E. , 1997. NKG2A complexed with CD94 defines a novel inhibitory natural killer cell receptor. J. Exp. Med. 185, 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M.G. , Dokun, A.O. , Heusel, J.W. , Smith, H.R. , Beckman, D.L. , Blattenberger, E.A. , Dubbelde, C.E. , Stone, L.R. , Scalzo, A.A. , Yokoyama, W.M. , 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 292, 934–937. [DOI] [PubMed] [Google Scholar]
- Charoudeh, H.N. , Terszowski, G. , Czaja, K. , Gonzalez, A. , Schmitter, K. , Stern, M. , 2013. Modulation of the natural killer cell KIR repertoire by cytomegalovirus infection. Eur. J. Immunol. 43, 480–487. [DOI] [PubMed] [Google Scholar]
- Cichocki, F. , Sitnicka, E. , Bryceson, Y.T. , 2014. NK cell development and function–plasticity and redundancy unleashed. Semin. Immunol. 26, 114–126. [DOI] [PubMed] [Google Scholar]
- Cichocki, F. , Verneris, M.R. , Cooley, S. , Bachanova, V. , Brunstein, C.G. , Blazar, B.R. , Wagner, J. , Schlums, H. , Bryceson, Y.T. , Weisdorf, D.J. , Miller, J.S. , 2015. The past, present, and future of NK cells in hematopoietic cell transplantation and adoptive transfer. Curr. Top. Microbiol. Immunol. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin, M. , Dickinson, R. , Bigley, V. , 2015. Haematopoietic and immune defects associated with GATA2 mutation. Br. J. Haematol. 169, 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, K.D. , Waggoner, S.N. , Whitmire, J.K. , 2014. NK cells and their ability to modulate T cells during virus infections. Crit. Rev. Immunol. 34, 359–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, Z.B. , Cooley, S.A. , Cichocki, F. , Felices, M. , Wangen, R. , Luo, X. , DeFor, T.E. , Bryceson, Y.T. , Diamond, D.J. , Brunstein, C. , Blazar, B.R. , Wagner, J.E. , Weisdorf, D.J. , Horowitz, A. , Guethlein, L.A. , Parham, P. , Verneris, M.R. , Miller, J.S. , 2015. Adaptive natural killer cell and killer cell immunoglobulin-like receptor-expressing T cell responses are induced by cytomegalovirus and are associated with protection against cytomegalovirus reactivation after allogeneic donor hematopoietic cell transplantation. Biol. Blood Marrow Transpl. 21, 1653–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijf, E.M. , Sajet, A. , van Nes, J.G. , Natanov, R. , Putter, H. , Smit, V.T. , Liefers, G.J. , van den Elsen, P.J. , van de Velde, C.J. , Kuppen, P.J. , 2010. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J. Immunol. 185, 7452–7459. [DOI] [PubMed] [Google Scholar]
- Della Chiesa, M. , Falco, M. , Bertaina, A. , Muccio, L. , Alicata, C. , Frassoni, F. , Locatelli, F. , Moretta, L. , Moretta, A. , 2014. Human cytomegalovirus infection promotes rapid maturation of NK cells expressing activating killer Ig-like receptor in patients transplanted with NKG2C-/- umbilical cord blood. J. Immunol. 192, 1471–1479. [DOI] [PubMed] [Google Scholar]
- Della Chiesa, M. , Falco, M. , Podesta, M. , Locatelli, F. , Moretta, L. , Frassoni, F. , Moretta, A. , 2012. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus?. Blood. 119, 399–410. [DOI] [PubMed] [Google Scholar]
- Dokun, A.O. , Kim, S. , Smith, H.R. , Kang, H.S. , Chu, D.T. , Yokoyama, W.M. , 2001. Specific and nonspecific NK cell activation during virus infection. Nat. Immunol. 2, 951–956. [DOI] [PubMed] [Google Scholar]
- Donnelly, R.P. , Loftus, R.M. , Keating, S.E. , Liou, K.T. , Biron, C.A. , Gardiner, C.M. , Finlay, D.K. , 2014. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J. Immunol. 193, 4477–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguizabal, C. , Zenarruzabeitia, O. , Monge, J. , Santos, S. , Vesga, M.A. , Maruri, N. , Arrieta, A. , Rinon, M. , Tamayo-Orbegozo, E. , Amo, L. , Larrucea, S. , Borrego, F. , 2014. Natural killer cells for cancer immunotherapy: pluripotent stem cells-derived NK cells as an immunotherapeutic perspective. Front. Immunol. 5, 439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, J.M. , Wahle, J.A. , Yokoyama, W.M. , 2010. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J. Exp. Med. 207, 2073–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmaagacli, A.H. , Steckel, N.K. , Koldehoff, M. , Hegerfeldt, Y. , Trenschel, R. , Ditschkowski, M. , Christoph, S. , Gromke, T. , Kordelas, L. , Ottinger, H.D. , Ross, R.S. , Horn, P.A. , Schnittger, S. , Beelen, D.W. , 2011. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 118, 1402–1412. [DOI] [PubMed] [Google Scholar]
- Fauriat, C. , Andersson, S. , Bjorklund, A.T. , Carlsten, M. , Schaffer, M. , Bjorkstrom, N.K. , Baumann, B.C. , Michaelsson, J. , Ljunggren, H.G. , Malmberg, K.J. , 2008. Estimation of the size of the alloreactive NK cell repertoire: studies in individuals homozygous for the group A KIR haplotype. J. Immunol. 181, 6010–6019. [DOI] [PubMed] [Google Scholar]
- Felices, M. , Lenvik, T.R. , Ankarlo, D.E. , Foley, B. , Curtsinger, J. , Luo, X. , Blazar, B.R. , Anderson, S.K. , Miller, J.S. , 2014. Functional NK cell repertoires are maintained through IL-2Ralpha and Fas ligand. J. Immunol. 192, 3889–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley, B. , Cooley, S. , Verneris, M.R. , Pitt, M. , Curtsinger, J. , Luo, X. , Lopez-Verges, S. , Lanier, L.L. , Weisdorf, D. , Miller, J.S. , 2012. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 119, 2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni, L. , Klebanoff, C.A. , Restifo, N.P. , 2012. Paths to stemness: building the ultimate antitumour T cell. Nat. Rev. Cancer. 12, 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel, S. , Locatelli, F. , Lamparelli, T. , Velardi, A. , Davies, S. , Frumento, G. , Maccario, R. , Bonetti, F. , Wojnar, J. , Martinetti, M. , Frassoni, F. , Giorgiani, G. , Bacigalupo, A. , Holowiecki, J. , 2003. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 102, 814–819. [DOI] [PubMed] [Google Scholar]
- Gill, S. , June, C.H. , 2015. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol. Rev. 263, 68–89. [DOI] [PubMed] [Google Scholar]
- Gineau, L. , Cognet, C. , Kara, N. , Lach, F.P. , Dunne, J. , Veturi, U. , Picard, C. , Trouillet, C. , Eidenschenk, C. , Aoufouchi, S. , Alcais, A. , Smith, O. , Geissmann, F. , Feighery, C. , Abel, L. , Smogorzewska, A. , Stillman, B. , Vivier, E. , Casanova, J.L. , Jouanguy, E. , 2012. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J. Clin. Invest. 122, 821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glienke, W. , Esser, R. , Priesner, C. , Suerth, J.D. , Schambach, A. , Wels, W.S. , Grez, M. , Kloess, S. , Arseniev, L. , Koehl, U. , 2015. Advantages and applications of CAR-expressing natural killer cells. Front. Pharmacol. 6, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooden, M. , Lampen, M. , Jordanova, E.S. , Leffers, N. , Trimbos, J.B. , van der Burg, S.H. , Nijman, H. , van Hall, T. , 2011. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8(+) T lymphocytes. Proc. Natl. Acad. Sci. U S A. 108, 10656–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge, J.P. , Onfelt, B. , Malmberg, K.J. , 2015. Newtonian cell interactions shape natural killer cell education. Immunol. Rev. 267, 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras Navarro, A. , Bjorklund, A.T. , Chekenya, M. , 2015. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front. Immunol. 6, 202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guma, M. , Angulo, A. , Vilches, C. , Gomez-Lozano, N. , Malats, N. , Lopez-Botet, M. , 2004. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 104, 3664–3671. [DOI] [PubMed] [Google Scholar]
- Guma, M. , Budt, M. , Saez, A. , Brckalo, T. , Hengel, H. , Angulo, A. , Lopez-Botet, M. , 2006. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 107, 3624–3631. [DOI] [PubMed] [Google Scholar]
- Hanna, S. , Beziat, V. , Jouanguy, E. , Casanova, J.L. , Etzioni, A. , 2015. A homozygous mutation of RTEL1 in a child presenting with an apparently isolated natural killer cell deficiency. J. Allergy Clin. Immunol. 136, 1113–1114. [DOI] [PubMed] [Google Scholar]
- Herberman, R.B. , Nunn, M.E. , Holden, H.T. , Lavrin, D.H. , 1975. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer. 16, 230–239. [DOI] [PubMed] [Google Scholar]
- Igarashi, T. , Wynberg, J. , Srinivasan, R. , Becknell, B. , McCoy, J.P. , Takahashi, Y. , Suffredini, D.A. , Linehan, W.M. , Caligiuri, M.A. , Childs, R.W. , 2004. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood. 104, 170–177. [DOI] [PubMed] [Google Scholar]
- Iguchi-Manaka, A. , Kai, H. , Yamashita, Y. , Shibata, K. , Tahara-Hanaoka, S. , Honda, S. , Yasui, T. , Kikutani, H. , Shibuya, K. , Shibuya, A. , 2008. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J. Exp. Med. 205, 2959–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, K. , Matsuyama, S. , Miyake, S. , Suga, K. , Nakachi, K. , 2000. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 356, 1795–1799. [DOI] [PubMed] [Google Scholar]
- Ivarsson, M.A. , Michaelsson, J. , Fauriat, C. , 2014. Activating killer cell Ig-like receptors in health and disease. Front. Immunol. 5, 184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joncker, N.T. , Shifrin, N. , Delebecque, F. , Raulet, D.H. , 2010. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J. Exp. Med. 207, 2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juelke, K. , Killig, M. , Luetke-Eversloh, M. , Parente, E. , Gruen, J. , Morandi, B. , Ferlazzo, G. , Thiel, A. , Schmitt-Knosalla, I. , Romagnani, C. , 2010. CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. Blood. 116, 1299–1307. [DOI] [PubMed] [Google Scholar]
- Juelke, K. , Killig, M. , Thiel, A. , Dong, J. , Romagnani, C. , 2009. Education of hyporesponsive NK cells by cytokines. Eur. J. Immunol. 39, 2548–2555. [DOI] [PubMed] [Google Scholar]
- Kadri, N. , Luu Thanh, T. , Hoglund, P. , 2015. Selection, tuning, and adaptation in mouse NK cell education. Immunol. Rev. 267, 167–177. [DOI] [PubMed] [Google Scholar]
- Kaiser, B.K. , Barahmand-Pour, F. , Paulsene, W. , Medley, S. , Geraghty, D.E. , Strong, R.K. , 2005. Interactions between NKG2x immunoreceptors and HLA-E ligands display overlapping affinities and thermodynamics. J. Immunol. 174, 2878–2884. [DOI] [PubMed] [Google Scholar]
- Karre, K. , 2002. Immunology. A perfect mismatch. Science. 295, 2029–2031. [DOI] [PubMed] [Google Scholar]
- Kiessling, R. , Klein, E. , Wigzell, H. , 1975. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 5, 112–117. [DOI] [PubMed] [Google Scholar]
- Knorr, D.A. , Bachanova, V. , Verneris, M.R. , Miller, J.S. , 2014. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin. Immunol. 26, 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorr, D.A. , Ni, Z. , Hermanson, D. , Hexum, M.K. , Bendzick, L. , Cooper, L.J. , Lee, D.A. , Kaufman, D.S. , 2013. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cell Transl. Med. 2, 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers, T.W. , Baars, P.A. , Dantin, C. , van den Burg, M. , van Lier, R.A. , Roosnek, E. , 2008. Human NK cells can control CMV infection in the absence of T cells. Blood. 112, 914–915. [DOI] [PubMed] [Google Scholar]
- Lanier, L.L. , Corliss, B. , Wu, J. , Phillips, J.H. , 1998. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 8, 693–701. [DOI] [PubMed] [Google Scholar]
- Lazetic, S. , Chang, C. , Houchins, J.P. , Lanier, L.L. , Phillips, J.H. , 1996. Human natural killer cell receptors involved in MHC class I recognition are disulfide-linked heterodimers of CD94 and NKG2 subunits. J. Immunol. 157, 4741–4745. [PubMed] [Google Scholar]
- Le Garff-Tavernier, M. , Beziat, V. , Decocq, J. , Siguret, V. , Gandjbakhch, F. , Pautas, E. , Debre, P. , Merle-Beral, H. , Vieillard, V. , 2010. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 9, 527–535. [DOI] [PubMed] [Google Scholar]
- Lee, J. , Zhang, T. , Hwang, I. , Kim, A. , Nitschke, L. , Kim, M. , Scott, J.M. , Kamimura, Y. , Lanier, L.L. , Kim, S. , 2015. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 42, 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, W. , 2014. Infusions of allogeneic natural killer cells as cancer therapy. Clin. Cancer Res. 20, 3390–3400. [DOI] [PubMed] [Google Scholar]
- Lim, D.P. , Jang, Y.Y. , Kim, S. , Koh, S.S. , Lee, J.J. , Kim, J.S. , Thi Phan, M.T. , Shin, D.J. , Shin, M.G. , Lee, S.H. , Yoon, M. , Kim, S.K. , Yoon, J.H. , Park, M.H. , Cho, D. , 2014. Effect of exposure to interleukin-21 at various time points on human natural killer cell culture. Cytotherapy. 16, 1419–1430. [DOI] [PubMed] [Google Scholar]
- Ljungman, P. , 2014. The role of cytomegalovirus serostatus on outcome of hematopoietic stem cell transplantation. Curr. Opin. Hematol. 21, 466–469. [DOI] [PubMed] [Google Scholar]
- Llano, M. , Lee, N. , Navarro, F. , Garcia, P. , Albar, J.P. , Geraghty, D.E. , Lopez-Botet, M. , 1998. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: preferential response to an HLA-G-derived nonamer. Eur. J. Immunol. 28, 2854–2863. [DOI] [PubMed] [Google Scholar]
- Lopez-Verges, S. , Milush, J.M. , Pandey, S. , York, V.A. , Arakawa-Hoyt, J. , Pircher, H. , Norris, P.J. , Nixon, D.F. , Lanier, L.L. , 2010. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 116, 3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Verges, S. , Milush, J.M. , Schwartz, B.S. , Pando, M.J. , Jarjoura, J. , York, V.A. , Houchins, J.P. , Miller, S. , Kang, S.M. , Norris, P.J. , Nixon, D.F. , Lanier, L.L. , 2011. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. U S A. 108, 14725–14732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetke-Eversloh, M. , Cicek, B.B. , Siracusa, F. , Thom, J.T. , Hamann, A. , Frischbutter, S. , Baumgrass, R. , Chang, H.D. , Thiel, A. , Dong, J. , Romagnani, C. , 2014. NK cells gain higher IFN-gamma competence during terminal differentiation. Eur. J. Immunol. 44, 2074–2084. [DOI] [PubMed] [Google Scholar]
- Luetke-Eversloh, M. , Hammer, Q. , Durek, P. , Nordstrom, K. , Gasparoni, G. , Pink, M. , Hamann, A. , Walter, J. , Chang, H.D. , Dong, J. , Romagnani, C. , 2014. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 10, e1004441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetke-Eversloh, M. , Killig, M. , Romagnani, C. , 2013. Signatures of human NK cell development and terminal differentiation. Front. Immunol. 4, 499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace, E.M. , Hsu, A.P. , Monaco-Shawver, L. , Makedonas, G. , Rosen, J.B. , Dropulic, L. , Cohen, J.I. , Frenkel, E.P. , Bagwell, J.C. , Sullivan, J.L. , Biron, C.A. , Spalding, C. , Zerbe, C.S. , Uzel, G. , Holland, S.M. , Orange, J.S. , 2013. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood. 121, 2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man, K. , Kallies, A. , 2015. Synchronizing transcriptional control of T cell metabolism and function. Nat. Rev. Immunol. 15, 574–584. [DOI] [PubMed] [Google Scholar]
- Marcais, A. , Cherfils-Vicini, J. , Viant, C. , Degouve, S. , Viel, S. , Fenis, A. , Rabilloud, J. , Mayol, K. , Tavares, A. , Bienvenu, J. , Gangloff, Y.G. , Gilson, E. , Vivier, E. , Walzer, T. , 2014. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 15, 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell, K.A. , Hank, J.A. , DeSantes, K.B. , Capitini, C.M. , Otto, M. , Sondel, P.M. , 2015. NK cell-based immunotherapies in pediatric oncology. J. Pediatr. Hematol. Oncol. 37, 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero, I. , Gaudernack, G. , Gerritsen, W. , Huber, C. , Parmiani, G. , Scholl, S. , Thatcher, N. , Wagstaff, J. , Zielinski, C. , Faulkner, I. , Mellstedt, H. , 2014. Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol. 11, 509–524. [DOI] [PubMed] [Google Scholar]
- Michaud, H.A. , Eliaou, J.F. , Lafont, V. , Bonnefoy, N. , Gros, L. , 2014. Tumor antigen-targeting monoclonal antibody-based immunotherapy: orchestrating combined strategies for the development of long-term antitumor immunity. Oncoimmunology. 3, e955684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J.S. , Cooley, S. , Parham, P. , Farag, S.S. , Verneris, M.R. , McQueen, K.L. , Guethlein, L.A. , Trachtenberg, E.A. , Haagenson, M. , Horowitz, M.M. , Klein, J.P. , Weisdorf, D.J. , 2007. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 109, 5058–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J.S. , Soignier, Y. , Panoskaltsis-Mortari, A. , McNearney, S.A. , Yun, G.H. , Fautsch, S.K. , McKenna, D. , Le, C. , Defor, T.E. , Burns, L.J. , Orchard, P.J. , Blazar, B.R. , Wagner, J.E. , Slungaard, A. , Weisdorf, D.J. , Okazaki, I.J. , McGlave, P.B. , 2005. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 105, 3051–3057. [DOI] [PubMed] [Google Scholar]
- Min-Oo, G. , Kamimura, Y. , Hendricks, D.W. , Nabekura, T. , Lanier, L.L. , 2013. Natural killer cells: walking three paths down memory lane. Trends Immunol. 34, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski, E.S. , 2004. Immune escape and exploitation strategies of cytomegaloviruses: impact on and imitation of the major histocompatibility system. Cell Microbiol. 6, 707–717. [DOI] [PubMed] [Google Scholar]
- Nandagopal, N. , Ali, A.K. , Komal, A.K. , Lee, S.H. , 2014. The critical role of IL-15-PI3K-mTOR pathway in natural killer cell effector functions. Front. Immunol. 5, 187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary, J.G. , Goodarzi, M. , Drayton, D.L. , von Andrian, U.H. , 2006. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7, 507–516. [DOI] [PubMed] [Google Scholar]
- Orr, M.T. , Murphy, W.J. , Lanier, L.L. , 2010. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat. Immunol. 11, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang, Q. , Baerlocher, G. , Vulto, I. , Lansdorp, P.M. , 2007. Telomere length in human natural killer cell subsets. Ann. N. Y Acad. Sci. 1106, 240–252. [DOI] [PubMed] [Google Scholar]
- Paust, S. , von Andrian, U.H. , 2011. Natural killer cell memory. Nat. Immunol. 12, 500–508. [DOI] [PubMed] [Google Scholar]
- Petitdemange, C. , Becquart, P. , Wauquier, N. , Beziat, V. , Debre, P. , Leroy, E.M. , Vieillard, V. , 2011. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog. 7, e1002268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittari, G. , Filippini, P. , Gentilcore, G. , Grivel, J.C. , Rutella, S. , 2015. Revving up natural killer cells and cytokine-induced killer cells against hematological malignancies. Front. Immunol. 6, 230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prod'homme, V. , Tomasec, P. , Cunningham, C. , Lemberg, M.K. , Stanton, R.J. , McSharry, B.P. , Wang, E.C. , Cuff, S. , Martoglio, B. , Davison, A.J. , Braud, V.M. , Wilkinson, G.W. , 2012. Human cytomegalovirus UL40 signal peptide regulates cell surface expression of the NK cell ligands HLA-E and gpUL18. J. Immunol. 188, 2794–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves, R.K. , Li, H. , Jost, S. , Blass, E. , Li, H. , Schafer, J.L. , Varner, V. , Manickam, C. , Eslamizar, L. , Altfeld, M. , von Andrian, U.H. , Barouch, D.H. , 2015. Antigen-specific NK cell memory in rhesus macaques. Nat. Immunol. 16, 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo, N.P. , Dudley, M.E. , Rosenberg, S.A. , 2012. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani, C. , Juelke, K. , Falco, M. , Morandi, B. , D'Agostino, A. , Costa, R. , Ratto, G. , Forte, G. , Carrega, P. , Lui, G. , Conte, R. , Strowig, T. , Moretta, A. , Munz, C. , Thiel, A. , Moretta, L. , Ferlazzo, G. , 2007. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J. Immunol. 178, 4947–4955. [DOI] [PubMed] [Google Scholar]
- Romee, R. , Schneider, E.S. , Leong, J. , Chase, J.M. , Keppel, C.R. , S, R.P. , Cooper, M.A. , Fehniger, T.A. , 2012. Cytokine activation induces human memory-like NK cells. Blood. 120, 4751–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri, L. , Capanni, M. , Casucci, M. , Volpi, I. , Tosti, A. , Perruccio, K. , Urbani, E. , Negrin, R.S. , Martelli, M.F. , Velardi, A. , 1999. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 94, 333–339. [PubMed] [Google Scholar]
- Ruggeri, L. , Capanni, M. , Tosti, A. , Urbani, E. , Posati, S. , Aversa, F. , Martelli, M.F. , Velardi, A. , 2002. Innate immunity against hematological malignancies. Cytotherapy. 4, 343–346. [DOI] [PubMed] [Google Scholar]
- Ruggeri, L. , Capanni, M. , Urbani, E. , Perruccio, K. , Shlomchik, W.D. , Tosti, A. , Posati, S. , Rogaia, D. , Frassoni, F. , Aversa, F. , Martelli, M.F. , Velardi, A. , 2002. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 295, 2097–2100. [DOI] [PubMed] [Google Scholar]
- Schlums, H. , Cichocki, F. , Tesi, B. , Theorell, J. , Beziat, V. , Holmes, T.D. , Han, H. , Chiang, S.C. , Foley, B. , Mattsson, K. , Larsson, S. , Schaffer, M. , Malmberg, K.J. , Ljunggren, H.G. , Miller, J.S. , Bryceson, Y.T. , 2015. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 42, 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, T.N. , Schreiber, R.D. , 2015. Neoantigens in cancer immunotherapy. Science. 348, 69–74. [DOI] [PubMed] [Google Scholar]
- Siegler, U. , Meyer-Monard, S. , Jorger, S. , Stern, M. , Tichelli, A. , Gratwohl, A. , Wodnar-Filipowicz, A. , Kalberer, C.P. , 2010. Good manufacturing practice-compliant cell sorting and large-scale expansion of single KIR-positive alloreactive human natural killer cells for multiple infusions to leukemia patients. Cytotherapy. 12, 750–763. [DOI] [PubMed] [Google Scholar]
- Sun, J.C. , Beilke, J.N. , Lanier, L.L. , 2009. Adaptive immune features of natural killer cells. Nature. 457, 557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J.C. , Beilke, J.N. , Lanier, L.L. , 2010. Immune memory redefined: characterizing the longevity of natural killer cells. Immunol. Rev. 236, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons, H.J. , Leffell, M.S. , Rossiter, N.D. , Zahurak, M. , Jones, R.J. , Fuchs, E.J. , 2010. Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol. Blood Marrow Transpl. 16, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasec, P. , Braud, V.M. , Rickards, C. , Powell, M.B. , McSharry, B.P. , Gadola, S. , Cerundolo, V. , Borysiewicz, L.K. , McMichael, A.J. , Wilkinson, G.W. , 2000. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 287, 1031 [DOI] [PubMed] [Google Scholar]
- Ulbrecht, M. , Martinozzi, S. , Grzeschik, M. , Hengel, H. , Ellwart, J.W. , Pla, M. , Weiss, E.H. , 2000. Cutting edge: the human cytomegalovirus UL40 gene product contains a ligand for HLA-E and prevents NK cell-mediated lysis. J. Immunol. 164, 5019–5022. [DOI] [PubMed] [Google Scholar]
- Valiante, N.M. , Uhrberg, M. , Shilling, H.G. , Lienert-Weidenbach, K. , Arnett, K.L. , D'Andrea, A. , Phillips, J.H. , Lanier, L.L. , Parham, P. , 1997. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 7, 739–751. [DOI] [PubMed] [Google Scholar]
- Vivier, E. , Raulet, D.H. , Moretta, A. , Caligiuri, M.A. , Zitvogel, L. , Lanier, L.L. , Yokoyama, W.M. , Ugolini, S. , 2011. Innate or adaptive immunity? The example of natural killer cells. Science. 331, 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier, E. , Ugolini, S. , Blaise, D. , Chabannon, C. , Brossay, L. , 2012. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 12, 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner, S.N. , Cornberg, M. , Selin, L.K. , Welsh, R.M. , 2012. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 481, 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng, N. , 2001. Interplay between telomere length and telomerase in human leukocyte differentiation and aging. J. Leukoc. Biol. 70, 861–867. [PubMed] [Google Scholar]
- Weng, N.P. , Palmer, L.D. , Levine, B.L. , Lane, H.C. , June, C.H. , Hodes, R.J. , 1997. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol. Rev. 160, 43–54. [DOI] [PubMed] [Google Scholar]
- Yawata, M. , Yawata, N. , Draghi, M. , Partheniou, F. , Little, A.M. , Parham, P. , 2008. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood. 112, 2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood, B. , Hale, J.S. , Ahmed, R. , 2013. T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology. 139, 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T. , Scott, J.M. , Hwang, I. , Kim, S. , 2013. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J. Immunol. 190, 1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]


