Abstract
The term ‘inhibitory checkpoint’ refers to the broad spectrum of co‐receptors expressed by T cells that negatively regulate T cell activation thus playing a crucial role in maintaining peripheral self‐tolerance. Co‐inhibitory receptor ligands are highly expressed by a variety of malignancies allowing evasion of anti‐tumour immunity. Recent studies demonstrate that manipulation of these co‐inhibitory pathways can remove the immunological brakes that impede endogenous immune responses against tumours. Antibodies that block the interactions between co‐inhibitory receptors and their ligands have delivered very promising clinical responses, as has been shown by recent successful trials targeting the CTLA‐4 and PD‐1 pathways. In this review, we discuss the mechanisms of action and expression pattern of co‐inhibitory receptors on different T cells subsets, emphasising differences between CD4+ and CD8+ T cells. We also summarise recent clinical findings utilising immune checkpoint blockade.
Keywords: Inhibitory receptors on T cells, Inhibitory checkpoints, Cancer immunotherapy, CTLA-4, PD-1
Highlights
Expression of co‐inhibitory receptors differs between the subsets of CD4 and CD8 T cells.
Manipulation of co‐inhibitory pathways can remove the immunological brakes that impede immune responses against tumours.
Antibodies against CTLA‐4 and PD‐1 were approved for clinical use by FDA.
1. Introduction
T cell‐mediated immunity is tightly regulated by a series of complex mechanisms that range from the elimination of self‐reactive T cell clones in the thymus to the fine‐tuning of T cell activation by co‐receptors. Co‐stimulatory and co‐inhibitory receptors demonstrate great diversity in structure, function and expression pattern, and their functions are largely context dependent. They regulate a broad spectrum of T cells activity from proliferation and motility to the expression of cytokines and cytotoxic molecules, such as granzyme B and perforin. Co‐inhibitory receptors play a crucial role in curtailing T‐cell activation and defects in their function lead to aberrant immune responses such as autoimmunity. The same regulatory mechanisms limiting T cell activation following chronic exposure to antigen or preventing autoimmune responses may, however, diminish adequate immune responses against cancer. Supported by pre‐clinical and clinical evidence, novel immunotherapeutic strategies have focused on monoclonal antibodies designed to block the interaction between co‐inhibitory receptors expressed on T cells and their respective ligands, with the aim of increasing the anti‐tumour immune response. Optimisation of these therapies has evolved thanks to the understanding of the function of the various checkpoints, their expression landscape and their effect on both the effector and regulatory T cell (Treg) subpopulations prevalent in many malignancies.
In this review, we specifically focus on the role of negative immune checkpoints in T cell responses, the adaptive immune resistance and the complex interplay between the receptors and their multiple ligands in the tumour microenvironment. We highlight the differences in co‐inhibitory receptor expression in different subsets of CD4+ and CD8+ T cells and summarise both early clinical experience with the use of monoclonal antibodies and the outcomes of late‐stage clinical trials with anti‐CTLA‐4 and anti‐PD‐1 antibodies.
2. Inhibitory receptors: mechanism of action and targeted therapies
2.1. 1 CTLA‐4
2.1.1. CTLA‐4 structure, endo‐ and exocytosis, mechanism of action
CTLA‐4 (cytotoxic T‐lymphocyte‐associated protein 4, CD152) is a CD28 homologue and genes for these molecules are localised in the same chromosome regions in human and mouse. The overall homology between the human and murine CTLA‐4 proteins is 76%, whereas cytoplasmic domains show complete identity. CTLA‐4 is a type 1 transmembrane glycoprotein of the immunoglobulin superfamily (IgSF) and exhibits a dimer interface similar to that present in the constant (c‐type) family members (Dariavach et al., 1988; Ostrov et al., 2000). The full length CTLA‐4 consists of 4 exons, in humans in addition transcripts skipping exon 2–3 can be detected and in mouse transcripts lacking exon 2 encoding ligand‐binding domain were described (Ling et al., 1999).
CTLA‐4 is expressed mainly by T cells. Some studies report its expression on a variety of immune cells including B cells, fibroblasts and embryonic cells, but the role of CTLA‐4 remains unknown for these non‐T cell subsets (Quandt et al., 2007). Whereas both human and murine natural regulatory T cells (nTreg) cells constitutively express high levels of CTLA‐4 on their surface, naive T cells upregulate CTLA‐4 only after activation, reaching the maximum level 2–3 days post in vitro activation with anti‐CD3 antibodies (Walunas et al., 1994). In non‐activated T cells, expression of CTLA‐4 is practically undetectable (Perkins et al., 1996). Upon activation in vitro, both CD4+ and CD8+ T cells express CTLA‐4, although CD4+ T cells are reported to express more CTLA‐4 than CD8+ T cells on both the mRNA and protein level (Table 1). Unfortunately it is not clear if Foxp3‐positive cells were excluded from these analyses (Chan et al., 2014). Although CTLA‐4 upregulation upon TCR engagement is well described, the transcription factors driving its expression are not completely elucidated. Indeed, only NFATc1 (nuclear factor for activated T cells) has been reported to bind CTLA‐4 promoter in human primary T lymphocytes (Gibson et al., 2007; Chan et al., 2014). The mechanisms regulating surface expression level of CTLA‐4 are better understood: exocytosis and endocytosis. CTLA‐4 is mainly located in intracellular vesicles and interacts with the μ2 subunit of the clathrin adaptor protein complex AP2 (Follows et al., 2001). Redistribution of CTLA‐4 to the plasma membrane is dependent on the GTPase ADP ribosylation factor‐1 and on phospholipase D activity as inhibition of these pathways prevents its membrane expression (Mead et al., 2005). Endocytosis (internalisation) of CTLA‐4 utilises a clathrin‐ and dynamin‐sensitive pathway and it is not impaired during T cell activation. CTLA‐4 is degraded in lysosomes (Mead et al., 2005).
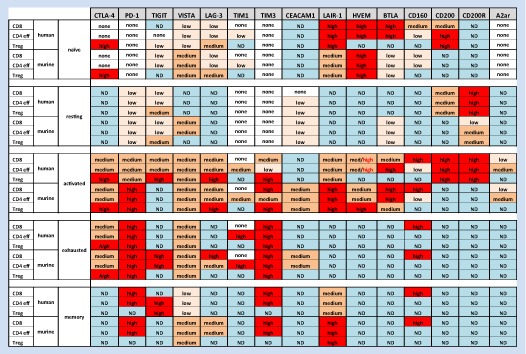
Table 1.
Simplified expression of inhibitory molecules on T cells.
Being a CD28 homologue, CTLA‐4 also binds to the same ligands as this co‐stimulatory receptor. However, CTLA‐4 interacts with CD80 and CD86 with 10 times higher affinity than CD28. Furthermore, CD28 recruitment to the immunological synapse can be disrupted by CTLA‐4, which forms extended high affinity lattices of alternating CTLA‐4 and CD80 homodimers (Greene et al., 1996). Thus, CTLA‐4 competes with CD28 ligands and thereby prevents CD28‐mediated T cell activation. Both ligands are expressed by antigen presenting cells (APCs). CD86 is constitutively expressed and upregulated after activation while CD80 is expressed only after activation (Hathcock et al., 1994). Mice that are deficient for either CD80 or CD86 show that these molecules have partially overlapping function. So far, there are no reports suggesting any unique biochemical or molecular differences in interactions of CTLA‐4 with either of the ligands. Despite extensive studies, little is known about the intracellular signalling pathways initiated upon CTLA‐4 engagement by its ligands. In a sharp contrast to CD28 or ICOS, engagement of CTLA‐4 in vitro leads to only relatively small changes in the transcriptional profile (Wakamatsu et al., 2013). In agreement with these results, the Allison team showed that only 9 genes besides CTLA‐4 itself were differentially expressed between CTLA‐4 sufficient and deficient T cells upon in vivo antigenic stimulation (Corse and Allison, 2012). These data suggest that there is no obvious inhibitory signalling pathway initiated by the engagement of CTLA‐4 (further reading: (Walker and Sansom, 2015)). A recent report focusing on Treg cells showed that the CTLA‐4 cytoplasmic tail interacts with the protein kinase C‐η (PKC‐η) in this T cells subset and that PKC‐η‐deficient Treg cells were impaired in contact‐dependent suppressive activity, which was associated with a grossly defective activation of the transcription factors NFAT and NF‐κB in these cells. In addition, this study demonstrated that CTLA‐4/PKC association mediates recruitment of focal adhesion disassembly complex (GIT2‐aPIX‐PAK) and hence plays a role in T cell motility (Kong et al., 2014; Walker and Sansom, 2015).
In 2011, Qureshi et al. characterised the cell‐extrinsic function of CTLA‐4. They showed that CTLA‐4 captures CD80 (B7‐1) and CD86 (B7‐2) from neighbouring cells by a unidirectional process called trans‐endocytosis. With a mutant lacking the conserved C‐terminus domain of CTLA‐4, they defined the interaction involved in this process. The acquisition of CD80 and CD86 by CTLA‐4 was enhanced upon TCR stimulation. Interestingly, in vivo data showed that both Foxp3+ and Foxp3‐ are capable of trans‐endocytosis (Qureshi et al., 2011).
2.1.2. CTLA‐4, tumour immunity: pre‐clinical data
Numerous studies with different disease models show that CTLA‐4 is a crucial molecule for T cell homoeostasis and function, but is also vital for maintaining peripheral tolerance. CTLA‐4‐deficient mice suffer from early onset aggressive autoimmune diseases with multi‐organ lymphocytic infiltration and organ destruction and in consequence premature death by 3–4 weeks of age (Tivol et al., 1995). Further studies with CTLA‐4 KO mice show that CTLA‐4 may have different impact on CD4+ vs. CD8+ T cells homoeostasis and function. In this model CTLA4‐deficient CD8+ T cells do not get activated and expand when CTLA‐4 KO CD4+ T cells are depleted but CTLA‐4 KO CD4+ T cells do in the absence of CTLA‐4 KO CD8+ (Chambers et al., 1997). Similar results are found in human T cells: blocking of CTLA‐4 in vitro on T cells results in a significant increase in proliferation of CD4+ but not CD8+ T cells (Chan et al., 2014). Nonetheless, despite the lack of evidence supporting a relevant role for CTLA‐4 on primary CD8 responses, CTLA‐4 has been demonstrated to modulate secondary responses in CD8+ T cells (Chambers et al., 1998).
High levels of CTLA‐4 expression on regulatory T cells suggested that CTLA‐4 may play a crucial role in Treg‐mediated suppression. One of the major functions of Treg cells is the inhibition of priming and differentiation of effector T cells (Josefowicz et al., 2012). Among many mechanisms employed by Treg cells, CTLA‐4‐mediated suppression is considered to be the most crucial one in vivo. The absence of CTLA‐4 specifically on Treg cells (Foxp3‐Cre × CTLA‐4fl/fl model) is sufficient for the development of systemic autoimmune disease, which confirms the importance of CTLA‐4 inhibiting overt immune response (Kajsa, 2008). These genetically modified regulatory T cells were not able to suppress in vivo tumour rejection, leading to enhanced tumour immunity (Kajsa, 2008).
Data from numerous in vitro and in vivo experiments demonstrate that CTLA‐4 is a negative regulator of T‐cell mediated responses in tumours. The first successful attempt at blocking the CTLA‐4 pathway to increase anti‐tumour immunity was reported by J. Allison's group in 1996 where in vivo administration of anti‐CTLA‐4 antibody induced the rejection of established murine colon carcinoma (Leach et al., 1996). Subsequently, anti‐CTLA‐4 treatment was tested in several highly immunogenic murine tumour models including prostatic carcinoma, lymphoma, and renal carcinoma (Kwon et al., 1997b). Anti‐CTLA‐4 treatment was shown to enhance anti‐tumour responses by CD8+ OT‐I cells against EG.7 Ova‐expressing tumours. The effect appeared to be dependent on CD4+ T cells (Shrikant et al., 1999). Studies with gp100‐specific TCR transgenic mice (Pmel) crossed to the CTLA‐4 KO strain also confirmed that autoimmunity and tumour immunity mediated by these CD8+ T cells required CTLA‐4 –deficient CD4+ T cells (Gattinoni et al., 2006).
As a monotherapy, anti‐CTLA‐4 mAbs failed to promote rejection of established poorly immunogenic tumours leading to a number of studies assessing potential additive or synergistic activity in combination with other approaches. Both conventional approaches such as radio and chemotherapy as well as immune modulatory interventions targeting innate and adoptive immunity were proposed and evaluated (Peggs et al., 2008). Successful outcome brought studies that combined anti‐CTLA‐4 treatment with administration of cytokines that were reported to enhance T cell priming, infiltration of innate cells to tumour or INF‐γ expression. CTLA‐4‐blockade in combination with granulocyte‐macrophage colony stimulating factor (GM‐CSF) – expressing tumour cell‐based vaccine (GVAX) proved to be very efficient in treatment of established tumour e.g. B16 melanoma (Quezada et al., 2006; van Elsas et al., 1999). The GVAX/anti‐CTLA‐4 combined treatment was also shown to elicit potent immune responses that decreased the incidence of prostate cancer in TRAMP mice (Hurwitz et al., 2000). Increased T cell‐mediated immunity was also described when intratumoural IL‐12 application was combined with systemic blockade of CTLA‐4 to cure established Gl261 glioblastoma (Vom Berg et al., 2013).
Despite much investigation, the precise mechanisms underpinning the in vivo activity of anti‐CTLA‐4 mAbs have remained elusive. Whilst the prevailing hypothesis had been that CTLA‐4‐blockade “released the breaks” on effector T cells, the demonstration that the antibody needed to target both Teff (effector T cells) and Treg for maximal anti‐tumour activity supported the potential existence of an additional mechanism of action (Peggs et al., 2009). Most recently, it was demonstrated that in addition to its immune‐modulatory activity on effector T cells, anti‐CTLA‐4 also promoted site‐dependent Treg depletion in vivo. Depletion was restricted to the tumour and mediated by macrophages in the tumour expressing high levels of FcγRIV (Simpson et al., 2013). Interestingly, depletion was not restricted to Tregs but to cells expressing high levels of CTLA‐4 on their surface. Both CD8+ and CD4+Foxp3‐ cells expressed lower levels of surface CTLA‐4, hence favouring depletion of tumour infiltrating Tregs, which express high levels of surface CTLA‐4 (Simpson et al., 2013). Similar observations for other tumour models were published by Selby et al.; the authors showed that only a mIgG2a anti‐CTLA‐4 mAb with capacity for ADCC (antibody‐dependent cell‐mediated cytotoxicity) was able to deplete Tregs‐infiltrating CT26 and M38 tumours but that efficacy was lost once the Fc portion of the mAb was engineered to a mIgG1 isotype with reduced ADCC capacity ((Selby et al., 2013); further reading: (Furness et al., 2014)). Thus, it has been become clear that even if the target of immunotherapy is solely expressed by T cells, the role of tumour microenvironment cannot be underestimated. Characterisation of innate cells infiltrating tumour, especially effector macrophages in terms of expression of Fc receptors will likely be necessary to better understand and predict the outcome of treatment with antibody of a certain isotype.
2.1.3. Anti‐CTLA4 antibody based therapies. Clinical trials with ipilimumab and tremelimumab
Two fully human monoclonal anti‐CTLA‐4 antibodies have entered clinical trials: ipilimumab (MDX‐010, Medarex) and tremelimumab (Pfizer). Up to 42 studies for clinical trials phase I/II and 3 phase III clinical trials with ipilimumab as a single agent or with combined therapies have been completed (http://clinicltrials.gov) (Table 2).
Table 2.
Clinical trials targeting inhibitory checkpoints pathways.
| Target | Antibody/Isotype/Activity | Disease | Therapy (Mono or combained) | Phase/Owner/Sponsor | No. patients | Results | Adverse events and immune‐related adverse events (irAEs) | Reference |
|---|---|---|---|---|---|---|---|---|
| CTLA‐4 | Ipilimumab (IgG1κ, human), Antagonist | Stage IV melanoma | Monotherapy | III (NCT00094653)/Bristol‐Myers Squibb | 676 | ORR: 10,9%; OS: ipilimumab + gp100: 10.0 months, gp100: 6.4 months | 60% (ipilimumab) 32% (gp100) Grade 3–4: 10–15% (ipilimumab) 3% (gp100) Dermatological,gastrointestinal, endocrine and hepatic side effects | Hodi et al., 2010 |
| Melanoma with brain metastasis | Monotherapy | II (NCT00623766)/Bristol‐Myers Squibb/Medarex | 72 | Disease control within the brain: 24% of patients in Cohort A (neurologically asymptomatic patients) and 10% of patients B (symptomatic patients receiving systemic corticosteroids); OS at 1 year (A) 31%, (B) 19%, at 2 years (A) 26% (B) 10% | Grade 3–4 similar in both cohorts: fatigue, diarrhoea, nausea, headache, rash and pruritus | Margolin et al., 2012 | ||
| Stage IV melanoma | Combined (dacarbazine) | III (NCT00324155)/Bristol‐Myers Squibb/Medarex | 502 | ORR: 15.2%; OS: ipilimumam + dacarbazine: 11.2 months vs. dacarbazine 9.1 months, OS at 1 year, respectively (47.3% vs. 36.3%), 2 years (28.5% vs. 17.9%), and 3 years (20.8% vs. 12.2%) | Similar to prior studies with ipilimumab, except higher rate for liver‐function and lower rats for gastrointestinal | Robert et al., 2011 | ||
| Metastatic castration‐resistant prostate cancer | Monotherapy | III (NCT00861614)/Bristol‐Myers Squibb | 799 | No significant effect in terms of overall survival | Grade 3–4: diarrhoea (16% of patients in the ipilimumab group vs 2% in the placebo group), fatigue (11% vs 9%), anaemia (10% vs 11%), and colitis (5%vs 0). 4 (1%) deaths from toxic effects of the ipilimumab | Kwon et al., 2014 | ||
| Tremelimumab (IgG2, human), Antagonist | Stage IV melanoma | Monotherapy | III (NCT00257205)/Pfizer | 655 | ORR: 10.7%; OS: tremelimumab: 12.6 months, chemotherapy: 10.7 months | Diarrhoea, pruritus and rash in the tremelimumab group, endocrine toxicities: 7.4%, 7 deaths in the tremelimumab group | Ribas et al., 2013 | |
| Anti‐PD‐1 | Nivolumab (IgG4 fully human), Antagonist | Advanced renal carcinoma | VEGFR TKIs (98%) mTOR inhibitors (38%) and immunotherapy (24%) | II (NCT01354431)/Bristol‐Myers Squibb | 168 | 19/35 responders (54%) OR lasting >12–20 + months/Median OS 18.2 months/4.2 months (10 mg kg−1) | Grade 3–4: 17% | Motzer RJ, J Clin Oncol (Meeting Abstracts) 32:5s. |
| Metastatic melanoma (MM) | Monotherapy | III (NCT01721772)/Bristol‐Myers Squibb | 418 | ORR nivolumab group: 40.0%, dacarbazine group: 13.9% At 1 year, OS nivolumab group: 72.9%, dacarbazine group: 42.1% in the/PFS nivolumab group: 5.1 months, dacarbazine group: 2.2 months | Grade 3–4: nivolumab group 11.7%, dacarbazine group 17.6% | Robert et al., 2015 | ||
| Metastatic melanoma | Nivolumab (n = 268) or ICC = dacarbazine or carboplatin + paclitaxel (n = 102) | III (NCT01721746) open‐label trial/Bristol‐Myers Squibb | 268 | ORR 32%/median time to response of 2.1Reduction of ≥50% in target lesion burden occurred in 82% (31/38) of nivolumab responders and 60% (3/5) of responders | Grade 3–4: 9% | Weber JS et al. Eur Soc Med Oncol Congr (Meeting Abstracts) 728. | ||
| Pembrolizumab (Humanised IgG4), Antagonist | MM ipilimumab refractory | Monotherapy | II (NCT01295827)/Merck Sharp & Dohme Corp. | 411 | ORR: 34%, PFS: 5.5 months, OS: 69% at one year and 62% at 18 months | Ribas A, Wolchok JD et al. Soc Melanoma Res (Meeting Abstracts). | ||
| Pidilizumab (CT‐011, Humanised IgG1k), Antagonist | Metastatic melanoma | Monotherapy | II (NCT01435369)/CureTech Ltd | 103 | ORR prior Ipi: 10.0% vs 5.9%, PFS prior Ipi: 2.8 vs 1.9 months, OS at 1 year 64.5%, without Ipi 55.7% B‐RAF V600 WT tumours 69.3% | Fatigue (43%), diarrhoea (22.5%), arthralgia (21%) pneumonia (5%) and dyspnoea (3%) | Atkins et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 9001) | |
| Follicular lymphoma | Rituximab | II (NCT00904722)/M.D. Anderson Cancer Center | 29 | ORR 66%, PFS 21.1%; complete responses in 15 (52%) patients and partial responses in 4 (14%) | Grade 3–4: 0% | Westin et al., 2014 | ||
| CD200 | Samalizumab (ALXN6000, Humanised IgG2/G4 κ), Antagonist | Advanced CLL or Multiple Myeloma | Monotherapy | I/II (NCT00648739)/Alexion pharmaceutics | 26 | 19/20 with 81%–98% reduction in peripheral CD200+ CD4+ T cells, (14/21) with 64%–75% CD200 loss on B‐CLL cells. Overall, 36% (8/22) of evaluable patients experienced at least a 10% reduction in bulky disease | Grade 3–5: anaemia and neutropenia (8%), thrombocytopaenia, reduced visual acuity, respiratory syncytial virus infection and rash (4%) | Alexander, 2011 P T. 2011 Feb; 36(2): 100–103 (Meeting abstract) |
Early phase studies with ipilimumab in previously vaccinated metastatic melanoma and ovarian carcinoma patients (Hodi et al., 2003) demonstrated tolerability and suggested that ipilimumab could amplify a long‐lived memory response in patients, thus justifying further evaluation. Another pilot study using ipilimumab as a monotherapy in patients with metastatic hormone‐refractory prostate cancer showed decrease in prostate‐specific‐antigen (PSA) level ≥ 50% in two out of fourteen patients (Small et al., 2007); for details about phase I and II clinical trials please see review: (Peggs et al., 2008).
In the first randomised phase III controlled trial of ipilimumab 676 patients with unresectable stage III or IV melanoma were recruited to test its activity as a monotherapy or in combination with a peptide vaccine targeting the melanoma associated antigen gp100 (glycoprotein 100). Patients were randomly assigned in a 3:1:1 ratio to receive ipilimumab (3 mg/kg) plus gp100, or single agent ipilimumab or gp100. The median overall survival was 10.1 months among patients receiving ipilimumab compared to 6.4 months in those receiving gp100 alone (HR 0.66; p = 0.003). The response rate was significantly higher in the ipilimumab monotherapy group compared to the gp100 group (10.9% versus 1.5%; p = 0.001). The responses to ipilimumab were durable with the 1‐year and 2‐year survival rates being 46% and 24%, respectively, in the ipilimumab monotherapy group compared to 25% and 14%, respectively, in the gp100 group (Hodi et al., 2010) (Table 2). In keeping with murine studies in CTLA‐4 deficient mice and phase I/II clinical trials, immune‐related adverse effects were observed in up to 60% of the patients, most of them being reversible when appropriate treatment with steroids or other immunosuppressive drugs applied. The outcome of that trial was the basis for the approval of ipilimumab for the treatment of advanced melanoma by the FDA in 2011 and in 2013 by EMA.
Another phase III clinical trial in metastatic melanoma evaluated ipilimumab monotherapy against ipilimumab with dacarbazine treatment (chemotherapy) in previously untreated patients. The combination therapy resulted in higher ORR (>15%) than the dacarbazine treatment only (Robert et al., 2011). Ipilimumab was also tested in melanoma patients with brain metastasis in a phase II clinical trial. Ipilimumab showed activity in some patients particularly when metastases were small and asymptomatic. No unexpected toxic effects in this population were observed (Margolin et al., 2012) (Table 2).
Despite promising results from phase I/II clinical trials with tremelimumab (monotherapy) the phase III randomised trial with advanced melanoma patients failed to demonstrate a statistically significant survival advantage of treatment with tremelimumab over standard‐of‐care chemotherapy in first‐line treatment of patients with metastatic melanoma (Ribas et al., 2013). On the basis of pre‐clinical studies one may hypothesise that the lack of ADCC capacity shown by tremelimumab (IgG2 isotype) could be one of the reasons of its inferior activity to ipilimumab. However, several other points need to be considered: the unexpectedly good outcome in the control arm, possibly due to the enrolment of patients with a more favourable prognosis and use of another CTLA4‐blocking agent (ipilimumab) as salvage therapy for patients in the comparator arm (Ribas et al., 2013).
Clinical trials with CTLA‐4 blockade suggested that conventional Response Evaluation Criteria in Solid Tumours (RECIST) might be not effective as a method for evaluating patients undergoing immune‐modulatory treatment. In initial phases of treatment some patients who subsequently achieved disease response presented with increased tumour growth due to the ongoing inflammatory reaction. Therefore additional criteria have been proposed (immune‐related response criteria (irRC)) that consider total tumour burden regardless of the growth of new disease, with higher maximum tumour growth allowed within the bounds of stable disease (Weber et al., 2012).
Although clinical trials with ipilimumab show promising results the exact mechanism of its action is still not completely resolved. Analysis of PBMC from four NY‐ESO‐1 seropositive melanoma patients treated with ipilimumab demonstrated an expansion of NY‐ESO‐1 antigen‐specific cytotoxic CD4+ T cells that show increased granzyme B and perforin production (Kitano et al., 2013). Recently the analysis of innate cells isolated from peripheral blood from the patient with advanced melanoma treated with ipilimumab revealed an increase of nonclassical CD14+CD16++ monocytes in responding patients. These cells expressed high level of FcγRIIIa and were able to lyse Treg cells in vitro in the presence of ipilimumab (Romano et al., 2015). Earlier reports showed that patients treated with anti‐CTLA‐4 do not have decreased numbers of regulatory T cells when changes in peripheral blood were studied (Maker et al., 2005). Clearly further investigations are required to explain the mechanistic basis of anti‐CTLA‐4 therapies in human malignancies. Since most of the work done so far has focused on peripheral blood, a thorough analysis of tumour‐infiltrating immune cells will be crucial for better understanding of the mechanisms of anti‐CTLA‐4‐mediated therapy. To be able to better predict the in vivo activity of immune‐modulatory antibodies in cancer patients, analysis of target molecule density on tumour‐infiltrating T effector and Treg cells rather than cells present in peripheral blood will likely be vital. Moreover, the different outcomes of clinical trials with anti‐CTLA‐4 antibodies of different isotypes highlight the importance of FcγR‐expressing innate cells in antibody‐mediated immunotherapies.
2.2. The PD‐1/PD‐L1 axis
2.2.1. PD‐1 expression
First identified as a protein upregulated in a murine T‐cell hybridoma undergoing programmed cell death (Ishida et al., 1992), PD‐1 is an IgSF member. PD‐1 possesses an N‐terminal IgV‐like domain, a transmembrane domain and a cytoplasmic tail containing an immunoreceptor tyrosine‐based inhibitory motif (ITIM) and an immunoreceptor tyrosine‐based switch motif (ITSM). PD‐1 is a monomeric molecule on the cell surface lacking the extracellular cysteine found in CD28, CTLA‐4, and ICOS, which allows these proteins to homodimerise (Zhang et al., 2004). PD‐1 is absent on naïve cells but is highly expressed on activated T cells, B cells (Good‐Jacobson et al., 2010), NK cells (Terme et al., 2011) and myeloid‐derived cells (Keir et al., 2008). Its expression can be induced by TCR signalling (Agata et al., 1996) and by the common γ chain cytokines (interleukin‐2 (IL‐2), IL‐7, IL‐15, and IL‐21) (Kinter et al., 2008). Recently, NFATc1 binding site was identified in the PD‐1 promoter region making NFATc1 a critical factor for PD‐1 expression on T cells (Oestreich et al., 2008).
2.2.2. PD‐L1/PD‐L2 expression
Like PD‐1, its two known ligands PD‐L1 (programmed cell death protein 1 ligand 1; B7H1 and CD274) (Dong et al., 1999) and PD‐L2 (B7DC and CD273) (Latchman et al., 2001; Tseng et al., 2001) display a complex expression pattern. PD‐L1 is constitutively expressed at low levels and further up‐regulated upon cell activation on both haematopoietic cells, including T, B, myeloid, and dendritic cells, and non‐hematopoietic cells as in the lung, heart, and especially on a wide range of cancer cells (Chen et al., 2012a; Liang et al., 2003; Rodig et al., 2003; Sznol and Chen, 2013). PD‐L1 can be upregulated on tumours by activation of key oncogenic pathways [phosphoinositide 3‐kinase (PI3K) and mitogen‐activated protein kinase (MAPK)] or by IFN‐γ production in the tumour microenvironment by T‐cell responses (Pardoll, 2012; Parsa et al., 2007; Taube et al., 2012). PD‐L1 hampers antitumour immunity by tolerising tumour‐reactive T cells through PD1 interaction on activated T cells (Dong et al., 2002; Latchman et al., 2004), by rendering tumour cells resistant to CD8+ T cell‐mediated destruction and refractory to apoptosis induced by Fas ligation (Azuma et al., 2008), by promoting the development and maintenance of induced T regulatory cells (Francisco et al., 2009), and by tolerising T cells by reverse signalling through T cell–expressed CD80 (Butte et al., 2007; Park et al., 2010). Interestingly, the bidirectional inhibitory interaction reported between PD‐L1 and CD80 demonstrate that CD80 may serve as a biomarker and functional checkpoint for T cell anergy. Whether this interaction is involved in the immune evasion by tumour cells needs to be further investigated in human cells. Nevertheless, a role of CD80 on T cells as an inhibitory receptor to deliver outside‐in signal is concordant with previous findings in mice, including: increased cytokine productions in CD80‐KO T cells (Schweitzer and Sharpe, 1999), an enhanced severity of graft‐versus‐host disease by CD80/CD86‐KO donor T cells (Taylor et al., 2004), as well as resistance of CD80‐KO T cells to inhibitory effects of Tregs (Paust et al., 2004).
PD‐L2 has a distinct expression pattern and was initially thought to be restricted to macrophages and dendritic cells (DCs) (Latchman et al., 2001). Recently, however, PD‐L2 expression has been extended to some solid tumours, such as ovarian carcinoma (Hamanishi et al., 2007), small cell lung cancer (Konishi et al., 2004), Oesophageal cancer (Ohigashi et al., 2005) and tumour‐associated‐fibroblast, enhanced by treatment with IFN‐γ (Nazareth et al., 2007). Furthermore, PD‐L2 has also been found on activated CD4+ and CD8+ T cell subsets that bring an additional level of complexity. Engagement of PD‐L2, predominantly expressed on T helper type 2 cells (Th2), was able to down‐modulate cytokine production and cell proliferation (Messal et al., 2011; Lesterhuis et al., 2011). It has been demonstrated that PD‐L1 and PD‐L2 cross‐compete to bind to PD‐1 (Ghiotto et al., 2010) although the relative affinity of PD‐L2 to PD‐1 is 2–6‐fold higher than that of PD‐L1 (Youngnak et al., 2003). However, PD‐L2 is generally expressed at a lower level, which favours PD‐L1 as the primary binding ligand of PD‐1, except for Th2 responses.
2.2.3. PD‐1 signalling pathway‐molecular mechanisms
It was demonstrated that the signalling pathway initiated by interactions of PD‐1 with its ligands (PD‐L1, PD‐L2) depends on the phosphorylation of two tyrosines at the intracellular tail of PD‐1. The recruitment of SH2‐domain containing protein tyrosine phosphatases (SHP‐1 and/or SHP‐2) to the ITSM cytoplasmic region of PD‐1 then inhibits downstream signals of the T‐cell receptor, particularly PI3K/AKT activation (Riley, 2009; Okazaki et al., 2001; Plas et al., 1996). Genetic ablation of PD‐1 in mice has highlighted its crucial function in maintaining peripheral tolerance and T cell‐exhaustion. PD‐1‐deficient mice present various autoimmune conditions, including autoantibody‐induced cardiomyopathy (Nishimura et al., 2001; Okazaki et al., 2003), arthritis and lupus‐like disease (Nishimura et al., 1999) and type 1 diabetes (Wang et al., 2005; Yao et al., 2013). In the periphery, these suppressive functions of PD‐1 seem to be mediated by PD‐L1 (Ansari et al., 2003; Tsushima et al., 2007). This crucial function of the PDL‐1/PD‐1 axis in the control of human T cell activation can be exploited by tumour cells (Zou and Chen, 2008). Indeed, tumour‐induced PD‐Ls utilise several mechanisms to suppress and facilitate the evasion of host immune surveillance, including the promotion of T cell anergy and exhaustion (Crespo et al., 2013), unresponsiveness and apoptosis (Dong et al., 2002) and inducing the expansion of Treg cells (Amarnath et al., 2011; Wang et al., 2008). Moreover, upon PD‐1 ligation, PD‐L1 expressing tumours can receive an anti‐apoptotic signal, which renders them resistant to lysis by cytotoxic T lymphocytes (CTLs) and FAS‐induced apoptosis (Azuma et al., 2008; Dong et al., 2002; Hirano et al., 2005; Yao and Chen, 2006).
2.2.4. PD‐1 pathway blockade and clinical relevance
PD‐1 blockade in vivo by specific mAbs promotes CTLs expansion (Strome et al., 2003) and can reduce tumour growth or induce spontaneous tumour rejection (Blank et al., 2004; Crespo et al., 2013; Kryczek et al., 2006). PD‐1 blockade is clinically relevant since exhausted PD‐1+CD8+ T cells have been found in patients with melanoma (Baitsch et al., 2011), ovarian cancer (Curiel et al., 2003), hepatocellular carcinoma (Wu et al., 2009) and prostate cancer (Sfanos et al., 2009). CD4+ tumour‐infiltrating lymphocytes (TILs) also showed high expression of PD‐1 associated with impaired effector functions. Moreover, PD‐1 expression has shown prognostic value in breast cancer (Ahmadzadeh et al., 2009; Ghebeh et al., 2008; Gu‐Trantien et al., 2013). Interestingly, tumour‐infiltrating T cells exhibited persistently up‐regulated expression of the activator protein 1 (AP‐1) subunit c‐Fos during tumour progression. This unexpected immunosuppressive effect of c‐Fos was mediated through the induced expression of PD‐1 via the direct binding of c‐Fos to the AP‐1‐binding site in the Pdcd1 (gene encoding PD‐1) promoter (Xiao et al., 2012a). Because many tumours are highly infiltrated with Treg cells, PD‐1 blockade may also enhance anti‐tumour immune responses by diminishing their number and/or suppressive activity (Pardoll, 2012). PD‐L1 blockade, however, has been shown to improve myeloid dendritic cell‐mediated (MDC) anti‐tumour immunity through enhanced MDC‐mediated T cell activation, accompanied by downregulation of T‐cell IL‐10 and upregulation of IL‐2 and IFN‐γ (Curiel et al., 2003).
2.3. Clinical trials
There are currently 101 clinical trials investigating PD‐1 and PD‐L1 blocking clinical efficacy in a variety of cancers. First evaluated in large phase 1 studies, nivolumab (BMS‐936558) and pembrolizumab (MK‐3475), humanised‐monoclonal immunoglobulin G4 (IgG4) antibodies to PD‐1, demonstrated durable response rates with acceptable toxicity in patients with advanced melanoma, non‐small‐cell lung cancer (NSCLC), renal cell carcinoma or Hodgkin lymphoma (Ansell et al., 2015; Hamid et al., 2013; Topalian et al., 2014). Nivolumab and pembrolizumab are now both approved by the FDA for the treatment of patients with melanoma who have received ipilimumab and, if relevant, a BRAF inhibitor. Recently, nivolumab demonstrated a higher rate of objective response than dacarbazine in a phase III study in patients with ipilimumab‐refractory metastatic melanoma, thus improving the overall survival of patients treated with immunotherapy compared to chemotherapy (Robert et al., 2015) (Table 2). Nivolumab is now being evaluated in a large phase III trial in patients with other solid tumours, such as non–small cell lung cancer and renal cell carcinoma (Topalian et al., 2012). Although most of the clinical trials have investigated PD‐1 and PD‐L1 clinical efficacy in patients with solid tumours, anti–PD‐1 therapies have also demonstrated promising outcomes in patients with haematologic malignancies, such as Hodgkin Lymphoma (Ansell et al., 2015; Berger et al., 2008; Lesokhin et al., 2015). BMS‐936559, an anti‐PDL1 humanised IgG4 mAb was evaluated in a total of 207 patients with advanced cancers, including NSCLC, melanoma, and renal‐cell cancer. It induced durable tumour regression at 24 weeks in a number of these patients (6–17%) (Brahmer et al., 2012).
2.4. TIGIT
The co‐inhibitory molecule TIGIT (T cell immunoglobulin and immunoreceptor tyrosine‐based inhibitory motif [ITIM] domain, also known as Vstm3, Vsig9 or WUCAM) is a protein of the IgSF and consists of two ITIMs, a transmembrane domain and an immunoglobulin variable (IgV) domain (Levin et al., 2011; Yu et al., 2009). TIGIT is partially conserved between species and has 58% homology between mouse and human amino acid sequences (Yu et al., 2009). TIGIT expression patterns are also similar between both species: it is expressed on various T cell subsets such as resting and activated Tregs, activated CD8+ and CD4+ effectors and particularly on memory T cells (Boles et al., 2009; Levin et al., 2011; Stanietsky et al., 2009; Yu et al., 2009). Furthermore, TIGIT expression is associated with other exhaustion markers such as PD‐1 and TIM‐3 on murine melanoma specific CD4+ T effector and Treg cells during tumour recurrence (Goding et al., 2013). Similarly, Johnston and colleagues recently demonstrated that high TIGIT expression strongly correlated with PD‐1 expression on exhausted CD8+ and CD4+ T cells but could not be detected on non‐haematopoietic, myeloid or tumour cells (Johnston et al., 2014) (Table 1).
TIGIT binds with high affinity to Ig‐like molecules called nectins, such as CD155 (poliovirus receptor (PVR), NECL5) and CD112 (PVRL2, NECTIN‐2) and with lower affinity to CD113 (PVRL3, NECTIN‐3) (Yu et al., 2009). TIGIT outcompetes CD226 and CD96 for the binding of CD155 due to its higher binding affinity for the two ligands (Clark et al., 2009; Stanietsky et al., 2013; Yu et al., 2009). CD155, CD112 and CD113 demonstrate similar expression patterns: they are expressed on fibroblasts, endothelial and epithelial cells, on dendritic cells (DCs) and also on many human tumours (Carlsten et al., 2007, 2009, 2004, 2009, 2007, 2006, 2002, 1998, 2012, 2013, 2015, 2006, 2005, 2004, 2005). The third TIGIT ligand CD113 is, however, the only Nectin molecule expressed on T cells (Devilard et al., 2013).
There are two separate T cell inhibitory mechanisms mediated by TIGIT: one T cell intrinsic and the other dependent on interaction with its ligand on APCs (Joller et al., 2011; Yu et al., 2009). Engagement of TIGIT by one of its ligands or an agonistic antibody results in the inhibition of CD4+ T cell proliferation and pro‐inflammatory cytokine production in vitro and in vivo (Joller et al., 2011; Levin et al., 2011; Yu et al., 2009). TIGIT can additionally directly inhibit cytotoxic activity of human and murine NK cells (Li et al., 2014; Stanietsky et al., 2009). In human peripheral blood, interaction of TIGIT with CD155 on monocyte derived DCs has been shown to increase their IL‐10 and decrease IL‐12, IL‐6 and IL‐18 secretion (Yu et al., 2009). T cell intrinsic function of mouse and human TIGIT was demonstrated by the use of agonistic antibodies that can directly suppress T cell proliferation and production of pro‐inflammatory cytokines in the absence of APCs (Joller et al., 2011; Levin et al., 2011). In humans, engagement of TIGIT with an agonistic mAb inhibited the expression of early activation markers CD25 and CD69 and the production of IL‐2 and IFN‐γ (Levin et al., 2011). In mice, it was shown on transcriptional level that TIGIT suppresses T cell responses via down‐regulation of the expression of the TCR alpha chain and TCR components while simultaneously up‐regulating the expression of the anti‐apoptotic molecule BCL‐XL and cytokine receptors important for T cell maintenance such as IL‐2R, IL‐7R and IL‐15R (Joller et al., 2011; Levin et al., 2011; Lozano et al., 2012). TIGIT is also capable of inhibiting primary human and murine CD8+ T cell function by preventing the homodimerisation of the costimulatory receptor CD226 in cis and thereby impairing its function (Johnston et al., 2014). An anti‐TIGIT blocking antibody disrupts the TIGIT:CD226 interaction and restores CD226 homodimerisation and thus its co‐stimulatory function (Grogan et al., 2015).
There are distinct similarities between the CTLA‐4/CD28 axis and the relationship between TIGIT and CD226: TIGIT is upregulated upon T cell activation and is able to outcompete the activating receptor CD226. TIGIT might therefore contribute to suppression of immune responses and autoimmunity in a similar fashion as CTLA‐4. Chan and colleagues suggest the use of a TIGIT blocking antibody for immunotherapy for cancers in which T cell, CD155 and CD226 play an important role in controlling the tumour. It is not clear, however, if a blocking TIGIT mAb would be beneficial in the context of an NK cell‐controlled tumours (Chan et al., 2012). Although TIGIT was shown to be widely expressed on murine Tregs (Levin et al., 2011; Yu et al., 2009), recent reports illustrated that a specific Th1‐ and Th17‐ (but not Th2) response suppressing a subset of activated Tregs displayed significantly increased TIGIT expression (Joller et al., 2014). Upon TIGIT engagement, this Treg subset showed increased production of the anti‐inflammatory cytokine IL‐10, which specifically inhibits Th1 and promotes Th2 responses (Chan et al., 2003; Joller et al., 2014). An increased Th2 cytokine production was also observed when human T cells were cultured with TIGIT+ Tregs in vitro (Joller et al., 2014). Interestingly, TIGIT was also found to be expressed on another human Treg subset, Helios+ memory Tregs (Bin Dhuban et al., 2015). Furthermore, the TIGIT locus was found to be hypomethylated in activated human Tregs, facilitating increased TIGIT expression. It was therefore suggested that Foxp3 binding and demethylation can coordinate TIGIT expression in this T cell subset (Zhang et al., 2013).
Transgenic mice expressing TIGIT on T and B cells and TIGIT deficient mice do not develop any spontaneous autoimmune disorders (Joller et al., 2011; Levin et al., 2011). However, T cells from TIGIT KO mice produce more IL‐6, IFN‐γ and IL‐17 upon activation in comparison to wild‐type (wt) cells in model of induced autoimmune encephalitis (Burton et al., 2014; Joller et al., 2011). These results suggest that TIGIT limits autoimmunity and contributes to the development and maintenance of peripheral tolerance but is not indispensable in steady state immunity. It was recently demonstrated in a murine model of chronic LCMV infection that TIGIT was crucial in the regulation of inflammatory cytokine (IFN‐γ and TNFα) production of exhausted or chronically stimulated CD8+ and CD4+ T cells (Grogan et al., 2015). Grogan and colleagues further suggest that although there is a synergy between the inhibitory activity of PD‐1 and TIGIT, the latter does not exert an extensive inhibitory effect as PD‐1 but has a specific role of suppressing T cell effector function and cytokine production (Grogan et al., 2015). Several reports demonstrate that tumour infiltrating CD8+ T cells often co‐express TIGIT with PD‐1 (Chauvin et al., 2015; Johnston et al., 2014).
TIGIT is highly expressed at the RNA level in solid tumours such as breast carcinoma, colon adenocarcinoma, renal clear cell carcinoma, uterine corpus endometrioid carcinoma and expression correlates with CD8+ T cell infiltration of these tumours (Cancer Genome Atlas, 2012, 2012, 2012, 2013, 2014). These results were confirmed at protein level in murine models (mammary carcinoma EMT6 and colon carcinoma CT26) and tumour samples from patients with NSCLC or colorectal carcinoma. Johnston and colleagues further demonstrated that simultaneous blockade of TIGIT and PDL‐1 resulted in rejection of CT26 tumours and that 70% of the treated mice displayed long term complete tumour eradication (Johnston et al., 2014). CD8+ but not CD4+ tumour reactive T cells from anti‐TIGIT and anti‐PD‐L1 treated mice showed increased production of both TNFα and IFN‐γ. Interestingly, the effect of anti‐TIGIT and anti‐PD‐L1 treatment could be partially reversed by co‐injecting anti‐CD226, suggesting that TIGIT suppression of CD8+ T cell responses is dependent on CD226 (Johnston et al., 2014). Combination of TIGIT and PD‐L1 blocking antibodies also promoted complete remission of both MC38 (fibrosarcoma) and EMT6 tumours in in vivo (Grogan et al., 2015).
There are currently two patents published which support the role of modulation of TIGIT signalling in immunotherapy. Genentech's first patent suggests and outlines the suitability of blocking TIGIT in the treatment of immune related diseases such as psoriasis, arthritis, inflammatory bowel disease and breast cancer, and using aberrant TIGIT expression or activity as diagnostic tool for these diseases (Clark et al., 2009). The most recent patent by Genentech describes the efficacy of a combination treatment with an anti‐PD‐1 or anti‐PD‐L1 antibody (MPDL3280A) and an inhibitor for TIGIT expression/activity to treat or delay the progression of cancer or and immune related disease. The agents used to modulate TIGIT expression and/or activity were an inhibitory mAb, a Fab fragment, an aptamer, a small molecule inhibitor, an inhibitory nucleic acid or polypeptide (Grogan et al., 2015). Collectively, these data indicate that TIGIT shows great promise as one of the next targets in trials for immunotherapy in, for example, metastatic melanoma and breast cancer.
2.5. VISTA/PD‐1H (V‐domain immunoglobulin (Ig)‐containing suppressor of T‐cell activation)
VISTA (also called PD‐1H, Dies1 or Gi24) is a type I transmembrane protein with a single extracellular IgV domain containing three additional cysteine residues that are not present in other Ig superfamily members (Wang et al., 2011). Human and murine VISTA share 90% homology and are constitutively expressed on haematopoietic cell subsets, including T cells, macrophages and dendritic cells (Lines et al., 2014a). VISTA is present at a greater density on CD11bhigh myeloid cells than on CD4+, CD8+ T cells and Tregs (Flies et al., 2011; Le Mercier et al., 2014) (Table 1). While the extracellular domain of VISTA shares significant sequence homology (24%) with the B7 family ligand PD‐L1, it displays a distinct expression pattern. VISTA is not found on B cells, NK cells or non‐hematopoietic tumour cells (Flies et al., 2011; Le Mercier et al., 2014; Wang et al., 2011). First identified as an inhibitory ligand on APCs, suppressing CD4+ and CD8+ T cell proliferation and cytokine production, VISTA is now also considered as an inhibitory receptor on T cells (Flies et al., 2014; Wang et al., 2011). However, the binding partner(s) of VISTA responsible for the potential reverse signalling between T cells and APCs remains unknown (Lines et al., 2014b). Both naïve and antigen‐stimulated cells are sensitive to VISTA‐induced suppression, suggesting a constitutive expression on resting T cells (Lines et al., 2014b; Wang et al., 2011). Administration of an antagonistic VISTA mAb was shown to enhance proliferation and effector molecule production (i.e. IFN‐γ and granzyme B) in CD8+ T cells (Le Mercier et al., 2014). Similarly, experiments using VISTA–KO mice indicate that VISTA expressed on CD4+ T cells limits both their activation and function (Wang et al., 2011). Human and murine VISTA appear to have indistinguishable functional properties. First, VISTA was found to induce a durable suppression of human CD4+ and CD8+ T cell proliferation and to reduce the production of IL‐10, TNFα (both CD4+ and CD8+ T cells) and IFN‐γ (CD4+ T cells only) (Le Mercier et al., 2014; Lines et al., 2014a). Additionally, VISTA‐Ig fusion protein was effective at suppression of memory (CD45RO+) and effector (CD27−) CD4+ T cell subsets (Lines et al., 2014b). Finally, under conditions promoting T cell proliferation, an enhanced conversion of murine naïve T cells into Foxp3+ T cells was observed in vitro, an effect also seen on human CD4+ T cells treated with human‐VISTA‐Ig (Lines et al., 2014a).
Regarding VISTA function in vivo, depletion of CD4+ T cells in a GL261 brain tumour model has been shown to suppress the radiotherapy‐induced antitumour effect in VISTA–KO mice whereas depletion of CD8+ T cells had no impact on tumour growth or on the overall survival (Flies et al., 2014). Importantly, both human and murine VISTA are predominantly expressed on tumour‐infiltrating leukocytes such as myeloid derived suppressor cells, tumour associated macrophages and dendritic cells (Lines et al., 2014a). VISTA blockade enhances antitumour immunity in multiple tumour models as shown by suppression or delay of tumour growth of the MB49 bladder cancer cells, the B16‐OVA melanoma and the poorly‐immunogenic B16‐BL6 melanoma (Le Mercier et al., 2014). A reduction of tumour‐infiltrating monocytic myeloid‐derived suppressor cells (CD11b+Gr1+CD11C− MDSCs) was observed as well as an increase of tumour‐infiltrating CD4+ and CD8+ T cells.
Thus, VISTA's immunosuppressive and immunoregulatory functions, as well as its consistent expression on leukocytes within tumours make it a relevant target with a broad spectrum of clinical applications. As with PD‐L1, it would be interesting to assess VISTA expression on haematological cancer cells. Indeed, PD‐L1 overexpression, reported in multiple myeloma, Hodgkin Lymphoma or AML was associated with invasiveness and tumour cell resistance to T lymphocyte cytotoxicity (Iwai et al., 2002; Yamamoto et al., 2008). A clinical trial conducted with a PD‐L1 blocking antibody in patients with various haematologic malignancies also demonstrated some evidence of clinical activity (Berger et al., 2008). As VISTA displays significant homology with PD‐L1 and its expression is limited to hematopoietic cell subsets, a blocking antibody could also be an effective therapeutic strategy. Importantly, treatment with VISTA‐specific mAb has already shown a therapeutic value in manipulating immune responses, as a strategy to prevent the induction of GVHD (Flies et al., 2011).
Although VISTA has been observed within the tumour microenvironment (TME) in human tumours, a comprehensive study of the correlation of VISTA expression with patient outcome in different tumour types is warranted. Antibodies targeting VISTA for cancer immunotherapy are already under development by Johnson & Johnson and VISTA may soon be a part of the immunotherapy revolution (Lines et al., 2014b). Moreover, VISTA, PD1, and CTLA4 appear to induce distinct signalling pathways rendering the multiple blockade agents potentially synergistic.
2.6. LAG‐3
Human LAG‐3 (CD223, lymphocyte activation gene 3) is a type I membrane protein belonging to the IgSF. LAG‐3 forms four extracellular IgSF domains and its structure resembles the CD4 molecule. Genes encoding both proteins share 20% homology and show similar exon/intron organisation and chromosomal location, suggesting that both proteins are closely related (Triebel et al., 1990). Murine LAG‐3 also resembles CD4 in structure and sequence (Miyazaki et al., 1996; Workman et al., 2002).
Initially, it was shown that LAG‐3 was expressed by activated T and NK cells (Triebel et al., 1990). More detailed analysis demonstrated that mouse LAG‐3 is expressed by a small fraction of memory like T cells, 20% of γδ T cells and around 10% of NK cells. Both CD4+ and CD8+ T cells upregulate LAG‐3 expression upon activation in vitro (Workman et al., 2002). LAG‐3 is particularly highly expressed by activated regulatory T cells, although this was demonstrated at the mRNA rather than at the protein level (Huang et al., 2004). Exhausted CD8+ T cells in an LCMV infection model were also shown to express LAG‐3 highly along with PD‐1 (Wherry et al., 2007) (Table 1).
As suggested by the similarity to CD4, the MHC‐II molecule was considered an obvious candidate for a LAG‐3 ligand. Indeed, both human and mouse LAG‐3 were shown to interact with MHC‐II molecules with even higher affinity than CD4 (Baixeras et al., 1992; Workman et al., 2002). These discoveries suggested that LAG‐3 might be involved in regulation of T‐cell mediated immune responses. Results of the first in vitro studies showed that LAG‐3 mediates an inhibitory signal to T cells as blocking of LAG‐3 by a specific antibody increased proliferation of human T cells (Huard et al., 1994). Also the ectopic expression of LAG‐3 on mouse CD4+ T cells reduced their proliferation (Huang et al., 2004). Relatively high expression of LAG‐3 by Treg cells raised the question of possible involvement of this molecule in Treg homoeostasis and function. Indeed, the group of D. Vignalli demonstrated that LAG‐3 deficient Treg cells exhibit reduced regulatory activity (Huang et al., 2004). However, LAG‐3 KO mice do not develop spontaneous lymphoproliferative disease, in contrast to CTLA‐4 KO or CTLA‐4 Treg specific KO mice (Kajsa, 2008; Miyazaki et al., 1996; Workman and Vignali, 2005). In older LAG‐3 deficient animals, a cells‐intrinsic, Treg independent expansion of both CD4+ and CD8+ T cells was observed (Workman and Vignali, 2005).
Mechanistic studies on LAG‐3 function showed that it associates with TCR/CD3 complexes on T cells and LAG‐3 cross‐linking inhibited the calcium response to CD3 stimulation (Hannier et al., 1998; Hannier and Triebel, 1999) but the downstream signalling from LAG‐3 remains largely unknown. It was established that the regulation of T cell homoeostasis by LAG‐3 requires the conserved KIEELE motif in the cytoplasmic tail of the protein (Workman and Vignali, 2005). In 2001, the LAG‐3‐associated protein (LAP) was identified in activated human T cells and shown to bind to the Glu‐Pro (EP) repeated motifs in the intracellular domain of LAG‐3 (Iouzalen et al., 2001). Further binding partners of either LAP or LAG‐3 have not been discovered so far.
LAG‐3 appears to have a more complex role in immune homoeostasis than just inhibiting T cell activation. Binding of LAG‐3 to MHC‐II positive monocytes or dendritic cells leads to their activation and an increase of chemokine production (MDC, CCL22, TARC, CCL17) (Avice et al., 1999; Annunziato et al., 1996). LAG‐3 also induces the upregulation of the cell surface receptors CD40, CD80 and CD86 and the expression of the maturation marker CD83 on monocyte‐derived DCs. Furthermore, it was demonstrated that DCs activation mediated by MHC‐II engagement of LAG‐3 occurs through the kinase p72syk (Andreae et al., 2003). Interestingly, MHC‐II can be also expressed by certain types of tumour cells. Interaction of LAG‐3 with MHC II expressed on melanoma cells upregulates both MAPK/Erk and PI3K/Akt pathways, albeit with different kinetics. Inhibition studies using specific inhibitors of both pathways provided evidence of their involvement in the LAG‐3–induced protection from apoptosis (Hemon et al., 2011). The engagement of LAG‐3 with MHC‐II on tumour cells may therefore provide a survival signal to them.
In addition to the transmembrane protein, soluble LAG‐3 (sLAG‐3) formed by an alternatively spliced RNA can be detected in the serum of healthy individuals (Annunziato et al., 1996). Soluble LAG‐3 was shown to bind MHC‐II and activate APCs but it is not clear if sLAG‐3 has any direct impact on T cells. One could assume that binding of sLAG‐3 to MHC‐II blocks interaction of LAG‐3 with MHC‐II thus indirectly preventing T cell inhibition. The first attempts of using LAG‐3 in immunotherapies focused on the adjuvant function of the soluble form of LAG‐3 rather than on blocking its signalling to T cells. Multiple studies with sLAG‐3‐Ig (soluble LAG‐3 fused to human Ig) were performed with different mouse tumour models. Co‐administration of LAG‐3‐Ig with irradiated tumour cells [MCA205 (murine sarcoma), TS/A (mammary adenocarcinoma) and RENCA – (renal adenocarcinoma)] reduced the growth of primary tumours (Prigent et al., 1999). Addition of LAG‐3 to cultured human CD8+ T cells isolated from PBMCs from patients with malignancies increased the expansion of antigen specific T cells in vitro (Casati et al., 2006). Furthermore, soluble LAG‐3 was found to be a good prognostic factor in human breast cancer expressing oestrogen or progesterone receptors. High pre‐treatment serum level of sLAG‐3 serves as a marker for favourable prognosis for patients with this type of malignancy (Triebel et al., 2006).
Immutep (IMP321, soluble LAG‐3‐Ig fusion protein) entered clinical trials involving patients with advanced malignancies. Phase I trial with patients with advanced renal cell carcinoma showed an increase in numbers of activated CD8+ T cells in the group treated with IMP321 (Brignone et al., 2009). IMP321 was also tested in combination with chemotherapies or other immune therapies: sLAG‐3‐Ig together with paclitaxel was used in patients with metastatic breast carcinoma, and after a few months of application the group treated with IMP321 showed increased numbers of activated APCs and cytotoxic NK and CD8+ T cells (Brignone et al., 2010). The encouraging results from the phase I clinical trials supported further combined therapies with Immutep.
In parallel, antibody‐based therapies focusing on blocking the interaction between T cell‐expressed LAG‐3 with MHC‐II molecule expressed by APCs or tumour cells were developed. It was shown that TILs, especially CD8+ T cells, infiltrating murine renal cell carcinoma and melanoma express LAG‐3 (Demeure et al., 2001). Blocking of LAG‐3 with a specific antibody or genetic ablation of LAG‐3 in a mouse model of prostate cancer (ProHA × TRAMP) resulted in an accumulation of antigen specific, activated CD8+ T cells and the observed effect was independent of the presence of CD4+ T cells (Grosso et al., 2007). The impact of LAG‐3 on CD8+ T cells has been somewhat puzzling, as these cells do not bind to MHC‐II molecules. One possible explanation for these results would be the existence of an alternative ligand. Recently it was reported that LAG‐3 also binds Galectin‐3, a 31 kDa lectin. Depletion of Galectin‐3 resulted in an expansion of CD8+ T cells in the NT2.5 mammary tumour. In addition, galectin‐3 mediated suppression on IFN‐γ production by T cells was shown to be dependent on the interaction with LAG‐3 but not with PD‐1 (Kouo et al., 2015).
A set of human LAG‐3 binding antibodies was patented first by Medarex and the optimised version of the antibodies by Bristol‐Myers Squibb. A phase I clinical trial is currently testing single agent therapy with BMS‐986016 (anti‐LAG‐3 antibody, Bristol‐Myers Squibb, source: Clinicaltrial.gov) in patients with relapsed or refractory chronic lymphocytic leukaemia, lymphomas and multiple myeloma. The same agent is being evaluated in combination with nivolumab (anti‐PD1) in advanced solid tumours (Clinicaltrial.gov). LAG‐3, with its high expression on regulatory and activated T cells, has a great potential to be an important target for antibody‐based cancer immunotherapies.
2.7. TIMs (T cell Ig and mucin domain)
In mice, the TIM gene family is represented by four members (TIM‐1–TIM‐4) and four additional predicted genes (TIM‐5–TIM‐8) clustering on chromosome 11B1.1. In human, only three members (TIMs 1, 3 and 4) have been identified so far and they are expressed on chromosome 5q33.2. (Meyers et al., 2005b). The TIM molecules are type I transmembrane proteins, containing a single IgV domain followed by a variable length mucin domain and cytoplasmic tail with tyrosine‐based signalling motif, except for TIM‐4 (Chattopadhyay et al., 2009).
TIM‐1/TIM‐2 and TIM‐3 are expressed on T cells, while TIM‐4 is primarily expressed on antigen‐presenting cells (Rodriguez‐Manzanet et al., 2009). TIM‐1 is expressed on CD4+ T cells after activation and its expression is preferentially sustained in Th2 cells (Meyers et al., 2005a) (Table 1). TIM‐4 is the ligand for TIM‐1 and both are known to deliver co‐stimulating signals to T cells. The administration of soluble TIM‐1−Ig and TIM‐4−Ig in vivo induces hyperproliferation of T cells (Meyers et al., 2005a) and the use of agonist antibody to TIM‐1 upregulates the production of IL‐4 and IFN‐γ in unpolarised T cells (Umetsu et al., 2005). Nevertheless, an inhibitory potential for both TIM‐1 and TIM‐4 has been reported. They have been shown to inhibit the Th1 and Th17 responses of CD4+ T cells (Mizui et al., 2008) through a still unknown mechanism but independently of each other (Cao et al., 2011; Xiao et al., 2011). Interestingly, TIM‐1 seems to play a critical role in maintaining the suppressive function of regulatory B cells (Bregs) as shown by impaired IL‐10 production in B cells, associated with autoimmune disease in Tim‐1Δmucin mice (Xiao et al., 2012b). Of note, TIM‐1, TIM‐3 and TIM‐4 are phosphatidylserine (PtdSer) receptors involved in the engulfment of apoptotic cells and intercellular signalling with exosomes (DeKruyff et al., 2010; Nakayama et al., 2009). Yet, the importance of such interactions in the regulation of T cell responses as well as the TIM‐1/TIM4 inhibitory activity remain unclear.
It is now well established that TIM‐3 functions as a negative immune‐checkpoint molecule. This activation‐induced inhibitory molecule is involved in tolerance and induces T‐cell exhaustion in chronic viral infection and cancers (Jones et al., 2008; Sabatos et al., 2003; Sanchez‐Fueyo et al., 2003). TIM‐3 is selectively expressed on a subset of murine IFNγ‐secreting Th1 CD4+ and CD8+ T cells but not Th2 cells (Monney et al., 2002). In human, it is also expressed on a subset of activated CD4+, CD8+ and at lower levels on Th17 cells (Chen et al., 2006; Hastings et al., 2009; Nakae et al., 2007). Ex vivo stimulation indicates that Tim‐3 is more easily induced on CD8+ than on CD4+ T cells (Jones et al., 2008; Mujib et al., 2012). Furthermore, TIM‐3 expression can also be upregulated independently of TCR or antigenic stimulation in an inflammatory environment rich in cytokines (IL‐2, IL‐7, IL‐15, IL‐21), an effect that is blocked by a γc cytokine receptor‐neutralising antibody (Mujib et al., 2012) (Table 1). Additionally, TIM‐3 proteins can be produced in a soluble form or can be shed from the cell surface, cleaved by membrane‐associated proteases (Geng et al., 2006; Moller‐Hackbarth et al., 2013). As a cell‐free ligand, sTIM‐3 can still significantly impair T cell anti‐tumour immunity, as shown by decreased anti‐tumour CTL activity and reduced amount of tumour‐infiltrating lymphocytes (Geng et al., 2006).
In addition to the interaction with PtdSer, the IgV domain of TIM‐3 can bind to the alarmin protein HMGB1 (High‐Mobility Group Box 1) and suppress the activation of dendritic cells associated with tumours. TIM‐3 and HMGB1 interaction blocks the trafficking of nucleic acids into endosomes, thus decreasing toll like receptor stimulation (Chiba et al., 2012; Gorman and Colgan, 2014). Whether HMGB1 is being sequestered by TIM‐3‐expressing T cells or if such contact regulates T cell responses remains unknown.
A study from Zhu et al. was the first to demonstrate that galectin‐9, a soluble protein with specificity for carbohydrate chains, can be a ligand for TIM‐3, impacting T cells responses. TIM‐3 can indeed bind to galectin‐9 via its two carbohydrate recognition domains. This interaction has been shown to be critical for inducing cell death of antigen specific CD4+ (Zhu et al., 2005) and CD8+ T cells (Sehrawat et al., 2010), a process that is reduced by the ligation of Bat3 (leucocyte antigen B‐associated transcript 3; also known as BAG6) to the intracellular tail of TIM‐3 (Rangachari et al., 2012; Sabatos et al., 2003; Saresella et al., 2014). Even though galectin‐9 can mediate a calcium–calpain–caspase‐1 dependent apoptosis of Th1 cells (Kashio et al., 2003), the cell death is not completely abolished in TIM‐3‐deficient cells, suggesting that an additional receptor is possibly involved in this mechanism (Zhu et al., 2005). As galectins can induce both necrosis and apoptosis, the Fas–Fas ligand pathway has been considered as the supplementary target (Vercammen et al., 1998). A subsequent study demonstrated that galectin‐9 can induce cytokine secretion and apoptosis of Th1 and Th2 T cells independently of TIM‐3 (Su et al., 2011). A further report, however, provided evidence refuting a role for interactions between galectin‐9 and TIM‐3 in regulating T cell responses (Leitner et al., 2013). Finally, another mechanism explaining the TIM‐3/galectin‐9 mediated indirect inhibition of Th1 immune responses is the expansion of MDSCs resulting from TIM‐3 overexpression on T cells (Dardalhon et al., 2010; Sabatos et al., 2003). Galectin‐9 expression is widely distributed in tissues involved in the immune system and was first identified as a tumour antigen in Hodgkin lymphoma (Tureci et al., 1997). Interestingly, galectin‐9 levels vary when comparing normal and tumour tissue. A low or decreased expression has been reported in several solid tumours including breast, lung, melanoma, renal, adrenal and prostate tumour cells (Irie et al., 2005) while a high or increased galectin‐9 expression has been noted in leukaemia and colon cancer cell lines (Lahm et al., 2001). Altogether, most data demonstrate an inverse relation between galectin‐9 expression and cancer progression for the majority of tumours (Heusschen et al., 2013; Jiang et al., 2013). Expression of galectin‐9 expression and its splice variant remain to be characterised in the tumour microenvironment, and whether the loss of galectin‐9 allows tumours to escape from immune control needs to be investigated.
It has been shown that the TIM‐3 IgV domain interacts with at least one other ligand, expressed on CD4+, CD8+, regulatory T cells, B cells, macrophages, and dendritic cells (Cao et al., 2007). Recently, Huang et al., demonstrated that TIM‐3 co‐localises with the carcinoembryonic antigen cell adhesion molecule 1 (CEACAM1) (Huang et al., 2015), expressed on activated CD4+ and CD8+ T cells and known to be involved in T‐cell inhibition (Boulton and Gray‐Owen, 2002; Iijima et al., 2004; Nagaishi et al., 2006). Interestingly, Th1‐ polarised cells from TIM‐3‐overexpressing Ceacam1 −/− mice were resistant to galectin‐9‐induced apoptosis, suggesting a distinct role for these two ligands. However, the authors reported that the CEACAM1 and TIM‐3 heterodimeric complex is the major factor responsible for T‐cell inhibition and human leucocyte antigen‐B‐associated transcript 3 (BAT3) release. Importantly, TIM‐3 expression appears to be regulated by CEACAM1 since Ceacam1 −/− CD4+ T cells lack TIM‐3 expression after activation. The double‐positive (CEACAM1+ TIM‐3+) phenotype is essential for both CD4+ T cells and CD8+ T cells to exhibit the tolerance‐inducing function of TIM‐3 (Huang et al., 2015). Both CEACAM1‐L and TIM‐3 can recruit SHP‐1/SHP‐2 phosphatases, respectively, in an ITIM‐dependent manner and Lck, CD45, CD148 at the immune synapse to suppress T cell receptor (TCR) signalling (Izzi et al., 1999; Clayton et al., 2014). Downstream, TIM‐3 expression is regulated by the Th1 transcriptional protein T‐bet (Anderson et al., 2010). As T‐bet does not regulate the Th17 differentiation (Park et al., 2005), other transcription factors might be involved in the regulation of Tim‐3 expression in Th17 cells.
Anti‐tumour immunity is hindered by T cells with an exhausted phenotype (Fridman et al., 2012) and TIM‐3 has been reported to be co‐expressed with PD‐1 on tumour‐specific CD8+ T cells in mice (Sakuishi et al., 2010) and in patients with advanced melanoma (Baitsch et al., 2012; Fourcade et al., 2010). In both case, TIM‐3+ PD‐1+ TILs represent the most abundant and dysfunctional population of TILs with a severe exhausted phenotype as defined by the failure to proliferate and to produce IL‐2, TNF, and IFN‐γ. Recently, PD‐1+ TIM‐3+ CEACAM1+ T cells were also characterised by extremely low intracellular IL‐2 and TNF‐α expression consistent with exhaustion. However, TIM‐3 blockade by itself displays a modest therapeutic effect in a murine model of colorectal cancer (CT26) (Sakuishi et al., 2010). CEACAM1 and TIM‐3 co‐blockade is associated with increased CD8+ and CD4+ TILs, together with enhanced IFN‐γ production and decreased IL‐10 production. This dual‐blockade improved elimination of CT26 tumours (Huang et al., 2015). Finally, dual blockade of TIM‐3 and PD‐1 pathways can reverse T cell exhaustion and restore anti‐tumour immunity with restoration of anti‐tumour CD8+ T cell cytokine production and enhanced proliferation (Fourcade et al., 2010; Ngiow et al., 2011; Sakuishi et al., 2010). Similarly, the simultaneous targeting of TIM‐3 and PD‐1 has been shown to rescue CD8+ T cells from exhaustion in a model of chronic infection (Takamura et al., 2010). To conclude, manipulation of the TIM‐3 pathway could be a promising molecular target for therapeutic intervention in autoimmune disease, chronic viral infections and cancer.
2.8. Ceacam1
Carcinoembryonic antigen‐related cell adhesion molecule 1 (CEACAM1, CD66a, BGP) is a member of the IgSF and has been shown to have co‐inhibitory function on T cells in vitro in both human and murine cells and in vivo in a murine model of Th1‐mediated colitis (Chen and Shively, 2004; Iijima et al., 2004; Kammerer et al., 1998; Markel et al., 2002b; Nakajima et al., 2002). The human and murine Ceacam1 genes are highly conserved and can generate 12 and 4 alternative splice variants, respectively. The different splice variants exist as secreted molecules or transmembrane receptors with varying lengths of the cytosolic tail. The different isoforms of the transmembrane molecules are termed long (‐L) or short (‐S) due to their cytoplasmic domain which contains two ITIM domains in the long isoforms in mouse and human (Beauchemin et al., 1999; Tan et al., 2002). Semiquantitative analysis in T cells suggests that CEACAM1‐L isoforms predominate over CEACAM1‐S isoforms (Singer et al., 2002). The short isoform CEACAM1‐4S provides T cell co‐stimulation and can function independently of CEACAM1‐L isoforms and their inhibitory signalling (Chen et al., 2012, 2004, 2012, 2012). Unlike other family members, CEACAM1 is the only one expressed on CD8+ and CD4+ T cells, immediately after activation through the TCR‐CD3 complex ex vivo (Kammerer et al., 1998; Morales et al., 1999; Nakajima et al., 2002) (Table 1). The long CEACAM1 isoforms negatively regulate T cell function by ablating TCR‐CD3 complex signalling through ZAP‐70. The inhibition is initiated by CEACAM1‐L associating with the CD3 signalling complex which enables the phosphorylation of the CEACAM1‐L ITIMs by src‐related kinase p56lck, thus allowing the recruitment of SHP‐1 phosphatase. SHP‐1 then dephosphorylates CD3‐ζ and ZAP70 and thereby abrogates all downstream functions resulting in the down‐modulation of the immune response, including the exocytosis of cytotoxic granule proteins (Chen and Shively, 2004; Chen et al., 2008; Huber et al., 1999; Nakajima et al., 2002). CEACAM1 deficient mice did not display any change in the haematopoietic cell compartment, autoimmune disorder development or in spontaneous tumour growth (Leung et al., 2006). However, engagement of CEACAM1‐L with mAbs in vitro leads to inhibition of murine and human CD4+ and CD8+ T cell cytokines (IL‐2, IL‐4, IFN‐γ) production and proliferation (Boulton and Gray‐Owen, 2002; Chen and Shively, 2004; Morales et al., 1999; Nakajima et al., 2002). The overexpression of CEACAM1‐L on naive T cells led to the inhibition of in vitro differentiation to both Th1 and Th2 cells and to in vivo impaired cytokine secretion by these T cells subsets in an adoptive transfer colitis model (Nagaishi et al., 2006). However, Th1 pathways may be more sensitive to CEACAM1‐mediated suppression than Th2 pathways, a result that is similar to previous observations with PD‐1 and PD‐1 ligand interactions (Latchman et al., 2001). The overexpression of CEACAM1‐L also inhibited nuclear translocation of T‐bet, GATA‐3, STAT‐4, and STAT‐6 which suggests that CEACAM1 inhibits not only TCR‐CD3 signalling in T cells but also likely IL‐4 and IL‐12 receptor signalling (Nagaishi et al., 2006). The latter is consistent with previous studies showing CEACAM1 inhibition of IL‐2R signalling (Chen and Shively, 2004).
CEACAM1 was originally discovered as a colorectal cancer antigen. It has since been demonstrated that the expression of long‐ and short‐tailed CEACAM1 is vastly dysregulated on various cancers (Hinoda et al., 1988; Gaur et al., 2008; Turbide et al., 1997; Wang et al., 2000). CEACAM1‐L is highly expressed in gastric carcinoma, melanoma, metastatic colorectal carcinoma, NSCLC, bladder and thyroid cancer and CEACAM1 isoforms are downregulated in breast cancer, endometrial carcinomas and in the early stages of colon, prostate and liver cancer (Bamberger et al., 1998; Busch et al., 2002; Huang et al., 1998; Neumaier et al., 1993; Riethdorf et al., 1997; Rosenberg et al., 1993). Markel and colleagues demonstrated that CEACAM1 expression allows tumour cells to escape destruction by NK and T cells in vitro (Markel et al., 2002, 2006, 2002). NK and T cells from melanoma patients express CEACAM1 at high levels, which increases their potential sensitivity to CEACAM1‐dependent inhibition by tumour cells (Markel et al., 2002, 2010, 2006). This is consistent with human CEACAM1‐L overexpression in Jurkat cells, which is able to inhibit both Th1 and Th2 cytokine secretion in an ITIM‐dependent pathway.
In this context, Ortenberg and colleagues successfully developed an anti‐CEACAM1 antibody, which counteracts the CEACAM1‐mediated inhibition of T cells by other lymphocytes and melanoma cells in vitro and in vivo. Specifically, administration of the anti‐CEACAM mAb in a human‐melanoma xenograft murine model resulted in the inhibition of xenograft growth and elimination of malignant cells by T cells (Ortenberg et al., 2012). There are two patents published involving CEACAM1, one detailing the function and efficacy of newly synthesised peptides to modulate CD66 family member activity (Skubitz and Skubitz, 2001) while cCam Biotherapeutics patented an anti‐CEACAM1 therapeutic antibody for use in viral infection and cancer therapy (cCam Biotherapeutics LTD et al.). cCAM Biotherapeutics also recently started a phase I clinical trial with their patented anti‐CEACAM1 mAb CM‐24 (humanised IgG4) for treatment of advanced or recurrent NSCLC, melanoma, bladder, colorectal, gastric and ovarian cancer (cCAM Biotherapeutics Ltd. NCT0234695).
2.9. LAIR‐1
LAIR‐1 (leucocyte‐associated immunoglobulin‐like receptor‐1, CD305) was first identified as an inhibitory receptor expressed by human peripheral blood leukocytes in studies focusing on NK‐cell mediated toxicity. Human LAIR‐1, a type 1 membrane protein structurally related to KIRs, belongs to the IgSF. The intracellular part of LAIR‐1 contains the amino acid sequences VTYAQL and ITYAAV, which fit the consensus sequences for ITIMs (Meyaard et al., 1997). LAIR‐1 mediates negative signalling to cells through binding of SHP‐1 and SHP‐2 to ITIMs fragments (Meyaard et al., 1997). It can also function independently from SHP‐1 and 2 and signal through C‐terminal Src kinase (Csk) (Verbrugge et al., 2006). The murine homologue (mLAIR‐1) shares 40% sequence identity with human LAIR‐1 and also contains 2 ITIM motifs in the cytoplasmic tail that only associate with SHP‐2. LAIR2, another protein highly homologues to LAIR‐1 has been described in mice (Lebbink et al., 2004).
LAIR‐1 is broadly expressed on immune cells, including 70–80% of human CD4+ and CD8+ T cells (PBMCs), nearly all NK cells and monocytes in healthy individuals (Meyaard et al., 1997). It is expressed at higher levels on human CD8+ than CD4+ T cells. It is also reported that naïve human T cells have higher expression of LAIR‐1 than effector or memory cells (Maasho et al., 2005). Expression of LAIR‐1 on murine cells resembles the pattern shown in humans but it is not expressed on B cells in contrast to human cells (van der Vuurst de Vries et al., 1999). LAIR‐1 expression was recently shown to predict time‐to‐first‐treatment in patients presenting with chronic lymphocytic leukaemia (CLL) (Perbellini et al., 2014). In contrast to the human protein, mLAIR‐1 expression is higher on mouse memory than naïve T cells (Tang et al., 2012) (Table 1).
Engagement of the LAIR‐1 receptor by a specific antibody caused a decrease in human NK and T cells cytotoxicity, although the antibody did not inhibit T cell proliferation and activation (Meyaard et al., 1999). In contrast, Maasho et al. demonstrated that ligation of LAIR‐1 on human CD8+ T cells decreased calcium mobilisation but not CD8+ T cells mediated lysis (Maasho et al., 2005). In 2012, the LAIR‐1 knockout mouse was generated and no systemic autoimmune phenotype was observed. The impact on T cells was relatively mild with a slight increase of CD69 and CD44 expression in older animals and increased number of regulatory T cells (Tang et al., 2012).
Both human and murine LAIR‐1 bind with high affinity to collagen (Lebbink et al., 2006). In addition, collagens I and III are capable of cross‐linking with hLAIR‐1. Subsequently it was demonstrated that both soluble and cell surface bound LAIR‐1 bind in vitro to collagen‐expressing cell lines (Rygiel et al., 2011). Collagens are important component of the extracellular matrix (ECM) and various collagens, including collagen I, II, III, V, and IX, show increased deposition during tumour formation which opens a possibility for interventions in cancer immunotherapies.
Currently there are no clinical trials ongoing with antibodies modulating the LAIR‐1 pathway. There are several reasons why LAIR‐1 might not be considered an optimal target for checkpoint modulating therapies. Firstly, LAIR‐1 is highly expressed on naïve human T cells rather than activated ones. Secondly, the results obtained with LAIR‐1 KO cells show only slight impact on T cell compartment.
2.10. HVEM‐BTLA‐CD160 network
HVEM (herpes virus entry mediator) is both, a signalling receptor and ligand for specific inhibitory receptors expressed on T cells. HVEM was first shown to interact with the glycoprotein envelope D (gD) of the Herpes viruses 1 and 2 expressed on T cells and later shown to bind the TNFR family members lymphotoxin‐α (LT‐α) and lymphotoxin‐like, exhibits inducible expression, and competes with herpes simplex virus glycoprotein D for HVEM, a receptor expressed by T lymphocytes (LIGHT), as well as to the immunoglobulin superfamily (IgSF) members BTLA (CD272) and CD160. With a broad expression pattern, including haematopoietic and non‐haematopoietic cells, the interaction between these ligand/receptor pairs, either in cis (expression on the same cell) or in trans (expression on different cells), constitutes a complex co‐inhibitory and co‐stimulatory network. Thus, HVEM serves as a bidirectional switch for T cell activation, promoting either activation or regulation depending on the ligand engaged and the cellular context.
2.10.1. HVEM – receptor and ligand
HVEM is a member of the TNFR family molecules (TNFR14), also called LIGHTR in reference to one of its ligands (Montgomery et al., 1996). The HVEM open reading frame encodes a single transmembrane protein with two perfect and two imperfect TNFR‐like cysteine‐rich domains (CDR). LIGHT and LT‐α interact with the CDR2 and CDR3 domains of HVEM and the Ig superfamily molecules BTLA and CD160 bind to CDR1 and partially to CDR2 (Sarrias et al., 2000; Compaan et al., 2005; Cai and Freeman, 2009). HVEM has a short cytoplasmic tail which does not contain a death domain and shares some similarity with 4–1BB and CD40 (Kwon et al., 1997a), for detailed see review: (del Rio et al., 2010). Instead, there are PXQT and IPEEGD motifs that bind to TRAFs (TNFR associated factors) which activate the transcription factors NF‐kB and AP‐1 (Hsu et al., 1997).
HVEM is widely expressed on immune cells including T and B lymphocytes, NK cells, DCs and myeloid cells as well as non hematopoietic cells in liver, kidney and lungs (Marsters et al., 1997). It is highly expressed on naïve T cells, downregulated shortly upon activation and returns to its steady state levels a few days after activation in vitro (Morel et al., 2000; Sedy et al., 2005) (1, 3). This expression profile in response to in vitro stimulation has not yet been confirmed in human T cells but was demonstrated in human B cells, binding to LIGHT and in DCs after maturation (Duhen et al., 2004). The exact mechanisms underpinning HVEM downregulation is not completely understood but thought to depend on receptor/ligand interactions as monoclonal antibodies blocking HVEM/LIGHT binding prevent HVEM down‐regulation (Morel et al., 2000).
Table 3.
Expression of BTLA, HVEM and CD160 on T cells and tumour cells.
| Molecule | Ligand | Expression on haematopoetic cells | Expression on malignancies | Gene mutations and its expressions/SNPs | Ref. |
|---|---|---|---|---|---|
| HVEM | BTLA, CD160, LIGHT, lymphotoxin‐α, herpes simplex virus glycoprotein D | T cells, B cells, NK cells, DCs, myeloid cells; | Melanoma, hepatocellular carcinoma, colorectal cancer; MCL, ALL, CLL Melanoma (primary tumour and metastasis), Oesophageal squamous cell carcinoma, Primary plasma cells (Myeloma, plasma cell leukaemia) | Nonsynonymous substitutions in patients with follicular lymphoma ‐ poor prognosis or no difference | Derre et al., 2010, Costello et al., 2003 |
| T cells: Higher on naïve T cells, drops shortly after activation and than re‐expressed | |||||
| BTLA | HVEM | T cells, B cells, NK cells, DCs, myeloid cells | CLL, SLL, TA+ CD8 T cells in melanoma, MCL, Marginal zone lympohma, Follicular Lymphoma, Burkitt Lymphoma, Hodgkin's Lymphoma | No data | Watanabe et al., 2003, M'Hidi et al., 2009 |
| T cells: Increased upon activation (Human and Murine), Higher On Th1 than Th2, Higher expression by CD4 than CD8 T cells | |||||
| CD160 | HVEM, MHC‐I | NK T cells, NKT cells, T cells, γδTcell (transmembrane form only on subpopulation of IL‐15 activated human NK cells) | CLL, MCL, Hairy Cell leukaemia, | No data | Farren et al., 2011, Maiza et al., 1993 |
| T cells: Highest expression on exhausted and activated CD8 cells, low expression by CD4 T cells |
Recent studies have shown that HVEM overexpression and gene polymorphisms can both be associated with malignancies such as colorectal cancer, melanoma, and breast cancer as well as haematopoietical malignancies (Table 3). Whilst its role in tumourigenesis has not been completely understood, recent studies demonstrate that high levels of HVEM expression on tumour cells is associated with unfavourable prognosis in patients with oesophageal squamous cell carcinoma (Migita et al., 2014). High levels of HVEM on these patients tumours' correlated with larger tumour size, lymph node metastasis and lower 5‐year survival rate. Of relevance, tumours expressing high levels of HVEM were also found to be poorly infiltrated by both CD4+ and CD8+ T cells (Migita et al., 2014). In human colorectal cancer HVEM expression was also inversely correlated with the presence of tumour‐infiltrating T cells (Inoue et al., 2015). Similar outcomes were observed in patients suffering from hepatocellular carcinoma where high HVEM expression was linked to lower infiltration of CD4+, CD8+ and CD45RO+ T cells. Moreover, the expression of granzyme B, perforin and interferon‐c was also decreased in this group (Hokuto et al., 2015). Although the function of HVEM in other malignancies still remains unknown, the current data does support a role for tumour associated HVEM in the negative control of anti‐tumour immunity.
2.10.2. BTLA (CD272) B and T lymphocyte attenuator
BTLA (CD272) is an Ig‐domain‐containing transmembrane glycoprotein (Watanabe et al., 2003) that belongs to the CD28 IgSF. Whilst it was first cloned from murine Th1‐polarised CD4+ T cells, highest levels of expression are found on B cells, followed by T cells and antigen presenting cells.
Whereas BTLA is expressed at low levels on murine T cells, BTLA is rapidly upregulated during in vitro polarisation of T cells to either Th1 or Th2 with higher expression on the latter compartment (Watanabe et al., 2003). In steady state conditions, BTLA expression on murine Tregs is equivalent to that on naïve cells and it remains low even upon TCR stimulation (Tao et al., 2008). In healthy individuals BTLA is expressed on 90% of human T and B lymphocytes freshly isolated from blood (Otsuki et al., 2006). Human T cells differentiated in vitro into either to IFN‐γ or IL‐4 producing cells have shown the same level of BTLA expression (Otsuki et al., 2006). (1, 3).
The relevance of BTLA for T cell function and homoeostasis was demonstrated both in vitro and in vivo. Cross‐linking of BTLA on activated T cells results in decreased proliferation and cytokine production (i.e. IL‐2, IL‐4, IL‐10 and IFN‐γ). Interestingly, this effect is stronger in CD4+ than in CD8+ T cells which might be due to the differential expression of BTLA on these two subsets (Krieg et al., 2005; Watanabe et al., 2003; Otsuki et al., 2006). BTLA knockout mice did not develop an overt autoimmune phenotype although BTLA‐deficient T cells showed a heightened response to stimulation with anti‐CD3 (Watanabe et al., 2003).
Although early studies suggested that BTLA and B7x were binding partners, later studies identified HVEM as the ligand for BTLA, and which remains as the only known binding partner for BTLA (Sedy et al., 2005). Upon the interaction with HVEM BTLA delivers inhibitory signal to T cells mediated most likely by SHP‐1 and SHP‐2 proteins (Chemnitz et al., 2006). However, when BTLA binds to HVEM in trans (expressed by adjacent cell) both molecules signal – HVEM sends stimulatory signal through the NF‐kB pathway whilst BTLA delivers inhibitory signals as shown in 293T cells (Cheung et al., 2009). In addition, BTLA was shown to form heterodimeric complexes on the same cell on naïve human and mouse T cells, thus blocking the interaction in trans (Cheung et al., 2009).
There are several reports investigating BTLA expression in different malignancies. MART‐1 specific CD8+ T cells isolated from melanoma patients' peripheral blood expressed high levels of BTLA and showed decreased IFN‐γ production upon crosslinking with HVEM (Derre et al., 2010). Another group characterised BTLA expression by NY‐ESO‐1 specific CD8+ T cells isolated from melanoma patients' blood and showed that BTLA+ CD8+ T cells (which also expressed high levels of PD‐1 and TIM‐3) produced lower amounts of IFN‐γ and TNF‐α (Fourcade et al., 2012). Both studies focused on the expression of co‐inhibitory receptors on circulating T cells and there is still a paucity of data on the expression of BTLA by tumour infiltrating lymphocytes.
2.10.3. CD160
Human CD160 was initially described on NK cells as the target recognised by the BY55 monoclonal antibody (Anumanthan et al., 1998). CD160, which belongs to the IgSF, exists in two isoforms with either a transmembrane domain or a glycosphingolipid anchor to the cell membrane. Both isoforms contain a single immunoglobulin V (IgV)‐like domain with low degree of homology to other killer inhibitory receptors (Agrawal et al., 1999). The glycophosphatidylinositol (GPI)‐anchored isoform of CD160 is expressed by human CD56dimCD16+ NK, NKT cells, activated CD8+ T cells, and a small fraction of CD4+ T cells whereas the transmembrane isoform is only found in a small subset of IL‐15 activated NK cells (Giustiniani et al., 2009). Although CD160 is not expressed on B cells in physiological conditions, its expression was reported on B cell malignancies including CLL, monoclonal B‐cell lymphocytosis and hairy cell leukaemia. Most recently CD160 has been suggested as a marker for minimal residual disease in CLL (Farren et al., 2011, 2015) (Table 3).
Similarly, in mice, CD160 expression is highest on NK and memory CD8+ T cells and express at very low levels on CD4+ T cells (Agrawal et al., 1999; Cai et al., 2008; Le Bouteiller et al., 2002; Maeda et al., 2005). For some time, CD160 was considered a good marker for cytotoxic CD8+ T cells, however, later studies failed to demonstrate co‐localisation with cytotoxic molecules (Merino et al., 2007). A more recent publication shows that CD160 is upregulated on murine exhausted CD8+ T cells along with PD‐1, LAG‐3 and CD244 (Blackburn et al., 2009) (1, 3).
CD160 was first shown to bind MHC I and later on, its high affinity interaction with HVEM was demonstrated (Maeda et al., 2005; Cai et al., 2008). Binding of HVEM to CD160 expressed on CD4+ T cell was shown to inhibit their proliferation in vitro, thus supporting the co‐inhibitory nature of this ligand/pair interaction (Cai et al., 2008).
There are several reports describing the function of CD160 in NK cell development, homoeostasis and function, whereas full understanding of its role on T cells is rather scarce. CD160 knockout mice did not show any abnormalities in NK or NKT cell development, however CD160‐deficient mice were more susceptible to early onset of tumour growth. This was demonstrated to be due to decreased IFN‐γ production by NK cells rather than impaired cytotoxicity (Tu et al., 2015).
Without detailed understanding and characterisation of the expression pattern of HVEM and its ligands on TILs, myeloid cells and tumour cells, it will be very difficult to predict the results of antibody‐mediated therapy. Although in 2013 Medarex patented a set of fully human monoclonal anti‐BTLA and anti‐HVEM antibodies to date no clinical trial has been reported with their usage.
2.11. CD200/CD200R
CD200 (OX‐2) and CD200 receptor (CD200R) are type‐1 transmembrane glycoproteins that contain two immunoglobulin superfamily (IgSF) domains and are highly conserved between human and mouse (Wright et al., 2003, 2001). In mouse and human CD200 is robustly expressed by cellular components of the CNS as well as on B cells and activated CD8+ and CD4+ T cells (Barclay et al., 2002; Webb and Barclay, 1984; Wright et al., 2001). CD200 receptor (CD200R) is expressed on granulocytes, monocytes and CD4+ T cells in mice (Preston et al., 1997, 2003, 2000). Similarly, CD200R is mainly expressed in human peripheral blood CD4+ and CD8+ T cells, neutrophils, basophils and monocytes and in vitro differentiated DCs (Wright et al., 2003). Interestingly, the cytoplasmic tails of both mouse and human CD200 and CD200R do not contain an ITIM motives. The cytoplasmic region of CD200R, however, features an NPxY motif which contains three free tyrosine residues that are phosphorylated upon interaction of CD200R and CD200. The signalling is mediated via adaptor proteins Dok1 and Dok2 that bind the phosphorylated NPXY motif and recruit RasGAP. As RasGAP inhibits the Ras/MAPK pathways, activation of crucial molecules such as ERK, JNK, and p38 MAPK are inhibited upon engagement of CD200R with its ligand (Wright et al., 2003). There are four different isoforms of CD200R, but only CD200R1 expresses the long cytoplasmic tail with the NPXY motif (Gorczynski et al., 2004; Wright et al., 2003).
Characterisation of CD200 KO mice showed its major role as an inhibitory molecule for myeloid cell with no direct impact on T cells (Gorczynski et al., 1999; Hoek et al., 2000). Misstear and colleagues demonstrated, however, that antigen presenting cells expressing CD200 were able to suppress cognate antigen‐specific T cell activity such as IFN‐γ production and cytotoxic granule mobilisation in mouse and human (Misstear et al., 2012). Similarly, it was shown that human CD200 expressing B cells inhibited Th1 responses (IL‐2 and IFN‐γ production) (McWhirter et al., 2006). As in CD200 KO mice, there was also a lack of phenotypic changes in the CD200R1 deficient mouse. When immunologically challenged, however, the importance of CD200:CD200R1 in the suppression of immune responses became apparent: CD200R1 KO mice rejected allografts quickly, despite administration of CD200 Fc (Boudakov et al., 2007).
Recent studies have revealed that CD200 is broadly expressed in a variety of cancer cells. Increased expression of CD200 was detected on B cells of CLL patients and was shown to correlate with poor prognosis in acute myeloid leukaemia (AML) (McWhirter et al., 2006; Tonks et al., 2007). Also a large variety of solid tumours were shown to express high levels of CD200 such as (metastatic) melanoma, head and neck carcinoma, ovarian cancer, testicular cancer, malignant mesothelioma, colon carcinoma, multiple myeloma and renal carcinoma and CD200 expression was found to be associated with tumour progression in the majority of these cancers (Moreaux et al., 2006, 2008, 2007, 2008).
Kretz‐Rommel and colleagues demonstrated that in a murine model of CD200 expressing human Burkitt's lymphoma, blocking of the CD200‐CD200R interaction by anti‐CD200 mAb administration resulted in almost complete suppression of tumour growth and thus suggested the use of anti‐CD200 antibodies for the clinic (Kretz‐Rommel et al., 2008, 2007). In a mouse model of breast cancer, EMT6 cells increased CD200 expression dramatically only when transplanted into immunocompetent mice. Administration of neutralising anti‐CD200 mAbs improved the cytotoxic immune response and led to decreased tumour growth. Interestingly, a strong correlation was found between the levels of a soluble form of CD200 in the serum of EMT6 or EL4 (thymoma) tumour bearing mice and their respective tumour growth, which allowed a non‐invasive monitoring of the tumour burden (Gorczynski et al., 2010).
Trillium Therapeutics Inc. holds two patents disclosing different methods of inducing and preventing immune suppression by engaging CD200 or inhibiting CD200:CD200R interaction in the context of cancer, autoimmune diseases, graft rejection and allergies (patent: Gorczynski and Clark, 2005). Furthermore, an assay to detect high levels of soluble CD200 in the serum of cancer patients to diagnose and monitor the disease was established (patent: Gorczynski and Wong, 2009). In addition, Merck & Co (Schering Corp) has published three patents outlining the use of an agonist/antagonist anti‐CD200R/anti‐CD200 mAb/antibody fragment to induce CD200‐mediated T cell inhibition for the treatment inflammatory and autoimmune disorders (Presta, Cherwinski, and Phillips, 2008; Truitt, Rosenblum, and Olasz, 2005). Alexion Pharmaceuticals currently holds three patents concerning CD200 and its modulation for therapy of cancer or autoimmune disorders. Patented are also antagonistic monoclonal antibodies or antibody fragments against CD200 which either interfere with the CD200:CD200R interaction to block CD200R‐mediated immune suppression or directly target CD200 expressing tumour cells by marking them for ADCC/CDC or by using a fusion molecule of the anti‐CD200 mAb with a toxin (patent: Bowdish et al., [Link], [Link]). Furthermore, a combination therapy with anti‐CD200 mAb and anti‐CD20 mAb (or a CD200 and CD20 bispecific antibody) is suggested for CLL treatment (Rother and Yan, 2011). One of the published CD200 blocking mAbs, ALXN6000, was taken further into a phase I/II study to determine safety and best dose for the treatment of relapsing or refractory B‐CLL or multiple myeloma. The study was completed in November 2010 but no official outcome was presented so far.
2.12. A2aR
Soluble immune suppressive mediators can also be found in the tumour microenvironment and extracellular adenosine has been shown to be a critical and non‐redundant negative regulator of a variety of immune cells (Hasko et al., 2008; Idzko et al., 2014; Ohta et al., 2006; Ohta and Sitkovsky, 2001). During the process of inflammation, the destruction of host tissue by the immune cells combined with damaged microcirculation and hypoxia leads to an increase in extracellular ATP levels (Sitkovsky, 2003; Sitkovsky et al., 2004). A2A adenosine (purinergic) receptors (A2aR) were identified as sensors of excessive inflammatory tissue damage (Ohta and Sitkovsky, 2001) since normally nonlethal inflammation in wt mice led to excessive inflammation and death in A2aR‐null mice (Ohta et al., 2006).
The tumour microenvironment also presents high levels of adenosine, as a result of hypoxia and ectopic expression of CD39 and CD73 ectonucleotidases responsibles for the generation of adenosine from ATP by Tregs (Deaglio et al., 2007; Stagg and Smyth, 2010; Young et al., 2014). Adenosine can then interact with four G‐protein‐coupled receptor subtypes (A1, A2A, A2B, and A3) with variable affinity, thus allowing differential responses to adenosine levels (Olah and Stiles, 1995). Interestingly, in both human and mice, A2aR proteins and their mRNA levels are preferentially upregulated in CD4+ rather than upon activation (Lappas et al., 2005; Li et al., 2012; Koshiba et al., 1999). A2A adenosine receptors are potent inhibitors of T‐cell receptor (TCR)‐triggered proliferation as well as upregulation of interleukin‐2 receptor (Huang et al., 1997; Sitkovsky et al., 2004). Indeed, A2aR signalling to CD4+ and CD8+ T cells has been shown to limit their effector function (Erdmann et al., 2005; Fishman et al., 2009; Lappas et al., 2005). A2aR engagement can prevent the development of IL‐17 producing cells and promote the development of Foxp3+ and LAG‐3+ regulatory T‐cells in vivo (Zarek et al., 2008; Deaglio et al., 2007). Thus, Ohta and colleagues hypothesised that A2aR could protect cancerous cells by inhibiting the activity of tumour‐reactive lymphocytes. Experiments using A2aR‐null mice demonstrated an increased rejection of transplantable melanoma and lymphoma (Ohta et al., 2006) as well as a delay in the EL4 lymphoma cells growth associated with increased efficacy of tumour vaccines (Waickman et al., 2012). Activation of the A2aR results in inhibition of the immune response to tumours through several mechanisms, including suppression of T regulatory cell function, inhibition of natural killer cell cytotoxicity and tumour‐specific CD4+/CD8+ activity (Fishman et al., 2009). Adenosine inhibits production of various cytokines and chemokines (IFN‐γ, IL‐2, TNF‐α, GM‐CSF, MIP‐1α, MIP‐1β, IL‐13, and RANTES) in CD8+ and CD4+ Th1 cells, indicating that adenosine affects a common regulatory mechanism in their production (Raskovalova et al., 2007). The use of a specific agonist of A2aR indicates that the inhibitory effects of adenosine are mediated via increased levels of cyclic AMP (cAMP), leading to protein kinase A (PKA) activation (Raskovalova et al., 2007). The rise in intracellular cAMP does not only inhibit Th1 and Th17 cell generation but also promotes the generation of Foxp3+ and LAG‐3+ regulatory T cells, hence producing a self‐amplifying regulatory loop within the tumour (Zarek et al., 2008).
To conclude, A2aR‐adenosine binding enhances polarisation of T‐cell subsets to immunosuppressive phenotypes, leading to increase tumour growth. Therefore, pharmacological inhibition of A2aR activation by specific antagonists or antibodies that block adenosine binding is likely to synergise with other immunotherapies. Although this target has not yet been evaluated in cancer patients, the effect of agonistic antibodies to A2aR is currently been evaluated in atherosclerotic cardiovascular disease, and autoimmune syndromes such as systemic lupus erythematosus and rheumatoid arthritis (NCT01180361).
3. Conclusions and future perspectives
Essential for immune homoeostasis, inhibitory checkpoints predominate in the presence of active malignancy, and prevent effective anti‐tumour immune responses. Research over the past ten years has highlighted that manipulation of these immune checkpoints with therapeutic antibodies can control or even eliminate tumours, leading to novel approaches to tumour immunotherapy. Antibodies that block inhibitory pathways, such as those directed against CTLA‐4 and PD‐1 have been developed for clinical use and have resulted in promising clinical outcomes, exemplified by their FDA approval. Future studies should delineate the mechanisms underpinning immunologic checkpoint function within diverse tumour microenvironments, and provide the rationale for effective personalised therapeutic combinations with other approaches such as chemotherapy, radiotherapy, and targeted therapies. Gene editing strategies now also allow consideration of targeting these inhibitory pathways in a variety of cell therapies including TILs, CIK cell (cytokine induced killer), CAR – (chimeric antigen receptors) or TCR‐engineered T cells. Such cell‐intrinsic disruption of immune checkpoints in tumour‐specific T‐cells may display a better safety profile than the systemic administration of blocking antibodies.
Acknowledgements
S.A.Q. is funded by a Cancer Research UK Career Development Fellowship and a Cancer Research Institute Investigator Award. K.S.P. receives funding from Cancer Research UK, Leukaemia and Lymphoma Research, and the Medical Research Council UK. This work was undertaken at University College London Hospitals/University College London, which received support from the Department of Health and Cancer Research United Kingdom Funding Schemes for National Institute for Health Research Biomedical Research Centres and Experimental Cancer Medicine Centres.
We note that not all relevant results have been discussed and cited, owing to space limitations.
Śledzińska Anna, Menger Laurie, Bergerhoff Katharina, Peggs Karl S., Quezada Sergio A., (2015), Negative immune checkpoints on T lymphocytes and their relevance to cancer immunotherapy, Molecular Oncology, 9, doi: 10.1016/j.molonc.2015.10.008.
This is a contribution to the special issue edited by Johanna Olweus, Cancer Immunotherapy.
Contributor Information
Karl S. Peggs, Email: k.peggs@ucl.ac.uk
Sergio A. Quezada, Email: s.quezada@ucl.ac.uk
References
- Agata, Y. , Kawasaki, A. , Nishimura, H. , Ishida, Y. , Tsubata, T. , Yagita, H. , Honjo, T. , 1996. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 8, 765–772. [DOI] [PubMed] [Google Scholar]
- Agrawal, S. , Marquet, J. , Freeman, G.J. , Tawab, A. , Bouteiller, P.L. , Roth, P. , Bolton, W. , Ogg, G. , Boumsell, L. , Bensussan, A. , 1999. Cutting edge: MHC class I triggering by a novel cell surface ligand costimulates proliferation of activated human T cells. J. Immunol. 162, 1223–1226. [PubMed] [Google Scholar]
- Ahmadzadeh, M. , Johnson, L.A. , Heemskerk, B. , Wunderlich, J.R. , Dudley, M.E. , White, D.E. , Rosenberg, S.A. , 2009. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 114, 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarnath, S. , Mangus, C.W. , Wang, J.C. , Wei, F. , He, A. , Kapoor, V. , Foley, J.E. , Massey, P.R. , Felizardo, T.C. , Riley, J.L. , 2011. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci. Translational Med. 3, 111ra120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, A.C. , Lord, G.M. , Dardalhon, V. , Lee, D.H. , Sabatos-Peyton, C.A. , Glimcher, L.H. , Kuchroo, V.K. , 2010. T-bet, a Th1 transcription factor regulates the expression of Tim-3. Eur. J. Immunol. 40, 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreae, S. , Buisson, S. , Triebel, F. , 2003. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG-3 protein (CD223). Blood. 102, 2130–2137. [DOI] [PubMed] [Google Scholar]
- Annunziato, F. , Manetti, R. , Tomasevic, I. , Guidizi, M.G. , Biagiotti, R. , Gianno, V. , Germano, P. , Mavilia, C. , Maggi, E. , Romagnani, S. , 1996. Expression and release of LAG-3-encoded protein by human CD4+ T cells are associated with IFN-gamma production. FASEB J. 10, 769–776. [DOI] [PubMed] [Google Scholar]
- Ansari, M.J. , Salama, A.D. , Chitnis, T. , Smith, R.N. , Yagita, H. , Akiba, H. , Yamazaki, T. , Azuma, M. , Iwai, H. , Khoury, S.J. , 2003. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 198, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell, S.M. , Lesokhin, A.M. , Borrello, I. , Halwani, A. , Scott, E.C. , Gutierrez, M. , Schuster, S.J. , Millenson, M.M. , Cattry, D. , Freeman, G.J. , 2015. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. New Engl. J. Med. 372, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumanthan, A. , Bensussan, A. , Boumsell, L. , Christ, A.D. , Blumberg, R.S. , Voss, S.D. , Patel, A.T. , Robertson, M.J. , Nadler, L.M. , Freeman, G.J. , 1998. Cloning of BY55, a novel Ig superfamily member expressed on NK cells, CTL, and intestinal intraepithelial lymphocytes. J. Immunol. 161, 2780–2790. [PubMed] [Google Scholar]
- Avice, M.N. , Sarfati, M. , Triebel, F. , Delespesse, G. , Demeure, C.E. , 1999. Lymphocyte activation gene-3, a MHC class II ligand expressed on activated T cells, stimulates TNF-alpha and IL-12 production by monocytes and dendritic cells. J. Immunol. 162, 2748–2753. [PubMed] [Google Scholar]
- Azuma, T. , Yao, S. , Zhu, G. , Flies, A.S. , Flies, S.J. , Chen, L. , 2008. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 111, 3635–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baitsch, L. , Baumgaertner, P. , Devevre, E. , Raghav, S.K. , Legat, A. , Barba, L. , Wieckowski, S. , Bouzourene, H. , Deplancke, B. , Romero, P. , 2011. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baitsch, L. , Legat, A. , Barba, L. , Fuertes Marraco, S.A. , Rivals, J.P. , Baumgaertner, P. , Christiansen-Jucht, C. , Bouzourene, H. , Rimoldi, D. , Pircher, H. , 2012. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PloS One. 7, e30852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baixeras, E. , Huard, B. , Miossec, C. , Jitsukawa, S. , Martin, M. , Hercend, T. , Auffray, C. , Triebel, F. , Piatier-Tonneau, D. , 1992. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J. Exp. Med. 176, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger, A.M. , Riethdorf, L. , Nollau, P. , Naumann, M. , Erdmann, I. , Gotze, J. , Brummer, J. , Schulte, H.M. , Wagener, C. , Loning, T. , 1998. Dysregulated expression of CD66a (BGP, C-CAM), an adhesion molecule of the CEA family, in endometrial cancer. Am. J. Pathol. 152, 1401–1406. [PMC free article] [PubMed] [Google Scholar]
- Barclay, A.N. , Wright, G.J. , Brooke, G. , Brown, M.H. , 2002. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 23, 285–290. [DOI] [PubMed] [Google Scholar]
- Beauchemin, N. , Draber, P. , Dveksler, G. , Gold, P. , Gray-Owen, S. , Grunert, F. , Hammarstrom, S. , Holmes, K.V. , Karlsson, A. , Kuroki, M. , 1999. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 252, 243–249. [DOI] [PubMed] [Google Scholar]
- Berger, R. , Rotem-Yehudar, R. , Slama, G. , Landes, S. , Kneller, A. , Leiba, M. , Koren-Michowitz, M. , Shimoni, A. , Nagler, A. , 2008. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 14, 3044–3051. [DOI] [PubMed] [Google Scholar]
- Bin Dhuban, K. , d'Hennezel, E. , Nashi, E. , Bar-Or, A. , Rieder, S. , Shevach, E.M. , Nagata, S. , Piccirillo, C.A. , 2015. Coexpression of TIGIT and FCRL3 identifies Helios+ human memory regulatory T cells. J. Immunol. 194, 3687–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn, S.D. , Shin, H. , Haining, W.N. , Zou, T. , Workman, C.J. , Polley, A. , Betts, M.R. , Freeman, G.J. , Vignali, D.A. , Wherry, E.J. , 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank, C. , Brown, I. , Peterson, A.C. , Spiotto, M. , Iwai, Y. , Honjo, T. , Gajewski, T.F. , 2004. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 64, 1140–1145. [DOI] [PubMed] [Google Scholar]
- Boles, K.S. , Vermi, W. , Facchetti, F. , Fuchs, A. , Wilson, T.J. , Diacovo, T.G. , Cella, M. , Colonna, M. , 2009. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur. J. Immunol. 39, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudakov, I. , Liu, J. , Fan, N. , Gulay, P. , Wong, K. , Gorczynski, R.M. , 2007. Mice lacking CD200R1 show absence of suppression of lipopolysaccharide-induced tumor necrosis factor-alpha and mixed leukocyte culture responses by CD200. Transplantation. 84, 251–257. [DOI] [PubMed] [Google Scholar]
- Boulton, I.C. , Gray-Owen, S.D. , 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 3, 229–236. [DOI] [PubMed] [Google Scholar]
- Bowdish, Katherine S., Anke Kretz-Rommel, Susan Faas Mcknight, Jeremy P. Springhorn, and Dayang Wu. 2007. “Antibodies to OX-2/CD200 and Uses Thereof.” (Patent: WO2007084321A2).
- Bowdish, Katherine S., John McWhirter, Anke Kretz-Rommel, and Toshiaki Maruyama. 2009. “Polypeptides and Antibodies Derived from Chronic Lymphocytic Leukemia Cells and Uses Thereof.” (Patent: US7598353).
- Brahmer, J.R. , Tykodi, S.S. , Chow, L.Q. , Hwu, W.J. , Topalian, S.L. , Hwu, P. , Drake, C.G. , Camacho, L.H. , Kauh, J. , Odunsi, K. , 2012. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New Engl. J. Med. 366, 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignone, C. , Escudier, B. , Grygar, C. , Marcu, M. , Triebel, F. , 2009. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin. Cancer Res. 15, 6225–6231. [DOI] [PubMed] [Google Scholar]
- Brignone, C. , Gutierrez, M. , Mefti, F. , Brain, E. , Jarcau, R. , Cvitkovic, F. , Bousetta, N. , Medioni, J. , Gligorov, J. , Grygar, C. , 2010. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J. Transl Med. 8, 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, B.R. , Britton, G.J. , Fang, H. , Verhagen, J. , Smithers, B. , Sabatos-Peyton, C.A. , Carney, L.J. , Gough, J. , Strobel, S. , Wraith, D.C. , 2014. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat. Commun. 5, 4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch, C. , Hanssen, T.A. , Wagener, C. , öbrink, B. , 2002. Down-regulation of CEACAM1 in human prostate cancer: correlation with loss of cell polarity, increased proliferation rate, and Gleason grade 3 to 4 transition. Hum. Pathol. 33, 290–298. [DOI] [PubMed] [Google Scholar]
- Butte, M.J. , Keir, M.E. , Phamduy, T.B. , Sharpe, A.H. , Freeman, G.J. , 2007. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 27, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, G. , Anumanthan, A. , Brown, J.A. , Greenfield, E.A. , Zhu, B. , Freeman, G.J. , 2008. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat. Immunol. 9, 176–185. [DOI] [PubMed] [Google Scholar]
- Cai, G. , Freeman, G.J. , 2009. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol. Rev. 229, 244–258. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas, N, 2012. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 487, 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas, N, 2012. Comprehensive molecular portraits of human breast tumours. Nature. 490, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research, N, 2012. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 489, 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research, N, 2013. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 499, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, E. , Zang, X. , Ramagopal, U.A. , Mukhopadhaya, A. , Fedorov, A. , Fedorov, E. , Zencheck, W.D. , Lary, J.W. , Cole, J.L. , Deng, H. , 2007. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity. 26, 311–321. [DOI] [PubMed] [Google Scholar]
- Cao, W. , Ryan, M. , Buckley, D. , O'Connor, R. , Clarkson, M.R. , 2011. Tim-4 inhibition of T-cell activation and T helper type 17 differentiation requires both the immunoglobulin V and mucin domains and occurs via the mitogen-activated protein kinase pathway. Immunology. 133, 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsten, M. , Bjorkstrom, N.K. , Norell, H. , Bryceson, Y. , van Hall, T. , Baumann, B.C. , Hanson, M. , Schedvins, K. , Kiessling, R. , Ljunggren, H.G. , Malmberg, K.J. , 2007. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 67, 1317–1325. [DOI] [PubMed] [Google Scholar]
- Carlsten, M. , Norell, H. , Bryceson, Y.T. , Poschke, I. , Schedvins, K. , Ljunggren, H.G. , Kiessling, R. , Malmberg, K.J. , 2009. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J. Immunol. 183, 4921–4930. [DOI] [PubMed] [Google Scholar]
- Casati, C. , Camisaschi, C. , Rini, F. , Arienti, F. , Rivoltini, L. , Triebel, F. , Parmiani, G. , Castelli, C. , 2006. Soluble human LAG-3 molecule amplifies the in vitro generation of type 1 tumor-specific immunity. Cancer Res. 66, 4450–4460. [DOI] [PubMed] [Google Scholar]
- Castriconi, R. , Dondero, A. , Corrias, M.V. , Lanino, E. , Pende, D. , Moretta, L. , Bottino, C. , Moretta, A. , 2004. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 64, 9180–9184. [DOI] [PubMed] [Google Scholar]
- Chambers, C.A. , Sullivan, T.J. , Allison, J.P. , 1997. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 7, 885–895. [DOI] [PubMed] [Google Scholar]
- Chambers, C.A. , Sullivan, T.J. , Truong, T. , Allison, J.P. , 1998. Secondary but not primary T cell responses are enhanced in CTLA-4-deficient CD8+ T cells. Eur. J. Immunol. 28, 3137–3143. [DOI] [PubMed] [Google Scholar]
- Chan, C.J. , Andrews, D.M. , Smyth, M.J. , 2012. Receptors that interact with nectin and nectin-like proteins in the immunosurveillance and immunotherapy of cancer. Curr. Opin. Immunol. 24, 246–251. [DOI] [PubMed] [Google Scholar]
- Chan, C.W.Y. , Kay, L.S. , Khadaroo, R.G. , Chan, M.W.C. , Lakatoo, S. , Young, K.J. , Zhang, L. , Gorczynski, R.M. , Cattral, M. , Rotstein, O. , Levy, G.A. , 2003. Soluble fibrinogen-like protein 2/fibroleukin exhibits immunosuppressive properties: suppressing T cell proliferation and inhibiting maturation of bone marrow-derived dendritic cells. J. Immunol. 170, 4036–4044. [DOI] [PubMed] [Google Scholar]
- Chan, D.V. , Gibson, H.M. , Aufiero, B.M. , Wilson, A.J. , Hafner, M.S. , Mi, Q.S. , Wong, H.K. , 2014. Differential CTLA-4 expression in human CD4+ versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation. Genes Immun. 15, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay, K. , Lazar-Molnar, E. , Yan, Q. , Rubinstein, R. , Zhan, C. , Vigdorovich, V. , Ramagopal, U.A. , Bonanno, J. , Nathenson, S.G. , Almo, S.C. , 2009. Sequence, structure, function, immunity: structural genomics of costimulation. Immunological Rev. 229, 356–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvin, J.M. , Pagliano, O. , Fourcade, J. , Sun, Z. , Wang, H. , Sander, C. , Kirkwood, J.M. , Chen, T.H. , Maurer, M. , Korman, A.J. , Zarour, H.M. , 2015. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J. Clin. Invest. 125, 2046–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemnitz, J.M. , Lanfranco, A.R. , Braunstein, I. , Riley, J.L. , 2006. B and T Lymphocyte attenuator-mediated signal transduction provides a potent inhibitory signal to primary human CD4 T Cells that can Be initiated by multiple phosphotyrosine motifs. J. Immunol. 176, 6603–6614. [DOI] [PubMed] [Google Scholar]
- Chen, C.J. , Shively, J.E. , 2004. The cell-cell adhesion molecule carcinoembryonic antigen-related cellular adhesion molecule 1 inhibits IL-2 production and proliferation in human T cells by association with Src homology protein-1 and down-regulates IL-2 receptor. J. Immunol. 172, 3544–3552. [DOI] [PubMed] [Google Scholar]
- Chen, D. , Iijima, H. , Nagaishi, T. , Nakajima, A. , Russell, S. , Raychowdhury, R. , Morales, V. , Rudd, C.E. , Utku, N. , Blumberg, R.S. , 2004. Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and costimulate human T cell function. J. Immunol. 172, 3535–3543. [DOI] [PubMed] [Google Scholar]
- Chen, D.S. , Irving, B.A. , Hodi, F.S. , 2012. Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 18, 6580–6587. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Chen, Z. , Baker, K. , Halvorsen, E.M. , da Cunha, A.P. , Flak, M.B. , Gerber, G. , Huang, Y.H. , Hosomi, S. , Arthur, J.C. , 2012. The short isoform of the CEACAM1 receptor in intestinal T cells regulates mucosal immunity and homeostasis via Tfh cell induction. Immunity. 37, 930–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Langrish, C.L. , McKenzie, B. , Joyce-Shaikh, B. , Stumhofer, J.S. , McClanahan, T. , Blumenschein, W. , Churakovsa, T. , Low, J. , Presta, L. , 2006. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 116, 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Chen, L. , Qiao, S.W. , Nagaishi, T. , Blumberg, R.S. , 2008. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J. Immunol. 180, 6085–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, T.C. , Oborne, L.M. , Steinberg, M.W. , Macauley, M.G. , Fukuyama, S. , Sanjo, H. , D'Souza, C. , Norris, P.S. , Pfeffer, K. , Murphy, K.M. , 2009. T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding microenvironment. J. Immunol. 183, 7286–7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba, S. , Baghdadi, M. , Akiba, H. , Yoshiyama, H. , Kinoshita, I. , Dosaka-Akita, H. , Fujioka, Y. , Ohba, Y. , Gorman, J.V. , Colgan, J.D. , 2012. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 13, 832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, H. , Eaton, D. , Gonzalez, L. , Grogan, J.L. , Hackney, J. , Harden, K.D. , Yu, X. , 2009. Novel Compositions and Methods for the Treatment of Immune Related Diseases W.I.P. Organization, ed. (Genentech, Inc.) [Google Scholar]
- Clayton, K.L. , Haaland, M.S. , Douglas-Vail, M.B. , Mujib, S. , Chew, G.M. , Ndhlovu, L.C. , Ostrowski, M.A. , 2014. T cell Ig and mucin domain-containing protein 3 is recruited to the immune synapse, disrupts stable synapse formation, and associates with receptor phosphatases. J. Immunol. 192, 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compaan, D.M. , Gonzalez, L.C. , Tom, I. , Loyet, K.M. , Eaton, D. , Hymowitz, S.G. , 2005. Attenuating lymphocyte activity: the crystal structure of the BTLA-HVEM complex. J. Biol. Chem. 280, 39553–39561. [DOI] [PubMed] [Google Scholar]
- Corse, E. , Allison, J.P. , 2012. Cutting edge: CTLA-4 on effector T cells inhibits in trans. J. Immunol. 189, 1123–1127. [DOI] [PubMed] [Google Scholar]
- Costello, R.T. , Mallet, F. , Barbarat, B. , Schiano De Colella, J.M. , Sainty, D. , Sweet, R.W. , Truneh, A. , Olive, D. , 2003. Stimulation of non-Hodgkin's lymphoma via HVEM: an alternate and safe way to increase Fas-induced apoptosis and improve tumor immunogenicity. Leukemia. 17, 2500–2507. [DOI] [PubMed] [Google Scholar]
- Crespo, J. , Sun, H. , Welling, T.H. , Tian, Z. , Zou, W. , 2013. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 25, 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel, T.J. , Wei, S. , Dong, H. , Alvarez, X. , Cheng, P. , Mottram, P. , Krzysiek, R. , Knutson, K.L. , Daniel, B. , Zimmermann, M.C. , 2003. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 9, 562–567. [DOI] [PubMed] [Google Scholar]
- Dardalhon, V. , Anderson, A.C. , Karman, J. , Apetoh, L. , Chandwaskar, R. , Lee, D.H. , Cornejo, M. , Nishi, N. , Yamauchi, A. , Quintana, F.J. , 2010. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J. Immunol. 185, 1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dariavach, P. , Mattei, M.G. , Golstein, P. , Lefranc, M.P. , 1988. Human Ig superfamily CTLA-4 gene: chromosomal localization and identity of protein sequence between murine and human CTLA-4 cytoplasmic domains. Eur. J. Immunol. 18, 1901–1905. [DOI] [PubMed] [Google Scholar]
- Deaglio, S. , Dwyer, K.M. , Gao, W. , Friedman, D. , Usheva, A. , Erat, A. , Chen, J.F. , Enjyoji, K. , Linden, J. , Oukka, M. , 2007. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKruyff, R.H. , Bu, X. , Ballesteros, A. , Santiago, C. , Chim, Y.L. , Lee, H.H. , Karisola, P. , Pichavant, M. , Kaplan, G.G. , Umetsu, D.T. , 2010. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J. Immunol. 184, 1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio, M.L. , Lucas, C.L. , Buhler, L. , Rayat, G. , Rodriguez-Barbosa, J.I. , 2010. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J. Leukoc. Biol. 87, 223–235. [DOI] [PubMed] [Google Scholar]
- Demeure, C.E. , Wolfers, J. , Martin-Garcia, N. , Gaulard, P. , Triebel, F. , 2001. T lymphocytes infiltrating various tumour types express the MHC class II ligand lymphocyte activation gene-3 (LAG-3): role of LAG-3/MHC class II interactions in cell-cell contacts. Eur. J. Cancer. 37, 1709–1718. [DOI] [PubMed] [Google Scholar]
- Derre, L. , Rivals, J.P. , Jandus, C. , Pastor, S. , Rimoldi, D. , Romero, P. , Michielin, O. , Olive, D. , Speiser, D.E. , 2010. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J. Clin. Invest. 120, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilard, E. , Xerri, L. , Dubreuil, P. , Lopez, M. , Reymond, N. , 2013. Nectin-3 (CD113) interacts with Nectin-2 (CD112) to promote lymphocyte transendothelial migration. PLoS One. 8, e77424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaghy, H. , Bosnjak, L. , Harman, A.N. , Marsden, V. , Tyring, S.K. , Meng, T.C. , Cunningham, A.L. , 2009. Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J. Virol. 83, 1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, H. , Strome, S.E. , Salomao, D.R. , Tamura, H. , Hirano, F. , Flies, D.B. , Roche, P.C. , Lu, J. , Zhu, G. , Tamada, K. , 2002. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800. [DOI] [PubMed] [Google Scholar]
- Dong, H. , Zhu, G. , Tamada, K. , Chen, L. , 1999. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5, 1365–1369. [DOI] [PubMed] [Google Scholar]
- Duhen, T. , Pasero, C. , Mallet, F. , Barbarat, B. , Olive, D. , Costello, R.T. , 2004. LIGHT costimulates CD40 triggering and induces immunoglobulin secretion; a novel key partner in T cell-dependent B cell terminal differentiation. Eur. J. Immunol. 34, 3534–3541. [DOI] [PubMed] [Google Scholar]
- El-Sherbiny, Y.M. , Meade, J.L. , Holmes, T.D. , McGonagle, D. , Mackie, S.L. , Morgan, A.W. , Cook, G. , Feyler, S. , Richards, S.J. , Davies, F.E. , 2007. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 67, 8444–8449. [DOI] [PubMed] [Google Scholar]
- Erdmann, A.A. , Gao, Z.G. , Jung, U. , Foley, J. , Borenstein, T. , Jacobson, K.A. , Fowler, D.H. , 2005. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 105, 4707–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farren, T.W. , Giustiniani, J. , Fanous, M. , Liu, F. , Macey, M.G. , Wright, F. , Prentice, A. , Nathwani, A. , Agrawal, S.G. , 2015. Minimal residual disease detection with tumor-specific CD160 correlates with event-free survival in chronic lymphocytic leukemia. Blood Cancer J. 5, e273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farren, T.W. , Giustiniani, J. , Liu, F.T. , Tsitsikas, D.A. , Macey, M.G. , Cavenagh, J.D. , Oakervee, H.E. , Taussig, D. , Newland, A.C. , Calaminici, M. , 2011. Differential and tumor-specific expression of CD160 in B-cell malignancies. Blood. 118, 2174–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman, P. , Bar-Yehuda, S. , Synowitz, M. , Powell, J.D. , Klotz, K.N. , Gessi, S. , Borea, P.A. , 2009. Adenosine receptors and cancer. Handbook Exp. Pharmacol. 399–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flies, D.B. , Han, X. , Higuchi, T. , Zheng, L. , Sun, J. , Ye, J.J. , Chen, L. , 2014. Coinhibitory receptor PD-1H preferentially suppresses CD4(+) T cell-mediated immunity. J. Clin. Invest. 124, 1966–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flies, D.B. , Wang, S. , Xu, H. , Chen, L. , 2011. Cutting edge: a monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J. Immunol. 187, 1537–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follows, E.R. , McPheat, J.C. , Minshull, C. , Moore, N.C. , Pauptit, R.A. , Rowsell, S. , Stacey, C.L. , Stanway, J.J. , Taylor, I.W. , Abbott, W.M. , 2001. Study of the interaction of the medium chain mu 2 subunit of the clathrin-associated adapter protein complex 2 with cytotoxic T-lymphocyte antigen 4 and CD28. Biochem. J. 359, 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade, J. , Sun, Z. , Benallaoua, M. , Guillaume, P. , Luescher, I.F. , Sander, C. , Kirkwood, J.M. , Kuchroo, V. , Zarour, H.M. , 2010. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 207, 2175–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade, J. , Sun, Z. , Pagliano, O. , Guillaume, P. , Luescher, I.F. , Sander, C. , Kirkwood, J.M. , Olive, D. , Kuchroo, V. , Zarour, H.M. , 2012. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 72, 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco, L.M. , Salinas, V.H. , Brown, K.E. , Vanguri, V.K. , Freeman, G.J. , Kuchroo, V.K. , Sharpe, A.H. , 2009. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman, W.H. , Pages, F. , Sautes-Fridman, C. , Galon, J. , 2012. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer. 12, 298–306. [DOI] [PubMed] [Google Scholar]
- Fuchs, A. , Colonna, M. , 2006. The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin. Cancer Biol. 16, 359–366. [DOI] [PubMed] [Google Scholar]
- Furness, A.J. , Vargas, F.A. , Peggs, K.S. , Quezada, S.A. , 2014. Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies. Trends Immunol. 35, 290–298. [DOI] [PubMed] [Google Scholar]
- Gattinoni, L. , Ranganathan, A. , Surman, D.R. , Palmer, D.C. , Antony, P.A. , Theoret, M.R. , Heimann, D.M. , Rosenberg, S.A. , Restifo, N.P. , 2006. CTLA-4 dysregulation of self/tumor-reactive CD8+ T-cell function is CD4+ T-cell dependent. Blood. 108, 3818–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur, S. , Shively, J.E. , Yen, Y. , Gaur, R.K. , 2008. Altered splicing of CEACAM1 in breast cancer: identification of regulatory sequences that control splicing of CEACAM1 into long or short cytoplasmic domain isoforms. Mol. Cancer. 7, 46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng, H. , Zhang, G.M. , Li, D. , Zhang, H. , Yuan, Y. , Zhu, H.G. , Xiao, H. , Han, L.F. , Feng, Z.H. , 2006. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J. Immunol. 176, 1411–1420. [DOI] [PubMed] [Google Scholar]
- Ghebeh, H. , Barhoush, E. , Tulbah, A. , Elkum, N. , Al-Tweigeri, T. , Dermime, S. , 2008. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer. 8, 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiotto, M. , Gauthier, L. , Serriari, N. , Pastor, S. , Truneh, A. , Nunes, J.A. , Olive, D. , 2010. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int. Immunol. 22, 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, H.M. , Hedgcock, C.J. , Aufiero, B.M. , Wilson, A.J. , Hafner, M.S. , Tsokos, G.C. , Wong, H.K. , 2007. Induction of the CTLA-4 gene in human lymphocytes is dependent on NFAT binding the proximal promoter. J. Immunol. 179, 3831–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustiniani, J. , Bensussan, A. , Marie-Cardine, A. , 2009. Identification and characterization of a transmembrane isoform of CD160 (CD160-TM), a unique activating receptor selectively expressed upon human NK cell activation. J. Immunol. 182, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding, S.R. , Wilson, K.A. , Xie, Y. , Harris, K.M. , Baxi, A. , Akpinarli, A. , Fulton, A. , Tamada, K. , Strome, S.E. , Antony, P.A. , 2013. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J. Immunol. 190, 4899–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good-Jacobson, K.L. , Szumilas, C.G. , Chen, L. , Sharpe, A.H. , Tomayko, M.M. , Shlomchik, M.J. , 2010. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 11, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski, R. , Chen, Z. , Kai, Y. , Lee, L. , Wong, S. , Marsden, P.A. , 2004. CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J. Immunol. 172, 7744–7749. [DOI] [PubMed] [Google Scholar]
- Gorczynski, R.M. , Cattral, M.S. , Chen, Z. , Hu, J. , Lei, J. , Min, W.P. , Yu, G. , Ni, J. , 1999. An immunoadhesin incorporating the molecule OX-2 is a potent immunosuppressant that prolongs allo- and xenograft survival. J. Immunol. 163, 1654–1660. [PubMed] [Google Scholar]
- Gorczynski, R.M. , Chen, Z. , Diao, J. , Khatri, I. , Wong, K. , Yu, K. , Behnke, J. , 2010. Breast cancer cell CD200 expression regulates immune response to EMT6 tumor cells in mice. Breast Cancer Res. Treat. 123, 405–415. [DOI] [PubMed] [Google Scholar]
- Gorczynski, Reginald M., and David A. Clark. 2005. “Methods and Compositions for Modulating Immunity.” (patent: US20050048069).
- Gorczynski, Reginald M., and Karrie Ka Wai Wong. 2009. “Assay for Soluble CD200.” (patent: WO2009121162A1).
- Gorman, J.V. , Colgan, J.D. , 2014. Regulation of T cell responses by the receptor molecule Tim-3. Immunologic Res. 59, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene, J.L. , Leytze, G.M. , Emswiler, J. , Peach, R. , Bajorath, J. , Cosand, W. , Linsley, P.S. , 1996. Covalent dimerization of CD28/CTLA-4 and oligomerization of CD80/CD86 regulate T cell costimulatory interactions. J. Biol. Chem. 271, 26762–26771. [DOI] [PubMed] [Google Scholar]
- Grogan, J. , Johnston, R.J. , Irving, B. , Hackney, J. , Yu, X. , Eaton, D. , Bowles, K. , Comps-Agrar, L. , 2015. Methods of Treating Cancer Using PD-1 axis Binding Antagonists and TIGIT Inhibitors W.I.P. Organization, ed. (Genentech, Inc., Hoffmann-La Roche Ag) [Google Scholar]
- Grosso, J.F. , Kelleher, C.C. , Harris, T.J. , Maris, C.H. , Hipkiss, E.L. , De Marzo, A. , Anders, R. , Netto, G. , Getnet, D. , Bruno, T.C. , 2007. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Invest. 117, 3383–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu-Trantien, C. , Loi, S. , Garaud, S. , Equeter, C. , Libin, M. , de Wind, A. , Ravoet, M. , Le Buanec, H. , Sibille, C. , Manfouo-Foutsop, G. , 2013. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Invest. 123, 2873–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanishi, J. , Mandai, M. , Iwasaki, M. , Okazaki, T. , Tanaka, Y. , Yamaguchi, K. , Higuchi, T. , Yagi, H. , Takakura, K. , Minato, N. , 2007. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. U S A. 104, 3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid, O. , Robert, C. , Daud, A. , Hodi, F.S. , Hwu, W.J. , Kefford, R. , Wolchok, J.D. , Hersey, P. , Joseph, R.W. , Weber, J.S. , 2013. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. New Engl. J. Med. 369, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannier, S. , Tournier, M. , Bismuth, G. , Triebel, F. , 1998. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J. Immunol. 161, 4058–4065. [PubMed] [Google Scholar]
- Hannier, S. , Triebel, F. , 1999. The MHC class II ligand lymphocyte activation gene-3 is co-distributed with CD8 and CD3-TCR molecules after their engagement by mAb or peptide-MHC class I complexes. Int. Immunol. 11, 1745–1752. [DOI] [PubMed] [Google Scholar]
- Hasko, G. , Linden, J. , Cronstein, B. , Pacher, P. , 2008. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nature reviews. Drug Discov. 7, 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings, W.D. , Anderson, D.E. , Kassam, N. , Koguchi, K. , Greenfield, E.A. , Kent, S.C. , Zheng, X.X. , Strom, T.B. , Hafler, D.A. , Kuchroo, V.K. , 2009. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 39, 2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathcock, K.S. , Laszlo, G. , Pucillo, C. , Linsley, P. , Hodes, R.J. , 1994. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J. Exp. Med. 180, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemon, P. , Jean-Louis, F. , Ramgolam, K. , Brignone, C. , Viguier, M. , Bachelez, H. , Triebel, F. , Charron, D. , Aoudjit, F. , Al-Daccak, R. , Michel, L. , 2011. MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J. Immunol. 186, 5173–5183. [DOI] [PubMed] [Google Scholar]
- Heusschen, R. , Griffioen, A.W. , Thijssen, V.L. , 2013. Galectin-9 in tumor biology: a jack of multiple trades. Biochim. Biophys. Acta. 1836, 177–185. [DOI] [PubMed] [Google Scholar]
- Hinoda, Y. , Neumaier, M. , Hefta, S.A. , Drzeniek, Z. , Wagener, C. , Shively, L. , Hefta, L.J. , Shively, J.E. , Paxton, R.J. , 1988. Molecular cloning of a cDNA coding biliary glycoprotein I: primary structure of a glycoprotein immunologically crossreactive with carcinoembryonic antigen. Proc. Natl. Acad. Sci. U S A. 85, 6959–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano, F. , Kaneko, K. , Tamura, H. , Dong, H. , Wang, S. , Ichikawa, M. , Rietz, C. , Flies, D.B. , Lau, J.S. , Zhu, G. , 2005. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 65, 1089–1096. [PubMed] [Google Scholar]
- Hodi, F.S. , Mihm, M.C. , Soiffer, R.J. , Haluska, F.G. , Butler, M. , Seiden, M.V. , Davis, T. , Henry-Spires, R. , MacRae, S. , Willman, A. , 2003. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl. Acad. Sci. U S A. 100, 4712–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi, F.S. , O'Day, S.J. , McDermott, D.F. , Weber, R.W. , Sosman, J.A. , Haanen, J.B. , Gonzalez, R. , Robert, C. , Schadendorf, D. , Hassel, J.C. , 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek, R.M. , Ruuls, S.R. , Murphy, C.A. , Wright, G.J. , Goddard, R. , Zurawski, S.M. , Blom, B. , Homola, M.E. , Streit, W.J. , Brown, M.H. , 2000. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science. 290, 1768–1771. [DOI] [PubMed] [Google Scholar]
- Hokuto, D. , Sho, M. , Yamato, I. , Yasuda, S. , Obara, S. , Nomi, T. , Nakajima, Y. , 2015. Clinical impact of herpesvirus entry mediator expression in human hepatocellular carcinoma. Eur. J. Cancer. 51, 157–165. [DOI] [PubMed] [Google Scholar]
- Hsu, H. , Solovyev, I. , Colombero, A. , Elliott, R. , Kelley, M. , Boyle, W.J. , 1997. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. J. Biol. Chem. 272, 13471–13474. [DOI] [PubMed] [Google Scholar]
- Huang, C.T. , Workman, C.J. , Flies, D. , Pan, X. , Marson, A.L. , Zhou, G. , Hipkiss, E.L. , Ravi, S. , Kowalski, J. , Levitsky, H.I. , 2004. Role of LAG-3 in regulatory T cells. Immunity. 21, 503–513. [DOI] [PubMed] [Google Scholar]
- Huang, J. , Simpson, J.F. , Glackin, C. , Riethorf, L. , Wagener, C. , Shively, J.E. , 1998. Expression of biliary glycoprotein (CD66a) in normal and malignant breast epithelial cells. Anticancer Res. 18, 3203–3212. [PubMed] [Google Scholar]
- Huang, S. , Apasov, S. , Koshiba, M. , Sitkovsky, M. , 1997. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 90, 1600–1610. [PubMed] [Google Scholar]
- Huang, Y.H. , Zhu, C. , Kondo, Y. , Anderson, A.C. , Gandhi, A. , Russell, A. , Dougan, S.K. , Petersen, B.S. , Melum, E. , Pertel, T. , 2015. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 517, 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard, B. , Tournier, M. , Hercend, T. , Triebel, F. , Faure, F. , 1994. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur. J. Immunol. 24, 3216–3221. [DOI] [PubMed] [Google Scholar]
- Huber, M. , Izzi, L. , Grondin, P. , Houde, C. , Kunath, T. , Veillette, A. , Beauchemin, N. , 1999. The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J. Biol. Chem. 274, 335–344. [DOI] [PubMed] [Google Scholar]
- Hurwitz, A.A. , Foster, B.A. , Kwon, E.D. , Truong, T. , Choi, E.M. , Greenberg, N.M. , Burg, M.B. , Allison, J.P. , 2000. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 60, 2444–2448. [PubMed] [Google Scholar]
- Idzko, M. , Ferrari, D. , Eltzschig, H.K. , 2014. Nucleotide signalling during inflammation. Nature. 509, 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima, H. , Neurath, M.F. , Nagaishi, T. , Glickman, J.N. , Nieuwenhuis, E.E. , Nakajima, A. , Chen, D. , Fuss, I.J. , Utku, N. , Lewicki, D.N. , 2004. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J. Exp. Med. 199, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, T. , Sho, M. , Yasuda, S. , Nishiwada, S. , Nakamura, S. , Ueda, T. , Nishigori, N. , Kawasaki, K. , Obara, S. , Nakamoto, T. , 2015. HVEM expression contributes to tumor progression and prognosis in human colorectal cancer. Anticancer Res. 35, 1361–1367. [PubMed] [Google Scholar]
- Iouzalen, N. , Andreae, S. , Hannier, S. , Triebel, F. , 2001. LAP, a lymphocyte activation gene-3 (LAG-3)-associated protein that binds to a repeated EP motif in the intracellular region of LAG-3, may participate in the down-regulation of the CD3/TCR activation pathway. Eur. J. Immunol. 31, 2885–2891. [DOI] [PubMed] [Google Scholar]
- Irie, A. , Yamauchi, A. , Kontani, K. , Kihara, M. , Liu, D. , Shirato, Y. , Seki, M. , Nishi, N. , Nakamura, T. , Yokomise, H. , Hirashima, M. , 2005. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 11, 2962–2968. [DOI] [PubMed] [Google Scholar]
- Ishida, Y. , Agata, Y. , Shibahara, K. , Honjo, T. , 1992. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11, 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai, Y. , Ishida, M. , Tanaka, Y. , Okazaki, T. , Honjo, T. , Minato, N. , 2002. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. U S A. 99, 12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki, A. , Welker, R. , Mueller, S. , Linehan, M. , Nomoto, A. , Wimmer, E. , 2002. Immunofluorescence analysis of poliovirus receptor expression in Peyer's patches of humans, primates, and CD155 transgenic mice: implications for poliovirus infection. J. Infect. Dis. 186, 585–592. [DOI] [PubMed] [Google Scholar]
- Izzi, L. , Turbide, C. , Houde, C. , Kunath, T. , Beauchemin, N. , 1999. cis-Determinants in the cytoplasmic domain of CEACAM1 responsible for its tumor inhibitory function. Oncogene. 18, 5563–5572. [DOI] [PubMed] [Google Scholar]
- Jiang, J. , Jin, M.S. , Kong, F. , Cao, D. , Ma, H.X. , Jia, Z. , Wang, Y.P. , Suo, J. , Cao, X. , 2013. Decreased galectin-9 and increased Tim-3 expression are related to poor prognosis in gastric cancer. PloS One. 8, e81799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, R.J. , Comps-Agrar, L. , Hackney, J. , Yu, X. , Huseni, M. , Yang, Y. , Park, S. , Javinal, V. , Chiu, H. , Irving, B. , 2014. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 26, 923–937. [DOI] [PubMed] [Google Scholar]
- Joller, N. , Hafler, J.P. , Brynedal, B. , Kassam, N. , Spoerl, S. , Levin, S.D. , Sharpe, A.H. , Kuchroo, V.K. , 2011. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 186, 1338–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joller, N. , Lozano, E. , Burkett, P.R. , Patel, B. , Xiao, S. , Zhu, C. , Xia, J. , Tan, T.G. , Sefik, E. , Yajnik, V. , 2014. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 40, 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, R.B. , Ndhlovu, L.C. , Barbour, J.D. , Sheth, P.M. , Jha, A.R. , Long, B.R. , Wong, J.C. , Satkunarajah, M. , Schweneker, M. , Chapman, J.M. , 2008. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 205, 2763–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz, S.Z. , Lu, L.F. , Rudensky, A.Y. , 2012. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajsa, W. , 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 332, 271–275. [DOI] [PubMed] [Google Scholar]
- Kammerer, R. , Hahn, S. , Singer, B.B. , Luo, J.S. , von Kleist, S. , 1998. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur. J. Immunol. 28, 3664–3674. [DOI] [PubMed] [Google Scholar]
- Kashio, Y. , Nakamura, K. , Abedin, M.J. , Seki, M. , Nishi, N. , Yoshida, N. , Nakamura, T. , Hirashima, M. , 2003. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J. Immunol. 170, 3631–3636. [DOI] [PubMed] [Google Scholar]
- Keir, M.E. , Butte, M.J. , Freeman, G.J. , Sharpe, A.H. , 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter, A.L. , Godbout, E.J. , McNally, J.P. , Sereti, I. , Roby, G.A. , O'Shea, M.A. , Fauci, A.S. , 2008. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J. Immunol. 181, 6738–6746. [DOI] [PubMed] [Google Scholar]
- Kitano, S. , Tsuji, T. , Liu, C. , Hirschhorn-Cymerman, D. , Kyi, C. , Mu, Z. , Allison, J.P. , Gnjatic, S. , Yuan, J.D. , Wolchok, J.D. , 2013. Enhancement of tumor-reactive cytotoxic CD4+ T cell responses after ipilimumab treatment in four advanced melanoma patients. Cancer Immunol. Res. 1, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, K.F. , Fu, G. , Zhang, Y. , Yokosuka, T. , Casas, J. , Canonigo-Balancio, A.J. , Becart, S. , Kim, G. , Yates, J.R. , Kronenberg, M. , 2014. Protein kinase C-eta controls CTLA-4-mediated regulatory T cell function. Nat. Immunol. 15, 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi, J. , Yamazaki, K. , Azuma, M. , Kinoshita, I. , Dosaka-Akita, H. , Nishimura, M. , 2004. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 10, 5094–5100. [DOI] [PubMed] [Google Scholar]
- Koshiba, M. , Rosin, D.L. , Hayashi, N. , Linden, J. , Sitkovsky, M.V. , 1999. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol. Pharmacol. 55, 614–624. [PubMed] [Google Scholar]
- Kouo, T. , Huang, L. , Pucsek, A.B. , Cao, M. , Solt, S. , Armstrong, T. , Jaffee, E. , 2015. Galectin-3 shapes antitumor immune responses by suppressing CD8+ T cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol. Res. 3, 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz-Rommel, A. , Qin, F. , Dakappagari, N. , Cofiell, R. , Faas, S.J. , Bowdish, K.S. , 2008. Blockade of CD200 in the presence or absence of antibody effector function: implications for anti-CD200 therapy. J. Immunol. 180, 699–705. [DOI] [PubMed] [Google Scholar]
- Kretz-Rommel, A. , Qin, F. , Dakappagari, N. , Ravey, E.P. , McWhirter, J. , Oltean, D. , Frederickson, S. , Maruyama, T. , Wild, M.A. , Nolan, M.J. , 2007. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J. Immunol. 178, 5595–5605. [DOI] [PubMed] [Google Scholar]
- Krieg, C. , Han, P. , Stone, R. , Goularte, O.D. , Kaye, J. , 2005. Functional Analysis of B and T lymphocyte attenuator engagement on CD4+ and CD8+ T cells. J. Immunol. 175, 6420–6427. [DOI] [PubMed] [Google Scholar]
- Kryczek, I. , Zou, L. , Rodriguez, P. , Zhu, G. , Wei, S. , Mottram, P. , Brumlik, M. , Cheng, P. , Curiel, T. , Myers, L. , 2006. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 203, 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, E.D. , Drake, C.G. , Scher, H.I. , Fizazi, K. , Bossi, A. , van den Eertwegh, A.J. , Krainer, M. , Houede, N. , Santos, R. , Mahammedi, H. , 2014. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, B.S. , Tan, K.B. , Ni, J. , Oh, K.O. , Lee, Z.H. , Kim, K.K. , Kim, Y.J. , Wang, S. , Gentz, R. , Yu, G.L. , 1997. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J. Biol. Chem. 272, 14272–14276. [DOI] [PubMed] [Google Scholar]
- Kwon, E.D. , Hurwitz, A.A. , Foster, B.A. , Madias, C. , Feldhaus, A.L. , Greenberg, N.M. , Burg, M.B. , Allison, J.P. , 1997. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc. Natl. Acad. Sci. U S A. 94, 8099–8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm, H. , Andre, S. , Hoeflich, A. , Fischer, J.R. , Sordat, B. , Kaltner, H. , Wolf, E. , Gabius, H.J. , 2001. Comprehensive galectin fingerprinting in a panel of 61 human tumor cell lines by RT-PCR and its implications for diagnostic and therapeutic procedures. J. Cancer Res. Clin. Oncol. 127, 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas, C.M. , Rieger, J.M. , Linden, J. , 2005. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J. Immunol. 174, 1073–1080. [DOI] [PubMed] [Google Scholar]
- Latchman, Y. , Wood, C.R. , Chernova, T. , Chaudhary, D. , Borde, M. , Chernova, I. , Iwai, Y. , Long, A.J. , Brown, J.A. , Nunes, R. , 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2, 261–268. [DOI] [PubMed] [Google Scholar]
- Latchman, Y.E. , Liang, S.C. , Wu, Y. , Chernova, T. , Sobel, R.A. , Klemm, M. , Kuchroo, V.K. , Freeman, G.J. , Sharpe, A.H. , 2004. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. U S A. 101, 10691–10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouteiller, P. , Barakonyi, A. , Giustiniani, J. , Lenfant, F. , Marie-Cardine, A. , Aguerre-Girr, M. , Rabot, M. , Hilgert, I. , Mami-Chouaib, F. , Tabiasco, J. , 2002. Engagement of CD160 receptor by HLA-C is a triggering mechanism used by circulating natural killer (NK) cells to mediate cytotoxicity. Proc. Natl. Acad. Sci. U S A. 99, 16963–16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Mercier, I. , Chen, W. , Lines, J.L. , Day, M. , Li, J. , Sergent, P. , Noelle, R.J. , Wang, L. , 2014. VISTA regulates the development of protective antitumor immunity. Cancer Res. 74, 1933–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach, D.R. , Krummel, M.F. , Allison, J.P. , 1996. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 271, 1734–1736. [DOI] [PubMed] [Google Scholar]
- Lebbink, R.J. , de Ruiter, T. , Adelmeijer, J. , Brenkman, A.B. , van Helvoort, J.M. , Koch, M. , Farndale, R.W. , Lisman, T. , Sonnenberg, A. , Lenting, P.J. , Meyaard, L. , 2006. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J. Exp. Med. 203, 1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebbink, R.J. , de Ruiter, T. , Verbrugge, A. , Bril, W.S. , Meyaard, L. , 2004. The mouse homologue of the leukocyte-associated Ig-like Receptor-1 is an inhibitory receptor that recruits Src homology region 2-containing protein tyrosine phosphatase (SHP)-2, but not SHP-1. J. Immunol. 172, 5535–5543. [DOI] [PubMed] [Google Scholar]
- Leitner, J. , Rieger, A. , Pickl, W.F. , Zlabinger, G. , Grabmeier-Pfistershammer, K. , Steinberger, P. , 2013. TIM-3 does not act as a receptor for galectin-9. PLoS Pathog. 9, e1003253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesokhin, A.M. , Callahan, M.K. , Postow, M.A. , Wolchok, J.D. , 2015. On being less tolerant: enhanced cancer immunosurveillance enabled by targeting checkpoints and agonists of T cell activation. Sci. Translational Med. 7, 280sr281 [DOI] [PubMed] [Google Scholar]
- Lesterhuis, W.J. , Steer, H. , Lake, R.A. , 2011. PD-L2 is predominantly expressed by Th2 cells. Mol. Immunol. 49, 1–3. [DOI] [PubMed] [Google Scholar]
- Leung, N. , Turbide, C. , Olson, M. , Marcus, V. , Jothy, S. , Beauchemin, N. , 2006. Deletion of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) gene contributes to colon tumor progression in a murine model of carcinogenesis. Oncogene. 25, 5527–5536. [DOI] [PubMed] [Google Scholar]
- Levin, S.D. , Taft, D.W. , Brandt, C.S. , Bucher, C. , Howard, E.D. , Chadwick, E.M. , Johnston, J. , Hammond, A. , Bontadelli, K. , Ardourel, D. , 2011. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur. J. Immunol. 41, 902–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Xia, P. , Du, Y. , Liu, S. , Huang, G. , Chen, J. , Zhang, H. , Hou, N. , Cheng, X. , Zhou, L. , 2014. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. J. Biol. Chem. 289, 17647–17657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. , Mu, L. , Wang, J. , Zhang, J. , Xie, X. , Kong, Q. , Tang, W. , Yao, X. , Liu, Y. , Wang, L. , 2012. Activation of the adenosine A2A receptor attenuates experimental autoimmune myasthenia gravis severity. Eur. J. Immunol. 42, 1140–1151. [DOI] [PubMed] [Google Scholar]
- Liang, S.C. , Latchman, Y.E. , Buhlmann, J.E. , Tomczak, M.F. , Horwitz, B.H. , Freeman, G.J. , Sharpe, A.H. , 2003. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 33, 2706–2716. [DOI] [PubMed] [Google Scholar]
- Lines, J.L. , Pantazi, E. , Mak, J. , Sempere, L.F. , Wang, L. , O'Connell, S. , Ceeraz, S. , Suriawinata, A.A. , Yan, S. , Ernstoff, M.S. , Noelle, R. , 2014. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 74, 1924–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines, J.L. , Sempere, L.F. , Broughton, T. , Wang, L. , Noelle, R. , 2014. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol. Res. 2, 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, V. , Wu, P.W. , Finnerty, H.F. , Sharpe, A.H. , Gray, G.S. , Collins, M. , 1999. Complete sequence determination of the mouse and human CTLA4 gene loci: cross-species DNA sequence similarity beyond exon borders. Genomics. 60, 341–355. [DOI] [PubMed] [Google Scholar]
- Lopez, M. , Aoubala, M. , Jordier, F. , Isnardon, D. , Gomez, S. , Dubreuil, P. , 1998. The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood. 92, 4602–4611. [PubMed] [Google Scholar]
- Lozano, E. , Dominguez-Villar, M. , Kuchroo, V. , Hafler, D.A. , 2012. The TIGIT/CD226 axis regulates human T cell function. J. Immunol. 188, 3869–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maasho, K. , Masilamani, M. , Valas, R. , Basu, S. , Coligan, J.E. , Borrego, F. , 2005. The inhibitory leukocyte-associated Ig-like receptor-1 (LAIR-1) is expressed at high levels by human naive T cells and inhibits TCR mediated activation. Mol. Immunol. 42, 1521–1530. [DOI] [PubMed] [Google Scholar]
- Maeda, M. , Carpenito, C. , Russell, R.C. , Dasanjh, J. , Veinotte, L.L. , Ohta, H. , Yamamura, T. , Tan, R. , Takei, F. , 2005. Murine CD160, Ig-like receptor on NK cells and NKT cells, recognizes classical and nonclassical MHC class I and regulates NK cell activation. J. Immunol. 175, 4426–4432. [DOI] [PubMed] [Google Scholar]
- Alexander, W. 2011. American society of hematology, 52nd annual meeting and exposition. P T 36, 100–103. [PMC free article] [PubMed]
- Maiza, H. , Leca, G. , Mansur, I.G. , Schiavon, V. , Boumsell, L. , Bensussan, A. , 1993. A novel 80-kD cell surface structure identifies human circulating lymphocytes with natural killer activity. J. Exp. Med. 178, 1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maker, A.V. , Phan, G.Q. , Attia, P. , Yang, J.C. , Sherry, R.M. , Topalian, S.L. , Kammula, U.S. , Royal, R.E. , Haworth, L.R. , Levy, C. , 2005. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann. Surg. Oncol. 12, 1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniwa, Y. , Nishio, W. , Okita, Y. , Yoshimura, M. , 2012. Expression of nectin 3: novel prognostic marker of lung adenocarcinoma. Thorac. Cancer. 3, 175–181. [DOI] [PubMed] [Google Scholar]
- Margolin, K. , Ernstoff, M.S. , Hamid, O. , Lawrence, D. , McDermott, D. , Puzanov, I. , Wolchok, J.D. , Clark, J.I. , Sznol, M. , Logan, T.F. , 2012. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 13, 459–465. [DOI] [PubMed] [Google Scholar]
- Markel, G. , Lieberman, N. , Katz, G. , Arnon, T.I. , Lotem, M. , Drize, O. , Blumberg, R.S. , Bar-Haim, E. , Mader, R. , Eisenbach, L. , Mandelboim, O. , 2002. CD66a interactions between human melanoma and NK cells: a novel class I MHC-independent inhibitory mechanism of cytotoxicity. J. Immunol. 168, 2803–2810. [DOI] [PubMed] [Google Scholar]
- Markel, G. , Ortenberg, R. , Seidman, R. , Sapoznik, S. , Koren-Morag, N. , Besser, M.J. , Bar, J. , Shapira, R. , Kubi, A. , Nardini, G. , 2010. Systemic dysregulation of CEACAM1 in melanoma patients. Cancer Immunol. Immunother. 59, 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markel, G. , Seidman, R. , Stern, N. , Cohen-Sinai, T. , Izhaki, O. , Katz, G. , Besser, M. , Treves, A.J. , Blumberg, R.S. , Loewenthal, R. , 2006. Inhibition of human tumor-infiltrating lymphocyte effector functions by the homophilic carcinoembryonic cell adhesion molecule 1 interactions. J. Immunol. 177, 6062–6071. [DOI] [PubMed] [Google Scholar]
- Markel, G. , Wolf, D. , Hanna, J. , Gazit, R. , Goldman-Wohl, D. , Lavy, Y. , Yagel, S. , Mandelboim, O. , 2002. Pivotal role of CEACAM1 protein in the inhibition of activated decidual lymphocyte functions. J. Clin. Invest. 110, 943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsters, S.A. , Ayres, T.M. , Skubatch, M. , Gray, C.L. , Rothe, M. , Ashkenazi, A. , 1997. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J. Biol. Chem. 272, 14029–14032. [DOI] [PubMed] [Google Scholar]
- Martin, T.A. , Lane, J. , Harrison, G.M. , Jiang, W.G. , 2013. The expression of the Nectin complex in human breast cancer and the role of Nectin-3 in the control of tight junctions during metastasis. PLoS One. 8, e82696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter, J.R. , Kretz-Rommel, A. , Saven, A. , Maruyama, T. , Potter, K.N. , Mockridge, C.I. , Ravey, E.P. , Qin, F. , Bowdish, K.S. , 2006. Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc. Natl. Acad. Sci. U S A. 103, 1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead, K.I. , Zheng, Y. , Manzotti, C.N. , Perry, L.C.A. , Liu, M.K.P. , Burke, F. , Powner, D.J. , Wakelam, M.J.O. , Sansom, D.M. , 2005. Exocytosis of CTLA-4 is dependent on phospholipase D and ADP ribosylation Factor-1 and stimulated during activation of regulatory T cells. J. Immunol. 174, 4803–4811. [DOI] [PubMed] [Google Scholar]
- Merino, J. , Ramirez, N. , Moreno, C. , Toledo, E. , Fernandez, M. , Sanchez-Ibarrola, A. , 2007. BY55/CD160 cannot be considered a cytotoxic marker in cytomegalovirus-specific human CD8(+) T cells. Clin. Exp. Immunol. 149, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messal, N. , Serriari, N.E. , Pastor, S. , Nunes, J.A. , Olive, D. , 2011. PD-L2 is expressed on activated human T cells and regulates their function. Mol. Immunol. 48, 2214–2219. [DOI] [PubMed] [Google Scholar]
- Meyaard, L. , Adema, G.J. , Chang, C. , Woollatt, E. , Sutherland, G.R. , Lanier, L.L. , Phillips, J.H. , 1997. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 7, 283–290. [DOI] [PubMed] [Google Scholar]
- Meyaard, L. , Hurenkamp, J. , Clevers, H. , Lanier, L.L. , Phillips, J.H. , 1999. Leukocyte-associated Ig-like receptor-1 functions as an inhibitory receptor on cytotoxic T cells. J. Immunol. 162, 5800–5804. [PubMed] [Google Scholar]
- Meyers, J.H. , Chakravarti, S. , Schlesinger, D. , Illes, Z. , Waldner, H. , Umetsu, S.E. , Kenny, J. , Zheng, X.X. , Umetsu, D.T. , DeKruyff, R.H. , 2005. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat. Immunol. 6, 455–464. [DOI] [PubMed] [Google Scholar]
- Meyers, J.H. , Sabatos, C.A. , Chakravarti, S. , Kuchroo, V.K. , 2005. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol. Med. 11, 362–369. [DOI] [PubMed] [Google Scholar]
- M'Hidi, H. , Thibult, M.L. , Chetaille, B. , Rey, F. , Bouadallah, R. , Nicollas, R. , Olive, D. , Xerri, L. , 2009. High expression of the inhibitory receptor BTLA in T-follicular helper cells and in B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Am. J. Clin. Pathol. 132, 589–596. [DOI] [PubMed] [Google Scholar]
- Migita, K. , Sho, M. , Shimada, K. , Yasuda, S. , Yamato, I. , Takayama, T. , Matsumoto, S. , Wakatsuki, K. , Hotta, K. , Tanaka, T. , 2014. Significant involvement of herpesvirus entry mediator in human esophageal squamous cell carcinoma. Cancer. 120, 808–817. [DOI] [PubMed] [Google Scholar]
- Misstear, K. , Chanas, S.A. , Rezaee, S.A. , Colman, R. , Quinn, L.L. , Long, H.M. , Goodyear, O. , Lord, J.M. , Hislop, A.D. , Blackbourn, D.J. , 2012. Suppression of antigen-specific T cell responses by the Kaposi's sarcoma-associated herpesvirus viral OX2 protein and its cellular orthologue, CD200. J. Virol. 86, 6246–6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki, T. , Dierich, A. , Benoist, C. , Mathis, D. , 1996. LAG-3 is not responsible for selecting T helper cells in CD4-deficient mice. Int. Immunol. 8, 725–729. [DOI] [PubMed] [Google Scholar]
- Mizui, M. , Shikina, T. , Arase, H. , Suzuki, K. , Yasui, T. , Rennert, P.D. , Kumanogoh, A. , Kikutani, H. , 2008. Bimodal regulation of T cell-mediated immune responses by TIM-4. Int. Immunol. 20, 695–708. [DOI] [PubMed] [Google Scholar]
- Moller-Hackbarth, K. , Dewitz, C. , Schweigert, O. , Trad, A. , Garbers, C. , Rose-John, S. , Scheller, J. , 2013. A disintegrin and metalloprotease (ADAM) 10 and ADAM17 are major sheddases of T cell immunoglobulin and mucin domain 3 (Tim-3). J. Biol. Chem. 288, 34529–34544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monney, L. , Sabatos, C.A. , Gaglia, J.L. , Ryu, A. , Waldner, H. , Chernova, T. , Manning, S. , Greenfield, E.A. , Coyle, A.J. , Sobel, R.A. , 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 415, 536–541. [DOI] [PubMed] [Google Scholar]
- Montgomery, R.I. , Warner, M.S. , Lum, B.J. , Spear, P.G. , 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 87, 427–436. [DOI] [PubMed] [Google Scholar]
- Morales, V.M. , Christ, A. , Watt, S.M. , Kim, H.S. , Johnson, K.W. , Utku, N. , Texieira, A.M. , Mizoguchi, A. , Mizoguchi, E. , Russell, G.J. , 1999. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a). J. Immunol. 163, 1363–1370. [PubMed] [Google Scholar]
- Moreaux, J. , Hose, D. , Reme, T. , Jourdan, E. , Hundemer, M. , Legouffe, E. , Moine, P. , Bourin, P. , Moos, M. , Corre, J. , 2006. CD200 is a new prognostic factor in multiple myeloma. Blood. 108, 4194–4197. [DOI] [PubMed] [Google Scholar]
- Moreaux, J. , Veyrune, J.L. , Reme, T. , De Vos, J. , Klein, B. , 2008. CD200: a putative therapeutic target in cancer. Biochem. Biophys. Res. Commun. 366, 117–122. [DOI] [PubMed] [Google Scholar]
- Morel, Y. , Schiano de Colella, J.M. , Harrop, J. , Deen, K.C. , Holmes, S.D. , Wattam, T.A. , Khandekar, S.S. , Truneh, A. , Sweet, R.W. , Gastaut, J.A. , 2000. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J. Immunol. 165, 4397–4404. [DOI] [PubMed] [Google Scholar]
- Mujib, S. , Jones, R.B. , Lo, C. , Aidarus, N. , Clayton, K. , Sakhdari, A. , Benko, E. , Kovacs, C. , Ostrowski, M.A. , 2012. Antigen-independent induction of Tim-3 expression on human T cells by the common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway. J. Immunol. 188, 3745–3756. [DOI] [PubMed] [Google Scholar]
- Nagaishi, T. , Pao, L. , Lin, S.H. , Iijima, H. , Kaser, A. , Qiao, S.W. , Chen, Z. , Glickman, J. , Najjar, S.M. , Nakajima, A. , 2006. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity. 25, 769–781. [DOI] [PubMed] [Google Scholar]
- Nakae, S. , Iwakura, Y. , Suto, H. , Galli, S.J. , 2007. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J. Leukoc. Biol. 81, 1258–1268. [DOI] [PubMed] [Google Scholar]
- Nakajima, A. , Iijima, H. , Neurath, M.F. , Nagaishi, T. , Nieuwenhuis, E.E. , Raychowdhury, R. , Glickman, J. , Blau, D.M. , Russell, S. , Holmes, K.V. , Blumberg, R.S. , 2002. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J. Immunol. 168, 1028–1035. [DOI] [PubMed] [Google Scholar]
- Nakayama, M. , Akiba, H. , Takeda, K. , Kojima, Y. , Hashiguchi, M. , Azuma, M. , Yagita, H. , Okumura, K. , 2009. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 113, 3821–3830. [DOI] [PubMed] [Google Scholar]
- Nazareth, M.R. , Broderick, L. , Simpson-Abelson, M.R. , Kelleher, R.J. , Yokota, S.J. , Bankert, R.B. , 2007. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J. Immunol. 178, 5552–5562. [DOI] [PubMed] [Google Scholar]
- Neumaier, M. , Paululat, S. , Chan, A. , Matthaes, P. , Wagener, C. , 1993. Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc. Natl. Acad. Sci. U S A. 90, 10744–10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngiow, S.F. , von Scheidt, B. , Akiba, H. , Yagita, H. , Teng, M.W. , Smyth, M.J. , 2011. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 71, 3540–3551. [DOI] [PubMed] [Google Scholar]
- Nishimura, H. , Nose, M. , Hiai, H. , Minato, N. , Honjo, T. , 1999. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 11, 141–151. [DOI] [PubMed] [Google Scholar]
- Nishimura, H. , Okazaki, T. , Tanaka, Y. , Nakatani, K. , Hara, M. , Matsumori, A. , Sasayama, S. , Mizoguchi, A. , Hiai, H. , Minato, N. , Honjo, T. , 2001. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 291, 319–322. [DOI] [PubMed] [Google Scholar]
- Nishiwada, S. , Sho, M. , Yasuda, S. , Shimada, K. , Yamato, I. , Akahori, T. , Kinoshita, S. , Nagai, M. , Konishi, N. , Nakajima, Y. , 2015. Clinical significance of CD155 expression in human pancreatic cancer. Anticancer Res. 35, 2287–2297. [PubMed] [Google Scholar]
- Oestreich, K.J. , Yoon, H. , Ahmed, R. , Boss, J.M. , 2008. NFATc1 regulates PD-1 expression upon T cell activation. J. Immunol. 181, 4832–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohigashi, Y. , Sho, M. , Yamada, Y. , Tsurui, Y. , Hamada, K. , Ikeda, N. , Mizuno, T. , Yoriki, R. , Kashizuka, H. , Yane, K. , 2005. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 11, 2947–2953. [DOI] [PubMed] [Google Scholar]
- Ohta, A. , Gorelik, E. , Prasad, S.J. , Ronchese, F. , Lukashev, D. , Wong, M.K. , Huang, X. , Caldwell, S. , Liu, K. , Smith, P. , 2006. A2A adenosine receptor protects tumors from antitumor T cells. N. Engl. J. Med. 103, 13132–13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta, A. , Sitkovsky, M. , 2001. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 414, 916–920. [DOI] [PubMed] [Google Scholar]
- Okazaki, T. , Maeda, A. , Nishimura, H. , Kurosaki, T. , Honjo, T. , 2001. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. U S A. 98, 13866–13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki, T. , Tanaka, Y. , Nishio, R. , Mitsuiye, T. , Mizoguchi, A. , Wang, J. , Ishida, M. , Hiai, H. , Matsumori, A. , Minato, N. , Honjo, T. , 2003. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 9, 1477–1483. [DOI] [PubMed] [Google Scholar]
- Olah, M.E. , Stiles, G.L. , 1995. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu. Rev. Pharmacol. Toxicol. 35, 581–606. [DOI] [PubMed] [Google Scholar]
- Ortenberg, R. , Sapir, Y. , Raz, L. , Hershkovitz, L. , Ben Arav, A. , Sapoznik, S. , Barshack, I. , Avivi, C. , Berkun, Y. , Besser, M.J. , 2012. Novel immunotherapy for malignant melanoma with a monoclonal antibody that blocks CEACAM1 homophilic interactions. Mol. Cancer Ther. 11, 1300–1310. [DOI] [PubMed] [Google Scholar]
- Ostrov, D.A. , Shi, W. , Schwartz, J.C. , Almo, S.C. , Nathenson, S.G. , 2000. Structure of murine CTLA-4 and its role in modulating T cell responsiveness. Science. 290, 816–819. [DOI] [PubMed] [Google Scholar]
- Otsuki, N. , Kamimura, Y. , Hashiguchi, M. , Azuma, M. , 2006. Expression and function of the B and T lymphocyte attenuator (BTLA/CD272) on human T cells. Biochem. Biophys. Res. Commun. 344, 1121–1127. [DOI] [PubMed] [Google Scholar]
- Pardoll, D.M. , 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 12, 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H. , Li, Z. , Yang, X.O. , Chang, S.H. , Nurieva, R. , Wang, Y.H. , Wang, Y. , Hood, L. , Zhu, Z. , Tian, Q. , Dong, C. , 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.J. , Omiya, R. , Matsumura, Y. , Sakoda, Y. , Kuramasu, A. , Augustine, M.M. , Yao, S. , Tsushima, F. , Narazaki, H. , Anand, S. , 2010. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 116, 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa, A.T. , Waldron, J.S. , Panner, A. , Crane, C.A. , Parney, I.F. , Barry, J.J. , Cachola, K.E. , Murray, J.C. , Tihan, T. , Jensen, M.C. , 2007. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 13, 84–88. [DOI] [PubMed] [Google Scholar]
- Paust, S. , Lu, L. , McCarty, N. , Cantor, H. , 2004. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc. Natl. Acad. Sci. U S A. 101, 10398–10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peggs, K.S. , Quezada, S.A. , Allison, J.P. , 2008. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol. Rev. 224, 141–165. [DOI] [PubMed] [Google Scholar]
- Peggs, K.S. , Quezada, S.A. , Chambers, C.A. , Korman, A.J. , Allison, J.P. , 2009. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende, D. , Castriconi, R. , Romagnani, P. , Spaggiari, G.M. , Marcenaro, S. , Dondero, A. , Lazzeri, E. , Lasagni, L. , Martini, S. , Rivera, P. , 2006. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood. 107, 2030–2036. [DOI] [PubMed] [Google Scholar]
- Pende, D. , Spaggiari, G.M. , Marcenaro, S. , Martini, S. , Rivera, P. , Capobianco, A. , Falco, M. , Lanino, E. , Pierri, I. , Zambello, R. , 2005. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112). Blood. 105, 2066–2073. [DOI] [PubMed] [Google Scholar]
- Perbellini, O. , Falisi, E. , Giaretta, I. , Boscaro, E. , Novella, E. , Facco, M. , Fortuna, S. , Finotto, S. , Amati, E. , Maniscalco, F. , 2014. Clinical significance of LAIR1 (CD305) as assessed by flow cytometry in a prospective series of patients with chronic lymphocytic leukemia. Haematologica. 99, 881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins, D. , Wang, Z. , Donovan, C. , He, H. , Mark, D. , Guan, G. , Wang, Y. , Walunas, T. , Bluestone, J. , Listman, J. , Finn, P.W. , 1996. Regulation of CTLA-4 expression during T cell activation. J. Immunol. 156, 4154–4159. [PubMed] [Google Scholar]
- Petermann, K.B. , Rozenberg, G.I. , Zedek, D. , Groben, P. , McKinnon, K. , Buehler, C. , Kim, W.Y. , Shields, J.M. , Penland, S. , Bear, J.E. , 2007. CD200 is induced by ERK and is a potential therapeutic target in melanoma. J. Clin. Invest. 117, 3922–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas, D.R. , Johnson, R. , Pingel, J.T. , Matthews, R.J. , Dalton, M. , Roy, G. , Chan, A.C. , Thomas, M.L. , 1996. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 272, 1173–1176. [DOI] [PubMed] [Google Scholar]
- Presta, Leonard G., Holly M. Cherwinski, and Joseph H. Phillips. 2008. “Antibodies to CD200R.” (Patent: WO2008079352A3).
- Preston, S. , Wright, G.J. , Starr, K. , Barclay, A.N. , Brown, M.H. , 1997. The leukocyte/neuron cell surface antigen OX2 binds to a ligand on macrophages. Eur. J. Immunol. 27, 1911–1918. [DOI] [PubMed] [Google Scholar]
- Prigent, P. , El Mir, S. , Dreano, M. , Triebel, F. , 1999. Lymphocyte activation gene-3 induces tumor regression and antitumor immune responses. Eur. J. Immunol. 29, 3867–3876. [DOI] [PubMed] [Google Scholar]
- Quandt, D. , Hoff, H. , Rudolph, M. , Fillatreau, S. , Brunner-Weinzierl, M.C. , 2007. A new role of CTLA-4 on B cells in thymus-dependent immune responses in vivo. J. Immunol. 179, 7316–7324. [DOI] [PubMed] [Google Scholar]
- Quezada, S.A. , Peggs, K.S. , Curran, M.A. , Allison, J.P. , 2006. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Invest. 116, 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi, O.S. , Zheng, Y. , Nakamura, K. , Attridge, K. , Manzotti, C. , Schmidt, E.M. , Baker, J. , Jeffery, L.E. , Kaur, S. , Briggs, Z. , 2011. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 332, 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari, M. , Zhu, C. , Sakuishi, K. , Xiao, S. , Karman, J. , Chen, A. , Angin, M. , Wakeham, A. , Greenfield, E.A. , Sobel, R.A. , 2012. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat. Med. 18, 1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskovalova, T. , Lokshin, A. , Huang, X. , Su, Y. , Mandic, M. , Zarour, H.M. , Jackson, E.K. , Gorelik, E. , 2007. Inhibition of cytokine production and cytotoxic activity of human antimelanoma specific CD8+ and CD4+ T lymphocytes by adenosine-protein kinase A type I signaling. Cancer Res. 67, 5949–5956. [DOI] [PubMed] [Google Scholar]
- Ribas, A. , Kefford, R. , Marshall, M.A. , Punt, C.J. , Haanen, J.B. , Marmol, M. , Garbe, C. , Gogas, H. , Schachter, J. , Linette, G. , 2013. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 31, 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethdorf, L. , Lisboa, B.W. , Henkel, U. , Naumann, M. , Wagener, C. , Loning, T. , 1997. Differential expression of CD66a (BGP), a cell adhesion molecule of the carcinoembryonic antigen family, in benign, premalignant, and malignant lesions of the human mammary gland. J. Histochem. Cytochem. 45, 957–963. [DOI] [PubMed] [Google Scholar]
- Riley, J.L. , 2009. PD-1 signaling in primary T cells. Immunological Rev. 229, 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert, C. , Long, G.V. , Brady, B. , Dutriaux, C. , Maio, M. , Mortier, L. , Hassel, J.C. , Rutkowski, P. , McNeil, C. , Kalinka-Warzocha, E. , 2015. Nivolumab in previously untreated melanoma without BRAF mutation. New Engl. J. Med. 372, 320–330. [DOI] [PubMed] [Google Scholar]
- Robert, C. , Thomas, L. , Bondarenko, I. , O'Day, S. , Weber, J. , Garbe, C. , Lebbe, C. , Baurain, J.F. , Testori, A. , Grob, J.J. , 2011. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526. [DOI] [PubMed] [Google Scholar]
- Rodig, N. , Ryan, T. , Allen, J.A. , Pang, H. , Grabie, N. , Chernova, T. , Greenfield, E.A. , Liang, S.C. , Sharpe, A.H. , Lichtman, A.H. , Freeman, G.J. , 2003. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 33, 3117–3126. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzanet, R. , DeKruyff, R. , Kuchroo, V.K. , Umetsu, D.T. , 2009. The costimulatory role of TIM molecules. Immunological Rev. 229, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano, E. , Kusio-Kobialka, M. , Foukas, P.G. , Baumgaertner, P. , Meyer, C. , Ballabeni, P. , Michielin, O. , Weide, B. , Romero, P. , Speiser, D.E. , 2015. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl. Acad. Sci. U S A. 112, 6140–6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, M. , Nedellec, P. , Jothy, S. , Fleiszer, D. , Turbide, C. , Beauchemin, N. , 1993. The expression of mouse biliary glycoprotein, a carcinoembryonic antigen-related gene, is down-regulated in malignant mouse tissues. Cancer Res. 53, 4938–4945. [PubMed] [Google Scholar]
- Rother, Russell P., and Yan Yan. 2011. “Therapeutic Methods Using Anti-CD200 Antibodies.” (Patent: WO2011100538A1).
- Rygiel, T.P. , Stolte, E.H. , de Ruiter, T. , van de Weijer, M.L. , Meyaard, L. , 2011. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol. Immunol. 49, 402–406. [DOI] [PubMed] [Google Scholar]
- Sabatos, C.A. , Chakravarti, S. , Cha, E. , Schubart, A. , Sanchez-Fueyo, A. , Zheng, X.X. , Coyle, A.J. , Strom, T.B. , Freeman, G.J. , Kuchroo, V.K. , 2003. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 4, 1102–1110. [DOI] [PubMed] [Google Scholar]
- Sakisaka, T. , Takai, Y. , 2004. Biology and pathology of nectins and nectin-like molecules. Curr. Opin. Cell Biol. 16, 513–521. [DOI] [PubMed] [Google Scholar]
- Sakuishi, K. , Apetoh, L. , Sullivan, J.M. , Blazar, B.R. , Kuchroo, V.K. , Anderson, A.C. , 2010. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Fueyo, A. , Tian, J. , Picarella, D. , Domenig, C. , Zheng, X.X. , Sabatos, C.A. , Manlongat, N. , Bender, O. , Kamradt, T. , Kuchroo, V.K. , 2003. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4, 1093–1101. [DOI] [PubMed] [Google Scholar]
- Saresella, M. , Piancone, F. , Marventano, I. , La Rosa, F. , Tortorella, P. , Caputo, D. , Rovaris, M. , Clerici, M. , 2014. A role for the TIM-3/GAL-9/BAT3 pathway in determining the clinical phenotype of multiple sclerosis. FASEB J. Off. Publ. Fed. Am. Societies Exp. Biol. 28, 5000–5009. [DOI] [PubMed] [Google Scholar]
- Sarrias, M.R. , Whitbeck, J.C. , Rooney, I. , Ware, C.F. , Eisenberg, R.J. , Cohen, G.H. , Lambris, J.D. , 2000. The three HveA receptor ligands, gD, LT-alpha and LIGHT bind to distinct sites on HveA. Mol. Immunol. 37, 665–673. [DOI] [PubMed] [Google Scholar]
- Schweitzer, A.N. , Sharpe, A.H. , 1999. Mutual regulation between B7-1 (CD80) expressed on T cells and IL-4. J. Immunol. 163, 4819–4825. [PubMed] [Google Scholar]
- Sedy, J.R. , Gavrieli, M. , Potter, K.G. , Hurchla, M.A. , Lindsley, R.C. , Hildner, K. , Scheu, S. , Pfeffer, K. , Ware, C.F. , Murphy, T.L. , Murphy, K.M. , 2005. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 6, 90–98. [DOI] [PubMed] [Google Scholar]
- Sehrawat, S. , Reddy, P.B. , Rajasagi, N. , Suryawanshi, A. , Hirashima, M. , Rouse, B.T. , 2010. Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response. PLoS Pathog. 6, e1000882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby, M.J. , Engelhardt, J.J. , Quigley, M. , Henning, K.A. , Chen, T. , Srinivasan, M. , Korman, A.J. , 2013. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 1, 32–42. [DOI] [PubMed] [Google Scholar]
- Sfanos, K.S. , Bruno, T.C. , Meeker, A.K. , De Marzo, A.M. , Isaacs, W.B. , Drake, C.G. , 2009. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. The Prostate. 69, 1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrikant, P. , Khoruts, A. , Mescher, M.F. , 1999. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 11, 483–493. [DOI] [PubMed] [Google Scholar]
- Simpson, T.R. , Li, F. , Montalvo-Ortiz, W. , Sepulveda, M.A. , Bergerhoff, K. , Arce, F. , Roddie, C. , Henry, J.Y. , Yagita, H. , Wolchok, J.D. , 2013. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 210, 1695–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, B.B. , Scheffrahn, I. , Heymann, R. , Sigmundsson, K. , Kammerer, R. , Obrink, B. , 2002. Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes. J. Immunol. 168, 5139–5146. [DOI] [PubMed] [Google Scholar]
- Sitkovsky, M.V. , 2003. Use of the A(2A) adenosine receptor as a physiological immunosuppressor and to engineer inflammation in vivo. Biochem. Pharmacol. 65, 493–501. [DOI] [PubMed] [Google Scholar]
- Sitkovsky, M.V. , Lukashev, D. , Apasov, S. , Kojima, H. , Koshiba, M. , Caldwell, C. , Ohta, A. , Thiel, M. , 2004. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 22, 657–682. [DOI] [PubMed] [Google Scholar]
- Siva, A. , Xin, H. , Qin, F. , Oltean, D. , Bowdish, K.S. , Kretz-Rommel, A. , 2008. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol. Immunother. 57, 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz, Amy P. N., and Keith M. Skubitz. 2001. “Peptides Capable of Modulating the Function of CD66 (CEACAM) Family Members.” (patent: WO 2001013937 A1).
- Sloan, K.E. , Stewart, J.K. , Treloar, A.F. , Matthews, R.T. , Jay, D.G. , 2005. CD155/PVR enhances glioma cell dispersal by regulating adhesion signaling and focal adhesion dynamics. Cancer Res. 65, 10930–10937. [DOI] [PubMed] [Google Scholar]
- Small, E.J. , Tchekmedyian, N.S. , Rini, B.I. , Fong, L. , Lowy, I. , Allison, J.P. , 2007. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin. Cancer Res. 13, 1810–1815. [DOI] [PubMed] [Google Scholar]
- Stagg, J. , Smyth, M.J. , 2010. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 29, 5346–5358. [DOI] [PubMed] [Google Scholar]
- Stanietsky, N. , Rovis, T.L. , Glasner, A. , Seidel, E. , Tsukerman, P. , Yamin, R. , Enk, J. , Jonjic, S. , Mandelboim, O. , 2013. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur. J. Immunol. 43, 2138–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanietsky, N. , Simic, H. , Arapovic, J. , Toporik, A. , Levy, O. , Novik, A. , Levine, Z. , Beiman, M. , Dassa, L. , Achdout, H. , 2009. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. U S A. 106, 17858–17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome, S.E. , Dong, H. , Tamura, H. , Voss, S.G. , Flies, D.B. , Tamada, K. , Salomao, D. , Cheville, J. , Hirano, F. , Lin, W. , 2003. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 63, 6501–6505. [PubMed] [Google Scholar]
- Su, E.W. , Bi, S. , Kane, L.P. , 2011. Galectin-9 regulates T helper cell function independently of Tim-3. Glycobiology. 21, 1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznol, M. , Chen, L. , 2013. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 19, 1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura, S. , Tsuji-Kawahara, S. , Yagita, H. , Akiba, H. , Sakamoto, M. , Chikaishi, T. , Kato, M. , Miyazawa, M. , 2010. Premature terminal exhaustion of friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors. J. Immunol. 184, 4696–4707. [DOI] [PubMed] [Google Scholar]
- Tan, K. , Zelus, B.D. , Meijers, R. , Liu, J.H. , Bergelson, J.M. , Duke, N. , Zhang, R. , Joachimiak, A. , Holmes, K.V. , Wang, J.H. , 2002. Crystal structure of murine sCEACAM1a[1,4]: a coronavirus receptor in the CEA family. EMBO J. 21, 2076–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X. , Tian, L. , Esteso, G. , Choi, S.C. , Barrow, A.D. , Colonna, M. , Borrego, F. , Coligan, J.E. , 2012. Leukocyte-associated Ig-like receptor-1-deficient mice have an altered immune cell phenotype. J. Immunol. 188, 548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, R. , Wang, L. , Murphy, K.M. , Fraser, C.C. , Hancock, W.W. , 2008. Regulatory T cell expression of herpesvirus entry mediator suppresses the Function of B and T lymphocyte attenuator-positive effector T cells. J. Immunol. 180, 6649–6655. [DOI] [PubMed] [Google Scholar]
- Taube, J.M. , Anders, R.A. , Young, G.D. , Xu, H. , Sharma, R. , McMiller, T.L. , Chen, S. , Klein, A.P. , Pardoll, D.M. , Topalian, S.L. , Chen, L. , 2012. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Translational Med. 4, 127ra137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, P.A. , Lees, C.J. , Fournier, S. , Allison, J.P. , Sharpe, A.H. , Blazar, B.R. , 2004. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions [corrections]. J. Immunol. 172, 34–39. [DOI] [PubMed] [Google Scholar]
- Terme, M. , Ullrich, E. , Aymeric, L. , Meinhardt, K. , Desbois, M. , Delahaye, N. , Viaud, S. , Ryffel, B. , Yagita, H. , Kaplanski, G. , 2011. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 71, 5393–5399. [DOI] [PubMed] [Google Scholar]
- Tivol, E.A. , Borriello, F. , Schweitzer, A.N. , Lynch, W.P. , Bluestone, J.A. , Sharpe, A.H. , 1995. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 3, 541–547. [DOI] [PubMed] [Google Scholar]
- Tonks, A. , Hills, R. , White, P. , Rosie, B. , Mills, K.I. , Burnett, A.K. , Darley, R.L. , 2007. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 21, 566–568. [DOI] [PubMed] [Google Scholar]
- Topalian, S.L. , Hodi, F.S. , Brahmer, J.R. , Gettinger, S.N. , Smith, D.C. , McDermott, D.F. , Powderly, J.D. , Carvajal, R.D. , Sosman, J.A. , Atkins, M.B. , 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian, S.L. , Sznol, M. , McDermott, D.F. , Kluger, H.M. , Carvajal, R.D. , Sharfman, W.H. , Brahmer, J.R. , Lawrence, D.P. , Atkins, M.B. , Powderly, J.D. , 2014. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 32, 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel, F. , Hacene, K. , Pichon, M.F. , 2006. A soluble lymphocyte activation gene-3 (sLAG-3) protein as a prognostic factor in human breast cancer expressing estrogen or progesterone receptors. Cancer Lett. 235, 147–153. [DOI] [PubMed] [Google Scholar]
- Triebel, F. , Jitsukawa, S. , Baixeras, E. , Roman-Roman, S. , Genevee, C. , Viegas-Pequignot, E. , Hercend, T. , 1990. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 171, 1393–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt, Robert, Michael Rosenblum, and Edit Olasz. 2005. “Methods of Modulating CD200.” Retrieved (Patent: US20050169870).
- Tseng, S.Y. , Otsuji, M. , Gorski, K. , Huang, X. , Slansky, J.E. , Pai, S.I. , Shalabi, A. , Shin, T. , Pardoll, D.M. , Tsuchiya, H. , 2001. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 193, 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima, F. , Yao, S. , Shin, T. , Flies, A. , Flies, S. , Xu, H. , Tamada, K. , Pardoll, D.M. , Chen, L. , 2007. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 110, 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, T.C. , Brown, N.K. , Kim, T.J. , Wroblewska, J. , Yang, X. , Guo, X. , Lee, S.H. , Kumar, V. , Lee, K.M. , Fu, Y.X. , 2015. CD160 is essential for NK-mediated IFN-gamma production. J. Exp. Med. 212, 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbide, C. , Kunath, T. , Daniels, E. , Beauchemin, N. , 1997. Optimal ratios of biliary glycoprotein isoforms required for inhibition of colonic tumor cell growth. Cancer Res. 57, 2781–2788. [PubMed] [Google Scholar]
- Tureci, O. , Schmitt, H. , Fadle, N. , Pfreundschuh, M. , Sahin, U. , 1997. Molecular definition of a novel human galectin which is immunogenic in patients with Hodgkin's disease. J. Biol. Chem. 272, 6416–6422. [DOI] [PubMed] [Google Scholar]
- Umetsu, S.E. , Lee, W.L. , McIntire, J.J. , Downey, L. , Sanjanwala, B. , Akbari, O. , Berry, G.J. , Nagumo, H. , Freeman, G.J. , Umetsu, D.T. , DeKruyff, R.H. , 2005. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat. Immunol. 6, 447–454. [DOI] [PubMed] [Google Scholar]
- van der Vuurst de Vries, A.R. , Clevers, H. , Logtenberg, T. , Meyaard, L. , 1999. Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) is differentially expressed during human B cell differentiation and inhibits B cell receptor-mediated signaling. Eur. J. Immunol. 29, 3160–3167. [DOI] [PubMed] [Google Scholar]
- van Elsas, A. , Hurwitz, A.A. , Allison, J.P. , 1999. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge, A. , Rijkers, E.S. , de Ruiter, T. , Meyaard, L. , 2006. Leukocyte-associated Ig-like receptor-1 has SH2 domain-containing phosphatase-independent function and recruits C-terminal Src kinase. Eur. J. Immunol. 36, 190–198. [DOI] [PubMed] [Google Scholar]
- Vercammen, D. , Brouckaert, G. , Denecker, G. , Van de Craen, M. , Declercq, W. , Fiers, W. , Vandenabeele, P. , 1998. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 188, 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Berg, J. , Vrohlings, M. , Haller, S. , Haimovici, A. , Kulig, P. , Sledzinska, A. , Weller, M. , Becher, B. , 2013. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J. Exp. Med. 210, 2803–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waickman, A.T. , Alme, A. , Senaldi, L. , Zarek, P.E. , Horton, M. , Powell, J.D. , 2012. Enhancement of tumor immunotherapy by deletion of the A2A adenosine receptor. Cancer Immunol. Immunother. 61, 917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu, E. , Mathis, D. , Benoist, C. , 2013. Convergent and divergent effects of costimulatory molecules in conventional and regulatory CD4+ T cells. Proc. Natl. Acad. Sci. U S A. 110, 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, L.S. , Sansom, D.M. , 2015. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol. 36, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas, T.L. , Lenschow, D.J. , Bakker, C.Y. , Linsley, P.S. , Freeman, G.J. , Green, J.M. , Thompson, C.B. , Bluestone, J.A. , 1994. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1, 405–413. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Yoshida, T. , Nakaki, F. , Hiai, H. , Okazaki, T. , Honjo, T. , 2005. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc. Natl. Acad. Sci. U S A. 102, 11823–11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Lin, S.H. , Wu, W.G. , Kemp, B.L. , Walsh, G.L. , Hong, W.K. , Mao, L. , 2000. C-CAM1, a candidate tumor suppressor gene, is abnormally expressed in primary lung cancers. Clin. Cancer Res. 6, 2988–2993. [PubMed] [Google Scholar]
- Wang, L. , Pino-Lagos, K. , de Vries, V.C. , Guleria, I. , Sayegh, M.H. , Noelle, R.J. , 2008. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc. Natl. Acad. Sci. U S A. 105, 9331–9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Rubinstein, R. , Lines, J.L. , Wasiuk, A. , Ahonen, C. , Guo, Y. , Lu, L.F. , Gondek, D. , Wang, Y. , Fava, R.A. , 2011. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 208, 577–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N. , Gavrieli, M. , Sedy, J.R. , Yang, J. , Fallarino, F. , Loftin, S.K. , Hurchla, M.A. , Zimmerman, N. , Sim, J. , Zang, X. , 2003. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 4, 670–679. [DOI] [PubMed] [Google Scholar]
- Webb, M. , Barclay, A.N. , 1984. Localisation of the MRC OX-2 glycoprotein on the surfaces of neurones. J. Neurochem. 43, 1061–1067. [DOI] [PubMed] [Google Scholar]
- Weber, J.S. , Kahler, K.C. , Hauschild, A. , 2012. Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol. 30, 2691–2697. [DOI] [PubMed] [Google Scholar]
- Westin, J.R. , McLaughlin, P. , Romaguera, J. , Hagemeister, F.B. , Pro, B. , Dang, N.H. , Samaniego, F. , Rodriguez, M.A. , Fayad, L. , Oki, Y. , 2014. Paclitaxel, topotecan and rituximab: long term outcomes of an effective salvage programme for relapsed or refractory aggressive B-cell non-Hodgkin lymphoma. Br. J. Haematol. 167, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry, E.J. , Ha, S.J. , Kaech, S.M. , Haining, W.N. , Sarkar, S. , Kalia, V. , Subramaniam, S. , Blattman, J.N. , Barber, D.L. , Ahmed, R. , 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 27, 670–684. [DOI] [PubMed] [Google Scholar]
- Workman, C.J. , Dugger, K.J. , Vignali, D.A. , 2002. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J. Immunol. 169, 5392–5395. [DOI] [PubMed] [Google Scholar]
- Workman, C.J. , Vignali, D.A.A. , 2005. Negative Regulation of T Cell homeostasis by lymphocyte activation Gene-3 (CD223). J. Immunol. 174, 688–695. [DOI] [PubMed] [Google Scholar]
- Wright, G.J. , Cherwinski, H. , Foster-Cuevas, M. , Brooke, G. , Puklavec, M.J. , Bigler, M. , Song, Y. , Jenmalm, M. , Gorman, D. , McClanahan, T. , 2003. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. 171, 3034–3046. [DOI] [PubMed] [Google Scholar]
- Wright, G.J. , Jones, M. , Puklavec, M.J. , Brown, M.H. , Barclay, A.N. , 2001. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 102, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, G.J. , Puklavec, M.J. , Willis, A.C. , Hoek, R.M. , Sedgwick, J.D. , Brown, M.H. , Barclay, A.N. , 2000. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 13, 233–242. [DOI] [PubMed] [Google Scholar]
- Wu, K. , Kryczek, I. , Chen, L. , Zou, W. , Welling, T.H. , 2009. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 69, 8067–8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, G. , Deng, A. , Liu, H. , Ge, G. , Liu, X. , 2012. Activator protein 1 suppresses antitumor T-cell function via the induction of programmed death 1. Proc. Natl. Acad. Sci. U S A. 109, 15419–15424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, L. , Fu, Z.R. , Liu, F. , Zhang, L.D. , Shi, X.M. , Shen, X.Y. , Ni, Z.J. , Fu, H. , Li, R.D. , Cao, X.T. , 2011. Suppression of allograft rejection by Tim-1-Fc through cross-linking with a novel Tim-1 binding partner on T cells. PloS One. 6, e21697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, S. , Brooks, C.R. , Zhu, C. , Wu, C. , Sweere, J.M. , Petecka, S. , Yeste, A. , Quintana, F.J. , Ichimura, T. , Sobel, R.A. , 2012. Defect in regulatory B-cell function and development of systemic autoimmunity in T-cell Ig mucin 1 (Tim-1) mucin domain-mutant mice. Proc. Natl. Acad. Sci. U S A. 109, 12105–12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, R. , Nishikori, M. , Kitawaki, T. , Sakai, T. , Hishizawa, M. , Tashima, M. , Kondo, T. , Ohmori, K. , Kurata, M. , Hayashi, T. , Uchiyama, T. , 2008. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 111, 3220–3224. [DOI] [PubMed] [Google Scholar]
- Yao, S. , Chen, L. , 2006. Reviving exhausted T lymphocytes during chronic virus infection by B7-H1 blockade. Trends Mol. Med. 12, 244–246. [DOI] [PubMed] [Google Scholar]
- Yao, S. , Zhu, Y. , Chen, L. , 2013. Advances in targeting cell surface signalling molecules for immune modulation. Nature reviews. Drug Discov. 12, 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, A. , Mittal, D. , Stagg, J. , Smyth, M.J. , 2014. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov. 4, 879–888. [DOI] [PubMed] [Google Scholar]
- Youngnak, P. , Kozono, Y. , Kozono, H. , Iwai, H. , Otsuki, N. , Jin, H. , Omura, K. , Yagita, H. , Pardoll, D.M. , Chen, L. , Azuma, M. , 2003. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem. Biophys. Res. Commun. 307, 672–677. [DOI] [PubMed] [Google Scholar]
- Yu, X. , Harden, K. , Gonzalez, L.C. , Francesco, M. , Chiang, E. , Irving, B. , Tom, I. , Ivelja, S. , Refino, C.J. , Clark, H. , 2009. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 10, 48–57. [DOI] [PubMed] [Google Scholar]
- Zarek, P.E. , Huang, C.T. , Lutz, E.R. , Kowalski, J. , Horton, M.R. , Linden, J. , Drake, C.G. , Powell, J.D. , 2008. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 111, 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Schwartz, J.C. , Guo, X. , Bhatia, S. , Cao, E. , Lorenz, M. , Cammer, M. , Chen, L. , Zhang, Z.Y. , Edidin, M.A. , 2004. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 20, 337–347. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Maksimovic, J. , Naselli, G. , Qian, J. , Chopin, M. , Blewitt, M.E. , Oshlack, A. , Harrison, L.C. , 2013. Genome-wide DNA methylation analysis identifies hypomethylated genes regulated by FOXP3 in human regulatory T cells. Blood. 122, 2823–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, C. , Anderson, A.C. , Schubart, A. , Xiong, H. , Imitola, J. , Khoury, S.J. , Zheng, X.X. , Strom, T.B. , Kuchroo, V.K. , 2005. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6, 1245–1252. [DOI] [PubMed] [Google Scholar]
- Zou, W. , Chen, L. , 2008. Inhibitory B7-family molecules in the tumour microenvironment. Nature reviews. Immunology. 8, 467–477. [DOI] [PubMed] [Google Scholar]


