Abstract
Over the past few years melanoma incidence has been rising steadily, resulting in an increase in melanoma related mortality. Until recently, therapeutic options for metastatic melanoma were scarce. Chemotherapy and, in some countries, IL‐2 were the only registered treatment modalities. In the last five years, treatment with immunotherapy (anti CTLA‐4, anti PD‐1, or the combination of these antibodies) has shown very promising results and was able to improve survival in patients with metastatic melanoma. Adoptive cell therapy using tumor‐infiltrating lymphocytes is yet another, but highly promising, immunotherapeutic strategy for patients with metastatic melanoma. This review will discuss the development of TIL as a treatment option for melanoma, its mode of action and simplification over time, and the possibilities to expand this therapy to other types of cancer. Also, the future directions of TIL based therapies will be highlighted.
Keywords: Melanoma, Tumor-infiltrating lymphocytes, Adoptive cell transfer, Lymphodepletion, Interleukin-2
Highlights
TIL therapy shows promising results for the treatment of cancer.
Lymphodepletion prior to TIL depletes Tregs and removes cellular “sinks”.
Preselection of TIL can lead to enrichment of tumor‐reactive T cells.
Abbreviations
- ACT
adoptive cell transfer
- APC
antigen presenting cell
- CI
confidence interval
- CR
complete remission
- C/T
cancer/testis
- CTLA-4
cytotoxic t-lymphocyte-associated protein-4
- Cy
cyclophosphamide
- DNA
deoxyribonucleic acid
- DTIC
dacarbazine
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence-activated cell sorting
- FDA
Food and Drug Administration
- Flu
fludarabine
- gp100
glycoprotein 100
- Gy
gray
- HD-IL2
high dose interleukin-2
- HPV
human papilloma virus
- HLA
human leukocyte antigen
- IFN
interferon
- IL
interleukin
- IU
international unit
- i.v.
intravenous
- kg
kilogram
- LAK
lymphokine-activated killer
- MAGE
melanoma antigen
- MAPK
mitogen-activated protein kinase
- MDA
melanocyte differentiation antigens
- MHC
major histocompatibility complex
- mg
milligram
- MIU
million international units
- NCI
National Cancer Institute
- NK
natural killer (cell)
- NKI
Netherlands Cancer Institute
- NSCLC
non-small cell lung cancer
- NMA
non-myeloablative
- OR
objective response
- ORR
objective response rate
- PD
progressive disease
- PD-1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
- PFS
progression free survival
- PR
partial response
- RCC
renal cell carcinoma
- RCT
randomized controlled trial
- SAE
serious adverse events
- SB
Surgery Branch of the National Institutes of Health
- sBM
symptomatic brain metastases
- s.c.
subcutaneous
- TBI
total body irradiation
- TCR
T-Cell Receptor
- t.i.d
ter in die
- TIL
tumor-infiltrating lymphocytes
- RECIST
response evaluation criteria in solid tumors
- REP
rapid expansion protocol
1. Introduction
In 1863, Rudolf Virchow described the presence of lymphoid cells in neoplastic tissue and hypothesized a connection between inflammation and cancer (Virchow, 1863). Over the past two decades, clear correlations have been found between the presence of lymphocytic infiltrates within tumors and patients' clinical outcome in several tumor types, including metastatic melanoma, ovarian cancer, colorectal cancer and breast cancer subtypes (Clemente et al., 1996; Pages et al., 2010; Santoiemma and Powell, 2015; Tuthill et al., 2002; Zhang et al., 2003). The first attempts to isolate and characterize the lymphoid cells in cancerous tissue dates back to the 1970‐ies and revealed that many tumor tissues contained lymphocytes (Blazar and Heppner, 1978; Zettergren et al., 1973). Pioneering work in this field of research has been performed by Dr. Steven Rosenberg from the Surgery Branch (SB) of the National Institutes of Health (NIH), Bethesda, Maryland. Rosenberg and colleagues started by growing tumor‐infiltrating lymphocytes (TIL) from multiple murine tumors and demonstrated antitumor activity of these TILs in vivo (Spiess et al., 1987). In a murine sarcoma model, infusion of TIL in combination with T cell growth factor interleukin‐2 (IL‐2), appeared to be 50–100 times more effective in killing tumor cells than Lymphokine‐Activated Killer (LAK) cells, that were generated by culturing peripheral blood lymphocytes in the presence of high concentrations of IL‐2 (Rosenberg et al., 1986). Importantly, TIL cultured from human tumors were also able to lyse autologous but not allogeneic tumor cells in a major histocompatibility complex (MHC) dependent fashion in the majority of cases. This observation pointed towards some patient‐specificity of this treatment, while this was lacking completely in LAK cell therapy (Rosenberg et al., 1985). In a first TIL pilot study, twelve patients with metastatic cancer were treated with TIL, with or without the chemotherapeutic agent cyclophosphamide and IL‐2 (Topalian et al., 1988). Two partial responses were observed, one in a patient with melanoma and one in a patient with renal cell carcinoma. Both patients received cyclophosphamide prior to TIL infusion. This was the first indication that TIL therapy could induce clinical responses in patients with metastatic cancer and formed the basis for further studies, which will be discussed in this review.
During the past decade, a much better understanding of the working mechanism of TIL therapy has been gained, especially regarding the role of lymphodepleting conditioning of the host, the role of interleukin‐2 as a survival factor for the infused TIL, the optimal quality and quantity of the infused cells and their antigen recognition pattern. In addition, although growing TIL was for a long time only successful in metastatic melanoma, the current protocols of TIL outgrowth are now also being explored in other types of cancer as well. These aspects and future developments will be discussed here.
2. TIL therapy for metastatic melanoma
Since, the first clinical trial with TIL therapy by Rosenberg et al., a series of phase I/II clinical trials have shown that infusion of TIL combined with lymphodepleting preconditioning and followed by high dose bolus infusional IL‐2 can mediate objective responses in patients with metastatic melanoma (Dudley et al., 2002, 2005, 2008, 2010, 2013, 1994, 2011). Originally, the protocol consisted of a metastasectomy of one or more melanoma lesions. A total size of around 3 cm in diameter was required to be able to successfully grow TIL from these lesions. These resected melanomas were subsequently fragmented into microcultures in the presence of IL‐2. Once enough TIL were grown from these cultures, TIL were tested for recognition of autologous melanoma cells (usually melanoma cell lines or freshly frozen tumor digest), and if not available, reactivity to a panel of human leukocyte antigen (HLA) matched allogeneic melanoma cell lines. Readout was the measurement of interferon‐γ (IFN) secreted in the medium using an IFN‐γ enzyme‐linked immunosorbent assay (ELISA). Only those cultures containing melanoma‐reactive TIL were further propagated and rapidly expanded by stimulation with soluble anti‐CD3 monoclonal antibody, high concentration of IL‐2 (6000 IU/ml) and irradiated allogeneic or autologous feeder cells. Starting with approximately 50 × 106 TIL, these numbers were expanded in a 14‐day time period to 1–20 × 1010 CD3+ TIL. After concentration of the cells to a 200–300 ml suspension, the product was ready for infusion. It was convincingly shown that TILs selected for reactivity towards autologous melanoma cells displayed high functional activity in metastatic melanoma patients, with ORR varying between 34% and 72% of treated patients some of whom developed a long‐lasting complete remission, however, there were some important drawbacks associated with this elaborate TIL production protocol (Dudley et al., 2005, 2008, 1994). First, the selection of TIL for reactivity against autologous melanoma required the presence of an autologous melanoma cell line. With a success rate for growing cell lines from patient material of less than 50%, the selection step on autologous tumor could not be done in at least half of the patients (Dudley et al., 2003). Secondly, as only a fraction of cultures contained tumor‐reactive TILs, the total culture time to obtain enough cells for initiating rapid expansion (200 × 106 TIL) was long. The risk for these refractory melanoma patients to rapidly progress up to a stage that TIL therapy was no longer considered beneficial, increased with longer culture time. Thirdly, longer culture time also translated into obtaining TIL with a more terminally differentiated phenotype, decreasing their capacity to persist in vivo after infusion (Huang et al., 2005; Powell et al., 2005). Together with the inability to grow TIL from 20 to 25% of metastatic melanoma patients, the accumulative dropout rate amounted to 70% or more of patients that could not be treated with TIL in these early studies.
In their first clinical study with these so‐called “selected TILs”, Rosenberg et al. treated 86 metastatic melanoma patients, of whom 57 received a single dose of 25 mg/kg cyclophosphamide as a lymphodepleting regiment, followed by infusion of selected TIL and high‐dose intravenous bolus IL‐2 (Rosenberg et al., 1994). The overall ORR in this clinical trial was 34%. Significant differences in overall ORR were noted in patients who were treated with TIL from younger cultures (p = 0.0001), TIL with shorter doubling times (p = 0.03) and TIL that exhibited higher lytic activity against autologous tumor targets (p = 0.0008) (Figure 1).
Figure 1.
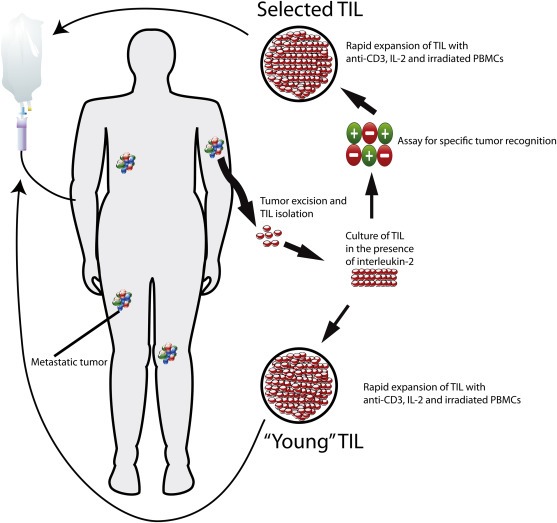
Schematic overview of the process for adoptive cell transfer of tumor‐infiltrating lymphocytes. After excision, the melanoma metastasis is digested into a single cell suspension in 24 well plates or fragmented. These suspensions/fragments are then cultured in the presence of IL‐2. In earlier days (selected TIL) cultures were tested for recognition of autologous melanoma cells (usually melanoma cell lines or freshly frozen tumor digest, and if not available a panel of HLA‐matched allogeneic melanoma cell lines), by measuring IFN‐γ secreted in the medium using an IFN‐γ ELISA. In the “young” TIL approach, this selection step for tumor reactivity has been omitted. TIL cultures are then expanded to treatment levels by stimulation with soluble anti‐CD3 monoclonal antibody and, high concentration of IL‐2 and irradiated allogeneic feeder cells. After concentration, the product is infused in the previously lymphodepleted host.
In 2008, Dudley et al. described three cohorts of patients with metastatic melanoma treated with selected TIL in combination with different lymphodepleting regimens (Dudley et al., 2008; Rosenberg et al., 2011). Lymphodepleting regimens consisted of “standard” non‐myeloablative (NMA) chemotherapy with cyclophosphamide and fludarabine (43 patients), or NMA chemotherapy with cyclophosphamide and fludarabine (over five instead of seven days) plus a single fraction of 2 Gray (Gy) TBI (25 patients). The third cohort of patients received the same NMA regimen as the second cohort, but instead of 2 Gy TBI, patients in this cohort received 12 Gy TBI; 2 Gy twice a day for three days (25 patients). All patients received high‐dose bolus IL‐2 t.i.d. to tolerance. The ORR for all 93 patients was 56%. NMA chemotherapy alone showed an ORR of 49%, when 2 or 12 Gy TBI was added, the response rates were 52% and 72%, respectively. Twenty complete remissions were seen in this clinical trial. A significant difference in ORR was noted in patients receiving less IL‐2 (p < 0.001), patients receiving TIL with longer telomeres and larger fractions of CD8+CD27+ cells (p < 0.001). Despite the differences seen in ORR, there appeared to be no significant difference in overall survival when comparing the three groups (p = 0.13). A separate early clinical trial was performed at the Moffitt Cancer Center, Tampa, Florida, with 19 patients of whom 13 were treated with selected TIL. The ORR was 38% for treated patients and 26% for the total group (Pilon‐Thomas et al., 2012).
These clinical data nicely illustrate the reproducible efficacy of TIL therapy for metastatic melanoma. However, as little is known about the exact dropout rate of patients that were intended to be treated, these exciting response rates were somewhat misleading. The studies pointed clearly towards the benefits of creating a TIL infusion product in the shortest possible culture time and infusion of as many as possible TIL, displaying a more central memory phenotype (CD27 and CD28 positive) and long telomeres. In order to fulfill these goals and decrease the dropout rate, the investigators at the SB amended the TIL production protocol by leaving out the selection step. Without the selection step for tumor reactivity, the culture time was decreased by on average three weeks, rendering the cells ‘younger’, hence the name ‘young TIL protocol’. As a result of this modification at least 50% of patients, who were referred for TIL therapy, could be treated.
The first clinical trial, in which patients with metastatic melanoma were treated with young TIL, also included a CD8 enrichment step (Dudley et al., 2010). This was considered prudent because of the risk that possibly Tregs were infused as well, if bulk TIL were given. In this trial, 122 patients with metastatic melanoma were enrolled, however only 56 patients could be treated, mainly due to either inability to grow TIL from tumor digests (17%), disease progression prior to TIL infusion (16%), or no evaluable disease after metastasectomy (11%). Although, the dropout rate was still high (50%), this was substantially less compared to the delivery of selected TIL. The ORR for all treated patients in this trial was 54%. Within the group of patients that received NMA TIL an ORR of 58% was observed, compared to 48% for patients treated with NMA + 6Gy TBI. The ORR for all 122 enrolled (intention to treat) patients was 25%.
The clinical protocol of using unselected young TIL in combination with NMA and high dose IL‐2 was subsequently implemented in TIL trials at other centers in and outside the US. The results from these trials are summarized here.
At the Ella Institute in Tel Aviv, Israel, 55 patients with metastatic melanoma, who had received at least prior high dose IL‐2, were enrolled in a phase II clinical trial with young TIL (Itzhaki et al., 2011). Thirty‐two patients received TIL infusion. The dropout rate was 42%, mostly due to development of brain metastases, rapid disease progression, and inability to grow TIL. The ORR for patients that had received TIL infusion was 47%, including four patients with a complete response (CR), whereas the ORR for the total cohort of 55 patients was 27%. These results were very much in line with the outcomes observed in the study with CD8‐enriched TIL at the SB. Also in agreement with prior studies was the finding of a significant correlation between patients receiving TIL with a shorter culture time (p = 0.0008), higher number of infused cells (p = 0.0251), or TIL cultures with a higher percentage of CD8+ T cells (p = 0.0144) and outcome (ORR).
This study was updated recently and reported on 80 patients, of whom 57 were treated with young TIL following NMA with cyclophosphamide and fludarabine and high dose bolus IL‐2 following TIL infusion (Besser et al., 2013). In the intention‐to‐treat analysis the ORR was 29% and for the treated group 40%. The total number of complete responders was 5%. The 3‐year overall survival of responding patients was 78%.
In another trial conducted at the MD Anderson Cancer Center, 31 patients with metastatic melanoma were treated with young TIL (Radvanyi et al., 2012). The biggest difference relative to the Ella Institute protocol was a second course of high‐dose (HD) IL‐2 three weeks after TIL infusion. ORR for the 31 treated patients was 42%. Significant differences in ORR were seen in patients receiving more TIL (p = 0.0003), patients receiving a higher percentage of CD8+ cells (p = 0.001) and patients receiving a higher absolute number of CD8+ cells (p = 0.0003). Two patients developed a complete response.
At the Herlev Hospital in Copenhagen, Denmark, we treated patients with TIL in two sequential studies. One pilot study in which NMA TIL was combined with low dose IL‐2 and a second phase II study with decrescendo IL‐2 dosing (see section on IL‐2). Thirty‐three patients were enrolled in the phase II trial of whom 25 were treated with TIL. Ten of 24 evaluable patients obtained an objective response, of which 3 CR (R. Andersen, manuscript submitted).
In 2013, Dudley et al. reported the results of a randomized controlled phase II clinical trial in patients with metastatic melanoma who were randomized to receive either CD8+ enriched young TIL or unselected young TIL (Dudley et al., 2013). Hundred and one patients were enrolled in this clinical trial of whom 69 were actually treated with TIL. Of these, 35 patients received CD8‐enriched TIL and 34 received unselected young TIL. ORR for the two arms of the study were 20% and 35% respectively, although this difference was not statistically significant due to the small number of patients that were enrolled in this study.
These selected clinical trials utilized young TIL for the treatment of metastatic melanoma. Although, the treatment protocols were not completely equal (use of TBI next to NMA, different schedules of IL‐2, CD8‐enrichment), the outcome of these trials conveyed very similar messages. When combining the 3 largest studies a total of 336 patients were enrolled. Of these 207 patients were actually treated with TIL, resulting in a dropout rate of 38% of patients, mostly due to rapid disease progression, development of symptomatic brain metastases, inability to generate TIL or due to withdrawal of informed consent. An objective response was seen in 82 patients, or 40% of treated patients and 24% of all enrolled patients. In all four clinical trials combined 18 complete responses were seen, this amounts to 9% of all treated patients, or 5% of all enrolled patients (see Table 1).
Table 1.
Studies evaluating the effect of tumor‐infiltrating lymphocytes in patients with metastatic melanoma.
| Reference | TIL production | Culture time (weeks) | Enrolled patients | Treated patients (%) | Reason dropout | Lymphodepleting chemotherapy regimen | IL‐2 regimen | Response according to RECIST | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PD or development sBM | No TIL | Other | OR (n) | % OR (enrolled patients) | % OR (treated patients) | |||||||
| (Rosenberg et al., 1994) | Selected | – | 86 | 86 (100) | – | – | – | 57 received Cy (25 mg/kg) as single infusion | 720,000 IU/kg t.i.d. to toleranceRepeated after 21 days | 29 | 34 | 34 |
| (Schwartzentruber et al., 1994) | Selected | 5–7 (without REP) | 41 | 43 (2 patients received multiple treatments) | – | – | – | 16 patients received Cy (25 mg/kg) as single infusion | 720,000 IU/kg t.i.d. to tolerance, or 216,000 IU/kg and IFN‐alpha 3 × 106 U/m2 t.i.d. to tolerance | 9 | 21 | 21 |
| (Dudley et al., 2008; Rosenberg et al., 2011) | Selected | 5–8 | 93 | 93 (100) | – | – | – | 1st cohort Cy 60 mg/kg (day −7&‐6) + Flu 25 mg/m2 (day −5 through −1)2nd cohort Cy 60 mg/kg (day −6&‐5) + Flu 25 mg/m2 (day −6 through −2) + 2 Gy TBI3rd cohort Cy 60 mg/kg (day −7&‐6) + Flu 25 mg/m2 (day −7 through −3) + 2 × 2 Gy TBI per day for 3 days | 720,000 IU/kg t.i.d. to tolerance Maximum of 15 doses | 1st cohort 21(5 CR, 16 PR)2nd cohort 13(5 CR, 8 PR)3rd cohort 18(10 CR, 8 PR) | 1st cohort 492nd cohort 523rd cohort 72Total: 56 | 1st cohort 492nd cohort 523rd cohort 72Total: 56 |
| (Dudley et al., 2010) | CD8+ enriched “young” | 4–5 | 122 | 53 (43)+3 additional patients from prior resections | 20 | 21 | 28 | 1st cohort Cy 60 mg/kg (day −7&−6) + Flu 25 mg/m2 (day −5 through −1)2nd cohort Cy 60 mg/kg (day −7&‐6) + Flu 25 mg/m2 (day −5 through −1) + 3 × 2 Gy TBI | 720,000 IU/kg t.i.d. to tolerance Maximum of 15 doses | 1st cohort 19(3 CR, 16 PR)2nd cohort11(2 CR, 9 PR)Total: 30 (5 CR, 25 PR) | 25 | 1st cohort 58 2nd cohort 48 Total: 54 |
| (Ellebaek et al., 2012) | Selected | 7–8 (including REP) | 11 | 6 (55) | 4 | 1 | – | Cy 60 mg/kg (day −7&‐6) + Flu 25 mg/m2 (day −5 through −1) | 2 MIU s.c. for 14 days | 2 (2 CR) | 18 | 33 |
| (Ullenhag et al., 2012) | Selected | 6 (not including REP) | 24 | 24 (100) | – | – | – | Cy 60 mg/kg (day −7&‐6) + Flu 25 mg/m2 (day −5 through −1) | 2.4 × 106 units/m2 until PD or toxicities | 5 (1 CR, 4 PR) | 21 | 21 |
| (Pilon‐Thomas et al., 2012) | Selected | 8–10 (including REP) | 19 | 13 (68) | 4 | 1 | 1 (SAE during chemotherapy) | Cy 60 mg/kg (day −7&‐6) + Flu 25 mg/m2 (day −5 through −1) | 720,000 IU/kg t.i.d. to tolerance Maximum of 15 doses | 5 (2 CR, 3 PR) | 26 | 38 |
| (Radvanyi et al., 2012) | “Young” | 7 (including REP) | 31 | 31 (100) | – | – | – | Cy 60 mg/kg (day −7&‐6) + Flu 25 mg/m2 (day −5 through −1) | 720,000 IU/kg t.i.d. to tolerance Maximum of 15 dosesSecond cycle of IL‐2 21 days post TIL infusion | 13 (2 CR, 11 PR) | 42 | 42 |
| (Dudley et al., 2013) | “Young”a | 3–7 | 101 | 69 (68) | 15 | 17 | – | Cy 60 mg/kg (day −7&‐6) + Flu 25 mg/m2 (day −5 through −1) | 720,000 IU/kg t.i.d. to toleranceMaximum of 15 doses | 1st cohort12 (2 CR, 10 PR)2nd cohort7 (3 CR, 4 PR)Total: 19 (5 CR, 14 PR) | 19 | 28 |
| (Besser et al., 2013) | “Young” | 4 (including REP) | 80 | 57 (71) | 11 | 8 | 3 refused | Cy 60 mg/kg (day −7&‐6) + F 25 mg/m2 (day −5 through −1) | 720,000 IU/kg t.i.d. to tolerance Maximum of 15 doses | 23 (5 CR, 18 PR)1 patient died during chemotherapy regimen | 29 | 40 |
| (Rikke Andersen, 2014) | “Young” | – | 33 | 25 (76) | 7 | 1 | 0 | Cy 60 mg/kg (day −7&‐6) + F 25 mg/m2 (day −5 through −1 | 18 MIU/m2 s.c. over 6, then 12 and then 24 h, followed by 4.5 MIU/m2 over 24 h q 3 days | 10 (3 CR, 7 PR)1 patient not yet evaluated | 30 | 40 |
Abbreviations: CR, complete remission; Cy, Cyclophosphamide; Flu, Fludarabine; Gy, Gray; IU, international unit; kg, kilogram; mg, milligram; OR, objective response; PD, progressive disease; PR, partial remission; REP, rapid expansion protocol; SAE, serious adverse even; sBM, symptomatic brain metastases; s.c., subcutaneous; t.i.d., ter in die; TIL, tumor‐infiltrating lymphocytes.
Either unselected “young” TIL or unselected “young” CD8+‐enriched TIL.
3. Role of lymphodepletion
Several mouse models have demonstrated that conditioning of the host by use of chemotherapy or total body irradiation (TBI) improved the response rate of adoptive T cell therapy. Berendt and North were the first to point out that immunosuppressive T cells from the host could prevent complete eradication of established transplanted tumors by adoptive T cell therapy (Berendt and North, 1980). Thus, only hosts that were T cell deficient by prior thymectomy demonstrated tumor rejection. Similarly, the use of cyclophosphamide and TBI in conjunction with adoptive cell therapy appeared much more effective in comparison to non‐pretreated mice (Berenson et al., 1975; Eberlein et al., 1982). Also in patients with metastatic cancer, lymphodepleting host conditioning resulted in high objective response rates (ORR) upon adoptive cell transfer and durable benefit for the treated patients (Dudley et al., 2002). By studying the immunological effects of host lymphodepletion in murine models, several mechanisms of action have been suggested. First, by inducing a temporary lymphopenic state in the host the remaining peripheral lymphocytes will restore the original lymphocyte pool by a process called homeostatic expansion. Under these conditions, the infused syngeneic lymphocytes were more likely to expand and engraft in vivo. Second, lymphodepletion could cause a decrease in competition with endogenous T cells for antigen‐presenting cell interaction. Recently, Gattinoni et al., demonstrated in a murine B16 melanoma model that infusion of gp100‐specific pmel‐1 T cells followed by IL‐2 was much more effective in non‐lethally irradiated animals than in non‐irradiated mice. Induction of lymphopenia did not result in increased expansion of adoptively transferred pmel‐1 T cells, but rendered these cells functionally much more active. This phenomenon could be explained by the depletion of regulatory and immunosuppressive CD4+, FoxP3+ regulatory T cells (Tregs), which is a potentially third effect of lymphodepletion.
Fourth, removing so‐called cellular sinks, especially NK cells that highly compete with the adoptively transferred T cells for the host homeostatic cytokines IL‐7 and IL‐15 is considered a very important contribution of lymphodepletion on efficacy of TIL therapy. Whereas IL‐7 appears to be required for the proliferation and survival of the T cells, IL‐15 critically serves to maintain or improve the functional quality of the pmel‐1 T cells (Gattinoni et al., 2005). Notably, in patients receiving lymphodepleting conditioning regimens the serum concentrations of IL‐7 and IL‐15 also increased (Dudley et al., 2008).
In patients with metastatic melanoma, lymphodepleting chemotherapy consisting of cyclophosphamide and fludarabine induces a temporary lympho‐ and leukopenic state lasting around 5–10 days. For bone marrow recovery, CD34+ peripheral bone marrow stem cell support is not required. Dudley et al. examined whether intensifying the lymphodepletion by adding TBI to the non‐myeloablative chemotherapy (NMA) regimen, would improve the outcome of TIL treated patients (Dudley et al., 2008). Two cohorts of 25 patients each were treated either with cyclophosphamide/fludarabine plus 2 Gy TBI, or 12 Gy TBI. In both groups, bone marrow recovery was supported by autologous peripheral blood stem cell transplantation. Compared to a cohort of patients treated with chemotherapy alone (ORR 48.8%), adding TBI resulted in ORR of 52% and 72% respectively for 2 Gy and 12 Gy TBI. As this was not a randomized controlled trial, these differences in outcome could be explained by variation in patient selection, however this outcome warranted direct comparison in a randomized controlled trial (RCT). This clinical trial (ClinicalTrials.gov Identifier: NCT01319565) is still ongoing, but preliminary results presented so far fail to show a difference in clinical outcome between patients treated with chemotherapy compared to chemotherapy plus 12 Gy TBI (Rosenberg, personal communication).
In summary, conditioning by depletion of lymphocytes and NK cells appears to be an important component in the success of TIL therapy for metastatic melanoma, through depletion of immunosuppressive cells from the host and tumor micro‐environment and removal of cellular sinks for homeostatic cytokines IL‐7 and IL‐15. So far, the necessity for increased lymphodepletion has not clearly been demonstrated. Obviously, a more stringent myeloablative conditioning regimen, requiring autologous CD34+ stem cell support, would complicate a wider application of TIL therapy considerably.
4. Interleukin‐2 dosing schedule
In the original TIL treatment regimen published by Rosenberg et al., a high‐dose (HD) bolus IL‐2 schedule of 720.000 IU/kg i.v. every 8 h was initiated immediately after TIL‐infusion and continued until treatment limiting toxicity (Rosenberg et al., 2011). This classical HD IL‐2 schedule has been used as standard of care for treatment of metastatic melanoma for several decades and the rationale for its use after TIL infusion is to support the continued growth and activity of the infused TIL (Atkins et al., 1999).
The HD IL‐2 regimen is associated with transient but severe systemic toxicity affecting multiple organ systems and restricting its use to highly specialized cancer centers with experienced clinicians and intensive care support (Schwartz et al., 2002). To this end, HD IL‐2 administration to patients experiencing pancytopenia after HD chemotherapy leads to a particularly vulnerable medical condition with the need of intensive monitoring and specialist care.
The requirement for repeated high doses of IL‐2 in order to obtain clinical efficacy after TIL based ACT has never been documented in the clinical setting. On the contrary, data from the SB showing that patients who experienced an objective response received fewer doses of HD IL‐2 as compared to non‐responders, have recently questioned the administration of multiple high doses of IL‐2 (Yao et al., 2012). This might be explained by the fact that IL‐2 administration significantly increased the number of Tregs with a direct correlation between the number of IL‐2 doses given and reconstitution of Treg numbers in the blood and an inverse correlation between reconstitution of the Tregs and the probability of achieving an anti‐tumor response (Yao et al., 2012).
At the Center for Cancer Immune Therapy, Herlev Hospital, University of Copenhagen, Denmark we have tested a low and an intermediate IL‐2 dose schedule TIL based ACT. In an initial pilot study including six melanoma patients a low‐dose regimen of IL‐2 was used, consisting of subcutaneous (s.c.) administration of IL‐2, 2 million international units (MIU)/day for 14 days. Two of these patients achieved complete and long‐lasting responses (Ellebaek et al., 2012). Both patients experienced recurrence of a solitary metastasis (1 and 3 years after therapy), which was surgically removed and are currently free of disease more than 4 years after therapy. In a subsequent phase II trial presented at ESMO 2015, the intermediate decrescendo IL‐2 schedule was used (Keilholz et al., 1997). This regimen consists of five days continuous intravenous (i.v.) infusion of decreasing IL‐2 doses: 18 MIU/m2 over six, then 12, and then 24 h followed by 4.5 MIU/m2 over 24 h for three days. In this study, 25 patients were treated, with an ORR of approximately 40% which is comparable to what has previously been published with high dose bolus IL‐2 (Rikke Andersen, 2014). Low‐dose subcutaneous IL‐2 was associated with very limited toxicity while i.v. decrescendo IL‐2 led to increased, but certainly manageable toxicity, without the requirement for intensive care support.
These studies indicate that objective and durable responses can in fact be induced without the use of HD IL‐2. Thus, the optimal dosing of IL‐2 after TIL transfer in regard to clinical efficacy as well as toxicity requires further investigation, which may likely lead to dose reduction of IL‐2 in the future. A randomized phase II trial, TIL therapy in metastatic melanoma and IL‐2 dose assessment (ClinicalTrials.gov Identifier: NCT01995344), testing HD versus low dose IL‐2 is planned at The Christie Hospital NHS Foundation Trust, Manchester, United Kingdom, but is not yet recruiting patients. Another non‐randomized phase II study at the SB, plans to assess the feasibility of TIL based ACT for melanoma without the use of IL‐2 (ClinicalTrials.gov Identifier: NCT01468818).
5. Quality and quantity of TIL
Preclinical models on adoptive cell therapy for the treatment of cancer demonstrated the absolute requirement for CD8+ T cells within the infusion product for anti‐tumor efficacy. In some models, the presence of CD4+ T cells was required as well (Antony et al., 2005). In addition, the absolute numbers of transferred T cells correlated with outcome in these models, showing that infusion of more cells resulted in better tumor control. Other factors such as lymphodepletion and combination with high dose of IL‐2 improved persistence of the TILs after transfer and efficacy of the treatment (Cheever et al., 1980; North, 1982). Based on these preclinical findings, clinical trials were designed and many aspects of preclinical evidence were found in human studies as well. In clinical trials performed at the SB and other centers, correlation between ORR and absolute number of infused T cells was very consistent (Itzhaki et al., 2011; Radvanyi et al., 2012). However, a clear correlation between in vitro antitumor reactivity of the TIL product and clinical response has not be demonstrated, suggesting that the TIL products with the highest fold expansion might hold the “fittest” cells with the highest antitumor activity.
Infusion of a less differentiated cell population is another important factor in improving the efficacy of TIL both in preclinical models and humans (Klebanoff et al., 2012). As TIL, by virtue of their presence within the tumor micro‐environment, are thought to be antigen experienced T cells, these cells have already gained effector function. Correlations with clinical outcome have been found for surface expression of the co‐stimulatory molecules CD27 and CD28 by the infused cells, which is indicative of a less terminally differentiated phenotype (Huang et al., 2005; Powell et al., 2005). In a report by Tran et al., the expression of CD27 and CD28 was measured by flow cytometry in young TIL and standard TIL cultures (Tran et al., 2008). Fourteen matched pairs of young (mean culture age of 12 days) and standard (mean culture age 25 days) TIL were generated from tumor specimens. Flow cytometry analysis demonstrated that phenotypic expression of CD27 and CD28 differed in young versus standard TIL. Young TIL had significantly higher expression of CD27 and CD28, p < 0.00001 and p = 0.003, respectively, confirming their less differentiated phenotype.
Indicative of a less differentiated T cell pool is also its proliferative capacity at the time of infusion, as determined by the length of telomeres. In several clinical studies, longer telomere length was associated with ORR (Rosenberg et al., 1994; Dudley et al., 2008; Itzhaki et al., 2011). It was shown that although telomere lengths varied widely at any given TIL age, there was an inverse correlation between culture time and mean telomere length of TIL (p < 0.001).
TIL products contain variable quantities of CD4+ T cells, but their role in mediating tumor regression has not been well clarified. Some studies suggested that a higher percentage of CD4+ TILs in the infusion products may be associated with worse outcomes after treatment (Prieto et al., 2010; Radvanyi et al., 2012). However, reports on single patient cases seem to indicate that effector CD4+ TILs may mediate antitumor effector functions (Friedman et al., 2012; Robbins et al., 2002; Tran et al., 2014). More recently, we showed that about 50% of patients with melanoma harbor tumor‐reactive CD4+ TILs. These cells can recognize MHC class II positive autologous melanoma cells but are largely monofunctional (Donia M et al., manuscript submitted). It seems therefore unlikely that, in the majority of patients, tumor‐specific CD4+ T cells mediate clinical effects.
More recently, in depth phenotypic analysis comparing characteristics of CD8+ TIL to peripheral blood CD8+ T cells from the same patients indicated that TIL have a distinct expression pattern of co‐inhibitory and co‐stimulatory molecules PD‐1, LAG3, TIM3 and 4‐1BB (CD137) (Gros et al., 2014). Although, the level of expression varied, CD8+ TIL invariably showed higher expression of these molecules compared to peripheral blood CD8+ T cells. Importantly, the in vitro tumor‐reactive T cells within TIL resided within this population. When stimulated with the autologous tumor cells, only PD1+, LAG3+ and TIM3+ TIL showed cytolytic activity, produced IFN‐γ and started to express 4‐1BB (as marker of T cell activation), whereas the PD1−, LAG3− and TIM3− cells failed to do so. Clonotypic frequencies measured by TCRVbeta sequencing between PD1+ and PD1− CD8+ TIL differed considerably, showing oligoclonal expansion within CD8+/PD1+ compared to CD8+/PD1− TIL, reminiscent of prior antigen encounter and antigen‐driven proliferation. The tumor‐reactive TIL resided within the CD8+/PD1+ clonotypes. In another study by Ye et al., 4‐1BB was mainly expressed on the tumor‐reactive lymphocyte subset within TILs (Ye et al., 2014). In this study, 4‐1BB+ and 4‐1BB− T cells from ovarian cancer were cultured overnight in median supplemented with IL‐7/IL‐15. The 4‐1BB+ and 4‐1BB− fractions were then cultured for 8–10 days in IL‐2 and tested for reactivity against autologous tumor cells. 4‐1BB+ TILs secreted IFN‐γ in response to autologous tumor cells, whereas 4‐1BB− TILs did not. These results strongly suggest that pre‐selection of TIL either by PD1 expression or 4‐1BB prior to rapid expansion, can lead to enrichment of tumor‐reactive T cells and increase the efficacy of this treatment. Not only does 4‐1BB play a role in the possible selection of tumor‐reactive T cells, 4‐1BB co‐stimulation could also be involved in improving TIL survival following ACT and potentially boost anti‐tumor cytolytic activity. It is known that the majority of post‐REP CD8+ T cells lose the expression of the co‐stimulatory molecule CD28 (Li et al., 2010). Furthermore, the expression of the co‐stimulatory molecule CD27 is lost after stimulation of TILs with IL‐2 (Huang et al., 2006). With the loss of both CD27 and CD28, alternative co‐stimulation pathways may have an important role in maintaining TIL survival after ACT, and 4‐1BB could be such a candidate. When the agonistic anti‐4‐1BB antibody was added during the initial tumor fragment cultures to provide 4‐1BB costimulation, this resulted in an accelerated expansion of CD8+ TIL. Furthermore, it also appeared that TIL expanded in the presence of anti‐4‐1BB antibody showed increased antitumor reactivity, as measured by INF‐γ release after a 24‐h tumor cell‐TIL co‐culture assay (Chacon et al., 2013, 2015).
6. TIL recognition of tumor antigens
T cells recognize antigens expressed at the cell surface presented by MHC class I and II molecules. For melanoma TIL, recognition of several classes of antigens have been described. First, there are antigens derived from melanocyte differentiation antigens (MDA), especially MART‐1 and gp100, but also tyrosinase and tyrosinase related peptides 1 and 2 (Bakker et al., 1994; Engelhard et al., 2002; Kawakami et al., 1994; Robbins et al., 1994; Romero et al., 1997). In many TIL, CD8+ T cells specific for MART‐1 and gp100 have be found (Castelli et al., 1995). Most melanomas express MART‐1 and gp100, and the fact that T cells specific for these antigens are sometimes abundantly present in TIL, at least suggest that these T cells have undergone antigen‐specific expansion. As these proteins are also expressed in normal melanocytes in skin, eye and inner ear as well, one could expect that following infusion of 1011 TIL harboring MART‐1 or gp100 specific T cells, patients would develop toxicities as a result of melanocyte destruction, such as skin rash, vitiligo, uveitis or even the Vogt–Koyanagi–Harada syndrome (uveitis, dermatitis, with also neurologic and inner ear involvement due to melanocyte destruction). Although, these toxicities were indeed observed in patients treated with T cells genetically modified to express high‐affinity MART‐1 or gp100‐specific T cell receptors, this was not the case in the many melanoma patients that have been treated with TIL, despite the (oftentimes low abundant) presence of MART‐1 or gp100‐specific cells, thereby perhaps questioning the relevance of these cells for melanoma rejection. A correlation between presence of these cells and outcome after TIL treatment has not been demonstrated (Kvistborg et al., 2012).
Another class of antigens that is recognized by melanoma TIL are Cancer/Testis (C/T) gene products. These genes are normally expressed during embryogenesis and in germ cells, however are silenced in other tissues. Many tumors can start to aberrantly express these genes. One example is the melanoma antigen (MAGE), first described by Boon and colleagues, expressed on melanoma cells and other tumors, but not on normal tissue (Traversari et al., 1992). Later, many more C/T antigens were discovered, including SSX2, NY‐eso‐1, RAGE and SAGE (Chen et al., 1998; Scanlan et al., 2002). Some of them have sub‐members, such as the MAGE antigens (MAGE‐A1 through 12, MAGE‐B and MAGE‐C) family members, and many are expressed on a wide variety of different tumor histologies. In a recently published study, we carefully examined the frequency of CD8+ T cells specific for previously described C/T epitopes within melanoma TIL infusion products from the SB and the Ella Institute. The screen, which utilized soluble peptide‐MHC multimers, (HLA‐A*0201 harboring known antigenic peptides from C/T antigens) in a flow‐cytometry based combinatorial encoding strategy (Hadrup et al., 2009; Toebes et al., 2006), revealed that C/T antigen specific T cells can oftentimes be found, although in the majority of patients tested, the frequency of these cells was rather low, seldom higher than 0.1% of CD8+ TIL (Kvistborg et al., 2012). That C/T antigen specific T cells can result in tumor rejection, was endorsed by an adoptive T cell transfer study using peripheral blood T cells genetically equipped with a NY‐eso‐1 specific TCR (Robbins et al., 2011). The role of C/T antigen specific TIL in tumor rejection is not yet fully appreciated, and may differ between tumor types.
Next to expressing C/T antigens, tumors may also overexpress proteins that give rise to antigen‐specific T cell responses. One example is Meloe‐1, encoded by a gene that is overexpressed as a result of epigenetic changes in the tumor (Chalopin et al., 2015; Godet et al., 2008). The aforementioned screen of melanoma TIL infusion products also included known overexpressed antigens. CD8+ T cells specific for these antigens were present within TIL coming from several patients. Again the frequency of these T cells was generally very low.
With current DNA technologies readily available, full exome sequencing of tumor derived DNA has become feasible in a limited period of time and to affordable costs. The Welcome Trust Sanger Institute recently published the results of high fidelity DNA sequencing of many human tumors and revealed the mutational load within these tumors (Alexandrov et al., 2013). On average, melanomas were found to contain the highest number of somatic mutations per megabase of DNA, followed by NSCLC, bladder cancer, stomach and esophageal cancer, whereas leukemias harbor only few mutations Already several decades ago, melanoma derived T cells specific for mutated antigens such as CDK4 and β‐catenin were described (Robbins et al., 1996; Wolfel et al., 1995), however their role as tumor rejection antigens has largely been ignored as these mutations are patient specific and rare. To identify potential neo‐epitopes, whole exome DNA sequence data of tumor and matching healthy cells need to be aligned in order to detect patient‐specific mutations. RNA expression data is used to subsequently assess whether a mutated gene is transcribed and its gene‐product potentially expressed on the tumor cell surface. Several approaches can be followed to assess whether the T‐cell based immune system is able to recognize and respond to these mutated antigens. One such approach followed by the SB utilizes synthesis of minigenes encoding fragments corresponding to the mutation flanked on both sides by four amino acids. These minigenes were transiently transfected into COS‐7 cells for stimulation of TIL (Lu et al., 2014). A different approach followed by our group at the Netherlands Cancer Institute (NKI) utilized peptide‐MHC binding algorithms to predict potential epitopes around these mutations for the different HLA molecules of the patients, followed by the generation of peptide‐MHC multimers and screening of TIL for the presence of neo‐antigen specific CD8+ T cells (van Rooij et al., 2013). Using a different approach, we were able to screen for neo‐antigen specific CD4+ T cells as well (Linnemann et al., 2015). In the vast majority of patients, both approaches led to the discovery of neo‐antigen specific T cell responses within TIL products. In most cases, the frequency of TIL reactive against mutated antigens appeared higher than what was previously observed for other antigen classes. However, despite the high number of nonsynonymous somatic DNA mutations found in melanoma, only very few appear to lead to a neo‐antigen specific T cell response. This may be explained by 1.) not all DNA mutations are in expressed genes, 2.) mutated proteins need to be properly processed to generate class I binding epitopes 3.) the TCR repertoire needs to cover these potential neoantigens, 4.) our technical set‐up may be far from optimal (incomplete RNA seq, imperfect prediction algorithms for binding to different HLA molecules). Importantly, the few neoantigen specific T cells responses found per patient so far are highly unique for every patient, indicating that the induction of a T cell response against mutated antigens appears to be a random process that can best be explained by a probabilistic lottery model (Schumacher and Schreiber, 2015) (see Figure 2).
Figure 2.
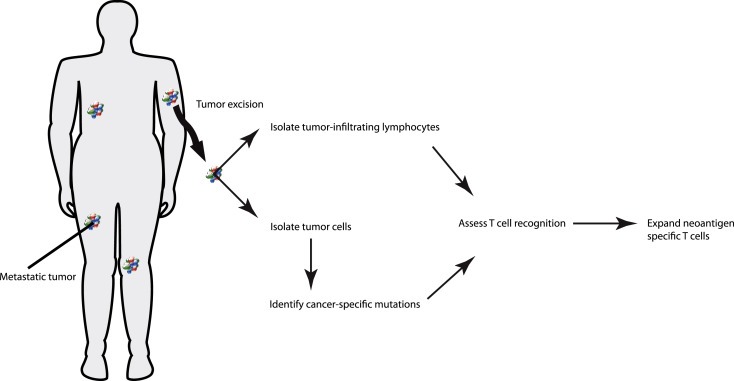
Schematic overview of the possible selection of neo‐antigen specific TIL. Excised metastatic melanoma is analyzed for cancer‐specific mutations. Resulting epitopes are used to identify neo‐antigen specific T cell responses within T cell populations. The neo‐antigen specific T cells are then further expanded and infused into the previously lymphodepleted patient.
In conclusion, within melanoma TIL reside T cells, both CD4+ and CD8+ that recognize tumor antigens. These antigens can be derived from MDA, C/T genes, overexpressed genes and mutated genes. Immunological tolerance is likely lacking for epitopes coming from mutated genes, which could result in higher affinity T cells than those specific for most other antigens. Whether these T cells are in general functionally superior still remains to be demonstrated.
7. Beyond melanoma – TIL for other tumor types
The presence of TILs and its association with improved survival has been documented in virtually every human cancer studied (Fridman et al., 2012; Gooden et al., 2011). It therefore seemed logical to test TIL therapy for any solid tumor, depending on the ability to grow TIL that are reactive to autologous tumors.
So far, adoptive cell transfer immunotherapy with TILs has been applied with consistent success only in metastatic melanoma. Indeed, early studies in melanoma showed that TILs could be expanded in vitro and recognize autologous tumors, but until recently it was difficult to demonstrate similar level of tumor recognition in other tumor histologies (Muul et al., 1987; Topalian et al., 1987). Here we describe the main advances in characterization of TIL and application of TIL therapy in other major types of solid tumors.
8. Cervical cancer
Persistent infection with human papilloma viruses (HPVs) is essential in the pathogenesis of virtually all cervical cancers (Walboomers et al., 1999), and prophylactic HPV vaccination is now recognized as a standard procedure for the prevention of cervical cancer and other HPV‐associated diseases (Wang et al., 2015). However, despite its immunogenicity and encouraging recent results in patients with high‐grade cervical intraepithelial neoplasia (Maldonado et al., 2014), an effective therapeutic vaccine for established cervical cancer has not yet been developed. It was suggested that the relatively low magnitude of vaccine‐induced immune responses, and the immune suppression mediated by large established tumors may represent a barrier to the induction of immune‐mediated regression of cervical cancers with classical immunization protocols (Zsiros et al., 2015).
In a recent study, Stevanovic et al. reported that out of nine patients with recurrent metastatic cervical cancer treated with TIL therapy (when possible, TIL microcultures were selected for HPV E6 and E7 oncoprotein reactivity), three patients experienced an objective response including two patients with complete tumor regression lasting over one year. Interestingly, responses occurred only when reactivity to E6 and E7 HPV‐related oncoproteins was demonstrated in TILs (Stevanovic et al., 2015). Though these findings may warrant further optimization, it is currently not known whether the T cell reactivity to E6 and E7 by itself is mediating tumor regression or, rather, whether this represents a biomarker of more potent antitumor immune responses directed towards other tumor antigens.
This pivotal study demonstrates the feasibility and efficacy of this approach in selected patients with metastatic cervical cancer, and will ensure further development of adoptive cell therapy in this malignancy.
9. Ovarian cancer
In ovarian cancer, the prognostic significance of tumor‐infiltrating T‐cells has been known for over a decade (Zhang et al., 2003) and several recent studies have confirmed and expanded on these results (Hwang et al., 2012). For instance, two recent studies from independent groups, demonstrated the presence of functional tumor‐antigen specific T‐cells in the tumor microenvironment of ovarian cancer (Landskron et al., 2015; Webb et al., 2015).
Through characterization of the tumor mutanome and comprehensive screening of mutation‐specific T‐cells obtained from the tumor microenvironment of three patients, Wick et al. demonstrated a highly specific CD8+ T cell response to a nonsynonymous mutation in one patient (Wick et al., 2014). Thus, these data demonstrate that some degree of immune surveillance to the tumor mutanome may be present in selected patients with ovarian cancer.
Encouraging clinical data from the use of TILs in ovarian cancer were already reported in the 90's (Aoki et al., 1991; Freedman and Platsoucas, 1996; Fujita et al., 1995). However, based on the current knowledge of the biology of immune responses to ovarian cancer, as well as new protocols for adoptive transfer, which consistently demonstrated efficacy in melanoma, the possibility to revisit TIL therapy for ovarian cancer is highly warranted. To this end, we are currently developing optimized protocols to apply TILs in ovarian cancer.
10. Kidney cancer
It has been known for decades that effective manipulation of the immune system can mediate durable complete responses in a small fraction of patients with advanced kidney cancer (Rosenberg et al., 1998). However, despite early demonstration that renal cell carcinoma (RCC) contain tumor‐antigen specific cytotoxic T‐lymphocytes (Becker et al., 2001; Schendel et al., 1997; Wang et al., 2012), previous clinical trials with TIL therapy in RCC has been quite disappointing and, in general, it has been particularly difficult to demonstrate any tumor reactivity of RCC TIL (reviewed in (Shablak et al., 2009)).
In recent years, two studies demonstrated the utilization of optimized methods for TIL manufacturing to generate high numbers of TILs with (at least to some extent) tumor reactivity.
Schachter and co‐workers (Sheba Medical Center, Tel‐Hashomer, Israel), who are very experienced in applying TIL therapy in metastatic melanoma, have tested the same exact methods of TIL manufacturing that have been used with success in melanoma in RCC (Markel et al., 2009). They demonstrated that TILs from some patients could exert antitumor functions. Indeed, in a few cases TILs secreted IFN‐γ upon recognition of autologous tumors, while in other cases TILs exerted killing activity. Surprisingly, none of the TIL cultures demonstrated simultaneous killing and IFN‐γ secretion (Markel et al., 2009). The relatively low sensitivity of some assays used in this study may explain this apparent paradox. However, functional deficiencies of tumor‐specific TILs in RCC have indeed been described, thus firm conclusions cannot be drawn (Wang et al., 2012).
The group of R. Hawkins (University of Manchester, UK), also with extensive experience in generating TILs from metastatic melanoma, was able to optimize the method of expansion by using anti‐CD3/anti‐CD28‐coated paramagnetic beads. With this method, IFN‐γ secretion from expanded TILs co‐cultured with uncultured tumor cells was shown in about 50% of patients (Baldan et al., 2015).
Despite the heterogeneous and, in some cases, conflicting results, these studies demonstrate that TIL therapy may be feasible in RCC and warrant additional clinical testing. Along this line of research, our group, at the Herlev Hospital in Copenhagen, is currently testing optimized methods of TIL manufacturing in order to apply TIL therapy in RCC.
11. Gastrointestinal cancers
A high TIL density is considered a good prognostic indicator in various gastrointestinal adenocarcinomas (Fridman et al., 2012). Several studies have established the prognostic discriminatory power of immune‐cell signatures (in particular cytotoxic CD8+ and memory CD45RO+ T cells) in colorectal cancer, including data clearly suggesting that the “immunoscore” is superior to standard staging systems (Galon et al., 2006; Mlecnik et al., 2011).
Thus, it is not surprising that two recent studies have demonstrated the presence of naturally occurring tumor‐reactive CD8+ T‐cells in the tumor microenvironment of patients with gastrointestinal cancers (Turcotte et al., 2013, 2014). With a very high efficiency, TILs could be cultured (Turcotte et al., 2013, 2014) and expanded to clinically relevant numbers (Turcotte et al., 2013).
In general, it seems that in gastrointestinal cancers the frequency of in vitro tumor‐reactive CD8+ T cells is relatively low (0–3% of TILs) as compared to melanoma (Turcotte et al., 2013). Therefore, it has been suggested that one of the main challenges in developing TIL therapy for gastrointestinal tumors may be the ability to selectively enrich and expand tumor‐reactive T‐cells.
Notably, the same group has recently reported that dramatic regression of liver and lung metastases could be induced in a patient with metastatic cholangiocarcinoma after treatment by in vitro enriched naturally occurring CD4+ T cells (isolated from autologous TILs) recognizing a mutated antigen (Tran et al., 2014).
12. Head and neck cancers
A high density of lymphocyte infiltration is associated with improved outcome in head and neck squamous cell carcinoma (Balermpas et al., 2014; Ward et al., 2014).
In a recent article, we characterized TILs obtained from head and neck cancer metastases. TILs were expanded with high efficiency (80% of patients, with massive expansion for up to 3500 folds), and recognition of tumor antigens could be demonstrated in 60% of patients (Junker et al., 2011). These data show that TIL therapy may be feasible for selected patients with head and neck squamous cell carcinoma, and pave the way for its clinical testing.
In summary, TIL therapy is now explored in cancers other than melanoma. Whether the same rules for efficacy as have been established for melanoma TIL apply to TIL treatment for other cancers, remains to be investigated. With the current technologies to enrich for tumor‐reactive TIL and to define the specificity of TIL, an even more personalized approach by expanding only tumor‐specific T cells for TIL infusion becomes feasible. Examples like the ability to grow tumor‐specific TIL, such as from the cholangiocarcinoma patient mentioned above, demonstrate that this approach is both feasible and efficacious.
13. Future perspectives for TIL
Until 2010, interleukin‐2 and the chemotherapeutic drug dacarbazine (DTIC) were the only Food and Drug Administration (FDA) registered treatments for metastatic melanoma, showing an objective response in a minority of treated patients without any impact on overall survival.
In 2011, the FDA approved the specific inhibitor vemurafenib of BRAF V600 for metastatic melanoma patients harboring this mutation in their malignancy (Chapman et al., 2011). Vemurafenib and later also dabrafenib (Hauschild et al., 2012) are highly active drugs resulting in impressive improvements in median PFS and OS in metastatic melanoma. Unfortunately, the tumor heterogeneity in metastatic disease prohibits these drugs from inducing long‐term remissions, due to early tumor escape mechanisms. More recently, it was demonstrated that combining BRAF inhibitors with MEK inhibitors (Larkin et al., 2014; Long et al., 2014; Robert et al., 2014) results in significant prolongation of PFS and probably also OS compared to BRAF inhibitors alone in BRAF V600 mutated metastatic melanoma.
In the same year, the CTLA4 checkpoint inhibitor ipilimumab was registered for the treatment of metastatic melanoma. Ipilimumab has shown ORR between 10 and 12% in patients with metastatic melanoma (Hodi et al., 2010; Postow et al., 2015; Robert et al., 2015b). Importantly, the overall survival following ipilimumab treatment reaches a plateau around 20% at 3 years, indicating that in contrast to BRAF inhibitors, ipilimumab treated patients may benefit long‐term (Schadendorf et al., 2015). In 2014, two other drugs, pembrolizumab and nivolumab, blocking the checkpoint molecule PD‐1, became available for metastatic melanoma and in 2015 nivolumab also for non‐small cell lung carcinoma (NSCLC). Follow‐up of patients treated with these drugs is still short, but 1‐, 2‐, and 3‐year survival rates appear higher compared to ipilimumab. In a direct comparison pembrolizumab outcompetes ipilimumab when treating a population of naïve metastatic melanoma patients with regard to ORR and progression free survival (PFS) (Robert et al., 2015b). The combination of ipilimumab plus nivolumab has also been reported to improve ORR and PFS compared to ipilimumab (Postow et al., 2015). With an impressive rate of complete remissions it has become clear that checkpoint inhibitors and combination of BRAF and MEK inhibitors are highly potent therapies (Table 2).
Table 2.
Overview of response rates of other treatment modalities for metastatic melanoma.
| Treatment | 1‐year survival rate (%) | ORR (%) | Median PFS | Median OS | CR (%) | |
|---|---|---|---|---|---|---|
| (Hodi et al., 2010) | Ipilimumab 3 mg/kg | 45.6 | 11.0 | 2.9 months (95% CI 2.76–3.02) | 10.1 months (95% CI 8.0–13.8) | 6.0 |
| (Robert et al., 2014) | Dabrafenib 150 mg 2dd + trametinib 2 mg 1dd | 72.0 (95% CI 67–77) | 64.0 (95% CI 59–69) | 11.4 months | Not yet reached | 13.0 |
| (Long et al., 2014) | Dabrafenib 150 mg 2dd + trametinib 2 mg 1dd | Yet unknown | 67.0 (95% CI 60–73) | 9.3 months | Not yet reached | 10.0 |
| (Larkin et al., 2014) | Vemurafenib 960 mg 2dd + cobimetinib 60 mg 1dd (for 21 days followed by 7 days rest) | Yet unknown | 68.0 (95% CI 61–73) | 9.9 months (95% CI 9.0 – NR) | Not yet reached | 10.0 |
| (Weber et al., 2015) | Nivolumab 3 mg/kg q2 weeks | Yet unknown | 31.7 (95% CI 23.5–40.8) | 4.7 months (95% CI 2.3–6.5) | Not yet reached | 3.3 |
| (Robert et al., 2015a) | Nivolumab 3 mg/kg q2 weeks | 72.9 (95% CI 65.5–78.9) | 40.0 (95% CI 33.3–47.0) | 5.1 months (95% CI 3.5–10.8) | Not yet reached | 7.6 |
| (Postow et al., 2015) | Ipilimumab 3 mg/kg + nivolumab 1 mg/kg q3 weeks for 4 cycles, followed by nivolumab 3 mg/kg q2 weeks | Yet unknown | BRAF WT 61.0 (95% CI 49–72)BRAF V600E mutated52.0 (95% CI 31–73)Total 58.9% | Not yet reached | Not yet reached | 22.0 |
| (Robert et al., 2015b) | Pembrolizumab 10 mg/kg q2 or q3 weeks | q2 weeks 74.1q3 weeks 68.4 | q2 weeks 33.7%q3 weeks 32.9% | q2 weeks 5.5 months (95% CI 3.4–6.9)q3 weeks 4.1 months (95% CI 2.9–6.9) | Not yet reached | q2 weeks 5.0q3 weeks 6.1 |
Abbreviations: CI, confidence interval; CR, complete response; mg, milligrams; kg, kilogram; ORR, objective response rate; OS, overall survival; PFS, progression free survival.
So how does TIL compare to these active treatments? When should TIL be given during the course of the cancer (Figure 3)? A direct comparison of TIL with either checkpoint inhibitors or targeted agents has not yet been done. In fact, apart from randomized controlled phase II trials comparing different TIL strategies, a RCT comparing TIL with standard of care has never been performed. In Europe, a first RCT comparing young TIL therapy to ipilimumab as first or second line treatment for metastatic melanoma has been started (ClinicalTrials.gov Identifier: NCT02278887). In the US, Lion Biotechnologies has obtained an exclusive license from the SB to develop and commercialize TIL for the treatment of metastatic melanoma and is preparing for a phase II trial in refractory patients. These strategies are directed at getting TIL therapy approved as a therapeutic option for metastatic melanoma. Compared to checkpoint inhibitors, TIL therapy has some advantages and disadvantages. TIL therapy consists of a single treatment course. Despite the toxicity that is coming from NMA, such as nausea, alopecia and bone marrow depression, and high dose bolus IL‐2 with short term high fever, chills, hypotension, oliguria, hypoxia and weight gain due to fluid accumulation, practically all treated patients tolerated the treatment well and very few treatment related deaths (n = 2) have been reported (Dudley et al., 2008) (Svane, personal communication). With young TIL, especially without additional TBI, no long‐term side effects have been observed, clearly showing the safety of this regimen. In up to 10% of treated, mostly refractory, patients complete remissions are induced with TIL. Especially, these CR patients tend to have an excellent prognosis. Some of the deep PR patients show similar long‐term survival. Prior treatment with ipilimumab does not impair subsequent treatment with TIL (Besser et al., 2013). Whether prior treatment with PD‐1 blocking agents influences subsequent TIL therapy remains to be established, but early results suggest that TIL may still be effective (Rosenberg, unpublished observation). A major disadvantage of TIL therapy is that it is laborious, patient‐specific, and time‐consuming, with a dropout rate of between 20 and 40%. As a large part of the infused TIL appear not tumor‐specific, strategies to enrich for tumor‐specific TIL, for instance by selecting cells based on their phenotype (PD‐1, 4‐1BB) or reactivity towards tumor antigens without severely increasing culture time, should be developed. Alternatively, combining TIL with either checkpoint inhibitors or boosting TIL with neo‐antigen based vaccines may increase the efficacy and outcome even further. We expect that in the coming years, these strategies are likely to be investigated and if proven safe and efficacious may replace the current standard young TIL protocol.
Figure 3.
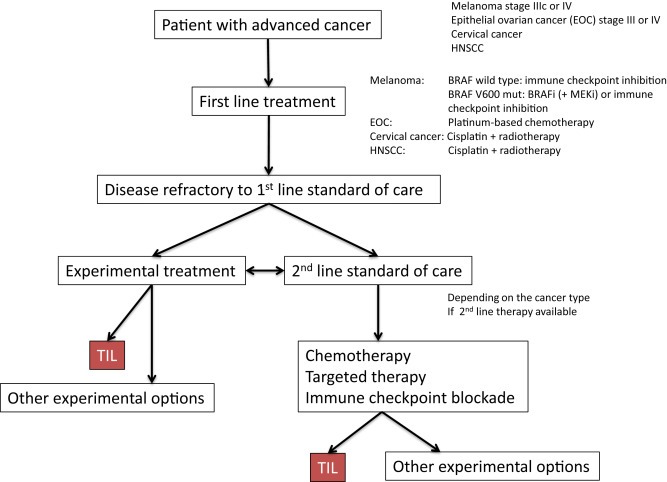
TIL and treatment algorithm for metastatic cancer.
TIL therapy has undergone a long history of development and is still being improved to obtain the best outcome for patients. Our increasing understanding of the immunohostile tumor micro‐environment, the tumor‐specificity of tumor‐infiltrating T cells and the development of manipulations to isolate the fittest and most tumor‐reactive cells for adoptive cell therapy, will further drive the field of adoptive T cell therapy for metastatic cancers in the years ahead.
Geukes Foppen M.H., Donia M., Svane I.M., Haanen J.B.A.G., (2015), Tumor‐infiltrating lymphocytes for the treatment of metastatic cancer, Molecular Oncology, 9, doi: 10.1016/j.molonc.2015.10.018.
This is a contribution to the special issue edited by Johanna Olweus, Cancer Immunotherapy.
Contributor Information
M.H. Geukes Foppen, Email: m.geukes@nki.nl
M. Donia, Email: Marco.Donia@regionh.dk
I.M. Svane, Email: Inge.Marie.Svane@regionh.dk
J.B.A.G. Haanen, Email: j.haanen@nki.nl
References
- Alexandrov, L.B. , Nik-Zainal, S. , Wedge, D.C. , Aparicio, S.A. , Behjati, S. , Biankin, A.V. , Bignell, G.R. , Bolli, N. , Borg, A. , Borresen-Dale, A.L. , Boyault, S. , Burkhardt, B. , Butler, A.P. , Caldas, C. , Davies, H.R. , Desmedt, C. , Eils, R. , Eyfjord, J.E. , Foekens, J.A. , Greaves, M. , Hosoda, F. , Hutter, B. , Ilicic, T. , Imbeaud, S. , Imielinski, M. , Jager, N. , Jones, D.T. , Jones, D. , Knappskog, S. , Kool, M. , Lakhani, S.R. , Lopez-Otin, C. , Martin, S. , Munshi, N.C. , Nakamura, H. , Northcott, P.A. , Pajic, M. , Papaemmanuil, E. , Paradiso, A. , Pearson, J.V. , Puente, X.S. , Raine, K. , Ramakrishna, M. , Richardson, A.L. , Richter, J. , Rosenstiel, P. , Schlesner, M. , Schumacher, T.N. , Span, P.N. , Teague, J.W. , Totoki, Y. , Tutt, A.N. , Valdes-Mas, R. , van Buuren, M.M. , van 't Veer, L. , Vincent-Salomon, A. , Waddell, N. , Yates, L.R. , Australian Pancreatic Cancer Genome Initiative, ICGC Breast Cancer Consortium, ICGC MMML-Seq Consortium, ICGC PedBrain, Zucman-Rossi, J. , Futreal, P.A. , McDermott, U. , Lichter, P. , Meyerson, M. , Grimmond, S.M. , Siebert, R. , Campo, E. , Shibata, T. , Pfister, S.M. , Campbell, P.J. , Stratton, M.R. , 2013. Signatures of mutational processes in human cancer. Nature. 500, 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, Rikke , Danio, Marco , Borch, Troels Holz , Steensgaard, Eva Ellebæk , Iversen, Trine Zeeberg , Kongsted, Per , Andersen, Mads Hald , thor Straten, Per , Svane, Inge Marie , 2014. Adoptive cell therapy with tumor infiltrating lymphocytes and intermediate dose IL-2 for metastatic melanoma. J. Immunother. Cancer. 2, 1 24829758 [Google Scholar]
- Antony, P.A. , Piccirillo, C.A. , Akpinarli, A. , Finkelstein, S.E. , Speiss, P.J. , Surman, D.R. , Palmer, D.C. , Chan, C.C. , Klebanoff, C.A. , Overwijk, W.W. , Rosenberg, S.A. , Restifo, N.P. , 2005. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 174, 2591–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki, Y. , Takakuwa, K. , Kodama, S. , Tanaka, K. , Takahashi, M. , Tokunaga, A. , Takahashi, T. , 1991. Use of adoptive transfer of tumor-infiltrating lymphocytes alone or in combination with cisplatin-containing chemotherapy in patients with epithelial ovarian cancer. Cancer Res. 51, 1934–1939. [PubMed] [Google Scholar]
- Atkins, M.B. , Lotze, M.T. , Dutcher, J.P. , Fisher, R.I. , Weiss, G. , Margolin, K. , Abrams, J. , Sznol, M. , Parkinson, D. , Hawkins, M. , Paradise, C. , Kunkel, L. , Rosenberg, S.A. , 1999. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 17, 2105–2116. [DOI] [PubMed] [Google Scholar]
- Bakker, A.B. , Schreurs, M.W. , de Boer, A.J. , Kawakami, Y. , Rosenberg, S.A. , Adema, G.J. , Figdor, C.G. , 1994. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J. Exp. Med. 179, 1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldan, V. , Griffiths, R. , Hawkins, R.E. , Gilham, D.E. , 2015. Efficient and reproducible generation of tumour-infiltrating lymphocytes for renal cell carcinoma. Br. J. Cancer. 112, 1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balermpas, P. , Michel, Y. , Wagenblast, J. , Seitz, O. , Weiss, C. , Rodel, F. , Rodel, C. , Fokas, E. , 2014. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br. J. Cancer. 110, 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, C. , Pohla, H. , Frankenberger, B. , Schuler, T. , Assenmacher, M. , Schendel, D.J. , Blankenstein, T. , 2001. Adoptive tumor therapy with T lymphocytes enriched through an IFN-gamma capture assay. Nat. Med. 7, 1159–1162. [DOI] [PubMed] [Google Scholar]
- Berendt, M.J. , North, R.J. , 1980. T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J. Exp. Med. 151, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson, J.R. , Einstein, A.B. , Fefer, A. , 1975. Syngeneic adoptive immunotherapy and chemoimmunotherapy of a friend leukemia: requirement for T cells. J. Immunol. 115, 234–238. [PubMed] [Google Scholar]
- Besser, M.J. , Shapira-Frommer, R. , Itzhaki, O. , Treves, A.J. , Zippel, D. , Levy, D. , Kubi, A. , Shoshani, N. , Zikich, D. , Ohayon, Y. , Ohayon, D. , Shalmon, B. , Markel, G. , Yerushalmi, R. , Apter, S. , Ben-Nun, A. , Ben-Ami, E. , Shimoni, A. , Nagler, A. , Schachter, J. , 2013. Adoptive transfer of tumor infiltrating lymphocytes in metastatic melanoma patients: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin. Cancer Res. 19, 4792–4800. [DOI] [PubMed] [Google Scholar]
- Blazar, B.A. , Heppner, G.H. , 1978. In situ lymphoid cells of mouse mammary tumors. I. Development and evaluation of a method for the separation of lymphoid cells from mouse mammary tumors. J. Immunol. 120, 1876–1880. [PubMed] [Google Scholar]
- Castelli, C. , Storkus, W.J. , Maeurer, M.J. , Martin, D.M. , Huang, E.C. , Pramanik, B.N. , Nagabhushan, T.L. , Parmiani, G. , Lotze, M.T. , 1995. Mass spectrometric identification of a naturally processed melanoma peptide recognized by CD8+ cytotoxic T lymphocytes. J. Exp. Med. 181, 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon, J.A. , Pilon-Thomas, S. , Sarnaik, A.A. , Radvanyi, L.G. , 2013. Continuous 4-1BB co-stimulatory signals for the optimal expansion of tumor-infiltrating lymphocytes for adoptive T-cell therapy. Oncoimmunology. 2, e25581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon, J.A. , Sarnaik, A.A. , Chen, J.Q. , Creasy, C. , Kale, C. , Robinson, J. , Weber, J. , Hwu, P. , Pilon-Thomas, S. , Radvanyi, L. , 2015. Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor-infiltrating lymphocytes for adoptive cell therapy. Clin. Cancer Res. 21, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalopin, B. , Florenceau, L. , Fradin, D. , Labarriere, N. , Moreau-Aubry, A. , 2015. A lineage-specific methylation pattern controls the transcription of the polycistronic mRNA coding MELOE melanoma antigens. Melanoma Res. 25, 279–283. [DOI] [PubMed] [Google Scholar]
- Chapman, P.B. , Hauschild, A. , Robert, C. , Haanen, J.B. , Ascierto, P. , Larkin, J. , Dummer, R. , Garbe, C. , Testori, A. , Maio, M. , Hogg, D. , Lorigan, P. , Lebbe, C. , Jouary, T. , Schadendorf, D. , Ribas, A. , O'Day, S.J. , Sosman, J.A. , Kirkwood, J.M. , Eggermont, A.M. , Dreno, B. , Nolop, K. , Li, J. , Nelson, B. , Hou, J. , Lee, R.J. , Flaherty, K.T. , McArthur, G.A. , 2011. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever, M.A. , Greenberg, P.D. , Fefer, A. , 1980. Specificity of adoptive chemoimmunotherapy of established syngeneic tumors. J. Immunol. 125, 711–714. [PubMed] [Google Scholar]
- Chen, Y.T. , Gure, A.O. , Tsang, S. , Stockert, E. , Jager, E. , Knuth, A. , Old, L.J. , 1998. Identification of multiple cancer/testis antigens by allogeneic antibody screening of a melanoma cell line library. Proc. Natl. Acad. Sci. U. S. A. 95, 6919–6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente, C.G. , Mihm, M.C. , Bufalino, R. , Zurrida, S. , Collini, P. , Cascinelli, N. , 1996. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 77, 1303–1310. [DOI] [PubMed] [Google Scholar]
- Dudley, M.E. , Wunderlich, J.R. , Robbins, P.F. , Yang, J.C. , Hwu, P. , Schwartzentruber, D.J. , Topalian, S.L. , Sherry, R. , Restifo, N.P. , Hubicki, A.M. , Robinson, M.R. , Raffeld, M. , Duray, P. , Seipp, C.A. , Rogers-Freezer, L. , Morton, K.E. , Mavroukakis, S.A. , White, D.E. , Rosenberg, S.A. , 2002. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 298, 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, M.E. , Wunderlich, J.R. , Shelton, T.E. , Even, J. , Rosenberg, S.A. , 2003. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J. Immunother. 26, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, M.E. , Wunderlich, J.R. , Yang, J.C. , Sherry, R.M. , Topalian, S.L. , Restifo, N.P. , Royal, R.E. , Kammula, U. , White, D.E. , Mavroukakis, S.A. , Rogers, L.J. , Gracia, G.J. , Jones, S.A. , Mangiameli, D.P. , Pelletier, M.M. , Gea-Banacloche, J. , Robinson, M.R. , Berman, D.M. , Filie, A.C. , Abati, A. , Rosenberg, S.A. , 2005. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 23, 2346–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, M.E. , Yang, J.C. , Sherry, R. , Hughes, M.S. , Royal, R. , Kammula, U. , Robbins, P.F. , Huang, J. , Citrin, D.E. , Leitman, S.F. , Wunderlich, J. , Restifo, N.P. , Thomasian, A. , Downey, S.G. , Smith, F.O. , Klapper, J. , Morton, K. , Laurencot, C. , White, D.E. , Rosenberg, S.A. , 2008. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 26, 5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, M.E. , Gross, C.A. , Langhan, M.M. , Garcia, M.R. , Sherry, R.M. , Yang, J.C. , Phan, G.Q. , Kammula, U.S. , Hughes, M.S. , Citrin, D.E. , Restifo, N.P. , Wunderlich, J.R. , Prieto, P.A. , Hong, J.J. , Langan, R.C. , Zlott, D.A. , Morton, K.E. , White, D.E. , Laurencot, C.M. , Rosenberg, S.A. , 2010. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin. Cancer Res. 16, 6122–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, M.E. , Gross, C.A. , Somerville, R.P. , Hong, Y. , Schaub, N.P. , Rosati, S.F. , White, D.E. , Nathan, D. , Restifo, N.P. , Steinberg, S.M. , Wunderlich, J.R. , Kammula, U.S. , Sherry, R.M. , Yang, J.C. , Phan, G.Q. , Hughes, M.S. , Laurencot, C.M. , Rosenberg, S.A. , 2013. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J. Clin. Oncol. 31, 2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberlein, T.J. , Rosenstein, M. , Rosenberg, S.A. , 1982. Regression of a disseminated syngeneic solid tumor by systemic transfer of lymphoid cells expanded in interleukin 2. J. Exp. Med. 156, 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellebaek, E. , Iversen, T.Z. , Junker, N. , Donia, M. , Engell-Noerregaard, L. , Met, O. , Holmich, L.R. , Andersen, R.S. , Hadrup, S.R. , Andersen, M.H. , thor Straten, P. , Svane, I.M. , 2012. Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients. J. Transl. Med. 10, 169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard, V.H. , Bullock, T.N. , Colella, T.A. , Sheasley, S.L. , Mullins, D.W. , 2002. Antigens derived from melanocyte differentiation proteins: self-tolerance, autoimmunity, and use for cancer immunotherapy. Immunol. Rev. 188, 136–146. [DOI] [PubMed] [Google Scholar]
- Freedman, R.S. , Platsoucas, C.D. , 1996. Immunotherapy for peritoneal ovarian carcinoma metastasis using ex vivo expanded tumor infiltrating lymphocytes. Cancer Treat. Res. 82, 115–146. [DOI] [PubMed] [Google Scholar]
- Fridman, W.H. , Pages, F. , Sautes-Fridman, C. , Galon, J. , 2012. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer. 12, 298–306. [DOI] [PubMed] [Google Scholar]
- Friedman, K.M. , Prieto, P.A. , Devillier, L.E. , Gross, C.A. , Yang, J.C. , Wunderlich, J.R. , Rosenberg, S.A. , Dudley, M.E. , 2012. Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes. J. Immunother. 35, 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, K. , Ikarashi, H. , Takakuwa, K. , Kodama, S. , Tokunaga, A. , Takahashi, T. , Tanaka, K. , 1995. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin. Cancer Res. 1, 501–507. [PubMed] [Google Scholar]
- Galon, J. , Costes, A. , Sanchez-Cabo, F. , Kirilovsky, A. , Mlecnik, B. , Lagorce-Pages, C. , Tosolini, M. , Camus, M. , Berger, A. , Wind, P. , Zinzindohoue, F. , Bruneval, P. , Cugnenc, P.H. , Trajanoski, Z. , Fridman, W.H. , Pages, F. , 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 313, 1960–1964. [DOI] [PubMed] [Google Scholar]
- Gattinoni, L. , Finkelstein, S.E. , Klebanoff, C.A. , Antony, P.A. , Palmer, D.C. , Spiess, P.J. , Hwang, L.N. , Yu, Z. , Wrzesinski, C. , Heimann, D.M. , Surh, C.D. , Rosenberg, S.A. , Restifo, N.P. , 2005. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 202, 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet, Y. , Moreau-Aubry, A. , Guilloux, Y. , Vignard, V. , Khammari, A. , Dreno, B. , Jotereau, F. , Labarriere, N. , 2008. MELOE-1 is a new antigen overexpressed in melanomas and involved in adoptive T cell transfer efficiency. J. Exp. Med. 205, 2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooden, M.J. , de Bock, G.H. , Leffers, N. , Daemen, T. , Nijman, H.W. , 2011. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br. J. Cancer. 105, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros, A. , Robbins, P.F. , Yao, X. , Li, Y.F. , Turcotte, S. , Tran, E. , Wunderlich, J.R. , Mixon, A. , Farid, S. , Dudley, M.E. , Hanada, K. , Almeida, J.R. , Darko, S. , Douek, D.C. , Yang, J.C. , Rosenberg, S.A. , 2014. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest. 124, 2246–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrup, S.R. , Bakker, A.H. , Shu, C.J. , Andersen, R.S. , van Veluw, J. , Hombrink, P. , Castermans, E. , Thor Straten, P. , Blank, C. , Haanen, J.B. , Heemskerk, M.H. , Schumacher, T.N. , 2009. Parallel detection of antigen-specific T-cell responses by multidimensional encoding of MHC multimers. Nat. Methods. 6, 520–526. [DOI] [PubMed] [Google Scholar]
- Hauschild, A. , Grob, J.J. , Demidov, L.V. , Jouary, T. , Gutzmer, R. , Millward, M. , Rutkowski, P. , Blank, C.U. , Miller, W.H. , Kaempgen, E. , Martin-Algarra, S. , Karaszewska, B. , Mauch, C. , Chiarion-Sileni, V. , Martin, A.M. , Swann, S. , Haney, P. , Mirakhur, B. , Guckert, M.E. , Goodman, V. , Chapman, P.B. , 2012. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 380, 358–365. [DOI] [PubMed] [Google Scholar]
- Hodi, F.S. , O'Day, S.J. , McDermott, D.F. , Weber, R.W. , Sosman, J.A. , Haanen, J.B. , Gonzalez, R. , Robert, C. , Schadendorf, D. , Hassel, J.C. , Akerley, W. , van den Eertwegh, A.J. , Lutzky, J. , Lorigan, P. , Vaubel, J.M. , Linette, G.P. , Hogg, D. , Ottensmeier, C.H. , Lebbe, C. , Peschel, C. , Quirt, I. , Clark, J.I. , Wolchok, J.D. , Weber, J.S. , Tian, J. , Yellin, M.J. , Nichol, G.M. , Hoos, A. , Urba, W.J. , 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Khong, H.T. , Dudley, M.E. , El-Gamil, M. , Li, Y.F. , Rosenberg, S.A. , Robbins, P.F. , 2005. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J. Immunother. 28, 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Kerstann, K.W. , Ahmadzadeh, M. , Li, Y.F. , El-Gamil, M. , Rosenberg, S.A. , Robbins, P.F. , 2006. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J. Immunol. 176, 7726–7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, W.T. , Adams, S.F. , Tahirovic, E. , Hagemann, I.S. , Coukos, G. , 2012. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol. Oncol. 124, 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki, O. , Hovav, E. , Ziporen, Y. , Levy, D. , Kubi, A. , Zikich, D. , Hershkovitz, L. , Treves, A.J. , Shalmon, B. , Zippel, D. , Markel, G. , Shapira-Frommer, R. , Schachter, J. , Besser, M.J. , 2011. Establishment and large-scale expansion of minimally cultured “young” tumor infiltrating lymphocytes for adoptive transfer therapy. J. Immunother. 34, 212–220. [DOI] [PubMed] [Google Scholar]
- Junker, N. , Andersen, M.H. , Wenandy, L. , Dombernowsky, S.L. , Kiss, K. , Sorensen, C.H. , Therkildsen, M.H. , Von Buchwald, C. , Andersen, E. , Straten, P.T. , Svane, I.M. , 2011. Bimodal ex vivo expansion of T cells from patients with head and neck squamous cell carcinoma: a prerequisite for adoptive cell transfer. Cytotherapy. 13, 822–834. [DOI] [PubMed] [Google Scholar]
- Kawakami, Y. , Eliyahu, S. , Delgado, C.H. , Robbins, P.F. , Sakaguchi, K. , Appella, E. , Yannelli, J.R. , Adema, G.J. , Miki, T. , Rosenberg, S.A. , 1994. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc. Natl. Acad. Sci. U. S. A. 91, 6458–6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz, U. , Stoter, G. , Punt, C.J. , Scheibenbogen, C. , Lejeune, F. , Eggermont, A.M. , 1997. Recombinant interleukin-2-based treatments for advanced melanoma: the experience of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. Cancer J. Sci. Am. 3, (Suppl. 1) S22–S28. [PubMed] [Google Scholar]
- Klebanoff, C.A. , Gattinoni, L. , Restifo, N.P. , 2012. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy?. J. Immunother. 35, 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvistborg, P. , Shu, C.J. , Heemskerk, B. , Fankhauser, M. , Thrue, C.A. , Toebes, M. , van Rooij, N. , Linnemann, C. , van Buuren, M.M. , Urbanus, J.H. , Beltman, J.B. , Thor Straten, P. , Li, Y.F. , Robbins, P.F. , Besser, M.J. , Schachter, J. , Kenter, G.G. , Dudley, M.E. , Rosenberg, S.A. , Haanen, J.B. , Hadrup, S.R. , Schumacher, T.N. , 2012. TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology. 1, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskron, J. , Helland, O. , Torgersen, K.M. , Aandahl, E.M. , Gjertsen, B.T. , Bjorge, L. , Tasken, K. , 2015. Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients. Cancer Immunol. Immunother. 64, 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, J. , Ascierto, P.A. , Dreno, B. , Atkinson, V. , Liszkay, G. , Maio, M. , Mandala, M. , Demidov, L. , Stroyakovskiy, D. , Thomas, L. , de la Cruz-Merino, L. , Dutriaux, C. , Garbe, C. , Sovak, M.A. , Chang, I. , Choong, N. , Hack, S.P. , McArthur, G.A. , Ribas, A. , 2014. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 371, 1867–1876. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Liu, S. , Hernandez, J. , Vence, L. , Hwu, P. , Radvanyi, L. , 2010. MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. J. Immunol. 184, 452–465. [DOI] [PubMed] [Google Scholar]
- Linnemann, C. , van Buuren, M.M. , Bies, L. , Verdegaal, E.M. , Schotte, R. , Calis, J.J. , Behjati, S. , Velds, A. , Hilkmann, H. , Atmioui, D.E. , Visser, M. , Stratton, M.R. , Haanen, J.B. , Spits, H. , van der Burg, S.H. , Schumacher, T.N. , 2015. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat. Med. 21, 81–85. [DOI] [PubMed] [Google Scholar]
- Long, G.V. , Stroyakovskiy, D. , Gogas, H. , Levchenko, E. , de Braud, F. , Larkin, J. , Garbe, C. , Jouary, T. , Hauschild, A. , Grob, J.J. , Chiarion Sileni, V. , Lebbe, C. , Mandala, M. , Millward, M. , Arance, A. , Bondarenko, I. , Haanen, J.B. , Hansson, J. , Utikal, J. , Ferraresi, V. , Kovalenko, N. , Mohr, P. , Probachai, V. , Schadendorf, D. , Nathan, P. , Robert, C. , Ribas, A. , DeMarini, D.J. , Irani, J.G. , Casey, M. , Ouellet, D. , Martin, A.M. , Le, N. , Patel, K. , Flaherty, K. , 2014. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 371, 1877–1888. [DOI] [PubMed] [Google Scholar]
- Lu, Y.C. , Yao, X. , Crystal, J.S. , Li, Y.F. , El-Gamil, M. , Gross, C. , Davis, L. , Dudley, M.E. , Yang, J.C. , Samuels, Y. , Rosenberg, S.A. , Robbins, P.F. , 2014. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin. Cancer Res. 20, 3401–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado, L. , Teague, J.E. , Morrow, M.P. , Jotova, I. , Wu, T.C. , Wang, C. , Desmarais, C. , Boyer, J.D. , Tycko, B. , Robins, H.S. , Clark, R.A. , Trimble, C.L. , 2014. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci. Transl. Med. 6, 221ra213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markel, G. , Cohen-Sinai, T. , Besser, M.J. , Oved, K. , Itzhaki, O. , Seidman, R. , Fridman, E. , Treves, A.J. , Keisari, Y. , Dotan, Z. , Ramon, J. , Schachter, J. , 2009. Preclinical evaluation of adoptive cell therapy for patients with metastatic renal cell carcinoma. Anticancer Res. 29, 145–154. [PubMed] [Google Scholar]
- Mlecnik, B. , Tosolini, M. , Kirilovsky, A. , Berger, A. , Bindea, G. , Meatchi, T. , Bruneval, P. , Trajanoski, Z. , Fridman, W.H. , Pages, F. , Galon, J. , 2011. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J. Clin. Oncol. 29, 610–618. [DOI] [PubMed] [Google Scholar]
- Muul, L.M. , Spiess, P.J. , Director, E.P. , Rosenberg, S.A. , 1987. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J. Immunol. 138, 989–995. [PubMed] [Google Scholar]
- North, R.J. , 1982. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J. Exp. Med. 155, 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages, F. , Galon, J. , Dieu-Nosjean, M.C. , Tartour, E. , Sautes-Fridman, C. , Fridman, W.H. , 2010. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 29, 1093–1102. [DOI] [PubMed] [Google Scholar]
- Pilon-Thomas, S. , Kuhn, L. , Ellwanger, S. , Janssen, W. , Royster, E. , Marzban, S. , Kudchadkar, R. , Zager, J. , Gibney, G. , Sondak, V.K. , Weber, J. , Mule, J.J. , Sarnaik, A.A. , 2012. Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma. J. Immunother. 35, 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow, M.A. , Chesney, J. , Pavlick, A.C. , Robert, C. , Grossmann, K. , McDermott, D. , Linette, G.P. , Meyer, N. , Giguere, J.K. , Agarwala, S.S. , Shaheen, M. , Ernstoff, M.S. , Minor, D. , Salama, A.K. , Taylor, M. , Ott, P.A. , Rollin, L.M. , Horak, C. , Gagnier, P. , Wolchok, J.D. , Hodi, F.S. , 2015. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 21, 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, D.J. , Dudley, M.E. , Robbins, P.F. , Rosenberg, S.A. , 2005. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 105, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto, P.A. , Durflinger, K.H. , Wunderlich, J.R. , Rosenberg, S.A. , Dudley, M.E. , 2010. Enrichment of CD8+ cells from melanoma tumor-infiltrating lymphocyte cultures reveals tumor reactivity for use in adoptive cell therapy. J. Immunother. 33, 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radvanyi, L.G. , Bernatchez, C. , Zhang, M. , Fox, P.S. , Miller, P. , Chacon, J. , Wu, R. , Lizee, G. , Mahoney, S. , Alvarado, G. , Glass, M. , Johnson, V.E. , McMannis, J.D. , Shpall, E. , Prieto, V. , Papadopoulos, N. , Kim, K. , Homsi, J. , Bedikian, A. , Hwu, W.J. , Patel, S. , Ross, M.I. , Lee, J.E. , Gershenwald, J.E. , Lucci, A. , Royal, R. , Cormier, J.N. , Davies, M.A. , Mansaray, R. , Fulbright, O.J. , Toth, C. , Ramachandran, R. , Wardell, S. , Gonzalez, A. , Hwu, P. , 2012. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 18, 6758–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, P.F. , el-Gamil, M. , Kawakami, Y. , Stevens, E. , Yannelli, J.R. , Rosenberg, S.A. , 1994. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 54, 3124–3126. [PubMed] [Google Scholar]
- Robbins, P.F. , El-Gamil, M. , Li, Y.F. , Kawakami, Y. , Loftus, D. , Appella, E. , Rosenberg, S.A. , 1996. A mutated beta-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J. Exp. Med. 183, 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, P.F. , El-Gamil, M. , Li, Y.F. , Zeng, G. , Dudley, M. , Rosenberg, S.A. , 2002. Multiple HLA class II-restricted melanocyte differentiation antigens are recognized by tumor-infiltrating lymphocytes from a patient with melanoma. J. Immunol. 169, 6036–6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, P.F. , Morgan, R.A. , Feldman, S.A. , Yang, J.C. , Sherry, R.M. , Dudley, M.E. , Wunderlich, J.R. , Nahvi, A.V. , Helman, L.J. , Mackall, C.L. , Kammula, U.S. , Hughes, M.S. , Restifo, N.P. , Raffeld, M. , Lee, C.C. , Levy, C.L. , Li, Y.F. , El-Gamil, M. , Schwarz, S.L. , Laurencot, C. , Rosenberg, S.A. , 2011. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert, C. , Karaszewska, B. , Schachter, J. , Rutkowski, P. , Mackiewicz, A. , Stroiakovski, D. , Lichinitser, M. , Dummer, R. , Grange, F. , Mortier, L. , Chiarion-Sileni, V. , Drucis, K. , Krajsova, I. , Hauschild, A. , Lorigan, P. , Wolter, P. , Long, G.V. , Flaherty, K. , Nathan, P. , Ribas, A. , Martin, A.M. , Sun, P. , Crist, W. , Legos, J. , Rubin, S.D. , Little, S.M. , Schadendorf, D. , 2014. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 372, 30–39. [DOI] [PubMed] [Google Scholar]
- Robert, C. , Long, G.V. , Brady, B. , Dutriaux, C. , Maio, M. , Mortier, L. , Hassel, J.C. , Rutkowski, P. , McNeil, C. , Kalinka-Warzocha, E. , Savage, K.J. , Hernberg, M.M. , Lebbe, C. , Charles, J. , Mihalcioiu, C. , Chiarion-Sileni, V. , Mauch, C. , Cognetti, F. , Arance, A. , Schmidt, H. , Schadendorf, D. , Gogas, H. , Lundgren-Eriksson, L. , Horak, C. , Sharkey, B. , Waxman, I.M. , Atkinson, V. , Ascierto, P.A. , 2015. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330. [DOI] [PubMed] [Google Scholar]
- Robert, C. , Schachter, J. , Long, G.V. , Arance, A. , Grob, J.J. , Mortier, L. , Daud, A. , Carlino, M.S. , McNeil, C. , Lotem, M. , Larkin, J. , Lorigan, P. , Neyns, B. , Blank, C.U. , Hamid, O. , Mateus, C. , Shapira-Frommer, R. , Kosh, M. , Zhou, H. , Ibrahim, N. , Ebbinghaus, S. , Ribas, A. , investigators, K.- , 2015. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532. [DOI] [PubMed] [Google Scholar]
- Romero, P. , Gervois, N. , Schneider, J. , Escobar, P. , Valmori, D. , Pannetier, C. , Steinle, A. , Wolfel, T. , Lienard, D. , Brichard, V. , van Pel, A. , Jotereau, F. , Cerottini, J.C. , 1997. Cytolytic T lymphocyte recognition of the immunodominant HLA-A*0201-restricted Melan-A/MART-1 antigenic peptide in melanoma. J. Immunol. 159, 2366–2374. [PubMed] [Google Scholar]
- Rosenberg, S.A. , Lotze, M.T. , Muul, L.M. , Leitman, S. , Chang, A.E. , Ettinghausen, S.E. , Matory, Y.L. , Skibber, J.M. , Shiloni, E. , Vetto, J.T. , 1985. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 313, 1485–1492. [DOI] [PubMed] [Google Scholar]
- Rosenberg, S.A. , Spiess, P. , Lafreniere, R. , 1986. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 233, 1318–1321. [DOI] [PubMed] [Google Scholar]
- Rosenberg, S.A. , Yannelli, J.R. , Yang, J.C. , Topalian, S.L. , Schwartzentruber, D.J. , Weber, J.S. , Parkinson, D.R. , Seipp, C.A. , Einhorn, J.H. , White, D.E. , 1994. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J. Natl. Cancer Inst. 86, 1159–1166. [DOI] [PubMed] [Google Scholar]
- Rosenberg, S.A. , Yang, J.C. , White, D.E. , Steinberg, S.M. , 1998. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann. Surg. 228, 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, S.A. , Yang, J.C. , Sherry, R.M. , Kammula, U.S. , Hughes, M.S. , Phan, G.Q. , Citrin, D.E. , Restifo, N.P. , Robbins, P.F. , Wunderlich, J.R. , Morton, K.E. , Laurencot, C.M. , Steinberg, S.M. , White, D.E. , Dudley, M.E. , 2011. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17, 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoiemma, P.P. , Powell, D.J. , 2015. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol. Ther. 0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan, M.J. , Gure, A.O. , Jungbluth, A.A. , Old, L.J. , Chen, Y.T. , 2002. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunolog. Rev. 188, 22–32. [DOI] [PubMed] [Google Scholar]
- Schadendorf, D. , Hodi, F.S. , Robert, C. , Weber, J.S. , Margolin, K. , Hamid, O. , Patt, D. , Chen, T.T. , Berman, D.M. , Wolchok, J.D. , 2015. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 33, 1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel, D.J. , Oberneder, R. , Falk, C.S. , Jantzer, P. , Kressenstein, S. , Maget, B. , Hofstetter, A. , Riethmuller, G. , Nossner, E. , 1997. Cellular and molecular analyses of major histocompatibility complex (MHC) restricted and non-MHC-restricted effector cells recognizing renal cell carcinomas: problems and perspectives for immunotherapy. J. Mol. Med. 75, 400–413. [DOI] [PubMed] [Google Scholar]
- Schumacher, T.N. , Schreiber, R.D. , 2015. Neoantigens in cancer immunotherapy. Science. 348, 69–74. [DOI] [PubMed] [Google Scholar]
- Schwartz, R.N. , Stover, L. , Dutcher, J. , 2002. Managing toxicities of high-dose interleukin-2. Oncology. 16, 11–20. [PubMed] [Google Scholar]
- Schwartzentruber, D.J. , Hom, S.S. , Dadmarz, R. , White, D.E. , Yannelli, J.R. , Steinberg, S.M. , Rosenberg, S.A. , Topalian, S.L. , 1994. In vitro predictors of therapeutic response in melanoma patients receiving tumor-infiltrating lymphocytes and interleukin-2. J. Clin. Oncol. 12, 1475–1483. [DOI] [PubMed] [Google Scholar]
- Shablak, A. , Hawkins, R.E. , Rothwell, D.G. , Elkord, E. , 2009. T cell-based immunotherapy of metastatic renal cell carcinoma: modest success and future perspective. Clin. Cancer Res. 15, 6503–6510. [DOI] [PubMed] [Google Scholar]
- Spiess, P.J. , Yang, J.C. , Rosenberg, S.A. , 1987. In vivo antitumor activity of tumor-infiltrating lymphocytes expanded in recombinant interleukin-2. J. Natl. Cancer Inst. 79, 1067–1075. [PubMed] [Google Scholar]
- Stevanovic, S. , Draper, L.M. , Langhan, M.M. , Campbell, T.E. , Kwong, M.L. , Wunderlich, J.R. , Dudley, M.E. , Yang, J.C. , Sherry, R.M. , Kammula, U.S. , Restifo, N.P. , Rosenberg, S.A. , Hinrichs, C.S. , 2015. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J. Clin. Oncol. 33, 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toebes, M. , Coccoris, M. , Bins, A. , Rodenko, B. , Gomez, R. , Nieuwkoop, N.J. , van de Kasteele, W. , Rimmelzwaan, G.F. , Haanen, J.B. , Ovaa, H. , Schumacher, T.N. , 2006. Design and use of conditional MHC class I ligands. Nat. Med. 12, 246–251. [DOI] [PubMed] [Google Scholar]
- Topalian, S.L. , Muul, L.M. , Solomon, D. , Rosenberg, S.A. , 1987. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J. Immunol. Methods. 102, 127–141. [DOI] [PubMed] [Google Scholar]
- Topalian, S.L. , Solomon, D. , Avis, F.P. , Chang, A.E. , Freerksen, D.L. , Linehan, W.M. , Lotze, M.T. , Robertson, C.N. , Seipp, C.A. , Simon, P. , 1988. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J. Clin. Oncol. 6, 839–853. [DOI] [PubMed] [Google Scholar]
- Tran, K.Q. , Zhou, J. , Durflinger, K.H. , Langhan, M.M. , Shelton, T.E. , Wunderlich, J.R. , Robbins, P.F. , Rosenberg, S.A. , Dudley, M.E. , 2008. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J. Immunother. 31, 742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, E. , Turcotte, S. , Gros, A. , Robbins, P.F. , Lu, Y.C. , Dudley, M.E. , Wunderlich, J.R. , Somerville, R.P. , Hogan, K. , Hinrichs, C.S. , Parkhurst, M.R. , Yang, J.C. , Rosenberg, S.A. , 2014. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 344, 641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversari, C. , van der Bruggen, P. , Luescher, I.F. , Lurquin, C. , Chomez, P. , Van Pel, A. , De Plaen, E. , Amar-Costesec, A. , Boon, T. , 1992. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J. Exp. Med. 176, 1453–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte, S. , Gros, A. , Hogan, K. , Tran, E. , Hinrichs, C.S. , Wunderlich, J.R. , Dudley, M.E. , Rosenberg, S.A. , 2013. Phenotype and function of T cells infiltrating visceral metastases from gastrointestinal cancers and melanoma: implications for adoptive cell transfer therapy. J. Immunol. 191, 2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte, S. , Gros, A. , Tran, E. , Lee, C.C. , Wunderlich, J.R. , Robbins, P.F. , Rosenberg, S.A. , 2014. Tumor-reactive CD8+ T cells in metastatic gastrointestinal cancer refractory to chemotherapy. Clin. Cancer Res. 20, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuthill, R.J. , Unger, J.M. , Liu, P.Y. , Flaherty, L.E. , Sondak, V.K. , Southwest Oncology, G. , 2002. Risk assessment in localized primary cutaneous melanoma: a Southwest Oncology Group study evaluating nine factors and a test of the Clark logistic regression prediction model. Am. J. Clin. Pathol. 118, 504–511. [DOI] [PubMed] [Google Scholar]
- Ullenhag, G.J. , Sadeghi, A.M. , Carlsson, B. , Ahlstrom, H. , Mosavi, F. , Wagenius, G. , Totterman, T.H. , 2012. Adoptive T-cell therapy for malignant melanoma patients with TILs obtained by ultrasound-guided needle biopsy. Cancer Immunol. Immunother. 61, 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij, N. , van Buuren, M.M. , Philips, D. , Velds, A. , Toebes, M. , Heemskerk, B. , van Dijk, L.J. , Behjati, S. , Hilkmann, H. , El Atmioui, D. , Nieuwland, M. , Stratton, M.R. , Kerkhoven, R.M. , Kesmir, C. , Haanen, J.B. , Kvistborg, P. , Schumacher, T.N. , 2013. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 31, e439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow, R. , 1863. Cellular Pathology. Philadelphia [Google Scholar]
- Walboomers, J.M. , Jacobs, M.V. , Manos, M.M. , Bosch, F.X. , Kummer, J.A. , Shah, K.V. , Snijders, P.J. , Peto, J. , Meijer, C.J. , Munoz, N. , 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189, 12–19. [DOI] [PubMed] [Google Scholar]
- Wang, Q.J. , Hanada, K. , Robbins, P.F. , Li, Y.F. , Yang, J.C. , 2012. Distinctive features of the differentiated phenotype and infiltration of tumor-reactive lymphocytes in clear cell renal cell carcinoma. Cancer Res. 72, 6119–6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.W. , Hung, C.F. , Huh, W.K. , Trimble, C.L. , Roden, R.B. , 2015. Immunoprevention of human papillomavirus-associated malignancies. Cancer Prevent. Res. 8, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, M.J. , Thirdborough, S.M. , Mellows, T. , Riley, C. , Harris, S. , Suchak, K. , Webb, A. , Hampton, C. , Patel, N.N. , Randall, C.J. , Cox, H.J. , Jogai, S. , Primrose, J. , Piper, K. , Ottensmeier, C.H. , King, E.V. , Thomas, G.J. , 2014. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer. 110, 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, J.R. , Milne, K. , Nelson, B.H. , 2015. PD-1 and CD103 are widely co-expressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol. Res. 3, 926–935. [DOI] [PubMed] [Google Scholar]
- Weber, J.S. , D'Angelo, S.P. , Minor, D. , Hodi, F.S. , Gutzmer, R. , Neyns, B. , Hoeller, C. , Khushalani, N.I. , Miller, W.H. , Lao, C.D. , Linette, G.P. , Thomas, L. , Lorigan, P. , Grossmann, K.F. , Hassel, J.C. , Maio, M. , Sznol, M. , Ascierto, P.A. , Mohr, P. , Chmielowski, B. , Bryce, A. , Svane, I.M. , Grob, J.J. , Krackhardt, A.M. , Horak, C. , Lambert, A. , Yang, A.S. , Larkin, J. , 2015. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 16, 375–384. [DOI] [PubMed] [Google Scholar]
- Wick, D.A. , Webb, J.R. , Nielsen, J.S. , Martin, S.D. , Kroeger, D.R. , Milne, K. , Castellarin, M. , Twumasi-Boateng, K. , Watson, P.H. , Holt, R.A. , Nelson, B.H. , 2014. Surveillance of the tumor mutanome by T cells during progression from primary to recurrent ovarian cancer. Clin. Cancer Res. 20, 1125–1134. [DOI] [PubMed] [Google Scholar]
- Wolfel, T. , Hauer, M. , Schneider, J. , Serrano, M. , Wolfel, C. , Klehmann-Hieb, E. , De Plaen, E. , Hankeln, T. , Meyer zum Buschenfelde, K.H. , Beach, D. , 1995. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 269, 1281–1284. [DOI] [PubMed] [Google Scholar]
- Yao, X. , Ahmadzadeh, M. , Lu, Y.C. , Liewehr, D.J. , Dudley, M.E. , Liu, F. , Schrump, D.S. , Steinberg, S.M. , Rosenberg, S.A. , Robbins, P.F. , 2012. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 119, 5688–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Q. , Song, D.G. , Poussin, M. , Yamamoto, T. , Best, A. , Li, C. , Coukos, G. , Powell, D.J. , 2014. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin. Cancer Res. 20, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettergren, J.G. , Luberoff, D.E. , Pretlow, T.G. , 1973. Separation of lymphocytes from disaggregated mouse malignant neoplasms by sedimentation in gradients of ficoll in tissue culture medium. J. Immunol. 111, 836–840. [PubMed] [Google Scholar]
- Zhang, L. , Conejo-Garcia, J.R. , Katsaros, D. , Gimotty, P.A. , Massobrio, M. , Regnani, G. , Makrigiannakis, A. , Gray, H. , Schlienger, K. , Liebman, M.N. , Rubin, S.C. , Coukos, G. , 2003. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 348, 203–213. [DOI] [PubMed] [Google Scholar]
- Zsiros, E. , Tsuji, T. , Odunsi, K. , 2015. Adoptive T-cell therapy is a promising salvage approach for advanced or recurrent metastatic cervical cancer. J. Clin. Oncol. 33, 1521–1522. [DOI] [PubMed] [Google Scholar]


