Abstract
Infiltration of the tumor microenvironment by nerve fibers is an understudied aspect of breast carcinogenesis. In this study, the presence of nerve fibers was investigated in a cohort of 369 primary breast cancers (ductal carcinomas in situ, invasive ductal and lobular carcinomas) by immunohistochemistry for the neuronal marker PGP9.5. Isolated nerve fibers (axons) were detected in 28% of invasive ductal carcinomas as compared to only 12% of invasive lobular carcinomas and 8% of ductal carcinomas in situ (p = 0.0003). In invasive breast cancers, the presence of nerve fibers was observed in 15% of lymph node negative tumors and 28% of lymph node positive tumors (p = 0.0031), indicating a relationship with the metastatic potential. In addition, there was an association between the presence of nerve fibers and the expression of nerve growth factor (NGF) in cancer cells (p = 0.0001). In vitro, breast cancer cells were able to induce neurite outgrowth in PC12 cells, and this neurotrophic activity was partially inhibited by anti‐NGF blocking antibodies. In conclusion, infiltration by nerve fibers is a feature of the tumor microenvironment that is associated with aggressiveness and involves NGF production by cancer cells. The potential participation of nerve fibers in breast cancer progression needs to be further considered.
Keywords: Nerve fibers (axons), Tumor microenvironment, Nerve growth factor, Breast cancer
Highlights
Single nerve fibers (axons) are a component of the breast tumor microenvironment, particularly in invasive ductal carcinomas.
The presence of nerve fibers is associated with lymph node invasion.
Breast cancer cell lines can induce nerve fibers outgrowth via the secretion of nerve growth factor.
Abbreviations
- DCIS
ductal carcinoma in situ
- IDC
invasive ductal carcinoma
- ILC
invasive lobular carcinoma
- EGF
epidermal growth factor
- IHC
immunohistochemistry
- NGF
nerve growth factor
- PGP9.5
protein gene product 9.5
- TMA
tumor microarrays
1. Introduction
The role of the nervous system in cancer etiology, and in particular the influence of nerve fibers in the tumor microenvironment, is an understudied aspect of cancer biology (Ondicova and Mravec, 2010). It is well established that cancer cells can grow around existing nerves and eventually invade them in a process called perineural invasion (Marchesi et al., 2010). This is generally associated with a poor prognosis and can cause pain as demonstrated in pancreatic cancer (Bapat et al., 2011). Conversely, the infiltration of tumors by growing nerves, or tumor axonogenesis, has only recently been suggested to actively participate in cancer progression. Indeed, a recent report (Magnon et al., 2013) has revealed that tumor infiltration by nerve fibers is essential for prostate cancer progression from early initiation to metastasis. The mechanism remains unclear but includes liberation of catecholamines and acetylcholine in the vicinity of cancer cells, resulting in the stimulation of tumor growth and invasion. In addition, another recent study has shown that denervation suppresses gastric tumorigenesis (Zhao et al., 2014a), further pointing to the role of the nervous system in carcinogenesis. These pioneering studies in prostate and gastric cancer suggest a potential value of anti‐neurogenic therapies (Jobling et al., 2015) and raise the possibility that tumor infiltration by nerve fibers may also be important in other types of cancer.
In breast cancer, little is known about nerve fibers in the tumor microenvironment. A study in mice has shown that nerve infiltration in bone metastases participates in the stimulation of metastatic growth (Campbell et al., 2012) and recent investigations have evidenced the presence of nerves in human primary breast tumors (Zhao et al., 2014b; Huang et al., 2014). Anatomical and histological studies have shown that the normal breast is innervated by both sympathetic and sensory fibers from the 4–6th thoracic nerves (Sarhadi et al., 1996). Sensory fibers supply the nipple and skin, whereas sympathetic fibers innervate blood vessels and ducts. Therefore, it is conceivable that nerve fibers could be attracted into breast tumors, especially in light of the evidence that breast cancer cells can produce neurotrophic growth factors such as the neurotrophins (Hondermarck, 2012). In particular, nerve growth factor (NGF), which can stimulate the growth of sympathetic and sensory nerves, is produced and secreted by breast cancer cells (Adriaenssens et al., 2008). During embryonic development, NGF plays a major role in directing nerves to their correct targets (Skaper, 2012) and similarly it can be hypothesized that the production of NGF by breast cancer cells may result in the attraction of nerve fibers into primary breast tumors.
In the present study, the hypothesis that nerve fibers are a significant component of the breast tumor microenvironment has been explored by analyzing a cohort of breast cancers. Nerve fibers were detected in a significant proportion of invasive breast cancers and their presence was associated with lymph node invasion, suggesting a relationship with metastatic potential. An association was found between the presence of nerve fibers and the expression of NGF by cancer cells, and in co‐culture with neuronal cells, breast cancer cells were able to induce neuronal outgrowth via the release of NGF.
2. Materials and methods
2.1. Breast cancer tissue samples and cell lines
High‐density tumor microarrays (TMAs) of invasive ductal carcinomas (IDC), invasive lobular carcinomas (ILC) and ductal carcinomas in situ (DCIS) of the breast were obtained from Biomax (Maryland, USA, catalog number BR1921, BR1921a and BR8011). They included 159 IDC, 160 ILC and 50 DCIS. Histopathological subtypes were reviewed by a pathologist (MMW). Clinical annotation included age at diagnosis, tumor size, lymph node status, estrogen receptor, progesterone receptor, human epidermal growth factor (EGF) receptor 2 (HER2). MDA‐MB‐231, MCF‐7, BT‐474, and SKBR‐3 breast cancer cell lines were from the American Type Culture Collection. JIMT‐1 cells were from DSMZ (Germany). Brain metastatic 231‐BR cells and HME human mammary epithelial cells (transformed but non‐tumorigenic) were a generous gift from Barbara Steeg (Bethesda, USA) and Robert Weinberg (Boston, USA), respectively. Individual cell line authentication was performed after DNA extraction (Promega genomic purification kit, catalog number A1120) and using the GenePrint 10 PCR amplification kit (Promega catalog number B9510). All breast epithelial cell lines were maintained in RPMI‐1640 with 10% foetal calf serum (JRH Biosciences) and 2 mM l‐glutamine. The neuron‐like PC12 cell line was from Ralph A. Bradshaw (University of California San Francisco). They were maintained in Dulbecco's modified eagle medium (DMEM) from Life Technologies (Australia) with 5% foetal calf serum, 10% horse serum (Sigma), and 2 mM l‐glutamine. All cell lines were grown in 75 cm2 tissue culture flasks in a humidified incubator at 37 °C with 5% CO2. The study was approved by the Human Research Ethic Committee of the University of Newcastle, Australia.
2.2. Immunohistochemistry
After deparaffinization and rehydration of the TMAs following standard procedures, heat induced epitope retrieval was carried out in a citrate based low pH buffer (Vector Laboratories) using a decloaking chamber (Biocare) at 95 °C for 20 min. Immunohistochemistry (IHC) was then performed using an ImmPRESS detection kit (Vector Laboratories) as per the manufacturer's recommendations. Briefly, after inactivation of endogenous peroxidases with H2O2, and blocking with 2.5% horse serum, rabbit PGP9.5 antibody (Abcam, catalog number ab15503), or rabbit NGF antibody (Abcam, catalog number ab52918), or non‐immune rabbit IgG control (Alpha Diagnostic, catalog number 20009‐1‐200) were applied at a 1:200 dilution. ImmPRESS HRP anti‐rabbit IgG (peroxidase) was then applied to the sections and revealed with DAB peroxidase substrate solution (Vector laboratories). Finally, TMA slides were counterstained with hematoxylin (Gill's formulation, Vector laboratories), dehydrated and cleared in Xylene before mounting in Ultramount #4 mounting media (Thermo Scientific). Imaging was performed using an Axioplan‐2 microscope fitted with an AxioCam Mrc5 digital camera (Carl Zeiss AG). The presence or absence of nerve fibers was recorded for each tumor sample of the TMAs by two independent observers including a pathologist (MMW).
2.3. Digital quantification of immunohistochemistry
For quantification of NGF staining, TMA slides were digitized at 200× absolute resolution using an Aperio AT2 scanner (Leica Biosystems). Quantitative IHC analyses were performed using the Halo™ image analysis platform (Indica Labs) under the supervision of a pathologist. Five random areas containing cancer cells were selected and the pixel intensities of DAB staining calculated using the Area Quantification algorithm. Pixel intensity values were then used to determine h‐scores for each core (index ranging from 0 to 300 calculated as the sum of 3 × % of pixels with strong staining + 2 × % of pixels with intermediate staining + 1 × % pixels with weak staining). To compare NGF levels across the cohort, the h‐scores were used to divide cases into 4 classifications (0 = h‐score <50; 1 = h‐score 50–100, 2 = h‐score 101–150, 3 = h‐score h > 150).
2.4. Association between nerve fibers and clinicopathological parameters
The presence of nerve fibers was compared with clinicopathological parameters (patient age at diagnosis, tumor size, histological subtype, lymph node invasion, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), molecular subtype) and NGF staining intensity. For statistical analysis, simple unadjusted associations between nerve fibers and other pathological variables were performed using a chi‐squared test. We used log‐linear models to adjust the various bivariate associations for other potential confounders. The log linear models provided a Chi‐squared test adjusted for all other variables in the model; these included cancer type (lobular vs ductal), lymph node involvement (yes/no), estrogen receptor positivity (yes/no), progesterone receptor positivity (yes/no), HER2+ (yes/no) and nerve fibers (negative/positive). The model was specified as a Poisson generalized linear model with a log‐link function. Using hierarchical nesting of models, all 3‐way then 2‐way interactions involving nerve fibers were examined. Goodness of fit was tested using G2 Chi‐squared statistics, as well as AIC and BIC. These models were fitted using SAS (SAS Institute, North Carolina, USA).
2.5. Preparation of conditioned medium and dot‐blot analysis
Subconfluent breast cancer cells were seeded at 5 × 106 cells per 100 mm culture dish and grown in 10 mL serum free media for 24 h. The collected medium was centrifuged (800× g for 5 min at 4 °C) and the supernatant was concentrated and desalted using 10‐kDa cut‐off Amicon Ultra‐15 filter unit (Millipore) for 30 min (4000× g, 4 °C). The recovered 250 μL concentrate was stored at −80 °C. Dot‐blot analysis was performed by spotting 20 μL of concentrated medium onto nitrocellulose membrane using the Bio‐Dot microfiltration system (Bio‐Rad). Then the membrane was saturated with blocking buffer (LI‐COR Biosciences) for 1 h at room temperature before incubation with rabbit anti‐NGF polyclonal IgG (Santa Cruz Biotechnology, catalog number sc548) overnight at 4 °C. After washing with PBS containing 0.1% Tween‐20, membranes were probed with goat anti‐rabbit IR‐Dye 680 secondary antisera (LI‐COR Biosciences, catalog number 926‐68073), then washed twice. Densitometric analysis was performed using the Odyssey infrared imaging system (LI‐COR Biosciences).
2.6. Neurite outgrowth assay
The neurotrophic ability of breast cancer cells was tested in co‐culture experiments with the neuronal‐like PC12 cells and neurite outgrowth was measured. PC12 cells are extensively used for studying neurite elongation (Suter and Miller, 2011). For co‐culture experiments, PC12 cells (5 × 104 in 1 ml) were seeded on bottom wells of 12‐well Transwell plates (Corning) coated with rat‐tail collagen I (Invitrogen). After 24 h, they were serum starved in DMEM containing 1% horse serum. Breast cancer cells were grown in Transwell inserts (12.0 mm in diameter with 0.4 μm pores, Corning). Differentiation of PC12 cells was allowed for 3 days, with or without anti‐NGF mouse monoclonal blocking antibody (Alomone, catalog number alm‐006) and neurite elongation was measured. PC12 cells exhibiting neurites of at least twice the size of the cell body were considered as differentiated. Pictures were taken using a Zeiss Axiovert 200 inverted microscope fitted with an AxioCam HRm digital camera (Zeiss AG). One‐way ANOVA statistical test (GraphPad Prism 5.01) was used.
3. Results
3.1. Breast tumors are infiltrated by nerve fibers
To test the hypothesis that nerve fibers could infiltrate breast tumors, we have explored TMAs containing 319 invasive breast cancers (IDC + ILC) and 50 DCIS by immunohistochemistry against the neuronal marker PGP9.5. Typical morphological features corresponding to both nerves and isolated nerve fibers (axons) were observed (Figure 1). The presence of nerve trunks (composed of many fibers or axons) with perineural invasion (Figure 1A) was occasionally observed in 6 out of the 319 cases of invasive cancers, but this does not constitute a reliable quantification due to the bias introduced by sampling in relation to the location of nerve trunks. Importantly, isolated nerve fibers (axons) were also observed (Figure 1B–H). The reactivity to the neuronal marker PGP9.5 and typical morphology were characteristic of axons. Nerve fibers were localized around cancer cells and adipocytes (Figure 1B), in the tumor stroma next to cancer cells (Figure 1C–F), around arterioles (Figure 1G) and blood vessels (Figure 1H). In Table 1, breast cancers were classified as nerve fiber positive versus nerve fiber negative tumors, and comparison was made with clinicopathological parameters. TMAs do not contain enough tissue for analyzing and dissecting precisely nerve fiber densities in tumors and therefore we have chosen to present the data in terms of presence versus absence of nerve fibers (this may lead to an underestimation of the innervation). The presence of nerve fibers was detected in 8% of DCIS, 12% of ILC and 28% of IDC (p = 0.0003), indicating that nerve fibers are predominantly associated with invasive ductal carcinomas. This association was confirmed in Log‐Linear modeling, two‐way analysis (p < 0.001). In invasive breast tumors, there was no association between the presence of nerve fibers and age at diagnosis, HER2, estrogen receptor, progesterone receptor, tumor size and molecular subtype (defined as TNBC: ER−/PR−/HER2−, luminal A: ER+ and/or PR+/HER2−, luminal B: ER+ and/or PR+/HER2+, HER2+: ER−/PR−/HER2+). The tumors that presented innervation were not enriched in any particular molecular subtypes. A more detailed analysis of innervation in function of ER/PR/HER2 status is shown in Supplementary Table 1 and indicates the absence of statistically significant differences between the subgroups.
Figure 1.
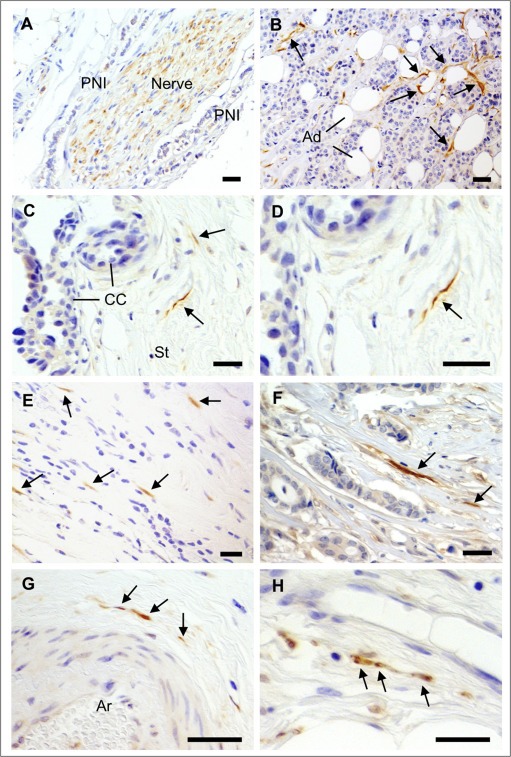
Detection of nerve fibers in breast cancers. IHC for the neuronal marker PGP9.5 was performed on a series of 319 breast cancer samples. A) Nerve trunks (composed of many nerve fibers), occasionally present in breast tumors, were positive for PGP9.5. Perineural invasion (PNI) could be observed, as shown here. B–H) In some breast cancers, isolated nerve fibers (axons) positive for PGP9.5 were observed and are indicated by arrows. B) Nerve fibers around cancer cells and adipocytes (Ad). C) Nerve fibers in the tumor stroma (St) adjacent to cancer cells (CC). D) Enlargement of C. E, F) Nerve fibers among scattered breast cancer cells and in tumor stroma. G) Nerve fibers around an arteriole (Ar). H) Nerve fibers close to a thin walled blood vessel in the tumor stroma. Scale bar = 50 μm.
Table 1.
Association between the presence of nerve fibers and clinicopathological parameters in breast carcinomas.
| Parameter | Nerve fibers negative | Nerve fibers positive | p‐value |
|---|---|---|---|
| All cases (n = 369) | 301 (82%) | 68 (18%) | |
| Pathological subtype | 0.0003 a | ||
| DCIS (n = 50) | 46 (92%) | 4 (8%) | |
| ILC (n = 160) | 140 (88%) | 20 (12%) | |
| IDC (n = 159) | 115 (72%) | 44 (28%) | |
| Clinical parameters in invasive carcinomas | |||
| Patient age | |||
| 50≤ (n = 183) | 142 (77%) | 41 (22%) | 0.1201 |
| >50 (n = 136) | 115 (84%) | 21 (15%) | |
| Lymph node status (N) | 0.0031 a | ||
| Negative (n = 164) | 140 (85%) | 24 (15%) | |
| Positive (n = 135) | 97 (72%) | 38 (28%) | |
| Undetermined (n = 20) | 16 (80%) | 4 (20%) | |
| HER2 | 0.2162 | ||
| HER2 negative (n = 252) | 205 (81%) | 47 (19%) | |
| HER2 positive (n = 67) | 50 (75%) | 17 (15%) | |
| Estrogen receptor | 0.3093 | ||
| ER negative (n = 182) | 149 (82%) | 33 (18%) | |
| ER positive (n = 137) | 106 (77%) | 31 (13%) | |
| Progesterone receptor | 0.8143 | ||
| PR negative (n = 208) | 167 (80%) | 41 (20%) | |
| PR positive (n = 111) | 88 (79%) | 23 (21%) | |
| Molecular subtype | 0.5801 | ||
| TNBC (n = 123) | 98 (80%) | 25 (20%) | |
| Luminal A (n = 129) | 107 (83%) | 22 (17%) | |
| Luminal B (n = 34) | 25 (74%) | 9 (26%) | |
| HER2+ (n = 33) | 25 (76%) | 8 (24%) | |
| Tumor size (T) | 0.5284 | ||
| 1 (n = 25) | 19 (76%) | 6 (14%) | |
| 2 (n = 228) | 183 (80%) | 45 (20%) | |
| 3 (n = 32) | 28 (88%) | 4 (12%) | |
| 4 (n = 30) | 22 (73%) | 8 (27%) | |
| Nerve growth factor | 0.0001 a | ||
| NGF negative (n = 167) | 149 (89%) | 18 (11%) | |
| NGF positive (n = 152) | 107 (70%) | 45 (30%) | |
DCIS = ductal carcinomas in situ; ER = estrogen receptor; HER2 = Human EGF receptor 2; IDC = invasive ductal carcinomas; ILC = invasive lobular carcinomas; NGF = nerve growth factor; TNBC = triple negative breast cancer.
Statistically significant p‐values (p < 0.05 using chi‐square test). Molecular subtypes were defined as TNBC: ER−/PR−/HER2−, luminal A: ER+ and/or PR+/HER2−, luminal B: ER+ and/or PR+/HER2+, HER2+: ER−/PR−/HER2+.
3.2. The presence of nerve fibers in invasive breast tumors is associated with lymph node invasion and NGF production
The presence of nerve fibers was associated with lymph node invasion and NGF production in cancer cells (Table 1). Individual nerve fibers were observed in only 15% of invasive tumors with no lymph node invasion whereas 28% of lymph node positive tumors contained nerve fibers (p = 0.0031), indicating a relationship between the presence of nerve fibers and metastatic potential/poorer prognosis. The association between nerve fibers and lymph node invasion was confirmed in Log‐linear modeling (p = 0.0064 in two‐way analysis). Together this association with lymph node invasion and the fact that only 8% of DCIS presented with nerve fibers indicate that the presence of nerve fibers in breast tumors is related to aggressiveness/invasiveness.
In addition, as we have previously shown that the neurotrophic growth factor NGF is expressed in breast tumors (Adriaenssens et al., 2008), we tested the hypothesis that the presence of nerve fibers in breast cancers could be related to NGF expression. Interestingly, there was an association between the presence of nerve fibers and the expression of NGF in cancer cells (Table 1). Nerve fibers were observed in only 11% of NGF negative tumors (h‐score<50), as compared to 30% of NGF positive tumors (h‐score≥50) (p = 0.0001). The digital quantification of NGF intensity staining is presented (Figure 2). NGF intensity staining (h‐score) was significantly higher in IDC than in DCIS and ILC (p < 0.0001) (Figure 2A), thus corroborating the higher proportion of IDC presenting with nerve fibers. The frequency distribution of NGF staining intensity in DCIS, ILC and IDC is presented as categories (Figure 2B), with 0 = h‐score <50, 1 = h‐score 50–100, 2 = h‐score 101–150, 3 = h‐score>150. The percentage of cases with NGF labeling was 2% in DCIS, 15% in ILC and 79% in IDC (p < 0.0001). In invasive tumors (Figure 2C), the proportion of cases with high NGF labeling (intensity labeling 2 and 3) was 12% in nerve fibers negative tumors and 31% in nerve fibers positive tumors (p < 0.0001), confirming the association between NGF and the presence of nerve fibers. Overall, the Spearman correlation factor between nerve fibers and NGF level was 0.28 (p < 0.0001). Furthermore, in serial sections with NGF and PGP9.5 staining, nerve fibers were observed around cancer cells producing NGF (Figure 3). Together, these results suggested that NGF produced by breast cancer cells could participate in tumor infiltration by nerves and have prompted an examination of a NGF‐mediated neurotrophic effect (ability to induce neuronal outgrowth) of breast cancer cells.
Figure 2.
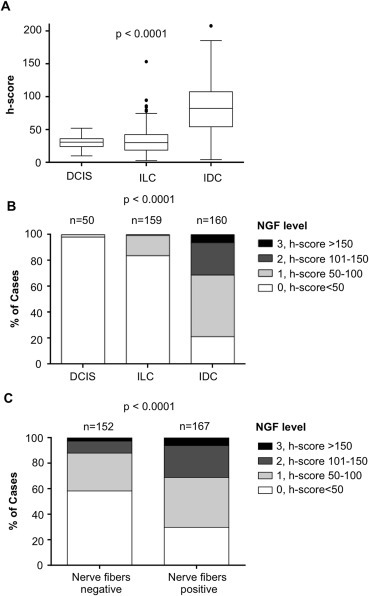
Frequency distribution of NGF level in breast cancers according to the presence of nerve fibers. NGF levels were obtained after digital quantification. A) Distribution of NGF intensity staining (h‐score) in ductal carcinomas in situ (DCIS), invasive lobular carcinomas (ILC) and invasive ductal carcinomas (IDC). Box and Whisker plots comparing median NGF levels using h‐scores as a measure of IHC staining (n = 50, 160 and 160, respectively). The box limits indicate the 25th and 75th percentiles with the whiskers extending 1.5 times the interquartile range from the 25th and 75th percentiles (outliers are represented by dots; prepared using BoxPlotR). B) Distribution of NGF staining intensity in DCIS, ILC and IDC. Categorization is presented as 0 = h‐score <50, 1 = h‐score 50–100, 2 = h‐score 101–150, 2 = h‐score>150. C) Distribution of NGF staining intensity in invasive tumors (nerve fibers positive versus nerve fibers negative tumors). Categories of NGF staining (0, 1, 2, 3) were the same as in B. Tumors presenting with nerve fibers were more likely to have higher NGF expression than tumors without nerve fibers. Number of cases (n) is indicated. ***One‐way ANOVA was used for A and Chi square for B and D.
Figure 3.
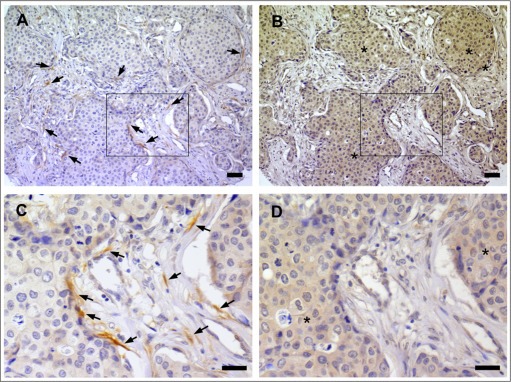
Co‐localization between nerve fibers and NGF in breast cancer. A) IHC for PGP9.5 indicating the presence of many nerve fibers in the stroma and along cancer cells of an invasive ductal carcinoma. Arrows point to few nerve fibers. B) IHC against NGF in a section serial to that presented in panel A. NGF immunoreactivity (indicated by stars) was observed in cancer cells adjacent to nerve fibers. C) Enlargement of the area boxed in panel A. D) Enlargement of the area boxed in panel B. Scale bar = 50 μm.
3.3. Breast cancer cells can induce neuronal outgrowth through NGF secretion
Given the association between the presence of nerve fibers and the production of NGF in breast tumors, the neurotrophic ability of breast cancer cells was investigated. Co‐cultures between breast cancer cell lines and the neuronal‐like PC12 cells (Figure 4A) were performed and neurite outgrowth of PC12 cells was measured as a percentage of cells with elongated neurites (Figure 4B). MCF‐7, MDA‐MB‐231 and 231‐BR induced a strong neurite outgrowth, whereas only a slight induction was observed with SKBR‐3 and JIMT‐1. In contrast, no neurite outgrowth was detected with BT‐474. Interestingly, the non‐tumorigenic HME cells were not able to induce neurite outgrowth. Representative pictures of PC12 cells co‐cultured in presence of breast cancer cells are shown (Figure 4C). NGF was quantified in dot blot analyses of conditioned media from breast cancer cell lines (Figure 4D). The dot blot was prepared from an equivalent number of cells and the densitometric quantification indicated different levels of NGF between cell lines. Interestingly, the levels of NGF in the conditioned media of breast cancer cells partly corroborated their ability to induce neurite outgrowth (as shown in Figure 4B), suggesting that NGF participates in the neurotrophic effect induced by breast cancer cells. MCF‐7 exhibited the highest level of NGF secretion and the highest neurotrophic effect (as reported in panel B). The non‐tumorigenic HME had a low level of NGF and exhibited a limited neurotrophic effect. However, MDA‐MB‐231 cells exhibited a significant neurotrophic effect whereas the level of NGF was low, and BT‐474 had an intermediate NGF level and only a low impact on neuritogenesis. This suggested that NGF is probably not the only neurotrophic factor produced by breast cancer cells and able to stimulate neuron outgrowth. The involvement of NGF in breast cancer‐induced neurite outgrowth was confirmed by use of blocking antibodies against NGF (Figure 4E). As shown with MCF‐7 cells, blocking antibodies against NGF could partly inhibit breast cancer cell‐induced neurite outgrowth whereas a control IgG antibody had no effect. Representative pictures showing the effect of anti‐NGF antibody on neurite outgrowth induced by MCF‐7 cells are shown (Figure 4F). The inhibitory activity of anti‐NGF blocking antibody for breast cancer‐induced neurite outgrowth has been confirmed using the 50B11 cell line (Supplementary Figure 1), which is derived from dorsal root ganglia and morphologically responsive to NGF (Bhattacherjee et al., 2014). Together these data indicate that breast cancer cells have the ability to stimulate axonogenesis through the production and release of NGF, and that other neurotrophic factors may also be involved.
Figure 4.
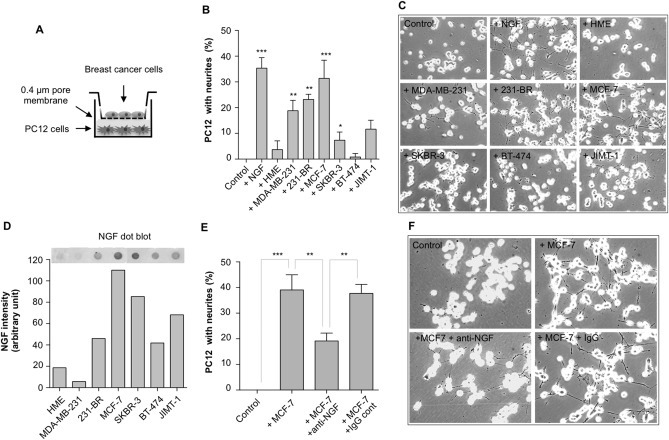
NGF‐mediated neurotrophic effect of breast cancer cells. A) Co‐culture experiments were performed in Transwell Boyden chambers with breast epithelial cells in the upper part and PC12 cells in the lower part. B) Some breast cancer cell lines were able to induce a neurotrophic effect on PC12 cells. Neurite outgrowth was induced in presence of MDA‐MB‐231, 231‐BR, MCF‐7, SKBR‐3, JIMT‐1, but not in presence of BT‐474 and the non‐tumorigenic HME. A negative control (with no breast cancer cells) and a positive control (addition of 50 ng/ml NGF) have been added. The results represent the mean of 3 independent experiments ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 for comparison with control. C) Representative pictures showing the effect of breast cancer cell lines and HME on PC12 cells. Quantifications are presented in panel B. D) Dot‐blot analysis for the detection of NGF in breast cancer cell conditioned media and quantification of NGF signal intensity in different breast epithelial cells. E) Impact of blocking anti‐NGF antibodies on breast cancer cell‐induced neurite outgrowth. MCF‐7 cells were co‐cultured for 72 h with PC12 cells in presence or absence of anti‐NGF blocking antibodies (1 μg/ml). Control was without MCF‐7 cells, and isotype antibodies were also tested. The results represent the mean of 3 independent experiments ± SD. ***p < 0.001. F) Representative pictures corresponding to the experiment described in panel E.
4. Discussion
The tumor microenvironment is crucial to breast cancer progression and the interaction of breast cancer cells with the components of the stroma, endothelial and immune cells, fibroblasts and extracellular matrix, is well established (Hanahan and Weinberg, 2011). In contrast, the presence of nerve fibers in the breast tumor microenvironment and a possible interaction with cancer cells has not been studied in detail. The present study demonstrates the presence of thin and isolated nerve fibers (axons) in the breast tumor microenvironment and their association with NGF production in cancer cells. In prostate cancer, nerve infiltration correlates with tumor aggressiveness (Magnon et al., 2013), and is driven by the production of proNGF (Pundavela et al., 2014) and granulocyte colony‐stimulating factor (G‐CSF) (Dobrenis et al., 2015). The results presented here suggest that a similar situation occurs in breast cancer as infiltration by nerve fibers was found associated to lymph node invasion. Lymph node status is the single most important prognostic variable for the management of patients with primary breast cancer. However, the occurrence of false negatives, along with heterogeneity of clinical outcomes among lymph node positive patients, highlights the need to improve the classification and management of invasive breast cancer. This study points to the potential value of using the presence of nerve fibers as a new predictive biomarker in breast cancer.
The production and release of neurotrophic factors by breast cancer cells has been described (Hondermarck, 2012). NGF expression is increased in breast cancer cells as compared to normal breast epithelial cells (Adriaenssens et al., 2008), resulting in an autocrine stimulation of breast cancer cells through the tyrosine kinase receptor TrkA and the TNF‐receptor family member p75NTR (Lagadec et al., 2009; Verbeke et al., 2010). However, until now, the possibility that NGF produced by breast cancer cells could stimulate tumor nerve infiltration had not been investigated. The present study reveals that breast cancer cells can activate neuronal outgrowth through a NGF‐mediated mechanism. In bone metastases of breast cancer, one study has already shown that NGF production by tumor cells can attract nerves (Bloom et al., 2011) and our study demonstrates that nerve infiltration is also a characteristic of primary breast tumors that is partially driven by NGF production. However, the correlation between NGF and nerve outgrowth, both in tumor samples and in cell cultures, was only partial, suggesting that NGF is not the only factor involved. There are many growth factors (neurotrophins and others) that can exhibit a neurotrophic activity, and several are produced in breast cancers. Brain‐derived neurotrophic factor (BDNF) and neurotrophin‐4/5 (Hernandez‐Bedolla et al., 2015; Vanhecke et al., 2011), Artemin (Kang et al., 2009) or fibroblast growth factors (Penault‐Llorca et al., 1995), as well as axon guidance molecules such as netrins (Harburg and Hinck, 2011) are expressed by breast cancer cells and could also contribute to the attraction of nerve fibers. The development of the nervous system involves a variety of neurotrophic molecules that act on different neuronal subtypes, and it is possible that a similar diversity of mechanisms also participates in tumor innervation. Further investigations are warranted to clarify the possible involvement of neurotrophic factors and axon guidance molecules in the infiltration of breast tumors by nerve fibers.
The presence of nerve fibers could be particularly relevant in terms of tumor growth and metastasis, via the secretion of active neuropeptides and neurotransmitters. Breast cancer cells have been reported to express receptors for a number of neuropeptides and neurotransmitters, like norepinephrine and epinephrine (Luthy et al., 2009) or substance P (Garcia‐Recio et al., 2013). As a consequence tumor cells are able to transduce neurotransmitter‐induced intracellular signaling pathways, which have been described to eventually lead to the activation of their growth and metastasis (Entschladen et al., 2004). The presence of nerve fibers in the breast tumor microenvironment suggests that they could liberate neurotransmitters directly in the vicinity of breast cancer cells. We have observed tyrosine hydroxylase positivity in some nerve trunks as well as axons (Supplementary Figure 2), indicating that some of the nerve fibers present in breast cancer are of sympathetic origin and could therefore liberate catecholamines. It has been shown that the expression of the beta‐adrenergic receptor for catecholamines is associated with a poor clinical outcome in breast cancer patients (Powe et al., 2011) and that stress‐induced activation of the sympathetic nervous system can induce a metastatic switch (Sloan et al., 2010). The mechanism involved the stimulation of beta‐adrenergic receptors at the surface of breast cancer cells by circulating catecholamines, but our study suggests that a local production by nerves may also be involved. In addition, epidemiological studies have suggested that blockers of the beta‐adrenergic receptors, traditionally used for the treatment of cardiovascular disorders and anxiety, can increase breast cancer patient survival (Barron et al., 2011; Melhem‐Bertrandt et al., 2011); the mechanism is unclear, but beta‐blockers could potentially inhibit the stimulatory effect of catecholamines liberated by nerves. Although further experiments are necessary to test these hypotheses and determine if infiltrating nerve fibers have an impact on cancer and stromal cells, our results open the theoretical possibility that, similarly to prostate cancer (Magnon et al., 2013), nerve fibers could be involved in breast cancer progression.
In conclusion, the nerve‐breast cancer connection described here bridges a gap in knowledge about the neuronal component of the breast tumor microenvironment and points to NGF as a driver of nerve infiltration. This opens a new perspective of crosstalk between nerves and breast cancer cells, and warrants more studies to investigate the impact of nerve fibers in breast cancer progression.
Contributions
SR performed the immunohistochemistry. Tissue slide analyses were performed by MMW (histopathologist), and confirmed by PJ (neuroanatomist) and HH. Digital quantification of IHC was performed by RT. JP performed all in vitro experiments and participated in IHC; he also prepared all Figures and Tables. JA supervised the statistical analyses. SF contributed to cell culture. HH and PJ conceived the study. HH, MMW, RJS, RAB and JFF supervised the study. HH drafted the manuscript. All authors have read and approved the final manuscript.
Conflict of interest
The authors disclosed no competing interests.
Supporting information
The following are the supplementary data related to this article:
Supplementary data
Supplementary Figure 1 NGF‐mediated neurotrophic effect of breast cancer cells for 50B11 cells from dorsal root ganglia. Immortalized dorsal root ganglia (DRG) neurons 50B11 were obtained from Dr A. Höke (John Hopkins University, Baltimore USA). The co‐culture with breast cancer cells was performed as described for PC12 cells in the Material and methods section. For co‐culture with the breast cancer cells, the same protocol as for PC12 cells was used (see Material and methods section), but the culture media included 5μM forskolin (necessary to obtain neurite outgrowth with these cells). A) MCF‐7 cells were able to induce neurite outgrowth in 50B11 cells. This neurotrophic effect was partially inhibited by addition of anti‐NGF antibody but not by IgG control. B) Representative pictures of each experimental condition are shown. The results represent the mean of 3 independent experiments ± SD. ***p < 0.001; **p < 0.05.
Supplementary Figure 2 Detection of tyrosine hydroxylase positive nerve fibers in breast cancer. Tyrosine hydroxylase was detected by IHC using the same protocol as described in Material and methods, with anti‐tyrosine hydroxylase (Millipore, catalog number AB152). A) Nerve trunk composed of many fibers positive for tyrosine hydroxylase is shown by an arrow. B) Individual nerve fibers (axons) positive for tyrosine hydroxylase are indicated by arrows. Scale bar = 50 μm.
Acknowledgments
This work was supported by the University of Newcastle, Australia and the Hunter Cancer Research Alliance. We thank Dr. Christopher Oldmeadow from the Clinical Research Design, IT, and Statistical Support (CReDITSS) Unit of the University of Newcastle.
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2015.05.001.
Pundavela Jay, Roselli Severine, Faulkner Sam, Attia John, Scott Rodney J., Thorne Rick F., Forbes John F., Bradshaw Ralph A., Walker Marjorie M., Jobling Phillip, Hondermarck Hubert, (2015), Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer, Molecular Oncology, 9, doi: 10.1016/j.molonc.2015.05.001.
References
- Adriaenssens, E. , Vanhecke, E. , Saule, P. , Mougel, A. , Page, A. , Romon, R. , Nurcombe, V. , Le Bourhis, X. , Hondermarck, H. , 2008. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 68, 346–351. [DOI] [PubMed] [Google Scholar]
- Bapat, A.A. , Hostetter, G. , Von Hoff, D.D. , Han, H. , 2011. Perineural invasion and associated pain in pancreatic cancer. Nat. Rev. Cancer. 11, 695–707. [DOI] [PubMed] [Google Scholar]
- Barron, T.I. , Connolly, R.M. , Sharp, L. , Bennett, K. , Visvanathan, K. , 2011. Beta blockers and breast cancer mortality: a population- based study. J. Clin. Oncol. 29, 2635–2644. [DOI] [PubMed] [Google Scholar]
- Bhattacherjee, A. , Liao, Z. , Smith, P.G. , 2014. Trophic factor and hormonal regulation of neurite outgrowth in sensory neuron-like 50B11 cells. Neurosci. Lett. 558, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom, A.P. , Jimenez-Andrade, J.M. , Taylor, R.N. , Castaneda-Corral, G. , Kaczmarska, M.J. , Freeman, K.T. , Coughlin, K.A. , Ghilardi, J.R. , Kuskowski, M.A. , Mantyh, P.W. , 2011. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J. Pain. 12, 698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, J.P. , Karolak, M.R. , Ma, Y. , Perrien, D.S. , Masood-Campbell, S.K. , Penner, N.L. , Munoz, S.A. , Zijlstra, A. , Yang, X. , Sterling, J.A. , Elefteriou, F. , 2012. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 10, e1001363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrenis, K. , Gauthier, L.R. , Barroca, V. , Magnon, C. , 2015. Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. Int. J. Cancer. 136, 982–988. [DOI] [PubMed] [Google Scholar]
- Entschladen, F. , Drell, T.L.t , Lang, K. , Joseph, J. , Zaenker, K.S. , 2004. Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncol. 5, 254–258. [DOI] [PubMed] [Google Scholar]
- Garcia-Recio, S. , Fuster, G. , Fernandez-Nogueira, P. , Pastor-Arroyo, E.M. , Park, S.Y. , Mayordomo, C. , Ametller, E. , Mancino, M. , Gonzalez-Farre, X. , Russnes, H.G. , Engel, P. , Costamagna, D. , Fernandez, P.L. , Gascon, P. , Almendro, V. , 2013. Substance P autocrine signaling contributes to persistent HER2 activation that drives malignant progression and drug resistance in breast cancer. Cancer Res. 73, 6424–6434. [DOI] [PubMed] [Google Scholar]
- Hanahan, D. , Weinberg, R.A. , 2011. Hallmarks of cancer: the next generation. Cell. 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Harburg, G.C. , Hinck, L. , 2011. Navigating breast cancer: axon guidance molecules as breast cancer tumor suppressors and oncogenes. J. Mammary Gland Biol. Neoplasia. 16, 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Bedolla, M.A. , Carretero-Ortega, J. , Valadez-Sanchez, M. , Vazquez-Prado, J. , Reyes-Cruz, G. , 2015. Chemotactic and proangiogenic role of calcium sensing receptor is linked to secretion of multiple cytokines and growth factors in breast cancer MDA-MB-231 cells. Biochim. Biophys. Acta. 1853, 166–182. [DOI] [PubMed] [Google Scholar]
- Hondermarck, H. , 2012. Neurotrophins and their receptors in breast cancer. Cytokine Growth Factor Rev. 23, 357–365. [DOI] [PubMed] [Google Scholar]
- Huang, D. , Su, S. , Cui, X. , Shen, X. , Zeng, Y. , Wu, W. , Chen, J. , Chen, F. , He, C. , Liu, J. , Huang, W. , Liu, Q. , Su, F. , Song, E. , Ouyang, N. , 2014. Nerve fibers in breast cancer tissues indicate aggressive tumour progression. Medicine (Baltimore). 93, e172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling, P. , Pundavela, J. , Oliverira, S.M.R. , Roselli, S. , Walker, M.M. , Hondermarck, H. , 2015. Nerve-cancer cell crosstalk: a novel promoter of tumour progression. Cancer Res. 75, 1–5. [DOI] [PubMed] [Google Scholar]
- Kang, J. , Perry, J.K. , Pandey, V. , Fielder, G.C. , Mei, B. , Qian, P.X. , Wu, Z.S. , Zhu, T. , Liu, D.X. , Lobie, P.E. , 2009. Artemin is oncogenic for human mammary carcinoma cells. Oncogene. 28, 2034–2045. [DOI] [PubMed] [Google Scholar]
- Lagadec, C. , Meignan, S. , Adriaenssens, E. , Foveau, B. , Vanhecke, E. , Romon, R. , Toillon, R.A. , Oxombre, B. , Hondermarck, H. , Le Bourhis, X. , 2009. TrkA overexpression enhances growth and metastasis of breast cancer cells. Oncogene. 28, 1960–1970. [DOI] [PubMed] [Google Scholar]
- Luthy, I.A. , Bruzzone, A. , Pinero, C.P. , Castillo, L.F. , Chiesa, I.J. , Vazquez, S.M. , Sarappa, M.G. , 2009. Adrenoceptors: non conventional target for breast cancer?. Curr. Med. Chem. 16, 1850–1862. [DOI] [PubMed] [Google Scholar]
- Magnon, C. , Hall, S.J. , Lin, J. , Xue, X. , Gerber, L. , Freedland, S.J. , Frenette, P.S. , 2013. Autonomic nerve development contributes to prostate cancer progression. Science. 341, 1236361 [DOI] [PubMed] [Google Scholar]
- Marchesi, F. , Piemonti, L. , Mantovani, A. , Allavena, P. , 2010. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 21, 77–82. [DOI] [PubMed] [Google Scholar]
- Melhem-Bertrandt, A. , Chavez-Macgregor, M. , Lei, X. , Brown, E.N. , Lee, R.T. , Meric-Bernstam, F. , Sood, A.K. , Conzen, S.D. , Hortobagyi, G.N. , Gonzalez-Angulo, A.M. , 2011. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 29, 2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondicova, K. , Mravec, B. , 2010. Role of nervous system in cancer aetiopathogenesis. Lancet Oncol. 11, 596–601. [DOI] [PubMed] [Google Scholar]
- Penault-Llorca, F. , Bertucci, F. , Adelaide, J. , Parc, P. , Coulier, F. , Jacquemier, J. , Birnbaum, D. , deLapeyriere, O. , 1995. Expression of FGF and FGF receptor genes in human breast cancer. Int. J. Cancer. 61, 170–176. [DOI] [PubMed] [Google Scholar]
- Powe, D.G. , Voss, M.J. , Habashy, H.O. , Zanker, K.S. , Green, A.R. , Ellis, I.O. , Entschladen, F. , 2011. Alpha- and beta-adrenergic receptor (AR) protein expression is associated with poor clinical outcome in breast cancer: an immunohistochemical study. Breast Cancer Res. Treat. 130, 457–463. [DOI] [PubMed] [Google Scholar]
- Pundavela, J. , Demont, Y. , Jobling, P. , Lincz, L.F. , Roselli, S. , Thorne, R. , Bond, D. , Bradshaw, R.A. , Walker, M.M. , Hondermarck, H. , 2014. ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer. Am. J. Pathol. 184, 3156–3162. [DOI] [PubMed] [Google Scholar]
- Sarhadi, N.S. , Shaw Dunn, J. , Lee, F.D. , Soutar, D.S. , 1996. An anatomical study of the nerve supply of the breast, including the nipple and areola. Br. J. Plast. Surg. 49, 156–164. [DOI] [PubMed] [Google Scholar]
- Skaper, S.D. , 2012. The neurotrophin family of neurotrophic factors: an overview. Methods Mol. Biol. 846, 1–12. [DOI] [PubMed] [Google Scholar]
- Sloan, E.K. , Priceman, S.J. , Cox, B.F. , Yu, S. , Pimentel, M.A. , Tangkanangnukul, V. , Arevalo, J.M. , Morizono, K. , Karanikolas, B.D. , Wu, L. , Sood, A.K. , Cole, S.W. , 2010. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70, 7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter, D.M. , Miller, K.E. , 2011. The emerging role of forces in axonal elongation. Prog. Neurobiol. 94, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhecke, E. , Adriaenssens, E. , Verbeke, S. , Meignan, S. , Germain, E. , Berteaux, N. , Nurcombe, V. , Le Bourhis, X. , Hondermarck, H. , 2011. Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin. Cancer Res. 17, 1741–1752. [DOI] [PubMed] [Google Scholar]
- Verbeke, S. , Meignan, S. , Lagadec, C. , Germain, E. , Hondermarck, H. , Adriaenssens, E. , Le Bourhis, X. , 2010. Overexpression of p75(NTR) increases survival of breast cancer cells through p21(waf1). Cell Signal. 22, 1864–1873. [DOI] [PubMed] [Google Scholar]
- Zhao, C.M. , Hayakawa, Y. , Kodama, Y. , Muthupalani, S. , Westphalen, C.B. , Andersen, G.T. , Flatberg, A. , Johannessen, H. , Friedman, R.A. , Renz, B.W. , Sandvik, A.K. , Beisvag, V. , Tomita, H. , Hara, A. , Quante, M. , Li, Z. , Gershon, M.D. , Kaneko, K. , Fox, J.G. , Wang, T.C. , Chen, D. , 2014. Denervation suppresses gastric tumorigenesis. Sci. Transl Med. 6, 250ra115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q. , Yang, Y. , Liang, X. , Du, G. , Liu, L. , Lu, L. , Dong, J. , Han, H. , Zhang, G. , 2014. The clinicopathological significance of neurogenesis in breast cancer. BMC Cancer. 14, 484 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data
Supplementary Figure 1 NGF‐mediated neurotrophic effect of breast cancer cells for 50B11 cells from dorsal root ganglia. Immortalized dorsal root ganglia (DRG) neurons 50B11 were obtained from Dr A. Höke (John Hopkins University, Baltimore USA). The co‐culture with breast cancer cells was performed as described for PC12 cells in the Material and methods section. For co‐culture with the breast cancer cells, the same protocol as for PC12 cells was used (see Material and methods section), but the culture media included 5μM forskolin (necessary to obtain neurite outgrowth with these cells). A) MCF‐7 cells were able to induce neurite outgrowth in 50B11 cells. This neurotrophic effect was partially inhibited by addition of anti‐NGF antibody but not by IgG control. B) Representative pictures of each experimental condition are shown. The results represent the mean of 3 independent experiments ± SD. ***p < 0.001; **p < 0.05.
Supplementary Figure 2 Detection of tyrosine hydroxylase positive nerve fibers in breast cancer. Tyrosine hydroxylase was detected by IHC using the same protocol as described in Material and methods, with anti‐tyrosine hydroxylase (Millipore, catalog number AB152). A) Nerve trunk composed of many fibers positive for tyrosine hydroxylase is shown by an arrow. B) Individual nerve fibers (axons) positive for tyrosine hydroxylase are indicated by arrows. Scale bar = 50 μm.


