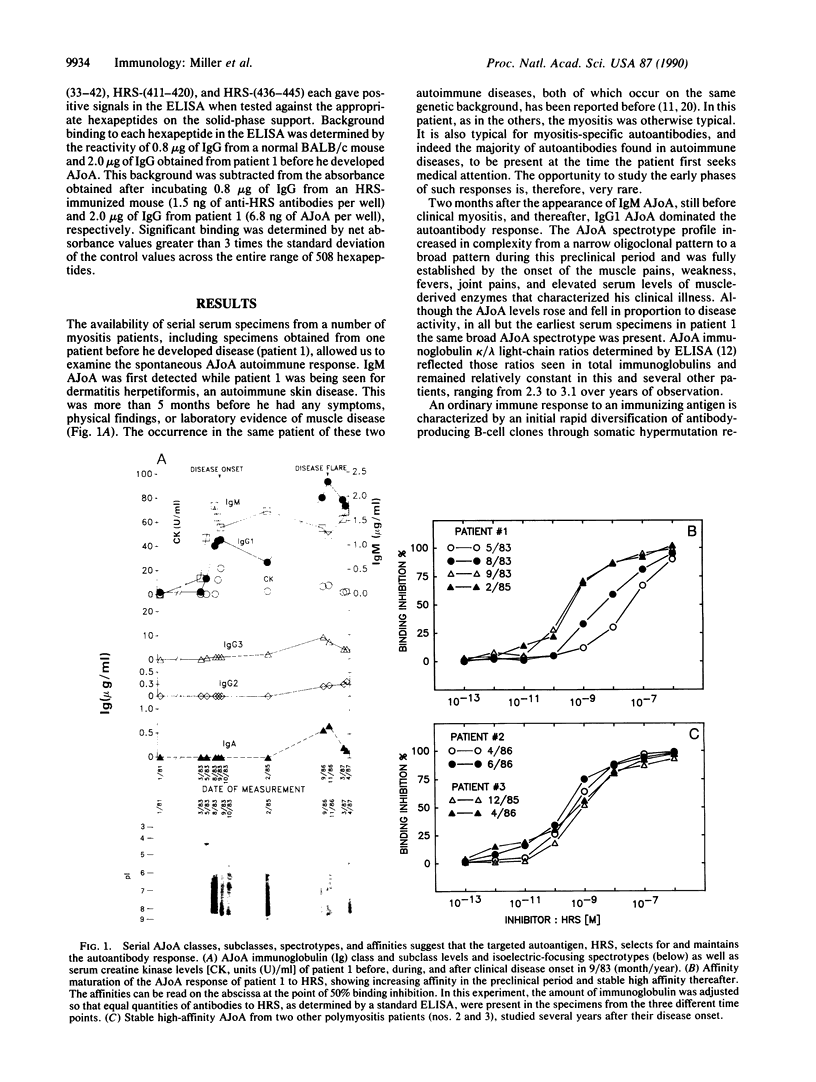
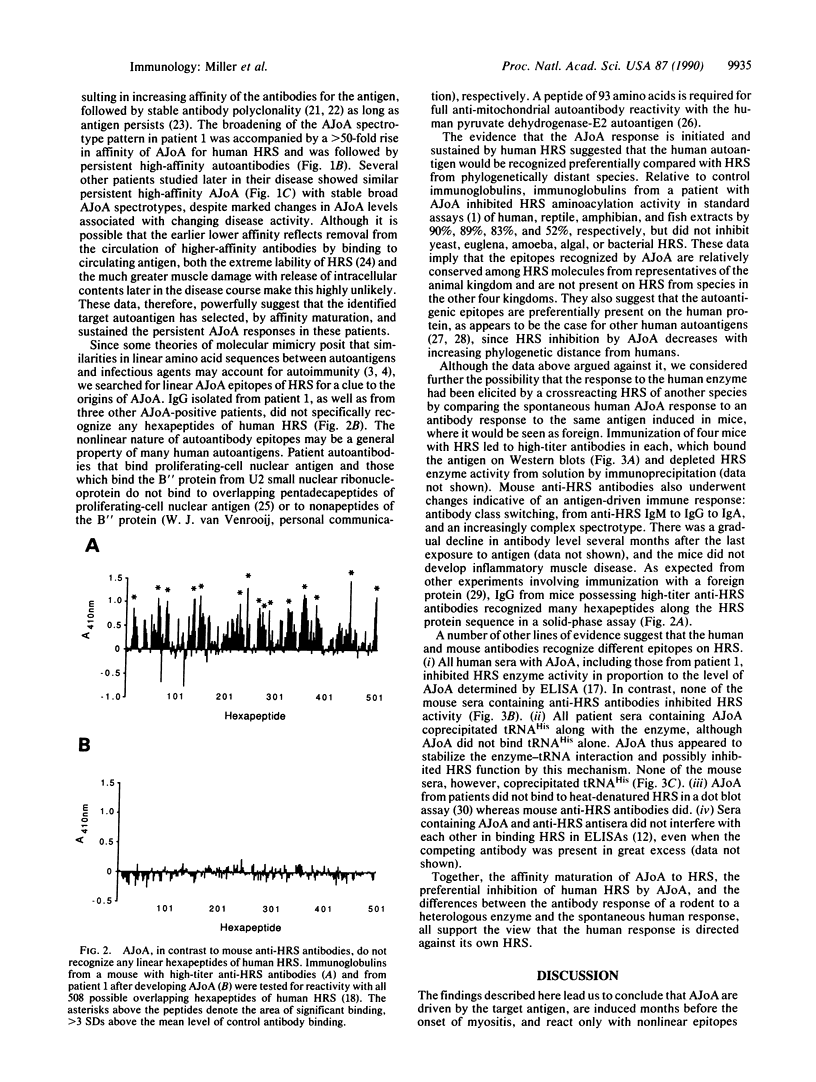
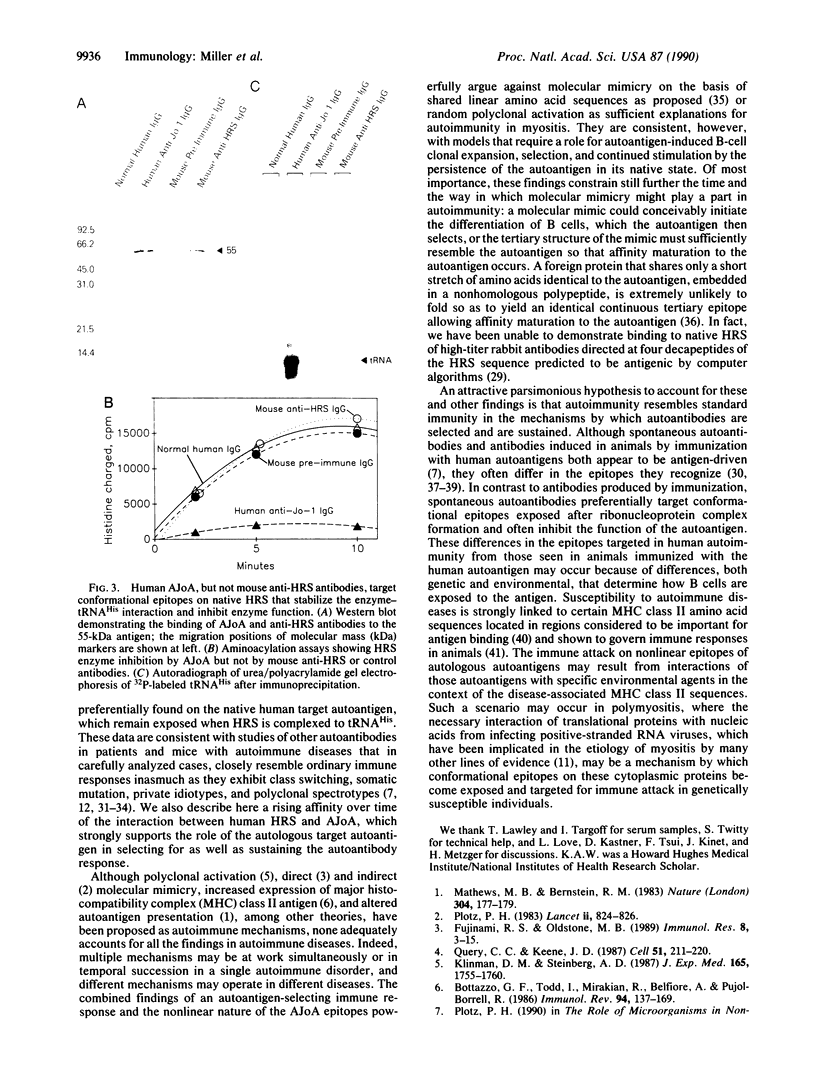
Abstract
Patients with systemic autoimmune diseases make specific autoantibodies that are directed against self structures. According to one view, these autoantibodies arise as a result of an immune response to foreign antigens such as infectious agents that share, by molecular mimicry, common structures with host proteins. An alternative view is that the target autoantigen itself initiates, selects, and sustains autoantibody synthesis. We show here that anti-Jo-1 autoantibodies directed against histidyl-tRNA synthetase in the human autoimmune muscle disease polymyositis undergo, in addition to spectrotype broadening and class switching, the sine qua non of an immune response to the target antigen--affinity maturation to that antigen. We demonstrate further that these autoantibodies, unlike anti-synthetase antibodies induced in mice immunized with heterologous antigen, bind only nonlinear epitopes on the native human synthetase that remain exposed when the enzyme is complexed to tRNA(His). These data suggest that the native target autoantigen itself has played a direct role in selecting and sustaining the autoantibody response and sharply restrict the time and the way in which a molecular mimic might act to provoke autoantibodies.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Williamson A. R., Wright B. E. Selection of a single antibody-forming cell clone and its propagation in syngeneic mice. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1398–1403. doi: 10.1073/pnas.67.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow D. J., Edwards M. S., Thornton J. M. Continuous and discontinuous protein antigenic determinants. Nature. 1986 Aug 21;322(6081):747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- Biswas T., Miller F. W., Plotz P. H. Stimulation and partial stabilization of human histidyl-tRNA synthetase by hemoglobin. FEBS Lett. 1988 Feb 29;229(1):203–205. doi: 10.1016/0014-5793(88)80827-5. [DOI] [PubMed] [Google Scholar]
- Biswas T., Miller F. W., Twitty S. A., Plotz P. H. An efficient method for enrichment of histidyl-tRNA synthetase (Jo-1 antigen) from HeLa cells. J Immunol Methods. 1987 Apr 16;98(2):235–241. doi: 10.1016/0022-1759(87)90010-x. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Todd I., Mirakian R., Belfiore A., Pujol-Borrell R. Organ-specific autoimmunity: a 1986 overview. Immunol Rev. 1986 Dec;94:137–169. doi: 10.1111/j.1600-065X.1986.tb01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E. K., Tan E. M. Human autoantibody-reactive epitopes of SS-B/La are highly conserved in comparison with epitopes recognized by murine monoclonal antibodies. J Exp Med. 1987 Dec 1;166(6):1627–1640. doi: 10.1084/jem.166.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Tan E. M., Traugh J. A. Myositis autoantibody reactivity and catalytic function of threonyl-tRNA synthetase. FASEB J. 1988 May;2(8):2376–2379. doi: 10.1096/fasebj.2.8.2452112. [DOI] [PubMed] [Google Scholar]
- Delves P. J., Roitt I. M. Long term spectrotypic and idiotypic stability of thyroglobulin autoantibodies in patients with Hashimoto's thyroiditis. Clin Exp Immunol. 1988 Mar;71(3):459–463. [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. A., Dyer K., Craven S. Y., Fuller C. R., Yount W. J. Subclass restriction and polyclonality of the systemic lupus erythematosus marker antibody anti-Sm. J Clin Invest. 1985 Apr;75(4):1270–1277. doi: 10.1172/JCI111826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoeur A. M., Peebles C. L., Gompper P. T., Tan E. M. Identification of Ki (Ku, p70/p80) autoantigens and analysis of anti-Ki autoantibody reactivity. J Immunol. 1986 Mar 1;136(5):1648–1653. [PubMed] [Google Scholar]
- Friguet B., Chaffotte A. F., Djavadi-Ohaniance L., Goldberg M. E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985 Mar 18;77(2):305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Molecular mimicry as a mechanism for virus-induced autoimmunity. Immunol Res. 1989;8(1):3–15. doi: 10.1007/BF02918552. [DOI] [PubMed] [Google Scholar]
- Getzoff E. D., Geysen H. M., Rodda S. J., Alexander H., Tainer J. A., Lerner R. A. Mechanisms of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1191–1196. doi: 10.1126/science.3823879. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Tainer J. A., Rodda S. J., Mason T. J., Alexander H., Getzoff E. D., Lerner R. A. Chemistry of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1184–1190. doi: 10.1126/science.3823878. [DOI] [PubMed] [Google Scholar]
- Gray D., Skarvall H. B-cell memory is short-lived in the absence of antigen. Nature. 1988 Nov 3;336(6194):70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- Hochman P. S., Huber B. T. A class II gene conversion event defines an antigen-specific Ir gene epitope. J Exp Med. 1984 Dec 1;160(6):1925–1930. doi: 10.1084/jem.160.6.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff J. P., Roos G., Peebles C. L., Houghten R., Sullivan K. F., Tan E. M. Insights into native epitopes of proliferating cell nuclear antigen using recombinant DNA protein products. J Exp Med. 1990 Aug 1;172(2):419–429. doi: 10.1084/jem.172.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalovidouris A. E., Miller F. W., Lawley T. J. Polymyositis/dermatomyositis associated with dermatitis herpetiformis. Arthritis Rheum. 1989 Sep;32(9):1179–1181. doi: 10.1002/anr.1780320920. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. Nature. 1983 Jul 14;304(5922):177–179. doi: 10.1038/304177a0. [DOI] [PubMed] [Google Scholar]
- Miller F. W., Twitty S. A., Biswas T., Plotz P. H. Origin and regulation of a disease-specific autoantibody response. Antigenic epitopes, spectrotype stability, and isotype restriction of anti-Jo-1 autoantibodies. J Clin Invest. 1990 Feb;85(2):468–475. doi: 10.1172/JCI114461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A., Steitz J. A. Characterization of the DNA-binding protein antigen Ku recognized by autoantibodies from patients with rheumatic disorders. J Biol Chem. 1986 Feb 15;261(5):2274–2278. [PubMed] [Google Scholar]
- Plotz P. H. Autoantibodies are anti-idiotype antibodies to antiviral antibodies. Lancet. 1983 Oct 8;2(8354):824–826. doi: 10.1016/s0140-6736(83)90740-7. [DOI] [PubMed] [Google Scholar]
- Plotz P. H., Dalakas M., Leff R. L., Love L. A., Miller F. W., Cronin M. E. Current concepts in the idiopathic inflammatory myopathies: polymyositis, dermatomyositis, and related disorders. Ann Intern Med. 1989 Jul 15;111(2):143–157. doi: 10.7326/0003-4819-111-2-143. [DOI] [PubMed] [Google Scholar]
- Portanova J. P., Cheronis J. C., Blodgett J. K., Kotzin B. L. Histone autoantigens in murine lupus. Definition of a major epitope within an accessible region of chromatin. J Immunol. 1990 Jun 15;144(12):4633–4640. [PubMed] [Google Scholar]
- Query C. C., Keene J. D. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987 Oct 23;51(2):211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- Reichlin M., Rader M., Harley J. B. Autoimmune response to the Ro/SSA particle is directed to the human antigen. Clin Exp Immunol. 1989 Jun;76(3):373–377. [PMC free article] [PubMed] [Google Scholar]
- Rosa M. D., Hendrick J. P., Jr, Lerner M. R., Steitz J. A., Reichlin M. A mammalian tRNAHis-containing antigen is recognized by the polymyositis-specific antibody anti-Jo-1. Nucleic Acids Res. 1983 Feb 11;11(3):853–870. doi: 10.1093/nar/11.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Aucoin A. H., Pisetsky D. S., Weigert M. G. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Marshak-Rothstein A., Wolfowicz C. B., Rothstein T. L., Weigert M. G. The role of clonal selection and somatic mutation in autoimmunity. 1987 Aug 27-Sep 2Nature. 328(6133):805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Kocks C., Rajewsky K., Dildrop R. Analysis of somatic mutation and class switching in naive and memory B cells generating adoptive primary and secondary responses. Cell. 1987 Mar 13;48(5):757–770. doi: 10.1016/0092-8674(87)90073-0. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh C. D., Coppel R., Gershwin M. E. Structural requirement for autoreactivity on human pyruvate dehydrogenase-E2, the major autoantigen of primary biliary cirrhosis. Implication for a conformational autoepitope. J Immunol. 1990 May 1;144(9):3367–3374. [PubMed] [Google Scholar]
- Targoff I. N., Arnett F. C., Berman L., O'Brien C., Reichlin M. Anti-KJ: a new antibody associated with the syndrome of polymyositis and interstitial lung disease. J Clin Invest. 1989 Jul;84(1):162–172. doi: 10.1172/JCI114136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targoff I. N., Arnett F. C., Reichlin M. Antibody to threonyl-transfer RNA synthetase in myositis sera. Arthritis Rheum. 1988 Apr;31(4):515–524. doi: 10.1002/art.1780310408. [DOI] [PubMed] [Google Scholar]
- Targoff I. N., Reichlin M. Measurement of antibody to Jo-1 by ELISA and comparison to enzyme inhibitory activity. J Immunol. 1987 May 1;138(9):2874–2882. [PubMed] [Google Scholar]
- Todd J. A., Acha-Orbea H., Bell J. I., Chao N., Fronek Z., Jacob C. O., McDermott M., Sinha A. A., Timmerman L., Steinman L. A molecular basis for MHC class II--associated autoimmunity. Science. 1988 May 20;240(4855):1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- Tsui F. W., Siminovitch L. Isolation, structure and expression of mammalian genes for histidyl-tRNA synthetase. Nucleic Acids Res. 1987 Apr 24;15(8):3349–3367. doi: 10.1093/nar/15.8.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E. J., Jeffrey P. D. Sequence homology between encephalomyocarditis virus protein VPI and histidyl-tRNA synthetase supports a hypothesis of molecular mimicry in polymyositis. Med Hypotheses. 1988 Jan;25(1):21–25. doi: 10.1016/0306-9877(88)90041-2. [DOI] [PubMed] [Google Scholar]