Abstract
Purpose
The KRAS gene frequently mutates in colorectal cancer (CRC). Here we investigated the prognostic and predictive role of KRAS mutation in patients with stage II or III CRC.
Experimental design
A consecutive cohort of patients with stage II or III CRC from a single center database was studied. The association between KRAS status, adjuvant FOLFOX therapy, and 3‐year disease‐free survival (3‐y DFS) was analyzed.
Results
Of our 433 patients, 166 (38.3%) exhibited the KRAS mutation. Among the 190 patients who did not receive adjuvant therapy, those with KRAS mutation tumors had a worse 3‐y DFS (hazard ratio [HR], 1.924; 95% confidence interval [CI], 1.078–3.435; P = 0.027). Among patients who received adjuvant chemotherapy, KRAS mutation was not correlated with worse 3‐y DFS (HR, 1.083; 95% CI, 0.618–1.899; P = 0.781). Adjuvant chemotherapy improved 3‐y DFS only among patients with KRAS mutant tumors (78.0% vs 69.2%) on multivariate analysis adjusted for age, stage, grade, site, vessel invasion, and carcinoembryonic antigen level (HR, 0.454; 95% CI, 0.229–0.901; P = 0.024). In contrast, there was no benefit of adjuvant chemotherapy in the KRAS wild‐type group (84.3% vs 82.0%).
Conclusions
KRAS mutation indicates poor prognosis. FOLFOX adjuvant chemotherapy benefits patients with stage II or III colorectal cancer with KRAS mutant tumors and is worth further investigation.
Keywords: KRAS, Adjuvant chemotherapy, Colorectal cancer, FOLFOX
Highlights
The results showed patients with KRAS mutant tumors have poorer survival than those with KRAS wild type tumors, but after adjuvant FOLFOX chemotherapy, the patients' prognosis gets improved.
Only patients with KRAS mutant tumors benefit from adjuvant chemotherapy in colorectal cancer.
It indicated that patients with KRAS mutant tumors, oxaliplatin‐based adjuvant therapy must be guaranteed.
1. Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide. Over 40% of patients with CRC carry a KRAS mutation (TCGA, 2012). KRAS is a proto‐oncogene that encodes for a guanosine triphosphate/guanosine diphosphate‐binding protein that is downstream of the epidermal growth factor receptor (EGFR) in the RAS/RAF/MAPK pathway.
KRAS mutation status is a demonstrated predictive biomarker of resistance to anti‐EGFR targeted therapies in patients with metastatic CRC (mCRC) (Bokemeyer et al., 2012; Peeters et al., 2013). Testing for KRAS mutations is increasingly common in clinical practice since it better directs CRC treatment. KRAS mutation is increasingly accepted as a poor prognostic factor as shown in several studies (Hutchins et al., 2011) and a meta‐analysis (Clarinda Wei Ling Chua et al., 2014), although not as strong prognostic factor as the BRAF mutation. However, the role of KRAS mutation as a predictive marker for cytotoxic drug response in CRC adjuvant setting is controversial (Roth et al., 2010; Lee et al., 2015). One PETTAC8 study showed that patients with a KRAS mutation had inferior prognosis under FOLFOX or cetuximab plus FOLFOX treatment (Yoon et al., 2014). However, as it is already known that anti‐EGFR therapy combination with FOLFOX in patients with KRAS mutation patients has a detrimental effect (Douillard et al., 2013), the prognostic role of KRAS in a FOLFOX group should be studied independently. To our understanding, although KRAS is an old story, its relationship with FOLFOX treatment in the adjuvant setting of CRC is still debatable and worth investigation.
In this study, we aim to identify whether KRAS status has predictive value for FOLFOX treatment as adjuvant chemotherapy for patients with stage II or III CRC. In the KRAS wild‐type and KRAS mutation subgroups, the impact of FOLFOX versus no treatment on 3‐year disease‐free survival (3‐y DFS) was compared and the impact of KRAS on DFS with or without FOLFOX treatment was compared.
2. Materials and methods
2.1. Patient population
Patients with stage II or III CRC who underwent a radical resection surgery between January 2007 and April 2012 were consecutively selected from the Gastrointestinal Hospital of Sun Yat‐sen University database. All participants provided informed written consent and the study was approved by the Medical Ethics Board of Gastrointestinal Hospital, Sun Yat‐sen University. Tumor stage was classified according to the 7th edition of the American Joint Committee on cancer staging system. All patients included in this study were treated by the same surgical team using the same protocol. Adjuvant chemotherapies were recommended for patients with stage III and high‐risk stage II disease after surgical resection. Mid to low rectal cancer patients received radiation therapy postoperatively according to the doctor's choice. Patients with the following conditions were excluded from the analysis in the present study: (A) presence of other malignancies, (B) underwent single agent chemotherapy, (C) underwent neo‐chemoradiotherapy before surgery, (D) died of complications or other diseases during the same hospitalization of the surgery, or (E) tumor recurrence within 3 months.
2.2. Clinical evaluation and follow‐up
All patients were reevaluated at 3‐month intervals for 2 years and every 6 months thereafter. The evaluations consisted of pertinent medical history, physical examination, blood cell counts, and blood chemistry including carcinoembryonic antigen (CEA) levels at every follow‐up visit. Colonoscopies (in patients treated with anterior resections) were performed at 12‐month intervals in the first 2 years and every 2 years thereafter. Follow‐up computed tomography of the chest, abdomen, and pelvis was scheduled every 6 months for the first 3 years and annually thereafter. Histological confirmation of local recurrence and distant relapse (defined as tumor manifestation outside the pelvis) was encouraged. Alternate acceptable criteria included sequential enlargement of a mass on radiological studies with a simultaneous increase in serum CEA levels or other tumor markers.
2.3. Chemotherapy regimens
Doublet adjuvant chemotherapy was started within 8 weeks postoperatively and consisted of an mFOLFOX6 regimen of 85 mg/m2 oxaliplatin intravenous infusion followed by 400 mg/m2 leucovorin, followed by a 5‐FU 0.4 g/m2 bolus intravenous injection and then a 2.4 g/m2 continuous intravenous infusion for 46–48 h. The regimen was repeated every 2 weeks. Because XELOX was proven to be identical to FOLFOX in treating patients with colorectal cancer in an adjuvant setting (Schmoll et al., 2014), to reduce selection bias, we pooled the 40 patients who received the XELOX regimen in the FOLFOX analysis. The median chemotherapy duration was 4 months. The common reason patients did not receive adjuvant chemotherapy was patient rejection due to fear of side effects, poor economic status, or comorbidities.
2.4. DNA preparation
Formalin‐fixed and paraffin‐embedded tumor tissues were collected from the Pathological Tissue Bank of Gastrointestinal Hospital of Sun Yat‐sen University. All tissue samples were confirmed independently by two gastrointestinal pathologists. Genomic DNA was extracted using a DNA FFPE Tissue Kit (BIOMIGA, CA) according to the manufacturer's recommendations.
2.5. KRAS mutational analysis
The primers for the amplification and Sanger dideoxy chain termination sequencing of KRAS gene exon 2 were forward 5′‐GTCCTGCACCAGTAATATGC‐3′ and reverse 5′‐ATGTTCTAATATAGTCACATTTTC‐3′ for codons 12 and 13. Polymerase chain reaction (PCR) was performed using 100 ng of genomic DNA as a template. Each mixture contained 10 pmol of each primer. The reactions were performed in a total volume of 31.5 μL. The amplification reaction were as follows: an initial denaturing cycle of 95 °C for 5 min; 45 cycles of 94 °C for 25 s, 58 °C for 25 s, 72 °C for 25 s; and a final extension cycle at 72 °C for 10 min. The PCR products were then purified and subjected to direct sequencing using an automatic sequencer (ABI‐3730 DNA Sequencer; Life Technologies, CA).
2.6. Association and survival analysis
Survival curves were generated using the Kaplan–Meier method, while univariate survival distributions were compared using the log‐rank test. Hazard ratios and 95% confidence intervals for uni‐ and multivariate models were computed using Cox proportional hazards regression. The chi‐square test was used to evaluate categorical variables. All reported P values are two‐sided, and P values <0.05 were considered statistically significance.
3. Results
3.1. Patient characteristics by KRAS status
Of the 473 patients with eligible tumor specimens, KRAS mutation analysis was successfully performed in 453 (95.7%). Among the 453 patients with an available KRAS status, 433 (95.6%) had follow‐up data. Median follow‐up time was 49 months (range, 24–78 months). Data on DFS were censored at 5 years. A total of 100 (23.1%) events were recorded. Among the 433 patients, 218 had stage II CRC, 215 had stage III CRC, and 113 had rectal cancer. A total of 243 (56.1%) patients received adjuvant chemotherapy, and 166 of 433 patients (38.3%) demonstrated a KRAS mutation (123 patients in codon 12, 43 in codon 13). The patients with tumors exhibiting a KRAS mutation were similar to those with tumors exhibiting KRAS wild‐type (Table 1). Patients not receiving adjuvant chemotherapy were more likely to have stage II disease (67.9%) and be older. The baseline characteristics of patients with or without chemotherapy did not differ significantly otherwise (Table 1).
Table 1.
Patient demographics and disease characteristics stratified by KRAS mutation and chemotherapy status.
| Factors | KRAS | Adjuvant chemotherapy | |||||
|---|---|---|---|---|---|---|---|
| wt | mt | P | No | Yes | P | ||
| Age | Median(Range) | 60.15(18–88) | 63.90(17–90) | 0.739 | 67.38(17–90) | 57.14(18–78) | 0.001 |
| Sex | 0.688 | 0.695 | |||||
| Male | 155 | 100 | 114 | 141 | |||
| Female | 112 | 66 | 76 | 102 | |||
| Stage | 0.332 | 0.001 | |||||
| Ⅱ | 123 | 95 | 129 | 89 | |||
| Ⅲ | 144 | 71 | 61 | 154 | |||
| T stage | 0.122 | 0.077 | |||||
| 1 | 3 | 1 | 0 | 4 | |||
| 2 | 9 | 6 | 1 | 14 | |||
| 3 | 236 | 156 | 179 | 213 | |||
| 4 | 18 | 3 | 9 | 12 | |||
| N stage | 0.043 | 0.001 | |||||
| 0 | 123 | 95 | 129 | 89 | |||
| 1 | 110 | 59 | 52 | 117 | |||
| 2 | 34 | 12 | 9 | 37 | |||
| Site | 0.574 | 0.058 | |||||
| colon | 200 | 120 | 128 | 192 | |||
| rectum | 67 | 46 | 62 | 51 | |||
| Grade | 0.460 | 0.102 | |||||
| 1 | 53 | 42 | 57 | 38 | |||
| 2 | 134 | 79 | 89 | 124 | |||
| 3 | 61 | 31 | 34 | 58 | |||
| mucinous | 19 | 14 | 10 | 23 | |||
| CEA | Median(Range) | 2.84(0–308) | 3.3(0–605) | 0.823 | 3.10(0–605) | 2.76(0–189) | 0.289 |
3.2. Correlation between KRAS and survival
The 3‐y DFS rate among patients with KRAS wild‐type tumors (81.3%) tended to be higher than that among patients with tumors exhibiting a KRAS mutation (73.6%, log rank P = 0.068). Among patients who did not receive adjuvant chemotherapy, those with KRAS wild‐type tumors had higher 3‐y DFS than those exhibiting a KRAS mutation (82.0% vs 69.2% months, respectively; HR, 1.924; 95% CI, 1.078–3.435, P = 0.027; Table 2, Figure 1A). However, the analysis of patients who did receive adjuvant therapy failed to show any significant differences in DFS by KRAS status (80.8% vs 78.0%; HR, 1.083; 95% CI, 0.618–1.899; P = 0.781; Table 2, Figure 1B). On multivariate analysis adjusted for age, stage, grade, site, vessel invasion, CEA level, and adjuvant chemotherapy, KRAS status was significantly associated with 3‐y DFS (HR, 1.572; 95% CI, 1.058–2.335; P = 0.025).
Table 2.
Univariate analysis of 3‐year disease‐free survival among patients with stage II and III and those with stage III only colorectal cancer according to KRAS mutation and adjuvant chemotherapy status.
| Analysis | Stage Ⅱ–Ⅲ patients | Stage Ⅲ patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No | 3y‐DFS(%) | HR(95% CI) | P value | No | 3y‐DFS(%) | HR(95% CI) | P value | ||
| According to KRAS status | |||||||||
| All patients | 433 | 215 | |||||||
| KRAS wt | 267 | 81.3 | 1 | 144 | 75.2 | ||||
| KRAS mt | 166 | 73.6 | 1.442(0.973–2.138) | 0.068 | 71 | 66.9 | 1.281(0.772–2.125) | 0.338 | |
| Patients receiving no adjuvant chemotherapy | 190 | 61 | |||||||
| KRAS wt | 107 | 82 | 38 | 75.9 | |||||
| KRAS mt | 83 | 69.2 | 1.924(1.078–3.435) | 0.027 | 23 | 50.2 | 2.455(1.052–5.73) | 0.038 | |
| Patients receiving adjuvant chemotherapy | 243 | 154 | |||||||
| KRAS wt | 160 | 80.8 | 106 | 74.9 | |||||
| KRAS mt | 83 | 78 | 1.083(0.618–1.899) | 0.781 | 48 | 74.4 | 0.88(0.45–1.719) | 0.708 | |
| According to adjuvant‐chemotherapy status | |||||||||
| All patients | 433 | 215 | |||||||
| No adjuvant chemotherapy | 190 | 76.4 | 61 | 66.4 | |||||
| Adjuvant chemotherapy | 243 | 79.8 | 0.842(0.568–1.248) | 0.392 | 154 | 74.7 | 0.701(0.418–1.174) | 0.077 | |
| KRAS wt | 267 | 144 | |||||||
| No adjuvant chemotherapy | 107 | 82 | 1 | 38 | 75.9 | ||||
| Adjuvant chemotherapy | 160 | 80.8 | 1.131(0.651–1.966) | 0.662 | 106 | 74.9 | 1.063(0.52–2.176) | 0.866 | |
| KRAS mt | 71 | ||||||||
| No adjuvant chemotherapy | 83 | 69.2 | 23 | 50.2 | |||||
| Adjuvant chemotherapy | 83 | 78 | 0.638(0.354–1.15) | 0.135 | 48 | 74.4 | 0.398(0.178–0.890) | 0.025 | |
Figure 1.
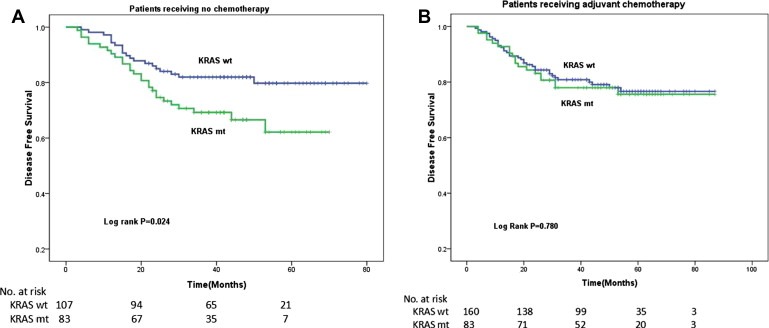
Kaplan–Meier estimates of disease‐free survival (DFS) among patients with stage II and III colorectal cancer. (A) In the absence of FOLFOX adjuvant chemotherapy, the patients with KRAS mutant tumors had significantly worse survival than patients with KRAS wild‐type tumors. (B) In the presence of FOLFOX adjuvant chemotherapy, patients with KRAS mutant tumors did not have a significant decrease in DFS compared with patients with KRAS wild‐type tumors.
3.3. Correlation between KRAS status and adjuvant chemotherapy benefit
We next tested whether response to FOLFOX chemotherapy differed between the KRAS wild‐type and mutation groups. Among patients with KRAS wild‐type tumors, FOLFOX chemotherapy did not improve outcomes compared with no chemotherapy (3‐y DFS, 84.3% vs 82.0%, log rank P = 0.661, Figure 2A). On the other hand, the trend of improved 3‐y DFS after FOLFOX adjuvant chemotherapy for patients with KRAS mutant tumors (3‐y DFS, 78.0% vs 69.2%; log rank P = 0.130; Figure 2B) became significant when confined to patients with stage III CRC (3‐y DFS, 50.2% vs 74.4%; log rank P = 0.020) and after adjustment for age, stage, grade, site, vessel invasion, and CEA level (HR, 0.454; 95% CI, 0.229–0.901; P = 0.024). Such an effect was not observed in patients with stage II CRC. Therefore, although KRAS mutation status is a prognostic biomarker associated with worse DFS without chemotherapy (Figure 1A), it is also a predictive biomarker associated with greater improvement of DFS under FOLFOX chemotherapy (Figure 2B). The combination of these two opposite effects of KRAS mutation may explain the lack of a significant difference in DFS between patients with KRAS mutation and wild‐type tumors under FOLFOX chemotherapy treatment. Since patients with stage III CRC contributed most of the effect, we examined the characteristics of patients with KRAS mutation stage III CRC; however, we found no difference in age, sex, CEA level, grade, tumor deposits, or vessel invasion (data shown in Supplementary Table 1). The same effects were observed in a rectal cancer subgroup analysis (data shown in Supplementary materials). To confirm the results, we randomly selected 80% and 40% of the 433 patients for the exact same analysis and obtained similar results. Same effects were observed between codon 12 and codon 13 mutations of KRAS exon 2 (data not shown).
Figure 2.
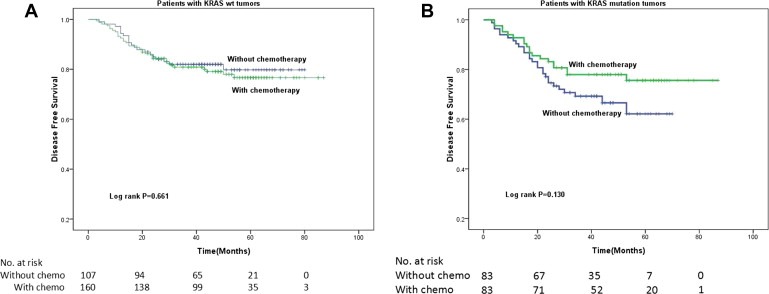
Kaplan–Meier estimates of disease‐free survival (DFS) among patients stage II and IIIII or stage III colorectal cancer. (A) with KRAS wild‐type tumors. FOLFOX adjuvant chemotherapy did not improve DFS compared with no adjuvant chemotherapy. (B) With KRAS mutation. FOLFOX adjuvant treatment improved DFS compared with no adjuvant treatment.
4. Discussion
The KRAS gene encodes a protein that acts in the extracellular signal‐regulated kinase/mitogen‐activated protein kinase signaling pathway. This pathway plays a critical role in the pathogenesis of colorectal cancer. Because KRAS is a downstream effector of EGFR in the signaling pathway, mutations in KRAS were predictive of non‐response to anti‐EGFR targeted therapies, such as cetuximab (Van Cutsem et al., 2009) and panitumumab (Andreyev et al., 2001), for patients with metastatic colorectal cancer. However, the significance of KRAS mutation as a predictive biomarker with regard to response to FOLFOX adjuvant chemotherapy was never reported. Although KRAS is an old story, most of the previous studies are conducted in Western populations and few data were available in Chinese populations. Our study showed that KRAS mutation status is a prognostic biomarker and the presence of KRAS mutations was associated with a worse DFS in stage II or III CRC patients without adjuvant chemotherapy. More importantly, the data presented demonstrate that KRAS tumor mutation status has predictive value for benefit from adjuvant chemotherapy in patients with stage II or III CRC. Only the subpopulation with KRAS mutations could benefit from FOLFOX adjuvant chemotherapies. However, KRAS wild‐type indicated good prognosis and less benefit from adjuvant chemotherapy.
Our results were in accordance with data from two large collaborative RASCAL studies of 2721 and 4268 patients with CRC (Andreyev et al., 2001, 1998, 2012). The first RASCAL study reported an increased risk of recurrence and death linked to KRAS mutations, while the second refined this observation to report significant prognostic value in failure‐free survival only with the specific codon 12 glycine/valine mutation in patients with Dukes' C (stage III) disease. Three recent studies also showed that KRAS mutations were associated with reduced survival (Samowitz et al., 2000; Wang et al., 2003; Imamura et al., 2012; Schmoll et al., 2014; Lee et al., 2015). However, an analysis of two prospective trials comparing 5‐FU and 5‐FU with irinotecan in patients with stage II and III CRC (PETTAC 3 trial) and those with stage III colon cancer (CALBG 89803) showed that KRAS mutational status had no significant influence on DFS or overall survival (Ogino et al., 2009). Since all of the patients in those two trials received adjuvant therapy, the prognostic effect of KRAS mutation might be reversed. Our results support that, although patients with the KRAS mutation had poor prognosis, their disease was sensitive to adjuvant FOLFOX therapy, which resulted in almost the same outcomes as those of patients with KRAS wild‐type tumors who received adjuvant chemotherapy.
Few studies have shown that KRAS mutation can predict response to conventional chemotherapy. KRAS mutation status failed to predict effects in patients with mCRC receiving cytotoxic drugs (Richman et al., 2009). A multivariate analysis demonstrated that KRAS mutation type could be a clinically useful molecular marker for the identification of CRC subgroups that are likely to benefit from 5‐FU–based adjuvant chemotherapy (Gnanasampanthan et al., 2001). Comparison of 5‐FU adjuvant chemotherapy with surgery alone in 1913 patients with stage II CRC in the QUASAR trial revealed a significantly higher risk of recurrence for patients with KRAS mutant tumors than those with KRAS wild‐type tumors (28% vs 21%), but the prognostic value of KRAS mutation was similar in the presence and absence of adjuvant chemotherapy (Hutchins et al., 2011). It is worth noting that the majority of patients in the aforementioned studies had stage II tumors and that only the effect of the single agent 5‐FU was evaluated.
Our results show that KRAS mutant tumors are more sensitive to adjuvant combination chemotherapy and that the effect is more profound in stage III CRC. The current study is the first of its kind to evaluate the role of KRAS mutation in predicting adjuvant FOLFOX treatment. Although it is a retrospective study, its results are convincing and valuable. First, no trials have compared FOLFOX and no chemotherapy in the adjuvant setting since such a study will have no chance of gaining ethical review committee approval; therefore, only a retrospective analysis is possible. Second, internal validation of 40% and 80% of the samples were done and found similar and repeatable results. As we know, even in patients with stage III disease, the adjuvant chemotherapy rejection rate is as high as 35–40%. The results of this study suggest that patients with KRAS mutant tumors should undergo adjuvant treatment, especially those with stage III CRC. Thus, we encourage routine checking of KRAS status in patients with stage II and III CRC.
The limitations of the study are as follows. First, this was a single center retrospective study. Second, the comparison of patients not receiving adjuvant chemotherapy to those who did has the potential to introduce bias. Therefore, prospective studies are warranted to verify KRAS as well as NRAS mutation status as predictive biomarkers for FOLFOX treatment response in patients with stage II and III CRC.
Conflict of interest
None declared.
Supporting information
The following is the supplementary data related to this article:
Supplementary data
Acknowledgments
The study was supported by the China National Natural Science Foundation (No. 81472249) and a Guangzhou Scientific Grant (2014J2200059).
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2015.03.006.
Deng Yanhong, Wang Li, Tan Shuyun, Kim George P., Dou Ruoxu, Chen Dianke, Cai Yue, Fu Xinhui, Wang Lei, Zhu Jun, Wang Jianping, (2015), KRAS as a predictor of poor prognosis and benefit from postoperative FOLFOX chemotherapy in patients with stage II and III colorectal cancer, Molecular Oncology, 9, doi: 10.1016/j.molonc.2015.03.006.
Contributor Information
Yanhong Deng, Email: dengyanh@mail.sysu.edu.cn.
Jianping Wang, Email: wangjpgz@126.com.
References
- Andreyev, H.J. , Norman, A.R. , Cunningham, D. , 2001. Kirsten ras mutations in patients with colorectal cancer: the 'RASCAL II' study. Br. J. Cancer 85, (5) 692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreyev, H.J. , Norman, A.R. , Cunningham, D. , Oates, J.R. , Clarke, P.A. , 1998. Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J. Natl. Cancer Inst. 90, (9) 675–684. [DOI] [PubMed] [Google Scholar]
- Bokemeyer, C. , Van Cutsem, E. , Rougier, P. , 2012. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur. J. Cancer 48, (10) 1466–1475. [DOI] [PubMed] [Google Scholar]
- Chua, Clarinda Wei Ling , Chong, Dawn Q.Q. , Kanesvaran, Ravindran , Tai, Wai Meng David , Tham, Chee Kian , Tan, Patrick , Earnest, Arul , Tan, Iain B. , 2014. The prognostic impact of KRAS mutation in colorectal cancer patients: a meta-analysis of phase III clinical trials. J. Clin. Oncol. 32, (suppl) abstr e14515 [Google Scholar]
- Douillard, J.Y. , Oliner, K.S. , Siena, S. , 2013. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 369, (11) 1023–1034. [DOI] [PubMed] [Google Scholar]
- Gnanasampanthan, G. , Elsaleh, H. , McCaul, K. , Iacopetta, B. , 2001. Ki-ras mutation type and the survival benefit from adjuvant chemotherapy in Dukes' C colorectal cancer. J. Pathol. 195, (5) 543–548. [DOI] [PubMed] [Google Scholar]
- Hutchins, G. , Southward, K. , Handley, K. , 2011. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J. Clin. Oncol. 29, (10) 1261–1270. [DOI] [PubMed] [Google Scholar]
- Imamura, Y. , Morikawa, T. , Liao, X. , 2012. Specific mutations in KRAS codons 12 and 13, and patient prognosis in 1075 BRAF wild-type colorectal cancers. Clin. Cancer Res. 18, (17) 4753–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D.W. , Kim, K.J. , Han, S.W. , 2015. KRAS mutation is associated with worse prognosis in stage III or high-risk stage II colon cancer patients treated with adjuvant FOLFOX. Ann. Surg. Oncol. 22, (1) 187–194. [DOI] [PubMed] [Google Scholar]
- Ogino, S. , Meyerhardt, J.A. , Irahara, N. , 2009. KRAS mutation in stage III colon cancer and clinical outcome following intergroup trial CALGB 89803. Clin. Cancer Res. 15, (23) 7322–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters, M. , Douillard, J.Y. , Van Cutsem, E. , 2013. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J. Clin. Oncol. 31, (6) 759–765. [DOI] [PubMed] [Google Scholar]
- Richman, S.D. , Seymour, M.T. , Chambers, P. , 2009. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J. Clin. Oncol. 27, (35) 5931–5937. [DOI] [PubMed] [Google Scholar]
- Roth, A.D. , Tejpar, S. , Delorenzi, M. , 2010. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J. Clin. Oncol. 28, (3) 466–474. [DOI] [PubMed] [Google Scholar]
- Samowitz, W.S. , Curtin, K. , Schaffer, D. , Robertson, M. , Leppert, M. , Slattery, M.L. , 2000. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol. Biomarkers Prev. 9, (11) 1193–1197. [PubMed] [Google Scholar]
- Schmoll, H.J. , Twelves, C. , Sun, W. , 2014. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol. 15, (13) 1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCGA, 2012. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, (7407) 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem, E. , Kohne, C.H. , Hitre, E. , 2009. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 360, (14) 1408–1417. [DOI] [PubMed] [Google Scholar]
- Wang, C. , van Rijnsoever, M. , Grieu, F. , 2003. Prognostic significance of microsatellite instability and Ki-ras mutation type in stage II colorectal cancer. Oncology 64, (3) 259–265. [DOI] [PubMed] [Google Scholar]
- Yoon, H.H. , Tougeron, D. , Shi, Q. , 2014. KRAS codon 12 and 13 mutations in relation to disease-free survival in BRAF-wild-type stage III colon cancers from an adjuvant chemotherapy trial (N0147 alliance). Clin. Cancer Res. 20, (11) 3033–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following is the supplementary data related to this article:
Supplementary data


