Abstract
Context
Evidence is inconclusive whether a nurse consultation can improve osteoporosis-related patient outcomes.
Objective
To evaluate whether a nurse consultation immediately after dual-energy x-ray absorptiometry (DXA) produced better osteoporosis-related outcomes than a simple intervention to activate adults in good bone health practices or usual care.
Design
Pilot randomized controlled trial, conducted within the larger Patient Activation After DXA Result Notification (PAADRN) trial (NCT01507662). After DXA, consenting adults age 50 years or older were randomly assigned to 3 groups: nurse consultation, PAADRN intervention (mailed letter with individualized fracture risk and an educational brochure), or usual care (control). Nurse consultation included reviewing DXA results, counseling on bone health, and ordering needed follow-up tests or physician referrals.
Main Outcome Measures
Change from baseline to 52 weeks in participant-reported osteoporosis-related pharmacotherapy, lifestyle, activation and self-efficacy, and osteoporosis care satisfaction.
Results
Nurse consultation participants (n = 104) reported 52-week improvements in strengthening and weight-bearing exercise (p = 0.09), calcium intake (p < 0.01), osteoporosis knowledge (p = 0.04), activation (p < 0.01), dietary self-efficacy (p = 0.06), and osteoporosis care satisfaction (p < 0.01). Compared with PAADRN intervention participants (n = 39), nurse consultation participants had improved dietary self-efficacy (p = 0.07) and osteoporosis care satisfaction (p = 0.05). No significant improvements in osteoporosis-related outcomes were achieved vs PAADRN controls (n = 70).
Conclusion
“Just-in-time” nurse consultation yielded a few improvements over 52 weeks in osteoporosis-related outcomes; however, most changes were not different from those obtained through the lower-cost PAADRN intervention or usual care.
INTRODUCTION
There are a number of steps between a patient receiving a screening test for disease—such as a dual-energy x-ray absorptiometry (DXA) test for assessment of bone density and diagnosis of osteoporosis—and when the screened patient is informed of results and started on a treatment plan to address related health issues. Between each step, there is potential for a “care gap,” which, singly or in the aggregate, may result in suboptimal changes to treatment and health outcomes that the screening test is intended to initiate.1
In the case of DXA tests, a radiology technologist or technician administers the test but does not interpret results or initiate a treatment plan. Results are forwarded to a physician (typically a radiologist or rheumatologist) for interpretation. That step might take several days. The interpretation is then frequently forwarded to the ordering physician, typically a patient’s primary care physician (PCP), who is responsible for ensuring that the patient is notified of the results and, if needed, for developing a treatment plan. These additional steps may take days or weeks. Each delay dilutes the effectiveness of what might have been a “teachable moment”—completion of the DXA, especially in terms of immediacy of results and an opportunity to inform and guide the patient on next steps in osteoporosis prevention or treatment.
Integrated health care delivery systems are well positioned to implement cost-effective solutions to closing these care gaps by “[g]etting work done by the right person at the right time.”2pS459 Nonphysician clinicians, such as nurses (registered and advanced practice), dietitians, and clinical pharmacists, often have scopes of practice, training, and clinical experience that would allow them to assist physicians in diagnosis and treatment of patients for maintaining good bone health.1,3
Greater involvement of nurses in osteoporosis-related care has involved a range of intervention strategies focusing on the following: 1) extent of role integration (standalone vs part of a multidisciplinary team); 2) mode of delivery (one-on-one patient consultations by visit or by telephone, patient groups); 3) clinical outcomes which are the focus of protocol (antiresorptive therapy, patient dietary intake, or exercise frequency); and 4) targeted patient population (young women, postmenopausal women, patients with confirmed osteoporosis, men and women).4–20 Evaluation designs for assessing impact of these nurse interventions include pre-/postintervention designs with or without controls, as well as randomized controlled trials (RCTs). Durations of health outcome assessments range from several months to a year or more. Evidence is inconclusive whether the nurse role can be adapted to close care gaps in osteoporosis-related care and to improve patient outcomes.
We conducted a pilot RCT to evaluate whether a registered nurse, working under the supervision of a rheumatologist, could improve patient adherence to behaviors that promote good bone health. The nurse was available immediately after DXA (“just in time”) and had a scope of practice that allowed for reviewing DXA results, counseling patients on self-management strategies and lifestyle to address osteoporosis and maintain good bone health, and entering orders (if needed) for additional diagnostic tests as well as follow-up visits to the patient’s PCP or other specialists. The intervention goal was to achieve improvements in guideline-concordant, osteoporosis-related pharmacotherapy, frequency of weight-bearing and strengthening exercise, calcium and Vitamin D intake, activation and osteoporosis self-efficacy related to diet and exercise, and satisfaction with osteoporosis care during a one-year period, compared with similar participants enrolled in either the Patient Activation After DXA Result Notification (PAADRN) trial (NCT01507662) intervention or usual care (control) group.
METHODS
Study Setting and Population
This nurse consultation pilot RCT was conducted at Kaiser Permanente Georgia (KPGA) within the context of the larger, multisite PAADRN study, a double-blinded, parallel, pragmatic RCT.21 The PAADRN protocol was reviewed, approved, and monitored by the institutional review board of each of the three participating sites. In addition, the nurse consultation pilot RCT protocol was reviewed, approved, and monitored by the KPGA institutional review board.
Eligible patients were KPGA members age 50 years or older who were scheduled for DXA between March 2013 and May 2014 at KPGA’s Crescent Center medical facility. Consistent with the PAADRN protocol, we excluded patients who were unable to read, speak, or understand English; prisoners; and those unable to provide informed consent because of perceived cognitive disabilities. Informed consent was obtained from all KPGA participants before we initiated the baseline interview.
Randomization and Allocation
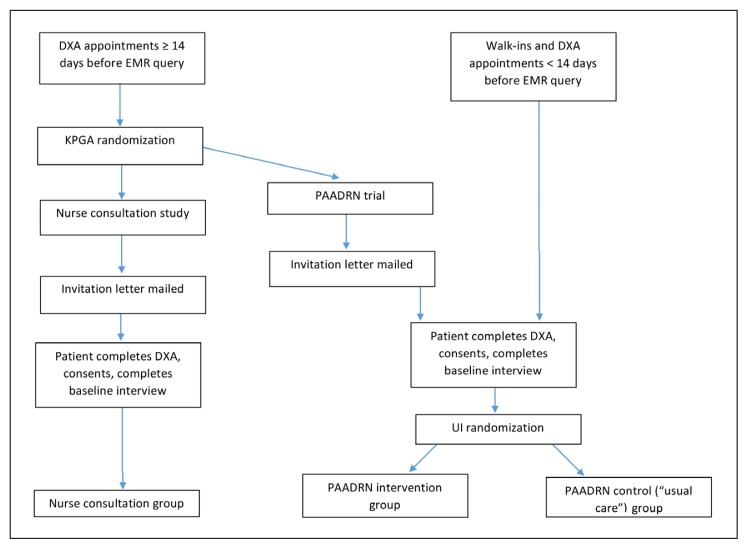
Randomization and allocation procedures for the nurse consultation study were coordinated with PAADRN study procedures (Figure 1). Each day during the study period, the DXA schedule was queried to identify eligible members who had DXA scheduled in the subsequent 2-week window on a day the nurse consultant was also scheduled to be available. Those members were randomly assigned at KPGA to the nurse consultation study group or to the PAADRN study using the random number function of analytic software (SAS 9.4, SAS Institute Inc, Cary, NC). Randomization was designed to yield approximately a 2:1 (pilot RCT study:PAADRN study) ratio and a sample size of approximately 150 participants receiving nurse consultation. Among those allocated to PAADRN at Crescent Center, randomization to the PAADRN intervention or usual care group was done at the University of Iowa coordinating center following standard PAADRN procedures.
Figure 1.
Participant contact, recruitment, and allocation process.
DXA = dual-energy x-ray absorptiometry; EMR = electronic medical record; KPGA = Kaiser Permanente Georgia; PAADRN = Patient Activation After DXA Result Notification; UI = University of Iowa.
Randomization and allocation to the nurse consultation pilot RCT or to PAADRN occurred before mailing an invitation letter to eligible KPGA members. This was necessary because the composition of the invitation letter needed to describe the specific time requirements, risks, and benefits of the nurse consultation pilot RCT (eg, allowing time for a baseline interview plus a consultation session after DXA completion) compared with PAADRN (eg, allowing time only for a baseline interview). Thus, from invitation through baseline interview, study assignment to the nurse consultation pilot RCT or PAADRN was not concealed to patients or project staff. Among PAADRN participants, however, allocation to the intervention or usual care (control) group (described in the “PAADRN Protocol” section) occurred after the baseline interview and was concealed to patients and project staff.
At approximately 52 weeks after the baseline interview, a subsequent interview was conducted by telephone by trained staff at the University of Iowa coordinating center. These interviewers were blinded to patients’ allocation to the 3 study groups (ie, nurse consultation, PAADRN intervention, PAADRN usual care).
Recruitment
After randomization, letters inviting participation in the nurse consultation pilot RCT or PAADRN study were mailed to patients’ home addresses approximately 7 to 10 days before the DXA appointment. Both letters stated the voluntary nature of study participation. The PAADRN study letter indicated that consent and 15 to 20 minutes after the DXA would be required for an interview with a research assistant. The nurse consultation pilot RCT letter indicated that consent and 30 to 45 minutes after the DXA would be required for the research assistant interview plus the nurse consultation. This letter also briefly described the nurse consultation. Both letters indicated that a $20 gift card would be provided to compensate for a participant’s time. The nurse consultation pilot RCT letter further indicated that the nurse consultation would not require a copayment, which is typically required of a nurse educator visit. Eligible participants who did not specifically decline participation were called by a research assistant approximately 2 days before the appointment either to ascertain interest or to remind them of the research appointment and procedures after their DXA.
Nurse Consultation Procedures
The nurse consultant for the pilot RCT was a senior licensed registered nurse who had practiced for many years with KPGA both in primary care and administration. Before conducting consultations for the pilot RCT, she shadowed the supervising physician (a senior KPGA rheumatologist) during his office visits with patients who presented for initial treatment of osteoporosis.
GUIDELINES FOR PHYSICIAN OVERSIGHT AND NURSE EDUCATION CONSULTATION IN THE PATIENT ACTIVATION AFTER DXA RESULT NOTIFICATION (PAADRN) STUDY: OSTEOPOROSIS MANAGEMENT DELEGATED TO REGISTERED NURSE.
A. OVERVIEW OF THE ENCOUNTER PROTOCOL
The purpose of the protocol is to provide guidelines for physician oversight of a registered nurse (RN) who will provide educational consultation to patients following a dual-energy x-ray absorptiometry (DXA). The RN, using standardized procedures and protocols, will be responsible to educate and to manage research study participants for both prevention and primary treatment of osteoporosis. Procedures and protocols for screening, diagnosis, and referrals for follow-up care will be consistent with the implementation of the Kaiser Permanente (KP) Georgia National Clinical Practice Guidelines: “Osteoporosis/Fracture Prevention Clinical Practice Guideline Summary.”1
B. PURPOSE OF THE ENCOUNTER PROTOCOL
The RN is delegated to perform all the medical acts in the protocol without the direct or immediate observation, supervision, or approval of the delegating physician. Immediate consultation with the delegating physician or his/her designee is available at all times. Critical laboratory results will be communicated to the delegating physician or his/her designee the same day received by the RN. Supervision will be provided by the delegating physician. Each chart will be forwarded on the day of the encounter to the delegating/supervising physician and each order will be co-signed by the delegating physician.
C. DETAILS OF THE ENCOUNTER PROTOCOL
1. Assessing osteoporosis during the RN consultation
Diagnosis of normal bone mass, low bone mass/osteopenia, or osteoporosis is made by the RN after review of clinical history, physical findings, and results of the DXA scan. The RN will initiate therapy on the basis of the treatment plan listed below and recommendations of supervising the physician.
Clinical history/physical findings:
Review DXA scan results and risk factors using Fracture Risk Assessment Tool (FRAX) model. Information should be obtained regarding risk factors including, but not limited to:
age
height/decreased height/weight
smoking/alcohol history
diet/history of poor calcium/Vitamin D intake
exercise level/tolerance/mobility
personal/family history fracture
personal/family history osteoporosis
history of osteoporosis medication
previous DXA results
pre-/postmenopause
low testosterone
kyphosis/back pain
fall assessment (history falling to ground past 6 months, review medication for balance risks)
history of medications that increase fracture risk (ie, leuprolide [Lupron], chemotherapy)
history of corticosteroid use (5 mg/d prednisone or equivalent for > 3 mos)
history of anticonvulsants, blood thinners, proton pump inhibitors, selective serotonin reuptake inhibitors, antacids
current medications/vitamins
drug allergies
pregnancy/nursing
hypocalcemia
dysphagia/reflux
history of hypertension, diabetes mellitus, chronic kidney disease, asthma
history of hyperthyroidism, anorexia, organ transplant, gastric bypass
history of liver disease
history of rheumatoid arthritis.
2. Education on osteoporosis and fracture prevention
Define and review pathophysiology of osteoporosis.
Review consequences of untreated osteoporosis and steps to mitigate osteoporosis
Review DXA results and fracture risk
-
Recommend calcium carbonate (OsCal-Calcium, Tums, Caltrate)
- Premenopausal: 1000 mg (elemental calcium) daily with food
- Postmenopausal: 1200 mg (elemental calcium) daily with food
- Men over 50 years of age: 1200 mg (elemental calcium) daily with food
- Do not exceed 1500 mg/d
- Use calcium carbonate/citrate/phosphate. Bone meal/dolomite/oyster shell may contain lead
- Take calcium carbonate with meals, but no more than 500 mg at one time
- Take with 8 oz water or juice
- Take with Vitamin D
- Separate calcium from other medicines, iron, zinc, or folic acid by 2 hours (thyroid by 4 hours)
- Avoid other antacids. Limit aspirin and nonsteroidal anti-inflammatory drugs
- Wheat bran, whole grain cereals, and foods high in oxalates decrease absorption of calcium
- Take calcium 1 hour before/2 hours after high-fiber meal
- Increase exercise, fluids, fiber, and fruit to avoid constipation
Recommend calcium citrate (Citracal, Calcitrate) for decreased gastric acidity (elevated pH), H2 receptor antagonist/proton pump inhibitor use, or bariatric surgery. Take calcium citrate on an empty stomach with 8 oz water
-
Recommend vitamin D3
- Pre-/postmenopausal women and men over 50 years of age: 1000 IU/d
- Do not take with cholestyramine, mineral oil, magnesium-containing antacids, orlistat, cimetidine (Tagamet), or vitamin A
-
Diet
- Food sources high in calcium include dairy, dark green vegetables, beans, and calcium-fortified orange juice
- Decrease caffeine, soda, sodium, and alcohol
-
Exercise
- Advise regular weight-bearing and muscle-building exercise daily
- Discuss exercise program with primary care physician (PCP)
-
Tobacco
- Advise tobacco cessation
- Refer to PCP and/or smoking cessation class
-
Regular eye examinations
- Maintain visual acuity to assist in fall prevention
-
Home safety proofing
- Advise assessment/removal of rugs
- Advise installation of grab bars as needed
- Advise use of nightlights/adequate lighting
- Advise securing electrical cords
-
Patient handouts
- Osteoporosis Treatment/Prevention Instructions (for patient after visit summary)
- Osteoporosis handouts from KP Clinical Library (all Regions) optional
- Osteoporosis handouts from National Osteoporosis Foundation and National Institute of Arthritis and Musculoskeletal and Skin Diseases optional
- Lexi-Patient Education (Lexicomp) handouts optional.
3. Laboratory work
Before recommending/initiating drug therapy, consider orders (if not done in past 12 months) for 25-hydroxyvitamin D, calcium, creatinine, Vitamin D, or as recommended by the supervising physician
For suspected secondary osteoporosis (Z-score -2.0 or lower) recommended workup will be provided to the RN by the supervising physician on case-by-case basis
-
Vitamin D assays for deficiency:
- If 21–30 ng/mL, recommend ergocalciferol 50,000 IU orally 3 times/wk for 4 weeks, then 1000 IU/d
- If < 20 ng/mL, postpone bisphosphonate until after prescription above.
4. Medications
-
Recommend initiation of osteoporosis medication for:
- Postmenopausal women with a history of fragility fracture
- Women aged 65 or older with T-score -2.5 or less
- Postmenopausal women with a FRAX 10-year risk of hip fracture 3% or greater or major osteoporotic fracture of 20% or higher. If T-score is below -1.0 but above -2.5, use FRAX score
- Optional for postmenopausal women younger than age 65 years or men younger than age 70 years with T-score -2.5 or lower, but without FRAX hip 3%/major osteoporotic fracture 20% or greater
- Men age 70 years or older with prior fragility fracture, T-score -2.5 or lower, or FRAX hip 3%/major osteoporotic fracture 20% or greater
- Steroid use 3 months or more and FRAX hip 3%/major osteoporotic fracture 20% or greater
Order PCP consultation for medication initiation and refills. All medications will be provided by member’s PCP
Present if requested and only after supervising physician consultation, with medication treatment options based on patient history and assessment of contraindications
If patient is receiving medication or if medication orders are pending, stress compliance with long-term therapy to reduce fracture risk
If patient is receiving medication or if medication orders are pending, instruct patient to report side effects/symptoms of medication; give handout. Instruct to stop medication and call PCP or 911 immediately for symptoms of anaphylaxis (itching/hives, swelling of face/hands/mouth/throat, tingling of mouth/throat, tightness in chest, trouble breathing), unusual/severe stomach pain, or jaw pain
Assess for treatment contraindications and secondary osteoporosis. Consult with/refer to endocrinology/rheumatology: hypocalcemia, chronic kidney disease Stage 4 or 5/last glomerular filtration rate (GFR) < 30, severe gastroesophageal reflux disease, pregnancy/nursing, difficulty swallowing or cannot stay upright for at least 30 minutes, current use of cancer chemotherapeutic agent or anticonvulsants, history/consideration of organ transplant, bariatric surgery, inability to follow instructions, poor prognosis, suspected secondary cause (Z-score -2.0 or lower), fracture during osteoporosis treatment/atypical or low-impact subtrochanteric stress fracture, contraindications/intolerances to first- or second-line therapies, premature menopause, history of corticosteroid use, history of gonadotropin-releasing hormone/leuprolide (Lupron) therapy or medroxyprogesterone (Depo-Provera) use, or hyperthyroidism.
5. Medication options for women
-
FIRST-LINE therapies
-
- Alendronate (Fosamax)
♦ Dose: 70 mg/wk; take with 8 oz water 30 min before activity/drink/medications; upright 30 minutes
♦ Contraindications: Women of childbearing age without contraception, GFR < 30–35, Vitamin D level 30 ng/mL or less, hypocalcemia, teeth/gum problems
♦ Precautions: Esophageal disease, gastritis, ulcers
-
- Risedronate (Actonel)
♦ Nonformulary alternative when alendronate is contraindicated or not tolerated
♦ Dose: 5 mg/d or 35 mg/wk; take with 8 oz water 30 minutes before activity/drink/medications; upright 30 minutes
♦ Contraindications: Women of childbearing age without contraception, GFR < 30–35, Vitamin D level 30 ng/mL or less, hypocalcemia, teeth/gum problems
-
-
SECOND-LINE therapies are used when first-line agents are contraindicated or cannot be tolerated. The following second-line therapies have not been shown to significantly reduce nonvertebral fractures of the hip or wrist, however. Consider specialist referral.
-
- Raloxifene (Evista)
♦ Option for postmenopausal women with low risk for thrombotic complications
♦ Option for women at high risk for breast cancer
♦ Dose: 60 mg/d orally without regard to meal
♦ Contraindications: Active/history thromboembolism, increased risk of stroke
♦ Precautions: Discontinue 72 hours before prolonged bedrest; avoid one position for long period. Report swelling, warmth, pain in calves
-
- Ibandronate (Boniva)
♦ Nonformulary option for postmenopausal women older than age 65 years with prior vertebral fracture
♦ Dose: 2.5 mg/d orally or 150 mg/mo; take 60 minutes before food/drink/medications; sit 60 minutes
♦ Precautions: See bisphosphonates
-
- Nasal calcitonin (Miacalcin)
♦ Nonformulary option for postmenopausal women older than age 65 years with prior vertebral fracture
♦ Dose: 200 IU/d (1 spray) alternating nostrils (activate pump before first dose)
♦ Precautions: Osteogenic sarcoma, pernicious anemia, renal disease, hypersensitivity to salmon protein or gelatin diluent
-
-
THIRD-LINE therapies for postmenopausal at high risk of fracture when first- and second-line therapies contraindicated or not tolerated. Refer to specialist.
-
- Zoledronic acid (Zometa)
♦ Nonformulary option for postmenopausal women older than age 65 years with high risk and prior vertebral fracture
♦ Hypercalcemia associated with malignancy
♦ Dose: 5 mg intravenously annually
♦ Precautions: Renal dysfunction, asthma
-
- Teriparatide (Forteo) (recombinant parathyroid hormone)
♦ Nonformulary option for high-risk women not tolerant of or responsive to other agents
♦ Dose: 20 μg/d subcutaneously in thigh/abdomen wall
♦ Contraindications: Paget disease, elevated alkaline phosphatase, open epiphyses, prior external beam or implant radiation involving the skeleton
♦ Precautions: Recent urolithiasis, digitalis; treatment should not exceed 18–24 months.
-
6. Medication options for men
-
Alendronate, 10 mg/d or 70 mg/wk
- For men age 70 years or older with prior fracture, osteoporosis/T-score -2.5 or less, or FRAX 3%/20% or greater
- Optional for men under age 70 with osteoporosis/T-score -2.5 or less, without FRAX 3%/20%.
7. Medication management for women and men taking corticosteroids
Men/women on prednisone 5 mg (or equivalent) for 3 months or greater and with FRAX 3%/20% or greater. Refer to specialist.
First-line: Alendronate 10 mg/d or 70 mg/wk, risedronate 5 mg/d or 35 mg/wk.
Second-line: Teriparatide for glucocorticoid-treated patients intolerant of or responsive to other agents.
8. Hormone Therapy
Initiating/continuing hormone therapy solely for the prevention of osteoporosis is not recommended.
D. ELECTRONIC MEDICAL RECORD DOCUMENTATION OF THE ENCOUNTER
Progress notes will be completed on all patient contacts and documented in the medical record. HealthConnect chart documentation will include chief complaint = “Clinical Research Study,” supervision type = “Clinical Trial” (vs RN Supervision), and primary diagnosis = “V70.7A Clinical Trial Participant Examination”
Diagnosis and KP codes will be coded in HealthConnect and documented in the progress note. (Medicare requires osteoporosis diagnosis must be in progress note and note must indicate some level of evaluation/treatment performed during encounter)
PCP will be notified of assessment findings and treatment electronically via HealthConnect progress note
Patients who cannot be managed under protocol will have a notation in the chart by both the RN and the supervising physician. Reasons for protocol deviation and off-protocol treatment plan will be documented.
Reference
- 1.Osteoporosis/fracture prevention clinical practice guideline summary [Internet] Oakland, CA: Kaiser Permanente Medical Care Program: Care Management Institute; 2010. Nov, [cited 2017 Jun 14]. Available from: https://providers.kaiserpermanente.org/info_assets/cpp_oh/oh_osteoporosisfracturepreventionguidelinesummary_dec2013.pdf. [Password protected.] [Google Scholar]
The nurse consultant was responsible for counseling pilot RCT participants for both prevention and primary treatment of osteoporosis after the DXA. She reviewed the patient’s medical history with attention to indications of fracture risk (including DXA T-scores) and provided general education about lifestyle (ie, dietary calcium, supplemental Vitamin D, strengthening and weight-bearing exercise) to maintain good bone health and to reduce fracture risk. She was provided with a National Institutes of Health brochure on effective osteoporosis self-management practices to distribute to patients at the time of consultation.22 After each visit, the visit was documented in the KPGA electronic medical record (EMR) as a “research visit” using an Epic SmartSet (Epic Systems Corp, Verona, WI) to help organize and standardize each visit’s documentation. At the end of each day, the nurse consultant’s EMR visit notes, including the DXA scan, and recommended orders were reviewed by the supervising physician. The nurse consultant also had discretion to phone the patient the following day and provide an additional limited phone consultation regarding the DXA results and treatment recommendations of the supervising physician.
The nurse consultant was delegated to perform specific medical acts in accordance with an approved, written clinical protocol without the direct or immediate observation, supervision, or approval of the delegating physician; however, immediate consultation with the supervising physician or his designee was available at all times. A clinical protocol for conducting the nurse consultation was developed by the research team and then reviewed and approved by physicians with the KPGA Preventive Medicine and Rheumatology Departments (see Sidebar: Guidelines for Physician Oversight and Nurse Education Consultation in the Patient Activation after DXA Result Notification [PAADRN] Study: Osteoporosis Management Delegated to Registered Nurse). Procedures and protocols for screening, diagnosis, and referrals for follow-up care were consistent with KPGA clinical practice guidelines: “Osteoporosis/Fracture Prevention Clinical Practice Guideline” (November 2008, November 2010) and its “Guideline Revision Summary” (2008–2010).
Tasks delegated to the nurse consultant included, but were not limited to, entering orders for appropriate laboratory and other diagnostic tests, and entering orders for other services, such as physician visits for further treatment and evaluation. Ordering prescription medications was specifically excluded from delegated medical acts.
The supervising physician provided oversight to the nurse consultant’s training, order entries, and visit documentation. He ensured that the nurse consultant was aware of regulatory requirements and acceptable standards for osteoporosis screening, diagnosis, and treatment. He met with the nurse consultant on a regular basis to review any national or KPGA-specific changes to osteoporosis-related protocols or procedures. At the end of each day, he reviewed and signed orders made by the nurse consultant. If he considered some orders to be incorrect or unnecessary, he informed the nurse consultant, including reasons for not approving the order. He also entered into the KPGA EMR any additional orders for patients as needed.
The KPGA EMR facilitated implementation of the study protocol and coordination of care between the nurse consultant and the supervising physician. Components of the protocol were implemented as a template in the EMR to standardize documentation. The EMR allowed entry of orders for approved services and rapid review of orders and consultation notes by the supervising physician.
PAADRN Protocol
Participants of PAADRN who were randomly assigned to the PAADRN intervention group received directly from the University of Iowa a mailed letter with individualized fracture risk and a bone health education brochure within approximately four weeks of the DXA. The letter described results of their DXA (lowest T-score) and interpretation (osteoporosis, low bone mineral density [osteopenia], or normal) and presented a graphic portrayal of the ten-year probability of sustaining a major osteoporotic fracture, calculated by the FRAX Fracture Risk Assessment Tool (available at www.shef.ac.uk/FRAX/). These materials are described elsewhere.23–25 The PAADRN intervention participants also received the usual KPGA mode of communicating DXA results and action steps, described in the next paragraph.
Participants who were randomly assigned to the control group received “usual care.” Communication of DXA results to controls followed a more circuitous route than the direct-to-patient, tailored letter received by intervention participants. After DXA, a rheumatologist’s clinical impression would be forwarded to the ordering practitioner, who might further review and possibly annotate the rheumatologist’s impression before eventually forwarding the summary interpretation and recommendations (if any) to the patient.
Members of KPGA assigned to the nurse consultation pilot RCT were excluded from participation in the PAADRN study. Thus, participants in the nurse consultation pilot RCT did not receive the direct-to-patient, tailored letter on fracture risk with an accompanying educational brochure.
Measures
Guideline-Concordant Pharmacotherapy
The primary PAADRN end point was guideline-concordant pharmacologic treatment at 52 weeks. The algorithm for determining guideline-concordant pharmacologic treatment was based on the 2010 National Osteoporosis Foundation guidelines in effect at the time of the PAADRN study26 and is described in detail elsewhere.27 Essentially, the algorithm is based on the 2-by-2 cross-classification of whether the patient was taking osteoporosis pharmacologic treatment (bisphosphonates, calcitonin, estrogen/hormone therapy, estrogen agonist/antagonist, parathyroid hormone, or denosumab) and whether this was guideline-concordant. In this pilot RCT, guideline-concordant pharmacotherapy was measured only at 52 weeks after enrollment.
Combined Exercise Frequency
Combined exercise frequency at baseline and at 52 weeks was assessed from 2 items: “In the past 30 days, how many times per week were you engaged in aerobic activity?” and “In the past 30 days, how many times per week were you engaged in strength training?” Examples of aerobic activity and strength training were provided with each item. Response categories were none, 1 to 2, 3 to 4, or 5 or more times per week. These categories were weighted 0, 1.5, 3.5, and 5, respectively. A combined exercise score was the sum of the weighted values and resulted in possible scores of: 0, 1.5, 3, 3.5, 5, 6.5, 7, 8.5, and 10. This score represents the relative number of sessions per week during which the recommended activities occurred.
Total Calcium
Total calcium (mg/d), at baseline and 52 weeks, was estimated from responses to food sources (4 items), calcium supplements (1 item), and daily multiple vitamins (1 item).28 Calcium from food sources was assessed by frequency (0–1, 2–3, 4–6, 7 or more units) per week for cups of milk, ounces of cheese, servings of yogurt, and cups of calcium-fortified beverages. Midpoints of response categories for fewer than 7 d/wk were used for intake estimation. The 7 d/wk category was scored as 7. Each amount was multiplied by the following quantities to obtain an estimated calcium intake: 300 mg per milk serving, 200 mg/oz (200 mg/28 g) cheese, 300 mg per yogurt serving, 80 mg/cup (80 mg/0.24 L) serving of calcium-fortified beverage. The sum of these quantities was divided by 7 to get an average estimate of milligrams per day. Two other survey items assessed calcium from supplements: “Do you take calcium supplements?” and “Do you take a daily multiple vitamin?” To the milligrams-per-day estimate from food sources, a “Yes” response to these items added 250 mg/d and 75 mg/d, respectively.
Supplemental Vitamin D
Vitamin D supplementation was assessed by the item assessing daily multiple vitamin use. The use of supplemental Vitamin D at baseline and 52 weeks was binary coded.
Osteoporosis Knowledge
We used the 10-item (true/false) scale of the “Osteoporosis and You” questionnaire to measure osteoporosis knowledge at baseline and at 52 weeks.29,30 Each item was a 5-response Likert scale (“strongly agree” to “strongly disagree”) that we recoded into correct (assigned a value of “1”) or incorrect (including a neutral response; assigned a value of “0”) knowledge of osteoporosis. Correct responses (for true statements, or strongly disagree or disagree for false statements) were coded “1” and incorrect responses were coded “0.” We summed the recoded values to the 10 items to create a summary score ranging from 0 to 10 (PAADRN baseline α = 0.68).
Activation
We used 6 of the 22 items from the Patient Activation Measure to measure general patient knowledge, confidence, and self-efficacy related to health at baseline and 52 weeks.31,32 Each item has 4 response options ranging from “strongly agree” to “strongly disagree.” Item responses were initially recoded from “1” (lowest activation) to “4” (highest activation), including reverse coding of responses where needed (PAADRN baseline α = 0.66). Item scores were summed and then subsequently converted to a score ranging from 0 (least activated) to 100 (most activated) following the same approach used for the 13-item shortened version of the Patient Activation Measure.
Osteoporosis Self-Efficacy
Osteoporosis self-efficacy (OSE) at baseline and 52 weeks was measured with 2 subscales: exercise (OSE-Exercise, 10 items, PAADRN baseline α = 0.97) and diet (OSE-Diet, 11 items, PAADRN baseline α = 0.96).33 These items represent attitudes toward initiation, maintenance, and persistence of osteoporosis-related behaviors. Each item was a 10-point Likert scale, ranging from 1 (“not at all confident”) to 10 (“very confident”). The OSE-Exercise and OSE-Diet subscale scores were each computed as the mean of the component item response scores.
Osteoporosis Care Satisfaction
Osteoporosis care satisfaction was measured both at baseline and 52 weeks for participants with prior DXA scans.34 This 5-item scale assessed patient satisfaction with DXA notification, understanding DXA results, understanding osteoporosis treatments, receiving adequate information to make an informed decision, and overall satisfaction with bone health care (PAADRN baseline α = 0.77). Each item was a 5-point Likert scale, ranging from 1 (“strongly dissatisfied”) to 5 (“strongly satisfied”). The scale score was the sum of the item scores, ranging from 5 to 25.
Participant Characteristics
At baseline, the following patient characteristics were collected: age, sex, race/ethnicity, educational attainment, literacy and numeracy, prior DXA, self-reported health status, DXA T-scores, height and weight, and comorbidities related to computing the FRAX score.
Statistical Analysis
To assess balance in the 3 study groups at baseline, we compared participant characteristics of pilot RCT participants at KPGA’s Crescent Center medical office with 1) PAADRN intervention participants and 2) PAADRN usual care participants who were also recruited during the duration of the pilot RCT at KPGA’s Crescent Center medical office (ie, between March 2013 and May 2014). Baseline characteristics were compared using a χ2 or t-test as appropriate to the measure’s distribution.
We assessed 52-week change for the 9 outcomes (ie, guideline-concordant pharmacotherapy, total calcium, supplemental Vitamin D, combined exercise, osteoporosis knowledge, activation, OSE-Diet, OSE-Exercise, osteoporosis care satisfaction) within each of the 3 study groups. We were primarily interested in change from baseline to 52 weeks in the outcome measures for pilot RCT participants. The null hypothesis was no significant difference between baseline and 52 weeks for participants in each group, and the alternative hypothesis was a significant improvement within each group.
We then assessed pairwise differences in 52-week change on each outcome between pilot RCT participants and either 1) PAADRN intervention participants or 2) PAADRN usual care participants. The null hypothesis was no significant difference in change between pilot RCT participants and either of the PAADRN study groups; the alternative hypothesis was a significant improvement in the nurse consultation group compared with either one of the PAADRN study groups.
This was a pilot RCT and not fully powered statistically at the level of the much larger PAADRN study; therefore, we initially considered any within- or between-group difference with p ≤ 0.10 to be statistically significant. We chose 2-tailed vs 1-tailed statistical tests to allow for bidirectional change in study outcomes because of recent concerns about 1) overuse of bisphosphonates among adults with low risk of fragility fracture35 and 2) excessive calcium intake particularly through use of supplements.36 Because we were making many pairwise comparisons within the nurse consultation group (ie, 52 weeks vs baseline) and between treatment groups (ie, pilot RCT vs PAADRN intervention or pilot RCT vs usual care) on 9 outcomes, we subsequently used a Bonferroni adjustment37 for each of these sets of comparisons to lower the critical p value from ≤ 0.10 to ≤ 0.01 (ie, 0.10/9). All tests of differences in outcomes were conducted on observations unadjusted for participant covariates.
Finally, we also examined effects as being potentially clinically significant at the level of an individual participant. A clinically significant effect was defined as a difference in outcome of one-half of a standard deviation (SD) of the baseline distribution.38–40
All data management and statistical analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
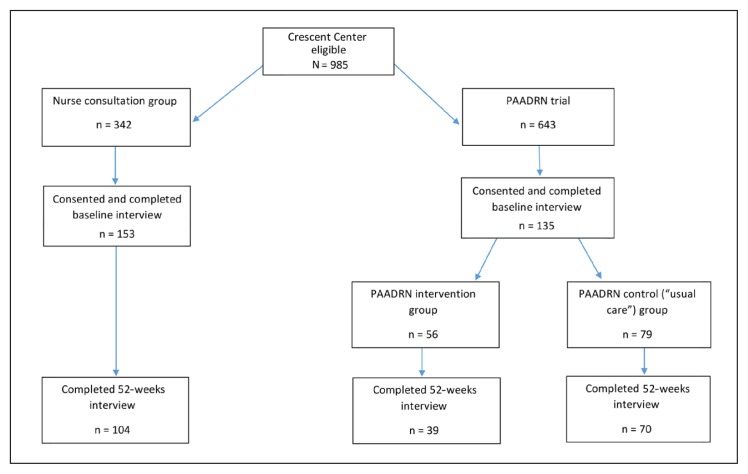
At baseline, the study sample initially included 153 participants in the nurse consultation group, 56 in the PAADRN intervention group, and 79 in the PAADRN usual care group (Figure 2). Of these, 68% (104 of 153) of nurse consultation participants, 70% (39 of 56) of PAADRN intervention participants, and 89% (70 of 79) of PAADRN control participants completed both baseline and 52-week interviews (Figure 2).
Figure 2.
Nurse consultation pilot study: randomization, allocation, and retention.
PAADRN = Patient Activation After DXA Result Notification.
Baseline characteristics of participants who completed both baseline and 52-week interview—and who comprise the final analytic sample—are displayed in Table 1 (Table 2 for characteristics of the initial study sample). Participants in each group, on average, were relatively well matched on baseline characteristics. Participants in the nurse consultation group, however, were several years older than those in the PAADRN intervention and usual care groups (p < 0.01 and p = 0.02, respectively) and had a higher proportion with “high” fracture risk than those in the PAADRN intervention group (p = 0.03).
Table 1.
Baseline characteristics of participants randomly assigned to a “just-in-time” nurse consultation and to PAADRN intervention and control groups who completed a 52-week follow-up interview
| Characteristic | Nurse consultation (n = 104) | PAADRN intervention (n = 39) | PAADRN control (n = 70) | p value (consultation vs intervention) | p value (consultation vs control) |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 70.7 (6.1) | 66.2 (8.7) | 68.7 (7.7) | 0.001 | 0.059 |
| Sex, no. (%) | |||||
| Men | 19 (18.3) | 6 (15.4) | 17 (24.3) | 0.686 | 0.337 |
| Women | 85 (81.7) | 33 (84.6) | 53 (75.7) | ||
| Race, no. (%) | |||||
| White | 69 (66.3) | 25 (64.1) | 43 (61.4) | 0.631 | 0.749 |
| Black | 33 (31.7) | 14 (35.9) | 26 (37.1) | ||
| Other/unknown | 2 (1.9) | 0 (0.0) | 1 (1.4) | ||
| Education, no. (%) | |||||
| High school or less | 18 (17.3) | 7 (17.9) | 10 (14.3) | 0.775 | 0.878 |
| Some college | 39 (37.5) | 14 (35.9) | 30 (42.9) | ||
| College graduate | 25 (24.0) | 7 (17.9) | 17 (24.3) | ||
| Postgraduate | 22 (21.2) | 11 (28.2) | 13 (18.6) | ||
| Literacy, mean (SD) | 4.4 (0.72) | 4.7 (0.52) | 4.5 (0.9) | 0.050 | 0.730 |
| Numeracy, mean (SD) | 4.6 (0.98) | 4.9 (0.65) | 4.7 (0.9) | 0.040 | 0.434 |
| Prior DXA, no. (%) | |||||
| No | 37 (35.6) | 19 (48.7) | 30 (42.9) | 0.152 | 0.333 |
| Yes | 67 (64.4) | 20 (51.3) | 40 (57.1) | ||
| Self-reported health, no. (%) | |||||
| Excellent/very good | 50 (48.1) | 18 (46.2) | 40 (57.1) | 0.227 | 0.499 |
| Good | 39 (37.5) | 19 (48.7) | 22 (31.4) | ||
| Fair/poor | 15 (14.4) | 2 (5.1) | 8 (11.4) | ||
| Baseline DXA, no. (%) | |||||
| Normal | 32 (30.8) | 15 (38.5) | 21 (30.0) | 0.233 | 0.871 |
| Low BMD | 56 (53.8) | 22 (56.4) | 40 (57.1) | ||
| Osteoporosis | 16 (15.4) | 2 (5.1) | 9 (12.9) | ||
| Fracture risk, no. (%) | |||||
| Low | 60 (57.7) | 30 (76.9) | 40 (57.1) | 0.083 | 0.848 |
| Moderate | 28 (26.9) | 7 (17.9) | 21 (30.0) | ||
| High | 16 (15.4) | 2 (5.1) | 9 (12.9) | ||
BMD = bone mineral density; DXA = dual-energy x-ray absorptiometry; PAADRN = Patient Activation After DXA Result Notification; SD = standard deviation.
Table 2.
Baseline characteristics of all consenting participants randomized to a “just-in-time” nurse consultation and to PAADRN intervention and control groups
| Characteristic | Nurse consultation (n = 153) | PAADRN intervention (n = 56) | PAADRN control (n = 79) | p value (consultation vs intervention) | p value (consultation vs control) |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 70.4 (6.3) | 66.6 (8) | 68.2 (7.9) | < 0.001 | 0.018 |
| Sex, no. (%) | |||||
| Men | 30 (19.6) | 9 (15.0) | 21 (23.6) | 0.434 | 0.463 |
| Women | 123 (80.4) | 51 (85.0) | 68 (76.4) | ||
| Race, no. (%) | |||||
| White | 97 (63.4) | 32 (53.3) | 50 (56.2) | 0.171 | 0.54 |
| Black | 53 (34.6) | 28 (46.7) | 37 (41.6) | ||
| Other/unknown | 3 (2.0) | 0 (0.0) | 2 (2.2) | ||
| Education, no. (%) | |||||
| High school or less | 30 (19.6) | 14 (23.3) | 18 (20.2) | 0.651 | 0.624 |
| Some college | 47 (30.7) | 21 (35.0) | 34 (38.2) | ||
| College graduate | 40 (26.1) | 11 (18.3) | 19 (21.3) | ||
| Postgraduate | 30 (19.6) | 14 (23.3) | 18 (20.2) | ||
| Literacy, mean (SD) | 4.5 (0.8) | 4.6 (0.8) | 4.5 (0.9) | 0.362 | 0.636 |
| Numeracy, mean (SD) | 4.7 (0.9) | 4.7 (0.9) | 4.7 (1.0) | 0.761 | 0.931 |
| Prior DXA, no. (%) | |||||
| No | 59 (38.6) | 30 (50.0) | 39 (43.8) | 0.128 | 0.422 |
| Yes | 94 (61.4) | 30 (50.0) | 50 (56.2) | ||
| Self-reported health, no. (%) | |||||
| Excellent/very good | 79 (51.6) | 30 (50.0) | 51 (57.3) | 0.735 | 0.648 |
| Good | 54 (35.3) | 24 (40.0) | 29 (32.6) | ||
| Fair/poor | 20 (13.1) | 6 (10.0) | 9 (10.1) | ||
| Baseline DXA, no. (%) | |||||
| Normal | 50 (32.9) | 21 (35) | 27 (30.3) | 0.205 | 0.623 |
| Low BMD | 78 (51.3) | 35 (58.3) | 51 (57.3) | ||
| Osteoporosis | 24 (15.8) | 4 (6.7) | 11 (12.4) | ||
| Fracture risk, no. (%) | |||||
| Low | 91 (59.5) | 47 (78.3) | 53 (59.6) | 0.028 | 0.945 |
| Moderate | 41 (26.8) | 10 (16.7) | 25 (28.1) | ||
| High | 21 (13.7) | 3 (5.0) | 11 (12.4) | ||
BMD = bone mineral density; DXA = dual-energy x-ray absorptiometry; PAADRN = Patient Activation After DXA Result Notification; SD = standard deviation.
During 52 weeks, participants in the nurse consultation group reported, on average, significant improvements in 6 of the 9 outcomes: total calcium (113 mg/d or 33% of baseline SD, uncorrected p < 0.01; Table 3); osteoporosis knowledge (0.35 points or 19% of baseline SD, p = 0.04); activation (16.12 points or 126% of baseline SD, p < 0.01); osteoporosis care satisfaction (2.41 points or 70% of baseline SD, p < 0.01); combined exercise frequency (0.46 points or 17% of baseline SD, p = 0.09); and OSE-Diet (0.29 points or 17% of baseline SD, p = 0.06). After Bonferroni corrections, 52-week improvements in total calcium, activation, and osteoporosis care satisfaction remained significant (adjusted p values ≤ 0.01). Of these, improvements in 2 outcomes were likely clinically significant (ie, change ≥ 50% of the baseline SD): activation and osteoporosis care satisfaction.
Table 3.
Effects of a “just-in-time” nurse consultation on osteoporosis pharmacotherapy and osteoporosis-related behaviors, knowledge, and self-efficacy compared with matched PAADRN intervention and control participantsa
| Outcome measure | Nurse consultation (n = 104) | PAADRN intervention (n = 39) | PAADRN control (n = 70) | p value (consultation vs intervention) | p value (consultation vs control) |
|---|---|---|---|---|---|
| Proportion with guideline-concordant pharmacotherapy | |||||
| 52 weeks | 0.70 (0.46) | 0.72 (0.46) | 0.67 (0.47) | 0.853 | 0.672 |
| Combined exercise (sessions of exercise per week) | |||||
| Baseline | 3.69 (2.77) | 4.63 (3.18) | 4.44 (2.09) | 0.085 | 0.056 |
| 52 weeks | 4.15 (3.18) | 4.33 (2.93) | 4.91 (2.66) | 0.753 | 0.101 |
| Δ52-BL | 0.46 (2.79) | −0.29 (2.85) | 0.47 (2.16) | 0.153 | 0.980 |
| p value (Δ52-BL) | 0.094 | 0.522 | 0.073 | — | — |
| Total calcium (average daily calcium in diet, mg/d) | |||||
| Baseline | 917.86 (341.81) | 920.97 (339.93) | 990.1 (325.15) | 0.961 | 0.165 |
| 52 weeks | 1030.94 (332.25) | 1012.45 (294.91) | 1026.53 (358.52) | 0.761 | 0.934 |
| Δ52-BL | 113.08 (314.48) | 91.48 (309.57) | 36.43 (293.91) | 0.714 | 0.107 |
| p value (Δ52-BL) | < 0.001 | 0.073 | 0.303 | — | — |
| Proportion with vitamin D supplementation | |||||
| Baseline | 0.58 (0.50) | 0.54 (0.51) | 0.63 (0.49) | 0.682 | 0.499 |
| 52 weeks | 0.59 (0.49) | 0.51 (0.51) | 0.60 (0.49) | 0.432 | 0.860 |
| Δ52-BL | 0.01 (0.43) | −0.03 (0.49) | −0.03 (0.42) | 0.674 | 0.561 |
| p value (Δ52-BL) | 0.820 | 0.744 | 0.567 | — | — |
| Osteoporosis knowledge | |||||
| Baseline | 7.61 (1.81) | 7.26 (2.00) | 7.66 (1.54) | 0.320 | 0.846 |
| 52 weeks | 7.95 (1.63) | 7.58 (1.57) | 7.78 (1.88) | 0.240 | 0.523 |
| Δ52-BL | 0.35 (1.65) | 0.39 (1.74) | 0.09 (1.70) | 0.895 | 0.324 |
| p value (Δ52-BL) | 0.035 | 0.19 | 0.67 | — | — |
| Activation (scale points) | |||||
| Baseline | 58.95 (12.81) | 62.58 (12.92) | 63.63 (14.64) | 0.135 | 0.027 |
| 52 weeks | 75.07 (16.38) | 80.36 (17.52) | 79.66 (17.37) | 0.094 | 0.079 |
| Δ52-BL | 16.12 (19.37) | 17.78 (20.49) | 16.03 (16.72) | 0.654 | 0.974 |
| p value (Δ52-BL) | < 0.001 | < 0.001 | < 0.001 | — | — |
| OSE-Exercise (scale points) | |||||
| Baseline | 8.11 (1.82) | 8.01 (1.61) | 8.3 (1.90) | 0.773 | 0.514 |
| 52 weeks | 8.11 (2.07) | 7.62 (2.15) | 8.43 (1.60) | 0.223 | 0.278 |
| Δ52-BL | 0 (1.63) | −0.39 (2.00) | 0.02 (1.47) | 0.235 | 0.934 |
| p value (Δ52-BL) | 0.985 | 0.232 | 0.895 | — | — |
| OSE-Diet (scale points) | |||||
| Baseline | 8.51 (1.73) | 8.41 (1.34) | 8.77 (1.43) | 0.752 | 0.286 |
| 52 weeks | 8.81 (1.54) | 8.18 (1.84) | 8.94 (1.23) | 0.043 | 0.551 |
| Δ52-BL | 0.29 (1.51) | −0.23 (1.42) | 0.18 (1.33) | 0.068 | 0.645 |
| p value (Δ52-BL) | 0.059 | 0.32 | 0.257 | — | — |
| Osteoporosis care satisfaction (scale points)b | |||||
| Baseline | 19.22 (3.45) | 18.78 (3.96) | 19.37 (3.04) | 0.645 | 0.834 |
| 52 weeks | 21.58 (2.67) | 23.45 (1.57) | 21.41 (3.12) | 0.004 | 0.769 |
| Δ52-BL | 2.41 (3.75) | 4.56 (4.26) | 2.11 (3.01) | 0.045 | 0.694 |
| p value (Δ52-BL) | < 0.001 | < 0.001 | < 0.001 | — | — |
Restricted to participants who completed baseline and 52-week interviews. Guideline-concordant pharmacotherapy was assessed only at 52 weeks.
Among participants with prior dual-energy x-ray absorptiometry (DXA): 56 in the nurse consultation group, 18 in the PAADRN intervention group, and 35 in the PAADRN control group.
OSE = osteoporosis self-efficacy; PAADRN = Patient Activation After DXA Result Notification; Δ52-BL = change between baseline and 52-week follow-up.
In contrast, participants in the PAADRN intervention or usual care group reported, on average, significant improvements over 52 weeks in 3 of the 9 outcomes. Both the PAADRN intervention and usual care participants reported improvements in activation and osteoporosis care satisfaction (both uncorrected p < 0.01). Additionally, PAADRN intervention participants reported improvements in calcium intake (p = 0.07); and, PAADRN usual care participants reported improvements in combined exercise frequency (p = 0.07). After Bonferroni corrections, 52-week improvements in activation and osteoporosis care satisfaction remained significant (adjusted p values < 0.01). As with nurse consultation group participants, PAADRN intervention participants and usual care participants had clinically significant improvements in activation (138% and 109% 52-week change as a percent of baseline SD, respectively) and osteoporosis care satisfaction (109% and 67% 52-week change as a percent of baseline SD, respectively).
Comparisons between study groups, however, yielded few statistically significant differences. The 52-week improvement in OSE-Diet was greater, on average, among nurse consultation participants than among PAADRN intervention participants (net change of 0.52, p = 0.07). However, the 52-week change in osteoporosis care satisfaction was lower among nurse consultation participants than among PAADRN intervention participants (net change of −2.15, p = 0.05). With Bonferroni corrections, neither of these effects was statistically significant.
DISCUSSION
In this pilot RCT of a nurse consultation immediately after completion of a patient’s DXA, we found that 52 weeks later participants reported significant improvements on 6 of 9 outcomes before adjustment for multiple comparisons (total calcium, exercise frequency, osteoporosis knowledge, dietary self-efficacy, activation, and osteoporosis care satisfaction), but significant improvements in only 2 outcomes after Bonferroni adjustments (activation and osteoporosis care satisfaction). The magnitude of improvement in activation and osteoporosis care satisfaction suggests that for many participants these improvements were clinically meaningful.
Among PAADRN intervention and usual care participants with similar baseline demographic and clinical characteristics, significant improvements in outcomes were achieved on only 3 of 9 outcomes before adjustment for multiple comparisons. After Bonferroni adjustments, only 2 of these outcomes remained significant; the magnitude of change in these 2 outcomes (activation and osteoporosis care satisfaction) suggested that for many participants these improvements were also clinically meaningful.
Although these within-group findings are encouraging of the potential benefits of a “just-in-time” nurse consultation after DXA, between-group comparisons were less encouraging. Compared with PAADRN intervention participants, nurse consultation participants experienced greater improvement in diet self-efficacy (p = 0.07). Yet, Bonferroni adjustments nullified the statistical significance of this finding, and the magnitude of the improvement in diet self-efficacy (17%) was not likely clinically meaningful for many nurse consultation participants. Furthermore, we found relatively less improvement in osteoporosis care satisfaction among nurse consultation participants compared with PAADRN intervention participants (and about the same relative improvement compared with usual care participants).
This latter finding is intriguing and generally consistent with the larger PAADRN study findings. The PAADRN intervention—a timely, tailored, direct-to-patient letter with personal fracture risk information and an educational brochure—appears to be particularly potent in improving osteoporosis care satisfaction compared with usual care.41 On other outcomes (eg, guideline-concordant pharmacotherapy, total calcium Vitamin D supplementation, frequency of weight-bearing and strengthening exercise), the PAADRN intervention was typically marginally better than usual care over a 52-week period.27,42
Two other integrated delivery systems that conducted RCTs of similar nurse consultations found somewhat more encouraging results. In a Geisinger Health System study, a brief nurse consultation with periodic phone follow-up found no improvements in osteoporosis pharmacologic treatment at 12 months compared with a control group, although, patients who received nurse consultation reported significant increases in calcium intake and exercise frequency compared with the control group.16 In a HealthPartners study, a 2-hour nurse educational consultation (with or without DXA), increased calcium and Vitamin D intake, but not exercise frequency at 12 weeks compared with a matched control group of postmenopausal women without consultation.15
This pilot RCT of a nurse consultation after DXA has limitations. It was conducted at only one of several DXA sites in a single health maintenance organization. One nurse conducted the consultations. Thus, it is not possible to disentangle the effects of a “just-in-time” nurse consultation after DXA that can be generally attributed to this model of care from effects specific to the study setting or practice style of the nurse consultant. Outcomes were self-reported by participants and may be subject to bias. We attempted to minimize participant bias by selecting either measures for validated scales or methods that generated averages consistent with those used for estimating similar outcomes, such as calcium intake and Vitamin D supplementation in the US national population.43–47 Patient satisfaction is an outcome contingent on patient expectations, which we did not measure. Patients may have expected something more from a nurse consultation (eg, medication prescribing, which was outside the nurse’s scope of practice) than what they expected from mailed materials (eg, the PAADRN intervention). If so, then patient satisfaction with the nurse consultation might have been attenuated compared with the PAADRN intervention or usual care.
CONCLUSION
This pilot RCT of a nurse consultation provided to older adults immediately after DXA yielded modest benefits to osteoporosis-related treatment, knowledge, self-efficacy, or lifestyle (diet and exercise) compared with either a lower-cost, tailored, direct-to-patient letter conveying personal fracture risk (through text and a figure) or the usual care (ie, a generic letter on DXA results without standardized annotation or display of fracture risk).
Acknowledgments
This work was supported by Grant R01 AG033035 to Dr Cram and Dr Wolinsky from the National Institute on Aging at the National Institutes of Health (NIH), Bethesda, MD. Dr Cram is also supported by a K24 AR062133 award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) at the NIH. Dr Saag is also supported by a K24 AR052361 award from the NIAMS at the NIH. The National Institute on Aging, NIAMS, and NIH had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Patient Activation After DXA Result Notification (PAADRN), NCT01507662, https://clinicaltrials.gov/ct2/show/NCT01507662.
Kathleen Louden, ELS, of Louden Heatlh Communications provided editorial assistance.
Footnotes
Disclosure Statement
Dr Saag has received grants from Amgen Inc, Thousand Oaks, CA; Eli Lilly and Co, Indianapolis, IN; and Merck & Co, Whitehouse Station, NJ, and has served as a paid consultant to Amgen, Eli Lilly, and Merck unrelated to this project. All other author(s) have no conflicts of interest to disclose.
References
- 1.Newman ED. Perspectives on pre-fracture intervention strategies: The Geisinger Health System Osteoporosis Program. Osteoporos Int. 2011 Aug;22(Suppl 3):451–5. doi: 10.1007/s00198-011-1695-x. DOI: https://doi.org/10.1007/s00198-011-1695-x. [DOI] [PubMed] [Google Scholar]
- 2.Dell R. Fracture prevention in Kaiser Permanente Southern California. Osteoporos Int. 2011 Aug;22(Suppl 3):457–60. doi: 10.1007/s00198-011-1712-0. DOI: https://doi.org/10.1007/s00198-011-1712-0. [DOI] [PubMed] [Google Scholar]
- 3.Adler RA, Bates DW, Dell RM, et al. Systems-based approaches to osteoporosis and fracture care: Policy and research recommendations from the workgroups. Osteoporos Int. 2011 Aug;22(Suppl 3):495–500. doi: 10.1007/s00198-011-1708-9. DOI: https://doi.org/10.1007/s00198-011-1708-9. [DOI] [PubMed] [Google Scholar]
- 4.Bohaty K, Rocole H, Wehling K, Waltman N. Testing the effectiveness of an educational intervention to increase dietary intake of calcium and Vitamin D in young adult women. J Am Acad Nurse Pract. 2008 Feb;20(2):93–9. doi: 10.1111/j.1745-7599.2007.00281.x. DOI: https://doi.org/10.1111/j.1745-7599.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- 5.Charalambous CP, Mosey C, Johnstone E, et al. Improving osteoporosis assessment in the fracture clinic. Ann R Coll Surg Engl. 2009 Oct;91(7):596–8. doi: 10.1308/003588409X432400. DOI: https://doi.org/10.1308/003588409x432400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: A randomized controlled trial. J Clin Endocrinol Metab. 2004 Mar;89(3):1117–23. doi: 10.1210/jc.2003-030501. DOI: https://doi.org/10.1210/jc.2003-030501. [DOI] [PubMed] [Google Scholar]
- 7.Dunniway DL, Camune B, Baldwin K, Crane JK. FRAX counseling for bone health behavior change in women 50 years of age and older. J Am Acad Nurse Pract. 2012 Jun;24(6):382–9. doi: 10.1111/j.1745-7599.2012.00700.x. DOI: https://doi.org/10.1111/j.1745-7599.2012.00700.x. [DOI] [PubMed] [Google Scholar]
- 8.El Miedany Y, Gardiner A, El Gaafary M, Toth M. Outcomes of a nurse-led osteoporosis and falls assessment. Br J Nurs. 2006 Oct-Nov;15(19):1070–6. doi: 10.12968/bjon.2006.15.19.22108. DOI: https://doi.org/10.12968/bjon.2006.15.19.22108. [DOI] [PubMed] [Google Scholar]
- 9.Gaboury I, Corriveau H, Boire G, et al. Partnership for fragility bone fracture care provision and prevention program (P4Bones): Study protocol for a secondary fracture prevention pragmatic controlled trial. Implement Sci. 2013 Jan 24;8:10. doi: 10.1186/1748-5908-8-10. DOI: https://doi.org/10.1186/1748-5908-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giles M, Van Der Kallen J, Parker V, et al. A team approach: Implementing a model of care for preventing osteoporosis related fractures. Osteoporosis Int. 2011 Aug;22(8):2321–8. doi: 10.1007/s00198-010-1466-0. DOI: https://doi.org/10.1007/s00198-010-1466-0. [DOI] [PubMed] [Google Scholar]
- 11.Huntjens KM, van Geel TC, Geusens PP, et al. Impact of guideline implementation by a fracture nurse on subsequent fractures and mortality in patients presenting with non-vertebral fractures. Injury. 2011 Sep;42(Suppl 4):S39–43. doi: 10.1016/S0020-1383(11)70011-0. DOI: https://doi.org/10.1016/s0020-1383(11)70011-0. [DOI] [PubMed] [Google Scholar]
- 12.Little EA, Eccles MP. A systematic review of the effectiveness of interventions to improve post-fracture investigation and management of patients at risk of osteoporosis. Implement Sci. 2010 Oct 22;5:80. doi: 10.1186/1748-5908-5-80. DOI: https://doi.org/10.1186/1748-5908-5-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majumdar SR, Johnson JA, Bellerose D, et al. Nurse case-manager vs multifaceted intervention to improve quality of osteoporosis care after wrist fracture: Randomized controlled pilot study. Osteoporos Int. 2011 Jan;22(1):223–30. doi: 10.1007/s00198-010-1212-7. DOI: https://doi.org/10.1007/s00198-010-1212-7. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen D, Ryg J, Nielsen W, Knold B, Nissen N, Brixen K. Patient education in groups increases knowledge of osteoporosis and adherence to treatment: A two-year randomized controlled trial. Patient Educ Couns. 2010 Nov;81(2):155–60. doi: 10.1016/j.pec.2010.03.010. DOI: https://doi.org/10.1016/j.pec.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Rolnick SJ, Kopher R, Jackson J, Fischer LR, Compo R. What is the impact of osteoporosis education and bone mineral density testing for postmenopausal women in a managed care setting? Menopause. 2001 Summer;8(2):141–8. doi: 10.1097/00042192-200103000-00010. DOI: https://doi.org/10.1097/00042192-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Schousboe JT, DeBold RC, Kuno LS, Weiss TW, Chen YT, Abbott TA., 3rd Education and phone follow-up in postmenopausal women at risk for osteoporosis: Effects on calcium intake, exercise frequency, and medication use. Disease Management and Health Outcomes. 2005 Dec;13(6):395–404. DOI: https://doi.org/10.2165/00115677-200513060-00004. [Google Scholar]
- 17.Sedlak CA, Doheny MO, Estok PJ, Zeller RA. Tailored interventions to enhance osteoporosis prevention in women. Orthop Nurs. 2005 Jul-Aug;24(4):270–8. doi: 10.1097/00006416-200507000-00007. DOI: https://doi.org/10.1097/00006416-200507000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Seuffert P, Sagebien CA, McDonnell M, O’Hara DA. Evaluation of osteoporosis risk and initiation of a nurse practitioner intervention program in an orthopedic practice. Arch Osteoporos. 2016;11:10. doi: 10.1007/s11657-016-0262-7. DOI: https://doi.org/10.1007/s11657-016-0262-7. [DOI] [PubMed] [Google Scholar]
- 19.Sewerynek E, Horst-Sikorska H, Stępień-Kłos W, et al. The role of counselling and other factors in compliance of postmenopausal osteoporotic patients to alendronate 70 therapy. Arch Med Sci. 2013 Apr 20;9(2):288–96. doi: 10.5114/aoms.2013.34575. DOI: https://doi.org/10.5114/aoms.2013.34575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CA. A systematic review of healthcare professional-led education for patients with osteoporosis or those at high risk for the disease. Orthopaedic Nursing. 2010 Mar-Apr;29(2):119–32. doi: 10.1097/NOR.0b013e3181d24414. DOI: https://doi.org/10.1097/nor.0b013e3181d24414. [DOI] [PubMed] [Google Scholar]
- 21.Edmonds SW, Wolinsky FD, Christensen AJ, et al. PAADRN Investigators. The PAADRN study: A design for a randomized controlled practical clinical trial to improve bone health. Contemp Clin Trials. 2013 Jan;34(1):90–100. doi: 10.1016/j.cct.2012.10.002. DOI: https://doi.org/10.1016/j.cct.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services. Bone health and osteoporosis: A report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General (US); 2004. [Google Scholar]
- 23.Edmonds SW, Solimeo SL, Lu X, Roblin DW, Saag KG, Cram P. Developing a bone mineral density test result letter to send to patients: A mixed-methods study. Patient Prefer Adherence. 2014 Jun;8:827–41. doi: 10.2147/PPA.S60106. DOI: https://doi.org/10.2147/ppa.s60106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edmonds SW, Cram P, Lu X, et al. PAADRN Investigators. Improving bone mineral density reporting to patients with an illustration of personal fracture risk. BMC Med Inform Decis Mak. 2014 Nov 25;14:101. doi: 10.1186/s12911-014-0101-y. DOI: https://doi.org/10.1186/s12911-014-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edmonds SW, Solimeo SL, Nguyen VT, et al. Understanding preferences for osteoporosis information to develop an osteoporosis patient education brochure. Perm J. 2017;21:16–024. doi: 10.7812/TPP/16-024. DOI: https://doi.org/10.7812/TPP/16-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Arlington, VA: National Osteoporosis Foundation; 2010. [Google Scholar]
- 27.Cram P, Wolinsky FD, Lou Y, et al. PAADRN Investigators. Patient-activation and guideline-concordant pharmacological treatment after bone density testing: The PAADRN randomized controlled trial. Osteoporos Int. 2016 Dec;27(12):3513–24. doi: 10.1007/s00198-016-3681-9. DOI: https://doi.org/10.1007/s00198-016-3681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Osteoporosis Foundation. Steps to estimate your calcium intake [Internet] Arlington, VA: National Osteoporosis Foundation; c2017. [cited 2016 Jul 11]. Available from: www.nof.org/patients/treatment/calciumvitamin-d/steps-to-estimate-your-calcium-intake. [Google Scholar]
- 29.Brenneman SK, Blau EM, Chen Y, Abbott TA. Validation of a patient questionnaire, “Osteoporosis and You,” designed to assess osteoporosis-related attitudes, knowledge and behavior. J Bone Miner Res. 2002 Sep;17(Suppl 1):S466. DOI: https://doi.org/10.1002/jbmr.5650170102. [Google Scholar]
- 30.Cadarette SM, Gignac MA, Beaton DE, Jaglal SB, Hawker GA. Psychometric properties of the “Osteoporosis and You” questionnaire: Osteoporosis knowledge deficits among older community-dwelling women. Osteoporos Int. 2007 Jul;18(7):981–9. doi: 10.1007/s00198-007-0326-z. DOI: https://doi.org/10.1007/s00198-007-0326-z. [DOI] [PubMed] [Google Scholar]
- 31.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004 Aug;39(4 Pt 1):1005–26. doi: 10.1111/j.1475-6773.2004.00269.x. DOI: https://doi.org/10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005 Dec;40(6 Pt 1):1918–30. doi: 10.1111/j.1475-6773.2005.00438.x. DOI: https://doi.org/10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horan ML, Kim KK, Gendler P, Froman RD, Patel MD. Development and evaluation of the Osteoporosis Self-Efficacy Scale. Res Nurs Health. 1998 Oct;21(5):395–403. doi: 10.1002/(sici)1098-240x(199810)21:5<395::aid-nur3>3.0.co;2-i. DOI: https://doi.org/10.1002/(sici)1098-240x(199810)21:5<395::aid-nur3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 34.Cram P, Schlechte J, Christensen A. A randomized trial to assess the impact of direct reporting of DXA scan results to patients on quality of osteoporosis care. J Clin Densitom. 2006 Oct-Dec;9(4):393–8. doi: 10.1016/j.jocd.2006.09.002. DOI: https://doi.org/10.1016/j.jocd.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 35.McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: Benefits, risks, and drug holiday. Am J Med. 2013 Jan;126(1):13–20. doi: 10.1016/j.amjmed.2012.06.023. DOI: https://doi.org/10.1016/j.amjmed.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 36.Anderson JJB, Rosen CJ. Reassessment of adult recommendations and supplements of calcium. Nutr Today. 2016 Jan-Feb;51(1):25–8. DOI: https://doi.org/10.1097/nt.0000000000000077. [Google Scholar]
- 37.Motulsky HJ. Intuitive biostatistics: A nonmathematical guide to statistical thinking. 3rd ed. New York, NY: Oxford University Press; 2013. [Google Scholar]
- 38.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR Clinical Significance Consensus Meeting Group. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002 Apr;77(4):371–83. doi: 10.4065/77.4.371. DOI: https://doi.org/10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 39.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003 May;41(5):582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. DOI: https://doi.org/10.1097/01.mlr.0000062554.74615.4c. [DOI] [PubMed] [Google Scholar]
- 40.Wyrwich KW, Wolinsky FD. Identifying meaningful intra-individual change standards for health-related quality of life measures. J Eval Clin Pract. 2000 Feb;6(1):39–49. doi: 10.1046/j.1365-2753.2000.00238.x. DOI: https://doi.org/10.1046/j.1365-2753.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 41.Edmonds SW, Cram P, Lou Y, et al. PAADRN Investigators. Effects of a DXA result letter on satisfaction, quality of life, and osteoporosis knowledge: A randomized controlled trial. BMC Musculoskelet Disord. 2016 Aug 26;17(1):369. doi: 10.1186/s12891-016-1227-0. DOI: https://doi.org/10.1186/s12891-016-1227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roblin DW, Wolinsky FD, Lou Y, et al. Change in dietary intake and physical activity following bone densitometry. Presentation at the 2016 Gerontological Society of America Annual Scientific Meeting; 2016 Nov 16–20; New Orleans, LA. Washington, DC: The Gerontological Society of America; 2016. [Google Scholar]
- 43.Dodd KW, Guenther PM, Freedman LS, et al. Statistical methods for estimating usual intake of nutrients and foods: A review of the theory. J Am Diet Assoc. 2006 Oct;106(10):1640–50. doi: 10.1016/j.jada.2006.07.011. DOI: https://doi.org/10.1016/j.jada.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Krebs-Smith SM, Kirkpatrick SI. Methodologic approaches influence assessment of calcium intakes. J Am Diet Assoc. 2011 May;111(5):683–6. doi: 10.1016/j.jada.2011.02.012. DOI: https://doi.org/10.1016/j.jada.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and Vitamin D intakes in the United States. J Nutr. 2010 Apr;140(4):817–22. doi: 10.3945/jn.109.118539. DOI: https://doi.org/10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey RL, Fulgoni VL, 3rd, Keast DR, Dwyer JT. Examination of vitamin intakes among US adults by dietary supplement use. J Acad Nutr Diet. 2012 May;112(5):657–663.e4. doi: 10.1016/j.jand.2012.01.026. DOI: https://doi.org/10.1016/j.jand.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mangano KM, Walsh SJ, Insogna KL, Kenny AM, Kerstetter JE. Calcium intake in the United States from dietary and supplemental sources across adult age groups: New estimates from the National Health and Nutrition Examination Survey 2003–2006. J Am Diet Assoc. 2011 May;111(5):687–95. doi: 10.1016/j.jada.2011.02.014. DOI: https://doi.org/10.1016/j.jada.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]