Abstract
Paraben esters and their salts are widely used as preservatives in cosmetics, personal care products, pharmaceuticals, and foods. We previously reported that parabens promoted adipocyte differentiation in vitro and increased adiposity but suppressed serum marker of bone formation in vivo. Here, we investigated the effects of parabens (methylparaben and butylparaben) on modulating cell fate of multipotent stem cell line C3H10T1/2. Both parabens modulated adipogenic, osteogenic, and chondrogenic differentiation of C3H10T1/2 cells in vitro. Butylparaben markedly promoted adipogenic differentiation, but suppressed osteogenic and chondrogenic differentiation whereas methylparaben showed similar but less pronounced effects. Moreover, butylparaben, but not methylparaben, was shown to activate peroxisome proliferator-activated receptor (PPAR) γ whereas neither of the paraben was shown to activate glucocorticoid receptor (GR) responsive reporter in C3H10T1/2 cells. The adipogenic effects of butylparaben were significantly attenuated by PPARγ knockdown, but not by GR knockdown. In contrast, paraben’s effects on osteoblast differentiation were affected by both knockdowns. Collectively, the results demonstrate opposing effects of parabens on adipogenic and osteoblastogenic/chondrogenic differentiation of multipotent stem cells. In light of the recent findings that parabens are detected in human placenta and milk, our studies provide rationales to study paraben exposure during early development of life in the future.
Keywords: Parabens, Endocrine disrupting chemicals, Adiposity, C3H10T1/2, Multipotent mesenchymal stem cell, Obesity
1. INTRODUCTION
Paraben esters and their salts are widely used as preservatives in cosmetics, personal care products [1], pharmaceuticals, and foods [2]. Systemic human exposure to parabens has been confirmed; parabens are detected in human serum and urine [3, 4], milk [5, 6], placental tissues [7], seminal plasma [4], and breast tumor tissue samples [8]. The extent of this exposure is further reflected by the frequent detection of free and conjugated forms of parabens in urine samples in the general population [9–11]. Moreover, recent detection of parabens in human breast milk and placenta [7, 12] has raised the concerns about potential impact of perinatal exposure to parabens on early development in humans.
Many in vitro and animal studies have suggested that parabens are endocrine disrupting compounds (EDCs) with estrogenic and anti-androgenic properties. Emerging evidence also suggested that parabens may be metabolic disruptors possibly due to their abilities to activate nuclear receptors, peroxisome proliferator-activated receptor (PPAR)γ in particular [13–15], which is one of the properties shared by many known EDCs with metabolic disrupting capabilities [16, 17]. We reported [14] and later confirmed by others [15] that parabens promoted adipocyte differentiation of 3T3-L1 cells and primary culture derived from human adipose tissue. These effects may be mediated through activation of PPARγ and/or glucocorticoid receptor (GR) [14, 15]. Moreover, we reported that post-weaning exposure to methyl- or butylparaben in the females of obesity-prone C57BL/6J mice under the chow diet-feeding induced changes in gene expression related to adipocyte differentiation and lipogenesis in the white adipose tissue (WAT) and the liver [18]. However, methylparaben exposed mice had significant increases of total white fat mass whereas butylparaben exposed mice did not show such increase. Remarkably, exposure to both parabens under the chow diet significantly decreased serum levels of procollagen type 1 N-terminal propeptide (P1NP, a marker of bone formation) but had no effects on serum levels of C-terminal telopeptide of type I collagen (CTX-I, a marker of bone resorption), suggesting that post-weaning exposure to parabens may negatively affect both fat tissue and bone formation in mice.
It is believed that adipocytes are derived from common multipotent mesenchymal stem cells that also give rise to osteocytes and chondrocytes [18]. Some EDCs are able to sensitize multipotent stem cells to undergo adipogenesis, and to suppress their osteoblast differentiation [19]. In light of our studies demonstrating opposing effects of parabens on fat tissue and bone [20], we hypothesize that parabens may modulate the multipotent stem cell fate by promoting adipocyte differentiation but suppressing differentiation of osteocyte and/or chondrocyte of stem cells.
In this study, we investigate effects of methylparaben and butylparaben on stem cell differentiation into adipocytes, osteoblasts, or chondrocytes. Moreover, we explore the role of PPARγ and GR in mediating the observed effects by parabens.
2. MATERIALS AND METHODS
2.1. Reagents
Methylisobutylxanthine (MIX), dexamethasone (DEX), insulin (Ins), PPARγ agonist rosiglitazone were purchased from Sigma-Aldrich (St. Louis, MO). Methyl-, butylparaben (>=99%) and dimethyl sulfoxide (DMSO) were purchased from Acros Organics (Thermo Fisher Scientific, Pittsburg, PA). Parabens were dissolved in DMSO at a stock concentration of 100 mM. The working concentration of parabens was 100 μM, which was obtained by diluting the stock with the culture medium (final concentration of DMSO was 0.1%).
2.2. C3H10T1/2 cell culture and differentiation
Multipotent stem cell line C3H10T1/2 (ATCC, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, Waltham, MA) containing 10% fetal bovine serum (FBS) (Atlas Biologicals, Fort Collins, CO) in 5% CO2, 37°C environment until confluence. Cells were differentiated into adipocytes, osteocytes, or chondrocytes in the presence or absence of methylparaben or butylparaben (100 μM) as follows:
Adipogenic differentiation
C3H10T1/2 cells were induced to differentiate using a modified 3T3-L1 differentiation protocol. Briefly, on the day that the cells reached confluence (designated as day 0), cells were induced to differentiate with differentiation DMEM medium containing 10% FBS, 10 μg/ml insulin, 1 μM DEX, and 0.5 mM MIX for 4 days. Cells were then switched to the maintenance DMEM containing 10% FBS and 10 μg/ml insulin for an additional 2 days followed by DMEM medium with 10% FBS for another 2 days before the cells were harvested for gene expression analysis or Oil red O (ORO) staining. Parabens or the vehicle control (0.1% DMSO in culture medium) were applied at the induction and at each change of the medium.
Osteoblastogenic differentiation
C3H10T1/2 cells were differentiated into osteoblasts as previously described by Ducy et al [21]. Briefly, the cells were seeded at a density of 2×104/cm2 in DMEM containing 10% FBS. After 24 h, the media was replaced with fresh medium supplemented with 200 ng/ml of human bone morphogenetic protein 7 (hBMP7) (R&D systems, Minneapolis, MI) when the cells reach confluence (designed as D0). Fresh medium with hBMP7 was replenished every two days until day 12. Parabens or the vehicle control (DMSO) were applied at the induction with hBMP7 and at each change of the medium.
Chondrogenic differentiation
C3H10T1/2 cells were differentiated into chondrocytes in a high-density mciromass culture as previously described by Ahrens et al [22]. Briefly, C3H10T1/2 cells were trypsinized and re-suspended in Ham’s F12K medium (CORNING, Tewksbury, MA) containing 10% FBS at a concentration of 107 cells/ml, and a 10 μl-drop of cell suspension was placed in the center of each well of 24-well cell culture plates. Cells were allowed to adhere for 1–2 h at 37°C under 5% CO2, then 1 ml of the medium containing 100 ng/ml of hBMP2 (R&D systems, Minneapolis, MI) was added to the culture (designed as D0). Medium was replenished every two days until day 6. Parabens or the vehicle control (DMSO) were applied at the induction with hBMP2 and at each change of the medium.
2.3. RNA preparation and quantitative real-time PCR analysis
At indicated times, total RNA was prepared from C3H10T1/2 cells using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH) according to the manufacturer’s instruction. Total RNA abundance was quantified using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Reverse transcription was carried out using High Capacity Reverse Transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instruction. mRNA expression of various genes, and the loading control 36B4 were measured by ABI 7300HT quantitatively using gene-specific primers and SYBR master mix (Thermo Fisher Scientific). The primer sequences are summarized in Supplemental Table 1. Cycle conditions were 50°C 2 min, 95°C 15 min, then 40 cycles of 95°C for 15 s/60°C for 1 min.
2.4. ORO staining and quantification
Lipid accumulation in differentiated adipocytes were stained with oil red O (ORO) and quantified by ORO absorbance, as previously described [14].
2.5. Alcian blue staining and quantification
Chondrogenic differentiation was assessed as previously described by Ji et al [23]. Briefly, C3H10T1/2 cells undergone chondrogenic differentiation were rinsed twice with PBS, and fixed in 4% (w/v) paraformaldehyde for 15 min. Alcian blue 8-GX (1% (w/v)) in 0.1 N HCl (pH 1.0) was applied to stain cells overnight. For quantitative analysis, guanidine HCl (6 M) were used to recover the Alcian blue staining overnight at room temperature. Absorbance at 650 nm in the extracted dye was read in Glomax multidetection system (Promega, Madison, WI).
2.6. Reporter gene assays
C3H10T1/2 cells were seeded and transiently transfected with PPARγ transactivation reporters or a GR responsive luciferase reporter (the mouse mammary tumor virus promoter driven luciferase reporter MMTV-Luc) and β-galactosidase (β-gal) control plasmid (for monitoring the transfection efficiency) with Lipofectamin 2000 according to the manufacturer’s instructions (ThermoFisher Scientific). PPARγ transactivation reporter system consists of a murine PPARγ ligand binding domain (LBD) coupled to the Gal4 DNA binding domain (DBD) (mPPARγ-Gal4) and a reporter construct containing an upstream activating sequence (UAS) linked luciferase, 4xUAS-TK-luc (TK: thymidine kinase). The cells were treated as indicated in the figure legends for 18 h, cell lysates were then prepared and luciferase/β-gal activity were measured using Glomax multidetection system (Promega, Madison, WI).
2.7. Lentiviral shRNA particle infection and generation of stably infected cell pools
C3H10T1/2 cells were plated at ~50% confluence in 6-well plates. The cells were then infected with MISSION shRNA lentiviral transduction particles for mouse PPARγ, GR, or scrambled non-targeting controls according to manufacturer’s instructions (Sigma-Aldrich). Stably infected cells were selected by puromycin (2 μg/ml) for two weeks.
2.8. Statistical analysis
All data were presented as group mean ± SEM (n=3). Analysis of variance (ANOVA) was performed to compare the effects of methyl and butylparabens on various endpoints followed by Student–Newman–Keuls post hoc test. Statistical analysis was performed using SigmaPlot 12.5 (Systat Software, Inc., San Jose, CA). The level of significance was set at p < 0.05.
3. RESULTS
3.1. Differential effects of methyl- and butylparaben on adipogenic differentiation of multipotent stem cell C3H10T1/2
We investigated the effects of methyl- and butylparaben on modulating cell fates using a multipotent stem cell line C3H10T1/2, which have the potentials to be differentiated into adipocytes, osteocytes, or chondrocytes [24]. First, we examined the effects of parabens on adipogenic differentiation of C3H10T1/2 cells. C3H10T1/2 cells were subjected to adipogenic differentiation in the presence or absence of methylparaben, butylparaben, or the vehicle control (DMSO) for 8 days (Fig. 1). As early as 12 h upon treatment, butylparaben increased mRNA expression of adipocyte differentiation marker PPARγ by 79% (p<0.01) and CCAAT/enhancer binding protein alpha (C/EBPα) by 132% (p<0.01) and decreased Runx2 mRNA level by 36% (p<0.01). Methylparaben increased C/EBPα mRNA by 35% (p<0.05) and decreased Runx2 mRNA by 32% (p<0.01); however, it did not change PPARγ mRNA at first 12 h of exposure (Fig. 1A top). At terminal differentiation (day 8), butylparaben increased mRNA level of PPARγ by 105% (p<0.05), C/EBPα by 174% (p<0.01), and fatty acid binding protein 4 (FABP4) by 82% (p<0.01) compared to the vehicle controls (Fig. 1A bottom). In contrast, methylparaben did not induce further increase on these markers compared to the controls at day 8. The findings of ORO-stained adipocyte morphology (Fig. 1B right) and quantification of lipid accumulation measured by ORO absorbance (Fig. 1B left) corroborated with the up-regulation of mRNA of adipocyte differentiation markers by butylparaben. Methylparaben, however, didn’t induce noticeable enhancement of ORO absorbance compared to the controls at day 8 (Fig. 1B right).
Figure 1.
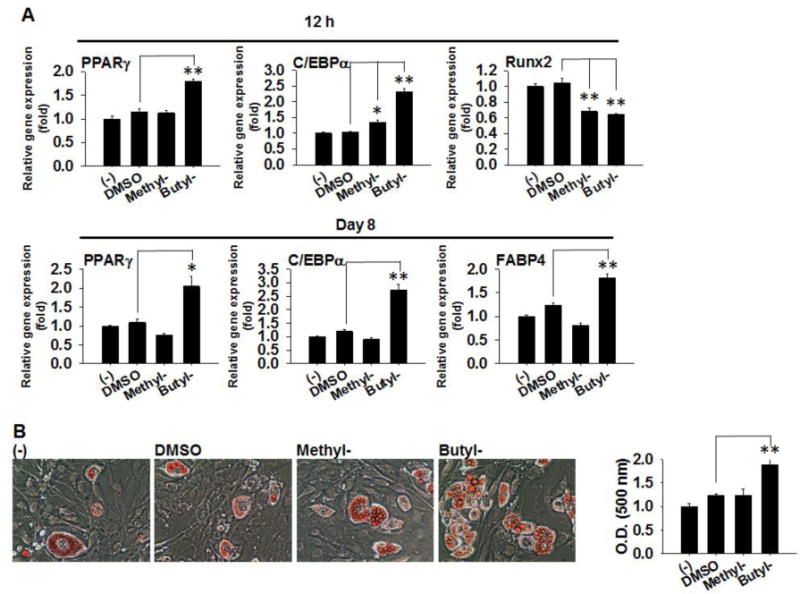
Effects of methyl- and butylparaben on adipogenic differentiation of multipotent stem cell C3H10T1/2. C3H10T1/2 cells were induced for adipogenic differentiation in the presence or absence of methyl- (100 μM), butylparaben (100 μM) or the vehicle control DMSO. (A) mRNA expression of PPARγ, C/EBPα Runx2, or FABP4 were analyzed 12 h (Top panels) and 8 days (Low panels) after initiation of differentiation. Expression was normalized to 36B4 and expressed as fold of the controls (set at 1). (B) ORO staining of cell morphology at day 8 (Left panels) and quantifications of ORO absorbance (Right panel) were shown. Data are mean ± SEM (n=3). *,**, p<0.05 and p<0.01 versus the vehicle control DMSO, respectively.
We also performed dose-response studies of methyl- and butylparaben on adipogenic differentiation of C3H10T1/2 cells. Butylparaben (0.1, 1, 10, and 100 μM) dose-dependently enhanced adipogenic differentiation as revealed by increased mRNA expression of PPARγ, C/EBPα, and FABP4 (Supplemental Fig. 1). In contrast, we did not detect dose dependent response of methylparaben using standard differentiation protocol (Supplemental Fig. 1).
3.2. Differential effects of methyl- and butylparaben on osteoblastogenic differentiation of C3H10T1/2
Next, we examined the effects of parabens on osteogenic differentiation of C3H10T1/2 cells. C3H10T1/2 cells were subjected to osteoblastogenic differentiation in the presence or absence of methylparaben, butylparaben or the DMSO for 12 days (Fig. 2). Upon initial 12 h treatment, methylparaben and butylparaben suppressed Runx 2 mRNA expression by 21% (p<0.05) and 32% (p<0.01), respectively (Fig. 2A left). At terminal differentiation on day 12, methylparaben suppressed mRNA expression of osteoblastogenic marker alkaline phosphatase (ALP) by 35% (p<0.01), osteocalcin (OCA) by 25% (p<0.05), and osteopontin (OPN) by 18% (p<0.05). Butylparaben suppressed mRNA expression of ALP by 41% (p<0.01), OCA by 81% (p<0.01), and OPN by 24% (p<0.01) (Fig. 2A right). Microscopic examination at day 12 revealed uniform osteoblasts with cytoplasmic storage materials in the controls. Methylparaben treated cells were less uniform and with less cytoplasmic storage materials whereas butylparaben treated cells were flat, not uniform, and almost with no cytoplasmic storage materials, indicating a strong suppression of differentiation by butylparaben and modest suppression by methylparaben compared with the differentiated control cells (Fig. 2B).
Figure 2.
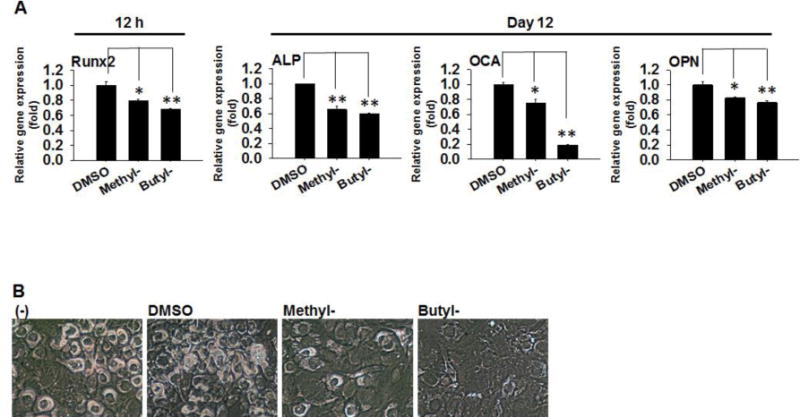
Effects of methyl- and butylparaben on osteoblastogenic differentiation of multipotent stem cell C3H10T1/2. C3H10T1/2 cells were induced for osteoblastogenic differentiation in the presence or absence of methyl- (100 μM), butylparaben (100 μM), or the vehicle control DMSO. (A) Runx 2 mRNA was analyzed 12 h (Left panel) and mRNA of ALP, OCA, and OPN was analyzed 12 days (Right panels) after the initiation of differentiation. Expression was normalized to 36B4 and expressed as fold of the controls (set at 1). (B) Microscopic images of the cells at day 12 were shown. Data are mean ± SEM (n=3). *,**, p<0.05 and p<0.01 versus the vehicle control DMSO, respectively.
We performed dose-response studies of methyl- and butylparaben on osteoblastogenic differentiation of C3H10T1/2 cells. When compared at day 12, there were no significant effects of methylparaben or butylparaben on mRNA of ALP, OCA, and OPN when used at doses less than 100 μM (Supplemental Fig. 2).
3.3. Differential effects of methyl- and butylparabens on chondrogenic differentiation of C3H10T1/2
Further, we examined the effects of parabens on chondrogenic differentiation of C3H10T1/2 cells. C3H10T1/2 cells were subjected to chondrogenic differentiation in the presence or absence of methylparaben, butylparaben or DMSO for 6 days (Fig. 3). After 12 h treatment, methylparaben suppressed mRNA expression of chondrogenic marker Runx2 by 42% (p<0.01), Col II by 59% (p<0.01), and Col X by 42% (p<0.01). Similarly, butylparaben suppressed mRNA expression of Runx2 by 30% (p<0.01), Col II by 61% (p<0.01), and Col X by 51% (p<0.01) (Fig. 3A top). At terminal differentiation on day 6, butylparaben suppressed mRNA expression of Agg to the basal level (~100%, p<0.01), Col II by 96% (p<0.01), and Col X by 43% (p<0.01) compared with the vehicle control (Fig. 3A bottom). In contrast, methylparaben did not induce any noticeable effects on these markers. The effects were confirmed by Alcian blue staining at termination (Fig. 3B left), which indicates that Alcian blue staining was much less in the micromass treated by butylparaben. The quantifications of the staining absorbance is shown in Fig. 3B right. Microscopic examination revealed (i) a large portion of undifferentiated cells in the periphery of the butylparaben-treated micromass; and (ii) a much smaller portion of undifferentiated cells in the periphery of methylparaben-treated micromass compared with the vehicle control (Fig. 3C).
Figure 3.
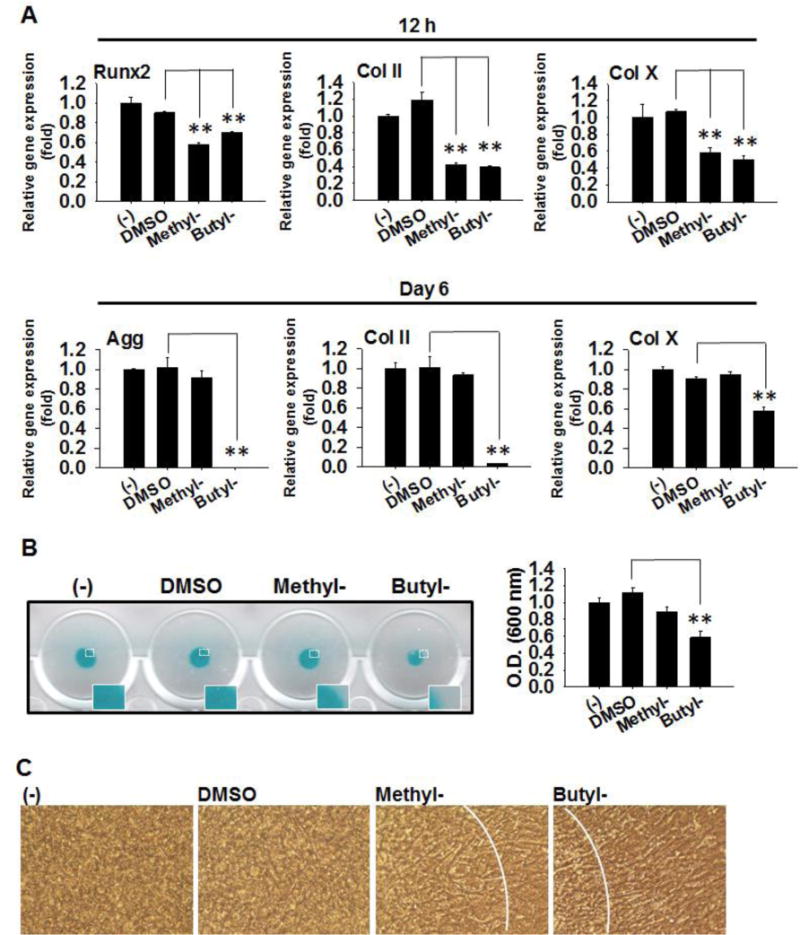
Effects of methyl- and butylparaben on chondrogenic differentiation of multipotent stem cell C3H10T1/2. Micromass of C3H10T1/2 cells were seeded and induced for chondrogenic differentiation in the presence or absence of methyl- (100 μM), butylparaben (100 μM), or the vehicle control DMSO. (A) mRNA expression of Runx2, Agg, Col II and Col X were analyzed at 12 h (Top panels) and 6 days (Low panels) after initiation of differentiation. Expression was normalized to 36B4 and expressed as fold of the controls (set at 1). (B) Alcian blue staining (Left panels) and quantifications of the absorbance (Right panel) were performed on day 6. (C) Microscopic images of the micromass at day 6 were shown. Data are mean ± SEM (n=3). **, p<0.01 versus the vehicle control DMSO.
3.4. Butylparaben, activates PPARγ, but not a GR responsive reporter, in C3H10T1/2 cells
We have previously reported that parabens, butylparaben in particular, activated PPARγ and GR using the PPARγ transactivation reporters and MMTV-Luc (a GR responsive reporter), respectively [14]. Moreover, the adipogenic effects of butylparaben was attenuated by the knockdown of PPARγ or GR [14]. Therefore, we further determined whether methylparaben or butylparaben activates PPARγ or GR in C3H10T1/2 cells using the same reporter assays (Fig. 4A). Rosiglitazone (Rosi), a synthetic ligand for PPARγ, and dexamethasone, a synthetic ligand for GR, were included as positive controls. We found that butylparaben, but not methylparaben, activated PPARγ in C3H10T1/2 cells (p<0.01) (Fig. 4A left). In contrast, we did not observe activation of MMTV-Luc by either paraben in C3H10T1/2 cells (Fig. 4A, right). We further examined parabens with various length in linear alkyl side chain. Activation of PPARγ by paraben in C3H10T1/2 cells increased with increasing length of the alkyl side chain, similar to what was reported in 3T3-L1 cells (Supplemental Fig. 1A). However, we did not observe activation of MMTV-Luc by any of the tested parabens in C3H10T1/2 cells (Supplemental Fig. 1B).
Figure 4.
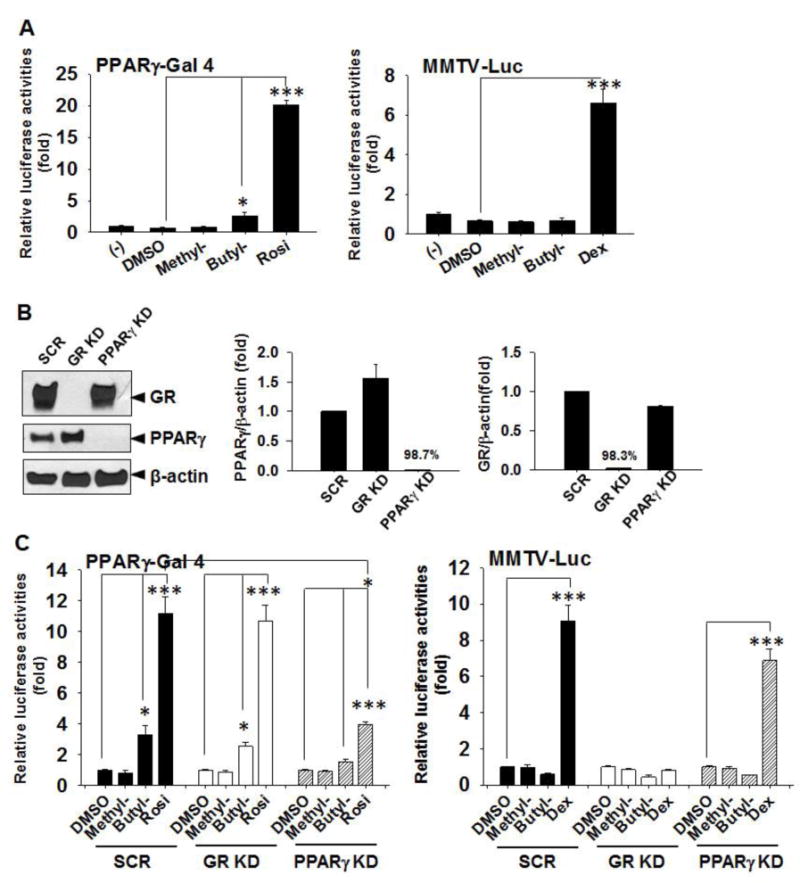
Butylparaben activates PPARγ, but not a GR responsive reporter, in C3H10T1/2 cells. (A) C3H10T1/2 cells were seeded and transiently transfected with PPARγ transactivation reporters (Left panel) or MMTV-Luc (Right panel) and β-gal control plasmid for 24 h and treated with or without methyl- (100 μM), butylparaben (100 μM), DMSO, Rosi (1 μM) or Dex (1 μM) as indicated for 18 h before the cells were lysed and reporter activities were measured. Relative luciferase activities are luciferase activities normalized by β-gal activities and are expressed as fold of the negative control (−) (set at 1). (B) C3H10T1/2 cells were stably infected with lentiviral shRNA particles targeting against GR, PPARγ, or a scrambled control (SCR). Knockdown (KD) efficiency was determined by western analysis (Left panel) and quantified by densitometry (Right panels). (C) C3H10T1/2 cells with stable knockdown of PPARγ (PPARγ KD), GR (GR KD), or the scrambled controls (SCR) were seeded and transiently transfected with PPARγ transactivation reporters (Left panels) or MMTV-Luc (Right panels) and β-gal control plasmid for 24 h and treated with or without methyl- (100 μM), butylparaben (100 μM), DMSO, Rosi (1 μM) or Dex (1 μM) for 18 h before the cells were lysed and reporter activities were measured. Relative luciferase activities are luciferase activities normalized by β-gal activities and are expressed as fold of the negative control (−) (set at 1). Data are mean ± SEM (n=3). *, ***, p<0.05 and p<0.01 versus DMSO, respectively.
To further determine whether PPARγ or GR is required for the observed effects of parabens in C3H10T1/2 cells, we generated C3H10T1/2 cells with stable knockdown (KD) of PPARγ, GR, or non-targeting controls by infecting the cells with lentiviral particles encoding shRNA targeting either PPARγ, GR, or a non-targeting scrambled (SCR) shRNA followed by puromycin selection (Fig. 4B). As determined by western analysis, the knockdown efficiency for PPARγ and GR was 98.7% and 98.3%, respectively (Fig. 4B right). We further demonstrated that butylparaben, but not methylparaben, activated PPARγ in the scrambled controls to a similar degree as shown in the non-infected cells (Fig. 4A). As expected, PPARγ activation by butylparaben or Rosi was significantly attenuated in PPARγ KD cells, but not in GR KD cells (Fig. 4C left). In contrast, both methylparaben and butylparaben did not activate GR in the scramble controls. GR activation by the positive control Dex was significantly attenuated in GR KD cells, but not in PPARγ KD cells (Fig. 4 C right).
3.5. Adipogenic effects of butylparaben are attenuated by PPARγ knockdown, but not by GR knockdown in C3H10T1/2 cells
To determine whether PPARγ or GR is required for the paraben’s adipogenic effects in C3H10T1/2 cells, we compared adipogenic effects of parabens among the PPARγ KD, GR KD, and the control SCR cells (Fig. 5). Increased mRNA expression of adipogenic marker C/EBPα, FABP4, and PLIN by butylparaben was significantly attenuated in PPARγ KD cells, but not in GR KD cells, compared to the SCR controls (p<0.05) (Fig. 5A). Consistently, increased lipid accumulation by butylparaben as assessed by ORO absorbance was also significantly attenuated in PPARγ KD cells, not in GR KD cells, compared to the SCR controls (Fig. 5B). Interestingly, GR knockdown potentiated the upregulation of FABP4 mRNA by both butylparaben and methylparaben compared to the SCR controls (Fig. 5A).
Figure 5.
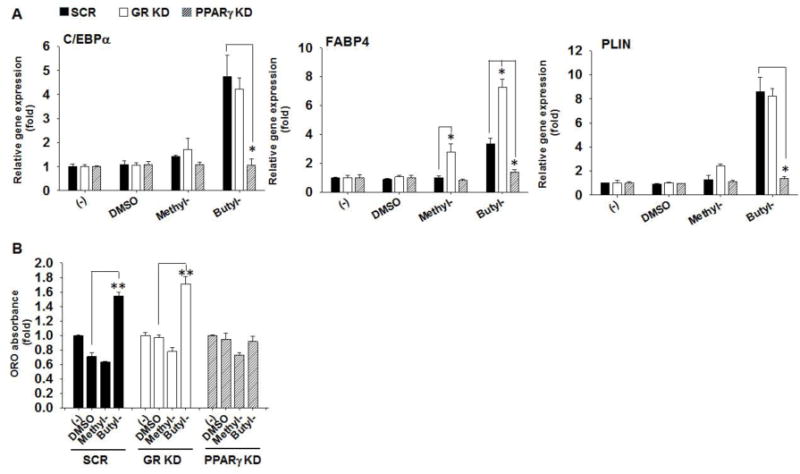
Adipogenic effects of butylparaben are attenuated by PPARγ knockdown, but not by GR knockdown in C3H10T1/2 cells. C3H10T1/2 cells with stable knockdown of PPARγ (PPARγ KD), GR (GR KD), or the scrambled controls (SCR) were seeded at the same density and induced for adipogenic differentiation for 8 days after reaching confluence in the presence or absence of methyl- (100 μM), butylparaben (100 μM) or the vehicle control DMSO. (A) mRNA expression of C/EBPα, FABP4, and PLIN were analyzed on day 8 after initiation of differentiation. Expression was normalized to 36B4 and expressed as fold of the controls (set at 1). (B) Quantifications of ORO absorbance at day 8 are shown. Data are mean ± SEM (n=3). *,**, p<0.05 and p<0.01 versus the SCR controls (A) or versus the vehicle control DMSO (B), respectively.
3.6. Suppression of osteoblastogenic differentiation by parabens is affected by knockdown of either PPARγ or GR in C3H10T1/2 cells
To determine whether PPARγ or GR is required for paraben’s suppression on osteoblastogenesis, we compared the effects of parabens on osteoblastogenic differentiation among the PPARγ KD, GR KD, and SCR cells (Fig. 6). Suppression of ALP mRNA by butylparaben was attenuated in both PPARγ KD (p<0.05) and GR KD cells (p<0.05). Similarly, suppression of ALP by methylparaben also appeared to be attenuated in PPARγ KD or GR KD cells, but neither reached statistical significance. Suppression of OCA mRNA by methylparaben was attenuated in PPARγ KD and GR KD cells; however, suppression of OCA mRNA by butylparaben was not affected in either PPARγ KD or GR KD cells. Further, suppression of OPN mRNA by butylparaben was attenuated in GR KD (p<0.05) cells but was potentiated in PPARγ KD cells (p<0.05). In contrast, suppression of OPN mRNA by methylparaben was not affected in either PPARγ KD or GR KD cells (Fig. 6A).
Figure 6.
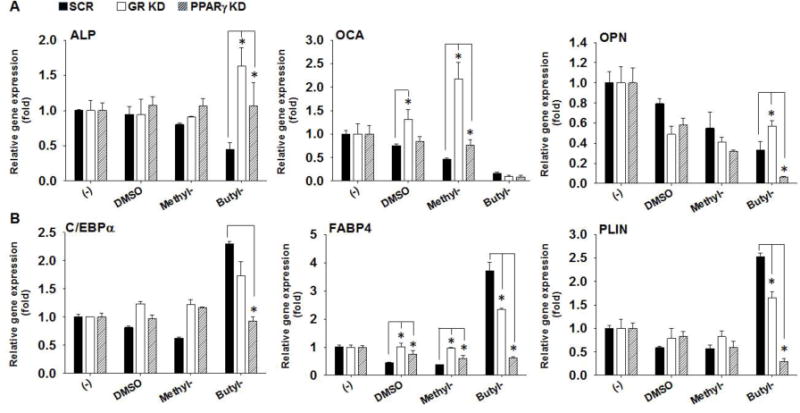
Suppression of osteoblastogenic differentiation by paraben was affected by knockdown of either PPARγ or GR in C3H10T1/2 cells. C3H10T1/2 cells with stable knockdown of PPARγ (PPARγ KD), GR (GR KD), or the scrambled controls (SCR) were seeded at the same density (~ 5.0×10(4) per well of 12-well plates) and induced for osteoblastogenic differentiation for 12 days after reaching confluence. C3H10T1/2 cells were induced for osteoblastogenic differentiation in the presence or absence of methyl- (100 μM), butylparaben (100 μM), or the vehicle control DMSO. (A) mRNA expression of osteoblastogenic marker ALP, OCA and OPN were analyzed on day 12 after the initiation of differentiation. (B) mRNA expression of adipogenic and lipid associated marker C/EBPα, FABP4, and PLIN were analyzed on day 12 after the initiation of osteoblastogenic differentiation. Expression was normalized to 36B4 and expressed as fold of the controls (set at 1). Data are mean ± SEM (n=3). *,**, p<0.05 and p<0.01 versus the SCR controls, respectively.
We noticed that butylparaben treatment was associated with lipid droplet formation in osteoblastogenically differentiated C3H10T1/2 cells; therefore, we further examined adipogenic marker expression in the osteoblastogenic differentiation of the stable knockdown cells and the controls. We confirmed that butylparaben increased mRNA expression of adipogenic marker C/EBPα, FABP4, and PLIN in the SCR controls, all of which were almost completely attenuated in PPARγ KD cells (p<0.05) (Fig. 6B). In contrast, butylparaben’s effects on C/EBPα mRNA were not attenuated and its effects on mRNA of FABP4 and PLN were only partially attenuated (p<0.05) in GR KD cells compared with the SCR controls (Fig. 6B). Methylparaben did not significantly affect adipogenic gene expression in the osteoblastogenic differentiation compared with the SCR contorls (Fig. 6B).
4. DISCUSSION
The multipotent stem cell line C3H10T1/2, which has the potential to be differentiated into adipocytes, chondrocytes, osteocytes or myocytes [24], is a widely used model to study the modulations of stem cell fate under nutritional and environmental influence [25–29]. Consistent with the effects of parabens on 3T3-L1 adipocyte differentiation [14], butylparaben markedly promoted adipogenic differentiation (Fig. 1) but suppressed osteoblastogenic (Fig. 2) and chondrogenic differentiation (Fig. 3) of C3H10T1/2 cells at both early (i.e., 12 h) and later time points (i.e., day 12 or day 6). Although methylparaben suppressed osteoblastogenic differentiation at both time points (Fig. 2), its suppression on chondrogenic (Fig. 3) and adipogenic differentiation (Fig. 1) was only detected at early time point and not detected at the end of the differentiation. Moreover, we demonstrated that while adipogenic effects by butylparaben on adipogenic differentiation (Fig. 5) and osteoblastogenic differentiation (Fig. 6B) of C3H10T1/2 cells may be mainly mediated by PPARγ, the effects on osteoblastogenic differentiation by both methyl- and butylparaben may be mediated by both PPARγ and GR (Fig. 6). To our knowledge, this is the first report demonstrating the opposing effects of parabens on adipogenic and osteoblastogenic or chondrogenic differentiation using a multipotent stem cell line. The results suggest that parabens may modulate stem cell fate by favoring the differentiation of adipocytes at the expense of osteoblasts or chondrocytes since these cell types are known to share the same stem cell population [30–32].
We have focused on methylparaben and butylparaben in the current studies because these two parabens not only represent parabens with either a short or long linear alkyl side chain, respectively, but also they are among the most commonly used parabens [33]. Adipogenic potency of parabens on 3T3-L1 adipocyte differentiation increases with the length of linear alkyl side chain with methylparaben being the weakest and butylparaben being the strongest, which correlated with their abilities to activate PPARγ and GR [14]. We found a similar trend of PPARγ activation by parabens in C3H10T1/2 cells (Supplemental Fig. 1A). In contrast to what was reported in 3T3-L1 cells, none of the tested parabens was shown to activate MMTV-Luc, a GR responsive reporter in C3H10T1/2 cells (Supplemental Fig. 1B). However, it was reported that propylparaben and butylparaben at the concentration of 1 μM induced 1.60 and 1.57 fold activation of a stably transfected MMTV-Luc reporter over the control in MDA-kb2 cells, which express high levels of functional endogenous GR [34]; therefore, it is possible that the lack of GR activation by paraben in C3H10T1/2 cells may be due to the low endogenous GR expression levels in these cells.
We previously reported critical roles of nuclear transcription factor PPARγ and GR in mediating parabens’ adipogenic effects in 3T3-L1 adipocyte differentiation [14]. In this report we explored their roles in mediating stem cell differentiation in the presence of parabens. Butylparaben activated PPARγ (Fig. 4) while knockdown of PPARγ almost completely attenuated butylparaben’s adipogenic effects as demonstrated by mRNA of adipocyte markers (C/EBPα, FABP4, and PLIN) (Fig. 5) and lipid accumulation as assessed by ORO absorbance (Fig. 5). These data suggest that butylparaben’s adipogenic effects on C3H10T1/2 stem cells may be primarily mediated through PPARγ. In contrast, the suppressive effects of methylparaben or butylparaben on osteoblastogenic differentiation were significantly affected by the knockdown of either PPARγ or GR depending on the specific osteoblast marker examined (Fig. 6). These data suggest that osteoblastogenic effects of parabens in C3H10T1/2 stem cells may be mediated through both PPARγ and GR.
The roles of PPARγ in EDCs’ opposing effects of adipogenic versus osteogenic differentiation of stem cells have been suggested. Phthalates and organotins have been reported to activate PPARγ and are adipogenic both in vitro and/or in vivo; therefore, they are recognized as environmental obesogens [35]. Interestingly, they also suppressed osteogenesis in mouse primary culture of bone marrow-derived stem cells and there was a strong correlation between their abilities to induce adipogenesis and the abilities to suppress osteogenesis [36]. Consistently, adipose tissue derived stromal stem cells (ADSCs) from mice exposed to tributyltin (TBT) in utero showed increased adipogenic capacity and reduced osteogenic capacity [19]. It is worth noting that activation of PPARγ by rosiglitazone or over expression of PPARγ was reported to promote adipogenesis but suppress osteogenesis both in vitro and in vivo [37, 38]. On the other hand, decreasing PPARγ expression or treatment with PPARγ antagonist led to reduced adipogenesis and increased bone mass [39, 40]. Together, our results added to the increasing evidence that some EDCs may elicit opposing effects on adipogenesis and osteogenesis through modulation of PPARγ.
The roles of GR activation on osteogenesis have also been reported. Low glucocorticoids (GC) concentrations promote mesenchymal stem cell commitment and osteoblastogenic differentiation [41, 42]; while high concentrations and long term treatments inhibit osteoblasts maturation and terminal differentiation [43, 44]. Even though we could not detect GR activation using MMTV-Luc reporter assays, knockdown of GR clearly attenuated the suppression of ALP mRNA, potentiated the suppression of OPN mRNA, and had no effects on suppression on OCA mRNA, by butylparaben. In addition to their reported activation of PPARγ and suppression on osteogenesis discussed above, phthalates (diethyl hexyl phthalate and diphenyl isophthalate) have been shown to activate a stably transfected MMTV-Luc reporter in MDA-kb2 cells (which express high levels of functional endogenous GR) [34, 45] whereas tributyltin did not activate MMTV-Luc in 3T3-L1 cells when used at 1 μM concentration [46]; however, the role of GR in their suppression on osteogenesis have not been reported. Therefore, more studies are needed to clarify the role of GR in EDC’s effects on osteogenesis.
How can parabens induce adipogenic differentiation but suppress osteogenic differentiation downstream of activation of PPARγ and/or GR? We demonstrated that both methyl- and butylparabens down-regulated Runx2 during differentiation of adipocytes, osteoblasts and chondrocytes of C3H10T1/2. Runx2 mRNA was suppressed by both parabens as early as 12 h upon the treatment during osteoblastogenic and chondrogenic differentiation (Fig. 2 and 3). Runx2, which belongs to the runt-domain gene family, is highly expressed in osteoblasts and chondrocytes, and its up-regulation is required for differentiation of osteoblasts [21, 47, 48] and chondrocytes [49, 50]. Moreover, Runx2 was shown to be an inhibitor for adipocyte differentiation as knockdown of Runx2 expression promoted whereas overexpression of Runx2 inhibited adipocyte differentiation [51]. Consistently, we found that Runx2 mRNA was also suppressed at 12 h upon the treatment in adipogenic differentiation of C3H10T1/2 cells (Fig. 1). Moreover, Runx2 has been identified as a downstream target of GR signaling [51]. Therefore, it is possible that parabens could modulate stem cell fate through downregulating Runx2 expression, which could be downstream events of GR and/or PPARγ activation. Future studies are necessary to further explore the role of Runx2 and delineate the pathways by which parabens modulate stem cell fates.
Opposing effects of paraben on adipogenic versus osteogenic differentiation of stem cells reported herein provides some explanation for the reported effects of post-weaning exposure to parabens in vivo [20]. Post-weaning exposure to methylparaben, but not butylparaben, significantly increased total white fat mass whereas both paraben exposure decreased serum P1NP levels [20]. It is known that WAT starts to develop shortly after birth and continues throughout life in rodents [52]. In contrast, endochondral bone development starts from early to midgestation in rodents [53]. Even though linear growth may stop after puberty, bone remodeling and repair continue throughout life [54]. Decrease of P1NP levels found in both methyl- and butylparaben exposed mice [20] are consistent with the suppressive effects of both parabens on osteoblatogenic differentiation of stem cells. However, discrepancy between the in vitro and in vivo adipogenic effects by parabens was noted. Post-weaning exposure to methylparaben significantly increased total white fat mass, which is in contrast to the modest adipogenic effects of methylparaben on C3H10T1/2 cells [20]. Although we did not detect PPARγ activation by methylparaben in C3H10T1/2 cells, methylparaben has been reported to dose-dependently activate a stably transfected PPRE-Luc, a luciferase reporter driven by a promoter containing peroxisome proliferator hormone response elements, in PPARγ-CALUX cells. This PPARγ-CALUX cells are human osteoblast cells with a stable transfection of human PPARγ cDNA [15]. Moreover, methylparaben was shown to dose-dependently promote lipid accumulation in 3T3-L1 differentiation induced by insulin alone [15]. Therefore, the fact that we could not detect PPARγ activation by methylparaben via transient transfection of PPARγ activation reporters in C3H10T1/2 cells may be due to low transfection efficiency of C3H10T1/2 cells. Moreover, higher basal adipogenic differentiation in the C3H10T1/2 cells induced by the standard program, which includes treatment with DEX, MIX, and insulin (see materials and methods), may have prevented us from detecting the differences between the methylparaben-treated cells and the controls at the end of the differentiation.
Why did butylparaben induce strong adipogenic effects in C3H10T1/2 cells, but not increase total white fat mass in the post-weaning mice? It is believed that parabens are subject to hydrolysis by non-specific esterase in the body, mostly in the gastrointestinal tract and the liver. In addition, the efficiency and pattern of hydrolysis depend on the length of alkyl side chain and the location of the hydrolysis [55, 56]. Butylparaben was found to be more efficiently hydrolyzed than methylparaben by microsomes prepared from rat liver and small intestine as well as by rat plasma [56]. Therefore, it is conceivable that higher hydrolysis of butylparaben in the exposed mice may have contributed to less pronounced effects of butylparaben on total white fat mass in vivo. Less efficient hydrolysis of methylparaben may have led to unmetabolized methylparaben accumulation in the plasma or tissue compartment, resulting in more pronounced in vivo effects. Future investigations are needed to confirm paraben’s opposing effects on adipogenic and osteogenic effects in vivo.
One limitation of our current studies is that we have focused on one dose of paraben (100 μM) for most of our studies. Although this dose of paraben falls in the range of doses reported for paraben studies in the current literature [14, 57–59], our knowledge about human systemic exposure to parabens remains to be limited. It has been suggested that the levels of paraben detected in human subjects vary depending on the age, gender, and ethnicity, possibly reflecting frequency of exposure, the level of parabens contained in the products, and the percentage of skin areas covered with paraben containing cosmetic products [11, 60]. Women have higher exposure to parabens compared to men probably due to the higher use frequency of paraben-containing cosmetic products [11, 60]. In addition, paraben exposure was reportedly higher in children with atopic dermatitis, possibly due to increased use of specific emollients for treatment and/or impaired skin barrier [61]. Future human studies are warranted to assess paraben exposure in various populations and the health impact of parabens exposure in the risk groups. Moreover, systematic examination of dose-response of methylparaben and butylparaben on adipogenic and osteoblastogenic differentiation of the stem cells are needed in the future. When examined at Day 8, the end of the adipogenic differentiation of C3H10T1/2 cells, butylparaben, not methylparaben, appeared to dose-dependently increase adipocyte marker gene expression (Supplemental Fig. 1). However, methylparaben significantly increased C/EBPα and suppressed Runx2 mRNA expression as early as 12 h upon treatment (Fig. 1A). On the other hand, based on gene expression analysis at Day 12 of the osteoblastogenic differentiation of C3H10T1/2 cells, there were no linear dose response by either methylparaben or butylparaben (Supplemental Figure 2). Therefore, it may be necessary to analyze more time points, examine various marker genes and endpoints, or even decrease the strength of the differentiation stimulation (e.g., in case of adipogenic differentiation) to assess dose-response of parabens, including methylparaben, on adipogenic and osteoblastogenic differentiation of the stem cells.
In conclusion, we have demonstrated that butylparaben markedly promoted adipogenic differentiation but suppressed osteogenic and chondrogenic differentiation of C3H10T1/2 cells whereas methylparaben showed similar but less pronounced effects. Mechanistically, we have demonstrated the role of PPARγ and GR in mediating the modulation of stem cell differentiation by parabens. In light of the recent findings that parabens are detected in human placenta and milk, our studies provide rationales to study paraben exposure during early development of life in the future.
Supplementary Material
Highlights.
Butylparaben modulates mesenchymal stem cell fate towards adipocytes
Methylparaben has similar effects but to a less extent
The effects of parabens are mediated through PPARγ and/or GR
Paraben exposure during early life development warrants further investigation
Acknowledgments
The studies were supported by the funding from University of Tennessee, Knoxville and from the National Center For Complementary & Integrative Health of the National Institutes of Health under Award Number R15AT008733. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary & Integrative Health or the National Institutes of Health.
ABBEVIATIONS
- ALP
Alkaline Phosphatase
- Agg
Aggrecan
- Col II
Collagen II
- Col X
Collagen X
- C/EBPα
CCAAT/enhancer binding protein alpha
- EDCs
endocrine disrupting chemicals
- FABP4
Fatty acid binding protein 4
- FAS
Fatty acid Synthase
- GR
glucocorticoid receptor
- OCA
Osteocalcin
- OPN
Osteopontin
- PLIN
perilipin
- PPARγ
peroxisome proliferator-activated receptor gamma
- Runx2
Runt-related transcription factor 2
- SCD
Stearoyl-CoA desaturase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
The authors declare no conflict of interest.
References
- 1.Final amended report on the safety assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(Suppl 4):1–82. doi: 10.1080/10915810802548359. [DOI] [PubMed] [Google Scholar]
- 2.Liao C, Chen L, Kannan K. Occurrence of parabens in foodstuffs from China and its implications for human dietary exposure. Environ Int. 2013;57–58:68–74. doi: 10.1016/j.envint.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Ye X, et al. Stability of the conjugated species of environmental phenols and parabens in human serum. Environ Int. 2009;35(8):1160–3. doi: 10.1016/j.envint.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Frederiksen H, Jorgensen N, Andersson AM. Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) J Expo Sci Environ Epidemiol. 2011;21(3):262–71. doi: 10.1038/jes.2010.6. [DOI] [PubMed] [Google Scholar]
- 5.Ye X, et al. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal Chim Acta. 2008;622(1–2):150–6. doi: 10.1016/j.aca.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 6.Hines EP, et al. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod Toxicol. 2014 doi: 10.1016/j.reprotox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jimenez-Diaz I, et al. A new liquid chromatography-tandem mass spectrometry method for determination of parabens in human placental tissue samples. Talanta. 2011;84(3):702–9. doi: 10.1016/j.talanta.2011.01.075. [DOI] [PubMed] [Google Scholar]
- 8.Darbre PD, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24(1):5–13. doi: 10.1002/jat.958. [DOI] [PubMed] [Google Scholar]
- 9.Ye X, et al. Parabens as urinary biomarkers of exposure in humans. Environ Health Perspect. 2006;114(12):1843–6. doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janjua NR, et al. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int J Androl. 2008;31(2):118–30. doi: 10.1111/j.1365-2605.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 11.Calafat AM, et al. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect. 2010;118(5):679–85. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlumpf M, et al. Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere. 2010;81(10):1171–83. doi: 10.1016/j.chemosphere.2010.09.079. [DOI] [PubMed] [Google Scholar]
- 13.Taxvig C, et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARgamma activation. Mol Cell Endocrinol. 2012;361(1–2):106–15. doi: 10.1016/j.mce.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Hu P, et al. Effects of parabens on adipocyte differentiation. Toxicol Sci. 2013;131(1):56–70. doi: 10.1093/toxsci/kfs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira-Fernandes A, et al. Evaluation of a screening system for obesogenic compounds: screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect. PLoS One. 2013;8(10):e77481. doi: 10.1371/journal.pone.0077481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3–L1 preadipocytes. J Steroid Biochem Mol Biol. 2011;127(1–2):9–15. doi: 10.1016/j.jsbmb.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bishop-Bailey D, Hla T, Warner TD. Bisphenol A diglycidyl ether (BADGE) is a PPARgamma agonist in an ECV304 cell line. Br J Pharmacol. 2000;131(4):651–4. doi: 10.1038/sj.bjp.0703628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Kirchner S, et al. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol. 2010;24(3):526–39. doi: 10.1210/me.2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu P, et al. Differential effects on adiposity and serum marker of bone formation by post-weaning exposure to methylparaben and butylparaben. Environ Sci Pollut Res Int. 2016 doi: 10.1007/s11356-016-7452-0. [DOI] [PubMed] [Google Scholar]
- 21.Ducy P, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–54. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 22.Ahrens PB, Solursh M, Reiter RS. Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol. 1977;60(1):69–82. doi: 10.1016/0012-1606(77)90110-5. [DOI] [PubMed] [Google Scholar]
- 23.Ji YH, et al. Quantitative proteomics analysis of chondrogenic differentiation of C3H10T1/2 mesenchymal stem cells by iTRAQ labeling coupled with on-line two-dimensional LC/MS/MS. Mol Cell Proteomics. 2010;9(3):550–64. doi: 10.1074/mcp.M900243-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinney DF, Emerson CP., Jr 10T1/2 cells: an in vitro model for molecular genetic analysis of mesodermal determination and differentiation. Environ Health Perspect. 1989;80:221–7. doi: 10.1289/ehp.8980221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takata T, Morimoto C. Raspberry ketone promotes the differentiation of C3H10T1/2 stem cells into osteoblasts. J Med Food. 2014;17(3):332–8. doi: 10.1089/jmf.2013.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biemann R, Fischer B, Navarrete Santos A. Adipogenic effects of a combination of the endocrine-disrupting compounds bisphenol A, diethylhexylphthalate, and tributyltin. Obes Facts. 2014;7(1):48–56. doi: 10.1159/000358913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chani B, et al. Epigallocatechin Gallate Inhibits Mouse Mesenchymal Stem Cell Differentiation to Adipogenic Lineage. J Stem Cells Regen Med. 2016;12(1):16–24. doi: 10.46582/jsrm.1201004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Choudhury M. The plasticizer BBP selectively inhibits epigenetic regulator sirtuin during differentiation of C3H10T1/2 stem cell line. Toxicol In Vitro. 2017;39:75–83. doi: 10.1016/j.tiv.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava S, et al. Role of vanadium (V) in the differentiation of C3H10t1/2 cells towards osteoblast lineage: a comparative analysis with other trace elements. Biol Trace Elem Res. 2013;152(1):135–42. doi: 10.1007/s12011-013-9602-2. [DOI] [PubMed] [Google Scholar]
- 30.Abdallah BM, Kassem M. New factors controlling the balance between osteoblastogenesis and adipogenesis. Bone. 2012;50(2):540–5. doi: 10.1016/j.bone.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 31.James AW. Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation. Scientifica (Cairo) 2013;2013:684736. doi: 10.1155/2013/684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23(7):1128–39. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Program, NB. Centers for Disease Control and Prevention: p. https://www.cdc.gov/BIOMONITORING/Parabens_BiomonitoringSummary.html, last updated Dec 23, 2016.
- 34.Klopcic I, Kolsek K, Dolenc MS. Glucocorticoid-like activity of propylparaben, butylparaben, diethylhexyl phthalate and tetramethrin mixtures studied in the MDA-kb2 cell line. Toxicol Lett. 2015;232(2):376–83. doi: 10.1016/j.toxlet.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 Suppl):S50–5. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 36.Watt J, Schlezinger JJ. Structurally-diverse, PPARgamma-activating environmental toxicants induce adipogenesis and suppress osteogenesis in bone marrow mesenchymal stromal cells. Toxicology. 2015;331:66–77. doi: 10.1016/j.tox.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lecka-Czernik B, et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem. 1999;74(3):357–71. [PubMed] [Google Scholar]
- 38.Rzonca SO, et al. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145(1):401–6. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akune T, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113(6):846–55. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marciano DP, et al. Pharmacological repression of PPARgamma promotes osteogenesis. Nat Commun. 2015;6:7443. doi: 10.1038/ncomms8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng SL, et al. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134(1):277–86. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 42.Hong D, et al. Osteoblastogenic effects of dexamethasone through upregulation of TAZ expression in rat mesenchymal stem cells. J Steroid Biochem Mol Biol. 2009;116(1–2):86–92. doi: 10.1016/j.jsbmb.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Ishida Y, Heersche JN. Glucocorticoid-induced osteoporosis: both in vivo and in vitro concentrations of glucocorticoids higher than physiological levels attenuate osteoblast differentiation. J Bone Miner Res. 1998;13(12):1822–6. doi: 10.1359/jbmr.1998.13.12.1822. [DOI] [PubMed] [Google Scholar]
- 44.Ito S, et al. Glucocorticoids induce the differentiation of a mesenchymal progenitor cell line, ROB-C26 into adipocytes and osteoblasts, but fail to induce terminal osteoblast differentiation. Bone. 2007;40(1):84–92. doi: 10.1016/j.bone.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Kolsek K, et al. Molecular docking revealed potential disruptors of glucocorticoid receptor-dependent reporter gene expression. Toxicol Lett. 2014;226(2):132–9. doi: 10.1016/j.toxlet.2014.01.047. [DOI] [PubMed] [Google Scholar]
- 46.Sargis RM, et al. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 2010;18(7):1283–8. doi: 10.1038/oby.2009.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otto F, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89(5):765–71. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 48.Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–64. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 49.Kim IS, et al. Regulation of chondrocyte differentiation by Cbfa1. Mech Dev. 1999;80(2):159–70. doi: 10.1016/s0925-4773(98)00210-x. [DOI] [PubMed] [Google Scholar]
- 50.Inada M, et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev Dyn. 1999;214(4):279–90. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 51.Zhang YY, et al. Down-regulation of type I Runx2 mediated by dexamethasone is required for 3T3-L1 adipogenesis. Mol Endocrinol. 2012;26(5):798–808. doi: 10.1210/me.2011-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Kovacs CS. Bone development in the fetus and neonate: role of the calciotropic hormones. Curr Osteoporos Rep. 2011;9(4):274–83. doi: 10.1007/s11914-011-0073-0. [DOI] [PubMed] [Google Scholar]
- 54.Gosman JH, Stout SD, Larsen CS. Skeletal biology over the life span: a view from the surfaces. Am J Phys Anthropol. 2011;146(Suppl 53):86–98. doi: 10.1002/ajpa.21612. [DOI] [PubMed] [Google Scholar]
- 55.Imai T, et al. Substrate specificity of carboxylesterase isozymes and their contribution to hydrolase activity in human liver and small intestine. Drug Metab Dispos. 2006;34(10):1734–41. doi: 10.1124/dmd.106.009381. [DOI] [PubMed] [Google Scholar]
- 56.Ozaki H, et al. Comparative study of the hydrolytic metabolism of methyl-, ethyl-, propyl-, butyl-, heptyl- and dodecylparaben by microsomes of various rat and human tissues. Xenobiotica. 2013;43(12):1064–72. doi: 10.3109/00498254.2013.802059. [DOI] [PubMed] [Google Scholar]
- 57.Kodani SD, et al. Parabens inhibit fatty acid amide hydrolase: A potential role in paraben-enhanced 3T3-L1 adipocyte differentiation. Toxicol Lett. 2016;262:92–99. doi: 10.1016/j.toxlet.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J, et al. Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol Appl Pharmacol. 2007;221(3):278–84. doi: 10.1016/j.taap.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Majewska N, et al. Methylparaben-induced decrease in collagen production and viability of cultured human dermal fibroblasts. J Appl Toxicol. 2017 doi: 10.1002/jat.3466. [DOI] [PubMed] [Google Scholar]
- 60.Smith KW, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect. 2012;120(11):1538–43. doi: 10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Overgaard LE, et al. Children with atopic dermatitis and frequent emollient use have increased urinary levels of low-molecular-weight phthalate metabolites and parabens. Allergy. 2017 doi: 10.1111/all.13157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


