Abstract
The brain includes multiple types of interconnected excitatory and inhibitory neurons that together allow us to move, think, feel and interact with the environment. Inhibitory interneurons comprise a small, heterogeneous fraction, but they exert a powerful and tight control over neuronal activity and consequently modulate the magnitude of neuronal output and, ultimately, information processing. Interneuronal abnormalities are linked to two pediatric psychiatric disorders with high comorbidity: autism and Tourette syndrome. Studies probing the basis of this link have been contradictory regarding whether the causative mechanism is a reduction in number, dysfunction or gene aberrant expression (or a combination thereof). Here, we integrate different theories into a more comprehensive view of interneurons as responsible for the symptomatology observed in these disorders.
Keywords: interneurons, autism, Tourette syndrome, cortex, striatum
The brain as a whole
Our brain is responsible for the titanic task of integrating all the external information surrounding us and producing a proportionate and adequate behavioral response. The brain achieves this by generating multiple connections between different types of neurons clustered in well-defined topologically organized regions. The information flow between regions occurs due to a fine balance between excitatory and inhibitory neurons that control the output signal. This kind of organization allows the brain to make local changes in micro circuitry that can have global and distal effects on regions involved in this neural circuit. However, this raises the question of which are the consequences at a behavioral level when this excitatory/inhibitory balance is disrupted. Herein we will review the role of a subtype of neurons called interneurons (INs), responsible for keeping the inhibitory tone in the brain and how dysregulation of these neurons can lead to two child psychiatric disorders: autism and Tourette syndrome.
Role of interneurons in the brain
INs are a sub group of inhibitory neurons that are widely spread through the brain. Even though they represent a small proportion of the total number of neurons in the brain, these cells keep neuronal timing, synchronicity and activity by generating inhibitory inputs over other neurons. Different types of INs modulate neural circuits by releasing various neurotransmitters, such as γ-Aminobutyric acid (GABA) or acetylcholine (Ach) (see glossary). GABA-releasing INs are mainly classified by a complex combination of morphological, connectivity, intrinsic electrophysiological properties and molecular content [1, 2].
The brain cortex is organized in a laminar way, consisting of six different layers composed by a heterogeneous group neurons with differential interconnection between each eah layer and other subcortical structures [3]. Besides, the complexity and functionality is also increased with an intricated network of projections between cortices that allow complex processing [4, 5]. Cortical neurons can be divided mainly in two subgroups, excitatory and inhibitory, the complexity and diversity of the different cortices emerges from a combination of the unique characteristics described above. Cortical GABAergic INs represent only 20% of the total number of neurons but they inhibit a complex network of excitatory and inhibitory neurons in multiple cortical circuits [6]. These INs are largely classified into six groups based on the expression of the following markers: parvalbumin (PV+), somatostatin (SST+), vasointestinal peptide (VIP+), neuronal nitric oxide synthase (nNOS+), calretinin (CR+) and REELIN (see glossary) [7]. Of note, this is a simplification for the purpose of this review because these markers sometimes overlap between each other depending on specific cortical layers or sub-regions [8]. INs are the main source of cortical modulation over glutamatergic pyramidal cells (PC) (see glossary). However, through the inhibition of other INs, they can generate cortical activation by disinhibition of PC. Overall, INs can regulate the activation state of local cortical networks but their effects can also be extended to other brain areas by modulating outgoing projections..
One of the regions of the basal ganglia that is densely innervated by cortical afferents is the striatum, a key region for motor programming, habit formation and social behaviors [9, 10]. The striatum is largely composed, by a vast majority, of projecting GABAergic spiny neurons (SPN), these neurons are categorized as striatonigral (direct pathway) or striatopallidal (indirect pathway) based on their target projections [11]. Activation of the direct pathway projections to the globus pallidus interna (GPi) and substantia nigra (SNr) exerts a powerful inhibition over these regions that in turn relieves the inhibitory brake on the thalamus. Then, the thalamus send glutamatergic projections that activates the frontal cortex. The indirect pathway activation inhibits the globus pallidus externa that produces decreased inhibition on the subthalamic nucleus, which increases its excitation over the GPi/SNr. The GPi/SNr then increases its inhibitory drive over the thalamus, resulting in a reduced glutamatergic output to the frontal cortex [12]. Either subgroup of SPNs is modulated by a small but influential group of local GABAergic and cholinergic INs [13]. GABA releasing INs in the striatum are mainly PV+ and CR+, but the striatum also has subtypes of INs that are positive for the expression of tyrosine hydroxylase [14], SST+ or nNOS+. There are no subgroups of striatal cholinergic INs and all of them express choline acetyltransferase (Chat) (see glossary). However, these cells can exert either excitatory or inhibitory effects depending on the cholinergic receptor expressed in the target neuron [15]. As a whole, cortical and striatal INs modulate neural circuits to coordinate and orchestrate a behavioral output according to the environmental challenges [16].
Interneurons in cortico- striatal circuits
The cortex is organized in hierarchical neural networks that allow a dynamic balance between excitation and inhibition (E/I) [17]. Across the cortical lamina (Layers I to VI) PCs are known to differentially and selectively project to multiple brain regions; INs subtypes are asymmetrically distributed in this architecture [1, 18]. Altogether, this allows cortical clusters of neurons to have specific roles in different behavioral outputs according to their topographical position. The flow of information between the different cortical layers is managed by interlaminar projections of PCs and INs.
Layer II-III (L2-3) PCs send projections to layers IV (L4) and V (L5), INs in this layer are heterogeneous, with PV+, VIP+ (some CR+ too) and SST+ present [19]. Although most of INs in this layer only produce intralaminar inhibition, VIP+ INs can also modulate L4 [20–22]. PCs present in L4 do not project to other non-cortical structures, they project exclusively to L2-3. Cortical projections to the striatum and midbrain neurons originate from L5 PCs, which are modulated by a group of PV+, SST+ and VIP+ INs [21]. Finally, Layer VI (L6) receives PC inputs from L2-3 PCs and then projects to the thalamus. Notably, L6 PCs can, at the same time, produce feedforward inhibition onto cortical PCs through PV+ INs[23]. Adding an extra dimension of complexity to this picture, it has been found that INs also differentially inhibit each other. SST+ and VIP+ INs inhibit each other and although PV+ INs modulate only PV+ INs, they can also be inhibited by SST+ INs [22, 24, 25]. In general, INs control E/I balance and the direction of information flow between different cortical layers.
The striatum does not have a highly-organized structure like the cortex. It is compartmentalized on a gross level, i.e., dorsal and ventral striatum, and neurochemically into the matrix/striosome system (see glossary) [26]. In this sense, both SPNs or INs are homogenously distributed across this region without any segregation patterns. Action initiation or suppression is triggered by SPNs through complex and coordinated activity [27]; under this framework SPNs are subject to strong modulation by either Chat+ or PV+ INs. Recent studies have shown that PV+ INs only inhibit SPN and that SST+ cells target SPNs and Chat+ INs [28]. Additionally, it was recently shown that there is also a cholinergic-evoked striatal inhibition derived from GABAergic INs[29].
Cortical and striatal circuits are integrated in multiple ways. For example, cortical afferents directly target and activate Chat+ INs, leading to increments in striatal dopamine (DA) levels [30]. Conversely, activity of striatal PV+ INs is enhanced after cortical stimulation that is independent of DA and Ach [31]. Regarding how cortical afferents affect SPNs circuitry, it has been shown that different cortices preferentially innervate either direct or indirect pathway SPNs [32]. The striatum does not have direct reciprocal projections to cortical structures; instead its effects are indirectly exerted through thalamic projections to the cortex.
Finally, there is a complex indirect influence of cortical INs on the striatum via modulation of the activity of the PCs innervating this region. Thus, INs are able to locally affect neurons to produce distal effects on other brain regions. These cortical-striatal projections also recruit striatal INs to regulate SPNs function. All this evidence opens up a question: what are the behavioral consequences of INs dysfunction in the cortex or striatum and what causes these abnormalities.
Pathophysiology of autism spectrum disorder and Tourette’s syndrome
Autism is a neurodevelopmental psychiatric disorder with an onset in early childhood; the core symptoms of this disease are social and communicational deficits, stereotypic behavior and, in some cases, intellectual disabilities [33]. Autism is 4.5 times more common in males than females [34]. Tourette’s syndrome (TS) is a neuropsychiatric disease that affects between 0.3–0.9% of children [35]; it is also characterized by stereotypic behaviors and male predominance. In addition to sharing some symptomatology and sexual dimorphism, these disorders are commonly comorbid [36–39]. Furthermore, abnormalities in cortico-striatal circuits are common in both disorders. Brain imaging studies in TS patients have shown aberrant hyper-connectivity between cortical regions and the striatum [40]. This is accompanied by aberrant structural organization and volumes in the cortico-striatal circuitry [41, 42]. ASD patients also have overconnectivity between cortico-striatal circuits, as well as abnormal striatal growth ratio and shape [43–45]. Importantly, the fact that these neurodevelopmental disorders share a dysfunction in specific neural circuits does not mean that the etiology is common.
Genetics and interneuron related dysfunction
Tourette’s Syndrome
TS and tic disorders appear to be among the most heritable neuropsychiatric disorders [46]. Multiple genes have been related to TS etiology, including the histidine decarboxilase (HDC), contactin associated protein-like 2 (CNTNAP2) and Neuroligin 4 (NLGN4), among others [47]. Although most of these are rare mutations with high penetrance that run in (some of) the families of the patients, this has brought valuable information regarding the genetics of TS. Altered cortico-striatal anatomy in TS is well documented, particularly in the striatum, orbital and medial prefrontal cortices [41, 48]. TS patients are also affected by a widespread GABAergic disruption [49]. Interestingly, patients with TS showed a reduction in the number of PV+, nNOS+ and cholinergic striatal INs[50, 51]. Finally, an exhaustive transcriptomic analysis of the striatum in TS patients showed decrements in the expression of GABA, cholinergic and SST levels, all key elements for proper neurotransmission [51].
Autism Spectrum Disorders
The ASD genetic framework is proposed to derive from a combination of common genetic variants and contributions from de novo and rare inherited mutations [52, 53]. Genetic studies have shown multiple genes with high penetrance in different forms of ASD like SH3 and multiple ankyrin repeat domains protein 3 (SHANK3) and CNTNAP2, among others [54]. Non-invasive measurement by magnetic resonance spectroscopy (MRS) in ASD patients showed a reduction of GABA levels in frontal, motor, somatosensory and auditory cortices [55–59]. Another set of studies has indicated a reduction of GABAA receptor binding in the frontal cortex and other subcortical region in patients with ASD [60]. Recent post mortem studies in ASD patients strikingly showed a decrease in the number of PV Ins in prefrontal cortex [61, 62]. Although this has brought some new insights into the pathophysiology of ASD, we still lack a mechanistic explanation of why, how, and when this aberrant GABAergic neurotransmission occurs.
Overlap and differences
Thus far, there has been little to no reported overlap between the genetics of TS and ASD, although they do share the fact that E/I imbalance occurs in cortico-striatal circuits [63, 64]. The origin of this imbalance is thought to be due to an interneuron dysfunction that leads to overall disinhibition of the areas compromised. Even though the aforementioned studies have offered more insight into the role of INs in the pathophysiology of TS and ASD, we still cannot elucidate precise molecular mechanisms altered in patients due to both technological and ethical limitations. For this purpose, multiple rodent models based on clinical and genetic data have been developed to unravel the pathophysiology of these disorders.
Pre-clinical models of ASD and TS with interneuronal alterations
Tourette’s Syndrome
Based on data from postmortem studies that found striatal INs alteration, rodent models of TS has been developed for further studying this disorder at different levels. The single striatal ablation of PV+ or Chat+ INs showed abnormal stereotypy after amphetamine administration [66, 67]. The growing body of evidence from clinical and preclinical studies points out that the etiology of stereotypic behavior and social deficits observed in these child psychiatric disorders comes in part from interneuron dysfunction. Perhaps one of the most intriguing questions is where and which kind of these INs modulates these behavioral outputs.
Autism Spectrum Disorder
Originally described as a deletion in a syndromic form of ASD, the shank3 deletion in different exons has been widely studied in rodent models. Shank3 deletions in mice produce social deficits, stereotypic behavior and aberrant cortical and striatal synaptic neurotransmission [68–70]. There is evidence that in these models the PV+ circuitry has a reduction in its perineuronal net over PC cells, resulting in a dysregulation in cortical networks due to an E/I imbalance [71]. Another genetic model that mimics the core symptoms of ASD is the CNTNAP2 knockout (KO) mouse [72]. This rodent model is characterized by asynchronous cortical neuronal firing, as well as a strong reduction in the number of GABAergic INs in the somatosensory cortex and striatum (with a highest reduction in the PV+ subtype). Furthermore, it has been shown that knockout of this gene affects spine density, glutamatergic receptor trafficking and perisomatic inhibition [73, 74]. In this sense, the pharmacological model of ASD based on prenatal exposure to valproate also showed a reduction of PV at the levels of protein, mRNA and cell number [75]. The BTBR mouse idiopathic model of ASD also has reduced GABAergic neurotransmission [76], as well as decreased levels of PV+ expression and PV+ contacts onto pyramidal cells in somatosensory cortex [71].
PV+ KO mice display core symptomatology of ASD, e.g., social and communicational deficits [77], but perhaps another important fact to highlight is that these mice exhibit an E/I imbalance as well. Rett syndrome is a syndromic form of ASD, with a well identified genetic mutation in the methyl CpG binding protein 2 (MECP2). Interestingly, specific deletion of this gene in PV+ INs produces social deficits, whereas in SST+ INs generates stereotypic behaviors [78].
A recent study [65] showed that PV+ and Chat+ striatal INs dual ablation produce social deficits and stereotypic behavior only in male mice during adulthood. Reduction of both types of INs produces an overall activation of the striatum, which explains the stereotypic behavior but not social deficits. Interestingly, single ablation of either PV or Chat does not induce spontaneous stereotypy or social deficits, being the aberrant behavior only attributable to the combined reduction of both cell types. Different preclinical models for both disorders have shown abnormalities in INs, proposing that dysfunction of this type of neuron is an important characteristic in social deficits and stereotypic behaviors (Table 1). Besides, this evidence arises the question regarding if the striatum is involved in social behaviors or if the behavioral output found is the result of a cortico-striatal dysfunction at circuit level.
Table 1.
| Preclinical model | Region | Behavioral abnormalities | INs abnormality |
|---|---|---|---|
| Tourette syndrome | |||
| PV+ INs ablation | Striatum | ↑ stereotypy behavior after amphetamine, anxiety | ↓ number of PV+ INs |
| Chat+ INs ablation | Striatum | ↑ stereotypy behavior after amphetamine | ↓ number of Chat+ INs |
| Austism | |||
| Shank3 | Striatum | Stereotypic behaviors, social deficits | ↓ PV expression |
| CNTNAP2 | Striatum and somatosensory cortex | Stereotypic behaviors, social deficits, seizures | ↓ number of PV INs |
| Prenatal Valproate | Striatum | Stereotypic behaviors, social deficits | ↓ PV expression and cell number |
| BTBR | Somatosensory cortex | Stereotypic behavior, social deficits, cognitive rigidity | ↓ PV expression |
| MecP2 deletion in PV+ INs | Whole brain | motor, sensory, memory, and social deficits | Null mecp2 expression in PV INs |
| Chat+ and PV+ INs ablation | Striatum | Stereotypic behaviors, social deficits, anxiety | ↓ number of PV+ and Chat+ INs |
Protein down regulation or cell loss? That is the questionThe studies using the rodent models discussed above raises an important question: are these disorders occurring due to cell-specific aberrant gene expression or reduced number of specific INs in cortico-striatal circuits? Both ASD and TS have a strong neurodevelopmental component. Based on the current literature, the evidence of a disruption in E/I balance due to interneuronal dysfunction is solid. However, there are multiple questions about when, how and where interneuron dysfunction occurs and leads to social deficits and/or stereotypic behavior.
Autism Spectrum Disorder
One question that immediately arises is when during development these anomalies in cortico-striatal INs occur. INs arise from different neuronal precursors in the brain, expressing a complex set of markers, that during development migrate, differentiate and integrate into cortico-striatal networks [7, 79]. Mutations in the Distal-less homeobox (Dlx) gene, which is critical for GABAergic interneuron migration, has been found in ASD patients [80]. Dlx genes are the only family of genes related to migration and differentiation found to be altered in ASD patients. Similarly, Dlx1 KO mice have their interneuron migratory system completely disrupted [81]. Absence of CNTNAP2 in mice produces aberrant migration of GABAergic INs [72], whereas patients with mutations in this gene present ectopic neurons [80, 82]. We have already described previous evidence that supports a reduction of PV+ cells in ASD in mice and humans. Thus, it seems plausible that reduction of PV+ INs in the ASD brain could be due to a deficiency in the migratory system during development. Nevertheless, we cannot discard the possibility that failure to integrate into functional neural circuits (and posterior cell death and removal) might result instead from mutations in scaffold proteins. Mild reductions in the levels of PV gene have been observed in humans with ASD [83], a similar observation was made in certain preclinical models (e.g., Shank3 and Shank1 KO mice) but without reduction in the number of PV+ INs [84]. Notably, there is still no rationale or suitable hypothesis about why mutations in a scaffold protein can lead to PV down regulation or migration abnormalities.
It seems clear that various GABAergic interneuron abnormalities result in similar effects on behavior. Unfortunately, the heterogeneity of genetic factors that lead to GABAergic abnormalities in ASD does not help to understand why interneuron pathology occurs. Although a full understanding of different putative mechanisms could open alternative clinical approaches to treatment, in general, reestablishing normal GABAergic tone is the ultimate therapeutic target. In this vein, there continue to be advances in stem cell therapies to transplant GABAergic INs to treat psychiatric disorders [85]. Importantly, this approach is valuable since it has been shown that stem cells can be reprogrammed to INs in vivo and in vitro [86–88]. Although, reduction of PV+ INsin ASD patients occurs in discrete brain regions [61, 62] we still cannot discard that is happening in other regions, being this is a substantial pitfall for treatment. Thinking that only interneuron dysfunction or a rare gene mutation are the sole cause for ASD would be a simplistic and narrow-minded way to approach this disorder. Studies in patients with TS showed that the number of PV+ cells is reduced without changes in the expression of the gene, whereas in the case of Chat+ INs there was a reduction in cell number and expression of the Chat gene [50, 51].
Tourette’s Syndrome
Relative to the evidence in ASD, it is clearer in the case of TS that loss of PV+ INs occur. Conversely, for Chat+INs in TS the date is harder to interpret because of a dual contribution of low expression of Chat and reduction in cell number. Thus, it will be of importance to identify, in TS patients, other genes (besides CTNAP2) that are not related to neuronal migration, especially since there are no studies investigating abnormalities and/or reporting disease linked modulation of factors that modulate striatal cholinergic differentiation, such as the Lhx7 gene. Perhaps one of the most intriguing questions regarding TS and ASD is that even though interneuron pathology is described in the cortico-striatal circuits in both neurodevelopmental disorders (Figure 1), patients with TS do not develop social deficits. Given that TS patients have cortical abnormalities in somatosensory, orbital and medial cortex similar to those reported in ASD [41, 89] there must be an additional disturbance in the neural circuits of patients with ASD to produce these additional symptoms.
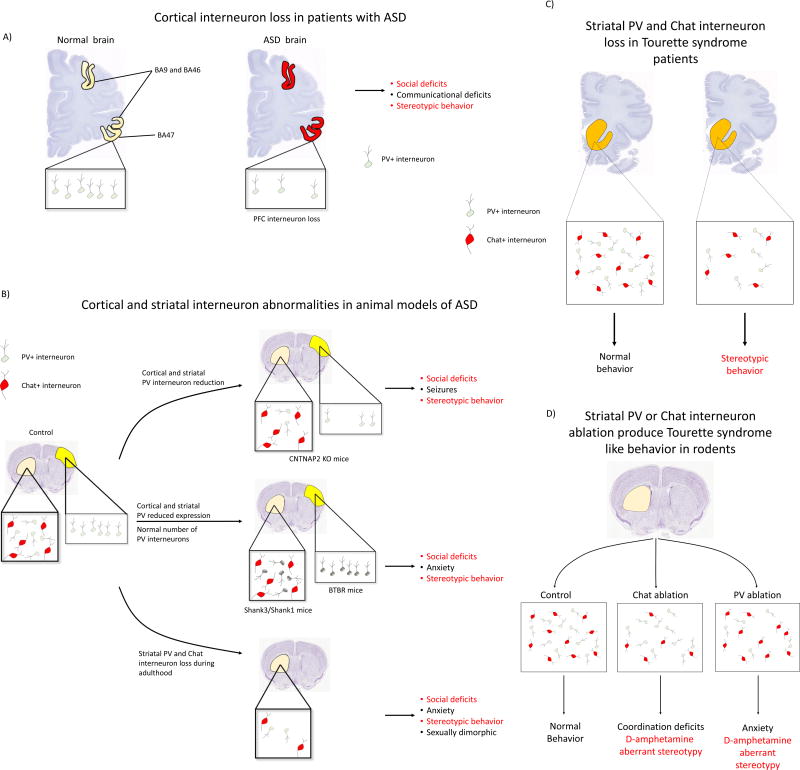
Figure 1.
INs alterations in ASD and TS, from humans to rodent models. A) Medial prefrontal cortex loss of PV+ INs in Broadmann areas (BA) BA46, BA47, and BA9 [62] has been found in postmortem studies in patients with ASD. B) Somatosensory cortices and striatum are affected by either PV interneuron death or PV lower levels of expression in rodent models of ASD. Different KO mice based on ASD genetic abnormalities found in ASD patients showed disruption in PV INs . Deletions in the shank3 and shank1 mice produces a reduction in the levels of PV expression without cell loss. In the CNTNAP2 KO mice, another well validated model of ASD, there is a reduction in the number of PV+ INs in the striatum and somatosensory cortex, where the main issue it seems to be migration impaired migration of progenitors. The idiopathic spontaneous inbred BTBR mice have a reduction in the expression of PV only in somatosensory cortices without alterations in other regions. Adulthood striatal ablation of PV and Chat INs showed to produces ASD behaviors like stereotypy and social deficits in a sexually dimorphic way, proposing a novel role of striatal INs in brain physiology. These different animals models of ASD shows a potential an exciting shared alteration that produces stereotypic behaviors and social deficits (in red): cortical and/or striatal INs abnormalities as a common denominator for ASD. C) Post mortem studies in TSpatients shows a significant reduction in the anumber of Chat and PV striatal INs. D) Striatal ablation of either PV or Chat INs mimics part of the core behaviors observed in TS like stereotypy. Single depletion of either type of these interneurons does not produce basal stereotypy, nevertheless this kind of behavior is strongly exacerbated after d-amphetamine administration.
Shared features
It is still an open question how PV+ and/or Chat+ INs contribute specifically to social deficits and stereotypic behaviors. It has been shown that both types contribute synergistically to produce those behaviors during adulthood when they are reduced in the striatum [65], but we need to know how reductions of only PV+ INs in cortical structures in ASD rodent models contribute to either of those behaviors. Interestingly, recent studies support a new alternative hypothesis about an autistic striatum [10]. These data showed that striatal restoration of shank3 during adulthood rescues social deficits and stereotypic behavior [90]. Disruption of striatal E/I balance during adulthood by PV and Chat interneuron ablation produced ASD-like behaviors [65], being this evidenced by striatal overactivation and aberrant signaling. Therefore, we and others suggest that ASD should no longer be seen as a disorder with an etiology predominantly cortical, but more as a cortico-striatal dysfunction (Figure 2). Nevertheless, the specific circuit disruption that results in disruption of the cortico-striatal neural circuits involved in social and stereotypic behaviors remains to be identified. There is also the necessity to study more thoroughly the role of the striatum as an alternative structure as trigger of ASD, or at least as a critical region to this pathology (Figure 2). This is not the case for TS, where we have a clearer idea where in the striatum the circuitry is altered and then triggers tics (Figure 2). However, we are still far from a full understanding of, and effective treatments too, this disorder.
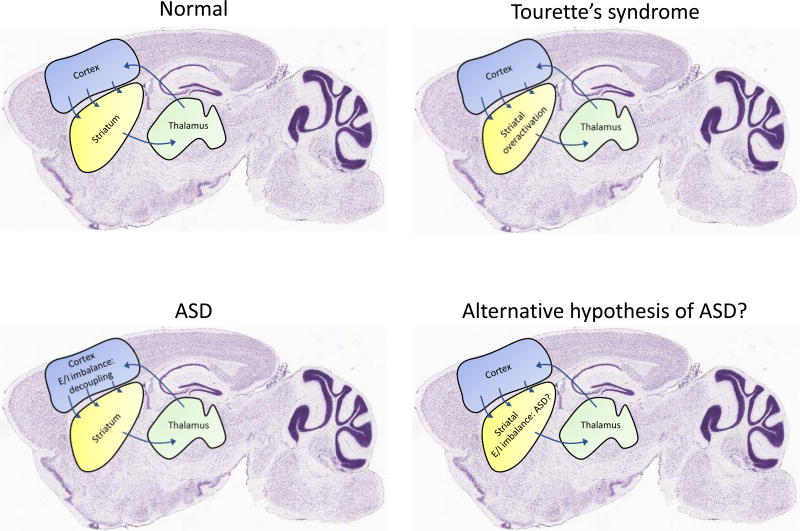
Figure 2.
Different hypothesis of ASD and TS brain functioning. Diagram of the cortico-striatal region specific alterations involved in TS and ASD. In the normal brain (top left panel) cortico-striatal-thalamic loops are balanced in such way to produce the appropriate behavioral output. It is proposed that TS symptomatology emerges from an overactivation in the striatum (top right), particularly due to an increment in the direct pathway activity and inhibition of the indirect pathway that eventually leads also to cortical processing deficits. ASD is characterized by having an excitatory and inhibitory imbalance in the cortex (shifted towards excitatory) that generate social deficits and stereotypic behavior. Another interesting hypothesis is the one that proposes a pivotal role of the striatum in the pathophysiology of ASD [10], and that this could occur due to INs dysfunction that triggers a E/I imbalance [65].
Conclusions and future directions
Based on the genetic studies performed in humans, the etiology of ASD and TS seems to be heterogeneous and complex making it hard to find an effective treatment. The use of preclinical models to formulate a better understanding of the progressive molecular mechanisms involved, from development to adulthood, and resultant neuronal abnormalities during different stages of development is critical to obtain better treatments in the short term. Preclinical studies will have to focus on how to find feasible early intervention strategies, either via pharmacological treatments or stem cell therapies (for return of PV+ and/or Chat+ interneuron number to normal range), to reestablish the E/I balance.. Particularly, work should focus on therapeutic approaches aimed at reducing the loss of INs, or at minimizing the impact of interneuron dysfunction, to ameliorate social deficits and stereotypic behaviors.
Trends.
Interneuron dysfunction in cortico-striatal circuits leads to TS and ASD.
Differences in the location of these abnormalities could be the key that can lead to either TS or ASD.
Reduced GABAergic tone in both disorders generates an E/I imbalance that produces the core behaviors in both disorders.
There is strong evidence for a critical role of the striatum in TS, but only recently, a series of studies have demonstrated a new hypothesis of the striatum as a key participant in ASD.
Outstanding Question Box.
Is normalization of GABAergic tone the main therapeutic feasible target for ASD?
Is interneuron dysfunction a consequence of genetic abnormalities not yet discovered?
Is the absence of PV interneurons or reduction of PV levels responsible for affecting the brain E/I balance in ASD?
How striatal circuitry and its interneurons modulate social behaviors?
Does TS and ASD have common circuit deficiencies that are differentially affected by environmental factor and then results to different symptomatology?
Acknowledgments
This work has been supported by Yale University and the State of Connecticut (CP). MR was supported 2016 NARSAD Young Investigator Award from Brain and Behavior Foundations.
GLOSSARY
- Parvalbumin
a small calcium binding protein that controls calcium transients highly expressed in fast spiking interneurons.
- Somatostatin
neuropeptide secreted by a specific subtype of brain interneurons that exerts an inhibitory effect through its multiple receptors. Specifically used as a marker to differentiate between interneurons.
- neuronal nitric oxide synthase
neuronal isoform of the enzyme that synthetizes nitric oxide in the brain. Expressed only in certain subtype of interneurons.
- pyramidal cells
principal type of excitatory neurons in cortical, thalamic and hippocampal regions.
- GABA
the main and most important inhibitory neurotransmitter in the brain.
- Acetylcholine
it is a molecule widely distributed in the body derived from choline acetylation, but in the brain, works as a neurotransmitter that can be either excitatory or inhibitory based on the target receptor.
- choline acetyltransferase
enzyme responsible for the synthesis of acetylcholine. It is also a marker for cholinergic interneurons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartolini G, et al. Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron. 2013;79(5):849–64. doi: 10.1016/j.neuron.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Petilla Interneuron Nomenclature, G et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9(7):557–68. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson C, et al. The mouse nervous system. 1. Elsevier Academic Press; 2012. [Google Scholar]
- 4.Zingg B, et al. Neural networks of the mouse neocortex. Cell. 2014;156(5):1096–111. doi: 10.1016/j.cell.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyagi R. Neural Networks of the Mouse Neocortex Cell. 2014 Feb 27;156(5):1096-111. Ann Neurosci. 2015;22(4):238. doi: 10.5214/ans.0972.7531.220409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 7.Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505(7483):318–26. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tricoire L, Vitalis T. Neuronal nitric oxide synthase expressing neurons: a journey from birth to neuronal circuits. Front Neural Circuits. 2012;6:82. doi: 10.3389/fncir.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helie S, et al. Learning robust cortico-cortical associations with the basal ganglia: an integrative review. Cortex. 2015;64:123–35. doi: 10.1016/j.cortex.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Fuccillo MV. Striatal Circuits as a Common Node for Autism Pathophysiology. Front Neurosci. 2016;10:27. doi: 10.3389/fnins.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox SM, et al. Striatal D1 and D2 signaling differentially predict learning from positive and negative outcomes. Neuroimage. 2015;109:95–101. doi: 10.1016/j.neuroimage.2014.12.070. [DOI] [PubMed] [Google Scholar]
- 12.Ahmari SE, Dougherty DD. Dissecting Ocd Circuits: From Animal Models to Targeted Treatments. Depress Anxiety. 2015;32(8):550–62. doi: 10.1002/da.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabresi P, et al. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci. 2014;17(8):1022–30. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- 14.Xenias HS, et al. Are striatal tyrosine hydroxylase interneurons dopaminergic? J Neurosci. 2015;35(16):6584–99. doi: 10.1523/JNEUROSCI.0195-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim SA, et al. Striatal cholinergic interneuron regulation and circuit effects. Front Synaptic Neurosci. 2014;6:22. doi: 10.3389/fnsyn.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin X, Costa RM. Shaping action sequences in basal ganglia circuits. Curr Opin Neurobiol. 2015;33:188–96. doi: 10.1016/j.conb.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dehghani N, et al. Dynamic Balance of Excitation and Inhibition in Human and Monkey Neocortex. Sci Rep. 2016;6:23176. doi: 10.1038/srep23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubota Y, et al. The Diversity of Cortical Inhibitory Synapses. Front Neural Circuits. 2016;10:27. doi: 10.3389/fncir.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen CC, Crochet S. Synaptic computation and sensory processing in neocortical layer 2/3. Neuron. 2013;78(1):28–48. doi: 10.1016/j.neuron.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Pronneke A, et al. Characterizing VIP Neurons in the Barrel Cortex of VIPcre/tdTomato Mice Reveals Layer-Specific Differences. Cereb Cortex. 2015;25(12):4854–68. doi: 10.1093/cercor/bhv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naka A, Adesnik H. Inhibitory Circuits in Cortical Layer 5. Front Neural Circuits. 2016;10:35. doi: 10.3389/fncir.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu Y, et al. A cortical circuit for gain control by behavioral state. Cell. 2014;156(6):1139–52. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, et al. Layer 6 corticothalamic neurons activate a cortical output layer, layer 5a. J Neurosci. 2014;34(29):9656–64. doi: 10.1523/JNEUROSCI.1325-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pi HJ, et al. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503(7477):521–4. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfeffer CK, et al. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16(8):1068–76. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzales KK, Smith Y. Cholinergic interneurons in the dorsal and ventral striatum: anatomical and functional considerations in normal and diseased conditions. Ann N Y Acad Sci. 2015;1349:1–45. doi: 10.1111/nyas.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tecuapetla F, et al. Complementary Contributions of Striatal Projection Pathways to Action Initiation and Execution. Cell. 2016;166(3):703–15. doi: 10.1016/j.cell.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Straub C, et al. Principles of Synaptic Organization of GABAergic Interneurons in the Striatum. Neuron. 2016;92(1):84–92. doi: 10.1016/j.neuron.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faust TW, et al. Neostriatal GABAergic Interneurons Mediate Cholinergic Inhibition of Spiny Projection Neurons. J Neurosci. 2016;36(36):9505–11. doi: 10.1523/JNEUROSCI.0466-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosillo P, et al. Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sciamanna G, et al. Optogenetic stimulation reveals distinct modulatory properties of thalamostriatal vs corticostriatal glutamatergic inputs to fast-spiking interneurons. Sci Rep. 2015;5:16742. doi: 10.1038/srep16742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wall NR, et al. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. 2013;79(2):347–60. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Association AP. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013. [Google Scholar]
- 34.CDC. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Surveillance Summaries. 2016;(63):1–21. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scharf JM, et al. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord. 2015;30(2):221–8. doi: 10.1002/mds.26089. [DOI] [PubMed] [Google Scholar]
- 36.Capuano A, Valeri G. Tics and Tourette Syndrome in Autism Spectrum Disorder. In: Mazzone L, Vitiello B, editors. Psychiatric Symptoms and Comorbidities in Autism Spectrum Disorder. Springer International Publishing; 2016. pp. 93–109. [Google Scholar]
- 37.Hanks CE, et al. Social Deficits and Autism Spectrum Disorders in Tourette’s Syndrome. Current Developmental Disorders Reports. 2015;2(4):285–292. [Google Scholar]
- 38.Kalyva E, et al. A review of co-occurrence of autism spectrum disorder and Tourette syndrome. Research in Autism Spectrum Disorders. 2016;24:39–51. [Google Scholar]
- 39.Eapen V, et al. Comorbidities, Social Impact, and Quality of Life in Tourette Syndrome. Front Psychiatry. 2016;7:97. doi: 10.3389/fpsyt.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Worbe Y, et al. Altered structural connectivity of cortico-striato-pallido-thalamic networks in Gilles de la Tourette syndrome. Brain. 2015;138(Pt 2):472–82. doi: 10.1093/brain/awu311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greene DJ, et al. Brain structure in pediatric Tourette syndrome. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller-Vahl KR, et al. Tics are caused by alterations in prefrontal areas, thalamus and putamen, while changes in the cingulate gyrus reflect secondary compensatory mechanisms. BMC Neurosci. 2014;15:6. doi: 10.1186/1471-2202-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conti E, et al. Network over-connectivity differentiates autism spectrum disorder from other developmental disorders in toddlers: A diffusion MRI study. Hum Brain Mapp. 2017 doi: 10.1002/hbm.23520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuetze M, et al. Morphological Alterations in the Thalamus, Striatum, and Pallidum in Autism Spectrum Disorder. Neuropsychopharmacology. 2016;41(11):2627–37. doi: 10.1038/npp.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langen M, et al. Changes in the development of striatum are involved in repetitive behavior in autism. Biol Psychiatry. 2014;76(5):405–11. doi: 10.1016/j.biopsych.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Mataix-Cols D, et al. Familial Risks of Tourette Syndrome and Chronic Tic Disorders. A Population-Based Cohort Study. JAMA Psychiatry. 2015;72(8):787–93. doi: 10.1001/jamapsychiatry.2015.0627. [DOI] [PubMed] [Google Scholar]
- 47.Georgitsi M, et al. The Genetic Etiology of Tourette Syndrome: Large-Scale Collaborative Efforts on the Precipice of Discovery. Front Neurosci. 2016;10:351. doi: 10.3389/fnins.2016.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Church JA, Schlaggar BL. Pediatric Tourette syndrome: insights from recent neuroimaging studies. J Obsessive Compuls Relat Disord. 2014;3(4):386–393. doi: 10.1016/j.jocrd.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lerner A, et al. Widespread abnormality of the gamma-aminobutyric acid-ergic system in Tourette syndrome. Brain. 2012;135(Pt 6):1926–36. doi: 10.1093/brain/aws104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kataoka Y, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518(3):277–91. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lennington JB, et al. Transcriptome Analysis of the Human Striatum in Tourette Syndrome. Biol Psychiatry. 2016;79(5):372–82. doi: 10.1016/j.biopsych.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson EB, et al. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet. 2016;48(5):552–5. doi: 10.1038/ng.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaugler T, et al. Most genetic risk for autism resides with common variation. Nat Genet. 2014;46(8):881–5. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de la Torre-Ubieta L, et al. Advancing the understanding of autism disease mechanisms through genetics. Nat Med. 2016;22(4):345–61. doi: 10.1038/nm.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puts NAJ, et al. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Research. 2016 doi: 10.1002/aur.1691. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Port RG, et al. Exploring the relationship between cortical GABA concentrations, auditory gamma-band responses and development in ASD: Evidence for an altered maturational trajectory in ASD. Autism Research. 2016 doi: 10.1002/aur.1686. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robertson CE, et al. Reduced GABAergic Action in the Autistic Brain. Curr Biol. 2016;26(1):80–5. doi: 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 58.Harada M, et al. Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J Autism Dev Disord. 2011;41(4):447–54. doi: 10.1007/s10803-010-1065-0. [DOI] [PubMed] [Google Scholar]
- 59.Gaetz W, et al. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 2014;86:1–9. doi: 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zurcher NR, et al. A systematic review of molecular imaging (PET and SPECT) in autism spectrum disorder: current state and future research opportunities. Neurosci Biobehav Rev. 2015;52:56–73. doi: 10.1016/j.neubiorev.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Ariza J, et al. The Number of Chandelier and Basket Cells Are Differentially Decreased in Prefrontal Cortex in Autism. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hashemi E, et al. The Number of Parvalbumin-Expressing Interneurons Is Decreased in the Medial Prefrontal Cortex in Autism. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson SB, Valakh V. Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron. 2015;87(4):684–98. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McBride SD, Parker MO. The disrupted basal ganglia and behavioural control: an integrative cross-domain perspective of spontaneous stereotypy. Behav Brain Res. 2015;276:45–58. doi: 10.1016/j.bbr.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 65.Rapanelli M, et al. Targeted Interneuron Depletion in the Dorsal Striatum Produces Autism-Like Behavioral Abnormalities in Male But not Female Mice. Biological Psychiatry. doi: 10.1016/j.biopsych.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu M, et al. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc Natl Acad Sci U S A. 2015;112(3):893–8. doi: 10.1073/pnas.1419533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu M. Ablation of fast-spiking interneurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience. 2016;324:321–9. doi: 10.1016/j.neuroscience.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaramillo TC, et al. Novel Shank3 mutant exhibits behaviors with face validity for autism and altered striatal and hippocampal function. Autism Res. 2017;10(1):42–65. doi: 10.1002/aur.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duffney LJ, et al. Autism-like Deficits in Shank3-Deficient Mice Are Rescued by Targeting Actin Regulators. Cell Rep. 2015;11(9):1400–13. doi: 10.1016/j.celrep.2015.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peca J, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472(7344):437–42. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gogolla N, et al. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron. 2014;83(4):894–905. doi: 10.1016/j.neuron.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Penagarikano O, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147(1):235–46. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jurgensen S, Castillo PE. Selective Dysregulation of Hippocampal Inhibition in the Mouse Lacking Autism Candidate Gene CNTNAP2. J Neurosci. 2015;35(43):14681–7. doi: 10.1523/JNEUROSCI.1666-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varea O, et al. Synaptic abnormalities and cytoplasmic glutamate receptor aggregates in contactin associated protein-like 2/Caspr2 knockout neurons. Proc Natl Acad Sci U S A. 2015;112(19):6176–81. doi: 10.1073/pnas.1423205112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lauber E, et al. Prenatal Valproate Exposure Differentially Affects Parvalbumin-Expressing Neurons and Related Circuits in the Cortex and Striatum of Mice. Front Mol Neurosci. 2016;9:150. doi: 10.3389/fnmol.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han S, et al. Enhancement of inhibitory neurotransmission by GABAA receptors having alpha2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron. 2014;81(6):1282–9. doi: 10.1016/j.neuron.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wohr M, et al. Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Transl Psychiatry. 2015;5:e525. doi: 10.1038/tp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ito-Ishida A, et al. Loss of MeCP2 in Parvalbumin-and Somatostatin-Expressing Neurons in Mice Leads to Distinct Rett Syndrome-like Phenotypes. Neuron. 2015;88(4):651–8. doi: 10.1016/j.neuron.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopes R, et al. Transcription factor LIM homeobox 7 (Lhx7) maintains subtype identity of cholinergic interneurons in the mammalian striatum. Proc Natl Acad Sci U S A. 2012;109(8):3119–24. doi: 10.1073/pnas.1109251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reiner O, et al. Regulation of neuronal migration, an emerging topic in autism spectrum disorders. J Neurochem. 2016;136(3):440–56. doi: 10.1111/jnc.13403. [DOI] [PubMed] [Google Scholar]
- 81.Cobos I, et al. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8(8):1059–68. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- 82.Strauss KA, et al. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354(13):1370–7. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- 83.Stoner R, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370(13):1209–19. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Filice F, et al. Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism. Molecular Brain. 2016;9:10. doi: 10.1186/s13041-016-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chohan MO, Moore H. Interneuron Progenitor Transplantation to Treat CNS Dysfunction. Front Neural Circuits. 2016;10:64. doi: 10.3389/fncir.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arber C, Li M. Cortical interneurons from human pluripotent stem cells: prospects for neurological and psychiatric disease. Front Cell Neurosci. 2013;7:10. doi: 10.3389/fncel.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oki K, et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30(6):1120–33. doi: 10.1002/stem.1104. [DOI] [PubMed] [Google Scholar]
- 88.Tang Y, et al. Cortical plasticity induced by transplantation of embryonic somatostatin or parvalbumin interneurons. Proc Natl Acad Sci U S A. 2014;111(51):18339–44. doi: 10.1073/pnas.1421844112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fahim C, et al. Somatosensory-motor bodily representation cortical thinning in Tourette: effects of tic severity, age and gender. Cortex. 2010;46(6):750–60. doi: 10.1016/j.cortex.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 90.Mei Y, et al. Adult restoration of Shank3 expression rescues selective autistic-like phenotypes. Nature. 2016;530(7591):481–4. doi: 10.1038/nature16971. [DOI] [PMC free article] [PubMed] [Google Scholar]