Abstract
Purpose
Satisfaction with social resources, or “social well-being,” relates to better adaptation and longer survival after breast cancer diagnosis. Biobehavioral mechanisms linking social well-being (SWB) to mental and physical health may involve inflammatory signaling. We tested whether reports of greater SWB were associated with lower levels of pro-inflammatory and pro-metastatic leukocyte gene expression after surgery for non-metastatic breast cancer.
Methods
Women (N = 50) diagnosed with non-metastatic (0–III) breast cancer were enrolled 2–8 weeks after surgery. SWB was assessed with the Social/Family Well-Being subscale of the FACT-B. Leukocyte gene expression for specific pro-inflammatory (cytokines, chemokines, and COX-2) and pro-metastatic genes (e.g., MMP-9) was derived from microarray analysis.
Results
Multiple regression analyses controlling for age, stage of disease, days since surgery, education, and body mass index (BMI) found higher levels of SWB related to less leukocyte pro-inflammatory and pro-metastatic gene expression (p < 0.05). Emotional well-being, physical well-being, and functional well-being did not relate to leukocyte gene expression (p > 0.05). Greater SWB remained significantly associated with less leukocyte pro-inflammatory and pro-metastatic gene expression after controlling for depressive symptoms.
Conclusions
Results have implications for understanding mechanisms linking social resources to health-relevant biological processes in breast cancer patients undergoing primary treatment.
Keywords: Breast cancer, social well-being, social support, inflammation, leukocyte gene expression
Introduction
Breast cancer is the second leading cause of cancer deaths among women [1]. Almost half these women experience significant adversity during diagnosis and treatment [2]. Smaller social networks and perceptions of inadequate social resources may deleteriously affect both their psychological adaptation [3] and survival [4, 5]. Limited social networks [6], low social support [7], and low social well-being (SWB) [8] are all associated with increased mortality in breast cancer. Additional research is needed to uncover processes that explain negative health outcomes in breast cancer patients reporting deficits in social resources.
Social isolation is associated with alterations in sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis hormones that influence tumor growth and clinical outcomes [9]. One pathway through which these relationships may be mediated is inflammatory signaling by stromal cells such as leukocytes that may interact with cancer cells to promote angiogenesis [10], invasion, extravasation into the circulatory system, and metastasis [11]. Although production of glucocorticoids is often associated with anti-inflammatory effects, chronic social isolation and adversity and accompanying glucocorticoid elevation are associated with upregulated inflammation wherein leukocytes become desensitized to chronically elevated cortisol release, and transcription of genes coding inflammatory cytokines is no longer inhibited [12].
Hughes et al. [13] demonstrated that lower perceived support before treatment for non-metastatic breast cancer predicted more serum interleukin-6 (IL-6), a pro-inflammatory cytokine, at 6-month follow-up. Upregulated leukocyte conserved transcriptional response to adversity (CTRA), which includes upregulation of pro-inflammatory genes, is associated with greater loneliness in older adults [14], and with low socioeconomic status (SES) in patients awaiting hematopoietic stem cell transplant [15]. High CTRA expression in turn relates to decreased leukemia-free survival [15]. This line of research suggests that chronic social adversity can impair a regulatory mechanism for inflammation, leading to increased levels of inflammation. It follows that a greater sense of SWB may be associated with better inflammatory control, though this has not been studied in the context of cancer generally or in breast cancer specifically.
The present study examined whether SWB related to less leukocyte gene expression for pro-inflammatory and pro-metastatic signaling in women who recently underwent surgery for non-metastatic breast cancer. We hypothesized that women reporting greater SWB would exhibit less expression of leukocyte genes for pro-inflammatory cytokines, chemokines and their receptors, and other pro-inflammatory and tumor-promoting factors. To explore potential pathways connecting SWB to leukocyte gene expression, we repeated the analyses controlling for depression, given prior links between depressive symptoms and inflammatory indicators [16]. Exploratory analyses assessed whether SWB mediated the relationship between depression and leukocyte gene expression. We also explored whether partnered versus non-partnered women would show different SWB by gene expression associations.
Materials and Methods
Participants
Women were recruited from cancer treatment centers and surgical oncologist offices in South Florida to participate in a larger parent study testing the effects of stress management in breast cancer. Women were eligible if they were 2–8 weeks post-surgery for non-metastatic (0–III) breast cancer. Phone screens excluded women who reported a prior history of cancer (except minor skin cancers); had already received radiation treatment, immunotherapy or chemotherapy; were diagnosed with stage IV cancer (metastatic disease); did not speak fluent English; had a severe psychiatric disorder (e.g., psychosis, major depressive disorder), or endorsed suicidality. Data from a subgroup of 78 women who had cryopreserved peripheral blood mononuclear cells (PBMCs) described previously [17] were used in the present analyses.
Procedures
Questionnaires that measured SWB and sociodemographic characteristics are detailed below. A blood sample was collected between 4:00pm and 6:30pm, to minimize the impact of diurnal fluctuations. Vacutainer tubes containing sodium heparin as an anticoagulant (BD catalog # 367874) were used for blood collection. The study was approved by the Institutional Review Board at the University of Miami, and women were compensated $50.
Measures
Demographics
Participants self-reported age, stage of disease, number of days since surgery, and education on a questionnaire at study entry. Body mass index (BMI) was calculated based on self-reported height and weight. Demographic, medical, and treatment-related information was validated through medical chart reviews.
Well-Being
The Functional Assessment of Cancer Therapy – Breast (FACT-B) Version 4 assessed self-reported well-being over the prior 7 [18]. The Social/Family Well-Being subscale measured SWB with items primarily assessing subjective feelings of being close to and generally supported by and satisfied with communication with family and friends. It consists of 7 items with 5 response choices ranging from 1 (not at all) to 5 (very much). Subscales were scored as described by Webster, Cella, & Yost [19]. It was found to be reliable and valid in women diagnosed with breast cancer [18], and the SWB subscale had adequate internal consistency (α = 0.79) in this sample. The SWB subscale was associated with less inflammatory cell-signaling in women diagnosed with ovarian cancer [20]. The Emotional Well-Being, Physical Well-Being, and Functional Well-Being subscales were administered and were analyzed to assess specificity of the relationship between SWB and leukocyte gene expression. Internal consistency of the Emotional Well-Being (α = 0.64), Physical Well-Being (α = 0.83), and Functional Well-Being (α = 0.85) subscales were adequate.
Depressive symptoms
Interviewers administered the 17-item Hamilton Rating Scale for Depression (HRSD) [21] to assess depressive symptoms. This measure has previously been used in studies of women with breast cancer [22] and reliability was adequate in this sample (α = 0.80).
Leukocyte Gene Expression
We examined leukocyte RNA expression of pro-inflammatory cytokine genes (IL1A, IL1B, IL6, TNFSF10, TNFRSF21, and PTGS2/COX-2), genes for pro-inflammatory chemokines and their receptors (CCL3, CCL7, CCL20, CCL3L1, CCL4L2, and CXCR7), and genes for other tumor-promoting factors (MMP9 and LMNA) in circulating peripheral blood mononuclear cells (PBMCs). Table 1 describes each gene’s function. Genes were selected due to their prior association with psychosocial processes [17], and their central function in inflammation and putative involvement in promoting cancer metastasis [23].
Table 1.
Gene symbols defined by description and function.
| Gene Symbol | Gene Description | Gene Function [47, 48]. |
|---|---|---|
| Pro-Inflammatory Cytokines | ||
| IL1A | Interleukin 1 Alpha | Encodes cytokine IL-1α, which is produced by white blood cells (leukocytes) in response to wounds and contributes to inflammation and programmed cell death (apoptosis). |
| IL1B | Interleukin 1 Beta | Encodes cytokine IL-1β, which is produced by leukocytes and contributes to inflammation and programmed cell death (apoptosis). |
| IL6 | Interleukin 6 | Encodes cytokine IL-6, which is produced during inflammation and induces further inflammatory transcription. |
| TNFSF10 | Tumor Necrosis Factor (Ligand) Superfamily, Member 10 | Encodes a cytokine that induces apoptosis in tumor cells. |
| TNFRSF21 | Tumor Necrosis Factor Receptor Superfamily, Member 21 | Encodes a member of the TNF Receptor Superfamily that induces apoptosis and regulates immune functioning. |
| PTGS2/COX-2 | Prostaglandin-Endoperoxide Synthase 2 | Encodes an enzyme involved in synthesis of a prostaglandin (cyclooxygenase-2; COX-2), which acts as a hormone to stimulate inflammation and cell division. |
| Pro-Inflammatory Chemokines | ||
| CCL3 | Chemokine (C-C motif) Ligand 3 | Encodes a chemokine that signals recruitment of immune cells to sites of inflammation. |
| CCL7 | Chemokine (C-C Motif) Ligand 7 | Encodes a chemokine that attracts macrophages during inflammation and metastasis. |
| CCL20 | Chemokine (C-C motif) Ligand 20 | Encodes a chemokine that signals movement of white blood cells; involved in inflammation. |
| CCL3L1 | Chemokine (C-C Motif) Ligand 3-Like 1 | Encodes a pro-inflammatory chemokine that regulates immune functioning. |
| CCL4L2 | Chemokine (C-C Motif) Ligand 4-Like 2 | Encodes a pro-inflammatory chemokine involved in immune regulation. |
| CXCR7 | C-X-C Chemokine Receptor Type 7 | Encodes a pro-inflammatory chemokine receptor; regulates migration of tumor cells. |
| Pro-Metastatic Factors | ||
| MMP9 | Matrix Metallopeptidase 9 | Encodes proteins that facilitate the breakdown of the extracellular matrix in the context of tissue remodeling and metastasis. |
| LMNA | Lamin A/C | Encodes proteins that provide structure near the inner nuclear membrane of a cell. Involved in tissue remodeling. |
To generate RNA expression units (log2), RNA was extracted from PBMCs, quality assured for mass and integrity, and assayed by Illumina Human HT-12 v3 Expression BeadChips, with gene abundance estimates derived from low-level fluorescence intensity values, quantile normalized with Illumina Genome Studio software, and log2-transformed for analysis, as previously described [24, 25]. Composite scores of gene expression for pro-inflammatory cytokines, pro-inflammatory chemokines and their receptors, and tumor-promoting factors were created by averaging the normalized log2-transformed transcript abundance estimates for genes in each of these three categories based on their known function.
Data Analytic Approach
Data were analyzed using IBM SPSS Version 22.00. Descriptive statistics characterized participants’ demographic, medical, and study variables. Outliers > 3 standard deviations outside the mean were winsorized [26] and variables were then analyzed for normal distribution (skewness < 3.0, kurtosis < 8.0) [27]. Independent sample t-tests and chi-square tests determined whether this subsample differed from the parent study’s sample of 240 women on demographic and medical variables. Bivariate correlations were conducted to determine associations between SWB and theoretically related variables, specifically depression.
Primary analyses used multiple regression to test whether SWB was associated with pro-inflammatory and pro-metastatic leukocyte gene expression. Age, stage of disease, days since surgery, education, and BMI were included as covariates based on theoretical associations with inflammation [28]. Analyses were repeated controlling for depression. Secondary analyses used multiple regression to test whether other psychosocial variables, emotional well-being, physical well-being, functional well-being, and depression, might also be related to gene expression using the same covariates.
Exploratory analyses were conducted to determine whether SWB mediated the association between depression and gene expression. To test the generality of the SWB and gene expression associations, moderation analyses examined whether associations between SWB and gene expression varied as a function of structural sources of social support (partner status). Step 1 consisted of covariates age, days since surgery, stage, education, and BMI. Step 2 consisted of SWB and partner status, and Step 3 contained the interaction of SWB and partner status. The Benjamini-Hochberg procedure [29] was applied to the results of each analysis to correct for multiple comparisons by controlling the false discovery rate to 0.10 [30].
Results
Sample Characterization
Sample characteristics are displayed in Table 2. Participants were middle-aged (M = 49.55, SD = 7.51) with an average of 15.86 years of education (SD = 2.58). The majority self-identified as non-Hispanic White (69.2%), and the sample also included Hispanic (20.5%) and African American/Black women (9.0%). Most women were married or partnered (67.9%). Approximately one third of participants had children (30.8%), consistent with Florida state population norms at the time of data collection [31]. Average number of children was 2.11 (SD =0.85).
Table 2.
Demographics, medical characteristics, and key study variables of the participants (N = 78).
| Variable | Mean (SD) | Range | |
|---|---|---|---|
| Sociodemographics | |||
|
| |||
| Age after surgery (years) | 49.55 (7.51) | 32.00 – 69.00 | |
|
| |||
| Years of Education | 15.86 (2.58) | 8.00 – 23.00 | |
|
| |||
| Employment | Employed full time | 64 (82.1%) | – |
| Not employed full time | 14 (17.9%) | – | |
|
| |||
| Income (thousands of US dollars) | 76.19 (49.20) | 15.00 – 300.00 | |
|
| |||
| Ethnic Identification | Non-Hispanic White | 54 (69.2%) | – |
| Hispanic/Latino | 16 (20.5%) | – | |
| African American/Black | 7 (9.0%) | – | |
| Other | 1 (1.3%) | – | |
|
| |||
| Marital Status | Married/Partnered | 53 (67.9%) | – |
| Separated | 2 (2.6%) | – | |
| Divorced | 19 (24.4%) | – | |
| Single | 4 (5.1%) | – | |
|
| |||
| Children | Yes | 24 (30.8%) | – |
| No | 54 (69.2%) | – | |
|
| |||
| Number of Children | 2.11 (0.85) | 1.00 – 5.00 | |
|
| |||
| Medical Status | |||
|
| |||
| Cancer Stagea | Stage 0 | 10 (12.8%) | – |
| Stage I | 37 (47.4%) | – | |
| Stage II | 24 (30.8%) | – | |
| Stage III | 7 (9.0%) | – | |
|
| |||
| Surgery | Lumpectomy | 34 (43.6%) | – |
| Mastectomy | 44 (56.4%) | – | |
|
| |||
| Days since Surgery | 38.58 (24.22) | 10.00 – 133.00 | |
|
| |||
| Estrogen Receptor Status | Positive | 43 (55.1%) | – |
| Negative | 8 (10.3%) | – | |
| Unknown | 27 (34.6%) | – | |
|
| |||
| Progesterone Receptor Status | Positive | 28 (35.9%) | – |
| Negative | 11 (14.1%) | – | |
| Unknown | 39 (50.0%) | – | |
|
| |||
| HER2/neu Status | Positive | 11 (14.1%) | – |
| Negative | 31 (39.7%) | – | |
| Unknown | 36 (46.2%) | – | |
|
| |||
| Medication Use | Anti-depressant | 5 (6.4%) | – |
| Anti-anxiety | 13 (16.7%) | – | |
| Sleep | 12 (15.4%) | – | |
| Pain | 22 (28.2%) | – | |
|
| |||
| Body Mass Index (kg/m2) | 26.96 (6.49) | 18.88 – 55.81 | |
|
| |||
| Gene Expression | |||
|
| |||
| Cytokine Composite | 10.67 (1.21) | 8.73 – 13.23 | |
|
| |||
| Chemokine Composite | 10.87 (1.29) | 7.84 – 13.04 | |
|
| |||
| Pro-Metastatic Composite | 9.58 (0.86) | 7.52 – 11.36 | |
|
| |||
| IL1A | 9.39 (1.83) | 6.77 – 12.75 | |
|
| |||
| IL1B | 13.05 (1.52) | 8.08 – 14.55 | |
|
| |||
| IL6 | 10.36 (2.03) | 7.26 – 14.95 | |
|
| |||
| TNFSF10 | 9.54 (1.15) | 6.87 – 13.17 | |
|
| |||
| TNFRSF21 | 10.25 (0.90) | 7.75 – 12.38 | |
|
| |||
| CCL3 | 12.83 (1.55) | 8.43 – 15.06 | |
|
| |||
| CCL7 | 10.22 (1.54) | 6.88 – 13.61 | |
|
| |||
| CCL20 | 10.58 (2.02) | 7.40 – 14.00 | |
|
| |||
| CCL3L1 | 12.11 (1.60) | 7.78 – 14.75 | |
|
| |||
| CCL4L2 | 11.35 (1.51) | 8.60 – 13.92 | |
|
| |||
| CXCR7 | 8.09 (0.78) | 7.09 – 10.61 | |
|
| |||
| PTGS2 | 11.45 (1.32) | 9.00 – 13.65 | |
|
| |||
| MMP9 | 9.20 (1.04) | 7.15 – 11.53 | |
|
| |||
| LMNA | 9.96 (0.84) | 7.89 – 11.85 | |
|
| |||
| Survey Data | |||
|
| |||
| Social Well-Being | 22.56 (4.71) | 8.17 – 28.00 | |
| Emotional Well-Being | 17.77 (4.12) | 4.00 – 24.00 | |
| Physical Well-Being | 20.90 (5.21) | 3.00 – 28.00 | |
| Functional Well-Being | 18.75 (5.73) | 2.00 – 28.00 | |
|
| |||
| Hamilton Depression Score | 6.68 (5.52) | 0.00 – 23.00 | |
TNM staging system.
SD = Standard deviation; HP = husband/partner; AW = adult women; CMAF = children and male adult family; FR = friends. Gene expression reported in RNA expression units (log2).
The greatest percentage of women were diagnosed with stage I breast cancer, over half underwent a mastectomy (56.4%) and the remainder a lumpectomy (43.6%). On average, participants were approximately 5 weeks post-surgery at study entry. According to their body mass index (BMI) scores, women were classified as overweight on average (M = 26.96kg/m2, SD = 6.49).
Average IL1A, IL1B, IL6, and CCL20 gene expression appeared higher in our sample than in prior studies of breast cancer survivors who had completed treatment [32], which may be because women in the current sample recently had surgery. Levels of social, emotional, physical, and functional well-being were similar to the sample on which the FACT-B was validated [18], and to a contemporary sample of women with non-metastatic breast cancer [33]. Average HRSD score was 6.68 (SD = 5.52), which is within the normal range for depressive symptoms. HRSD levels of depressive symptoms in this sample were higher than in several samples of healthy controls [34] and lower than in a sample of cancer patients diagnosed with major depressive disorder [35].
3.2. Preliminary Analyses
Independent samples two-tailed t-tests indicated that the subsample of women who provided blood samples for leukocyte gene expression data did not differ significantly from the parent sample on demographic variables and study variables including age, education, annual household income, number of children, days elapsed since breast cancer surgery, BMI, SWB scores, or HRSD scores (all ps > 0.05). The parent sample did not differ from the subsample on categorical variables such as employment status, race/ethnicity, marital status, having children, disease stage, surgery type, estrogen receptor status, progesterone receptor status, HER2/neu status, or use of depression, anxiety, sleep or pain medications (all ps > 0.05).
Within the subsample of 78 participants in the present study, data was complete for SWB, leukocyte gene expression, depression, stage, days since surgery, age, and education. BMI data were incomplete for 35.9% of cases, hence the effective sample size for covariate-adjusted analyses was 50. Women with versus without BMI data did not differ on the pro-inflammatory gene expression composite.
Primary Analyses
We hypothesized that SWB would be significantly related to a down-regulation of the expression of pro-inflammatory and pro-metastatic leukocyte genes when controlling for age, stage of disease, days since surgery, education, and BMI. Results of these multiple regression analyses are displayed in Table 3. With covariates entered in Step 1 and SWB in Step 2, greater SWB was related to lower levels of the pro-inflammatory cytokine gene expression composite (β = −0.33, p < 0.05), the chemokine and chemokine receptor gene expression composite (β = −0.31, p < 0.05), and the pro-metastatic leukocyte gene expression composite (β = −0.46, p < 0.01). At the level of individual genes, greater SWB was associated with lower expression of IL1A (β = −0.40, p < 0.05), CCL20 (β = −0.33, p < 0.05), PTGS2/COX-2 (β = −0.35, p < 0.05), MMP9 (β = −0.35, p < 0.05), and LMNA (β = −0.50, p < 0.01).
Table 3.
Regression analyses relating leukocyte pro-inflammatory and pro-metastatic gene expression and social well-being in multivariable analyses in models (1 – 2) controlling for specified covariates.
| Model | Independent Variable | Dependent Variable | β (SE) | t | p | R2 Change |
|---|---|---|---|---|---|---|
|
1 2 |
Social Well-Being Social Well-Being |
Cytokine Composite*† Cytokine Composite |
−0.328 (0.040) −0.282 (0.040) |
−2.127 −1.825 |
0.039 0.075 |
0.089 0.063 |
|
1 2 |
Social Well-Being Social Well-Being |
Chemokine Composite*† Chemokine Composite |
−0.311 (0.040) −0.271 (0.040) |
−2.032 −1.755 |
0.048 0.087 |
0.080 0.058 |
|
1 2 |
Social Well-Being Social Well-Being |
Pro-Metastatic Composite**† Pro-Metastatic Composite**† |
−0.458 (0.026) −0.394 (0.025) |
−3.081 −2.735 |
0.004 0.009 |
0.173 0.124 |
|
1 2 |
Social Well-Being Social Well-Being |
IL1A*† IL1A* |
−0.397 (0.059) −0.350 (0.059) |
−2.624 −2.317 |
0.012 0.025 |
0.130 0.098 |
|
1 2 |
Social Well-Being Social Well-Being |
ILIB ILIB |
−0.216 (0.047) −0.178 (0.047) |
−1.378 −1.122 |
0.175 0.268 |
0.038 0.025 |
|
1 2 |
Social Well-Being Social Well-Being |
IL6 IL6 |
−0.242 (0.071) −0.197 (0.072) |
−1.535 −1.245 |
0.132 0.220 |
0.048 0.031 |
|
1 2 |
Social Well-Being Social Well-Being |
TNFSF10 TNFSF10 |
−0.117 (0.041) −0.107 (0.042) |
−0.719 −0.639 |
0.476 0.526 |
0.011 0.009 |
|
1 2 |
Social Well-Being Social Well-Being |
TNFRSF21† TNFRSF21 |
−0.302 (0.026) −0.268 (0.026) |
−2.006 −1.755 |
0.051 0.087 |
0.075 0.057 |
|
1 2 |
Social Well-Being Social Well-Being |
PTGS2/COX-2*† PTGS2/COX-2 |
−0.353 (0.042) −0.304 (0.042) |
−2.313 −1.999 |
0.026 0.052 |
0.103 0.073 |
|
1 2 |
Social Well-Being Social Well-Being |
CCL3 CCL3 |
−0.251 (0.048) −0.234 (0.049) |
−1.639 −1.487 |
0.109 0.145 |
0.052 0.043 |
|
1 2 |
Social Well-Being Social Well-Being |
CCL7 CCL7 |
−0.188 (0.046) −0.149 (0.047) |
−1.207 −0.946 |
0.234 0.350 |
0.029 0.018 |
|
1 2 |
Social Well-Being Social Well-Being |
CCL20*† CCL20 |
−0.332 (0.065) −0.286 (0.065) |
−2.158 −1.854 |
0.037 0.071 |
0.091 0.065 |
|
1 2 |
Social Well-Being Social Well-Being |
CCL3L1 CCL3L1 |
−0.245 (0.050) −0.218 (0.051) |
−1.593 −1.392 |
0.118 0.171 |
0.049 0.038 |
|
1 2 |
Social Well-Being Social Well-Being |
CCL4L2 CCL4L2 |
−0.273 (0.050) −0.234 (0.051) |
−1.767 −1.501 |
0.084 0.141 |
0.062 0.044 |
|
1 2 |
Social Well-Being Social Well-Being |
CXCR7† CXCR7 |
−0.280 (0.023) −0.248 (0.024) |
−1.976 −1.724 |
0.055 0.092 |
0.065 0.049 |
|
1 2 |
Social Well-Being Social Well-Being |
MMP9*† MMP9 |
−0.353 (0.032) −0.292 (0.032) |
−2.338 −1.982 |
0.024 0.054 |
0.102 0.068 |
|
1 2 |
Social Well-Being Social Well-Being |
LMNA**† LMNA**† |
−0.504 (0.024) −0.448 (0.024) |
−3.531 −3.203 |
0.001 0.003 |
0.210 0.160 |
p < 0.05
p < 0.01
Statistically significant after application of the Benjamini-Hochberg procedure at a false discovery rate of 0.10.
SE = Standard error. Model 1 analyses controlled for age, stage of disease, days since surgery, education, and BMI. Model 2 analyses controlled for age, stage of disease, days since surgery, education, BMI, and depression. Gene expression reported in RNA expression units (log2).
When the Benjamini-Hochberg procedure was applied to these analyses to control for multiple comparisons, SWB remained significantly associated with the pro-inflammatory cytokine, pro-inflammatory chemokine, and pro-metastatic gene expression composites as well as with individual gene expression of IL1A, TNFRSF21, CCL20, CXCR7, PTGS2/COX-2, MMP9, and LMNA (see Table 3). Figure 1 depicts scatterplots of the association between SWB and gene expression composites, which suggest that the associations were not driven by extreme values. Independent samples t-tests showed that women scoring at the lowest quintile of SWB did not differ from the highest 80% on gene expression levels (all ps > 0.05). This finding suggests that the association between SWB and gene expression likely operates across the continuum of SWB.
Figure 1.
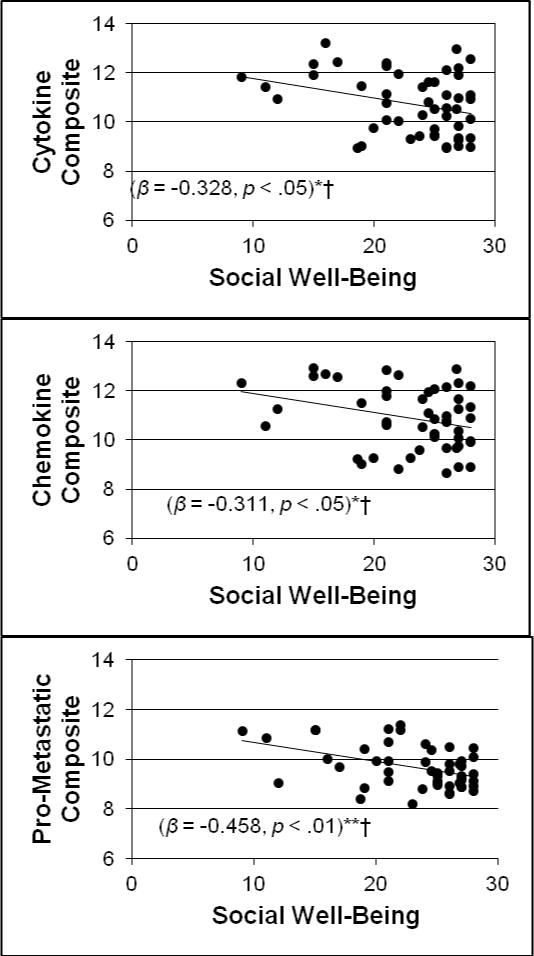
Scatterplots depicting the association between social well-being and pro-inflammatory and pro-metastatic gene expression composites after breast cancer surgery controlling for age, stage of disease, days since surgery, education, and BMI.
*p < .05 **p < .01
†Remains statistically significant after application of the Benjamini-Hochberg procedure at a false discovery rate of 0.10.
a Scatterplot depicts data after winsorization. Gene expression reported in RNA expression units (log2).
For descriptive purposes, Figure 2 depicts fold differences in pro-inflammatory and pro-metastatic gene expression in participants with low versus high SWB as determined by median split. The low SWB group had 2–2.5 times higher levels of expression for every pro-inflammatory cytokine, chemokine, and pro-metastatic gene and their respective composite scores than their counterparts who reported high SWB, suggesting effects that were meaningful.
Figure 2.
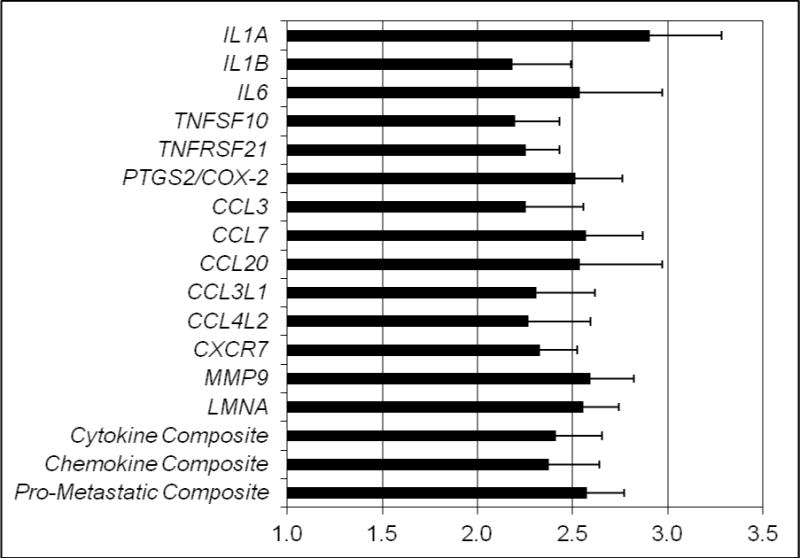
Fold differences in pro-inflammatory and pro-metastatic gene expression in participants with low (N = 25) versus high (N = 25) social well-being determined by median split.
a Cytokine Composite consisted of IL1A, IL1B, IL6, TNFSF10, TNFRSF21, and PTGS2/COX-2. Chemokine composite consisted of CCL3, CCL7, CCL20, CCL3L1, CCL4L2, and CXCR7. Pro-metastatic composite consisted of MMP9 and LMNA.
Leukocyte gene expression was not associated with the emotional, physical, or functional well-being scales of the FACT (all ps > .05).
Exploratory Analyses
Additional analyses examined whether depressive symptoms were related to SWB and leukocyte gene expression and whether SWB acted as an intermediary between depression and gene expression. HRSD depression was significantly negatively associated with SWB (r = −.25, p < .05), yet SWB associations with leukocyte gene expression held after controlling for HRSD, though some associations were attenuated (see Table 3, model 2). Since SWB was associated with depressive symptoms, we initiated mediation analyses to determine whether SWB mediated the effects of depression on gene expression. Depression was directly related to the pro-metastatic composite (Table 4, model 1 and 3 values). However, when SWB was controlled (Table 4, models 2 and 4), depression × pro-metastatic gene expression was attenuated. Examining whether expression of individual genes was directly associated with depression reveals a similar pattern (see Table 4). Here, depression was significantly associated with greater PTGS2/COX-2, MMP9, and LMNA (Table 4, model 1), but this association was non-significant when controlling for SWB after the Benjamini-Hochberg correction (Table 4, model 2). Therefore, no further steps of mediation analysis were conducted.
Table 4.
Regression analyses relating leukocyte pro-inflammatory and pro-metastatic gene expression and depression in multivariable analyses, with and without controlling for social well-being.
| Model | Independent Variable | Dependent Variable | β (SE) | t | p | R2 Change |
|---|---|---|---|---|---|---|
|
1 2 |
Depression Depression |
Cytokine Composite Cytokine Composite |
0.311 (0.035) 0.255 (0.035) |
1.909 1.578 |
0.063 0.122 |
0.073 0.047 |
|
1 2 |
Depression Depression |
Chemokine Composite Chemokine Composite |
0.275 (0.035) 0.221 (0.035) |
1.695 1.371 |
0.097 0.178 |
0.057 0.036 |
|
1 2 |
Depression Depression |
Pro-Metastatic Composite*† Pro-Metastatic Composite* |
0.430 (0.023) 0.352 (0.022) |
2.710 2.336 |
0.010 0.024 |
0.140 0.090 |
|
1 2 |
Depression Depression |
IL1A IL1A |
0.324 (0.053) 0.255 (0.052) |
1.993 1.614 |
0.053 0.144 |
0.079 0.047 |
|
1 2 |
Depression Depression |
ILIB ILIB |
0.244 (0.041) 0.209 (0.041) |
1.500 1.265 |
0.141 0.213 |
0.045 0.032 |
|
1 2 |
Depression Depression |
IL6 IL6 |
0.286 (0.062) 0.246 (0.062) |
1.748 1.491 |
0.088 0.143 |
0.062 0.044 |
|
1 2 |
Depression Depression |
TNFSF10 TNFSF10 |
0.076 (0.036) 0.055 (0.037) |
0.445 0.312 |
0.659 0.756 |
0.004 0.002 |
|
1 2 |
Depression Depression |
TNFRSF21 TNFRSF21 |
0.240 (0.023) 0.187 (0.023) |
1.500 1.175 |
0.141 0.247 |
0.044 0.026 |
|
1 2 |
Depression Depression |
PTGS2/COX-2* PTGS2/COX-2 |
0.329 (0.037) 0.269 (0.036) |
2.043 1.695 |
0.047 0.097 |
0.082 0.053 |
|
1 2 |
Depression Depression |
CCL3 CCL3 |
0.141 (0.043) 0.095 (0.043) |
0.863 0.577 |
0.393 0.567 |
0.015 0.007 |
|
1 2 |
Depression Depression |
CCL7 CCL7 |
0.246 (0.040) 0.216 (0.041) |
1.525 1.316 |
0.135 0.195 |
0.046 0.034 |
|
1 2 |
Depression Depression |
CCL20 CCL20 |
0.312 (0.057) 0.255 (0.057) |
1.922 1.588 |
0.061 0.120 |
0.074 0.048 |
|
1 2 |
Depression Depression |
CCL3L1 CCL3L1 |
0.189 (0.044) 0.146 (0.044) |
1.161 0.888 |
0.252 0.379 |
0.027 0.015 |
|
1 2 |
Depression Depression |
CCL4L2 CCL4L2 |
0.260 (0.044) 0.213 (0.044) |
1.598 1.307 |
0.117 0.198 |
0.051 0.033 |
|
1 2 |
Depression Depression |
CXCR7 CXCR7 |
0.227 (0.021) 0.178 (0.021) |
1.510 1.189 |
0.138 0.241 |
0.039 0.023 |
|
1 2 |
Depression Depression |
MMP9*† MMP9* |
0.390 (0.028) 0.332 (0.028) |
2.496 2.157 |
0.016 0.037 |
0.115 0.080 |
|
1 2 |
Depression Depression |
LMNA*† LMNA* |
0.397 (0.022) 0.309 (0.021) |
2.514 2.114 |
0.016 0.041 |
0.119 0.069 |
p < 0.05
p < 0.01
Statistically significant after application of the Benjamini-Hochberg procedure at a false discovery rate of 0.10.
SE = Standard error. Model 1 analyses controlled for age, stage of disease, days since surgery, education, and BMI. Model 2 analyses controlled for age, stage of disease, days since surgery, education, BMI, and social well-being. Gene expression reported in RNA expression units (log2).
The associations between SWB and leukocyte gene expression were not moderated by partner status (all ps > .05). This pattern of results suggests that SWB provides a generalizable association with inflammatory signaling that does not vary as a function of structural indicators of social support.
Discussion
Greater SWB was associated with less leukocyte expression of pro-inflammatory and pro-metastatic genes in women who recently underwent surgery for non-metastatic breast cancer and had not yet begun adjuvant therapy. These findings are consistent with prior literature demonstrating upregulation of pro-inflammatory genes in socially isolated individuals [25]. Results suggest a possible biobehavioral pathway relating SWB to gene transcripts associated with inflammation and pro-metastatic processes that might account for previously reported relations between social resources and survival time [36]. These findings suggest that social processes may influence cancer-promoting biological processes in the critical post-surgical period when any residual cancer cells may be impacted by inflammatory signaling [37, 38].
Interestingly, the associations between leukocyte gene expression and depression also became non-significant when we controlled for SWB. SWB may serve as an intermediary between depression and gene expression. However, given the cross-sectional nature of the study, depression may also serve as an intermediary between SWB and gene expression. We can conclude that SWB and depression overlap in their contribution to individual differences in leukocyte pro-inflammatory and pro-metastatic gene expression during the adverse period of breast cancer treatment.
These findings are consistent with research linking lack of social resources with inflammatory processes. Miller et al. [39] found down-regulation of leukocyte genes associated with inflammatory control in children raised in low SES environments. Women with ovarian cancer that reported lower SWB (and emotional support) showed increased levels of tumor promoters [vascular endothelial growth factor (VEGF), interleukin-6 (IL-6), matrix metalloproteases (MMPs) [20, 40], and greater inflammatory gene expression for these tumor promoters [40] than high SWB counterparts. MMPs, derived from monocytes, are involved in wound healing responses and are relevant in promoting changes in the tumor microenvironment (e.g., endothelial-mesenchymal transition [EMT]) that could favor entry of cancer cells into circulation and metastasis [40]. The present findings suggest greater SWB may contribute to less leukocyte expression of MMP-associated genes in breast cancer patients.
The association between SWB and less PTGS2/COX-2 expression suggests that lack of social resources may also relate to cancer progression through prostaglandins. Prostaglandins contribute to vascularization of tumor tissue and encourage tumor progression [41, 42]. Greater COX-2 expression, encoded by the PTGS2/COX-2 gene, is associated with tumor metastasis while inhibition of COX-2 is associated with increased tumor cell apoptosis [41]. Greater SWB in women with breast cancer may mitigate leukocyte signaling associated with inflammation, tumor proliferation, and metastasis though the precise mechanisms are unknown.
Alternatively, inflammation may increase social withdrawal, and consequently decrease SWB (and increase depression), as a “sickness behavior” response to illness. In response to infection, circulating pro-inflammatory cytokines may increase cytokine activation in the brain, which signals a reduction in social activity [43] to conserve energy for fighting infection [44]. The current results may be accounted for by leukocyte gene expression encoding pro-inflammatory cytokines that in turn activate social withdrawal and decrease SWB along with increasing depressive symptoms.
Strengths and Limitations
This study had several notable strengths. Women with non-metastatic breast cancer participated during the weeks after surgery while anticipating adjuvant treatment, a stressful time when social resources may be particularly important. That associations between SWB and leukocyte gene expression were tested when women had not yet begun adjuvant therapy also reduced the potentially confounding effects of radiation, chemotherapy and immunotherapy. Several other potential confounders were also controlled, including demographic characteristics, disease stage, point in treatment, and time since surgery. Significant effects persisted above and beyond the effects of age, education, and BMI, which are strongly and consistently associated with inflammation [28]. The statistically significant findings also survived correction for multiple comparisons using the Benjamini-Hochberg procedure, which is a recommended technique when analyzing medical data in the context of directional hypotheses [45].
Present findings are in line with research that sets a precedent for linking “well-being” indices to leukocyte gene expression [24]. Specificity of the relationship between leukocyte gene expression and SWB in women undergoing breast cancer treatment was established through findings that gene expression was not related to emotional, physical, or functional well-being. Finally, analyzing the impact of SWB on individual genes as well as on gene composite scores offers potential data reduction strategies, while simultaneously highlighting genes that may deserve special attention.
The design was a post-hoc secondary analysis of a previously examined cross-sectional dataset; therefore, the direction of the temporal connection between SWB and leukocyte gene expression cannot be determined. The small sample size may have limited ability to detect effects. Excluding participants missing key data (e.g. BMI) from analyses was considered a conservative strategy, though small sample size could have biased the results in the direction of false negatives. SWB was measured with retrospective self-report, and participants may have inaccurately remembered their experiences or underreported dissatisfaction with social resources to appear socially desirable. Although the sample was reasonably ethnically diverse, results may be less applicable to low-income women and women with metastatic disease.
Future Directions
Additional longitudinal studies with larger sample sizes are needed to examine the directionality of these relationships between SWB and pro-inflammatory indicators. It will be important to develop more nuanced scales of SWB for use with patients diagnosed with cancer to determine whether specific domains are strongly related to disease promoting factors. Studies of more diverse samples are needed to examine whether these findings hold across different ethnic and racial groups, especially given the culture-specific nature of social resources [46].
Implications
Low SWB may result, in part, from low utilization of available support. Patients may lack assertiveness skills, reducing their ability to request support from others during breast cancer treatment. This possibility provides directions for cognitive-behavioral interventions that could improve SWB by promoting assertiveness, engagement, and communication skills.
Conclusions
This study found robust cross-sectional relationships between SWB and less pro-inflammatory and pro-metastatic leukocyte gene expression in the period after breast cancer surgery, before adjuvant radiation or chemotherapy. Future longitudinal research should examine mechanisms linking SWB with pro-inflammatory and pro-metastatic leukocyte gene expression and longer-term clinical outcomes. Further research is needed to develop psychosocial interventions that enhance SWB for patients recently diagnosed with breast cancer.
Acknowledgments
Funding Information: This study was funded by the National Cancer Institute of the National Institutes of Health [R01-CA-064710], and the Sylvester Comprehensive Cancer Center.
Footnotes
Clinical Trial Registration Number: NCT01422551
Ethical standards: the experiments comply with the current laws of the country in which they were performed
Conflict of interest: The authors declare that they have no conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2013–2014. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Burgess C, Comelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: Five-year observational cohort study. BMJ. 2005;330(7493):702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom JR, Stewart SL, Johnston M, Banks P, Fobair P. Sources of social support and the physical and mental well-being of young women with breast cancer. Soc Sci Med. 2001;53(11):1513–24. doi: 10.1016/s0277-9536(00)00440-8. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds P, Boyd PT, Blacklow RS, Jackson JS, Greenberg RS, Austin DF, Chen VW, Edwards BK, the National Cancer Institute Black/White Cancer Survival Study Group The relationship between social ties and survival among black and white breast cancer patients. Cancer Epidemiol Biomarkers Prev. 1994;3(3):253–259. [PubMed] [Google Scholar]
- 5.Kroenke CH, Quesenberry C, Kwan ML, Sweeney C, Castillo A, Caan BJ. Social networks, social support, and burden in relationships, and mortality after breast cancer diagnosis in the Life After Breast Cancer Epidemiology (LACE) study. Breast Cancer Res Treat. 2013;137(1):261–271. doi: 10.1007/s10549-012-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. Journal of Clinical Oncology. 2006;24(7):1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 7.Beasley JM, Newcomb PA, Trentham-Dietz A, Hampton JM, Ceballos RM, Titus-Ernstoff L, Egan KM, Holmes MD. Social networks and survival after breast cancer diagnosis. J Cancer Surviv. 2010;4(4):372–380. doi: 10.1007/s11764-010-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epplein M, Zheng Y, Zheng W, Chen Z, Gu K, Penson D, Lu W, Shu X. Quality of life after breast cancer diagnosis and survival. J Clin Oncol. 2011;29(4):406–412. doi: 10.1200/JCO.2010.30.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol. 2010;28(26):4094–4099. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6(8):3282–3289. [PubMed] [Google Scholar]
- 11.Cohen EN, Gao H, Anfossi S, Mego M, Reddy NG, Debeb B, Giordano A, Tin S, Wu Qiong, Garza RJ, Cristofanilli M, Mani SA, Croix DA, Ueno NT, Woodward WA, Luthra R, Krishnamurthy S, Reuben JM. Inflammation mediated metastasis: immune induced epithelial-to-mesenchymal transition in inflammatory breast cancer cells. PLoS One. 2015;10(7):e0132710. doi: 10.1371/journal.pone.0132710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes S, Jaremka LM, Alfano CM, Glaser R, Povoski SP, Lipari AM, Agnese DM, Farrar WB, Yee LD, Carson WE, 3rd, Malarkey WB, Kiecolt-Glaser JK. Social support predicts inflammation, pain, and depressive symptoms: Longitudinal relationships among breast cancer survivors. Psychoneuroendocrinology. 2014;42:38–44. doi: 10.1016/j.psyneuen.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole SW, Levine ME, Arevalo JMG, Ma J, Weir DR, Crimmins EM. Loneliness, eudaimonia, and the human conserved transcriptional response to adversity. Psychoneuroendocrinology. 2015;62:11–17. doi: 10.1016/j.psyneuen.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight JM, Rizzo JD, Logan BR, Wang T, Arevalo JMG, Ma J, Cole SW. Low socioeconomic status, adverse gene expression profiles, and clinical outcomes in hematopoietic stem cell transplant recipients. Clin Cancer Res. 2016;22(1):69–78. doi: 10.1158/1078-0432.CCR-15-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouchard LC, Antoni MH, Blomberg BB, Stagl JM, Gudenkauf LM, Jutagir DR, Diaz A, Lechner S, Glück S, Derhagopian RP, Carver CS. Postsurgical depressive symptoms and proinflammatory cytokine elevations in women undergoing primary treatment for breast cancer. Psychosomatic Medicine. 2016;78(1):26–37. doi: 10.1097/PSY.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antoni MH, Lutgendorf SK, Blomberg B, Carver CS, Lechner S, Diaz A, Stagl J, Arevalo JMG, Cole SW. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71(4):366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 19.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1(79) doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutgendorf SK, Johnsen EL, Cooper B, Andersen B, Sorosky JI, Buller RE, Sood AK. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95(4):808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musselman DL, Somerset WI, Guo Y, Manatunga AK, Porter M, Penna S, Lewison B, Goodkin R, Lawson K, Lawson D, Evans DL, Nemeroff CB. A double-blind, multicenter, parallel-group study of paroxetine, desipramine, or placebo in breast cancer (stages I, II, III, and IV) with major depression. J Clin Psychiatry. 2006;67(2):288–296. doi: 10.4088/jcp.v67n0217. [DOI] [PubMed] [Google Scholar]
- 23.Cole SW. Social regulation of human gene expression: mechanisms and implications for public health. Am J Public Health. 2013;103(Sup 1):S84–92. doi: 10.2105/AJPH.2012.301183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredrickson BL, Grewen KM, Coffee KA, Algoe SB, Firestine AM, Arevalo JMG, Ma J, Cole SW. A functional genomic perspective on human well-being. Proc Natl Acad Sci. 2013;110(33):13684–13689. doi: 10.1073/pnas.1305419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilcox RR. Some results on a winsorized correlation-coefficient. Br J Math Stat Psychol. 1993;46:339–349. [Google Scholar]
- 27.Kline RB. Principles and Practice of Structural Equation Modeling. Fourth. New York: The Guilford Press; 2015. [Google Scholar]
- 28.O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav, Immun. 2009;23(7):887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 30.Jansen R, Penninx BWJH, Madar V, Xia K, Milaneschi Y, Hottenga JJ, Hammerschlag AR, Beekman A, van der Wee N, Smit JH, Brooks A, Tischfield J, Posthuma D, Schoevers R, van Grootheest G, Willemsen G, de Geus EJ, Boomsma DI, Wright FA, Zou F, Sun W, Sullivan PF. Gene expression in major depressive disorder. Mol Psychiatry. 2016;21(3):339–347. doi: 10.1038/mp.2015.94. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Census Bureau. Florida: 2000. 2002 Retrieved January 1, 2017, from https://www.census.gov/prod/2002pubs/c2kprof00-fl.pdf.
- 32.Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: Increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25(1):147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fenlon D, Powers C, Simmonds P, Clough J, Addington-Hall J. The JACS prospective cohort study of newly diagnosed women with breast cancer investigating joint and muscle pain, aches, and stiffness: pain and quality of life after primary surgery and before adjuvant treatment. BMC Cancer. 2014;14:467. doi: 10.1186/1471-2407-14-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmerman M, Chelminski I, Posternak M. A review of studies of the Hamilton depression rating scale in healthy controls: implications for the definition of remission in treatment studies of depression. J Nerv Ment Dis. 2004;192(9):595–601. doi: 10.1097/01.nmd.0000138226.22761.39. [DOI] [PubMed] [Google Scholar]
- 35.Brothers BM, Yang H, Strunk DR, Andersen BL. Cancer patients with major depressive disorder: testing a biobehavioral/cognitive behavior intervention. J Consult Clin Psychol. 2011;79(2):253–260. doi: 10.1037/a0022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroenke CH, Michael YL, Poole EM, Kwan ML, Nechuta S, Leas E, Caan BJ, Pierce J, Shu XO, Zheng Y, Chen WY. Postdiagnosis social networks and breast cancer mortality in the After Breast Cancer Pooling Project. Cancer Advance online publication. 2016 doi: 10.1002/cncr.30440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G, Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J, Nick AM, Stone RL, Lu C, Lutgendorf SK, Cole SW, Lokshin AE, Sood AK. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15(8):2695–2702. doi: 10.1158/1078-0432.CCR-08-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12(4):213–226. doi: 10.1038/nrclinonc.2014.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lutgendorf SK, Lamkin DM, Jennings NB, Arevalo JM, Penedo F, DeGeest K, Langley RR, Lucci JA, 3rd, Cole SW, Lubaroff DM, Sood AK. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res. 2008;14(21):6839–6846. doi: 10.1158/1078-0432.CCR-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neeman E, Zmora O, Ben-Eliyahu S. A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin Cancer Res. 2012;18(18):4895–4902. doi: 10.1158/1078-0432.CCR-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, Pimentel MA, Chai MG, Karnezis T, Rotmensz N, Renne G, Gandini S, Pouton CW, Ferrari D, Möller A, Stacker SA, Sloan EK. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;1(7):10634. doi: 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29(2):247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maes M, Berk M, Goehler L, Song C, Anderson G, Galecki P, Leonard B. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10(66) doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67(8):850–857. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Jutagir DR, Gudenkauf LM, Stagl JM, Carver CS, Bouchard LC, Lechner SC, Glück S, Blomberg BB, Antoni MH. Ethnic differences in types of social support from multiple sources after breast cancer surgery. Ethn Health. 2015;21(5):411–425. doi: 10.1080/13557858.2015.1066494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pruitt KD, Brown GR, Hiatt SM, Thibaud-Nissen F, Astashyn A, Ermolaeva O, Farrell CM, Hart J, Landrum MJ, McGarvey KM, Murphy MR, O’Leary NA, Pujar S, Rajput B, Rangwala SH, Riddick LD, Shkeda A, Sun H, Tamez P, Tully RE, Wallin C, Webb D, Weber J, Wu W, DiCuccio M, Kitts P, Maglott DR, Murphy TD, Ostell JM. RefSeq: an update on mammalian reference sequences. Nucleic Acids Res. 2014;42:D756–D763. doi: 10.1093/nar/gkt1114. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berahovich RD, Zabel BA, Lewén S, Walters MJ, Ebsworth K, Wang Y, Jaen JC, Schall TJ. Endothelial expression of CXCR7 and the regulation of systemic CXCL12 levels. Immunology. 2013;141(1):111–122. doi: 10.1111/imm.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]


