Introduction
An annual seasonal influenza vaccine is the main protection against influenza infection and its complications.1 Influenza infections have increased morbidity among HIV-infected children and adults, including pregnant women. This underscores the importance of vaccine-conferred protection. However, the effectiveness of the influenza vaccine depends on at least 2 factors, the characteristics of the vaccinated person (eg, their age and health) as well as the match between the influenza vaccine strain and the circulating strain spreading in the community at that time.2
HIV infection is a characteristic of a vaccinated person that can influence vaccine-conferred protection. Multiple studies have shown a poor antibody response to influenza vaccines in HIV-infected individuals.3-6 A previous study demonstrated only 38% of children had a complete seroprotective response of all strains of trivalent influenza vaccine (TIV) at both 1 month and 6 months.7 However, some studies demonstrated that the proportion of HIV-infected children generating a seroprotective response after influenza vaccination was as high as 79%.8
Case Report
A 17-year-old Thai female with human immunodeficiency virus type 1 (HIV-1) infection who had received TIV for 2012-2013 in August 2013 presented at the Bamrasnaradura Infectious Diseases Institute (BIDI) complaining of 2 days of fever, malaise, headache, cough, and sore throat in April 2014. She had been diagnosed with HIV infection confirmed by machine-based assay (ArchiTECT, Abbott, chemiluminescent test), particle agglutination test (Serodia HIV, Fujirebio), and immunochromatography test (Allere Determine or TriLine) at BIDI. Approximately 13 years prior to presentation, she received zidovudine (AZT) plus didanosine (ddI) from 2001 to 2005; AZT, ddI, and efavirenz (EFV) from 2005 to 2007; and has been receiving EFV plus lopinavir/ritonavir in combination (LPV/r) from 2007 to present. Before TIV was given in 2013, she had a CD4 count of 922 cell/µL (31%), plasma HIV-1 RNA <40 copies/mL by quantitative real-time polymerase chain reaction (RT-PCR), a body weight of 85.7 kg, and a height of 165 cm. She received TIV 2011-2012 on August 1, 2012, and TIV 2012-2013 on August 28, 2013. TIV in 2012-2013 included the A/California/7/2009 (H1N1)-derived strain using NYMC X-179A, the A/Victoria/361/2011 (H3N2)-like strain using IVR-65, and the B/Wisconsin/1/2010-like strain using NYMC BX-39 derived from the B/Hubei-Wujiagang/158/2009 strains. At presentation of influenza-like illness symptoms, the physical examination was unremarkable except for a body temperature 38.5°C and body mass index of 31.48 kg/m2. Because of a positive rapid influenza test (Quidel QuickVue Influenza A+B test), the patient was given oseltamivir for 5 days and the clinical symptoms resolved The positive rapid influenza test result was from the BIDI, Nonthaburi, Thailand, with a confirmatory PCR result from the Siriraj Influenza Cooperative Research Center, Department of Microbiology, Faculty of Medicine, Siriraj Hospital, Bangkok, Thailand. The specimen was identified by the amino acid sequence of full hemagglutinin (HA) gene to evaluate the match between the influenza infection and TIV strains.
To perform nucleotide sequencing, total RNA was extracted using a QIAamp viral RNA mini kit (Qiagen) from direct nasal swab specimens of a vaccinated person who had developed a PCR-confirmed influenza illness from the 2009 pandemic virus. The RNA sample was amplified for the complete influenza HA gene, and the amplified product was subjected to nucleotide sequencing. The sequence was edited, aligned, and translated into an amino acid sequence by BioEdit program (version 7.1.11; available at http://www.mbio.ncsu.edu/bioedit/bioedit.html). The sequence was submitted to the GenBank database with the accession number of KU144591.
Table 1 shows the percentage of identity match of amino acid sequences of the full HA gene. The specimen of the present case matches TIV strains CA 07 vaccine strain, H1N1 A/Nonthaburi/102/2009, and H1N1 A/Thailand/104/2009 by 97%, 98%, and 98%, respectively. The matches of the specimen for H1N1 2014 isolates are 99% to 100%.
Table 1.
Percentage of Identity Match of Amino Acid Sequences of Full HA Gene.
| Viruses | Percentage Match of Child |
|---|---|
| CA07a vaccine strain | 97.1 |
| A/Nonthaburi/102/2009 | 97.7 |
| A/Thailand/104/2009 | 97.7 |
| H1N1 2014 isolate #1 | 99.4 |
| H1N1 2014 isolate #2 | 99.6 |
California 2007.
To perform phylogenetic analysis, the HA amino acid sequence of virus from the case was analyzed for its correlation with the sequences from A/California/07/2009 (H1N1) vaccine strain, 2 Thai virus isolates obtained from the first epidemic wave (A/Nonthaburi/102/2009 and A/Thailand/104/2009), and 2 more virus isolates from 2014 (A/Thailand/NMA_1/2014 and A/Thailand/BKK_1/2014) See Figure 1. The results demonstrate a closer relationship between the causative virus and the circulating strains rather than the vaccine strain closely related to the Thai viruses from the first epidemic wave.
Figure 1.
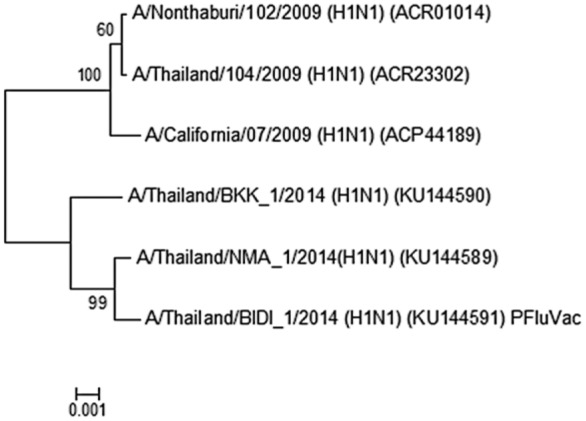
Phylogenetic analysis of HA amino acid sequences derived from 2009 pandemic influenza viruses by maximum likelihood technique using MEGA 5 software (PFuVac = patient).
Discussion
In this case, we were unable to determine the degree of vaccine-conferred protection of TIV For 2012-2013 given in August 2013 against the illness caused by the PCR-confirmed strain of influenza A virus in April 2014. The case illustrates the issues causing this uncertainty, including an issue of matching the vaccine virus strain and causative virus strain as well as other issues about the peculiar characteristics of the vaccinated person, some of which are relevant to HIV infection.
The results demonstrate a closer relationship between the causative virus and the circulating strains rather than the vaccine strain. Because the viral components of the TIV for 2012-2013 vaccine are not well matched with circulating influenza viruses of 2014 in Thailand, the benefits of influenza vaccination may have been reduced. However, the degree of antigenic drift of the circulating viruses of 2014 from vaccine viruses and the proportion of circulating drifted viruses could have varied. If the circulating virus of 2014 that caused the illness of the patient was mildly or moderately drifted compared to one of the strains in the TIV 2012-2013 used, some degree of protection may have occurred.9
There are several interesting characteristics of the vaccinated person in the present case. First, she was 17 years old. The seroprotection of TIV among HIV-infected children is reported to be low. A previous double-blinded, placebo randomized controlled trial of 410 young HIV-infected children demonstrated that the proportions of TIV-vaccinated children seroconverting after a second dose against the vaccine strains of H1N1, H3N2, and influenza B were as low as 47.5%, 50%, and 40%, respectively.10 However, a previous systematic review reported that TIV prevented laboratory-confirmed influenza with a pooled efficacy as low as 11% in young children and as high as 85% in adults.11 Second, the influenza A virus symptom of the present case occurred 216 days after TIV vaccination was given. The durability of the seroprotection for influenza vaccine often spans only an estimated 180 days.12 Third, she had a confirmed HIV infection. Thus, the response to the vaccine may have depended on the B-cell subset distribution rather than CD4 counts or viral load.13 The present case had a high CD4 count and viral suppression. Fourth, she had a high body mass index. The previous study demonstrated that a high body mass index was associated with a greater decline in influenza antibody titers at 12 months post TIV.14 Obesity may impair the ability to mount a protective immune response to influenza virus.
Conclusion
The closer relationship between the causative virus and the circulating strains, the low seroprotection of TIV among HIV-infected children, the durability of seroprotection against influenza vaccine that often spans only an estimated 180 days, and obesity may have reduced the effectiveness of the influenza vaccine in the present case.
Footnotes
Author Contributions: VM: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
SC: Contributed to design; contributed to acquisition and interpretation; drafted manuscript; critically revised manuscript.
JA: Contributed to conception; contributed to acquisition.
SSa: Contributed to conception; contributed to acquisition.
SL: Contributed to conception; contributed to acquisition.
SU: Contributed to conception; contributed to acquisition.
SSr: Contributed to conception; contributed to acquisition.
DC: Contributed to conception; contributed to acquisition.
HL: Contributed to conception; contributed to acquisition.
PP: Contributed to conception; contributed to acquisition, analysis, and interpretation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Centers for Disease Control and Prevention. Seasonal influenza: flu basics. https://www.cdc.gov/flu/about/disease/index.htm. Accessed June 23, 2017.
- 2. Centers for Disease Control and Prevention. Vaccine effectiveness—how well does the flu vaccine work? http://www.cdc.gov/flu/about/qa/vaccineeffect.htm. Accessed June 23, 2017.
- 3. Flynn PM, Nachman S, Muresan P, et al. Safety and immunogenicity of 2009 pandemic H1N1 influenza vaccination in perinatally HIV-1-infected children, adolescents, and young adults. J Infect Dis. 2012;206:421-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weinberg A, Song LY, Walker R, et al. Anti-influenza serum and mucosal antibody responses after administration of live attenuated or inactivated influenza vaccines to HIV-infected children. J Acquir Immune Defic Syndr. 2010;55:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madhi SA, Maskew M, Koen A, et al. Trivalent inactivated influenza vaccine in African adults infected with human immunodeficient virus: double blind, randomized clinical trial of efficacy, immunogenicity, and safety. Clin Infect Dis. 2011;52:128-137. [DOI] [PubMed] [Google Scholar]
- 6. Madhi SA, Kuwanda L, Venter M, Violari A: Prospective cohort study comparing seasonal and H1N1 (2009) pandemic influenza virus illnesses in HIV-infected children during 2009. Pediatr Infect Dis J. 2014;33:174-176. [DOI] [PubMed] [Google Scholar]
- 7. Moolasart V, Manosuthi W, Ausavapipit J, et al. Long-term seroprotective response of trivalent seasonal influenza vaccine in HIV-infected children, regardless of immunogenicity before immunisation. Int J STD AIDS. 2016;27:761-768. [DOI] [PubMed] [Google Scholar]
- 8. Pass RF, Nachman S, Flynn PM, et al. Immunogenicity of licensed influenza A (H1N1) 2009 monovalent vaccines in HIV-infected children and youth. J Pediatr Infect Dis Soc. 2013;2:352-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Flu vaccine effectiveness: questions and answers for health professionals. http://www.cdc.gov/flu/professionals/vaccination/effectivenessqa.htm. Accessed June 23, 2017.
- 10. Madhi SA, Dittmer S, Kuwanda L, et al. Efficacy and immunogenicity of influenza vaccine in HIV-infected children: a randomized, double-blind, placebo controlled trial. AIDS. 2013;27:369-379. [DOI] [PubMed] [Google Scholar]
- 11. Remschmidt C, Wichmann O, Harder T. Influenza vaccination in HIV-infected individuals: systematic review and assessment of quality of evidence related to vaccine efficacy, effectiveness and safety. Vaccine. 2014;32:5585-5592. [DOI] [PubMed] [Google Scholar]
- 12. Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1-62. [PubMed] [Google Scholar]
- 13. Curtis DJ, Muresan P, Nachman S, et al. Characterization of functional antibody and memory B-cell responses to pH1N1 monovalent vaccine in HIV-infected children and youth. PLoS One. 2015;10:e0118567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond). 2012;36:1072-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]


