Abstract
The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see http://journals.sagepub.com/doi/10.1177/2374289517715040.
Keywords: pathology competencies, organ system pathology, morphologic features, paraneoplastic syndrome, pulmonary neoplasia, squamous cell carcinoma
Primary Objective
Objective RS3.2: Morphologic Features of Lung Neoplasms
Discuss key gross and histopathologic features that may help differentiate between small cell, adenocarcinoma, and squamous cell carcinoma.
Competency 2: Organ System Pathology; Topic RS: Respiratory System; Learning Goal 3: Lung Neoplasia.
Secondary Objectives
Objective RS3.1: Lung Neoplasms
Describe the common locations for the different types of lung cancer.
Competency 2: Organ System Pathology; Topic RS: Respiratory System; Learning Goal 3: Lung Neoplasia.
Objective RS3.5: Environmental Factors Predisposing to Lung Cancer
Explain the environmental factors that predispose to the development of lung cancer and illustrate how these factors interact with genetic factors in the development of cancer.
Competency 2: Organ System Pathology; Topic RS: Respiratory System; Learning Goal 3: Lung Neoplasia.
Patient Presentation
A 62-year-old male presents to the clinic with a 4-month history of dysphagia to solids in addition to nausea and abdominal pain. In addition, he reports recent hemoptysis and the onset of hoarseness. He also has had unintentional 10-kg (22 lb) weight loss over the past 6 months. He has a history of emphysema. He takes no medication. He has a 26 pack-year history of cigarette smoking and has a 6-pack beer on weekends. Physical examination reveals a thin male who appears older than his stated age, oriented to time and place. Body mass index is 18. Vital signs are within normal limits. Chest auscultation reveals diminished breath sounds over the right lung fields.
Diagnostic Findings, Part 1
A chest X-ray reveals a 6-cm hilar mass on the right.
Laboratory studies show serum calcium 12 mg/dL (normal 8.5-10.5 mg/dL).
Questions/Discussion Points, Part 1
What is Your Differential Diagnosis Based on the Clinical History?
The patient presents with dysphagia, hemoptysis, and hoarseness with a significant weight loss history, which could be indications of an upper airway neoplasm such as laryngeal carcinoma, esophageal carcinoma, or a lung carcinoma. The chest X-ray findings of a hilar mass would make a lung lesion (neoplasm or infection) most likely, or a middle mediastinum neoplasm such as lymphoma. Central lung neoplasms would include a differential of squamous cell carcinoma, small cell carcinoma, or a carcinoid tumor. Less likely, this could be an adenocarcinoma. The hypercalcemia may be indicative of a paraneoplastic syndrome due to squamous cell carcinoma.
What Further Testing is Indicated for this Patient?
A biopsy of the lesion is indicated to identify the origin of this mass.
Diagnostic Findings, Part 2
A biopsy was performed because of the high clinical suspicion of a malignant lesion. Figures 1 to 3 show representative histologic features of the biopsy on low, medium, and high power.
Figure 1.
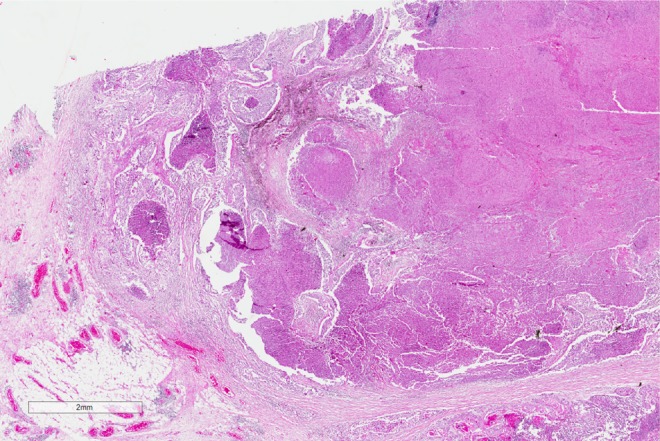
Histopathology of the lung biopsy. A low-power image showing extensive tumor on the right hand side, with nests of tumor cells infiltrating into the pulmonary parenchyma on the left.
Figure 2.
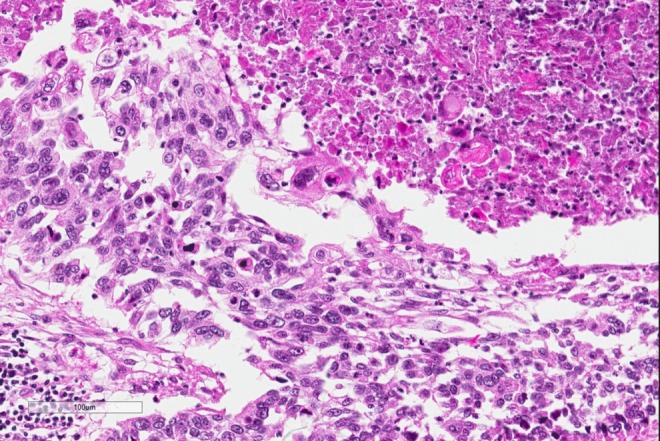
Higher power image of the tumor with neoplastic cells that have keratinization shown by brightly eosinophilic cytoplasm in the upper right hand side, while the neoplastic cells on the mid and left and lower image show pleomorphism and hyperchromasia.
Figure 3.
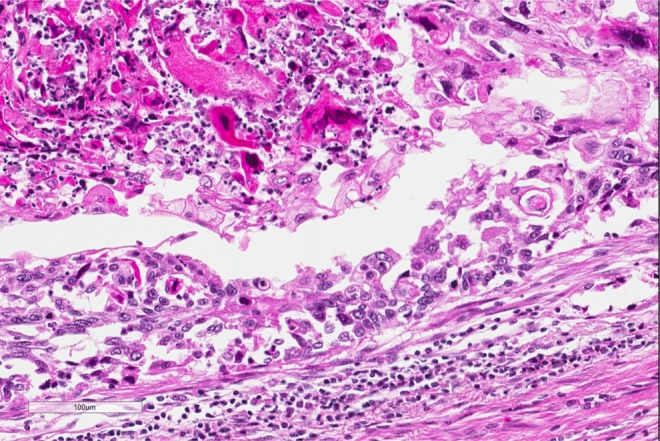
High power of another area of tumor cells showing many keratinized tumor cells that are hyperchromatic and eosinophilic.
Questions/Discussion Points, Part 2
Describe the Histologic Heatures of Figures 1 to 3. Based on the Histologic Findings and Clinical Presentation, What is Your Diagnosis?
The diagnosis, based on the morphologic features, is squamous cell carcinoma. On low power, there are multiple infiltrating nests of tumor cells, as well as a large area of necrosis in the upper right corner (Figure 1). On intermediate magnification (Figure 2), one can recognize sheets of polygonal cells with a high nuclear to cytoplasmic ratio, hyperchromatic and pleomorphic nuclei on the bottom and left of the image, and large keratinizing and atypical cells within the necrotic area on the upper right. Keratinization can be in the form of keratin pearls or as deeply eosinophilic dyskeratotic malignant cells (Figure 3). If one observes intercellular bridges between the polygonal cells, this can be helpful in confirming squamous cell carcinoma.
Histologically one will find a progression of epithelial cells from normal respiratory bronchial epithelium to squamous metaplasia from the exposure of cigarette smoke. With time and continued exposure, the epithelium will progress to dysplasia, then carcinoma in situ, and finally, invasive squamous cell carcinoma once tumor cells have broken through the basement membrane.
Other common lung tumors include adenocarcinoma and small cell carcinoma. Adenocarcinoma has a glandular pattern, it often contains larger cells with lacy cytoplasm and large pleomorphic nuclei. These cells may produce mucin. The cells from small cell carcinoma are medium sized, with a high nuclear to cytoplasmic ratio, and have hyperchromatic nuclei with a granular or salt and pepper appearance. Since the small cells are deficient in intermediate filaments, they conform to the shape of adjoining cells in a pattern that is referred to as molding.
If the Tumor Spreads locally to the Recurrent Laryngeal Nerve, Which Clinical Feature would you Probably Elicit from the Patient History?
Local spread of lung tumors may produce multiple different clinical features depending on the structures that are affected by the invasion of the tumor. Over 50% of patients will present with a cough if the tumor involves the central airways. About 24% to 50% of patients will present with hemoptysis if there is hemorrhage secondary to tumor invasion in the airway. Patients may present with hoarseness if there is recurrent laryngeal nerve invasion or diaphragm paralysis with phrenic nerve invasion and pleural effusions with pleural invasion.1
Although Chronic Smoking is a Well-known Carcinogen Associated with Lung Cancer, Cancer Only Develops in 11% of Heavy Smokers. What Genetic Risk Factors Most Commonly Promote the Development of Squamous Cell Carcinoma within Heavy Smokers?
Carcinogenesis is the process by which normal cells are transformed in carcinoma. Multiple mutations accumulate over years of growth affecting many aspects of cell development and regulation until one or more clones emerge with all hallmarks of cancer. With continued tumor growth, cells compete for nutrients in the microenvironment, additional mutations accumulate, and progression leads to a genetically heterogeneous neoplasm.2
The duration and the amount of exposure to cigarette smoke are well associated with lung cancer risk. In cigarettes, particularly, there are multiple procarcinogens present, which can be converted to carcinogens via the P-450 monooxygenase system. Some patients with P-450 system polymorphisms have an even greater propensity to activate these cigarette smoke procarcinogens to carcinogens.
In addition, the normal mucosa cells of patients with lung squamous cell carcinoma frequently have tumor protein p53 (TP53) mutations. TP53 mutations are early events in the progression from in situ to invasive squamous cell carcinoma. TP53 mutations are found in 10% to 50% of the cells of squamous cell dysplasia and in 60% to 90% of carcinoma in situ cells.3 TP53 mutations are also identified in 75% to 90% of small cell lung carcinoma cells.
Which of the Findings Described in the Patient Presentation Above is Most Closely Associated with the Specific Histologic Type of the Patient’S Tumor?
This patient exhibits symptoms of a paraneoplastic syndrome. Such syndromes arise when the cancer cells produce systemic effects by acquiring the ability to secrete hormones not usually associated with the tissue of origin. The process of why particular syndromes are often associated with particular cancers is not entirely understood.
Paraneoplastic syndromes are important to recognize for several reasons including: these may cause significant clinical consequences, such as hypercalcemia, these may be the first clinical sign of an underlying malignancy, or these may confuse the clinical picture. Hypercalcemia is a common paraneoplastic syndrome that can be seen with multiple different types of neoplasms including squamous cell carcinoma of the lung, breast carcinomas, renal cell carcinoma, ovarian neoplasm, or adult T-cell leukemia/lymphoma. The mechanism of the production of the paraneoplastic hypercalcemia is due to parathyroid hormone-related protein (PTHRP) production, which can be induced by the primary cancer or in the bone metastasis. Alternatively, it may be related to other factors secondary to the malignancy such as transforming growth factor α, tumor necrosis factor, or interleukin 1. Clinically, the hypercalcemia may present with nausea and vomiting in addition to abdominal cramps. Other hypercalcemia symptoms include constipation, frequent urination, and confusion.
Other common paraneoplastic syndromes include polycythemia secondary to erythropoietin production as seen in renal cell carcinoma, hypoglycemia due to insulin or insulin-like substance production as seen in ovarian carcinomas, or Cushing syndrome due to adrenocorticotropic hormone (ACTH) production as seen in small cell carcinoma of the lung.4
In Considering The General Category of Lung Neoplasms, How Does the Location of the Neoplasm in the Lungs Help You Narrow Your Differential of the Specific Type of Lung Cancer?
Common lung neoplasms have a predilection for specific areas of the lung. A central or hilar mass is most likely to be a squamous cell carcinoma or a small cell tumor, less commonly an adenocarcinoma. These have distinctive morphologies that are important to differentiate for adequate treatment. In addition, classifying a lung malignancy as adenocarcinoma has the added requirement for molecular testing to be performed in order to determine whether the patient is eligible for targeted therapy. If tumors arise in the periphery of the lungs, then they tend to be adenocarcinomas, especially if they arise at a previous scar site. Differentiating the exact cell type of a lung tumor is important for treatment considerations. Squamous cell carcinomas and adenocarcinomas tend to be treated with surgical resection as a primary treatment, while small cell carcinoma is treated with chemotherapy as a first line.
Teaching Points
Squamous cell carcinoma is often a centrally located tumor close to the hilum.
Histologic hallmarks of squamous cell carcinoma are polygonal cells with intercellular bridges, crisp eosinophilic cytoplasm. Tumors may also contain keratin pearls, and larger tumors may have extensive necrosis.
The histologic progression of normal mucosa to squamous cell carcinoma includes normal respiratory epithelium, squamous metaplasia, dysplasia, carcinoma in situ, and then invasive squamous cell carcinoma once the basement membrane is breached.
Squamous cell carcinoma may present with hypercalcemia in a paraneoplastic syndrome due to PTHRP production.
Adenocarcinoma of the lung is often found at the periphery of the lung, perhaps in a prior scar, and shows a sheet of larger cells with lacey cytoplasm often within a glandular pattern.
Small cell carcinoma of the lung is often found centrally in the lung and has cells that have a very high nuclear to cytoplasmic ratio and hyperchromatic with granular chromatin, and the cells often mold against one another.
Cigarette smoke is well associated with lung cancer risk and contains multiple procarcinogens which can be converted to carcinogens via the P-450 monooxygenase system. Some patients with P-450 system polymorphisms have an even greater propensity to activate these cigarette smoke procarcinogens to carcinogens.
Footnotes
Authors’ Note: The opinions expressed herein are those of the authors and are not necessarily representative of those of the Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DOD), or the United States Army, Navy, or Air Force.
References
- 1. Husain A. The lung In: Kumar V, Abbas A, Aster A, eds., Robbins and Cotran Pathologic Basis of Disease. 9th ed Philadelphia, PA: Elsevier Saunders; 2015:718. [Google Scholar]
- 2. Neoplasia. In: Kumar V, Abbas A, Aster A, eds. Robbins and Cotran Pathologic Basis of Disease. 9th ed Philadelphia, PA: Elsevier Saunders; 2015:280–281. [Google Scholar]
- 3. Husain A. The lung In: Kumar V, Abbas A, Aster A, eds. Robbins and Cotran Pathologic Basis of Disease. 9th ed Philadelphia, PA: Elsevier Saunders; 2015:712–715. [Google Scholar]
- 4. Neoplasia. In: Kumar V, Abbas A, Aster A, eds. Robbins and Cotran Pathologic Basis of Disease. 9th ed Philadelphia, PA: Elsevier Saunders; 2015:330–331. [Google Scholar]


