Abstract
5‐Hydroxytryptamine (5‐HT), a neurotransmitter and vasoactive factor, has been reported to promote proliferation of serum‐deprived hepatocellular carcinoma (HCC) cells but the detailed intracellular mechanism is unknown. As Wnt/β‐catenin signalling is highly dysregulated in a majority of HCC, this study explored the regulation of Wnt/β‐catenin signalling by 5‐HT. The expression of various 5‐HT receptors was studied by quantitative real‐time polymerase chain reaction (qPCR) in HCC cell lines as well as in 33 pairs of HCC tumours and corresponding adjacent non‐tumour tissues. Receptors 5‐HT1D (21/33, 63.6%), 5‐HT2B (12/33, 36.4%) and 5‐HT7 (15/33, 45.4%) were overexpressed whereas receptors 5‐HT2A (17/33, 51.5%) and 5‐HT5 (30/33, 90.1%) were reduced in HCC tumour tissues. In vitro data suggests 5‐HT increased total β‐catenin, active β‐catenin and decreased phosphorylated β‐catenin protein levels in serum deprived HuH‐7 and HepG2 cells compared to control cells under serum free medium without 5‐HT. Activation of Wnt/β‐catenin signalling was evidenced by increased expression of β‐catenin downstream target genes, Axin2, cyclin D1, dickoppf‐1 (DKK1) and glutamine synthetase (GS) by qPCR in serum‐deprived HCC cell lines treated with 5‐HT. Additionally, biochemical analysis revealed 5‐HT disrupted Axin1/β‐catenin interaction, a critical step in β‐catenin phosphorylation. Increased Wnt/β‐catenin activity was attenuated by antagonist of receptor 5‐HT7 (SB‐258719) in HCC cell lines and patient‐derived primary tumour tissues in the presence of 5‐HT. SB‐258719 also reduced tumour growth in vivo. This study provides evidence of Wnt/β‐catenin signalling activation by 5‐HT and may represent a potential therapeutic target for hepatocarcinogenesis.
Keywords: 5‐HT, Wnt/β‐catenin signalling, SB‐258719
Highlights
5‐HT increases β‐catenin protein levels in serum deprived HuH‐7 and HepG2 cells.
Receptor 5‐HT7 is overexpressed in 45.4% of HCC tissues.
Antagonist of receptor 5‐HT7 attenuates β‐catenin protein levels in vitro and in vivo.
Abbreviations
- 5‐HT
serotonin
- HCC
hepatocellular carcinoma
- qPCR
quantitative real‐time polymerase chain reaction
- DKK1
dickoppf‐1
- GS
glutamine synthetase
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- SFM
serum free medium
1. Introduction
Liver cancer is ranked as the second leading cause of cancer death in men worldwide. There were an estimated 745,500 deaths in 2012 (Torre et al., 2015). HCC is the primary malignancy of liver cancer. Men have a higher incidence of developing HCC than women. Endemic in south‐east Asia and sub‐Saharan Africa, the incidence of HCC is also on the rise in the western world due to an increase in hepatitis C virus (HCV) infection (Altekruse et al., 2009). HCC suffers from poor prognosis due to lack of effective diagnostic markers for early detection combined with a high rate of post‐operative recurrence. Chronic hepatitis B virus (HBV) and HCV infections, exposure to aflatoxins, chronic alcohol abuse, non‐alcoholic steatohepatitis and pre‐existing diabetes are well documented risk factors for HCC (Schutte et al., 2009; Tseng et al., 2012) but the molecular and cellular mechanisms leading to hepatocarcinogenesis are not well understood.
Serotonin, also known as 5‐hydroxytryptamine (5‐HT), is a monoamine hormone and neurotransmitter. It is mainly synthesized in the gastrointestinal tract (enterochromaffin cells), but a small percentage is also synthesized within the nervous system. 5‐HT also regulates several physiological functions in the gastrointestinal tract. Recent studies have shed light on its role in liver diseases, including liver fibrosis (Ruddell et al., 2006), mediation of oxidative stress in non‐alcoholic steatohepatitis (Nocito et al., 2007), and aggravation of liver hepatitis (Lang et al., 2008). All of these conditions being risk factors for HCC.
5‐HT is a potent mitogen for many different cell types (Cattaneo et al., 1993; Hambek et al., 2006; Siddiqui et al., 2006), including hepatocytes (Soll et al., 2010) and it is also crucial for liver regeneration (Lesurtel et al., 2006). There are 7 different 5‐HT receptors with 14 subtypes, suggesting an extraordinary level of functional complexity. Soll et al. (2012) reported an increased expression of 5‐HT1B and 5‐HT2B in 32% and 35% of HCC patients, respectively, and their increased expression has been associated to increased tumour size and proliferative index. 5‐HT2B receptor has also shown to stimulate hepatic stellate cells, which are negative regulators of liver regeneration. In healthy liver, the expression of 5‐HT2B is reduced whereas it is increased in patients with chronic liver disease. Furthermore, antagonism of 5‐HT2B has shown to attenuate fibrogenesis and improves liver function in fibrotic disease models (Ruddell et al., 2006).
Of these 14 receptor subtypes, 5‐HT receptors 5‐HT1A, 5‐HT1B, 5‐HT2A, 5‐HT2B and 5‐HT7 are expressed in the human gastrointestinal (GI) tract and involved in normal liver function (Ruddell et al., 2008; Siddiqui et al., 2006). Receptors 5‐HT4 and 5‐HT5 are either not expressed or poorly expressed in the liver (Mader et al., 2006). In this study, we investigated 5‐HT receptors that are expressed in the human gastrointestinal tract and showed differential expression in HCC cell lines and immortalized human hepatocytes (MIHA). We did not observe differential expression of 5‐HT4, 5‐HT1A and 5‐HT1B in MIHA and HCC cell lines. We also studied 5‐HT1D as this has been shown to be expressed in bladder (Siddiqui et al., 2006) and pancreatic (Gurbuz et al., 2014) cancers, which are also part of the GI tract and we were interested to know its expression in HCC cell lines and tissues.
5‐HT has also been reported as an important factor for Wnt/β‐catenin signalling during axis formation in Xenopus (Beyer et al., 2012). Through a series of complex experiments Beyer et al. (2012) implicated an interaction between 5‐HT and Wnt/β‐catenin signalling at the level of ligand and/or receptors. As Wnt/β‐catenin signalling is a hallmark of several cancers including HCC we investigated the effect of 5‐HT on Wnt/β‐catenin signalling in HCC.
The Wnt/β‐catenin pathway is critical for embryonic development, self‐renewal and liver regeneration (Monga et al., 2001). However, it is highly dysregulated in HCC with β‐catenin accumulation reported in 40–70% of HCC cases (Monga et al., 2001; Nhieu et al., 1999; Suzuki et al., 2002; Wong et al., 2001). Cytoplasmic levels of β‐catenin are controlled by phosphorylation by a destruction complex consisting of GSK3β/Axin/APC. The phosphorylated β‐catenin is then targeted for ubiquitination and degradation by the proteasome. However, activation of Wnt signalling in a majority of HCC tumours is achieved through either mutation in β‐catenin or due to aberrant expression of the different components of the Wnt/β‐catenin pathway. When the Wnt pathway is dysregulated stabilization of β‐catenin in the cytoplasm enhances its nuclear translocation where it cooperates with TCF/LEF family of transcription regulators and induces transcription of downstream target genes such as cyclin D1, c‐Myc (Huang and He, 2008).
Considering the role of the Wnt/β‐catenin pathway and 5‐HT in HCC tumourigenesis, we studied the relationship between the two in HCC and also investigated the effect of 5‐HT receptor antagonists on Wnt/β‐catenin signalling.
2. Materials and methods
2.1. HCC cell lines and tissues
MIHA and 6 HCC cell lines, PLC/PRF/5 (PLC), MHCC97L (97L) and MHCC97H (97H), HuH‐7, Hep3B and HepG2 were grown in Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen, Grand Island, NY, USA) supplemented with 10% foetal bovine serum (FBS) (Invitrogen) and 1% penicillin and streptomycin (Invitrogen) at 37 °C in a humidified atmosphere with 5% carbon dioxide.
Thirty‐three pairs of tumour and adjacent non‐tumour liver tissues were obtained from patients with primary HCC resected at Queen Mary Hospital, Hong Kong. Consent regarding the use of clinical specimens for research was obtained from Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB).
2.2. Chemicals and reagents
5‐Hydroxytryptamine (Tocris Bioscience, Bristol, UK) was dissolved in ddH2O with a final concentration of 10 mM and stored in −20 °C prior to use. Antibodies against Axin1, Axin2, phosphorylated β‐catenin (p‐β‐catenin), GSK3β, phosphorylated GSK3β (p‐GSK3β), and β‐actin were purchased from Cell Signalling Technology (Danvers, MA, USA); β‐catenin was purchased from BD Biosciences (San Jose, CA, USA) and active β‐catenin was purchased from Millipore (Billerica, MA, USA). SB‐258719, a 5‐HT7 inhibitor, and cycloheximide were purchased from Tocris Bioscience. MG132 was obtained from Sigma Aldrich (St Louis, MO, USA). ICG‐001 was purchased from Selleck Chemicals.
2.3. Experimental conditions
HCC cell lines, HuH‐7 and HepG2 cells were harvested, seeded in culture plates/dish and allowed to adhere overnight. The cells were cultured in serum free medium (SFM) for 48 h, then the medium was replaced with medium containing 50 μM 5‐HT for 24 h or otherwise indicated. For experiments involving ICG‐001, the cells were incubated with ICG‐001 for 24 h followed by the addition of 5‐HT for another 24 h. For experiments involving 5‐HT and SB‐258719, the cells were incubated with 50 μM SB‐258719 for 2 h followed by the addition of 50 μM 5‐HT for 24 h (Liang et al., 2013; Soll et al., 2010).
2.4. MTT
Cell viability was assessed by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2, 5‐diphenyltetrazolium bromide (MTT) assay. 3000/well HCC cells were seeded in 96‐well plates. 24 hours after the cells adhered to the plates, medium was removed and cells were incubated in SFM for another 48 h followed by the addition of 5‐HT or SB‐258719. 10 μl of MTT solution (5 mg/ml) was dispensed into each well and plates were incubated for 4 h at 37 °C. After incubation, 100 μl dimethyl sulfoxide was added per well and colour formation was quantified in a plate reader at 570 nm wavelength with 655 nm being the reference wavelength.
2.5. qPCR
Total RNA was isolated using TRIzol reagent (Invitrogen) and treated with DNase I (Qiagen) to remove residual genomic DNA. RNA was then dissolved in diethylpyrocarbonate (DEPC)‐treated water and stored at −80 °C prior to use. Reverse transcription and qPCR were performed as described previously (Fatima et al., 2012). The primers used for the study are as follows:
5‐HT1D forward: 5‐GTCGGACTGTCTGGTGAACA‐3, 5‐HT1D reverse: 5‐GAGTGAGGGTGGATTCAGGA‐3; 5‐HT2A forward: 5‐TCCTACACAGGCAGG AGGAC‐3, 5‐HT2A reverse: 5‐TGCAGGACTCTTTGCAGATG‐5; 5‐HT2B forward: 5‐GCCTTCTTCACACCTCTTGC‐3, 5‐HT2B reverse: 5‐AACCATGTTAGGCGT TGAGG‐3; 5‐HT5 forward: 5‐CTGTGTGGTGCTCTTCGTGT‐3, 5‐HT5 reverse: 5‐GCAGAGTCCTTCACCTCCAC‐3; 5‐HT7 forward: 5‐TCAGCCAGGACTTTG GCTAT‐3, 5‐HT7 reverse: 5‐TGTGTTTGGCAGCACTCTTC‐3.
2.6. Preparation of cytoplasmic and nuclear extracts
Cytoplasmic and nuclear extracts were prepared using the NE‐PER Nuclear and Cytoplasmic Extraction Reagents kit from Thermo Fisher Scientific (Rockford, IL, USA) according to the manufacturer's protocol.
2.7. Immunoprecipitation and western blot
After treatment with 5‐HT for 24 h, cells were washed twice with PBS and lysed with IP lysis buffer (Tris 50 mM, NaCl 150 mM, EGTA 2 mM, EDTA 1 mM, NP‐40 1%) on ice for 30 min. Cellular lysates were collected after centrifugation at 14,000 rpm 4 °C, 10 min and were pre‐cleared with protein‐A/G beads (Roche, Mannheim, Germany) for at least 3 h at 4 °C. Immunoprecipitation of the complexes was performed by incubating the cellular lysates with β‐catenin antibody at 4 °C overnight. The immunocomplexes were washed with cold PBS thrice and re‐suspended in SDS sample buffer followed by analysis by western blot which was done as described previously (Fatima et al., 2012).
2.8. Patient‐derived primary HCC culture
Liver tumour specimens, obtained from the liver patients in People's Hospital of Shenzhen, were cut into small pieces and re‐suspended in pre‐warmed 0.1% Collagenase IV (Sigma Aldrich, St Louis, MO, USA) and then shaken in 37 °C incubator for 40–60 min to fully dissociate the tissue specimen into single cells. The cells were filtered through 70 μM strainer, washed with ice‐cold PBS for three times and then re‐suspended in DMEM/F12 (Invitrogen) supplemented with 10% FBS (Invitrogen) and EGF (Invitrogen). The cells were then seeded in 12‐well plates coated with rat tail collagen type I (diluted with PBS) and allowed to adhere for a few hours. The medium was discarded and the cells were incubated with DMEM/F12 without FBS for 24 h and then treated with 5‐HT and/or SB‐258719 for MTT experiment or collection of protein for western blot.
2.9. Immunohistochemistry
Paraffin‐embedded tumours were sectioned (4 μM) and mounted on glass slides. Immunohistochemistry analysis was performed as described previously (Fatima et al., 2012).
2.10. Animal experiment
Male athymic Balb/c nude male mice between the ages of 7–9 weeks were bred in 12‐h day/light cycle environment with free access to food and water. HuH‐7 cells were trypsinized (Invitrogen), washed and re‐suspended in PBS. 2 × 106 HuH‐7 cells in 200 μl PBS were injected subcutaneously into the flanks of the mice. When the tumours reached about 100 mm3, the mice were randomly divided into two groups (6 animals per group); one group received 20 mg/kg SB‐258719 in 200 μl water subcutaneously twice a day and the other group of 200 μl PBS daily. Tumour growth was calculated with calipers every day, and the volume was calculated according to the formula: volume = (width)2 × length/2. Other indicators such as body weight, feeding behaviour, stool and motor activity were also observed. The animals were killed two weeks after subcutaneous injection.
2.11. Statistical analysis
All experiments were carried out in triplicates and the data was expressed as mean ± standard deviation (SD) unless otherwise indicated. Statistical analysis was done using Prism for Windows (version 5.01) (GraphPad Software, La Jolla, CA, USA) and SPSS17.0 for Windows (version 16.0) (IBM, New York, NY, USA). Two‐way analysis of variance (ANOVA) or the Student's t‐test was used to study significant difference in treatments. For clinicopathological analysis, Pearson's Χ2 was used to calculate the P‐value. Statistical significance was defined as P < 0.05.
3. Results
3.1. Differential expression of 5‐HT receptor subtypes in HCC cell lines and tissues
Expression of various 5‐HT receptors were evaluated in six HCC cell lines by qPCR and compared with immortalized normal hepatocytes (MIHA). All the five 5‐HT receptors studied are differentially expressed in the 6 different types of HCC cell lines (Figure 1A). 5‐HT1D has higher expression in the metastatic cell lines (97L and 97H) and reduced expression in the primary cell lines (HepG2, Hep3B, HuH‐7, and PLC) compared to MIHA. Receptors 5‐HT2A, 5‐HT2B and 5‐HT5 are reduced in almost all the cell lines compared to MIHA, with the exception of increased expression of 5‐HT2A in PLC. Receptor 5‐HT7 has higher expression in the two primary cell lines (HepG2 and HuH‐7) and reduced expression in the remaining cell lines compared to MIHA. The varied expression of the receptors in different HCC cell lines ranging from primary cell lines to metastatic cell lines is suggestive of their differing functional roles.
Figure 1.
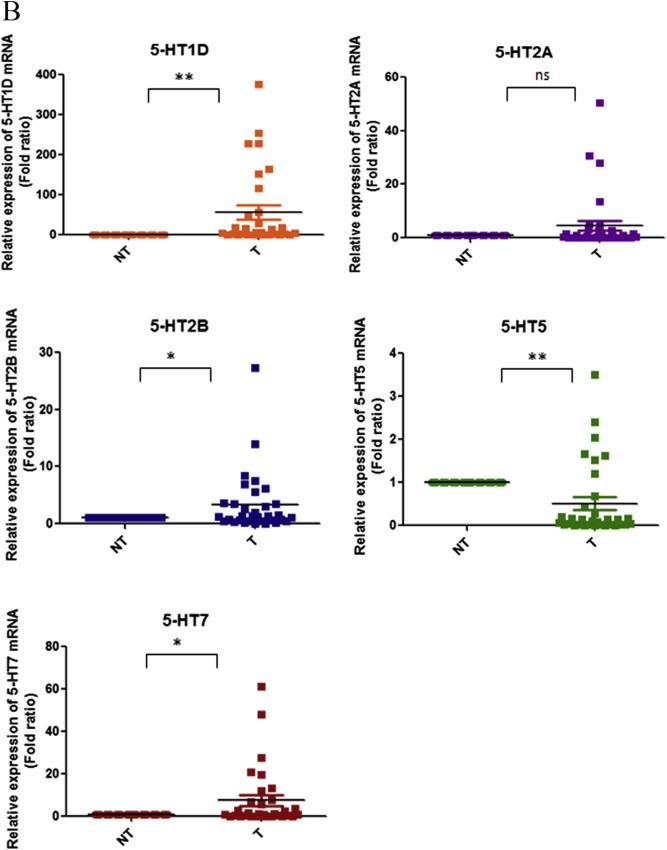
Expression of different 5‐HT receptors on HCC cell lines and tissues. qPCR analysis of 5‐HT receptor subtypes in (A) HCC cell lines and (B) 33 pairs of HCC tissues (T) and their corresponding adjacent non‐tumour tissues (NT). Receptor expression of each sample is presented by the fold ratio against MIHA or all NT tissues (*P < 0.05, **P < 0.01, ns denotes no significant difference, t‐test).
We next checked their expression in 33 pairs of matched HCC tumours and their corresponding adjacent non‐tumour liver tissues. Receptors 5‐HT1D (21/33, 63.6%), 5‐HT2B (12/33, 36.4%) and 5‐HT7 (15/33, 45.4%) were significantly overexpressed in HCC tumour tissues whereas receptor 5‐HT5 (30/33, 90.1%) was reduced in HCC tumour tissues. Receptor 5‐HT2A did not show any significant statistical difference between HCC tumour and corresponding non‐tumour tissues (Figure 1B). Overexpression was defined as 5‐HT receptor having a tumour/non‐tumour expression ratio greater than twofold, and under expression was defined as having this ratio less than twofold.
We next analysed the association of 5‐HT receptor expression to clinicopathological parameters. Receptor 5‐HT1D was associated with non‐tumorous liver histology and tumour size (Table 1). Receptor 5‐HT2A was associated with non‐tumorous liver histology (Table 2). No association was found between receptors 5‐HT2B (Table 3) and 5‐HT5 (Table 4) with any of the clinicopathological parameters. Receptor 5‐HT7 (Table 5) was found to be associated to non‐tumorous liver histology and venous infiltration.
Table Table 1.
Clinical correlation of 5‐HT1D gene expression in HCC.
| Clinicopathological features | Frequency (%) | Receptor 5‐HT1D | P value | |
|---|---|---|---|---|
| Mean ± SD/number of patients | ||||
| Overexpression | ||||
| − | + | |||
| Age (year) | 0.261 | |||
| <53 | 15 (45.5) | 7 | 8 | |
| ≥53 | 18 (54.5) | 5 | 13 | |
| Sex | 0.614 | |||
| Male | 29 (87.9) | 11 | 18 | |
| Female | 4 (12.1) | 1 | 3 | |
| Non‐tumorous liver histology | 0.032 ∗ | |||
| Non‐cirrhotic | 2 (6.1) | 0 | 2 | |
| Chronic hepatitis | 16 (48.5) | 3 | 13 | |
| Cirrhotic | 15 (45.5) | 9 | 6 | |
| Tumour size (cm) a | 0.027 ∗ | |||
| <5 | 9 (27.3) | 6 | 3 | |
| ≥5 | 24 (72.7) | 6 | 18 | |
| Tumour recurrence | 0.784 | |||
| Absence | 12 (36.4) | 4 | 8 | |
| Presence | 21 (63.6) | 8 | 13 | |
| Venous infiltration b | 0.692 | |||
| Absence | 15 (45.5) | 6 | 9 | |
| Presence | 18 (54.5) | 6 | 12 | |
| Number of tumour nodules | 0.443 | |||
| 1 | 25 (75.8) | 10 | 15 | |
| ≥2 | 8 (24.2) | 2 | 6 | |
| Cellular differentiation c , d | 0.688 | |||
| Well differentiated | 11 (33.3) | 3 | 8 | |
| Moderately differentiated | 14 (42.4) | 6 | 8 | |
| Poorly differentiated | 7 (21.2) | 3 | 4 | |
| AJCC stage | 0.064 | |||
| I and II | 15 (45.5) | 8 | 7 | |
| III and IV | 18 (54.5) | 4 | 14 | |
*P < 0.05.
Tumour size was defined as the length of the largest tumour nodule.
Venous infiltration was defined based on findings by microscopic and major pathologic examination.
Well differentiated (Edmonson grade 0–2); moderately differentiated (Edmonson grade 3); poorly differentiated (Edmonson grade 4).
Partial data is not available and statistics were based on available data of 32 patients.
Table Table 2.
Clinical correlation of 5‐HT2A gene expression in HCC.
| Clinicopathological features | Frequency (%) | Receptor 5‐HT2A | P value | |
|---|---|---|---|---|
| Mean ± SD/number of patients | ||||
| Overexpression | ||||
| − | + | |||
| Age (year) | 0.101 | |||
| <53 | 15 (45.5) | 13 | 2 | |
| ≥53 | 18 (54.5) | 11 | 7 | |
| Sex | 0.191 | |||
| Male | 29 (87.9) | 20 | 9 | |
| Female | 4 (12.1) | 4 | 0 | |
| Non‐tumorous liver histology | 0.031 ∗ | |||
| Non‐cirrhotic | 2 (6.1) | 0 | 2 | |
| Chronic hepatitis | 16 (48.5) | 11 | 5 | |
| Cirrhotic | 15 (45.5) | 13 | 2 | |
| Tumour size (cm) a | 0.202 | |||
| <5 | 9 (27.3) | 8 | 1 | |
| ≥5 | 24 (72.7) | 16 | 8 | |
| Tumour recurrence | 0.825 | |||
| Absence | 12 (36.4) | 9 | 3 | |
| Presence | 21 (63.6) | 15 | 6 | |
| Venous infiltration b | 0.475 | |||
| Absence | 15 (45.5) | 10 | 5 | |
| Presence | 18 (54.5) | 14 | 4 | |
| Number of tumour nodules | 0.097 | |||
| 1 | 25 (75.8) | 20 | 5 | |
| ≥2 | 8 (24.2) | 4 | 4 | |
| Cellular differentiation c,d | 0.524 | |||
| Well differentiated | 11 (33.3) | 9 | 2 | |
| Moderately differentiated | 14 (42.4) | 10 | 4 | |
| Poorly differentiated | 7 (21.2) | 4 | 3 | |
| AJCC stage | 0.392 | |||
| I and II | 15 (45.5) | 12 | 3 | |
| III and IV | 18 (54.5) | 12 | 6 | |
*P < 0.05.
Tumour size was defined as the length of the largest tumour nodule.
Venous infiltration was defined based on findings by microscopic and major pathologic examination.
Well differentiated (Edmonson grade 0–2); moderately differentiated (Edmonson grade 3); poorly differentiated (Edmonson grade 4).
Partial data is not available and statistics were based on available data of 32 patients.
Table Table 3.
Clinical correlation of 5‐HT2B gene expression in HCC.
| Clinicopathological features | Frequency (%) | Receptor 5‐HT2B | P value | |
|---|---|---|---|---|
| Mean ± SD/number of patients | ||||
| Overexpression | ||||
| − | + | |||
| Age (year) | 0.741 | |||
| <53 | 15 (45.5) | 10 | 5 | |
| ≥53 | 18 (54.5) | 11 | 7 | |
| Sex | 0.545 | |||
| Male | 29 (87.9) | 19 | 10 | |
| Female | 4 (12.1) | 2 | 2 | |
| Non‐tumorous liver histology | 0.204 | |||
| Non‐cirrhotic | 2 (6.1) | 1 | 1 | |
| Chronic hepatitis | 16 (48.5) | 8 | 8 | |
| Cirrhotic | 15 (45.5) | 12 | 3 | |
| Tumour size (cm) a | 0.065 | |||
| <5 | 9 (27.3) | 8 | 1 | |
| ≥5 | 24 (72.7) | 13 | 11 | |
| Tumour recurrence | 0.632 | |||
| Absence | 12 (36.4) | 7 | 5 | |
| Presence | 21 (63.6) | 14 | 7 | |
| Venous infiltration b | 0.692 | |||
| Absence | 15 (45.5) | 9 | 6 | |
| Presence | 18 (54.5) | 12 | 6 | |
| Number of tumour nodules | 0.357 | |||
| 1 | 25 (75.8) | 17 | 8 | |
| ≥2 | 8 (24.2) | 4 | 4 | |
| Cellular differentiation c,d | 0.222 | |||
| Well differentiated | 11 (33.3) | 8 | 3 | |
| Moderately differentiated | 14 (42.4) | 7 | 7 | |
| Poorly differentiated | 7 (21.2) | 6 | 1 | |
| AJCC stage | 0.290 | |||
| I and II | 15 (45.5) | 11 | 4 | |
| III and IV | 18 (54.5) | 10 | 8 | |
*P < 0.05.
Tumour size was defined as the length of the largest tumour nodule.
Venous infiltration was defined based on findings by microscopic and major pathologic examination.
Well differentiated (Edmonson grade 0–2); moderately differentiated (Edmonson grade 3); poorly differentiated (Edmonson grade 4).
Partial data is not available and statistics were based on available data of 32 patients.
Table Table 4.
Clinical correlation of 5‐HT5 gene expression in HCC.
| Clinicopathological features | Frequency (%) | Receptor 5‐HT5 | P value | |
|---|---|---|---|---|
| Mean ± SD/number of patients | ||||
| Overexpression | ||||
| − | + | |||
| Age (year) | 0.658 | |||
| <53 | 15 (45.5) | 14 | 1 | |
| ≥53 | 18 (54.5) | 16 | 2 | |
| Sex | 0.238 | |||
| Male | 29 (87.9) | 27 | 2 | |
| Female | 4 (12.1) | 3 | 1 | |
| Non‐tumorous liver histology | 0.767 | |||
| Non‐cirrhotic | 2 (6.1) | 2 | 0 | |
| Chronic hepatitis | 16 (48.5) | 14 | 2 | |
| Cirrhotic | 15 (45.5) | 14 | 1 | |
| Tumour size (cm) a | 0.266 | |||
| <5 | 9 (27.3) | 9 | 0 | |
| ≥5 | 24 (72.7) | 21 | 3 | |
| Tumour recurrence | 0.170 | |||
| Absence | 12 (36.4) | 12 | 0 | |
| Presence | 21 (63.6) | 18 | 3 | |
| Venous infiltration b | 0.658 | |||
| Absence | 15 (45.5) | 14 | 1 | |
| Presence | 18 (54.5) | 16 | 2 | |
| Number of tumour nodules | 0.072 | |||
| 1 | 25 (75.8) | 24 | 1 | |
| ≥2 | 8 (24.2) | 6 | 2 | |
| Cellular differentiation c, d | 0.254 | |||
| Well differentiated | 11 (33.3) | 11 | 0 | |
| Moderately differentiated | 14 (42.4) | 12 | 2 | |
| Poorly differentiated | 7 (21.2) | 7 | 0 | |
| AJCC stage | 0.097 | |||
| I and II | 15 (45.5) | 15 | 0 | |
| III and IV | 18 (54.5) | 15 | 3 | |
*P < 0.05.
Tumour size was defined as the length of the largest tumour nodule.
Venous infiltration was defined based on findings by microscopic and major pathologic examination.
Well differentiated (Edmonson grade 0–2); moderately differentiated (Edmonson grade 3); poorly differentiated (Edmonson grade 4).
Partial data is not available and statistics were based on available data of 32 patients.
Table Table 5.
Clinical correlation of 5‐HT7 gene expression in HCC.
| Clinicopathological features | Frequency (%) | Receptor 5‐HT7 | P value | |
|---|---|---|---|---|
| Mean ± SD/number of patients | ||||
| Overexpression | ||||
| − | + | |||
| Age (year) | 0.898 | |||
| <53 | 15 (45.5) | 8 | 7 | |
| ≥53 | 18 (54.5) | 10 | 8 | |
| Sex | 0.846 | |||
| Male | 29 (87.9) | 16 | 13 | |
| Female | 4 (12.1) | 2 | 2 | |
| Non‐tumorous liver histology | 0.000 ∗ | |||
| Non‐cirrhotic | 2 (6.1) | 0 | 2 | |
| Chronic hepatitis | 16 (48.5) | 4 | 12 | |
| Cirrhotic | 15 (45.5) | 14 | 1 | |
| Tumour size (cm) a | 0.101 | |||
| <5 | 9 (27.3) | 7 | 2 | |
| ≥5 | 24 (72.7) | 11 | 13 | |
| Tumour recurrence | 0.692 | |||
| Absence | 12 (36.4) | 6 | 6 | |
| Presence | 21 (63.6) | 12 | 9 | |
| Venous infiltration b | 0.025 ∗ | |||
| Absence | 15 (45.5) | 5 | 10 | |
| Presence | 18 (54.5) | 13 | 5 | |
| Number of tumour nodules | 0.054 | |||
| 1 | 25 (75.8) | 16 | 9 | |
| ≥2 | 8 (24.2) | 2 | 6 | |
| Cellular differentiationc,d | 0.462 | |||
| Well differentiated | 11 (33.3) | 6 | 5 | |
| Moderately differentiated | 14 (42.4) | 6 | 8 | |
| Poorly differentiated | 7 (21.2) | 5 | 2 | |
| AJCC stage | 0.202 | |||
| I and II | 15 (45.5) | 10 | 5 | |
| III and IV | 18 (54.5) | 8 | 10 | |
*P < 0.05.
Tumour size was defined as the length of the largest tumour nodule.
Venous infiltration was defined based on findings by microscopic and major pathologic examination.
Well differentiated (Edmonson grade 0–2); moderately differentiated (Edmonson grade 3); poorly differentiated (Edmonson grade 4).
Partial data is not available and statistics were based on available data of 32 patients.
3.2. 5‐HT enhances growth of serum‐deprived HCC cells
To determine whether 5‐HT can promote HCC cell proliferation we measured cell viability of HuH‐7 and HepG2 cells both in the presence and absence of 10% FBS in combination with 5‐HT. As shown in Figure 2A, HuH‐7 and HepG2 cells demonstrate reduced growth in SFM compared to cells grown in 10% FBS (SFM vs FBS, ***P < 0.001, two‐way ANOVA). However, 5‐HT enhanced cell viability of serum‐deprived HuH‐7 and HepG2 cells (SFM vs SFM + 50 μm 5‐HT, ***P < 0.001, two‐way ANOVA). Furthermore, other than HuH‐7 and HepG2, the remaining HCC cell lines, MIHA, Hep3B, PLC, 97L and 97H did not respond to 5‐HT under serum deprivation (Supplementary Figure 1A). To confirm the proliferative effect of 5‐HT we also investigated the effect of 5‐HT in the presence of 10% FBS whereby 5‐HT did not promote proliferation of HuH‐7 and HepG2 cells (Supplementary Figure 1B). Furthermore, as shown in Supplementary Figures 2A–C, 5‐HT also did not influence cell migration, apoptosis or cell cycle. However, treatment with 5‐HT inhibited autophagy in HuH‐7 and HepG2 cells (Supplementary Figure 2D). In both cell lines, microtubule‐associated protein light chain 3 (LC3B) protein, required for autophagosome assembly and a widely used marker for autophagy (Mizushima et al., 2010), increased after 24 h of SFM but in the presence of 5‐HT its levels decreased. Secondly, p62 protein levels, an additional marker of autophagy, increased 24 h after 5‐HT treatment. Specific interaction between p62 and LC3 is critical for specific degradation of autophagy. As degradation of p62 is dependent on autophagy, p62 protein levels are increased upon inhibition of autophagy (Itakura and Mizushima, 2011).
Figure 2.
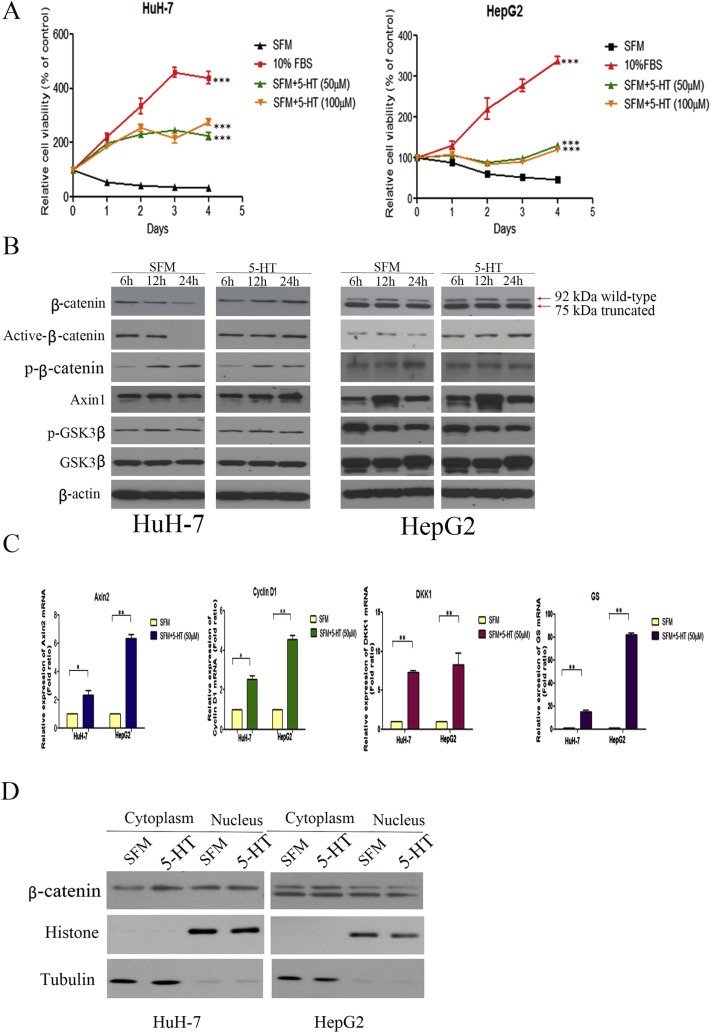
5‐HT can promote proliferation of serum‐deprived HuH‐7 and HepG2 cells and upregulate β‐catenin. (A) The relative viability of HuH‐7 and HepG2 cells cultured in 10% FBS, or SFM with or without 5‐HT (50 μM and 100 μM 5‐HT) was detected by MTT. 5‐HT increased proliferation of serum‐deprived HuH‐7 and HepG2 cells (SFM vs SFM + 5‐HT, ***P < 0.001, two‐way ANOVA). (B) HuH‐7 and HepG2 cells were cultured in SFM for 48 h followed by addition of 5‐HT for indicated time points. 5‐HT increased total β‐catenin, active β‐catenin and decreased p‐β‐catenin protein levels. Axin1, total GSK3β and p‐GSK3β protein levels remain unchanged. (C) Relative mRNA expression of β‐catenin downstream targets was assessed by qPCR. 5‐HT increased mRNA levels of Axin2, cyclin D1, DKK1, and GS in HuH‐7 and HepG2 cells (*P < 0.05, **P < 0.01, t‐test). (D) 5‐HT increased cytoplasmic β‐catenin protein levels in serum‐deprived HuH‐7 and HepG2 cells.
3.3. 5‐HT enhances β‐catenin in serum‐deprived cells
Since Wnt/β‐catenin signalling is highly dysregulated in HCC, we first asked whether 5‐HT affects β‐catenin protein levels in serum‐deprived cells. HepG2 cells contain a heterozygous deletion in exon 3 of the β‐catenin gene, resulting in two β‐catenin proteins, the wild‐type β‐catenin (92 kDa) and the truncated form (75 kDa). As shown in Figure 2B, 5‐HT increased total β‐catenin protein levels in both serum‐deprived cell lines in a time‐dependent manner compared to control cells under serum starvation only. Previous studies have suggested that phosphorylation of β‐catenin at specific Ser/Thr residues followed by its ubiquitination results in β‐catenin degradation (Amit et al., 2002). Therefore we examined the phosphorylation status of β‐catenin in response to 5‐HT. Compared to serum‐deprived control cells, 5‐HT increased active form of β‐catenin (dephosphorylated on Ser37 or Thr41) and reduced p‐β‐catenin protein levels in HuH‐7 and HepG2 after 24 h. The active form of β‐catenin mediates target gene activation whereas p‐β‐catenin is targeted for degradation. However, in both cell lines 5‐HT had no effect on Axin1, total GSK3β, and p‐GSK3β. 5‐HT had no effect on the Wnt/β‐catenin pathway under FBS condition (Supplementary Figure 3).
5‐HT also enhanced mRNA levels of β‐catenin downstream targets, e.g. Axin2, cyclin D1, DKK1, and GS in both cell lines (Figure 2C). After observing an increase in total β‐catenin levels, we next investigated the distribution of cytoplasmic and the nuclear β‐catenin protein levels. 5‐HT increased cytoplasmic β‐catenin levels in HuH‐7 and HepG2 cells after 24 h but nuclear β‐catenin levels remain unchanged in both cell lines (Figure 2D).
To confirm the role of β‐catenin in the proliferation of HCC cells induced by 5‐HT, we used a Wnt pathway inhibitor, ICG‐001, which inhibits the association of cAMP‐responsive element binding (CREB)‐binding protein (CBP) and β‐catenin leading to down regulation of β‐catenin‐TCF4 responsive genes e.g. cyclin D1 (Emami et al., 2004). As shown in Supplementary Figure 4A, treatment with ICG‐001 reduced cyclin D1 protein levels and also inhibited the proliferation of HuH‐7 and HepG2 cells both alone and/or in combination with 5‐HT (5‐HT vs ICG‐001 + 5‐HT, ***P < 0.001, two‐way ANOVA) (Supplementary Figure 4B). These results suggest the importance of the Wnt/β‐catenin pathway in the proliferation of HCC induced by 5‐HT.
3.4. Increased β‐catenin due to inhibition of β‐catenin degradation
To determine whether the increased β‐catenin by 5‐HT in serum‐derived HuH‐7 and HepG2 cells is a result of its increased synthesis or decreased degradation, HuH‐7 and HepG2 cells were treated with 5‐HT and cycloheximide (CHX, inhibitor of de novo protein synthesis) alone or in combination. Serum‐deprived HuH‐7 and HepG2 cells were treated with 5‐HT for 2 h followed by treatment with 100 μM CHX for the indicated time points. According to Figure 3A, β‐catenin is up regulated after treatment with 5‐HT and CHX compared to CHX alone, suggesting that increase of β‐catenin by 5‐HT is a result of decreased degradation. Furthermore, we also checked if inhibition of β‐catenin degradation is influenced by the proteasome system. Serum‐deprived HuH‐7 and HepG2 cells were pre‐treated with 0.5 μM MG132 (proteasome inhibitor) for 2 h, followed by treatment with 5‐HT for 24 h. As shown in Figure 3B, combination of MG132 and 5‐HT resulted in the same increase in β‐catenin levels as after MG132 alone.
Figure 3.
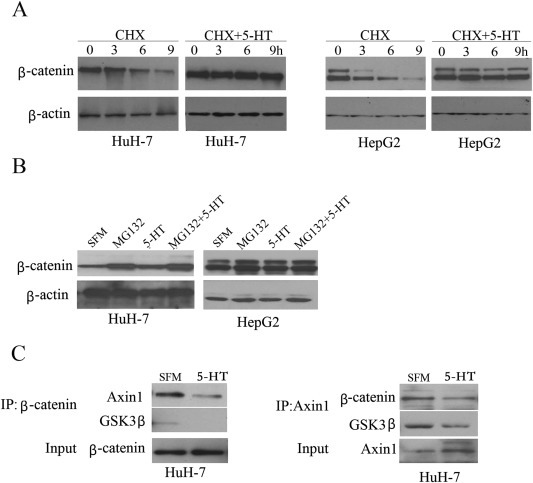
5‐HT enhances β‐catenin protein level via decreased degradation and disrupts Axin1/β‐catenin interaction. (A) Serum‐deprived HuH‐7 cells and HepG2 cells were first cultured with 5‐HT for 2 h followed by 100 μM CHX treatment for the indicated time points. β‐Catenin levels decreased in SFM cells in the presence of CHX only but treatment with CHX and 5‐HT in serum‐deprived cells resulted in increased β‐catenin protein levels as did 5‐HT treatment alone. (B) Serum‐deprived HuH‐7 and HepG2 cells were pre‐treated with 0.5 μM MG132 for 2 h and followed by 5‐HT for another 24 h 5‐HT increased β‐catenin protein levels in the presence of MG312. (C) Protein from HuH‐7 cells under SFM and 5‐HT treatment was immunoprecipitated with Axin1 or β‐catenin antibodies and immunoblotted for Axin1, β‐catenin and GSK3β by western blot.
3.5. 5‐HT disrupts Axin1/β‐catenin interaction
GSK3β‐mediated β‐catenin phosphorylation and its subsequent degradation is regulated by interaction of Axin1 with GSK3β and β‐catenin (Huang and He, 2008). As 5‐HT increased β‐catenin but did not significantly affect total GSK3β and Axin1 protein levels, we asked whether 5‐HT disrupted the interaction between Axin1/β‐catenin, which represents a pivotal step in β‐catenin degradation. In HuH‐7 cells, co‐immunoprecipitation with β‐catenin antibody demonstrated that 5‐HT decreased the association between β‐catenin and Axin1. The result was repeated upon co‐immunoprecipitation with Axin1 antibody. Furthermore, 5‐HT also disrupted the interaction between GSKβ and Axin1/β‐catenin (Figure 3C). Thus, increase in β‐catenin in serum‐deprived HuH‐7 cells may be due to disruption of the Axin1/GSK3β/β‐catenin complex resulting in β‐catenin stabilization.
3.6. Receptor 5‐HT7 antagonist, SB‐258719, attenuates β‐catenin
As receptors 5‐HT1D and 5‐HT7 were significantly overexpressed in HCC clinical tissues and that their expression was correlated to liver histology and tumour size or venous infiltration, respectively, we next investigated if antagonists of 5‐HT receptors attenuated 5‐HT induced growth of HCC cells and β‐catenin protein levels. Although 97L cells overexpressed receptor 5‐HT1D mRNA, they did not respond to 5‐HT, thus we focused on receptor 5‐HT7. Both cell lines, HuH‐7 and HepG2, expressed receptor 5‐HT7 and responded to 5‐HT treatment (Figure 2A).
We first checked the effect of 5‐HT7 antagonist, SB‐258719, on the growth of serum‐deprived cells in the presence of 5‐HT. Serum‐deprived HuH‐7 and HepG2 cells were treated with different concentrations of SB‐258719 for 2 h followed by addition of 5‐HT. As shown in Figure 4A, SB‐258719 reduced the cell viability of serum‐deprived HuH‐7 and HepG2 cells, compared to cells treated with 5‐HT alone (Figure 4, 5‐HT vs 5‐HT + SB‐258719 50 μM, ***P < 0.001, two‐way ANOVA). This suggests that 5‐HT may regulate cell proliferation via 5‐HT7 subtype. We next assessed whether SB‐258719 affected β‐catenin protein levels. As shown in Figure 4B, serum‐deprived HuH‐7 and HepG2 cells treated with 5‐HT alone show increased β‐catenin levels but treatment with SB‐258719 alone had no effect. However, when the serum‐deprived cell lines were pre‐treated with SB‐258719 for 2 h followed by 5‐HT for 24 h, β‐catenin levels decreased in both cell lines.
Figure 4.
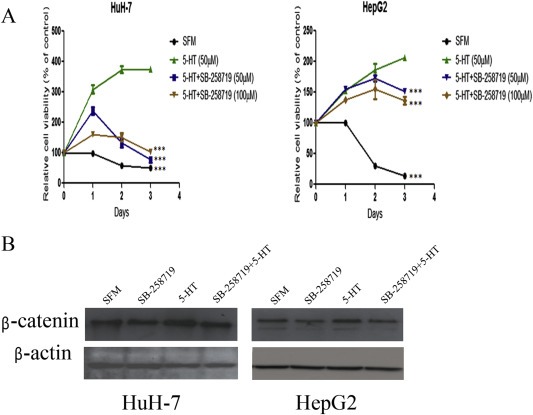
5‐HT7 receptor antagonist, SB‐258719, reduces proliferation of HuH‐7 and HepG2 cells and also down regulates β‐catenin. (A) Serum deprived HuH‐7 and HepG2 cells were subjected to different concentrations of SB‐258719 for 2 h followed by incubation with 5‐HT. Cell viability was detected by MTT. SB‐258719 inhibited the proliferative effect of 5‐HT (5‐HT vs 5‐HT + SB‐258719, ***P < 0.001, two‐way ANOVA). (B) SB‐258719 reduced β‐catenin protein levels in serum‐deprived HCC cells in the presence of 5‐HT compared to 5‐HT treatment alone in both HuH‐7 and HepG2 cells.
3.7. SB‐258719 attenuates proliferation and β‐catenin protein levels of patient‐derived primary HCC cultures
To further confirm reduced cell growth and attenuation of β‐catenin protein levels by SB‐258719, we investigated the effect of SB‐258719 in primary HCC tumour cell cultures which closely mimic in vivo tumour microenvironment. Following extraction of primary hepatocytes, the cells were serum‐starved for 48 h and pre‐treated with SB‐258719 for 2 h before incubation with 5‐HT and analysed for cell viability by MTT. Of the six specimen collected, three cases demonstrated reduced cell growth after treatment with SB‐258719 with the IC50 around 100 μM (Figure 5A). Of these three cases, unfortunately only one case had sufficient cells for detection of β‐catenin protein levels in which SB‐258719 reduced β‐catenin protein levels (Figure 5B).
Figure 5.
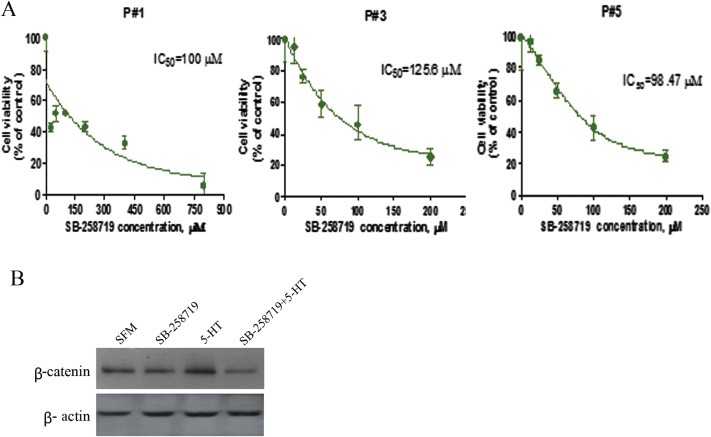
The effect of SB‐258719 on patient‐derived primary HCC cultures. (A) SB‐258719 attenuated the growth of patient‐derived primary HCC cultures in the presence of 5‐HT and cell viability was detected by MTT. (B) SB‐258719 down‐regulated the expression of β‐catenin in patient‐derived primary HCC cultures compared to 5‐HT treatment only.
3.8. SB‐258719 suppresses in vivo tumourigenicity
To assess the in vivo anti‐cancer activity of 5‐HT7 antagonist SB‐258719, tumour xenograft mouse model was constructed by subcutaneous injection of HuH‐7 cells and when the tumour reached about 80–100 mm3 after three weeks, the mice were randomly divided into two groups, control and SB‐258719 group. Tumour growth in the SB‐258719 group was significantly reduced compared to the control group (Figure 6A and B), while the motor, feeding habit and body weight did not show any obvious difference between the two groups (data not shown). The SB‐258719 group also had reduced tumour weight compared to the control group but this could not exhibit any statistical significance due to the limited sample number (Figure 6C). We next investigated the effect of SB‐258719 on the Wnt/β‐catenin pathway in vivo. Immunohistochemical analysis of tumour xenografts showed that tumours in the control group had strong cytoplasmic and nuclear β‐catenin accumulation and weakly stained for GSK3β levels. On the other hand, tumours from the SB‐258719 group had reduced β‐catenin accumulation in both the cytoplasm and the nucleus and exhibited strong GSK3β levels (Figure 6D). These results imply that SB‐258719 attenuates Wnt/β‐catenin signalling and plays an important regulatory role in tumourigenesis.
Figure 6.
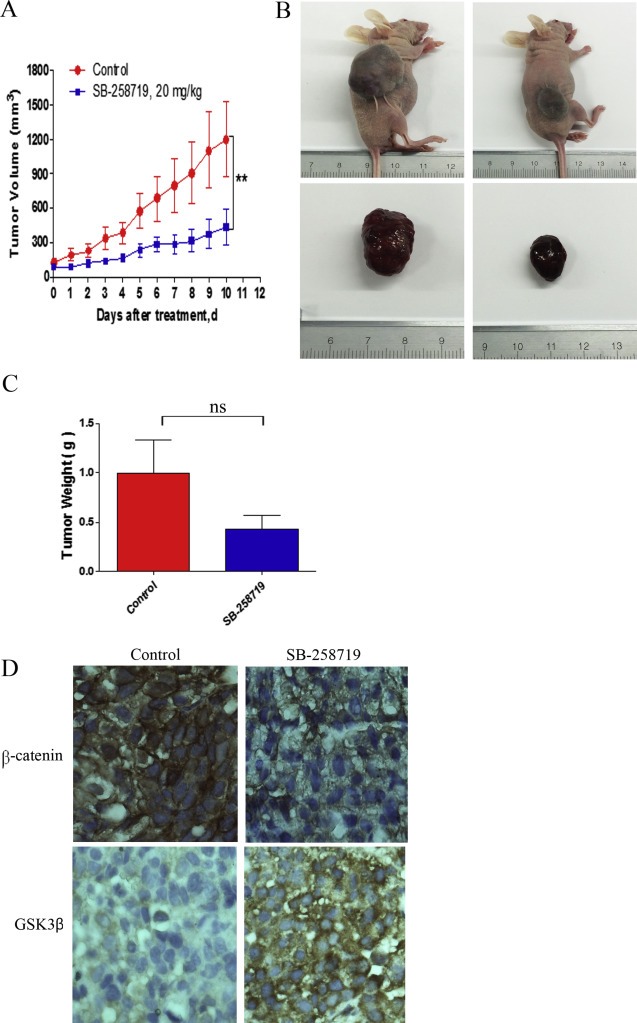
SB‐258719 suppresses tumorigenicity of HCC xenografts in vivo. (A) HuH‐7 cells were subcutaneously injected into BALB/c nude mice. The xenograft tumours were treated with vehicle or SB‐258719 20 mg/kg twice a day subcutaneously for 10 days and tumour growth was measured every day. Compared to the control group, SB‐258719 treatment reduced (B) tumour growth (**P < 0.01, two‐way ANOVA) and (C) tumour weight (ns denotes no significant difference, t‐test). (D) Immunohistochemical analysis of β‐catenin and GSK3β in the SB‐258719 treatment and control group. Tumour xenografts from SB‐258719 treatment group had reduced accumulation of β‐catenin and increased accumulation of GSK3β.
4. Discussion
Deregulation of the Wnt/β‐catenin signalling is characteristic of several cancers including HCC and accumulation of β‐catenin has been associated to poor HCC prognosis (Chen et al., 2014). Recently, 5‐HT has been reported to promote proliferation of different cell types but its mechanism of action remains to be elucidated. To our knowledge, the present study is the first to demonstrate the influence of 5‐HT on Wnt/β‐catenin signalling in HCC. Our results show that 5‐HT promoted proliferation of serum‐deprived HCC cells by influencing β‐catenin.
We first confirmed the differential expression of 5‐HT receptors, 5‐HT1D, 5‐HT2A, 5‐HT2B, 5‐HT5 and 5‐HT7 in HCC tissues and their corresponding non‐tumour tissues. The expression of 5‐HT1D, 5‐HT2A and 5‐HT7 significantly correlated to non‐tumorous liver histology (non‐cirrhotic, chronic hepatitis, cirrhosis). This is contrary to a study by Soll et al. (2012) who reported significant correlation of 5‐HT2B expression to higher proliferative index and however did not observe any difference in 5‐HT7 expression between HCC tumour and non‐tumour tissues (Soll et al., 2012). Thus, this is the first study to report overexpression of 5‐HT7 in HCC. The difference in 5‐HT receptor expression between the two studies may be due to different underlying etiology of the HCC patient cases and the genetic heterogeneity of HCC. In the study by Soll et al. (2012), the patient pool consisted of HCC cases with various underlying etiologies including HBV infection, HCV infection, alcohol abuse, haemochromatosis and there were many patients with unknown etiologies. Our patient cases were mostly HCC with underlying cirrhosis and HBV infection. The correlation of 5‐HT1D, 5‐HT2A and 5‐HT7 to non‐tumorous liver histology may imply that the different receptors could play a role in the different histological stages of HCC, warranting further studies to check any association of the 5‐HT receptors to a distinct etiology. Furthermore, a large patient cohort will be important to investigate the diagnostic and prognostic significance of the 5‐HT receptors. Using genome‐wide analysis including cDNA microarrays, several studies have reported distinguished gene expression profiles between HBV and HCV associated HCC (Honda et al., 2006; Iizuka et al., 2002; Kim et al., 2003; Lee et al., 2008), suggesting that hepatitis viruses affect gene expression in a type‐specific manner leading to hepatocarcinogenesis by different mechanisms.
Similar to results reported by Soll et al. (2010), we only observed proliferation of HCC cells and inhibition of autophagy upon treatment of HCC cells with 5‐HT (Soll et al., 2010). We did not observe difference in cell migration, apoptosis, or cell cycle between serum‐deprived cells and cells treated with 5‐HT. Autophagy has been associated to several diseases including cancer (Levine and Kroemer, 2008; Li et al., 2013). Several studies have reported attenuation of Wnt/β‐catenin signalling by autophagy via interaction of Wnt/β‐catenin signalling components with LC3 or p62 (Gao et al., 2010; Petherick et al., 2013). As both autophagy and Wnt/β‐catenin signalling play a critical role in hepatocarcinogenesis (Li et al., 2013; Takigawa and Brown, 2008), it will be important to investigate how activation of Wnt/β‐catenin signalling by 5‐HT inhibits autophagy.
Cytoplasmic/nuclear accumulation of β‐catenin is a hallmark of Wnt/β‐catenin signalling either due to mutation in β‐catenin or due to aberrant expression of the different components of the Wnt/β‐catenin pathway. Nuclear β‐catenin in HCC has been associated to tumour progression and poor prognosis (Cieply et al., 2009; Inagawa et al., 2002). Our results show that 5‐HT increased cytoplasmic β‐catenin levels as well as β‐catenin downstream targets, Axin2, cyclin D1, DKK1 and GS in serum‐deprived cells. Being a downstream target of β‐catenin, DKK1 is also a reported antagonist of the Wnt/β‐catenin signalling supporting a negative feedback mechanism to regulate the Wnt/β‐catenin pathway. However, increased DKK1 has been reported in HCC implying disruption of its negative feedback mechanism. Increased expression of DKK1 mRNA in HCC has been correlated to cytoplasmic/nuclear β‐catenin accumulation resulting in poor over‐all survival and prognosis (Yu et al., 2009). GS is another liver‐related Wnt‐target gene (Cadoret et al., 2002) that has been suggested as a marker for the identification of β‐catenin mutations in HCC (Austinat et al., 2008).
Although 5‐HT increased cytoplasmic β‐catenin levels and not nuclear β‐catenin levels in the HCC cell lines, it may imply that 5‐HT on its own may not be sufficient to promote Wnt/β‐catenin signalling and may require other components. Import and export of β‐catenin to and from the nucleus is a regulated process. Several studies have reported that activation of Wnt/β‐catenin signalling does not influence β‐catenin translocation to the nucleus and that cytoplasmic‐nuclear shuffling of β‐catenin may be governed by other cytoplasmic and nuclear retention factors (Everly et al., 2004; Henderson and Fagotto, 2002; Krieghoff et al., 2006). Krieghoff et al. (2006) reported nuclear localization of β‐catenin to be regulated via nuclear import by TCF4 and BCL9 and via nuclear export by APC and Axin (Krieghoff et al., 2006). Recently Sharma et al. (2012) identified Armadillo repeats (10–12) on β‐catenin that facilitate its nuclear transport by direct interaction with nuclear pore complex proteins e.g. Nup62, Nup98 and Nup153 (Sharma et al., 2012). Further studies are warranted to understand the role of 5‐HT in the nucleus and whether it affects any cytoplasmic/nuclear retention factors that influence Wnt/β‐catenin signalling. We have also previously reported increased cytoplasmic β‐catenin as well as cyclin D1 protein levels without concurrent increase in nuclear β‐catenin protein levels in HCC cells (Fatima et al., 2012).
In an attempt to delineate the mechanism of how 5‐HT increases β‐catenin levels, other components of the Wnt/β‐catenin pathway were studied. In serum‐deprived medium, presence of 5‐HT increased active β‐catenin levels and decreased phosphorylated β‐catenin, which is a prerequisite for subsequent ubiquitination and degradation, suggesting that increased β‐catenin in the cytoplasm could be a result of β‐catenin stabilization and decreased β‐catenin degradation. Supporting this, when serum‐deprived cells were treated with CHX, the presence of 5‐HT masked the effect of CHX, thereby preventing β‐catenin degradation. Stabilization of β‐catenin also results from the disruption of the interaction between the β‐catenin destruction complexes. The scaffolding protein, Axin1, interacts with GSK3β and β‐catenin and orchestrates phosphorylation of β‐catenin at Ser45 by casein kinase Iα (CKIα) followed by phosphorylation by GSK3β at Ser33/Ser37/Thr41 and its subsequent degradation (Orford et al., 1997). Based on these results we propose that 5‐HT disrupts the association between Axin1/GSK3β/β‐catenin, thereby inhibiting GSK3β‐mediated phosphorylation of β‐catenin. The unphosphorylated β‐catenin is not degraded and thus accumulates in the cytoplasm.
To confirm the in vitro effect of 5‐HT on Wnt/β‐catenin signalling, the treatment of HCC cells with 5‐HT7 receptor antagonist, SB‐258719, reduced proliferation of serum‐deprived cells in the presence of 5‐HT, and attenuated β‐catenin protein levels in both HCC cell lines and patient‐derived primary HCC cultures, which closely mimic tumour micro‐environment. Reduced cytoplasmic and nuclear β‐catenin levels were also observed in tissues from in vivo xenograft model. Together, these results suggest the 5‐HT7 subtype may influence aberrant activation of the Wnt/β‐catenin pathway in HCC and may also act as a potential therapeutic target for HCC. Given the presence of various 5‐HT receptors in HCC, 5‐HT may exert its effect via differing mechanisms and also only 5‐HT2B and its antagonist has been studied in some detail in HCC. Soll et al. (2010) and Liang et al. (2013) both observed varying mechanism of action of 5‐HT2B receptor subtype in HCC. Soll et al. (2010) reported that 5‐HT increased mTOR downstream targets, p70S6K and 4E‐BP1, facilitating survival and inhibiting autophagy in serum‐deprived HCC cells. On the other hand, Liang et al. (2013) observed that 5‐HT promoted proliferation of serum‐deprived HCC cells via upregulation of Foxo3a. Furthermore, Soll et al. (2010) and Liang et al. (2013) reported decreased p70S6K and 4E‐BP1, and Foxo3a, respectively, following treatment of HCC cells with 5‐HT2B antagonist. Thus the activation of different pathways by 5‐HT suggests its complex role in HCC proliferation and represents a complex cross‐talk between signalling pathways.
It will also be worthy to investigate if the receptors function redundantly. As 5‐HT proliferates HCC cells via upregulation of Foxo3a (Liang et al., 2013) and that Foxo3a has shown to interact with β‐catenin (Hoogeboom et al., 2008), further studies are warranted to study the interaction of β‐catenin and Foxo3a under the influence of 5‐HT and its antagonists. Oncogenic phosphoinositide 3‐kinase (PI3K/AKT) suppresses the function of Foxo3a by phosphorylating Foxo3a and sequestering Foxo3a to the cytoplasm. In colon cancer, Tenbaum et al. (2012) reported that in the presence of nuclear β‐catenin accumulation, activation of FOXO3a by PI3K/AKT inhibitors, which are caused in the treatment of colon cancer, can promote metastasis (Tenbaum et al., 2012). Thus it would be important to study if 5‐HT leads PI3K/AKT and the Wnt/β‐catenin pathways to converge to regulate cell cycle progression in HCC cells.
We conclude that expression of various 5‐HT receptors is involved in HCC hepatocarcinogenesis. This study identified dysregulation of the Wnt/β‐catenin signalling pathway under the influence of 5‐HT resulting in β‐catenin stabilization. However, β‐catenin accumulation was reversed by 5‐HT7 antagonist. Thus, 5‐HT mediated Wnt/β‐catenin signalling in HCC may serve as a potential therapeutic target.
Supporting information
The following are the supplementary data related to this article:
Supplementary data
Supplementary Figure 1 5‐HT does not promote proliferation in all HCC cell lines. (A) The relative viability of five HCC cell lines cultured in 10% FBS, or SFM with or without 5‐HT (50 μM and 100 μM 5‐HT) was detected by MTT. (B) The relative viability of HuH‐7 and HepG2 cells cultured in 10% FBS with or without 5‐HT (50 μM and 100 μM 5‐HT) was detected by MTT.
Supplementary Figure 2 Affect of 5‐HT on various HCC characteristics. 5‐HT does not affect (A) cell migration as demonstrated by wound‐healing assay, (B) apoptosis, and (C) cell cycle as demonstrated by flow cytometry. 5‐HT inhibited (D) autophagy as shown by western blot of autophagy related protein markers, p62 and LC3B.
Supplementary Figure 3 5‐HT does not affect the Wnt/β‐catenin signalling in the presence of 10% FBS. HuH‐7 and HepG2 cells were cultured in 10% FBS with 50 μM 5‐HT for the indicated time points. Total β‐catenin, active β‐catenin, p‐β‐catenin, Axin1, p‐GSK3β, and total GSK3β protein levels remain unchanged.
Supplementary Figure 4 Wnt/β‐catenin pathway inhibitor, ICG‐001, attenuates HCC proliferation in the presence of 5‐HT. (A) ICG‐001 reduced cyclin D1 protein levels in HuH‐7 and HepG2 cell lines with and without the presence of 5‐HT. (B) The relative viability of HuH‐7 and HepG2 cells was detected by MTT. The cells were cultured in SFM and treated with ICG‐001 and/or 5‐HT. ICG‐001 inhibited HCC proliferation in both cell lines (ICG‐001 + 5‐HT vs 5‐HT, ***P < 0.001, two‐way ANOVA).
Supplementary Figure 5 IgG were used as negative control for Co‐IP detection in HuH‐7 cells.
Acknowledgements
The present work was supported by Hong Kong Baptist University (IRMC/11‐12/02‐SCM).
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2015.09.008.
Fatima Sarwat, Shi Xiaoke, Lin Zesi, Chen Guo-qing, Pan Xiao-hua, Wu Justin Che-Yuen, Ho John W., Lee Nikki P., Gao Hengjun, Zhang Ge, Lu Aiping, Bian Zhao Xiang, (2016), 5‐Hydroxytryptamine promotes hepatocellular carcinoma proliferation by influencing β‐catenin, Molecular Oncology, 10, doi: 10.1016/j.molonc.2015.09.008.
References
- Altekruse, S.F. , McGlynn, K.A. , Reichman, M.E. , 2009. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. 27, 1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit, S. , Hatzubai, A. , Birman, Y. , Andersen, J.S. , Ben-Shushan, E. , Mann, M. , Ben-Neriah, Y. , Alkalay, I. , 2002. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 16, 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austinat, M. , Dunsch, R. , Wittekind, C. , Tannapfel, A. , Gebhardt, R. , Gaunitz, F. , 2008. Correlation between beta-catenin mutations and expression of Wnt-signaling target genes in hepatocellular carcinoma. Mol. Cancer. 7, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer, T. , Danilchik, M. , Thumberger, T. , Vick, P. , Tisler, M. , Schneider, I. , Bogusch, S. , Andre, P. , Ulmer, B. , Walentek, P. , Niesler, B. , Blum, M. , Schweickert, A. , 2012. Serotonin signaling is required for Wnt-dependent GRP specification and leftward flow in Xenopus. Curr. Biol. 22, 33–39. [DOI] [PubMed] [Google Scholar]
- Cadoret, A. , Ovejero, C. , Terris, B. , Souil, E. , Levy, L. , Lamers, W.H. , Kitajewski, J. , Kahn, A. , Perret, C. , 2002. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 21, 8293–8301. [DOI] [PubMed] [Google Scholar]
- Cattaneo, M.G. , Codignola, A. , Vicentini, L.M. , Clementi, F. , Sher, E. , 1993. Nicotine stimulates a serotonergic autocrine loop in human small-cell lung carcinoma. Cancer Res. 53, 5566–5568. [PubMed] [Google Scholar]
- Chen, J. , Liu, J. , Jin, R. , Shen, J. , Liang, Y. , Ma, R. , Lin, H. , Liang, X. , Yu, H. , Cai, X. , 2014. Cytoplasmic and/or nuclear expression of beta-catenin correlate with poor prognosis and unfavorable clinicopathological factors in hepatocellular carcinoma: a meta-analysis. PloS One. 9, e111885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieply, B. , Zeng, G. , Proverbs-Singh, T. , Geller, D.A. , Monga, S.P. , 2009. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 49, 821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami, K.H. , Nguyen, C. , Ma, H. , Kim, D.H. , Jeong, K.W. , Eguchi, M. , Moon, R.T. , Teo, J.L. , Kim, H.Y. , Moon, S.H. , Ha, J.R. , Kahn, M. , 2004. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc. Natl. Acad. Sci. U. S. A. 101, 12682–12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everly, D.N. , Kusano, S. , Raab-Traub, N. , 2004. Accumulation of cytoplasmic beta-catenin and nuclear glycogen synthase kinase 3beta in Epstein-Barr virus-infected cells. J. Virol. 78, 11648–11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima, S. , Lee, N.P. , Tsang, F.H. , Kolligs, F.T. , Ng, I.O. , Poon, R.T. , Fan, S.T. , Luk, J.M. , 2012. Dickkopf 4 (DKK4) acts on Wnt/beta-catenin pathway by influencing beta-catenin in hepatocellular carcinoma. Oncogene. 31, 4233–4244. [DOI] [PubMed] [Google Scholar]
- Gao, C. , Cao, W. , Bao, L. , Zuo, W. , Xie, G. , Cai, T. , Fu, W. , Zhang, J. , Wu, W. , Zhang, X. , Chen, Y.G. , 2010. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat. Cell Biol. 12, 781–790. [DOI] [PubMed] [Google Scholar]
- Gurbuz, N. , Ashour, A.A. , Alpay, S.N. , Ozpolat, B. , 2014. Down-regulation of 5-HT1B and 5-HT1D receptors inhibits proliferation, clonogenicity and invasion of human pancreatic cancer cells. PloS One. 9, e110067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambek, M. , Werner, C. , Baghi, M. , Gstottner, W. , Knecht, R. , 2006. Prestimulation of head and neck cancer cells with growth factors enhances treatment efficacy. Anticancer Res. 26, 1091–1095. [PubMed] [Google Scholar]
- Henderson, B.R. , Fagotto, F. , 2002. The ins and outs of APC and beta-catenin nuclear transport. EMBO Rep. 3, 834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, M. , Yamashita, T. , Ueda, T. , Takatori, H. , Nishino, R. , Kaneko, S. , 2006. Different signaling pathways in the livers of patients with chronic hepatitis B or chronic hepatitis C. Hepatology. 44, 1122–1138. [DOI] [PubMed] [Google Scholar]
- Hoogeboom, D. , Essers, M.A. , Polderman, P.E. , Voets, E. , Smits, L.M. , Burgering, B.M. , 2008. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J. Biol. Chem. 283, 9224–9230. [DOI] [PubMed] [Google Scholar]
- Huang, H. , He, X. , 2008. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr. Opin. Cell Biol. 20, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka, N. , Oka, M. , Yamada-Okabe, H. , Mori, N. , Tamesa, T. , Okada, T. , Takemoto, N. , Tangoku, A. , Hamada, K. , Nakayama, H. , Miyamoto, T. , Uchimura, S. , Hamamoto, Y. , 2002. Comparison of gene expression profiles between hepatitis B virus- and hepatitis C virus-infected hepatocellular carcinoma by oligonucleotide microarray data on the basis of a supervised learning method. Cancer Res. 62, 3939–3944. [PubMed] [Google Scholar]
- Inagawa, S. , Itabashi, M. , Adachi, S. , Kawamoto, T. , Hori, M. , Shimazaki, J. , Yoshimi, F. , Fukao, K. , 2002. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 8, 450–456. [PubMed] [Google Scholar]
- Itakura, E. , Mizushima, N. , 2011. p62 Targeting to the autophagosome formation site requires self-oligomerization but not LC3 binding. J. Cell Biol. 192, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, W. , Oe Lim, S. , Kim, J.S. , Ryu, Y.H. , Byeon, J.Y. , Kim, H.J. , Kim, Y.I. , Heo, J.S. , Park, Y.M. , Jung, G. , 2003. Comparison of proteome between hepatitis B virus- and hepatitis C virus-associated hepatocellular carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 9, 5493–5500. [PubMed] [Google Scholar]
- Krieghoff, E. , Behrens, J. , Mayr, B. , 2006. Nucleo-cytoplasmic distribution of beta-catenin is regulated by retention. J. Cell Sci. 119, 1453–1463. [DOI] [PubMed] [Google Scholar]
- Lang, P.A. , Contaldo, C. , Georgiev, P. , El-Badry, A.M. , Recher, M. , Kurrer, M. , Cervantes-Barragan, L. , Ludewig, B. , Calzascia, T. , Bolinger, B. , Merkler, D. , Odermatt, B. , Bader, M. , Graf, R. , Clavien, P.A. , Hegazy, A.N. , Lohning, M. , Harris, N.L. , Ohashi, P.S. , Hengartner, H. , Zinkernagel, R.M. , Lang, K.S. , 2008. Aggravation of viral hepatitis by platelet-derived serotonin. Nat. Med. 14, 756–761. [DOI] [PubMed] [Google Scholar]
- Lee, C.F. , Ling, Z.Q. , Zhao, T. , Lee, K.R. , 2008. Distinct expression patterns in hepatitis B virus- and hepatitis C virus-infected hepatocellular carcinoma. World J. Gastroenterol. 14, 6072–6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesurtel, M. , Graf, R. , Aleil, B. , Walther, D.J. , Tian, Y. , Jochum, W. , Gachet, C. , Bader, M. , Clavien, P.A. , 2006. Platelet-derived serotonin mediates liver regeneration. Science. 312, 104–107. [DOI] [PubMed] [Google Scholar]
- Levine, B. , Kroemer, G. , 2008. Autophagy in the pathogenesis of disease. Cell. 132, 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Yang, B. , Zhou, Q. , Wu, Y. , Shang, D. , Guo, Y. , Song, Z. , Zheng, Q. , Xiong, J. , 2013. Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition. Carcinogenesis. 34, 1343–1351. [DOI] [PubMed] [Google Scholar]
- Liang, C. , Chen, W. , Zhi, X. , Ma, T. , Xia, X. , Liu, H. , Zhang, Q. , Hu, Q. , Zhang, Y. , Bai, X. , Liang, T. , 2013. Serotonin promotes the proliferation of serum-deprived hepatocellular carcinoma cells via upregulation of FOXO3a. Mol. Cancer. 12, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader, R. , Kocher, T. , Haier, J. , Wieczorek, G. , Pfannkuche, H.J. , Ito, M. , 2006. Investigation of serotonin type 4 receptor expression in human and non-human primate gastrointestinal samples. Eur. J. Gastroenterol. Hepatol. 18, 945–950. [DOI] [PubMed] [Google Scholar]
- Mizushima, N. , Yoshimori, T. , Levine, B. , 2010. Methods in mammalian autophagy research. Cell. 140, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga, S.P. , Pediaditakis, P. , Mule, K. , Stolz, D.B. , Michalopoulos, G.K. , 2001. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 33, 1098–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhieu, J.T. , Renard, C.A. , Wei, Y. , Cherqui, D. , Zafrani, E.S. , Buendia, M.A. , 1999. Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am. J. Pathol. 155, 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocito, A. , Dahm, F. , Jochum, W. , Jang, J.H. , Georgiev, P. , Bader, M. , Renner, E.L. , Clavien, P.A. , 2007. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis. Gastroenterology. 133, 608–618. [DOI] [PubMed] [Google Scholar]
- Orford, K. , Crockett, C. , Jensen, J.P. , Weissman, A.M. , Byers, S.W. , 1997. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J. Biol. Chem. 272, 24735–24738. [DOI] [PubMed] [Google Scholar]
- Petherick, K.J. , Williams, A.C. , Lane, J.D. , Ordonez-Moran, P. , Huelsken, J. , Collard, T.J. , Smartt, H.J. , Batson, J. , Malik, K. , Paraskeva, C. , Greenhough, A. , 2013. Autolysosomal beta-catenin degradation regulates Wnt-autophagy-p62 crosstalk. EMBO J. 32, 1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddell, R.G. , Mann, D.A. , Ramm, G.A. , 2008. The function of serotonin within the liver. J. Hepatol. 48, 666–675. [DOI] [PubMed] [Google Scholar]
- Ruddell, R.G. , Oakley, F. , Hussain, Z. , Yeung, I. , Bryan-Lluka, L.J. , Ramm, G.A. , Mann, D.A. , 2006. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am. J. Pathol. 169, 861–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte, K. , Bornschein, J. , Malfertheiner, P. , 2009. Hepatocellular carcinoma–epidemiological trends and risk factors. Dig. Dis. (Basel, Switzerland). 27, 80–92. [DOI] [PubMed] [Google Scholar]
- Sharma, M. , Jamieson, C. , Johnson, M. , Molloy, M.P. , Henderson, B.R. , 2012. Specific armadillo repeat sequences facilitate beta-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358. J. Biol. Chem. 287, 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Siddiqui, E.J. , Shabbir, M.A. , Mikhailidis, D.P. , Mumtaz, F.H. , Thompson, C.S. , 2006. The effect of serotonin and serotonin antagonists on bladder cancer cell proliferation. BJU Int. 97, 634–639. [DOI] [PubMed] [Google Scholar]
- Soll, C. , Jang, J.H. , Riener, M.O. , Moritz, W. , Wild, P.J. , Graf, R. , Clavien, P.A. , 2010. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology. 51, 1244–1254. [DOI] [PubMed] [Google Scholar]
- Soll, C. , Riener, M.O. , Oberkofler, C.E. , Hellerbrand, C. , Wild, P.J. , DeOliveira, M.L. , Clavien, P.A. , 2012. Expression of serotonin receptors in human hepatocellular cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 18, 5902–5910. [DOI] [PubMed] [Google Scholar]
- Suzuki, T. , Yano, H. , Nakashima, Y. , Nakashima, O. , Kojiro, M. , 2002. Beta-catenin expression in hepatocellular carcinoma: a possible participation of beta-catenin in the dedifferentiation process. J. Gastroenterol. Hepatol. 17, 994–1000. [DOI] [PubMed] [Google Scholar]
- Takigawa, Y. , Brown, A.M. , 2008. Wnt signaling in liver cancer. Curr. Drug Targets. 9, 1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenbaum, S.P. , Ordonez-Moran, P. , Puig, I. , Chicote, I. , Arques, O. , Landolfi, S. , Fernandez, Y. , Herance, J.R. , Gispert, J.D. , Mendizabal, L. , Aguilar, S. , Ramon y Cajal, S. , Schwartz, S. , Vivancos, A. , Espin, E. , Rojas, S. , Baselga, J. , Tabernero, J. , Munoz, A. , Palmer, H.G. , 2012. Beta-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat. Med. 18, 892–901. [DOI] [PubMed] [Google Scholar]
- Torre, L.A. , Bray, F. , Siegel, R.L. , Ferlay, J. , Lortet-Tieulent, J. , Jemal, A. , 2015. Global cancer statistics, 2012. CA. Cancer J. Clin. 65, 87–108. [DOI] [PubMed] [Google Scholar]
- Tseng, T.C. , Liu, C.J. , Yang, H.C. , Su, T.H. , Wang, C.C. , Chen, C.L. , Kuo, S.F. , Liu, C.H. , Chen, P.J. , Chen, D.S. , Kao, J.H. , 2012. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 142, 1140–1149.e1143; quiz e1113–e1144 [DOI] [PubMed] [Google Scholar]
- Wong, C.M. , Fan, S.T. , Ng, I.O. , 2001. Beta-catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 92, 136–145. [DOI] [PubMed] [Google Scholar]
- Yu, B. , Yang, X.R. , Xu, Y. , Yao, G.F. , Shu, H.Q. , Lin, B.Y. , Hoods, L. , Wang, H.Y. , Yang, S.L. , Gu, J.R. , Fan, J. , Qin, W.X. , 2009. Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta-catenin accumulation and poor prognosis in hepatocellular carcinomas. J. Hepatol. 50, 948–957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data
Supplementary Figure 1 5‐HT does not promote proliferation in all HCC cell lines. (A) The relative viability of five HCC cell lines cultured in 10% FBS, or SFM with or without 5‐HT (50 μM and 100 μM 5‐HT) was detected by MTT. (B) The relative viability of HuH‐7 and HepG2 cells cultured in 10% FBS with or without 5‐HT (50 μM and 100 μM 5‐HT) was detected by MTT.
Supplementary Figure 2 Affect of 5‐HT on various HCC characteristics. 5‐HT does not affect (A) cell migration as demonstrated by wound‐healing assay, (B) apoptosis, and (C) cell cycle as demonstrated by flow cytometry. 5‐HT inhibited (D) autophagy as shown by western blot of autophagy related protein markers, p62 and LC3B.
Supplementary Figure 3 5‐HT does not affect the Wnt/β‐catenin signalling in the presence of 10% FBS. HuH‐7 and HepG2 cells were cultured in 10% FBS with 50 μM 5‐HT for the indicated time points. Total β‐catenin, active β‐catenin, p‐β‐catenin, Axin1, p‐GSK3β, and total GSK3β protein levels remain unchanged.
Supplementary Figure 4 Wnt/β‐catenin pathway inhibitor, ICG‐001, attenuates HCC proliferation in the presence of 5‐HT. (A) ICG‐001 reduced cyclin D1 protein levels in HuH‐7 and HepG2 cell lines with and without the presence of 5‐HT. (B) The relative viability of HuH‐7 and HepG2 cells was detected by MTT. The cells were cultured in SFM and treated with ICG‐001 and/or 5‐HT. ICG‐001 inhibited HCC proliferation in both cell lines (ICG‐001 + 5‐HT vs 5‐HT, ***P < 0.001, two‐way ANOVA).
Supplementary Figure 5 IgG were used as negative control for Co‐IP detection in HuH‐7 cells.


