Abstract
The use of stem cells has enabled the successful generation of simple organs. However, anatomically complicated organs such as the kidney have proven more refractory to stem-cell-based regenerative techniques. Given the limits of allogenic organ transplantation, an ultimate therapeutic solution is to establish self-organs from autologous stem cells and transplant them as syngrafts back into donor patients. To this end, we have striven to establish an in vitro organ factory to build up complex organ structures from autologous adult stem cells by using the kidney as a target organ. Cultivation of human mesenchymal stem cells in growing rodent embryos enables their differentiation within a spatially and temporally appropriate developmental milieu, facilitating the first step of nephrogenesis. We show that a combination of whole-embryo culture, followed by organ culture, encourages exogenous human mesenchymal stem cells to differentiate and contribute to functional complex structures of the new kidney.
Keywords: organogenesis, regeneration
Organ regeneration has recently attracted considerable attention as a new therapeutic strategy. The potential for regenerative medicine has been gradually realized with the discovery of tissue stem cells and the reported therapeutic benefits of their implantation or systemic delivery for the regeneration of several tissues such as neurons (1), β-islet cells (2), myocytes (3) and vessels (4). However, success using such strategies to date has been limited to cells and simple tissues. Anatomically complicated organs such as the kidney and lung, which are comprised of several different cell types and have a sophisticated 3-dimensional organization and cellular communication, have proven more refractory to stem cell-based regenerative techniques. Allogenic tissue transplantation by using a scaffold is an alternative strategy to replace whole organs. However, the scarcity of suitable organs has prevented organ transplantation from becoming a practical solution in most cases of organ failure. Furthermore, chronic rejection of the allograft remains a common cause of graft failure after organ transplantation despite life-long administration of immunosuppressive agents (5). One of the ultimate therapeutic aims is therefore to establish self-organs from autologous tissue stem cells and transplant the in vitro-derived organ as a syngraft back into the donor individual.
Human mesenchymal stem cells (hMSCs) found in adult bone marrow were recently shown to maintain plasticity and to differentiate into several different cell types, depending on their microenvironment (6). In contrast to embryonic stem cells, adult MSCs can be isolated from autologous bone marrow and applied for therapeutic use without any serious ethical issues or immunologic consequences (7). Primary hMSCs were obtained from the bone marrow of healthy volunteers and used throughout this study.
The kidney was selected as the target organ for this study, because it represents a complicated organ, comprising several different cell types, has a sophisticated 3D organization, and its embryonic development has been well researched. Kidney development is initiated when the metanephric mesenchyme at the caudal portion of the nephrogenic cord (8) induces the nearby Wolffian duct to produce a ureteric bud (9). Development proceeds as a result of reciprocal epithelial-mesenchymal signaling between the ureteric bud and metanephric mesenchyme (10). To test whether hMSCs could participate in kidney development, they were initially cocultured with either rodent Wolffian duct extracted at the embryonic stage immediately before formation of the kidney primordia, or with established metanephric rudiment. However, this procedure was not sufficient to achieve kidney organogenesis or even integration of hMSCs into the developing rodent metanephros (T.Y., unpublished data). This finding suggests that hMSCs must be placed in a specific, defined embryonic niche to allow for exposure to the repertoire of nephrogenic signals required to generate the organ. This outcome can best be achieved by implanting hMSCs into the nephrogenic site of a developing embryo. However, it is difficult to implant cells prenatally at the exact site of organogenesis by a transuterus approach. Equally, once embryos are removed for cell implantation, they cannot be returned to the uterus for further development. Therefore, embryos were isolated from uteri for cell implantation, after which they were further developed in vitro, using whole-embryo culture. Here, we show that by using this culture combination, hMSCs develop into morphologically identical cells to endogenous renal cells and are able to contribute to complex kidney structures.
Methods
Animals. Wild-type Sprague–Dawley rats were purchased from Sankyo Lab Services (Tokyo). A breeding colony of Fabry mice was established at the Laboratory Animal Center of the Jikei University School of Medicine from breeding pairs that were kindly donated by R. O. Brady (National Institutes of Health, Bethesda). The midpoint on the day when a vaginal plug was observed was designated as day 0.5. Animals were housed in a ventilated (positive airflow) rack and bred and maintained under pathogen-free conditions. All experimental procedures were approved by The Committee for Animal Experiments of the Jikei University School of Medicine.
Culture and Manipulation of hMSCs. Bone marrow-derived hMSCs that were confirmed to be CD105-, CD166-, CD29-, and CD44-positive and CD14-, CD34-, and CD45-negative, were purchased from Cambrex Bio Science Walkersville (Walkersville, MD) and cultured according to the manufacturer's instructions. The hMSCs were used within five cell passages to avoid phenotypic changes. A replication-defective recombinant adenovirus carrying human glial cell line-derived neurotrophic factor (GDNF) cDNA (AxCAhGDNF) was generated and purified as described (11). Packaging cells (Ψ-crip) that produce a recombinant retrovirus bearing the bacterial LacZ gene, MFG-LacZ, were a gift from H. Hamada (Sapporo Medical University, Sapporo, Japan). Adenoviral and retroviral infection were performed as described (12, 13). The cells were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI) (Molecular Probes) at 0.25% (wt/vol) in 100% dimethylformamide and injected by using micropipettes at the site of ureteric bud sprouting.
Whole-Embryo Culture and Organ Culture. Whole embryos were cultured in vitro according to a method described (14), with several modifications. Using a surgical microscope at low-power magnification, uteri were dissected from anaesthetized mothers. Stage embryonic day (E)11.5 rat embryos and stage E9.5 mouse embryos were freed from the uterine wall, decidua, and the outer-membrane layer, including Reichert's membrane. The yolk sac and amnion were opened to enable injection but the chorioallantoic placenta was left intact. Successfully injected embryos were immediately cultivated in 15-ml culture bottles containing 3 ml of culture media consisting of 100% centrifuged rat serum supplemented with glucose (10 mg/ml), penicillin G (100 units/ml), streptomycin (100 μg/ml), and amphotericin B (0.25 μg/ml). The culture bottles were allowed to rotate in an incubator (model no. RKI10–0310, Ikemoto, Tokyo). Ex vivo development of the rat embryos was assessed after 24- and 48-h cultivation periods and compared with E12.5 and E13.5 rat embryos. Forty-eight hours later, embryos were assessed for heartbeat, whole-body blood circulation, and general morphology. Kidney rudiments were dissected and cultured as described (15). To enhance the accumulation of globotriaosylceramide (Gb3) in the kidney primordia, the cultivated metanephroi were cultured in the presence of ceramide trihexoside (1 nmol, Sigma) (16). α-galactosidase A (α-gal A) enzymatic activity in metanephroi was fluorometrically assessed as described (17).
Histology. Two-color staining of metanephroi were performed essentially as described (17) by using mouse anti-β-gal (Promega) and rabbit anti-human WT-1 (Santa Cruz Biotechnology) as the primary antibodies. A monoclonal mouse anti-Gb3 antibody (Seikagaku, Tokyo) was also used. Whole-mount in situ hybridization with digoxigenin UTP-labeled c-ret riboprobes was performed as described (15). In situ hybridization was also performed on histological sections by using biotin-labeled human genomic AluI/II probes (Invitrogen) according to the manufacturer's protocol. An X-gal assay was used to assess expression of the LacZ gene as described (13).
Identification of hMSC-Positive Cells. Metanephroi generated by relay culture were digested in collagenase type I (1 mg/ml) for 30 min and labeled with fluorescein digalactoside (Molecular Probes) by using transient permeabilization by hypotonic shock as described by Fiering et al. (18) (FACS-galactosidase assay). LacZ-positive cells were sorted by using a cell sorter (Becton Dickinson). Total RNA was extracted and subjected to RT-PCR to analyze expression of aquaporin-1 (AQP-1), parathyroid hormone (PTH) receptor 1, 1α hydroxylase, 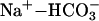 cotransporter 1 (NBC-1), nephrin, podocine, and glomerular epithelial protein 1 (GLEPP-1). A list of primer sequences and reaction conditions used can be found in Table 1, which is published as supporting information on the PNAS web site. For the analysis of cell ploidy, cells were stained with propidium iodide, and DNA content was assessed by using a flow cytometer.
cotransporter 1 (NBC-1), nephrin, podocine, and glomerular epithelial protein 1 (GLEPP-1). A list of primer sequences and reaction conditions used can be found in Table 1, which is published as supporting information on the PNAS web site. For the analysis of cell ploidy, cells were stained with propidium iodide, and DNA content was assessed by using a flow cytometer.
Statistical Analysis. Data were expressed as the mean ± SE. Statistical analysis was performed by using the two-sample t test to compare data in different groups. P < 0.05 was taken to be statistically significant.
Results and Discussion
The whole-embryo culture system was first optimized to allow a defined concentration of oxygen to be supplied continuously to rotating culture bottles, thus improving embryonic development ex utero (14). Using this system, rat embryos (E11.5), together with the yolk sac, amnion, and chorioallantoic placenta were cultured in the media consisting of 100% freshly centrifuged rat serum supplemented with glucose (10 mg/ml) at 37°C in the culture bottle (see Movie 1, which is published as supporting information on the PNAS web site). Forty-eight hours later, embryos were assessed for heartbeat, whole-body blood circulation, and general morphology. Based on the resultant somite number and general morphology, the developmental age of rat embryos cultured in this way appeared consistent with E13 embryos that had developed in utero (Fig. 1a). At this stage, ureteric buds were elongated and initial branching was completed (data not shown), indicating that during culture, the metanephric mesenchyme had been stimulated to undertake the initial step of commitment toward nephrogenesis. However, embryos could not develop further and died soon after 48 h because of insufficient development of the placenta in vitro (19). To overcome this limitation, whole-embryo culture was followed by organ culture. After whole-embryo culture for 48 h, metanephroi were dissected from embryos and subjected to organ culture for 6 days. Using this combination, which will be referred to as relay culture, kidney rudiments continued to grow in vitro, as assessed by the observation of fine tubulogenesis and ureteric bud branching (Fig. 1 b and c). Thus, the metanephros can complete development ex utero, even if the embryo is dissected before the stage at which the ureteric bud sprouts.
Fig. 1.
Ex utero development of kidney primordia by using the relay culture system. Rat embryos (E11.5) just before ureteric bud sprouting were cultured in vitro, using a whole-embryo culture system for 48 h. After 24 and 48 h in culture, ex utero development of the rat embryos was assessed by comparing them with those that had grown in the uterus for E11.5, E12.0, E12.5, E13.0 and E13.5. Embryos that were developed in the culture bottle reached stages of development that were consistent with E13 embryos that had developed in utero. (a). At the end of the 48-h culture period, kidney rudiments were isolated and subjected to metanephric organ culturing for 6 days. To confirm the extent of tubulogenesis and ureteric bud branching, hematoxylin/eosin staining (b) and whole-mount in situ hybridization for c-ret (c) were performed. Fine tubulogenesis and ureteric bud branching can be observed. Experiments were performed in triplicate and representative pictures are shown.
Using this system, hMSCs were injected into rodent embryos at the site of organogenesis. To distinguish the donor-derived cells from host cells, hMSCs were labeled with the LacZ gene and DiI. A total of 1 × 103/embryo of labeled cells were then injected into the intermediate mesoderm between the somite and the lateral plate at the level of somite 29 for rat and somite 26 for mouse, which we previously estimated by in situ hybridization for c-ret, to be the ureteric budding sites (15). Successful injection was confirmed by in situ hybridization for human genomic AluI/II, which identifies exclusively human cells, and injected hMSCs-derived cells were detected along the Wolffian duct (see Fig. 6, which is published as supporting information on the PNAS web site). After relay culture, LacZ-positive cells were detected in the metanephros (5.0 ± 4.2%), as measured by a FACS-galactoside assay of single cells derived from a dissected metanephros (Fig. 2a). No LacZ-positive cells were detected in the isolated kidney if the injection site was altered by >1 somite in length. In control embryos, injection of labeled mouse fibroblasts instead of hMSCs resulted in only a negligible number of LacZ-positive cells detected in the metanephros (data not shown). To enhance the number of integrated donor-derived cells, the hMSCs were further modified before injection to temporally express GDNF by using the adenovirus AxCAh-GDNF (11). GDNF is normally expressed in metanephric mesenchyme at this stage, and the interaction between GDNF and its receptor, c-ret, is required for epithelial-mesenchymal signaling to occur (10). The FACS-galactosidase assay revealed a significant increase in the number of LacZ-positive cells that were detected after transient GDNF expression (29.8 ± 9.2%, Fig. 2b). Importantly, 68.8 ± 11.4% of LacZ-positive cells in the neogenerated metanephros were euploid (Fig. 2c). The number of LacZ-positive cells were significantly increased (2.84 ± 0.49 × 105/metanephros) compared with the starting number of injected cells (1 × 103/embryo), suggesting that the remaining polyploid cells were mostly undergoing cell division. Furthermore, FISH, using the human and rat Y chromosome showed that a negligible number of cells were doubly positive for the Y chromosome (≈0.1%, see Fig. 7, which is published as supporting information on the PNAS web site). These data strongly suggest that, if any, only a small percentage of hMSC cells undergo cell fusion during differentiation.
Fig. 2.
Proportion of donor-derived cells in culture-derived metanephroi and assessment of their DNA ploidy. hMSCs expressing the LacZ gene were retrovirally transfected with GDNF (b) or without GDNF (a) and injected into rodent embryos at the site of budding. After relay culture, the neogenerated metanephroi were digested with collagenase, and single cells were subjected to a FACS-galactosidase assay. M, the informative peak. The use of GDNF was found to significantly increase the number of hMSCs that were incorporated into the developing metanephroi. (c) LacZ-positive cells were sorted, and their DNA content was assessed by using propidium iodide intensity. These cells were found to be euploid, and thus did not represent transplanted hMSCs that had fused with host metanephric cells. Ten thousand cells were subjected to flow cytometric analysis. Experiments were performed in quadruplicate and representative figures are shown.
During metanephric organ culture, DiI-positive cells migrated toward the medulla and dispersed in the kidney primordia (see Fig. 8, which is published as supporting information on the PNAS web site), suggesting that the transplanted cells become integrated in the host kidney. To confirm that these cells contribute to renal structures, the kidney primordia was subjected to an X-gal assay. LacZ-positive cells were scattered throughout the metanephric rudiment and were morphologically identical to the resident glomerular epithelial cells, tubular epithelial cells, and interstitial cells (Fig. 3a). Serial sections of metanephric rudiment showed glomerular epithelial cells linked to tubular epithelial cells (Fig. 3b, arrowhead). Some β-gal-positive cells of the S-shaped bodies were also positive for WT-1, which is strongly expressed in glomerular podocytes at this stage (20) (Fig. 3c). RT-PCR of FACS-sorted metanephric cells revealed that LacZ-positive cells expressed podocyte-specific genes (nephrin, podocine, and GLEPP-1) and tubular epithelial cell-specific genes (AQP-1, 1α hydroxylase, PTH receptor 1, and NBC-1) (Fig. 3d). In contrast to endogenous renal cells, ATP-sensitive K+ channel subunit, Kir6.1/SUR2 (21), which is expressed in hMSCs, was still expressed after relay culture. Furthermore, when hMSCs were injected into cultured metanephroi (E13), cell dispersal was not observed, and the hMSCs remained aggregated. After 6 days in organ culture, the hMSCs failed to contribute to kidney structures (Fig. 4a), and did not express kidney-specific genes (Fig. 4b). These data suggest that during whole-embryo culture, hMSCs complete an initial step essential for commitment to a renal fate and that during organ culture, they further undergo a mesenchyme-to-epithelium transition or stromogenic differentiation.
Fig. 3.
Differentiation of transplanted hMSCs into organized, resident renal cells. (a) After relay culturing, the resulting metanephros was subjected to an X-gal assay to trace the transplanted hMSCs. The morphology of these LacZ-positive cells (shown under high magnification; ×400) and the renal structures to which they contributed, were consistent with them being glomerular epithelial cells (lane 1), tubular epithelial cells (lane 2), and interstitial cells (lane 3). (b) Serial sections were examined by light microscopy. Glomerular epithelial cells were linked to tubular epithelial cells (arrow head), and some of these cells formed a continuous tubular extension toward the medulla (arrow). gl, glomerulus. (c) Tissue sections were subjected to two-color immunofluorescent staining for β-gal (Left) and WT-1 (Right). (Center) A merged image is presented. (d) After relay culture, the resulting metanephroi were digested, and single cells were subjected to the FACS-galactosidase assay. LacZ-positive cells were sorted and subjected to RT-PCR for expression analysis of Kir6.1, SUR2, AQP-1, PTH receptor 1, 1α hydroxylase, NBC-1, nephrin, podocin, GLEPP1, human-specific β2 microgloblin (MG) and rat GAPDH. Lane 1, control rat metanephros; lane 2, hMSCs; lanes 3–5, regenerated metanephros from three individual experiments. Representative photographs are shown.
Fig. 4.
Injection and culture of hMSCs in isolated metanephroi. hMSCs expressing the LacZ gene were retrovirally transfected with GDNF and injected into the cultured metanephroi (E13). (a) After 6 days of organ culture, the resulting metanephroi were subjected to an X-gal assay. (Inset) LacZ-positive cells at a high magnification are shown. Note that hMSCs-derived cells remain aggregated and do not form recognizable kidney structures. (b) RNAs were extracted and subjected to RT-PCR. Neogenerated kidney rudiment before (lane 2) and after (lane 3) organ culturing is shown. Mixture of metanephroi and hMSCs before (lane 4) and after (lane 5) organ culture is shown. Note that only the hMSCs differentiated in the whole embryo are able to express kidney-specific gene after organ culture. Lane 1, maker (ϕX174/HaeIII).
To examine whether the hMSC-positive nephrons are viable, hMSCs were developed in the Fabry mouse (22), which does not express the gene encoding α-gal A enzyme. This defect leads to the abnormal accumulation of glycosphinogolipid mainly in glomerular podocytes and tubular epithelial cells, leading to renal failure after birth. The hMSCs were injected into E9.5 Fabry mouse embryos and subjected to relay culturing to regenerate the kidney. Compared with the wild-type mouse (655.0 ± 199.6 nmol per mg per hour), the basal level of α-gal A bioactivity in the metanephros from the Fabry mouse is low (19.7 ± 5.5 nmol per mg per hour), whereas chimeric kidney primordia expressed significantly higher amounts of α-gal A bioactivity (204.2 ± 98.8 nmol per mg per hour, P < 0.05, Fig. 5a). Furthermore, accumulation of the glycosphinogolipid Gb3 within the ureteric buds and S-shaped bodies (Fig. 5b Right) in the metanephros of the Fabry mouse was markedly resolved by integration of the hMSC-derived nephrons that possess α-gal A and act to normalize substrate metabolism in the surrounding host cells (Fig. 5b Center). This result indicates that the neogenerated nephrons were biologically viable.
Fig. 5.
Therapeutic kidney regeneration in an α-Gal A-null Fabry mouse. hMSCs were transplanted into E9.5 embryos of Fabry mice lacking the α-gal A gene and subjected to relay culture. (a) The α-gal A enzymatic bioactivity of resulting metanephroi was fluorometrically assessed as described (19). For the controls, metanephros from wild-type mice (Left) and Fabry mice (Right) were also subjected to the same protocol. The data are shown as the means ± SE. Asterisks indicate statistically significant differences (P < 0.05) between the two groups. (b) To confirm the potency of Gb3 clearance in resulting metanephroi, organ culture was performed in the presence of Gb3, and accumulation in the metanephroi was assessed by immunostaining for Gb3. Control metanephroi from wild-type mice (Left) and Fabry mice (Right) were subjected to the same analysis.
In this study, we show that allowing hMSCs to grow in a specific organ location in whole-embryo culture can commit them to the fate of that organ. Injection of GDNF-transfected hMSCs into embryos followed by relay culture enables the generation of chimeric kidneys. In some cases, entire nephrons are hMSC-derived. These hMSC-derived cells are functional as tested by their ability to metabolize Gb3.
Thus, hMSCs could be reprogrammed for other fates and organ structures, depending on the embryonic environment into which they are. An added advantage of using hMSCs is that although they are mesodermal in origin, they have the potential to differentiate into cell types that are normally derived from ectoderm or endoderm (23). Thus, it might be possible to reconstitute organs such as liver and pancreas that, unlike the kidney, are derived from the endodermal germ layer. Furthermore, by changing the conditions of organ culture after the initiation of organ development during whole-embryo culture, a specific cell or simple-structured tissue, such as an endocrine gland, may be generated from autologous MSCs. Importantly, the host immune system is not sufficiently developed during this stage of whole-embryo culture, thus facilitating the tolerance to xenogenic cells. Here, we have demonstrated a system that might provide the means to generate self-organs from autologous MSCs by using the inherent developmental system of an immunocompromised xenogeneic host.
Supplementary Material
Acknowledgments
We thank Dr. M. Okabe (MRC Centre for Developmental Neurobiology, King's College, London) for critical reading of this manuscript. This work was supported by a grant from the Ministry of Health, Labour, and Welfare of Japan, the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and the Takeda Scientific Foundation.
Author contributions: T.Y., T.O., Y.M., Y.U., and Y.E. designed research; T.Y., J.S.S., and K.S. performed research; T.K. and T.H. analyzed data.; and T.Y. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: hMSC, human mesenchymal stem cell; GDNF, glial cell line-derived neurotrophic factor; En, embryonic day n; α-gal A, α-galactosidase A; Gb3, globotriaosylceramide; AQP-1, aquaporin-1; PTH, parathyroid hormone; NBC-1,  cotransporter 1; GLEPP-1, glomerular epithelial protein 1; DiI, 1,1′-dioctadecyl-3,3,3′, 3′-tetramethylindocarbocyanine.
cotransporter 1; GLEPP-1, glomerular epithelial protein 1; DiI, 1,1′-dioctadecyl-3,3,3′, 3′-tetramethylindocarbocyanine.
References
- 1.Ogawa, Y., Sawamoto, K., Miyata, T., Miyao, S., Watanabe, M., Nakamura, M., Bregman, B. S., Koike, M., Uchiyama, Y., et al. (2002) J. Neurosci. Res. 69, 925-933. [DOI] [PubMed] [Google Scholar]
- 2.Ramiya, V. K., Maraist, M., Arfors, K. E., Schatz, D. A. & Cornelius, J. G. (2000) Nat. Med. 6, 278-282. [DOI] [PubMed] [Google Scholar]
- 3.Orlic, D., Kajstura, J., Chimenti, S., Jakoniuk, I., Anderson, S. M., Li, B., Pickel, J., McKay, R., Nadal-Ginard, B., Bodine, D. M., et al. (2001) Nature 410, 701-705. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi, T., Kalka, C., Masuda, H., Chen, D., Silver, M., Kearney, M., Magner, M., Isner, J. M. & Asahara, T. (1999) Nat. Med. 5, 434-438. [DOI] [PubMed] [Google Scholar]
- 5.Fung, J. J. (2004) Transplantation 77, S41-S43. [DOI] [PubMed] [Google Scholar]
- 6.Prockop, D. J. (1997) Science 276, 71-74. [DOI] [PubMed] [Google Scholar]
- 7.Barry, F. P. (2003) Birth Defects Res. 69, 250-256. [DOI] [PubMed] [Google Scholar]
- 8.Saxen, L. (1987) Organogenesis of the Kidney (Cambridge Univ. Press, Cambridge, U.K.)
- 9.Davies, J. A. & Fisher, C. E. (2002) Exp. Nephrol. 10, 102-113. [DOI] [PubMed] [Google Scholar]
- 10.Lipschuts, J. H. (1998) Am. J. Kidney Dis. 31, 383-397. [DOI] [PubMed] [Google Scholar]
- 11.Watabe, K., Ohashi, T., Sakamoto, T., Kawazoe, Y., Takeshima, T., Oyanagi, K., Inoue, K., Eto, Y. & Kim, S. U. (2000) J. Neurosci. Res. 60, 511-519. [DOI] [PubMed] [Google Scholar]
- 12.Yokoo, T., Ohashi, T., Utsunomiya, Y., Shen, J.-S., Hisada, Y., Eto, Y., Kawamura, T. & Hosoya, T. (2001) Blood 98, 57-64. [DOI] [PubMed] [Google Scholar]
- 13.Yokoo, T., Ohashi, T., Utsunomiya, Y., Shiba, H., Shen, J.-S., Hisada, Y., Eto, Y., Kawamura, T. & Hosoya, T. (2001) J. Am. Soc. Nephrol. 11, 2330-2337. [DOI] [PubMed] [Google Scholar]
- 14.Osumi, N. & Inoue, T. (2001) Methods 24, 35-42. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki, Y., Oshima, K., Fogo, A., Hogan, B. L. M. & Ichikawa, I. (2000) J. Clin. Invest. 105, 868-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi, T., Shinnoh, N. & Kuroiwa, Y. (1984) J. Neurol. Sci. 65, 169-177. [DOI] [PubMed] [Google Scholar]
- 17.Yokoo, T., Ohashi, T., Utsunomiya, Y., Okamoto, A., Suzuki, T., Shen, J.-S., Tanaka, T., Kawamura, T. & Hosoya, T. (2003) Kidney Int. 64, 102-109. [DOI] [PubMed] [Google Scholar]
- 18.Fiering, S. N., Roederer, M., Molan, G. P., Micklem, D. R., Parks, D. R. & Herzenberg, L. A. (1991) Cytometry 12, 291-301. [DOI] [PubMed] [Google Scholar]
- 19.Eto, E. & Osumi-Yamashita, N. (1995) Dev. Growth Differ. 37, 123-132. [DOI] [PubMed] [Google Scholar]
- 20.Huber, S. M., Braun, G. S., Segerer, S., Veh, R. W. & Horster, M. F. (2000) Am. J. Physiol. 279, F65-F76. [DOI] [PubMed] [Google Scholar]
- 21.Braun, G. S., Veh, R. W., Segerer, S., Horster, M. F. & Huber, S. M. (2002) Eur. J. Physiol. 445, 321-330. [DOI] [PubMed] [Google Scholar]
- 22.Takenaka, T., Murray, G. J., Qin, G., Quirk, J. M., Ohshima, T., Qasba, P., Clark, K., Kulkarni, A. B., Brady, R. O. & Medin, J. A. (2000) Proc. Natl. Acad. Sci. USA 97, 7515-7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang, Y., Jahagirdar, B. N., Reinhardt, R. L., Schwartz, R. E., Keene, C. D., Ortiz-Gonzalez, X. R., Reyes, M., Lenvik, T., Lund, T., Blackstad, M., et al. (2002) Nature 418, 41-49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







