Abstract
During the process of metastasis, which is the leading cause of cancer‐related death, cancer cells dissociate from primary tumors, migrate to distal sites, and finally colonize, eventually leading to the formation of metastatic tumors. The migrating tumor cells in circulation, e.g., those found in peripheral blood (PB) or bone marrow (BM), are called circulating tumor cells (CTCs). CTCs in the BM are generally called disseminated tumor cells (DTCs). Many studies have reported the detection and characterization of CTCs to facilitate early diagnosis of relapse or metastasis and improve early detection and appropriate treatment decisions. Initially, epithelial markers, such as EpCAM and cytokeratins (CKs), identified using immunocytochemistry or reverse transcription polymerase chain reaction (RT‐PCR) were used to identify CTCs in PB or BM. Recently, however, other markers such as human epidermal growth factor receptor 2 (HER2), estrogen receptor (ER), and immuno‐checkpoint genes also have been examined to facilitate detection of CTCs with metastatic potential. Moreover, the epithelial‐to‐mesenchymal transition (EMT) and cancer stem cells (CSCs) have also received increasing attention as important CTC markers owing to their roles in the biological progression of metastasis. In addition to these markers, researchers have attempted to develop detection or capture techniques for CTCs. Notably, however, the establishment of metastasis requires cancer‐host interactions. Markers from host cells, such as macrophages, mesenchymal stem cells, and bone marrow‐derived cells, which constitute the premetastatic niche, may become novel biomarkers for predicting relapse or metastasis or monitoring the effects of treatment. Biological studies of CTCs are still emerging. However, recent technical innovations, such as next‐generation sequencing, are being used more commonly and could help to clarify the mechanism of metastasis. Additionally, biological findings are gradually being accumulated, adding to our body of knowledge on CTCs. In this review, we will summarize recent approaches to detect or capture CTCs. Moreover, we will introduce recent studies of the clinical and biological importance of CTCs and host cells.
Keywords: Circulating tumor cells, Epithelial markers, Epithelial-to-mesenchymal transition, Cancer stem cell, Premetastatic niche
Highlights
Clinical and biological characteristics of circulating tumor cells (CTCs) are gradually being accumulated.
Development of sensitive and useful detection technologies of CTCs is ongoing.
Novel host‐side markers FBXW7/CCL2 for predicting cancer metastasis are proposed.
Targeting CTCs markers would be the most successful therapeutic strategy to eliminate cancer cells with metastatic potential.
1. Introduction
In the field of cancer research, liquid biopsy has attracted much attention as a new screening or monitoring tool for patients with cancer. Liquid biopsies are noninvasive tests using blood or fluids that can detect circulating tumor cells (CTCs) or the products of tumors, such as fragments of nucleotides or proteins that are shed into biological fluids from primary or metastatic tumors. Such biopsies are expected to be informative or easily accessible tools to provide comprehensive information regarding cancers beyond conventional biopsies. Moreover, these biopsies may enable us to collect information reflecting different phases of the metastatic process and may help clinicians to achieve early diagnosis of malignancies or accurately predict metastasis or recurrence. This methodology has developed gradually since it was first proposed by Mandel and Metais (1948) around 70 years ago because the accuracy of diagnosis must be at least as good as that of conventional biopsy.
There are 3 materials that may be detected in liquid biopsy: CTCs, cell‐free DNA (cfDNA), and exosomes (Figure 1). CTCs are circulating cancer cells that are shed into vessels from tumors and have the potential to develop metastatic lesions. cfDNA is short, fragmented DNA released from cancer cells after apoptosis or necrosis (Bettegowda et al., 2014; Schwarzenbach et al., 2011). The study of cfDNA is an active area of cancer research because cfDNA can provide large amounts of information regarding the patient's cancer and can be analyzed comprehensively using next‐generation sequencing. Finally, exosomes, a hot topic in the field of intercellular communication, are small membrane‐enveloped vesicles containing functional biomolecules (i.e., protein, RNA, and DNA) that can be transferred to recipient cells (Raposo and Stoorvogel, 2013). Tumor‐derived exosomes are thought to function to prepare a favorable microenvironment at future sites of metastasis (called the premetastatic niche) (Hoshino et al., 2015). Moreover, exosomes are expected to be useful biomarkers because they are remarkably stable in fluids.
Figure 1.
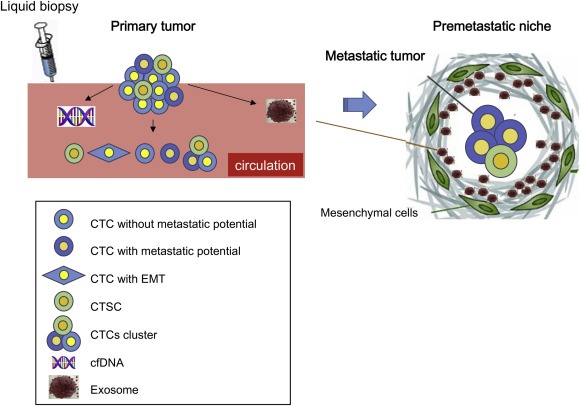
Circulating biomarkers in tumor metastasis. Cancer cells dissociate from primary tumors, migrate to distal sites, and finally colonize at the premetastatic niche, where mesenchymal cells facilitate metastasis by communication between cancer cells and mesenchymal cells using intercellular messengers, such as exosomes.
Currently, CTCs have some advantages over cfDNA and exosomes in clinical applications. For example, CTCs can be identified morphologically, and molecular characterization of CTCs can be performed using a variety of modalities (Ilie et al., 2014). It is possible to detect cancer cells by virtue of DNA alterations, such as somatic mutations and copy number aberrations, using cfDNA or exosomes. However, these methods are technically difficult and expensive owing to the low amounts of these materials in circulation. Furthermore, the biological signatures of CTCs, such as the epithelial‐to‐mesenchymal transition (EMT) or cancer stemness, which are thought to dictate tumorigenic or metastatic phenotype and resistance to therapies in circulating systems, can be useful in clinical and diagnostic applications. Thus, CTCs have the potential to provide large amounts of biological information.
In this review, we will focus on CTCs in the peripheral blood (PB) or bone marrow (BM), which have been well studied and are currently being used in the clinical setting. Additionally, we will discuss detection methods and the clinical and biological significance of CTCs in cancer.
2. Markers of CTCs in the PB or BM and clinical significance of these CTCs
2.1. Epithelial markers
Epithelial markers are effective targets that can be used to detect CTCs because most epithelial cells in the PB or BM are believed to be derived from epithelial malignancies, i.e., cancer.
-
1)
Immunocytology
Immunocytology with antibodies for epithelial markers, such as EpCAM or cytokeratins (CKs), is a common method used for detection of CTCs. The CellSearch system (Veridex, Warren, NJ, USA), an automated enrichment and immunocytochemical detection system for CTCs, was developed in 2002 (Allard et al., 2004). This system defines “CTCs” as EpCAM+/CK+/CD45‐ cells. Cristofanilli et al. (2004, 2005) and Hayes et al. (2006) first reported the detection and clinical applications of CTCs using this system and showed that CTCs were a significant indicator of prognosis and effectiveness of treatment in metastatic breast cancer. Moreover, based on these previous studies, this system was approved as a method to “monitor breast cancer treatment and indicate its effectiveness” by the United States Food and Drug Administration and now can be used clinically to monitor patients with metastatic breast, colorectal and prostate cancer (Ignatiadis et al., 2014).
Many reports have described the clinical significance of CTCs identified using the CellSearch system. In patients with metastatic breast cancer, 911 of 1944 eligible cases (46.9%) were shown to have 5 CTCs in 7.5 mL PB; the presence of these CTCs was associated with decreased progression‐free survival (PFS) and overall survival (OS) (Bidard et al., 2014). Riethdorf et al. (2007) reported that CTCs could be detected in approximately 70% of patients with metastatic breast cancer. Moreover, Rack et al. (2014) showed that CTCs could be used as an independent prognostic factor in early breast cancer. Hiraiwa et al. (2008) demonstrated that the presence of CTCs could predict disease progression and chemotherapeutic effects in patients with gastrointestinal cancers. In lung cancer, Tanaka et al. (2009) reported that the CTC count was higher in patients with lung cancer than in patients without malignant disease (30.6% versus 12.0%, respectively) and that the CTC count was higher in patients with distant metastasis in a cohort of 150 patients with lung cancer. In gastric cancer, Okabe et al. (2015) showed that CTCs were positive in 18.4% of patients with gastric cancer; additionally, the detection of CTCs was an independent predictor of short PFS in patients with advanced gastric cancer. In nonmetastatic colorectal cancer (CRC), pre‐operative CTC detection was reported to be an independent prognostic marker (Bork et al., 2015). In testicular germ cell tumors, the presence of CTCs in the PB was shown to be correlated with clinical stage in 41% of CTC‐positive patients with metastasized tumors and in 100% of patients with relapsed and chemotherapy‐refractory disease (Nastaly et al., 2014). Similar results were reported in esophageal cancer (Reeh et al., 2015) and squamous cell carcinoma of the oral cavity (Grover et al., 2014). However, the detectability of CTCs depends on the type of malignancy (Bao et al., 2013). Furthermore, some large‐scale meta‐analyses have shown the prognostic significance of CTCs detected using the CellSearch system in metastatic breast cancer or CRC (Bidard et al., 2014; Lv et al., 2015; Huang et al., 2014). In contrast, a recent large study using this system showed that there was no prognostic significance of CTCs in CRC (Sotelo et al., 2015). Pantel et al. (2012) reported that the CellSearch system gave false‐positive results in patients with benign colorectal diseases, such as inflammatory colon diseases, and demonstrated that further molecular characterization of circulating epithelial cells was needed for the use of this CTC testing modality (Pantel et al., 2012).
The BM is a secondary organ in which CTCs may be found (Joosse et al., 2015). Several studies have shown the prognostic significance of CTCs in the BM of patients with breast cancer. Of 4703 patients, 31% were positive for CTCs using immunocytochemistry, and the positive rate of CTCs was inversely related to disease‐free survival (DFS) or OS in breast cancer without overt metastasis (Braun et al., 2005). Wiedswang et al. (2003) also showed that 13% of 817 cases of breast cancer without overt metastasis were positive for CTCs using immunocytochemistry and that the CTC‐positive rate was inversely related to DFS. In 178 cases of metastatic breast cancer, CTCs were predictors of poor prognosis (Hartkopf et al., 2014). According to these solid data, the presence of CTCs in the BM at the time of diagnosis of breast cancer is thought to be associated with a poor prognosis. With respect to CRC, a large‐scale meta‐analysis of CTCs in the BM or PB of patients with metastatic CRC also showed that the detection of CTCs was associated with DFS and OS (Groot Koerkamp et al., 2013).
-
2)
Reverse transcription polymerase chain reaction (RT‐PCR)
Another representative detection method for CTCs is RT‐PCR, which can be used to detect the mRNA expression of epithelial markers, such as EpCAM or CKs (Becker et al., 2009). We have also reported the identification of CTCs and their clinical significance in the PB or BM of patients with gastric, colorectal, and breast cancers using RT‐PCR for epithelial markers such as carcinoembryonic antigen (CEA), CK‐7, and CK‐19 (Masuda et al., 2005, 2008, 1995, 1996, 1996, 1997, 1998). These assays are sensitive; however, false positive results may occur due to pseudogenes or transcripts from nonmalignant cells. Multiplex PCR, such as the AdnaTest (AdnaGen AG), could overcome this limitation (Fehm et al., 2009). This system is based on detection of 3 cancer‐associated transcripts, including estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) status, by RT‐PCR after immunomagnetic enrichment of cancer cells (Fehm et al., 2009). The AdnaTest is used to detect CTCs in the BM in patients with CRC (Musella et al., 2015) or breast cancer (Cabinakova et al., 2015; Fina et al., 2015; Hartkopf et al., 2016).
-
3)
Oligonucleotide aptamers
Aptamers are synthetic oligonucleotide ligands that are composed of RNA or single‐stranded DNA oligonucleotides and have high specificity and affinity for their targets by recognizing specific structures. In contrast to protein antibodies, aptamers exhibit unique chemical and biological characteristics based on their oligonucleotide properties. Therefore, they are expected to be more suitable for the development of novel clinical applications than the conventional detection techniques described above (Dickey and Giangrande, 2015; Sun et al., 2014). Zamay et al. (2015) developed aptamers that bound to CK‐positive and ‐negative tumor elements in the PB of patients with lung adenocarcinoma and squamous cell carcinoma.
Despite some shortcomings, CTCs detected by the CellSearch system are thought to be useful biomarkers for diagnosis (Beije et al., 2015). However, CTCs have not yet been fully accepted in clinical practice for guiding treatment decisions in individual cancer patients because of problems associated with sensitivity and specificity (Hardingham et al., 2015). As discussed below, further studies are needed to overcome these limitations.
2.2. Additional markers that can be used to characterize the malignant phenotype of CTCs
Epithelial markers are theoretically available for the detection of all CTCs. However, not all CTCs will lead to the formation of metastatic lesions (Wicha and Hayes, 2011). Additional specific markers that can detect only CTCs with metastatic potential may be better for use in clinical applications. The following have been reported as additional markers to detect CTCs in high‐grade malignancies.
1) HER2: HER2 is a member of the human epidermal growth factor receptor (HER/EGFR/ERBB) family. The amplification or overexpression of HER2 plays a critical role in the development and progression of certain aggressive types of breast cancer or gastric cancer.
In recent years, HER2 has been used clinically as a biomarker and molecular therapeutic target for approximately 30% of patients with breast or gastric cancer (Ahmed et al., 2015). The prognostic significance of HER2‐positive CTCs in breast cancer has been evaluated using enrichment and separation of epithelial cells such as the CellSearch system elsewhere. The patients with HER2‐positive CTCs were reported to have poorer PFS compared with those with HER2‐negative CTCs (Wulfing et al., 2006; Munzone et al., 2010). Riethdorf and colleagues (2010) reported that HER2‐positive CTCs were observed in 14 of 58 (24.1%) CTC‐positive patients with breast cancer, including 8 patients with HER2‐negative primary tumors using the CellSearch system. Fehm et al. (2010) also found that HER2‐positive CTCs could be detected in patients with HER2‐negative primary tumors (CellSearch system: 25/78 [32%], AdnaTest BreastCancer: 28/57 [49%]). Therefore, Riethdorf et al. (2010) suggested that the evaluation of HER2 status in CTCs could help to determine the appropriate treatment in patients with breast cancer, which may lead to improvements in current treatment strategies.
2) ER: ER is expressed in approximately 70% of patients with breast cancer; these cases are referred to as ER‐positive cancers. The ER status is determined by immunohistochemical analysis of cancer tissues. Two hypotheses have been proposed to explain why ER induces tumorigenesis. First, binding of estrogen to the ER stimulates proliferation of mammary cells, resulting in increased cell division and DNA replication, thereby leading to mutations. Second, estrogen metabolism produces genotoxic waste. Notably, ER‐positive breast cancers have the most favorable prognosis of all subtypes (Crowe et al., 1991), and ER‐positive cancers typically respond to endocrine therapy (ET). However, approximately 20% of patients with ER‐positive disease do not benefit from therapy and exhibit further metastatic progression. Babayan et al. (2013) found that CTCs frequently lacked ER expression in patients with metastatic breast cancer who had ER‐positive primary tumors. Furthermore, Paoletti et al. (2015) showed that the ET index of CTCs based on the expression of multiple parameters (ER, BCL‐2, HER2, and Ki67) could predict resistance to ET. Therefore, determination of the ER expression status of CTCs would be useful in decision making regarding the use of ET.
3) Programmed death ligand 1 (PD‐L1): Immune response represents a complex phenomenon based on a balance between activator and inhibitor pathways that regulate tumor‐infiltrating lymphocytes. PD‐L1 binds PD‐1 to reduce the cellular immune response by inducing T‐cell tolerance which helps prevent autoimmunity (Patel and Kurzrock, 2015). This system is considered to promote tumor progression. An analysis of 196 tumor specimens from patients with renal cell carcinoma demonstrated that high expression of PD‐L1 in tumors was associated with increased tumor aggressiveness and a 4.5‐fold increased risk of death (Thompson et al., 2004). Patients with ovarian cancer having higher expression of PD‐L1 exhibit significantly poorer prognosis than patients with lower expression of PD‐L1, and PD‐L1 expression is inversely correlated with the intraepithelial CD8+ T‐lymphocyte count, suggesting that PD‐L1 on tumor cells may suppress antitumor CD8+ T cells (Hamanishi et al., 2007).
Ali et al. (2015) conducted the large‐scale clinical analysis of PD‐L1 expression in breast cancer by immunohistochemistry, and found that PD‐L1 on tumor cells was expressed in just 1.7% (66/3916 cases) of breast cancer and 7.9% (24/302 cases) of basal‐like breast cancer which belongs to triple‐negative tumors. However, Mazel et al. (2015) performed flow cytometric and immunocytochemical analysis and showed that PD‐L1 was frequently expressed on metastatic cells circulating in the PB of patients with hormone receptor‐positive, HER2‐negative breast cancer. Moreover, they found that 11 of 16 (68.8%) patients with CTCs were PD‐L1 positive. Thus, PD‐L1 may be useful as an additional marker of CTCs.
2.3. CTCs with cancer stem cell (CSC) properties
CSCs are believed to develop cancers through the stem cell processes of self‐renewal and differentiation into multiple cell types. Such cells are hypothesized to persist in cancers as a distinct population and cause recurrence and metastasis. CSC markers are expected to be useful targets for CTCs. These cells are termed circulating tumor stem cells (CTSCs) (Grover et al., 2014).
1) Aldehyde dehydrogenase 1 (ALDH1): Kasimir‐Bauer assessed the expression of the stem cell marker ALDH1 in CTCs from patients with primary breast cancer using the AdnaTest BreastCancer system. They found that 5% of CTC‐negative patients were positive for ALDH1. However, there was no association between the presence of CTCs and ALDH1 expression and the prognosis of breast cancer (Aktas et al., 2009; Kasimir‐Bauer et al., 2012).
2) EpCAM+/CD44+/CD47+/MET + cells: Baccelli et al. (2013) reported that metastasis‐initiating cells containing CTC populations expressed EpCAM, CD44, CD47, and hepatocyte growth factor tyrosine kinase receptor (MET) by flow cytometry. MET has been suggested to activate a migration and putative invasion program (Trusolino et al., 2010). In a small cohort of patients with metastases, the number of EpCAM+/CD44+/CD47+/MET + CTCs, but not of bulk EPCAM + CTCs, correlated with lower overall survival and an increased number of metastatic sites (Baccelli et al., 2013). However, CTCs isolated using the epithelial cell surface marker EpCAM in hepatocellular carcinoma showed CSC properties, and their presence was associated with a higher incidence of recurrence (Sun et al., 2013).
3) CEA/CK/CD133 mRNA‐positive cells: CD133 (also known as Prominin‐1) is a key marker of CSCs in CRC (O'Brien et al., 2007; Ricci‐Vitiani et al., 2007). We found that the detection of CEA/CK/CD133 mRNA by quantitative RT‐PCR in the PB was a useful tool for determining which patients with CRC were at higher risk for recurrence and poor prognosis. In particular, CEA/CK/CD133 mRNA status has significant prognostic value in patients with Dukes' stage B and C (Iinuma et al., 2011).
2.4. CTCs with mesenchymal cell properties
The EMT is a process through which epithelial cells lose their cell polarity and cell–cell adhesion and gain migratory and invasive properties to become mesenchymal cells. Initiation of metastasis requires invasion, which is enabled by the EMT; however, the EMT is not always needed for tumor cell motility (Aceto et al., 2014) and, recently, EMT was reported to be dispensable for metastasis but contribute to chemoresistance (Fischer et al., 2015; Zheng et al., 2015). Anyway, mesenchymal markers would be potential targets of CTCs. Bitting et al. (2013) developed a method to isolate CTCs using a mesenchymal‐based capture method with the CellSearch system (OB‐cadherin‐positive cells).
Pecot et al. (2011) assessed the population of complex aneuploid CTCs that did not express CK or EpCAM epithelial antigen in patients with breast, ovarian, or colorectal cancer. They found a conversion to an EMT phenotype in the captured cells (Pecot et al., 2011). Recently, Yu et al. (2013) reported that mesenchymal CTCs occurred as both single cells and multicellular clusters, expressing known EMT regulators, including transforming growth factor (TGF)‐β pathway components and the FOXC1 transcription factor. Moreover, the EMT was suggested to have a role in the blood‐borne dissemination of human breast cancer (Yu et al., 2013). According to these findings, the EMT leads to loss of epithelial characteristics and to acquisition of mesenchymal features, which may hinder the detection of CTCs using epithelial markers.
We recently identified a novel CTCs marker, Plastin3 (PLS3), which is expressed in cancer cells with either the epithelial or mesenchymal phenotype (Sugimachi et al., 2014; Yokobori et al., 2013). PLS3 is expressed in CTCs with an EpCAM + epithelial phenotype and in EpCAM‐mesenchymal cells (Yokobori et al., 2013). We recently reported the clinical significance of PLS3 mRNA expression in the PB of various cancers, such as CRC (Sugimachi et al., 2014; Yokobori et al., 2013) and breast cancer (Ueo et al., 2015). The PLS3 gene encodes an actin bundling protein that may be important for processes enabling tumor cells to form metastases, such as escape from anoikis, resistance to chemotherapy, increased cancer stemness, and induction of the EMT (Yokobori et al., 2009). Interestingly, aberrant PLS3 gene expression may be caused by DNA copy number gains in CTCs from primary tumors (Sugimachi et al., 2014). Multivariate analysis showed that the frequency of PLS3‐positive CTCs was independently associated with poor prognosis. The association between the frequency of PLS3‐positive CTCs and prognosis was particularly strong in patients in Duke's B and C categories (Yokobori et al., 2013). This finding is important for determining which patients should receive adjuvant chemotherapy even in cases of Duke's B CRC because more than 10% of cases of Duke's B CRC exhibit recurrence without adjuvant chemotherapy.
Liu et al. (2014) reported that breast CSCs (BCSCs) exist in distinct mesenchymal‐like (CD24‐/CD44+) and epithelial‐like states (ALDH+) and that mesenchymal‐like or epithelial‐like BCSCs are localized at the cancer invasive front or more centrally, respectively. Additionally, they proposed that the plasticity of BCSCs endows these cells with the capacity for invasion and metastasis. It may be necessary to target alternative CSC states to detect or eliminate these clinically important CTCs.
3. Molecular and biological characteristics of CTCs
Analysis of the molecular and biological characteristics of CTCs has been a challenging task within the last few decades because CTCs are very rare. However, methods for analysis of CTCs have become more feasible with the development of enrichment techniques for CTCs and single‐cell analysis methods using next‐generation sequencers.
Baccelli et al. (2013) first developed a xenograft assay using CTCs from patients with metastatic breast cancer and verified the tumorigenicity of CTCs. Moreover, they identified the existence and phenotype of metastasis‐initiating cells (MICs) in CTCs. MICs expressed EpCAM, CD44, CD47, and MET, which could be biomarkers for relapse or metastasis, as described above (Baccelli et al., 2013). Additionally, Hodgkinson et al. (2014) demonstrated that CTCs from patients with metastatic small‐cell lung cancer were tumorigenic in immunodeficient mice. Toyoshima et al. (2015) also used immunodeficient mice inoculated with CTCs from patients with advanced gastric cancer and showed that the CD44+/high fraction among CTCs exhibited distinct tumorigenicity. Cayrefourcq et al. (2015) established a permanent cell line from CTCs from patients with metastatic CRC and characterized these cells at the genomic, transcriptomic, proteomic, and secretomic levels. They reported that the CTC line displayed an intermediate epithelial/mesenchymal phenotype, stem cell‐like properties, and an osteomimetic signature; furthermore, this cell line could induce endothelial cell tube formation and form tumors in xenografts.
Khoo et al. (2015) cultured CTCs from metastatic breast cancer and showed that these cells exhibit DNA copy number increases in the FGFR1, Myc, CCND1, HER2, TOP2A, and ZNF217 genes using fluorescence in situ hybridization (FISH) analysis. They also examined phenotypes in these cells, e.g., tumorigenicity, as analyzed using sphere formation assays, and the EMT phenotype, as analyzed by immunostaining (Khoo et al., 2015). Kanwar et al. (2015) performed copy‐number profiling of CTCs from breast cancer and identified 3 signatures of recurrent gains in CTCs: the dormancy‐related signature, tumor‐aggressiveness related signature (mainly on chromosome 19), and common signature (on chromosome 19q13.13 and 21q21). Neves et al. (2014) performed comparative genomic hybridization (aCGH) using CTCs from patients with metastatic breast cancer and identified amplification in the cyclin D1 locus. Additionally, Polzer et al. (2014) performed aCGH using single CTCs from patients with metastatic breast cancer and found genomic disparity of potentially high relevance between primary tumors and CTCs. Steinert et al. (2014) described a genomic analysis of single CRC‐derived CTCs by aCGH, mutational profiling, and microsatellite instability analysis and found mutational heterogeneity between primary tumors and CTCs in CRC. Moreover, Ni et al. (2013) also reported copy number variations among single CTCs from patients with lung cancer using exome sequencing.
Lohr et al. (2014) performed a comprehensive analysis of CTCs genomes from patients with metastatic prostate cancer using whole‐exome sequencing and identified 10 early trunk and 56 metastatic trunk mutations.
The elucidation of these molecular and biological features of CTCs will allow us to understand the mechanisms of cancer metastasis, which may lead to the identification of real biomarkers of early diagnosis or therapeutic molecular targets to prevent metastasis.
4. Host‐side factors indicating poor clinical outcomes
The “premetastatic niche” has attracted attention as a new concept of metastasis or recurrence. Based on this concept, the primary cancer is thought to emit signaling molecules that prime certain organs for cancer metastasis. Host cells such as hematopoietic progenitor cells in the BM or endothelial progenitor cells in the PB are recruited to future metastatic sites, namely the premetastatic niche, by signals from primary cancer cells. Then, isolated cancer cells from primary cancers are guided to the premetastatic niche by chemokines from the host cells (Kaplan et al., 2005; Lyden et al., 2001). Mimori et al. (2008) provided supportive data obtained by quantitative PCR for vascular endothelial growth factor receptor 1 (VEGFR1) and CKs in the PB or BM of patients with gastric cancer. Therefore, markers of premetastatic niche‐related host cells, such as surface markers of BM‐derived cells or chemokines and their receptors, may be useful biomarkers for the prediction or detection of metastasis or recurrence.
Recently, we reported that FBXW7 expression in the host environment is a key determinant of cancer metastasis using FBXW7‐deficient mice. Metastasis was found to be enhanced in mice lacking FBXW7 in the BM compared with that in control mice. Deletion of Fbxw7 from murine BM‐derived stromal cells induced accumulation of NOTCH and consequent transcriptional activation of CCL2. The increased production of CCL2 by these cells likely promoted the formation of metastatic niches through recruitment of monocytes and macrophages. Inhibition of CCL2/CCR2 signaling reduced the frequency of metastasis in FBXW7‐deficient mice (Yumimoto et al., 2015). These findings suggest that the FBXW7/NOTCH/CCL2 pathway plays a central role in the regulation of cancer metastasis through controlling the formation of the premetastatic niche. Therefore, studies aimed at detecting host‐side markers may provide important insights into the functions and characteristics of the premetastatic niche.
5. Conclusion
Identification of CTCs is clinically and biologically important and may facilitate the early detection and treatment of metastatic cancer. Moreover, further elucidation of the features of CTCs may provide important insights into the mechanisms of metastasis, one of the leading causes of cancer‐related death. Recent technological innovations, such as next‐generation sequencing for the detection of DNA from CTCs or cfDNA in circulation, may provide large amounts of data regarding the molecular features of CTCs. However, if we aim primarily at eliminating cancer cells with metastatic potential, which would contribute to improving the survival of patients with cancer, targeting cellular markers of CTCs would likely be the most successful strategy based on currently available technologies and methods.
Masuda Takaaki, Hayashi Naoki, Iguchi Tomohiro, Ito Shuhei, Eguchi Hidetoshi, Mimori Koshi, (2016), Clinical and biological significance of circulating tumor cells in cancer, Molecular Oncology, 10, doi: 10.1016/j.molonc.2016.01.010.
This is a contribution to the special issue edited by Klaus Pantel and Catherine Alix‐Panabieres, Liquid Biopsies.
References
- Aceto, N. , Bardia, A. , Miyamoto, D.T. , Donaldson, M.C. , Wittner, B.S. , Spencer, J.A. , Yu, M. , Pely, A. , Engstrom, A. , Zhu, H. , Brannigan, B.W. , Kapur, R. , Stott, S.L. , Shioda, T. , Ramaswamy, S. , Ting, D.T. , Lin, C.P. , Toner, M. , Haber, D.A. , Maheswaran, S. , 2014. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 158, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, S. , Sami, A. , Xiang, J. , 2015. HER2-directed therapy: current treatment options for HER2-positive breast cancer. Breast Cancer. 22, 101–116. [DOI] [PubMed] [Google Scholar]
- Aktas, B. , Tewes, M. , Fehm, T. , Hauch, S. , Kimmig, R. , Kasimir-Bauer, S. , 2009. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 11, R46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, H.R. , Glont, S.E. , Blows, F.M. , Provenzano, E. , Dawson, S.J. , Liu, B. , Hiller, L. , Dunn, J. , Poole, C.J. , Bowden, S. , Earl, H.M. , Pharoah, P.D. , Caldas, C. , 2015. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann. Oncol. 26, 1488–1493. [DOI] [PubMed] [Google Scholar]
- Allard, W.J. , Matera, J. , Miller, M.C. , Repollet, M. , Connelly, M.C. , Rao, C. , Tibbe, A.G. , Uhr, J.W. , Terstappen, L.W. , 2004. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 10, 6897–6904. [DOI] [PubMed] [Google Scholar]
- Baccelli, I. , Schneeweiss, A. , Riethdorf, S. , Stenzinger, A. , Schillert, A. , Vogel, V. , Klein, C. , Saini, M. , Bauerle, T. , Wallwiener, M. , Holland-Letz, T. , Hofner, T. , Sprick, M. , Scharpff, M. , Marme, F. , Sinn, H.P. , Pantel, K. , Weichert, W. , Trumpp, A. , 2013. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 31, 539–544. [DOI] [PubMed] [Google Scholar]
- Bao, H. , Burke, P.A. , Huang, J. , Chen, X. , Brohawn, P.Z. , Yao, Y. , Lechleider, R.J. , Sikorski, R.S. , Buzoianu, M. , Zhang, J. , Shi, X. , Richman, L.K. , Lavallee, T.M. , 2013. Circulating tumor cells: application as a biomarker for molecular characterization and predictor of survival in an all-comer solid tumor phase I clinical study. PLoS One. 8, e58557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, S. , Becker-Pergola, G. , Banys, M. , Krawczyk, N. , Wallwiener, D. , Solomayer, E. , Schuetz, C. , Fehm, T. , 2009. Evaluation of a RT-PCR based routine screening tool for the detection of disseminated epithelial cells in the bone marrow of breast cancer patients. Breast Cancer Res. Treat. 117, 227–233. [DOI] [PubMed] [Google Scholar]
- Beije, N. , Jager, A. , Sleijfer, S. , 2015. Circulating tumor cell enumeration by the CellSearch system: the clinician's guide to breast cancer treatment?. Cancer Treat. Rev. 41, 144–150. [DOI] [PubMed] [Google Scholar]
- Bettegowda, C. , Sausen, M. , Leary, R.J. , Kinde, I. , Wang, Y. , Agrawal, N. , Bartlett, B.R. , Wang, H. , Luber, B. , Alani, R.M. , Antonarakis, E.S. , Azad, N.S. , Bardelli, A. , Brem, H. , Cameron, J.L. , Lee, C.C. , Fecher, L.A. , Gallia, G.L. , Gibbs, P. , Le, D. , Giuntoli, R.L. , Goggins, M. , Hogarty, M.D. , Holdhoff, M. , Hong, S.M. , Jiao, Y. , Juhl, H.H. , Kim, J.J. , Siravegna, G. , Laheru, D.A. , Lauricella, C. , Lim, M. , Lipson, E.J. , Marie, S.K. , Netto, G.J. , Oliner, K.S. , Olivi, A. , Olsson, L. , Riggins, G.J. , Sartore-Bianchi, A. , Schmidt, K. , Shih, L.M. , Oba-Shinjo, S.M. , Siena, S. , Theodorescu, D. , Tie, J. , Harkins, T.T. , Veronese, S. , Wang, T.L. , Weingart, J.D. , Wolfgang, C.L. , Wood, L.D. , Xing, D. , Hruban, R.H. , Wu, J. , Allen, P.J. , Schmidt, C.M. , Choti, M.A. , Velculescu, V.E. , Kinzler, K.W. , Vogelstein, B. , Papadopoulos, N. , Diaz, L.A. , 2014. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 6, 224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidard, F.C. , Peeters, D.J. , Fehm, T. , Nole, F. , Gisbert-Criado, R. , Mavroudis, D. , Grisanti, S. , Generali, D. , Garcia-Saenz, J.A. , Stebbing, J. , Caldas, C. , Gazzaniga, P. , Manso, L. , Zamarchi, R. , de Lascoiti, A.F. , De Mattos-Arruda, L. , Ignatiadis, M. , Lebofsky, R. , van Laere, S.J. , Meier-Stiegen, F. , Sandri, M.T. , Vidal-Martinez, J. , Politaki, E. , Consoli, F. , Bottini, A. , Diaz-Rubio, E. , Krell, J. , Dawson, S.J. , Raimondi, C. , Rutten, A. , Janni, W. , Munzone, E. , Caranana, V. , Agelaki, S. , Almici, C. , Dirix, L. , Solomayer, E.F. , Zorzino, L. , Johannes, H. , Reis-Filho, J.S. , Pantel, K. , Pierga, J.Y. , Michiels, S. , 2014. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 15, 406–414. [DOI] [PubMed] [Google Scholar]
- Bitting, R.L. , Boominathan, R. , Rao, C. , Kemeny, G. , Foulk, B. , Garcia-Blanco, M.A. , Connelly, M. , Armstrong, A.J. , 2013. Development of a method to isolate circulating tumor cells using mesenchymal-based capture. Methods. 64, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork, U. , Rahbari, N.N. , Scholch, S. , Reissfelder, C. , Kahlert, C. , Buchler, M.W. , Weitz, J. , Koch, M. , 2015. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br. J. Cancer. 112, 1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, S. , Vogl, F.D. , Naume, B. , Janni, W. , Osborne, M.P. , Coombes, R.C. , Schlimok, G. , Diel, I.J. , Gerber, B. , Gebauer, G. , Pierga, J.Y. , Marth, C. , Oruzio, D. , Wiedswang, G. , Solomayer, E.F. , Kundt, G. , Strobl, B. , Fehm, T. , Wong, G.Y. , Bliss, J. , Vincent-Salomon, A. , Pantel, K. , 2005. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802. [DOI] [PubMed] [Google Scholar]
- Cabinakova, M. , Mikulova, V. , Malickova, K. , Vrana, D. , Pavlista, D. , Petruzelka, L. , Zima, T. , Tesarova, P. , 2015. Predictive factors for the presence of tumor cells in bone marrow and peripheral blood in breast cancer patients. Neoplasma. 62, 259–268. [DOI] [PubMed] [Google Scholar]
- Cayrefourcq, L. , Mazard, T. , Joosse, S. , Solassol, J. , Ramos, J. , Assenat, E. , Schumacher, U. , Costes, V. , Maudelonde, T. , Pantel, K. , Alix-Panabieres, C. , 2015. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 75, 892–901. [DOI] [PubMed] [Google Scholar]
- Cristofanilli, M. , Budd, G.T. , Ellis, M.J. , Stopeck, A. , Matera, J. , Miller, M.C. , Reuben, J.M. , Doyle, G.V. , Allard, W.J. , Terstappen, L.W. , Hayes, D.F. , 2004. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791. [DOI] [PubMed] [Google Scholar]
- Cristofanilli, M. , Hayes, D.F. , Budd, G.T. , Ellis, M.J. , Stopeck, A. , Reuben, J.M. , Doyle, G.V. , Matera, J. , Allard, W.J. , Miller, M.C. , Fritsche, H.A. , Hortobagyi, G.N. , Terstappen, L.W. , 2005. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J. Clin. Oncol. 23, 1420–1430. [DOI] [PubMed] [Google Scholar]
- Crowe, J.P. , Gordon, N.H. , Hubay, C.A. , Shenk, R.R. , Zollinger, R.M. , Brumberg, D.J. , McGuire, W.L. , Shuck, J.M. , 1991. Estrogen receptor determination and long term survival of patients with carcinoma of the breast. Surg. Gynecol. Obstet. 173, 273–278. [PubMed] [Google Scholar]
- Dickey, D.D. , Giangrande, P.H. , 2015. Oligonucleotide aptamers: a next-generation technology for the capture and detection of circulating tumor cells. Methods. (in press). http://dx.doi.org/10.1016/j.ymeth.2015.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehm, T. , Hoffmann, O. , Aktas, B. , Becker, S. , Solomayer, E.F. , Wallwiener, D. , Kimmig, R. , Kasimir-Bauer, S. , 2009. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res. 11, R59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehm, T. , Muller, V. , Aktas, B. , Janni, W. , Schneeweiss, A. , Stickeler, E. , Lattrich, C. , Lohberg, C.R. , Solomayer, E. , Rack, B. , Riethdorf, S. , Klein, C. , Schindlbeck, C. , Brocker, K. , Kasimir-Bauer, S. , Wallwiener, D. , Pantel, K. , 2010. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res. Treat. 124, 403–412. [DOI] [PubMed] [Google Scholar]
- Fina, E. , Reduzzi, C. , Motta, R. , Di Cosimo, S. , Bianchi, G. , Martinetti, A. , Wechsler, J. , Cappelletti, V. , Daidone, M.G. , 2015. Did circulating tumor cells tell us all they could? the missed circulating tumor cell message in breast cancer. Int. J. Biol. Markers. 30, e429–433. [DOI] [PubMed] [Google Scholar]
- Fischer, K.R. , Durrans, A. , Lee, S. , Sheng, J. , Li, F. , Wong, S.T. , Choi, H. , El Rayes, T. , Ryu, S. , Troeger, J. , Schwabe, R.F. , Vahdat, L.T. , Altorki, N.K. , Mittal, V. , Gao, D. , 2015. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 527, 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot Koerkamp, B. , Rahbari, N.N. , Buchler, M.W. , Koch, M. , Weitz, J. , 2013. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann. Surg. Oncol. 20, 2156–2165. [DOI] [PubMed] [Google Scholar]
- Grover, P.K. , Cummins, A.G. , Price, T.J. , Roberts-Thomson, I.C. , Hardingham, J.E. , 2014. Circulating tumour cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann. Oncol. 25, 1506–1516. [DOI] [PubMed] [Google Scholar]
- Hamanishi, J. , Mandai, M. , Iwasaki, M. , Okazaki, T. , Tanaka, Y. , Yamaguchi, K. , Higuchi, T. , Yagi, H. , Takakura, K. , Minato, N. , Honjo, T. , Fujii, S. , 2007. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. U.S.A. 104, 3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham, J.E. , Grover, P. , Winter, M. , Hewett, P.J. , Price, T.J. , Thierry, B. , 2015. Detection and clinical significance of circulating tumor cells in colorectal cancer-20 tears of progress. Mol. Med. 21, (Suppl. 1) S25–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartkopf, A.D. , Stefanescu, D. , Wallwiener, M. , Hahn, M. , Becker, S. , Solomayer, E.F. , Fehm, T.N. , Brucker, S.Y. , Taran, F.A. , 2014. Tumor cell dissemination to the bone marrow and blood is associated with poor outcome in patients with metastatic breast cancer. Breast Cancer Res. Treat. 147, 345–351. [DOI] [PubMed] [Google Scholar]
- Hartkopf, A.D. , Wallwiener, M. , Hahn, M. , Fehm, T.N. , Brucker, S.Y. , Taran, F.A. , 2016. Simultaneous detection of disseminated and circulating tumor cells in primary breast cancer patients. Cancer Res. Treat. 48, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, D.F. , Cristofanilli, M. , Budd, G.T. , Ellis, M.J. , Stopeck, A. , Miller, M.C. , Matera, J. , Allard, W.J. , Doyle, G.V. , Terstappen, L.W. , 2006. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin. Cancer Res. 12, 4218–4224. [DOI] [PubMed] [Google Scholar]
- Hiraiwa, K. , Takeuchi, H. , Hasegawa, H. , Saikawa, Y. , Suda, K. , Ando, T. , Kumagai, K. , Irino, T. , Yoshikawa, T. , Matsuda, S. , Kitajima, M. , Kitagawa, Y. , 2008. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann. Surg. Oncol. 15, 3092–3100. [DOI] [PubMed] [Google Scholar]
- Hodgkinson, C.L. , Morrow, C.J. , Li, Y. , Metcalf, R.L. , Rothwell, D.G. , Trapani, F. , Polanski, R. , Burt, D.J. , Simpson, K.L. , Morris, K. , Pepper, S.D. , Nonaka, D. , Greystoke, A. , Kelly, P. , Bola, B. , Krebs, M.G. , Antonello, J. , Ayub, M. , Faulkner, S. , Priest, L. , Carter, L. , Tate, C. , Miller, C.J. , Blackhall, F. , Brady, G. , Dive, C. , 2014. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 20, 897–903. [DOI] [PubMed] [Google Scholar]
- Hoshino, A. , Costa-Silva, B. , Shen, T.L. , Rodrigues, G. , Hashimoto, A. , Tesic Mark, M. , Molina, H. , Kohsaka, S. , Di Giannatale, A. , Ceder, S. , Singh, S. , Williams, C. , Soplop, N. , Uryu, K. , Pharmer, L. , King, T. , Bojmar, L. , Davies, A.E. , Ararso, Y. , Zhang, T. , Zhang, H. , Hernandez, J. , Weiss, J.M. , Dumont-Cole, V.D. , Kramer, K. , Wexler, L.H. , Narendran, A. , Schwartz, G.K. , Healey, J.H. , Sandstrom, P. , Jorgen Labori, K. , Kure, E.H. , Grandgenett, P.M. , Hollingsworth, M.A. , de Sousa, M. , Kaur, S. , Jain, M. , Mallya, K. , Batra, S.K. , Jarnagin, W.R. , Brady, M.S. , Fodstad, O. , Muller, V. , Pantel, K. , Minn, A.J. , Bissell, M.J. , Garcia, B.A. , Kang, Y. , Rajasekhar, V.K. , Ghajar, C.M. , Matei, I. , Peinado, H. , Bromberg, J. , Lyden, D. , 2015. Tumour exosome integrins determine organotropic metastasis. Nature. 527, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X. , Gao, P. , Song, Y. , Sun, J. , Chen, X. , Zhao, J. , Liu, J. , Xu, H. , Wang, Z. , 2014. Relationship between circulating tumor cells and tumor response in colorectal cancer patients treated with chemotherapy: a meta-analysis. BMC Cancer. 14, 976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatiadis, M. , Riethdorf, S. , Bidard, F.C. , Vaucher, I. , Khazour, M. , Rothe, F. , Metallo, J. , Rouas, G. , Payne, R.E. , Coombes, R. , Teufel, I. , Andergassen, U. , Apostolaki, S. , Politaki, E. , Mavroudis, D. , Bessi, S. , Pestrin, M. , Di Leo, A. , Campion, M. , Reinholz, M. , Perez, E. , Piccart, M. , Borgen, E. , Naume, B. , Jimenez, J. , Aura, C. , Zorzino, L. , Cassatella, M. , Sandri, M. , Mostert, B. , Sleijfer, S. , Kraan, J. , Janni, W. , Fehm, T. , Rack, B. , Terstappen, L. , Repollet, M. , Pierga, J.Y. , Miller, C. , Sotiriou, C. , Michiels, S. , Pantel, K. , 2014. International study on inter-reader variability for circulating tumor cells in breast cancer. Breast Cancer Res. 16, R43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iinuma, H. , Watanabe, T. , Mimori, K. , Adachi, M. , Hayashi, N. , Tamura, J. , Matsuda, K. , Fukushima, R. , Okinaga, K. , Sasako, M. , Mori, M. , 2011. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes' stage B and C colorectal cancer. J. Clin. Oncol. 29, 1547–1555. [DOI] [PubMed] [Google Scholar]
- Ilie, M. , Hofman, V. , Long, E. , Bordone, O. , Selva, E. , Washetine, K. , Marquette, C.H. , Hofman, P. , 2014. Current challenges for detection of circulating tumor cells and cell-free circulating nucleic acids, and their characterization in non-small cell lung carcinoma patients. What is the best blood substrate for personalized medicine?. Ann. Transl. Med. 2, 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosse, S.A. , Gorges, T.M. , Pantel, K. , 2015. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar, N. , Hu, P. , Bedard, P. , Clemons, M. , McCready, D. , Done, S.J. , 2015. Identification of genomic signatures in circulating tumor cells from breast cancer. Int. J. Cancer. 137, 332–344. [DOI] [PubMed] [Google Scholar]
- Kaplan, R.N. , Riba, R.D. , Zacharoulis, S. , Bramley, A.H. , Vincent, L. , Costa, C. , MacDonald, D.D. , Jin, D.K. , Shido, K. , Kerns, S.A. , Zhu, Z. , Hicklin, D. , Wu, Y. , Port, J.L. , Altorki, N. , Port, E.R. , Ruggero, D. , Shmelkov, S.V. , Jensen, K.K. , Rafii, S. , Lyden, D. , 2005. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 438, 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasimir-Bauer, S. , Hoffmann, O. , Wallwiener, D. , Kimmig, R. , Fehm, T. , 2012. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res. 14, R15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo, B.L. , Lee, S.C. , Kumar, P. , Tan, T.Z. , Warkiani, M.E. , Ow, S.G. , Nandi, S. , Lim, C.T. , Thiery, J.P. , 2015. Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy. Oncotarget. 6, 15578–15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. , Cong, Y. , Wang, D. , Sun, Y. , Deng, L. , Liu, Y. , Martin-Trevino, R. , Shang, L. , McDermott, S.P. , Landis, M.D. , Hong, S. , Adams, A. , D'Angelo, R. , Ginestier, C. , Charafe-Jauffret, E. , Clouthier, S.G. , Birnbaum, D. , Wong, S.T. , Zhan, M. , Chang, J.C. , Wicha, M.S. , 2014. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2, 78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr, J.G. , Adalsteinsson, V.A. , Cibulskis, K. , Choudhury, A.D. , Rosenberg, M. , Cruz-Gordillo, P. , Francis, J.M. , Zhang, C.Z. , Shalek, A.K. , Satija, R. , Trombetta, J.J. , Lu, D. , Tallapragada, N. , Tahirova, N. , Kim, S. , Blumenstiel, B. , Sougnez, C. , Lowe, A. , Wong, B. , Auclair, D. , Van Allen, E.M. , Nakabayashi, M. , Lis, R.T. , Lee, G.S. , Li, T. , Chabot, M.S. , Ly, A. , Taplin, M.E. , Clancy, T.E. , Loda, M. , Regev, A. , Meyerson, M. , Hahn, W.C. , Kantoff, P.W. , Golub, T.R. , Getz, G. , Boehm, J.S. , Love, J.C. , 2014. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat. Biotechnol. 32, 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, Q. , Gong, L. , Zhang, T. , Ye, J. , Chai, L. , Ni, C. , Mao, Y. , 2015. Prognostic value of circulating tumor cells in metastatic breast cancer: a systemic review and meta-analysis. Clin. Transl. Oncol. (in press). http://dx.doi.org/10.1007/s12094-015-1372-1 [DOI] [PubMed] [Google Scholar]
- Lyden, D. , Hattori, K. , Dias, S. , Costa, C. , Blaikie, P. , Butros, L. , Chadburn, A. , Heissig, B. , Marks, W. , Witte, L. , Wu, Y. , Hicklin, D. , Zhu, Z. , Hackett, N.R. , Crystal, R.G. , Moore, M.A. , Hajjar, K.A. , Manova, K. , Benezra, R. , Rafii, S. , 2001. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 7, 1194–1201. [DOI] [PubMed] [Google Scholar]
- Mandel, P. , Metais, P. , 1948. Les acides nucléiques du plasma sanguin chez l'homme. C. R. Seances Soc. Biol. Fil. 142, 241–243. [PubMed] [Google Scholar]
- Masuda, T.A. , Kataoka, A. , Ohno, S. , Murakami, S. , Mimori, K. , Utsunomiya, T. , Inoue, H. , Tsutsui, S. , Kinoshita, J. , Masuda, N. , Moriyama, N. , Mori, M. , 2005. Detection of occult cancer cells in peripheral blood and bone marrow by quantitative RT-PCR assay for cytokeratin-7 in breast cancer patients. Int. J. Oncol. 26, 721–730. [PubMed] [Google Scholar]
- Mazel, M. , Jacot, W. , Pantel, K. , Bartkowiak, K. , Topart, D. , Cayrefourcq, L. , Rossille, D. , Maudelonde, T. , Fest, T. , Alix-Panabieres, C. , 2015. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 9, 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori, K. , Fukagawa, T. , Kosaka, Y. , Kita, Y. , Ishikawa, K. , Etoh, T. , Iinuma, H. , Sasako, M. , Mori, M. , 2008. Hematogenous metastasis in gastric cancer requires isolated tumor cells and expression of vascular endothelial growth factor receptor-1. Clin. Cancer Res. 14, 2609–2616. [DOI] [PubMed] [Google Scholar]
- Mori, M. , Barnard, G.F. , Mimori, K. , Ueo, H. , Akiyoshi, T. , Sugimachi, K. , 1995. Overexpression of matrix metalloproteinase-7 mRNA in human colon carcinomas. Cancer. 75, 1516–1519. [DOI] [PubMed] [Google Scholar]
- Mori, M. , Inoue, H. , Mimori, K. , Shibuta, K. , Baba, K. , Nakashima, H. , Haraguchi, M. , Tsuji, K. , Ueo, H. , Barnard, G.F. , Akiyoshi, T. , 1996. Expression of MAGE genes in human colorectal carcinoma. Ann. Surg. 224, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, M. , Mimori, K. , Ueo, H. , Karimine, N. , Barnard, G.F. , Sugimachi, K. , Akiyoshi, T. , 1996. Molecular detection of circulating solid carcinoma cells in the peripheral blood: the concept of early systemic disease. Int. J. Cancer. 68, 739–743. [DOI] [PubMed] [Google Scholar]
- Mori, M. , Mimori, K. , Tanaka, F. , Ueo, H. , Sugimachi, K. , Akiyoshi, T. , 1997. Molecular diagnosis of circulating cancer cells using MAGE gene assays. JAMA. 278, 476–477. [PubMed] [Google Scholar]
- Mori, M. , Mimori, K. , Ueo, H. , Tsuji, K. , Shiraishi, T. , Barnard, G.F. , Sugimachi, K. , Akiyoshi, T. , 1998. Clinical significance of molecular detection of carcinoma cells in lymph nodes and peripheral blood by reverse transcription-polymerase chain reaction in patients with gastrointestinal or breast carcinomas. J. Clin. Oncol. 16, 128–132. [DOI] [PubMed] [Google Scholar]
- Munzone, E. , Nole, F. , Goldhirsch, A. , Botteri, E. , Esposito, A. , Zorzino, L. , Curigliano, G. , Minchella, I. , Adamoli, L. , Cassatella, M.C. , Casadio, C. , Sandri, M.T. , 2010. Changes of HER2 status in circulating tumor cells compared with the primary tumor during treatment for advanced breast cancer. Clin. Breast Cancer. 10, 392–397. [DOI] [PubMed] [Google Scholar]
- Musella, V. , Pietrantonio, F. , Di Buduo, E. , Iacovelli, R. , Martinetti, A. , Sottotetti, E. , Bossi, I. , Maggi, C. , Di Bartolomeo, M. , de Braud, F. , Daidone, M.G. , Cappelletti, V. , 2015. Circulating tumor cells as a longitudinal biomarker in patients with advanced chemorefractory, RAS-BRAF wild-type colorectal cancer receiving cetuximab or panitumumab. Int. J. Cancer. 137, 1467–1474. [DOI] [PubMed] [Google Scholar]
- Nastaly, P. , Ruf, C. , Becker, P. , Bednarz-Knoll, N. , Stoupiec, M. , Kavsur, R. , Isbarn, H. , Matthies, C. , Wagner, W. , Hoppner, D. , Fisch, M. , Bokemeyer, C. , Ahyai, S. , Honecker, F. , Riethdorf, S. , Pantel, K. , 2014. Circulating tumor cells in patients with testicular germ cell tumors. Clin. Cancer Res. 20, 3830–3841. [DOI] [PubMed] [Google Scholar]
- Neves, R.P. , Raba, K. , Schmidt, O. , Honisch, E. , Meier-Stiegen, F. , Behrens, B. , Mohlendick, B. , Fehm, T. , Neubauer, H. , Klein, C.A. , Polzer, B. , Sproll, C. , Fischer, J.C. , Niederacher, D. , Stoecklein, N.H. , 2014. Genomic high-resolution profiling of single CKpos/CD45neg flow-sorting purified circulating tumor cells from patients with metastatic breast cancer. Clin. Chem. 60, 1290–1297. [DOI] [PubMed] [Google Scholar]
- Ni, X. , Zhuo, M. , Su, Z. , Duan, J. , Gao, Y. , Wang, Z. , Zong, C. , Bai, H. , Chapman, A.R. , Zhao, J. , Xu, L. , An, T. , Ma, Q. , Wang, Y. , Wu, M. , Sun, Y. , Wang, S. , Li, Z. , Yang, X. , Yong, J. , Su, X.D. , Lu, Y. , Bai, F. , Xie, X.S. , Wang, J. , 2013. Reproducible copy number variation patterns among single circulating tumor cells of lung cancer patients. Proc. Natl. Acad. Sci. U.S.A. 110, 21083–21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, C.A. , Pollett, A. , Gallinger, S. , Dick, J.E. , 2007. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 445, 106–110. [DOI] [PubMed] [Google Scholar]
- Okabe, H. , Tsunoda, S. , Hosogi, H. , Hisamori, S. , Tanaka, E. , Tanaka, S. , Sakai, Y. , 2015. Circulating tumor cells as an independent predictor of survival in advanced gastric cancer. Ann. Surg. Oncol. 22, 3954–3961. [DOI] [PubMed] [Google Scholar]
- Pantel, K. , Deneve, E. , Nocca, D. , Coffy, A. , Vendrell, J.P. , Maudelonde, T. , Riethdorf, S. , Alix-Panabieres, C. , 2012. Circulating epithelial cells in patients with benign colon diseases. Clin. Chem. 58, 936–940. [DOI] [PubMed] [Google Scholar]
- Paoletti, C. , Muniz, M.C. , Thomas, D.G. , Griffith, K.A. , Kidwell, K.M. , Tokudome, N. , Brown, M.E. , Aung, K. , Miller, M.C. , Blossom, D.L. , Schott, A.F. , Henry, N.L. , Rae, J.M. , Connelly, M.C. , Chianese, D.A. , Hayes, D.F. , 2015. Development of circulating tumor cell-endocrine therapy index in patients with hormone receptor-positive breast cancer. Clin. Cancer Res. 21, 2487–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, S.P. , Kurzrock, R. , 2015. PD-L1 expression as a predictive biomarker in Cancer immunotherapy. Mol. Cancer Ther. 14, 847–856. [DOI] [PubMed] [Google Scholar]
- Pecot, C.V. , Bischoff, F.Z. , Mayer, J.A. , Wong, K.L. , Pham, T. , Bottsford-Miller, J. , Stone, R.L. , Lin, Y.G. , Jaladurgam, P. , Roh, J.W. , Goodman, B.W. , Merritt, W.M. , Pircher, T.J. , Mikolajczyk, S.D. , Nick, A.M. , Celestino, J. , Eng, C. , Ellis, L.M. , Deavers, M.T. , Sood, A.K. , 2011. A novel platform for detection of CK+ and CK- CTCs. Cancer Discov. 1, 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzer, B. , Medoro, G. , Pasch, S. , Fontana, F. , Zorzino, L. , Pestka, A. , Andergassen, U. , Meier-Stiegen, F. , Czyz, Z.T. , Alberter, B. , Treitschke, S. , Schamberger, T. , Sergio, M. , Bregola, G. , Doffini, A. , Gianni, S. , Calanca, A. , Signorini, G. , Bolognesi, C. , Hartmann, A. , Fasching, P.A. , Sandri, M.T. , Rack, B. , Fehm, T. , Giorgini, G. , Manaresi, N. , Klein, C.A. , 2014. Molecular profiling of single circulating tumor cells with diagnostic intention. EMBO Mol. Med. 6, 1371–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack, B. , Schindlbeck, C. , Juckstock, J. , Andergassen, U. , Hepp, P. , Zwingers, T. , Friedl, T.W. , Lorenz, R. , Tesch, H. , Fasching, P.A. , Fehm, T. , Schneeweiss, A. , Lichtenegger, W. , Beckmann, M.W. , Friese, K. , Pantel, K. , Janni, W. , Group, S.S. , 2014. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J. Natl. Cancer Inst. 106, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo, G. , Stoorvogel, W. , 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeh, M. , Effenberger, K.E. , Koenig, A.M. , Riethdorf, S. , Eichstadt, D. , Vettorazzi, E. , Uzunoglu, F.G. , Vashist, Y.K. , Izbicki, J.R. , Pantel, K. , Bockhorn, M. , 2015. Circulating tumor cells as a biomarker for preoperative prognostic staging in patients with esophageal cancer. Ann. Surg. 261, 1124–1130. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani, L. , Lombardi, D.G. , Pilozzi, E. , Biffoni, M. , Todaro, M. , Peschle, C. , De Maria, R. , 2007. Identification and expansion of human colon-cancer-initiating cells. Nature. 445, 111–115. [DOI] [PubMed] [Google Scholar]
- Riethdorf, S. , Fritsche, H. , Muller, V. , Rau, T. , Schindlbeck, C. , Rack, B. , Janni, W. , Coith, C. , Beck, K. , Janicke, F. , Jackson, S. , Gornet, T. , Cristofanilli, M. , Pantel, K. , 2007. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin. Cancer Res. 13, 920–928. [DOI] [PubMed] [Google Scholar]
- Riethdorf, S. , Muller, V. , Zhang, L. , Rau, T. , Loibl, S. , Komor, M. , Roller, M. , Huober, J. , Fehm, T. , Schrader, I. , Hilfrich, J. , Holms, F. , Tesch, H. , Eidtmann, H. , Untch, M. , von Minckwitz, G. , Pantel, K. , 2010. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin. Cancer Res. 16, 2634–2645. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach, H. , Hoon, D.S. , Pantel, K. , 2011. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer. 11, 426–437. [DOI] [PubMed] [Google Scholar]
- Sotelo, M.J. , Sastre, J. , Maestro, M.L. , Veganzones, S. , Vieitez, J.M. , Alonso, V. , Gravalos, C. , Escudero, P. , Vera, R. , Aranda, E. , Garcia-Alfonso, P. , Gallego-Plazas, J. , Lopez, C. , Pericay, C. , Arrivi, A. , Vicente, P. , Ballesteros, P. , Elez, E. , Lopez-Ladron, A. , Diaz-Rubio, E. , 2015. Role of circulating tumor cells as prognostic marker in resected stage III colorectal cancer. Ann. Oncol. 26, 535–541. [DOI] [PubMed] [Google Scholar]
- Steinert, G. , Scholch, S. , Niemietz, T. , Iwata, N. , Garcia, S.A. , Behrens, B. , Voigt, A. , Kloor, M. , Benner, A. , Bork, U. , Rahbari, N.N. , Buchler, M.W. , Stoecklein, N.H. , Weitz, J. , Koch, M. , 2014. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 74, 1694–1704. [DOI] [PubMed] [Google Scholar]
- Sugimachi, K. , Yokobori, T. , Iinuma, H. , Ueda, M. , Ueo, H. , Shinden, Y. , Eguchi, H. , Sudo, T. , Suzuki, A. , Maehara, Y. , Mori, M. , Mimori, K. , 2014. Aberrant expression of plastin-3 via copy number gain induces the epithelial-mesenchymal transition in circulating colorectal cancer cells. Ann. Surg. Oncol. 21, 3680–3690. [DOI] [PubMed] [Google Scholar]
- Sun, H. , Zhu, X. , Lu, P.Y. , Rosato, R.R. , Tan, W. , Zu, Y. , 2014. Oligonucleotide aptamers: new tools for targeted cancer therapy. Mol. Ther. Nucleic Acids. 3, e182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y.F. , Xu, Y. , Yang, X.R. , Guo, W. , Zhang, X. , Qiu, S.J. , Shi, R.Y. , Hu, B. , Zhou, J. , Fan, J. , 2013. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 57, 1458–1468. [DOI] [PubMed] [Google Scholar]
- Tanaka, F. , Yoneda, K. , Kondo, N. , Hashimoto, M. , Takuwa, T. , Matsumoto, S. , Okumura, Y. , Rahman, S. , Tsubota, N. , Tsujimura, T. , Kuribayashi, K. , Fukuoka, K. , Nakano, T. , Hasegawa, S. , 2009. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin. Cancer Res. 15, 6980–6986. [DOI] [PubMed] [Google Scholar]
- Thompson, R.H. , Gillett, M.D. , Cheville, J.C. , Lohse, C.M. , Dong, H. , Webster, W.S. , Krejci, K.G. , Lobo, J.R. , Sengupta, S. , Chen, L. , Zincke, H. , Blute, M.L. , Strome, S.E. , Leibovich, B.C. , Kwon, E.D. , 2004. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. U.S.A. 101, 17174–17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima, K. , Hayashi, A. , Kashiwagi, M. , Hayashi, N. , Iwatsuki, M. , Ishimoto, T. , Baba, Y. , Baba, H. , Ohta, Y. , 2015. Analysis of circulating tumor cells derived from advanced gastric cancer. Int. J. Cancer. 137, 991–998. [DOI] [PubMed] [Google Scholar]
- Trusolino, L. , Bertotti, A. , Comoglio, P.M. , 2010. MET signalling: principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 11, 834–848. [DOI] [PubMed] [Google Scholar]
- Ueo, H. , Sugimachi, K. , Gorges, T.M. , Bartkowiak, K. , Yokobori, T. , Muller, V. , Shinden, Y. , Ueda, M. , Ueo, H. , Mori, M. , Kuwano, H. , Maehara, Y. , Ohno, S. , Pantel, K. , Mimori, K. , 2015. Circulating tumour cell-derived plastin3 is a novel marker for predicting long-term prognosis in patients with breast cancer. Br. J. Cancer. 112, 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha, M.S. , Hayes, D.F. , 2011. Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. J. Clin. Oncol. 29, 1508–1511. [DOI] [PubMed] [Google Scholar]
- Wiedswang, G. , Borgen, E. , Karesen, R. , Kvalheim, G. , Nesland, J.M. , Qvist, H. , Schlichting, E. , Sauer, T. , Janbu, J. , Harbitz, T. , Naume, B. , 2003. Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J. Clin. Oncol. 21, 3469–3478. [DOI] [PubMed] [Google Scholar]
- Wulfing, P. , Borchard, J. , Buerger, H. , Heidl, S. , Zanker, K.S. , Kiesel, L. , Brandt, B. , 2006. HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin. Cancer Res. 12, 1715–1720. [DOI] [PubMed] [Google Scholar]
- Yokobori, T. , Iinuma, H. , Shimamura, T. , Imoto, S. , Sugimachi, K. , Ishii, H. , Iwatsuki, M. , Ota, D. , Ohkuma, M. , Iwaya, T. , Nishida, N. , Kogo, R. , Sudo, T. , Tanaka, F. , Shibata, K. , Toh, H. , Sato, T. , Barnard, G.F. , Fukagawa, T. , Yamamoto, S. , Nakanishi, H. , Sasaki, S. , Miyano, S. , Watanabe, T. , Kuwano, H. , Mimori, K. , Pantel, K. , Mori, M. , 2013. Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial-mesenchymal transition and is associated with colorectal cancer prognosis. Cancer Res. 73, 2059–2069. [DOI] [PubMed] [Google Scholar]
- Yokobori, T. , Mimori, K. , Iwatsuki, M. , Ishii, H. , Onoyama, I. , Fukagawa, T. , Kuwano, H. , Nakayama, K.I. , Mori, M. , 2009. p53-Altered FBXW7 expression determines poor prognosis in gastric cancer cases. Cancer Res. 69, 3788–3794. [DOI] [PubMed] [Google Scholar]
- Yu, M. , Bardia, A. , Wittner, B.S. , Stott, S.L. , Smas, M.E. , Ting, D.T. , Isakoff, S.J. , Ciciliano, J.C. , Wells, M.N. , Shah, A.M. , Concannon, K.F. , Donaldson, M.C. , Sequist, L.V. , Brachtel, E. , Sgroi, D. , Baselga, J. , Ramaswamy, S. , Toner, M. , Haber, D.A. , Maheswaran, S. , 2013. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 339, 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumimoto, K. , Akiyoshi, S. , Ueo, H. , Sagara, Y. , Onoyama, I. , Ueo, H. , Ohno, S. , Mori, M. , Mimori, K. , Nakayama, K.I. , 2015. F-box protein FBXW7 inhibits cancer metastasis in a non-cell-autonomous manner. J. Clin. Invest. 125, 621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamay, G.S. , Kolovskaya, O.S. , Zamay, T.N. , Glazyrin, Y.E. , Krat, A.V. , Zubkova, O. , Spivak, E. , Wehbe, M. , Gargaun, A. , Muharemagic, D. , Komarova, M. , Grigorieva, V. , Savchenko, A. , Modestov, A.A. , Berezovski, M.V. , Zamay, A.S. , 2015. Aptamers selected to postoperative lung adenocarcinoma detect circulating tumor cells in human blood. Mol. Ther. 23, 1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X. , Carstens, J.L. , Kim, J. , Scheible, M. , Kaye, J. , Sugimoto, H. , Wu, C.C. , LeBleu, V.S. , Kalluri, R. , 2015. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 527, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]


