Abstract
Atherosclerosis is characterized by inflammation and proliferation of vascular cells. The intracellular bacterium Chlamydia (Chlamydophila) pneumoniae uses blood monocytes [peripheral blood mononuclear cells (PBMCs)] for dissemination, has been found to persist in atherosclerotic lesions, and has been implicated in atherogenesis by small GTPase activation and T lymphocyte recruitment. Infection of human coronary artery smooth muscle cells with C. pneumoniae significantly induced mRNA and protein for the angiogenic transcription factor Egr-1, resulting in enhanced coronary artery smooth muscle cell proliferation, which was reduced by transfection with small interfering RNA duplexes targeted at Egr-1 mRNA. These effects required viable chlamydiae and depended on p44/42 mitogen-activated protein kinase activity but not on the p38 mitogen-activated protein kinase pathway. Postinfectious Egr-1 mRNA up-regulation in arterial vessels was confirmed ex vivo in a rat aortic ring model of focal vascular chlamydial infection. An in vivo model based on the injection of C. pneumoniae-infected PBMCs into mice confirmed Egr-1 mRNA up-regulation within 24 h of endovascular infection. Arterial injury from repeated direct chlamydial infections and cell-to-cell contact with C. pneumoniae-infected PBMCs might represent a chronic focus of proliferative activity linked to the media proliferation seen in advanced atherosclerosis. Overall, chlamydial infection induces a proliferative phenotype in vascular cells via transcription factor Egr-1 activation in vitro, ex vivo, and in vivo.
Keywords: atherosclerosis, cell proliferation
The comprehension of the pathogenesis of atherosclerosis has changed fundamentally. Models suggesting atherogenesis as a static accumulation of lipid-loaded macrophages and adherent thrombocytes were expanded by a more dynamic view of the mechanisms involved (1). Inflammation and proliferation of vascular smooth muscle cells were established as the central pathomechanisms (2), suggesting that injurious stimuli other than the known risk factors may initiate and trigger atherosclerosis. Epidemiological studies indicated that persistent infectious agents like Chlamydia (Chlamydophila) pneumoniae or herpes-viruses might be involved in perpetuating coronary artery disease. Although diagnostic assays have produced controversial results on its frequency in vascular infection, C. pneumoniae remains the only replicative pathogen that has been recovered from atherosclerotic lesions, where it seems to be deposited by infected circulating blood monocytes (3–6). Vascular C. pneumoniae infection has recently been linked to the inflammatory and procoagulatory component of atherosclerosis by demonstrating small GTPase-mediated NF-κB activation (7). Chlamydial heat-shock protein (8) and a soluble factor derived from infected endothelial cells are known to trigger smooth muscle cell proliferation (9). The molecular mechanisms leading to postinfectious proliferation of vascular cells, however, are unknown, although the entry into and the progression through the cell cycle are key events leading to the prominent smooth muscle cell proliferation of advanced vascular disease (1, 2).
Recent studies implicated a central role of early growth response gene 1 (Egr-1) in the induction of a proatherosclerotic and proliferative phenotype in vascular cells (10), the latter through the regulation of cell progress from the G0 to G1 cell cycle phase (11). Previously described only in acute vascular damage under physical injury or hypoxia, enhanced Egr-1 expression levels have now been consistently found in atherosclerotic lesions (12). Because of its dual role as mitogenic stimulus for vascular cells and proatherogenic transcription factor, Egr-1 is now assumed to play a pivotal role in the initiation and progression of atherosclerosis. Bea et al. (13) recently showed that C. pneumoniae infection of mouse macrophages induced secretion of tissue factor and enhanced their procoagulatory activity via Egr-1 activation. Thus, we were interested to analyze whether C. pneumoniae infection may trigger proliferation of coronary artery smooth muscle cells (CASMCs) by means of Egr-1-dependent signaling. In this study, we show that duplexes specific for Egr-1 mRNA [Egr-1 small interfering RNA (siEgr)] blocked C. pneumoniae-induced proliferation of smooth muscle cells.
In vitro results were confirmed ex vivo by using a rat aortic ring model of vascular chlamydial infection and in vivo in a mouse model, where vascular infection was initiated via infected peripheral blood mononuclear cells (PBMCs), the supposed vehicles for chlamydial dissemination within the circulation (14–16).
Materials and Methods
Culture of C. pneumoniae. The coronary artery isolate C. pneumoniae CV-6 was grown on HEp-2 monolayers, as described (16). Chlamydiae were free from mycoplasma contamination, as shown by PCR (Stratagene). HEp-2 cells cultured under identical conditions served as mock control.
Infection with C. pneumoniae and Fluorescence Microscopy. Primary nonimmortalized CASMCs were obtained from Clonetics (Walkersville, MD) and maintained in SmGm2 medium (Clonetics) in 1% gelatin-coated culture flasks (Greiner, Frickenhausen, Germany) for three to six passages before use. Cells were infected with C. pneumoniae at an infection rate of four inclusion-forming units (IFU) per cell with or without p44/42 mitogen-activated protein (MAP) kinase inhibitor UO126 (10 μM) or p38 MAP kinase inhibitor SB203580 (20 μM; Merck Biosciences), as described (7). Successful infection was monitored after 48 h by using a FITC-labeled anti-Chlamydia LPS antibody (DAKO). Coincubation controls with heat-killed C. pneumoniae did not result in replicative infection. The transfection rate of small interfering RNA in CASMCs was controlled with Cy3-labeled double-stranded RNA complexes (IBA, Goettingen, Germany). Viability of CASMCs after chlamydial infection was proven by live/dead staining with two fluorescent nucleic acid dyes (both from Molecular Probes), Syto9 (green fluorescence, viable cells), and propidium iodide (red fluorescence, dead cells). Egr-1 staining was performed on paraformaldehyde-treated cells with rabbit polyclonal anti-Egr-1 antibody (2 μg/ml; Santa Cruz Biotechnology) and visualized with FITC anti-rabbit antibody (Molecular Probes) after a 10,000-ms exposure time.
Real-Time PCR. Total RNA was isolated by using the NucleoSpin RNA II kit (Macherey-Nagel, Duren, Germany) and reverse-transcribed into cDNA (First-Strand PCR kit, Roche, Mannheim, Germany). PCR amplification was performed by using the LightCycler Detection System (Roche Molecular Biochemicals). Relative quantification of Egr-1 mRNA (forward, CCGCAGAGTCTTTTCCTGAC; reverse, TGGGTTGGTCATGCTCACTA) expression was performed against the endogenous control 18S rRNA gene, as described (17).
Preparation of Nuclear Extracts. Nuclear extracts were prepared from CASMCs according to commonly used protocols. Briefly, 3 × 106 cells were washed with cold PBS, resuspended in 1 ml of cell lysis buffer (10 mM Hepes, pH 7.9/10 mM KCl/0.1 mM EDTA/0.1 mM EGTA/0.5 mM PMSF/1mMDTT/0.4% Triton X-100), and allowed to swell on ice for 20 min, followed by vigorous vortex mixing for 30 s. The homogenate was centrifuged at 10,000 × g for 3 min. The supernatant was discarded, and the nuclear pellet was resuspended in 50 μl of nuclear extraction buffer (20 mM Hepes, pH 7.9/0.4 M NaCl/1 mM EDTA/1 mM EGTA/0.5 mM PMSF). The tube was mixed intermittently for 60 min. The nuclear extract was obtained by centrifuging at 10,000 × g for 15 min at 4°C.
EMSA for Transcription Factor Egr-1. The following oligonucleotides were used as probe for the EMSA: 5′-GGATCCAGCGGGGGCGAGCGGGGGCGA-3′ and 3′-CCTAGGTCGCCCCCGCTCGCCCCCGCT-5′ (18). Probes were 5′ end-labeled with 100 μCi (1 Ci = 37 GBq) of [γ-32P]ATP (NEN) by using T4-polynucleotide kinase (Roche Molecular Biochemicals). The DNA-binding reaction mixture contained nuclear proteins (3 μg), 32P-labeled double-stranded oligonucleotide probe (≈20,000 cpm), and 2 μg of poly(dIdC) (Roche Molecular Biochemicals). To demonstrate the specificity of the DNA–protein interaction, competition experiments were performed with a 50-fold excess of unlabeled double-stranded oligonucleotide Egr-1 probe (cold probe) and an oligonucleotide showing no sequence homology to Egr-1 (control probe). In supershift assays, nuclear extracts were preincubated for 20 min at room temperature with 2 μg of the specific antibody before addition of radiolabeled probe. The DNA–protein complex was then size-fractionated from the free DNA probe by electrophoresis in a 5% nondenaturing polyacrylamide gel. The gel was vacuum-dried and exposed to x-ray film.
Transient Transfection of CASMCs with siEgr-1 Duplex. A 21-nt sequence for siEgr was derived from the human Egr-1 mRNA sequence (GenBank accession no. GI:5420378), and was targeted to the coding region 1,239–1,259 relative to the start codon of the Egr-1 gene: small interfering RNA against Egr-1, siEgr (sense, 5′-GCAAGTGGATCTTGGTATGTT-3′; antisense, 5′-CATACCAAGATCCACTTGCTT-3′), and scrambled RNA (scRNA) (sense, 5′-TCGACAACATCACTGAGATTT-3′; antisense, 5′-ATCTCAGTGATGTTGTCGATT-3′) (18). CASMCs were transfected with either siEgr or scRNA duplexes by using Lipofectamine (Invitrogen). Briefly, RNA duplex (0.25 μg/ml) was incubated in serum-free DMEM (Invitrogen) containing 10 μl of Lipofectamine for 15 min. The complex was added to the CASMC cell suspension (5 × 105 cells per ml) and incubated at 37°C in 5% CO2 atmosphere for 4 h. Then, the medium was aspirated and replaced by RPMI medium 1640 with 10% FCS.
Proliferative Activity of Human CASMCs. To determine the proliferative activity of CASMCs, we measured the capacity of [3H]thymidine (Amersham Pharmacia Biotech) incorporation. To achieve a quiescent stage, the vascular cells were allowed to attach for 24 h before stimulation experiments were carried out. [3H]Thymidine incorporation was performed by an additional incubation step with 1 μCi of [methyl-3H]thymidine for 48 h, as described (19).
Rat Aorta in 72-h Organ Culture. Rat thoracic aortae were isolated under sterile conditions from Wistar rats. Surrounding tissue was removed, and the aortae were cut in 3- to 4-mm rings, as described (20). For infection experiments, aortic rings were cultivated in RPMI medium 1640 (Sigma) at 37°C in 5% CO2 atmosphere. Aortic rings were coincubated with mock control, the vascular chlamydial strain CV-6 (1 × 106 IFU) or heat-killed chlamydiae (1 × 106 IFU). After 2 h, rings were stored at -70°C for evaluation of Egr-1 mRNA expression (forward, CAGGAG TGATGAACGCAAGA; reverse, AGCCCGGAGAGGAGTAAGAG) by RT-PCR (LightCycler Detection System). Detection of chlamydiae was performed after 72 h by immunohistochemical staining (CF-2; Washington Research Foundation, Seattle) with a DAB Substrate kit (Vector Laboratories) and by in situ hybridization targeting the chlamydial PstI fragment (21).
Mouse Model of Vascular Chlamydial Infection. Murine PBMCs were obtained from eight 10-week-old female C57BL/6 mice and isolated by Ficoll (PAA Laboratories, Coelbe, Germany) gradient centrifugation. PBMCs were either infected with 106 IFU of chlamydiae (n = four mice), leading to an infection rate of 45–55% (FITC-labeled anti-Chlamydia LPS antibody), or cultured in RPMI medium 1640 alone (n = four mice) for 4 h. PBMCs with and without C. pneumoniae infection were reinjected intravenously into the tails of eight female C57BL/6 mice. Mice were killed after 24 h, and aortae were stored at -70°C. Aortae from four mice without reinjection of PBMCs served as control. Egr-1 mRNA expression in homogenized mice aortae was evaluated by RT-PCR as described for rat aortic rings. Vascular infection was confirmed by nested PCR targeting the chlamydial PstI fragment as described (3, 21).
Statistical Analysis. All data were expressed as mean + SEM. Significant differences between means were determined by using Student's t test for paired samples. Data analysis was performed by using spss for windows (SPSS, Chicago).
Results
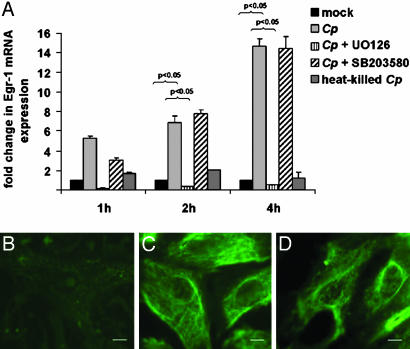
Chlamydial Infection Induces Egr-1 mRNA and Protein. Infection of CASMCs (53–63% after 48 h) with viable C. pneumoniae resulted in rapid (1-h) and sustained (up to 4-h) induction of Egr-1 mRNA; heat-killed C. pneumoniae had no such effect (Fig. 1A). Preincubation of CASMCs with the p44/42 MAP kinase inhibitor UO126 completely blocked Egr-1 mRNA expression in C. pneumoniae-infected CASMCs, whereas p38 MAP kinase inhibitor SB203580 had no impact on Egr-1 mRNA, even in concentrations up to 100 μM (data not shown). Stimulation with platelet-derived growth factor at a concentration of 20 ng/ml resulted in a 7- to 10-fold increase of Egr-1 mRNA and served as a positive control for the establishment of gene expression analysis (data not shown). A parallel increase of Egr-1 protein was shown with fluorescence staining: In contrast to the quiescent smooth muscle cell control (Fig. 1B), chlamydial infection resulted in enhanced expression (2 h, Fig. 1C) and perinuclear accumulation (4 h, Fig. 1D) of Egr-1.
Fig. 1.
The increasing expression of Egr-1 mRNA within 4 h of C. pneumoniae (Cp) infection was completely abrogated by p44/42 MAP kinase inhibitor UO126 but not by p38 inhibitor SB203580. (A) Mean + SEM (n = 3 experiments). (B–D) In parallel, fluorescence microscopy demonstrated enhanced Egr-1 protein expression in C. pneumoniae-infected (C) vs. mock-treated (B) CASMCs after 2 h and distinct perinuclear accumulation after 4 h (D). (Scale bar, 1 μm.)
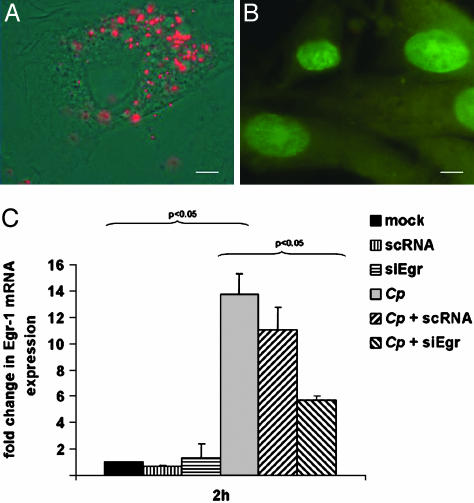
Transfection of CASMCs with Small Interfering RNA Duplexes Against Egr-1. To analyze transfection rates of small RNA duplexes, CASMCs were transfected with Cy3-labeled double-stranded RNAs. After an incubation period of 4 h, >75% of CASMCs showed incorporation of fluorescent RNAs (Fig. 2A). Live/dead staining proved that >90% of the cells remained alive after 24 h (Fig. 2B). Transfection with siEgr significantly reduced C. pneumoniae-related Egr-1 mRNA expression by 60% within 4 h (P < 0.05; Fig. 2C). A control experiment with scRNA complexes missing a complementary sequence to Egr-1 mRNA excluded nonspecific effects due to RNA complexes.
Fig. 2.
Transfection of CASMCs with Cy3-labeled double stranded-RNA. (A) Red fluorescence. (B) Green staining of nuclei proved CASMC viability 24 h after the transfection process by live/dead staining. (C) Transfection of CASMCs with siEgr duplexes but not with scRNA duplexes reduced Egr-1 mRNA expression by 60% 4 h after chlamydial infection (mean + SEM, n = 3 experiments).
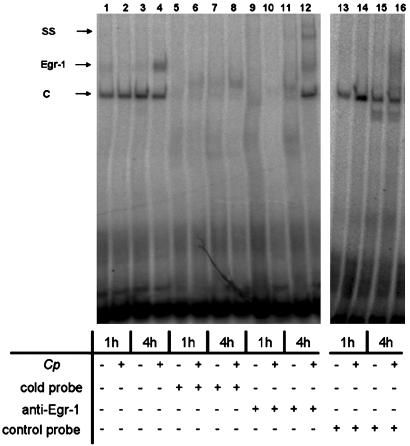
C. pneumoniae Activates DNA-Binding Properties of Egr-1. Gel mobility-shift assays were performed to examine Egr-1-binding activity in CASMCs after 1 and 4 h of infection with C. pneumoniae in comparison with noninfected cells. EMSAs revealed inducible DNA binding of Egr-1 in nuclear extracts from CASMCs 4 h after C. pneumoniae infection (Fig. 3). Specificity of the binding reaction was demonstrated by antigen supershift after coincubation with anti-Egr-1 antibody. Thus, chlamydial infection induces biological activation of Egr-1.
Fig. 3.
Inducible DNA-binding activity of the transcription factor Egr-1 (Egr-1) in C. pneumoniae (Cp)-infected CASMCs is first seen after 4 h (lane 4). Besides the inducible Egr-1 signal, constitutive DNA binding (C) is present after 1 and 4 h (lanes 1–4). Competition experiments with a 50-fold excess of unlabeled cold probe (lanes 5–8) but not of control probe (lanes 13–16) prevented DNA–protein interaction of both constitutive and inducible DNA binding. Supershifts with anti-Egr-1 antibody proved specificity of the inducible DNA binding (lanes 9–12). The data represent three independent experiments. SS, supershifted signal.
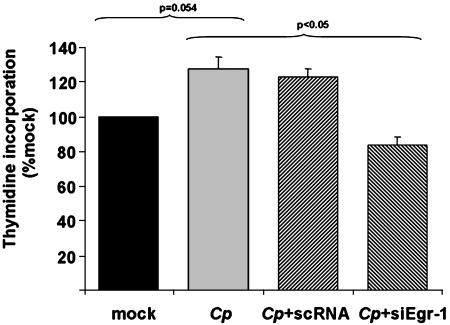
C. pneumoniae Increases the Proliferative Activity of CASMCs. The capacity of C. pneumoniae to induce proliferative activity of CASMCs was determined by measurement of [3H]thymidine incorporation with respect to the regulatory role of Egr-1 activation. Enhanced [3H]thymidine uptake was detectable in C. pneumoniae-infected CASMCs compared with noninfected vascular cells after 24 (data not shown) and 48 (Fig. 4) h. Transfection of CASMCs with siEgr but not with scRNA significantly abrogated cell proliferation after 48 h (P < 0.05; Fig. 4). In addition, C. pneumoniae-induced CASMC proliferation was completely blocked by inhibition of the p44/42, but not the p38, MAP kinase (data not shown).
Fig. 4.
Proliferation of CASMCs subsequent to Egr-1 up-regulation as determined by [3H]thymidine incorporation. The stimulatory effect of C. pneumoniae (Cp) infection on CASMC proliferation was completely blocked by preincubation with siEgr but not with scRNA after 48 h (mean + SEM, n = 3 experiments).
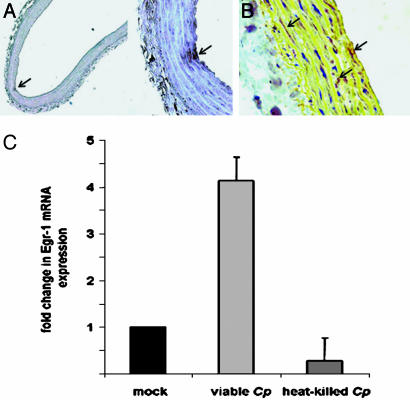
Postinfectious Egr-1 Expression in Rat Aortic Rings. To verify the direct Egr-1-inducing effect of C. pneumoniae on CASMCs in cell culture, we analyzed Egr-1 mRNA expression in rat aortic ring sections ex vivo. Rings acquired focal infection of the vascular wall, as shown by immunohistochemistry and in situ hybridization after a 72-h coincubation with viable chlamydiae (Fig. 5 A and B). Enhanced Egr-1 mRNA expression levels were detected by RT-PCR in homogenized aortic tissues within 2 h of chlamydial infection (Fig. 5C). Coincubation with heat-killed chlamydiae did not result in Egr-1 mRNA stimulation, indicating that viable pathogens are required for this process.
Fig. 5.
Aortic ring sections were cultured for up to 72 h after isolation from Wistar rats and infected with the vascular chlamydial strain CV-6 (106 IFU/ml), which resulted in focal chlamydial infection. (A and B) Arrows indicate focal chlamydial infection detectable by immunohistochemistry (A) and in situ hybridization (B). (C) In this ex vivo model, Egr-1 mRNA expression in aortic tissues as determined by real-time PCR was increased 2 h after stimulation with viable C. pneumoniae (Cp) but not with heat-killed Chlamydiae (mean + SEM, n = 3 experiments).
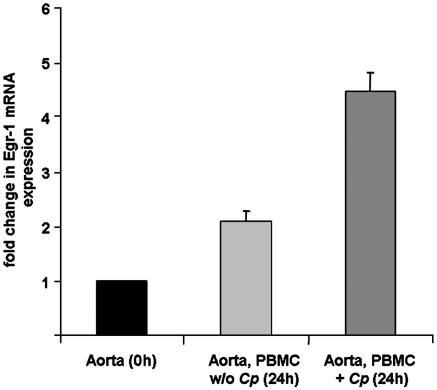
Postinfectious Egr-1 Expression in Vivo. The concept of vascular chlamydial infection is based on a finding of persistently infected blood monocytes in humans, which might serve as a vector for systemic dissemination of the pathogen and promote the formation of a proatherosclerotic phenotype in the vasculature. To accommodate these findings, we analyzed chlamydial capability to induce Egr-1 expression in the aorta in a mouse model of vascular chlamydial infection. For these purposes, C. pneumoniae-infected and noninfected PBMCs from C57BL/6 mice were reinjected intravenously into the tails of eight consecutive mice. Injection of C. pneumoniae-infected, but not of noninfected, PBMCs resulted in a 4.5-fold increase of Egr-1 mRNA expression in mice aortae after 24 h, compared with that in the aortae of untreated mice (Fig. 6).
Fig. 6.
Mouse aortae were isolated 24 h after i.v. injection of C. pneumoniae (Cp)-infected or noninfected PBMCs. RT-PCR from homogenized aorta specimens showed enhanced Egr-1 mRNA expression in the vasculature of mice carrying C. pneumoniae-infected PBMCs in their circulation (mean + SEM, n = 4 experiments).
Discussion
Inflammation and angiogenesis are central pathomechanisms in early and advanced atherosclerosis. In addition to known risk factors, C. pneumoniae has been linked to atherosclerosis epidemiologically, by detection of viable bacteria in lesions and by activating proinflammatory NF-κB signaling in vitro, although causality for atherogenesis has not yet been established (7, 22). We were interested in investigating whether C. pneumoniae might also promote the proliferative phenotype characteristic for atherosclerosis via up-regulation of transcription factor Egr-1 (12).
Egr-1 has been shown to bind to promoters of cytokines, chemokines, and proatherosclerotic factors like tissue factor and fibroblast growth factor 2 (23), as well as to regulate cell proliferation via its DNA-binding properties (24). Contact of viable, but not of heat-killed, Chlamydiae with smooth muscle cells resulted in rapid (within 1 h) and statistically significant induction of Egr-1 mRNA expression after 2 and 4 h. The brief period of Egr-1 up-regulation was invariably followed by smooth muscle cell proliferation, shown by increased [3H]thymidine uptake. This pattern confirms Egr-1 activity as a short-lived process fully sufficient to mediate entry into a proliferative phase (25). This study shows induction of a proproliferative transcription factor through a bacterial pathogen in vascular cells. The effect depended completely on the presence of viable metabolically active pathogen and was also observed in human coronary artery endothelial cells (data not shown). In contrast, chlamydial induction of foam cell formation, for example, can be promoted by chlamydial LPS alone (26). Proliferation of vascular cells has been characterized earlier as an integral part of atherosclerosis (27) and represents a general mechanism of arterial injury as observed in neointima formation after coronary angioplasty (28). The effects seen cannot be regarded as specific for C. pneumoniae or even bacteria. For example, Gram-positive (Staphylococcus aureus) or Gram-negative (Escherichia coli) infections of vascular cells may also promote Egr-1 upregulation, although this acute infection results in cell death within 24 h and not in proliferation (data not shown). In contrast to Chlamydia, other bacterial pathogens have not been found reproducibly in plaques. However, postinfectious proliferation may be a general mechanism in chronic vascular infection, relevant for bacterial as well as viral pathogens.
The immediate early gene Egr-1, a zinc finger transcription factor, was addressed in our experiments because of its central involvement in cellular mitosis and differentiation (25). It is associated with the promoter regions of genes encoding cytokines, adhesion molecules, and growth factors (10, 29) and has been implicated in smooth muscle cell proliferation in airway remodeling of asthmatic patients (30). Only a few stimuli of vascular Egr-1 expression, such as VEGF (31), hypoxia, and shear stress, are known, and none are of microbial origin (32). Our observations indicate that Egr-1 expression and the subsequent proliferation in vascular cells are sensitive to infection. Egr-1 expression may thus link proliferative activity of vascular cells and infection in a manner similar to that by which NF-κB activation links inflammation and infection in atherosclerosis.
Induction of Egr-1 has been described as regulated by the p38 and p44/42 MAP kinase signaling cascades in environmental conditions characterized by mechanical stress or hypoxia (33, 34). In our study, C. pneumoniae mediated Egr-1 up-regulation, and the resulting proliferative phenotype was exclusively dependent on p44/42 MAP kinase activity. This leaves only minor roles for other potential proproliferative factors and regulatory cascades (35). Using small inhibitory RNA duplexes specific for Egr-1, we could show that inhibition of transcription factor Egr-1 activation not only inhibits inflammatory immune response (18) but also abrogates proliferative activity of vascular cells to infectious stimuli.
To provide a better link to in vivo processes, we sought confirmation in a rat aortic ring model commonly used to examine vascular responses to injurious stimuli ex vivo (20) and a mouse model of vascular chlamydial infection (14). The advantage of the aortic ring is the good control of the infection initiated. Unfortunately, ring sections do not remain vital for prolonged periods and, thus, cannot demonstrate the media proliferation expected to follow the Egr-1 stimulus, which is a limitation of this arterial injury model. Only small amounts of bacteria in the tissue were required to induce proliferative signaling ex vivo, which is in line with the in vivo observation that infected cells in atheromatous lesions are scarce and may evolve to persistent forms, making cultural recovery of chlamydiae so difficult.
Current data indicate that the pathogen may use PBMCs as the vector for systemic dissemination to the vascular wall where it appears to be persistent and refractory to antibiotics (14–16). Our results obtained in the mouse model let us hypothesize that augmented Egr-1 expression within the vasculature may be a consequence not only of the direct chlamydial infection but also of indirect effects provoked during cell-to-cell contact with C. pneumoniae-infected PBMCs. Chlamydially infected monocyte-derived macrophages within the atherosclerotic plaques might thus represent a chronic focus of proliferative activity linked to media proliferation in advanced atherosclerosis (36).
Atherosclerosis is a progressive inflammatory process initiated and maintained by multiple factors. Mechanisms of innate and adaptive immunity, as well as pathological regulation of vascular cell activity, contribute to its pathogenesis (37). However, C. pneumoniae seems to be somewhat unique among the potential causal factors, because it may have simultaneously a part in several of the most prominent features of atherogenesis: It may initiate a proinflammatory vascular response (7, 22), recruit T cells (38), modify the lipid metabolism (39), and promote a proliferative phenotype of the vasculature, as shown here. In conclusion, the finding of postinfectious proliferation of vascular cells makes an infectious component in atherogenesis more plausible, but causality remains to be established.
Acknowledgments
We thank A. Gravenhorst, A. Hellberg, and T. Luedemann for excellent technical assistance; B. Weidtmann (University of Luebeck) and T. Goldmann (Research Center Borstel) for support in establishing the aortic ring model; and R. Kretschmer-Kazemi (Institute of Molecular Medicine, University of Luebeck) for help with small interfering RNA transfection experiments. This work was supported by Deutsche Forschungsgemeinschaft Grants SPP1130, Ma2070/4-1, and SFB3671811.
Author contributions: J.R. and M.M. designed research; J.R., T.H.-B., V.W., U.S., and E.B. performed research; J.R., T.H.-B., and M.M. analyzed data; and J.R. and M.M. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CASMC, coronary artery smooth muscle cell; siEgr, Egr-1 small interfering RNA; PBMC, peripheral blood mononuclear cell; IFU, inclusion-forming unit(s); MAP kinase, mitogen-activated protein kinase; scRNA, scrambled RNA.
References
- 1.Ross, R. (1999) N. Engl. J. Med. 340, 115-126. [DOI] [PubMed] [Google Scholar]
- 2.Libby, P., Ridker, P. M. & Maseri A. (2002) Circulation 105, 1135-1143. [DOI] [PubMed] [Google Scholar]
- 3.Maass, M., Bartels, C., Engel, P. M., Mamat, U. & Sievers, H. H. (1998) J. Am. Coll. Cardiol. 31, 827-832. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez, J. A. (1996) Ann. Intern. Med. 125, 979-982. [DOI] [PubMed] [Google Scholar]
- 5.Boman, J. & Hammerschlag, M. R. (2002) Clin. Microbiol. Rev. 15, 1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalayoglu, M. V., Libby, P. & Byrne, G. I. (2002) J. Am. Med. Assoc. 288, 2724-2731. [DOI] [PubMed] [Google Scholar]
- 7.Dechend, R., Gieffers, J., Dietz, R., Joerres, A., Rupp, J., Luft, F. C. & Maass, M. (2003) Circulation 108, 261-265. [DOI] [PubMed] [Google Scholar]
- 8.Sasu, S., LaVerda, D., Qureshi, N., Golenbock, D. T. & Beasley, D. (2001) Circ. Res. 89, 244-250. [DOI] [PubMed] [Google Scholar]
- 9.Coombes, B. K. & Mahony, J. B. (1999) Infect. Immun. 67, 2909-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khachigian, L. M., Lindner, V., Williams, A. J. & Collins, T. (1996) Science 271, 1427-1431. [DOI] [PubMed] [Google Scholar]
- 11.Molnar, G., Crozat, A. & Pardee, A. B. (1994) Mol. Cell. Biol. 14, 5242-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCaffrey, T. A., Fu, C., Du, B., Eksinar, S., Kent, K. C., Bush, H., Jr., Kreiger, K., Rosengart, T., Cybulsky, M. I., Silverman, E. S. & Collins, T. (2000) J. Clin. Invest. 105, 653-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bea, F., Puolakkainen, M. H., McMillen, T., Hudson, F. N., Mackman, N., Kuo, C. C., Campbell, L. A. & Rosenfeld, M. E. (2003) Circ. Res. 92, 394-401. [DOI] [PubMed] [Google Scholar]
- 14.May, A. E., Redecke, V., Gruner, S., Schmidt, R., Massberg, S., Miethke, T., Ryba, B., Prazeres Da Costa, C., Schomig, A. & Neumann, F. J. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 789-794. [DOI] [PubMed] [Google Scholar]
- 15.Gieffers, J., van Zandbergen, G., Rupp, J., Sayk, F., Kruger, S., Ehlers, S., Solbach, W. & Maass, M. (2004) Eur. Respir. J. 23, 506-510. [DOI] [PubMed] [Google Scholar]
- 16.Gieffers, J., Füllgraf, H., Jahn, J., Klinger, M., Dalhoff, K., Katus, H. A., Solbach, W. & Maass, M. (2001) Circulation 103, 351-356. [DOI] [PubMed] [Google Scholar]
- 17.Winer, J., Jung, C. K., Shackel, I. & Williams, P. M. (1999) Anal. Biochem. 270, 41-49. [DOI] [PubMed] [Google Scholar]
- 18.Giri, R. K., Selvaraj, S. K. & Kalra, V. K. (2003) J. Immunol. 170, 5281-5294. [DOI] [PubMed] [Google Scholar]
- 19.Yang, Z., Oemar, B. S., Carrel, T., Kipfer, B., Julmy, F. & Luscher, T. F. (1998) Circulation 97, 181-187. [DOI] [PubMed] [Google Scholar]
- 20.Chin, J. H., Okazaki, M., Hu, Z. W., Miller, J. W. & Hoffman, B. B. (1996) J. Clin. Invest. 97, 2316-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell, L. A. (1993) in Diagnostic Molecular Microbiology: Principles and Applications, eds. Persing, D. H., Smith, T. F., Tenover, F. C. & White, T. J. (Am. Soc. Microbiol., Washington, DC), pp. 247-252.
- 22.Grayston, J. T. (2000) J. Infect. Dis. 181, Suppl. 3, S402-S410. [DOI] [PubMed] [Google Scholar]
- 23.Fahmy, R. G., Dass, C. R., Sun, L. Q., Chesterman, C. N. & Khachigian, L. M. (2003) Nat. Med. 9, 1026-1032. [DOI] [PubMed] [Google Scholar]
- 24.Fahmy, R. G. & Khachigian, L. M. (2004) Nucleic Acids Res. 32, 2281-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sukhatme, V. P., Cao, X. M., Chang, L. C., Tsai-Morris, C. H., Stamenkovich, D., Ferreira, P. C., Cohen, D. R., Edwards, S. A., Shows, T. B., Curran, T., et al. (1988) Cell 53, 37-43. [DOI] [PubMed] [Google Scholar]
- 26.Kalayoglu, M. V. & Byrne, G. I. (1998) Infect. Immun. 66, 5067-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rekhter, M. D. & Gordon, D. (1995) Am. J. Pathol. 147, 668-677. [PMC free article] [PubMed] [Google Scholar]
- 28.Malik, N., Francis, S. E., Holt, C. M., Gunn, J., Thomas, G. L., Shepherd, L., Chamberlain, J., Newman, C. M., Cumberland, D. C. & Crossman, D. C. (1998) Circulation 98, 1657-1665. [DOI] [PubMed] [Google Scholar]
- 29.Bavendiek, U., Libby, P., Kilbride, M., Reynolds, R., Mackman, N. & Schonbeck, U. (2002) J. Biol. Chem. 277, 25032-25039. [DOI] [PubMed] [Google Scholar]
- 30.Hjoberg, J., Le, L., Imrich, A., Subramaniam, V., Mathew, S. I., Vallone, J., Haley, K. J., Green, F. H., Shore, S. A. & Silverman, E. S. (2004) Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L817-L825. [DOI] [PubMed] [Google Scholar]
- 31.Abe, M. & Sato, Y. (2001) Angiogenesis 4, 289-298. [DOI] [PubMed] [Google Scholar]
- 32.Morawietz, H., Ma, Y. H., Vives, F., Wilson, E., Sukhatme, V. P., Holtz, J. & Ives, H. E. (1999) Circ. Res. 84, 678-687. [DOI] [PubMed] [Google Scholar]
- 33.Chiu, J. J., Wung, B. S., Hsieh, H. J., Lo, L. W. & Wang, D. L. (1999) Circ. Res. 85, 238-246. [DOI] [PubMed] [Google Scholar]
- 34.Rolli, M., Kotlyarov, A., Sakamoto, K. M., Gaestel, M. & Neininger, A. (1999) J. Biol. Chem. 274, 19559-19564. [DOI] [PubMed] [Google Scholar]
- 35.Chang, L. & Karin, M. (2001) Nature 410, 37-40. [DOI] [PubMed] [Google Scholar]
- 36.Kol, A., Sukhova, G. K., Lichtman, A. H. & Libby, P. (1998) Circulation 98, 300-307. [DOI] [PubMed] [Google Scholar]
- 37.Hansson, G. K., Libby, P., Schonbeck, U & Yan, Z. Q. (2002) Circ. Res. 91, 281-291. [DOI] [PubMed] [Google Scholar]
- 38.Benagiano, M., Azzurri, A., Ciervo, A., Amedei, A., Tamburini, C., Ferrari, M., Telford, J. L., Baldari, C. T., Romagnani, S., Cassone, A., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 6658-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dittrich, R., Dragonas, C., Mueller, A., Maltaris, T., Rupp, J., Beckmann, M. W. & Maass, M. (2004) Biochem. Biophys. Res. Commun. 319, 501-505. [DOI] [PubMed] [Google Scholar]