Abstract
Despite numerous clinical studies indicating the clinical relevance of circulating tumor cells (CTCs) in blood and disseminated tumor cells (DTCs) in the bone marrow of cancer patients, the functional properties of these cells are largely unknown. The focus of this review is to emphasize how functional studies on viable CTCs and DTCs can enlarge the spectrum of applications of “liquid biopsies”. The low number of CTCs in the peripheral blood and DTCs in the bone marrow and the fact that carcinoma cells are difficult to culture are major challenges. Significant advances in the in vitro and in vivo expansion of CTCs and DTCs from cancer patients have been achieved, which enable us now to study the functional properties of these cells. Here, we discuss published data about functional studies on CTCs and DTCs using in vitro cultivation and in vivo xenograft models. Functional analyses on CTCs and DTCs offer the possibility to identify the metastasis‐initiating cells. Moreover, CTC‐derived cell lines and xenografts might point to new therapeutic targets and can be used for drug development.
Keywords: CTCs, DTCs, Cell lines, Solid tumors, Biomarkers, Liquid biopsy
1. Introduction
Numerous clinical studies have demonstrated strong correlations between circulating tumor cells (CTCs) and disseminated tumor cells (DTCs) counts and clinical outcome in large multi‐centre cohort studies on patients with different epithelial tumors such as breast and prostate cancer (Bidard et al., 2014; Goldkorn et al., 2014; Scher et al., 2015). In contrast, the functional properties of CTCs and DTCs are under investigated because these cells occur at very low concentrations in the peripheral blood and bone marrow of cancer patients (Alix‐Panabieres and Pantel, 2014). A prerequisite for functional analyses was, therefore, the recent advances in our ability to culture epithelial tumor cells in vitro and establish patient‐derived xenografts.
Short‐term culture of CTCs after leukocytes depletion has been already realized for a decade by the EPISPOT technology (Deneve et al., 2013; Ramirez et al., 2014). Cells are cultured for a short time on a membrane coated with antibodies that capture the secreted/released/shed tumor associated proteins that are subsequently detected by secondary antibodies labeled with fluorochromes. Moreover, temporary cultivation of DTCs over several weeks was established 20 years ago (Pantel et al., 1995) and the proliferative activity of DTCs in culture predicted an unfavorable outcome (Solakoglu et al., 2002). More recently, several groups have developed appropriate conditions for long‐term culture of CTCs and applied them to cancer patients at very advanced stages with higher amounts of CTCs. In addition, patient‐derived xenografts have become a valuable tool in cancer research and have entered the stage of clinical use for testing drug sensitivity in individual cancer patients (Hodgkinson et al., 2014). Extending this technology to CTCs has led to the development of unique in vivo models for several tumor entities.
Here, we will emphasize the possibilities and current limitations of functional studies on CTCs and DTCs using in vitro and in vivo models. We will focus on breast, prostate, colon and lung cancer as the major tumor entities in industrialized countries. However, pilot studies indicate that CTC cultivation can be also achieved in other tumor entities such as pancreatic cancer (Bobek et al., 2014a; Kolostova et al., 2015b), esophageal cancer (Bobek et al., 2014b) and gastric cancer (Kolostova et al., 2015a). Besides insights into the complex biology of metastasis functional CTC and DTC studies can point to new targets and novel strategies for more efficient anti‐metastatic therapies.
2. Functional studies in solid cancers
2.1. Breast cancer
2.1.1. Short‐term culture of CTCs (EPISPOT assay)
Using the EPISPOT assay, the release of cytokeratin‐19 (CK19) and mucin‐1 (MUC1) by breast cancer cells was measured, and the results demonstrated that many breast cancer patients harbored viable DTCs in their bone marrow, even if the tumors were classified as localized (stage M0: 54%) (Alix‐Panabieres et al., 2009). Most interestingly, patients with DTC‐releasing CK19 in their bone marrow had an unfavorable outcome. In the subsequent study, peripheral blood samples from 194 M1 breast cancer patients were analyzed by the EPISPOT assay. CTCs were identified as CK19‐releasing cells (CK19‐RC) and were correlated to an unfavorable clinical outcome (Ramirez et al., 2014).
The establishment of primary cultures from CTCs of breast cancer patients were reported for the first time by Zhang et al.; in this study CTCs from patients in advanced stage with brain metastases were cultured over weeks but no permanent cell lines were established (Zhang et al., 2013). In EpCAM(−) CTCs, a potential signature of brain metastasis comprising “brain metastasis selected markers (BMSMs)” HER2(+)/EGFR(+)/HPSE(+)/Notch1(+) was identified. Cultured CTCs that expressed the BMSM signature were highly invasive and able to generate brain and lung metastases after xenografting into nude mice.
2.1.2. Establishment of CTC lines
Recently, Yu et al. reported on oligoclonal CTC cultures that were sustained in vitro for more than 6 months. The cultured CTCs were isolated from 6 patients with metastatic luminal subtype breast cancer (Yu et al., 2014). Three of the five CTC lines tested were tumorigenic in mice. CTC lines revealed pre‐existing mutations in the PIK3CA gene and newly acquired mutations in the estrogen receptor gene (ESR1), PIK3CA gene, and fibroblast growth factor receptor gene (FGFR2). Through drug sensitivity testing of the established CTC lines multiple mutations could be revealed as potential new therapeutic targets.
2.1.3. Xenograft CTC assays (“avatars”)
In the first xenograft model, CTCs from patients with metastatic luminal breast cancer were injected into the tibial bone of immunodeficient mice and gave rise to bone, lung and liver metastases (Baccelli et al., 2013). These metastases uniformly expressed EpCAM, CD44, CD47 and MET, which might be important for engraftment and metastatic outgrowth of CTCs. In a subsequent validation cohort, the number of EpCAM(+)CD44(+)CD47(+)MET(+) CTCs, but not all EpCAM(+) CTCs, correlated with increased number of metastatic sites and poor prognosis. Although this report suggests that a special subset of CTCs might have potential metastasis‐initiating activity, it should be noted that xenografts could be only obtained from advanced stage patients with high count of CTCs. Thus, future studies need to include early stage patients correlate their potential metastasis‐initiator phenotype to the development of metastases. Obviously, this investigation will require long‐term follow‐up as well as more sensitive CTC assays.
Rossi et al. confirmed that CTCs have the potential to grow in immunodeficient mice (NOD/SCID) in a small pilot study on two breast cancer patients (Rossi et al., 2014). In contrast to Baccelli et al. who injected CTCs into the tibia, Rossi et al. injected CTCs subcutaneously. Thus, both routes of injection seem to work for the establishment of CTC xenografts in breast cancer and CTCs appear to sustain their migratory capacity in immunodeficient mice models.
2.2. Prostate cancer
2.2.1. Short‐term culture of CTCs (EPISPOT assay)
In the EPISPOT assay, prostate‐specific antigen (PSA) secretion was used as marker to detect PSA‐secreting cells in prostate cancer patients. In total, 83% and 42% of M1 & M0 cancer patients, respectively, and higher CTC counts were observed in metastatic patients as compared to earlier disease stages (Alix‐Panabieres et al., 2005). Importantly, a fraction of CTCs secreted fibroblast growth factor‐2 (FGF‐2), a known stem cell growth factor (Alix‐Panabieres et al., 2005, 2007). Further studies will show whether this subset of CTCs has an increased ability to initiate overt metastases.
Recently, Kolostova et al. used size‐based filtration of CTCs and were able to culture CTCs from patients with localized prostate cancer for 7–28 days (Kolostova et al., 2014). Cytokeratin‐positive cells with a proliferative capacity were observed in 18 of 28 CTC‐positive patients.
2.2.2. Establishment of CTC lines
The few cell lines available in biobanks prove that prostate cancer is very difficult to expand in cell culture. Recently, the use of a novel 3D organoid system allowed developing long‐term culture of prostate cancer from biopsy specimens and CTCs (Gao et al., 2014) recapitulated the molecular diversity of prostate cancer, including TMPRSS2‐ERG fusion, SPOP mutation, SPINK1 overexpression, and CHD1 loss. Although a CTC line from one metastatic prostate cancer patient was obviously established, there was no background information on the success rate of establishing a CTC line, i.e., how many times did the culture conditions failed to establish primary CTC cultures or a cell line. Moreover, it remained unclear whether the cell line has tumorigenic properties in a xenograft model.
2.2.3. Xenograft CTC assays
In a small pilot study, Rossi et al. isolated EpCAM(+) CTCs from metastatic prostate cancer patients (n = 6) and developed xenografts in NOD/SCID mice (Rossi et al., 2014). CTCs were found in the peripheral blood, bone marrow and spleen of these mice, which demonstrated the migratory capacity of EpCAM‐positive CTCs.
2.3. Colon cancer
2.3.1. Short‐term culture of CTCs (EPISPOT assay)
Our previous study demonstrated that a considerable portion of viable CTCs detectable by the EPISPOT assay is trapped in the liver as the first filter organ in colon cancer. In 75 colorectal cancer patients, CK19‐RC were enumerated by the CK19‐Epispot assay; viable CTCs were detected in 65.9% and 55.4% (p = 0.04) patients in mesenteric and peripheral blood, respectively. In contrast, the CellSearch® system detected CTCs in 55.9% and 29.0% (p = 0.0046) patients, respectively. The CTC count was significantly higher in mesenteric blood than in the peripheral blood. Follow‐up analysis revealed that localized colon cancer patients with high CTC counts have an unfavorable outcome (n = 60) (Deneve et al., 2013).
2.3.2. Establishment of CTC lines
The first experimental proof that CTCs isolated from the blood of a colon cancer patient are able to give rise to a permanent cell line was provided by Cayrefourcq et al. (Cayrefourcq et al., 2015). Thus far, no other group has published on the establishment of a permanent cell CTC line or even transient CTC cultures from patients with colon cancer (Pantel and Alix‐Panabières, 2015). It is well known that the frequency of CTCs is lower in peripheral blood of colon cancer patients as compared to breast or prostate cancer patients, making it even more difficult to find and grow CTCs in colon cancer. Besides the establishment of a permanent cell line, the cell line established by Cayrefourcq et al. named CTC‐MCC‐41 has tumorigenic properties in SCID mice (Cayrefourcq et al., 2015).
Interestingly, the CTC‐MCC‐41 line showed (i) epithelial properties with stem cell‐like characteristics, (ii) an intermediate epithelial/mesenchymal phenotype, (iii) a potential to induce quickly in vitro angiogenesis, (iv) an osteomimetic signature and (v) tumorigenic properties in SCID mice. Moreover, this CTC line shares the main features of the original primary tumor and lymph nodes metastasis of the colon cancer patient. Importantly, as we have established 9 different CTC lines during treatment and cancer progression of this colon cancer patient, there is no doubt that we will find precious and crucial information about the clonal selection among THE metastases‐initiator disseminating cells and their strong potential to survive chemotherapies and targeted therapies.
2.4. Lung cancer
2.4.1. In vitro expansion of CTCs
A novel in situ capture and culture methodology for ex vivo expansion of CTCs using a 3D co‐culture model was recently developed by Zhang et al., who simulated a tumor microenvironment to support CTC development (Zhang et al., 2014). CTCs were isolated from 14 of 19 early stage lung cancer patients and expanded tumor cells carried mutations of the TP53 gene identical to those observed in the matched primary tumors. Next‐generation sequencing revealed additional matched mutations between primary tumor and CTCs of cancer‐related genes.
2.4.2. Xenograft CTC assays
Many lung cancer patients are inoperable and biopsies to investigate lung cancer biology are difficult to obtain. Previous studies have shown that patients with small cell lung cancer (SCLC) have the highest CTC counts among all solid tumors, which provides the best conditions for developing functional models (Hou et al., 2012). Hodgkinson et al. demonstrated that CTCs from SCLC patients are tumorigenic in immunodeficient mice, and the CTC‐derived xenografts mirror the donor patient's response to chemotherapy (Hodgkinson et al., 2014). However, the number of xenografts is still limited and future studies will show whether xenografts from CTCs of patients with non‐small cell lung cancer (NSCLC), which present with lower CTC counts than SCLC patients, can be established. NSCLC harbor a series of druggable mutations present in small cohorts of patients and CTC‐derived models for individual drug testing are therefore desirable.
3. Bone marrow DTC lines from carcinoma patients
DTCs from the bone marrow of cancer patients can be isolated in only extremely low numbers (1 DTC per 105 – 106 normal cells). Therefore, in‐depth analyses of DTCs from cancer patients are restricted to methods that are applicable to single cell analyses. Pantel et al. generated DTC cell lines from single tumor cells isolated in the bone marrow of cancer patients, including breast, prostate and lung cancer patients (Pantel et al., 1995; Putz et al., 1999). These DTC lines were CD45(−) and showed expression of relevant proteins in cancer, such as ErbB‐2, PSA or E‐Cadherin. Since these DTC lines were generated from cancer patients that did not show overt metastasis (Putz et al., 1999), they may represent a DTC phenotype that reflects tumor cell dissemination prior to visible metastatic outgrowth. Similar to ‘standard’ cancer cell lines, these DTC lines can be cultured in vitro providing sufficient cell numbers for structural and functional analyses. The most frequently applied DTC cell lines are BC‐M1 and BC‐S1 (breast cancer), PC‐E1 and PC‐M1 (prostate cancer) as well as LC‐M1 (lung cancer) (Bartkowiak et al., 2010, 2009, 2011, 2005).
The phenotype of the majority of these DTC lines displays properties of an epithelial–mesenchymal transition (strong vimentin expression, weak cytokeratin expression), as well as attributes of cancer stem cells (CD44+/CD24−) (Bartkowiak et al., 2010; Willipinski‐Stapelfeldt et al., 2005). These properties of the DTC lines suit well to an assumed DTC phenotype with mesenchymal attributes that significantly contributes to tumor dormancy in the bone marrow before any mesenchymal‐to‐epithelial transition leading then to metastases growth (Tam and Weinberg, 2013). Analyses of these DTC lines were performed on the receptor tyrosine kinase signaling of the ErbB family members and the downstream AKT and Map‐kinase pathways. LC‐M1 was analyzed with focus on the AKT isoform specific cellular signaling (Grabinski et al., 2011). BC‐M1 and BC‐S1 were analyzed with focus on the EGFR/ErbB2 and ErbB2/ErbB3 heterodimer signaling (Balz et al., 2012).
An interesting feature of all analyzed DTC cell lines is their strong activation of the cytoprotective program unfolded protein response (UPR) (Bartkowiak et al., 2010). The UPR grants cytoprotection under hostile microenvironmental conditions like hypoxia, suggesting that such a DTC phenotype is well protected from microenvironmental stress. Detailed analyses of the DTC cell lines BC‐M1, LC‐M1 and PC‐E1 revealed a dynamic network of interconnected cellular programs like induction of the UPR chaperones and oxidoreductases in dependence of EGFR/ErbB2 expression under hypoxia (Bartkowiak et al., 2015). These findings support the view that DTCs in cancer patients are able to cope with a variety of different cell stress factors like the hypoxic conditions of the hematopoietic stem cell niche.
In addition, it was recently noticed that BC‐M1 and PC‐E1 display high levels of the protein PD‐L1 (programmed death‐ligand 1) (David, 2015; Mazel et al., 2015). PD‐L1 protects tumor cells from immunosurveillance, suggesting that such DTCs are well able to slip through the meshwork of the immune system to reach secondary organs.
In conclusion, the DTC lines serve as useful model systems for DTCs with mesenchymal attributes in vivo. The application of the DTC cell lines may range from biomarker validation like plastin‐3 on single cell level (Ueo et al., 2015) to approaches that require large amounts of cells like proteome analyses (Bartkowiak et al., 2010).
4. Conclusions
Functional analyses of whole tumor cells offer the possibility to identify the biological properties of metastatic cells and their origin through in‐depth in vitro and vivo analyses, which opens new avenues for basic and translational research. It is now possible to develop primary cell cultures from CTCs or DTCs and in some instances even permanent cancer cell lines have been established from rare tumor cells isolated in the blood or the bone marrow from patients with solid tumors (Figure 1). Besides in vitro studies several groups were also able to graft tumors after injection into immunodeficient mice and the first drug testing analyses suggest that these models might be useful predictors of the response of cancer patients (Figure 1) (Maheswaran and Haber, 2015).
Figure 1.
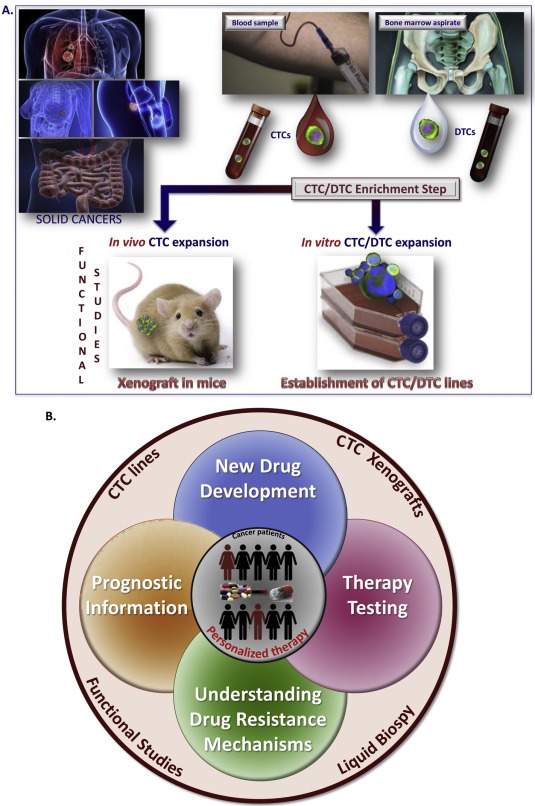
Functional studies with CTCs and DTCs using in vitro and/or in vivo models for personalized clinical management of patients with solid cancers. A. CTCs and DTCs are first enriched from blood samples or bone marrow aspirates of cancer patients (i.e., lung, breast, prostate, colon cancer), respectively. CTCs or DTCs are subsequently expanded in special culture medium or in immunodeficient mice. The last step is getting CTC/DTC lines or xenografts, respectively. B. Viable CTCs can be enumerated using a functional assay (EPISPOT assay) leading to prognostic information. CTCs can be cultured in vitro and an in‐depth characterization of established CTC lines may identify metastasis‐initiator cells, a crucial point for new drug development. To potentially eradicate metastatic disease, CTCs can be expanded in vivo for therapy testing and better understanding of drug resistance mechanisms.
Most researchers achieved to establish CTC lines from blood of patients with advanced metastatic stages with high CTC counts. It was indeed a difficult challenge to enrich THE rare metastasis‐initiator cells among CTCs and to grow them in vitro when it is already known to be difficult to get permanent cancer cell lines from primary tumors when millions of tumor cells are available. Thus, it is really a major achievement in cancer research to develop CTC lines or xenografts from hundreds of CTCs. Why are so few cells needed to establish CTC lines compared to primary tumors? This can be explained by the assumption that viable CTCs might be already selected for better survival and growth properties, which is consistent with published reports demonstrating that tumor cell dissemination and survival of DTCs and CTCs is not a random process (Woelfle et al., 2003; Wrage et al., 2009) and CTC cell lines and xenografts express cancer stem cell properties (Baccelli et al., 2013; Cayrefourcq et al., 2015). These studies nourish the hope that in‐depth functional analysis of these CTC lines will lead to the identification of metastasis‐initiator cells. However, to achieve this important goal it is crucial to extend the current studies to patients at earlier stages and correlate the findings with the development of metastatic relapse. In this context, the analysis of viable CTCs in short‐term culture assays as used in the EPISPOT technology might pave the road for individual drug testing in cancer patients.
It is now possible to study the development of metastatic relapse by sequential analysis of CTCs taken at different time points between primary surgery and clinical signs of overt metastases. Because of the short lifetime of CTCs in the blood, detection of these cells months or years after resection of the primary tumor in patients without evidence for overt metastases clearly indicates the presence of occult micrometastases. The molecular and functional analysis of CTCs derived from micrometastases may provide novel insights into the stage of cancer dormancy (Kang and Pantel, 2013), which may lead to novel strategies for the prevention of metastatic progression.
Disclosure
The authors disclose no potential conflicts of interest.
Acknowledgment
We apologize that we could not quote all CTC/DTC assays published in the literature because of space restriction.
The authors receive support from CANCER‐ID, an Innovative Medicines Initiative Joint Undertaking under grant agreement n° 115749, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007–2013) and EFPIA companies' in kind contribution. This work was further supported by the FEDER plus the Region Languedoc‐Roussillon (GEPETOS project) and the National Institute of Cancer (INCA) (CAP) and the European Research Council Advanced Investigator grant 269081 DISSECT (KP).
Alix‐Panabières Catherine, Bartkowiak Kai, Pantel Klaus, (2016), Functional studies on circulating and disseminated tumor cells in carcinoma patients, Molecular Oncology, 10, doi: 10.1016/j.molonc.2016.01.004.
This is a contribution to the special issue edited by Klaus Pantel and Catherine Alix‐Panabieres, Liquid Biopsies.
References
- Alix-Panabieres, C. , Pantel, K. , 2014. Challenges in circulating tumour cell research. Nat. Rev. Cancer. 14, 623–631. [DOI] [PubMed] [Google Scholar]
- Alix-Panabieres, C. , Rebillard, X. , Brouillet, J.P. , Barbotte, E. , Iborra, F. , Segui, B. , Maudelonde, T. , Jolivet-Reynaud, C. , Vendrell, J.P. , 2005. Detection of circulating prostate-specific antigen-secreting cells in prostate cancer patients. Clin. Chem. 51, 1538–1541. [DOI] [PubMed] [Google Scholar]
- Alix-Panabieres, C. , Vendrell, J.P. , Pelle, O. , Rebillard, X. , Riethdorf, S. , Muller, V. , Fabbro, M. , Pantel, K. , 2007. Detection and characterization of putative metastatic precursor cells in cancer patients. Clin. Chem. 53, 537–539. [DOI] [PubMed] [Google Scholar]
- Alix-Panabieres, C. , Vendrell, J.P. , Slijper, M. , Pelle, O. , Barbotte, E. , Mercier, G. , Jacot, W. , Fabbro, M. , Pantel, K. , 2009. Full-length cytokeratin-19 is released by human tumor cells: a potential role in metastatic progression of breast cancer. Breast Cancer Res. 11, R39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccelli, I. , Schneeweiss, A. , Riethdorf, S. , Stenzinger, A. , Schillert, A. , Vogel, V. , Klein, C. , Saini, M. , Bauerle, T. , Wallwiener, M. , Holland-Letz, T. , Hofner, T. , Sprick, M. , Scharpff, M. , Marme, F. , Sinn, H.P. , Pantel, K. , Weichert, W. , Trumpp, A. , 2013. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 31, 539–544. [DOI] [PubMed] [Google Scholar]
- Balz, L.M. , Bartkowiak, K. , Andreas, A. , Pantel, K. , Niggemann, B. , Zanker, K.S. , Brandt, B.H. , Dittmar, T. , 2012. The interplay of HER2/HER3/PI3K and EGFR/HER2/PLC-gamma1 signalling in breast cancer cell migration and dissemination. J. Pathol. 227, 234–244. [DOI] [PubMed] [Google Scholar]
- Bartkowiak, K. , Kwiatkowski, M. , Buck, F. , Gorges, T.M. , Nilse, L. , Assman, V. , Andreas, A. , Müller, V. , Wikman, H. , Riethdorf, S. , Schlüter, H. , Pantel, K. , 2015. Disseminated tumor cells persist in the bone marrow of breast cancer patients through sustained activation of the unfolded protein response. Cancer Res. 75, 5367–5377. [DOI] [PubMed] [Google Scholar]
- Bartkowiak, K. , Effenberger, K.E. , Harder, S. , Andreas, A. , Buck, F. , Peter-Katalinic, J. , Pantel, K. , Brandt, B.H. , 2010. Discovery of a novel unfolded protein response phenotype of cancer stem/progenitor cells from the bone marrow of breast cancer patients. J. Proteome Res. 9, 3158–3168. [DOI] [PubMed] [Google Scholar]
- Bartkowiak, K. , Wieczorek, M. , Buck, F. , Harder, S. , Moldenhauer, J. , Effenberger, K.E. , Pantel, K. , Peter-Katalinic, J. , Brandt, B.H. , 2009. Two-dimensional differential gel electrophoresis of a cell line derived from a breast cancer micrometastasis revealed a stem/progenitor cell protein profile. J. Proteome Res. 8, 2004–2014. [DOI] [PubMed] [Google Scholar]
- Bidard, F.C. , Peeters, D.J. , Fehm, T. , Nole, F. , Gisbert-Criado, R. , Mavroudis, D. , Grisanti, S. , Generali, D. , Garcia-Saenz, J.A. , Stebbing, J. , Caldas, C. , Gazzaniga, P. , Manso, L. , Zamarchi, R. , de Lascoiti, A.F. , De Mattos-Arruda, L. , Ignatiadis, M. , Lebofsky, R. , van Laere, S.J. , Meier-Stiegen, F. , Sandri, M.T. , Vidal-Martinez, J. , Politaki, E. , Consoli, F. , Bottini, A. , Diaz-Rubio, E. , Krell, J. , Dawson, S.J. , Raimondi, C. , Rutten, A. , Janni, W. , Munzone, E. , Caranana, V. , Agelaki, S. , Almici, C. , Dirix, L. , Solomayer, E.F. , Zorzino, L. , Johannes, H. , Reis-Filho, J.S. , Pantel, K. , Pierga, J.Y. , Michiels, S. , 2014. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 15, 406–414. [DOI] [PubMed] [Google Scholar]
- Bobek, V. , Gurlich, R. , Eliasova, P. , Kolostova, K. , 2014. Circulating tumor cells in pancreatic cancer patients: enrichment and cultivation. World J. Gastroenterol. 20, 17163–17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobek, V. , Matkowski, R. , Gurlich, R. , Grabowski, K. , Szelachowska, J. , Lischke, R. , Schutzner, J. , Harustiak, T. , Pazdro, A. , Rzechonek, A. , Kolostova, K. , 2014. Cultivation of circulating tumor cells in esophageal cancer. Folia histochem. et cytobiol./Pol. Acad. Sci. Pol. Histochem. Cytochem. Soc. 52, 171–177. [DOI] [PubMed] [Google Scholar]
- Cayrefourcq, L. , Mazard, T. , Joosse, S. , Solassol, J. , Ramos, J. , Assenat, E. , Schumacher, U. , Costes, V. , Maudelonde, T. , Pantel, K. , Alix-Panabieres, C. , 2015. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 75, 892–901. [DOI] [PubMed] [Google Scholar]
- David, R. , 2015. PD-L1 expression by circulating breast cancer cells. Lancet Oncol. 16, e321 [DOI] [PubMed] [Google Scholar]
- Deneve, E. , Riethdorf, S. , Ramos, J. , Nocca, D. , Coffy, A. , Daures, J.P. , Maudelonde, T. , Fabre, J.M. , Pantel, K. , Alix-Panabieres, C. , 2013. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin. Chem. 59, 1384–1392. [DOI] [PubMed] [Google Scholar]
- Gao, D. , Vela, I. , Sboner, A. , Iaquinta, P.J. , Karthaus, W.R. , Gopalan, A. , Dowling, C. , Wanjala, J.N. , Undvall, E.A. , Arora, V.K. , Wongvipat, J. , Kossai, M. , Ramazanoglu, S. , Barboza, L.P. , Di, W. , Cao, Z. , Zhang, Q.F. , Sirota, I. , Ran, L. , MacDonald, T.Y. , Beltran, H. , Mosquera, J.M. , Touijer, K.A. , Scardino, P.T. , Laudone, V.P. , Curtis, K.R. , Rathkopf, D.E. , Morris, M.J. , Danila, D.C. , Slovin, S.F. , Solomon, S.B. , Eastham, J.A. , Chi, P. , Carver, B. , Rubin, M.A. , Scher, H.I. , Clevers, H. , Sawyers, C.L. , Chen, Y. , 2014. Organoid cultures derived from patients with advanced prostate cancer. Cell. 159, 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldkorn, A. , Ely, B. , Quinn, D.I. , Tangen, C.M. , Fink, L.M. , Xu, T. , Twardowski, P. , Van Veldhuizen, P.J. , Agarwal, N. , Carducci, M.A. , Monk, J.P. , Datar, R.H. , Garzotto, M. , Mack, P.C. , Lara, P. , Higano, C.S. , Hussain, M. , Thompson, I.M. , Cote, R.J. , Vogelzang, N.J. , 2014. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 32, 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabinski, N. , Bartkowiak, K. , Grupp, K. , Brandt, B. , Pantel, K. , Jucker, M. , 2011. Distinct functional roles of Akt isoforms for proliferation, survival, migration and EGF-mediated signalling in lung cancer derived disseminated tumor cells. Cell Signal. 1952–1960. [DOI] [PubMed] [Google Scholar]
- Hodgkinson, C.L. , Morrow, C.J. , Li, Y. , Metcalf, R.L. , Rothwell, D.G. , Trapani, F. , Polanski, R. , Burt, D.J. , Simpson, K.L. , Morris, K. , Pepper, S.D. , Nonaka, D. , Greystoke, A. , Kelly, P. , Bola, B. , Krebs, M.G. , Antonello, J. , Ayub, M. , Faulkner, S. , Priest, L. , Carter, L. , Tate, C. , Miller, C.J. , Blackhall, F. , Brady, G. , Dive, C. , 2014. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 20, 897–903. [DOI] [PubMed] [Google Scholar]
- Hou, J.M. , Krebs, M.G. , Lancashire, L. , Sloane, R. , Backen, A. , Swain, R.K. , Priest, L.J. , Greystoke, A. , Zhou, C. , Morris, K. , Ward, T. , Blackhall, F.H. , Dive, C. , 2012. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 30, 525–532. [DOI] [PubMed] [Google Scholar]
- Kang, Y. , Pantel, K. , 2013. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell. 23, 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolostova, K. , Broul, M. , Schraml, J. , Cegan, M. , Matkowski, R. , Fiutowski, M. , Bobek, V. , 2014. Circulating tumor cells in localized prostate cancer: isolation, cultivation in vitro and relationship to T-stage and Gleason score. Anticancer Res. 34, 3641–3646. [PubMed] [Google Scholar]
- Kolostova, K. , Matkowski, R. , Gurlich, R. , Grabowski, K. , Soter, K. , Lischke, R. , Schutzner, J. , Bobek, V. , 2015. Detection and cultivation of circulating tumor cells in gastric cancer. Cytotechnology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolostova, K. , Spicka, J. , Matkowski, R. , Bobek, V. , 2015. Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am. J. Translat. Res. 7, 1203–1213. [PMC free article] [PubMed] [Google Scholar]
- Maheswaran, S. , Haber, D.A. , 2015. Ex vivo culture of CTCs: an emerging resource to guide cancer therapy. Cancer Res. 75, 2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel, M. , Jacot, W. , Pantel, K. , Bartkowiak, K. , Topart, D. , Cayrefourcq, L. , Rossille, D. , Maudelonde, T. , Fest, T. , Alix-Panabieres, C. , 2015. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 9, 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel, K. , Alix-Panabières, C. , 2015. Cell lines from circulating tumor cells. Oncoscience. 2, 815–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel, K. , Dickmanns, A. , Zippelius, A. , Klein, C. , Shi, J. , Hoechtlen-Vollmar, W. , Schlimok, G. , Weckermann, D. , Oberneder, R. , Fanning, E. , 1995. Establishment of micrometastatic carcinoma cell lines: a novel source of tumor cell vaccines. J. Natl. Cancer Inst. 87, 1162–1168. [DOI] [PubMed] [Google Scholar]
- Putz, E. , Witter, K. , Offner, S. , Stosiek, P. , Zippelius, A. , Johnson, J. , Zahn, R. , Riethmuller, G. , Pantel, K. , 1999. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res. 59, 241–248. [PubMed] [Google Scholar]
- Ramirez, J.M. , Fehm, T. , Orsini, M. , Cayrefourcq, L. , Maudelonde, T. , Pantel, K. , Alix-Panabieres, C. , 2014. Prognostic relevance of viable circulating tumor cells detected by EPISPOT in metastatic breast cancer patients. Clin. Chem. 60, 214–221. [DOI] [PubMed] [Google Scholar]
- Rossi, E. , Rugge, M. , Facchinetti, A. , Pizzi, M. , Nardo, G. , Barbieri, V. , Manicone, M. , De Faveri, S. , Chiara Scaini, M. , Basso, U. , Amadori, A. , Zamarchi, R. , 2014. Retaining the long-survive capacity of Circulating Tumor Cells (CTCs) followed by xeno-transplantation: not only from metastatic cancer of the breast but also of prostate cancer patients. Oncoscience. 1, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher, H.I. , Heller, G. , Molina, A. , Attard, G. , Danila, D.C. , Jia, X. , Peng, W. , Sandhu, S.K. , Olmos, D. , Riisnaes, R. , McCormack, R. , Burzykowski, T. , Kheoh, T. , Fleisher, M. , Buyse, M. , de Bono, J.S. , 2015. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 33, 1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solakoglu, O. , Maierhofer, C. , Lahr, G. , Breit, E. , Scheunemann, P. , Heumos, I. , Pichlmeier, U. , Schlimok, G. , Oberneder, R. , Kollermann, M.W. , Kollermann, J. , Speicher, M.R. , Pantel, K. , 2002. Heterogeneous proliferative potential of occult metastatic cells in bone marrow of patients with solid epithelial tumors. Proc. Natl. Acad. Sci. U S A. 99, 2246–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, W.L. , Weinberg, R.A. , 2013. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 19, 1438–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueo, H. , Sugimachi, K. , Gorges, T.M. , Bartkowiak, K. , Yokobori, T. , Muller, V. , Shinden, Y. , Ueda, M. , Mori, M. , Kuwano, H. , Maehara, Y. , Ohno, S. , Pantel, K. , Mimori, K. , 2015. Circulating tumour cell-derived plastin3 is a novel marker for predicting long-term prognosis in patients with breast cancer. Br. J. Cancer. 112, 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willipinski-Stapelfeldt, B. , Riethdorf, S. , Assmann, V. , Woelfle, U. , Rau, T. , Sauter, G. , Heukeshoven, J. , Pantel, K. , 2005. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin. Cancer Res. 11, 8006–8014. [DOI] [PubMed] [Google Scholar]
- Woelfle, U. , Cloos, J. , Sauter, G. , Riethdorf, L. , Janicke, F. , van Diest, P. , Brakenhoff, R. , Pantel, K. , 2003. Molecular signature associated with bone marrow micrometastasis in human breast cancer. Cancer Res. 63, 5679–5684. [PubMed] [Google Scholar]
- Wrage, M. , Ruosaari, S. , Eijk, P.P. , Kaifi, J.T. , Hollmen, J. , Yekebas, E.F. , Izbicki, J.R. , Brakenhoff, R.H. , Streichert, T. , Riethdorf, S. , Glatzel, M. , Ylstra, B. , Pantel, K. , Wikman, H. , 2009. Genomic profiles associated with early micrometastasis in lung cancer: relevance of 4q deletion. Clin. Cancer Res. 15, 1566–1574. [DOI] [PubMed] [Google Scholar]
- Yu, M. , Bardia, A. , Aceto, N. , Bersani, F. , Madden, M.W. , Donaldson, M.C. , Desai, R. , Zhu, H. , Comaills, V. , Zheng, Z. , Wittner, B.S. , Stojanov, P. , Brachtel, E. , Sgroi, D. , Kapur, R. , Shioda, T. , Ting, D.T. , Ramaswamy, S. , Getz, G. , Iafrate, A.J. , Benes, C. , Toner, M. , Maheswaran, S. , Haber, D.A. , 2014. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 345, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Ridgway, L.D. , Wetzel, M.D. , Ngo, J. , Yin, W. , Kumar, D. , Goodman, J.C. , Groves, M.D. , Marchetti, D. , 2013. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl Med. 5, 180ra148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Shiratsuchi, H. , Lin, J. , Chen, G. , Reddy, R.M. , Azizi, E. , Fouladdel, S. , Chang, A.C. , Lin, L. , Jiang, H. , Waghray, M. , Luker, G. , Simeone, D.M. , Wicha, M.S. , Beer, D.G. , Ramnath, N. , Nagrath, S. , 2014. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget. 5, 12383–12397. [DOI] [PMC free article] [PubMed] [Google Scholar]


