Abstract
We tested the hypothesis that testosterone-induced increase in blood pressure was due to changes in aquaporin (AQP) expression in kidneys. In this study, expression level of kidney AQPs was investigated under testosterone influence. Adult normotensive Wistar Kyoto (WKY) and hypertensive SHR male and female rats underwent gonadectomy. For female rats, testosterone was given for six weeks duration, two weeks following ovariectomy via subcutaneous silastic implant. Mean arterial pressure (MAP) was measured in all the rats after eight weeks via carotid artery cannulation and the rats were then sacrificed and kidneys were harvested for analyses of AQP-1, 2, 3, 4, 6, and 7 mRNA and protein expressions by quantitative real-time PCR and Western blotting, respectively. Distribution of AQP subunits’ protein in kidneys was observed by immunofluorescence. In male WKY rats, MAP, AQP-1, 2, 4, and 7 protein; and mRNA expression decreased however AQP-3 protein and mRNA expression increased following orchidectomy. The vice versa effects were observed in testosterone-treated ovariectomized female WKY rats. However, no changes in AQP-6 expression were observed. Meanwhile, in adult male SHR rats, MAP and expression level of all AQP subunits decreased following orchidectomy. The opposite effects were seen in ovariectomized female SHR rats following testosterone treatment. Immunofluorescence study showed AQP-1 and AQP-7 were distributed in the proximal convoluted tubules (PCT) while AQP-2, AQP-4, and AQP-6 were distributed in the collecting ducts (CDs). AQP-3 was distributed in the PCT and CD. In conclusion, changes in AQP subunit expression in kidneys could explain changes in blood pressure under testosterone influence.
Impact statement
This study provides fundamental understanding on the mechanisms underlying testosterone-induced increase in blood pressure which involve regulation of aquaporin channel subunits in the kidneys. A better understanding of this issue can help to explain the reason for higher blood pressure in males as compared to females and may explain the reason for higher blood pressure in females after menopause than females before menopause, the former most probably related to the changes in female androgen.
Keywords: Testosterone, aquaporin, kidneys, normotensive, hypertensive, rats
Introduction
Gender difference in blood pressure regulation has long been observed.1 Prior to menopause, female blood pressure was found to be lower than the age-matched male. However, after menopause, these differences diminish.2 Further, in postmenopausal females, the blood pressure can even be higher than the age-matched males.3 These differences can be due to the effect of sex steroid hormones, namely estrogen and androgen. Both hormones have been reported to affect expression and activity level of the components of the renin–angiotensin system.2 Besides, changes in estrogen/androgen ratios, increase in endothelin and oxidative stress levels, and possible activation of the sympathetic nervous system4 might also account for these differences. Additionally, increase in blood volume could also cause the blood pressure to increase, and this is known to involve the kidney.5
Kidney plays an important role in the hormonal regulation of blood pressure. Expression of epithelial Na+ channel (ENaC) which is involved in Na+ and H2O reabsorption in the kidney was found to be up-regulated by aldosterone.6 Under aldosterone influence, ENaC expression at the apical membrane of collecting ducts (CD) is markedly increased, associated with increased expression of the basolaterally located Na+/K+-ATPase.7 Increase in Na+ reabsorption as a result of increased ENaC expression then drives secondary H2O reabsorption. These will ultimately result in increase in blood volume, and subsequently blood pressure.8 Following Na+ reabsorption, H2O moves from the lumen into the blood partly via aquaporins (AQPs).9 Expression of AQP in renal CD was also found to be influenced by aldosterone and antidiuretic hormone.10,11 Exaggerated AQP expression in kidneys can result in H2O retention as observed in hypothyroidism and in glucocorticoid-induced hypertension.12,13
Thirteen AQP isoforms have been identified in mammals and are classified into classical AQPs and aquaglyceroporins, based on the pore selectivity.14 Classical AQPs facilitate permeation of H2O molecules while aquaglyceroporins facilitate permeation of H2O molecules and non-ionic small compounds including urea and glycerol.14 AQP provides a pathway for passive movement of H2O and small solutes across the cell membrane, down the osmotic gradient.15 Expression of AQP-1, 2, 3, 4, 6, and 7 has been reported in the kidney of rats and humans.16,17 In this study, we hypothesized that testosterone affects AQP subunit expression in kidneys, thereby causing changes in H2O reabsorption and subsequently the blood pressure. Therefore, the aims of this study were to investigate expression levels of AQP subunits in kidneys under testosterone influence, in both normotensive and hypertensive conditions.
Materials and methods
Animal preparation and hormonal treatment
Male and female normotensive Wistar Kyoto (WKY) and hypertensive spontaneously hypertensive (SHR) rats, aged eight weeks were kept in a well-ventilated standardized environment with temperature of 22℃ and 12:12 h light–dark cycle. Animals had free access to standard laboratory rodent diet (Teklad diet, Rossdoff, Germany) and tap water ad libitum. Animals were divided into eight groups as follows:
Sham-operated intact male WKY rats – WMS
Orchidectomized male WKY rats – WMO
Ovariectomized female WKY rats – WFO
Ovariectomized plus testosterone-treated female WKY rats – WFT
Sham-operated intact male SHR rats – SMS
Orchidectomized male SHR rats – SMO
Ovariectomized female SHR rats – SFO
Ovariectomized plus testosterone-treated female SHR rats – SFT
At eight weeks of age, animals were subjected to sham operation, orchidectomies, or ovariectomies under ketamine:xylazine (80:8 mg/kg; intraperitoneal) anesthesia. Following two weeks of recovery, WFT and SFT rats were implanted with 19 mm long silastic tubing (0.062 in ID, 9.125 in OD; Dow Corning) containing 10 mg testosterone propionate (Sigma-Aldrich, MO, USA) subcutaneously behind the neck scruff, following the method as described by Reckelhoff et al.18 The remaining groups were implanted with empty silastic tubing, also for six-week duration. In all treatment groups, the tubing was replaced at 13 weeks of age. All procedures were approved by Faculty of Medicine Institutional Animal Care and Use Committee, University of Malaya (Ethics number 2014-05-07/physio/R/NS).
Measurement of mean arterial pressure (MAP)
Upon completion of hormonal treatment, animals were anesthetized with pentobarbital sodium (60 mg/kg; intraperitoneal). A small incision was made near the trachea and a cannula prefilled with heparinized normal saline (0.5 IU/mL) was inserted directly into the carotid artery. The cannula was preconnected to a blood pressure transducer and a PowerLab data acquisition system (ADInstrument). Blood pressure was recorded for at least 5 min after stabilization for 10 min. Data was analyzed by using a LabChart V6.0. Systolic and diastolic blood pressures were obtained. The MAP was then calculated based on the following equation: MAP = DBP + [(SBP−DBP)/3].
Sample collection
Six weeks following initiation of hormonal treatment, i.e. at 16 weeks of age, rats were sacrificed by decapitation by using a guillotine (Harvard Apparatus, USA). Whole kidneys were collected and immediately frozen in dry ice. The frozen samples were stored at −80℃.
mRNA extraction and cDNA synthesis
The kidney was weighted and then disrupted by using a rotor-stator homogenizer (Heidolph DIAX 600) in Qiazol lysis reagent (Qiagen, NY, USA). Chloroform (EMPARTA MERCK, Mumbai, India) was added to the homogenates and the upper aqueous phase was removed. Seventy percent ethanol was added to the samples and immediately transferred into RNA-binding silica spin columns (Qiagen RNeasy Mini Kit; Qiagen, NY, USA). The following RNA cleanup method was performed following the manufacturer's protocol. Nanodrop (Thermo Scientific NanoDrop 2000, CA, USA) was used to determine the concentration of the extracted RNA. Complementary DNA (cDNA) was synthesized from 500 to 600 ng total RNA by using Quantitect reverse transcription kit (Qiagen, NY, USA), according to the manufacturer’s guidelines.
Quantitative real-time PCR (qRT-PCR)
mRNA levels for AQP in kidneys were quantified by using a qRT-PCR. All primers were designed by using NCBI Primer-BLAST tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/), which was synthesized by Integrated DNA Technologies. These primers are Aqp-1 (5′-ACCCACTGGAGAGAAACCAG-3′ and 5′-AGAGTAGCGATGCTCAGACC-3′), Aqp-2 (5′-AACTACCTGCTGTTCCCCTC-3′ and 5′-ACTTCACGTTCCTCCCAGTC-3′), Aqp-3 (5′-GAACCCTGCTGTGACCTTTG-3′ and 5′-AGTGTGTAGATGGGCAGCTT-3′), Aqp-4 (5′-ACACGAAAGATCAGCATCGC-3′ and 5′-TGACCAGGTAGAGGATCCCA-3′), Aqp-6 (5′- GGATCTTCTGGGTAGGACCG-3′ and 5′-ACGGTCTTGGTGTCAGGAAA-3′), Aqp-7 (5′-TATCTTCGCCATCACGGACA-3′ and 5′-CCCAAGAACGCAAACAAGGA-3′), and Gapdh (5′-GCTACACTGAGGACCAGGTT-3′ and 5′-TCATTGAGAGCAATGCCAGC-3′). Earlier synthesized cDNAs were used as template and primer optimization was performed prior to the experiments. RT-PCR has been validated with a set of primers. All experiments were performed in duplicate by using QuantiNova SYBR Green PCR Kit (Qiagen) for StepOne Plus Real-Time PCR Systems (Applied Biosystems). All data were analyzed by StepOne Software version 2.3. Gapdh was used as reference genes, where relative gene quantifications were obtained based on Comparative 2−ΔΔCt method.
Protein extraction and quantification
Kidneys were weighted and disrupted by using a rotor-stator homogenizer (Heidolph DIAX 600) in RIPA buffer (BioVision, CA, USA), containing protease inhibitor (BioVision, CA, USA). Homogenates were left on ice 30 min prior to centrifugation at 14,000 g for 15 min at 4℃. The concentration of total proteins was quantified by using BCA protein kit (Thermo Scientific, Rockford, USA). The standard curve was produced by using bovine serum albumin and all assays were performed in triplicate.
Western blotting
Equal amount of proteins were loaded and separated by using 12% SDS-PAGE. The separated proteins were then transferred onto polyvinylidene fluoride membrane (BIORAD, US). After 1 h blocking in 2% (w/v) Amersham ECL prime blocking reagent (GE Healthcare) at room temperature, membranes were then incubated with primary antibodies which were diluted in PBS-T (phosphate buffer saline containing 0.1% v/v Tween 20) overnight at 4℃. Primary antibodies used were goat polyclonal anti-AQP-1 (sc-9878), rabbit polyclonal anti-AQP-2 (sc-28629), rabbit polyclonal anti-AQP-3 (sc-20811), goat polyclonal anti-AQP-4 (sc-9888), goat polyclonal anti-AQP-6 (sc-14969), rabbit polyclonal anti-AQP-7 (sc-28625), and rabbit polyclonal anti-GAPDH (sc-25778) (Santa Cruz Biotechnology, CA, USA). After three washes, membranes were probed with appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, CA, USA) for 1 h at room temperature. The bands were visualized using enhanced chemiluminescence reagent (Thermo Scientific, Rockford, USA). Chemiluminescence signals were detected and captured using a highly sensitive CCD camera-based imager (BioSpectrum Imaging System, USA). The intensity of the bands was quantified using Image J software (NIH, NY, USA) and GAPDH was used as reference. All experiments were repeated three times and the average band intensities were obtained.
Immunofluorescence
At the end of the treatment, rats were anaesthetized by using ketamine:xylazine (80:8 mg/kg; intraperitoneal) anesthesia. The rats then underwent transcardial perfusion with PBS and 4% (w/v) paraformaldehyde (PFA). Kidneys were collected and postfixed in 30% (w/v) sucrose in PFA for overnight to three days at 4℃. Following tissue processing and embedding in paraffin, samples were coronally sliced into 5 µm thickness using a microtome. Sections were then mounted onto poly-l-lysine-coated glass slides. The slides were deparaffinized and subjected to antigen retrieval using 1 mM EDTA (BioVision InC.), pH 8.0 containing 0.05% (v/v) Tween20 (Sigma Aldrich). After 1 h blocking with appropriate 10% (v/v) normal serum at room temperature, the slides were incubated with primary antibodies (as above) at ratio of 1:100 overnight at 4℃. After three washes, slides were incubated with appropriate fluorophore-conjugated secondary antibodies (Thermo Scientific, Rockford, USA; DyLight 488 donkey anti-goat lgG, SA5-10086 and DyLight 550 goat anti-rabbit lgG, SA5-10033) at a ratio of 1:200 for 1 h at room temperature. Coverslips were mounted onto the slides in UltraCruz mounting medium (Santa Cruz Biotechnology, CA, USA) to prevent photo-bleaching and then permanently sealed with a nail polisher. Fluorescence signals were visualized and captured by using confocal laser scanning fluorescent microscope equipped with argon/krypton laser (Leica TCS SP5 II).
All experiments were performed in four biological replicates and the representative images were presented. High magnification images were taken with 63× oil immersion objective lancer. All images were subjected to postprocess by using LAS AF Lite software (Leica Microsystems, USA) and Adobe Photoshop (Adobe Systems Incorporated, USA). Nephron segments, i.e. renal corpuscle (G), distal convoluted tubule (DCT), proximal convoluted tubule (PCT) and CD were identified and labeled in all the representative immunofluorescence images based on the atlas of histology.
Statistical analysis
All data were expressed as mean ± standard error of mean (SEM). Statistical analysis was performed by GraphPad Prism (Graphpad Software®) and statistical differences between experimental groups were assessed by using unpaired t-test followed by one-way ANOVA. p < 0.05 was considered as significant.
Results
Changes in MAP in male and female normotensive WKY and hypertensive SHR rats
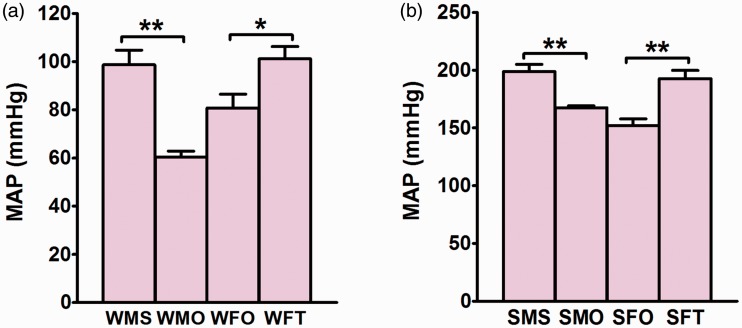
Orchidectomy significantly decreased the MAP in male WKY rats (p < 0.01) while testosterone treatment caused the MAP in ovariectomized female WKY rats to increase (p < 0.05) (Figure 1(a)). Similarly, in male SHR rats, MAP significantly decreased following orchidectomy (p < 0.01) however testosterone treatment to ovariectomized female SHR rats resulted in increase in MAP (p < 0.01) (Figure 1(b)).
Figure 1.
Mean arterial pressure (MAP) in different groups. Effects of testosterone on MAP in (a) normotensive WKY rats and (b) hypertensive SHR rats. Data are expressed as mean ± SEM (N = 6; *p < 0.05; **p < 0.01). (A color version of this figure is available in the online journal.)
Expression of Aqp subunits’ mRNAs in kidney of normotensive male and female WKY rats
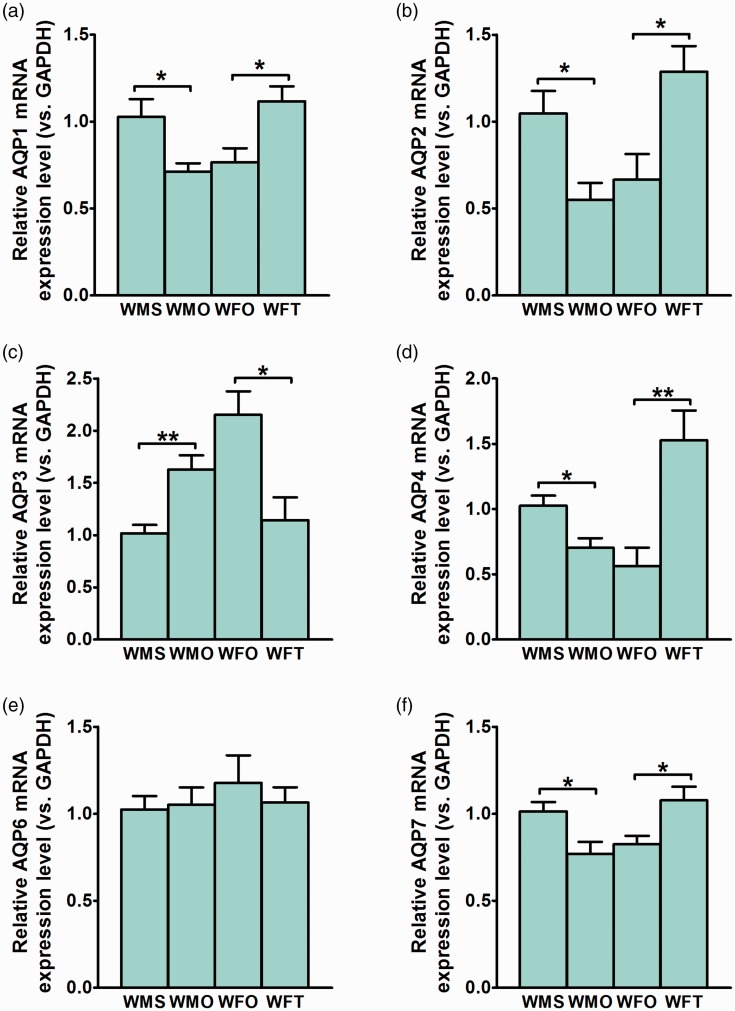
Aqp-1 mRNA level in male WKY rats’ kidney markedly decreased by orchidectomy (p < 0.05) (Figure 2(a)). In female WKY rats, testosterone treatment resulted in Aqp-1 mRNA level to significantly increase (p < 0.05). Aqp2-mRNA level in male WKY rats’ kidney markedly decreased by orchidectomy (p < 0.05) (Figure 2(b)). In ovariectomized female WKY rats, testosterone treatment resulted in Aqp2-mRNA level in the kidney to increase (p < 0.05).
Figure 2.
Expression level of Aqp subunits’ mRNAs in the kidney of normotensive WKY rats. Effects of testosterone on (a) Aqp-1, (b) Aqp-2, (c) Aqp-3, (d) Aqp-4, (e) Aqp-6, and (f) Aqp-7 mRNAs in the kidney of normotensive WKY rats. Data are expressed as mean ± SEM (N = 6; *p < 0.05; **p < 0.01). (A color version of this figure is available in the online journal.)
Aqp3 mRNA level in the kidney of male WKY rats significantly increased by orchidectomy (p < 0.01) (Figure 2(c)). In ovariectomized female WKY rats, testosterone treatment resulted in Aqp3 mRNA level to decrease (p < 0.05). Aqp-4 mRNA level significantly decreased in male WKY rats following orchidectomy (p < 0.05) (Figure 2(d)). In ovariectomized female WKY rats, testosterone treatment resulted in Aqp-4 mRNA level to significantly increase (p < 0.01).
No significant changes in Aqp-6 mRNA level were observed in male WKY rats following orchidectomy (Figure 2(e)). In ovariectomized female WKY rats, testosterone treatment did not cause significant changes in Aqp-6 mRNA level in kidneys. Orchidectomy caused marked reduction in Aqp-7 mRNA level in male WKY rats’ kidney (p < 0.05) (Figure 2(f)). In ovariectomized female WKY rats, testosterone treatment resulted in significant increase in Aqp-7 mRNA level in the kidney (p < 0.01c).
Expression of AQP subunits’ proteins in kidney of normotensive male and female WKY rats
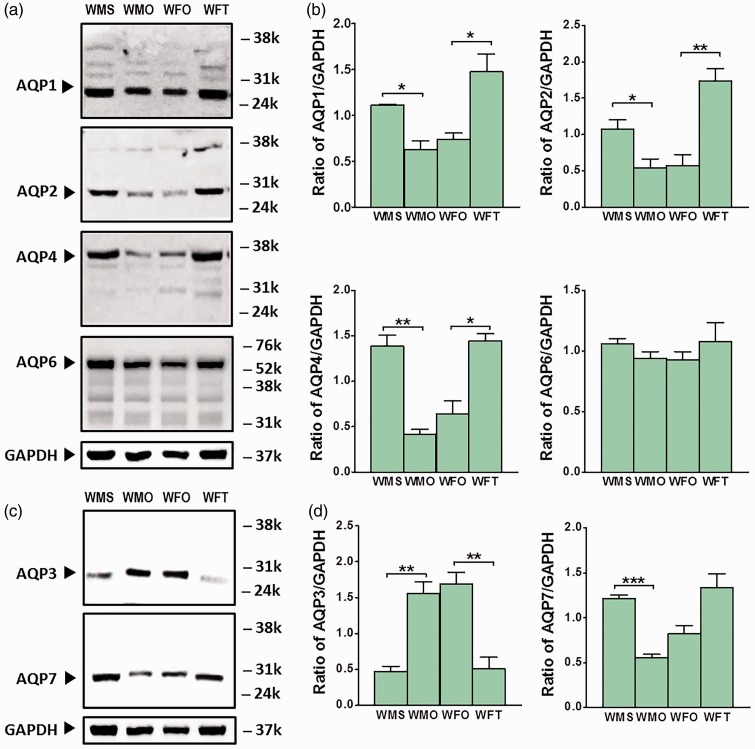
Levels of AQP-1 protein in the kidney of male WKY rats significantly decreased following orchidectomy (p < 0.05) (Figure 3(a) and (b)). In ovariectomized female WKY rats, testosterone treatment resulted in AQP-1 protein level in the kidney to increase (p < 0.05). AQP-2 protein level in the kidney of male WKY rats significantly decreased following orchidectomy (p < 0.05). A significant increase in AQP-2 protein level was observed in the kidney of ovariectomized female WKY rats receiving testosterone treatment (p < 0.01).
Figure 3.
Expression level of AQP subunits’ proteins in the kidney of normotensive WKY rats. (a) Representative Western blot band images for AQP-1, AQP-2, AQP-4, and AQP-6. (b) Relative ratio of band intensities of AQP-1, AQP-2, AQP-4, and AQP-6 over GAPDH. (c) Representative Western blot band images for AQP-3 and AQP-7. (d) Relative ratio of band intensities for AQP-3 and AQP-7 over GAPDH. Data are expressed as mean ± SEM (N = 6; *p < 0.05; **p < 0.01; ***p < 0.001). (A color version of this figure is available in the online journal.)
AQP-4 protein levels in male WKY rats significantly decreased following orchidectomy (p < 0.01). In ovariectomized female WKY rats, testosterone treatment resulted in AQP-4 protein level in the kidney to increase (p < 0.05). A slight but no significant changes in AQP-6 protein levels were observed in orchidectomized male WKY rats. Likewise, testosterone treatment in ovariectomized female WKY rats did not cause significant changes in AQP-6 protein level in kidneys.
AQP-3 protein level in the kidney of male WKY rats significantly increased following orchidectomy (p < 0.01) (Figure 3(c) and (d)). In ovariectomized female WKY rats, testosterone treatment resulted in significant decrease in AQP-3 protein level (p < 0.01). The levels of AQP-7 protein in the kidney of male WKY rats significantly decreased following orchidectomy (p < 0.01). In ovariectomized normotensive female WKY rats, AQP-7 protein level showed a trend to increase following testosterone treatment.
Distribution of AQP subunits’ proteins in kidney of normotensive male and female WKY rats
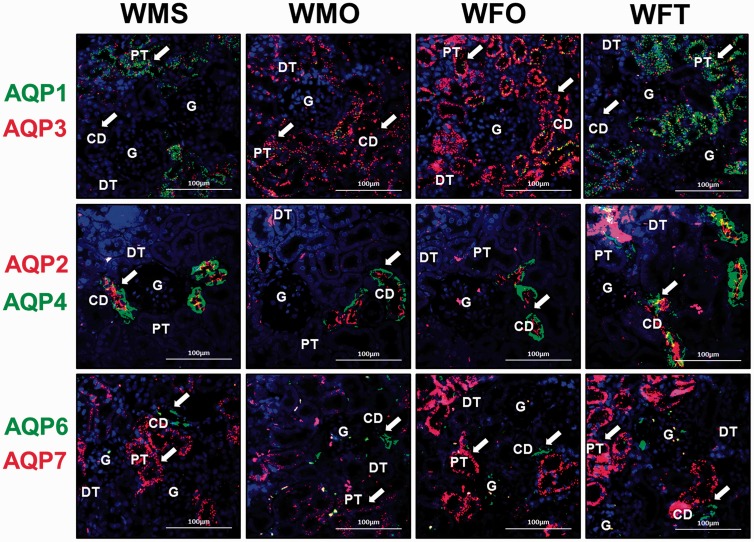
In Figure 4, AQP-1 protein (green fluorescent signal) is predominantly distributed in PCT. AQP-3 (red fluorescent signal) is distributed in PCT and CD. AQP-1 is not found in the glomerulus (G), DCTs, and CD in male and female WKY rats. AQP-3 is also not found in G and DCT of WKY rats. AQP-1 is distributed at a relatively higher level in the kidney of sham-operated male WKY rats compared to orchidectomized male WKY rats. In contrast, AQP-3 is distributed at a relatively higher level in orchidectomized male rats and in non-testosterone-treated ovariectomized female WKY rats compared to sham-operated male and testosterone-treated ovariectomized female WKY rats, respectively.
Figure 4.
Distribution of aquaporin subunits’ proteins in the nephron of normotensive WKY rats. Distribution of AQP-1, AQP-2, AQP-3, AQP-4, AQP-6, and AQP-7 in the kidney of normotensive WKY as shown by double immunofluorescent staining. First row: AQP-1 (green) and AQP-3 (red). Second row: AQP-2 (red) and AQP-4 (green). Third row: AQP-6 (green) and AQP-7 (red). White arrow heads show the protein distribution (n = 4; Scale bar = 100 µm; CD: collecting duct; DCT: distal convoluted tubule; G: glomerulus; PCT: proximal convoluted tubule). (A color version of this figure is available in the online journal.)
AQP-2 (red) and AQP-4 (green) were distributed exclusively in CD of normotensive male and female WKY rats. AQP-2 is distributed at apical membrane while AQP-4 is distributed at basolateral membrane. AQP-2 and AQP-4 are not found in G, DCT, and PCT. AQP-2 is distributed at a relatively higher level in CD of sham-operated male and testosterone-treated ovariectomized female WKY rats compared to orchidectomized male and non-testosterone-treated ovariectomized female WKY rats, respectively. A relatively higher distribution of AQP-4 protein was observed in CD of sham-operated males and testosterone-treated female WKY rats when compared to orchidectomized males and non-testosterone-treated ovariectomized female WKY rats, respectively.
AQP-6 (green) and AQP-7 (red) are distributed predominantly in PCT and CD of WKY rats, respectively. AQP-6 is not found in G, DCT, and CD while AQP-7 is not found in G, DCT, and PCT. Relatively lower AQP-7 distribution is observed in orchidectomized male WKY rats when compared to sham-operated male WKY rats. In ovariectomized female WKY rats receiving testosterone treatment, relatively higher AQP-7 distribution is observed when compared to non-testosterone-treated ovariectomized female WKY rats.
Expression of Aqp subunits’ mRNAs in kidney of male and female hypertensive SHR rats
Levels of Aqp-1 mRNA in the kidney of male SHR rats significantly decreased following orchidectomy (p < 0.01) (Figure 5(a)). In ovariectomized female SHR rats, testosterone treatment resulted in Aqp-1 mRNA level to increase (p < 0.05). Aqp-2 mRNA level in male SHR rats markedly decreased following orchidectomy (p < 0.05) (Figure 5(b)). In ovariectomized female SHR rats, Aqp-2 mRNA level significantly increased following testosterone treatment (p < 0.05).
Figure 5.
Expression level of Aqp subunits’ mRNAs in the kidney of hypertensive SHR rats. Effects of testosterone on (a) Aqp-1, (b) Aqp-2, (c) Aqp-3, (d) Aqp-4, (e) Aqp-6, and (f) Aqp-7 mRNA level in the kidney of SHR rats. Data are expressed as mean ± SEM (N = 6; *p < 0.05; **p < 0.01; ***p < 0.01). (A color version of this figure is available in the online journal.)
Aqp-3 mRNA level in kidney of hypertensive male SHR rats significantly decreased following orchidectomy (p < 0.05) (Figure 5(c)). In ovariectomized female SHR rats, testosterone treatment resulted in Aqp-3 mRNA level to significantly increase (p < 0.05). Aqp-4 mRNA level in hypertensive male SHR rats significantly decreased following orchidectomy (p < 0.05) (Figure 5(d)). In ovariectomized female SHR rats, testosterone treatment resulted in Aqp-4 mRNA level in kidney to significantly increase (p < 0.05).
Aqp-6 mRNA level in male SHR rats significantly decreased following orchidectomy (p < 0.05) (Figure 5(e)). In ovariectomized female SHR rats, Aqp-6 mRNA levels significantly increased following testosterone treatment, (p < 0.001). The level of Aqp-7 mRNA in kidneys of sham-operated male SHR rats was significantly higher than orchidectomized male SHR rats (p < 0.01) (Figure 5(f)). In ovariectomized female SHR rats, Aqp-7 mRNA level significantly increased following testosterone treatment (p < 0.01).
Expression of AQP subunits’ proteins in kidneys of hypertensive male and female SHR rats
Levels of AQP-1 protein in kidney of male hypertensive SHR rats significantly decreased following orchidectomy (p < 0.05) (Figure 6(a) and (b)). In ovariectomized female SHR rats, testosterone treatment resulted in AQP-1 protein level to significantly increase (p < 0.05). A significant decrease in AQP-2 protein level was observed in male SHR rats following orchidectomy (p < 0.05). In ovariectomized female SHR rats, testosterone treatment resulted in AQP-2 protein level to significantly increase (p < 0.05). Orchidectomy caused significant decrease in the level of AQP-4 protein in male SHR rats’ kidney (p < 0.05). In ovariectomized female SHR rats, testosterone treatment resulted in the level of this protein to significantly increase (p < 0.05). In male SHR rats, AQP-6 protein level significantly decreased following orchidectomy (p < 0.01). The level of AQP-6 protein in kidneys of ovariectomized female SHR rats significantly increased following testosterone treatment (p < 0.01).
Figure 6.
Expression level of AQP subunits’ proteins in the kidney of hypertensive SHR rats. (a) Representative Western blot band images of AQP-1, AQP-2, AQP-4, and AQP-6. (b) The relative band intensity ratio of AQP-1, AQP-2, AQP-4, and AQP-6 over GAPDH. (c) Representative Western blot band images of AQP-3 and AQP-7. (d) Relative ratio of band intensities of AQP-3 and AQP-7 over GAPDH. Data are expressed as mean ± SEM (N = 6; *p < 0.05; **p < 0.01; ***p< 0.001). (A color version of this figure is available in the online journal.)
The level of AQP-3 protein in the kidney of male SHR rats significantly decreased following orchidectomy (p < 0.001) (Figure 6(c) and (d)). In ovariectomized female SHR rats, AQP-3 protein level significantly increased following testosterone treatment (p < 0.01). The level of AQP-7 protein in male SHR rats significantly decreased following orchidectomy (p < 0.05). In ovariectomized female SHR rats, AQP-7 protein level significantly increased following testosterone treatment (p < 0.05).
Distribution of AQP subunits’ proteins in kidney of hypertensive male and female SHR rats
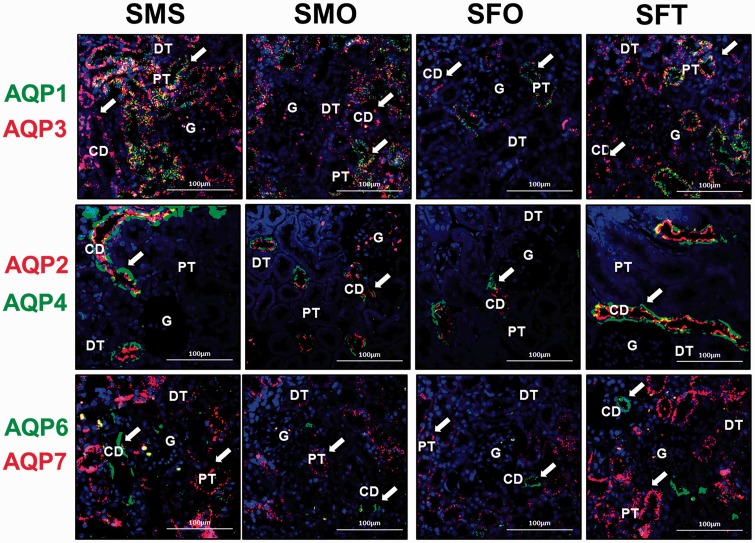
Localization of AQP-1, 2, 3, 4, 6, and 7 in the kidney of SHR rats is shown in Figure 7. In male and female SHR rats, AQP-1 protein (green fluorescent signal) can be seen to be distributed in PCT while AQP-3 protein (red fluorescent signal) can be seen to be distributed in PCT and CD. AQP-1 protein is not found in G, DCT, and CD while AQP-3 is also not found in G and DCT. Distribution of AQP-1 protein is relatively lower in orchidectomized male SHR rats when compared to sham-operated rats and in non-testosterone-treated ovariectomized female SHR rats as compared to testosterone-treated ovariectomized female SHR rats.
Figure 7.
Distribution of aquaporin subunits’ proteins in the nephron of hypertensive SHR rats. Distribution of AQP-1, AQP-2, AQP-3, AQP-4, AQP-6, and AQP-7 in cortical nephrons of SHR rats as shown by double immunofluorescence staining. First row: AQP-1 (green) and AQP-3 (red). Second row: AQP-2 (red) and AQP-4 (green). Third row: AQP-6 (green) and AQP-7 (red). White arrow heads show the protein distribution (N = 4; Scale bar = 100 µm; CD: collecting duct; DCT: distal convoluted tubule; G: glomerulus; PCT: proximal convoluted tubule). (A color version of this figure is available in the online journal.)
A relatively higher AQP-3 distribution is observed in sham-operated male rats when compared to orchidectomized male SHR rats. Relatively lower AQP-3 distribution can be seen in the kidney of non-testosterone treated as compared to testosterone-treated ovariectomized female rats.
AQP-2 (red fluorescent signal) and AQP-4 (green fluorescent signal) are distributed at the apical and basolateral membranes of CD, respectively. These proteins are not found in G, DCT, and PCT. AQP-2 distribution is relatively higher in sham-operated male rats compared to orchidectomized male SHR rats. Likewise, higher AQP-4 distribution is seen in CD of sham-operated male rats relative to orchidectomized male SHR rats. Distribution of AQP-2 and AQP-4 in testosterone-treated ovariectomized female rats is relatively higher when compared to non-testosterone-treated ovariectomized female rats.
In hypertensive male and female SHR rats, AQP-6 protein (green fluorescent signal) is distributed in CD while AQP-7 protein (red fluorescent signal) is distributed in PCT. AQP-6 and AQP-7 are not found in G and DCT. Relatively higher AQP-6 distribution is seen in sham-operated male SHR rats when compared to orchidectomized male SHR rats. AQP-7 protein is distributed at a relatively higher level in the kidney of sham-operated male SHR rat compared to orchidectomized male SHR rats. In ovariectomized female SHR rats receiving testosterone treatment, relatively higher AQP-6 and 7 distribution can be seen in the nephrons when compared to non-testosterone-treated ovariectomized female SHR rats.
Discussion
In the present study, testosterone was found to cause increase in MAP and expression of AQP-1, 2, 4, and 7 in normotensive female WKY rats. Testosterone was also found to cause increase in MAP and expression of AQP-1, 2, 3, 4, 6, and 7 in ovariectomized, hypertensive female SHR rats. The effect of testosterone on MAP and AQP expression in kidneys when administered to female rats with sex hormone deficiency were similar to the changes in MAP and AQP expression in intact male rats with high endogenous testosterone level. To support the latter observations, removal of testes, a major source of endogenous testosterone in intact male rats resulted in decreased expression level of AQP-1, 2, 4, and 7 in normotensive WKY rats with additional decrease in expression level of AQP-3 and 6 in hypertensive SHR rats. On the other hand, levels of AQP-3 in the kidney of normotensive WKY female rats were decreased following testosterone treatment. In intact normotensive male rats known to have high level of endogenous testosterone, levels of AQP-3 were lower than in orchidectomized normotensive male rats. However, in hypertensive male and female rats, AQP-3 level increased under testosterone influence. Together with increased expression of AQP-6, testosterone may contribute toward greater MAP in hypertensive condition.
The findings from this study indicate the possibility that testosterone affects kidney H2O handling. Increased expression of most of the AQP subunits could facilitate greater amount of H2O being reabsorbed from the tubular lumen of the CD, whereby this effect could lead to increase in plasma volume and subsequently blood pressure. In addition to testosterone, other sex steroid hormones such as estrogen and progesterone have also been shown to affect kidney H2O handling. Androgen, estrogen, and progesterone receptors have been reported to be expressed in the kidney,19–22 suggesting that kidney functions are controlled by sex steroids. Estrogen was found to directly affect AQP expression in the kidney.23,24 Meanwhile, testosterone has been found to up-regulate expression of AQP-4 in astrocytes25 and AQP-1 26,27 and AQP-727 in uterus.
In this study, AQP-2 expression in the kidney was markedly increased under testosterone influence. Under this condition, AQP-2 is found to be highly distributed at the apical membrane of CD. Up-regulation of AQP-2 by testosterone was found to be greater in hypertensive condition than in normotensive condition. The elevated level of AQP-2 under testosterone influence could lead to higher amount of H2O being reabsorbed from the tubular lumen. The involvement of AQP-2 in H2O reabsorption in the kidney is well documented. Expression of AQP-2 subunit in the principal cells of CD has been reported to be up-regulated by vasopressin15 which acutely regulates the permeability of CD to H2O via controlling the number of AQP-2 channel trafficked from the intracellular vesicles to the apical membrane.28
This study has shown that expression of AQP-4 at the basolateral membrane of the CD was increased by testosterone. This finding is consistent with the observations that the basolaterally located AQP-4 serves as an exit pathway for H2O that enters the CD epithelial cells via AQP-2.15 It has been shown in mice with Aqp-4 gene knockout that this channel is responsible for the majority of H2O movement across the basolateral membrane of the inner medullary CD. In these mice, defective AQP-4 expression could affect ability of kidney to concentrate the urine.29
In addition to AQP-2 and AQP-4, testosterone was also found to up-regulate the expression of AQP-1 in the kidney. However, in contrast to the two former subunits, AQP-1 is found to be distributed in the PCT. Since reabsorption of the filtered H2O occurs mostly in PCT,30 increase in AQP-1 expression could help to increase the amount of H2O being reabsorbed from the PCT lumen. This would ultimately cause increase in plasma volume and blood pressure. Schnermann et al.30 reported that in mice, deficiency in AQP-1 at the apical and basolateral membranes of PCT markedly reduced the transcellular H2O movement.
The findings from this study showed that AQP-3 expression in the kidney and its distribution at the basolateral membrane of CD were decreased under testosterone influence in normotensive condition. However, the opposite effects were observed under hypertensive conditions whereby expression of this subunit markedly increased under testosterone influence. In normotensive condition, the significant of this effect on kidney H2O reabsorption and the blood pressure is unknown, however in hypertensive condition, this effect might lead to increase in H2O reabsorption from tubular lumen. AQP-3 has been reported to be expressed at the basolateral membrane of the principal cells of CD and has been shown to be regulated by vasopressin.31
Meanwhile, AQP-6 is found to be expressed in CD and its level is highly increased under testosterone influence in hypertensive but not in normotensive conditions. In CD, AQP-6 has been reported to reside in the membrane of intracellular vesicles of type A intercalated cells.32 Therefore, based on its distribution and its apparent absent from the plasma membrane, this subunit most likely not involved in transepithelial H2O transport in this nephron segment.32
AQP-7 is found to be expressed exclusively in PCT, a finding which is consistent with the report that this subunit is distributed at the apical membrane of PCT and serves as the pathway for glycerol reabsorption in the nephron.33 A study in Aqp-1/Aqp-7 double gene knockout mice indicates that urine concentrating ability of these mice markedly reduced when compared to Aqp-1 only gene knockout mice, indicating a substantial contribution of AQP-7 toward H2O reabsorption in PCT.33 Increased AQP-7 expression under testosterone influence could lead to enhanced H2O and glycerol transports that could increase the blood volume and plasma glycerol concentration.
In conclusion, testosterone-induced increase in expression of AQP-2 and AQP-4 in the CD of the kidney in both normotensive and hypertensive conditions could result in enhanced H2O reabsorption from the tubular lumen into the blood plasma, which subsequently cause increase in blood pressure. Despite down-regulation of AQP-3 subunit expression in the kidney in normotensive condition, no significant changes in H2O exit through the basolateral membrane is expected since expression of AQP-4, which is also distributed at the basolateral membrane was increased by testosterone. Testosterone-induced up-regulation of additional AQP subunits, i.e. AQP-3 and 6 might contribute toward greater blood pressure in hypertensive as compared to normotensive conditions. In the meantime, up-regulation of AQP-1 and AQP-7 in PCT by testosterone could result in enhanced reabsorption of the filtered H2O, resulting in plasma volume expansion. Collectively, the effect of testosterone on AQP subunit expression in the kidney could explain higher blood pressure in males as compared to age-matched females prior to menopause, in which the former possesses higher testosterone levels. The observed testosterone effects might also help to explain higher blood pressure in women postmenopause with altered estrogen/testosterone level.34
Acknowledgements
This study is supported by University of Malaya Research Grant (UMRG RP011-13HTM) and Postgraduate Research Fund (PPP) PG002-2014B from the University of Malaya, Kuala Lumpur, Malaysia.
Authors’ contribution
NS designed the study, wrote, and reviewed the manuscript. SYL performed the experiment, wrote the draft, and conducted data analysis while NG performed the experiment and conducted data analysis.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension 2001; 37: 1199–208. [DOI] [PubMed] [Google Scholar]
- 2.Maric-Bilkan C, Manigrasso MB. Sex differences in hypertension: contribution of the renin–angiotensin system. Gender Med 2012; 9: 287–91. [DOI] [PubMed] [Google Scholar]
- 3.Barton M, Meyer MR. Postmenopausal hypertension mechanisms and therapy. Hypertension 2009; 54: 11–8. [DOI] [PubMed] [Google Scholar]
- 4.Reckelhoff JF. Basic research into the mechanisms responsible for postmenopausal hypertension. Int J Clin Pract Suppl 2004; 139: 13–9. [PubMed] [Google Scholar]
- 5.Laragh J. The endocrine control of blood volume, blood pressure and sodium balance: atrial hormone and renin system interactions. J Hypertens 1986; 4: S143–56. [PubMed] [Google Scholar]
- 6.Quinn S, Harvey BJ, Thomas W. Rapid aldosterone actions on epithelial sodium channel trafficking and cell proliferation. Steroids 2014; 81: 43–8. [DOI] [PubMed] [Google Scholar]
- 7.Dooley R, Angibaud E, Yusef YR, Thomas W, Harvey BJ. Aldosterone-induced ENaC and basal Na+/K+-ATPase trafficking via protein kinase D1-phosphatidylinositol 4-kinaseIIIβ trans Golgi signalling in M1 cortical collecting duct cells. Mol Cell Endocrinol 2013; 372: 86–95. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y-B, Leroy V, Maunsbach AB, Doucet A, Hasler U, Dizin E, Ernandez T, de Seigneux S, Martin P-Y, Féraille E. Sodium transport is modulated by p38 kinase–dependent cross-talk between ENaC and Na, K-ATPase in collecting duct principal cells. J Am Soc Nephrol 2014; 25: 250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE. Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 2014; 10: 135–146–135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takata K, Matsuzaki T, Tajika Y. Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem 2004; 39: 1–83. [DOI] [PubMed] [Google Scholar]
- 11.Lee B-H, Kwon T-H. Regulation of AQP2 in collecting duct: an emphasis on the effects of angiotensin II or aldosterone. Electrolyte Blood Press 2007; 5: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeum CH, Kim SW, Kim NH, Choi KC, Lee J. Increased expression of aquaporin water channels in hypothyroid rat kidney. Pharmacol Res 2002; 46: 85–8. [DOI] [PubMed] [Google Scholar]
- 13.Chen M, Cai H, Klein JD, Laur O, Chen G. Dexamethasone increases aquaporin-2 protein expression in ex vivo inner medullary collecting duct suspensions. Front Physiol 2015; 6: 310–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nature Rev Mol Cell Biol 2004; 5: 687–98. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen S, Frøkiær J, Marples D, Kwon T-H, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 2002; 82: 205–44. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal SK, Gupta A. Aquaporins: the renal water channels. Indian J Nephrol 2008; 18: 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen S, Kwon T, Christensen BM, Promeneur D, Frøkiær J, Marples D. Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol 1999; 10: 647–63. [DOI] [PubMed] [Google Scholar]
- 18.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 1998; 31: 4359–4359. [DOI] [PubMed] [Google Scholar]
- 19.Wenner MM, Stachenfeld NS. Blood pressure and water regulation: understanding sex hormone effects within and between men and women. J Physiol 2012; 590: 5949–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stachenfeld NS. Sex hormone effects on body fluid regulation. Exerc Sport Sci Rev 2008; 36: 152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis KS. Estrogen and the central control of body fluid balance. Physiol Behav 2009; 97: 180–92. [DOI] [PubMed] [Google Scholar]
- 22.Dubey RK, Jackson EK. Estrogen-induced cardiorenal protection: potential cellular, biochemical, and molecular mechanisms. Am J Physiol Renal Physiol 2001; 280: F365–88. [DOI] [PubMed] [Google Scholar]
- 23.Cheema MU, Irsik DL, Wang Y, Miller-Little W, Hyndman KA, Marks ES, Frokiaer J, Boesen EI, Norregaard R. Estradiol regulates AQP2 expression in the collecting duct: a novel inhibitory role for estrogen receptor alpha. Am J Physiol Renal Physiol 2015; 309: F305–17. [DOI] [PubMed] [Google Scholar]
- 24.Wei H, Wang G, Xu J, Yuan W, Wei J. Effects of reduced estrogen and progesterone on renal aquaporin expression in rats. J Zhengzhou Univ (Med Sci) 2014;5:R692.
- 25.Gu F, Hata R, Toku K, Yang L, Ma Y-J, Maeda N, Sakanaka M, Tanaka J. Testosterone up-regulates aquaporin-4 expression in cultured astrocytes. J Neurosci Res 2003; 72: 709–15. [DOI] [PubMed] [Google Scholar]
- 26.Herak-Kramberger CM, Breljak D, Ljubojevic M, Matokanovic M, Lovric M, Rogic D, Brzica H, Vrhovac I, Karaica D, Micek V, Dupor JI, Brown D, Sabolic I. Sex-dependent expression of water channel AQP1 along the rat nephron. Am J Physiol Renal Physiol 2015; 308: F809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salleh N, Mokhtar HM, Kassim NM, Giribabu N. Testosterone induces increase in aquaporin (AQP)-1, 5, and 7 expressions in the uteri of ovariectomized rats. J Membr Biol 2015; 248: 1097–105. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen S, Kwon T-H, Christensen BM, Promeneur D, Frokler J, Marples D. Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol 1999; 10: 647–63. [DOI] [PubMed] [Google Scholar]
- 29.Chou C, Ma T, Yang B, Knepper MA, Verkman A. Fourfold reduction of water permeability in inner medullary collecting duct of aquaporin-4 knockout mice. Am J Physiol Cell Physiol 1998; 274: C549–C54. [DOI] [PubMed] [Google Scholar]
- 30.Schnermann J, Huang Y, Mizel D. Fluid reabsorption in proximal convoluted tubules of mice with gene deletions of claudin-2 and/or aquaporin1. Am J Physiol Renal Physiol 2013; 305: F1352–F64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper M. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol Renal Physiol 1995; 269: F663–F72. [DOI] [PubMed] [Google Scholar]
- 32.Yasui M, Kwon T-H, Knepper MA, Nielsen S, Agre P. Aquaporin-6: an intracellular vesicle water channel protein in renal epithelia. Proc Natl Acad Sci 1999; 96: 5808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sohara E, Rai T, Sasaki S, Uchida S. Physiological roles of AQP7 in the kidney: lessons from AQP7 knockout mice. Biochim Biophys Acta 2006; 1758: 1106–10. [DOI] [PubMed] [Google Scholar]
- 34.Ablamowicz AF, Nichols JJ, Nichols KK. Association between serum levels of testosterone and estradiol with meibomian gland assessments in postmenopausal womenhormones and MG assessments in women. Invest Ophthalmol Vis Sci 2016; 57: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]