Abstract
Surfactant Protein B Deficiency is a rare but lethal monogenetic, congenital lung disease of the neonate that is unresponsive to any treatment except lung transplantation. Based on the potential that gene therapy offers to treat such intractable diseases, our objective was to test whether an electroporation-based gene delivery approach could restore surfactant protein B expression and improve survival in a compound knockout mouse model of surfactant protein B deficiency. Surfactant protein B expression can be shut off in these mice upon withdrawl of doxycycline, resulting in decreased levels of surfactant protein B within four days and death due to lung dysfunction within four to seven days. Control or one of several different human surfactant protein B-expressing plasmids was delivered to the lung by aspiration and electroporation at the time of doxycycline removal or four days later. Plasmids expressing human surfactant protein B from either the UbC or CMV promoter expressed surfactant protein B in these transgenic mice at times when endogenous surfactant protein B expression was silenced. Mean survival was increased 2- to 5-fold following treatment with the UbC or CMV promoter-driven plasmids, respectively. Histology of all surfactant protein B treated groups exhibited fewer neutrophils and less alveolar wall thickening compared to the control groups, and electron microscopy revealed that gene transfer of surfactant protein B resulted in lamellar bodies that were similar in the presence of electron-dense, concentric material to those in surfactant protein B-expressing mice. Taken together, our results show that electroporation-mediated gene delivery of surfactant protein B-expressing plasmids improves survival, lung function, and lung histology in a mouse model of surfactant protein B deficiency and suggest that this may be a useful approach for the treatment of this otherwise deadly disease.
Impact statement
Surfactant protein B (SP-B) deficiency is a rare but lethal genetic disease of neonates that results in severe respiratory distress with no available treatments other than lung transplantation. The present study describes a novel treatment for this disease by transferring the SP-B gene to the lungs using electric fields in a mouse model. The procedure is safe and results in enough expression of exogenous SP-B to improve lung histology, lamellar body structure, and survival. If extended to humans, this approach could be used to bridge the time between diagnosis and lung transplantation and could greatly increase the likelihood of affected neonates surviving to transplantation and beyond.
Keywords: Disease, epithelial, gene, lung, pediatrics, therapy
Introduction
Pulmonary surfactant is a phospholipid-rich lipid–protein complex that stabilizes lung alveoli by reducing the surface tension at the alveolar air–liquid interface to facilitate gas exchange. Surfactant protein B (SP-B) is particularly important to facilitate lipid adsorption and the formation of the surface active film.1 SP-B deficiency is a rare but lethal disease of neonates caused by an autosomal recessive mutation on chromosome 2, resulting in loss of SP-B.2,3 There are an estimated 1 in 1 million infants affected in the United States. The classic presentation of SP-B deficiency is severe respiratory distress in term infants resembling respiratory distress syndrome.2 This congenital surfactant deficiency is refractory to exogenous surfactant administration and currently there are no treatments available for SP-B deficiency other than extracorporeal membrane oxygenation (ECMO) as a bridge to lung transplantation.4–6 Given that SP-B deficiency is a monogenetic disorder, it is an excellent target for gene therapy.
While the generally perceived goal of gene therapy would be to correct this gene deficiency for the lifetime of the affected individuals, gene therapy also could prove to be a novel treatment option to improve morbidity and mortality until time of lung transplantation. Following diagnosis of SP-B deficiency, the mean time to transplant is 2.5 months, with near certain mortality by six months without transplant.5,6 Thus, gene therapy could be used effectively in the short term as a means to bridge this gap and ensure higher likelihood of transplantation and improved condition at time of transfer. In the past, several attempts using adenoviral vectors for gene delivery were studied in a mouse model.7,8 Low levels of transgene expression and a lack of studies on therapeutic efficacy make this approach a less than attractive option. Further, viral vectors used to transfer the gene for SP-B can induce mild inflammation,9 and in the setting of a very sick, fragile infant, any additional inflammation could be devastating.
We have developed an electroporation-based technique that involves using mild electric fields to deliver genes to the lungs of living animals.10,11 The method is safe, fast, inexpensive, and results in high-level expression of transgenes. Most importantly, the approach causes little to no inflammation and is extremely well tolerated, even in animals with existing lung injury.10–14 In the present study, we evaluated this approach to deliver SP-B-expressing plasmids to the lungs to restore SP-B production in a compound knockout mouse model of SP-B deficiency. The mouse model uses mice that have been genetically engineered to have both copies of their SP-B gene knocked out but carry an additional tetracycline-inducible copy of the SP-B gene.15,16 When these mice are fed doxycycline, they produce SP-B and show no phenotype, but when doxycycline is removed, SP-B levels fall, resulting in respiratory failure and death within five to seven days. Our studies show that electroporation-mediated gene delivery of SP-B-expressing plasmids results in increased levels of SP-B and a clear survival benefit in the SP-B-deficient mice.
Materials and methods
Plasmids
The human SP-B coding sequence was amplified by PCR from human lung cDNA (Stratagene, San Diego, CA; 5′ACGTACGTAAGCTTATGGCTGAGTCACACCTG-CTGCAGTGGC-3′ and 5′ TAGTAGCCGAATTCTCAAAGGTCGGGGCTGTGGATA CACTGG-3′). The product was digested and cloned into the EcoRI and HindIII sites of the appropriate plasmids. pUbC-SPB expresses SP-B from the Ubiquitin C promoter in pUB6/V5 (Invitrogen, Carlsbad, CA) and pCMV-SPB expresses human SP-B from the CMV promoter. All plasmids were verified by DNA sequencing, purified from E. coli using Qiagen gigapreps (Qiagen, Chatsworth, CA) and suspended in 10 mM Tris (pH 8.0), 1 mM EDTA, and 140 mM NaCl.
In vivo gene transfer to the lung
Six- to eight-week-old female and male compound transgenic SPB mice were anesthetized with 3% isofluorane. A volume of 50 µl of plasmid (100 µg in 10 mM Tris, pH8/1 mM EDTA/140 mM NaCl) was administered in the oropharynx with immediate closure of nares, causing the mice to aspirate the solution. The mice were placed under 3% isofluorane again and pediatric cutaneous pacemaker electrodes (Quick-Combo RTS; Medtronic Physio-Control Corporation, Redmond, WA) were placed on either side of the chest under the forelimbs. A small amount of Surgilube (E. Fougera and Company, Melville, NY) was applied to the electrodes, which are held in place by surgical tape. Immediately following aspiration and placement of the electrodes, a series of eight square-wave electric pulses at a 10 msec/pulse were administered at 1 Hz using an ECM830 electroporator (BTX, San Diego, CA), applying a field strength of 200 V/cm.10 The mice were allowed to recover from anesthesia in room air and were then returned to the vivarium. Mice were observed for signs of distress at least twice daily. At the specified time point or when mice began to show signs of impending respiratory failure, mice were euthanized and the lungs were removed and analyzed. In all experiments except survival studies, 4 or 5 mice were used per condition as indicated; survival experiments used 18 mice per condition. All experiments were repeated at least twice. All experiments were conducted in accordance with institutional guidelines in compliance with the recommendations of the Guide for the Care and Use of Laboratory Animals and with approval from the Institutional Animal Care and Use Committee.
Histological analysis
Paraffin embedded thin sections (5 µm) were cut from lungs inflated to total lung capacity with 10% buffered formalin immediately after mice were euthanized. Slides were deparaffinized and stained with hematoxylin and eosin. Immunofluorescent staining was carried out on deparaffinized sections as described,17 using a rabbit polyclonal antibody against pro- and mature SP-B (#40876; Abcam, Cambridge, MA) and a mouse monoclonal antibody against ABCA3 (clone 13-H2-57; Seven Hills Bioreagents, Cincinnati, OH), followed by fluorescently-labeled secondary antibodies, and mounted with DAPI.
Transmission electron microscopy methods
Mouse lungs were inflated to total lung capacity with 2.5% glutaraldehyde and 4.0% paraformaldehyde in 0.1 M sodium cacodylate buffer. Each lung was trimmed from the same lobe into smaller pieces, rinsed in buffer and post-fixed 90 min in 1.0% KFeCN/1.0% osmium tetroxide in 0.1 M sodium cacodylate buffer. The tissue was dehydrated at 45 min intervals through a graded series of ethanol up to 100% (×3), transitioned through propylene oxide (twice at 60 min each), then propylene oxide/EPON araldite (1:1 for 60 min) prior to infiltration with 100% EPON/araldite epoxy resin (60 min, then overnight). The next day, the lung tissue was embedded into BEEM capsules containing fresh epoxy resin and polymerized for 48 h at 60℃. Polymerized blocks were cut at 1 µm and stained with Toluidine blue to evaluate areas to be thin-sectioned (70 nm) using a diamond knife and a Boeckler PTXL ultramicrotome. Thin-sections were placed onto nickel formvar/carbon-coated slot grids and stained with aqueous uranyl acetate and lead citrate. A Hitachi 7650 transmission electron microscope with an attached Gatan Erlangshen 11 megapixel digital camera was used for ultrastructural analysis and photography. To quantitatively analyze lamellar bodies, between 70 and 110 type II pneumocytes were imaged at 15,000× magnification for each condition. The number of lamellar bodies in each cell was counted, their size measured, and their phenotype was assessed as either containing packed electron-dense, concentric surfactant, or having a bubbly appearance with no organized lamellae as previously described.18
SP-B analysis
Lung tissue was homogenized in DTT-free Promega passive lysis buffer (Promega) using a Qiagen TissueLyserII (Qiagen, Chatsworth, CA). Total lung protein (35 µg) was separated under non-reducing conditions on Bio-Rad Ready Gel 4–20% gradient precast gels and transferred onto nitrocellulose membranes. SP-B was detected with a polyclonal rabbit antiserum (#40876; Abcam, Cambridge, MA), followed by chemiluminescence detection and analysis of digitized films using ImageJ (ImageJ U.S. NIH, Bethesda, MD).
Lung myeloperoxidase analysis
MPO assays were carried out on lung homogenates (n = 4 per condition) as previously described.19
Statistical analysis
Data are presented as the mean ± SEM and P < 0.05 (two tailed) was considered statistically significant. Western blot densitometry and Kaplan survival analysis were analyzed using Graphpad Prism Software 5.0 (Graphpad Software, La Jolla, CA).
Results
Expression of SP-B in an SPB deficient mouse model after electroporation-mediated gene delivery
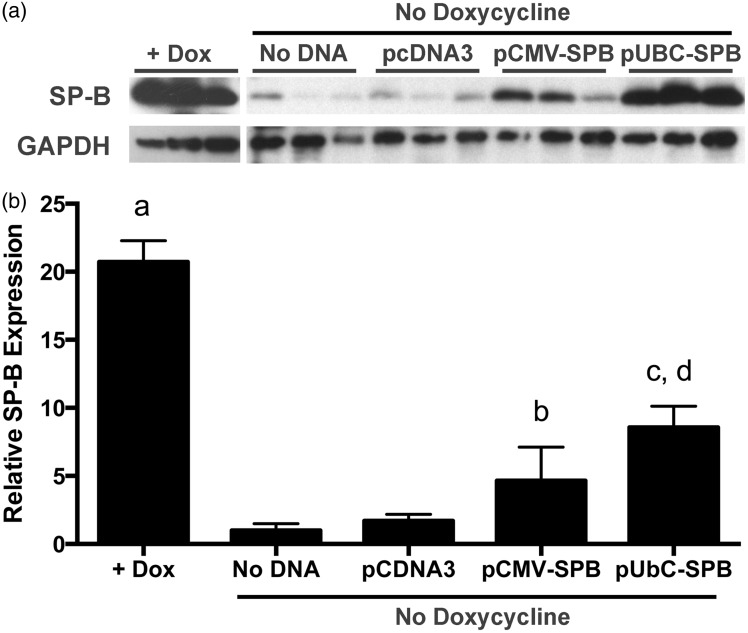
To determine whether electroporation could be used as a suitable method for gene delivery of a human SP-B plasmid, we assayed gene delivery in a mouse model that demonstrates SP-B deficiency. The human SP-B coding sequence was cloned behind one of two promoters for these studies: the CMV immediate early promoter/enhancer for short-term robust expression (<7 days)20,21 and the UbC promoter for sustained expression (≥6 months).22–24 Doxycycline was removed from the diet and gene delivery was performed on the same day. Mice (n = 4) were then fed a normal diet (no doxycycline) for the remainder of the experiment. Since the compound knock-out mice die between five and seven days without maintenance on doxycycline,15 we evaluated expression of SP-B four days after gene delivery, at a time before onset of severe respiratory distress.15 Removal of doxycycline from the diet resulted in almost a 20-fold drop in mature SP-B protein in lung homogenates (Figure 1). Electroporation-mediated gene transfer of plasmids expressing SP-B from the Ubiquitin C promoter gave the highest levels of expression in mice, almost 8-fold above that seen in mice that received either no DNA or the empty plasmid pCDNA3 alone (Figure 1), but was still less than half that seen in animals maintained on doxycycline. SP-B expression was somewhat more variable and slightly less (∼5-fold) in animals that received the CMV promoter-driven construct pCMV-SPB, but expression was still statistically significantly above that seen in untreated mice. In all mice, only the mature 18 kdal SP-B dimer was detected on blots from non-reducing gels; no 23 kdal or 42 kdal precursors were detected.
Figure 1.
Electroporation-mediated SP-B gene transfer in compound SP-B mice 4 days after gene transfer and removal of doxycycline. (a) Western blots of SP-B protein expression. Compound SP-B transgenic mice (n = 5) were either maintained on doxycycline (+Dox) or were taken off doxycycline and 100 µg of each plasmid in 50 µl of 10 mM Tris, pH 8/1 mM EDTA/140 mM NaCl were delivered to the lungs by aspiration and electroporation using eight square wave pulses of 10 ms duration at a field strength of 200 V/cm as described in Materials and Methods. Four days later, lungs were removed from animals and a portion of the lungs was used for preparation of lysates for protein analysis by SDS-PAGE and Western blot using antibodies against mature SP-B. Blots were also reacted with antibodies against GAPDH to ensure appropriate loading. (b) Normalized densitometry of Western blot data. Intensities (mean ± st. dev.) of 18 kdal SP-B reactive bands from A were normalized to GAPDH expression. a, P < 0.005 compared to all other groups; b, P < 0.05 compared to No DNA group; c, P < 0.005 compared to No DNA group; and d, P < 0.005 compared to pcDNA3, by one-way ANOVA and post hoc Tukey multiple comparisons test
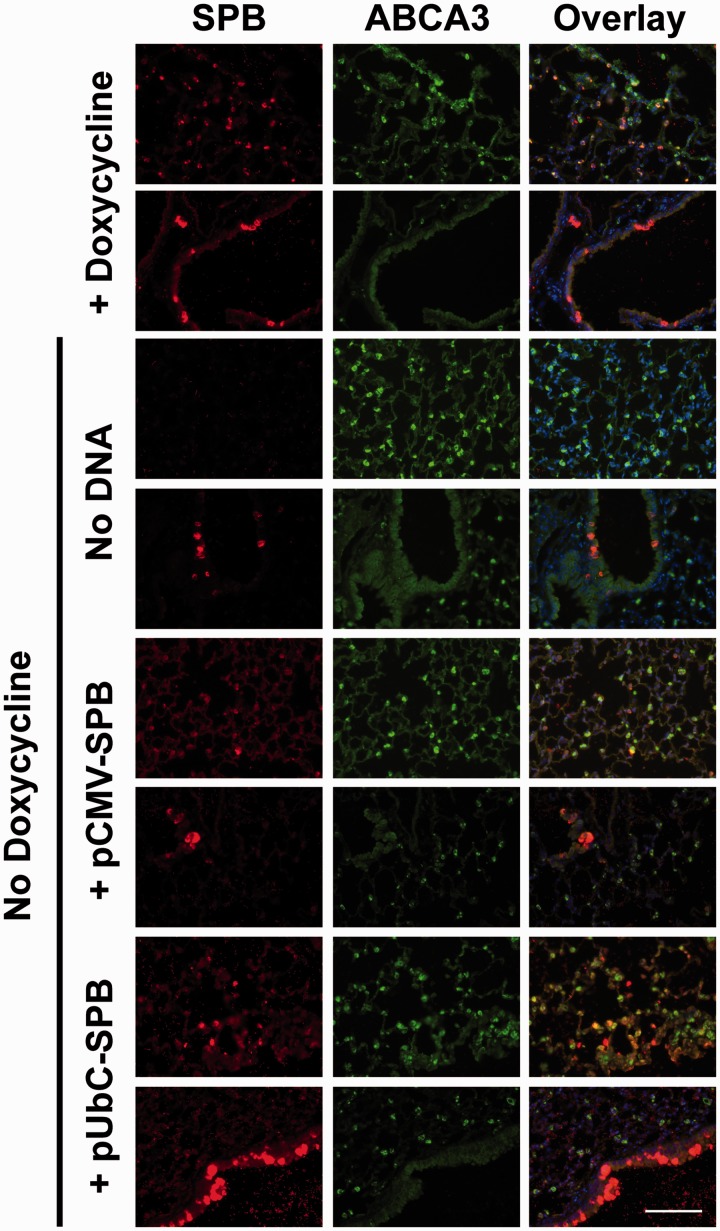
In addition to assaying the total SP-B protein expressed in lung homogenates four days after gene delivery and doxycycline removal in the compound transgenic mice, immunofluorescence staining for SP-B was performed on thin sections from the lungs of the animals. Since electroporation-mediated gene transfer to the lungs has been shown to deliver DNA to all cell types throughout the lung, we wanted to determine whether the delivered SP-B transgenes caused expression in multiple cell types in the lung as well. Since alveolar epithelial type II cells are the only cells in the lung shown to express correctly processed SP-B, we co-stained for these cells using an antibody against ABCA3 (Figure 2). In animals maintained on doxycycline, SP-B protein was detected exclusively in alveolar epithelial type II cells in the parenchyma. However, there also were a limited number of epithelial cells in the airways that were not ABCA3 positive but did show very high levels of SP-B protein. When doxycycline was removed, almost no SP-B protein was detected in the parenchyma, and a reduced number of airway epithelial cells moderately stained for SP-B compared to the doxycycline-fed mice. Electroporation of pCMV-SPB plasmids resulted in high numbers of SP-B positive cells, almost all of which were type II cells (based on ABCA3 staining), although the intensity of the staining was not as great as in doxycycline-fed mice. As in the mice receiving no DNA and no doxycycline, there were relatively few SP-B positive cells in the airway, although they were detected. Finally, electroporation of pUbC-SPB plasmids also resulted in a number of alveolar cells showing SP-B expression, although a number of these cells gave intense SP-B staining but did not co-localize with ABCA3, indicating that at least some of the cells receiving and expressing SP-B in the parenchyma were not type II cells. Further, the number of SP-B positive cells in the airways of these mice was much greater than seen under any other condition. Taken together, the results are consistent with the levels of total SP-B protein expression seen by Western blot.
Figure 2.
Immunofluorescent staining of SP-B in compound SP-B mice maintained on doxycycline or following gene transfer and removal of doxycycline. Compound SP-B transgenic mice (n = 4) were either maintained on doxycycline or were taken off doxycycline and 100 µg of each plasmid was delivered to the lungs by electroporation as in Figure 1. Four days later, lungs were removed from animals and inflation-fixed for paraffin embedding and thin sectioning. Sections were reacted with antibodies against SP-B (red) and ABCA3 (green), a marker of alveolar epithelial type II cells, followed by fluorescently-labeled secondary antibodies. Sections were reacted with DAPI (blue) to visualize nuclei. Two representative fields are shown for each experimental group. All images were taken at the same exposure and settings for each antibody. Bar = 100 µm
Effects of SP-B gene delivery on lung histology in an SPB deficient mouse model
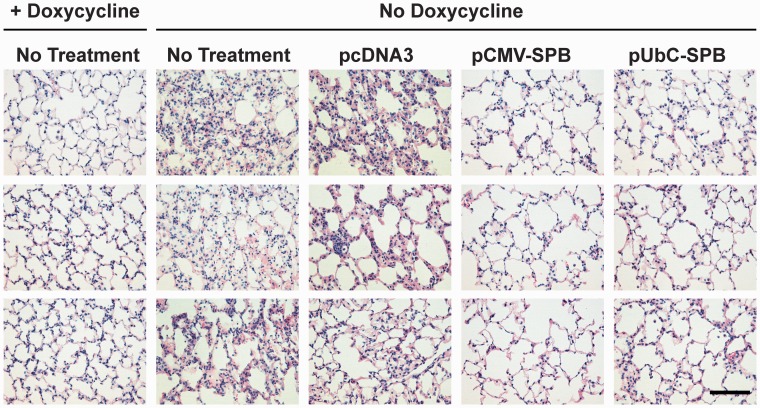
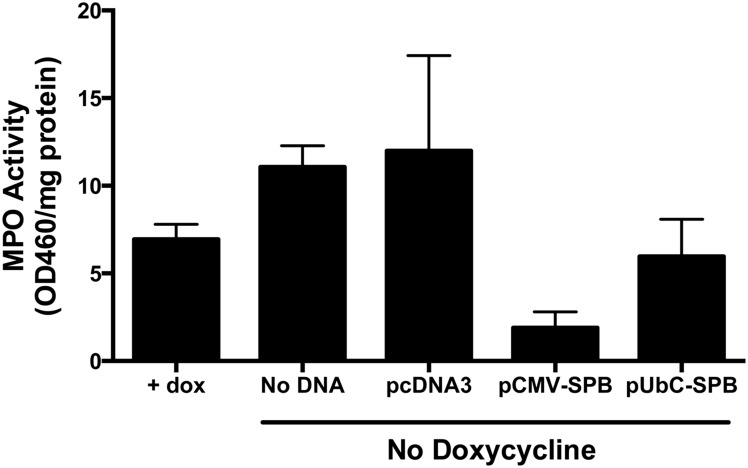
Differences between animals receiving the different plasmids were noted when histology was performed on lung sections that were stained with Hematoxylin and Eosin (Figure 3). When analyzed four days after removal of doxycycline, mice that had received the empty plasmid consistently showed thickened alveolar walls, increased cellularity, and interstitial edema, as would be expected in animals with partial SP-B deficiency,18 compared to the mice that had received SP-B-expressing plasmids. Further, the increased inflammatory infiltrates seen by histology in mice receiving no DNA or empty plasmid without doxycycline were confirmed by measuring MPO activity in lung homogenates (Figure 4). Mice that had received pUbC-SPB continued to show some alveolar wall thickening, but this was focal and heterogeneous and the majority of the lungs had more normal looking alveoli, compared to those receiving the empty plasmids or no DNA. MPO activity in these mice was also reduced compared to those receiving empty plasmid or no DNA. Mice receiving the SP-B plasmid driven by the CMV promoter had the healthiest appearing lungs with enlarged alveoli, thin alveolar walls, very little cellularity or interstitial edema, and low MPO activity.
Figure 3.
Histology of lungs isolated from compound SP-B mice following gene transfer and removal of doxycycline. The left lobes of the mouse lungs were inflation fixed with 4% paraformaldehyde and then paraffin-embedded for thin sectioning. Eight micron sections were de-paraffinized and stained with hematoxylin and eosin. Representative sections from three different mice are shown for each condition. Compound SP-B transgenic mice maintained on a doxycycline diet that received no additional treatment and those that received a diet containing no doxycycline for 4 days without DNA electroporation are also shown for comparison.
Figure 4.
Lung myeloperoxidase activity in compound SP-B mice following gene transfer and removal of doxycycline. Compound SP-B transgenic mice (n = 5) were either maintained on doxycycline or were taken off doxycycline and 100 µg of each plasmid (or no DNA) was delivered to the lungs by electroporation as in Figure 1. Four days later, lungs were removed from animals and portions were homogenized for quantitation of myeloperoxidase activity and normalized to total cell protein (mean ± st. dev.)
Effects of SP-B overexpression on lamellar body structure in SPB deficient mice
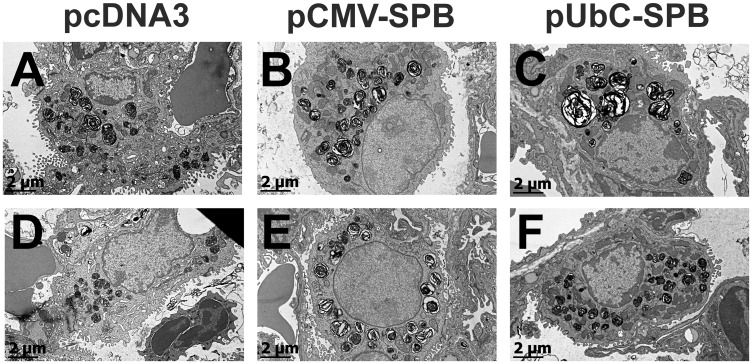
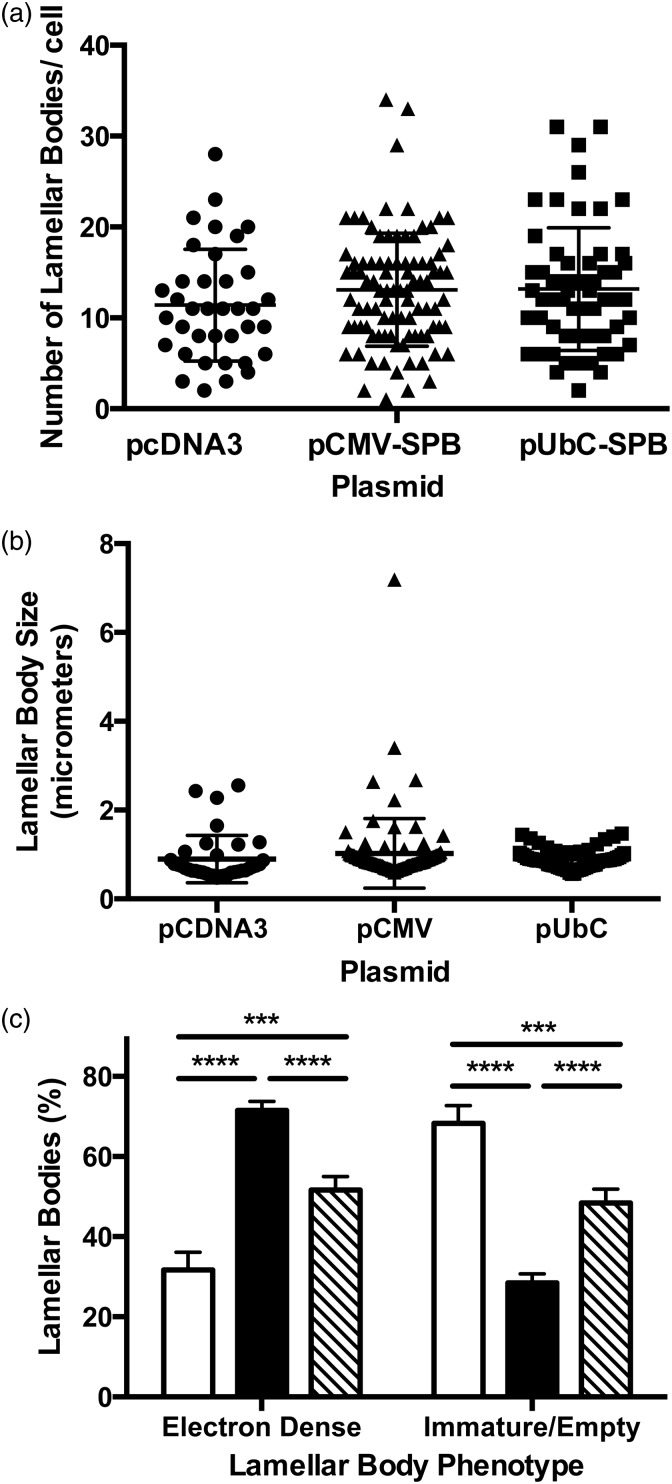
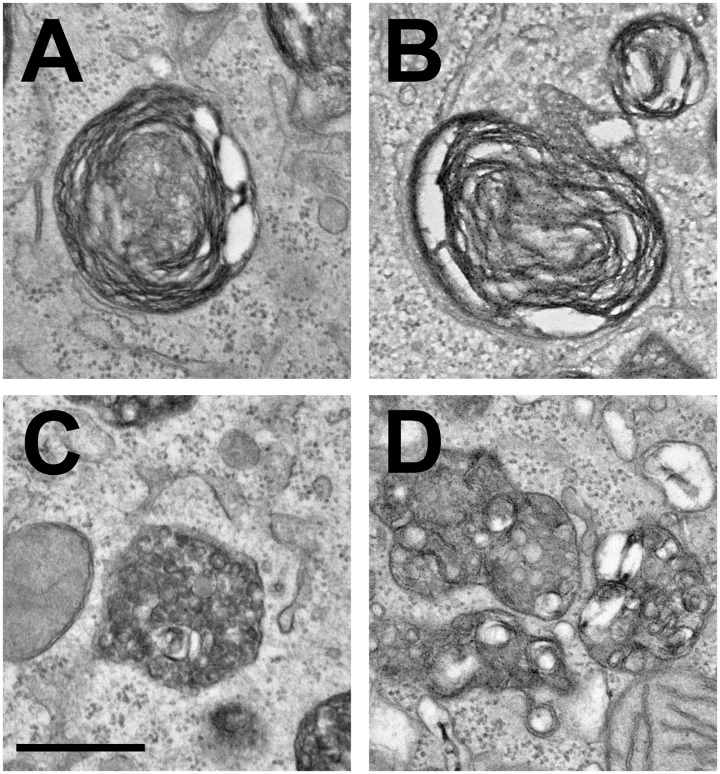
Transmission electron microscopy was carried out on thin sections of lung from compound transgenic SP-B mice that received either an empty plasmid (pcDNA3) or SP-B-expressing plasmids pCMV-SPB or pUbC-SPB. Gene transfer occurred at day 0, simultaneous with removal of doxycycline from the diet and lungs were removed and inflation-fixed four days later. To determine the effects of SP-B gene transfer on lamellar body structure, between 70 and 110 type II pneumocytes from each condition were imaged at the same magnification and contrast by electron microscopy (Figure 5). Although this simple method of analyzing lamellar bodies (or any subcellular organelle) in such two-dimensional images is inherently biased toward large lamellar bodies, because the probability that an organelle is sampled on a 2 dimensional section depends on the particle size,25 many studies have taken a similar approach.18,26,27 When lamellar bodies in each cell were analyzed, no differences were seen in the numbers or size distribution of lamellar bodies per cell (Figure 6(a) and (b)). However, when the phenotypes of the lamellar bodies were observed and quantified into two categories based on the presence of normal concentric, packed surfactant within the lamellar bodies, or those without organized lamellae or electron-dense inclusions with a bubbly appearance that denote immature lamellar bodies18,27(Figure 7), significant differences were noted (Figure 6(c)). Lamellar bodies from mice receiving the empty pcDNA3 plasmid had high numbers of empty and immature lamellar bodies, whereas those from mice receiving pCMV-SPB or pUbC-SPB had a greater percentage of electron-dense lamellar bodies containing surfactant, with the pCMV-SPB plasmid showing statistically more dense and light lamellar bodies than the pUbC-SPB plasmid (Figure 6(c)). Taken together with the overexpression and histology data, these results suggest that SPB expression may provide a physiological benefit to the mice.
Figure 5.
Electron microscopy of alveolar epithelial type II cells in lungs of compound SP-B mice following gene transfer and removal of doxycycline. Compound SP-B transgenic mice received either empty (pcDNA3; (a) and (d)), pCMV-SPB (b) and (e), or pUbC-SPB (c) and (f) plasmids by electroporation on the same day that doxycycline was removed from their diet and the lungs were fixed four days later for transmission electron microscopy as described in Materials and Methods. Two separate representative alveolar type II cells are shown for each condition
Figure 6.
Analysis of lamellar body phenotypes in mice following gene transfer. Compound SP-B transgenic taken off doxycycline and electroporated with pcDNA3, pCMV-SPB, or pUbC-SPB as in Figure 5 were analyzed for lamellar body phenotype. Between 70 and 110 type II pneumocytes were imaged at 15,000× magnification for each condition. The number of lamellar bodies in each cell was counted and their phenotype assessed as either containing packed electron-dense, concentric surfactant (“electron-dense”), or having a bubbly appearance with no organized lamellae as previously described (“immature/empty”).18 The total number of lamellar bodies in each cell (a), the average size of lamellar bodies in each cell (b) and the phenotype of lamellar bodies in each condition (c) were measured. Open bars represent mice receiving pcDNA3, closed bars represent those receiving pCMV-SPB, and hatched bars represent those receiving pUbC-SPB. ***P < 0.001 and ****P < 0.0001 by 2-way ANOVA and post hoc Tukey multiple comparisons test
Figure 7.
Representative lamellar body phenotypes in compound SP-B mice following removal of doxycycline. Electron-dense (a and b) and immature/empty (c and d) lamellar bodies representing the two phenotypes quantified in Figure 6 are shown at 50,000× magnification. Bar = 0.5 µm
Survival of SP-B deficient mouse model after gene delivery and doxycycline removal
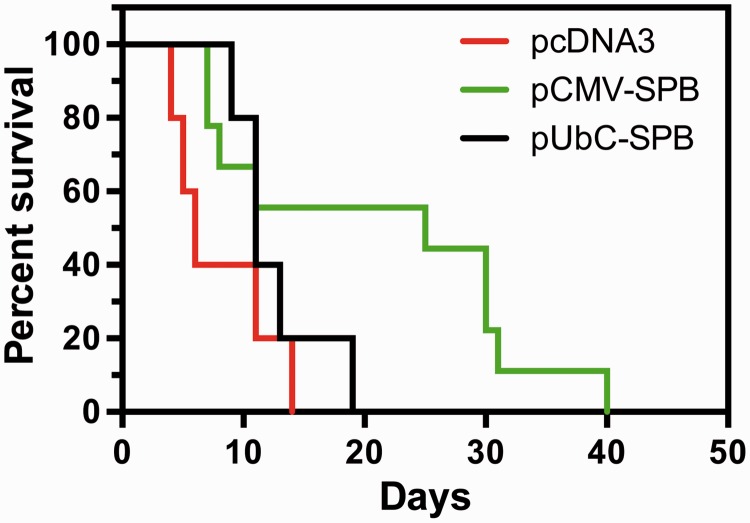
To evaluate the benefit of gene delivery on survival, the doxycycline diet was removed to shut off endogenous expression of SP-B, and mice were electroporated with SP-B plasmids on the same day. These mice were returned to the vivarium, maintained on a normal diet (no doxycycline), and followed at least twice daily for signs of impending respiratory failure. In all groups, death was preceded by severe respiratory distress and upon necropsy, lungs were edematous and showed signs of hemorrhage consistent with acute lung injury.13 As previously reported, mice receiving no intervention became moribund between day 5 and 7 with a median survival time of 5.5 days,15,16 as did mice receiving empty plasmid (median survival of 6 days, Figure 8). Mice receiving pUbC-SPB had a median survival of 11 days with several animals living to 13 days and one even to 19 days. Mice receiving pCMV-SPB showed the greatest survival, with a median survival of 25 days with 100% of the mice surviving beyond 7 days (P < 0.02) and several mice surviving until day 40.
Figure 8.
Electroporation-mediated gene transfer of hSP-B improves survival of compound SP-B transgenic mice. Compound SP-B transgenic mice (n = 18 per condition) were taken off doxycycline and plasmids were delivered to the lungs by aspiration and electroporation as described in Materials and Methods. Mice were maintained off of doxycycline for the remainder of the experiment and observed at least twice daily for signs of respiratory distress. Animals deemed moribund or in extreme respiratory failure were euthanized. P < 0.05 by log-rank (Mantel–Cox) test
Discussion
Gene therapy for SP-B deficiency is an attractive treatment option. In the current study, we have demonstrated that electroporation-mediated gene delivery of human SP-B can improve median survival by 2- to 5-fold (5–25 days) in a compound SP-B knockout mouse model, without any adverse effects. Not only were improvements seen in survival, but also in lung histology at four days after gene transfer and at time of death. Electroporation has multiple advantages, including quick and easy application, simple and inexpensive production of plasmid with the gene of interest, and minimal inflammatory or trauma to the lungs of animals.28,29 To our knowledge, this is the first time electroporation has been studied to treat neonatal lung disease and may prove to be a promising treatment for other neonatal genetic lung diseases, such as cystic fibrosis, alpha antitrypsin1 deficiency, surfactant protein C (SP-C) deficiency or ABCA3 mutations.
Previous studies aimed at SP-B gene therapy have used adenoviral-mediated gene delivery to produce human SP-B expression in both in vitro and in vivo experiments.8 In vitro experiments using adenoviral gene delivery to MLE 12 cells, which retain properties similar to alveolar type II cells but have low endogenous SP-B expression, showed increased SPB expression. In vivo experiments were performed in cotton rats that were infected with recombinant adenoviral vectors expressing hSP-B. Maximum expression of hSP-B mRNA was detected at 48–96 h post-infection and subsequently decreased to undetectable levels. Mouse and human SP-B mRNA production were differentiated and there was no change in mouse SPB of the infected group, nor were there any changes in SP-C expression or processing.8 A mild, inflammatory response was noted in the lung samples of the adenovirus infected group, which resolved by three to four weeks post-infection. Clearly, in neonates that are sick and fragile, any such inflammation could be devastating. Unfortunately, in the absence of any disease model for SP-B deficiency or physiological data, no clinically applicable conclusion could be made.
Several more recent studies have focused on newer gene technologies for treatment of SP-B deficiency. In one, modified mRNA for hSP-B was used as a treatment for SP-B deficiency in the SP-B compound knockout mouse model used in the current study and had very promising results.30 Kormann and his co-workers31 delivered modified mRNA for hSP-B to the lungs of the SP-B compound knockout mice via an intratracheal spray method. By performing gene delivery twice weekly, survival improved up to 28 days off doxycycline. Improved survival, normalized lung function, minimal cytokine production, and 72% of normal SP-B protein expression were among the findings. At four days post mRNA, the histological and IHC findings were similar to ours, with increased edema and cellularity seen four days after gene delivery in the negative controls. However, there is still much to be learned about the stability and immune response of the body to mRNA delivery and complementary approaches are valuable. A second study from the same group developed a novel zinc finger nuclease to target the SP-B cassette and delivered this to the lung as a modified mRNA using chitosan-coated poly(lactic-co-glycolic) acid nanoparticles.31 When delivered intratracheally along with Adeno-Associated virus 6 carrying a wild type copy of the intact SP-B gene as a donor template, gene correction was achieved and resulted in high SP-B protein levels for at least 20 days and enhanced survival for out to 35 days. While this approach did not elicit significant inflammation, the combined use of multiple delivery methods (non-viral and viral) could be cumbersome or a regulatory difficulty.
Our studies show that electroporation can be used to direct high level gene expression in the lung.10–12,32 The group of transgenic mice treated with either pUbC-hSPB or pCMV-hSPB had improved survival, strong protein expression, improved histology, and a higher percentage of lamellar bodies filled with electron-dense, concentric material than the transgenic mice that received an empty plasmid. Most striking was the effects on survival, in which plasmids expressing SP-B from the UbC or CMV promoter increased median survival time by 2- or 5-fold, respectively. While animals receiving the UbC-SPB plasmid showed a classic survival curve, those receiving the CMV promoter-driven construct displayed a bimodal distribution of animals, in which approximately 40% of the animals lived four to five days longer on average than those receiving empty plasmids, and the remaining animals survived 20 to 30 days longer than their controls. One possibility is that there were two populations of SP-B mice with differing degrees of endogenous SP-B loss at the time of intervention, and in those that lost endogenous SP-B expression sooner showed less benefit from gene transfer at early times. However, if this were the case, similar curves would be expected for control and UbC plasmids as well. Alternatively, this could be due to the differential distribution of gene transfer and/or expression in these animals or to absolute levels of gene transfer and expression in each animal, which remains to be seen. Alternatively, it is possible that the UbC promoter-driven plasmids were delivered to and expressed in cells that are not normally SP-B producers. In support of this, animals receiving the pUbC-SPB plasmids showed some degree of high level gene expression in non-alveolar epithelial type II cells in the parenchyma and a large number of airway epithelial cells. By contrast, mice receiving the CMV promoter-driven plasmid showed most of their expression in alveolar epithelial type II cells and just a few airway epithelial cells. It is possible that this high level, potentially “off-target” expression in non-type II cells seen with the UbC construct, much of which could be non-processed proSP-B precursors, could lead to cell stress and outweigh the beneficial effects of correctly processed SP-B.
In the presence of doxycycline, we found that most alveolar epithelial type II cells (as defined by ABCA3 staining) also show positive staining for SP-B in these compound SP-B transgenic mice. However, we also detect occasional cells in the airway epithelium that are highly SP-B positive with greatly increased numbers of these cells in animals receiving the UbC promoter-driven plasmids. Club cells have been shown to express SP-B but secrete an incorrectly processed SP-B species and these could account for the SP-B positive cells we see.33–36 Other precursor progenitor cell populations exist in the airways that can give rise to alveolar epithelial type II cells but since they proliferate and differentiate only upon significant injury to the alveolar epithelium, it is unlikely that these cells account for those seen in our sections.33,37 However, it is also possible that our antibody, while only detecting the 18 kdal SP-B dimer on non-reducing gels also detects other forms of the protein in thin sections and that expression of high levels of SP-B in the airway represents mis-processed or non-processed SP-B.
Since both the UbC and the CMV promoters are ubiquitously active in most mammalian cells and electroporation delivers DNA to all cell types in the lung,10 it is highly likely that the SP-B transgene is expressed in a number of non-type II cells throughout the lung. Since only alveolar type II cells have the appropriate machinery to properly process SP-B,38 the consequences of immature SP-B expression in other lung cells are unclear. While the SP-B expressed in these non-type II cells will not be processed, it may be secreted since the transgene carries the endogenous signal peptide.34 If secreted, it is possible that this immature, full length SP-B protein could be endocytosed by type II pneumocytes throughout the lung and subsequently correctly processed in the endosomes/lamellar bodies, as such surfactant recycling occurs as part of the normal physiology in the lung.38 Thus, even if expressed in these other cell types and not correctly processed, the exogenously expressed SP-B could produce functional surfactant.
While the ultimate goal of most gene therapy approaches would be to permanently correct the genetic defect, this may not always be necessary. Indeed, in the case of the surfactant deficiencies, lung transplantation is a viable and highly successful treatment option resulting in approximately 40–50% survival at five years with normal lung function and quality of life in those infants that survive to transplantation. The average time of diagnosis of a genetic surfactant deficiency is 19 days post-delivery and the time between placing an infant on the transplant list and receiving an organ is 76 days.5,6 Since 30% of these infants die before transplantation,6 it could be very efficacious to use gene therapy as a bridge between diagnosis and transplantation. If gene therapy using this or any approach can restore surfactant activity to the lungs even for one or more weeks, this could greatly increase their likelihood of surviving to transplantation.
Authors’ contributions
The authors made a substantial contribution to the concept and design, acquisition of data and analysis and interpretation of data (RB, XL, MB, RN, KB, FF, JY, DD); drafted the article (RB and DD) and revised it critically for important intellectual content (RB, XL, JY, and DD); and approved the version to be published (RB, XL, MB, RN, KB, FF, JY, DD).
Acknowledgements
We would like to thank Patricia Chess and Daria Krenitsky for technical assistance, insightful discussions and advice and Gayle Schneider of the URMC Electron Microscope Shared Resource. We would like to thank Tim Weaver (Cincinnati Children’s Research Foundation) and Rick Wetsel (University of Texas Health Science Center at Houston) for providing us with the compound SPB mice, and Guirong Wang (State University of New York, Upstate Medical Center) and Larry Nogee (Johns’ Hopkins University) for gifts of surfactant protein B antisera. This work was supported in part by postdoctoral training grant T32 HD57821, and grants EB9903, HL81148, HL92801, and HL107331 from the National Institutes of Health.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Walther FJ, Waring AJ, Sherman MA, Zasadzinski JA, Gordon LM. Hydrophobic surfactant proteins and their analogues. Neonatology 2007; 91: 303–10. [DOI] [PubMed] [Google Scholar]
- 2.Nogee LM, de Mello DE, Dehner LP, Colten HR. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med 1993; 328: 406–10. [DOI] [PubMed] [Google Scholar]
- 3.Nogee LM, Garnier G, Dietz HC, Singer L, Murphy AM, deMello DE, Colten HR. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest 1994; 93: 1860–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamvas A, Cole FS, deMello DE, Moxley M, Whitsett JA, Colten HR, Nogee LM. Surfactant protein B deficiency: antenatal diagnosis and prospective treatment with surfactant replacement. J Pediatr 1994; 125: 356–61. [DOI] [PubMed] [Google Scholar]
- 5.Hamvas A, Nogee LM, Mallory GB, Jr., Spray TL, Huddleston CB, August A, Dehner LP, deMello DE, Moxley M, Nelson R, Cole FS, Colten HR. Lung transplantation for treatment of infants with surfactant protein B deficiency. J Pediatr 1979; 130: 231–9. [DOI] [PubMed] [Google Scholar]
- 6.Palomar LM, Nogee LM, Sweet SC, Huddleston CB, Cole FS, Hamvas A. Long-term outcomes after infant lung transplantation for surfactant protein B deficiency related to other causes of respiratory failure. J Pediatr 2006; 149: 548–53. [DOI] [PubMed] [Google Scholar]
- 7.Vincent MC, Trapnell BC, Baughman RP, Wert SE, Whitsett JA, Iwamoto HS. Adenovirus-mediated gene transfer to the respiratory tract of fetal sheep in utero. Hum Gene Ther 1995; 6: 1019–28. [DOI] [PubMed] [Google Scholar]
- 8.Yei S, Bachurski CJ, Weaver TE, Wert SE, Trapnell BC, Whitsett JA. Adenoviral-mediated gene transfer of human surfactant protein B to respiratory epithelial cells. Am J Respir Cell Mol Biol 1994; 11: 329–36. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther 2003; 10: 935–40. [DOI] [PubMed] [Google Scholar]
- 10.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level non-viral gene transfer to the lung. Gene Ther 2003; 10: 1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GRS, Yeldandi A, Sznajder JI, Dean DA. Gene transfer of the Na+,K+-ATPase b1 subunit using electroporation increases lung liquid clearance in rats. Am J Respir Crit Care Med 2005; 171: 204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutlu GM, Machado-Aranda D, Norton JE, Bellmeyer A, Urich D, Zhou R, Dean DA. Electroporation-mediated gene transfer of the Na+,K+-ATPase rescues endotoxin-induced lung injury. Am J Respir Crit Care Med 2007; 176: 582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin X, Barravecchia M, Kothari P, Young JL, Dean DA. beta1-Na(+),K(+)-ATPase gene therapy upregulates tight junctions to rescue lipopolysaccharide-induced acute lung injury. Gene Ther 2016; 23: 489–99. [DOI] [PubMed] [Google Scholar]
- 14.Emr BM, Roy S, Kollisch-Singule M, Gatto LA, Barravecchia M, Lin X, Young JL, Wang G, Liu J, Satalin J, Snyder K, Nieman GF, Dean DA. Electroporation-mediated gene delivery of Na+,K+ -ATPase, and ENaC subunits to the lung attenuates acute respiratory distress syndrome in a two-hit porcine model. Shock 2015; 43: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melton KR, Nesslein LL, Ikegami M, Tichelaar JW, Clark JC, Whitsett JA, Weaver TE. SP-B deficiency causes respiratory failure in adult mice. Am J Physiol Lung Cell Mol Physiol 2003; 285: L543–9. [DOI] [PubMed] [Google Scholar]
- 16.Clark JC, Wert SE, Bachurski CJ, Stahlman MT, Stripp BR, Weaver TE, Whitsett JA. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci U S A 1995; 92: 7794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottfried L, Lin X, Barravecchia M, Dean DA. Identification of an alveolar type I epithelial cell-specific DNA nuclear import sequence for gene delivery. Gene Ther 2016; 23: 734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nesslein LL, Melton KR, Ikegami M, Na CL, Wert SE, Rice WR, Whitsett JA, Weaver TE. Partial SP-B deficiency perturbs lung function and causes air space abnormalities. Am J Physiol Lung Cell Mol Physiol 2005; 288: L1154–61. [DOI] [PubMed] [Google Scholar]
- 19.Bijli KM, Kanter BG, Minhajuddin M, Leonard A, Xu L, Fazal F, Rahman A. Regulation of endothelial cell inflammation and lung polymorphonuclear lymphocyte infiltration by transglutaminase 2. Shock 2014; 42: 562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee AH, Suh YS, Sung JH, Yang SH, Sung YC. Comparison of various expression plasmids for the induction of immune response by DNA immunization. Mol Cells 1997; 7: 495–501. [PubMed] [Google Scholar]
- 21.Norman JA, Hobart P, Manthorpe M, Felgner P, Wheeler C. Development of improved vectors for DNA-based immunization and other gene therapy applications. Vaccine 1997; 15: 801–3. [DOI] [PubMed] [Google Scholar]
- 22.Yew NS, Przybylska M, Ziegler RJ, Liu D, Cheng SH. High and sustained transgene expression in vivo from plasmid vectors containing a hybrid ubiquitin promoter. Mol Ther 2001; 4: 75–82. [DOI] [PubMed] [Google Scholar]
- 23.Gill DR, Smyth SE, Goddard CA, Pringle IA, Higgins CF, Colledge WH, Hyde SC. Increased persistence of lung gene expression using plasmids containing the ubiquitin C or elongation factor 1alpha promoter. Gene Ther 2001; 8: 1539–46. [DOI] [PubMed] [Google Scholar]
- 24.Gazdhar A, Bilici M, Pierog J, Ayuni EL, Gugger M, Wetterwald A, Cecchini M, Schmid RA. In vivo electroporation and ubiquitin promoter – a protocol for sustained gene expression in the lung. J Gene Med 2006; 8: 910–8. [DOI] [PubMed] [Google Scholar]
- 25.Hsia CC, Hyde DM, Ochs M, Weibel ER. ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 2010; 181: 394–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark JC, Wert SE, Bachurski CJ, Stahlman MT, Stripp BR, Weaver TE, Whitsett JA. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci U S A 1995; 92: 7794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stahlman MT, Gray MP, Falconieri MW, Whitsett JA, Weaver TE. Lamellar body formation in normal and surfactant protein B-deficient fetal mice. Lab Invest 2000; 80: 395–403. [DOI] [PubMed] [Google Scholar]
- 28.Murakami T, Sunada Y. Plasmid DNA gene therapy by electroporation: principles and recent advances. Curr Gene Ther 2011; 11: 447–56. [DOI] [PubMed] [Google Scholar]
- 29.Lin X, Dean DA. Gene therapy for ALI/ARDS. Crit Care Clin 2011; 27: 705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kormann MS, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S, Huppmann M, Mays LE, Illenyi M, Schams A, Griese M, Bittmann I, Handgretinger R, Hartl D, Rosenecker J, Rudolph C. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat Biotechnol 2011; 29: 154–7. [DOI] [PubMed] [Google Scholar]
- 31.Mahiny AJ, Dewerth A, Mays LE, Alkhaled M, Mothes B, Malaeksefat E, Loretz B, Rottenberger J, Brosch DM, Reautschnig P, Surapolchai P, Zeyer F, Schams A, Carevic M, Bakele M, Griese M, Schwab M, Nurnberg B, Beer-Hammer S, Handgretinger R, Hartl D, Lehr CM, Kormann MS. In vivo genome editing using nuclease-encoding mRNA corrects SP-B deficiency. Nat Biotechnol 2015; 33: 584–6. [DOI] [PubMed] [Google Scholar]
- 32.Degiulio JV, Kaufman CD, Dean DA. The SP-C promoter facilitates alveolar type II epithelial cell-specific plasmid nuclear import and gene expression. Gene Ther 2010; 17: 541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee M, Domm W, Gelein R, de Mesy Bentley KL, Kottmann RM, Sime PJ, Lawrence BP, O’Reilly MA. Alternative progenitor lineages regenerate the adult lung depleted of type II cells. Am J Respir Cell Mol Biol 2017; 56: 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Reilly MA, Weaver TE, Pilot-Matias TJ, Sarin VK, Gazdar AF, Whitsett JA. In vitro translation, post-translational processing and secretion of pulmonary surfactant protein B precursors. Biochim Biophys Acta 1989; 1011: 140–8. [DOI] [PubMed] [Google Scholar]
- 35.Phelps DS, Floros J. Localization of surfactant protein synthesis in human lung by in situ hybridization. Am Rev Respir Dis 1988; 137: 939–42. [DOI] [PubMed] [Google Scholar]
- 36.Lin S, Na CL, Akinbi HT, Apsley KS, Whitsett JA, Weaver TE. Surfactant protein B (SP-B) −/− mice are rescued by restoration of SP-B expression in alveolar type II cells but not clara cells. J Biol Chem 1999; 274: 19168–74. [DOI] [PubMed] [Google Scholar]
- 37.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BLM. The Role of Scgb1a1+ clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009; 4: 525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weaver TE, Whitsett JA. Processing of hydrophobic pulmonary surfactant protein B in rat type II cells. Am J Physiol 1989; 257: L100–8. [DOI] [PubMed] [Google Scholar]