Abstract
Objective
Post-operative delirium is associated with pre-operative cognitive difficulties and diminished functional independence, both of which suggest that brain pathology may be present in affected individuals prior to surgery. Currently, there are few studies that have examined imaging correlates of post-operative delirium. To our knowledge, none have examined the association of delirium with existing structural pathology in pre-operative cancer patients. Here, we present a novel, retrospective strategy to assess pre-operative structural brain pathology and its association with post-operative delirium. Standard of care structural magnetic resonance imaging (MRIs) from a cohort of surgical candidates prior to surgery were analyzed for white matter hyperintensities and cerebral atrophy.
Methods
We identified 23 non-small cell lung cancer patients with no evidence of metastases in the brain pre-operatively, through retrospective chart review, who met criteria for post-operative delirium within 4 days of surgery. 24 age- and gender-matched control subjects were identified for comparison to the delirium sample. T1 and fluid-attenuated inversion recovery sequences were collected from standard of care pre-operative MRI screening and assessed for white matter pathology and atrophy.
Results
We found significant differences in white matter pathology between groups with the delirium group exhibiting significantly greater white matter pathology than the non-delirium group. Measure of cerebral atrophy demonstrated no significant difference between the delirium and non-delirium group.
Conclusions
In this preliminary study utilizing standard of care pre-operative brain MRIs for assessment of structural risk factors to delirium, we found white matter pathology to be a significant risk factor in post-operative delirium. Limitations and implications for further investigation are discussed.
Background
Delirium following surgical procedures is of particular concern given its association with longer hospital stays [1], higher rates of admission to post-acute care facilities, higher rates of mortality [2–4], and long-term decline in functional and cognitive status [5–16]. The frequency of delirium following hip surgery ranges from 5% to 62% [17–22] and following vascular or cardiac surgery from 23% to 42% [23–28]. Although little information is available for rates of post-operative delirium in cancer patients, a recent retrospective study found 50% of patients undergoing surgery for esophageal cancer exhibited post-operative delirium [29]. Prior research has found that patient age, cognitive decline, and poor functional status prior to surgery are highly significant predictors of post-operative delirium [30–33]. A potential underlying factor that may link these predictors is identified in pre-operative brain pathology, either in the form of age appropriate changes in brain structure, or in clinically significant neurodegeneration consistent with dementing illnesses. A better understanding of pre-operative brain pathology and its potential role in post-operative delirium may shed light on how and why delirium develops and may lead to targets for intervention and treatment.
Prior research investigating predictors of delirium have found premorbid cognitive dysfunction and decreased activities of daily living to be a significant predictor to development of delirium [4,34] and potentially independent of other risk factors such as age and premorbid vascular pathology [35]. Such findings suggest a predisposition to delirium associated with pre-existing cognitive decline and raise the question of pre-existing neuropathology that may lower the threshold for emergent post-operative delirium. Structural neuroimaging has been previously identified as a potentially powerful method to be utilized in understanding the pathogenesis, course, and long-term outcome of episodic delirium [36], although, to date, only a handful of investigations have been undertaken utilizing this method either pre-operatively or post-operatively.
In an early prospective magnetic resonance imaging (MRI) study investigating the association of delirium with anti-depressant medication treatment [37], 5 of 60 individuals went on to exhibit delirium that proved to be reversible once medication was ceased; basal ganglia infarcts were demonstrated in all five individuals who developed delirium. In a prospective CT/MRI study by the same group investigating delirium following electroconvulsive treatment in the depressed elderly [38], 6 of 36 individuals receiving treatment developed delirium; basal ganglia and white matter lesions were found in all affected individuals. In a follow-up prospective MRI study also investigating electroconvulsive treatment-induced delirium in depressed elderly [39], 10 of 87 individuals went on to exhibit delirium; similar to these previous studies, basal ganglia lesions (9/10) were exhibited in individuals with delirium, together with moderate periventricular hyperintensities (6/10), and moderate deep white matter hyperintensities (WMHs) (8/10) similar to previous investigations. In a larger study [40] that included 69 cases of mixed etiology delirium and 31 controls, delirium cases exhibited a greater number of focal pathological findings (41/69) versus controls (3/31) with the predominance of lesions found in the right parieto-occipital area (19/69), and significant pathology distributed both cortically and in deep right and left hemispheric areas. Work utilizing diffusion tensor imaging, which allows for analysis of regional white matter integrity, found an independent association of frontal and thalamic white matter integrity with post-operative delirium [41]. Recently, results of the first study to investigate the association of pre-operative brain pathology with delirium in patients undergoing cardiac surgery reported significantly greater white matter burden in the delirium group [42]. Although focal lesions appear to be associated with greater risk of delirium, evidence of diffuse, structural differences in individuals with delirium, marked by cortical atrophy and ventricular enlargement [38,40,43] have also been found. Despite general agreement in findings of greater neuropathology in delirium, questions regarding the association and specificity of structural pathology with delirium remain.
Functional studies into the structural changes associated with delirium are few and have been limited to regional and global cerebral perfusion [36,44] in the acute and sub-acute phases of a delirium episode. Early reports of single cases, primarily utilizing single positron emission computed tomography, generally found cortical hypoperfusion with varying degrees of specificity over cortical sites including prefrontal [45,46], temporal [46–48], parietal [46,49], and occipital cortex [47]. Studies incorporating larger samples with heterogeneous delirium etiologies (e.g., cardiac surgery, hepatic encephalopathy, substance withdrawal, and cirrhosis) find similar results of diffuse hypoperfusion in delirium with some evidence of more regionally specific cortical decreases [50–58]. In line with the structural studies discussed previously, a subset of studies also find focal changes in non-cortical regions, including both increases and decreases in basal ganglia perfusion [51,57–59], as well as thalamic decreases [60].
With some exceptions, the functional and structural studies described previously are generally hampered by small sample size, inclusion of mixed delirium etiologies (pharmacologic, post-operative, electroconvulsive therapy, and emergency room admissions), and varying criteria for identifying delirium status, limiting the interpretation of findings. Further, only three studies utilize structural neuroimaging prior to delirium onset and none on post-operative delirium exclusively, although to what extent postoperative delirium represents a homogenous entity in its own right is open to question. Despite these limitations, results of structural and functional neuroimaging studies suggest both structural correlates that may predispose to delirium, as well as significant changes in brain perfusion that are broadly consistent with structural pathology and global function following resolution of delirium.
Here, we report findings from a preliminary investigation of structural brain predictors of post-operative delirium in individuals undergoing surgical procedures for non-small cell lung cancer. The goal of this study was to assess the association of pre-operative structural brain pathology, including cerebral atrophy and white matter pathology, with post-operative delirium. This study utilized a novel strategy for retrospective data collection, image analysis, and patient identification that capitalized on standard of care, clinically acquired imaging data in our non-small cell lung cancer patient population that may be usefully applied in prospective follow-up analyses. Individuals undergoing surgery for lung cancer are commonly imaged pre-operatively with MRI if considered at risk for brain metastases. As a result, a large retrospective dataset of structural brain MRI studies that characterizes brain pathology evident in these patients prior to surgery is available. Given the high cost of obtaining structural MRI data in a prospective cohort in which only a subset will exhibit delirium post-operatively, this retrospective strategy allows for analysis of a larger cohort of known delirium cases who will already have had an extensive, pre-operative, standard of care MRI study of the brain. Although previous neuroimaging studies of delirium suggest the involvement of focal lesions, as a first step in characterizing pathology in our cohort, we decided to calculate a global WMH burden together with global cerebral atrophy. This decision was in part due to the lack of previous studies in this cohort, together with limitations on analysis due to the imaging data available. On the basis of previous studies that found both atrophic and white matter changes to be associated with incident delirium, we predicted that individuals with post-operative delirium would exhibit a greater proportion of both cerebral atrophy and white matter pathology than unaffected control cases.
Methods
Data collection
Medical records of patients who underwent pre-operative MRI of the brain before anatomic lung resection for non-small cell carcinoma were reviewed through the Memorial Sloan Kettering Health Care Information System. Relevant information, including history, diagnosis, surgical procedure, duration of surgery, type of anesthetic, and peri-operative, post-operative, and course of stay variables were recorded. This research was approved by the Memorial Sloan Kettering Cancer Center IRB. Given that this study was retrospective, a waiver of authorized consent was utilized for collection of data.
Patient identification and categorization
Patients who underwent anatomic lung resection as treatment for non-small cell lung cancer after pre-operative MRI imaging of the brain were included for review. Exclusion criteria included evidence of current or prior metastatic central nervous system disease, other neurological disease that would affect brain imaging analysis (e.g., multiple sclerosis), or evidence of significant pre-operative cognitive decline consistent with a dementing illness. Patient selection was performed utilizing a chart-based method used by previous researchers in retrospective identification of patients meeting criteria for delirium [61]. In a first pass, medical records were reviewed for “high likelihood” post-operative delirium status as indicated by record of delirium diagnosis, order for psychiatric consult, order for one-to-one companion sitter, or novel prescription of anti-psychotic within 4 days post-surgery; identified patients were then reviewed by trained personnel in detail to confirm clinical status as indicated by attending, psychiatry, and nursing staff notes. Identified cases were then confirmed by an independent rater for presence of confusion, disorientation, hyperactivity, and/or impulsivity post-operatively. Following characterization of the delirium patient group, an unaffected control group was drawn from records previously identified as low-likelihood of delirium and who had no evidence of delirium in detailed chart review. Unaffected controls were age and gender matched to the delirium group to ensure comparability of groups.
Image collection
All sequences were acquired on clinically dedicated 1.5T General Electric magnets at Memorial Sloan Kettering Cancer Center. Because image data were collected from routine clinical scans from multiple scanners, scan parameters for both T1 and fluid-attenuated inversion recovery (FLAIR) sequences varied somewhat between individual scans, and the range for each parameter, if variable, is reported in parentheses following the mean value of the parameter. Standard of care, pre-surgical MRI screens include an axial, T1-weighted, spin echo sequence [slice thickness 5, gap 2.5 (24 cases), gap 0 (21 cases), number of slices 25 (20–32), TR 497 (range 400–633), TE 11 (range 8–21)], and a FLAIR sequence [slice thickness 5 mm, gap 2.5 (24 cases), gap 0 (21 cases), number of slices 25 (range 20–32), TR 9209 (range 8002–10002), TE 150 (range 120–172), TI 2160 (range 2000–2250)]. Retrospective imaging data of identified patients, consisting of original dicom format T1 and FLAIR-weighted image sequences, were collected utilizing the picture archiving communication system and stored in the neuroanesthesiology laboratory for processing and analysis.
Image processing
Semi-automated image processing analysis was performed utilizing Statistical Parametric Mapping Software (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/) in the Matlab 7 environment (The MathWorks), together with the MRIcron software package (http://www.mccauslandcenter.sc.edu/mricro/mricron/). Gray, white, and cerebrospinal fluid (CSF) quantification – All T1 sequences were segmented using SPM8, and segmentation quality was reviewed in both SPM8 and mricron: T1 sequences were segmented in native space into gray, white, and CSF with resulting maps intensity filtered in MRIcron, with total number of voxels falling in each tissue class entered into analysis. WMH quantification – FLAIR sequences for each patient were processed with a semi-automated routine as described by Gurol et al. [62] using the MRIcron software package: (i) a variable intensity filter was applied to each FLAIR volume and adjusted for optimal identification of WMH voxels to generate a mask image; (ii) a second mask image was defined by coarse manual tracing of the brain volume to exclude extra-brain tissue; and (iii) masks from steps 1 and 2 were combined to create a conjunction mask, and total number of voxels representing WMHs entered into analysis.
Image Analysis
Primary endpoints consisted of total white matter hyperintensity burden (WMHB) and cerebral atrophy (CA) in both groups. Total values for tissue volumes and WMHs were analyzed in the Statistical Package for the Social Sciences (SPSS) software package. CA was calculated from the ratio of CSF to total intracranial volume (gray, white, and CSF tissues combined). WMHB was calculated by the ratio of WMH to total intracranial volume (gray, white, and CSF tissue classes combined). Outliers for each group were trimmed such that any outlier values were adjusted to two standard deviations above the mean for each group [63]. For this analysis, two outlier values for WMHB were identified that were three standard deviations above the group mean for the control group and trimmed to two standard deviations above the mean for that group. Both CA and WMHB values were entered into a one-way, between subjects ANCOVA to compare the effect of group (Delirium versus Control) on WMHB and CA, with patient age and gender included as covariates
Results
Patient characteristics
For the delirium group, 24 patients were initially identified through medical record review. One patient was excluded as a result of prior resection. The remaining 23 patients in the delirium group had a mean age of 73.39 years (range 54–86; 13 women; 10 men), had received orders for anti-psychotic treatment or bedside companion following surgery (Table 1), and exhibited presence of confusion, disorientation, hyperactivity, and/or impulsivity in chart review. For the control group, 24 patients were identified through medical record review with a mean age of 73.63 (13 women; 11 men) for whom no diagnosis of delirium, order for anti-psychotic treatment, or bedside companion was recorded (Table 1). Procedures for both groups consisted of partial removal of lung, bilobectomy, segmentectomy, sleeve lobectomy, and thoracoscopy.
Table 1.
Patient demographics and treatment variables
| Delirium (n = 23) | Control (n = 24) | |
|---|---|---|
| Age | 73.39 (54–86) | 73.63 (54–85) |
| Gender | 13 female | 13 female |
| Procedure | ||
| Partial removal of lung | 13 | 15 |
| Bilobectomy | 2 | 1 |
| Segmentectomy | 4 | 4 |
| Sleeve lobectomy | 1 | 1 |
| Thoracoscopy | 3 | 3 |
| Duration hours (sd) | 2:53 (1:08) | 3:24 (1:24) |
| Anesthetic | ||
| Propofol | 2 | 3 |
| Isoflurane | 1 | 2 |
| Sevoflurane | 1 | 2 |
| Propofol isoflurane | 8 | 5 |
| Propofol sevoflurane | 9 | 12 |
| Propofol isoflurane sevoflurane | 1 | 0 |
| Midazolam | 14 | 16 |
| Dexmedetomidine | 1 | 0 |
| Epidural | 17 | 16 |
| Minimum systolic blood pressure | 97.91 (14.703) | 96.42 (7.723) |
| Treatment | ||
| Anti-psychotic only | 4 | – |
| Time to anti-psychotic treatment in days (range) | 2 (2–3) | |
| Bedside companion | 12 | – |
| Time to bedside companion in days (range) | 2.5 (0–4) | |
| Anti-psychotic and bedside companion | 7 | – |
| Time to treatment in days (range) | 2.13 (1.17) | – |
White matter hyperintensity burden
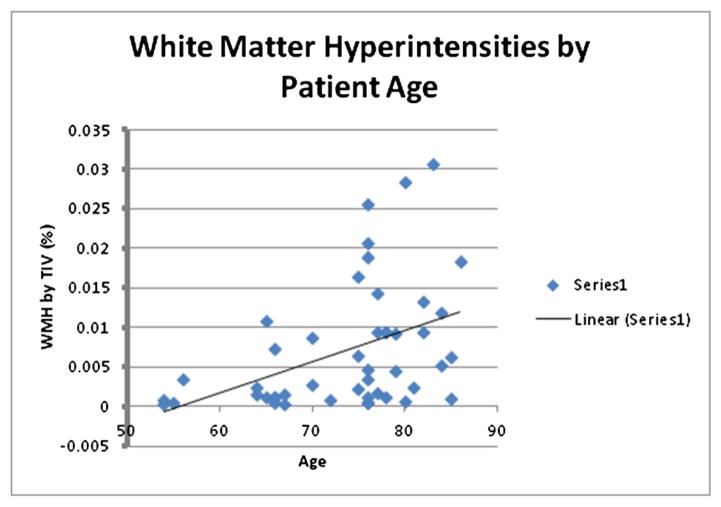
For the analysis of WMH burden, a one-way, between subjects ANCOVA was conducted to compare the effect of group (Delirium versus Control) on WMHB with patient age and gender included as covariates (Table 2). There was a significant main effect of group [F(1, 43) = 6.14, p = 0.017] on WMHB, with the delirium group exhibiting greater pathology than the control group (Figure 1(a)), and a significant effect of age, [F(1, 43) = 11.10, p = 0.002], with advancing age being significantly associated with greater WMHB (Figure 2).
Table 2.
White matter hyperintensities and cerebral atrophy differences between groups
| Delirium (n = 23) | Control (n = 24) | Significance | |
|---|---|---|---|
| WMH by cranial volume | Mean (sd): 0.01 (0.01) 95% CI: (0.005–0.015) | Mean (sd): 0.005 (0.005) 95% CI: (0.003–0.007) | F(3) = 6.144 p = 0.017* |
| CSF by cranial volume | Mean (sd): 0.37 (0.05)95% CI: 0.35–0.39 | Mean (sd): 0.35 (0.04) 95% CI: 0.33–0.37 | F(3) = 2.551 p = 0.118 |
WMH, white matter hyperintensity; CSF, cerebrospinal fluid.
p <0.05.
Figure 1.
(a) White matter hyperintensities by group. The delirium group exhibited a greater proportion of white matter hyperintensities, corrected for total intracranial volume (TIV), than the control group (p = 0.017); (b) cerebral atrophy by group. Cerebral atrophy did not differ significantly between groups (p = 0.118)
Figure 2.
White matter hyperintensities by patient age. Patient age was significantly associated with white matter hyperintensity burden (p = 0.002)
Cerebral atrophy
As in the WMHB analysis, for the analysis of CA, a one-way between subjects ANCOVA was conducted to compare the effect of group (Delirium versus Control) on CA with patient age and gender included as covariates of no interest (Table 2). Delirium and control groups did not differ significantly in CA [F(1, 43) = 2.61, p = 0.113] (Figure 1(b)), whereas advancing age was significantly associated with greater CA [F(1, 43) = 7.89, p = 0.007] (Figure 3).
Figure 3.
Cerebral atrophy by age. Patient age was significantly associated with cerebral atrophy (p = 0.007)
Discussion
To our knowledge, this is the first study to investigate pre-existing brain pathology prior to surgical intervention and its association with post-operative delirium in diagnosed non-small cell cancer patients. Results indicate that individuals in the post-operative delirium group exhibited greater cerebral white matter pathology than unaffected controls pre-operatively. This association appears to be specific to white matter pathology, and is not due to a general effect of normal aging, given that delirium and control groups were matched for age and CA was not significantly associated with post-operative delirium. These results suggest that pre-operative white matter pathology in the brain may predispose surgical candidates to relatively greater risk of post-operative delirium, although pathology alone does not fully account for delirium outcome as indicated by the fact that a subset of unaffected controls had similar levels of pathology. Although CA was not significantly associated with an increased risk of post-operative delirium, it is noted that the delirium group did exhibit greater CA than the unaffected control group. Although this difference did not reach statistical significance, it may be that a larger sample of each group, better clinical characterization, and research-dedicated MRI, which would allow more accurate measures of brain volume, would have yielded a significant result.
The association of pre-operative white matter pathology with delirium is consistent with previous research that finds that pre-operative cognitive and functional declines are also predictive of post-operative delirium. We believe that white matter pathology, in the form of WMHs, likely represents an imaging biomarker linking pre-operative cognitive and functional decline with post-operative delirium. The role of white matter pathology in diverse behavioral and neurological syndromes is well established, with several previous studies finding an association of white matter pathology with sub-clinical cognitive and functional decline. White matter pathology has been found to be associated with declines in mobility and functional independence [64–67], cognition [68,69], longitudinal change in cognitive ability [70], stroke, and mortality (see [71] for meta-analysis and review). Although the current analysis does not allow for localized, regional predictions of white matter pathology and its role in post-operative delirium, a potential contribution of frontostriatal regions is of particular interest given what is known regarding this region’s contribution to higher cognitive function and prior research that found evidence of basal ganglia pathology in delirium cases [37–39]. White matter integrity in frontal areas has been previously theorized to be involved in disparate neuropsychiatric disorders (see [72] for review) and is particularly vulnerable to age-related neurodegeneration (see [73] for review). Behavioral disinhibition, disorganization, impulsivity, and disorientation, all behavioral changes that are common in hyperactive post-operative delirium, are consistent with a potential frontostriatal focus and suggest that these regions will be important areas of future study in understanding delirium pathogenesis and risk.
It is clear from analysis of this dataset that white matter pathology alone cannot fully explain the emergence of post-operative delirium. Rather, pre-operative white matter pathology likely represents a vulnerability, which is exploited by surgical stress and physiologic response to surgical intervention. This model would be consistent with a brain-reserve [74] explanation of delirium, which would predict, given equivalent stressors, that extent or proportion of existing brain pathology would be a key determinant in whether post-operative delirium is exhibited.
One candidate mechanism by which surgical stress may exploit or interact with pre-existing brain pathology to cause delirium is an inflammatory response syndrome that is induced by surgery under general anesthesia [75]. The heightened inflammatory response to surgery stimulates macrophages with resultant release of inflammatory mediators including TNF-alpha, IL-6, and IL-1. These inflammatory mediators cross the blood brain barrier and in turn lead to microglial activation, neuroinflammation, and oxidative stress. While factors such as age and inflammation [76] are likely contributors, white matter pathology may play a more specific role in blood–brain permeability. Specifically, in regard to white matter pathology and blood–brain permeability, one model [77] holds that hypoperfusion, demyelination, and WMHs result from chronic leakage of plasma into the white matter as a result of a breakdown in the blood–brain barrier. This model has been supported by imaging studies that find markers of increased permeability in patients with subcortical ischemic vascular disease (SIVD) [78], together with finding of increased proteases (matrix metalloproteinases) that disrupt the blood brain barrier in SIVD [79] and vascular dementia [80,81]. As a result, dysregulation of neuronal function and, possibly, direct neuronal injury in the central nervous system may occur. Translational work provides some support for the role of inflammatory response in delirium. Oxidative stress may promote a cholinergic deficit, consistent with the cholinergic deficiency hypothesis of delirium, with associated imbalances in other neurotransmitters including dopamine, serotonin, and norepinephrine (see [82,83]). Recent animal models found that mild to moderate systemic inflammation in a normal animal is insufficient to induce cognitive deficits, but when similar inflammatory change was caused in animals with prior degenerative pathology, acute and transient cognitive deficits were exhibited [84]. A second animal study showed that systemic inflammation induces cognitive deficits only in animals with prior pathology in the basal forebrain cholinergic nuclei, the source of most acetylcholine in the forebrain [85].
Several unanswered questions remain. Although this study relied upon global measures of WMHB, it is likely that regionally specific white matter pathology may have a more specific association with delirium outcome. Another important and unanswered question is whether the manifestation of a delirium might either lead to cognitive decline or accelerate pre-existing cognitive deterioration. Prior research finds difficulties in long-term adjustment following episodic delirium, and several studies have now been completed tracking long-term cognitive outcomes (see [86,87] for review). Evidence for cognitive decline following post-operative delirium appears early in the course of recovery (3–6 months) [5,7], and although there is some debate whether short-term cognitive difficulties are due to a continuing sub-clinical unresolved delirium or represent chronic cognitive difficulties [88], follow-up at longer intervals continues to indicate chronic cognitive difficulties [12,14,89] compared with unaffected individuals. At longer intervals, individuals previously diagnosed with delirium continue to exhibit depressed cognitive performance [6,13,90] and also exhibit a higher incidence of diagnosed mild cognitive impairment and dementia [9,10,15,16,91,92] compared with unaffected individuals. Whether these outcomes represent the natural course of pre-existing progressive cognitive deterioration, new cognitive changes as a result of delirium, or an acceleration of pre-existing issues, will be an important focus of future research.
Given the retrospective nature of this study and its focus on standard of care MRI scans, limitations that will need to be addressed in future studies emerge. Patients were assigned to either delirium or control groups by means of proxy indicators of delirium, including anti-psychotic treatment and/or bedside companion orders, with subsequent in depth record review to confirm the presence of confusion, disinhibition, impulsivity, and disorientation. We relied on these indicators as actual delirium diagnoses in the immediate post-operative period were rare, likely because patients exhibiting behavioral disruption are treated emergently without a formal psychiatric evaluation or resulting diagnosis. Although similar techniques were developed by leading delirium researchers and have been successfully validated [61], our delirium sample may over-represent hyperactive or mixed delirium, while under-representing hypoactive delirium cases, because these are more likely to be under-recognized and under-treated. Reliance on standard of care pre-operative brain MRIs also limits the methods of analysis and specificity of findings that may be possible given other MRI sequences. The clinical scans analyzed in this study are two-dimensional and are of low resolution compared with what would be a standard, research-dedicated sequence. As a result, segmentation of tissue classes derived from these sequences are experimental, and normalization of individual patient brains to a standard template, most typical in research analyses, is not possible. High resolution, three-dimensional sequences would allow for better segmentation of images and normalization of patient brains to a standard space that would allow for testing of regionally specific hypotheses. We believe the limitations described above would only have the effect of decreasing the observed association of structural pathology with post-operative delirium and its specificity, and would likely increase our chances of a Type II error. We believe that without these limitations, the observed association would likely be stronger.
Despite these limitations, this study presents a novel approach to studying pre-operative structural risk factors to delirium and finds a significant and specific association of white matter pathology with post-operative delirium in the absence of a prominent role for age-related CA. Future studies will include a prospective strategy that will include research-dedicated sequences in the standard of care MRI screen, comprehensive geriatric assessment of pre-operative cognition, functional independence, and frailty to better characterize pre-operative status and risk factors, and formal post-operative assessment of delirium status to better characterize both hyperactive and hypoactive delirium cases. Follow-up imaging at longer intervals post-delirium resolution would allow for testing whether a prior delirium accelerates the rate of acquired brain pathology post-resolution. Regional hypotheses, specifically the role of frontostriatal white matter integrity in post-operative delirium, will be of particular interest, as well as further assessment of the potential interaction of age-related CA with white matter degeneration.
Acknowledgments
The authors thank Fern Panchana for assistance in review of medical records. This research was supported by a grant from the Seaver Foundation.
References
- 1.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fields SD. Review: delirium predicts 12-month mortality independent of dementia status. ACP J Club. 2003;139(3):80. [PubMed] [Google Scholar]
- 3.McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457–463. doi: 10.1001/archinte.162.4.457. [DOI] [PubMed] [Google Scholar]
- 4.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249(1):173–178. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 5.Benoit AG, Campbell BI, Tanner JR, et al. Risk factors and prevalence of perioperative cognitive dysfunction in abdominal aneurysm patients. J Vasc Surg. 2005;42(5):884–890. doi: 10.1016/j.jvs.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Bickel H, Gradinger R, Kochs E, et al. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement Geriatr Cogn Disord. 2008;26(1):26–31. doi: 10.1159/000140804. [DOI] [PubMed] [Google Scholar]
- 7.Duppils GS, Wikblad K. Cognitive function and health-related quality of life after delirium in connection with hip surgery. A six-month follow-up. Orthop Nurs. 2004;23(3):195–203. doi: 10.1097/00006416-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Gruber-Baldini AL, Zimmerman S, Morrison RS, et al. Cognitive impairment in hip fracture patients: timing of detection and longitudinal follow-up. J Am Geriatr Soc. 2003;51(9):1227–1236. doi: 10.1046/j.1532-5415.2003.51406.x. [DOI] [PubMed] [Google Scholar]
- 9.Kat MG, Vreeswijk R, de Jonghe JF, et al. Long-term cognitive outcome of delirium in elderly hip surgery patients. A prospective matched controlled study over two and a half years. Dement Geriatr Cogn Disord. 2008;26(1):1–8. doi: 10.1159/000140611. [DOI] [PubMed] [Google Scholar]
- 10.Lundstrom M, Edlund A, Bucht G, et al. Dementia after delirium in patients with femoral neck fractures. J Am Geriatr Soc. 2003;51(7):1002–6. doi: 10.1046/j.1365-2389.2003.51315.x. [DOI] [PubMed] [Google Scholar]
- 11.Wacker P, Nunes PV, Cabrita H, et al. Postoperative delirium is associated with poor cognitive outcome and dementia. Dement Geriatr Cogn Disord. 2006;21(4):221–227. doi: 10.1159/000091022. [DOI] [PubMed] [Google Scholar]
- 12.Katz IR, Curyto KJ, TenHave T, et al. Validating the diagnosis of delirium and evaluating its association with deterioration over a one-year period. Am J Geriatr Psychiatry. 2001;9(2):148–159. [PubMed] [Google Scholar]
- 13.Francis J, Kapoor WN. Prognosis after hospital discharge of older medical patients with delirium. J Am Geriatr Soc. 1992;40(6):601–606. doi: 10.1111/j.1532-5415.1992.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 14.McCusker J, Cole M, Dendukuri N, et al. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. Cmaj. 2001;165(5):575–583. [PMC free article] [PubMed] [Google Scholar]
- 15.Rahkonen T, Eloniemi-Sulkava U, Halonen P, et al. Delirium in the non-demented oldest old in the general population: risk factors and prognosis. Int J Geriatr Psychiatry. 2001;16(4):415–421. doi: 10.1002/gps.356. [DOI] [PubMed] [Google Scholar]
- 16.Rockwood K, Cosway S, Carver D, et al. The risk of dementia and death after delirium. Age Ageing. 1999;28(6):551–556. doi: 10.1093/ageing/28.6.551. [DOI] [PubMed] [Google Scholar]
- 17.Olofsson B, Lundstrom M, Borssen B, et al. Delirium is associated with poor rehabilitation outcome in elderly patients treated for femoral neck fractures. Scand J Caring Sci. 2005;19(2):119–127. doi: 10.1111/j.1471-6712.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 18.Andersson EM, Gustafson L, Hallberg IR. Acute confusional state in elderly orthopaedic patients: factors of importance for detection in nursing care. Int J Geriatr Psychiatry. 2001;16(1):7–17. doi: 10.1002/1099-1166(200101)16:1<7::aid-gps261>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Edelstein DM, Aharonoff GB, Karp A, et al. Effect of postoperative delirium on outcome after hip fracture. Clin Orthop Relat Res. 2004;(422):195–200. doi: 10.1097/01.blo.0000128649.59959.0c. [DOI] [PubMed] [Google Scholar]
- 20.Kagansky N, Rimon E, Naor S, et al. Low incidence of delirium in very old patients after surgery for hip fractures. Am J Geriatr Psychiatry. 2004;12(3):306–14. [PubMed] [Google Scholar]
- 21.Marcantonio E, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 22.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58(1):76–81. doi: 10.1093/gerona/58.1.m76. [DOI] [PubMed] [Google Scholar]
- 23.Bohner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238(1):149–156. doi: 10.1097/01.sla.0000077920.38307.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minden SL, Carbone LA, Barsky A, et al. Predictors and outcomes of delirium. Gen Hosp Psychiatry. 2005;27(3):209–214. doi: 10.1016/j.genhosppsych.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Rothenhausler HB, Grieser B, Nollert G, et al. Psychiatric and psychosocial outcome of cardiac surgery with cardiopulmonary bypass: a prospective 12-month follow-up study. Gen Hosp Psychiatry. 2005;27(1):18–28. doi: 10.1016/j.genhosppsych.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Rudolph JL, Babikian VL, Birjiniuk V, et al. Atherosclerosis is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2005;53(3):462–466. doi: 10.1111/j.1532-5415.2005.53165.x. [DOI] [PubMed] [Google Scholar]
- 27.Santos FS, Velasco IT, Fraguas R., Jr Risk factors for delirium in the elderly after coronary artery bypass graft surgery. Int Psychogeriatr. 2004;16(2):175–193. [PubMed] [Google Scholar]
- 28.Schneider F, Bohner H, Habel U, et al. Risk factors for postoperative delirium in vascular surgery. Gen Hosp Psychiatry. 2002;24(1):28–34. doi: 10.1016/s0163-8343(01)00168-2. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi M, Takeuchi H, Fujisawa D, et al. Incidence and risk factors of postoperative delirium in patients with esophageal cancer. Ann Surg Oncol. 2012;19:3963–3970. doi: 10.1245/s10434-012-2432-1. [DOI] [PubMed] [Google Scholar]
- 30.Tognoni P, Simonato A, Robutti N, et al. Preoperative risk factors for postoperative delirium (POD) after urological surgery in the elderly. Arch Gerontol Geriatr. 2011;52(3):e166–169. doi: 10.1016/j.archger.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Litaker D, Locala J, Franco K, et al. Preoperative risk factors for postoperative delirium. Gen Hosp Psychiatry. 2001;23(2):84–89. doi: 10.1016/s0163-8343(01)00117-7. [DOI] [PubMed] [Google Scholar]
- 32.Juliebo V, Bjoro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57(8):1354–1361. doi: 10.1111/j.1532-5415.2009.02377.x. [DOI] [PubMed] [Google Scholar]
- 33.Dyer CB, Ashton CM, Teasdale TA. Postoperative delirium. A review of 80 primary data-collection studies. Arch Intern Med. 1995;155(5):461–5. doi: 10.1001/archinte.155.5.461. [DOI] [PubMed] [Google Scholar]
- 34.Korevaar JC, van Munster BC, de Rooij SE. Risk factors for delirium in acutely admitted elderly patients: a prospective cohort study. BMC Geriatr. 2005;5:6. doi: 10.1186/1471-2318-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudolph JL, Jones RN, Rasmussen LS, et al. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120(9):807–13. doi: 10.1016/j.amjmed.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Alsop DC, Fearing MA, Johnson K, et al. The role of neuroimaging in elucidating delirium pathophysiology. J Gerontol A Biol Sci Med Sci. 2006;61(12):1287–1293. doi: 10.1093/gerona/61.12.1287. [DOI] [PubMed] [Google Scholar]
- 37.Figiel GS, Krishnan KR, Breitner JC, et al. Radiologic correlates of antidepressant-induced delirium: the possible significance of basal-ganglia lesions. J Neuropsychiatry Clin Neurosci. 1989;1(2):188–190. doi: 10.1176/jnp.1.2.188. [DOI] [PubMed] [Google Scholar]
- 38.Figiel GS, Krishnan KR, Doraiswamy PM. Subcortical structural changes in ECT-induced delirium. J Geriatr Psychiatry Neurol. 1990;3(3):172–176. doi: 10.1177/089198879000300309. [DOI] [PubMed] [Google Scholar]
- 39.Figiel GS, Coffey CE, Djang WT, et al. Brain magnetic resonance imaging findings in ECT-induced delirium. J Neuropsychiatry Clin Neurosci. 1990;2(1):53–58. doi: 10.1176/jnp.2.1.53. [DOI] [PubMed] [Google Scholar]
- 40.Koponen H, Hurri L, Stenback U, et al. Computed tomography findings in delirium. J Nerv Ment Dis. 1989;177(4):226–231. doi: 10.1097/00005053-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Shioiri A, Kurumaji A, Takeuchi T, et al. White matter abnormalities as a risk factor for postoperative delirium revealed by diffusion tensor imaging. Am J Geriatr Psychiatry. 2010;18(8):743–753. doi: 10.1097/JGP.0b013e3181d145c5. [DOI] [PubMed] [Google Scholar]
- 42.Hatano Y, Narumoto J, Shibata K, et al. White-matter hyperintensities predict delirium after cardiac surgery. Am J Geriatr Psychiatry. 2012 doi: 10.1016/j.jagp.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 43.Henon H, Lebert F, Durieu I, et al. Confusional state in stroke: relation to preexisting dementia, patient characteristics, and outcome. Stroke. 1999;30(4):773–779. doi: 10.1161/01.str.30.4.773. [DOI] [PubMed] [Google Scholar]
- 44.Soiza RL, Sharma V, Ferguson K, et al. Neuroimaging studies of delirium: a systematic review. J Psychosom Res. 2008;65(3):239–248. doi: 10.1016/j.jpsychores.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Bogousslavsky J, Ferrazzini M, Regli F, et al. Manic delirium and frontal-like syndrome with paramedian infarction of the right thalamus. J Neurol Neurosurg Psychiatry. 1988;51(1):116–119. doi: 10.1136/jnnp.51.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pittock SJ, Rabinstein AA, Edwards BS, et al. OKT3 neurotoxicity presenting as akinetic mutism. Transplantation. 2003;75(7):1058–1060. doi: 10.1097/01.TP.0000057830.67416.CE. [DOI] [PubMed] [Google Scholar]
- 47.Doyle M, Warden D. Use of SPECT to evaluate postcardiotomy delirium. Am J Psychiatry. 1996;153(6):838–839. doi: 10.1176/ajp.153.6.838b. [DOI] [PubMed] [Google Scholar]
- 48.Shih WJ, Hyatt M. Volume and surface three-dimensional displays of Tc-99m HMPAO brain SPECT imaging in a chronic hypnosedative abuser. Clin Nucl Med. 1993;18(6):506–509. doi: 10.1097/00003072-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Ohta H, Matsumoto R, Kato T, et al. Tc-99m HMPAO brain perfusion SPECT images in a patient with portal-systemic encephalopathy. Clin Nucl Med. 1998;23(9):634–636. doi: 10.1097/00003072-199809000-00025. [DOI] [PubMed] [Google Scholar]
- 50.Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57(3):337–339. doi: 10.1046/j.1440-1819.2003.01126.x. [DOI] [PubMed] [Google Scholar]
- 51.Catafau AM, Kulisevsky J, Berna L, et al. Relationship between cerebral perfusion in frontal-limbic-basal ganglia circuits and neuropsychologic impairment in patients with subclinical hepatic encephalopathy. J Nucl Med. 2000;41(3):405–410. [PubMed] [Google Scholar]
- 52.Fong TG, Bogardus ST, JR, Daftary A, et al. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. J Gerontol A Biol Sci Med Sci. 2006;61(12):1294–1299. doi: 10.1093/gerona/61.12.1294. [DOI] [PubMed] [Google Scholar]
- 53.Gokgoz L, Gunaydin S, Sinci V, et al. Psychiatric complications of cardiac surgery postoperative delirium syndrome. Scand Cardiovasc J. 1997;31(4):217–222. doi: 10.3109/14017439709041749. [DOI] [PubMed] [Google Scholar]
- 54.Gunaydin B, Babacan A. Cerebral hypoperfusion after cardiac surgery and anesthetic strategies: a comparative study with high dose fentanyl and barbiturate anesthesia. Ann Thorac Cardiovasc Surg. 1998;4(1):12–17. [PubMed] [Google Scholar]
- 55.Ikeda S, Yazaki M, Takei Y, et al. Type II (adult onset) citrullinaemia: clinical pictures and the therapeutic effect of liver transplantation. J Neurol Neurosurg Psychiatry. 2001;71(5):663–670. doi: 10.1136/jnnp.71.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jalan R, Olde Damink SW, Lui HF, et al. Oral amino acid load mimicking hemoglobin results in reduced regional cerebral perfusion and deterioration in memory tests in patients with cirrhosis of the liver. Metab Brain Dis. 2003;18(1):37–49. doi: 10.1023/a:1021978618745. [DOI] [PubMed] [Google Scholar]
- 57.Strauss GI, Hogh P, Moller K, et al. Regional cerebral blood flow during mechanical hyperventilation in patients with fulminant hepatic failure. Hepatology. 1999;30(6):1368–1373. doi: 10.1002/hep.510300608. [DOI] [PubMed] [Google Scholar]
- 58.Trzepacz PT, Tarter RE, Shah A, et al. SPECT scan and cognitive findings in subclinical hepatic encephalopathy. J Neuropsychiatry Clin Neurosci. 1994;6(2):170–175. doi: 10.1176/jnp.6.2.170. [DOI] [PubMed] [Google Scholar]
- 59.O’Carroll RE, Hayes PC, Ebmeier KP, et al. Regional cerebral blood flow and cognitive function in patients with chronic liver disease. Lancet. 1991;337(8752):1250–1253. doi: 10.1016/0140-6736(91)92920-w. [DOI] [PubMed] [Google Scholar]
- 60.Yazgan Y, Narin Y, Demirturk L, et al. Value of regional cerebral blood flow in the evaluation of chronic liver disease and subclinical hepatic encephalopathy. J Gastroenterol Hepatol. 2003;18(10):1162–1167. doi: 10.1046/j.1440-1746.2003.03141.x. [DOI] [PubMed] [Google Scholar]
- 61.Inouye SK, Leo-Summers L, Zhang Y, et al. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 62.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66(1):23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 63.Tukey JW. Exploratory Data Analysis. Addison-Wesley; 1977. [Google Scholar]
- 64.Benson RR, Guttmann CR, Wei X, et al. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58(1):48–55. doi: 10.1212/wnl.58.1.48. [DOI] [PubMed] [Google Scholar]
- 65.Guttmann CR, Benson R, Warfield SK, et al. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54(6):1277–1283. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- 66.Moscufo N, Guttmann CR, Meier D, et al. Brain regional lesion burden and impaired mobility in the elderly. Neurobiol Aging. 2011;32(4):646–654. doi: 10.1016/j.neurobiolaging.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolfson L, Wei X, Hall CB, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005;232(1–2):23–27. doi: 10.1016/j.jns.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 68.Parks CM, Iosif AM, Farias S, et al. Executive function mediates effects of white matter hyperintensities on episodic memory. Neuropsychologia. 2011;49(10):2817–2824. doi: 10.1016/j.neuropsychologia.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meier IB, Manly JJ, Provenzano FA, et al. White matter predictors of cognitive functioning in older adults. J Int Neuropsychol Soc. 2012;18(3):414–427. doi: 10.1017/S1355617712000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maillard P, Carmichael O, Fletcher E, et al. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology. 2012;79(5):442–448. doi: 10.1212/WNL.0b013e3182617136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 73.Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging. 2002;23(3):421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 74.Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12(1):11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- 75.Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165(7):803–812. doi: 10.1176/appi.ajp.2008.08020181. [DOI] [PubMed] [Google Scholar]
- 76.Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308(1):73–81. doi: 10.1001/jama.2012.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wardlaw JM, Sandercock PA, Dennis MS, et al. Is breakdown of the blood–brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34(3):806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 78.Taheri S, Gasparovic C, Huisa BN, et al. Blood–brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42(8):2158–2163. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Candelario-Jalil E, Thompson J, Taheri S, et al. Matrix metalloproteinases are associated with increased blood–brain barrier opening in vascular cognitive impairment. Stroke. 2011;42(5):1345–1350. doi: 10.1161/STROKEAHA.110.600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosenberg GA. Inflammation and white matter damage in vascular cognitive impairment. Stroke. 2009;40(3 Suppl):S20–23. doi: 10.1161/STROKEAHA.108.533133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenberg GA, Sullivan N, Esiri MM. White matter damage is associated with matrix metalloproteinases in vascular dementia. Stroke. 2001;32(5):1162–1168. doi: 10.1161/01.str.32.5.1162. [DOI] [PubMed] [Google Scholar]
- 82.Cerejeira J, Nogueira V, Luis P, et al. The cholinergic system and inflammation: common pathways in delirium pathophysiology. J Am Geriatr Soc. 2012;60(4):669–675. doi: 10.1111/j.1532-5415.2011.03883.x. [DOI] [PubMed] [Google Scholar]
- 83.Hshieh TT, Fong TG, Marcantonio ER, et al. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63(7):764–772. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murray C, Sanderson DJ, Barkus C, et al. Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol Aging. 2012;33(3):603–616. e603. doi: 10.1016/j.neurobiolaging.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Field RH, Gossen A, Cunningham C. Prior pathology in the basal forebrain cholinergic system predisposes to inflammation-induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium. J Neurosci. 2012;32(18):6288–294. doi: 10.1523/JNEUROSCI.4673-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30–42. doi: 10.1080/09540260802675031. [DOI] [PubMed] [Google Scholar]
- 87.Jackson JC, Gordon SM, Hart RP, et al. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14(2):87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 88.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koponen H, Stenback U, Mattila E, et al. Delirium among elderly persons admitted to a psychiatric hospital: clinical course during the acute stage and one-year follow-up. Acta Psychiatr Scand. 1989;79(6):579–585. doi: 10.1111/j.1600-0447.1989.tb10306.x. [DOI] [PubMed] [Google Scholar]
- 90.Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55(9):M527–534. doi: 10.1093/gerona/55.9.m527. [DOI] [PubMed] [Google Scholar]
- 91.Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 92.Davis DH, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135(Pt 9):2809–16. doi: 10.1093/brain/aws190. [DOI] [PMC free article] [PubMed] [Google Scholar]