Abstract
The role of reactive oxygen species (ROS) in cell communication, control of gene expression, and oxygen sensing is well established. Inappropriate regulation of ROS levels can damage cells, resulting in a diseased state. In Colletotrichum trifolii, a fungal pathogen of alfalfa, the mutationally activated oncogenic fungal Ras (DARas) elevates levels of ROS, causing abnormal fungal growth and development and eventual apoptotic-like cell death but only when grown under nutrient-limiting conditions. Remarkably, restoration to the wild-type phenotype requires only proline. Here, we describe a generally unrecognized function of proline: its ability to function as a potent antioxidant and inhibitor of programmed cell death. Addition of proline to DARas mutant cells effectively quenched ROS levels and prevented cell death. Treating cells with inhibitors of ROS production yielded similar results. In addition, proline protected wild-type C. trifolii cells against various lethal stresses, including UV light, salt, heat, and hydrogen peroxide. These observations appear to be general because proline also protected yeast cells from lethal levels of the ROS-generating herbicide methyl viologen (paraquat), suggesting a common protective role for proline in response to oxidative stress. The ability of proline to scavenge intracellular ROS and inhibit ROS-mediated apoptosis may be an important and broad-based function of this amino acid in responding to cellular stress, in addition to its well established role as an osmolyte.
Keywords: oxidative stress, programmed cell death, reactive oxygen species
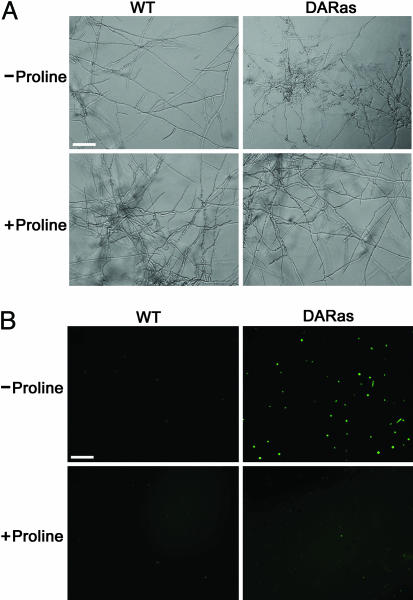
The small GTP-binding protein Ras regulates cellular signal transduction processes leading to cell growth, differentiation, and survival (1). In mammals, the importance of Ras in regulating growth is underscored by the observation that mutations conferring constitutive Ras activation are found in nearly 30% of all human tumors (2). Moreover, expression of constitutively active Ras in primary cells generally leads to cell-cycle arrest or apoptosis (3). Recently, the role of Ras in filamentous fungi has been studied. Truesdell et al. (4) found that an activating mutation of the unique ras gene (Ct-ras) from Colletotrichum trifolii, a fungal pathogen of alfalfa, causes oncogenic phenotypes in nu/nu mice, suggesting that this fungal ras gene has the genetic capability to function as a bona fide oncogene. More interestingly, we found that the dominant activated “oncogenic” Ras (denoted DARas), when expressed in C. trifolii, yielded a nutrient-dependent response. Under conditions of nutrient deprivation (minimal medium), the DARas mutant induced aberrant hyphal proliferation, defects in polarized growth, and, significantly, reduced differentiation such as conidiation and appressorium formation (4). Because these mutants showed normal hyphal growth and development in rich medium, it is possible that Ct-Ras regulates a signal transduction pathway that senses and responds to nutrients, similar to what has been observed in Saccharomyces cerevisiae (5). Growth of C. trifolii in minimal medium with various regimes of carbon, nitrogen, heat, and osmoticum failed to complement the DARas mutant (6). Based on the observation that peptone restored the wild-type (WT) phenotype, we found that proline alone, when added to minimal medium at the concentration found in peptone (1.6 mM), was sufficient to fully revert the WT phenotype, including restoration of normal hyphal morphology, polarized growth, and conidiation (Fig. 1) (6). However, the mechanism(s) by which proline restores the WT phenotype of the DARas mutant is unclear.
Fig. 1.
Effect of proline on hyphal morphology and intracellular ROS production of both WT and DARas mutant on minimal medium. (A) Cell morphology of WT and the DARas mutant after 6 days of growth on minimal medium with (1.6 mM) or without proline. (B) WT and the DARas mutant were grown at room temperature in minimal medium amended with or without proline. After 6 days of incubation, protoplasts were generated from each strain, and aliquots of protoplast cells were incubated with 50 μM H2O2-sensitive fluorophore 2′,7′-dichlorofluorescin diacetate and then photographed with an epifluorescence microscope. Pictures shown are representative of three independent experiments. (Bars, 20 μm.)
Proline differs from all other standard amino acids in that it is a α-imino acid. Proline is an osmoprotectant in plants, able to balance drought stress (7). In a variety of plants, stresses such as cold, heat, salt, drought, UV, and heavy metals significantly increase endogenous proline concentrations (7, 8). One common feature from these stresses is the production of reactive oxygen species (ROS). ROS encompass a variety of partially reduced oxygen metabolites (e.g., superoxide anions, H2O2, and hydroxyl radicals) and mediate diverse effects on normal cell functions (9). Of particular note is that mitogenic signals induced by activated Ras are mediated by ROS production (9, 10). ROS may act as second messengers to induce signaling cascades required for the proliferative response to oncogenic Ras (10). Consistent with this observation, we have shown that DARas mutant, but not WT strain, harbors high amounts of intracellular ROS as determined by 2′,7′-dichlorodihydrofluorescein diacetate fluorescence when grown in minimal medium (11). In this strain, ROS generation was via a Ras/Rac/cPLA2-dependent pathway (11). Treatment of the DARas mutant with inhibitors of ROS production such as N-acetyl cysteine or diphenylene iodonium decreased ROS levels and concomitantly restored the WT phenotype, similar to what was observed with proline addition (11). These findings thus suggest that ROS production contributes to the aberrant hyphal morphology observed in the DARas mutant grown in minimal medium and that proline may restore normal growth by reducing ROS levels.
In this study, we sought to explore the mechanism(s) involved in the phenotypic restoration of DARas mutant by proline. In minimal medium, treatment of DARas with proline significantly inhibited intracellular ROS production. Moreover, high amounts of ROS induced by DARas trigger an apoptotic-like programmed cell death (PCD), because apoptotic features including DNA condensation and DNA fragmentation as well as phosphatidylserine (PS) externalization were observed. Importantly, proline prevents this apoptotic response functioning in a cytoprotective manner. In addition, we found that various stresses, including UV light, salt, heat, and H2O2, promote an apoptotic-like PCD when WT C. trifolii was exposed to these treatments and proline inhibited these stress-induced apoptotic responses. Finally, the protective role of proline was extended to the budding yeast S. cerevisiae. Proline was able to protect yeast cells from lethal levels of ROS generated by paraquat. Taken together, our data suggest that proline can function as a potent antioxidant to scavenge intracellular ROS generation and thereby inhibit ROS-mediated apoptotic-like PCD, which may be an important and general function of this amino acid in response to cellular stress, in addition to its well established role as an osmolyte.
Materials and Methods
Strains. The following strains were used in this study: WT C. trifolii race 1, isolated by M.B.D. (12); DARas mutant (WT C. trifolii strain transformed with a dominant active form of Ct-Ras), which was constructed by Truesdell et al. (4); and WT S. cerevisiae strain HA0 (MATa).
Medium and Growth Conditions. C. trifolii cultures were routinely grown at 25°C on yeast extract–phosphate–soluble starch agar medium or Czapek–Dox minimal medium (0.2% sodium nitrate/0.1% potassium phosphate dibasic/0.05% magnesium sulfate/0.05% potassium chloride/0.001% ferric sulfate/2% agar). When needed, proline was added to the medium at a final concentration of 1.6 mM. S. cerevisiae strain HA0 was maintained at 30°C in Minimal Vitamin medium (0.15% Difco Bacto Yeast Nitrogen Base without amino acids/0.52% ammonium sulfate/2% dextrose/2% agar). When needed, methyl viologen (MV; paraquat) or proline was added to the medium at the indicated concentration.
Stress Treatments and Viability Assays. Conidia from the appropriate strains were diluted and treated in one of the following ways. For UV viability assays, conidia were plated at ≈100 per plate on minimal medium amended with or without 1.6 mM proline and allowed to germinate for 3 h before UV irradiation. Plates were incubated for 3 days at room temperature, and the number of survivors on each plate was counted. For salt stress, conidia (104 per milliliter) were directly plated on minimal medium containing the appropriate concentrations of sodium chloride, in the presence or absence of 1.6 mM proline. For heat stress, conidia (106 per milliliter) were exposed to heat (55°C) for 30 min and then immediately plated on minimal medium amended with or without 1.6 mM proline. Viability was determined as the percentage of colonies on treated plates compared with untreated controls. For chemical stress in yeast, early logarithmic phase yeast cultures (A600 = 0.5) were diluted to a density of A600 = 0.05, and then an aliquot of yeast cells (5 μl) was plated out on MV plates amended with paraquat (1 and 2 mM), proline (1.6 mM), or both. The viable colonies were photographed 3 days after inoculation at 30°C. All assays were carried out in triplicate.
Detection of Intracellular H2O2. Intracellular H2O2 levels in C. trifolii were monitored with the oxidant-sensitive probe 2′,7′-dichlorofluorescin diacetate (Molecular Probes) as described in ref. 11.
Evans Blue Staining. Conidia of DARas were inoculated to coverslips overlaid with a thin layer of minimal medium, in the presence or absence of 1.6 mM proline. After 6 days of incubation at room temperature, the cultures were incubated with 0.05% Evans blue for 45 min at room temperature and washed with PBS. Both proline-treated and untreated hyphae were observed by light microscopy.
DAPI Staining. Nuclei to be observed by fluorescence microscopy were stained with DAPI. After 6 days of growth, the DARas mutant cells were fixed briefly in 70% (vol/vol) ethanol and incubated with 1 μg/ml DAPI in PBS for 15 min at room temperature, rinsed twice with PBS, and then observed under an epifluorescence microscope (Zeiss Axioskop).
TUNEL. TUNEL reaction was determined by using the In Situ Cell Death Detection kit (Roche Diagnostics) as described by Madeo et al. (13). Prodium iodide (PI) staining was used to identify the nuclei.
Annexin V Staining. To examine cellular integrity and PS externalization, we stained the protoplasts of C. trifolii with PI and FITC-conjugated annexin V by using the Annexin V–FITC Apoptosis Detection kit (Oncogene Research Products, Boston). PI is a fluorochrome that cannot cross the membrane of living cells. However, PI can readily penetrate dead cells to stain DNA. Annexin V binding assays were carried out according to the protocol described by Madeo et al. (13). Each assay was repeated at least three times.
Measurement of Antioxidant Enzymes Activity. CAT activity was determined spectrophotometrically by monitoring disappearance of H2O2 at 240 nm (14). Superoxide dismutase (SOD) activity was measured by the nitroblue tetrazolium reduction method of Beauchamp and Fridovich (15).
Results
Proline Inhibits ROS Production by the DARas Mutant on Minimal Medium. Our previous data have shown that proline alone, when supplemented to the DARas mutant, is sufficient to restore a WT hyphal phenotype under nutrient-limiting conditions (6) (Fig. 1 A). We also found that that the DARas mutant, when grown in minimal medium, produces high amounts of ROS that contribute to the aberrant hyphal morphology because treatment of the DARas mutant with inhibitors of ROS production, such as N-acetyl cysteine or diphenylene iodonium, decreased ROS levels and concomitantly restored the WT phenotype, similar to addition of proline (11). Moreover, when the DARas mutant was treated with proline analogs (e.g., thiazolidine-2-carboxylic acid, d-proline, 2-azetidinecarboxylic acid, and thiazolidine-4-carboxylic acid), only thiazolidine-4-carboxylic acid mimicked the effect of proline (6). Interestingly, thiazolidine-4-carboxylic acid also is an antioxidant (16). Thus, it was reasonable to hypothesize that proline may act as a ROS scavenger, providing a plausible explanation for the ability of proline to revert the activated ras phenotype. To test this hypothesis, we monitored the intracellular ROS levels of the DARas mutant on minimal medium with or without proline, by using 2′,7′-dichlorodihydrofluorescein diacetate, a cell-permeable ROS indicator that penetrates live cells but does not fluoresce unless oxidized by ROS (17). As expected, supplementation with proline decreased ROS production by the DARas mutant (Fig. 1B). These results suggest that proline can ameliorate oxidative stress in C. trifolii and encourage us to ask whether cytoprotection by means of scavenging ROS may be a more generalized function of proline.
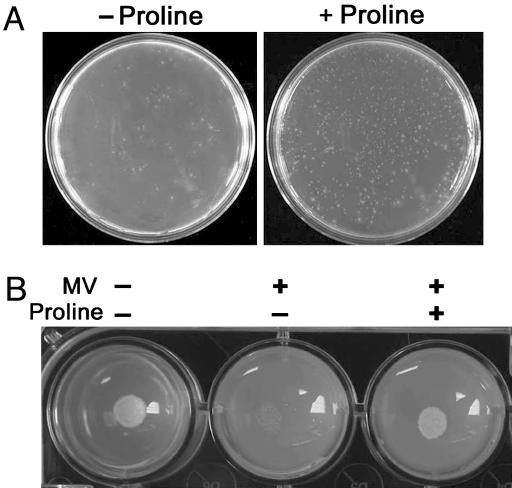
Proline Protects DARas Mutant Cells Against Various Abiotic Stresses. In plants, a positive correlation between free proline accumulation and osmotic stress tolerance has been well documented (7). Importantly, these osmotic stresses, including drought, salinity, cold, and UV radiation, are tightly linked with ROS generation (18). We therefore tested whether proline also protects fungal cells from various abiotic stresses. To this end, spores derived from both WT and DARas strains were exposed to heat (Fig. 2A), UV, or salt (Fig. 6, which is published as supporting information on the PNAS web site) stresses and then grown in minimal medium amended with or without proline. Viability assays indicated the following: (i) DARas strains were more sensitive to these stresses than WT; and (ii) proline protected both WT and the DARas mutants against these stresses, although more significant protection occurred with the mutants. These results indicate involvement of proline in the physiology of stress protection in C. trifolii.
Fig. 2.
Proline protects DARas mutant cells against heat stress and yeast cells against paraquat. (A) Conidia of DARas mutants were pretreated at 55°C for 30 min and then inoculated to minimal medium supplemented with or without proline. Pictures shown are representative of three independent experiments. (B) Yeast cells were inoculated to minimal vitamin medium containing 1 mM MV (paraquat), in the presence or absence of 1.6 mM proline. The colonies were photographed 4 days after inoculation at 30°C. The experiment was repeated in triplicate.
High Amounts of ROS Induced by DARas Trigger an Apoptotic-Like PCD, and Proline Inhibits This Apoptotic Response. Accumulating evidence from mammalian systems suggests that ROS regulate apoptosis, a morphologically distinct form of PCD (19). Thus, one model for phenotypic restoration of the DARas mutant by proline is that relatively high amounts of ROS may induce a PCD-like apoptosis, and proline may inhibit/limit this apoptotic response by reducing oxidative stress. To test this hypothesis, we first used Evans blue staining to evaluate membrane integrity of the DARas mutant with or without proline. Evans blue is a membrane-impermeable stain in normal, healthy cells but readily penetrates the membranes of dead cells. In minimal medium, Evans blue did not stain germ tubes and young hyphae (Fig. 3A) but did stain older hyphae of the DARas mutant (Fig. 3A). In contrast, growth on minimal medium plus proline prevented Evans blue accumulation in all developmental stages (Fig. 3A). As a control, no Evans blue staining was detected in the WT strain with or without proline (Fig. 3A). Thus, the aberrant hyphal growth induced by activated Ras is associated with increased cell death that is inhibited by proline.
Fig. 3.
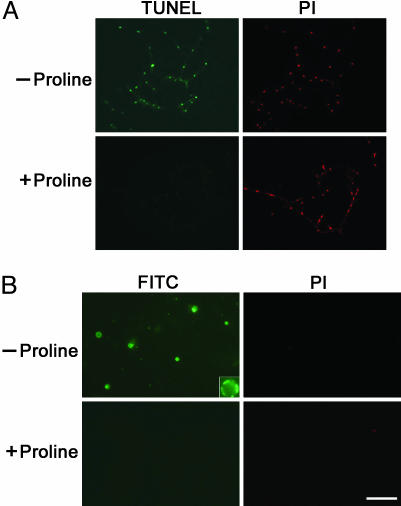
Proline inhibits apoptotic responses in DARas mutants. (A) The DARas mutant strain was grown for 6 days on minimal medium with or without proline. Hyphae were treated with Evans blue dye (0.4%) for 24 h. After extensive washes with PBS, the samples were observed by light microscopy. Pictures shown are representative of three independent experiments. (Bar, 20 μm.) (B) Spores of DARas mutant (Upper) and WT strain (Lower) were grown for 6 days in minimal medium with or without proline and were fixed and stained with DAPI to visualize DNA. (Bar, 20 μm.) (C) DNA fragmentation of DARas mutant cells. Cells were grown as in A followed by TUNEL assays. (Bar, 20 μm.) (D) Protoplasts produced from the same cultures as in A were stained for PS with FITC-conjugated annexin V. Note that only annexin(+)/PI(-) cells undergo apoptotic cell death. One protoplast showing this phenotype was enlarged for observation (Upper Left, upper right corner). The arrow indicates a cell undergoing necrosis, because it also stains for PI. (Bar, 20 μm.)
Although Evans blue is a reliable vital stain, it does not distinguish between necrotic or apoptotic (programmed) cell death. Apoptosis is a genetically controlled type of PCD characterized by distinct morphological and biochemical changes, including cell shrinkage, chromatin condensation, DNA fragmentation, and membrane externalization of PS on the cell surface (20). These morphological features serve as diagnostic markers for apoptosis. To determine whether ROS induced by DARas trigger apoptotic-like responses in C. trifolii, we assayed chromatin condensation, DNA fragmentation, and PS externalization. After DAPI staining assays to visualize DNA and nuclear morphology, we found that DARas cells grown on minimal medium showed diffuse nuclear staining indicating chromatin fragmentation, whereas cells grown on proline-containing minimal medium were like WT cells, displaying compact single nuclei (Fig. 3B).
DNA fragmentation is a commonly used marker for apoptosis and is generally detected in situ by the TUNEL assay (21). Strong TUNEL staining was observed in the hyphae of the DARas strain on minimal medium (Fig. 3C). In contrast, staining was only rarely detected in similar hyphae pretreated with proline (Fig. 3C). Thus, the majority of the DARas cells exhibited TUNEL staining under nutrient-limiting condition, and proline inhibited DNA fragmentation.
Another hallmark of apoptosis is the “flipping” or externalization of PS from the inner to the outer leaflet of the plasma membrane. Once exposed, PS can be detected by binding of annexin V to the cell surface (13). As with the TUNEL results, we observed FITC–annexin V binding to fungal protoplasts derived from the DARas mutant but no staining in proline-treated cells (Fig. 3D). Taking into account these observations, we propose that proline may function as an active cytoprotectant to suppress an apoptotic-like PCD in DARas mutant.
Proline Inhibits Apoptotic-Like PCD in WT C. trifolii Cells When Exposed to Lethal Abiotic Stresses. PCD has been observed in various organisms when exposed to a variety of abiotic stresses, including UV irradiation, salt, and heat (22–24). Because proline conferred protection to C. trifolii from these stresses, we investigated whether these stresses also could induce apoptosis in WT C. trifolii and whether the apoptotic responses could be suppressed by proline. By using TUNEL assays, we observed that the WT C. trifolii cells, when exposed to lethal levels (determined by Evans blue staining, data not shown) of UV (Fig. 4A), salt, or heat (Fig. 7 A and B, which is published as supporting information on the PNAS web site), displayed fragmented DNA. No cell death above background occurred under the same conditions in the presence of proline. These data suggest that proline inhibits apoptosis triggered by UV, salt, or heat.
Fig. 4.
Proline inhibits the apoptotic-like cell death triggered by UV and H2O2 in WT strains. (A) After 24 h of incubation on minimal medium with or without proline, the WT hyphae were exposed to UV for 1 min, and a TUNEL analysis was performed. Fluorescence micrographs showed TUNEL (+) and TUNEL (-) hyphae in response to addition of proline. (B) Hyphae of WT strains were collected from medium with or without proline amendment and treated with 1 mM H2O2 for 6 h. PS exposure was assessed by using a FITC-conjugated annexin V binding assay. (Inset) An enlarged apoptotic cell positively staining with annexin V. (Bar, 20 μm.)
To test the hypothesis that proline protects C. trifolii cells against ROS-mediated cell death, we treated the WT C. trifolii cells with 1 mM H2O2. As expected, both TUNEL (Fig. 7C) and annexin V staining assays (Fig. 4B) indicated that H2O2 treatment rapidly induced apoptosis in WT cells, and proline prevented the apoptotic response, suggesting that proline ameliorates oxidative stress and prevents PCD in C. trifolii.
Catalase (CAT), but Not SOD, May Mediate Proline-Dependent Protection in C. trifolii. Antioxidants, including CAT and SOD protect cells against oxidative stress by maintaining 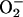 and H2O2 at low levels (19). The loss of SOD enzyme activity was sufficient to induce apoptosis in cultured motor neurons (25), and CAT prevented apoptosis of the human CEM T cell line in serum-free medium (26), consistent with antioxidant effects on apoptosis. We therefore were interested in evaluating the status of the activity of these scavenging enzymes in DARas strains with and without proline. After growth in minimal medium for 6 days, when compared to the WT strain (which harbors extremely low concentrations of ROS), the DARas cells (which harbor significantly higher concentrations of ROS) showed a slight increase in CAT activity. Interestingly, treatment of DARas cells with proline caused a nearly 4-fold increase in CAT activity compared with untreated cells, and this high CAT activity was maintained for up to 14 days (Fig. 5A). SOD activity in the DARas mutant was consistently higher than that of the WT (Fig. 5B). However, addition of proline did not increase SOD activity (Fig. 5B). In fact, SOD activity of the DARas mutant was similar to WT levels after 14 days of incubation, independent of proline addition (Fig. 5B). These data suggest that proline influences CAT activity, but not SOD, during oxidative stress.
and H2O2 at low levels (19). The loss of SOD enzyme activity was sufficient to induce apoptosis in cultured motor neurons (25), and CAT prevented apoptosis of the human CEM T cell line in serum-free medium (26), consistent with antioxidant effects on apoptosis. We therefore were interested in evaluating the status of the activity of these scavenging enzymes in DARas strains with and without proline. After growth in minimal medium for 6 days, when compared to the WT strain (which harbors extremely low concentrations of ROS), the DARas cells (which harbor significantly higher concentrations of ROS) showed a slight increase in CAT activity. Interestingly, treatment of DARas cells with proline caused a nearly 4-fold increase in CAT activity compared with untreated cells, and this high CAT activity was maintained for up to 14 days (Fig. 5A). SOD activity in the DARas mutant was consistently higher than that of the WT (Fig. 5B). However, addition of proline did not increase SOD activity (Fig. 5B). In fact, SOD activity of the DARas mutant was similar to WT levels after 14 days of incubation, independent of proline addition (Fig. 5B). These data suggest that proline influences CAT activity, but not SOD, during oxidative stress.
Fig. 5.
Addition of proline results in rapid and prolonged induction of CAT activity but does not affect SOD activity. (A) CAT activity of C. trifolii WT and DARas mutant strains when grown in minimal medium with or without proline. CAT activity was measured spectrophotometrically by absorbance at 240 nm (48). (B) SOD activity was measured by the nitroblue tetrazolium reduction (15). Results indicate the mean and SD from three independent experiments.
Proline Protects Yeast Cells Against Paraquat Killing. Recent studies in the budding yeast S. cerevisiae have shown that the appearance of apoptotic markers is accompanied by the production of ROS (13). To explore the generality of our findings, and extend the role of proline as an antioxidant, we treated yeast cells with MV (paraquat). MV is a contact herbicide that uncouples electron transport generating lethal levels of ROS (27). WT yeast cells treated with 1 mM MV were unable to grow, whereas incubation of MV-treated yeast cells with proline restored normal growth (Fig. 2B). Thus, the stress-protective effects of proline extend to yeast.
Discussion
We have identified proline as a potent ROS scavenger associated with prevention of apoptotic-like PCD in C. trifolii. Growth on minimal medium plus proline significantly suppressed intracellular ROS induced by dominant active Ras and inhibited the progression of a ROS-mediated apoptosis. Moreover, proline also inhibits the apoptotic responses triggered by a variety of abiotic stresses. Importantly, the protective role of proline extends to yeast because proline protects yeast cells against lethal effects of paraquat, a ROS generator. Therefore, we propose that the ability of proline to scavenge intracellular ROS and thereby inhibit ROS-mediated apoptosis may be a general function of this amino acid, in addition to its well established role as an osmolyte.
All aerobic organisms generate ROS as metabolic byproducts mainly through aerobic respiration. ROS can damage DNA, lipids, and proteins resulting in cytotoxicity. In mammals, ROS have been viewed as second messengers to influence numerous intracellular signaling pathways, including a variety of Ras-mediated cellular effects (28, 29). Of particular note is that ROS act as a downstream effector of Ras to potentially mediate or initiate an apoptotic process. Irani et al. (10) found that dominant active Ras-transformed NIH 3T3 cells generated larger amounts of superoxide than normal NIH 3T3 cells under basal conditions. In yeast, S. cerevisiae, the oncogenic Ras2val19 mutant exhibited significantly higher concentrations of ROS, which caused elevated stress sensitivity, increased oxidative damage, and a reduced replicative lifespan (30). These data suggest a linkage between ROS production and Ras signaling. Moreover, it is reported that dominant active Ras promotes apoptosis in several cell lines, including proliferating Drosophila imaginal tissue (31) and fibroblasts (32). Thus, these studies establish Ras as a modulator of apoptosis by regulating intracellular ROS production. Consistent with these observations, our results suggest that in C. trifolii, dominant active Ras expression also generates high concentrations of ROS that trigger a PCD-like apoptosis under nutrient-limiting conditions. Moreover, our findings further indicate that when WT C. trifolii was treated with lethal levels of abiotic stresses, including UV radiation, salt, heat, and H2O2, apoptosis was induced. PCD has been observed in budding yeast after oxidative stress (13). Stress-induced apoptosis also has been noted in Aspergillus nidulans (33) and Candida albicans (34). Our studies add C. trifolii to the growing list of fungi known to exhibit apoptosis.
In plants, proline constitutes <5% of the total pool of free amino acids under normal conditions. After stress this level can increase to up to 80% of the amino acid pool (35). This observation raises the question regarding the general role of proline under stress conditions. The ability of proline to confer stress protection usually is accounted for by its osmoprotective functions (7). Transgenic plants that cannot produce proline have reduced stress tolerance (36). In addition, other positive roles of proline have been proposed, which include stabilization of proteins (possibly by quenching ROS) (37), regulation of the cytosolic pH (38), and regulation of NAD/NADH ratio (39). In this work, we establish a generally unrecognized function of proline: its ability to inhibit ROS-mediated apoptosis. To our knowledge, this is the first report showing that proline inhibits apoptosis. Our results thus provide important insights into the mechanism of proline-mediated survival during oxidative stress. In C. trifolii, proline appears to function as a potent antioxidant to scavenge intracellular ROS produced by DARas. The cytoprotective role of proline is specific because all other amino acids or osmolytes were ineffective (6). Thus, proline may not be just a by-product of stress defense, but a chemically active compound, crucially involved in the physiology of stress protection.
Recently, proline was proposed to scavenge free radicals in plants (40) and has been reported to quench singlet oxygen (41). However, the mechanism of ROS amelioration by proline is unknown. Our preliminary results suggest that CAT, but not SOD, mediates the proline-dependent prevention of ROS generation and apoptotic initiation in the DARas mutant, because CAT activity was significantly induced when the DARas mutant was grown on minimal medium supplemented with proline. Previous studies showed that mutated H-ras transfected rat fibroblasts have elevated levels of CAT that inhibit apoptosis, suggesting that a CAT-dependent antioxidant mechanism mediated by mutated H-ras is able to protect cells from apoptosis (42). Moreover, expression of the antiapoptotic gene bcl-2 in the rat pheochromocytoma PC12 cell line was found to inhibit apoptotic cell death, and a 2-fold increase in CAT activity was observed when compared with control cells, suggesting the possible involvement of CAT activity in Bcl-2-mediated inhibition of ROS-mediated apoptosis (43). Similar to these observations, we found that in C. trifolii, inhibition of apoptosis by proline also correlated with a significant increase in CAT activity, supporting the notion that proline may mediate a CAT-dependent antioxidant pathway to influence the onset of stress-induced apoptosis in C. trifolii. In addition, our results revealed that exogenous H2O2, a direct target of CAT, triggered an apoptotic-like PCD in WT C. trifolii. Thus, the involvement of CAT coupled with high levels of dichlorofluorescein fluorescence that was observed with DARas suggests that H2O2 may be the key ROS species responsible for the aberrant hyphal morphology as well as the apoptotic-like cell death of the fungus. It will be of interest to examine in more detail the mechanistic relationship between proline and CAT activity as well as potential roles of other antioxidants. Of note, we found that exogenous proline is able to increase CAT activity in S. cerevisiae when cells were exposed to H2O2 (data not shown).
There are still a number of mechanistic details that remain unexplained. Evidence from mammalian studies suggests the possible involvement of the proline utilization pathway in growth inhibition and apoptosis. Proline utilization occurs inside the mitochondria, where two nuclear-encoded enzymes, proline dehydrogenase (ProDH) and Δ1-pyrroline-5-carboxylate dehydrogenase, are required to convert proline into glutamate (44). Both ProDH and Δ1-pyrroline-5-carboxylate exhibited the ability to suppress cell growth and to induce apoptosis in a lung carcinoma cell line (45). Moreover, ProDH is able to generate ROS (46). To determine whether or not ProDH is involved in the protective role of proline in ROS-mediated apoptotic response, we recently used the budding yeast S. cerevisiae as a model. Our preliminary results indicated that overexpression of a yeast ProDH gene, put1, exhibited a significantly higher sensitivity to H2O2 and paraquat treatment, but accumulation of proline in put1 deletion mutant protected yeast cells from these oxidative stresses (data not shown). Thus, enzymatic removal of proline results in increased sensitivity to oxidative stress.
In summary, proline has been shown to possess a potent cell-protective function by ameliorating oxidative stress. Because many biotic (pathogens) and abiotic (e.g., UV and high and low temperatures) stresses involve oxidative stress and PCD, the ability of proline to quench ROS and function as a cytoprotectant may have important implications beyond those observed in C. trifolii as evidenced by the ability of proline to also protect yeast and its association with stress protection in plants (47). Moreover, abnormalities in proline metabolism have been associated with a number of mammalian diseases. For example, ProDH catalyzes the generation of proline-dependent ROS and promotes apoptosis in human colon cancer cell line (46); ProDH mutations were associated with hyperprolinemia in the schizophrenic patients (20). Modulation of proline levels may be an effective means for protecting cells against environmental insults and disease.
Supplementary Material
Acknowledgments
We thank R. French and L. Lane for comments. This work was supported in part by the University of Nebraska Redox Biology Center and National Science Foundation Grant IBN 0133078.
Author contributions: M.B.D. designed research; C.C. performed research; C.C. and M.B.D. analyzed data; and M.B.D. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CAT, catalase; MV, methyl viologen; PCD, programmed cell death; PI, prodium iodide; ProDH, proline dehydrogenase; PS, phosphatidylserine; ROS, reactive oxygen species; SOD, superoxide dismutase.
See Commentary on page 3175.
References
- 1.Hall, A. (1993) Curr. Opin. Cell. Biol. 5, 265-268. [DOI] [PubMed] [Google Scholar]
- 2.Bos, J. L. (1989) Cancer Res. 49, 4682-4689. [PubMed] [Google Scholar]
- 3.Cox, A. D. & Der, C. J. (2003) Oncogene 22, 8999-9006. [DOI] [PubMed] [Google Scholar]
- 4.Truesdell, G. M., Jones, C., Holt, T., Henderson, G. & Dickman, M. B. (1999) Mol. Gen. Genet. 262, 46-54. [DOI] [PubMed] [Google Scholar]
- 5.Mbonyi, K., Beullens, M., Detremerie, K., Geerts, L. & Thevelein, J. M. (1988) Mol. Cell. Biol. 8, 3051-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Memmott, S. D., Ha, Y. S. & Dickman, M. B. (2002) Appl. Environ. Microbiol. 68, 1647-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshiba, Y., Kiyosue, T., Nakashima, K., Yamaguchi-Shinozake, K. & Shinozaki, K. (1997) Plant Cell Physiol. 38, 1095-1102. [DOI] [PubMed] [Google Scholar]
- 8.Chen, T. H. & Murata, N. (2002) Curr. Opin. Plant Biol. 5, 250-257. [DOI] [PubMed] [Google Scholar]
- 9.Droge, W. (2002) Physiol. Rev. 82, 47-95. [DOI] [PubMed] [Google Scholar]
- 10.Irani, K., Xia, Y., Zweier, J. L., Sollott, S. J., Der, C. J., Fearon, E. R., Sundaresan, M., Finkel, T. & Goldschmidt-Clermont, P. J. (1997) Science 275, 1649-1652. [DOI] [PubMed] [Google Scholar]
- 11.Chen, C. & Dickman, M. B. (2004) Mol. Microbiol. 51, 1493-1507. [DOI] [PubMed] [Google Scholar]
- 12.Dickman, M. B. (1988) Curr. Genet. 14, 241-246. [Google Scholar]
- 13.Madeo, F., Frohlich, E. & Frohlich, K. U. (1997) J. Cell Biol. 139, 729-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsuwon, J. & Anderson, A. J. (1992) Can. J. Microbiol. 38, 1026-1032. [Google Scholar]
- 15.Beauchamp, C. & Fridovich, I. (1971) Anal. Biochem. 44, 276-287. [DOI] [PubMed] [Google Scholar]
- 16.Weber, H. U., Fleming, J. F. & Miquel, J. (1982) Arch. Gerontol. Geriatr. 1, 299-310. [DOI] [PubMed] [Google Scholar]
- 17.LeBel, C. P., Ischiropoulos, H. & Bondy, S. C. (1992) Chem. Res. Toxicol. 5, 227-231. [DOI] [PubMed] [Google Scholar]
- 18.Apel, K. & Hirt, H. (2004) Annu. Rev. Plant Biol. 55, 373-399. [DOI] [PubMed] [Google Scholar]
- 19.Kannan, K. & Jain, S. K. (2000) Pathophysiology 7, 153-163. [DOI] [PubMed] [Google Scholar]
- 20.Jacquet, H., Raux, G., Thibaut, F., Hecketsweiler, B., Houy, E., Demilly, C., Haouzir, S., Allio, G., Fouldrin, G., Drouin, V., et al. (2002) Hum. Mol. Genet. 11, 2243-2249. [DOI] [PubMed] [Google Scholar]
- 21.Collins, J. A., Schandi, C. A., Young, K. K., Vesely, J. & Willingham, M. C. (1997) J. Histochem. Cytochem. 45, 923-934. [DOI] [PubMed] [Google Scholar]
- 22.Del Carratore, R., Della Croce, C., Simili, M., Taccini, E., Scavuzzo, M. & Sbrana, S. (2002) Mutat. Res. 513, 183-191. [DOI] [PubMed] [Google Scholar]
- 23.Huh, G. H., Damsz, B., Matsumoto, T. K., Reddy, M. P., Rus, A. M., Ibeas, J. I., Narasimhan, M. L., Bressan, R. A. & Hasegawa, P. M. (2002) Plant J. 29, 649-659. [DOI] [PubMed] [Google Scholar]
- 24.Yin, Y., Stahl, B. C., DeWolf, W. C. & Morgentaler, A. (1998) Apoptosis 3, 281-287. [DOI] [PubMed] [Google Scholar]
- 25.Estevez, A. G., Grow, J. P., Sampson, J. B., Reiter, C., Zhuang, Y., Richardson, G. J., Tarpey, M. M., Barbeito, L. & Beckman, J. S. (1999) Science 286, 2498-2500. [DOI] [PubMed] [Google Scholar]
- 26.Sandstrom, P. A. & Buttke, T. M. (1993) Proc. Natl. Acad. Sci. USA 90, 4708-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suntres, Z. E. (2002) Toxicology 180, 65-77. [DOI] [PubMed] [Google Scholar]
- 28.Irani, K. & Goldschmidt-Clermont, P. J. (1998) Biochem. Pharmacol. 55, 1339-1346. [DOI] [PubMed] [Google Scholar]
- 29.Adler, V., Yin, Z., Tew, K. D. & Ronai, Z. (1999) Oncogene 18, 6104-6111. [DOI] [PubMed] [Google Scholar]
- 30.Hlavatá, L., Aguilaniu, H., Pichová, A. & Nyström, T. (2003) EMBO J. 22, 3337-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karim, F. D. & Rubin, G. M. (1988) Development (Cambridge, U.K.) 125, 1-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen, C. Y. & Faller, D. V. (1995) Oncogene 11, 1487-1498. [PubMed] [Google Scholar]
- 33.Cheng, J., Park, T. S., Chio, L. C., Fischl, A. S. & Ye, X. S. (2003) Mol. Cell. Biol. 23, 163-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips, A. J., Sudbery, I. & Ramsdale, M. (2003) Proc. Natl. Acad. Sci. USA 100, 14327-14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aspinall, D. & Paleg, L. G., eds. (1981) The Physiology and Biochemistry of Drought Resistance in Plants (Academic, Sydney), pp. 205-241.
- 36.Nanjo, T., Kobayashi, M., Yoshiba, Y., Sanada, Y., Wada, K., Tsukaya, H., Kakubari, Y., Yamaguchi-Shinozazki, K. & Shinozaki, K. (1999) Plant J. 18, 185-193. [DOI] [PubMed] [Google Scholar]
- 37.Schobert, B. & Tschesche, H. (1978) Biochim. Biophys. Acta 541, 270-277. [DOI] [PubMed] [Google Scholar]
- 38.Venekamp, J. H., Lampe, J. E. M. & Koot, J. T. M. (1989) J. Plant Physiol. 133, 654-659. [Google Scholar]
- 39.Monticello, D. J. & Costilow, R. N. (1981) Can. J. Microbiol. 27, 942-948. [DOI] [PubMed] [Google Scholar]
- 40.Saradhi, P. P., Alia, Arora, S. & Prasad, K. V. S. K. (1995) Biochem. Biophy. Res. Commun. 209, 1-5. [DOI] [PubMed] [Google Scholar]
- 41.Alia, Mohanty, P. & Matysik, J. (2001) Amino Acids 21, 195-200. [DOI] [PubMed] [Google Scholar]
- 42.Fernandes, R. S., McGowan, A. J. & Cotter, T. G. (1996) Anticancer Res. 16, 1691-1705. [PubMed] [Google Scholar]
- 43.Ellerby, L. M., Ellerby, H. M., Park, S. M., Holleran, A. L., Murphy, A. N., Fiskum, G., Kane, D. J., Testa, M. P., Kayalar, C. & Bredesen, D. E. (1996) J. Neurochem. 67, 1259-1267. [DOI] [PubMed] [Google Scholar]
- 44.Phang, J. M. (1985) Curr. Top. Cell. Regul. 25, 91-132. [DOI] [PubMed] [Google Scholar]
- 45.Maxwell, S. A. & Davis, G. E. (2000) Proc. Natl. Acad. Sci. USA 97, 13009-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donald, S. P., Sun, X. Y., Hu, C. A., Yu, J., Mei, J. M., Valle, D. & Phang, J. M. (2001) Cancer Res. 61, 1810-1815. [PubMed] [Google Scholar]
- 47.Delauney, A. J. & Verma, D. P. S. (1993) Plant J. 4, 215-223. [Google Scholar]
- 48.Katsuwon, J. & Anderson, A. J. (1989) Appl. Environ. Microbiol. 55, 2985-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.