Abstract
Donor organ shortage is the main limitation to liver transplantation as a treatment for end-stage liver disease (ESLD) and acute liver failure (ALF). Liver regenerative medicine may in the future offer an alternative form of therapy for these diseases, be it through cell transplantation, bioartificial liver (BAL) devices, or bioengineered whole organ liver transplantation. All three strategies have shown promising results in the past decade. However, before they are incorporated into widespread clinical practice, the ideal cell type for each treatment modality must be found, and an adequate amount of metabolically active, functional cells must be able to be produced. Research is ongoing in hepatocyte expansion techniques, use of xenogeneic cells, and differentiation of stem cell-derived hepatocyte-like cells (HLCs). HLCs are a few steps away from clinical application, but may be very useful in individualized drug development and toxicity testing, as well as disease modeling. Finally, safety concerns including tumorigenicity and xenozoonosis must also be addressed before cell transplantation, BAL devices, and bioengineered livers occupy their clinical niche. This review aims to highlight the most recent advances and provide an updated view of the current state of affairs in the field of liver regenerative medicine.
Keywords: Liver regeneration, cell therapy, cell transplantation, bioartificial liver, tissue engineering
Introduction
Liver transplantation is to date the only proven treatment for ESLD and ALF. Due to the shortage of transplantable organs, however, alternatives to liver transplantation have long been sought after. With the advent of regenerative medicine, these alternative forms of treatment are becoming distinct possibilities – be it through cell and stem cell therapy, BAL devices, or organ bioengineering. The liver is particularly amenable to these forms of therapy due to its innate capacity for intense regeneration and self-repair.
The oldest form of cell therapy is cell transplantation, which has been tested on a myriad of different liver diseases with uneven results. Primary hepatocyte transplantation, however, shares many of the limitations of whole-organ liver transplantation: scarcity of donor livers from which high-quality primary hepatocytes can be isolated, and possibility of allogeneic rejection. For this reason, the focus of liver cell therapy has shifted slightly onto the therapeutic potential of stem cells. Stem cell transplantation is especially promising in inherited liver disease- where it may be able, in combination with gene therapy, to offer permanent correction of metabolic deficiencies. Stem cell-derived HLCs also provide the opportunity for non-invasive metabolic profiling and drug toxicity testing. With individualized medicine on the rise, these therapeutic strategies will soon gain visibility in the field of hepatology.
The next step in liver regenerative medicine was the creation of a BAL system capable of bridging a patient either to liver transplantation or to recovery of the native liver through endogenous regeneration. These devices, as opposed to artificial liver support systems, contain live, functioning hepatocytes, and so are able to perform synthetic functions as well as blood detoxification by way of albumin dialysis. This holds great promise for the treatment of ALF. Finally, the paradigm of regenerative medicine is considered by many to be tissue engineering. Considerable advances have been made in the past decade toward the construction of a bioengineered liver through the de- and recellularization of a three-dimensional liver scaffold.
The aim of this review is to highlight the most recent advances made in the field of regenerative medicine for the treatment of liver disease, and to address the role that these new technologies may play in the clinical setting over the next few years.
Use of Primary Hepatocytes vs. Stem Cells
Isolated primary hepatocytes were the first and most obvious candidate for use in cell therapy, but they have several limitations related to both the nature of the cell and the scarcity of its source. Hepatocytes are not easily cultured in vitro and are susceptible to freeze-thaw damage (1). The most important restriction to their use, however, is the difficulty that isolation of a sufficient quantity of metabolically active, high quality cells presents (2). Not only is there a universal shortage of donor livers on which cell harvests may be performed, but the fact that hepatocytes are typically harvested from livers not suitable for transplantation makes quantity and quality of cells obtained highly variable (3). Alternatives to the use of primary human hepatocytes are porcine hepatocytes and stem cell-derived HLCs. Due to xenozoonosis concerns, the use of porcine cells in trials applicable to clinical practice has typically been limited to BAL devices, where there is no direct contact between patient and porcine hepatocyte.
Research in the field of regenerative medicine has lately set its focus on the generation of HLCs, namely from induced pluripotent stem cells (iPSCs). Several groups have developed standardized, efficient protocols for the development and isolation of iPSC-derived HLCs through soluble factors (4-6), and direct reprogramming of fibroblasts to HLCs has also been achieved (7, 8). Cell reprogramming for the production of autologous hepatocytes potentially allows these therapies to bypass the scarcity of human donor livers, as well as avoid allogeneic rejection (9). However, reprogrammed cells are not without their disadvantages. As of today, fully mature HLCs have not yet been produced: studies have shown that stem cell-derived HLCs are phenotypically and functionally more similar to fetal than adult human hepatocytes (10). Notwithstanding this, their metabolic profile and CYP activity is sufficient to provide human in vitro models for toxicity testing and drug studies (11), a use which will be discussed more in depth in the following section. The main safety concern these cells pose is their possible tumorigenesis (12), and although this issue has been partly bypassed through the avoidance of viral vectors (13), the altered expression of the basal reprogramming factors involved in their differentiation has also been reported to be associated with cancer (14-16).
Stem cells have been used not only to produce HLCs, but to create a favorable environment for HLCs or primary hepatocytes to grow. Coculture with mesenchymal stem cells (MSCs) provides primary human hepatocytes with direct structural and paracrine trophic support, resulting in improved viability and function (17). Another strategy that may improve hepatocyte functionality is three-dimensional culture (18). Aggregation into organoid-like structures has been tested in primary hepatocytes and stem cell-derived HLCs with and without MSC coculture, yielding promising results both in terms of functionality and engraftment (19, 20).
In conclusion, to date the ideal cell type for use in hepatic regenerative medicine remains the primary hepatocyte. Nevertheless, progress is rapidly being made in the development and maturation of stem cell-derived HLCs, which may lead to the experimental introduction of these cells into the clinical setting in the near future.
Individualized Pharmaceutical Testing
The most immediate application of iPSC-derived HLCs will foreseeably take place in the field of pharmaceutical development and individualization. Drug development is a long and expensive process with up to 90% of developed drugs failing clinical trials or being withdrawn from the market due to unexpected toxicity (21). This underwhelming efficiency is likely due to imperfect toxicity correlations in vivo between animal model physiology and human patients, as well as irrelevant positive toxicity-related responses from in vitro assays that rely on primary cell cultures or immortalized cell lines (22, 23).
Drug assays in iPSC-derived HLCs provide a powerful in vitro alternative to existing methods for drug toxicity testing and metabolic profiling. iPSCs constitute a non-invasive, personalized approach to pharmacodynamic and pharmaceutical testing, since they can be produced from a blood sample rather than a liver biopsy (24). They allow for prediction of interindividual differences in hepatic metabolism and drug sensitivity mediated by genetic polymorphisms, which influence both drug efficacy and adverse reactions (25), especially idiosyncratic drug-induced liver injury (26). Disease-specific iPSC have also been created that allow for a disease-in-a-dish approach to modeling (27) that may be applicable to a variety of genetic diseases (28) and may offer the opportunity not only for drug screening, but for gene correction as well (29). Although standardization is necessary before these cells can be applied in routine pharmacotoxicology (30), the ability to test for undesired outcomes in a relevant biological model during the early stages of drug development will enhance the efficiency and affordability of the novel drug approval process.
Cell Transplantation
Cell transplantation has been tested in a number of patients with various forms of liver disease: most commonly metabolic diseases, but also acute and chronic liver disease. These clinical interventions have occurred as a result of the significant therapeutic benefit achieved in a number of preclinical models, mostly rodent. Cell transplantation has a number of key advantages. First, the procedure is less invasive than organ transplantation and multiple cell transplants can occur over time. Second, the native liver is left in place, allowing it the possibility of self-regeneration in the case of acute liver failure (ALF). Third, with the promise of gene therapy and stem cell technology slowly coming to realization, the opportunity exists for an individualized, autologous approach to regenerative medicine.
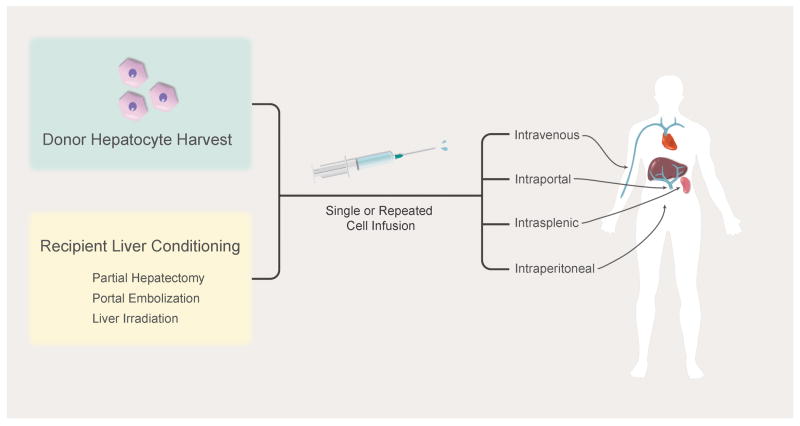
Therapeutic benefit in a number of small animal models of metabolic liver diseases has been demonstrated using hepatocyte transplantation (31, 32). The general concept behind these successes has been infusion of hepatocytes from highly inbred, syngeneic donors into recipients through intrasplenic or portal vein injection; the ultimate goal is to achieve a high enough engraftment so that a sufficient amount of the missing enzyme/protein is produced that a therapeutic effect is achieved – generally estimated to be 5-10% for many diseases. While cell engraftment is initially low, estimated to be < 1% of total liver mass (33), a number of strategies have been used to increase engraftment and/or proliferation of transplanted cells. Some diseases provide an inherent natural selective advantage for transplanted cells, as is the case in hereditary tyrosinemia type 1 (34) and alpha-1 antitrypsin deficiency (35). Other methods have been tested in the case of diseases without a selective advantage for transplanted cells, including genetic modification of the donor cells (36, 37) and injury to the recipient liver (38, 39). These methods have been successful in increasing donor cell expansion to sufficient quantities to produce a biological effect. While the safety of clinical hepatocyte transplantation has clearly been established, demonstration of therapeutic benefit has been modest and short-lived (40). A major focus of ongoing clinical trials is improving engraftment and proliferation of donor hepatocytes using one or a combination of partial hepatectomy (41), portal embolization (42), liver irradiation (38), and repeated cell infusion (43) (Figure 1).
Figure 1.
Infusion and Engraftment Strategies in Cell Transplantation
The goal of cell transplantation in ALF is to provide time for (a) the native liver to regenerate or (b) liver transplantation to occur. Because of the differences in regeneration of rodent and human livers, it is difficult to interpret the positive results seen in ALF rodent models (44). Notwithstanding this, significant improvements in survival have been demonstrated in various drug-induced rodent models of liver failure (45). Clinically, a number of studies have occurred studying the impact of hepatocyte transplantation on various forms of ALF, most commonly drug and viral-induced (40, 46). However, as all of these studies were non-controlled, it is not possible to draw definitive conclusions from them. Variations within these studies included: delivery method of cells (intravenous, intraperitoneal, intrasplenic, and portal vein infusion); dose of cells injected; and use of fresh or frozen cells. Therefore, it seems imperative that clinically-relevant models of ALF are utilized in future studies, and for these it would appear that large animal models will play a key role (47, 48).
In a number of small animal models of chronic liver failure, significant improvements were documented after hepatocyte transplantation (49, 50). Clinically, a range of responses have been reported, and interpretation of results is difficult given the nature of the uncontrolled experiments (51). Two general approaches have been attempted: allogeneic transplantation of donor hepatocytes from noncirrhotic liver donors; or autologous transplantation of hepatocytes isolated from a single lobe of the recipient's cirrhotic liver. Given the increased risk of portal hypertension after portal infusion of cells into a cirrhotic liver, intrasplenic injection has been the most common method of cell delivery (52). More recently, preclinical data has indicated that extrahepatic lymph nodes may provide a superior niche for hepatocyte engraftment than the cirrhotic liver, in which cell engraftment and function is limited (53).
Successful hepatocyte transplantation in animal models of liver disease, primarily rodent, has not been replicated clinically. While each of the three groups of diseases (metabolic, acute, and chronic) may have different etiologies, a few common hurdles will need to be overcome before significant and reproducible clinical success is achieved. The first major challenge is the shortage of high quality primary hepatocytes. As the shortage of donor organs is unlikely to improve in the future, alternative sources of high quality, engraftable cells will be needed. Ongoing research in this field includes the use of animal bioreactors to expand human hepatocytes (54), as well as identifying alternative sources of expandable cells. Recent data from several groups have identified populations of liver progenitor cells that allow expansion ex vivo (55). While reproducible correction of mouse models has not been achieved, the data indicates that under the right conditions these progenitor cells can engraft and function in vivo in rodent models of metabolic disease and liver failure. The second major challenge is the immune response against transplanted allogeneic cells (41). In the case of metabolic disease, this issue can be bypassed through the use of genetically-corrected autologous hepatocytes, a procedure that has been attempted only once for liver disorders (56). Given the encouraging clinical success observed in the treatment of primary immunodeficiencies using ex vivo gene therapy with lentiviral vectors (57), it appears highly warranted that continued evaluation of ex vivo hepatocyte-directed gene therapy occurs. Although primary hepatocytes isolated from liver resection remain the optimal cell population for re-transplantation following gene therapy, the advent of nuclear reprogramming technology may allow for an alternative cell population in which to perform gene therapy and subsequent hepatocyte differentiation. While the utility of this approach to model “disease in a dish” is irrefutable, evidence to support the reprogrammed HLCs' ability to engraft and expand in vivo is still limited. However, encouraging results in mouse models of metabolic disease and liver failure have been reported (8, 58, 59).
Bioartificial Liver Systems
Liver transplantation remains the only definitive treatment for ALF, with an overall one year survival rate for this disease of about 65% (60). However, there are several important limitations to liver transplantation, namely the nation-wide shortage of donor organs and the possibility of allogeneic rejection, together with the many long-term adverse effects of immunosuppressant medication. For this reason, alternative therapies are being sought out, with artificial and bioartificial liver support systems constituting one of the most promising solutions currently under development.
The ideal liver support system should detoxify waste molecules such as ammonia, provide synthetic function of albumin and coagulation factors, decrease inflammation, and promote cell regeneration. A BAL system incorporates hepatocytes into a purely mechanical, albumin dialysis-based artificial liver support device to achieve the aforementioned goals. Ideally, a BAL support system would use primary human hepatocytes. However, large amounts of high-quality human hepatocytes are not readily available. Therefore, a number of different cell lines currently being used, including immortalized hepatocytes, stem cell-derived HLCs, and xenogeneic hepatocytes. Research is ongoing to achieve mass-production of stem cell-derived fully mature, functional HLCs. Human fibroblasts have been successfully reprogrammed into functional human induced hepatocytes (hiHeps) (7, 8). These cells have been used to repopulate mouse livers, and have also been performance tested in a hiHep-based BAL for treatment of a drug-induced porcine ALF model. Their results showed improvement in prothrombin time (PT) and ammonia levels, as well as a statistically significant improvement in survival (61). To date, no stem cell-based BAL system has undergone human trial. The two commercially available BAL systems that have undergone the most extensive human clinical trials are the Extracorporeal Liver Assist Device (ELAD) and the HepatAssist. They use C3A immortalized human cells derived from the HepG2 hepatoblastoma cell line and porcine hepatocytes respectively.
The ELAD uses a HepG2/C3A cell line, and its first human trial was reported in 1994, demonstrating safety with the use of ELAD in ALF patients (62). In 1996, a small randomized controlled trial using ELAD was conducted in 24 patients, but were not able to show a significant difference in survival between the treatment and control groups (63). More recently, the Stabilization of Liver Failure (SILVER) trial was initiated in patients with ALF and acute-on-chronic liver failure (ACLF) in multiple centers in the United States, United Kingdom, China, and Saudi Arabia; patients were randomized to ELAD plus standard medical therapy (SMT) vs SMT alone. Its interim results from China were presented in 2007: they showed a significant increase in survival in the treatment group (86%, 35 patients) versus the control group (47%, 19 patients) (64). The US trial presented its results in 2015: they showed no statistically significant difference in overall survival at 28 and 91 days between the treatment (96 patients) and control (107 patients) groups. However, stratified results showed that ELAD improved outcomes in patients under 50 years of age with Model for End-stage Liver Disease (MELD) scores below 30. More randomized controlled trials are underway to confirm these results (65).
HepatAssist uses primary hepatocytes from healthy pig donors, and its largest clinical trial to date was published in 2004 (66). This trial was conducted at 20 institutions in the US and Europe, where patients were randomized prospectively to BAL plus SMT (85 patients) or SMT alone (85 patients), with no statistically significant difference in 30-day survival between the treatment (71%) and control (62%) groups. A further post hoc analysis of 83 patients with known causes of ALF did show a significant difference in survival with BAL therapy as compared to SMT (p<0.009).
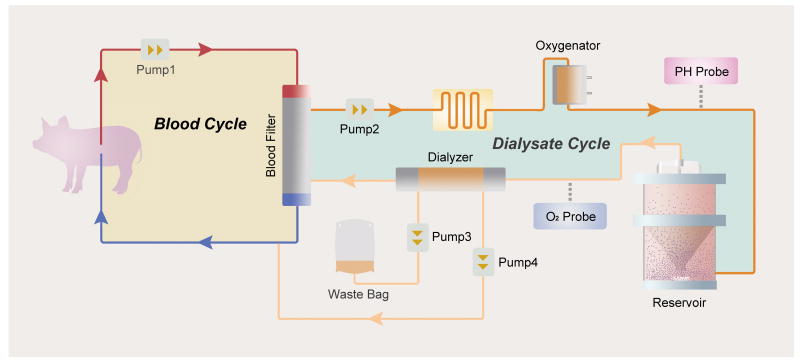
Both BAL systems have disadvantages. Since ELAD uses tumor cells, a theoretical risk exists of tumor migration into the patient's circulation. In addition, HepG2/C3A tumor cells show decreased hepatic functions when compared to normal hepatocytes, especially in terms of ureagenesis and drug metabolism (67). On the other hand, porcine hepatocyte-based BAL systems pose concern for xenozoonosis, although no zoonotic infection was observed in the HepatAssist trial (66). However, with primary hepatocytes the issue of their loss of function and tendency to apoptose in the ex vivo condition remains. The second-generation porcine hepatocyte BAL system, or Spheroid Reservoir Bioartificial Liver (SRBAL), resolved this issue by culturing the cells in three-dimensional spheroids instead of monolayers. This spheroid configuration allows for a higher number of hepatocytes per volume, and longer functional and survival times with decreased apoptosis (68, 69). A recent study showed a statistically significant survival benefit to treatment with the SRBAL in a drug-induced ALF pig model (47) (Figure 2), although no human trial has been performed thus far.
Figure 2.
Schematic Representation of the Spheroid Reservoir Bioartificial Liver (SRBAL) Device
Going forward, a method for large-scale production of fully functional hepatocytes must be developed in order for BAL systems to be incorporated into clinical use. To this effect, a re-population model was created in which a mouse model of hereditary tyrosinemia type 1 were used to expand transplanted human hepatocytes by virtue of the graft's selective advantage over the native fumarylacetoacetate hydrolase (FAH)-deficient cells (54). A similar porcine model has recently been developed that could potentially allow for large-scale production of high-quality and readily available human hepatocytes in an animal bioreactor (48). Besides these re-population models, continuous advancements in the hepatocytic differentiation of iPSCs, embryonic stem cells (ESCs), and human fibroblasts may be able to create the metabolically active, functional HLCs needed for BAL systems in the future (7, 70).
Organ Bioengineering
Tissue engineered regenerated whole organs have the unique potential to overcome two major issues facing the field of transplantation: the shortage of donor organs as well as the need for ongoing chronic immunosuppression. Tissue engineering strategies have been to date been applied in building a biological substitute for a number of failing tissues including the heart (71), lungs (72), bladder, intestines, trachea, kidney, and liver (73).
A tissue engineered liver requires a scaffold on which to build a functional organ. A variety of scaffolds have been trialed, including biodegradable polymer matrices (74), two dimensional hepatic tissue sheets (75), and decellularized xenogeneic liver matrices (76), with greatest success being observed in xenogeneic (porcine or murine) liver scaffolds. In this case, the presence of an intact native extracellular matrix (ECM) is of central importance, as it not only provides a platform for cell ingrowth, but is also thought to mediate biochemical and molecular signaling (77).
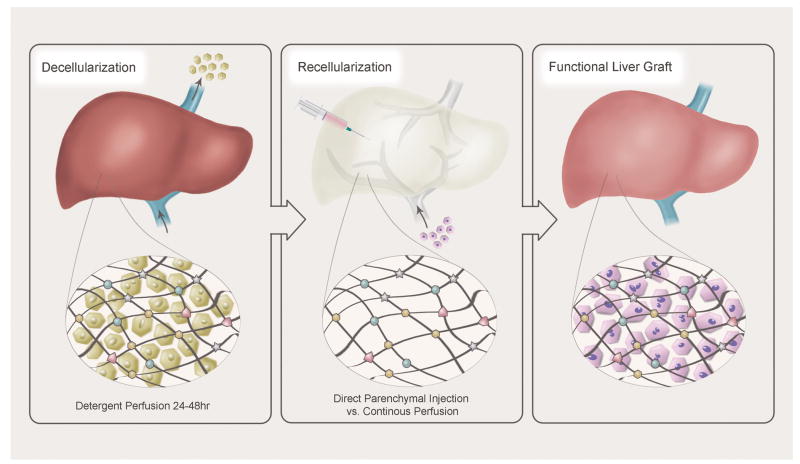
In order to obtain a xenogeneic scaffold, complete decellularization of the native organ must be achieved. Detergent perfusion at physiologic pressures via the native vasculature for 24-48 hours has proven to be successful in attaining complete decellularization. Livers are first flushed with large volumes of isotonic saline solution, then perfused with detergents, which include sodium dodecyl sulphate (SDS), ethylene glycol tetraacetic acid (EGTA), Triton X-100 1%, 2%, or 3%, and DNase (76). Some protocols also utilize gamma irradiation to further reduce immunogenic properties (78). The key to a successful decellularization is preservation of the ECM through maintenance of the collagen matrix together with destruction of the native organ DNA. In order to avoid immune reactions, a decellularized scaffold should have under 50 ng double stranded DNA per mg ECM (79). Immunogenicity has been tested via implantation of naked scaffolds into allogeneic or xenogeneic hosts without evidence of increased immune response as measured by total white blood cell count, lymphocyte count, monocyte count, and lack of CD3+ T cell activation at the site of implantation (80).
Recellularization of the xenogeneic scaffold requires a large quantity of readily available, highly functional hepatocytes. Human survival is possible with 10-30% (200-600 grams) of residual hepatic parenchyma. Therefore, 2.5-7.5 billion individual hepatocytes are needed. These cells must be capable of proliferation and safe for transplantation. Adult human hepatocytes harvested from deceased donor grafts or partial hepatectomy are a potential cell source; however, adequate volumes of suitable cells are difficult to obtain. Furthermore, although the adult human liver is capable of significant regeneration following major hepatectomy, this process is poorly understood (81), and the adult hepatocyte demonstrates minimal in vitro proliferation proving thus far to be a poor candidate for organ regeneration. In contrast, human fetal liver cells demonstrate in vitro proliferation; however, they are not readily available and their hepatocytic functions remain relatively low (77). Hepatoblastoma-derived cell lines (HepG2) offer unlimited expansion potential and have shown promise in clinical trials of BAL devices (82). Unfortunately, the risk for uncontrolled metastatic spread prevents the use of these cells in an implantable liver. Xenogeneic cells sources such as porcine hepatocytes have also been trialed with success in BAL devices. The question of zoonotic viral transmission has been raised, but has not been realized in several clinical trials (83). Autologous stem cells also show significant promise as a readily available and functional cell source: human iPSCs have been utilized to create an organ bud capable of liver specific protein production and drug metabolism (84).
In order to achieve successful recellularization, both direct parenchymal injections as well as single or multistep continuous perfusion at physiologic pressures have been explored, facilitated by the construction of a sterile organ chamber in which the scaffold is mounted and supported in tissue culture. A rat model was used to demonstrate proof of concept of whole liver decellularization and recellularization with mature rat hepatocytes (78). Proliferation was confirmed via Ki67 antibody staining; albumin production and CYP1A1/2 activity confirmed ongoing metabolic function. Since hepatocytes demonstrate a significant increase in function with cell-cell and cell-matrix interactions, in vitro liver progenitor cell spheroids were produced which showed increased cell survival and differentiation when compared to single cell suspensions (85). A rat hepatocyte spheroid tissue engineered liver was transplanted into a 90% rat hepatectomy model with an increase in overall survival from 16 to 72 hours; however, transplanted rats ultimately perished from small-for-size syndrome (86).
Regardless of the cell type selected, an intact vascular network is required to support the necessary cell mass. Therefore, a major challenge to hepatocyte recellularization has been the conservation of a functional vascular infrastructure. Rat anti-mouse CD31 was used to enhance reendothelialization of a liver scaffold with murine endothelial cells (MS1), and demonstrated in vivo patency at 24 hours in a porcine recipient (87). Significant reendothelialization and in vivo vascular patency has also been demonstrated through the use of porcine umbilical vein endothelial cells (PUVECs) at 72 hours following implantation in a porcine recipient (Mao et al 2017).
Organ bioengineering represents a promising frontier for the creation of readily available and sustainable organs for transplantation. The decellularized xenogeneic scaffold with an intact ECM has proven an effective backbone on which to create a tissue engineered organ, and additional efforts aimed at selecting an ideal cell type for human application are ongoing. Still, further research into optimal cell seeding techniques and cell volumes required to sustain function is necessary.
Conclusion
A regenerative medicine approach to liver disease may in the future be a solution to the current shortage of donor livers available for transplantation. Cell transplantation has been tested in a number of pre-clinical and clinical models of various forms of liver disease, yielding promising results for the treatment of metabolic disorders in particular. Similarly, BAL systems may soon start playing a role in the treatment of ALF by either allowing for regeneration of the native liver or bridging the patient to liver transplantation. At the same time, major advances are also being made towards the creation of bioengineered, transplantable organs.
However, several important challenges must still be overcome before these therapeutic strategies are incorporated into clinical practice. First and foremost, the optimal cell type for each therapy must be determined, and be able to be obtained or produced in quantities sufficient for large-scale clinical application. Secondly, these cells must be able to be cultured efficiently in vitro, and in the case of cell transplantation and bioengineered livers engraft successfully in vivo. Thirdly, each cell type must demonstrate safety in humans, with a special focus on concerns for xenozoonosis and tumorigenicity. Notwithstanding this, stem cell-derived HLCs are already being used in individualized medicine for the development and toxicity testing of new drugs.
Figure 3.
De- and Recellularization Process for the Creation of Bioengineered Livers
Footnotes
Clara T. Nicolas: Conception and design, manuscript writing
Raymond D. Hickey: Manuscript writing
Harvey S. Chen: Manuscript writing
Shennen A. Mao: Manuscript writing
Manuela Lopera Higuita: Manuscript writing
Yujia Wang: Figure preparation
Scott L. Nyberg: Conception and design, final approval of manuscript
References
- 1.Nicolas C, Wang Y, Luebke-Wheeler J, Nyberg SL. Stem Cell Therapies for Treatment of Liver Disease. Biomedicines. 2016;4(1) doi: 10.3390/biomedicines4010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibars EP, Cortes M, Tolosa L, Gomez-Lechon MJ, Lopez S, Castell JV, et al. Hepatocyte transplantation program: Lessons learned and future strategies. World J Gastroenterol. 2016;22(2):874–86. doi: 10.3748/wjg.v22.i2.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolas CT, Wang Y, Nyberg SL. Cell therapy in chronic liver disease. Curr Opin Gastroenterol. 2016 doi: 10.1097/MOG.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma X, Duan Y, Tschudy-Seney B, Roll G, Behbahan IS, Ahuja TP, et al. Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells. Stem Cells Transl Med. 2013;2(6):409–19. doi: 10.5966/sctm.2012-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asplund A, Pradip A, van Giezen M, Aspegren A, Choukair H, Rehnstrom M, et al. One Standardized Differentiation Procedure Robustly Generates Homogenous Hepatocyte Cultures Displaying Metabolic Diversity from a Large Panel of Human Pluripotent Stem Cells. Stem Cell Rev. 2016;12(1):90–104. doi: 10.1007/s12015-015-9621-9. [DOI] [PubMed] [Google Scholar]
- 6.Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Ishige N. Hepatocyte selection medium eliminating induced pluripotent stem cells among primary human hepatocytes. World J Methodol. 2015;5(3):108–14. doi: 10.5662/wjm.v5.i3.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang P, Zhang L, Gao Y, He Z, Yao D, Wu Z, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell stem cell. 2014;14(3):370–84. doi: 10.1016/j.stem.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhu S, Rezvani M, Harbell J, Mattis A, Wolfe A, Benet L, et al. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature. 2014;508(7494):93–7. doi: 10.1038/nature13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell stem cell. 2013;12(4):407–12. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Baxter M, Withey S, Harrison S, Segeritz CP, Zhang F, Atkinson-Dell R, et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol. 2015;62(3):581–9. doi: 10.1016/j.jhep.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulvestad M, Nordell P, Asplund A, Rehnstrom M, Jacobsson S, Holmgren G, et al. Drug metabolizing enzyme and transporter protein profiles of hepatocytes derived from human embryonic and induced pluripotent stem cells. Biochemical pharmacology. 2013;86(5):691–702. doi: 10.1016/j.bcp.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 13.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 14.Kuttler F, Mai S. c-Myc, Genomic Instability and Disease. Genome Dyn. 2006;1:171–90. doi: 10.1159/000092507. [DOI] [PubMed] [Google Scholar]
- 15.Park ET, Gum JR, Kakar S, Kwon SW, Deng G, Kim YS. Aberrant expression of SOX2 upregulates MUC5AC gastric foveolar mucin in mucinous cancers of the colorectum and related lesions. Int J Cancer. 2008;122(6):1253–60. doi: 10.1002/ijc.23225. [DOI] [PubMed] [Google Scholar]
- 16.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121(3):465–77. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Fitzpatrick E, Wu Y, Dhadda P, Hughes RD, Mitry RR, Qin H, et al. Coculture with mesenchymal stem cells results in improved viability and function of human hepatocytes. Cell Transplant. 2015;24(1):73–83. doi: 10.3727/096368913X674080. [DOI] [PubMed] [Google Scholar]
- 18.Rebelo SP, Costa R, Silva MM, Marcelino P, Brito C, Alves PM. Three-dimensional co-culture of human hepatocytes and mesenchymal stem cells: improved functionality in long-term bioreactor cultures. J Tissue Eng Regen Med. 2015 doi: 10.1002/term.2099. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran SD, Schirmer K, Munst B, Heinz S, Ghafoory S, Wolfl S, et al. In Vitro Generation of Functional Liver Organoid-Like Structures Using Adult Human Cells. PLoS ONE. 2015;10(10):e0139345. doi: 10.1371/journal.pone.0139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song W, Lu YC, Frankel AS, An D, Schwartz RE, Ma M. Engraftment of human induced pluripotent stem cell-derived hepatocytes in immunocompetent mice via 3D co-aggregation and encapsulation. Sci Rep. 2015;5:16884. doi: 10.1038/srep16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin LL. Stem cells and drug discovery: the beginning of a new era? Cell. 2008;132(4):549–52. doi: 10.1016/j.cell.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Anson BD, Kolaja KL, Kamp TJ. Opportunities for use of human iPS cells in predictive toxicology. Clin Pharmacol Ther. 2011;89(5):754–8. doi: 10.1038/clpt.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mann DA. Human induced pluripotent stem cell-derived hepatocytes for toxicology testing. Expert Opin Drug Metab Toxicol. 2015;11(1):1–5. doi: 10.1517/17425255.2015.981523. [DOI] [PubMed] [Google Scholar]
- 24.Dvorak Z. Opportunities and challenges in using human hepatocytes in cytochromes P450 induction assays. Expert Opin Drug Metab Toxicol. 2016;12(2):169–74. doi: 10.1517/17425255.2016.1125881. [DOI] [PubMed] [Google Scholar]
- 25.Takayama K, Morisaki Y, Kuno S, Nagamoto Y, Harada K, Furukawa N, et al. Prediction of interindividual differences in hepatic functions and drug sensitivity by using human iPS-derived hepatocytes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(47):16772–7. doi: 10.1073/pnas.1413481111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J, Einhorn S, Venkatarangan L, Miller M, Mann DA, Watkins PB, et al. Morphological and Functional Characterization and Assessment of iPSC-Derived Hepatocytes for In Vitro Toxicity Testing. Toxicol Sci. 2015;147(1):39–54. doi: 10.1093/toxsci/kfv117. [DOI] [PubMed] [Google Scholar]
- 27.Soldner F, Jaenisch R. Medicine. iPSC disease modeling. Science. 2012;338(6111):1155–6. doi: 10.1126/science.1227682. [DOI] [PubMed] [Google Scholar]
- 28.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi SM, Kim Y, Shim JS, Park JT, Wang RH, Leach SD, et al. Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology. 2013;57(6):2458–68. doi: 10.1002/hep.26237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wobus AM, Loser P. Present state and future perspectives of using pluripotent stem cells in toxicology research. Arch Toxicol. 2011;85(2):79–117. doi: 10.1007/s00204-010-0641-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, et al. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976;192(4242):892–4. doi: 10.1126/science.818706. [DOI] [PubMed] [Google Scholar]
- 32.Hickey R, Mao SA, Amiot B, Suksanpaisan L, Miller A, Nace R, et al. Noninvasive 3D imaging of liver regeneration in a mouse model of hereditary tyrosemia type 1 using the sdium iodide symporter gene. Liver Transplantation. 2015 doi: 10.1002/lt.24057. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponder KP, Gupta S, Leland F, Darlington G, Finegold M, DeMayo J, et al. Mouse hepatocytes migrate to liver parenchyma and function indefinitely after intrasplenic transplantation. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(4):1217–21. doi: 10.1073/pnas.88.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, et al. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1996;12(3):266–73. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- 35.Ding J, Yannam GR, Roy-Chowdhury N, Hidvegi T, Basma H, Rennard SI, et al. Spontaneous hepatic repopulation in transgenic mice expressing mutant human alpha1-antitrypsin by wild-type donor hepatocytes. J Clin Invest. 2011;121(5):1930–4. doi: 10.1172/JCI45260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yovchev M, Jaber FL, Lu Z, Patel S, Locker J, Rogler LE, et al. Experimental Model for Successful Liver Cell Therapy by Lenti TTR-YapERT2 Transduced Hepatocytes with Tamoxifen Control of Yap Subcellular Location. Sci Rep. 2016;6:19275. doi: 10.1038/srep19275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiang D, Liu CC, Wang MJ, Li JX, Chen F, Yao H, et al. Non-viral FoxM1 gene delivery to hepatocytes enhances liver repopulation. Cell Death Dis. 2014;5:e1252. doi: 10.1038/cddis.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanouchi K, Zhou H, Roy-Chowdhury N, Macaluso F, Liu L, Yamamoto T, et al. Hepatic irradiation augments engrfatment of donor cells following hepatocyte transplantation. Hepatology. 2009;49(1):258–67. doi: 10.1002/hep.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jirtle RL, Michalopoulos G. Effects of partial hepatectomy on transplanted hepatocytes. Cancer Res. 1982;42(8):3000–4. [PubMed] [Google Scholar]
- 40.Dhawan A, Puppi J, Hughes R, Mitry R. Human hepatocyte transplantation: current experience and future challenges. Nature Reviews Gastroenterology Hepatology. 2010;7:288–98. doi: 10.1038/nrgastro.2010.44. [DOI] [PubMed] [Google Scholar]
- 41.Jorns C, Nowak G, Nemeth A, Zemack H, Mork LM, Johansson H, et al. De Novo Donor-Specific HLA Antibody Formation in Two Patients With Crigler-Najjar Syndrome Type I Following Human Hepatocyte Transplantation With Partial Hepatectomy Preconditioning. Am J Transplant. 2016;16(3):1021–30. doi: 10.1111/ajt.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dagher I, Boudechiche L, Branger J, Coulomb-Lhermine A, Parouchev A, Sentilhes L, et al. Efficient hepatocyte engraftment in a nonhuman primate model after partial portal vein embolization. Transplantation. 2006;82(8):1067–73. doi: 10.1097/01.tp.0000236103.99456.8f. [DOI] [PubMed] [Google Scholar]
- 43.Darwish AA, Sokal E, Stephenne X, Najimi M, de Goyet Jde V, Reding R. Permanent access to the portal system for cellular transplantation using an implantable port device. Liver Transpl. 2004;10(9):1213–5. doi: 10.1002/lt.20228. [DOI] [PubMed] [Google Scholar]
- 44.Belanger M, Butterworth RF. Acute liver failure: a critical appraisal of available animal models. Metab Brain Dis. 2005;20(4):409–23. doi: 10.1007/s11011-005-7927-z. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland DE, Numata M, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation in acute liver failure. Surgery. 1977;82(1):124–32. [PubMed] [Google Scholar]
- 46.Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Seminars in liver disease. 1999;19(1):39–48. doi: 10.1055/s-2007-1007096. [DOI] [PubMed] [Google Scholar]
- 47.Glorioso J, Mao S, Rodysill B, Mounajjed T, Kremers W, Elgilani F, et al. Pivotal preclinical trial of the spheroid reservoir bioartificial liver. J Hepatology. 2015 doi: 10.1016/j.jhep.2015.03.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hickey R, Mao S, Glorioso J, Lillegard J, Fisher J, Amiot B, et al. Fumarylacetoacetate hydrolase deficient pigs are a novel large animal model of metabolic liver disease. Stem Cell Res. 2014;13:144–53. doi: 10.1016/j.scr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribeiro J, Nordlinger B, Ballet F, Cynober L, Coudray-Lucas C, Baudrimont M, et al. Intrasplenic hepatocellular transplantation corrects hepatic encephalopathy in portacaval-shunted rats. Hepatology. 1992;15(1):12–8. doi: 10.1002/hep.1840150104. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi N, Ito M, Nakamura J, Cai J, Gao C, Hammel JM, et al. Hepatocyte transplantation in rats with decompensated cirrhosis. Hepatology. 2000;31(4):851–7. doi: 10.1053/he.2000.5636. [DOI] [PubMed] [Google Scholar]
- 51.Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82(4):441–9. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- 52.Mito M, Kusano M. Hepatocyte transplantation in man. Cell Transplantation. 1993;2:65–74. [PubMed] [Google Scholar]
- 53.Komori J, Boone L, DeWard A, Hoppo T, Lagasse E. The mouse lymph node as an ectopic transplantation site for multiple tissues. Nature biotechnology. 2012;30(10):976–83. doi: 10.1038/nbt.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, et al. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nature biotechnology. 2007;25(8):903–10. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494(7436):247–50. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grossman M, Raper SE, Kozarsky K, Stein EA, Engelhardt JF, Muller D, et al. Successful ex vivo gene therapy directed to liver in a patient with familial hypercholesterolaemia. Nat Genet. 1994;6(4):335–41. doi: 10.1038/ng0494-335. [DOI] [PubMed] [Google Scholar]
- 57.Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341(6148):1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagamoto Y, Takayama K, Ohashi K, Okamoto R, Sakurai F, Tachibana M, et al. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y, Li Y, Wang X, Zhang W, Sauer V, Chang CJ, et al. Amelioration of Hyperbilirubinemia in Gunn Rats after Transplantation of Human Induced Pluripotent Stem Cell-Derived Hepatocytes. Stem Cell Reports. 2015;5(1):22–30. doi: 10.1016/j.stemcr.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee WM, Larson AM, Stravitz RT. AASLD position paper: the management of acute liver failure: update 2011. Hepatology. 2012;55:965–7. doi: 10.1002/hep.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi XL, Gao Y, Yan Y, Ma H, Sun L, Huang P, et al. Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes. Cell Res. 2016;26(2):206–16. doi: 10.1038/cr.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly JH, Sussman NL. The hepatix extracorporeal liver assist device in the treatment of fulminant hepatic failure. ASAIO Journal. 1994;40(1):83–5. [PubMed] [Google Scholar]
- 63.Ellis AJ, Hughes RD, Wendon JA, Dunne J, Langley PG, Kelly JH, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24(6):1446–51. doi: 10.1002/hep.510240625. [DOI] [PubMed] [Google Scholar]
- 64.Duan Z, Zhang J, Xin S, Chen J, He D, Brotherton J, et al. Interim Results of Randomized Controlled Trial of ELADTM in Acute on Chronic Liver Disease. Hepatology. 2007;46(4, Suppl. 1):1. [Google Scholar]
- 65.Reich DJ. The Effect of Extracorporeal C3A Cellular Therapy in Severe Alcoholic Hepatitis –The VTI-208 ELAD Trial. AASLD; San Francisco, CA: 2015. [Google Scholar]
- 66.Demetriou AA, Brown RS, Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239(5):660–7. doi: 10.1097/01.sla.0000124298.74199.e5. discussion 7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nyberg SL, Remmel RP, Mann HJ, Peshwa MV, Hu WS, Cerra FB. Primary hepatocytes outperform Hep G2 cells as the source of biotransformation functions in a bioartificial liver. Ann Surg. 1994;220(1):59–67. [PMC free article] [PubMed] [Google Scholar]
- 68.Nyberg SL, Hardin J, Amiot B, Argikar UA, Remmel RP, Rinaldo P. Rapid, large-scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transpl. 2005;11(8):901–10. doi: 10.1002/lt.20446. [DOI] [PubMed] [Google Scholar]
- 69.Brophy CM, Luebke-Wheeler JL, Amiot BP, Khan H, Remmel RP, Rinaldo P, et al. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology. 2009;49(2):578–86. doi: 10.1002/hep.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin I, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularization matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 72.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329(5991):538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nature medicine. 2010;16(7):814–20. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim S, Sundback CA, Kaihara S, Benvenuto MS, Kim BS, Mooney DJ, Vacanti JP. Dynamic Seeding and in Vitro Culture of Hepatocytes in a Flow Perfusion System. Tissue Eng. 2000;6(1):39–44. doi: 10.1089/107632700320874. [DOI] [PubMed] [Google Scholar]
- 75.Ohashi K, Yokoyama T, Yamato M, Kuge H, Kanehiro H, Okano T, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nature Medicine. 2007;13:880–5. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 76.Buhler NE, Schulze-Osthoff K, Konigsrainer A, Schenk M. Controlled processing of a full-sized porcine liver to a decellularized matrix in 24 h. J Biosci Bioeng. 2015;119(5):609–13. doi: 10.1016/j.jbiosc.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 77.Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, et al. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res. 173(1):e11–25. doi: 10.1016/j.jss.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 78.Soto-Gutierrez A, Zhang L, Medberry C, Fukumitsu K, Faulk D, Jiang H, Reing J, Gramignoli R, Komori J, Ross M, Nagaya M, Lagasse E, Stolz D. A Whole-Organ Regenerative Medicine Approach for Liver Replacement. Tissue Eng. 2011;17(6) doi: 10.1089/ten.tec.2010.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leyh RG, Wilhelmi M, Walles T, Kallenbach K, Rebe P, Oberbeck A, et al. Acellularized porcine heart valve scaffolds for heart valve tissue engineering and the risk of cross-species transmission of porcine endogenous retrovirus. J Thorac Cardiovasc Surg. 2003;126(4):1000–4. doi: 10.1016/s0022-5223(03)00353-2. [DOI] [PubMed] [Google Scholar]
- 80.Mirmalek-Sani SH, Sullivan DC, Zimmerman C, Shupe TD, Petersen BE. Immunogenicity of decellularized porcine liver for bioengineered hepatic tissue. Am J Pathol. 2013;183(2):558–65. doi: 10.1016/j.ajpath.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bohm F, Kohler UA, Speicher T, Werner S. Regulation of liver regeneration by growth factors and cytokines. EMBO Mol Med. 2010;2(8):294–305. doi: 10.1002/emmm.201000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Enosawa S, Miyashita T, Fujita Y, Suzuki S, Amemiya H, Omasa T, et al. In vivo estimation of bioartificial liver with recombinant HepG2 cells using pigs with ischemic liver failure. Cell transplantation. 2001;10(4-5):429–33. [PubMed] [Google Scholar]
- 83.Wang HH, Wang YJ, Liu HL, Liu J, Huang YP, Guo HT, et al. Detection of PERV by polymerase chain reaction and its safety in bioartificial liver support system. World J Gastroenterol. 2006;12(8):1287–91. doi: 10.3748/wjg.v12.i8.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takebe T, Enomura M, Koike H, Kimura M, et al. Vascularized and functional human liver from an iPS-derived organ bud transplant. Nature. 2013 doi: 10.1038/nprot.2014.020. [DOI] [PubMed] [Google Scholar]
- 85.Yap KK, Dingle AM, Palmer JA, Dhillon RS, Lokmic Z, Penington AJ, et al. Enhanced liver progenitor cell survival and differentiation in vivo by spheroid implantation in a vascularized tissue engineering chamber. Biomaterials. 2013;34(16):3992–4001. doi: 10.1016/j.biomaterials.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 86.Bao J, Shi Y, Sun H, Yin X, Yang R, Li L, et al. Construction of a Portal Implantable Functional Tissue Engineered Liver using Perfusion-decellularized Matrix and Hepatocytes in Rats. Cell Transplantation. 2011;20:753–66. doi: 10.3727/096368910X536572. [DOI] [PubMed] [Google Scholar]
- 87.Ko IK, Peng L, Peloso A, Smith CJ, Dhal A, Deegan DB, et al. Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials. 2015;40:72–9. doi: 10.1016/j.biomaterials.2014.11.027. [DOI] [PubMed] [Google Scholar]