Temporal fluctuations in species richness are frequently regulated, exhibiting a tendency to return toward a central level.
Abstract
Many theoretical models of community dynamics predict that species richness (S) and total abundance (N) are regulated in their temporal fluctuations. We present novel evidence for widespread regulation of biodiversity. For 59 plant and animal assemblages from around the globe monitored annually for a decade or more, the majority exhibited regulated fluctuations compared to the null hypothesis of an unconstrained random walk. However, there was little evidence for statistical artifacts, regulation driven by correlations with average annual temperature, or local-scale compensatory fluctuations in S or N. In the absence of major environmental perturbations, such as urbanization or cropland transformation, species richness and abundance may be buffered and exhibit some resilience in their temporal trajectories. These results suggest that regulatory processes are occurring despite unprecedented environmental change, highlighting the need for community-level assessment of biodiversity trends, as well as extensions of existing theory to address open source pools and shifting environmental conditions.
INTRODUCTION
Life is regulated at many levels: from elemental composition within cells, to physiological homeostasis within individuals, to constraints on population growth (1) and per-capita demographic rates (2). These lower-level regulatory processes may even contribute to emergent properties of stability, feedback loops, and resilience at the organizational scale of food webs and ecosystems (3–5). But are entire ecological communities also regulated? Community-level regulation (6–8) is important because it may dampen biodiversity fluctuations in the face of environmental change (9) and may cause species richness (S) or total abundance (N) to return toward a central level following a strong perturbation. Thus, understanding the prevalence of community regulation is critical for monitoring and interpreting biodiversity change in the Anthropocene. Here, we use a global survey of communities to show, for the first time, that community-level regulation is surprisingly common for both species richness and total abundance.
We use a broad statistical definition of regulation, which is that a regulated community exhibits a constant mean and variance in N or S with an autocorrelation function that decays quickly to 0 (10). We test for this pattern with the Augmented Dickey-Fuller (ADF) test (11), in which the null hypothesis is an unconstrained random walk that leads to a nonconstant variance in a time series. This test is widely used for time series analysis of econometric data (12). Rejecting the ADF test means that the time series is centered on a long-term mean (or a long-term trend line) and will return toward it if displaced, rather than drifting freely. We analyzed with the ADF test 59 high-quality data sets from across the globe in which multispecies communities of plants and animals have been monitored for 10 or more years with standardized census methods (see Materials and Methods).
RESULTS
In more than 90% of the communities analyzed, the pattern was in the direction of a regulated trajectory (z scores less than 0). By the P < 0.05 criterion, 55% of the species richness time series and 54% of the total abundance trajectories showed evidence of a stationary time series with a constant variance (Fig. 1 and tables S1 to S3). There was a significant correlation between the z scores for abundance and the z scores for species richness (r = 0.68, P <0.001): Communities that showed strong regulation in species richness also tended to show strong regulation in total abundance. Similar results were found for an analysis that controls for linear temporal trends of the time series (see Supplementary Text). We used a variety of ancillary tests and simulation models (fig. S10) to show that observation error (figs. S11 to S13), portfolio effects (Box 1 and Supplementary Text), or environmental tracking of annual sea or air temperatures (tables S4 and S5) cannot easily account for these results.
Fig. 1. Regulated and unregulated time series of species richness and total abundance.
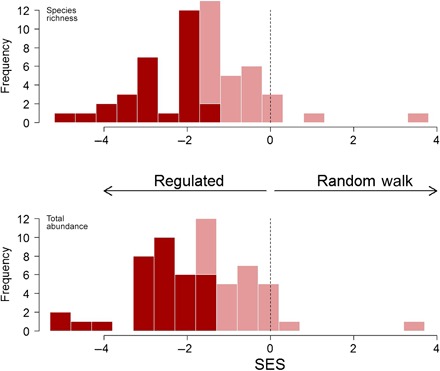
Histograms of statistically significant (dark hue) and nonsignificant (light hue) ADF test results for species richness (top) and total abundance (bottom) of 59 monitored assemblages. Individual P values for each assemblage were converted to standardized deviates for plotting on a continuous scale. Standardized effect sizes (SES) of less than ~−2.0 are statistically significant at P < 0.05 and indicated a pattern of regulated temporal fluctuations. The vertical black zero line, which indicates a tail probability of 0.50, is highlighted for comparison.
Box 1. Definitions.
ADF test. A statistical test for detecting regulation in a time series of observations (11). The test fits an autoregressive (AR) time series model with a lag of one time step (AR1) to a data series. The coefficient φ in the AR1 model reflects the degree of regulation. The extreme cases are φ = 0, which represents a white noise (Gaussian) distribution that shows strong regulation following a perturbation, and φ = 1, which represents an unregulated random walk that does not recover or return to a central value following a perturbation. The ADF test estimates the probability that the fitted value of |φ| < 1, which corresponds to a regulated process. The null hypothesis is that the time series represents a random walk with φ = 1.
AR time series model. A statistical model for a variable (such as abundance or species richness) that changes through time. In any AR model, the system has a “memory” so that current fluctuations are mathematically dependent on all previous values, although the strength of the effect diminishes as two observations are separated further in time. The behavior of an AR model depends on the parameter φ. If φ = 1, the system is completely dominated by the value it had at the last time step, corresponding to an unregulated random walk. If φ = 0, the system is unaffected by past values and will return sharply toward its equilibrium level in the next time step (white noise distribution). In between these extremes, an AR1 process will “remember” previous values and display a tendency to return to a central value whenever it is perturbed, but the degree of regulation is weaker as φ approaches 1.
Beta diversity. A measure of the degree to which species composition differs among sites or within a site among times. Pairwise beta diversity is quantified by the number of shared and unique species in two communities. It can be partitioned into a component that is caused by changes in species turnover and changes in total species richness (29).
Community-wide regulation. A variable quantified for an entire community, such as species richness, total abundance, or biomass, is measured repeatedly at a site through time. If the variable is regulated, it will have a long-term constant mean and a bounded variance (10). If the variable is pushed above the mean, it will be more likely to decrease than to increase, and if the variable is pushed below the mean, it will be more likely to increase than to decrease. Community-wide regulation does not imply a single equilibrium point but a distribution that is constrained so that the time series does not resemble a random walk. Community-wide regulation may occur when a universally shared resource, such as energy, is in limited supply (14). If environmental conditions are changing through time, community-wide regulation may be accompanied by changes in species composition and species traits (15).
Compensatory fluctuations. If species pairs in an assemblage are not independent, but covary negatively (because of negative species interactions or changing environmental conditions), the variance of the sum of their abundances will be less than the sum of the variances of their abundances. These measures form the basis for variance ratio tests for compensatory fluctuations, compared to a null hypothesis of species independence (23, 24). These tests are based on randomization of observed abundance or species richness data collected through time, so they assume that the source pool is constant and that population processes (colonization, extinction, and changes in abundance) do not change through time. Compensatory fluctuations represent one kind of community regulation, but statistical tests for compensatory fluctuations will not detect all cases of regulation in which the response variable is constrained and does not follow a random walk.
Environmental tracking. If an assemblage of species show a similar response to an abiotic variable, such as temperature, their abundances may exhibit correlated fluctuations by tracking the variable through time. In this case, total abundance may still be regulated, but the time series will not show evidence of compensatory fluctuations because of positive covariation between the abundances of many of the species (25).
MacArthur-Wilson equilibrium model. A model in which a mainland source pool of species can potentially colonize an island or discrete patches. Populations on the islands fluctuate independently and stochastically so that extinction and recolonization are common. The model links extinction rates to island area and immigration rates to island isolation or distance (19), but the concept of species-specific colonization and extinction rates can be generalized to other dynamic communities (50).
Markov patch model. A discrete-time transition model in which the replacement of one species by another (or one community by another) is specified as a probability that reflects species-specific interactions and probabilities of colonization, extinction, or persistence in a patch (21, 22).
Niche. The set of abiotic conditions (temperature, moisture, pH, etc.) and biotic conditions (presence of predators, parasites, competitors, prey, etc.) that jointly determine whether a species can colonize a site and achieve positive population growth (dN/dt > 0) (51).
Portfolio effect. In financial investments, a diversified portfolio will usually fluctuate less in value than an investment in a single vehicle. In analyses of community regulation, metrics such as the coefficient of variation in total abundance will have smaller values for assemblages composed of more species. This artifact can be avoided by using statistical tests that are not sensitive to the species richness or total abundance of an assemblage (52).
Random walk. A variable that increases or decreases with equal probability in each step of a time series. Random walks do not maintain a constant long-term average or bounded variance and serve as an appropriate null model for detecting community-wide regulation. If an assemblage that is unregulated and following a random walk is perturbed by a single shock (such as the removal of most species), it will subsequently fluctuate at a new, reduced level (Fig. 3D). In contrast, a regulated assemblage will begin to recover and show rapid or gradual increases in species richness and abundance following a single perturbation (Fig. 3C).
Zero-sum assumption. Classic neutral models of species assemblages explicitly assume that total abundance is constant so that when an individual dies, it is immediately replaced by another randomly selected individual (18). In a constant environment, this assumption reflects a constraint on total energy and will generate a constrained time series of species richness and total abundance. A strict “zero-sum” assumption is not necessary to achieve community regulation, and a bounded distribution of abundance and total species richness will result if species colonization and extinction probabilities are constant through time. These conditions arise in the MacArthur-Wilson equilibrium model (19), in which species colonize and become extinct at random (20). These conditions also arise in Markov patch models, in which the probability of colonization and extinction is determined by the identity of the species currently occupying a patch (21, 22). In the neutral model, the MacArthur-Wilson model, and Markov patch models, an empty landscape will be colonized and rise to a bounded distribution of species richness and total abundance. If abundance and species richness are pushed above this distribution, they will decline back toward it. In a closed system with a constant source pool, all three models will exhibit a pattern of community-wide regulation and may show evidence for compensatory fluctuations. In a changing environment, the distributions will still be bounded, but there may no longer be a simple pattern of compensatory fluctuations because colonization and extinction probabilities are changing through time.
DISCUSSION
Community-level regulation of species richness, total abundance, biomass, or energy flux (Box 1) can arise from a variety of mechanisms in two broad categories: (i) regulation caused by a shared universal resource, such as energy (6, 7, 13, 14), and (ii) regulation accompanied by shifting environmental conditions and open source pools (15), which may lead to species replacement and turnover (16, 17). Regulation by a shared universal resource is embodied in the zero-sum assumption of the neutral model (18), but regulation is also implied by the constant colonization and extinction probabilities in the MacArthur-Wilson equilibrium model (19, 20) and the assumption of constant species-specific replacement probabilities in Markov patch models (21, 22).
Previous “variance ratio” tests for regulation at the community level have focused on the idea that compensatory fluctuations in abundance or compensatory replacements of species (Box 1) should generate a smaller variance in S or N than would be expected without compensation (23, 24). For these communities, we applied the variance ratio test to total abundance and an analogous test for compensatory colonizations and extinction to species richness (see Supplementary Text). As in previous meta-analyses (24, 25), there were very few communities in which there was evidence for local-scale compensatory regulation of S or N (tables S6 and S7 and Supplementary Text).
How then do we account for the paradox that assemblage-level time series of species richness and total abundance appear to be stationary (Fig. 1) despite little evidence for local-scale compensatory fluctuations? The time series in these analyses cover 10 years or longer, and the communities were monitored relatively recently, during periods of unprecedented environmental change (26–28). With changing environmental conditions, shifts in species composition and in the ecological niches represented by each species are expected to predominate. These shifts represent important species replacements but will not necessarily be reflected in statistical tests for compensatory dynamics, which assume a constant source pool.
To further analyze the pattern of species change in these communities, we partitioned species composition into components of species turnover and species richness (29). For most of these communities, the dominant fraction of change came from species turnover, which could lead to stationary distributions of species richness and total abundance (Fig. 2, blue fraction). However, most communities also contained some component of beta diversity attributed to a change in species richness (Fig. 2, green fraction), which may have obscured the signature of regulation in statistical tests for compensatory fluctuations.
Fig. 2. Beta diversity partition of assemblage time series.
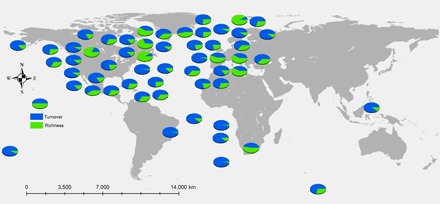
Each pie chart represents a different assemblage, plotted at its jittered location on the globe. Beta diversity was partitioned using the method of Baselga (29). Blue fraction, proportion of beta diversity attributable to changes in species composition; green fraction, proportion of beta diversity attributable to changes in species richness.
We note that, even with a constant source pool, the trajectories of random walks versus regulated assemblages may be very hard to distinguish (Fig. 3A versus Fig. 3B). However, if these communities are perturbed by reducing abundance or species richness to a low level, the differences are clear (Fig. 3C versus Fig. 3D). Regulated communities begin to trend upward, with a slow or fast return toward a stationary distribution (Fig. 3C). In contrast, random walks remain on average at chronically low levels following a perturbation—and may even decline stochastically to 0—but do not consistently rebound (Fig. 3D). A myriad of empirical studies, including controlled removal experiments (30), unintended anthropogenic perturbations (31), and comparisons of terrestrial vegetation structure from chronosequences (32), show that communities frequently do trend upward initially in S and N following species loss.
Fig. 3.
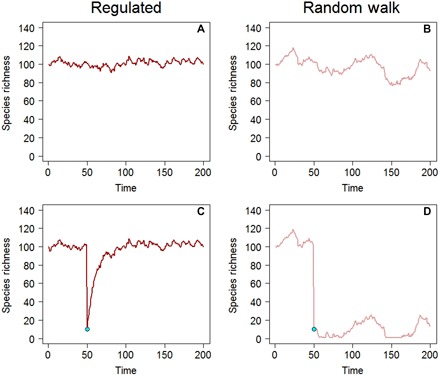
Contrasting dynamics of regulated assemblages versus random walks. In the absence of a perturbation, it is difficult to visually distinguish the dynamics of a regulated assemblage (A) versus an unconstrained random walk (B), although they are discriminated by the ADF test [P = 0.020 (A) and P = 0.545 (B)]. However, if the assemblage is reduced in a single time step from its equilibrium level of 100 species to 10 species, the regulated assemblage recovers (C), whereas the unregulated assemblage does not (D). Trajectories were simulated with an AR1 autoregressive model, ΔN = Nt + 1 − Nt = − (Nt − c)(1 − φ) + εt, where c = 100 and εt ~ N(0, σ = 2). For the regulated trajectories, φ = 0.900, and for the random walk trajectories, φ = 0.999. To simplify the appearance of (D), simulated values that were less than 1 were redrawn as 1. The ADF test is a one-tailed statistical test for whether |φ| < 1.0.
With long-term environmental change, a variety of scenarios for short- and long-term changes in species richness, composition, and abundance are possible (33). A scenario of environmental change triggering changes in species composition and niches, accompanied by relative constancy in species number or abundance, is consistent with more detailed studies of long-term fluctuations in desert rodent assemblages of the southeastern United States (6) and groundfish assemblages of the Northeast Atlantic (34).
There are some caveats and limitations to our analysis. The 59 studies used here represent the longest time series available from the compilation of Dornelas et al. (35). As in many other ecological meta-analyses, the surveys in this compilation are dominated by temperate-zone communities in North America and Europe, with relatively few examples of tropical communities in Asia, Africa, and Australia. Because the same community has been monitored with standardized census methods for 10 years or longer, the compilation does not include landscapes that have been radically transformed by human activity such as urbanization or crop planting (36). However, it would be a mistake to suggest that the communities were sampled from “habitats that are mostly intact and yet to be fully exploited by humans” (37). Some of these studies were conducted near nuclear power plants; in suburban landscapes of mixed forest, agriculture, and housing; and in coastal areas that are heavily affected by fisheries (38). Human domination of the biosphere implies indirect effects on biodiversity that extend well beyond areas of obvious anthropogenic transformation. For this reason, it is especially interesting to see a signature of community-level regulation in these high-quality long-term data sets.
Although we found evidence for widespread community-level regulation, note that almost half of the trajectories were unregulated and could not be distinguished from a random walk. What rules or factors determine whether a community is regulated or not? We stratified the data set by latitudinal zones, taxonomic groups, and habitat and then tested for differences in the strength of community regulation among subsets of communities. The only pattern that emerged was that the total abundance (but not species richness) of marine communities was more strongly regulated than that of terrestrial communities. However, even this difference accounted for only 16% of the variation in effect size (fig. S14). Moreover, the strength of regulation was not related to the length of the time series or the number of species in the community (see Supplementary Text).
The answer may lie at a lower level of analysis. If a community is subdivided into trait-based functional groups of species (39), strong interactions and species replacements within these groups might be driving temporal trajectories of species richness and total abundance. However, the functional status of most species in these surveys is currently unknown, although this knowledge gap could diminish with the continued development of public-domain databases of species ecological traits (40). Spatial and temporal heterogeneity is another source of variation that may affect community-level regulation (41). The temporal grain of most of these studies was roughly 1 year, and different patterns of regulation may be expected to appear at both shorter and longer time scales (42). In a similar way, the temporal trajectories may also change with the spatial grain and extent of sampling (33).
Resilient recovery and bounded trajectories of species richness and abundance should not be confused with a stasis of biodiversity. On the contrary, these patterns of weak constancy in S and N and recovery from perturbation are often accompanied by very strong changes in species composition that cannot be explained by classic equilibrium community models (18, 19). The evidence that substantial change can occur in communities while key properties such as total abundance and species richness show a stationary distribution with a constant mean is counterintuitive and likely an important signal of underlying processes and perhaps represents a previously unrecognized general pattern in community ecology (43, 44).
Current theory seems inadequate to explain the observed phenomenon of widespread community regulation. A better understanding of why communities are regulated is important to discern and predict whether communities can persist in the face of large anthropogenic impacts (33, 45) or whether they are about to collapse or disassemble. Better understanding of which aspects of communities are regulated (abundance and richness in this paper) and not regulated [species composition in Dornelas et al. (35)] is also important in predicting how the ecosystem functions that humans depend on will be altered. Finally, the existence of regulation at the community level highlights the need to study human impacts on whole communities, not just on selected species or populations. Long-term measurements of key shared resources and physiological tolerances of the species that appear and disappear through time should provide new insights into the details of community regulation and may guide strategies for managing assemblages in the face of strong environmental change.
MATERIALS AND METHODS
Data selection
We searched the scientific literature and online biodiversity databases for publicly available time series of species abundance estimates for consistently sampled ecological assemblages [sensu Fauth et al. (46)]. Our criteria for inclusion of a data set were that (i) it included 10 years (not necessarily consecutive) of sampling or longer, (ii) the sampling methods were described and relatively consistent through time, and (iii) the abundance estimates of all species in the sample were reported (that is, assemblage data rather than population data). The 59 data sets we used are a subset of the 100 data sets originally compiled by Dornelas et al. (35). A full list of the data sets used in this study, their characteristics, and sources is included in table S8 (separate file). Data sets were checked for duplicates, for species with zero abundance, and for nonorganismal records, which were deleted before any analysis.
For access to the data sets, contact the authors directly. Some of the data sets are proprietary, and we do not have permission to release all of them to third parties. However, we are currently assembling an expanded database of community time series that will be available in the form of a published data paper.
Most biodiversity metrics are affected by sampling effort, and sampling effort was often not constant throughout the time series. To prevent variation in sampling effort from obscuring temporal biodiversity patterns, we used sample-based rarefaction within each time series. Specifically, we used year as the temporal grain, and for each time series, we found the minimum number of samples in a year. We bootstrap-resampled the data from each other year to obtain a constant number of samples at each sampling time. Species abundances were then pooled within each year. Some time series included years with an unusually low number of samples. To assess whether this was causing an excessive loss of information, we individually assessed each time series and removed any years with less than half of the average number of samples before performing the sample-based rarefaction described above. This process did not affect the results of our analysis [fig. S7 of Dornelas et al. (35)], and hence, only the first type of rarefaction was used in these analyses.
Marine temperature data
The Extended Reconstructed Sea Surface Temperature (ERSST) data set is a global monthly sea surface temperature data set derived from the International Comprehensive Ocean–Atmosphere Data Set. It is produced on a 2° × 2° grid with spatial completeness enhanced using statistical methods. This monthly analysis begins in January 1854 and continues to the present and includes anomalies computed with respect to a 1971–2000 monthly climatology. The newest version of ERSST, version 4 (47), is based on optimally tuned parameters using the latest data sets and improved analysis methods.
The monthly analysis extends from January 1854 to the present, but because of sparse data in the early years, there was damping of the analyzed signal before 1880. After 1880, the strength of the signal has become more consistent over time. ERSST is suitable for long-term global and basin-wide studies, and smoothed local and short-term variations were used in the data set.
Monthly NetCDF format gridded data from 1854 to the present are available (48). These were imported and converted to feature layers using the ArcGIS Multidimensional Tools to link with central data points for analysis.
Terrestrial (and freshwater) temperature data
Climate simulations from the Community Climate System Model version 4 (CCSM4) are generated on a Gaussian grid, where each grid point can be uniquely accessed by one-dimensional latitude and longitude arrays (that is, the coordinates are orthogonal). In the CCSM4 model output, the longitudes are equally spaced at 1.25°, whereas the latitudes vary in spacing slightly around 0.94°. Therefore, approximate spatial resolution of global climate projections is 105 km. Because of the irregular grid in the CCSM, this portal distributes data in a point shapefile format, where each point represents a centroid of a corresponding CCSM grid cell. A shapefile of irregular rectangular polygons of the original model output is also available. Data from 1850 to 2005 are available (49).
Because the data set contains both marine and terrestrial assemblage time series from both hemispheres, we used the average of the July and January temperatures for all data sets. These two months correspond to midsummer in the Northern Hemisphere and the Southern Hemisphere, respectively; thus, the temperature measurements are comparable for both hemispheres. Air temperature from the CCSM4 model was measured as bulk temperature of the air in kelvin and was converted to degrees Celsius for analysis.
To calculate the temperature time series associated with each data set, we used the geographic midpoint of the study and chose the closest temperature point associated with it (see additional data for table S1). The distance to this closest point varied among studies but was always less than 2° in latitude and longitude. For both terrestrial and marine data, the temperature time series created for each assemblage is based on the geographic midpoint of the study location and covers the same years where the assemblage was censused.
Supplementary Material
Acknowledgments
Funding: N.J.G. was supported by the NSF (DEB 1257625, DEB 1144055, and DEB 1136644). A.E.M. acknowledges support from ERC AgG BioTIME (250189), ERC PoC BioCHANGE (727440), and the Royal Society. M.D. is grateful for the support from the Scottish Funding Council (Marine Alliance for Science and Technology for Scotland grant reference HR09011). Author contributions: N.J.G. led the project and wrote the initial manuscript draft. The data sets were compiled by M.D., A.E.M., and F.M. Analyses were conducted by H.S., B.M., M.D., F.M., and N.J.G. All authors contributed equally to the interpretation of the results, the development of the ideas, and the revision of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Some data sets used in these analyses are proprietary, so contact the authors for access to the data. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/7/e1700315/DC1
Supplementary Text
fig. S1. Time series of uncorrelated white noise.
fig. S2. Time series of uncorrelated white noise with a linear temporal trend.
fig. S3. Time series of uncorrelated white noise with a one-time perturbation.
fig. S4. Time series of random walk.
fig. S5. Time series of a random walk with a linear temporal trend.
fig. S6. Time series of a random walk with a one-time perturbation.
fig. S7. Time series of a regulated autoregressive process.
fig. S8. Time series of a regulated autoregressive process with a linear temporal trend.
fig. S9. Time series of a regulated autoregressive process with a one-time perturbation.
fig. S10. Logic tree for analysis and interpretation of community time series.
fig. S11. Benchmark analysis of ADF test.
fig. S12. Benchmark analysis of ADF test.
fig. S13. Benchmark analysis of ADF test.
fig. S14. Statistical tests for effects of latitudinal band (=climate), taxonomic group, and realm on standardized effect sizes (z scores) of species richness and total abundance.
table S1. Number of significant (P < 0.05) and nonsignificant test results for assemblage-level regulation of species richness or abundance.
table S2. Number of significant (P < 0.05) and nonsignificant test results for assemblage-level regulation of species richness or abundance.
table S3. Number of significant (P < 0.05) and nonsignificant test results for assemblage-level regulation of species richness or abundance.
table S4. Results of ADF tests for temperature time series.
table S5. Correlations of species richness and abundance with air or seawater temperature.
table S6. Variance ratio tests for patterns of compensatory fluctuations in total abundance.
table S7. Null model tests for the slope of the relationship between the observed number of colonizations at time t and the observed number of extinctions at time t + x.
table S8. Primary references and metadata for 59 assemblage time series data sets.
REFERENCES AND NOTES
- 1.Sibly R. M., Hone J., Population growth rate and its determinants: An overview. Philos. Trans. R. Soc. London Ser. B 357, 1153–1170 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leirs H., Stenseth N. C., Nichols J. D., Hines J. E., Verhagen R., Verheyen W., Stochastic seasonality and nonlinear density-dependent factors regulate population size in an African rodent. Nature 389, 176–180 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Levin S. A., Ecosystems and the biosphere as complex adaptive systems. Ecosystems 1, 431–436 (1998). [Google Scholar]
- 4.Patten B. C., Odum E. P., The cybernetic nature of ecosystems. Am. Nat. 118, 886–895 (1981). [Google Scholar]
- 5.Ulanowicz R. E., Aristotelean causalities in ecosystem development. Oikos 57, 42–48 (1990). [Google Scholar]
- 6.Brown J. H., Ernest S. K. M., Parody J. M., Haskell J. P., Regulation of diversity: Maintenance of species richness in changing environments. Oecologia 126, 321–332 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Ernest S. K. M., Brown J. H., Thibault K. M., White E. P., Goheen J. R., Zero sum, the niche, and metacommunities: Long-term dynamics of community assembly. Am. Nat. 172, E257–E269 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez A., Loreau M., The causes and consequences of compensatory dynamics in ecological communities. Annu. Rev. Ecol. Evol. Syst. 40, 393–414 (2009). [Google Scholar]
- 9.Connell S. D., Ghedini G., Resisting regime-shifts: The stabilising effect of compensatory processes. Trends Ecol. Evol. 30, 513–515 (2015). [DOI] [PubMed] [Google Scholar]
- 10.P. Turchin, Complex Population Dynamics: A Theoretical/Empirical Synthesis (Princeton Univ. Press, 2003), 456 pp. [Google Scholar]
- 11.Said S. E., Dickey D. A., Testing for unit roots in autoregressive-moving average models of unknown order. Biometrika 71, 599–607 (1984). [Google Scholar]
- 12.W. H. Green, Econometric Analysis (Prentice Hall, ed. 5, 2002). [Google Scholar]
- 13.Van Valen L., A new evolutionary law. Evol. Theor. 1, 1–30 (1973). [Google Scholar]
- 14.Wright D. H., Species-energy theory: An extension of species-area theory. Oikos 41, 496–506 (1983). [Google Scholar]
- 15.Rabosky D. L., Hurlbert A. H., Species richness at continental scales is dominated by ecological limits. Am. Nat. 185, 572–583 (2015). [DOI] [PubMed] [Google Scholar]
- 16.HilleRisLambers J., Adler P. B., Harpole W. S., Levine J. M., Mayfield M. M., Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. Syst. 43, 227–248 (2012). [Google Scholar]
- 17.Tilman D., Biodiversity: Population versus ecosystem stability. Ecology 77, 350–363 (1996). [Google Scholar]
- 18.Allouche O., Kadmon R., A general framework for neutral models of community dynamics. Ecol. Lett. 12, 1287–1297 (2009). [DOI] [PubMed] [Google Scholar]
- 19.R. H. MacArthur, E. O. Wilson, The Theory of Island Biogeography (Princeton Univ. Press, 1967). [Google Scholar]
- 20.Simberloff D., When is an island community in equilibrium? Science 220, 1275–1277 (1983). [DOI] [PubMed] [Google Scholar]
- 21.Diamond S. E., Nichols L. M., Pelini S. L., Penick C. A., Barber G. W., Cahan S. H., Dunn R. R., Ellison A. M., Sanders N. J., Gotelli N. J., Climate warming destabilizes forest ant communities. Sci. Adv. 2, e1600842 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.H. Horn, Markovian processes in forest succession, in Ecology and Evolution of Communities, M. L. Cody, J. M. Diamond, Eds. (Harvard Univ. Press, 1975), pp. 196–213. [Google Scholar]
- 23.Hallett L. M., Hsu J. S., Cleland E. E., Collins S. L., Dickson T. L., Farrer E. C., Gherardi L. A., Gross K. L., Hobbs R. J., Turnbull L., Suding K. N., Biotic mechanisms of community stability shift along a precipitation gradient. Ecology 95, 1693–1700 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Schluter D., A variance test for detecting species associations, with some example applications. Ecology 65, 998–1005 (1984). [Google Scholar]
- 25.Houlahan J. E., Currie D. J., Cottenie K., Cumming G. S., Ernest S. K. M., Findlay C. S., Fuhlendorf S. D., Gaedke U., Legendre P., Magnuson J. J., McArdle B. H., Muldavin E. H., Noble D., Russell R., Stevens R. D., Willis T. J., Woiwod I. P., Wondzell S. M., Compensatory dynamics are rare in natural ecological communities. Proc. Natl. Acad. Sci. U.S.A. 104, 3273–3277 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bongaarts J., Development: Slow down population growth. Nature 530, 409–412 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Burrows M. T., Schoeman D. S., Buckley L. B., Moore P., Poloczanska E. S., Brander K. M., Brown C., Bruno J. F., Duarte C. M., Halpern B. S., Holding J., Kappel C. V., Kiessling W., O’Connor M. I., Pandolfi J. M., Parmesan C., Schwing F. B., Sydeman W. J., Richardson A. J., The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Mack R. N., Simberloff D., Lonsdale W. M., Evans H., Clout M., Bazzaz F. A., Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 10, 689–710 (2000). [Google Scholar]
- 29.Baselga A., Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 19, 134–143 (2010). [Google Scholar]
- 30.Wardle D. A., Bonner K. I., Barker G. M., Yeates G. W., Nicholson K. S., Bardgett R. D., Watson R. N., Ghani A., Plant removals in perennial grassland: Vegetation dynamics, decomposers, soil biodiversity, and ecosystem properties. Ecol. Monogr. 69, 535–568 (1999). [Google Scholar]
- 31.Benedetti-Cecchi L., Pannacciulli F., Bulleri F., Moschella P. S., Airoldi L., Relini G., Cinelli F., Predicting the consequences of anthropogenic disturbance: Large-scale effects of loss of canopy algae on rocky shores. Mar. Ecol. Prog. Ser. 214, 137–150 (2001). [Google Scholar]
- 32.Walker L. R., Wardle D. A., Bardgett R. D., Clarkson B. D., The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 98, 725–736 (2010). [Google Scholar]
- 33.McGill B. J., Dornelas M., Gotelli N. J., Magurran A. E., Fifteen forms of biodiversity trend in the Anthropocene. Trends Ecol. Evol. 30, 104–113 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Magurran A. E., Dornelas M., Moyes F., Gotelli N. J., McGill B., Rapid biotic homogenization of marine fish assemblages. Nat. Commun. 6, 8405 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dornelas M., Gotelli N. J., McGill B., Shimadzu H., Moyes F., Sievers C., Magurran A. E., Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Gonzales A., Cardinale B. J., Allington G. R. H., Byrnes J., Arthur Endsley K., Brown D. G., Hooper D. U., Isbell F., O’Connor M. I., Loreau M., Estimating local biodiversity change: A critique of papers claiming no net loss of local diversity. Ecology 97, 1949–1960 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Cardinale B., Overlooked local biodiversity loss. Science 344, 1098 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Dornelas M., Gotelli N. J., McGill B., Magurran A. E., Overlooked local biodiversity loss—Response. Science 344, 1098–1099 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Violle C., Navas M.-L., Vile D., Kazakou E., Fortunel C., Hummel I., Garnier E., Let the concept of trait be functional!. Oikos 116, 882–892 (2007). [Google Scholar]
- 40.Kattge J., Díaz S., Lavorel S., Prentice I. C., Leadley P., Bönisch G., Garnier E., Westoby M., Reich P. B., Wright I. J., Cornelissen J. H. C., Violle C., Harrison S. P., Van Bodegom P. M., Reichstein M., Enquist B. J., Soudzilovskaia N. A., Ackerly D. D., Anand M., Atkin O., Bahn M., Baker T. R., Baldocchi D., Bekker R., Blanco C. C., Blonder B., Bond W. J., Bradstock R., Bunker D. E., Casanoves F., Cavender-Bares J., Chambers J. Q., Chapin F. S. III, Chave J., Coomes D., Cornwell W. K., Craine J. M., Dobrin B. H., Duarte L., Durka W., Elser J., Esser G., Estiarte M., Fagan W. F., Fang J., Fernández-Méndez F., Fidelis A., Finegan B., Flores O., Ford H., Frank D., Freschet G. T., Fyllas N. M., Gallagher R. V., Green W. A., Gutierrez A. G., Hickler T., Higgins S. I., Hodgson J. G., Jalili A., Jansen S., Joly C. A., Kerkhoff A. J., Kirkup D., Kitajima K., Kleyer M., Klotz S., Knops J. M. H., Kramer K., Kühn I., Kurokawa H., Laughlin D., Lee T. D., Leishman M., Lens F., Lenz T., Lewis S. L., Lloyd J., Llusià J., Louault F., Ma S., Mahecha M. D., Manning P., Massad T., Medlyn B. E., Messier J., Moles A. T., Müller S. C., Nadrowski K., Naeem S., Niinemets Ü., Nöllert S., Nüske A., Ogaya R., Oleksyn J., Onipchenko V. G., Onoda Y., Ordoñez J., Overbeck G., Ozinga W. A., Patiño S., Paula S., Pausas J. G., Peñuelas J., Phillips O. L., Pillar V., Poorter H., Poorter L., Poschlod P., Prinzing A., Proulx R., Rammig A., Reinsch S., Reu B., Sack L., Salgado-Negret B., Sardans J., Shiodera S., Shipley B., Siefert A., Sosinski E., Soussana J.-F., Swaine E., Swenson N., Thompson K., Thornton P., Waldram M., Weiher E., White M., White S., Wright S. J., Yguel B., Zaehle S., Zanne A. E., Wirth C., TRY—A global database of plant traits. Glob. Chang. Biol. 17, 2905–2935 (2011). [Google Scholar]
- 41.Wu J., Loucks O. L., From balance of nature to hierarchical patch dynamics: A paradigm shift in ecology. Quart. Rev. Biol. 70, 439–466 (1995). [Google Scholar]
- 42.Pimm S. L., Redfearn A., The variability of population-densities. Nature 334, 613–614 (1988). [Google Scholar]
- 43.W. Dodds, Laws, Theories, and Patterns in Ecology (University of California Press, 2011). [Google Scholar]
- 44.S. M. Scheiner, M. R. Willig, The Theory of Ecology (University of Chicago Press, 2011). [Google Scholar]
- 45.Dormann C. F., Schweiger O., Arens P., Augenstein I., Aviron S., Bailey D., Baudry J., Billeter R., Bugter R., Bukácek R., Burel F., Cerny M., De Cock R., De Blust G., DeFilippi R., Diekötter T., Dirksen J., Durka W., Edwards P. J., Frenzel M., Hamersky R., Hendrickx F., Herzog F., Klotz S., Koolstra B., Lausch A., Le Coeur D., Liira J., Maelfait J. P., Opdam P., Roubalova M., Schermann-Legionnet A., Schermann N., Schmidt T., Smulders M. J. M., Speelmans M., Simova P., Verboom J., van Wingerden W., Zobel M., Prediction uncertainty of environmental change effects on temperate European biodiversity. Ecol. Lett. 11, 235–244 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Fauth J. E., Bernardo J., Camara M., Resetarits W. J. Jr., Van Buskirk J., McCollum S. A., Simplifying the jargon of community ecology: A conceptual approach. Am. Nat. 147, 282–286 (1996). [Google Scholar]
- 47.Extended Reconstructed Sea Surface Temperature (ERSST) v4 (2016); www.ncdc.noaa.gov/data-access/marineocean-data/extended-reconstructed-sea-surface-temperature-ersst-v4.
- 48.NetCDF (2016); www1.ncdc.noaa.gov/pub/data/cmb/ersst/v4/netcdf/.
- 49.GIS Climate Change Scenarios (2016); http://www.gisclimatechange.org.
- 50.Gilpin M. E., Diamond J. M., Immigration and extinction probabilities for individual species: Relation to incidence functions and species colonization curves. Proc. Natl. Acad. Sci. U.S.A. 78, 392–396 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.A. T. Peterson, J. Soberón, R. G. Pearson, R. P. Anderson, E. Martínez-Meyer, M. Nakamursa, M. B. Araújo, Ecological Niches and Geographic Distributions (Princeton Univ. Press, 2011). [Google Scholar]
- 52.Doak D. F., Bigger D., Harding E. K., Marvier M. A., O’Malley R. E., Thomson D., The statistical inevitability of stability-diversity relationships in community ecology. Am. Nat. 151, 264–276 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Benjamini Y., Hochberg Y., Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B Methodol. 57, 289–300 (1995). [Google Scholar]
- 54.Shenk T. M., White G. C., Burnham K. P., Sampling-variance effects on detecting density dependence from temporal trends in natural populations. Ecol. Monogr. 68, 445–463 (1998). [Google Scholar]
- 55.Freckleton R. P., Watkinson A. R., Green R. E., Sutherland W. J., Census error and the detection of density dependence. J. Anim. Ecol. 75, 837–851 (2006). [DOI] [PubMed] [Google Scholar]
- 56.de Valpine P., Hastings A., Fitting population models incorporating process noise and observation error. Ecol. Monogr. 72, 57–76 (2002). [Google Scholar]
- 57.H. G. Andrewartha, L. C. Birch, The Distribution and Abundance of Animals (University of Chicago Press, 1954). [Google Scholar]
- 58.Dennis B., Taper M. L., Density dependence in time series observations of natural populations: Estimation and testing. Ecol. Monogr. 64, 205–224 (1994). [Google Scholar]
- 59.M. J. Angilletta, Thermal Adaptation: A Theoretical and Empirical Synthesis (Oxford Univ. Press, 2009). [Google Scholar]
- 60.Zachmann L., Moffet C., Adler P., Mapped quadrats in sagebrush steppe: Long-term data for analyzing demographic rates and plant–plant interactions. Ecology 91, 3427 (2010). [Google Scholar]
- 61.Widdicombe C. E., Eloire D., Harbour D., Harris R. P., Somerfield P. J., Long-term phytoplankton community dynamics in the Western English Channel. J. Plankton Res. 32, 643–655 (2010). [Google Scholar]
- 62.Holmes R. T., Sherry T. W., Sturges F. W., Bird community dynamics in a temperate deciduous forest: Long-term trends at Hubbard Brook. Ecol. Monogr. 56, 201–220 (1986). [Google Scholar]
- 63.K. J. Gaston, T. M. Blackburn, Pattern and Process in Macroecology (Wiley-Blackwell, 2000). [Google Scholar]
- 64.D. W. Gibbons, J. B. Reid, R. A. Chapman, The New Atlas of Breeding Birds in Britain and Ireland: 1988–1991 (Poyser, 1994). [Google Scholar]
- 65.P. Lack, The Atlas of Wintering Birds in Britain and Ireland (A&C Black, 2010). [Google Scholar]
- 66.P. Standley, A. Swash, R. Gillmor, The Birds of Berkshire (Berkshire Atlas Group, 1996). [Google Scholar]
- 67.Stone B. H., Sears J., Cranswick P. A., Gregory R. D., Gibbons D. W., Rehfisch M. M., Aebischer N. J., Reid J. B., Population estimates of birds in Britain and in the United Kingdom. British Birds 90, 1–22 (1997). [Google Scholar]
- 68.M. Williamson, Are communities ever stable?, in Colonization, Succession and Stability, A. J. Gray, M. J. Crawley, P. J. Edwards, Eds. (Blackwell Scientific Publications, 1987), pp. 353–371. [Google Scholar]
- 69.Beven G., Changes in breeding bird populations of an oak-wood on Bookham Common, Surrey, over twenty-seven years. Lond. Nat. 55, 23–42 (1976). [Google Scholar]
- 70.Halpern C. B., Lutz J. A., Data from: Canopy closure exerts weak controls on understory dynamics: A 30-year study of overstory–understory interactions. Dryad Digital Repository 10.5061/dryad.1q88j (2013). [Google Scholar]
- 71.Halpern C. B., Lutz J. A., Canopy closure exerts weak controls on understory dynamics: A 30-year study of overstory–understory interactions. Ecol. Monogr. 83, 221–237 (2013). [Google Scholar]
- 72.C. B. Halpern, C. T. Dyrness, “Plant succession and biomass dynamics following logging and burning in Watersheds 1 and 3, Andrews Experimental Forest, 1962 to Present,” Long-Term Ecological Research, Forest Science Data Bank (2010); http://andlter.forestry.oregonstate.edu/data/abstract.aspx?dbcode=TP073.
- 73.Williamson M., The land-bird community of Skokholm: Ordination and turnover. Oikos 41, 378–384 (1983). [Google Scholar]
- 74.Vickery W. L., Nudds T. D., Detection of density-dependent effects in annual duck censuses. Ecology 65, 96–104 (1984). [Google Scholar]
- 75.Lindén H., Rajala P., Fluctuations and long-term trends in the relative densities of tetraonid populations in Finland 1964–1977. Finn. Game Res. 39, 13–34 (1981). [Google Scholar]
- 76.“Fluctuations and long-term in the relative densities of tetraonid populations in Finland, 1964-77,” NERC Centre for Population Biology, Imperial College, The Global Population Dynamics Database v2.0; www.imperial.ac.uk/cpb/gpdd2/secure/register.aspx [accessed 2012].
- 77.Pulliainen E., A transect survey of small land carnivore and red fox populations on a subarctic fell in Finnish Forest Labland over 13 winters. Ann. Zool. Fenninci 18, 270–278 (1981). [Google Scholar]
- 78.“A transect survey of small land carnivore and red fox populations on a subarctic fell in Finnish forest Lapland over 13 winters,” NERC Centre for Population Biology, Imperial College, The Global Population Dynamics Database v2.0; www.imperial.ac.uk/cpb/gpdd2/secure/register.aspx [accessed 2012].
- 79.C. P. Bloch, M. Willig, “Community Ecology of Land Snails Survey Dataset,” San Juan, Puerto Rico: Luquillo Long Term Ecological Research Site Database: El Verde Grid Invertebrate data LTER DBAS107 (2007); http://luq.lternet.edu/data/luqmetadata107/7427 [accessed 2012].
- 80.M. Friggens, “Sevilleta LTER Small Mammal Population Data,” Albuquerque, NM: Sevilleta Long Term Ecological Research Site Database: SEV008 (2008); http://sev.lternet.edu/data/sev-8 [accessed 2012].
- 81.E. Stanley, “NTLFI02 North Temperate Lakes LTER: Fish Abundance 1981 - current,” North Temperate Lakes Long Term Ecological Research program, NSF, Center for Limnology, University of Wisconsin-Madison; https://lter.limnology.wisc.edu/dataset/north-temperate-lakes-lter-fish-abundance-1981-current [accessed 2012].
- 82.R. B. Waide, “Bird abundance - point counts,” El Verde Field Station, Puerto Rico: Luquillo Long Term Ecological Research Site Database: Data Set 23; http://luq.lternet.edu/data/luqmetadata23 [accessed 2012].
- 83.Ernest S. K. M., Valone T. J., Brown J. H., Long-term monitoring and experimental manipulation of a Chihuahuan Desert ecosystem near Portal, Arizona, USA. Ecology 90, 1708 (2009). [DOI] [PubMed] [Google Scholar]
- 84.Moore N. W., The development of dragonfly communities and the consequences of territorial behaviour: A 27 year study on small ponds at Woodwalton Fen, Cambridgeshire, United Kingdom. Odonatologica 20, 203 (1991). [Google Scholar]
- 85.N. W. Moore, “The development of dragonfly communities and the consequences of territorial behaviour: A 27-year study on small ponds at Woodwalton Fen, Cambridgeshire, United Kingdom,” NERC Centre for Population Biology, Imperial College, The Global Population Dynamics Database v2.0 (1991); www.imperial.ac.uk/cpb/gpdd2/secure/register.aspx [accessed 2012].
- 86.“Animal Demography Unit - Coordinated Waterbird Counts (CWAC) - AfrOBIS”; www.iobis.org/ [accessed 2012].
- 87.“Marine and Coastal Management - Copepod Surveys - AfrOBIS”; www.iobis.org/ [accessed 2012].
- 88.B. Vanholder, “Belgian Migrating Lepidoptera,” NERC Centre for Population Biology, Imperial College, The Global Population Dynamics Database v2.0; www.imperial.ac.uk/cpb/gpdd2/secure/register.aspx [accessed 2012].
- 89.M. L. Zettler, “Macrozoobenthos Baltic sea (1980–2005) as part of the IOW-Monitoring,” Institut für Ostseeforschung Warnemünde, Germany (2005). “IOW Macrozoobenthos monitoring Baltic Sea (1980–2005) (EurOBIS)”; www.iobis.org [accessed 2012].
- 90.K. Robinson, “CRRU (Cetacean Research and Rescue Unit) Cetacean sighting in Scotland waters” (2010); www.emodnet-biology.eu/data-catalog?module=dataset&dasid=2819 [accessed 2012].
- 91.N. A. Milchakova, V. G. Ryabogina, E. B. Chernyshova, “Macroalgae of the Crimean coastal zone (the Black Sea, 1967–2007),” Sevastopol, IBSS (2011); www.emodnet-biology.eu/data-catalog?module=dataset&dasid=2690 [accessed 2012].
- 92.W. Addinck, M. de Kluijver, “North Sea observations of Crustacea, Polychaeta, Echinodermata, Mollusca and some other groups between 1986 and 2003,” Expert Centre for Taxonomic Idenditification (ETI), the Netherlands (2003); www.emodnet-biology.eu/data-catalog?module=dataset&dasid=1037 [accessed 2012].
- 93.A. Naumov, “Benthos of the White Sea. A database,” White Sea Biological Station, Zoological Institute RAS; www.emodnet-biology.eu/data-catalog?module=dataset&dasid=2769 [accessed 2012].
- 94.E. L. Markhaseva, A. A. Golikov, T. A. Agapova, A. A. Beig, “Archives of the Arctic Seas Zooplankton 1” (1985); www.iobis.org [accessed 2012].
- 95.Henderson P. A., Magurran A. E., Data from: Direct evidence that density-dependent regulation underpins the temporal stability of abundant species in a diverse animal community. Dryad Data Repository 10.5061/dryad.3090c (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Henderson P. A., Magurran A. E., Direct evidence that density-dependent regulation underpins the temporal stability of abundant species in a diverse animal community. Proc. Biol. Sci. 281, 20141336 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.E. Woehler, “Seabirds of the Southern and South Indian Ocean - Australian Antarctic Data Centre”; www.iobis.org [accessed 2012].
- 98.R. Ostler, “Marine Nature Conservation Review (MNCR) and associated benthic marine data held and managed by JNCC - EurOBIS,” Joint Nature Conservation Committee, Centre for Ecology and hydrology, Aberdeenshire, UK; www.emodnet-biology.eu/data-catalog?module=dataset&dasid=621 [accessed 2012].
- 99.Southeast Fisheries Science Center, National Oceanic and Atmospheric Administration. NOAA Southeast Fishery Science Center (SEFSC) Fisheries Log Book System (FLS) Commercial Pelagic Logbook Data; www.iobis.org [accessed 2012].
- 100.P. Pugh, “Discovery Collections Midwater Database,” National Oceanography Centre, Southampton, UK (2000); https://gcmd.nasa.gov/KeywordSearch/Metadata.do?Portal=GCMD&MetadataView=Full&EntryId=OBIS.Discovery.Collections.Midwater [accessed 2012].
- 101.“South Western Pacific Regional OBIS Data Asteroid Subset,” NIWA (National Institute of Water and Atmospheric Research - New Zealand) MBIS (Marine Biodata Information System) accessed through South Western Pacific OBIS; www.iobis.org [accessed 2012].
- 102.D. Clark, B. Branton, “DFO Maritimes Research Vessel Trawl Surveys, OBIS Canada Digital Collections,” Bedford Institute of Oceanography, Dartmouth, Nova Scotia, Canada, OBIS Canada (2007); www.iobis.org [accessed 2012].
- 103.M. Reichert, “MARMAP Chevron Trap Survey 1990-2009,” SCDNR/NOAA MARMAP Program, SCDNR MARMAP Aggregate Data Surveys, The Marine Resources Monitoring, Assessment, and Prediction (MARMAP) Program, Marine Resources Research Institute, South Carolina Department of Natural Resources (2009); www.usgs.gov/obis-usa/data_search_and_access/participants.html [accessed 2012].
- 104.“Previous_fisheries_REVIZEE_Program,” Tropical and Subtropical Western South Pacific OBIS; www.iobis.org [accessed 2012].
- 105.“South Western Pacific Regional OBIS Data Bryozoan Subset,” South Western Pacific OBIS; www.iobis.org [accessed 2012].
- 106.“South Western Pacific Regional OBIS Data provider for the NIWA Marine Biodata Information System,” NIWA (National Institute of Water and Atmospheric Research - New Zealand) MBIS (Marine Biodata Information System), South Western Pacific OBIS; www.iobis.org [accessed 2012].
- 107.“CMarZ (Census of Marine Zooplankton)-Asia Database,” OBIS-SCAR-MarBIN; www.scarmarbin.be [accessed 2012].
- 108.“EPA’s EMAP Database,” U.S. Environmental Protection Agency, Environmental Monitoring and Assessment Program (EMAP); www.iobis.org [accessed 2012].
- 109.“The Observer Program database,” OBIS-USA North Pacific Groundfish Observer (North Pacific Research Board); www.iobis.org [accessed 2012].
- 110.Halpin P. N., Read A. J., Fujioka E., Best B. D., Donnelly B., Hazen L. J., Kot C., Urian K., Labrecque E., Dimatteo A., Cleary J., Good C., Crowder L. B., Hyrenbach K. D., OBIS-SEAMAP: The world data center for marine mammal, sea bird, and sea turtle distributions. Oceanography 22, 104–115 (2009). [Google Scholar]
- 111.R. G. B. Brown, D. N. Nettleship, P. Germain, C. E. Tull, T. Davis, Atlas of Eastern Canadian Seabirds (Canadian Wildlife Service, 1975). [Google Scholar]
- 112.Diamond A. W., Gaston A. J., Brown R. G. B., Converting PIROP counts of seabirds at sea to absolute densities. Can. Wildl. Serv. Progr. 164, 21 (1986). [Google Scholar]
- 113.F. Huettmann, An ecological GIS research application for the northern Atlantic—The PIROP database software, environmental data sets and the role of the internet/WWW, in Hypermedia im Umweltschutz Proceedings of Deutsche Gesellschaft für Informatik (GI) and Forschungsinstitut für anwendungsorientierte Wissensverarbeitung (FAW) Ulm, W.-F. Riekert, K. Tochtermann, Eds. (Umwelt-Informatik aktuell, Bd.17, Metropolis Verlag, 1998), pp. 213–217.
- 114.“PIROP Northwest Atlantic 1965–1992 - OBIS SEAMAP”; www.iobis.org [accessed 2012].
- 115.A. J. Read, P. N. Halpin, L. B. Crowder, B. D. Best, E. Fujioka, Eds., “OBIS-SEAMAP: Mapping marine mammals, birds and turtles” (2011); http://seamap.env.duke.edu [accessed 2012].
- 116.Yen P. P. W., Sydeman W. J., Bograd S. J., Hyrenbach K. D., Spring-time distributions of migratory marine birds in the southern California Current: Oceanic eddy associations and coastal habitat hotspots over 17 years. Deep Sea Res. Part 2 Oceanogr. Res. Pap. 53, 399–418 (2006). [Google Scholar]
- 117.J. Jahncke, C. Rintoul, “CalCOFI and NMFS Seabird and Marine Mammal Observation Data, 1987-2006,” California Cooperative Oceanic Fisheries Investigations (CalCOFI) and National Marine Fisheries Service (NMFS) cruises, 1987–2006 - OBIS SEAMAP (2006); www.iobis.org [accessed 2012].
- 118.C. Rintoul, B. Schlagenhauf-Langabeer, K. D. Hyrenbach, K. H. Morgan, W. J. Sydeman, Atlas of California Current Marine Birds and Mammals: Version 1 (unpublished report, PRBO Conservation Science, 2006). [Google Scholar]
- 119.Yen P. P. W., Sydeman W. J., Hyrenbach K. D., Marine bird and cetacean associations with bathymetric habitats and shallow-water topographies: Implications for trophic transfer and conservation. J. Mar. Syst. 50, 79–99 (2004). [Google Scholar]
- 120.“Bahamas Marine Mammal Research Organisation Opportunistic Sightings - OBIS SEAMAP”; www.iobis.org [accessed 2012].
- 121.M. Machete, R. S. Santos, Azores Fisheries Observer Program (POPA): A case study of the multidisciplinary use of observer data, in Proceedings of the 5th International Fisheries Observer Conference, Victoria, Canada, T. A. McVea, S. J. Kennelly, Eds. (2007).
- 122.Morato T., Varkey D. A., Damaso C., Machete M., Santos M., Prieto R., Santos R. S., Pitcher T. J., Evidence of a seamount effect on aggregating visitors. Mar. Ecol. Prog. Ser. 357, 23–32 (2008). [Google Scholar]
- 123.Amorim P., Figueiredo M., Machete M., Morato T., Martins A., Santos R. S., Spatial variability of seabird distribution associated with environmental factors: A case study of marine important bird areas in the Azores. ICES J. Mar. Sci. 66, 29–40 (2009). [Google Scholar]
- 124.“POPA cetacean, seabird, and sea turtle sightings in the Azores area 1998–2009 - OBIS SEAMAP”; www.iobis.org [accessed 2012].
- 125.“Marine Biological Sample Database, JAMSTEC,” OBIS_JAPAN; www.godac.jamstec.go.jp/bio-sample/index_e.html [accessed 2012].
- 126.M. K. Kennedy, J. A. Spry, “Atlantic Zone Monitoring Program Maritimes Region plankton datasets.” Fisheries and Oceans Canada - BioChem Archive, OBIS Canada, Bedford Institute of Oceanography, Dartmouth, Nova Scotia, Canada (2011); www.iobis.org [accessed 2012].
- 127.J. A. Boutillier, “Pacific Shrimp Trawl Survey.” ShrimpTrawl Bio Database, Fisheries and Oceans Canada PBS Shellfish Data Unit, OBIS Canada Digital Collections, Bedford Institute of Oceanography, Dartmouth, Nova Scotia, Canada (2007); www.iobis.org [accessed 2012].
- 128.“East Coast North America Strategic Assessment Project, Groundfish Atlas for the East Coast of North America”; www.iobis.org [accessed 2012].
- 129.E. J. Wade, “Snow crab research trawl survey database (Southern Gulf of St. Lawrence, Gulf region, Canada) from 1988 to 2010,” OBIS Canada, Bedford Institute of Oceanography, Dartmouth, Nova Scotia, Canada (2011); www.iobis.org [accessed 2012].
- 130.J. M. Tremblay, B. Branton, “DFO Maritimes Research Vessel Trawl Surveys Invertebrates,” OBIS Canada Digital Collections, Bedford Institute of Oceanography, Dartmouth, Nova Scotia, Canada (2007); www.iobis.org [accessed 2012].
- 131.“National Benthic Infaunal Database (NBID),” NOAA/NOS/NCCOS/CCEHBR/Coastal Ecology Program, NOAA’s Ocean Service, National Centers for Coastal Ocean Science (NCCOS) (2003); https://data.noaa.gov/dataset/national-benthic-infaunal-database-nbid [accessed 2012].
- 132.“NEFSC Benthic Database (OBIS-USA),” Northeast Fisheries Science Center, National Marine Fisheries Service, NOAA, U.S. Department of Commerce (2010); www.iobis.org [accessed 2012].
- 133.“Whale Catches in Southern Ocean,” OBIS - Australian Antarctic Data Centre; www.iobis.org [accessed 2013].
- 134.USGS Patuxent Wildlife Research Center, North American Breeding Bird Survey ftp data set, version 2014.0; ftp://ftpext.usgs.gov/pub/er/md/laurel/BBS/DataFiles/ [accessed 2013].
- 135.J. J. Moore, C. M. Howson, “Survey of the rocky shores in the region of Sullom Voe, Shetland, A report to SOTEAG from Aquatic Survey & Monitoring Ltd,” Cosheston, Pembrokeshire, 29 pp.; www.soteag.org.uk [accessed 2013].
- 136.“Scottish West Coast Survey for Commercial Fish Species 1985-2013”; http://datras.ices.dk/Data_products/Download/Download_Data_public.aspx [accessed 2013].
- 137.“ICES Baltic International Trawl Survey for Commercial Fish Species (1991-2013)”; http://datras.ices.dk/Data_products/Download/Download_Data_public.aspx [accessed 2013].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/7/e1700315/DC1
Supplementary Text
fig. S1. Time series of uncorrelated white noise.
fig. S2. Time series of uncorrelated white noise with a linear temporal trend.
fig. S3. Time series of uncorrelated white noise with a one-time perturbation.
fig. S4. Time series of random walk.
fig. S5. Time series of a random walk with a linear temporal trend.
fig. S6. Time series of a random walk with a one-time perturbation.
fig. S7. Time series of a regulated autoregressive process.
fig. S8. Time series of a regulated autoregressive process with a linear temporal trend.
fig. S9. Time series of a regulated autoregressive process with a one-time perturbation.
fig. S10. Logic tree for analysis and interpretation of community time series.
fig. S11. Benchmark analysis of ADF test.
fig. S12. Benchmark analysis of ADF test.
fig. S13. Benchmark analysis of ADF test.
fig. S14. Statistical tests for effects of latitudinal band (=climate), taxonomic group, and realm on standardized effect sizes (z scores) of species richness and total abundance.
table S1. Number of significant (P < 0.05) and nonsignificant test results for assemblage-level regulation of species richness or abundance.
table S2. Number of significant (P < 0.05) and nonsignificant test results for assemblage-level regulation of species richness or abundance.
table S3. Number of significant (P < 0.05) and nonsignificant test results for assemblage-level regulation of species richness or abundance.
table S4. Results of ADF tests for temperature time series.
table S5. Correlations of species richness and abundance with air or seawater temperature.
table S6. Variance ratio tests for patterns of compensatory fluctuations in total abundance.
table S7. Null model tests for the slope of the relationship between the observed number of colonizations at time t and the observed number of extinctions at time t + x.
table S8. Primary references and metadata for 59 assemblage time series data sets.


